Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - IMMUNE DESIGN CORP. | d261314d8k.htm |
Exhibit 99.1
As used in this Exhibit 99.1, “Immune Design,” “Immune Design Corp.,” the “Company,” “we,” “us” and “our” refer to Immune Design Corp., a Delaware corporation. ZVex is a U.S. registered trademark and GLAAS is an unregistered U.S. trademark of Immune Design Corp. All other trademarks, service marks and trade names included in this Exhibit 99.1 are the property of their respective owners.
FORWARD-LOOKING STATEMENTS
This Exhibit 99.1 contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These statements relate to future events or to our future operating or financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “plan,” “expect” and similar expressions, or the negative or plural of these words or expressions, are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, but are not limited to, statements concerning the following:
| • | our estimates regarding our expenses, revenues, anticipated capital requirements and our needs for additional financing; |
| • | the implementation of our business model and strategic plans for our business and technology; |
| • | the timing of the commencement, progress and receipt of data from any of our preclinical studies and clinical trials; |
| • | the expected results of any clinical trial and the impact on the likelihood or timing of any regulatory approval; |
| • | the scope of protection we establish and maintain for intellectual property rights covering our technology; |
| • | the timing or likelihood of regulatory filings and approvals; |
| • | the outcome of any current or future litigation; |
| • | developments relating to our competitors and our industry; and |
| • | our expectations regarding licensing, acquisitions and strategic operations. |
In addition, you should refer to the “Risk Factors” section in the Company’s Annual Report on Form 10-K for the year ended December 31, 2015 (“Form 10-K”) and the Company’s most recent Quarterly Report on Form 10-Q for the quarter ended June 30, 2016 (“Form 10-Q”) for a discussion of other important factors, risks and uncertainties that may cause our actual results to differ materially from those expressed or implied by these forward-looking statements. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included herein and in our Form 10-K and Form 10-Q that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. We qualify all of the forward-looking statements in the foregoing documents by these cautionary statements. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make. Except as required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments.
Our Company
Overview
We are a clinical-stage immunotherapy company with next-generation, diversified in vivo approaches designed to enable the body’s immune system to fight disease. Although we believe our approaches have broad potential across multiple therapeutic areas, we are focused in oncology and have designed our technologies to activate the immune system’s natural ability to create tumor-specific cytotoxic T cells to fight cancer via distinct mechanisms. Our two lead product candidates, CMB305 and G100, utilize distinct immuno-oncology approaches that, we believe, address the shortcomings of existing therapies and have the potential to treat a broad patient population either as individual therapies or in combination with other mechanisms of action. We have also been executing a strategy to partner individual indications outside of oncology in infectious and allergic diseases, which provide potential downstream economics while preserving growth opportunity in the future.
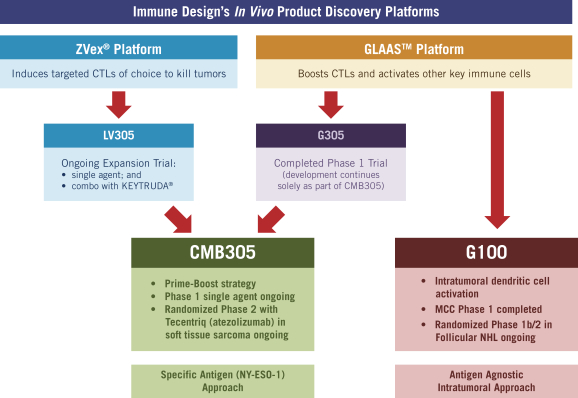
The following is our primary oncology product development pipeline produced by our two discovery platforms, ZVex® and GLAASTM:
Antigen Specific: Next-Generation Cancer Vaccines
CMB305 is a prime-boost approach targeting the NY-ESO-1 tumor antigen, in which an agent called LV305 from our ZVex platform is dosed sequentially with an agent from our GLAAS platform, G305. Both LV305 and G305 completed Phase 1 trials in 2014, the data for which we presented at the American Society of Clinical Oncology, or ASCO, annual meeting in 2015. CMB305 is currently being evaluated in multiple clinical trials, including, pursuant to a collaboration with Genentech, a randomized Phase 2 trial in patients with soft tissue sarcoma who receive either CMB305 combined with Genentech’s cancer immunotherapy, TecentriqTM (atezolizumab, anti-PD-L1) or Tecentriq alone. In June 2016, we disclosed that patient data from a completed first-in-human dose-escalation trial and an early subset of patients from an expansion trial of CMB305 in patients with soft tissue sarcoma showed that CMB305 had a favorable safety profile with only grade 1 and 2 adverse events, or AEs, and without dose-limiting toxicities. In addition, patients who responded immunologically had a greater degree of antigen-specific T cell response than previously reported in the Phase 1 trial of LV305 alone, which is consistent with the rationale of the prime boost approach. We also observed preliminary clinical benefit in the form of a median progression-free survival, or PFS, of 5.5 months, with a 93% patient survival as of the data review date. We continue to enroll patients in the ongoing randomized Phase 2 trial and, if afforded the opportunity, intend to present data from the trial at the ASCO annual meeting in 2017. We have received orphan drug designation in the US for CMB305, and in the US and EU for each component of CMB305, in each case, for soft tissue sarcoma. If the ongoing trials produce a sufficiently robust clinical benefit for patients, we plan to discuss an appropriate development path with the regulatory authorities and pursue soft tissue sarcoma as the first indication for which we would seek approval for CMB305. We are also developing a companion diagnostic in connection with our CMB305 development program to identify NY-ESO-1 expressing tumors, which test we believe would require U.S. Food and Drug Administration, or FDA, approval contemporaneously with CMB305.
