Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Minerva Neurosciences, Inc. | d255045dex991.htm |
| 8-K - FORM 8-K - Minerva Neurosciences, Inc. | d255045d8k.htm |
MIN-101C03 Update with Open Label, Extension Phase Data October 26, 2016 Exhibit 99.2

This presentation contains certain forward-looking statements about Minerva Neurosciences that are intended to be covered by the safe harbor for “forward-looking statements” provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. Words such as “expect(s),” “feel(s),” “believe(s),” “will,” “may,” “anticipate(s)” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to: the benefits, efficacy and safety of our new formulations; whether studies performed on analogs or backups of our compounds are a good predictor of the clinical efficacy of our compounds; the timing and results of future clinical milestones; the timing of future clinical trials and results of such clinical trials; statements regarding our ability to successfully develop and commercialize our therapeutic products; our ability to expand our long-term business opportunities; our expectations regarding approval for our products by the U.S. Food and Drug Administration or equivalent foreign regulatory agencies; estimates regarding the market potential for our products; financial projections and estimates and their underlying assumptions; and future performance. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking statements. These risks and uncertainties include, but are not limited to: the benefits, efficacy and safety of our new formulations; whether analogs or backups of our compounds are a good predictor of the clinical efficacy of our compounds; the timing and results of future clinical milestones; the timing of future clinical trials and results of such clinical trials; whether any of our therapeutic candidates will advance further in the clinical trials process and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether any of our therapeutic candidates will be successfully marketed if approved; whether our therapeutic product discovery and development efforts will be successful; our ability to achieve the results contemplated by our collaboration agreements; the strength and enforceability of our intellectual property rights; competition from pharmaceutical and biotechnology companies; the development of and our ability to take advantage of the market for our therapeutic products; our ability to raise additional capital to fund our operations on terms acceptable to us; general economic conditions; and the other risk factors contained in our periodic and