Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - Sorrento Tech, Inc. | pipeexhibit992.htm |
| EX-3.1 - EXHIBIT 3.1 - Sorrento Tech, Inc. | pipeexhibit31.htm |
| EX-10.4 - EXHIBIT 10.4 - Sorrento Tech, Inc. | pipeexhibit104.htm |
| EX-10.3 - EXHIBIT 10.3 - Sorrento Tech, Inc. | pipeexhibit103.htm |
| EX-10.2 - EXHIBIT 10.2 - Sorrento Tech, Inc. | pipeexhibit102.htm |
| EX-10.1 - EXHIBIT 10.1 - Sorrento Tech, Inc. | pipeexhibit101.htm |
| 8-K - 8-K - Sorrento Tech, Inc. | pipe8-k.htm |

CONFIDENTIAL
ROKA BIOSCIENCE
June 2016

CONFIDENTIAL
Cautionary Note Regarding Forward-Looking Statements
This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E
of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act, as amended (the “Exchange Act”). These
forward-looking statements are based on current expectations, estimates, forecasts and projections about the industry and markets in
which we operate and management’s current beliefs and assumptions. Any statements contained herein (including, without limitation,
statements to the effect that we “believe”, “expect”, “anticipate”, “plan” and similar expressions) that are not statements of historical fact
should be considered forward-looking statements. These statements relate to future events or our financial performance and involve
known and unknown risks, uncertainties and other factors that could cause our actual results, levels of activity, performance or
achievement to differ materially from those expressed or implied by these forward looking statements. There are a number of important
factors that could cause actual results to differ materially from those indicated by such forward-looking statements. Such factors include
those set forth in the company’s filings with the Securities and Exchange Commission. We expressly disclaim any obligation to update
any forward-looking statements, except as may be required by law. Given these uncertainties, you should not place undue reliance on
these forward-looking statements. These forward-looking statements represent our estimates and assumptions only as of the date of this
presentation and, except as required by law, we undertake no obligation to update or review publicly any forward-looking statements,
