Attached files
As filed with the Securities and Exchange Commission on July 1, 2016
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
________________________
Form S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
________________________
Guided Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
________________________
| Delaware | 3845 | 58-2029543 | ||
| (State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
5835 Peachtree Corners East, Suite D
Norcross, Georgia 30092
(770) 242-8723
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
________________________
Gene S. Cartwright, Ph.D
President and Chief Executive Officer
Guided Therapeutics, Inc.
5835 Peachtree Corners East, Suite D
Norcross, Georgia 30092
(770) 242-8723
(Name, address, including zip code, and telephone number, including area code, of agent for service)
________________________
Copy to:
Joel T. May, Esq. and
Heith D. Rodman, Esq.
Jones Day
1420 Peachtree Street, N.E.
Suite 800
Atlanta, Georgia 30309-3053
(404) 581-3939
________________________
Approximate date of commencement of proposed sale to the public: From time to time following the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. R
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | Non-accelerated filer | ¨ | Smaller reporting company | ☒ |
________________________
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered (1) |
Proposed Maximum Aggregate Offering Price (2) |
Amount of Registration Fee (3) | |||||
| Series D preferred stock, par value $0.001 | $ | $ | |||||
| Warrants to purchase shares of common stock (4) | $ | $ | |||||
| Shares of common stock issuable upon conversion of Series D preferred stock | $ | $ | |||||
| Shares of common stock issuable upon exercise of the Warrants | $ | $ | |||||
| Total: | $ | 5,000,000 | $ | 503.50 | |||
| (1) | In the event of a stock split, stock dividend or other similar transaction involving the registrant’s common stock, the number of shares of common stock registered hereby shall be automatically increased to cover the additional common shares in accordance with Rule 416(a) under the Securities Act of 1933. |
| (2) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(g) under the Securities Act. |
| (3) | Calculated pursuant to Rule 457(o) under the Securities Act of 1933, on the basis of the maximum aggregate offering price of all of the securities to be registered. |
| (4) | No separate registration fee is payable pursuant to Rule 457(g) under the Securities Act. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell or offer these securities until the registration statement filed with the Securities and Exchange Commission is declared effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS
Subject to completion, dated July 1, 2016
$5,000,000

5,000 Shares of Series D Convertible Preferred Stock
Warrants to Purchase up to _________ Shares of Common Stock
_________ Shares of Common Stock Underlying the Series D Preferred Stock
_________ Shares of Common Stock Underlying the Warrants
________________________
We are offering up to 5,000 shares of our Series D convertible preferred stock, together with warrants to purchase __________ shares of common stock, at a purchase price of $1,000 (and the shares issuable from time to time upon conversion of the Series D preferred stock and the exercise of the warrants) pursuant to this prospectus. The shares of Series D preferred stock and warrants are immediately separable and will be separately issued.
Subject to certain ownership limitations, the Series D preferred stock will be convertible at any time at the holder’s option into shares of our common stock at an initial conversion price of $____ per share. Subject to similar ownership limitations, each warrant will be immediately exercisable for _____ shares of our common stock, have an exercise price of $____ per share, and expire five years from the date of issuance. The warrants will be issued in book-entry form pursuant to a warrant agency agreement between us and our transfer agent.
Our common stock is quoted on the OTCQB marketplace under the symbol “GTHP.” The last reported sale price of our common stock on the OTCQB on June 15, 2016 was $0.01 per share. We will use our best efforts to have the warrants quoted on the OTCQB marketplace on or before the closing.
| Per Share/Warrant | Total | ||||||
| Offering Price | $ | 1,000 | $ | 5,000,000 | |||
| Placement Agent’s Fees (1) | $ | 90 | $ | 450,000 | |||
| Offering Proceeds, Before Expenses | $ | 910 | $ | 4,550,000 | |||
_____________________________
| (1) | We have agreed to issue the placement agent a warrant to purchase shares of common stock equal to an aggregate of up to 5% of the total shares of common stock underlying the Series D preferred stock sold in the offering and to reimburse to the placement agent for its reasonable, out-of-pocket expenses. See “Plan of Distribution” on page 18 for more information. |
We have retained Ladenburg Thalmann & Co., Inc. to act as our exclusive placement agent in connection with this offering and to use its “best efforts” to solicit offers to purchase the securities. The placement agent is not required to sell any specific number or dollar amount of securities but will use its best efforts to sell the securities offered. This best-efforts offering does not have a minimum purchase requirement and therefore is not certain to raise any specific amount.