interim reports filed with the Securities and Exchange Commission which are available on the SEC website at www.sec.gov. Our audience is cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. All trademarks, trade names and service marks appearing in this presentation are the property of their respective owners. Forward-Looking Statement Safe-Harbor
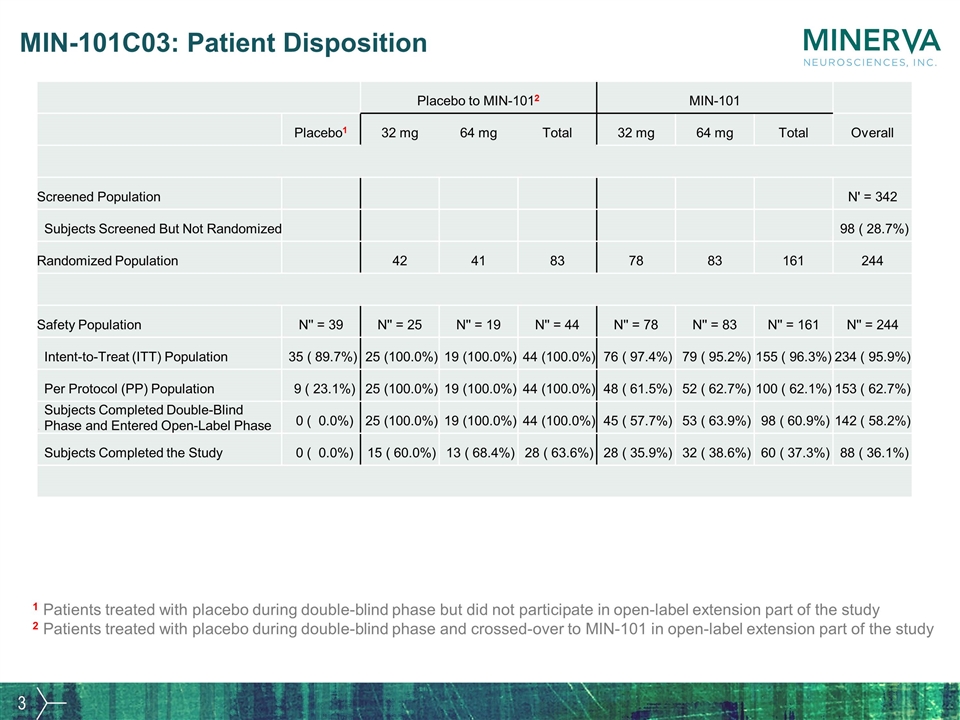
MIN-101C03: Patient Disposition Placebo to MIN-1012 MIN-101 Placebo1 32 mg 64 mg Total 32 mg 64 mg Total Overall Screened Population N' = 342 Subjects Screened But Not Randomized 98 ( 28.7%) Randomized Population 42 41 83 78 83 161 244 Safety Population N'' = 39 N'' = 25 N'' = 19 N'' = 44 N'' = 78 N'' = 83 N'' = 161 N'' = 244 Intent-to-Treat (ITT) Population 35 ( 89.7%) 25 (100.0%) 19 (100.0%) 44 (100.0%) 76 ( 97.4%) 79 ( 95.2%) 155 ( 96.3%) 234 ( 95.9%) Per Protocol (PP) Population 9 ( 23.1%) 25 (100.0%) 19 (100.0%) 44 (100.0%) 48 ( 61.5%) 52 ( 62.7%) 100 ( 62.1%) 153 ( 62.7%) Subjects Completed Double-Blind . Phase and Entered Open-Label Phase 0 ( 0.0%) 25 (100.0%) 19 (100.0%) 44 (100.0%) 45 ( 57.7%) 53 ( 63.9%) 98 ( 60.9%) 142 ( 58.2%) Subjects Completed the Study 0 ( 0.0%) 15 ( 60.0%) 13 ( 68.4%) 28 ( 63.6%) 28 ( 35.9%) 32 ( 38.6%) 60 ( 37.3%) 88 ( 36.1%) 1 Patients treated with placebo during double-blind