whether as a result of new information, future events or otherwise after the date of this presentation.
2

CONFIDENTIAL
Key Highlights
Founded in 2009 via
acquisition of the industrial
testing assets of Gen-Probe,
a global leader in molecular
diagnostics
Pure Play in Growing $2 Billion Food Safety
Market*
IPO completed in July 2014
Early Adoption By Strategic Customers
Full Menu of Molecular Pathogen tests on
Fully Automated Instrument Platform
Positioned to Benefit from Market
Consolidation and Regulatory Initiatives
Insider Participation by Leading Investors
3
*Capella Advisors, Company Estimates
CONFIDENTIAL
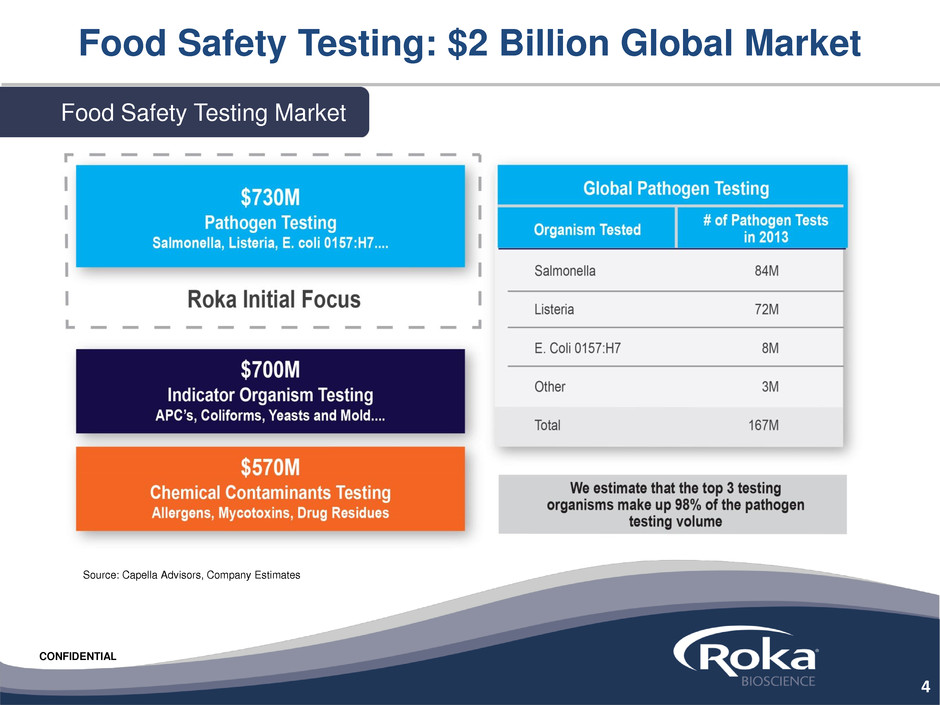
Food Safety Testing: $2 Billion Global Market
Source: Capella Advisors, Company Estimates
Food Safety Testing Market
4
CONFIDENTIAL
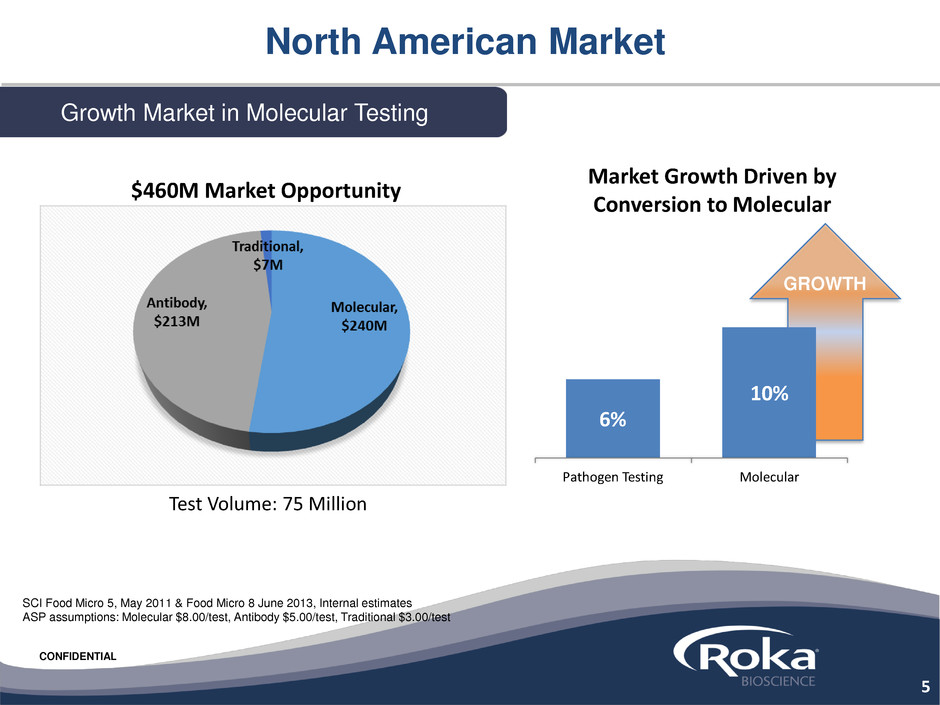
North American Market
Growth Market in Molecular Testing
Market Growth Driven by
Conversion to Molecular
Test Volume: 75 Million
SCI Food Micro 5, May 2011 & Food Micro 8 June 2013, Internal estimates
ASP assumptions: Molecular $8.00/test, Antibody $5.00/test, Traditional $3.00/test
5
$460M Market Opportunity
6%
10%
Pathogen Testing Molecular
GROWTH
CONFIDENTIAL
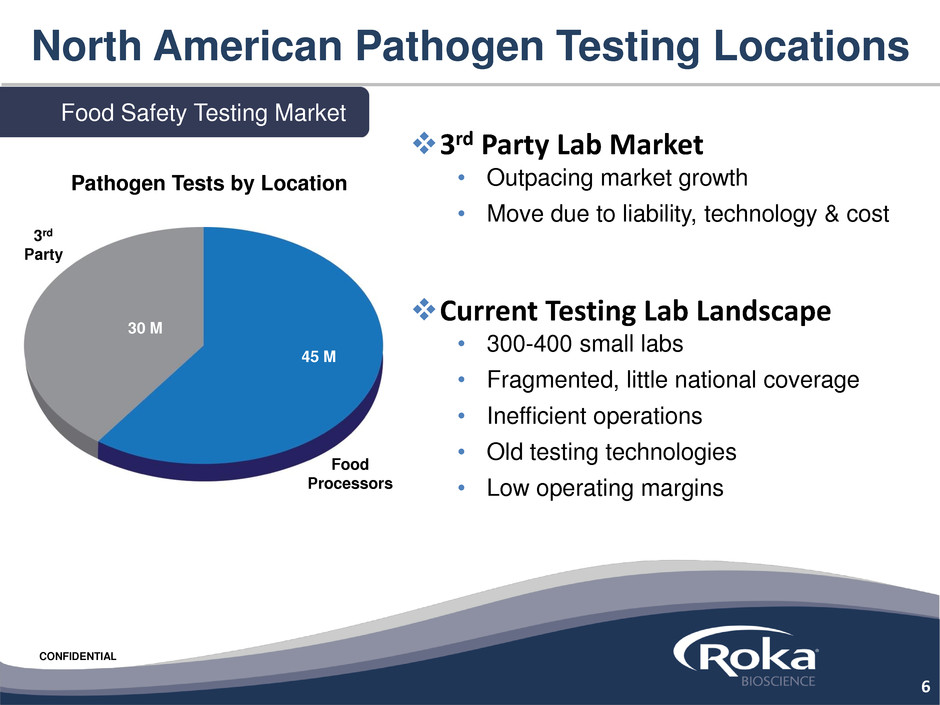
North American Pathogen Testing Locations
Food Safety Testing Market
45 M
30 M
Food
Processors
3rd
Party
Pathogen Tests by Location
3rd Party Lab Market
• Outpacing market growth
• Move due to liability, technology & cost
Current Testing Lab Landscape
• 300-400 small labs
• Fragmented, little national coverage
• Inefficient operations
• Old testing technologies
• Low operating margins
6
CONFIDENTIAL
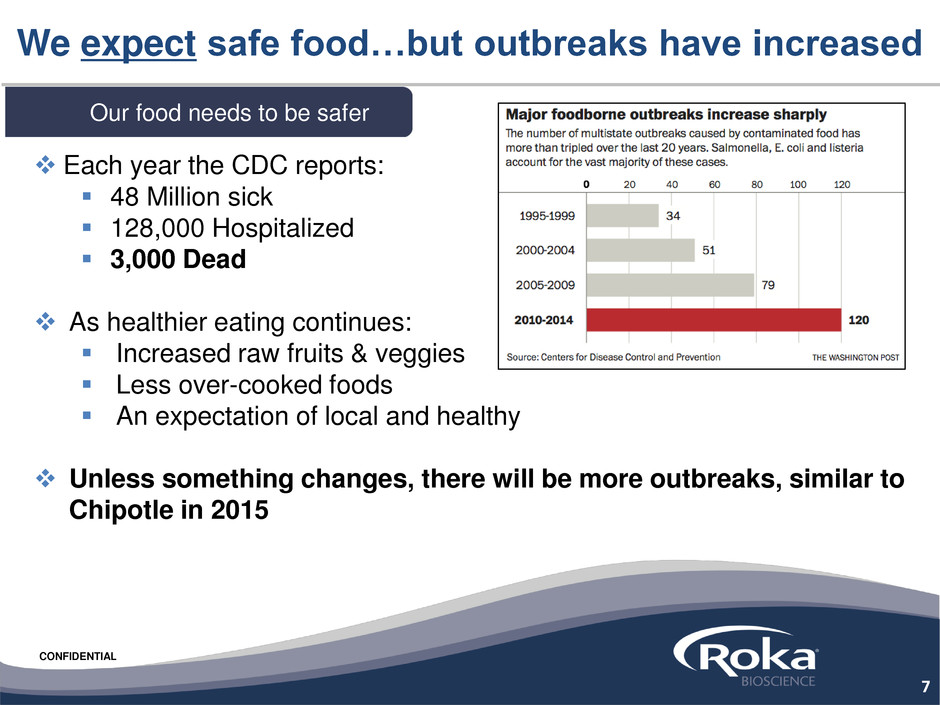
Each year the CDC reports:
48 Million sick
128,000 Hospitalized
3,000 Dead
As healthier eating continues:
Increased raw fruits & veggies
Less over-cooked foods
An expectation of local and healthy
Unless something changes, there will be more outbreaks, similar to
Chipotle in 2015
We expect safe food…but outbreaks have increased
Our food needs to be safer
7

CONFIDENTIAL
Finding pathogens means additional cost of operations, e.g.
