Attached files
| file | filename |
|---|---|
| 8-K - 8-K FINAL ILLUMENATE DATA - SPECTRANETICS CORP | a8-k2016finalillumenatedata.htm |
| EX-99.1 - EXHIBIT 99.1 PRESS RELEASE - SPECTRANETICS CORP | ex9912016finalillumenateda.htm |

ILLUMENATE European
Randomized Clinical Trial:
Prof. Dr. Marianne Brodmann
12-Month Final Results for the
Stellarex DCB
Division of Angiology, Medical University
Graz, Austria
On behalf of Dr. Henrik Schröder
Jewish Hospital, Berlin, Germany

Disclosures
• Consulting/Honoraria for
– Medtronic
– BARD
– Spectranetics
– Intact Vascular
– Avinger
– Soundbite Medical
– Rexgenero
– Biotronik
– Bayer
– Daiichi
– Böhringer Ingelheim
– Astra Zeneca
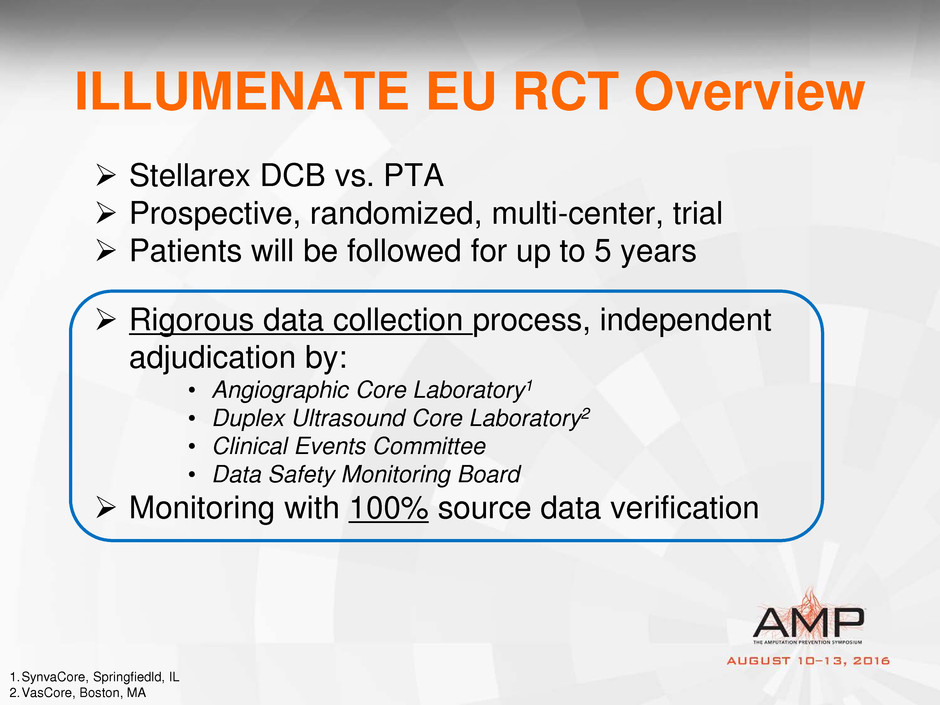
ILLUMENATE EU RCT Overview
Stellarex DCB vs. PTA
Prospective, randomized, multi-center, trial
Patients will be followed for up to 5 years
Rigorous data collection process, independent
adjudication by:
• Angiographic Core Laboratory1
• Duplex Ultrasound Core Laboratory2
• Clinical Events Committee
• Data Safety Monitoring Board
Monitoring with 100% source data verification
1.SynvaCore, Springfiedld, IL
2.VasCore, Boston, MA
ILLUMENATE EU RCT
Investigators
Dr. Henrik Schroeder :Jewish Hospital, Berlin
National Principal Investigator
Prof. Marianne Brodmann: LKH University Hospital Graz
Dr. Beata Lux: St Joseph Hospital, Berlin Prof. Peter Reimer:Clinical Center, Karlsruhe
Dr. Dirk-Roelfs Meyer: Lutheran Hospital Hubertus, Berlin Prof. Markus Duex: Hospital Nordwest GmbH, Frankfurt a. Main
Dr. Karsten Krueger: Vivantes Humboldt Hospital, Berlin Dr. Goetz Voshage: Robert Koch Clinical Center, Gehrden
Dr. Karsten Krueger: Vivantes Clinic Spandau, Berlin Prof. Giovanni Torsello: Saint Francis Hospital GmbH, Münster
Dr. Volker Sesselmann: SHR Central Clinic, Suhl Prof. Gunnar Tepe: RoMed Hospital, Rosenheim
Prof. Martin Zwaan: Ammerland-Clinic GmbH, Westerstede Prof. Claus Nolte-Ernsting: Lutheran Hospital, Mülheim
Prof. Thomas Albrecht: Vivantes Clinic Neukölln, Berlin Prof. Christian Loewe: University Hospital, Vienna
Prof. Roman Fischbach: Altona-Asklepios Clinic, Hamburg Dr. Martin Werner: Hanusch Hospital of WGKK Group, Vienna
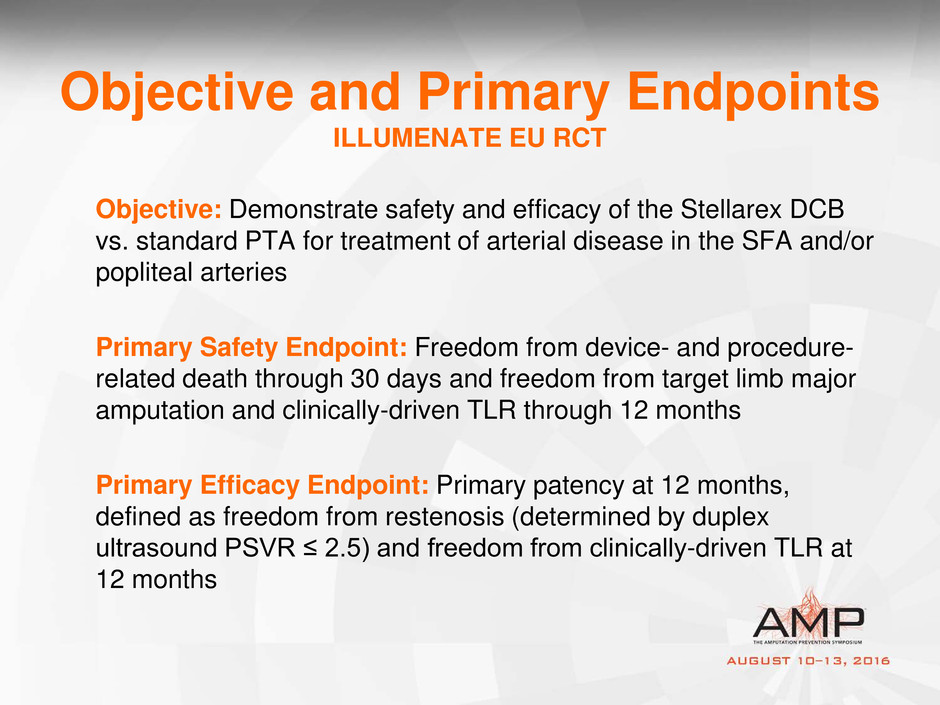
Objective and Primary Endpoints
ILLUMENATE EU RCT
Objective: Demonstrate safety and efficacy of the Stellarex DCB
vs. standard PTA for treatment of arterial disease in the SFA and/or
popliteal arteries
Primary Safety Endpoint: Freedom from device- and procedure-
related death through 30 days and freedom from target limb major
amputation and clinically-driven TLR through 12 months
Primary Efficacy Endpoint: Primary patency at 12 months,
defined as freedom from restenosis (determined by duplex
ultrasound PSVR ≤ 2.5) and freedom from clinically-driven TLR at
12 months

Study Device: StellarexTM DCB
(Spectranetics)
CAUTION: Investigational device. Not for sale or distribution in the United States.