2
Antigen Agnostic: Intratumoral Immune Activation
G100 was developed from the GLAAS platform and, in contrast to CMB305, leverages the range of endogenous antigens found in the tumor microenvironment, including neoantigens. At the ASCO annual meeting in 2016, we presented data on 10 patients from a completed Phase 1 trial in patients with Merkel cell carcinoma, or MCC, which showed: (1) an objective response rate, or ORR, of 50% per protocol; (2) a favorable safety profile; and (3) that G100 significantly altered the tumor microenvironment in responding patients. We are further developing G100 and, pursuant to a collaboration with Merck & Co., Inc., or Merck, we are enrolling patients in the Phase 2 portion of a randomized Phase 1b/2 trial in patients with follicular non-Hodgkin Lymphoma, or NHL, that will evaluate G100 in combination with local radiation and Merck’s anti-PD-1 agent, KEYTRUDA®. If afforded the opportunity, we intend to present data from this trial at the ASCO annual meeting in 2017.
Our Strategy
| • | Develop product candidates to treat a broad patient population. We believe our product candidates should benefit a wide range of patients in both orphan diseases and large indications because they are designed to create tumor-killing CTLs, could potentially target any tumor and have potential utility as both individual and multiple combination therapies. |
| • | Rapidly advance first-in-class immuno-oncology product candidates through clinical development. We intend to continue to execute a focused clinical development plan that takes selected product candidates through approval. We are initially focused on indications with a significant unmet need in targeted patient populations, such as CMB305 in soft tissue sarcoma. We are currently focusing our initial development efforts on CMB305 and G100, while preserving the ability to separately develop LV305. |
| • | Leverage our platforms’ ability to address multiple tumor types to build a robust product pipeline. Our ZVex and GLAAS platforms allow us to select both conserved tumor antigens and neoantigens and create separate product candidates for potentially any tumor type. We believe this ability, and the potential of our vectors to simultaneously express antigens and immuno-regulatory molecules, will be a driver of our future growth beyond the current product candidates. |
| • | Position Immune Design to potentially play a broad role in the immuno-oncology treatment paradigm. Our agents are designed to work either individually or together, as well as with multiple other mechanisms of action. In addition to our ongoing clinical collaborations combining CMB305 and G100 with checkpoint inhibitors, we intend to explore additional combinations with other immuno-oncology approaches to demonstrate this broad potential benefit. |
| • | Selectively monetize non-oncology indications, while retaining optionality for future internal development. Both ZVex and GLAAS also have potential application in infectious disease and allergy. We have licensed the right to use the GLAAS platform in specific infectious and allergic disease indications to large pharmaceutical companies. These collaborations provide us with both near- and long-term potential revenue and external validation of our technology, while preserving optionality for future growth beyond oncology. |
| • | Establish infrastructure and capabilities to support the future commercialization of our products. Our management team has extensive experience commercializing pharmaceutical products and as our product candidates advance, we intend to add the appropriate additional regulatory and commercial expertise to maximize the potential for successful product launches and franchise management. In certain instances, we will seek partners to maximize the commercial potential of our product candidates. |
ZVex and GLAAS: Complementary and Productive Product Discovery Platforms
We believe our approach to fighting cancer is the first of its kind. We utilize ZVex and GLAAS to develop product candidates that work in vivo and are designed to create and expand diverse armies of immune cells known as cytotoxic T lymphocytes, or CTLs, to fight tumors. An in vivo approach is preferred because it addresses both the cumbersome administration and the need for patient customization inherent in ex vivo approaches, such as engineered CD8 T cells. Although they have distinct mechanisms of action, we
3
designed both CMB305 and G100 to convert “cold” tumors, or those without CTLs, to “hot” tumors, or those with CTLs specific for the antigens expressed by the tumor. Although they are designed to share this effect on tumors, the agents’ distinct mechanisms of action may produce different clinical benefit profiles. For example, it has been noted in literature that although a cancer vaccine therapy may not result in an immediate or early change in tumor burden like cytotoxic approaches such as chemotherapy, a vaccine may induce a delayed anti-tumor response resulting in longer survival than that seen with the cytotoxic approach. Similarly, and based on our clinical studies to date, we believe that although we may not observe a short-term surrogate endpoint like ORR or PFS, ZVex-based cancer vaccines such as CMB305 may nonetheless confer a potentially meaningful overall survival, or OS, benefit in these patients.