Investing in our common stock involves a high degree of risk. These risks are described under the caption “Risk Factors” that begins on page 5 of this prospectus.
Neither the Securities and Exchange Commission, or SEC, nor any state securities commission has approved or disapproved of the securities that may be offered under this prospectus, nor have any of these organizations determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Ladenburg Thalmann
The date of this prospectus is , 2016.

TABLE OF CONTENTS
| FORWARD-LOOKING STATEMENTS | ii |
| SUMMARY | 1 |
| RISK FACTORS | 5 |
| USE OF PROCEEDS | 15 |
| DILUTION | 15 |
| CAPITALIZATION | 17 |
| PLAN OF DISTRIBUTION | 18 |
| DESCRIPTION OF SECURITIES WE ARE OFFERING | 20 |
| DESCRIPTION OF SECURITIES | 22 |
| OUR BUSINESS | 26 |
| PROPERTIES | 34 |
| LEGAL PROCEEDINGS | 34 |
| MARKET FOR OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS | 34 |
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 35 |
| DIRECTORS AND EXECUTIVE OFFICERS | 42 |
| CORPORATE GOVERNANCE | 44 |
| EXECUTIVE COMPENSATION | 45 |
| SHARE OWNERSHIP OF DIRECTORS, OFFICERS AND CERTAIN BENEFICIAL OWNERS | 47 |
| CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE | 47 |
| LEGAL MATTERS | 48 |
| EXPERTS | 48 |
| WHERE YOU CAN GET MORE INFORMATION | 48 |
| i |
ABOUT THIS PROSPECTUS
You should rely only on the information contained in this prospectus or a prospectus supplement. We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. This prospectus is an offer to sell only the common stock offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. You should assume that the information appearing in this prospectus is accurate only as of the date hereof. Our business, financial condition, results of operations and prospects may have changed.
The terms “Guided Therapeutics,” “Company,” “our,” “we,” and “us,” as used in this prospectus, refer to Guided Therapeutics, Inc. and its wholly owned subsidiary.
FORWARD-LOOKING STATEMENTS
Statements in this prospectus, which express “belief,” “anticipation” or “expectation,” as well as other statements that are not historical facts, are forward-looking statements. These forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from historical results or anticipated results, including those identified in the foregoing “Risk Factors” and elsewhere in this prospectus. Examples of these uncertainties and risks include, but are not limited to:
| · | access to sufficient debt or equity capital to meet our operating and financial needs; |
| · | the extent of dilution of the holdings of our existing stockholders upon the issuance, conversion or exercise of securities issued as part of our capital raising efforts; |
| · | whether and when we or any potential strategic partners will obtain required regulatory approvals in the markets in which we plan to operate; |
| · | the effectiveness and ultimate market acceptance of our products and our ability to generate sufficient sales revenues to sustain our growth and strategy plans; |
| · | whether our products in development will prove safe, feasible and effective; |
| · | our need to achieve manufacturing scale-up in a timely manner, and our need to provide for the efficient manufacturing of sufficient quantities of our products; |
| · | the lack of immediate alternate sources of supply for some critical components of our products; |
| · | our ability to establish and protect the proprietary information on which we base our products, including our patent and intellectual property position; |
| · | the need to fully develop the marketing, distribution, customer service and technical support and other functions critical to the success of our product lines; |
| · | the dependence on potential strategic partners or outside investors for funding, development assistance, clinical trials, distribution and marketing of some of our products; and |
| · | other risks and uncertainties described from time to time in our reports filed with the SEC. |
Forward-looking statements should not be read as a guarantee of future performance or results, and will not necessarily be accurate indications of the times at, or by which, such performance or results will be achieved. Forward-looking information is based on information available at the time and/or management’s good faith belief with respect to future events, and is subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in the statements.