EnduraCoatTM technology:
• Low dose paclitaxel, 2 µg/mm2
• Excipient: Polyethylene Glycol (PEG)
• Proprietary open-folded coating technology
Balloon catheter features:
• Catheter shaft designed for pushability
• Low 0.039” tip entry profile
• Flexible balloon and tip for tracking through tortuous
anatomy
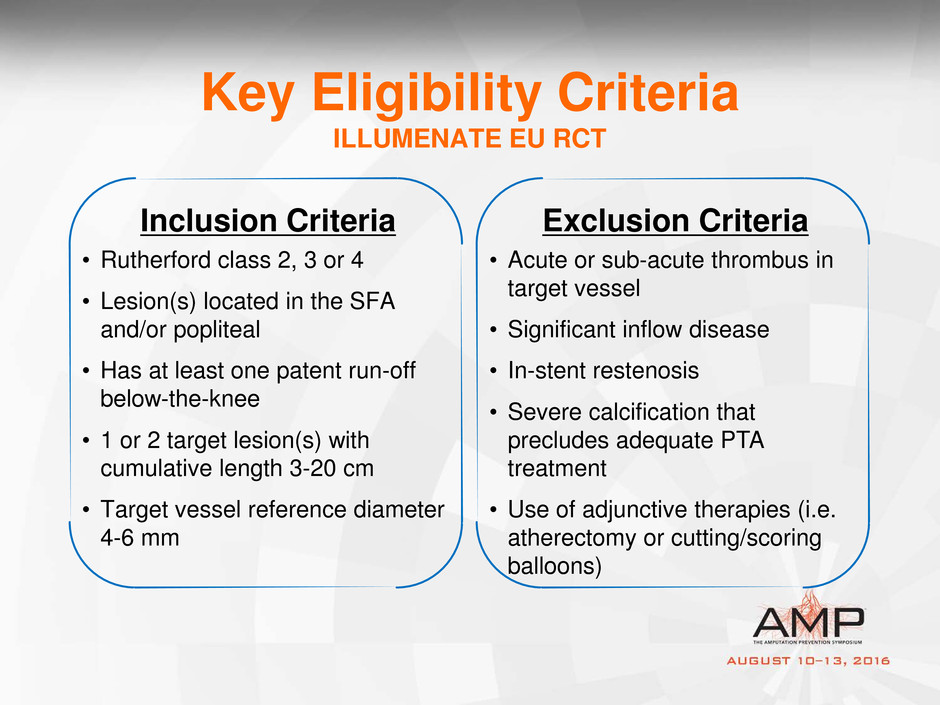
Key Eligibility Criteria
ILLUMENATE EU RCT
Inclusion Criteria
• Rutherford class 2, 3 or 4
• Lesion(s) located in the SFA
and/or popliteal
• Has at least one patent run-off
below-the-knee
• 1 or 2 target lesion(s) with
cumulative length 3-20 cm
• Target vessel reference diameter
4-6 mm
Exclusion Criteria
• Acute or sub-acute thrombus in
target vessel
• Significant inflow disease
• In-stent restenosis
• Severe calcification that
precludes adequate PTA
treatment
• Use of adjunctive therapies (i.e.
