Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - MyoKardia, Inc. | d214197dex991.htm |
| 8-K - FORM 8-K - MyoKardia, Inc. | d214197d8k.htm |

Data Supplement to Press Release July 11, 2016 MyoKardia Announces Topline Clinical Data Supporting Advancement of MYK-461 to Phase 2 PIONEER-HCM Study in Symptomatic, Obstructive Hypertrophic Cardiomyopathy Patients Exhibit 99.2

Safe-Harbor and Forward Looking Statements Statements in this presentation may include statements which are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, which are usually identified by the use of words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “seeks,” “should,” “will,” and variations of such words or similar expressions. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. These forward-looking statements, including statements regarding the anticipated safety profile and clinical and therapeutic potential of MYK-461, the anticipated dose for MYK-461 in the Company’s Phase 2 PIONEER-HCM study, our ability to advance MYK-461 into Phase 2 clinical development and the anticipated timing thereof, reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control including, without limitation, risks associated with the development and regulation of our product candidates, as well as those set forth in our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, our Quarterly Report on Form 10-Q for the quarter ended March 31, 2016 and our other filings with the SEC. Except as required by law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
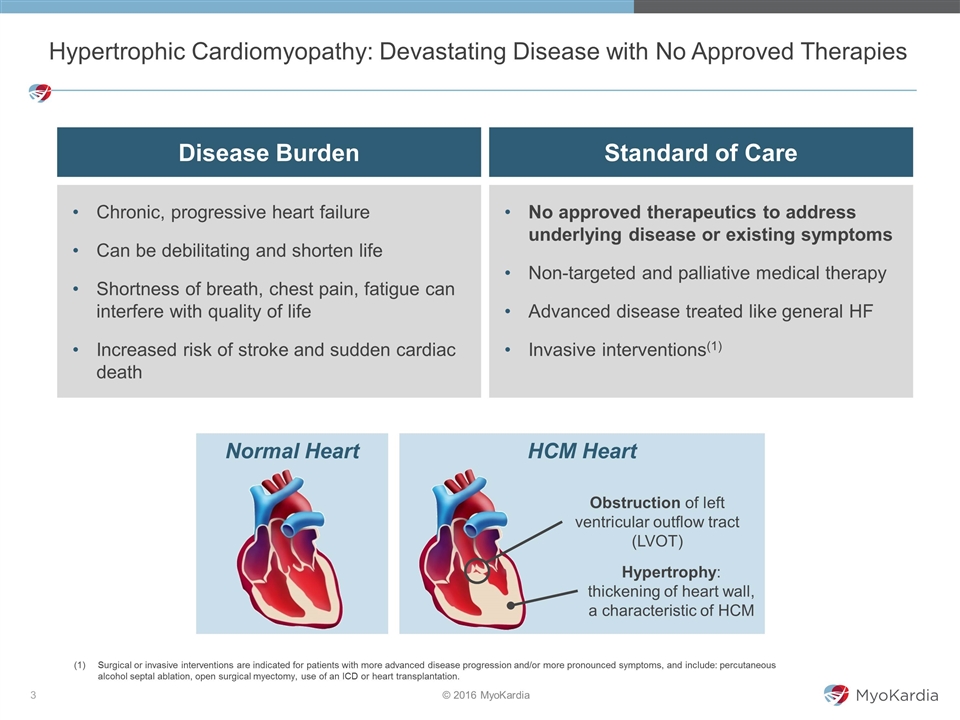
Hypertrophic Cardiomyopathy: Devastating Disease with No Approved Therapies Disease Burden Standard of Care Chronic, progressive heart failure Can be debilitating and shorten life Shortness of breath, chest pain, fatigue can interfere with quality of life Increased risk of stroke and sudden cardiac death No approved therapeutics to address underlying disease or existing symptoms Non-targeted and palliative medical therapy Advanced disease treated like general HF Invasive interventions(1) Surgical or invasive interventions are indicated for patients with more advanced disease progression and/or more pronounced symptoms, and include: percutaneous alcohol septal ablation, open surgical myectomy, use of an ICD or heart transplantation. HCM Heart Normal Heart Obstruction of left ventricular outflow tract (LVOT) Hypertrophy: thickening of heart wall, a characteristic of HCM
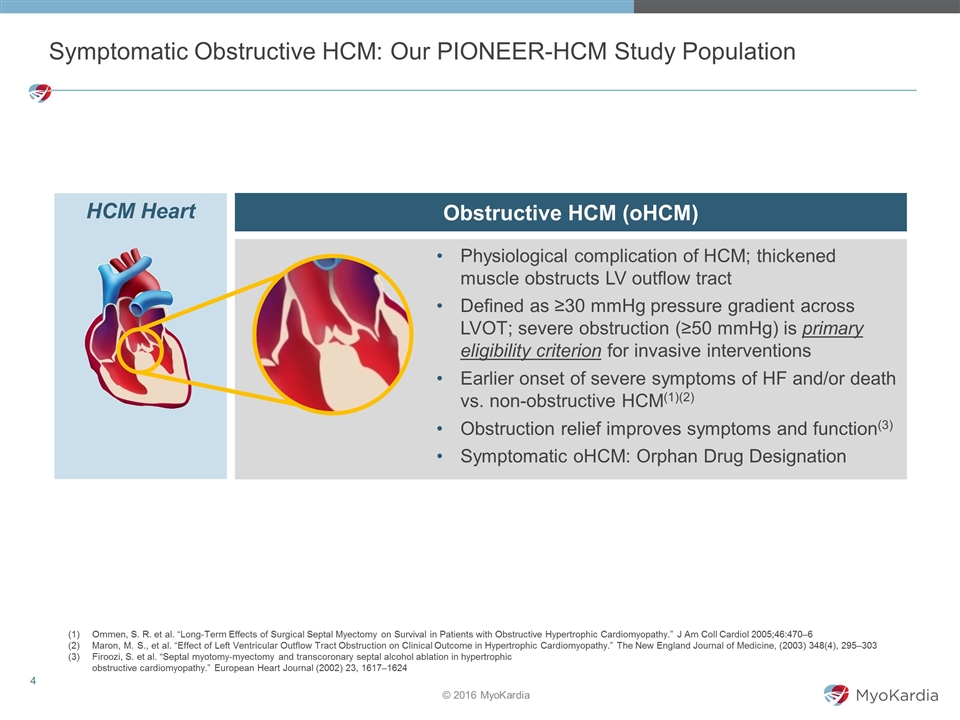
Symptomatic Obstructive HCM: Our PIONEER-HCM Study Population Physiological complication of HCM; thickened muscle obstructs LV outflow tract Defined as ≥30 mmHg pressure gradient across LVOT; severe obstruction (≥50 mmHg) is primary eligibility criterion for invasive interventions Earlier onset of severe