Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - CHEMBIO DIAGNOSTICS, INC. | v444858_ex99-1.htm |
| 8-K - CURRENT REPORT - CHEMBIO DIAGNOSTICS, INC. | v444858_8k.htm |
Exhibit 99.2
NASDAQ: CEMI July 2016

2 Forward - Looking Statement Statements contained herein that are not historical facts are forward - looking statements within the meaning of the Securities Act of 1933, as amended. Those statements include statements regarding the intent, belief or current expectations of Chembio and its management. Such statements reflect management's current views, are based on certain assumptions, and involve risks and uncertainties. Actual results, events, or performance may differ materially from the above forward - looking statements due to a number of important factors, and will be dependent upon a variety of factors, including, but not limited to, Chembio’s ability to develop, manufacture, market and finance new products and the demand for Chembio's products. Chembio undertakes no obligation to publicly update these forward - looking statements to reflect events or circumstances that occur after the date hereof or to reflect any change in Chembio's expectations with regard to these forward - looking statements or the occurrence of unanticipated events. Other factors that may impact Chembio's success are more fully disclosed in Chembio's most recent public filings with the U.S. Securities and Exchange Commission.

3 Investment Highlights ▪ A global leader in point - of - care (POC) infectious disease – Sales in 40+ countries, including direct sales in the United States – Core business in POC HIV testing; 8% revenue growth (2010 - 2015) ▪ Groundbreaking patented DPP ® technology platform – Superior