diverting product, cleaning facilities, etc.
Most used environmental tests (antibody-based) misses up to
50% of pathogenic bacteria*
Consistent negative results satisfied regulators until now
Rarely caught for poor testing, until now…
Technology adoption has not happened….why?
Processors have tested to get negative results
8
*Study Outline: Thirty‐five environmental sponges were collected each week for 6 weeks from areas in a
plant known to have high positivity for environmental Listeria. All ELFA testing was conducted by an
accredited third party commercial laboratory. Results: VIDAS 51.5% False Negatives; Atlas 4%
The CDC, the DoJ and new regulations are driving change of this culture

CONFIDENTIAL
Processors are now caught for poor testing
NEW ability to trace illness back to food
9
FSMA mandated
prevention-based
controls in all food
processors
2015 & 2016 starts the
enforcement phase
Beginning in 2013 the CDC
utilizes Whole Genome
Sequencing (WGS) to
build database to track and
trace foodborne illness
Identifies outbreaks faster
and ties pathogen to a
specific food source and
often a specific
processing plant (new
capability by matching
gene sequences)
Dept. of Justice now
involved in foodborne
outbreaks. Example:
Peanut Corp. ex-CEO gets
24 years in prison (2016)
Enforcement now includes
fines and jail time

CONFIDENTIAL
WGS will be a change agent for the industry
Listeria WGS launched in
2013; Salmonella, others to
follow
Testing to get a negative is no longer acceptable
10
Whole Genome Sequencing Impact
6 to 93 cases linked to food source in first 2 years
Traceability: Now linking outbreak to specific processor
Dramatically increases risk
of poor food safety to
processor
FEAR is a great motivator of
change
We expect processors to
increase testing with more
accurate tests
Reference: CDC 2016
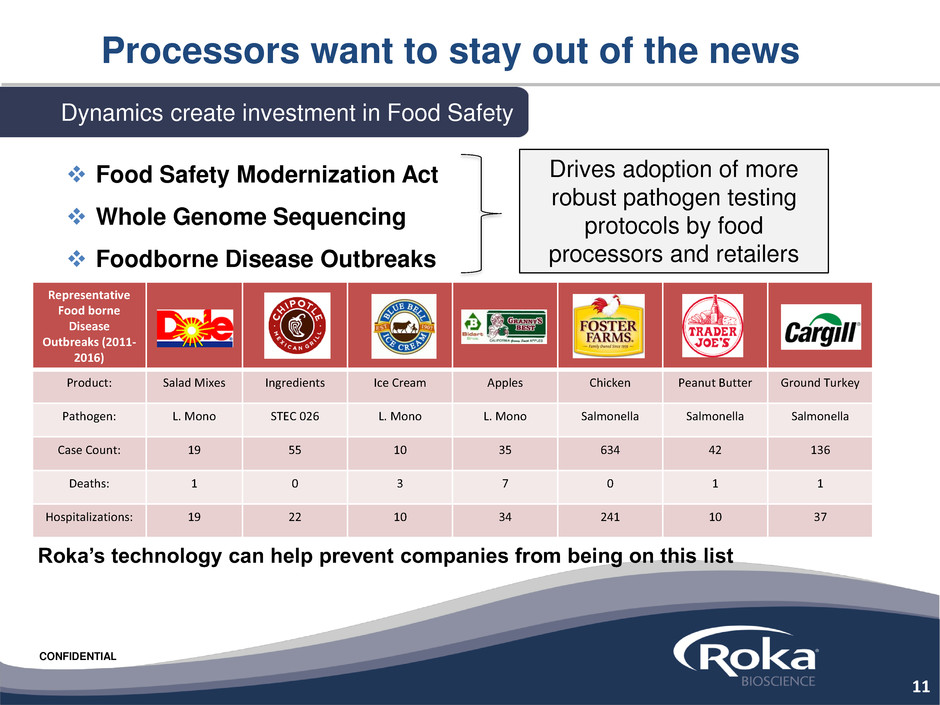
CONFIDENTIAL
Processors want to stay out of the news
Representative
Food borne
Disease
Outbreaks (2011-
2016)
Product: Salad Mixes Ingredients Ice Cream Apples Chicken Peanut Butter Ground Turkey
Pathogen: L. Mono STEC 026 L. Mono L. Mono Salmonella Salmonella Salmonella
Case Count: 19 55 10 35 634 42 136
Deaths: 1 0 3 7 0 1 1
Hospitalizations: 19 22 10 34 241 10 37
Dynamics create investment in Food Safety
Drives adoption of more
robust pathogen testing
protocols by food
processors and retailers
Food Safety Modernization Act
Whole Genome Sequencing
Foodborne Disease Outbreaks
11
Roka’s technology can help prevent companies from being on this list

CONFIDENTIAL
Commercial Timeline
Contamination issue with Listeria encountered Q3-2014
– Significant impact on stock price and valuation
Re-development of Listeria assay
– Increased robustness of assay, allowing handling by labs
– Commercial launch late 2015
Commercial status
– Con-Agra evaluation positive but lengthy; others in progress
– Positive market momentum re Listeria
• Whole Genome Sequencing
• Recalls (Blue Bell, Dole, Frozen Vegetables, etc.)
