Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99-1 - Emmaus Life Sciences, Inc. | s104172_ex99-1.htm |
| EX-10.1 - EXHIBIT 10-1 - Emmaus Life Sciences, Inc. | s104172_ex10-1.htm |
| EX-3.1 - EXHIBIT 3-1 - Emmaus Life Sciences, Inc. | s104172_ex3-1.htm |
| 8-K - 8-K - Emmaus Life Sciences, Inc. | s104172_8k.htm |
Exhibit 99.2
 |
September 21, 2016 |
To our shareholders:
We appreciate your continued support. We have produced disruptive results for one of the most challenging problems in medicine: reducing trial and error treatment in mental health. I’m pleased to report that we’ve now built the largest clinical registry in Psychiatry, the most predictive algorithms, and have achieved unprecedented clinical trial results in real-world military and civilian clinical trials.
The market for predictive medicine
Analysts have identified predictive medicine as one of the hottest investment markets in healthcare, “particularly, healthcare startups using advanced machine learning algorithms for medical imaging & diagnostics, remote patient monitoring, and risk prediction.”1 Predictive analytics to improve treatment response are projected to become a $4 billion market by 2020. We see it in Google’s $1 billion Baseline project to collect outcome data on 10,000 employees over the next 5 years, in IBM Watson’s brain initiative, and in Myriad Genetics’ recent acquisition of Assurex Health. The market for personalized treatment prediction is arriving, and we have put MYnd Analytics in the middle of it.
With the recent publication of our military results, and the 42 independent, confirming studies being reported in this month’s Biological Psychiatry, we now move our focus to commercialization and growth. Here’s our summary of how we intend to do that.
Our competitive advantage
Our product utilizes the largest database of longitudinal patient outcomes, collected from our subscribing physicians and patients over more than a decade. Because our data “learns”, we are uniquely positioned to build the gold standard for personalizing treatment in mental health. PEER offers practical advantages to physicians and patients, including:
| 1 | CB Insights, Artificial Intelligence Sub-Industries Heatmap: Healthcare Emerges As Hottest Area Of Investment, 6/18/16 |
| • | Higher Efficacy - Findings just presented at the MHSR Symposium included pooled results from all 4 randomized trials of PEER, with an average 47% improvement (mean change from baseline) for PEER-guided treatments, compared to only 16% average improvement in the standard of care group. In other words, physicians with PEER information have three times higher medication efficacy than physicians treating as usual without the benefit of PEER. |
Pooled Results of Randomized Clinical Trials
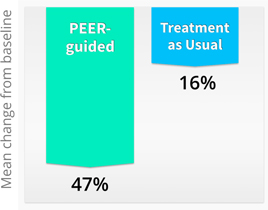
| • | Clinical utility — PEER results are available same-day and provide objective, actionable data to support treating physicians. A retrospective study by Medco found that 92% of physicians changed therapies based on information provided by PEER. |
| • | A Learning System — a core attribute of the PEER Registry approach is that it “learns”, meaning that machine learning algorithms improve the accuracy of recommendations as outcomes are added to the database. In the last three years, an additional 1,500 patients were added to the PEER Registry, improving overall predictive accuracy ranging from 86% to 91%. |

| • | Pharmacogenomics — Currently, the most proven targets for pharmacogenomics are in the liver — i.e. CYP450 drug metabolism — which apply to less than 15% of Americans.2 Conversely, PEER is based on functional brain activity and therefore, is more broadly applicable. Accordingly, we executed testing agreements this year with two national laboratories to include pharmacogenomic testing in our approved SMART-MD protocol. Outcome data from genomic testing may further improve the accuracy of PEER: the data from the SMART-MD trial should pinpoint the contribution of each modality to predictive accuracy and we will continue to look for strategic relationships with genomic partners. |
Latest Clinical Results
The Walter Reed PEER Interactive Trial is the fourth randomized, controlled trial of PEER technology, designed to be one of the largest psychiatric treatment trials in recent history, with 1,922 subjects. Reflecting the military’s need for a generalizable, real-world evidence trial we consequently included patients with active suicidal ideation, although in most drug trials these suicidal ideation patients would have been specifically excluded.
| 2 | Verbeurgt P1, Mamiya T, Oesterheld J. How common are drug and gene interactions? Prevalence in a sample of 1143 patients with CYP2C9, CYP2C19 and CYP2D6 genotyping. Pharmacogenomics. 2014 Apr;15(5):655-65. doi: 10.2217/pgs.14.6. |
Statistically significant results were obtained sooner than expected, at 150 patients, and interim results were prepared at the request of Congress in April, 2014. Ten of the study’s twelve endpoints had been achieved at this early stage in the study, leaving the possibility that the entire study may be completed with little more than 50 additional subjects.
