Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a16-16435_1ex99d1.htm |
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a16-16435_18k.htm |
Exhibit 99.2
2Q16 Financial Results and Program Updates August 9, 2016

2016: Significant Progress with Key Strategic Priorities 2016 Strategic Priorities Achieved EU Approval of Galafold International commercial launch of Galafold Accelerated regulatory path for Galafold in Japan Dosing of Pompe patients with ATB200/AT2221 Expansion of preclinical pipeline with CDKL5 program Completion of $130M of financing 2Q16 Overview 2 We Remain Sharply Focused on 5 Strategic Priorities for 2016 as We Continue to Build a Leading Global Biotechnology Company Focused on Rare and Devastating Diseases 2016 Strategic Priorities for 2H16 Launching Galafold internationally Pursuing Galafold regulatory approvals (U.S., Japan, and Rest-of-World) Advancing clinical programs (Epidermolysis Bullosa (EB) and Pompe) Maintaining a strong balance sheet Building the pipeline
Galafold™ (Migalastat) Precision Medicine for Fabry Disease International Launch Underway

European Commission Granted Full Approval for Galafold Galafold: Precision Medicine for Fabry Disease 4 Galafold Indicated for Long-Term Treatment of Adults and Adolescents Aged > 16 years with a Confirmed Diagnosis of Fabry Disease and Who have an Amenable Mutation The evaluation of EMA’s Committee for Medicinal Products for Human Use (CHMP) was based on the results of two phase III clinical trials in about 110 patients with Fabry disease who had a genetic mutation which responds to migalastat. Galafold demonstrated its efficacy compared to placebo (a dummy treatment) and to ERT in a long-term comparative study. - EMA Press Release Hard Capsules Oral Use 14 Hard Capsules

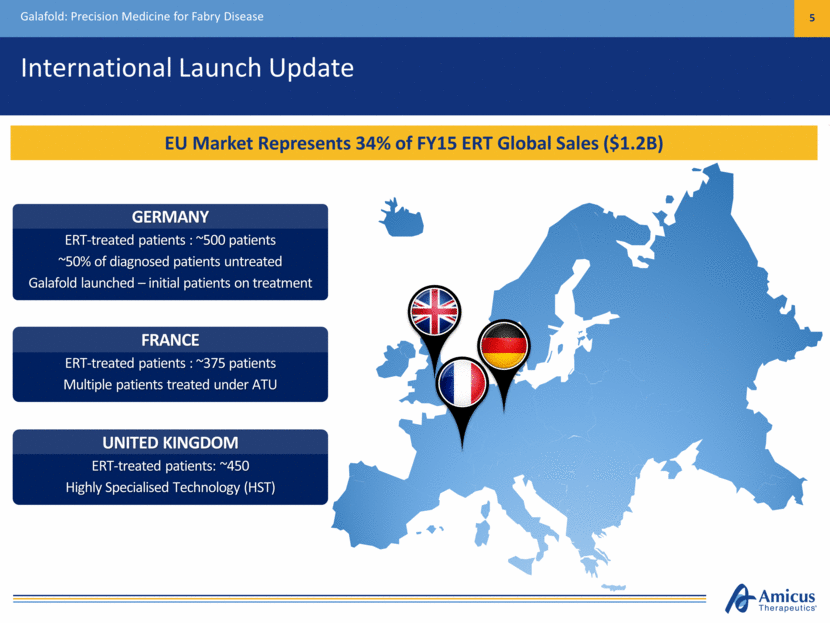
International Launch Update Galafold: Precision Medicine for Fabry Disease 5 EU Market Represents 34% of FY15 ERT Global Sales ($1.2B) UNITED KINGDOM ERT-treated patients: ~450 Highly Specialised Technology (HST) FRANCE ERT-treated patients : ~375 patients Multiple patients treated under ATU GERMANY ERT-treated patients : ~500 patients ~50% of diagnosed patients untreated Galafold launched – initial patients on treatment
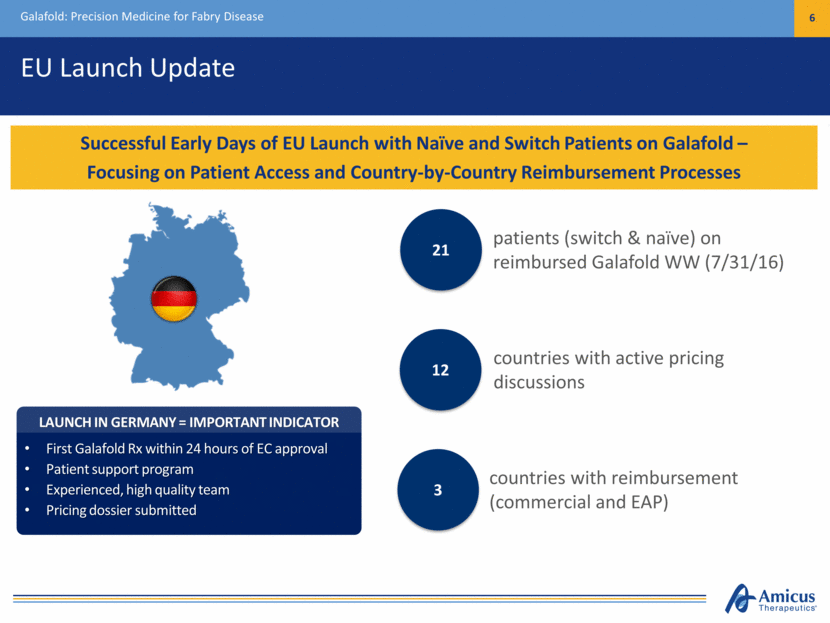
