Attached files
| file | filename |
|---|---|
| 8-K - ATRS-INVESTOR PRESENTATION - ANTARES PHARMA, INC. | atrs-8k_20160927.htm |

NASDAQ: ATRS Ladenburg Thalmann 2016 Healthcare Conference September 27, 2016 Robert F. Apple President and Chief Executive Officer Exhibit 99.1

Safe Harbor Statement This presentation contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: FDA approval of Teva’s Abbreviated New Drug Application (ANDA) filed for the Exenatide pen and future revenue from the same; the timing and results of the phase 3 studies for QuickShot® Testosterone (QS T) and acceptance of the data by the U.S. Food and Drug Administration (FDA); the timing and Company’s ability to successfully complete a New Drug Application (NDA) for QS T, acceptance of the NDA for QS T by the FDA and approval of the same by the FDA; Teva’s ability to adequately and timely respond to the Complete Response Letter received from the FDA for the VIBEX® epinephrine pen ANDA and approval by the FDA of the same, the timing and therapeutic equivalence rating thereof, and any future purchase orders and revenue pre or post FDA approval; the market acceptance and revenue from VIBEX® Sumatriptan Injection USP; the outcome of the pending patent litigation between Teva Pharmaceutical Industries, Ltd. (Teva) and Eli Lilly and Company regarding the Teriparatide multi-dose pen; the timing and approval, if any, by the FDA of Teva’s ANDA for the Teriparatide multi-dose pen and any future revenue resulting therefrom; continued growth of prescriptions and sales of OTREXUP™; the timing and results of the development project with AMAG Pharmaceuticals for an auto injector for Makena; the timing and results of research projects, clinical trials, and product candidates in development; actions by the FDA or other regulatory agencies with the respect to the Company’s products or product candidates of its partners; continued growth in product, development, licensing and royalty revenue; the Company’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities and other statements regarding matters that are not historical facts, and involve predictions. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, achievements or prospects expressed in or implied by such forward-looking statements. In some cases you can identify forward-looking statements by terminology such as ''may'', ''will'', ''should'', ''would'', ''expect'', ''intend'', ''plan'', ''anticipate'', ''believe'', ''estimate'', ''predict'', ''potential'', ''seem'', ''seek'', ''future'', ''continue'', or ''appear'' or the negative of these terms or similar expressions, although not all forward-looking statements contain these identifying words. Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward-looking statements is contained in the "Risk Factors" section of the Company's Annual Report on Form 10-K for the year ended December 31, 2015, and in the Company's other periodic reports and filings with the Securities and Exchange Commission. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this presentation. All forward-looking statements are based on information currently available to the Company on the date hereof, and the Company undertakes no obligation to revise or update these forward-looking statements to reflect events or circumstances after the date of this presentation, except as required by law. ©2016 Copyright Antares Pharma, Inc. All Rights Reserved.

Antares Pharma A Growing, Revenue Generating State-of-the-Art Specialty Pharmaceutical Company An Innovative Leader In Self-Administered Injection Technology Two combination products approved and on the market (OTREXUP™, Sumatriptan) Three ANDA drug device combination products under review with first to file status (Epinephrine pen, Exenatide, Teriparatide) Two Additional Drug Device Combination Products in Advanced Clinical Development (QuickShot® Testosterone, Amag’s Makena®) Novel Drug Delivery Technology Can Provide Life Cycle Management Solutions Auto-injector platform Multi-dose pen platform
2016 Potential Value Drivers Sumatriptan Launch QST NDA Filing 4 Alliance Business Growth OTREXUP™ Growth
Value Driver #1 – VIBEX® Sumatriptan Launch December 15, 2015 FDA approval; 4mg & 6mg doses commercially available 6/27/16 Therapeutically Equivalent to Imitrex® STATdose addressing a ~$200 million* retail injectable market – Dr. Reddy’s, Sun and Sandoz 50/50 profit split with Teva Antares produces final packaged product & sells to Teva at cost Teva distributes to market; profit split to Antares will be recorded as product revenue with one quarter delay VIBEX® Sumatriptan *Source: Symphony Health Solutions 2015 Retail PHAST Legacy 2.0 TRx Dollars,

