Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ImmunoGen, Inc. | a16-16097_18k.htm |
| EX-99.1 - EX-99.1 - ImmunoGen, Inc. | a16-16097_1ex99d1.htm |
Exhibit 99.2
August 4, 2016 Quarterly Update

Executive Director, Investor Relations & Corporate Communications Carol Hausner 2

These slides include forward-looking statements based on management's current expectations. These statements include, but are not limited to, ImmunoGen's expectations related to: the occurrence, timing and outcome of potential pre-clinical, clinical and regulatory events related to the Company's and its collaboration partners' product programs; the presentation of preclinical and clinical data on the Company’s and its collaboration partners’ product candidates; and financial guidance for the Company’s 2016 fiscal year. For these statements, ImmunoGen claims the protection of the safe harbor for forward-looking statements provided by the Private Securities Litigation Reform Act of 1995. Various factors could cause ImmunoGen's actual results to differ materially from those discussed or implied in the forward-looking statements, and you are cautioned not to place undue reliance on these forward-looking statements, which are current only as of the date of these slides. Factors that could cause future results to differ materially from such expectations include, but are not limited to: the timing and outcome of ImmunoGen's and its collaboration partners' research and clinical development processes; the difficulties inherent in the development of novel pharmaceuticals, including uncertainties as to the timing, expense and results of