Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - YUMANITY THERAPEUTICS, INC. | d249365d8k.htm |

Proteostasis Therapeutics, Inc. (Nasdaq: PTI) NACFC Update Conference Call October 27, 2016 8:00 a.m. ET Exhibit 99.1

Safe Harbor and Disclaimer To the extent that statements in this presentation are not historical facts, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Examples of forward-looking statements made in this presentation include, without limitation, statements regarding the status of, and our expected timelines for, our ongoing and expected pre-clinical and clinical development programs. Forward-looking statements made in this presentation involve substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by the forward-looking statements, and we therefore cannot assure you that our plans, intentions, expectations or strategies will be attained or achieved. Such risks and uncertainties include, without limitation, uncertainties inherent in the execution and completion of clinical trials, in the timing of availability of trial data, in the actions of regulatory agencies, and those set forth in our Registration Statement on Form S-1 effective in September 2016, and our other SEC filings. We assume no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to, among other items, disease incidence, market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of risk and uncertainty. New risks emerge from time to time, and neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such date after the date of this presentation. By attending or receiving this presentation you acknowledge you are solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and are solely responsible for forming your own view of the potential future performance of our business. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the Company or its securities. 2
Clinical Data and Timeline Update PTI-428 Phase 1 in CF patients and healthy volunteers (HV) recruiting well Data across both arms of study showed: Increases in CFTR mRNA and protein activity at threshold doses No safety or tolerability issues of concern Food effect study in HV showed increased exposure as expected and confirms consistent biomarker response HV arm: 3rd cohort of MAD expected to conclude in November CF arm: remaining SAD cohorts and all MAD cohorts expected to be completed in November 28 day POC to begin this quarter; FEV1 data expected in Q1 17

Phase 1 Initial Results Evaluating Safety, Tolerability, PK, and Biomarker Data Using PTI-428, A Novel CFTR Modulator Poster #187

