Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Aldeyra Therapeutics, Inc. | d208194dex992.htm |
| 8-K - 8-K - Aldeyra Therapeutics, Inc. | d208194d8k.htm |

Results of a randomized, double-blind, vehicle-controlled clinical trial of topical dermatologic NS2 in patients with ichthyosis due to Sjögren-Larsson Syndrome August 9, 2016 Exhibit 99.1

Disclaimers and Forward-Looking Statements This presentation contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations and expenses, business strategies and plans, research and development plans or expectations, trends, market sizing, competitive position, industry environment and potential growth opportunities, among other things. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan” or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development and clinical plans for NS2, in Sjogren Larsson Syndrome. Aldeyra is at an early stage of development and may not ever have any products that generate significant revenue. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements include, among others, the timing of commencement, enrollment and completion of Aldeyra’s clinical trials; the timing and success of preclinical studies and clinical trials conducted by Aldeyra and its development partners; the ability to obtain and maintain regulatory approval to commercialize Aldeyra’s product candidates, and the labeling for any approved products; the scope, progress, expansion, and costs of developing and commercializing Aldeyra’s product candidates; the size and growth of the potential markets for Aldeyra’s product candidates and the ability to serve those markets; Aldeyra’s expectations regarding its expenses and revenue, the sufficiency or use of Aldeyra’s cash resources and needs for additional financing; the rate and degree of market acceptance of any of Aldeyra’s product candidates; Aldeyra’s expectations regarding competition; Aldeyra’s anticipated growth strategies; Aldeyra’s ability to attract or retain key personnel; Aldeyra’s ability to establish and maintain development partnerships; Aldeyra’s expectations regarding federal, state and foreign regulatory requirements; regulatory developments in the United States and foreign countries; Aldeyra’s