• 3rd Party Lab Consolidation
12

CONFIDENTIAL
Relaunch with full suite of high quality assays
Listeria – NEW assay
Salmonella
E. coli O157:H7
Listeria Monocytogenes
Shiga toxin-producing
E. coli (STEC)
Atlas Detection AssaysAtlas Instrument Full Commercial Menu
Roka Value Proposition
Accurate, Rapid, Fully-Automated Test Results
As market need for quality increases – Roka is positioned to win
Current AOAC Certified Menu
covers >98% of Pathogen
testing volume
13
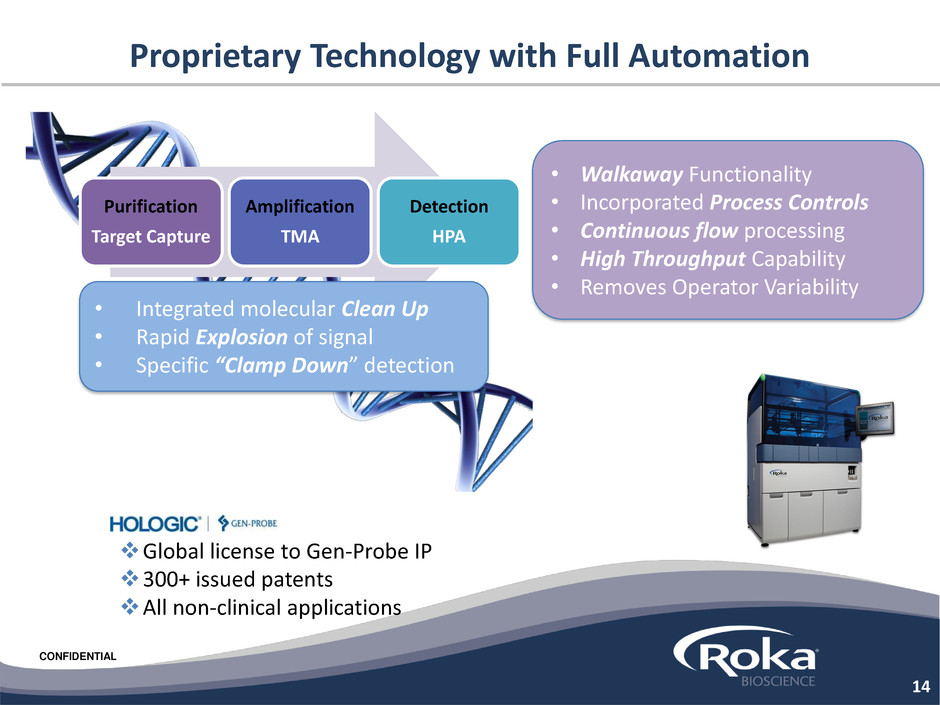
CONFIDENTIAL
Proprietary Technology with Full Automation
• Walkaway Functionality
• Incorporated Process Controls
• Continuous flow processing
• High Throughput Capability
• Removes Operator Variability
Purification
Target Capture
Amplification
TMA
Detection
HPA
• Integrated molecular Clean Up
• Rapid Explosion of signal
• Specific “Clamp Down” detection
Global license to Gen-Probe IP
300+ issued patents
All non-clinical applications
14
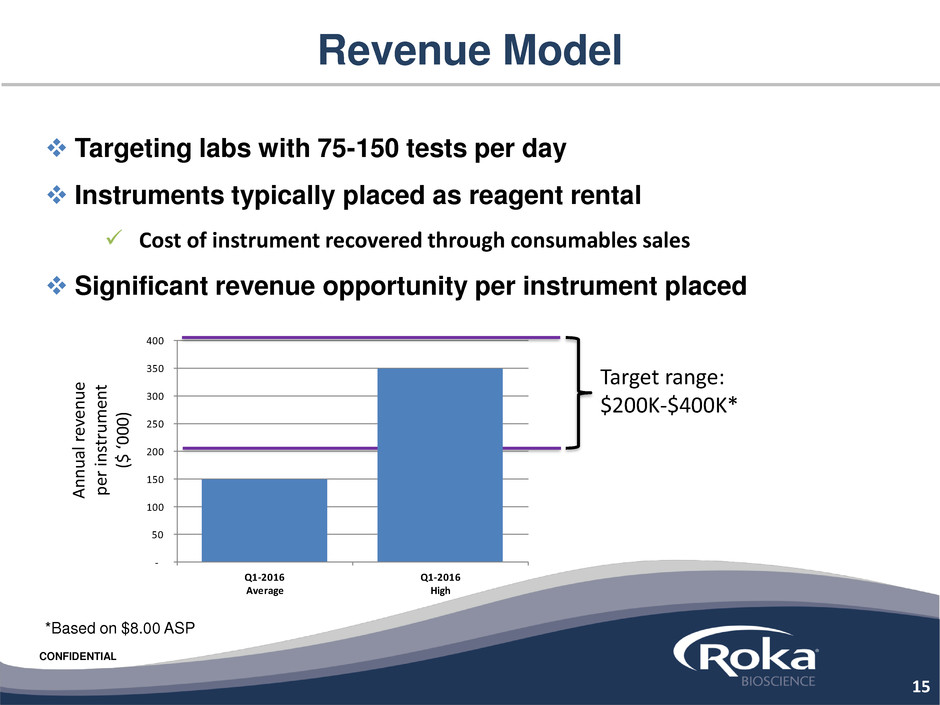
CONFIDENTIAL
-
50
100
150
200
250
300
350
400
Q1-2016
Average
Q1-2016
High
Revenue Model
Targeting labs with 75-150 tests per day
Instruments typically placed as reagent rental
Cost of instrument recovered through consumables sales
Significant revenue opportunity per instrument placed
15
*Based on $8.00 ASP
Target range:
$200K-$400K*
An
n
u
al
r
ev
en
u
e
p
er
in
str
u
m
en
t
($ ‘00
0)

CONFIDENTIAL
Commercial Success to Date
16
DoD Food Analysis & Diagnostics
Laboratory
*Actual customers
*

CONFIDENTIAL
Commercial Strategy
Capitalize on new market drivers
Drive a segment focused organization
Focused promotional activities
