Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - PIERIS PHARMACEUTICALS, INC. | d276876dex992.htm |
| 8-K - FORM 8-K - PIERIS PHARMACEUTICALS, INC. | d276876d8k.htm |
| Exhibit 99.1
|
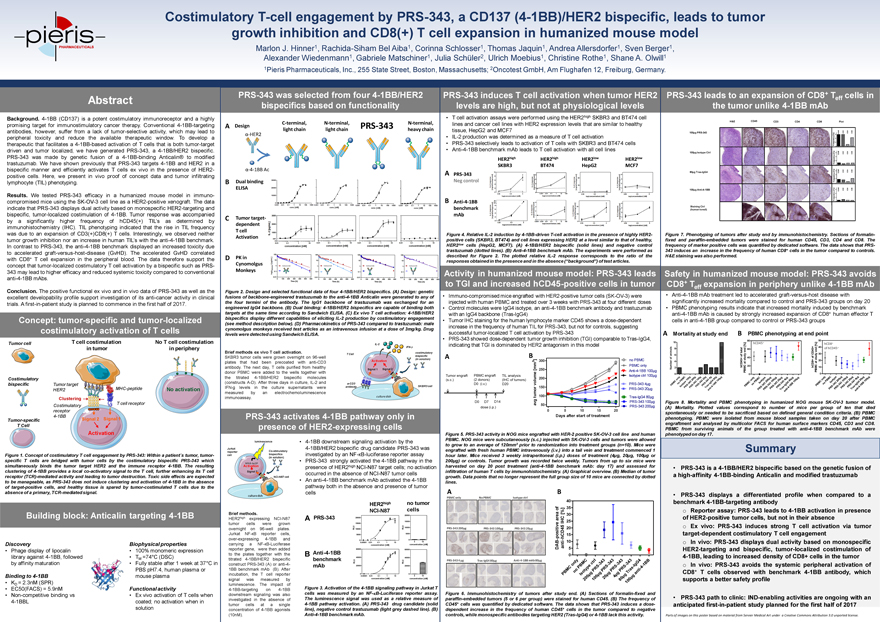
Costimulatory T-cell engagement by PRS-343, a CD137 (4-1BB)/HER2 bispecific, leads to tumor growth inhibition and CD8(+) T cell expansion in humanized mouse model Marlon J. Hinner1, Rachida-Siham Bel Aiba1, Corinna Schlosser1, Thomas Jaquin1, Andrea Allersdorfer1, Sven Berger1, Alexander Wiedenmann1, Gabriele Matschiner1, Julia Schüler2, Ulrich Moebius1, Christine Rothe1, Shane A. Olwill1 1Pieris Pharmaceuticals, Inc., 255 State Street, Boston, Massachusetts; 2Oncotest GmbH, Am Flughafen 12, Freiburg, Germany. Abstract Background. 4-1BB (CD137) is a potent costimulatory immunoreceptor and a highly promising target for immunostimulatory cancer therapy. Conventional 4-1BB-targeting antibodies, however, suffer from a lack of tumor-selective activity, which may lead to peripheral toxicity and reduce the available therapeutic window. To develop a therapeutic that facilitates a 4-1BB-based activation of T cells that is both tumor-target driven and tumor localized, we have generated PRS-343, a 4-1BB/HER2 bispecific. PRS-343 was made by genetic fusion of a 4-1BB-binding Anticalin® to modified trastuzumab. We have shown previously that PRS-343 targets 4-1BB and HER2 in a bispecific manner and efficiently activates T cells ex vivo in the presence of HER2-positive cells. Here, we present in vivo proof of concept data and tumor infiltrating lymphocyte (TIL) phenotyping. Results. We tested PRS-343 efficacy in a humanized mouse model in immuno-compromised mice using the SK-OV-3 cell line as a HER2-positive xenograft. The data indicate that PRS-343 displays dual activity based on monospecific HER2-targeting and bispecific, tumor-localized costimulation of 4-1BB. Tumor response was accompanied by a significantly higher frequency of hCD45(+) TIL’s as determined by immunohistochemistry (IHC). TIL phenotyping indicated that the rise in TIL frequency was due to an expansion of CD3(+)CD8(+) T cells. Interestingly, we observed neither tumor growth inhibition nor an increase in human TIL’s with the anti-4-1BB benchmark. In contrast to PRS-343, the anti-4-1BB benchmark displayed an increased toxicity due to accelerated graft-versus-host-disease (GvHD). The accelerated GvHD correlated with CD8+ T cell expansion in the peripheral blood. The data therefore support the concept that tumor-localized costimulatory T cell activation by a bispecific such as PRS-343 may lead to higher efficacy and reduced systemic toxicity compared to conventional anti-4-1BB mAbs. Conclusion. The positive functional ex vivo and in vivo data of PRS-343 as well as the excellent developability profile support investigation of its anti-cancer activity in clinical trials. A first-in-patient study is planned to commence in the first half of 2017. PRS-343 was selected from four 4-1BB/HER2 bispecifics based on functionality C-terminal, N-terminal, N-terminal, A Design PRS-343 light chain light chain heavy chain -HER2 -4-1BB Ac B Dual binding ELISA C Tumor target-dependent T cell Activation D PK in Cynomolgus Monkeys Figure 2. Design and selected functional data of four 4-1BB/HER2 bispecifics. (A) Design: genetic fusions of backbone-engineered trastuzumab to the anti-4-1BB Anticalin were generated to any of the four termini of the antibody. The IgG1 backbone of trastuzumab was exchanged for an engineered IgG4 backbone. (B) Dual binding: 