phase but did not participate in open-label extension part of the study 2 Patients treated with placebo during double-blind phase and crossed-over to MIN-101 in open-label extension part of the study
Baseline for Patients who Crossed from Placebo to MIN-101 is Start of Open Label (Week 12)
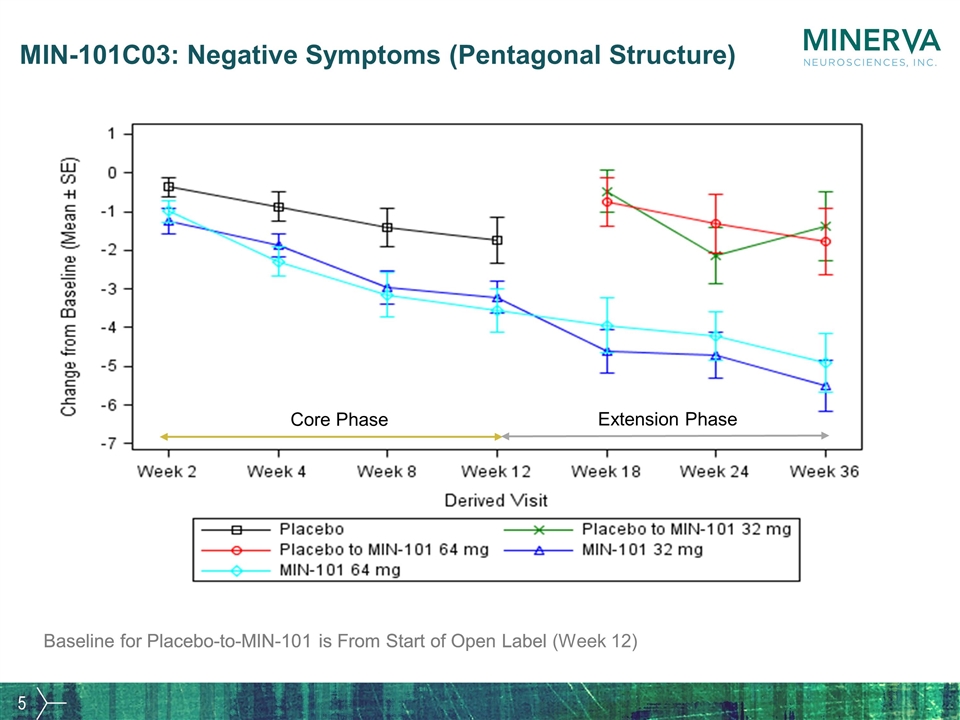
MIN-101C03: Negative Symptoms (Pentagonal Structure) Baseline for Placebo-to-MIN-101 is From Start of Open Label Core Phase Extension Phase Baseline for Placebo-to-MIN-101 is From Start of Open Label (Week 12)
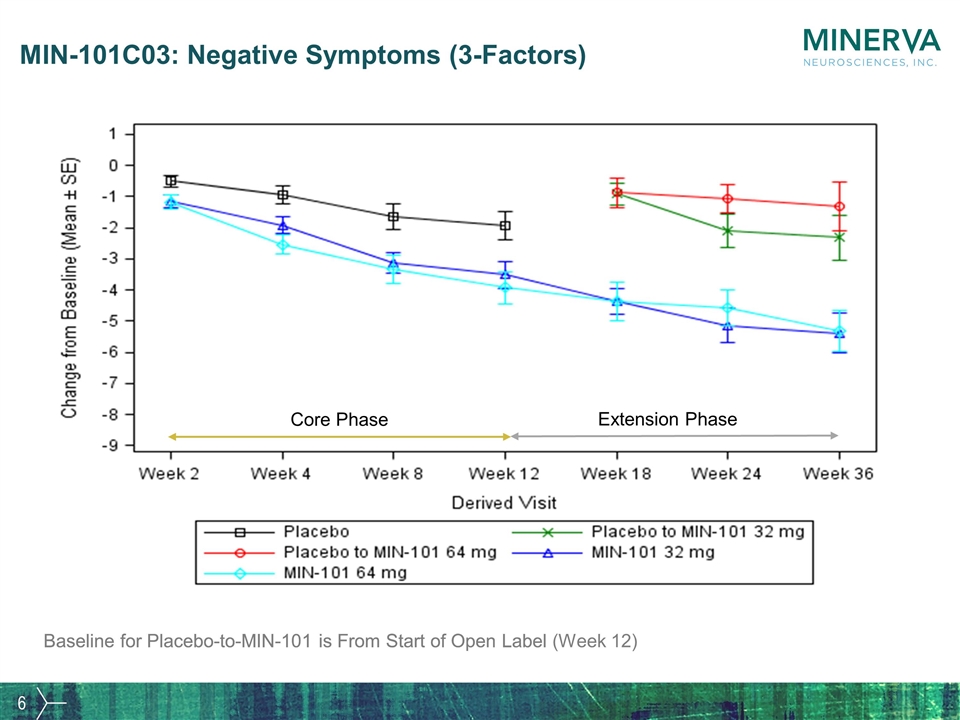
MIN-101C03: Negative Symptoms (3-Factors) Baseline for Placebo-to-MIN-101 is From Start of Open Label Core Phase Extension Phase Baseline for Placebo-to-MIN-101 is From Start of Open Label (Week 12)
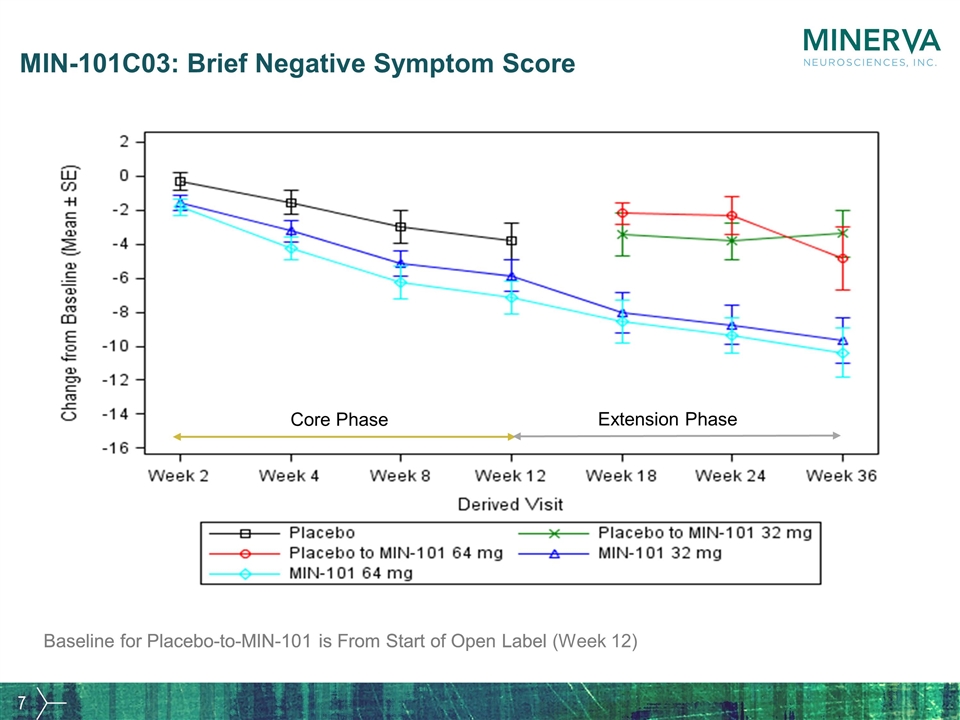
MIN-101C03: Brief Negative Symptom Score Baseline for Placebo-to-MIN-101 is From Start of Open Label Core Phase Extension Phase Baseline for Placebo-to-MIN-101 is From Start of Open Label (Week 12)
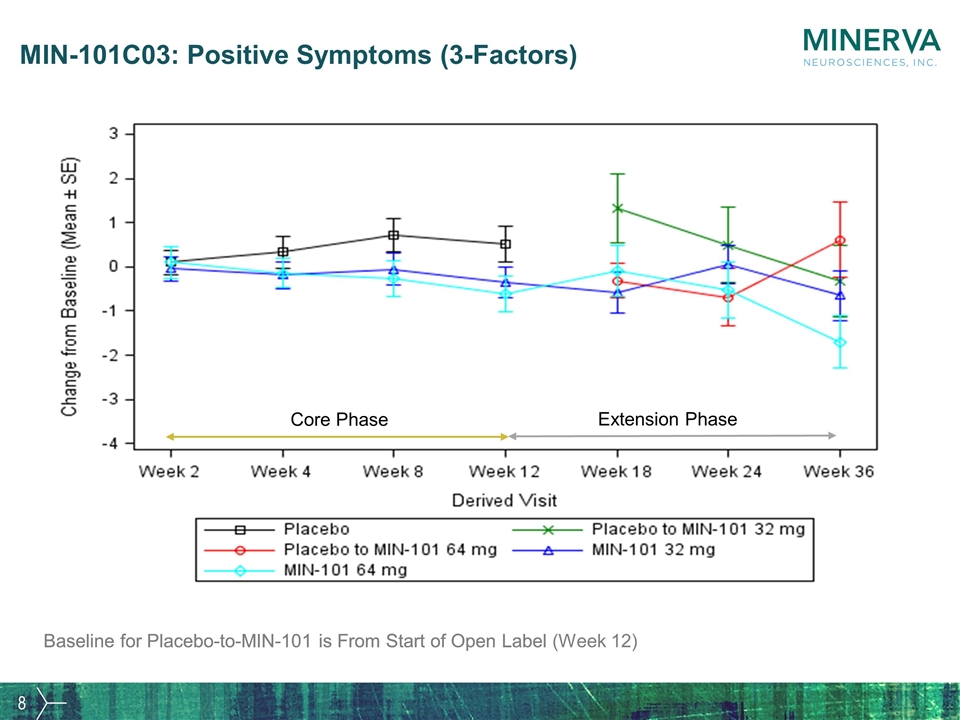
MIN-101C03: Positive Symptoms (3-Factors) Baseline for Placebo-to-MIN-101 is From Start of Open Label Core Phase Extension Phase Baseline for Placebo-to-MIN-101 is From Start of Open Label (Week 12)
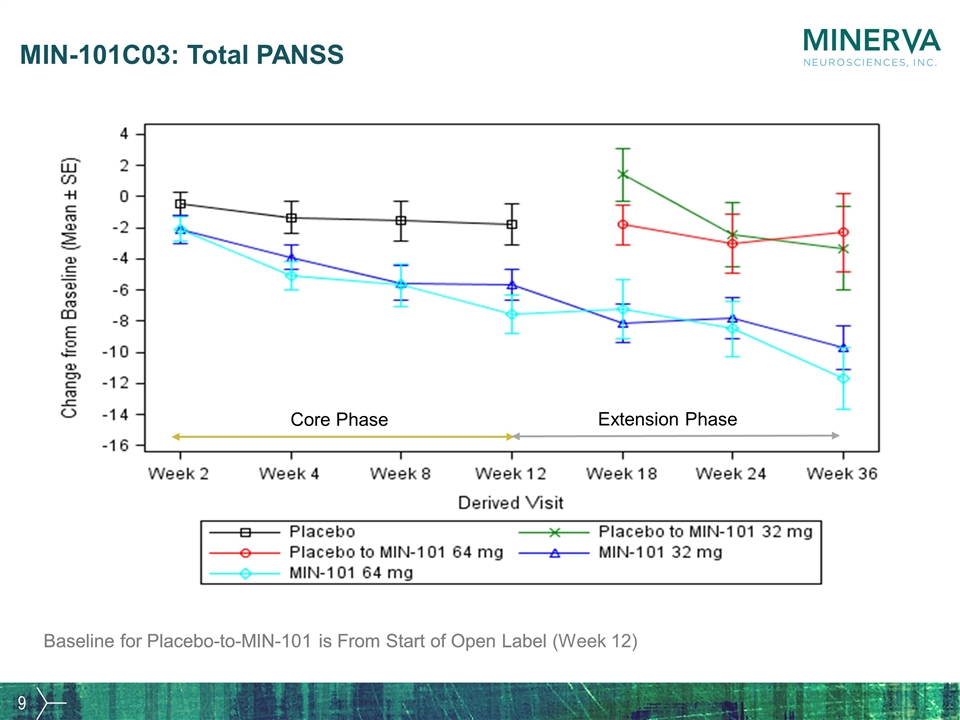