EU Launch Update Galafold: Precision Medicine for Fabry Disease 6 Successful Early Days of EU Launch with Naïve and Switch Patients on Galafold – Focusing on Patient Access and Country-by-Country Reimbursement Processes LAUNCH IN GERMANY = IMPORTANT INDICATOR First Galafold Rx within 24 hours of EC approval Patient support program Experienced, high quality team Pricing dossier submitted patients (switch & naïve) on reimbursed Galafold WW (7/31/16)) 21 countries with reimbursement (commercial and EAP) 3 countries with active pricing discussions 12
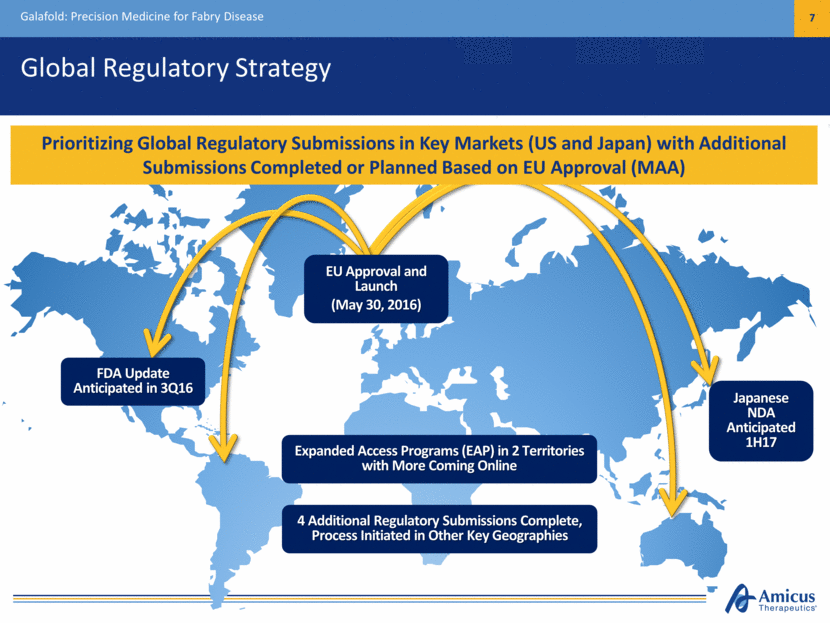
Global Regulatory Strategy Galafold: Precision Medicine for Fabry Disease 7 EU Approval and Launch (May 30, 2016) Expanded Access Programs (EAP) in 2 Territories with More Coming Online Prioritizing Global Regulatory Submissions in Key Markets (US and Japan) with Additional Submissions Completed or Planned Based on EU Approval (MAA) 4 Additional Regulatory Submissions Complete, Process Initiated in Other Key Geographies FDA Update Anticipated in 3Q16 Japanese NDA Anticipated 1H17
ATB200 Novel ERT for Pompe Disease A Proprietary, Clinical-Stage Biologics Program

Pompe Clinical Study ATB200-02 Data Cascade Novel ERT for Pompe Disease – ATB200 + Chaperone 9 Data in initial ambulatory ERT-switch patients (Cohort 1) Data in ERT-naïve patients (Cohort 2) Additional extension study data (all Cohorts) A Cascade of Data Points from 4Q16 through 2017 Offer Clear Parameters to Define Success and Differentiate ATB200/AT2221 Pompe Data Cascade 4Q16 Through 2017 Additional data & initial extension data in Cohort 1 Data in non-ambulatory ERT-switch patients (Cohort 3) 18-WEEK DATA Safety / tolerability Pharmacokinetics (PK) Biomarkers Immunogenicity EXTENSION DATA Motor/pulmonary function

SD-101 for Epidermolysis Bullosa (EB) Poised to deliver pivotal data for a devastating rare disease

PHASE 3 ESSENCE STUDY STATUS >50% of target enrollment achieved 100% conversion to extension study (SD-006) Top-line Phase 3 data anticipated 4Q16/1H17 EB Program Update - Phase 3 ESSENCE Study (SD-005) SD-101 for EB 11 Following Recent Meeting with FDA, Amicus has Elevated Time to Wound Closure From Secondary to Co-Primary Endpoint. We Believe This Change Improves the Overall Likelihood of Study Success while ESSENCE Study Remains Blinded.