The fundamental discoveries underlying ZVex originated with one of our founders, Nobel laureate David Baltimore, Ph.D. Dr. Baltimore and his colleagues theorized that a lentivirus, which is a virus that works in immune cells such as dendritic cells, or DCs, could be engineered to selectively deliver the specific genetic information of a tumor marker, called an antigen, directly to DCs in the skin. The expression of this antigen would trigger an immune response of CTLs to eliminate the tumor. In comparison, the core of the GLAAS platform is a highly potent synthetic stimulator of a specific cellular receptor called TLR4 that is present in DCs. Activation of DCs through TLR4 can safely trigger an anti-tumor immune response and synergize with either pre-existing CTLs or those generated by ZVex for what we believe will be a greater degree of tumor killing than either approach alone. We believe ZVex- and GLAAS-based product candidates have broad combination potential across the oncology landscape, such as in combination with checkpoint inhibitors in our two ongoing randomized studies and with other approaches, such as engineered T cells.
ZVex is a discovery platform that uses a first-in-class vector to generate product candidates designed to create CTLs in vivo. CTLs are essential because their primary function is the selective recognition and destruction of tumor cells. The ZVex vector is a delivery system based on a hybrid, re-engineered virus designed to carry the genetic information of selected tumor antigen(s) (in whole or selected epitopes) safely and selectively to
4
dendritic cells, or DCs, in the skin. We believe that DCs are the most important immune cells to target because they initiate the specific immune response that generates CTLs to kill the tumor. When injected into a cancer patient, a ZVex-based product candidate is designed to interact only with these DCs, delivering the tumor antigen(s) in the form of RNA. The DC then processes the RNA into a protein, splits it and presents the protein fragments outside of the cell to neighboring resting CD8 T cells, which then activate to become fully functional CD8+ CTLs. When a CD8 T cell is activated and starts dividing, the result is millions of CTLs that will kill tumor cells bearing that same specific tumor antigen epitopes. ZVex product candidates have the potential to carry the genetic material of different tumor antigens, including neoantigens, as well as immuno-modulatory agents, and therefore have the potential to target multiple types of cancers.
GLAAS, which stands for GLA Adjuvant Systems, is a discovery platform that also works in vivo and is based on a small synthetic molecule called GLA, which stands for glucopyranosyl lipid A. GLA selectively binds to the TLR4 receptor and causes potent activation of the DC. When GLA is accompanied by a tumor antigen and injected into a patient, the combination is taken up by DCs and leads to the production and expansion of immune cells called CD4 T helper lymphocytes, or CD4 T cells. Similar to CTLs, these CD4 T cells will be specific to a tumor antigen, but unlike CTLs, they generally cannot kill antigen-bearing tumor cells. They do, however, play a key role in boosting the anti-tumor immune response by: (1) expanding the number and function of existing CTLs that are specific to the same tumor antigen; and (2) providing help to other immune cells, including B lymphocytes that produce antibodies and natural killer, or NK, cells that are also important in the overall anti-tumor immune response. We therefore believe that product candidates leveraging GLAAS with one or more tumor antigens will be effective in amplifying the anti-tumor activity of CTLs, as well as other beneficial anti-tumor mechanisms. In addition, we can leverage GLAAS to use a specific formulation of GLA alone, without an antigen, for direct tumor microenvironment immune activation. Like ZVex, GLAAS product candidates have the potential to target multiple types of cancers.
The combination of ZVex and GLAAS is expected to synergize to yield a more potent immune response called a heterologous prime-boost. The ZVex vector primes the immune system by triggering the generation of CTLs, while the GLA-activated CD4 T cells boost the immune response by expanding and enhancing the function of CTLs and other anti-tumor immune mechanisms. We believe a more potent immune response should translate to clinical benefit for patients.