Value Driver #2 – NDA Filing For QuickShot® Testosterone NDA filing currently targeted for late Q416 Possible launch in late 2017 / early 2018 Final safety data from 26 and 52 week studies reported – most common AE’s in one or both studies included increased hematocrit, hypertension, upper respiratory tract infection, sinusitis, injection site bruising, PsA and headache. There were 7 SAE’s reported. Of 2,484 injections assessed for pain in the 003 and 005 studies, there were 10 reported instances of mild pain and 2,474 injections with no pain Human Factors study completed – all clinical work completed QuickShot® Testosterone
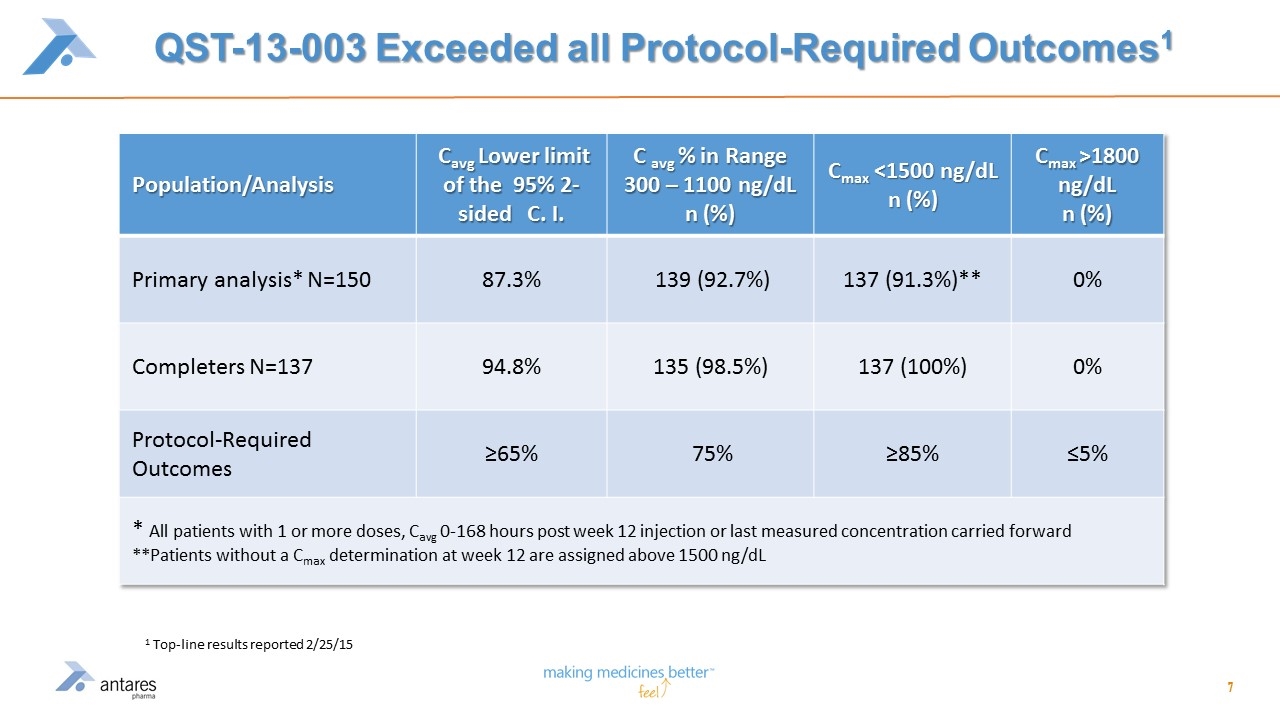
QST-13-003 Exceeded all Protocol-Required Outcomes1 Population/Analysis Cavg Lower limit of the 95% 2-sided C. I. C avg % in Range 300 – 1100 ng/dL n (%) Cmax <1500 ng/dL n (%) Cmax >1800 ng/dL n (%) Primary analysis* N=150 87.3% 139 (92.7%) 137 (91.3%)** 