Attached files
| file | filename |
|---|---|
| 8-K - 8-K - EAGLE PHARMACEUTICALS, INC. | a16-14715_38k.htm |
Exhibit 99.1
2015 ANNUAL REPORT EAGLE PHARMACEUTICALS, INC. (Nasdaq: EGRX)

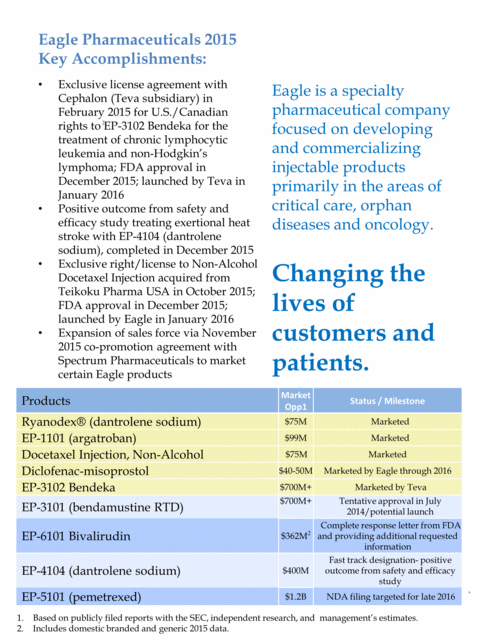
Eagle is a specialty pharmaceutical company focused on developing and commercializing injectable products primarily in the areas of critical care, orphan diseases and oncology. Changing the lives of customers and patients. Exclusive license agreement with Cephalon (Teva subsidiary) in February 2015 for U.S./Canadian rights to EP-3102 Bendeka for the treatment of chronic lymphocytic leukemia and non-Hodgkin’s lymphoma; FDA approval in December 2015; launched by Teva in January 2016 Positive outcome from safety and efficacy study treating exertional heat stroke with EP-4104 (dantrolene sodium), completed in December 2015 Exclusive right/license to Non-Alcohol Docetaxel Injection acquired from Teikoku Pharma USA in October 2015; FDA approval in December 2015; launched by Eagle in January 2016 Expansion of sales force via November 2015 co-promotion agreement with Spectrum Pharmaceuticals to market certain Eagle products Eagle Pharmaceuticals 2015 Key Accomplishments: Products Market Opp1 Status / Milestone Ryanodex® (dantrolene sodium) $75M Marketed EP-1101 (argatroban) $99M Marketed Docetaxel Injection, Non-Alcohol $75M Marketed Diclofenac-misoprostol $40-50M Marketed by Eagle through 2016 EP-3102 Bendeka $700M+ Marketed by Teva EP-3101 (bendamustine RTD) $700M+ Tentative approval in July 2014/potential launch EP-6101 Bivalirudin $362M2 Complete response letter from FDA and providing additional requested information EP-4104 (dantrolene sodium) $400M Fast track designation- positive outcome from safety and efficacy study EP-5101 (pemetrexed) $1.2B NDA filing targeted for late 2016 Based on publicly filed reports with the SEC, independent research, and management’s estimates. Includes domestic branded and generic 2015 data.
To my fellow stockholders:
2015 was a successful year for Eagle across many fronts. We transformed from a development-stage drug delivery company to a fully commercial and profitable specialty pharmaceutical company. We achieved several important milestones, including accomplishments in our product pipeline and the implementation of our commercialization strategy. To that end, we formed an internal and dedicated sales force that is now in place, presently selling Ryanodex®, and that will drive sales of future products as they come to market. This strategy will allow us to maximize the success of our new product launches.
For the year ended December 2015, revenue totaled $66.2 million, supported by our expanded in-market products and our sales and royalty income, up from $19.1 million for the year ended September 2014. For the full-year 2015, Eagle posted net income of $2.6 million, or $0.17 per basic and $0.16 per diluted share, compared with a net loss of $18.0 million, or $1.97 per basic and diluted share, for the period ended September 30, 2014. Our balance sheet remains strong. We closed 2015 with $79.1 million in cash and cash equivalents, no debt and $90.3 million in stockholders’ equity.