• Listeria Environmental Monitoring – all markets
• Capitalize on processors fear of WGS
Key Strategic Initiatives
• Partnership with significant third party lab
• Beef industry
17
CONFIDENTIAL
How to Win in the Lab Market
Sample Pick-Up Logistics
Leverage Existing Food Safety Business
Strong Balance Sheet
Efficient Lab Operations
Automation, flexibility
High volume, scalable instruments
Direct labor reductions
18
Enabled by Roka’s
Atlas

CONFIDENTIAL
Significant Lab Partnership
Opportunity to Drive Market
Competitive pricing of molecular tests
Competitors will experience price erosion or lose business
Leverage existing courier infrastructure for national coverage
Offer a fast turn around time, despite shipping
Lab efficiencies and margins enabled by Atlas
Roka is the only automated, high volume platform on the market
19
Lab already uses Gen-Probe Panther (Atlas) for Clinical Testing
CONFIDENTIAL
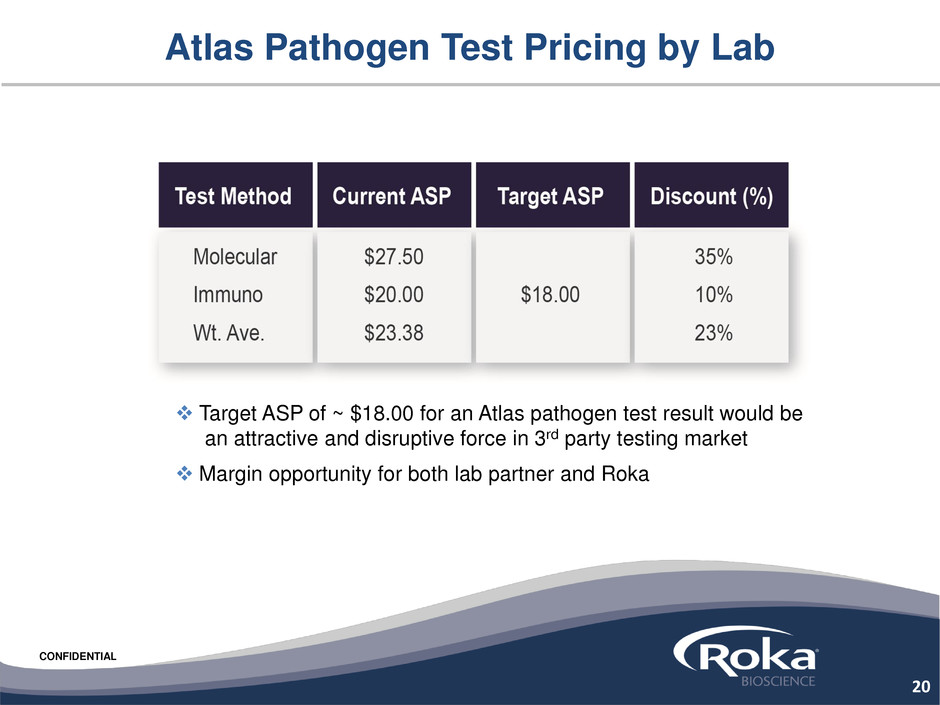
Atlas Pathogen Test Pricing by Lab
45.2 M
30.2 M
4%
31%
65%
Target ASP of ~ $18.00 for an Atlas pathogen test result would be
an attractive and disruptive force in 3rd party testing market
Margin opportunity for both lab partner and Roka
20
CONFIDENTIAL
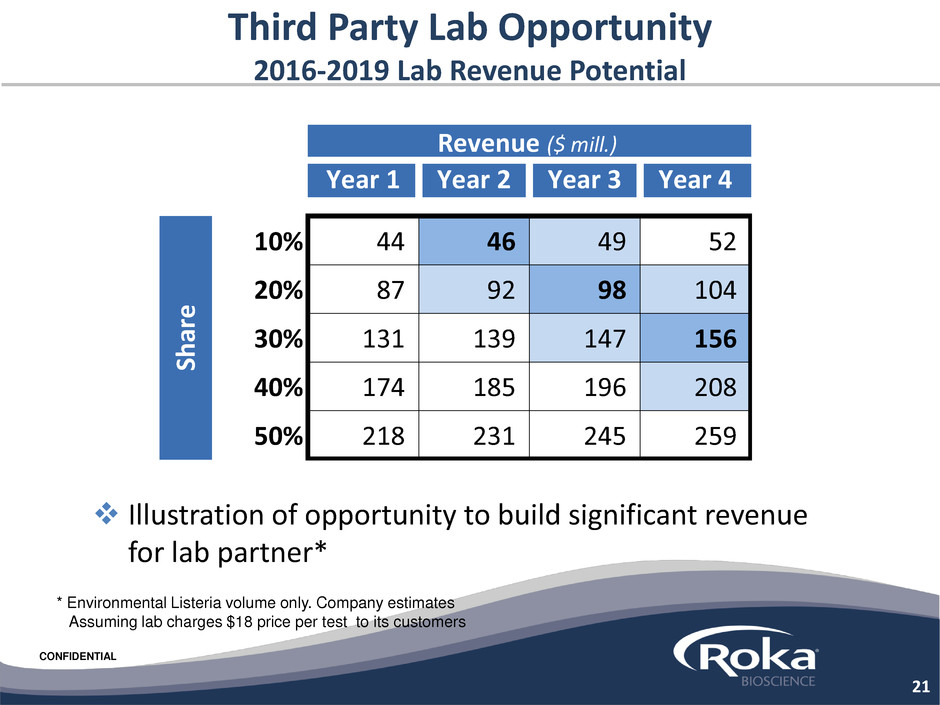
Third Party Lab Opportunity
2016-2019 Lab Revenue Potential
Illustration of opportunity to build significant revenue
for lab partner*
21
* Environmental Listeria volume only. Company estimates
Assuming lab charges $18 price per test to its customers
Year 1 Year 2 Year 3 Year 4
10% 44 46 49 52
20% 87 92 98 104
30% 131 139 147 156
40% 174 185 196 208
50% 218 231 245 259
Sh
are
Revenue ($ mill.)