0% Completers N=137 94.8% 135 (98.5%) 137 (100%) 0% Protocol-Required Outcomes ≥65% 75% ≥85% ≤5% * All patients with 1 or more doses, Cavg 0-168 hours post week 12 injection or last measured concentration carried forward **Patients without a Cmax determination at week 12 are assigned above 1500 ng/dL 1 Top-line results reported 2/25/15
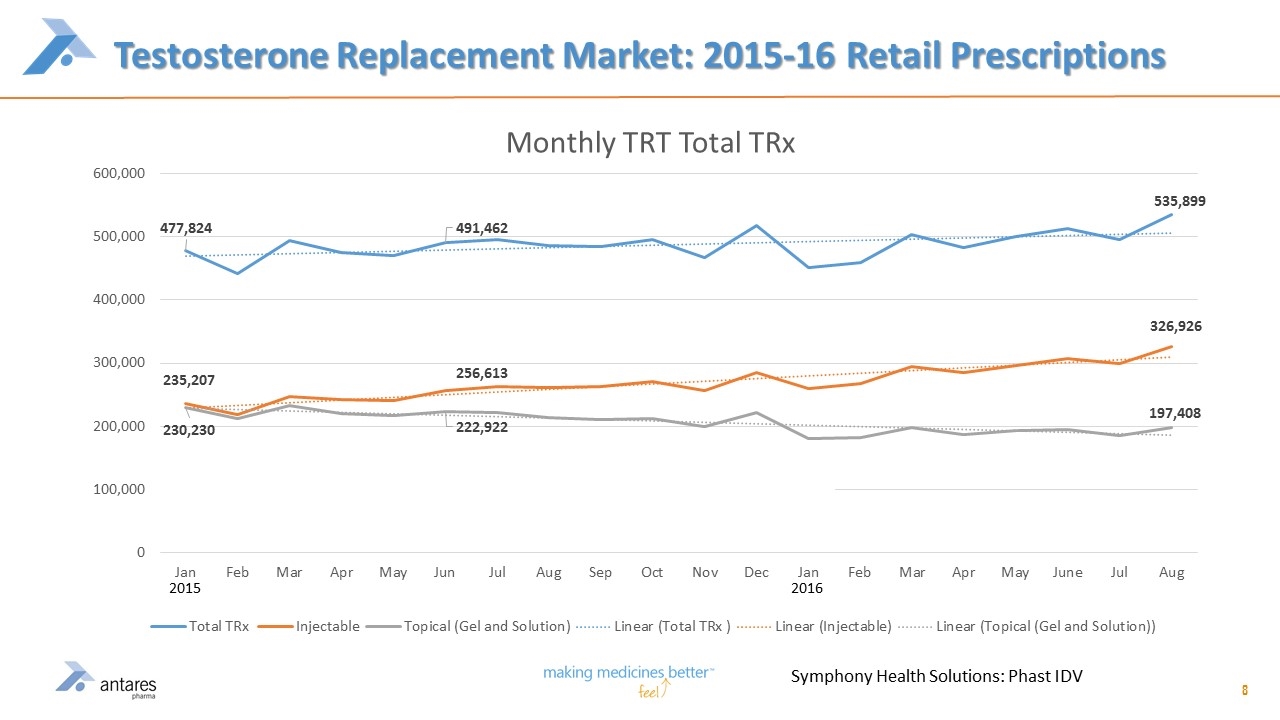
Testosterone Replacement Market: 2015-16 Retail Prescriptions 8 Symphony Health Solutions: Phast IDV 2016 2015
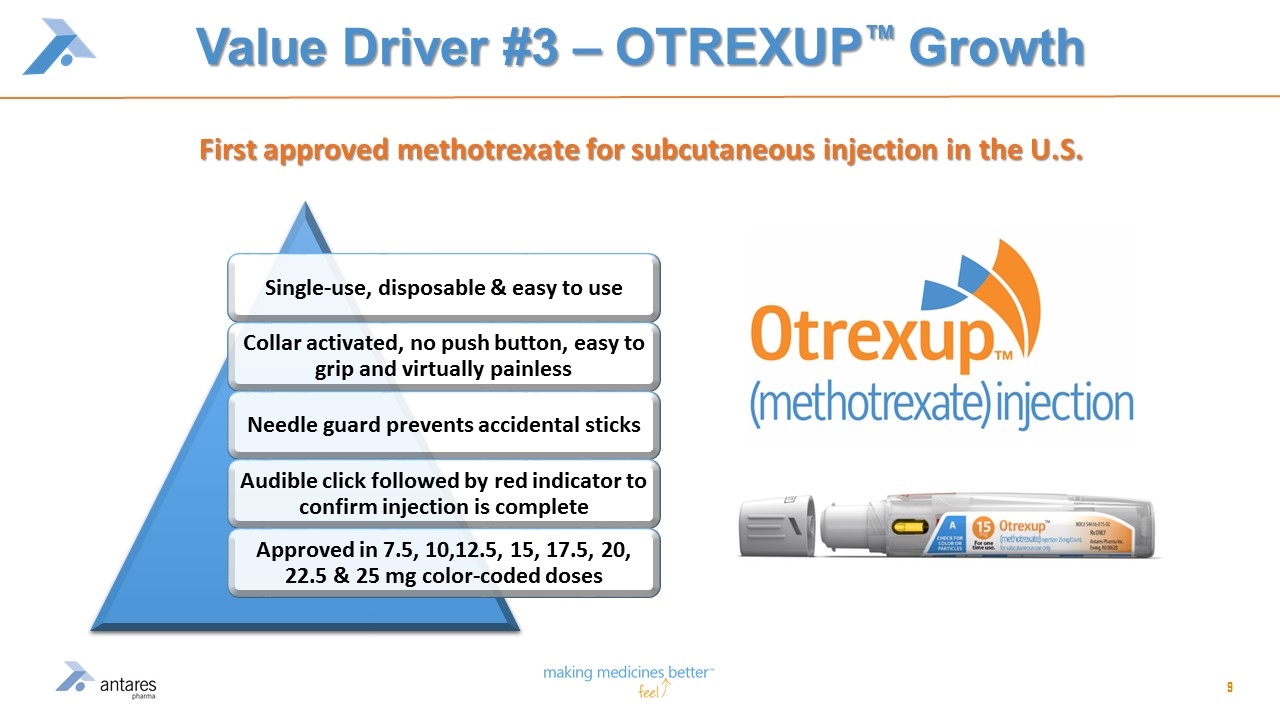
Value Driver #3 – OTREXUP™ Growth First approved methotrexate for subcutaneous injection in the U.S. Single-use, disposable & easy to use Collar activated, no push button, easy to grip and virtually painless Needle guard prevents accidental sticks Audible click followed by red indicator to confirm injection is complete Approved in 7.5, 10,12.5, 15, 17.5, 20, 22.5 & 25 mg color-coded doses