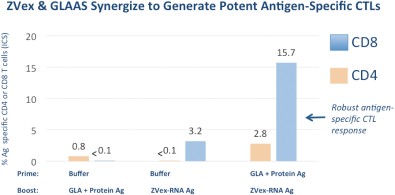
The following data from an in vivo rodent model illustrate the effect on antigen-specific CTL generation when combining the ZVex and GLAAS platforms in a prime-boost. When used alone, the ZVex agent increased the CTLs from 0.05% to 3.16%, and when used in combination with GLAAS, the percentage of antigen-specific CTLs in the rodents increased to 15.7%.
We are also studying a different combination of both platforms in a “prime-pull” strategy where a ZVex vector primes the immune system and G100 ‘pulls’ CTLs to the tumor via intratumoral injection. Further combination potential also exists with other immuno-oncology modalities, such as the use of G100 to “pull” engineered
5
T cells to a tumor. We believe that these combinations of different technologies have the potential to be best-in-class approaches that generate and expand CTLs and recruit them to the tumor.
Our Approaches to Treating Cancer
Immuno-oncology broadly refers to the modulation of the immune system to eradicate tumor cells, and is often colloquially divided into two categories: “create and expand” the anti-tumor immune response and “remove the brakes” placed on the immune response by the tumor’s defenses.
We believe alteration of the tumor microenvironment and trafficking of CTLs into the tumor are increasingly being recognized as important for the efficacy of any immunotherapy. Our platforms focus on the “create and expand” category and are designed to generate strong, tumor-specific CTLs and effector cells in vivo while addressing many of the shortcomings of previous approaches. Our platforms can generate individual product candidates, such as G100, or product candidates administered in sequence, such as CMB305. Additionally, we designed our therapies to be combined with other immuno-oncology therapeutic mechanisms such as checkpoint inhibitors from the “remove the brakes” category, which we believe will generate a greater anti-tumor response.
Our immuno-oncology product candidates are being developed in two separate strategies that we designate as the Antigen Specific and Antigen Agnostic approaches.
Antigen Specific
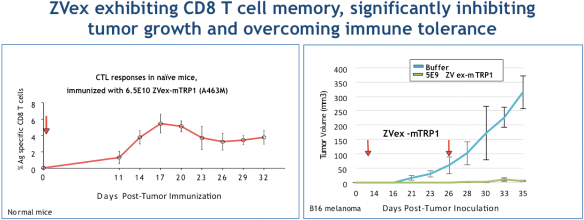
Our Antigen Specific approach is based on the observation that human tumor cells make a variety of antigens that are not found in normal tissues, but are present in the patient’s tumor, so there is an opportunity to educate the immune system to recognize the tumor antigen and kill tumor cells expressing it. ZVex products carry RNA of a chosen antigen or selected epitopes of multiple antigens, including neoantigens, whereas GLAAS products are accompanied by a full-length protein of the same antigen or, potentially, a peptide representing the selected epitopes. We have generated a significant amount of preclinical data illustrating the desirable qualities of this approach. The following graph illustrates the ability of ZVex in an in vivo rodent tumor model to generate an immune response against a protein the body recognizes as “self,” and therefore against which it would not normally mount an immune response. This experiment demonstrates the ability of ZVex to overcome immune tolerance, which is an important element of any potential cancer immunotherapy treatment.
For our first Antigen Specific product candidates, we have chosen a tumor-associated antigen named NY-ESO-1 that is expressed in a large number of solid and liquid tumors in varying degrees. We conducted an extensive search to choose NY-ESO-1, and we believe it is an attractive target for cancer immunotherapy due to its frequent expression in tumors, limited expression in normal tissue and its immunogenic potential. Among the antigens selected by the National Cancer Institute as the best targets for immunotherapy, only NY-ESO-1 and
6
one other antigen have been shown to be tumor-specific. Our first two clinical programs targeting NY-ESO-1 from ZVex and GLAAS were LV305 and G305, respectively. LV305 delivers the RNA for NY-ESO-1, while G305 consists of a specific formulation of GLA and the full-length NY-ESO-1 protein. We administer LV305 and G305 in sequence to become CMB305, the heterologous prime-boost therapy. Although G305 may have the potential to be an effective therapy with patients who have insufficient immune responses prior to treatment, we do not intend to develop it as a stand-alone product and believe it is more effective as part of CMB305. Also, although we have seen initial clinical benefit from LV305 as a single agent in patients with soft tissue sarcoma, because we believe CMB305 should be more effective than LV305 alone, we intend to focus our development efforts on CMB305. We may, however, decide to develop LV305 as the data continue to develop. For future product candidates, we are investigating the use of ZVex in the emerging neoantigen field, or ZVexNeo, where we believe we could deliver neoepitopes or selected epitopes from neoantigens, to DCs to induce CTLs specific for these potentially immunogenic targets. In May 2016, we announced a collaboration with Gritstone Oncology, or Gritstone, to apply ZVexNeo with Gritstone’s proprietary genomics and proteomics platform to develop neoantigen-based immunotherapies. The collaboration will likely focus initially in non-small cell lung cancer, with the first clinical trial expected to commence in 2017.
