Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Theravance Biopharma, Inc. | a16-20204_1ex99d1.htm |
| 8-K - 8-K - Theravance Biopharma, Inc. | a16-20204_18k.htm |
Exhibit 99.2
October 20, 2016 Revefenacin (TD-4208) Phase 3 Efficacy Results Once-daily, Nebulized Long-Acting Muscarinic Antagonist (LAMA)

Cautionary Statement Regarding Forward-Looking Statements Under the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, the company cautions investors that any forward-looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward-looking statements or projections. Examples of forward-looking statements in this presentation include statements relating to the company’s business plans and objectives, including financial and operating results, potential partnering transactions and sales targets, the company’s regulatory strategies and timing and results of clinical studies, the potential benefits and mechanisms of action of the company’s product and product candidates (including their potential as components of combination therapies and the timing and use of the net proceeds from the proposed offering). The company’s forward-looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective (including when our product candidates are studied in combination with other compounds), delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products, risks associated with establishing and maintaining sales, marketing and distribution capabilities, and market conditions that may affect whether the offering will be made or consummated on the proposed terms, if at all. Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Form 10-Q filed with the Securities and Exchange Commission (SEC) on August 9, 2016, and other periodic reports filed with the SEC.
Revefenacin: Positive Results in Replicate Phase 3 Efficacy Studies Robust and sustained improvements in FEV1 Effective as monotherapy and as add-on to LABA or LABA/ICS Generally well tolerated FEV1 = Forced expiratory volume in one second LABA = Long-acting-beta-agonist; ICS – Inhaled corticosteroid Primary Endpoint Met in Both Studies and at Both Doses
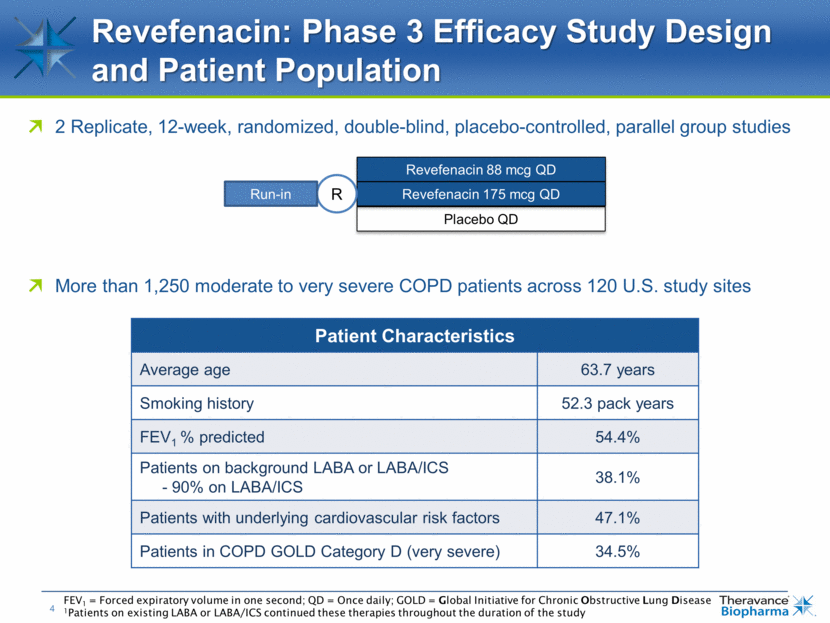
2 Replicate, 12-week, randomized, double-blind, placebo-controlled, parallel group studies Revefenacin: Phase 3 Efficacy Study Design and Patient Population More than 1,250 moderate to very severe COPD patients across 120 U.S. study sites Revefenacin 88 mcg QD Placebo QD Revefenacin 175 mcg QD R Run-in FEV1 = Forced expiratory volume in one second; QD = Once daily; GOLD = Global Initiative for Chronic Obstructive Lung Disease 1Patients on existing LABA or LABA/ICS continued these therapies throughout the duration of the study Patient Characteristics Average age 63.7 years Smoking history 52.3 pack years FEV1 % predicted 54.4% Patients on background LABA or LABA/ICS - 90% on LABA/ICS 38.1% Patients with underlying cardiovascular risk factors 47.1% Patients in COPD GOLD Category D (very severe) 34.5%
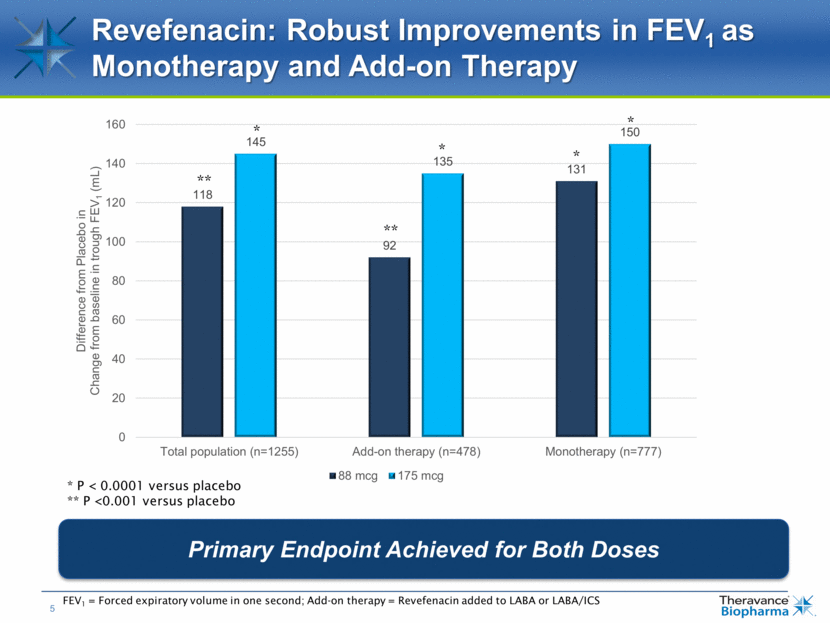
Revefenacin: Robust Improvements in FEV1 as Monotherapy and Add-on Therapy Primary Endpoint Achieved for Both Doses FEV1 = Forced expiratory volume in one second; Add-on therapy = Revefenacin added to LABA or LABA/ICS * P < 0.0001 versus placebo ** P <0.001 versus placebo * ** * * * **
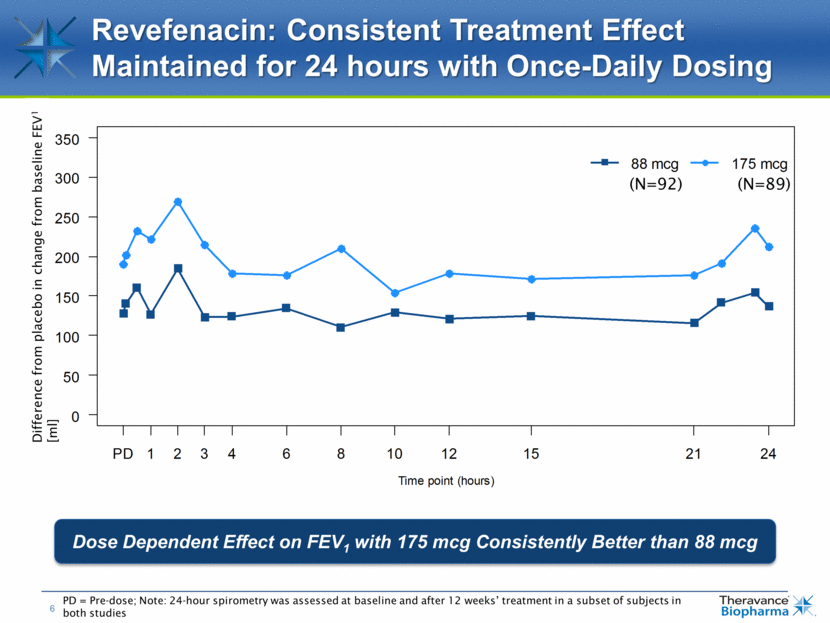
Revefenacin: Consistent Treatment Effect Maintained for 24 hours with Once-Daily Dosing PD = Pre-dose; Note: 24-hour spirometry was assessed at baseline and after 12 weeks’ treatment in a subset of subjects in both studies Dose Dependent Effect on FEV1 with 175 mcg Consistently Better than 88 mcg (N=92) (N=89) Difference from placebo in change from baseline FEV1 [ml]