symptoms of HF and/or death vs. non-obstructive HCM(1)(2) Obstruction relief improves symptoms and function(3) Symptomatic oHCM: Orphan Drug Designation HCM Heart Obstructive HCM (oHCM) Ommen, S. R. et al. “Long-Term Effects of Surgical Septal Myectomy on Survival in Patients with Obstructive Hypertrophic Cardiomyopathy.” J Am Coll Cardiol 2005;46:470–6 Maron, M. S., et al. “Effect of Left Ventricular Outflow Tract Obstruction on Clinical Outcome in Hypertrophic Cardiomyopathy.” The New England Journal of Medicine, (2003) 348(4), 295–303 Firoozi, S. et al. “Septal myotomy-myectomy and transcoronary septal alcohol ablation in hypertrophic obstructive cardiomyopathy.” European Heart Journal (2002) 23, 1617–1624
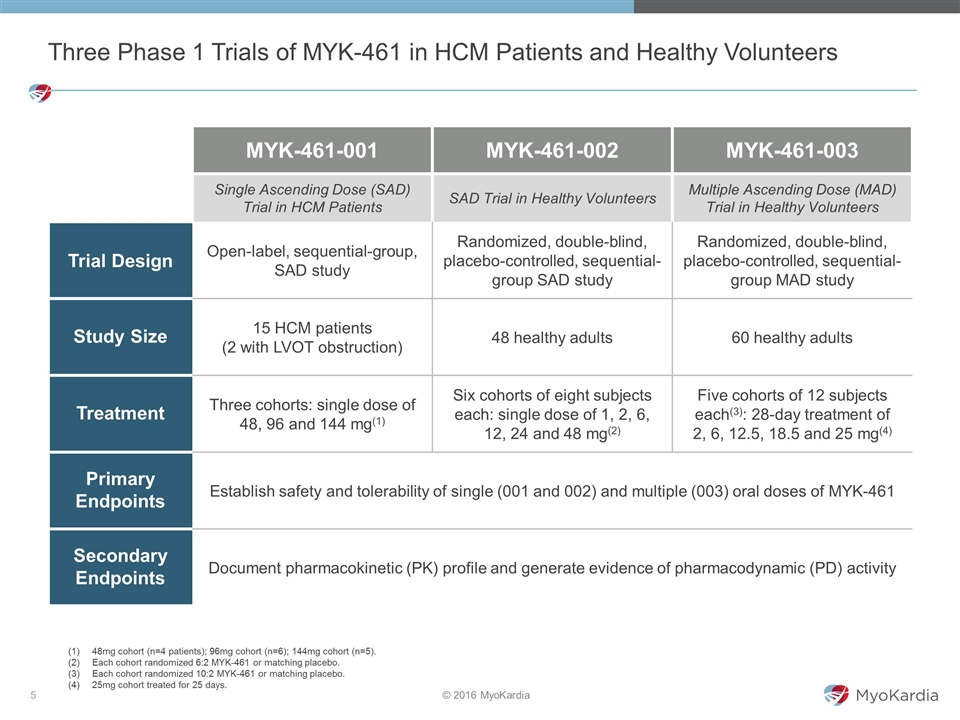
MYK-461-001 MYK-461-002 MYK-461-003 Single Ascending Dose (SAD) Trial in HCM Patients SAD Trial in Healthy Volunteers Multiple Ascending Dose (MAD) Trial in Healthy Volunteers Trial Design Open-label, sequential-group, SAD study Randomized, double-blind, placebo-controlled, sequential-group SAD study Randomized, double-blind, placebo-controlled, sequential-group MAD study Study Size 15 HCM patients (2 with LVOT obstruction) 48 healthy adults 60 healthy adults Treatment Three cohorts: single dose of 48, 96 and 144 mg(1) Six cohorts of eight subjects each: single dose of 1, 2, 6, 12, 24 and 48 mg(2) Five cohorts of 12 subjects each(3): 28-day