In addition to ZVexNeo, we are also investigating the potential of a next-generation of ZVex vectors, or ZVex2.0, to deliver multiple antigens with the intent to induce CTLs targeting multiple tumors or a single tumor that expresses the antigens of interest, as the case may be. ZVex2.0 may also contain modifications to the vector backbone and be designed to express immunostimulatory molecules. We intend to designate the first ZVex2.0 product candidate in 2017.
Antigen Agnostic
Unlike the Antigen Specific approach, the Antigen Agnostic approach does not require a selected tumor antigen present in the cancer. It instead relies on endogenous conserved antigens or neoantigens released during tumor lysis by treatments such as chemotherapy or local radiation. G100, our lead product under this approach, is injected directly into the tumor and neighboring GLA-activated DCs then capture the diverse set of released antigens and generate a broad and varied immune response. Because local radiation is an effective way to cause tumor cell lysis in accessible tumors, we plan initially to evaluate tumors that are accessible to both local radiation and intratumoral administration.
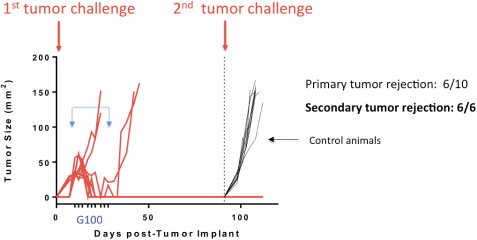
In collaboration with Dr. Ronald Levy’s lab at Stanford University, we examined the administration of intratumorally-injected G100 in the A20 murine model that is used to represent lymphoma. In an oral presentation at the 2015 American Society of Hematology, or ASH, annual meeting, Dr. Levy’s lab presented data showing tumor growth inhibition in both injected tumors as well as uninjected tumors, known as an abscopal effect. In addition, G100 had an impact on the tumor microenvironment, changing it from a non-inflammatory state, or cold, to an inflamed state, or hot. Specifically, as shown in the image below, responding animals remained tumor-free at least three months post G100 treatment and, without administration of additional G100, were resistant to secondary challenge with the same tumor type.
7
G100 is being evaluated in multiple clinical trials, including a completed Phase 1 trial in patients with MCC and an ongoing investigator-sponsored trial at the Fred Hutchinson Cancer Research Center, or FHCRC, in combination with radiation in patients with sarcoma. At the ASCO annual meeting in 2016, we presented data on all 10 patients in the MCC trial, which showed: (1) an ORR of 50% per protocol; (2) a favorable safety profile with no treatment-related serious adverse events observed; and (3) that G100 significantly altered the tumor microenvironment in responding patients. Further, the 50% ORR included a pathologic complete response after two doses of G100 alone, and we reported two patients with durable partial responses, or PRs, of greater than 17 and 18 months. We plan to assess the potential impact on PFS and OS beyond the stated ORR. We are further developing G100 and, pursuant to a collaboration with Merck, we are enrolling a randomized Phase 2 trial in patients with follicular NHL that will evaluate G100 in combination with local radiation and KEYTRUDA. The low incidence rates of NHL and MCC qualify each as an orphan disease, and if we are able to obtain orphan drug designation from the FDA for G100 for NHL or MCC, we may be able to obtain certain benefits such as research tax credits and grant funding. Either disease could be an excellent setting to show that G100 can provide clinical benefit and may provide separate registration paths.
In addition to G100, we are also investigating the potential use of our ZVex platform for intratumoral injection. For example, we presented preclinical data at the American Association for Cancer Research Annual Meeting in 2016 describing the intratumoral administration of a ZVex vector designed to generate localized expression of IL-12, a potent modulator of innate and adaptive immune responses. The results demonstrated strong local and systemic anti-tumor efficacy in multiple murine models, and this use of the ZVex platform offers a potential expansion opportunity of our Antigen Agnostic approach beyond G100.