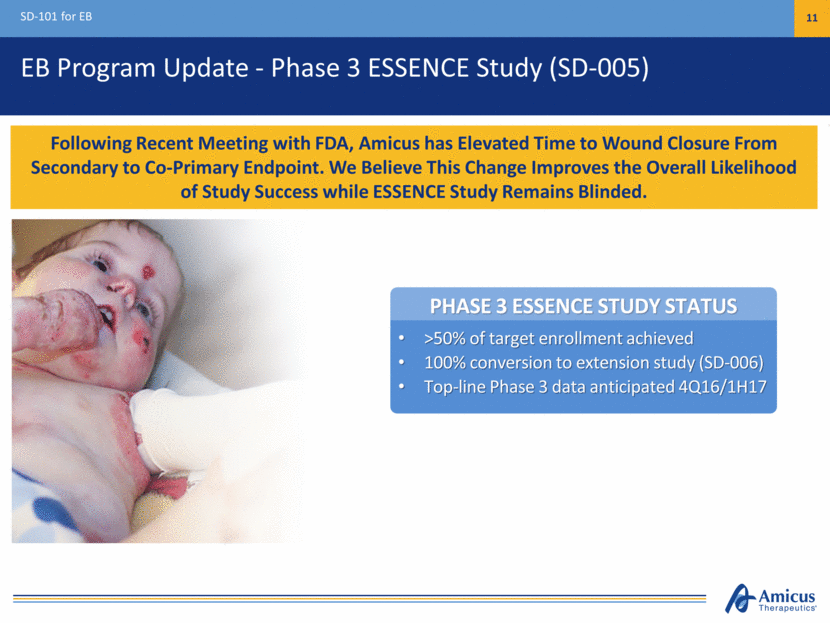
Elevation of Time to Wound Closure as Co-primary Endpoint SD-101 for EB 12 FDA 2006 Guidance Document1 States Time to Wound Closure is an Acceptable Primary Efficacy Endpoint Median Time to Wound Closure (Days) Median Time to Wound Closure (Days) 91 Days 86 Days 40 Days ITT Population (n=48) Evaluable Population (n=45) Placebo SD-101 3% SD-101 6% 91 Days 86 Days 30 Days N=17 N=15 N=16 N=17 N=12 N=16 Time to Wound Closure Encouraging results in SD-101 Phase 2b study Measuring healing over time vs. one time point may further control for placebo response Results correlate with incidence of complete wound closure Statistical simulations indicate addition of time to wound closure increases probability of study success Median Time to Wound Closure in Phase 2b Study 1http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071324.pdf
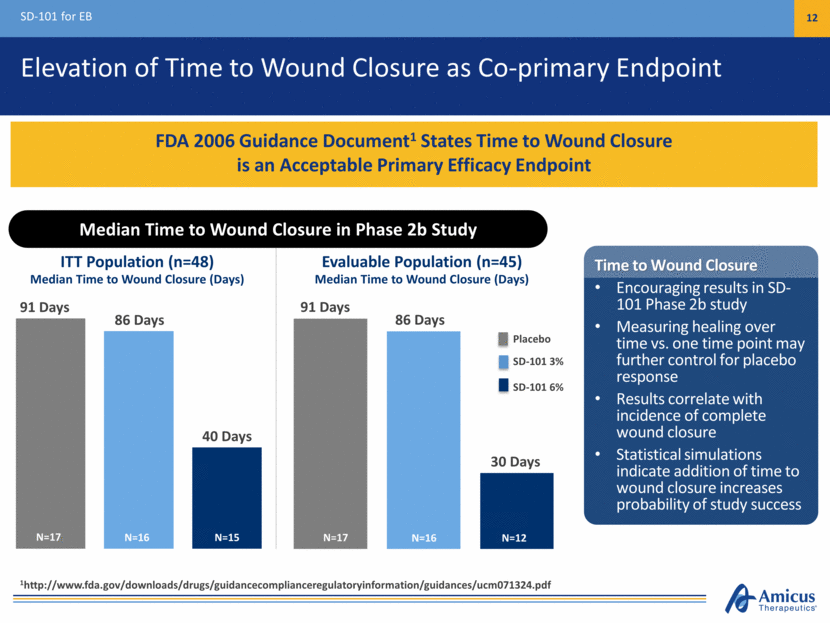
Phase 3 ESSENCE Study Design (SD-005) SD-101 for EB 13 Study Success Potentially Based on Achievement of One or Both Co-Primary Endpoints ~150 EB patients (age > 1 month) Baseline wound: Chronic (> 21 days), size >10 cm2 Placebo SD-101 