treatment of 2, 6, 12.5, 18.5 and 25 mg(4) Primary Endpoints Establish safety and tolerability of single (001 and 002) and multiple (003) oral doses of MYK-461 Secondary Endpoints Document pharmacokinetic (PK) profile and generate evidence of pharmacodynamic (PD) activity Three Phase 1 Trials of MYK-461 in HCM Patients and Healthy Volunteers 48mg cohort (n=4 patients); 96mg cohort (n=6); 144mg cohort (n=5). Each cohort randomized 6:2 MYK-461 or matching placebo. Each cohort randomized 10:2 MYK-461 or matching placebo. 25mg cohort treated for 25 days.
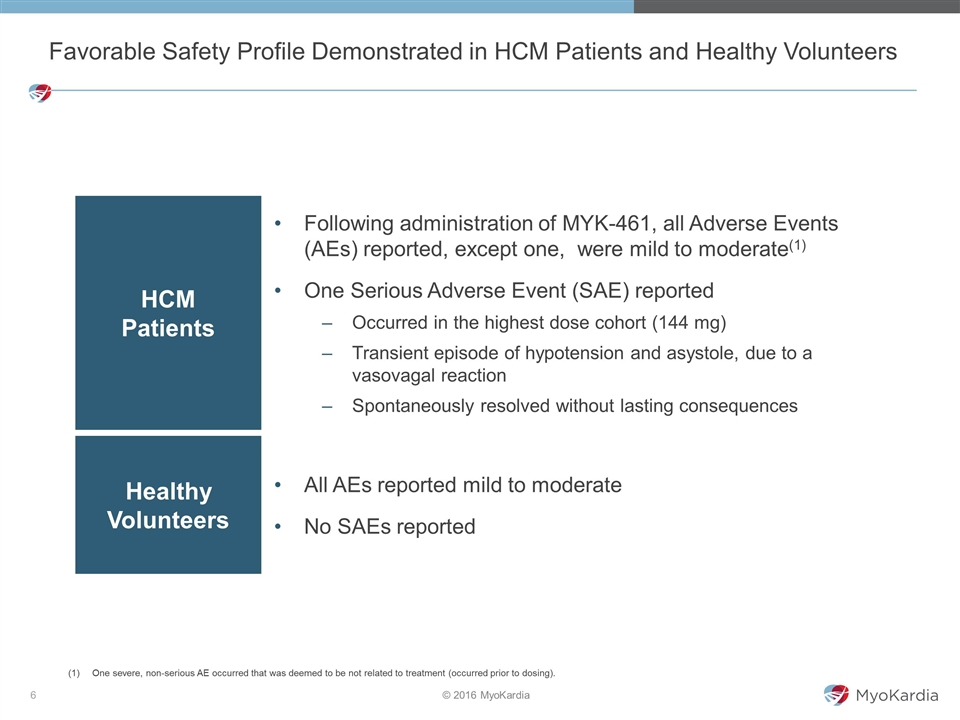
Favorable Safety Profile Demonstrated in HCM Patients and Healthy Volunteers HCM Patients Following administration of MYK-461, all Adverse Events (AEs) reported, except one, were mild to moderate(1) One Serious Adverse Event (SAE) reported Occurred in the highest dose cohort (144 mg) Transient episode of hypotension and asystole, due to a vasovagal reaction Spontaneously resolved without lasting consequences Healthy Volunteers All AEs reported mild to moderate No SAEs reported One severe, non-serious AE occurred that was deemed to be not related to treatment (occurred prior to dosing).
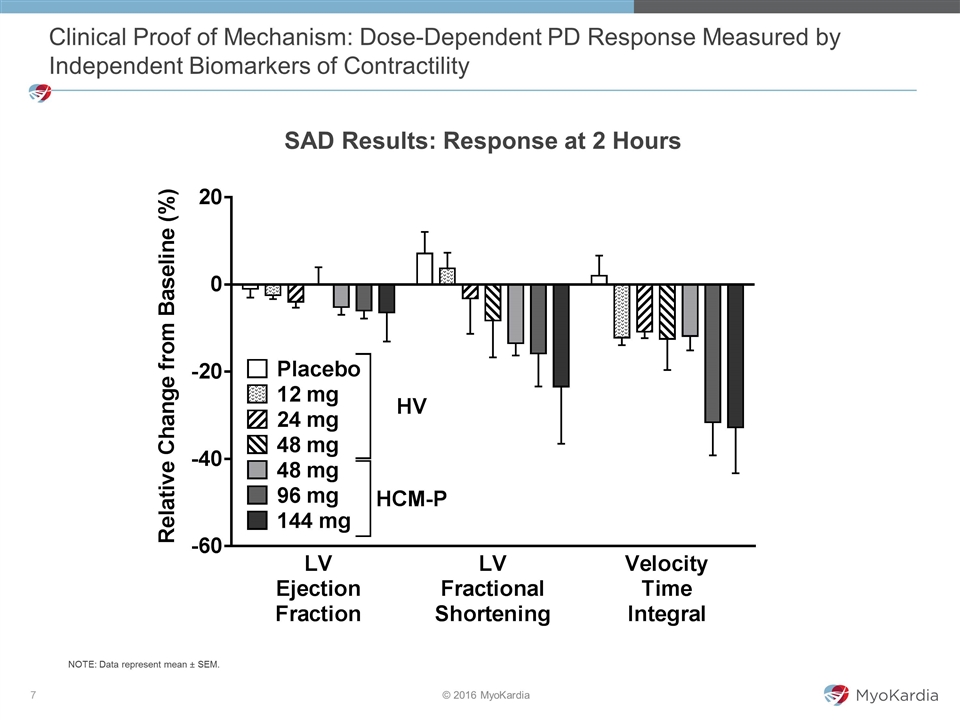
Clinical Proof of Mechanism: Dose-Dependent PD Response Measured by Independent Biomarkers of Contractility SAD Results: Response at 2 Hours NOTE: Data represent mean ± SEM.