8
Our Product Candidates in Development
Our clinical-stage oncology product candidates are depicted in the following diagram:
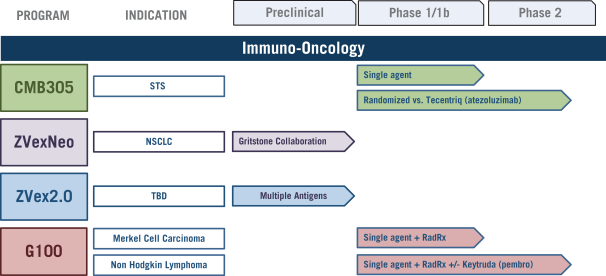
CMB305
We believe that prime-boost therapies are an optimal way to trigger a robust immune response. This is particularly true when distinct, but complementary, parts of the immune response are stimulated. Based on the predicted mechanisms of action in the prime-boost, our relevant preclinical studies and early CMB305 clinical data, we expect CMB305 to have synergistic effects and induce a stronger anti-tumor CTL response than either of its components alone. In addition to increasing the magnitude of the CTL response, we expect this approach to generate memory CTLs with long-term immune surveillance, as well as enhance other immune system anti-tumor mechanisms. Memory CTLs function as surveillance cells, ready to target persisting or new cancer cells with the same antigen signature, which may provide a longer-term benefit to patients.
We plan to test CMB305 first in two types of sarcoma, called synovial sarcoma and myxoid round cell liposarcoma. Synovial sarcoma is a rare form of cancer in the joints. Certain studies of outcomes relating to synovial sarcoma have shown a five-year and ten-year survival for people with grade 3 tumors or metastatic disease of less than 25% and 15%, respectively. Myxoid round cell liposarcoma is a rare malignant tumor that most often occurs in the deep-seated soft tissues of the extremities. Approximately 50% of patients with synovial sarcoma and approximately 90% myxoid round cell liposarcoma express the NY-ESO-1 protein. The low incidence rates of these sarcomas qualifies each as an orphan disease, and we have received orphan drug designation for soft tissue sarcoma for CMB305 in the US and for both components of CMB305 in the US and EU. Orphan drug designation provides certain benefits, such as research tax credits and waivers of certain regulatory fees, but does not provide any assurance of regulatory approval or expedite regulatory review. However, these soft tissue sarcomas could be an ideal setting to show that CMB305 can provide clinical benefit to patients and, if the data are sufficiently robust and CMB305 is determined to be safe, this may provide an accelerated registration path. We believe that there is an opportunity to improve upon both the safety and clinical benefit profiles of approved chemotherapy agents in soft tissue sarcoma, which to date have demonstrated poor toxicity and produced limited impact on patient survival.
In separate Phase 1 trials presented at the ASCO annual meeting in 2015, each of CMB305’s components demonstrated an acceptable safety profile and, we believe, sufficient immunogenicity as individual agents. In addition, all of the patients in the LV305 dose-escalation study had types of soft tissue sarcoma, and we saw initial signs of clinical benefit. Because of these results and the supportive preclinical data, we are evaluating CMB305 in a Phase 1 expansion trial and randomized Phase 2 trial. As announced in June 2016, data from
9
an early subset of patients from the Phase 1 trial showed CMB305 as a single agent was without dose-limiting toxicities, as reviewed by an independent data safety monitoring board, or DSMB. CMB305 induced an antigen-specific T cell response in 50% of the patients, and patients who did respond immunologically had a greater degree of antigen-specific T cell response than that previously reported with LV305 alone, as well as a fully integrated response inducing antigen-specific CD4, CD8 and antibodies, both of which are consistent with the rationale of the prime-boost approach. We also observed initial data showing that the immune response induced in this approach may take time to emerge, as evidenced by the following CMB305 patient data revealing an integrated immune response (CD8s, CD4s and antibodies that were NY-ESO-1 specific) occurring over a period up to 112 days:
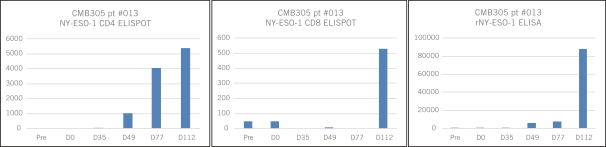
The time required to generate an immune response may be a contributing factor in the observation that cancer vaccines, although not likely to result in a significant ORR or PFS, may confer a better OS benefit than cytotoxic agents. Finally, preliminary clinical benefit in patients with soft tissue sarcoma was observed in the form of a median PFS of 5.5 months, with 93% patient survival as of the data review date. Chemotherapeutic agents approved to treat sarcoma have shown an OS of 12.4 to 13.5 months.