Mechanism of Action
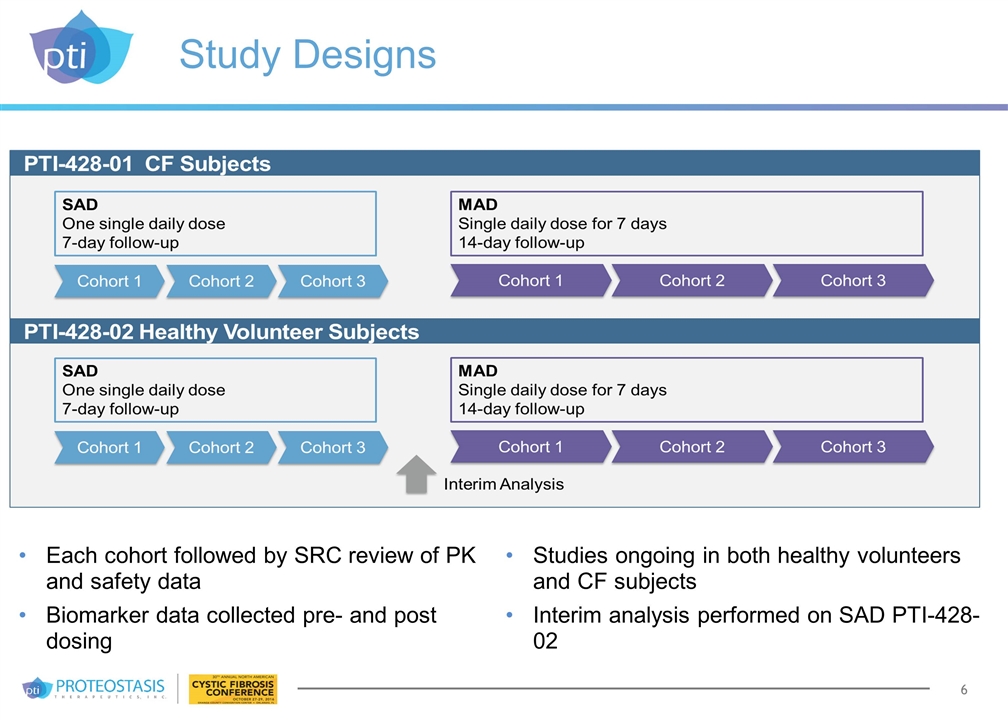
Study Designs Each cohort followed by SRC review of PK and safety data Biomarker data collected pre- and post dosing Studies ongoing in both healthy volunteers and CF subjects Interim analysis performed on SAD PTI-428-02
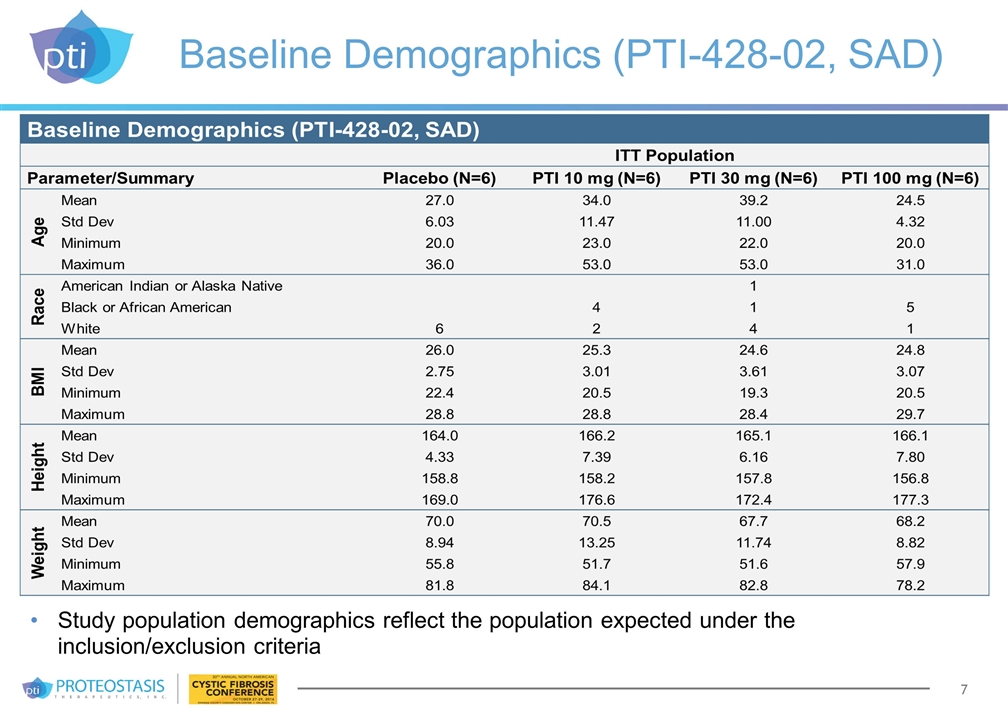
Baseline Demographics (PTI-428-02, SAD) Study population demographics reflect the population expected under the inclusion/exclusion criteria
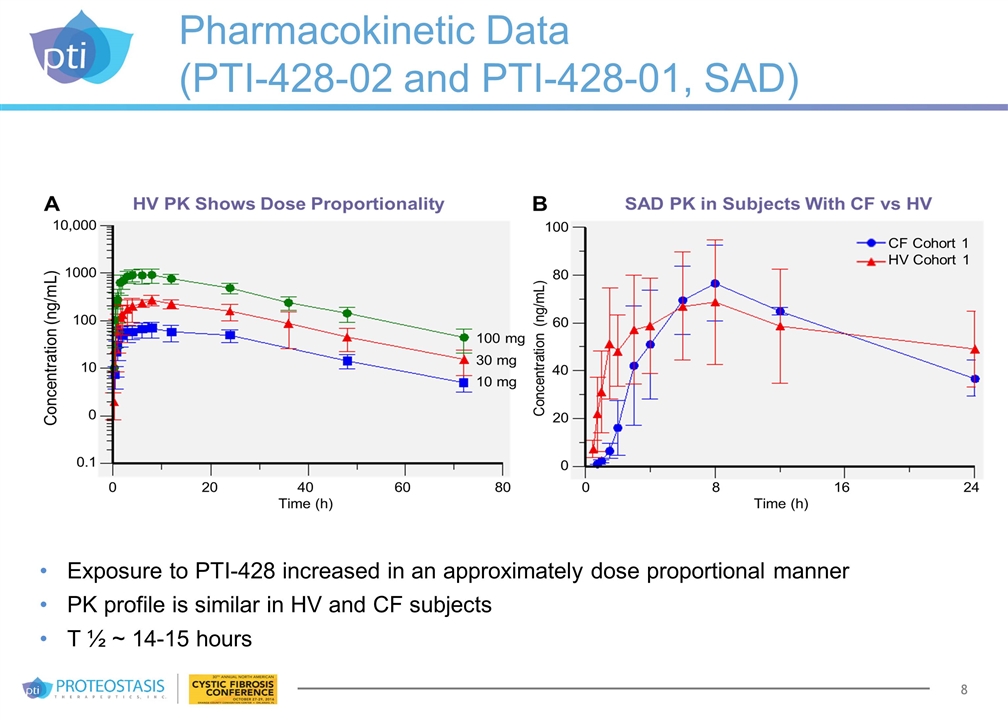
Pharmacokinetic Data (PTI-428-02 and PTI-428-01, SAD) Exposure to PTI-428 increased in an approximately dose proportional manner PK profile is similar in HV and CF subjects T ½ ~ 14-15 hours
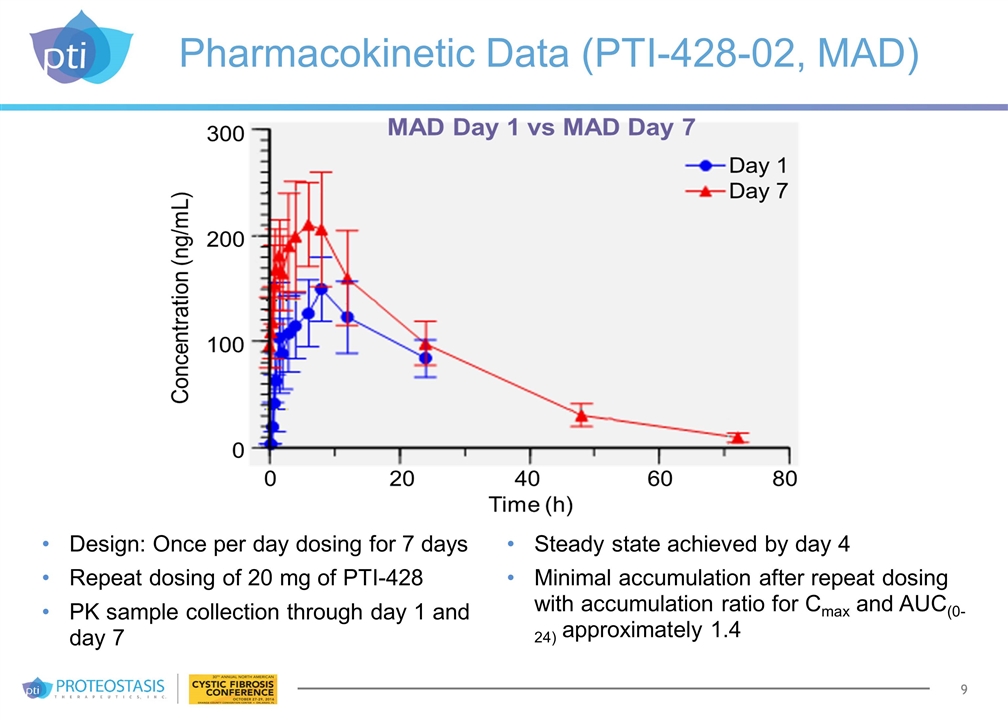
Pharmacokinetic Data (PTI-428-02, MAD) Design: Once per day dosing for 7 days Repeat dosing of 20 mg of PTI-428 PK sample collection through day 1 and day 7 Steady state achieved by day 4 Minimal accumulation after repeat dosing with accumulation ratio for Cmax and AUC(0-24) approximately 1.4
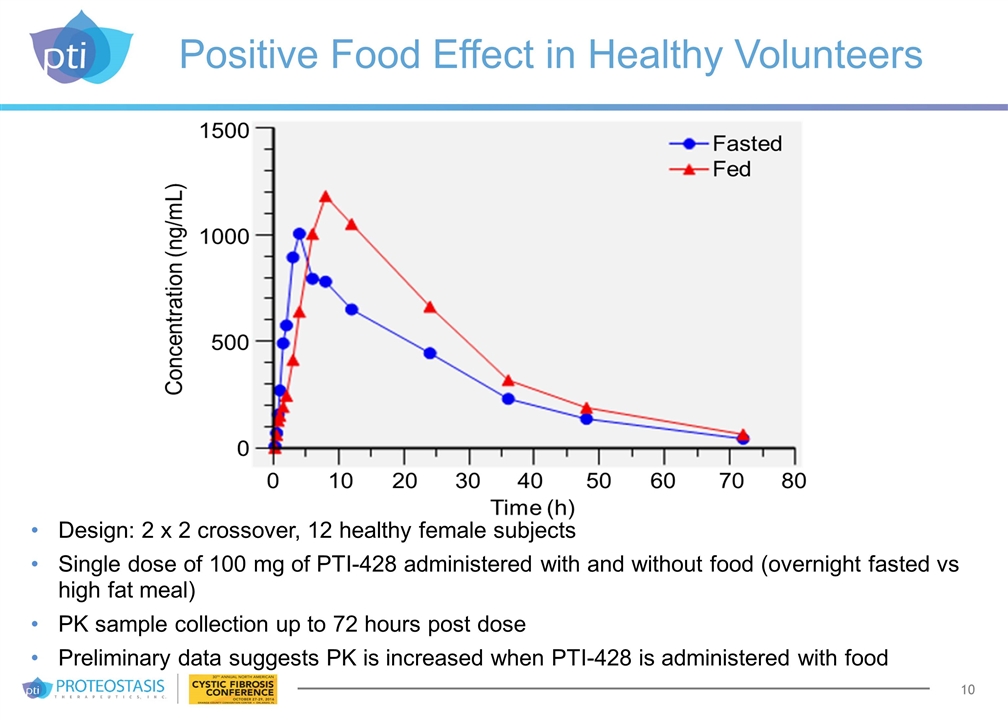
Positive Food Effect in Healthy Volunteers Design: 2 x 2 crossover, 12 healthy female subjects Single dose of 100 mg of PTI-428 administered with and without food (overnight fasted vs high fat meal) PK sample collection up to 72 hours post dose Preliminary data suggests PK is increased when PTI-428 is administered with food