6% Primary Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed on primary endpoint Baseline wound: Chronic (> 21 days), size >10 cm2 Primary Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed on primary endpoint Baseline wound: Chronic (> 21 days), size >10 cm2 Co-Primary Endpoints Complete closure of target wound (previously specified primary endpoint) Time to target wound closure (elevated from secondary to co-primary) Secondary Endpoints Include: Change in Body Surface Area (BSA) of lesions and blisters Patient-reported itching Patient-reported pain 3-Month, Double-Blind Treatment Period Open-Label SD-101 6% Optional Extension (SD-006) 100% Participation in Extension Study (August 1, 2016) Covariates include age of patient and size of wound at baseline Average Baseline Target Wound Size in Phase 3 Population: ~20 cm2 (August 1, 2016)
Financial Summary & Milestones Strong Balance Sheet to Invest in Rare Disease Pipeline

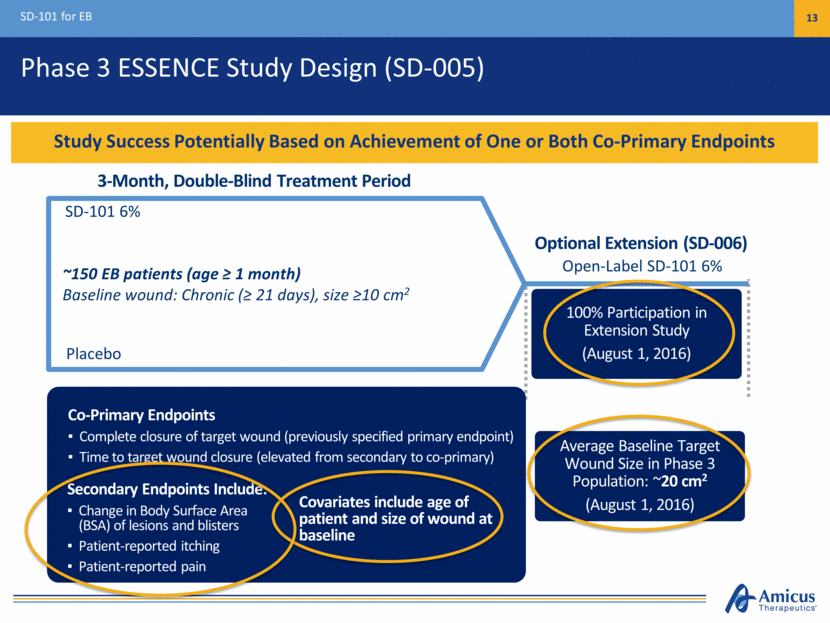
Strong Balance Sheet Financial Summary 15 Financial Position June 30, 2016 Cash: $214.2M Debt $80.0M FY16 Net Cash Spend Guidance: $135-$155M (maintained) Cash Runway Into 2H17 Full Allotment Raised in ATM (average