Forward-looking statements speak only as of the date the statements are made. We assume no obligation to update forward-looking statements to reflect actual results, changes in assumptions or changes in other factors affecting forward-looking information except to the extent required by applicable securities laws. If we update one or more forward-looking statements, no inference should be drawn that we will make additional updates with respect thereto or with respect to other forward-looking statements.
| ii |
SUMMARY
This summary highlights information contained elsewhere in this prospectus. This summary is not complete and may not contain all of the information that may be important to you. We urge you to read the entire prospectus carefully, including the ‘‘Risk Factors’’ section, before making an investment decision. Unless otherwise noted, all share and per share data in this prospectus give effect to the 1:___ reverse stock split of our common stock implemented on ___________, 2016, and is based on 67,901,547 pre-reverse split shares outstanding as of June 15, 2016. For more information about our reverse stock split, see “Recent Developments.”
Our Company
We are a medical technology company focused on developing innovative medical devices that have the potential to improve healthcare. Our primary focus is the sales and marketing of our LuViva® Advanced Cervical Scan non-invasive cervical cancer detection device. The underlying technology of LuViva primarily relates to the use of biophotonics for the non-invasive detection of cancers. LuViva is designed to identify cervical cancers and precancers painlessly, non-invasively and at the point of care by scanning the cervix with light, then analyzing the reflected and fluorescent light.
LuViva provides a less invasive and painless alternative to conventional tests for cervical cancer screening and detection. Additionally, LuViva improves patient well-being not only because it eliminates pain, but also because it is convenient to use and provides rapid results at the point of care. We focus on two primary applications for LuViva: first, as a cancer screening tool in the developing world, where infrastructure to support traditional cancer-screening methods is limited or non-existent, and second, as a triage following traditional screening in the developed world, where a high number of false positive results cause a high rate of unnecessary and ultimately costly follow-up tests.
Screening for cervical cancer represents one of the most significant demands on the practice of diagnostic medicine. As cervical cancer is linked to a sexually transmitted disease—the human pampillomavirus (HPV)—every woman essentially becomes “at risk” for cervical cancer simply after becoming sexually active. In the developing world there are approximately 2.0 billion women aged 15 and older who are potentially eligible for screening with LuViva. Guidelines for screening intervals vary across the world, but U.S. guidelines call for screening every three years. Traditionally, the Pap smear screening test, or Pap test, is the primary cervical cancer screening methodology in the developed world. However, in developing countries, cancer screening using Pap tests is expensive and requires infrastructure and skill not currently existing, and not likely to be developed in the near future, in these countries.
We believe LuViva is the answer to the developing world’s cervical cancer screening needs. Screening for cervical cancer in the developing world often requires working directly with foreign governments or non-governmental agencies (NGOs). By partnering with governments or NGOs, we are able to provide immediate access to cervical cancer detection to large segments of a nation’s population as part of national or regional governmental healthcare programs, eliminating the need to develop expensive and resource-intensive infrastructures.
Manufacturing, Sales Marketing and Distribution
We manufacture LuViva at our Norcross, Georgia facility. Most of the components of LuViva are custom made for us by third-party manufacturers. We adhere to ISO 13485:2003 quality standards in our manufacturing processes.
Research, Development and Engineering
We have been engaged primarily in the research, development and testing of our LuViva non-invasive cervical cancer detection product and our core biophotonic technology. Since 2013, we have incurred about $7.0 million in research and development expenses, net of about $927,000 reimbursed through collaborative arrangements and government grants. Research and development costs were approximately $1.5 million and $2.8 million in 2015 and 2014, respectively
Patents
We have pursued a course of developing and acquiring patents and patent rights and licensing technology. Our success depends in large part on our ability to establish and maintain the proprietary nature of our technology through the patent process and to license from others patents and patent applications necessary to develop our products. As of December 31, 2015, we have 24 granted U.S. patents relating to our biophotonic cancer detection technology and six pending U.S. patent applications. We also have three granted patents that apply to our interstitial fluid analysis system.
We are a Delaware corporation, originally incorporated in 1992 under the name “SpectRx, Inc.,” and, on February 22, 2008, changed our name to Guided Therapeutics, Inc. At the same time, we renamed our wholly owned subsidiary, InterScan, which originally had been incorporated as “Guided Therapeutics.”
Since our inception, we have raised capital through the public and private sale of debt and equity, funding from collaborative arrangements, and grants.