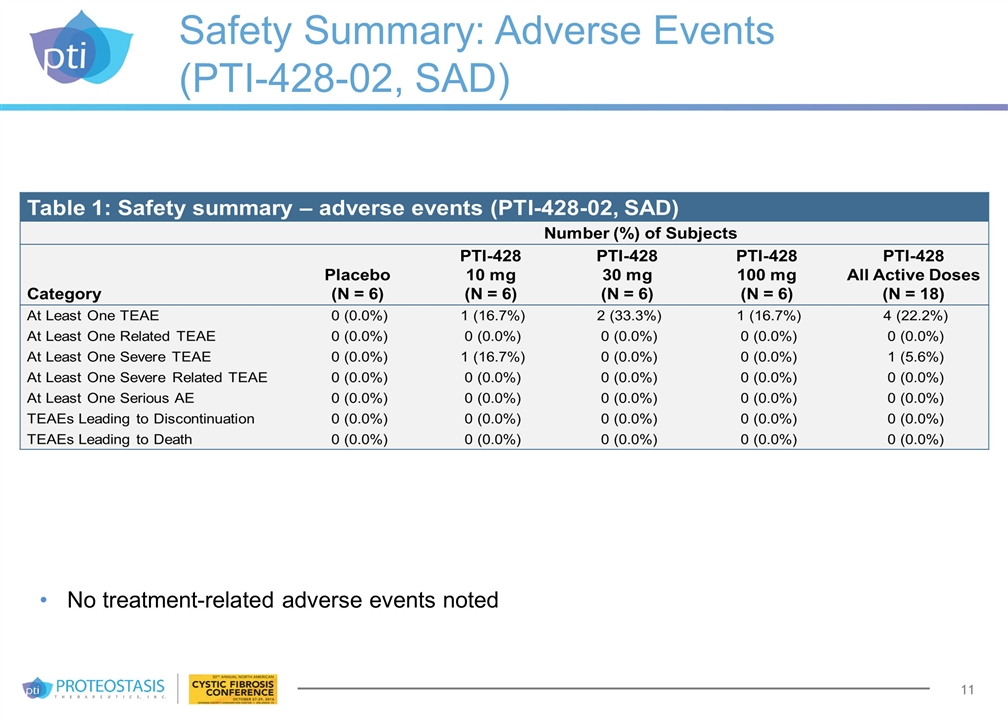
Safety Summary: Adverse Events (PTI-428-02, SAD) No treatment-related adverse events noted
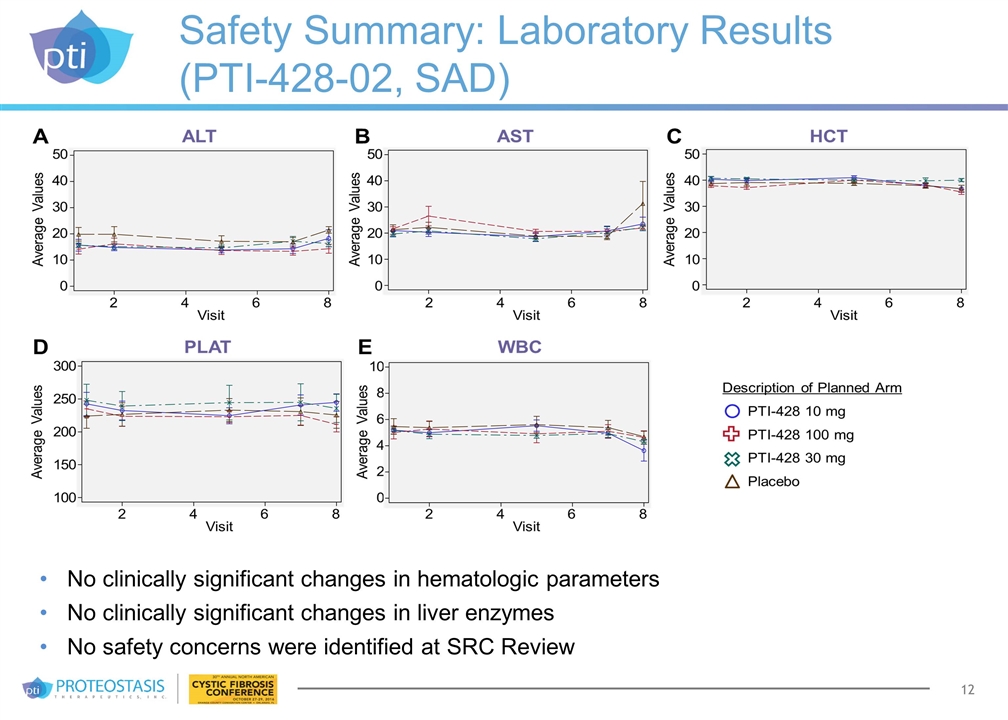
Safety Summary: Laboratory Results (PTI-428-02, SAD) No clinically significant changes in hematologic parameters No clinically significant changes in liver enzymes No safety concerns were identified at SRC Review
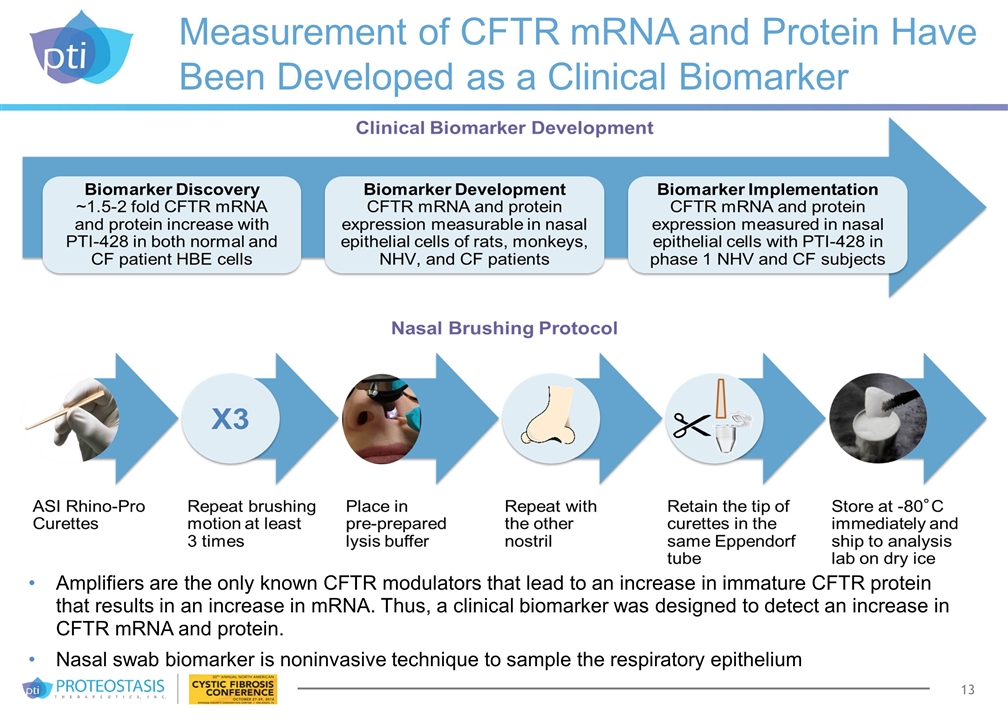
Measurement of CFTR mRNA and Protein Have Been Developed as a Clinical Biomarker Amplifiers are the only known CFTR modulators that lead to an increase in immature CFTR protein that results in an increase in mRNA. Thus, a clinical biomarker was designed to detect an increase in CFTR mRNA and protein. Nasal swab biomarker is noninvasive technique to sample the respiratory epithelium
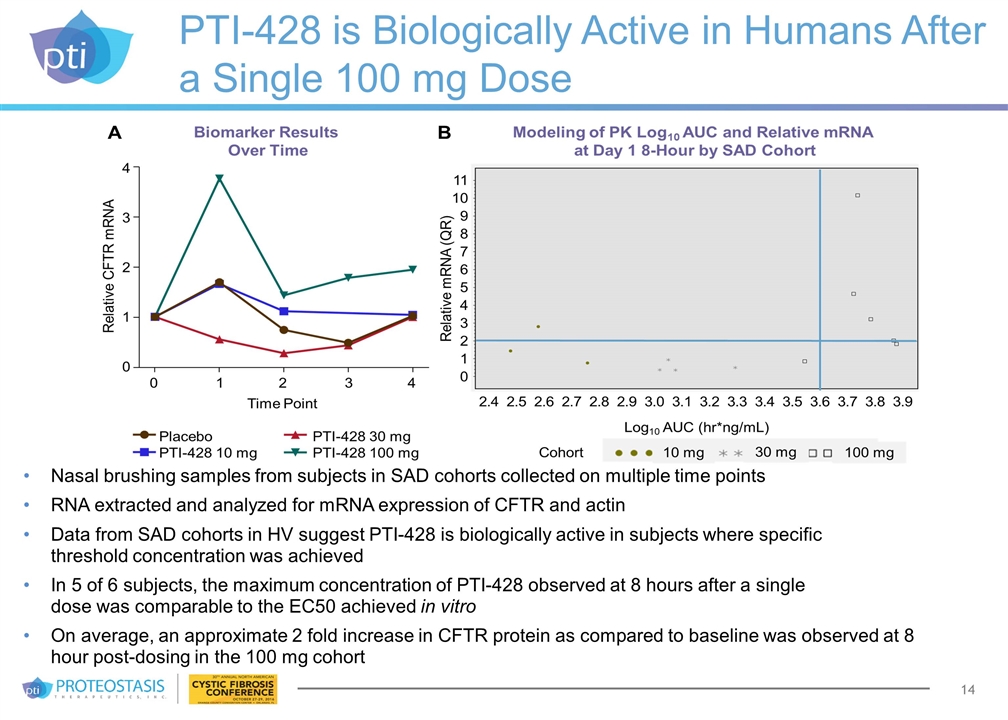
PTI-428 is Biologically Active in Humans After a Single 100 mg Dose Nasal brushing samples from subjects in SAD cohorts collected on multiple time points RNA extracted and analyzed for mRNA expression of CFTR and actin Data from SAD cohorts in HV suggest PTI-428 is biologically active in subjects where specific threshold