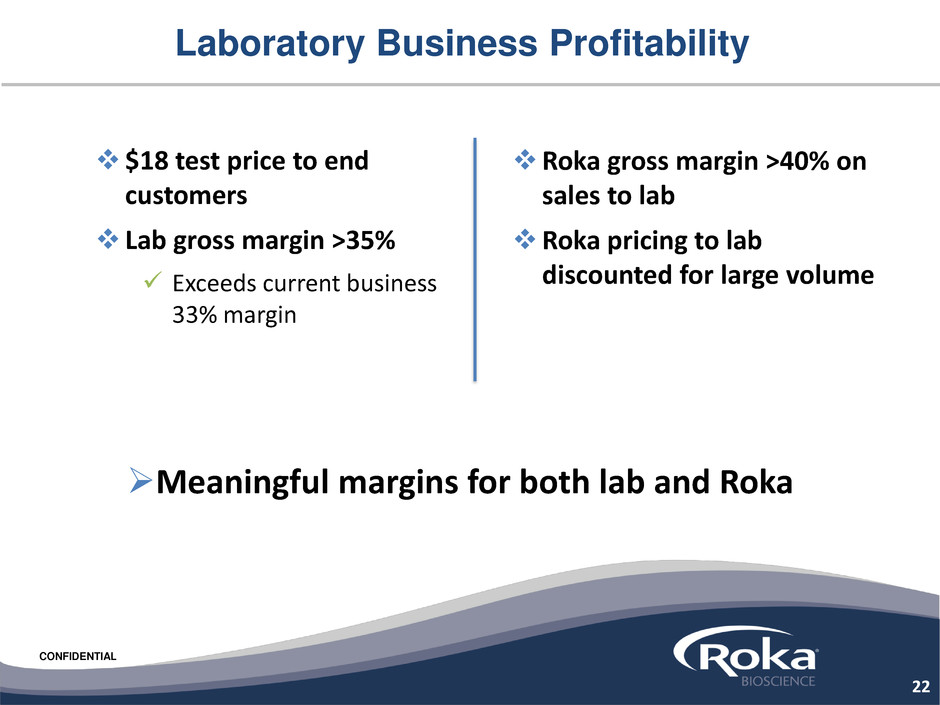
CONFIDENTIAL
$18 test price to end
customers
Lab gross margin >35%
Exceeds current business
33% margin
Laboratory Business Profitability
22
Roka gross margin >40% on
sales to lab
Roka pricing to lab
discounted for large volume
Meaningful margins for both lab and Roka
CONFIDENTIAL
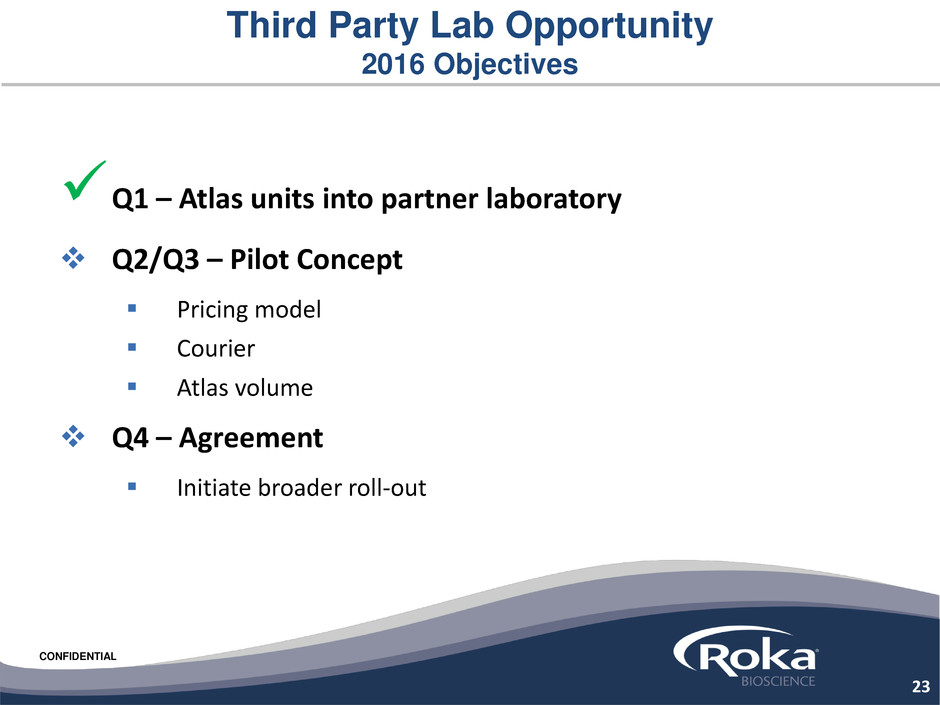
Third Party Lab Opportunity
2016 Objectives
Q1 – Atlas units into partner laboratory
Q2/Q3 – Pilot Concept
Pricing model
Courier
Atlas volume
Q4 – Agreement
Initiate broader roll-out
23
CONFIDENTIAL
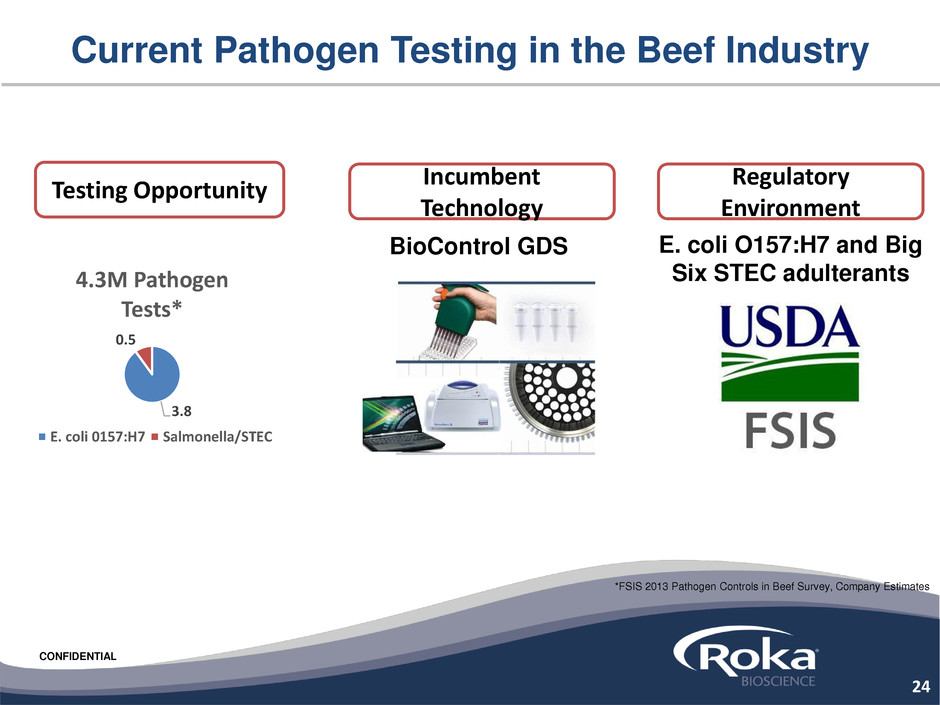
Current Pathogen Testing in the Beef Industry
Testing Opportunity
3.8
0.5
4.3M Pathogen
Tests*
E. coli 0157:H7 Salmonella/STEC
Incumbent
Technology
BioControl GDS
Regulatory
Environment
E. coli O157:H7 and Big
Six STEC adulterants
24
*FSIS 2013 Pathogen Controls in Beef Survey, Company Estimates