Improvement Over Standard of Care

The most important clinical finding indicated a 75% greater reduction in suicidality when military physicians followed PEER recommendations. No other military studies have achieved this level of improvement, simply by improved targeting of current therapies. By comparison, a 1% increase in suicidality among children and young adults was sufficient to cause the addition of black box warning labels by the FDA for all antidepressants.
Finally, treatments which followed PEER recommendations resulted in 2.5 times greater adherence to therapy, with a median of 5 follow-up visits for subjects on PEER-recommended therapies, compared with 2 visits for those on non-PEER treatments.
The findings of the PEER Interactive Trial were clearly disruptive: the study data has been validated and revalidated by internal and external groups and has not changed in two years. The military reported to Congress that “no quality or safety issues” had been present in the course of the study, and in 2016, the FDA completed a full on-site inspection of study data and procedures which “revealed no significant concerns”.
We expect additional publications around our study results and to be included in a Biological Psychiatry review article being published this month: the article summarizes 42 independent, controlled clinical trials using EEG and pharmacologic interventions in mental health.
Current research
Just as we have validated our military data, we have also committed to rapidly replicate our findings and grow our database asset:
| • | Canadian Armed Forces this year began their own clinical trial (n = 150) with a substantially similar protocol to that used in the U.S. Military’s PEER Interactive Trial. Additional NATO partners may join the clinical trial in 2017. |
| • | The SMART-MD trial was IRB approved and is expected to include 468 people. Plans are underway to begin enrollment in Southern California and North Carolina. This will be the first prospective trial to study the individual contributions of pharmacogenomics and QEEG, as well as providing useful data for updating of PEER classifiers. |
| • | The beta version of the MYnd Mobile App has launched, making outcome data collection easier and more granular for patients whose doctors use PEER. |
Commercial strategy
We plan to drive adoption of our technology and secure sustained profitability through the following four-pronged plan:
| 1) | Military and veterans: due to the high visibility of their problem, their ability to bring sustained demand and their need for intervention. As Dr. David J. Shulkin, the Veterans Administration Undersecretary for Health said in July: “One veteran suicide is one too many, and this collaborative effort provides both upside and comprehensive data that allows us to make better informed decisions on how to prevent this national tragedy.” |
| 2) | Commercial growth strategy outside of the US: the Canadian Armed Forces trial is now underway, which will provide both NATO and Health Canada (single payer system) experience with our PEER technology. It will also increase the size of our data base, and, hopefully, move toward PEER being adopted as a standard of care by Health Canada. |
| 3) | Payer and self-insured markets: management’s goal is to have one payer pilot program implemented before year end to collect clinical and economic data showing the efficacy of using PEER as a tool to get mental health patients on the appropriate medication quicker, thereby lowering utilization of health care costs and improving outcomes. Technology assessment/coverage submissions are underway with multiple commercial health plans and managed care organizations. |
CMS, the largest US payer, is moving 50% of reimbursement to be value-based by 2018. With this trend, and growing enforcement of Mental Health Parity, the payer market has changed in ways which favor our product. Our PEER technology focuses on patient outcomes.
| 4) | Market entry of provider groups: We’ve seen significant growth in formation of outpatient psychiatric groups, which concentrate both risk and purchasing power. We are actively pursuing group purchasing agreements with a number of outpatient, multicenter and multi physician groups. Provider certification with CMS is in process, and we will move forward with submission for national coverage determination. |
Capital structure
In order to make it more attractive for investors to purchase its stock on the open market, we have recently: 1) extinguished our $6m dollars of debt, 2) reduced the number of issued and outstanding shares, 3) eliminated 99% of issued and outstanding warrants, 4) eliminated the need for complex derivative accounting, and 5) settled a legal dispute with the former CEO.
On August 24, 2016, our Board approved and subsequently a majority of over 80% of note holders agreed to the conversion into common stock of $6 million (plus interest) of noteholder debt, along with the cancellation of all warrants issued to these noteholders. The cancelled warrants comprised 99% of all previously issued warrants issued. Furthermore, the Company undertook a 1:200 reverse stock-split, following which there are outstanding approximately 1,800,000 common shares, 7,700 warrants and options to purchase 79,400 shares with a strike price significantly out of the money and which expire by 2021 and 2026 respectively (see SEC filings for more details with link to 8k).
We look forward to keeping you apprised of our progress in commercializing our product as well as adding new shareholders to MYnd by telling our story at investor conferences, by receiving media coverage from our more recent clinical trial and by capitalizing on an exciting and growing sector of health care. Our PEER technology can provide help to the large number of people suffering from anxiety, depression, PTSD and multiple other non-psychotic behavioral disorders, both domestic and worldwide.