atherectomy or cutting/scoring
balloons)
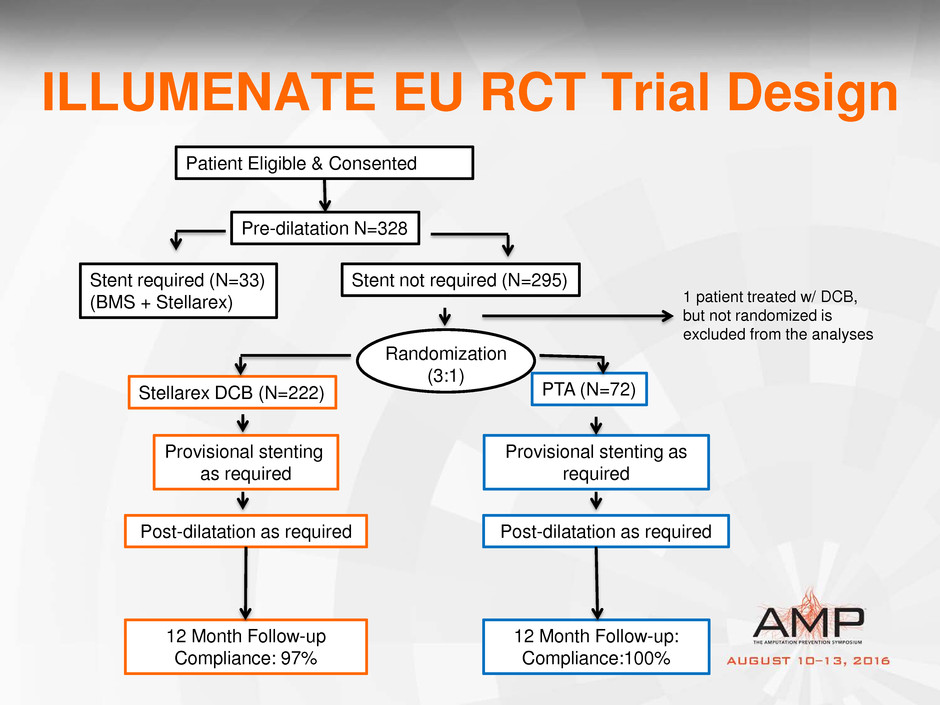
ILLUMENATE EU RCT Trial Design
Patient Eligible & Consented
Pre-dilatation N=328
Stent required (N=33)
(BMS + Stellarex)
Stent not required (N=295)
Randomization
(3:1)
Stellarex DCB (N=222) PTA (N=72)
Provisional stenting
as required
Post-dilatation as required
Provisional stenting as
required
Post-dilatation as required
1 patient treated w/ DCB,
but not randomized is
excluded from the analyses
12 Month Follow-up
Compliance: 97%
12 Month Follow-up:
Compliance:100%
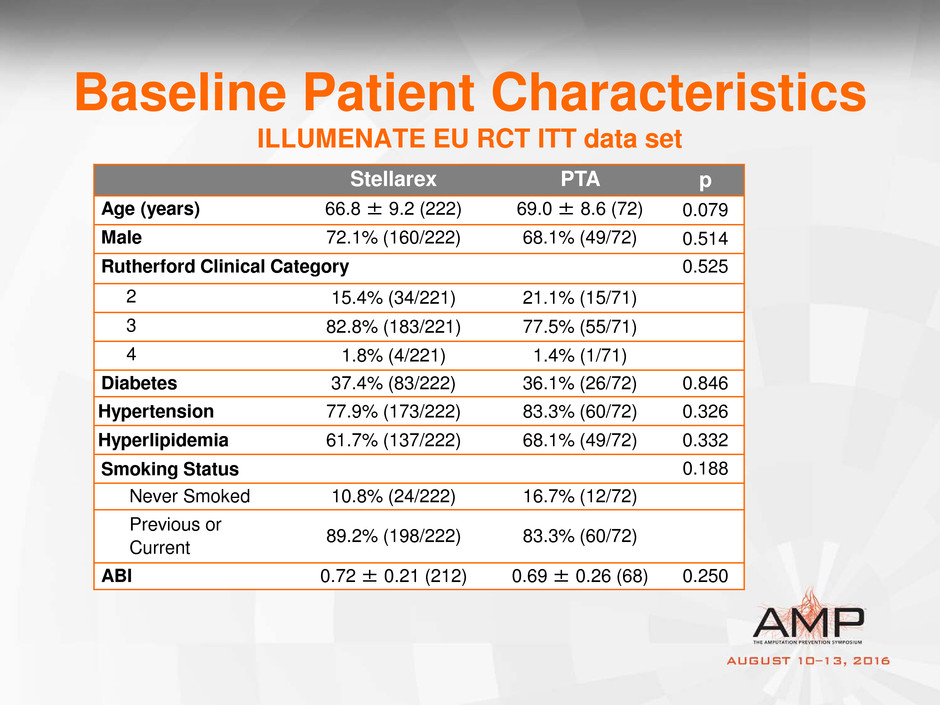
Baseline Patient Characteristics
ILLUMENATE EU RCT ITT data set
Stellarex PTA p
Age (years) 66.8 ± 9.2 (222) 69.0 ± 8.6 (72) 0.079
Male 72.1% (160/222) 68.1% (49/72) 0.514
Rutherford Clinical Category 0.525
2 15.4% (34/221) 21.1% (15/71)
3 82.8% (183/221) 77.5% (55/71)
4 1.8% (4/221) 1.4% (1/71)
Diabetes 37.4% (83/222) 36.1% (26/72) 0.846
Hypertension 77.9% (173/222) 83.3% (60/72) 0.326
Hyperlipidemia 61.7% (137/222) 68.1% (49/72) 0.332
Smoking Status 0.188
Never Smoked 10.8% (24/222) 16.7% (12/72)
Previous or
Current
89.2% (198/222) 83.3% (60/72)
ABI 0.72 ± 0.21 (212) 0.69 ± 0.26 (68) 0.250
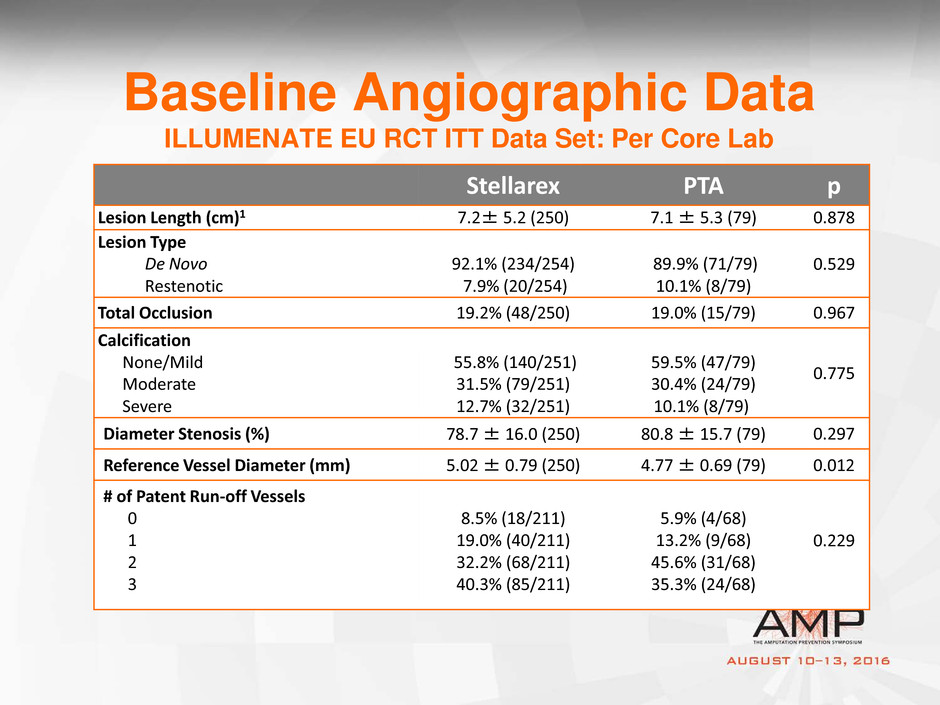
Baseline Angiographic Data
ILLUMENATE EU RCT ITT Data Set: Per Core Lab
Stellarex PTA p
Lesion Length (cm)1 7.2± 5.2 (250) 7.1 ± 5.3 (79) 0.878
