Attached files
| file | filename |
|---|---|
| 8-K - 8-K - NephroGenex, Inc. | nrx8-ksupplementalinvestor.htm |

Supplemental
Corporate Presentation
July 2016

Forward-Looking Statements
This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-
looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,”
“anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative
or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They
appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections,
outlook, analyses or current expectations concerning, among other things, the strength and breadth of our intellectual property,
expectations regarding financial condition, liquidity, the length of time that we will be able to continue to fund our operating
expenses and capital expenditures, our previously announced bankruptcy proceeding and our ability to sell our assets pursuant
to Section 363 of the United States Bankruptcy Code.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics,
and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in
the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for
each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not
guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development
of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a
result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the
year ended December 31, 2015 filed with the Securities and Exchange Commission on March 29, 2016. Any forward-looking
statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to
update such statements to reflect events or circumstances after the date of this presentation, except as required by law.
You should read carefully our Forward-Looking Statements and the factors described in the “Risk Factors” section of our Annual
Report on Form 10-K for the year ended December 31, 2015 filed with the Securities and Exchange Commission on March 29,
2016, to better understand the risks and uncertainties inherent in our business.
2
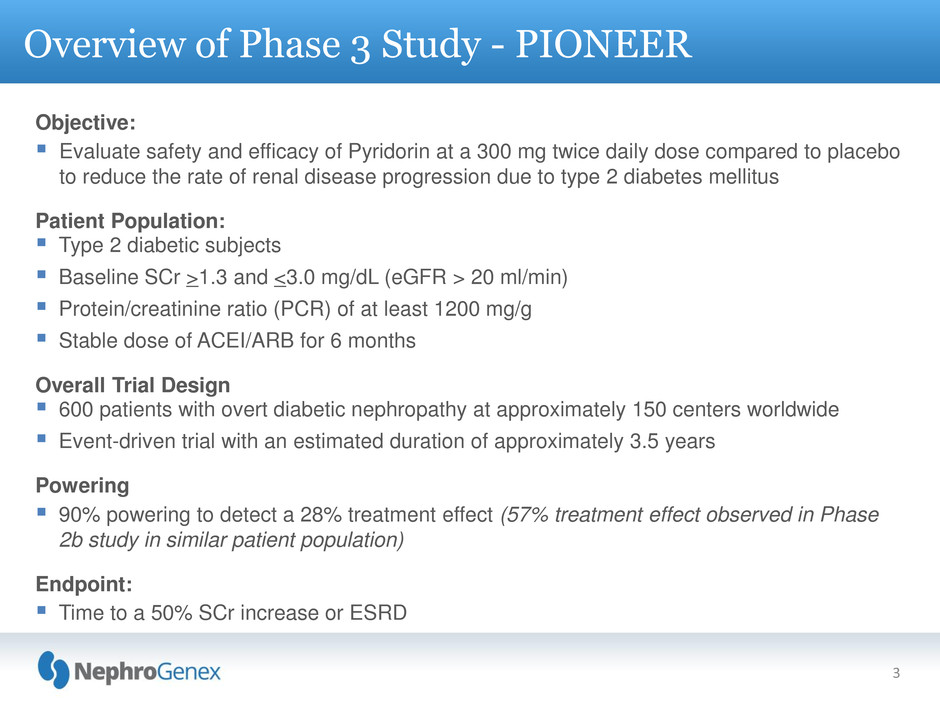
Objective:
Evaluate safety and efficacy of Pyridorin at a 300 mg twice daily dose compared to placebo
to reduce the rate of renal disease progression due to type 2 diabetes mellitus
Patient Population:
Type 2 diabetic subjects
Baseline SCr >1.3 and <3.0 mg/dL (eGFR > 20 ml/min)
Protein/creatinine ratio (PCR) of at least 1200 mg/g
Stable dose of ACEI/ARB for 6 months
Overall Trial Design
600 patients with overt diabetic nephropathy at approximately 150 centers worldwide
Event-driven trial with an estimated duration of approximately 3.5 years
Powering
90% powering to detect a 28% treatment effect (57% treatment effect observed in Phase
2b study in similar patient population)
Endpoint:
Time to a 50% SCr increase or ESRD
Overview of Phase 3 Study - PIONEER
3

4
Phase 3 Progress
As of April 30, 2016, 328 patients (out of 600) were enrolled during the first of two
phase 3 trials required for approval (total of 1,200 patients)
The study successfully passed three DSMB reviews (2/21/2015; 7/29/2015;
1/21/2016)
The study was halted due to financial considerations by the Company in February
2016
The Company unblinded the Phase 3 study to analyze the interim data collected
to corroborate its hypothesis of the Phase 2b trial