sensitivity and specificity vs lateral flow technology – Multiple tests from a single oral fluid or blood sample (multiplexing) ▪ Robust pipeline of new DPP ® POC assays in development – DPP ® HIV - Syphilis Combination Assay (U.S. version) – DPP ® Fever Assays (Malaria, Dengue, Zika , Chikungunya , Ebola, Lassa, Marburg) – DPP ® Technology Collaborations (Traumatic Brain Injury, Cancer, Micro Reader) ▪ Multiple high - value collaborations – Paul G. Allen Ebola Program: Fever Panel , Zika – Bill & Melinda Gates Foundation: Malaria Oral Fluid/Saliva – Centers for Disease Control & Prevention (CDC): Malaria, Ebola, Flu Immunostatus ▪ Experienced leadership team
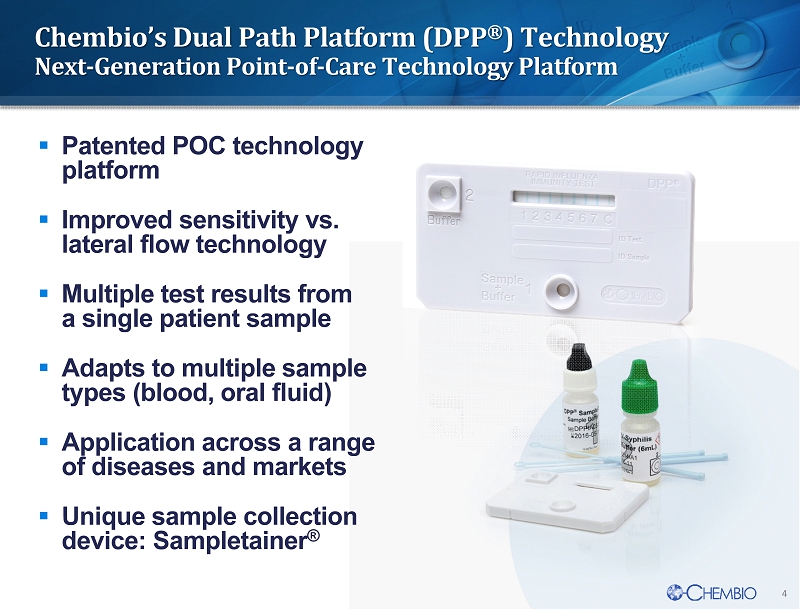
4 Chembio’s Dual Path Platform (DPP ® ) Technology Next - Generation Point - of - Care Technology Platform 4 ▪ P atented POC technology platform ▪ Improved sensitivity vs. lateral flow technology ▪ Multiple test results from a single patient sample ▪ Adapts to multiple sample types (blood, oral fluid) ▪ Application across a range of diseases and markets ▪ Unique sample collection device: Sampletainer ®
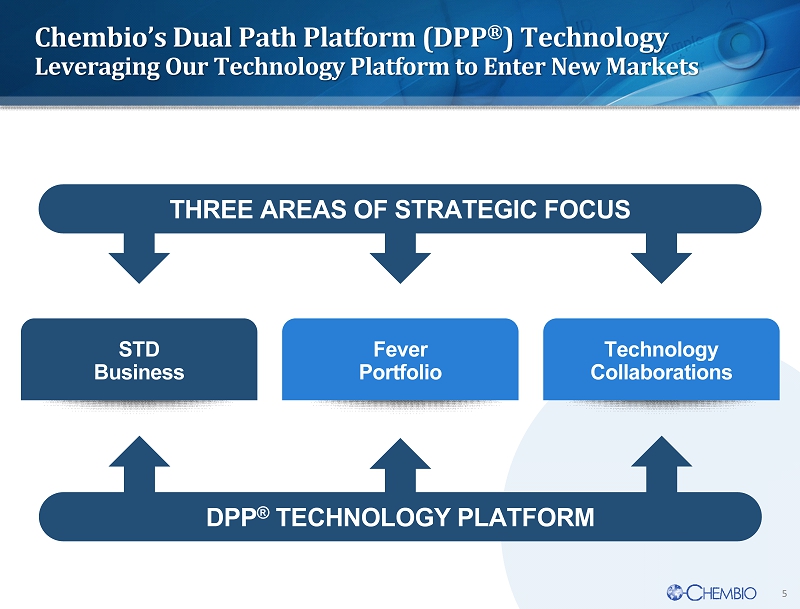
5 STD Business Technology Collaborations Fever Portfolio THREE AREAS OF STRATEGIC FOCUS DPP ® TECHNOLOGY PLATFORM Chembio’s Dual Path Platform (DPP ® ) Technology Leveraging Our Technology Platform to Enter New Markets
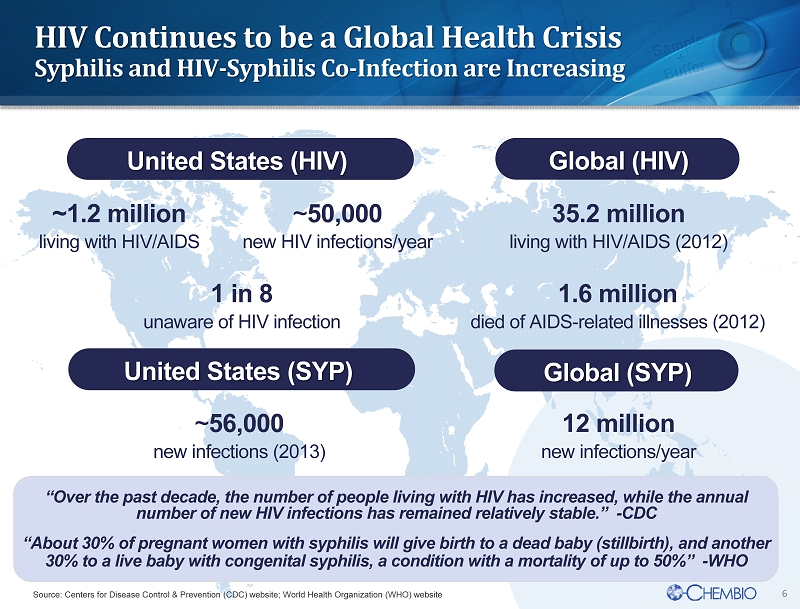
6 HIV Continues to be a Global Health Crisis Syphilis and HIV - Syphilis Co - Infection are Increasing 1.6 million died of AIDS - related illnesses (2012) “Over the past decade, the number of people living with HIV has increased, while the annual number of new HIV infections has remained relatively