Lesion Type
De Novo
Restenotic
92.1% (234/254)
7.9% (20/254)
89.9% (71/79)
10.1% (8/79)
0.529
Total Occlusion 19.2% (48/250) 19.0% (15/79) 0.967
Calcification
None/Mild
Moderate
Severe
55.8% (140/251)
31.5% (79/251)
12.7% (32/251)
59.5% (47/79)
30.4% (24/79)
10.1% (8/79)
0.775
Diameter Stenosis (%) 78.7 ± 16.0 (250) 80.8 ± 15.7 (79) 0.297
Reference Vessel Diameter (mm) 5.02 ± 0.79 (250) 4.77 ± 0.69 (79) 0.012
# of Patent Run-off Vessels
0
1
2
3
8.5% (18/211)
19.0% (40/211)
32.2% (68/211)
40.3% (85/211)
5.9% (4/68)
13.2% (9/68)
45.6% (31/68)
35.3% (24/68)
0.229
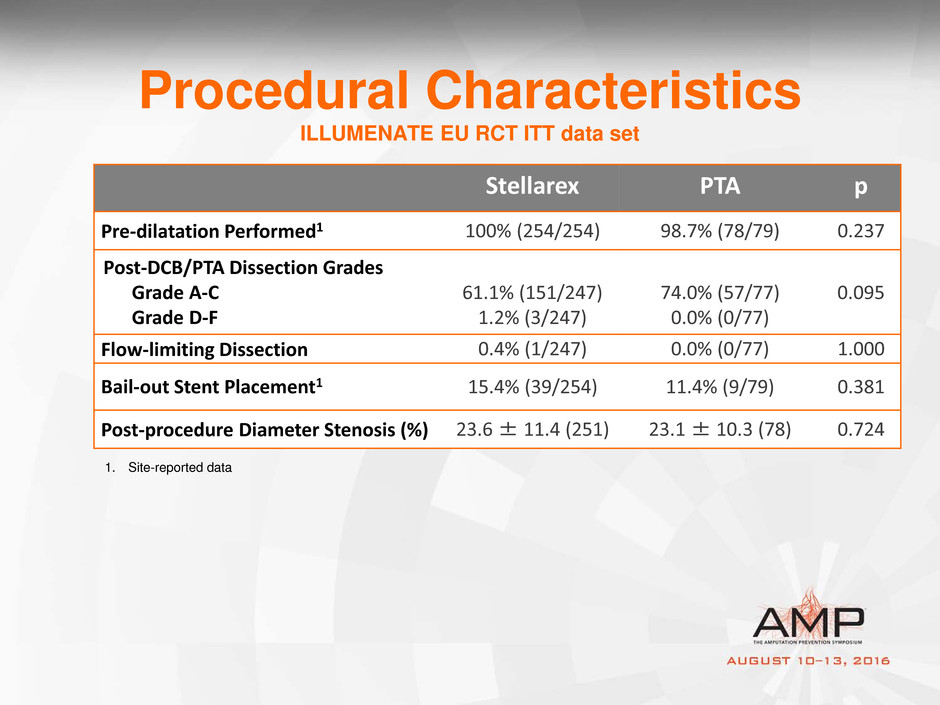
Procedural Characteristics
ILLUMENATE EU RCT ITT data set
Stellarex PTA p
Pre-dilatation Performed1 100% (254/254) 98.7% (78/79) 0.237
Post-DCB/PTA Dissection Grades
Grade A-C
Grade D-F
61.1% (151/247)
1.2% (3/247)
74.0% (57/77)
0.0% (0/77)
0.095
Flow-limiting Dissection 0.4% (1/247) 0.0% (0/77) 1.000
Bail-out Stent Placement1 15.4% (39/254) 11.4% (9/79) 0.381
Post-procedure Diameter Stenosis (%) 23.6 ± 11.4 (251) 23.1 ± 10.3 (78) 0.724
1. Site-reported data
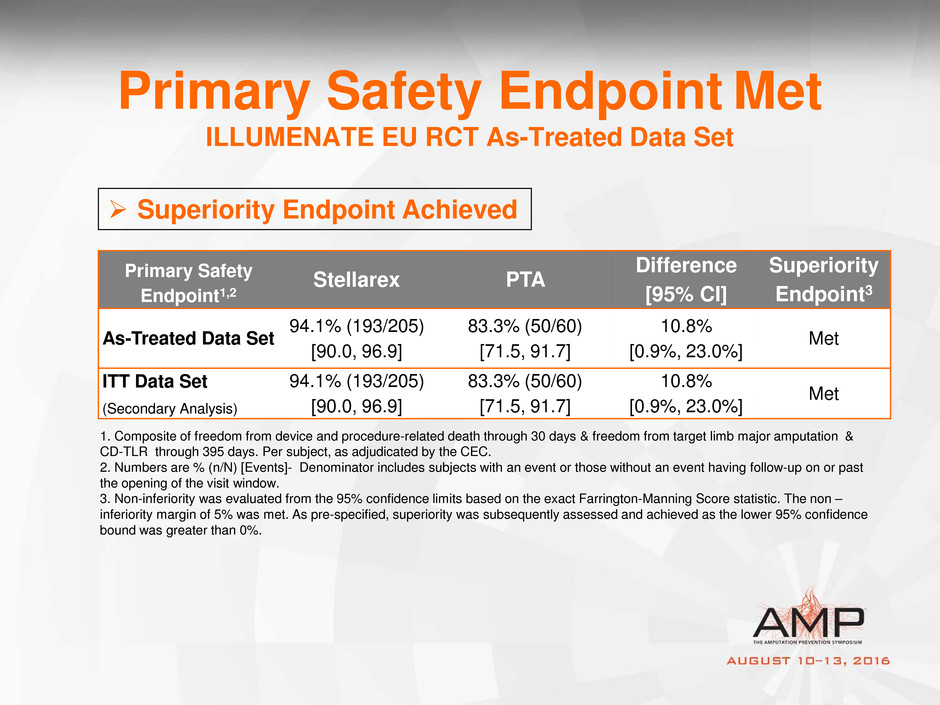
Primary Safety Endpoint Met
ILLUMENATE EU RCT As-Treated Data Set
1. Composite of freedom from device and procedure-related death through 30 days & freedom from target limb major amputation &
CD-TLR through 395 days. Per subject, as adjudicated by the CEC.