5
Phase 3 Data – Change from Baseline in SCr
(Unblinded Data From Terminated Phase 3 Trial – PYR-311 )
P 300mg bd P 300mg bd P 300mg bd
S
C
r
m
g/
dL
+
S
E
(
C
hange
fr
om
B
aseline
)
0.0
0.1
0.2
0.3
0.4
0.5
0.6
n= 92 n=94 n= 55 n=50 n= 23 n=21
6 months 9 months 12 months
All randomized subjects
P=0.27
P=0.89
P=0.57
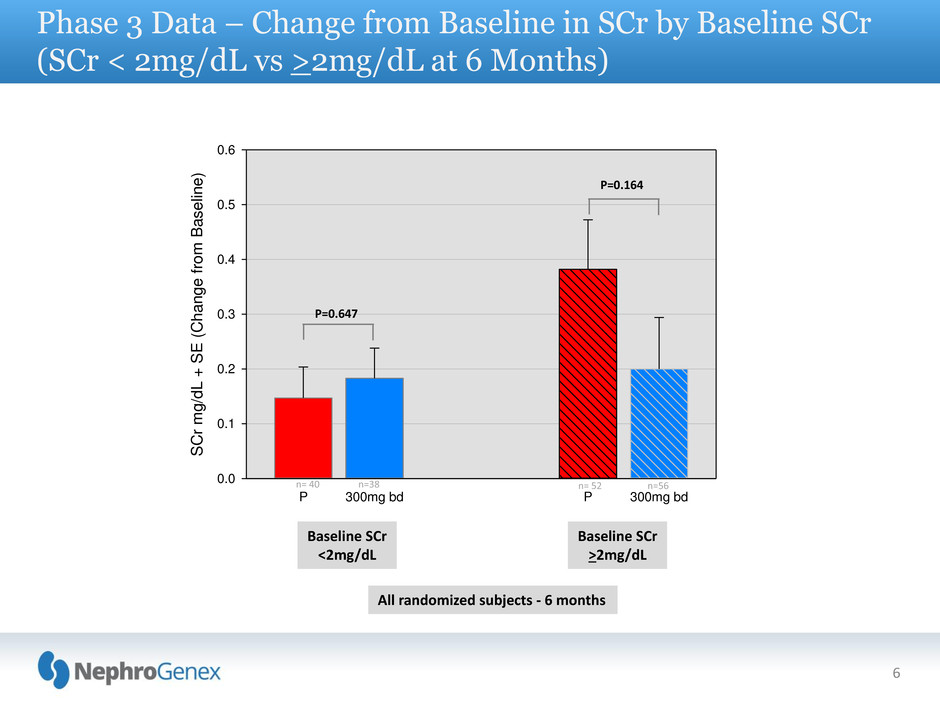
Phase 3 Data – Change from Baseline in SCr by Baseline SCr
(SCr < 2mg/dL vs >2mg/dL at 6 Months)
6
P 300mg bd P 300mg bd
S
C
r
m
g/
dL
+
S
E
(
C
hange
fr
om
B
aseline
)
0.0
0.1
0.2
0.3
0.4
0.5
0.6
P=0.164
P=0.647
n= 40 n=38 n= 52 n=56
Baseline SCr
<2mg/dL
Baseline SCr
>2mg/dL
All randomized subjects - 6 months

Phase 3 Data - Slope Analysis: Pyridorin vs Placebo
7
Baseline to Month 9 Baseline to Month 12
Month 3 to Month 12
Estimate (SE) -0.0010 (0.00218) -0.0002 (0.00182) -0.0004 (0.00215)
95% CI (-0.0053, 0.0032) (-0.0037, 0.0034) (-0.0046, 0.0038)
P-value 0.6314 0.9272 0.8522

Phase 2b & 3 Data: Historical Comparison at 6 months
8
PYR 311 at 26 weeks
(Terminated Phase 3 Study)
NS, P=0.27
n=92 n=94

Phase 2b & 3 Data: Historical Comparison at 12 months
9
PYR-210
(Phase 2b – Month 12)
PYR-311
(Terminated phase 3 – Month 12)
P=0.57
n=23 n=21

Phase 3 – Safety Data Summary
Phase 3 study was actively monitored by a Data Safety Monitoring Board and no safety issues were
highlighted. At the time of study termination, three reviews had been undertaken with recommendations
to continue the study as planned at each review (2/21/2015; 7/29/2015; 1/21/2016).
Adverse experiences:
• 168 subjects experienced Treatment Emergent Adverse Events (TEAE) (54.4%) defined as onset
on or after the first dose of study medication;
• 23 subjects (7.4%) with drug-related Adverse Events,
• 18 subjects (5.8%) with TEAEs leading to discontinuation.
Serious Adverse Events (SAE)
• There were 69 subjects (22.3%) with SAEs and one subject with a drug-related SAE (subject 200-
001 with elevated liver transaminases).
• 7 deaths were reported during the study from:
• Sepsis
• Lung cancer
• Pancreatic cancer
• Septic shock
• Acute respiratory failure
• Choking
• Unknown cause (history of COPD exacerbation, acute renal failure, pneumonia)
10