concentration was achieved In 5 of 6 subjects, the maximum concentration of PTI-428 observed at 8 hours after a single dose was comparable to the EC50 achieved in vitro On average, an approximate 2 fold increase in CFTR protein as compared to baseline was observed at 8 hour post-dosing in the 100 mg cohort
Conclusions Amplifiers are a new class of CFTR modulators Exposure to PTI-428 increased in a dose proportional manner Steady state achieved by day 4 with repeated dosing PK profile was comparable in healthy volunteers and CF subjects No safety concerns identified based on SRC review of laboratory and AE data to date Two-fold increase in CFTR mRNA and protein observed 8 hours following a single dose of PTI-428

Novel CFTR Modulator Combination of Amplifier, Corrector, and Potentiator Provides Advantages Over Two Corrector- Based Combinations Poster #39 A Novel Modulator of CFTR Chloride Ion Mobility With a Distinct Mutation-Specific Profile to Existing CFTR Modulators Poster #67
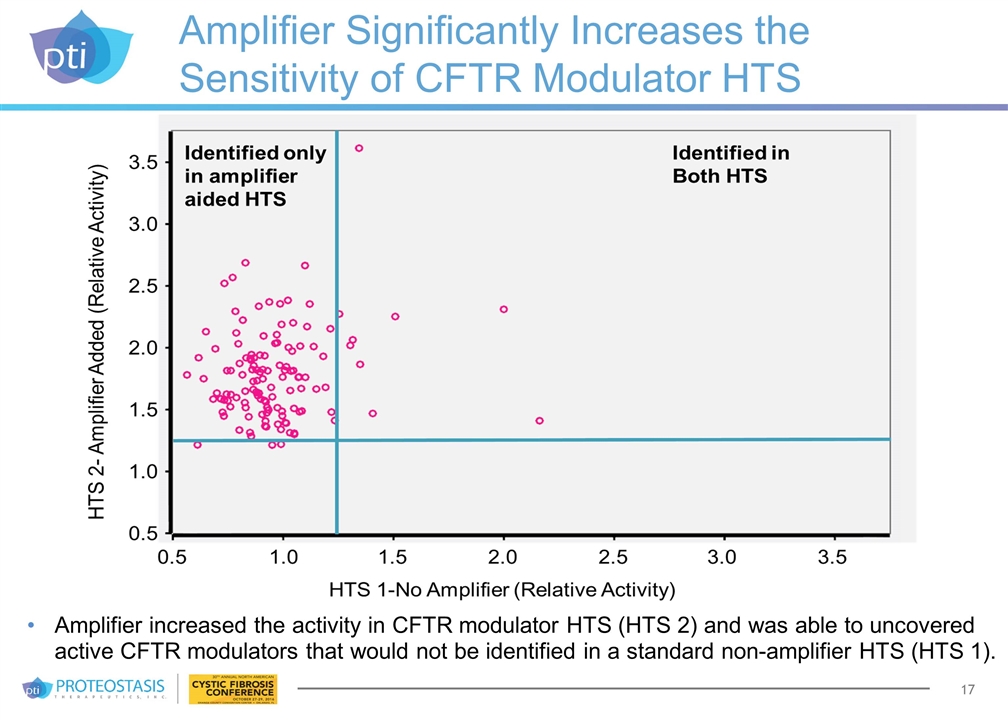
Amplifier Significantly Increases the Sensitivity of CFTR Modulator HTS Amplifier increased the activity in CFTR modulator HTS (HTS 2) and was able to uncovered active CFTR modulators that would not be identified in a standard non-amplifier HTS (HTS 1).