2. Numbers are % (n/N) [Events]- Denominator includes subjects with an event or those without an event having follow-up on or past
the opening of the visit window.
3. Non-inferiority was evaluated from the 95% confidence limits based on the exact Farrington-Manning Score statistic. The non –
inferiority margin of 5% was met. As pre-specified, superiority was subsequently assessed and achieved as the lower 95% confidence
bound was greater than 0%.
Superiority Endpoint Achieved
Primary Safety
Endpoint1,2
Stellarex PTA
Difference
[95% CI]
Superiority
Endpoint3
As-Treated Data Set
94.1% (193/205)
[90.0, 96.9]
83.3% (50/60)
[71.5, 91.7]
10.8%
[0.9%, 23.0%]
Met
ITT Data Set
(Secondary Analysis)
94.1% (193/205)
[90.0, 96.9]
83.3% (50/60)
[71.5, 91.7]
10.8%
[0.9%, 23.0%]
Met
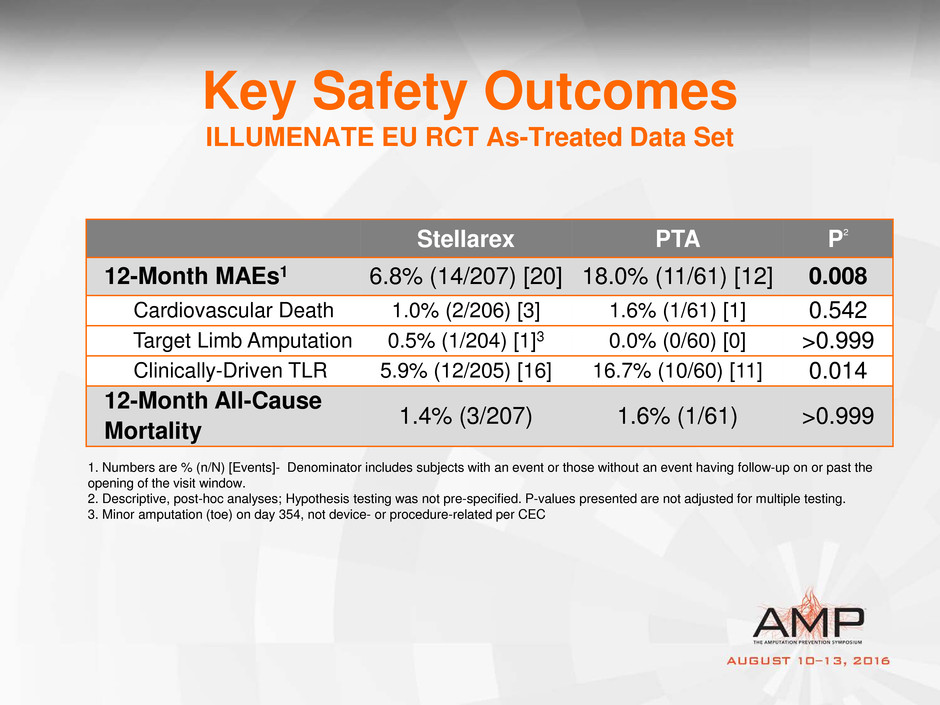
Key Safety Outcomes
ILLUMENATE EU RCT As-Treated Data Set
Stellarex PTA P2
12-Month MAEs1 6.8% (14/207) [20] 18.0% (11/61) [12] 0.008
Cardiovascular Death 1.0% (2/206) [3] 1.6% (1/61) [1] 0.542
Target Limb Amputation 0.5% (1/204) [1]3 0.0% (0/60) [0] >0.999
Clinically-Driven TLR 5.9% (12/205) [16] 16.7% (10/60) [11] 0.014
12-Month All-Cause
Mortality
1.4% (3/207) 1.6% (1/61) >0.999
1. Numbers are % (n/N) [Events]- Denominator includes subjects with an event or those without an event having follow-up on or past the
opening of the visit window.
2. Descriptive, post-hoc analyses; Hypothesis testing was not pre-specified. P-values presented are not adjusted for multiple testing.
3. Minor amputation (toe) on day 354, not device- or procedure-related per CEC
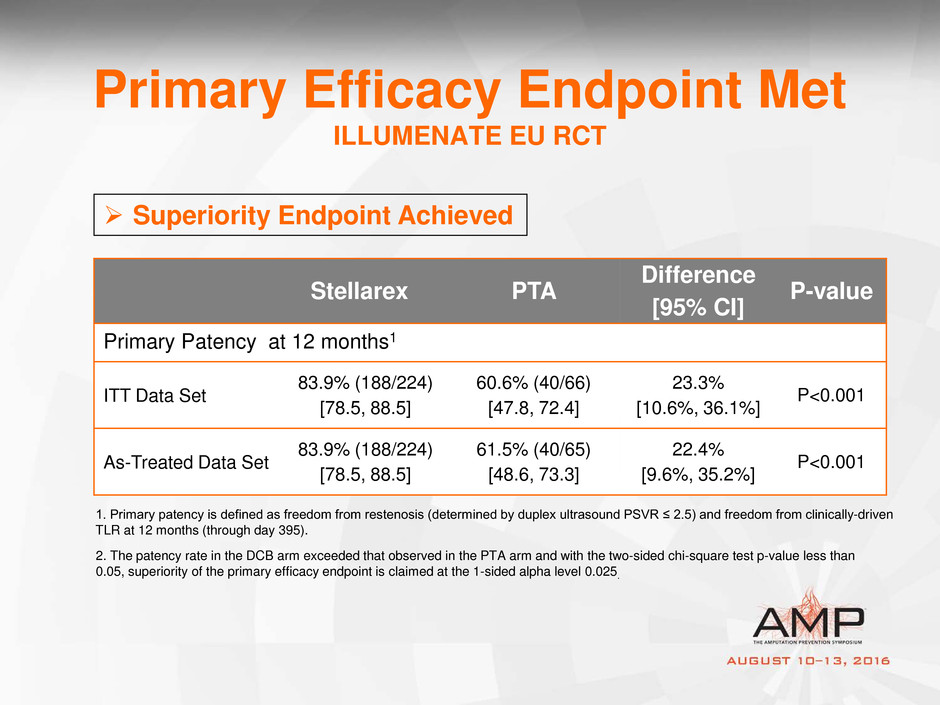
Primary Efficacy Endpoint Met
ILLUMENATE EU RCT
Stellarex PTA
Difference
[95% CI]
P-value
Primary Patency at 12 months1
ITT Data Set
83.9% (188/224)
[78.5, 88.5]
60.6% (40/66)
[47.8, 72.4]
23.3%
[10.6%, 36.1%]
P<0.001
As-Treated Data Set
83.9% (188/224)
[78.5, 88.5]
61.5% (40/65)
[48.6, 73.3]
22.4%
[9.6%, 35.2%]
P<0.001
2. The patency rate in the DCB arm exceeded that observed in the PTA arm and with the two-sided chi-square test p-value less than
0.05, superiority of the primary efficacy endpoint is claimed at the 1-sided alpha level 0.025.