preclinical studies, clinical trials and regulatory processes; ImmunoGen's ability to financially support its product programs; the Company’s dependence on its collaborative partners; industry merger and acquisition activity; and other factors more fully described in ImmunoGen's Annual Report on Form 10-K for the fiscal year ended June 30, 2015 and other reports filed with the Securities and Exchange Commission. 3 Forward-Looking Statements

President and Chief Executive Officer Mark Enyedy 4

ImmunoGen Today: Positioned for Sustainable Value Creation 5 Leadership in antibody-drug conjugates (ADCs) Lead program entering Phase 3 before year end Platform generating novel clinical candidates Technology validated clinically and through partnerships Strong cash position Experienced management team

Strategic Direction & Priorities 6 Platinum-resistant ovarian cancer Execute on speed-to-market for mirvetuximab soravtansine IMGN779, IMGN632, IMGN529 Accelerate earlier-stage portfolio Payloads, linkers, methods of conjugation Continue to drive innovation in ADCs as cancer therapies Lever partnerships to expand impact of innovations Enhanced financial discipline Build a fully-integrated biotech delivering innovative ADC therapies that meaningfully improve the lives of cancer patients
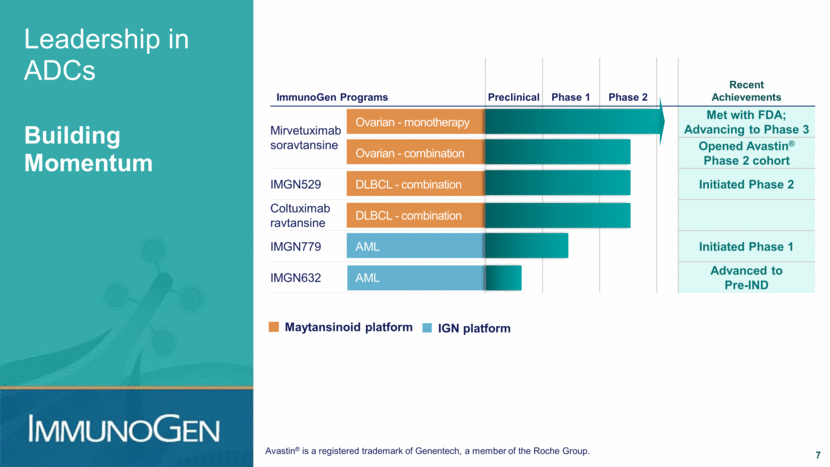
Leadership in ADCs Building Momentum ImmunoGen Programs Preclinical Phase 1 Phase 2 Recent Achievements Mirvetuximab soravtansine Ovarian - monotherapy Met with FDA; Advancing to Phase 3 Ovarian - combination Opened Avastin® Phase 2 cohort IMGN529 DLBCL - combination Initiated Phase 2 Coltuximab ravtansine DLBCL - combination IMGN779 AML Initiated Phase 1 IMGN632 AML Advanced to Pre-IND Maytansinoid platform IGN platform Avastin® is a registered trademark of Genentech, a member of the Roche Group. 7