ability to obtain and maintain intellectual property protection for its product candidates; the anticipated trends and challenges in Aldeyra’s business and the market in which it operates; and other factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Aldeyra’s Annual Report on Form 10-K for the year ended December 31, 2015 and Quarterly Report on Form 10-Q for the quarter ended March 31, 2016, which are on file with the Securities and Exchange Commission (SEC) and available on the SEC's website at www.sec.gov. Additional factors may be set forth in Aldeyra’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2016, to be filed with the SEC in the third quarter of 2016. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this presentation is provided only as of August 9, 2016, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.
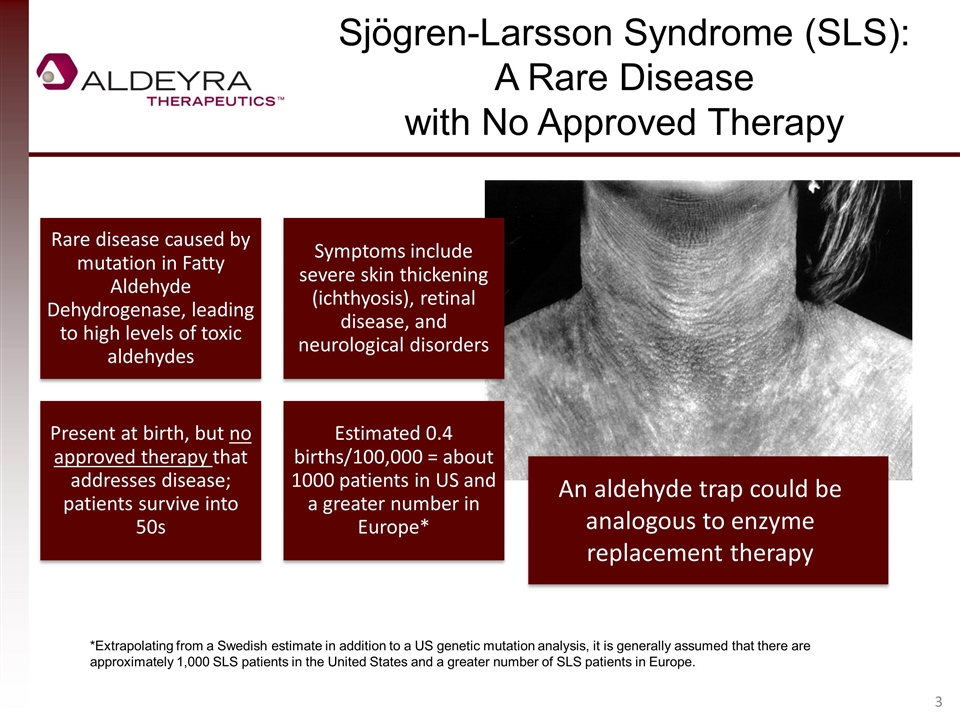
Sjögren-Larsson Syndrome (SLS): A Rare Disease with No Approved Therapy An aldehyde trap could be analogous to enzyme replacement therapy *Extrapolating from a Swedish estimate in addition to a US genetic mutation analysis, it is generally