Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - MERIT MEDICAL SYSTEMS INC | a07272016-8kpressreleaseex.htm |
| 8-K - 8-K - MERIT MEDICAL SYSTEMS INC | a7272016-8k.htm |

1
2nd Quarter 2016 Results
FRED LAMPROPOULOS
Chairman & CEO
BERNARD BIRKETT
CFO

2
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This presentation includes “forward-looking statements” as defined within applicable securities laws and regulations. All statements in this presentation, other than statements of historical
fact, are “forward-looking statements”, including projections of earnings, revenues or other financial items, any statements regarding our plans and objectives for future operations, any
statements concerning proposed new products or services, any statements regarding the integration, development or commercialization of our business or any business, assets or operations
we may acquire, any statements regarding future economic conditions or performance, and any statements of assumptions underlying any of the foregoing. All forward-looking statements,
including financial projections, included in this presentation are made as of the date of this presentation, and are based on information available to us as of such date. We assume no
obligation to update or disclose revisions to any forward-looking statement. In some cases, forward-looking statements can be identified by the use of terminology such as “may,” “will,”
“likely,” “expects,” “plans,” “anticipates,” “intends,” “believes,” “estimates,” “projects,” ”forecast,” “potential,” “plan” or “continue,” or other comparable terminology. Forward-looking
statements are based on our current beliefs, expectations and assumptions regarding our business, domestic and global economies, regulatory and competitive environments and other
future conditions. There can be no assurance that such beliefs, expectations or assumptions or any of the forward-looking statements will prove to be correct. Actual results will likely differ,
and may differ materially, from those projected or assumed in the forward-looking statements. Our future financial and operating results and condition, as well as any forward-looking
statements, are subject to inherent risks and uncertainties such as those described in our Annual Report on Form 10-K for the year ended December 31, 2015 and other filings with the U.S.
Securities and Exchange Commission. Such risks and uncertainties include risks relating to our potential inability to successfully manage growth through acquisitions; product recalls and
product liability claims; expenditures relating to research, development, testing and regulatory approval of our products and risks that such products may not be developed successfully or
approved for commercial use; regulation of the medical device industry; restrictions on our liquidity or our ability to operate our business in compliance with our current debt agreements;
possible infringement of our technology or the assertion that our technology infringes the rights of other parties; potential fines, penalties or other adverse consequences if our employees or
agents violate the U.S. Foreign Corrupt Practices Act or other laws and regulations; changes in tax laws and regulations in the United States or other countries; changes in the prices or supply
of commodity components; changes in economic and industry conditions in the United States and other countries; termination or interruption of relationships with our suppliers, or failure of
such suppliers to perform; fluctuations in exchange rates; our need to generate sufficient cash flow to fund our debt obligations, capital expenditures, and ongoing operations; development
of new products and technology that could render our existing products obsolete; market acceptance of new products; modification or limitation of governmental or private insurance
reimbursement policies; changes in health care markets related to health care reform initiatives; changes in key personnel; work stoppage or transportation risks; uncertainties associated
with potential healthcare policy changes; introduction of products in a timely fashion; price and product competition; availability of labor and materials; cost increases; and fluctuations in
and obsolescence of inventory. All subsequent forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by these cautionary
statements.
The financial projections set forth in this presentation are based on a number of assumptions, estimates and forecasts. The inaccuracy of any one of those assumptions, estimates or
forecasts could materially impact our actual financial results. Inevitably, some of those assumptions, estimates or forecasts will not occur and unanticipated events and circumstances will
occur subsequent to the date of this presentation. In addition to changes in the underlying assumptions, our future performance is subject to a number of risks and uncertainties with
respect to our existing and proposed business, and other factors that may cause our actual results or performance to be materially different from any predicted or implied. Although we
have attempted to identify important assumptions in the financial projections, there may be other factors that could materially affect our actual financial performance, and no assurance can
be given that all material factors have been considered in the preparation of the financial projections. Accordingly, you should not place undue reliance on such projections. Future
operating results are, in fact, impossible to predict.
3
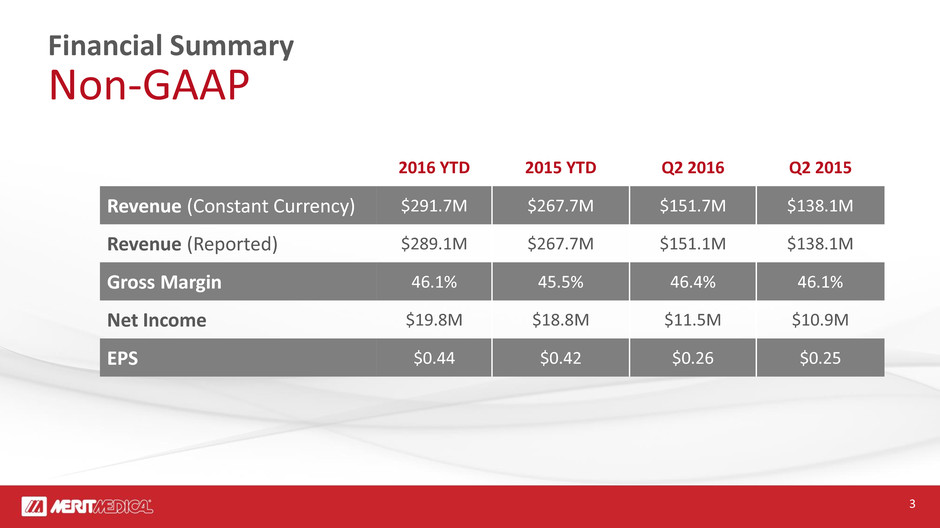
Financial Summary
Non-GAAP
2016 YTD 2015 YTD Q2 2016 Q2 2015
Revenue (Constant Currency) $291.7M $267.7M $151.7M $138.1M
Revenue (Reported) $289.1M $267.7M $151.1M $138.1M
Gross Margin 46.1% 45.5% 46.4% 46.1%
Net Income $19.8M $18.8M $11.5M $10.9M
EPS $0.44 $0.42 $0.26 $0.25