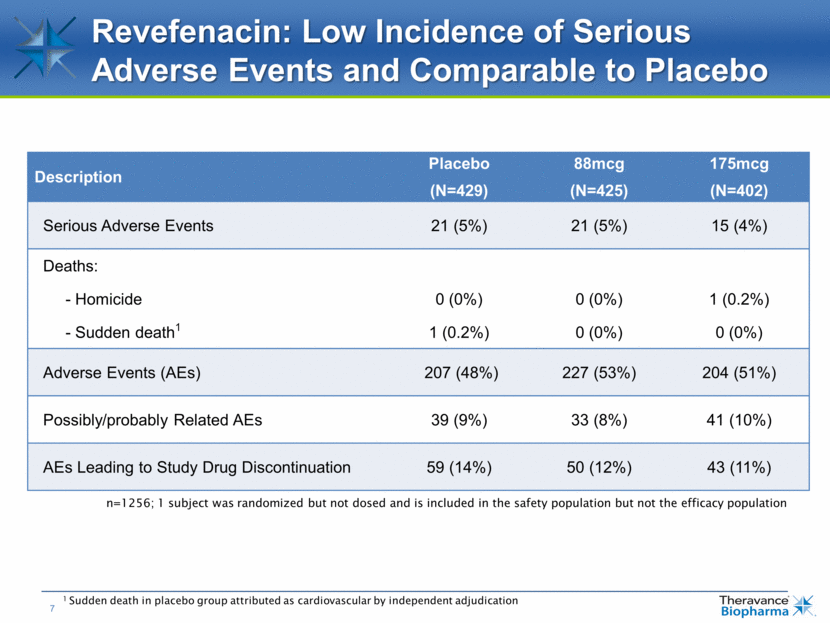
Revefenacin: Low Incidence of Serious Adverse Events and Comparable to Placebo n=1256; 1 subject was randomized but not dosed and is included in the safety population but not the efficacy population Description Placebo (N=429) 88mcg (N=425) 175mcg (N=402) Serious Adverse Events 21 (5%) 21 (5%) 15 (4%) Deaths: - Homicide 0 (0%) 0 (0%) 1 (0.2%) - Sudden death1 1 (0.2%) 0 (0%) 0 (0%) Adverse Events (AEs) 207 (48%) 227 (53%) 204 (51%) Possibly/probably Related AEs 39 (9%) 33 (8%) 41 (10%) AEs Leading to Study Drug Discontinuation 59 (14%) 50 (12%) 43 (11%) 1 Sudden death in placebo group attributed as cardiovascular by independent adjudication
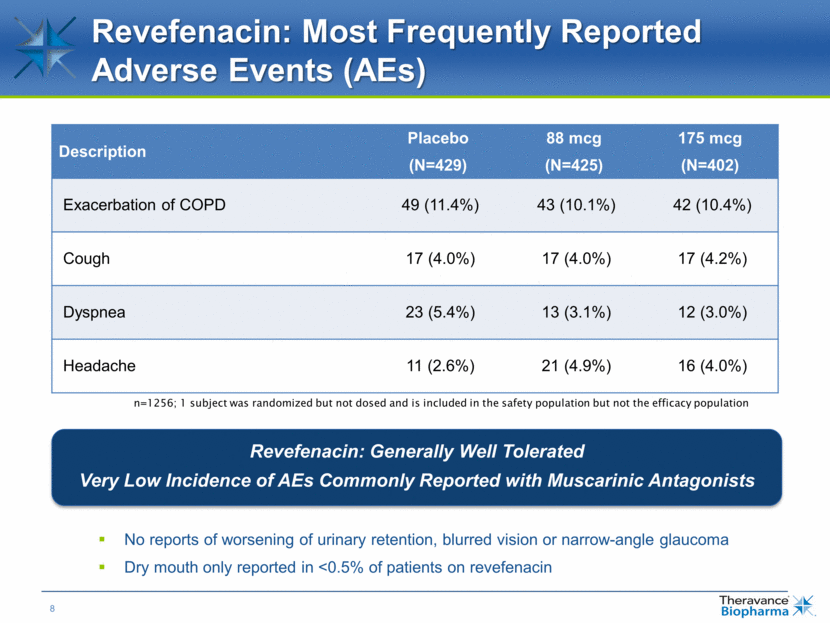
Revefenacin: Most Frequently Reported Adverse Events (AEs) Description Placebo (N=429) 88 mcg (N=425) 175 mcg (N=402) Exacerbation of COPD 49 (11.4%) 43 (10.1%) 42 (10.4%) Cough 17 (4.0%) 17 (4.0%) 17 (4.2%) Dyspnea 23 (5.4%) 13 (3.1%) 12 (3.0%) Headache 11 (2.6%) 21 (4.9%) 16 (4.0%) n=1256; 1 subject was randomized but not dosed and is included in the safety population but not the efficacy population No reports of worsening of urinary retention, blurred vision or narrow-angle glaucoma Dry mouth only reported in <0.5% of patients on revefenacin Revefenacin: Generally Well Tolerated Very Low Incidence of AEs Commonly Reported with Muscarinic Antagonists
Revefenacin: Positive Results in Replicate Phase 3 Efficacy Studies Robust and sustained improvements in FEV1 Effective as monotherapy and as add-on to LABA or LABA/ICS Generally well tolerated FEV1 = Forced expiratory volume in one second LABA = Long-acting-beta-agonist; ICS – Inhaled corticosteroid Primary Endpoint Met in Both Studies and at Both Doses