4-1BB/HER2 bispecifics are capable of binding both targets at the same time according to Sandwich ELISA. (C) Ex vivo T cell activation: 4-1BB/HER2 bispecifics display different capabilities of eliciting IL-2 production by costimulatory engagement (see method description below). (D) Pharmacokinetics of PRS-343 compared to trastuzumab: male cynomolgus monkeys received test articles as an intravenous infusion at a dose of 3mg/kg. Drug levels were detected using Sandwich ELISA. IL-2 IFN-g Brief methods ex vivo T cell activation. costimulatory T Cell SKBR3 tumor cells were grown overnight on 96-well bispecific (in solution) plates that had been precoated with anti-CD3 Activation antibody. The next day, T cells purified from healthy donor PBMC were added to the wells together with Signal 1 Signal 2 the titrated 4-1BB/HER2 bispecific molecules (constructs A-D). After three days in culture, IL-2 and a-CD3 IFN-g levels in the culture supernatants were antibody SKBR3 cell measured by an electrochemoluminescence immunoassay. culture dish PRS-343 induces T cell activation when tumor HER2 levels are high, but not at physiological levels T cell activation assays were performed using the HER2high SKBR3 and BT474 cell lines and cancer cell lines with HER2 expression levels that are similar to healthy tissue, HepG2 and MCF7 IL-2 production was determined as a measure of T cell activation PRS-343 selectively leads to activation of T cells with SKBR3 and BT474 cells Anti-4-1BB benchmark mAb leads to T cell activation with all cell lines HER2high HER2high HER2low HER2low SKBR3 BT474 HepG2 MCF7 A PRS-343 Neg control B Anti-4-1BB benchmark mAb Figure 4. Relative IL-2 induction by 4-1BB-driven T-cell activation in the presence of highly HER2-positive cells (SKBR3, BT474) and cell lines expressing HER2 at a level similar to that of healthy, HER2low cells (HepG2, MCF7). (A) 4-1BB/HER2 bispecific (solid lines) and negative control trastuzumab (dotted lines). (B) Anti-4-1BB benchmark mAb. The experiments were performed as described for Figure 2. The plotted relative IL-2 response corresponds to the ratio of the responses obtained in the presence and in the absence (“background”) of test articles. PRS-343 leads to an expansion of CD8+ T cells in effthe tumor unlike 4-1BB mAb H&E CD45 CD3 CD4 CD8 Plot 100µg PRS-343 ] CD45 CD3 CD4 CD8[% 30ency 20requ 10F0] 100µg Isotype Ctrl [% 30 CD3 CD4 CD8 CD4520Frequency 100] [% 3080µg Tras-IgG4 CD45 CD3 CD4 CD8ency 20requ 10F0] 100µg Anti-4-1BB [% 30 CD3 CD4 CD8cy CD4520Frequen 100Staining Ctrl (human tonsil) Figure 7. Phenotyping of tumors after study end by immunohistochemistry. Sections of formalin-fixed and paraffin-embedded tumors were stained for human CD45, CD3, CD4 and CD8. The frequency of marker positive cells was quantified by dedicated software. The data shows that PRS-343 induces an increase in the frequency of human CD8+ cells in the tumor compared to controls. H&E staining was also performed. Concept: tumor-specific and tumor-localized costimulatory activation of T cells Tumor cell T cell costimulation No T cell costimulation in tumor in periphery Costimulatory bispecific Tumor target HER2 MHC-peptide No activation Clustering T cell receptor Costimulatory receptor 4-1BB Tumor-specific Signal 2 Signal 1 T Cell Activation Figure 1. Concept of costimulatory T cell engagement by PRS-343: Within a patient´s tumor, tumor-specific T cells are bridged with tumor cells by the costimulatory bispecific PRS-343 which simultaneously binds the tumor target HER2 and the immune receptor 4-1BB. The