In addition to the ongoing Phase 1 trial examining CMB305 as a single agent, we have an ongoing randomized Phase 2 trial in patients with synovial or myxoid round cell liposarcoma. The patient’s cancer must be locally advanced, relapsed, or metastatic and express NY-ESO-1, and they must have had an inadequate response to, relapse from, and/or unacceptable toxicity with one or more prior systemic, surgical, or radiation cancer therapies. Pursuant to a collaboration with Genentech, Inc., or Genentech, these patients receive either CMB305 combined with Tecentriq, or Tecentriq alone. Clinical benefit will be evaluated by analyzing tumor responses and progression through short and long-term follow-up via clinical and radiological assessments.
We believe our “create and expand” the immune response approach embodied in CMB305, when combined with an agent such as Tecentriq that is designed to shut down certain tumor defenses, is potentially an excellent combination to bring greater clinical benefit than either approach alone, and may qualify for an accelerated approval path, either via an expansion of the ongoing Phase 2 trial or new trial, if the clinical benefit data are sufficiently robust and CMB305 is determined to be safe. In addition, we may expand the CMB305 Phase 1 trial further and examine safety and OS versus historical data as a potential additional registration path in patients with soft tissue sarcoma. If the data are sufficiently robust and if supported by the FDA, we intend to file a Biologics License Application, as soon as reasonably possible, and potentially by the end of 2018.
LV305 (the Prime in CMB305’s Prime Boost)
We have completed dosing in a Phase 1 trial to evaluate the safety of escalating doses of single agent LV305 in patients with locally advanced, recurrent, or metastatic cancer expressing the NY-ESO-1 tumor antigen. Although the trial was open to patients with multiple tumor types, all of the patients who enrolled had sarcoma. When available, we took post-treatment tumor biopsies to assist in clarifying the mechanisms that may mediate a treatment effect, such as the generation of NY-ESO-1 specific CTLs. Clinical benefit was evaluated by analyzing tumor responses and progression through short and long-term follow-up via clinical and radiological assessments. We also are conducting an ongoing expansion trial of LV305 at the highest dose
10
studied in the Phase 1 dose escalation trial. The expansion trial is open to patients with locally advanced, relapsed or metastatic melanoma, sarcoma, ovarian cancer or non-small cell lung cancer that express NY-ESO-1. A portion of this trial will explore, among other things, the use of LV305 with KEYTRUDA in melanoma patients who have an inadequate response to anti-PD1 therapy, pursuant to a collaboration with Merck.
At the ASCO meeting in 2016, we presented data on 24 patients from the LV305 Phase 1 trial with advanced or metastatic sarcoma cancers expressing NY-ESO-1. These patients had a median PFS of 4.6 months, and 58% of patients had clinical benefit in the form of stable disease and one patient showed a partial response, or PR. The safety profile remained very favorable with only grade 1 and 2 AEs. In addition, a median OS had not yet been reached, with 81% survival at one year. As set forth in the figures below, in accordance with gradual onset of immune response observed in the CMB305 patient data disclosed in June and set forth above, the observed PR occurred at approximately 18 months and was accompanied by NY-ESO-1 specific T cell memory. We believe the nature of the mechanism of action taken with these combined data may be evidence that a ZVex product, although not likely to produce to a near-term ORR or PFS in sarcoma patients, may result in a clinical benefit over time.
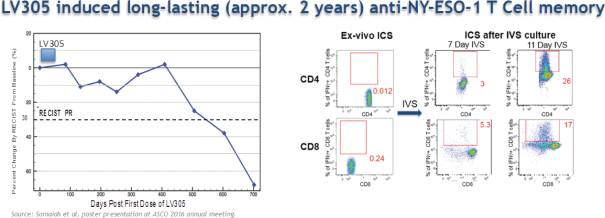
In all of these Antigen Specific trials, we will be collecting blood and tumor samples to measure CTL generation against NY-ESO-1 and to determine epitope mapping, antigen spreading and T cell receptor repertoire. If afforded the opportunity, we intend to present data for the ongoing CMB305 trials, as well as any follow-up data from the LV305 trials, at the ASCO annual meeting in 2017.
Antigen Agnostic
G100
We are evaluating our lead Antigen Agnostic approach product candidate, G100, in a recently completed Phase 1 trial and an ongoing Phase 1b/2 trial. In the Phase 1 trial, we treated in patients with MCC, which is a rare and aggressive type of skin cancer associated with a polyomavirus infection and UV exposure. The majority of patients present with localized disease in the skin, although the disease can readily spread to regional and distant sites. The accessibility of most MCC tumors makes them excellent for intratumoral dosing and obtaining skin lesion biopsies to determine changes in the tumor microenvironment following G100 treatment. The Phase 1 trial was designed to evaluate the safety and immunogenicity and provide preliminary indications of efficacy of G100 in MCC patients with either loco-regional or metastatic disease. At the ASCO annual meeting in 2016, we presented data on all 10 patients in the MCC trial showing: (1) an ORR of 50% per protocol; (2) a favorable safety profile; and (3) that G100 significantly altered the tumor microenvironment in responding patients. Further, the 50% ORR included a pathologic complete response after two doses of G100 alone, and we reported two patients with durable PRs of greater than 17 and 18 months. We intend to follow
11
these patients to further evaluate the safety of G100 and durability of these responses, and may elect to continue development of G100 in MCC. We plan to assess the potential impact on PFS and OS beyond the stated ORR.