assumed that there are approximately 1,000 SLS patients in the United States and a greater number of SLS patients in Europe. Rare disease caused by mutation in Fatty Aldehyde Dehydrogenase, leading to high levels of toxic aldehydes Symptoms include severe skin thickening (ichthyosis), retinal disease, and neurological disorders Present at birth, but no approved therapy that addresses disease; patients survive into 50s Estimated 0.4 births/100,000 = about 1000 patients in US and a greater number in Europe*

Sjögren-Larsson Syndrome Clinical Trial Design Dosing NS2 1% Topical Dermatologic Once Daily on 4x10 inch area Randomization Vehicle-Controlled 1:1 Enrollment 12 Patients with Moderate to Severe Ichthyosis Treatment Time 8 Weeks Endpoints Ichthyosis Severity Score (Investigator Exam and Masked Central Review of Photography) Dermal Biomarkers Further information can be found on www.clinicaltrials.gov: Trial #NCT02402309.
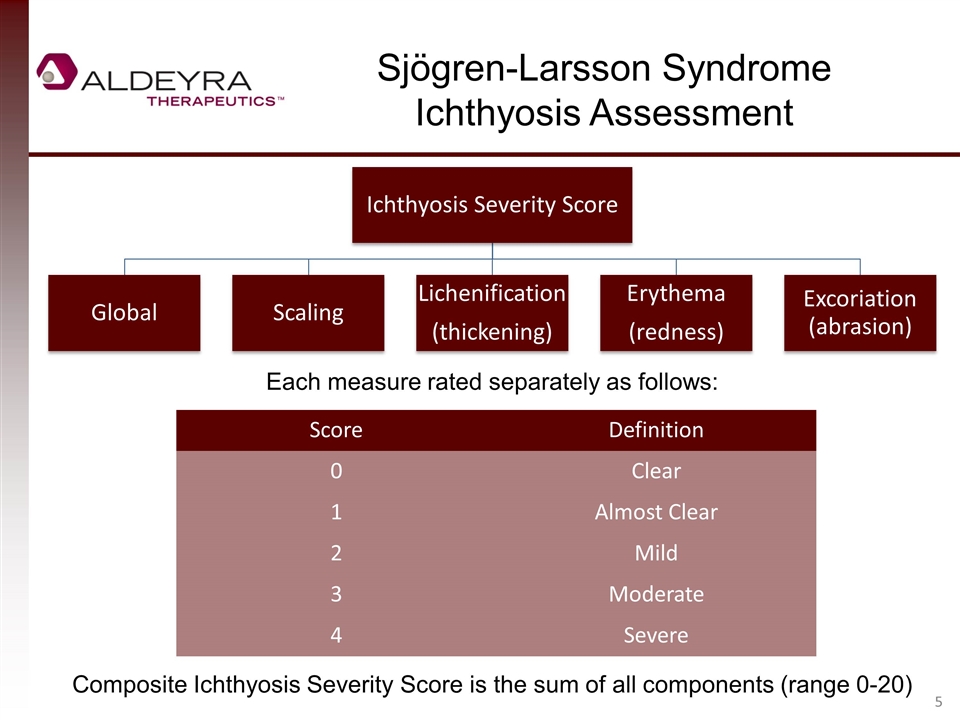