CONFIDENTIAL
Pathway to Market Adoption
Roka engaged with leading beef processor over the
past two years to meet external & internal
adoption requirements
Gain Atlas Adoption by one of the Big Four Beef
Producers
25
CONFIDENTIAL
Current Status
Customer already uses Atlas for all Poultry testing and recognizes
efficiencies
Industry conservative on making testing change due to recent
litigations on E.Coli O157:H7
Risk of change offset by significant cost savings benefit
Significant cost savings estimated by beef processor
Cost savings primary driver as discussion moves to implementation
Implementation may be Sept. 2016 – post ‘hot-season’
26
CONFIDENTIAL
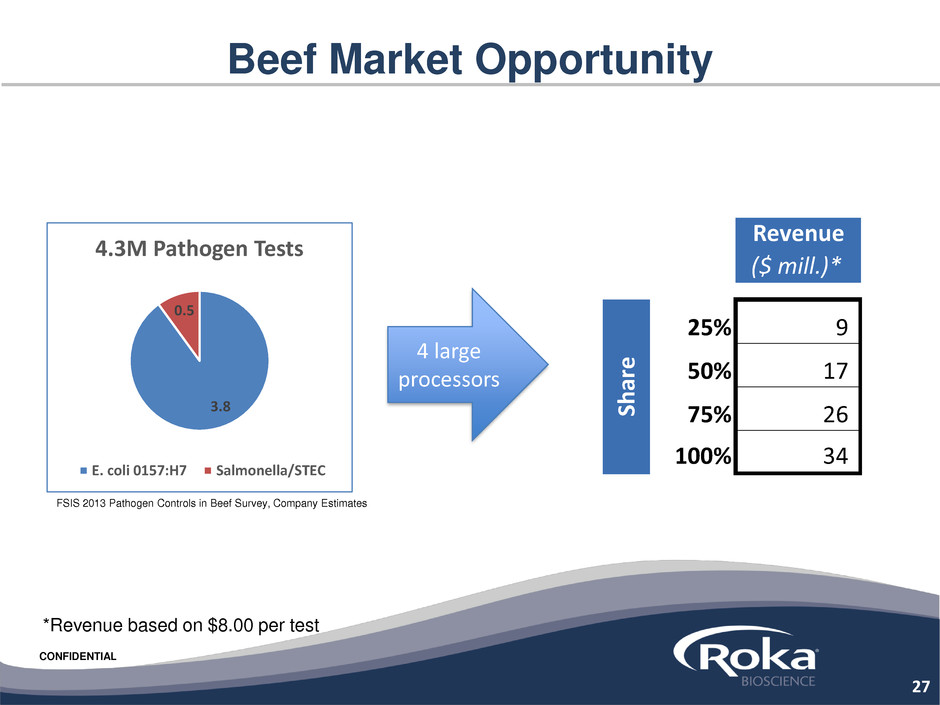
Beef Market Opportunity
27
3.8
0.5
4.3M Pathogen Tests
E. coli 0157:H7 Salmonella/STEC
4 large
processors
*Revenue based on $8.00 per test
Revenue
($ mill.)*
25% 9
50% 17
75% 26
100% 34