Our prospects must be considered in light of the substantial risks, expenses and difficulties encountered by entrants into the medical device industry. This industry is characterized by an increasing number of participants, intense competition and a high failure rate. We have experienced operating losses since our inception and, as of March 31, 2016 we have an accumulated deficit of about $122.9 million. To date, we have engaged primarily in research and development efforts and the early stages of marketing our products. We do not have significant experience in manufacturing, marketing or selling our products. We may not be successful in growing sales for our products. Moreover, required regulatory clearances or approvals may not be obtained in a timely manner, or at all. Our products may not ever gain market acceptance and we may not ever generate significant revenues or achieve profitability. The development and commercialization of our products requires substantial development, regulatory, sales and marketing, manufacturing and other expenditures. We expect our operating losses to continue through at least the end of 2016 as we continue to expend substantial resources to complete commercialization of our products, obtain regulatory clearances or approvals, build our marketing, sales, manufacturing and finance capabilities, and conduct further research and development.
Our product revenues to date have been limited. In 2015 and 2014, the majority of our revenues were from the sale of LuViva devices and disposables, as well as some revenue from grants from the NIH and licensing agreement fees received. We expect that the majority of our revenue in 2016 will be derived from revenue from the sale of LuViva devices and disposables.
Our principal executive and operations facility is located at 5835 Peachtree Corners East, Suite D, Norcross, Georgia 30092, and our telephone number is (770) 242-8723.
Recent Developments
Reverse Stock Split. On May 18, 2016, our board determined to recommend that at our 2016 annual stockholders’ meeting, our stockholders grant the board the authority, in its discretion, to effect a reverse stock split by a ratio of not less than 1:10 and not more than 1:400, with no change in the number of authorized shares of our common stock, and all fractional shares created by the reverse stock split to be rounded to the nearest whole share. On May 26, 2016, our largest stockholder, John Imhoff, who is also one of our directors, agreed to vote his shares of common stock in favor of the reverse stock split. As of the record date for the 2016 annual meeting, Dr. Imhoff held approximately 19.26% of the outstanding shares of common stock. The reverse stock split will not be effective unless and until the board files an amendment to our certificate of incorporation, which must occur prior to the first anniversary of the 2016 annual meeting. It is our intent to effect the reverse stock split prior to the closing of this offering.
Warrant Restructuring. Between June 13, 2016 and June 14, 2016, we entered into various agreements with holders of certain warrants (including John Imhoff, the chairman of our board of directors) originally issued in May 2013, and with GPB Debt Holdings II LLC, holder of a warrant issued February 12, 2016, pursuant to which each holder separately agreed to exchange warrants for either (1) shares of common stock equal to 166% of the number of shares of common stock underlying the surrendered warrants, or (2) new warrants exercisable for 200% of the number of shares underlying the surrendered warrants, but without certain anti-dilution protections included with the surrendered warrants. As a result of the exchanges, we effectively eliminate any potential exponential increase in the number of underlying shares issuable upon exercise of our outstanding warrants. In total, for surrendered warrants then-exercisable for an aggregate of 115,237,788 shares of common stock (but subject to exponential increase upon operation of certain anti-dilution provisions), we issued or are obligated to issue 13,517,342 shares of common stock and new warrants that, if exercised as of June 14, 2016, would be exercisable for an aggregate of 214,189,622 shares of common stock.
In certain circumstances, in lieu of presently issuing all of the shares (for each holder that opted for shares of common stock), we and the holder further agreed that we will, subject to the terms and conditions set forth in the applicable warrant exchange agreement, from time to time, be obligated to issue the remaining shares to the holder. No additional consideration will be payable in connection with the issuance of the remaining shares.
The holders that elected to receive shares for their surrendered warrants have agreed that they will not sell shares on any trading day in an amount, in the aggregate, exceeding 20% of the composite aggregate trading volume of the common stock for that trading day. The holders that elected to receive new warrants will be required to surrender their old warrants upon consummation of this offering. The new warrants will have an initial exercise price equal to the exercise price of the surrendered warrants as of immediately prior to consummation of this offering, subject to customary “downside price protection” for as long as our common stock is not listed on a national securities exchange, and will expire five years from the date of issuance.
| 2 |
Shenghuo License Agreement. On June 5, 2016, we entered into a license agreement with Shenghuo Medical, LLC pursuant to which we granted Shenghuo an exclusive license to manufacture, sell and distribute LuViva in Taiwan, Brunei Darussalam, Cambodia, Laos, Myanmar, Philippines, Singapore, Thailand, and Vietnam. Shenghuo was already our exclusive distributor in China, Macau and Hong Kong, and the license extends to manufacturing in those countries as well. Under the terms of the license agreement, once Shenghuo is capable of manufacturing LuViva in accordance with ISO 13485 for medical devices, Shenghuo will pay us a royalty equal to $2.00 or 20% of the distributor price (subject to a discount under certain circumstances), whichever is higher, per disposable distributed within Shenghuo’s exclusive territories.