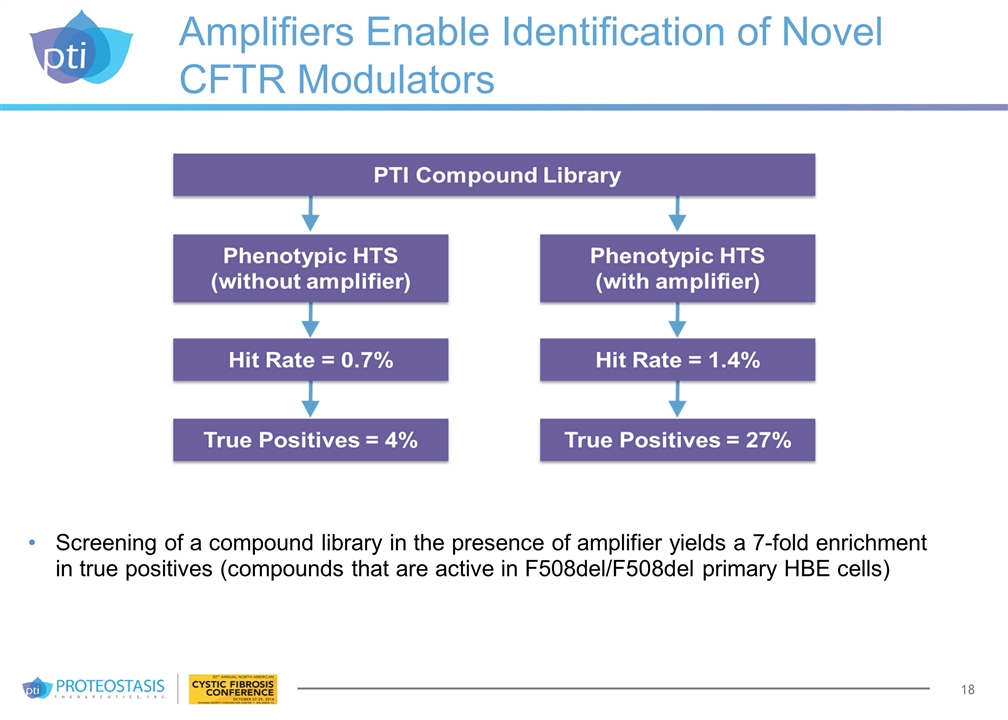
Amplifiers Enable Identification of Novel CFTR Modulators Screening of a compound library in the presence of amplifier yields a 7-fold enrichment in true positives (compounds that are active in F508del/F508del primary HBE cells)
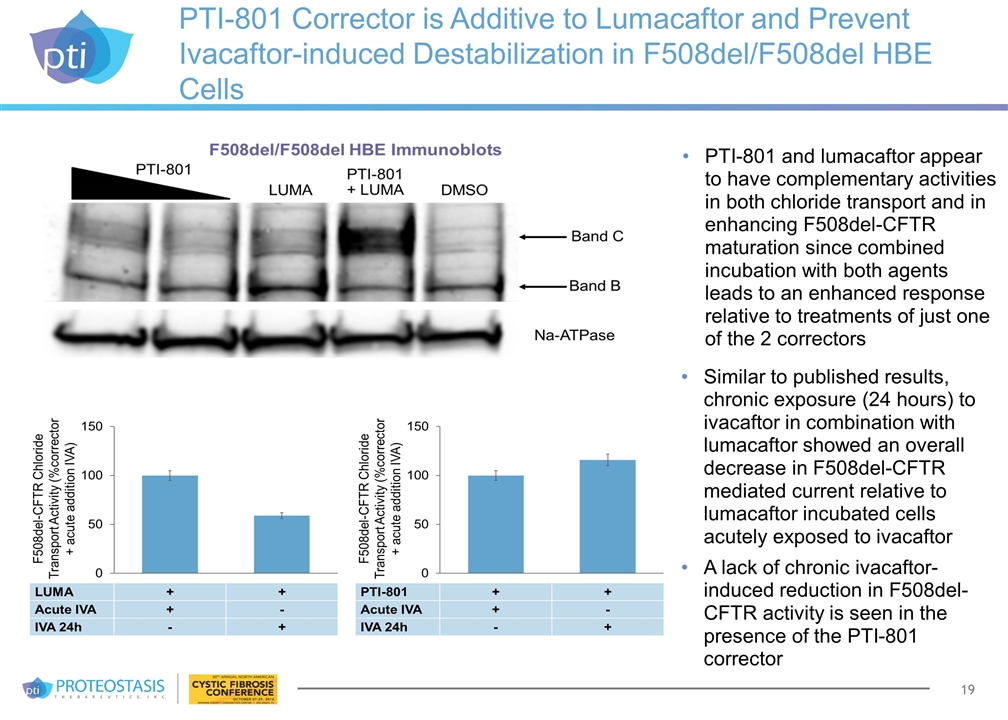
PTI-801 Corrector is Additive to Lumacaftor and Prevent Ivacaftor-induced Destabilization in F508del/F508del HBE Cells PTI-801 and lumacaftor appear to have complementary activities in both chloride transport and in enhancing F508del-CFTR maturation since combined incubation with both agents leads to an enhanced response relative to treatments of just one of the 2 correctors Similar to published results, chronic exposure (24 hours) to ivacaftor in combination with lumacaftor showed an overall decrease in F508del-CFTR mediated current relative to lumacaftor incubated cells acutely exposed to ivacaftor A lack of chronic ivacaftor-induced reduction in F508del-CFTR activity is seen in the presence of the PTI-801 corrector
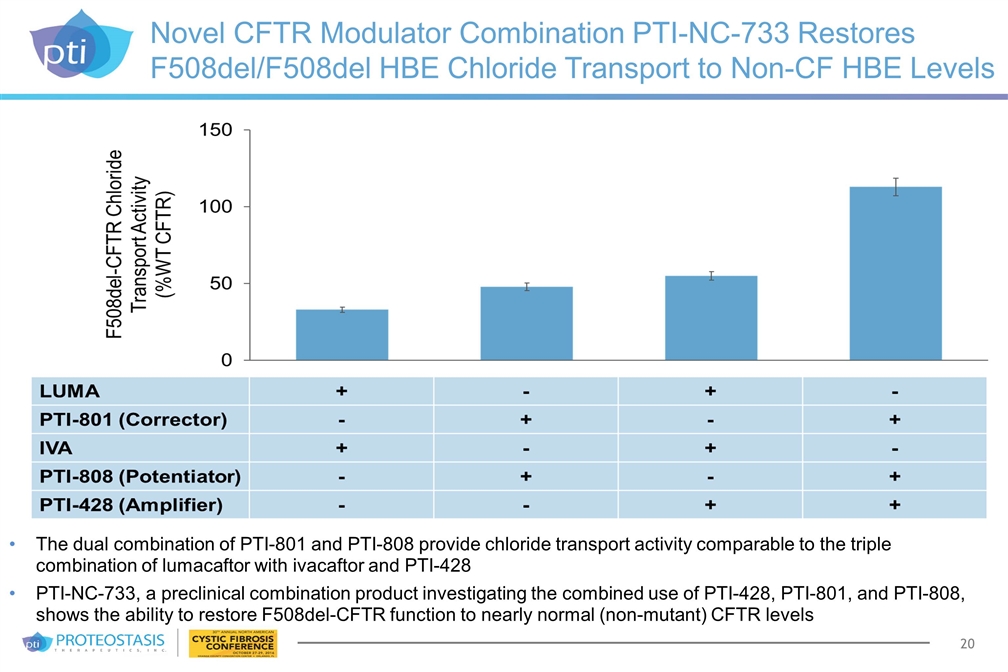