4
Financial Summary
GAAP
2016 YTD 2015 YTD Q2 2016 Q2 2015
Revenue $289.1M $267.7M $151.1M $138.1M
Gross Margin 43.9% 43.4% 44.3% 44.1%
Net Income $11.6M $12.6M $7.3M $7.4M
EPS $0.26 $0.28 $0.16 $0.17
5
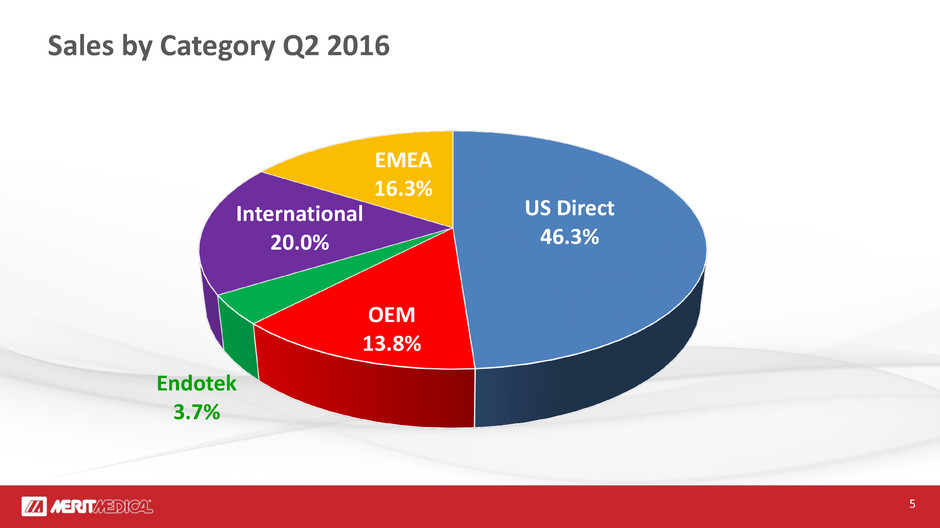
Sales by Category Q2 2016
US Direct
46.3%
OEM
13.8%
Endotek
3.7%
International
20.0%
EMEA
16.3%