In connection with the license grant, Shenghuo will underwrite the cost of securing approval of LuViva with Chinese Food and Drug Administration. At its option, Shenghuo also will provide up to $1.0 million in furtherance of our efforts to secure regulatory approval for LuViva from the U.S. Food and Drug Administration, in exchange for the right to receive payments equal to 2% of our future sales in the United States, up to an aggregate of $4.0 million.
Pursuant to the license agreement, Shenghuo has the option, after our next annual meeting of stockholders, to have a designee appointed to our board of directors.
As partial consideration for, and as a condition to, the license, and to further align the strategic interests of the parties, we have agreed to issue a convertible note to Shenghuo, in exchange for an aggregate cash investment of $200,000. The note will provide for a payment to Shenghuo of $240,000, due upon consummation of any capital raising transaction by us within 90 days and with net cash proceeds of at least $1.0 million. Absent such a transaction, the payment will increase to $300,000 and will be payable by December 31, 2016. The note will accrue interest at 20% per year on any unpaid amounts due after that date. The note will be convertible into shares of our common stock at a conversion price per share of $0.017402, subject to customary anti-dilution adjustment. The note will be unsecured, and is expected to provide for customary events of default. We will also issue Shenghuo a five-year warrant exercisable immediately for approximately 13.8 million shares of common stock at an exercise price equal to the conversion price of the note, subject to customary anti-dilution adjustment.
Short Term Promissory Notes. On May 4, 2016 and May 26, 2016, Aquarius Opportunity Fund advanced us a total of $87,500 for 2% simple interest notes due the earlier of December 31, 2016 or consummation of this offering. Also on May 26, 2016, GPB Debt Holdings II LLC, holder of our outstanding senior secured convertible note, advanced as an additional $87,500, on the same terms as their note. We intend to offer each of them the opportunity to participate in the offering at least up to the extent of the outstanding principal and interest on these cash advances, by extinguishing all or a portion of the debt on a dollar-for-dollar basis.
Series C Exchanges. Between April 27, 2016 and May 3, 2016, we entered into various agreements with certain holders of our Series C preferred stock, including John Imhoff, one of our directors, pursuant to which those holders separately agreed to exchange each share of Series C preferred stock held for 2.25 shares of our newly created Series C1 preferred stock and 9,600 shares of our common stock. In connection with these exchanges, each holder also agreed to exchange the $1,000 stated value per share of the holder’s shares of Series C1 preferred stock for new securities that we issue in the next qualifying financing we undertake on a dollar-for-dollar basis. We expect this offering will be a qualifying financing. Finally, each holder agreed, except in the event of an additional $50,000 cash investment in the financing by the holder, to execute a customary “lockup” agreement in connection with the qualifying financing. In total, for 1,916 shares of Series C preferred stock surrendered, we issued 4,311 shares of Series C1 preferred stock and 18,396,800 shares of common stock.
The Series C1 preferred stock has terms that are substantially the same as the Series C preferred stock, except that the Series C1 preferred stock does not pay dividends (unless and to the extent declared on the common stock) or at-the-market “make-whole payments.” See “Description of Securities” for a description of the material terms of the Series C1 preferred stock.
Separately, on April 27, 2016, we entered into a rollover and amendment agreement with another holder of Series C preferred stock, Aquarius Opportunity Fund, pursuant to which Aquarius agreed to exchange any shares of Series C preferred stock (stated value plus make-whole dividend), as well as any remaining principal and accrued interest on our convertible promissory note Aquarius holds, for new securities that we issue in our next financing, all on a dollar-for-dollar basis, as long as the next financing involves at least $1 million in cash from investors unaffiliated with Aquarius. We expect this offering will be a qualifying financing. Aquarius also agreed to return to us for cancelation warrants exercisable for 722,211 shares of our common stock that it held. Except in the event of an additional $50,000 cash investment by Aquarius in the qualifying financing, Aquarius has agreed to execute a customary “lockup” agreement in connection with the financing. Finally, Aquarius, as the holder of a majority of the outstanding Series C preferred stock, agreed to amend the Series C stock purchase agreement to eliminate any participation rights held by the Series C shareholders and to waive operation of certain anti-dilution provisions of the Series C that would otherwise be triggered by the transactions described in “—Recent Developments.”