We are also developing G100 to treat patients with a follicular NHL. Pursuant to a collaboration with Merck, we recently dosed the first patient in a randomized Phase 2 trial in patients with follicular NHL in combination with local radiation and KEYTRUDA. These patients must be either treatment naïve or relapsed or refractory following at least one prior treatment. We plan to inject a single tumor after the administration of local radiation, and then evaluate the local immune environment and the potential clinical effect on distant tumors. We expect data from a subset of patients to be available by the first half of 2017 and, if afforded the opportunity, intend to present the data at the ASCO annual meeting in 2017. As potential registration paths in NHL, we may decide to further pursue G100 in combination with checkpoint inhibitors as well as G100 with radiation alone.
We are also developing G100 for the treatment of other types of tumors where preclinical data suggests there may be opportunities for the Antigen Agnostic approach, such as an ongoing an investigator-sponsored sarcoma clinical trial at the Fred Hutchinson Cancer Research Center, or FHCRC, which we expect to complete by year-end 2016.
Therapeutic Applications Outside Oncology
Although immuno-oncology development is robust with therapies for an estimated 10 liquid and 18 solid tumors in development and with a market for immuno-oncology therapies projected to approach $35 billion by 2023, the broader market for immunotherapy applications also includes infectious and allergic diseases. The worldwide infectious diseases vaccine market garnered approximately $30 billion in sales in 2014 and the market for allergy therapies and diagnostics is projected to reach $41 billion by 2022. Beyond oncology, we believe our technologies offer several promising applications in the fields of infectious and allergic diseases.
Infectious Diseases
Historically, antigens have been used with sub-optimal immune adjuvants and have mainly focused on generating antibodies, which have been limited by low affinity and a narrow spectrum of activity. We believe using glucopyranosyl lipid adjuvant, or GLA, a novel molecular adjuvant, combined with infectious diseases antigens will boost pre-existing T cells and trigger a broad antibody response, allowing for diverse antigen recognition. To date, GLA has been studied in human clinical trials involving over 1,400 subjects. The results of these trials that we have reviewed to date support the finding of increased magnitude and breadth of the antibody response.
We have a preclinical vaccine product candidate called G103 to treat herpes simplex virus type 2, or HSV2. G103 consists of several recombinantly expressed proteins adjuvanted with a specific formulation of GLA. In October 2014, we announced a collaboration with Sanofi Pasteur, the vaccines division of Sanofi, to develop G103 along with additional assets contributed by us and Sanofi Pasteur. In addition to the G103 program, we have granted several licenses under the GLAAS platform to partners developing a range of infectious disease vaccines, including a license to MedImmune LLC, to develop a vaccine for respiratory syncytial virus, which began Phase 2 trials in October 2015.
Allergic Diseases
We believe allergy represents an exciting area for the application of GLAAS. Allergies to pollen or food often occur because of aberrant immune reactions, which are characterized by helper T cells producing signals that induce other immune cells to cause the allergy symptoms. We have a large set of preclinical data demonstrating that certain formulations of GLAAS, when given prophylactically or therapeutically with or without the allergen, can shift the responses in a way that results in significant protection from allergy symptoms. In essence, the immune system can be taught to redirect the T cells to respond in better ways. In August 2014, we announced a licensing agreement with Sanofi pursuant to which we granted Sanofi the right to use the GLAAS platform to develop therapeutic agents to treat peanut allergies.
12
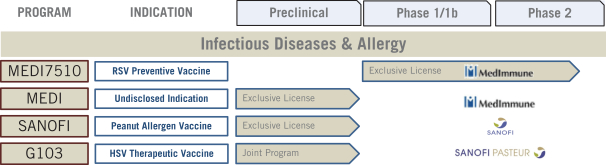
Infectious and Allergic Disease Immunotherapy Programs
We have been executing a strategy to partner the use of our GLAAS platform in individual indications outside of oncology in infectious and allergic diseases, which provide potential downstream revenue while preserving growth opportunity in the future. The following chart details our existing infectious disease programs and collaborations:
13