Some of our key achievements in 2015 and 2016 include:
Last December, the FDA approved Bendeka™, our rapidly infused bendamustine product. Our marketing partner, Teva Pharmaceuticals, launched the product in January 2016. We are confident that Teva is committed to enabling Bendeka to reach its full market potential. In addition, we expect our 20% royalty from Teva on those sales to be an important earnings driver for Eagle. Bendeka offers several key benefits, both to patients and to health care providers. For patients, the 10-minute infusion time means there is less time in the chair. Therefore, there is less nursing time, and more patients can be treated in a clinic. And from a safety perspective, there is a lower incidence of observed treatment emergent adverse events. Importantly, in February, Eagle was granted an additional patent pertaining to our liquid bendamustine hydrochloride formulations, further strengthening our franchise and bringing the total number of Orange Book listed patents to six. These patents extend from 2026 to 2033, with additional patents pending.
Sales of Ryanodex, our FDA-approved treatment for malignant hyperthermia, increased in 2015. This improvement reflects the efforts of our dedicated sales force in communicating the message about its effectiveness. We are pleased to see this sales momentum, and we will continue to seek additional growth. We are now focused on the potential for Ryanodex to be approved for additional new indications, including Exertional Heat Stroke (EHS), MDMA (Ecstasy) and Methamphetamine intoxication.
We have already received fast-track and orphan designation from the FDA for Ryanodex for the treatment of EHS. Results from our 2015 study at the Hajj were
promising. They showed a 122% incremental improvement in the Glasgow Coma Scale score at 90 minutes, in a subset of non-intubated subjects, supporting that the use of Ryanodex drives the improvement of cognitive function. These outcomes serve to support our view that the use of Ryanodex in combination with the current standard of care, can lead to improved patient results for this dangerous and often-life threatening condition. We have the potential to be the first drug approved by the FDA for the treatment of Exertional Heat Stroke.
We are delighted to share that in March 2016, we signed an agreement with the National Institutes of Health (NIH)/National Institute on Drug Abuse (NIDA) to explore the use of Ryanodex in the treatment of hyperthermia related to Ecstasy and Methamphetamine intoxication. Ecstasy and Methamphetamine intoxication can result in life-threatening brain hyperthermia and accounted for roughly 125,000 emergency room visits in 2011, the last year for which data is available. Preclinical studies, using a well-characterized animal model, will be conducted by NIDA beginning later this summer. We look forward to the results of this exciting collaboration in late 2016 or early 2017.
Turning to other products in the Eagle portfolio, we received FDA approval in December 2015 for our docetaxel injection, non-alcohol formulation, which we launched in January. Notably, this is the first and only alcohol-free formulation in the United States, and meets an important need in the market. Our sales force is actively marketing this new product, which we believe has the potential to improve the lives of patients and resolve concerns among health care professionals at hospitals and infusion centers.
In March 2016, we received a Complete Response Letter from the FDA for our New Drug Application for our ready-to-use (RTU), stable, liquid intravenous formulation of bivalirudin. We remain committed to this important new formulation, which is intended for use as an anticoagulant in patients, and believe it offers multiple benefits for patients and caregivers. We are currently working directly with the FDA to determine an appropriate path forward for this important product.
In addition, Eagle intends to submit a New Drug Application for our ready-to-use/dilute, liquid form of pemetrexed in late 2016.
At Eagle, we are always mindful of our end goal: to improve outcomes by offering differentiated solutions for the conditions our products treat. We remain committed to investing in research & development (R&D) and are focused on ensuring the strength of our portfolio pipeline – both internally and through strategic partnerships. As part of that effort, we are engaged in a joint development effort with Albany Molecular Research Inc. (AMRI), a global contract research and manufacturing organization, to develop, manufacture and commercialize several parenteral drugs in the United States. We are very excited about this collaboration and believe that this will add value going forward.
At Eagle, we believe that a strong and diverse product portfolio, protected by a solid patent estate, is essential to delivering important new therapies to patients and building long-term value for Eagle stockholders. We will continue to scale our investment in R&D in accordance with our growth.