| 3 |
The Offering
| Issuer | Guided Therapeutics, Inc. |
| Securities offered | Up to 5,000 shares of Series D preferred stock and warrants exercisable for an aggregate of ____________ shares of our common stock. Each share of Series D preferred stock will be accompanied by a warrant to purchase _____ of a share of common stock. The shares of Series D preferred stock and the warrants are immediately separable and will be issued separately, but will be purchased together in this offering. This prospectus also covers the shares of common stock issuable upon conversion of the Series D preferred stock and upon the exercise of the warrants. |
| Offering price | $1,000 combined price for each share of Series D preferred stock and accompanying warrant |
| Description of Series D preferred stock | Series D preferred stock has a liquidation preference senior to our common stock but junior to our Series C preferred stock, is convertible at the option of the holder (subject to certain ownership limitations) into shares of our common stock at a conversion price of $_____ per share, subject to anti-dilution adjustment, and is redeemable under certain circumstances at our option. See “Description of Securities We Are Offering—Series D Preferred Stock” on page 20 . |
| Shares of common stock underlying the shares of Series D preferred stock | ____________ shares* |
| Description of warrants | The warrants will be immediately exercisable upon issuance (subject to certain ownership limitations) at an exercise price per share equal to $______ (__% of the closing bid price preceding pricing of the offering). The warrants will expire five years after the date of issuance. See “Description of Securities We Are Offering—Warrants” on page 20. |
| Shares of common stock underlying the warrants | ____________ shares* |
| Shares of common stock outstanding before this offering | 67,901,547 shares.* See “Dilution” on page 15. |
| Common stock to be outstanding after this offering | ____________ shares, assuming all shares of Series D preferred stock are sold.* See “Dilution” on page 15. |
| Use of proceeds | We intend to apply any proceeds received in connection with the offering to increase inventory of LuViva to meet current demand for the product, and to support general working capital and operations. However, we will retain broad discretion over the use of the net proceeds and may use the money for other corporate purposes. See “Use of Proceeds” on page 15. |
| Market for the common stock | Our common stock is quoted on the OTCQB marketplace under the symbol “GTHP.” See “Market for Our Common Stock and Related Stockholder Matters” on page 34. |
| Risk factors | You should read “Risk Factors” on page 5 for an explanation of the risks of investing in this offering. |
| * | Share numbers are based on an assumed conversion or exercise price per share of the Series D preferred stock of $0.01, which was the last reported sale price for our common stock on June 15, 2016 and, with regard to shares outstanding, are as of such date, and exclude: |
| · | 104,356 shares of common stock reserved for future issuance under our 1995 Stock Plan; |
| · | 433,385,611 shares of common stock reserved for issuance upon conversion of, or issuance as dividends on, our Series C and Series C1 convertible preferred stock, which we assume will be exchanged for shares of Series D preferred stock and warrants as part of this offering; |
| · | 151,583,843 shares reserved for issuance upon conversion of our outstanding convertible debt; |
| · | 233,615,051 shares of common stock issuable upon the exercise of outstanding warrants or warrants to be exchanged for outstanding warrants |
| · | 5,205,101 shares reserved for issuance pursuant to contractual obligations; and |
| · | up to ______ shares of common stock issuable upon exercise of warrants sold as part of this offering,
including up to ______ shares of common stock issuable upon exercise of warrants to be issued to the placement agent. |
| 4 |
RISK FACTORS
Your investment in shares of our common stock involves substantial risks. In consultation with your own advisers, you should carefully consider, among other matters, the factors set forth below before deciding whether an investment in shares of our common stock is suitable for you. If any of the risks contained in this prospectus develop into actual events, our business, financial condition, liquidity, results of operations and prospects could be materially and adversely affected, the market price of our common stock could decline and you may lose all or part of your investment. Some statements in this prospectus, including statements in the following risk factors, constitute forward-looking statements. See “Forward-Looking Statements” in this prospectus.