price per share: $6.67) $100M ($61.7M in 2Q; $39.3M in 3Q) Capitalization Shares Outstanding 134,408,526 Cash Position Provides Runway Under Current Operating Plan into mid-2017 Balance Sheet Strengthened with $130M in Equity and Debt Proceeds Since March 31 with Cash Runway into 2H17
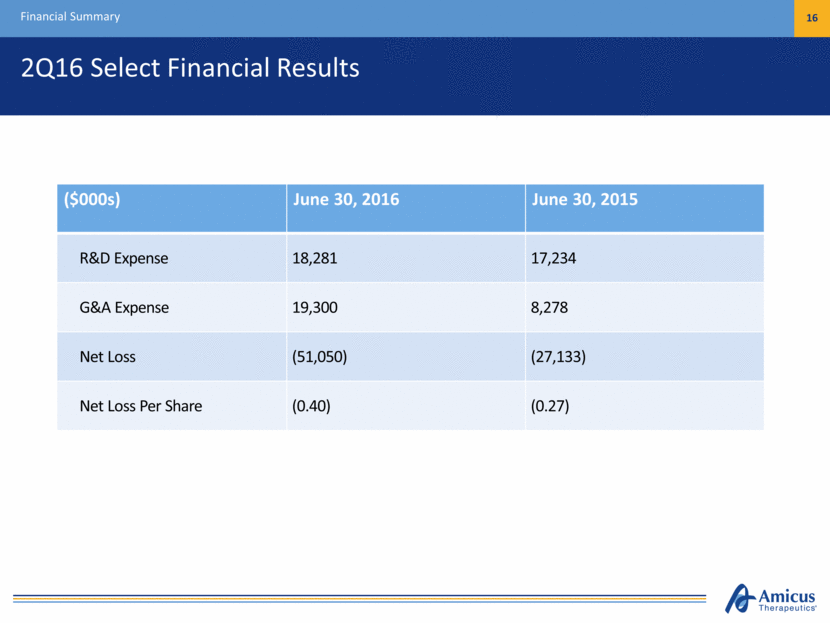
2Q16 Select Financial Results Financial Summary 16 ($000s) June 30, 2016 June 30, 2015 R&D Expense 18,281 17,234 G&A Expense 19,300 8,278 Net Loss (51,050) (27,133) Net Loss Per Share (0.40) (0.27)
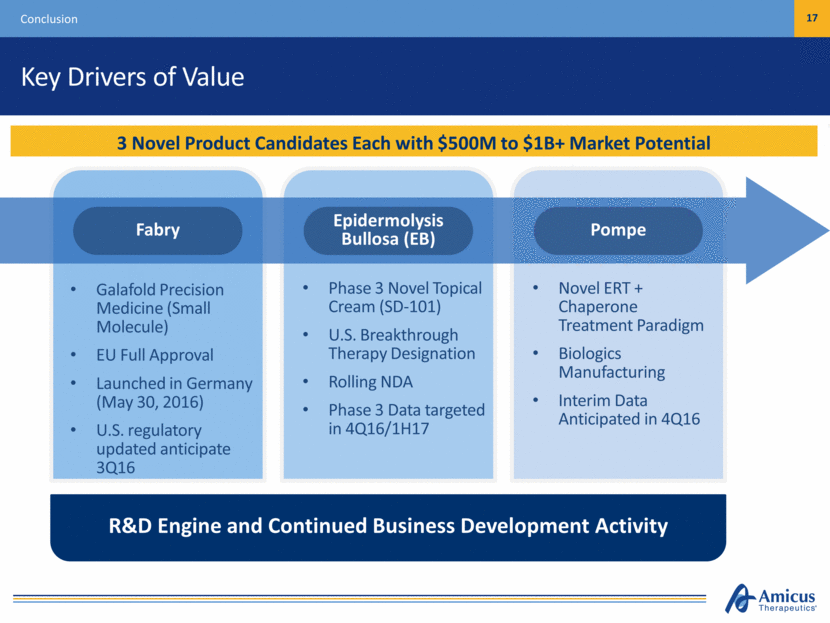
Key Drivers of Value Conclusion 17 3 Novel Product Candidates Each with $500M to $1B+ Market Potential Galafold Precision Medicine (Small Molecule) EU Full Approval Launched in Germany (May 30, 2016) U.S. regulatory updated anticipate 3Q16 Phase 3 Novel Topical Cream (SD-101) U.S. Breakthrough Therapy Designation Rolling NDA Phase 3 Data targeted in 4Q16/1H17 Novel ERT + Chaperone Treatment Paradigm Biologics Manufacturing Interim Data Anticipated in 4Q16 R&D Engine and Continued Business Development Activity Pompe Epidermolysis Bullosa (EB) Fabry
Thank You ©AMICUS THERAPEUTICS. CRANBURY, NJ. 2016