stable.” - CDC “About 30% of pregnant women with syphilis will give birth to a dead baby (stillbirth), and another 30% to a live baby with congenital syphilis, a condition with a mortality of up to 50%” - WHO 1 in 8 unaware of HIV infection 35.2 million living with HIV/AIDS (2012) ~1.2 million living with HIV/AIDS ~ 50,000 new HIV infections/year Source: Centers for Disease Control & Prevention (CDC) website; World Health Organization (WHO) website ~ 56,000 new infections (2013) 12 million new infections/year Global (SYP) United States (SYP) United States (HIV) Global (HIV)

7 Chembio Lateral Flow HIV Tests Foundational HIV Product Suite Chembio SURE CHECK ® HIV 1/2* ▪ Product Features & Benefits – FDA (PMA) approved, CLIA - waived – CE marked, WHO pre - qualified – 2.5 - 5.0 μL blood sample – 15 - 20 minute test time – Specificity: 99.9%, Sensitivity: 99.7% ▪ Commercialization – High quality brands, marketed globally since 2007 – Sold to Public Health Clinics, POLs, Hospitals, Self Test (EU) – Distribution Partners (US): Fisher, McKesson/PSS, H. Schein, Medline Chembio HIV 1/2 STAT - PAK ®
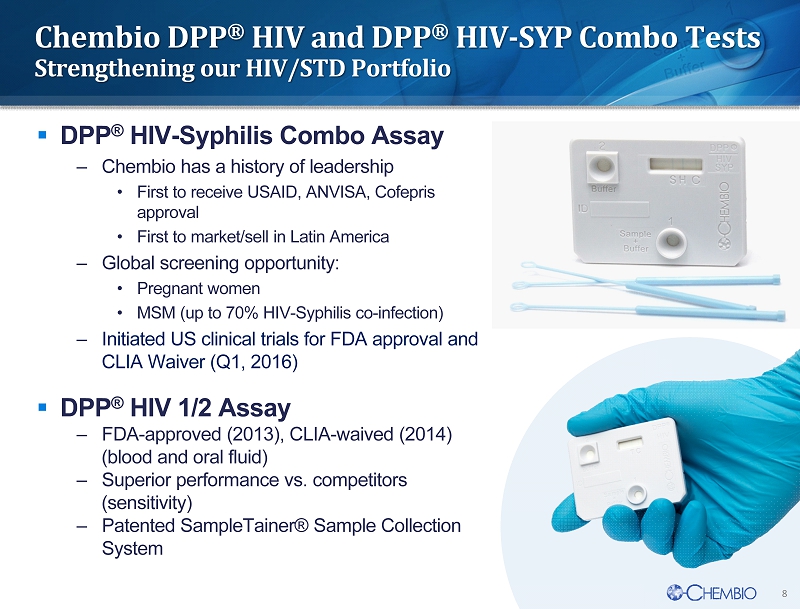
8 Chembio DPP ® HIV and DPP ® HIV - SYP Combo Tests Strengthening our HIV/STD Portfolio ▪ DPP ® HIV - Syphilis Combo Assay – Chembio has a history of leadership • First to receive USAID, ANVISA, Cofepris approval • First to market/sell in Latin America – Global screening opportunity: • Pregnant women • MSM (up to 70% HIV - Syphilis co - infection ) – Initiated US clinical trials for FDA approval and CLIA Waiver (Q1, 2016) ▪ DPP ® HIV 1/2 Assay – FDA - approved (2013), CLIA - waived (2014) (blood and oral fluid) – Superior performance vs . competitors ( sensitivity) – Patented SampleTainer® Sample Collection System