Risks Related to this Offering
As a new investor, you will incur substantial dilution as a result of this offering and future equity issuances.
Our net tangible book value deficiency as of March 31, 2016 was approximately $7.3 million, or $1.37 per share of common stock. Net tangible book value per share represents total tangible assets less total liabilities, divided by the number of shares of common stock outstanding. On a pro form basis after giving effect to the assumed sale of 5,000 shares of Series D preferred stock in this offering at a public offering price of $1,000 per share, and assuming the conversion of all the shares of Series D preferred stock sold in the offering at an assumed conversion price of $0.01 per share, which was the last reported sale price of our common stock on June 15, 2016 (and excluding shares of common stock issuable upon exercise of the warrants), and after deducting estimated placement agent fees and estimated offering expenses payable by us, if you purchase securities in this offering, you will suffer immediate and substantial dilution of approximately $0.006 per share in the net tangible book value of the common stock underlying the Series D preferred stock that you acquire. See “Dilution” for a more detailed discussion of the dilution you will incur if you participate in this offering. In addition to this offering, subject to market conditions and other factors, it is likely that we will pursue additional capital to finance our operations. Accordingly, we may conduct substantial future offerings of equity securities. The exercise of outstanding options and warrants and future equity issuances, including future public offerings or future private placements of equity securities, would result in further dilution to investors.
The actual offering amount, the offering price and the net proceeds to us, if any, in this offering may be substantially less than the amounts set forth above.
Because there is no minimum offering amount or offering price required as a condition to closing in this offering, the actual public offering amount, offering price and net proceeds to us, if any, in this offering are not presently determinable and may be substantially less than the amounts set forth above. We are not required to sell any specific number or dollar amount of the securities offered in this offering, but the placement agent will use its best efforts to sell the securities offered.
Sales of a significant number of shares of our common stock in the public markets, or the perception that such sales could occur, could depress the market price of our common stock.
If our stockholders (including those persons who may become stockholders upon conversion of outstanding convertible securities or exercise of outstanding warrants or options) sell substantial amounts of our common stock, or the public market perceives that stockholders might sell substantial amounts of our common stock, the market price of our common stock could decline significantly. Such sales also might make it more difficult for us to sell equity or equity-related securities in the future at a time and price that our management deems appropriate.
We will have broad discretion over the use of the proceeds of this offering and may not realize a return.
We will have considerable discretion in the application of the net cash proceeds of this offering. We intend to use the net cash proceeds to increase inventory of LuViva to meet current demand for the product, and to support general working capital and operations. However, we will retain broad discretion over the use of the net proceeds and may use the money for other corporate purposes. We may use the net cash proceeds for purposes that do not yield a significant return, if any, for our stockholders.
There is no public market for the Series D preferred stock or warrants being offered in this offering.
There is no established public trading market for the Series D preferred stock or warrants being offered in this offering. We do not expect a market to develop for the Series D preferred stock or the warrants. In addition, we do not intend to apply for listing of the Series D preferred stock or warrants on any securities exchange or expect the Series D preferred stock to trade on the OTCQB marketplace, although we will use our best efforts to have the warrants quoted on the OTCQB marketplace on or before the closing. Without an active market, the liquidity of the Series D preferred stock and warrants will be limited.
| 5 |
Risks Related to Our Business
Although we are required to raise funds in this offering in order to continue as a going concern, there is no assurance that such funds can be raised on terms that we would find acceptable, on a timely basis, or at all.
Additional debt or equity financing is required for us to continue as a going concern. If this offering is unsuccessful or insufficient, we may seek to obtain additional funds for the financing of our cervical cancer detection business through additional debt or equity financings and/or new collaborative arrangements. Any required additional funding may not be available on terms attractive to us, on a timely basis, or at all.
If we cannot obtain additional funds or achieve profitability, we may not be able to continue as a going concern.
Because we must obtain additional funds through financing transactions or through new collaborative arrangements in order to grow the revenues of our cervical cancer detection product line, there exists substantial doubt about our ability to continue as a going concern. Therefore, it will be necessary to raise additional funds. There can be no assurance that we will be able to raise these additional funds. If we do not secure additional funding when needed, we will be unable to conduct all of our product development efforts as planned, which may cause us to alter our business plan in relation to the development of our products. Even if we obtain additional funding, we will need to achieve profitability thereafter.