MIN-101C03: Total PANSS Baseline for Placebo-to-MIN-101 is From Start of Open Label Core Phase Extension Phase Baseline for Placebo-to-MIN-101 is From Start of Open Label (Week 12)

Safety Results Findings
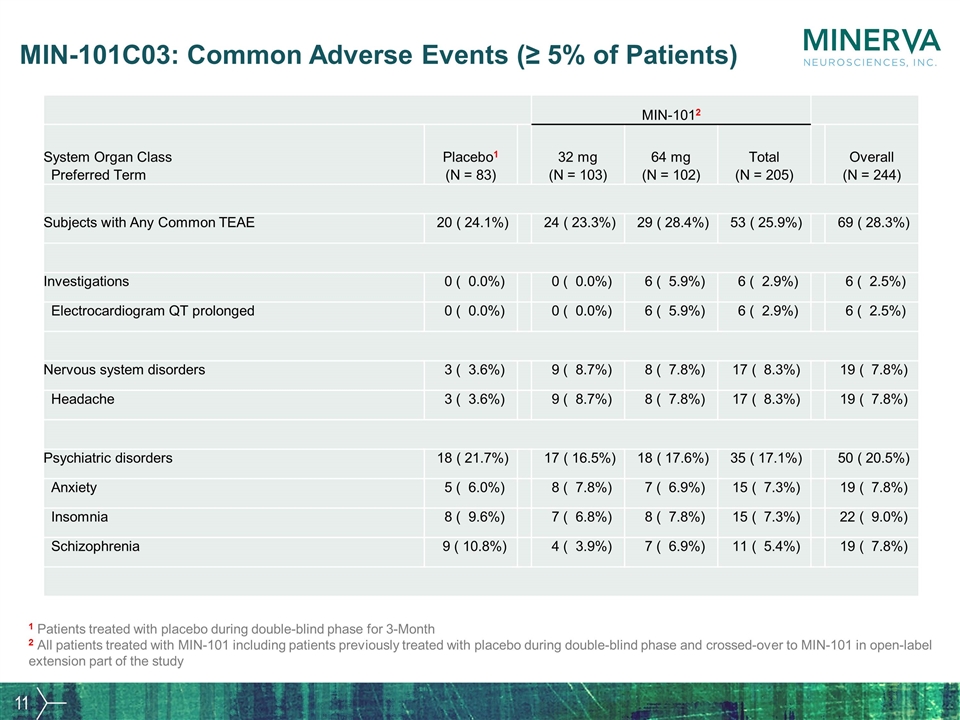
MIN-101C03: Common Adverse Events (≥ 5% of Patients) MIN-1012 System Organ Class Preferred Term Placebo1 (N = 83) 32 mg (N = 103) 64 mg (N = 102) Total (N = 205) Overall (N = 244) Subjects with Any Common TEAE 20 ( 24.1%) 24 ( 23.3%) 29 ( 28.4%) 53 ( 25.9%) 69 ( 28.3%) Investigations 0 ( 0.0%) 0 ( 0.0%) 6 ( 5.9%) 6 ( 2.9%) 6 ( 2.5%) Electrocardiogram QT prolonged 0 ( 0.0%) 0 ( 0.0%) 6 ( 5.9%) 6 ( 2.9%) 6 ( 2.5%) Nervous system disorders 3 ( 3.6%) 9 ( 8.7%) 8 ( 7.8%) 17 ( 8.3%) 19 ( 7.8%) Headache 3 ( 3.6%) 9 ( 8.7%) 8 ( 7.8%) 17 ( 8.3%) 19 ( 7.8%) Psychiatric disorders 18 ( 21.7%) 17 ( 16.5%) 18 ( 17.6%) 35 ( 17.1%) 50 ( 20.5%) Anxiety 5 ( 6.0%) 8 ( 7.8%) 7 ( 6.9%) 15 ( 7.3%) 19 ( 7.8%) Insomnia 8 ( 9.6%) 7 ( 6.8%) 8 ( 7.8%) 15 ( 7.3%) 22 ( 9.0%) Schizophrenia 9 ( 10.8%) 4 ( 3.9%) 7 ( 6.9%) 11 ( 5.4%) 19 ( 7.8%) 1 Patients treated with placebo during double-blind phase for 3-Month 2 All patients treated with MIN-101 including patients previously treated with placebo during double-blind phase and crossed-over to MIN-101 in open-label extension part of the study