9 STD Business Technology Collaborations Fever Portfolio THREE AREAS OF STRATEGIC FOCUS DPP ® TECHNOLOGY PLATFORM Chembio’s Dual Path Platform (DPP ® ) Technology Leveraging Our Technology Platform to Enter New Markets

10 Fever Disease - Product Development Chembio is Collaborating with World Leading Organizations
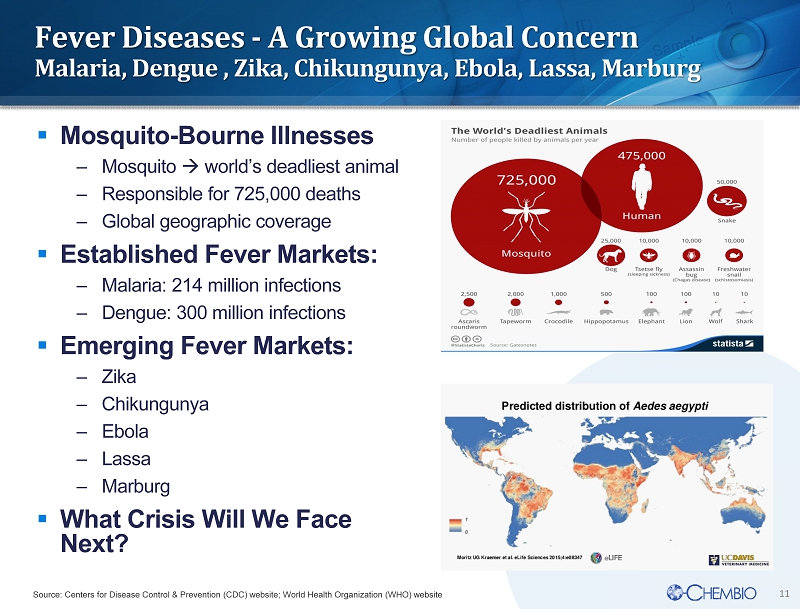
11 Fever Diseases - A Growing Global Concern Malaria, Dengue , Zika, Chikungunya, Ebola, Lassa, Marburg ▪ Mosquito - B ourne I llnesses – Mosquito world’s deadliest animal – Responsible for 725,000 deaths – Global g eographic coverage ▪ Established Fever Markets: – Malaria: 214 million infections – Dengue: 300 million infections ▪ Emerging Fever Markets: – Zika – Chikungunya – Ebola – Lassa – Marburg ▪ What Crisis Will We Face Next? Source: Centers for Disease Control & Prevention (CDC) website; World Health Organization (WHO) website
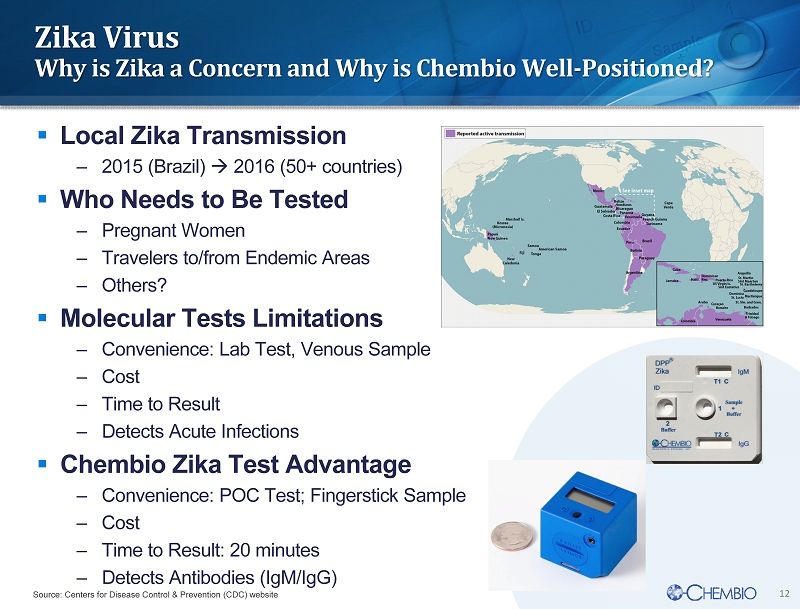
12 Zika Virus Why is Zika a Concern and Why is Chembio Well - Positioned? ▪ Local Zika Transmission – 2015 (Brazil) 2016 (50+ countries) ▪ Who Needs to Be Tested – Pregnant Women – Travelers to/from Endemic Areas – Others? ▪ Molecular Tests Limitations – Convenience: Lab Test, Venous Sample – Cost – Time to Result – Detects Acute Infections ▪ Chembio Zika Test Advantage – Convenience: POC Test; Fingerstick Sample – Cost – Time to Result: 20 minutes – Detects Antibodies (IgM/IgG) Source: Centers for Disease Control & Prevention (CDC) website