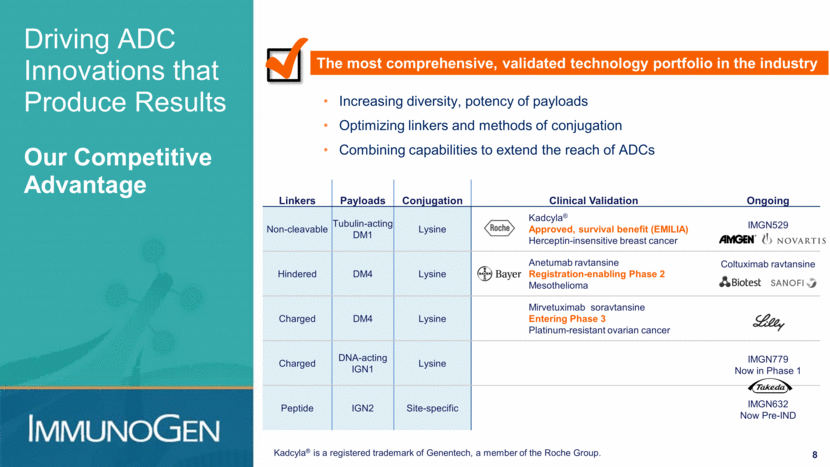
8 Driving ADC Innovations that Produce Results Our Competitive Advantage The most comprehensive, validated technology portfolio in the industry Linkers Payloads Conjugation Clinical Validation Ongoing Non-cleavable Tubulin-acting DM1 Lysine Kadcyla® Approved, survival benefit (EMILIA) Herceptin-insensitive breast cancer IMGN529 Hindered DM4 Lysine Anetumab ravtansine Registration-enabling Phase 2 Mesothelioma Coltuximab ravtansine Charged DM4 Lysine Mirvetuximab soravtansine Entering Phase 3 Platinum-resistant ovarian cancer Charged DNA-acting IGN1 Lysine IMGN779 Now in Phase 1 Peptide IGN2 Site-specific IMGN632 Now Pre-IND Increasing diversity, potency of payloads Optimizing linkers and methods of conjugation Combining capabilities to extend the reach of ADCs Kadcyla® is a registered trademark of Genentech, a member of the Roche Group.
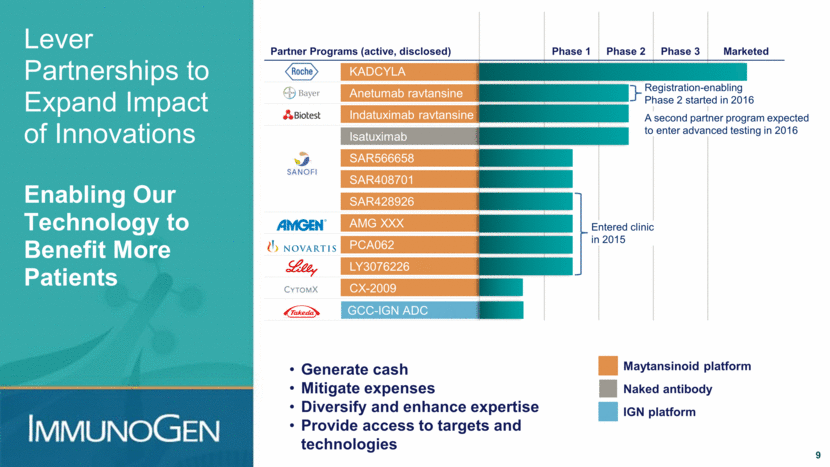
Lever Partnerships to Expand Impact of Innovations Enabling Our Technology to Benefit More Patients Partner Programs (active, disclosed) Phase 1 Phase 2 Phase 3 Marketed KADCYLA Anetumab ravtansine Indatuximab ravtansine Isatuximab SAR566658 SAR408701 SAR428926 AMG XXX PCA062 LY3076226 CX-2009 LY3076226 GCC-IGN ADC Entered clinic in 2015 9 Registration-enabling Phase 2 started in 2016 A second partner program expected to enter advanced testing in 2016 Maytansinoid platform IGN platform Naked antibody Generate cash Mitigate expenses Diversify and enhance expertise Provide access to targets and technologies