Sjögren-Larsson Syndrome Ichthyosis Assessment Score Definition 0 Clear 1 Almost Clear 2 Mild 3 Moderate 4 Severe Each measure rated separately as follows: Composite Ichthyosis Severity Score is the sum of all components (range 0-20) Ichthyosis Severity Score Global Scaling Lichenification (thickening) Erythema (redness) Excoriation (abrasion)
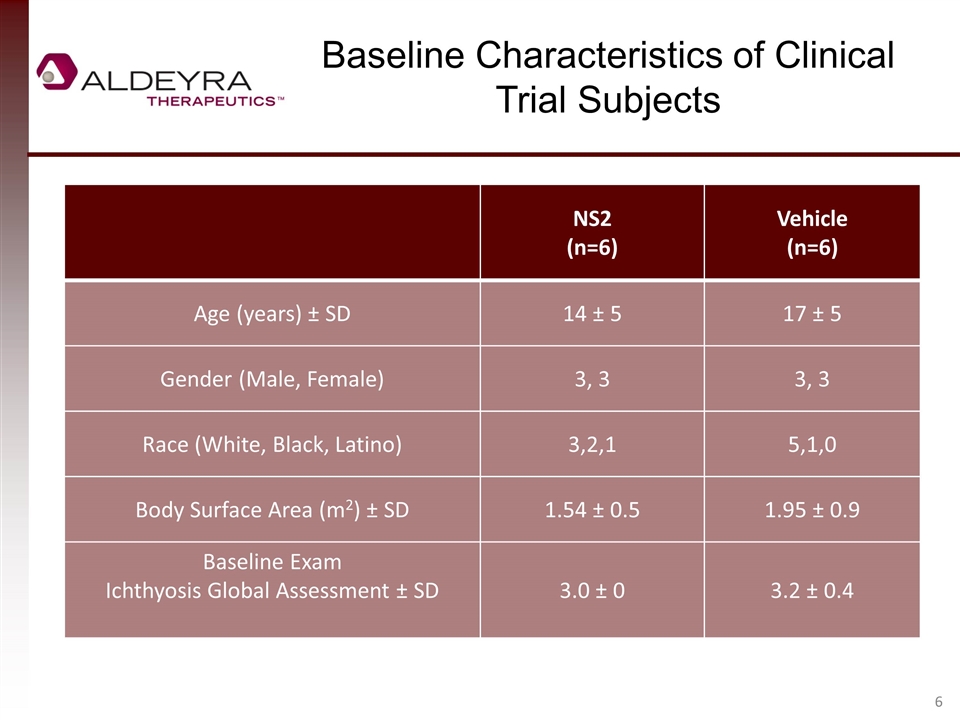
NS2 (n=6) Vehicle (n=6) Age (years) ± SD 14 ± 5 17 ± 5 Gender (Male, Female) 3, 3 3, 3 Race (White, Black, Latino) 3,2,1 5,1,0 Body Surface Area (m2) ± SD 1.54 ± 0.5 1.95 ± 0.9 Baseline Exam Ichthyosis Global Assessment ± SD 3.0 ± 0 3.2 ± 0.4 Baseline Characteristics of Clinical Trial Subjects
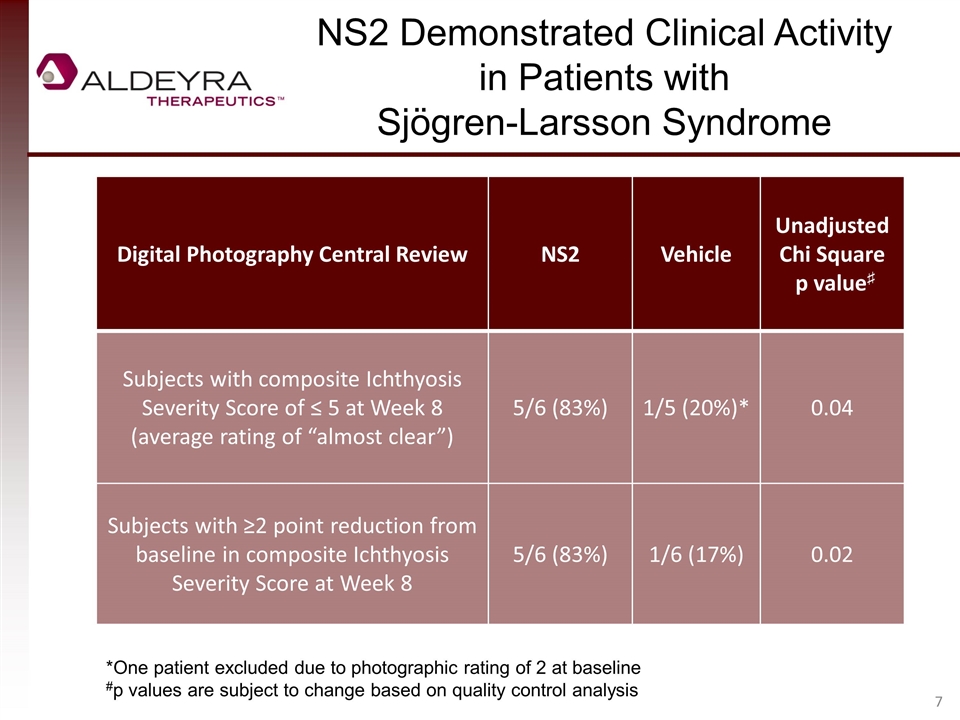
Digital Photography Central Review NS2 Vehicle Unadjusted Chi Square p value♯ Subjects with composite Ichthyosis Severity Score of ≤ 5 at Week 8 (average rating of “almost clear”) 5/6 (83%) 1/5 (20%)* 0.04 Subjects with ≥2 point reduction from baseline in composite Ichthyosis Severity Score at Week 8 5/6 (83%) 1/6 (17%) 0.02 NS2 Demonstrated Clinical Activity in Patients with Sjögren-Larsson Syndrome *One patient excluded due to photographic rating of 2 at baseline #p values are subject to change based on quality control analysis