13 ▪ Accelerated DPP ® Zika Assay Development – R eceived grant from Paul G. Allen Foundation and initiated project – 2/16 – A nnounced Zika collaboration with Bio - Manguihos /Fiocruz (Brazil) – 3/16 – C ompleted testing of >1,000 samples (including 600 pregnant women) – 4/16 – A nnounced regulatory filings with FDA - EUA (US, PR), ANVISA (Brazil ) – 5/16 – A nnounced regulatory filings with WHO - EUA, Cofepris (Mexico), CE mark – 7/16 ▪ Development of Zika - related DPP ® Assays – Continued development of DPP ® Dengue IgM/IgG Assay – Initiated development of DPP ® Chikungunya IgM/IgG Assay – Initiated development of DPP ® Zika/Chikungunya/Dengue IgM/IgG Combo Assay DPP ® Zika/Dengue/Chikungunya - Development Zika Virus represents the latest global health crisis
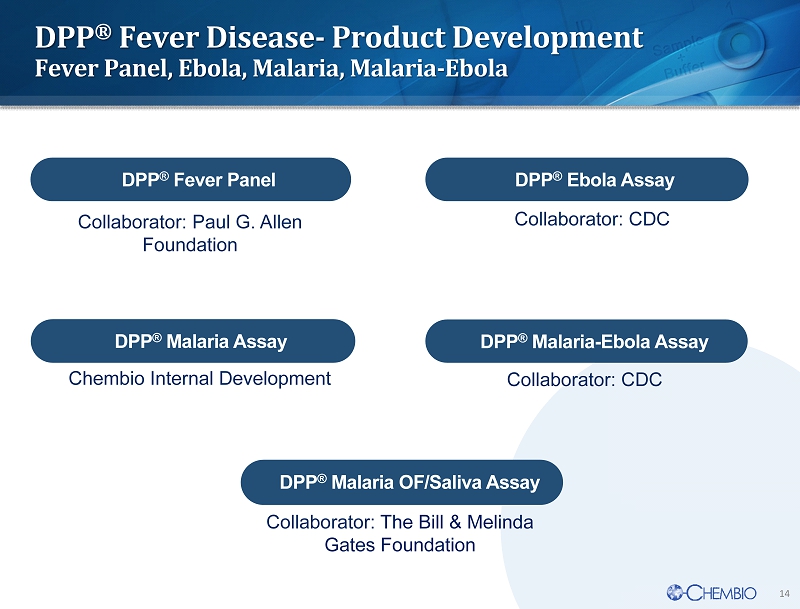
14 DPP ® Fever Disease - Product Development Fever Panel, Ebola, Malaria, Malaria - Ebola DPP ® Ebola Assay DPP ® Malaria - Ebola Assay DPP ® Fever Panel Collaborator: CDC Collaborator: CDC Collaborator: Paul G. Allen Foundation DPP ® Malaria Assay DPP ® Malaria OF/Saliva Assay Collaborator : The Bill & Melinda Gates Foundation Chembio Internal Development
15 STD Business Technology Collaborations Fever Portfolio THREE AREAS OF STRATEGIC FOCUS DPP ® TECHNOLOGY PLATFORM Chembio’s Dual Path Platform (DPP ® ) Technology Leveraging Our Technology Platform to Enter New Markets

16 ▪ Bio - Rad Geenius ™ System: HIV - 1 and HIV - 2 Confirmation – Multiplex DPP ® Assay – Developed by Chembio – Licensed by Bio - Rad – Marketed/sold by Bio - Rad (ex - Brazil) ▪ Chembio DPP ® Micro Reader: Improves Results & Data Mgmt. – Improves DPP ® Performance – Provides quantitative results – Standardizes result interpretation – Data capture, storage, transmission – Key features: Simple, Palm - Sized, Battery - Operated, Cost - Effective Technology Collaborations: Bio - Rad (NYSE: BIO) and opTricon (Berlin, Germany)