6
Q2 2016 Revenue Growth in Constant Currency Compared to Q2 2015
0%
5%
10%
15%
20%
25%
US Direct OEM Endotek International EMEA
10.3%
2.3%
4.8%
21.1%
5.3%

7
Q2 2016 Highlights
• Transferred production of
HeRO®Graft product line to
Utah
• FDA and CE approval of
Corvocet™ Biopsy System
• Ramped up catheter capacity
in Pearland, Texas
• Negotiated acquisition of
DFINE, Inc. (closed July 6,
2016)
• Canada office and
warehouse operational
• Mexico facility surpassed
break-even point

8
2016 Growth Drivers
• New Products
- HeRO® Graft
- Corvocet™ Biopsy Device
- SwiftNINJA® Steerable Microcatheter
- Pedal Access
- Micropuncture
- Centesis Catheters
- Amplatz Guide Wires
- 40 ATM BasixTouch™ Inflation Device
- Prelude® SNAP Hydrophilic
- Wire Guided & Pulmonary Balloons
• Wholesale to Retail
- Australia – January 1
- Canada – April 1
• ThinkRadial™ & Think HeRO® Graft Programs
• DFINE, Inc. Integration
9
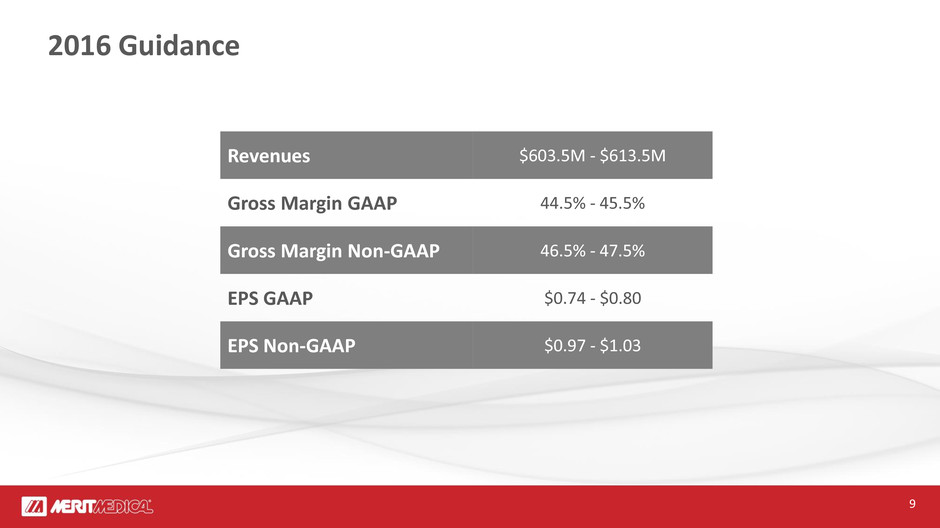
2016 Guidance
Revenues $603.5M - $613.5M
Gross Margin GAAP 44.5% - 45.5%
Gross Margin Non-GAAP 46.5% - 47.5%
EPS GAAP $0.74 - $0.80
EPS Non-GAAP $0.97 - $1.03

10
1 2 3 4
Disciplined,
customer-focused
enterprise
Guided by strong core
values to globally
address unmet or
underserved
healthcare needs
Target high-growth,
high-return
opportunities
Through understanding,
innovating, and delivering
in peripheral, cardiac,
OEM, and endoscopy
business lines
Optimize operational
capability
Through lean processes,
cost effective
environments, and asset
utilization
Enhance growth
and profitability
Through R&D, sales
model optimization,
cost discipline, and
operational focus
10

11