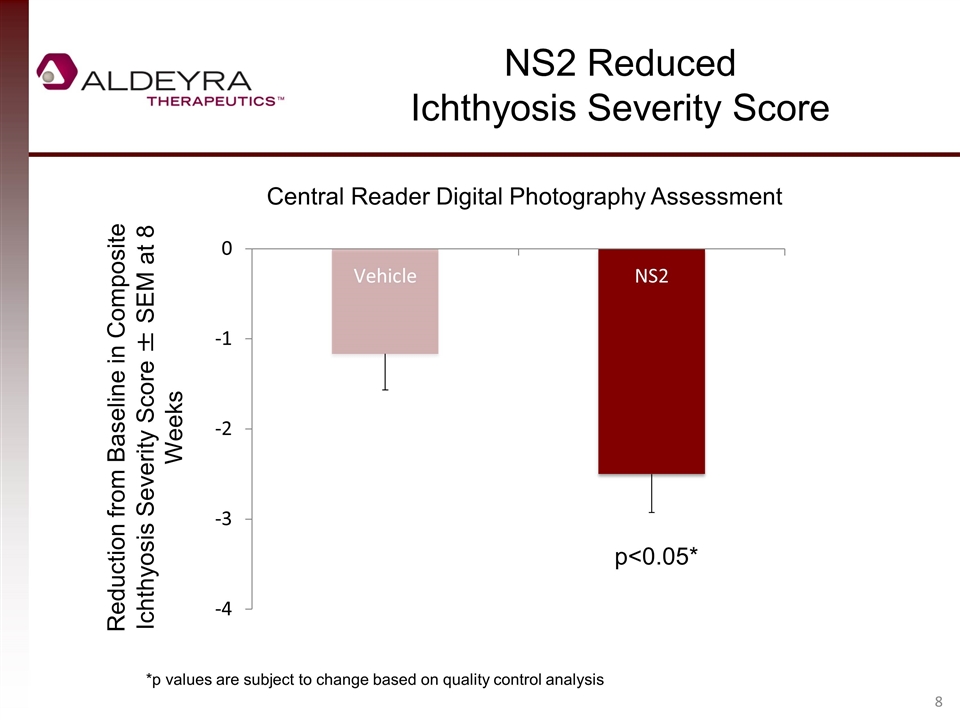
NS2 Reduced Ichthyosis Severity Score Central Reader Digital Photography Assessment Reduction from Baseline in Composite Ichthyosis Severity Score ± SEM at 8 Weeks p<0.05* *p values are subject to change based on quality control analysis
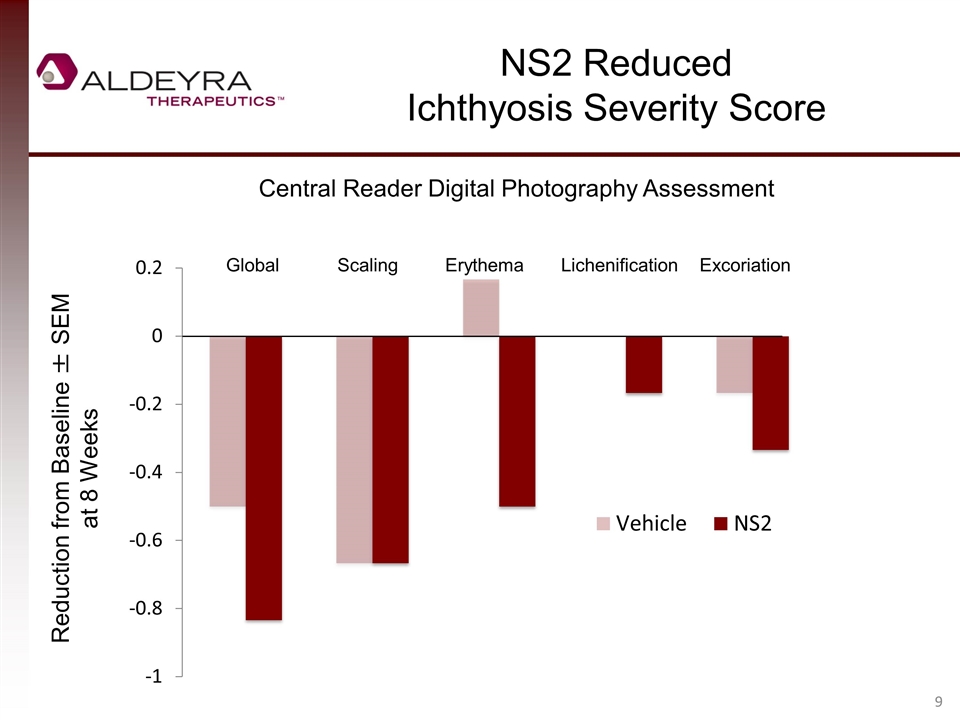
NS2 Reduced Ichthyosis Severity Score Central Reader Digital Photography Assessment Reduction from Baseline ± SEM at 8 Weeks Global Scaling Erythema Lichenification Excoriation
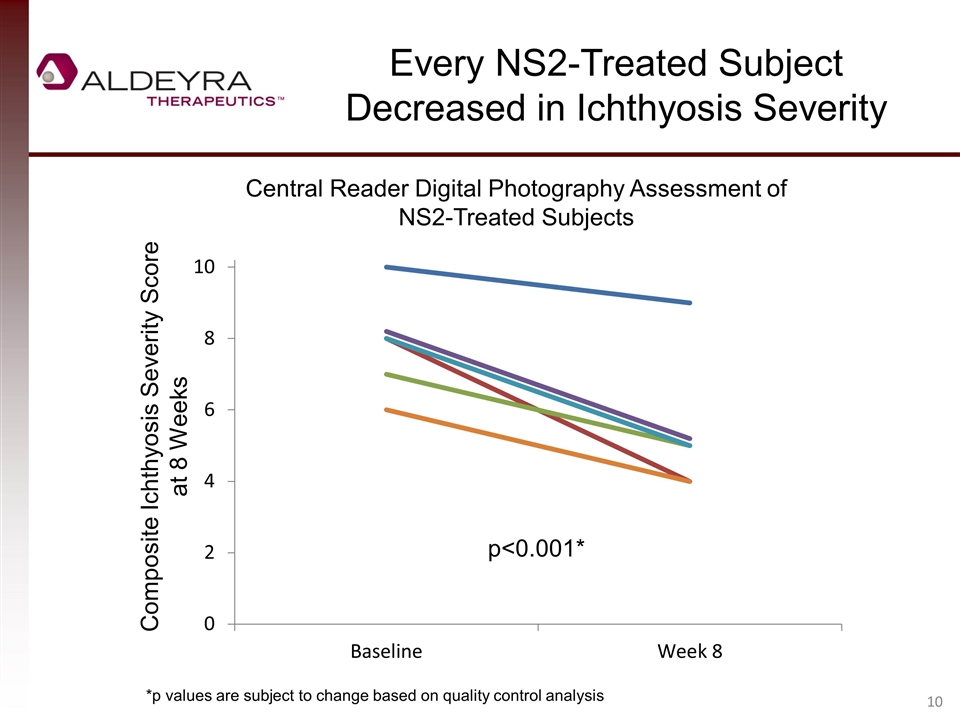
Every NS2-Treated Subject Decreased in Ichthyosis Severity Central Reader Digital Photography Assessment of NS2-Treated Subjects Composite Ichthyosis Severity Score at 8 Weeks p<0.001* *p values are subject to change based on quality control analysis

No significant adverse events, serious adverse events, or discontinuations in either group Itching was reported in two subjects (33%) in the NS2 treated group Moderate itching in one subject classified as “possibly related to drug” Mild itching in one subject classified as “unlikely related to drug” Overall, NS2 was well tolerated with no safety issues raised for future studies of topical dermatological NS2 NS2 Observed to be Well Tolerated