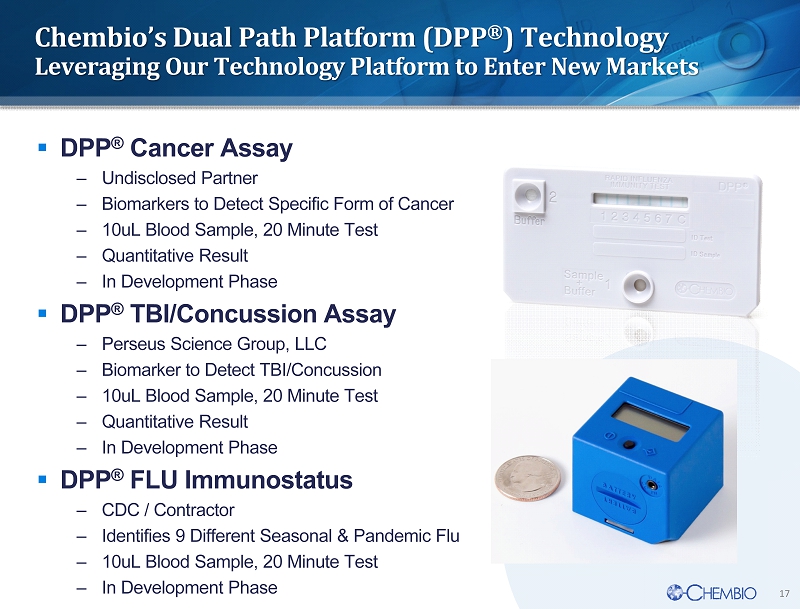
17 ▪ DPP ® Cancer Assay – Undisclosed Partner – Biomarkers to Detect Specific Form of Cancer – 10uL Blood Sample, 20 Minute Test – Quantitative Result – In Development P hase ▪ DPP ® TBI/Concussion Assay – Perseus Science Group, LLC – Biomarker to D etect TBI/Concussion – 10uL Blood Sample , 20 Minute Test – Quantitative Result – In Development Phase ▪ DPP ® FLU Immunostatus – CDC / Contractor – Identifies 9 Different Seasonal & Pandemic Flu – 10uL Blood Sample , 20 Minute Test – In Development Phase Chembio’s Dual Path Platform (DPP ® ) Technology Leveraging Our Technology Platform to Enter New Markets
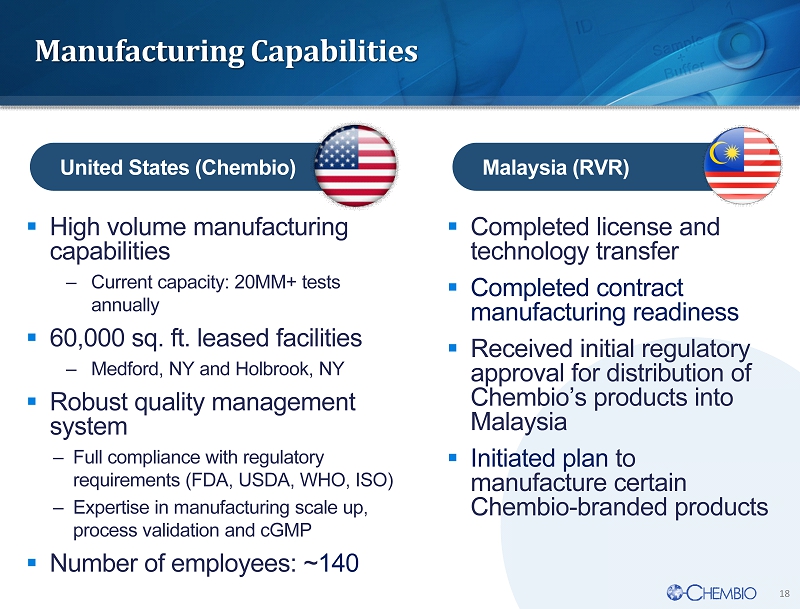
18 Manufacturing Capabilities ▪ High volume manufacturing capabilities – Current capacity: 20MM + tests annually ▪ 60,000 sq. ft. leased facilities – Medford, NY and Holbrook, NY ▪ Robust quality management system – Full compliance with regulatory requirements (FDA, USDA, WHO, ISO) – Expertise in manufacturing scale up, process validation and cGMP ▪ Number of employees: ~ 140 ▪ Completed license and technology transfer ▪ Completed contract manufacturing readiness ▪ Received initial regulatory approval for distribution of Chembio’s products into Malaysia ▪ I nitiated plan to manufacture certain Chembio - branded products United States (Chembio) Malaysia (RVR)
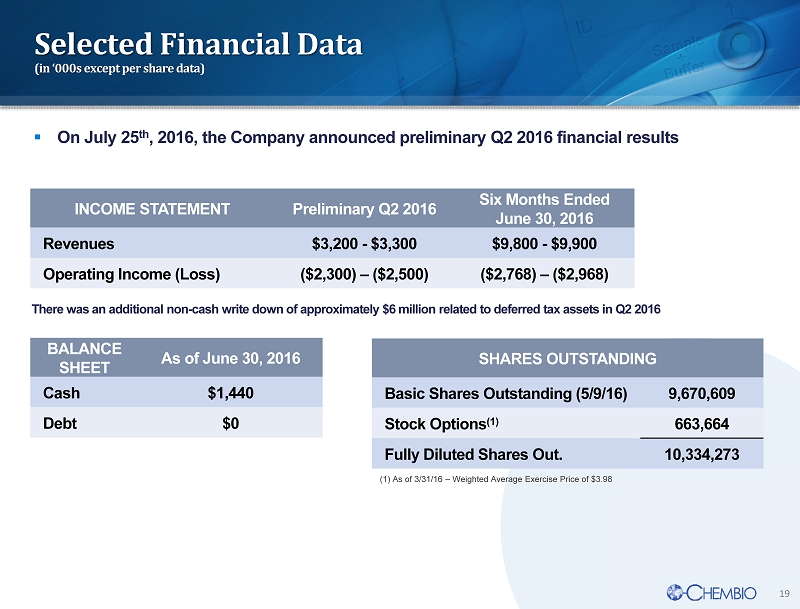
19 Selected Financial Data (in ‘000s except per share data) INCOME STATEMENT Preliminary Q2 2016 Six Months Ended June 30, 2016 Revenues $3,200 - $3,300 $9,800 - $9,900 Operating Income (Loss) ($2,300) – ($2,500) ($2,768) – ($2,968) SHARES OUTSTANDING Basic Shares Outstanding (5/9/16) 9,670,609 