Novel CFTR Modulator Combination PTI-NC-733 Restores F508del/F508del HBE Chloride Transport to Non-CF HBE Levels The dual combination of PTI-801 and PTI-808 provide chloride transport activity comparable to the triple combination of lumacaftor with ivacaftor and PTI-428 PTI-NC-733, a preclinical combination product investigating the combined use of PTI-428, PTI-801, and PTI-808, shows the ability to restore F508del-CFTR function to nearly normal (non-mutant) CFTR levels
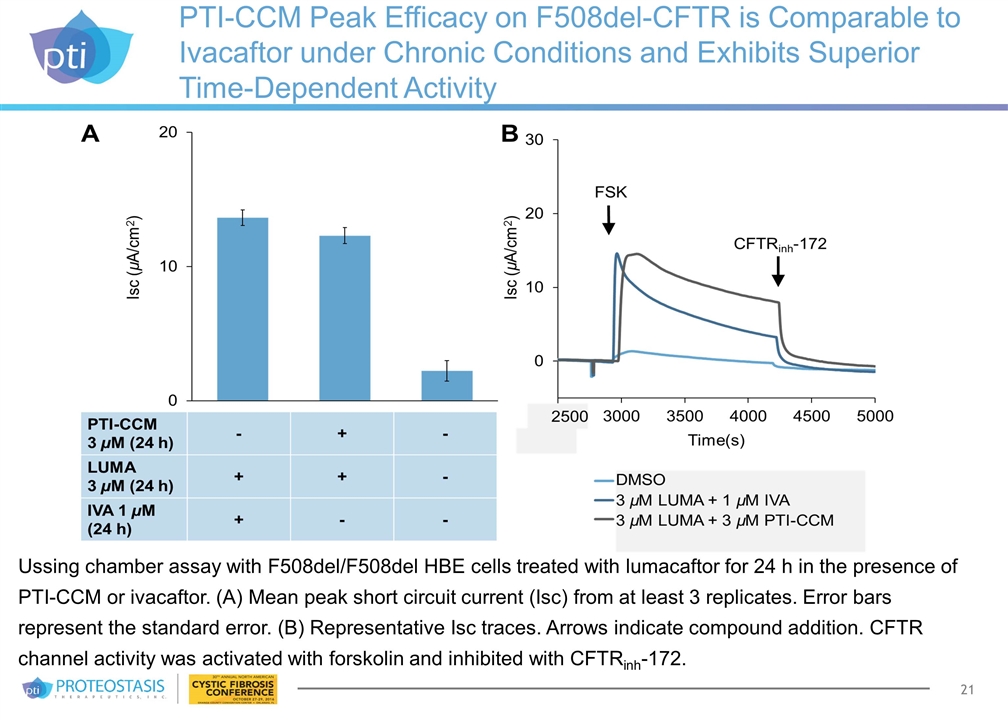
PTI-CCM Peak Efficacy on F508del-CFTR is Comparable to Ivacaftor under Chronic Conditions and Exhibits Superior Time-Dependent Activity Ussing chamber assay with F508del/F508del HBE cells treated with lumacaftor for 24 h in the presence of PTI-CCM or ivacaftor. (A) Mean peak short circuit current (Isc) from at least 3 replicates. Error bars represent the standard error. (B) Representative Isc traces. Arrows indicate compound addition. CFTR channel activity was activated with forskolin and inhibited with CFTRinh-172.
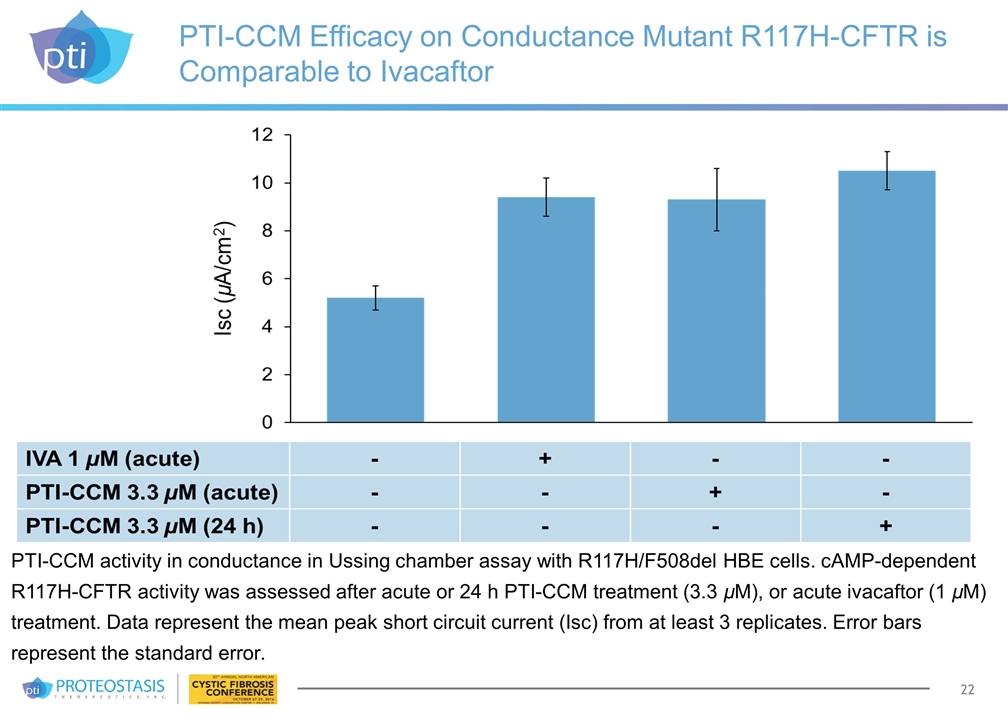
PTI-CCM Efficacy on Conductance Mutant R117H-CFTR is Comparable to Ivacaftor PTI-CCM activity in conductance in Ussing chamber assay with R117H/F508del HBE cells. cAMP-dependent R117H-CFTR activity was assessed after acute or 24 h PTI-CCM treatment (3.3 µM), or acute ivacaftor (1 µM) treatment. Data represent the mean peak short circuit current (Isc) from at least 3 replicates. Error bars represent the standard error.
Conclusions Amplifier is a powerful tool to identify CFTR modulators in an HTS PTI-801, a corrector, and PTI-808, a potentiator, could be additive to lumacaftor, consistent with the hypothesis that distinct mechanisms of action lead to a functional synergy PTI-801 prevented the chronic ivacaftor-mediated destabilization of F508del-CFTR in HBE Amplifier-based triple combinations have a potential therapeutic benefit in F508del homozygote and compound heterozygote CF patient populations PTI-NC-733, a novel combinational approach, restored F508del-CFTR to near normal CFTR activity in F508del/F508del HBE cells PTI-CCM is a novel CFTR modulator that has shown in-vitro activity when dosed continuously or acutely PTI-CCM F508del-CFTR efficacy was comparable to ivacaftor when dosed continuously and exhibited superior time-dependent activity PTI-CCM appears to possess a unique theratyping profile and likely could stimulate CFTR activity through a mechanism different from ivacaftor

Q&A