These are also exciting times for the leadership of Eagle. We recently announced the appointments of Douglas L. Braunstein, co-founder of Hudson Executive Capital LP and former Vice Chairman and Chief Financial Officer, JPMorgan Chase & Co., and Robert Glenning, President Financial Services Division and Chief Financial Officer, Hackensack Meridian Health to our Board of Directors. We look forward to their vision and guidance as we move forward to expand our product portfolio and pipeline. In addition, Michael Graves has been appointed Chairman of the Board.
In conclusion, I am extremely proud of the Eagle Pharmaceuticals team and want to thank all of our employees for their dedication and commitment to making Eagle a truly special company. We believe our business outlook remains bright. We are encouraged by the potential of our products to deliver solutions that address life-threatening conditions and improve treatment outcomes, and we look forward to some upcoming milestones that we expect will drive meaningful growth. To that end, we are excited about the future and look forward to continuing to deliver value to Eagle stockholders. Thank you for your confidence.
|
Sincerely, |
|
|
|
|
|
|
|
|
/s/ Scott Tarriff |
|
|
Scott Tarriff |
|
|
President and Chief Executive Officer |
|
|
Eagle Pharmaceuticals, Inc. |
|
|
BOARD OF DIRECTORS |
REGISTRAR & TRANSFER AGENT |
|
Michael Graves Chairman of the Board Scott Tarriff President and Chief Executive Officer Sander Flaum Steven Ratoff David M. Pernock Douglas L. Braunstein Robert Glenning
EXECUTIVE OFFICERS & MANAGEMENT TEAM Scott Tarriff President and Chief Executive Officer, Director Adrian Hepner, MD, Ph.D. Executive Vice President and Chief Medical Officer John LaRocca Executive Vice President and General Counsel Steven L. Krill, Ph.D. Executive Vice President and Chief Scientific Officer David E. Riggs Chief Financial Officer Sherry Korczynski Senior Vice President, Sales and Marketing Daniel O’Connor Vice President, Finance & Operations Michael Moran Vice President, Sales Damian Bonkowski Controller, Director of Accounting |
American Stock Transfer & Trust Company 6201 15th Avenue Brooklyn, NY 11219 718-921-8200
SECURITIES & RELATED INFORMATION The Company’s common stock is listed on the Nasdaq Stock Exchange under the ticker symbol “EGRX”.
ANNUAL MEETING The annual meeting of stockholders will be held on Tuesday, August 2, 2016 at 10 a.m. at the offices of our outside counsel, Cooley LLP, The Grace Building, 1114 Avenue of the Americas, New York, New York 10036-7798.
STOCKHOLDER INFORMATION Stockholders, investors, and analysts interested in corporate information may contact: In-Site Communications Lisa M. Wilson 212-452-2793
WEBSITE Information about Eagle Pharmaceuticals, Inc. may be obtained on our website at www.eagleus.com. Investors interested in Eagle Pharmaceuticals, Inc. stock quotes, news releases, SEC filings and other corporate information may click on the Investors link on our website. |
Safe Harbor Statement
This annual report contains forward-looking statements regarding Eagle Pharmaceuticals’ strategic direction, prospects and future results, which statements are indicated by the words “may,” “will,” “plans,” “believes,” “expects,” “potential,” “should,” and similar expressions. Such forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause actual results to differ materially from those projected in the forward-looking statements. These include all statements relating to expected financial performance and future business or product developments. Management believes that these forward-looking statements are reasonable as and when made on July 12, 2016. However, such forward-looking statements involve known and unknown risks, uncertainties, and other factors that may cause actual results to differ materially from those projected in the forward-looking statements. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date on which they are made. These statements are based on management’s current expectations and Eagle Pharmaceuticals does not undertake any responsibility to revise or update any forward-looking statements contained herein, except as expressly required by law. For a discussion of certain risks and uncertainties associated with Eagle Pharmaceuticals’ forward-looking statements, please review Eagle Pharmaceuticals’ reports filed with the SEC, including, but not limited to, its Annual Report on Form 10-K, as amended, for the period ended December 31, 2015.
Eagle Pharmaceuticals, Inc. 50 Tice Boulevard Suite 315 Woodcliff Lake, NJ 07677 Phone: 201.326.5300 Fax: 201.391.2430 Email: info@eagleus.com www.eagleus.com Ryanodex ® and Bendeka® are registered trademarks of Eagle Pharmaceuticals, Inc.