resulting clustering of 4-1BB provides a local co-activatory signal to the T cell, further enhancing its T cell receptor (TCR)-mediated activity and leading to tumor destruction. Toxic side effects are expected to be manageable, as PRS-343 does not induce clustering and activation of 4-1BB in the absence of target-positive cells, and healthy tissue is spared by tumor-costimulated T cells due to the absence of a primary, TCR-mediated signal. Building block: Anticalin targeting 4-1BB Discovery Biophysical properties Phage display of lipocalin 100% monomeric expression library against 4-1BB, followed TM =74°C (DSC) by affinity maturation Fully stable after 1 week at 37°C in PBS pH7.4, human plasma or Binding to 4-1BB mouse plasma KD = 2.3nM (SPR) EC50(FACS) = 5.9nM Functional activity Non-competitive binding vs Ex vivo activation of T cells when 4-1BBL coated; no activation when in solution PRS-343 activates 4-1BB pathway only in presence of HER2-expressing cellsluminescence4-1BB downstream signaling activation by theJurkat4-1BB/HER2 bispecific drug candidate PRS-343 wasreporterCo-stimulatorycellbispecificsinvestigated by an NF-?B-luciferase reporter assay(in solution)PRS-343 strongly activated the 4-1BB pathway in theNFkB-luc2PActivationpresenceof HER2high NCI-N87 target cells; no activationSignal 2occurred in the absence of NCI-N87 tumor cellsNCI-N87 cellAn anti-4-1BB benchmark mAb activatedthe 4-1BBpathway both in the absence and presence of tumorcellsculture dishHER2highno tumorBrief methods.NCI-N87cellsHER2highexpressingNCI-N87APRS-343tumorcellsweregrownovernighton96 -wellplates.JurkatNF-?Breportercells,over-expressing4-1BBandcarryingaNF-?B-Luciferasereporter gene,were then addedto theplates together with theBAnti -4-1BBtitrated4-1BB/HER2bispecificbenchmarkconstruct PRS-343 (A) or anti-4 -mAb1BB benchmark mAb(B). Afterincubation, theTcellreportersignalwasmeasuredbyluminescence.The impactof4-1BB-targetingon4 -1BBFigure 3. Activation of the 4-1BB signaling pathway in Jurkat Tdownstream signaling was alsocells was measured by an NF-kB-Luciferase reporter assay.investigated intheabsenceofThe luminescence signal was used as a relative measure oftumorcellsatasingle4-1BB pathway activation. (A) PRS-343drug candidate (solidconcentrationof4-1BB agonistsline), negative control trastuzumab (light grey dashed line). (B)(10nM).Anti-4-1BB benchmark mAb.Activity in humanized mouse model: PRS-343 leads to TGI and increased hCD45-positive cells in tumor Immuno-compromised mice engrafted with HER2-positive tumor cells (SK-OV-3) were injected with human PBMC and treated over 3 weeks with PRS-343 at four different doses Control molecules were IgG4 isotype, an anti-4-1BB benchmark antibody and trastuzumab with an IgG4 backbone (Tras-IgG4) Tumor IHC staining for the human lymphocyte marker CD45 shows a dose-dependent increase in the frequency of human TIL for PRS-343, but not for controls, suggesting successful tumor-localized T cell activation by PRS-343 PRS-343 showed dose-dependent tumor growth inhibition (TGI) comparable to Tras-IgG4, indicating that TGI is dominated by HER2 antagonism in this model A B 3 ] 300 no PBMC [m m PBMC only 250 Anti-4-1BB 100µg Isotype ctrl 100µg lume 200 v o PRS-343 4µg 150 mor PRS-343 20µg tu 100 Tras-IgG4 80µg g v PRS-343 100µg a 50 PRS-343 200µg0 5 10 15 20Days after start of treatmentFigure 5. PRS-343 activity in NOG mice engrafted with HER-2 positive SK-OV-3 cell line and human PBMC. NOG mice were subcutaneously (s.c.) injected with SK-OV-3 cells and tumors were allowed to grow to an average