Sh
ar
e
FSIS 2013 Pathogen Controls in Beef Survey, Company Estimates

CONFIDENTIAL
Funding Summary
Well positioned to benefit from a changing food safety
market
Lab partnership and beef present exciting opportunities
$25M Financing sought to fund significant revenue growth
Insider participation by leading investors
28
CONFIDENTIAL
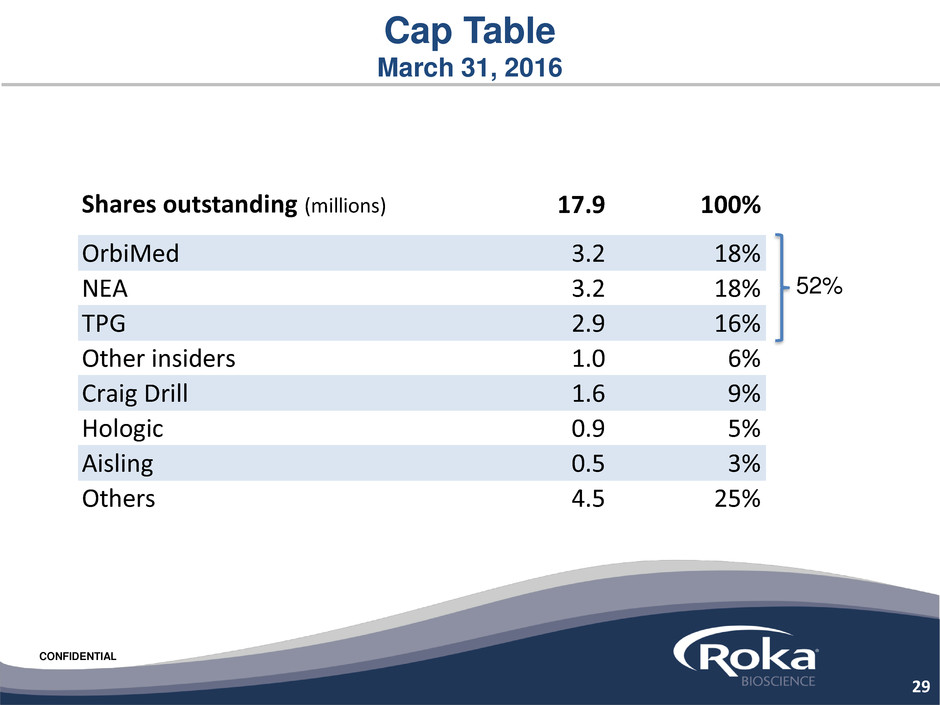
Cap Table
March 31, 2016
52%
29
Shares outstanding (millions) 17.9 100%
OrbiMed 3.2 18%
NEA 3.2 18%
TPG 2.9 16%
Other insiders 1.0 6%
Craig Drill 1.6 9%
Hologic 0.9 5%
Aisling 0.5 3%
Others 4.5 25%
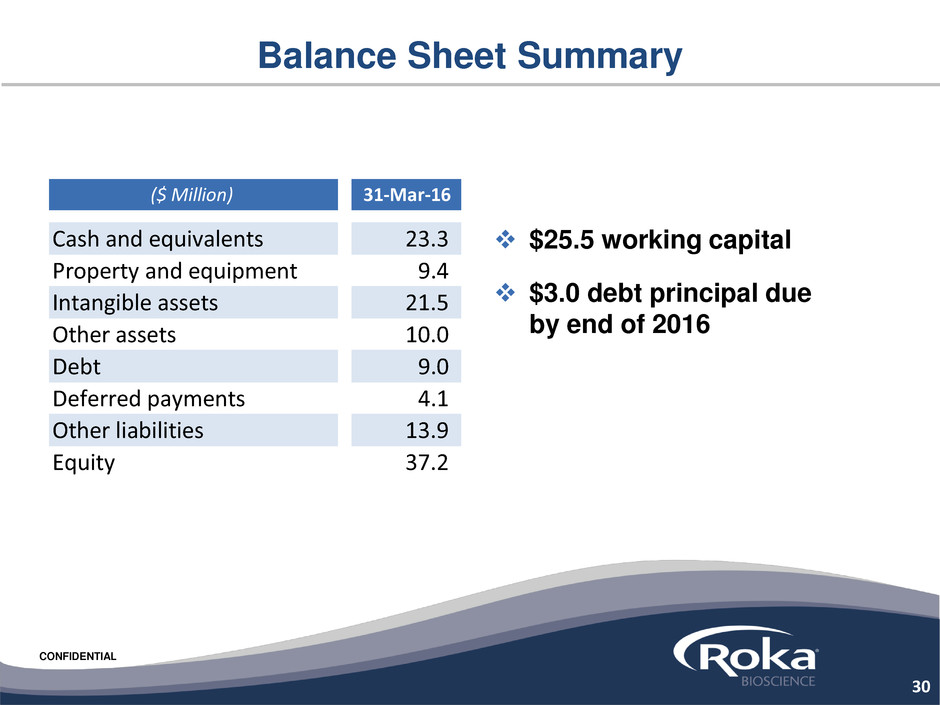
CONFIDENTIAL
Balance Sheet Summary
$25.5 working capital
$3.0 debt principal due
by end of 2016
($ Million) 31-Mar-16
Cash and equivalents 23.3
Property and equipment 9.4
Intangible assets 21.5
Other assets 10.0
Debt 9.0
Deferred payments 4.1
Other liabilities 13.9
Equity 37.2
30