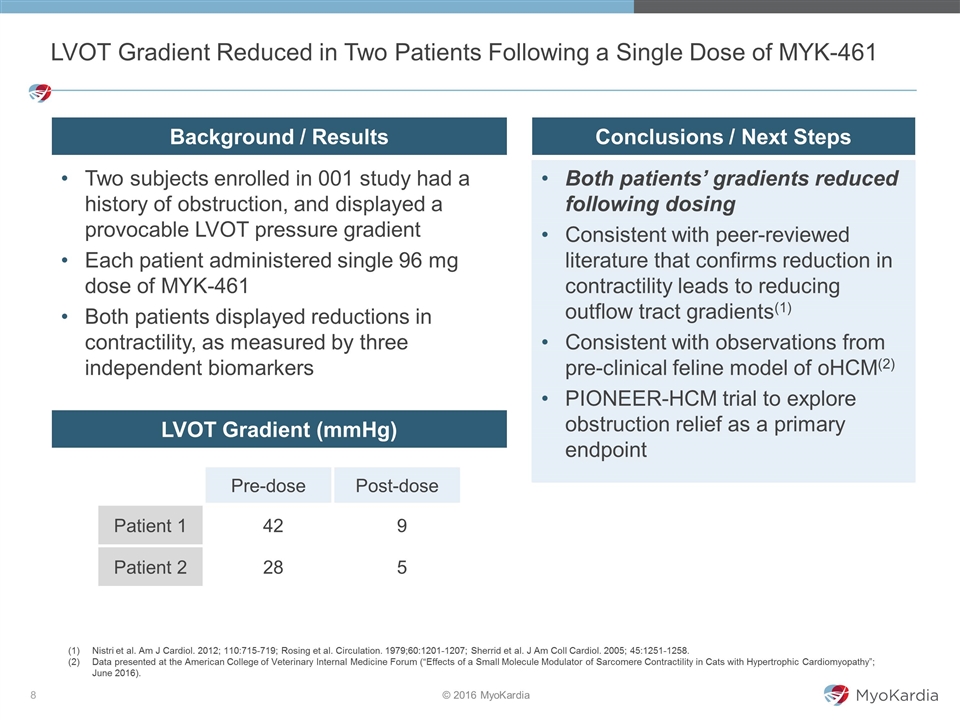
Two subjects enrolled in 001 study had a history of obstruction, and displayed a provocable LVOT pressure gradient Each patient administered single 96 mg dose of MYK-461 Both patients displayed reductions in contractility, as measured by three independent biomarkers LVOT Gradient Reduced in Two Patients Following a Single Dose of MYK-461 Pre-dose Post-dose Patient 1 42 9 Patient 2 28 5 LVOT Gradient (mmHg) Background / Results Conclusions / Next Steps Nistri et al. Am J Cardiol. 2012; 110:715-719; Rosing et al. Circulation. 1979;60:1201-1207; Sherrid et al. J Am Coll Cardiol. 2005; 45:1251-1258. Data presented at the American College of Veterinary Internal Medicine Forum (“Effects of a Small Molecule Modulator of Sarcomere Contractility in Cats with Hypertrophic Cardiomyopathy”; June 2016). Both patients’ gradients reduced following dosing Consistent with peer-reviewed literature that confirms reduction in contractility leads to reducing outflow tract gradients(1) Consistent with observations from pre-clinical feline model of oHCM(2) PIONEER-HCM trial to explore obstruction relief as a primary endpoint
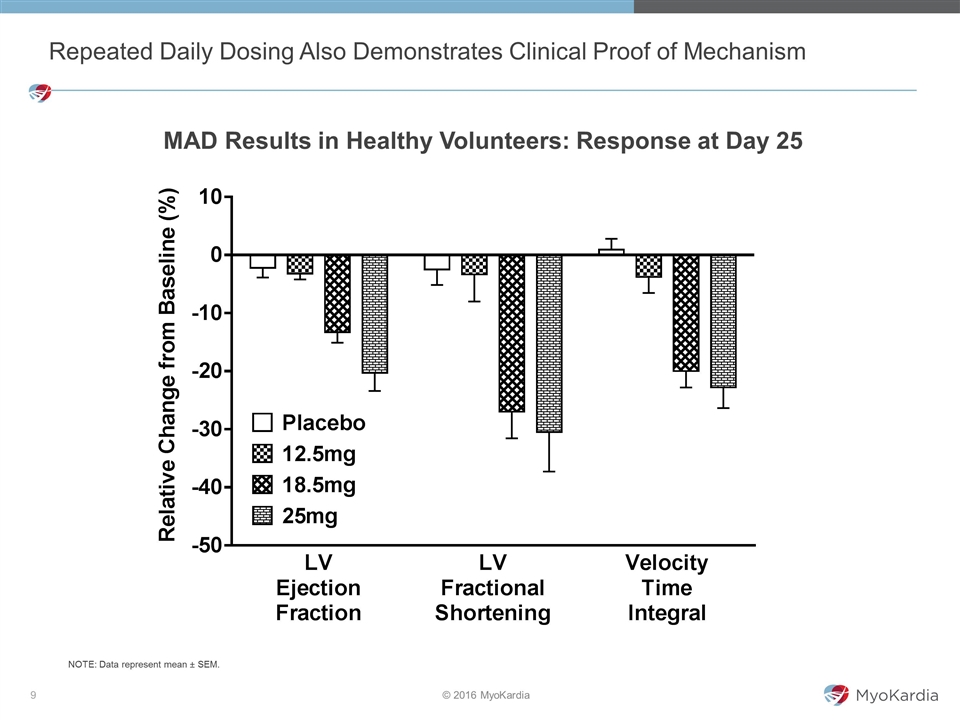
Repeated Daily Dosing Also Demonstrates Clinical Proof of Mechanism MAD Results in Healthy Volunteers: Response at Day 25 NOTE: Data represent mean ± SEM.