OTREXUP™ Q216 Revenues of $3.8 million were up 14% vs. Q215; and up 15% sequentially vs. Q116 Committed to growing OTREXUP™: Leadership changes in sales and marketing organizations Modifying certain marketing tactics Take advantage of new interim dosage strengths that have recently been launched
Near Term Alliance Business Opportunities Epinephrine Exenatide Makena 11 Teriparatide
VIBEX® Epinephrine Teva filed ANDA amendment with FDA in December 2014, Complete Response Letter (CRL) issued February 2016, Teva and Antares working together to answer FDA questions Shipped $1.0 million in devices to Teva in Q216 and ~$17 million to date Agreement with Teva – ATRS will receive margins on device sales and mid-to-high single digit royalty on overall product sales High visibility for the need of a generic epi pen EpiPen® is a registered trademark of the Mylan Companies
Generic Byetta® (exenatide) Multi-Dose Pen Teva announced settlement with AstraZeneca and Amylin which allows Teva to launch on October 15, 2017, pending FDA approval Teva filed ANDA in December 2014 and it is under review – Antares believes Teva has first to file status and 180 day marketing exclusivity Symphony retail sales of Byetta in 2015 ~$300 million Managed care plans may require Bydureon patients (extended release Byetta) to step through generic Byetta; Symphony 2015 retail sales of Bydureon ~$1 billion ATRS will supply devices at reasonable margin plus receive high single digit to mid-teens royalty on Teva end sales
Makena® AMAG/Makena® alliance began in 2014 Antares is using the QuickShot device to develop a once-weekly subcutaneous injection of Makena Better patient compliance Potentially less painful injection (small gauge needle) and easier administration Currently administered with a large gauge needle from a single dose vial Makena 2015 revenue was ~$250 million, expected to grow to approximately $310 - $340* in 2016 AMAG estimates sNDA filing in 2Q17 with a 10 month regulatory review ATRS will supply devices at reasonable margin plus receive high single to low double digit royalties and sales milestones *AMAG 2016 Revenue Guidance Issued 1/11/16
Generic Forteo® (teriparatide) Multi-Dose Pen Teva ANDA accepted by FDA 2/16; Lilly filed a lawsuit in response to Teva’s Paragraph IV notice, 30 month stay expires in August 2018 Lilly has agreed not to sue Teva on the device patent (which expires in 2025) - last to expire patent is now August 2019* Based on available information, Antares believes Teva may have first to file status and may be entitled to 180 day marketing exclusivity According to Lilly’s 2015 form 10k, Forteo® full year revenues were $1.3 billion, including U.S. revenues of $0.6 billion ATRS will supply devices at reasonable margin plus receive high single digit to mid-teens royalty on Teva end sales *Bloomberg Intelligence Litigation Watch: Eli Lilly Forteo Suit

Device Technology Platforms Allow For Multiple Product Development and Business Alliance Opportunities
Investment Considerations A growing, revenue generating company – $12.2 million in Q216 and $24.5 million through the six month period ended 6/30/16 Several potential value drivers in 2016: Sumatriptan launched 6/27/16 Anticipated QST NDA filing late Q4 2016 Growth of OTREXUP™ Growth in Alliance Business (Epinephrine, Exenatide, Makena®, Teriparatide) Development pipeline of products targeting large therapeutic markets over the next five years Strong balance sheet – $36.6 million in cash & investments and no debt at 6/30/16

NASDAQ: ATRS Ladenburg Thalmann 2016 Healthcare Conference September 27, 2016 Robert F. Apple President and Chief Executive Officer