EVP and Chief Development Officer Dr. Charles Morris 10

Execute on Speed-to-Market for Lead Program Mirvetuximab Soravtansine 11 First ADC to enter registration testing for ovarian cancer MOA distinct from other approaches (PARPs, I/Os, Avastin ) Different clinical profile 1WHO GLOBOCAN 2012, ACS Cancer Facts and Figures. 2Published data and prescribing information Ovarian cancer globally: 240,000 new cases; 140,000 deaths/yr1 Typically diagnosed at advanced stage Current single-agent therapies: 15-20% ORR; 3.5-4 months PFS2 Differentiated Significant Need Ready for Phase 3
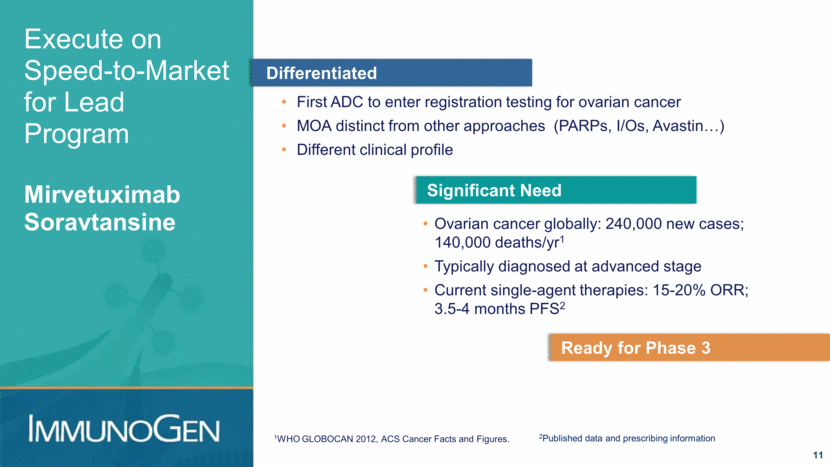
Mirvetuximab Soravtansine Ready for Phase 3 12 Data presented at ASCO 2016 (abstract #5567) Tumor cells with 2+/3+ FRα expression High: at least 75%; Medium: 50-74%; Low: 25-49% Positive meeting with FDA Sizeable safety database Single-agent activity Defined population ≤5 prior regimens ≥ low FRα ≤3 prior reg ≥ low Frα ≤3 prior regimens ≥ medium Frα 5000-7000 patients/year US, comparable in W. Europe All patients (N=46) ORR - confirmed responses only ORR 26% 39% 44% PFS 4.8 mo 6.7 mo 6.7 months
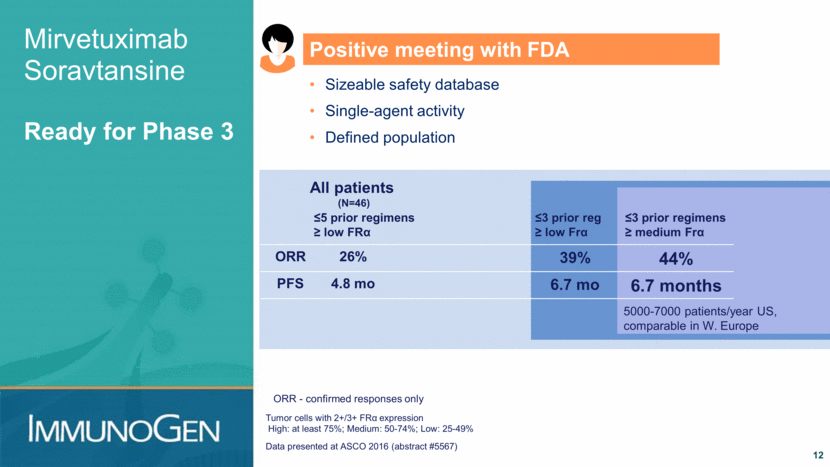
Mirvetuximab Soravtansine FORWARD I Phase 3 Trial 13 Mirvetuximab soravtansine Physician’s choice single agent chemo* with platinum-resistant ovarian cancer treated with up to 3 prior regimens; high or medium FRα levels *Pegylated liposomal doxorubicin (PLD), topotecan, weekly paclitaxel. 333 patients 2:1 randomization Endpoint: Significant improvement in PFS High expressers only, or All patients Indication: For patients with FRα-positive (high/medium) platinum-resistant ovarian cancer treated with up to 3 prior regimens for whom single-agent therapy is appropriate