1. Primary patency is defined as freedom from restenosis (determined by duplex ultrasound PSVR ≤ 2.5) and freedom from clinically-driven
TLR at 12 months (through day 395).
Superiority Endpoint Achieved
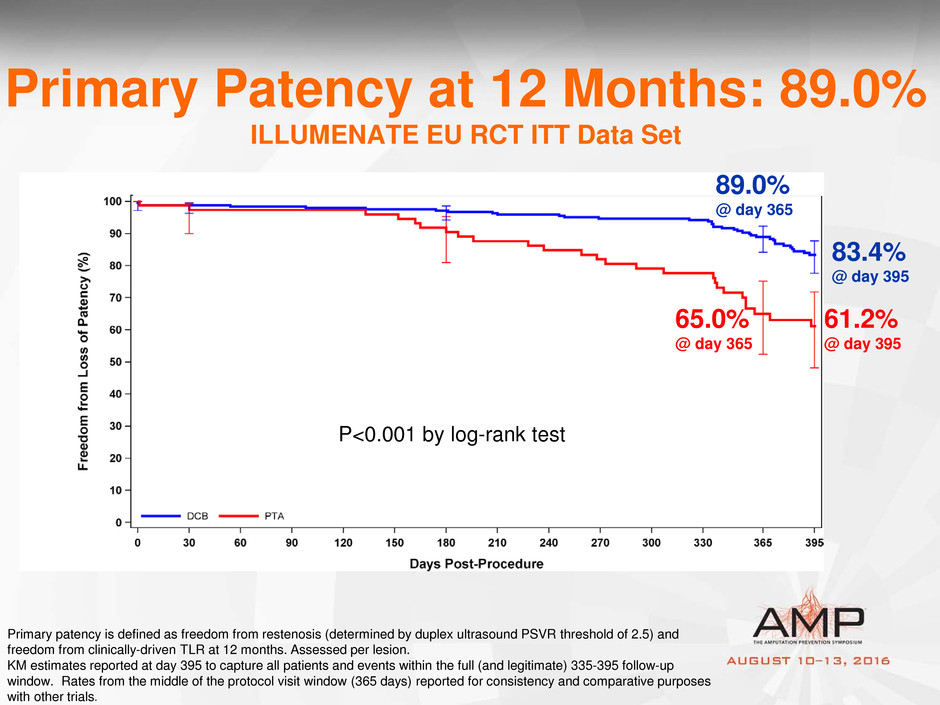
Primary Patency at 12 Months: 89.0%
ILLUMENATE EU RCT ITT Data Set
P<0.001 by log-rank test
65.0%
@ day 365
Primary patency is defined as freedom from restenosis (determined by duplex ultrasound PSVR threshold of 2.5) and
freedom from clinically-driven TLR at 12 months. Assessed per lesion.
KM estimates reported at day 395 to capture all patients and events within the full (and legitimate) 335-395 follow-up
window. Rates from the middle of the protocol visit window (365 days) reported for consistency and comparative purposes
with other trials.
83.4%
@ day 395
89.0%
@ day 365
61.2%
@ day 395
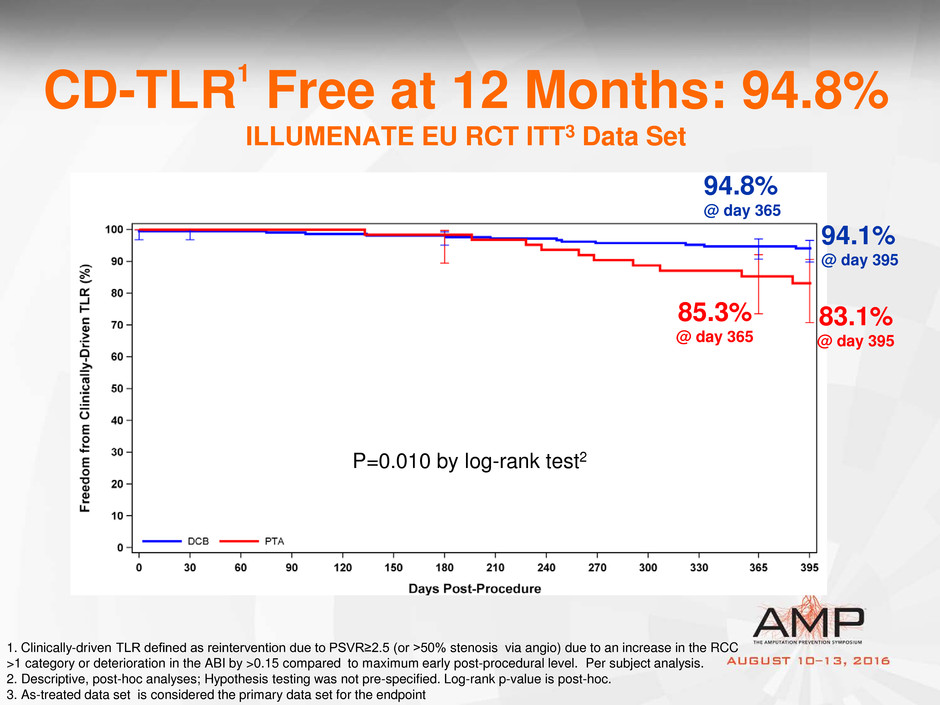
CD-TLR1 Free at 12 Months: 94.8%
ILLUMENATE EU RCT ITT3 Data Set
1. Clinically-driven TLR defined as reintervention due to PSVR≥2.5 (or >50% stenosis via angio) due to an increase in the RCC
>1 category or deterioration in the ABI by >0.15 compared to maximum early post-procedural level. Per subject analysis.