Stock Options (1) 663,664 Fully Diluted Shares Out. 10,334,273 (1) As of 3/31/16 – Weighted Average Exercise Price of $3.98 There was an additional non - cash write down of approximately $6 million related to deferred tax assets in Q2 2016 BALANCE SHEET As of June 30 , 2016 Cash $1,440 Debt $0 ▪ On July 25 th , 2016, the Company announced preliminary Q2 2016 financial results

20 Experienced Executive Leadership Team EXECUTIVE JOINED CHEMBIO PREVIOUS EXPERIENCE John Sperzel Ill President & CEO 2014 2011 - 2013, President and CEO of ITC.; 1987 - 2011 Axis Shield, Bayer Diagnostics, Instrumentation Laboratory and Boehringer Mannheim Richard Larkin, CPA Chief Financial Officer 2003 2000 - 2003, CFO of Visual Technology Group; 1987 - 2000 CFO of Protex International Corp. Sharon Klugewicz, M.S. Chief Operating Officer 2012 2009 - 2012, Sr. VP Scientific & Laboratory Services of Pall Corporation; 1991 - 2009 Pall Corporation Javan Esfandiari, M.S. Chief Science & Technology Officer 2000 1997 - 2000, Co - Founder of Sinovus Biotech AB (Sweden), acquired by Chembio in 2000; 1993 - 1997 R&D Director On - Site Biotech Thomas lppolito VP Regulatory & Clinical Affairs 2005 2000 - 2005, VP Quality & Regulatory of Biospecific Technologies Corp.; 1984 - 2000 United Biomedical Inc., Analytab Products Inc. and Eastern Long Island Hospital Michael Steele VP Sales, Marketing & Bus. Dev. 2012 2008 - 2011, VP Business Development of SeraCare Life Sciences; 1992 - 2008 Corautus Genetics, Life Therapeutics and Serologicals, Inc. Paul Lambotte VP Product Development 2014 2009 - 2014, President of PLC Inc.; 2009 - 2012, Chief Science Officer Axxin Pty Ltd.; 2000 - 2009, VP of R&D and Business Development Quidel, Inc.