of 120mm3 prior to randomization into treatment groups (n=10). Mice were engrafted with fresh human PBMC intravenously (i.v.) into a tail vein and treatment commenced 1 hour later. Mice received 3 weekly intraperitoneal (i.p.) doses of treatment (4µg, 20µg, 100µg or 200µg) or controls. Tumor growth was recorded twice weekly. Tumors from up to six mice were harvested on day 20 post treatment (anti-4-1BB benchmark mAb: day 17) and assessed for infiltration of human T cells by immunohistochemistry. (A) Graphical overview. (B) Median of tumor growth. Data points that no longer represent the full group size of 10 mice are connected by dotted lines. A B PBMC only No PBMC Isotype ctrl 40 f 35 o %] a [ 30 e r C a H 25 eI5 20PRS-343 200µg PRS-343 100µg PRS-343 20µg tiv0 0 2 0 02 1 8 0 1Figure 6. Immunohistochemistry of tumors after study end. (A) Sections of formalin-fixed and paraffin-embedded tumors (5 or 6 per group) were stained for human CD45. (B) The frequency of CD45+ cells was quantified by dedicated software. The data shows that PRS-343 induces a dose-dependent increase in the frequency of human CD45+ cells in the tumor compared to negative controls, while monospecific antibodies targeting HER2 (Tras-IgG4) or 4-1BB lack this activity. Safety in humanized mouse model: PRS-343 avoids CD8+ T expansion in periphery unlike 4-1BB mAb eff Anti-4-1BB mAb treatment led to accelerated graft-versus-host disease with significantly increased mortality compared to control and PRS-343 groups on day 20 PBMC phenotyping results indicate that increased mortality induced by benchmark anti-4-1BB mAb is caused by strongly increased expansion of CD8+ human effector T cells in anti-4-1BB group compared to control or PRS-343 groups Mortality at study end B PBMC phenotyping at end point 80 5010 hCD45+ + [%] [%] hCD8 + 40ls l of hCD45+ 8 nd 60 5 endetota nima y CD4 30a 6 of + tud 40 ofof sstudy 204 atathCD45 20 hCD8+Number 2 PBMC PBMC 10Figure 8. Mortality and PBMC phenotyping in humanized NOG mouse SK-OV-3 tumor model. (A) Mortality. Plotted values correspond to number of mice per group of ten that died spontaneously or needed to be sacrificed based on defined general condition criteria. (B) PBMC phenotyping. PBMC were isolated from mouse blood samples taken on day 20 after PBMC engraftment and analysed by multicolor FACS for human surface markers CD45, CD3 and CD8. PBMC from surviving animals of the group treated with anti-4-1BB benchmark mAb were phenotyped on day 17. Summary PRS-343 is a 4-1BB/HER2 bispecific based on the genetic fusion of a high-affinity 4-1BB-binding Anticalin and modified trastuzumab PRS-343 displays a differentiated profile when compared to a benchmark 4-1BB-targeting antibody o Reporter assay: PRS-343 leads to 4-1BB activation in presence of HER2-positive tumor cells, but not in their absence o Ex vivo: PRS-343 induces strong T cell activation via tumor target-dependent costimulatory T cell engagement o In vivo: PRS-343 displays dual activity based on monospecific HER2-targeting and bispecific, tumor-localized costimulation of 4-1BB, leading to increased density of CD8+ cells in the tumor o In vivo: PRS-343 avoids the systemic peripheral activation of CD8+ T cells observed with benchmark 4-1BB antibody, which supports a better safety profile PRS-343 path to clinic: IND-enabling activities are ongoing with an anticipated first-in-patient study planned for the first half of 2017 Parts of images on this poster based on material from Servier Medical Art under a Creative Commons Attribution 3.0 unported license.