2. Descriptive, post-hoc analyses; Hypothesis testing was not pre-specified. Log-rank p-value is post-hoc.
3. As-treated data set is considered the primary data set for the endpoint
85.3%
@ day 365
P=0.010 by log-rank test2
83.1%
@ day 395
94.8%
@ day 365
94.1%
@ day 395
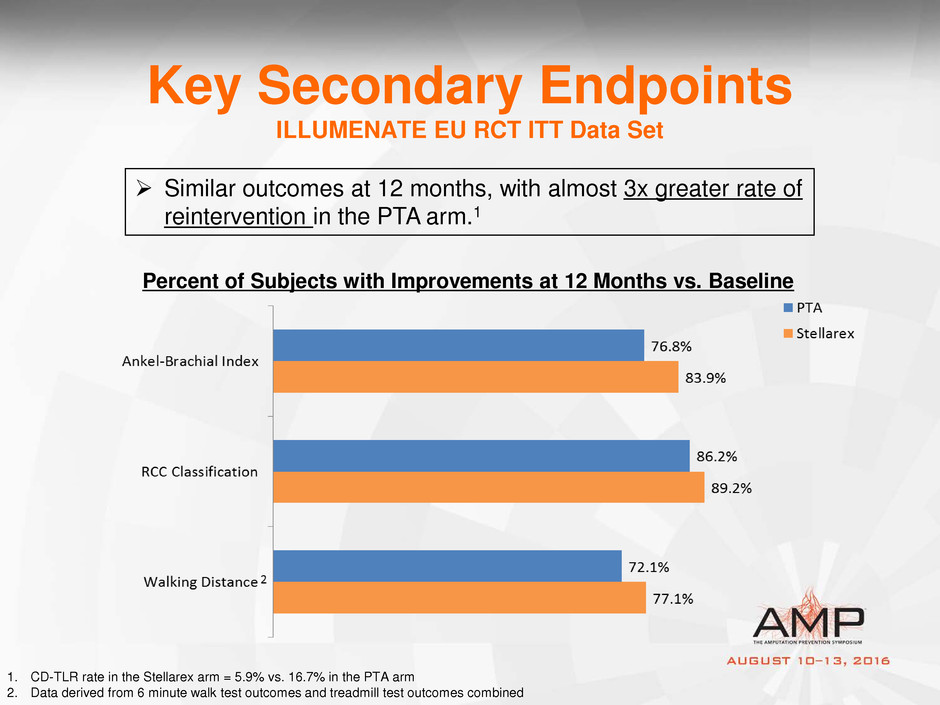
Key Secondary Endpoints
ILLUMENATE EU RCT ITT Data Set
Percent of Subjects with Improvements at 12 Months vs. Baseline
Similar outcomes at 12 months, with almost 3x greater rate of
reintervention in the PTA arm.1
1. CD-TLR rate in the Stellarex arm = 5.9% vs. 16.7% in the PTA arm
2. Data derived from 6 minute walk test outcomes and treadmill test outcomes combined
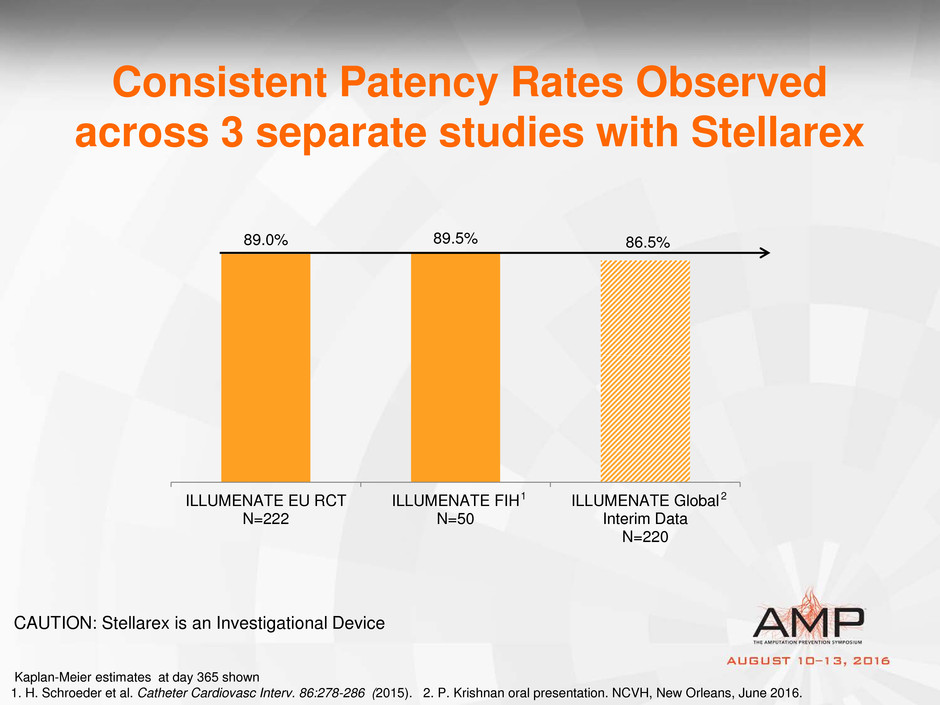
89.0% 89.5% 86.5%
ILLUMENATE EU RCT
N=222
ILLUMENATE FIH
N=50
ILLUMENATE Global
Interim Data
N=220
Consistent Patency Rates Observed
across 3 separate studies with Stellarex
Kaplan-Meier estimates at day 365 shown
1. H. Schroeder et al. Catheter Cardiovasc Interv. 86:278-286 (2015). 2. P. Krishnan oral presentation. NCVH, New Orleans, June 2016.