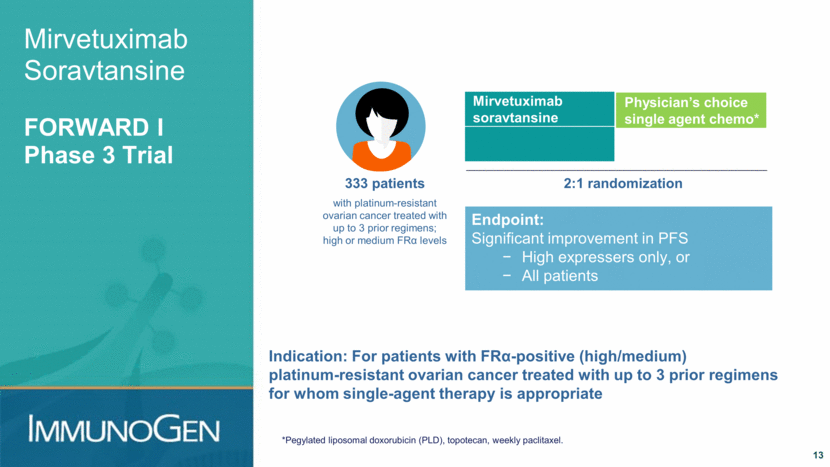
Mirvetuximab Soravtansine FORWARD II: Expanding for Ovarian Cancer 14 Platinum-based regimen Potential for Avastin+paclitaxel Once platinum-resistant Single agent chemotherapy Recurrence Treatment paradigm in US Phase 1/2 trial, Combination regimens to benefit more patients, longer Registration trial, single-agent therapy
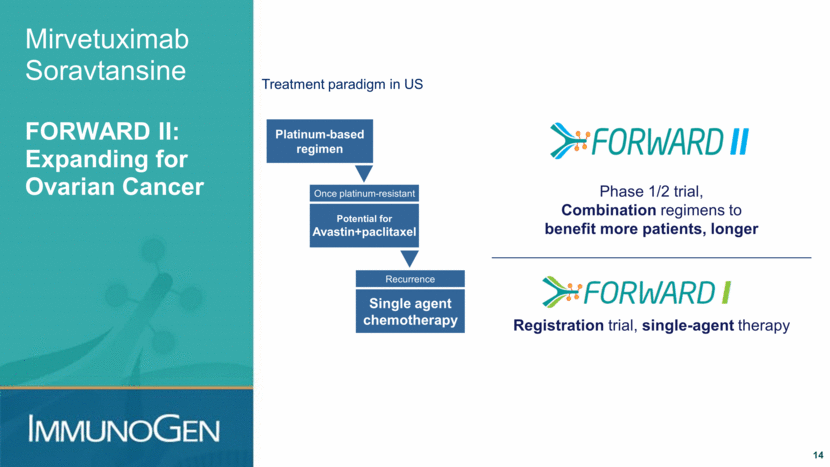
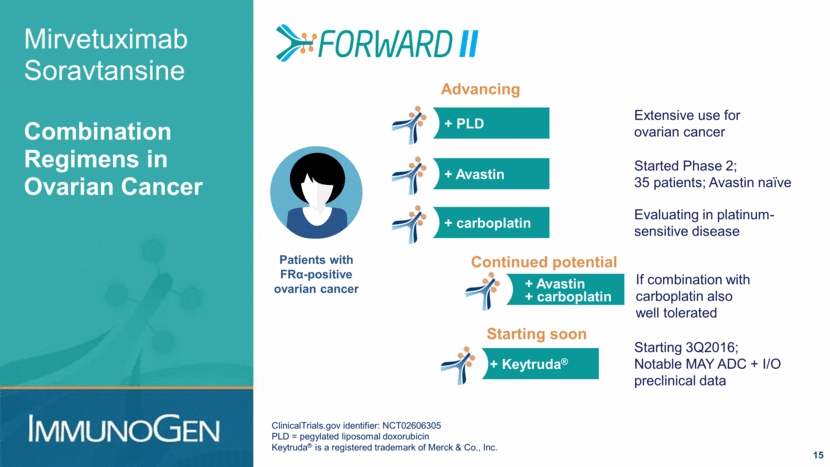
+ PLD Extensive use for ovarian cancer Mirvetuximab Soravtansine Combination Regimens in Ovarian Cancer 15 ClinicalTrials.gov identifier: NCT02606305 PLD = pegylated liposomal doxorubicin Keytruda® is a registered trademark of Merck & Co., Inc. Patients with FRα-positive ovarian cancer + Avastin Started Phase 2; 35 patients; Avastin naïve + carboplatin Evaluating in platinum- sensitive disease Advancing + Keytruda® Starting 3Q2016; Notable MAY ADC + I/O preclinical data Starting soon + Avastin + carboplatin If combination with carboplatin also well tolerated Continued potential
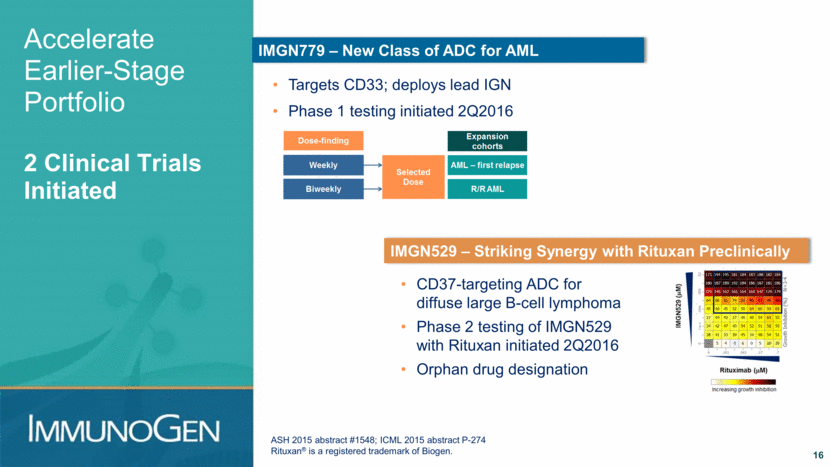
Accelerate Earlier-Stage Portfolio 2 Clinical Trials Initiated 16 Targets CD33; deploys lead IGN Phase 1 testing initiated 2Q2016 IMGN779 – New Class of ADC for AML IMGN529 – Striking Synergy with Rituxan Preclinically ASH 2015 abstract #1548; ICML 2015 abstract P-274 Rituxan® is a registered trademark of Biogen. CD37-targeting ADC for diffuse large B-cell lymphoma Phase 2 testing of IMGN529 with Rituxan initiated 2Q2016 Orphan drug designation