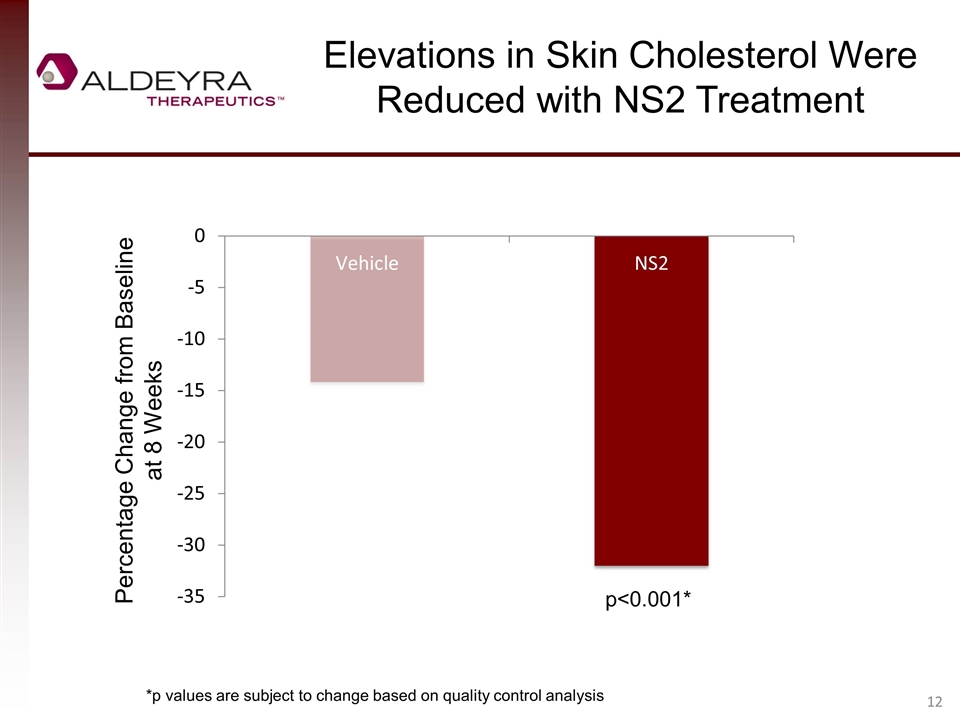
Elevations in Skin Cholesterol Were Reduced with NS2 Treatment Percentage Change from Baseline at 8 Weeks *p values are subject to change based on quality control analysis
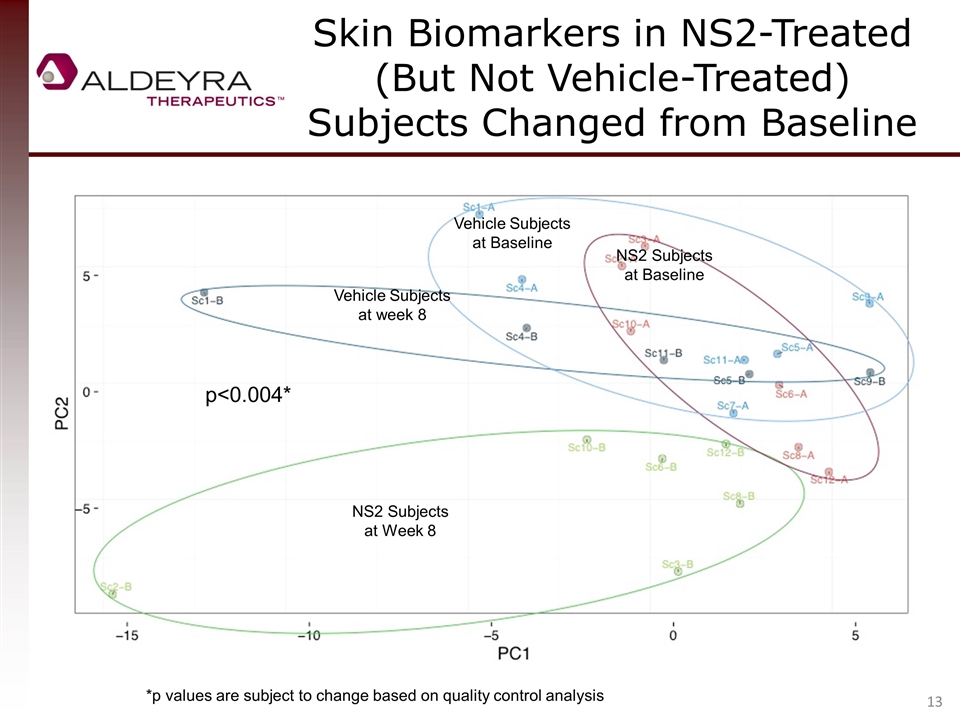
Skin Biomarkers in NS2-Treated (But Not Vehicle-Treated) Subjects Changed from Baseline NS2 Subjects at Week 8 NS2 Subjects at Baseline Vehicle Subjects at Baseline Vehicle Subjects at week 8 p<0.004* *p values are subject to change based on quality control analysis

In summary, this highly controlled clinical trial demonstrated: Clinically meaningful and statistically significant changes with NS2 relative to vehicle on the Ichthyosis Severity Score, as evidenced by moderate disease at baseline reduced to mild disease after 8 weeks of treatment with NS2; Clinical effects that correlated with dermal biomarkers; and, An acceptable safety and tolerability profile for NS2. NS2 Demonstrated Clinical Activity in Patients with Sjögren-Larsson Syndrome