CAUTION: Stellarex is an Investigational Device
2 1
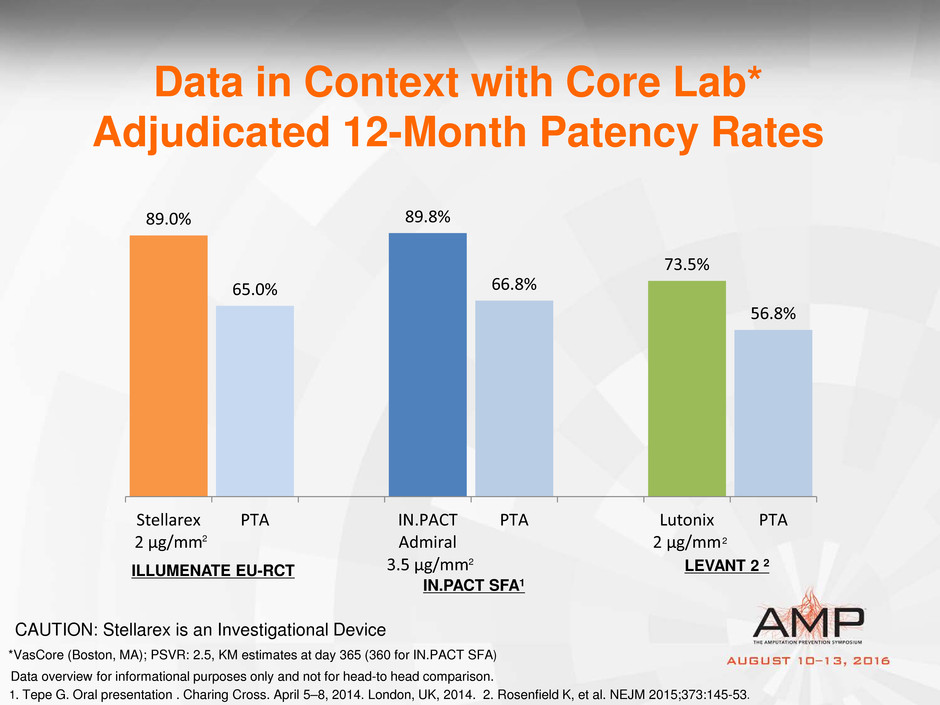
89.0%
65.0%
89.8%
66.8%
73.5%
56.8%
Stellarex
2 µg/mm
PTA IN.PACT
Admiral
3.5 µg/mm
PTA Lutonix
2 µg/mm
PTA
Data in Context with Core Lab*
Adjudicated 12-Month Patency Rates
ILLUMENATE EU-RCT
IN.PACT SFA1
LEVANT 2 2
1. Tepe G. Oral presentation . Charing Cross. April 5–8, 2014. London, UK, 2014. 2. Rosenfield K, et al. NEJM 2015;373:145-53.
Data overview for informational purposes only and not for head-to head comparison.
*VasCore (Boston, MA); PSVR: 2.5, KM estimates at day 365 (360 for IN.PACT SFA)
CAUTION: Stellarex is an Investigational Device
2
2
2
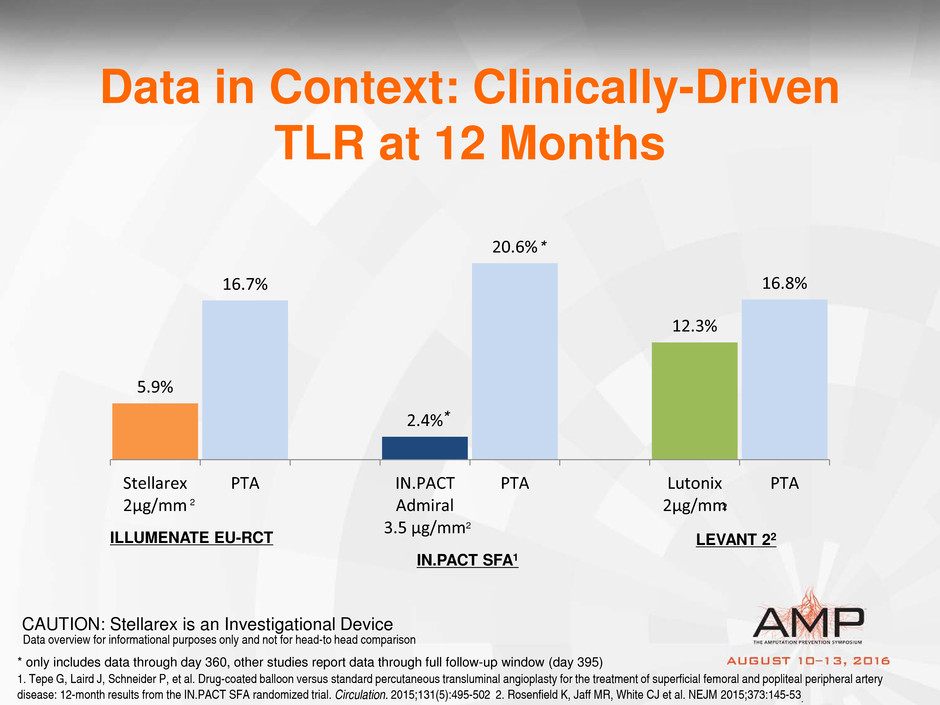
5.9%
16.7%
2.4%
20.6%
12.3%
16.8%
Stellarex
2µg/mm
PTA IN.PACT
Admiral
3.5 µg/mm
PTA Lutonix
2µg/mm
PTA
Data in Context: Clinically-Driven
TLR at 12 Months
ILLUMENATE EU-RCT
IN.PACT SFA1
LEVANT 22
1. Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery
disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131(5):495-502 2. Rosenfield K, Jaff MR, White CJ et al. NEJM 2015;373:145-53.
Data overview for informational purposes only and not for head-to head comparison
CAUTION: Stellarex is an Investigational Device
2
2
2
*
*
* only includes data through day 360, other studies report data through full follow-up window (day 395)

Conclusions
• This robust randomized study confirms First-in-
Human study results
• Primary safety and efficacy endpoints met and
superiority was demonstrated
• Stellarex is a low-dose DCB with consistently
positive results
– Primary Patency= 89.0% at day 365
– Freedom from CD-TLR= 94.8% at day 365
• ILLUMENATE Pivotal Data will be released Fall 2016