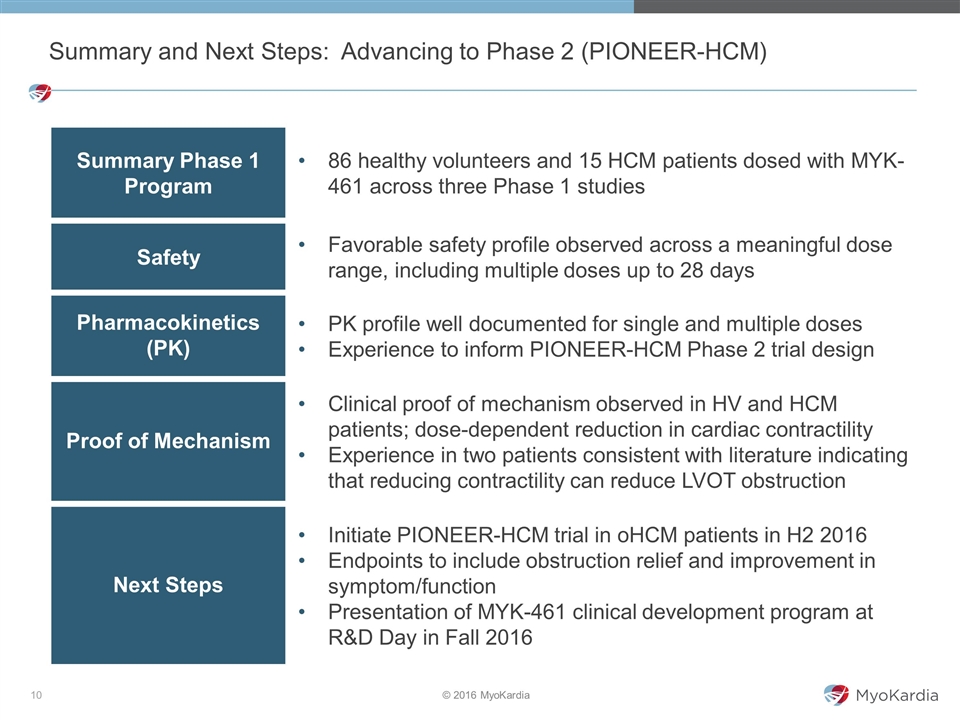
Summary Phase 1 Program 86 healthy volunteers and 15 HCM patients dosed with MYK-461 across three Phase 1 studies Safety Favorable safety profile observed across a meaningful dose range, including multiple doses up to 28 days Pharmacokinetics (PK) PK profile well documented for single and multiple doses Experience to inform PIONEER-HCM Phase 2 trial design Proof of Mechanism Clinical proof of mechanism observed in HV and HCM patients; dose-dependent reduction in cardiac contractility Experience in two patients consistent with literature indicating that reducing contractility can reduce LVOT obstruction Next Steps Initiate PIONEER-HCM trial in oHCM patients in H2 2016 Endpoints to include obstruction relief and improvement in symptom/function Presentation of MYK-461 clinical development program at R&D Day in Fall 2016 Summary and Next Steps: Advancing to Phase 2 (PIONEER-HCM)