EVP and Chief Financial Officer David Johnston 17

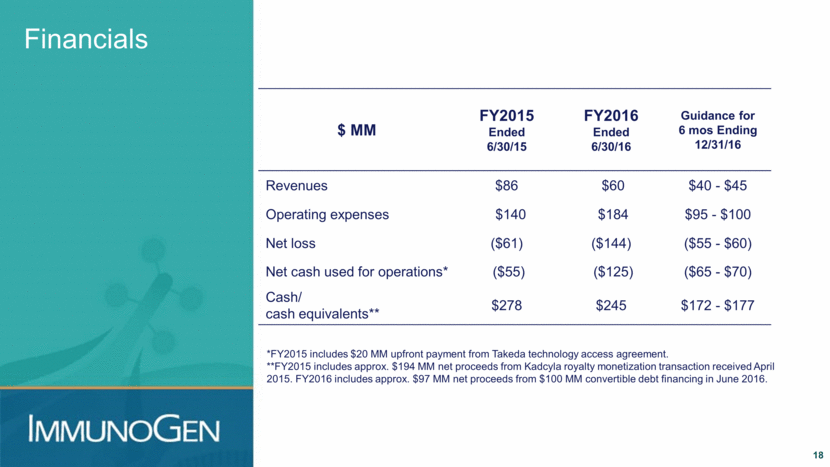
Financials *FY2015 includes $20 MM upfront payment from Takeda technology access agreement. **FY2015 includes approx. $194 MM net proceeds from Kadcyla royalty monetization transaction received April 2015. FY2016 includes approx. $97 MM net proceeds from $100 MM convertible debt financing in June 2016. $ MM FY2015 Ended 6/30/15 FY2016 Ended 6/30/16 Guidance for 6 mos Ending 12/31/16 Revenues $86 $60 $40 - $45 Operating expenses $140 $184 $95 - $100 Net loss ($61) ($144) ($55 - $60) Net cash used for operations* ($55) ($125) ($65 - $70) Cash/ cash equivalents** $278 $245 $172 - $177 18
President and Chief Executive Officer Mark Enyedy 19

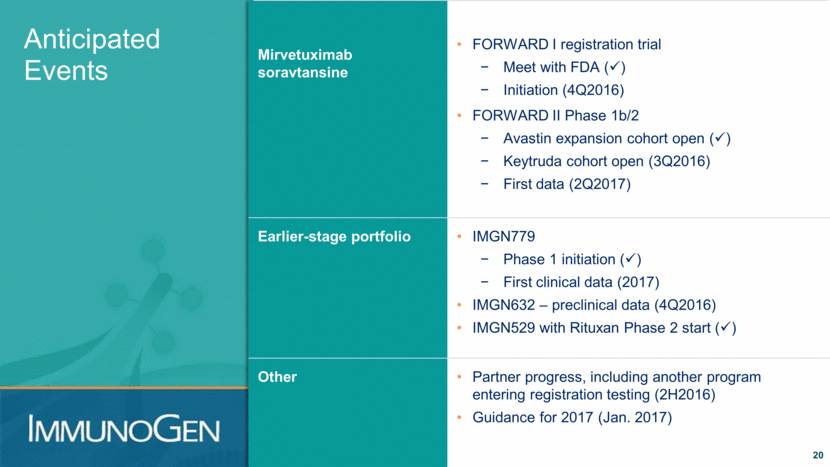
Mirvetuximab soravtansine FORWARD I registration trial Meet with FDA ( ) Initiation (4Q2016) FORWARD II Phase 1b/2 Avastin expansion cohort open ( ) Keytruda cohort open (3Q2016) First data (2Q2017) Earlier-stage portfolio IMGN779 Phase 1 initiation ( ) First clinical data (2017) IMGN632 – preclinical data (4Q2016) IMGN529 with Rituxan Phase 2 start ( ) Other Partner progress, including another program entering registration testing (2H2016) Guidance for 2017 (Jan. 2017) Anticipated Events 20
ImmunoGen Today: Positioned for Sustainable Value Creation 21 Cutting edge technology in growing field Rich pipeline of differentiated product candidates Enhanced financial resources Experienced team Strong partnerships
Carol Hausner Q & A 22