Our independent registered public accountants’ report on our consolidated financial statements as of and for the year ended December 31, 2015, indicated that there was substantial doubt about our ability to continue as a going concern because we had suffered recurring losses from operations and had an accumulated deficit of $122.6 million at December 31, 2015. At March 31, 2016, we had an accumulated deficit of 122.9 million, summarized as follows:
| Accumulated deficit, from inception to 12/31/2013 | $ | 103.0 million |
| Preferred dividends | $ | 0.2 million |
| Net Loss for fiscal year 2014, ended 12/31/2014 | $ | 9.9 million |
| Accumulated deficit, from inception to 12/31/2014 | $ | 113.1 million |
| Preferred dividends and deemed dividends | $ | 2.6 million |
| Net Loss for fiscal year 2015, ended 12/31/2015 | $ | 6.9 million |
| Accumulated deficit, from inception to 12/31/2015 | $ | 122.6 million |
| Net Income for year to date ended 3/31/2016 | $ | 0.1 million |
| Accumulated deficit, from inception to 3/31/2016 | $ | 122.9 million |
Our management has implemented reductions in operating expenditures and reductions in some development activities. We have determined to make cervical cancer detection the focus of our business. We are managing the development of our other programs only when funds are made available to us via grants or contracts with government entities or strategic partners. However, there can be no assurance that we will be able to successfully implement or continue these plans.
If we cannot obtain additional funds when needed, we will not be able to implement our business plan.
We require substantial additional capital to develop our products, including completing product testing and clinical trials, obtaining all required regulatory approvals and clearances, beginning and scaling up manufacturing, and marketing our products. We have historically financed our operations though the public and private sale of debt and equity, funding from collaborative arrangements, and grants. Any failure to achieve adequate funding in a timely fashion would delay our development programs and could lead to abandonment of our business plan. To the extent we cannot obtain additional funding, our ability to continue to manufacture and sell our current products, or develop and introduce new products to market, will be limited. Further, financing our operations through the public or private sale of debt or equity may involve restrictive covenants or other provisions that could limit how we conduct our business or finance our operations. Financing our operations through collaborative arrangements generally means that the obligations of the collaborative partner to fund our expenditures are largely discretionary and depend on a number of factors, including our ability to meet specified milestones in the development and testing of the relevant product. We may not be able to obtain an acceptable collaboration partner, and even if we do, we may not be able to meet these milestones, or the collaborative partner may not continue to fund our expenditures.
We do not have a long operating history, especially in the cancer detection field, which makes it difficult to evaluate our business.
Although we have been in existence since 1992, we have only recently begun to commercialize our cervical cancer detection technology. Because limited historical information is available on our revenue trends and manufacturing costs, it is difficult to evaluate our business. Our prospects must be considered in light of the substantial risks, expenses, uncertainties and difficulties encountered by entrants into the medical device industry, which is characterized by increasing intense competition and a high failure rate.
| 6 |
We have a history of losses, and we expect losses to continue.
We have never been profitable and we have had operating losses since our inception. We expect our operating losses to continue as we continue to expend substantial resources to complete commercialization of our products, obtain regulatory clearances or approvals, build our marketing, sales, manufacturing and finance capabilities, and conduct further research and development. The further development and commercialization of our products will require substantial development, regulatory, sales and marketing, manufacturing and other expenditures. We have only generated limited revenues from product sales. Our accumulated deficit was approximately $122.9 million at March 31, 2016.
Our ability to sell our products is controlled by government regulations, and we may not be able to obtain any necessary clearances or approvals.
The design, manufacturing, labeling, distribution and marketing of medical device products are subject to extensive and rigorous government regulation in most of the markets in which we sell, or plan to sell, our products, which can be expensive and uncertain and can cause lengthy delays before we can begin selling our products in those markets.
In foreign countries, including European countries, we are subject to government regulation, which could delay or prevent our ability to sell our products in those jurisdictions.
In order for us to market our products in Europe and some other international jurisdictions, we and our distributors and agents must obtain required regulatory registrations or approvals. We must also comply with extensive regulations regarding safety, efficacy and quality in those jurisdictions. We may not be able to obtain the required regulatory registrations or approvals, or we may be required to incur significant costs in obtaining or maintaining any regulatory registrations or approvals we receive. Delays in obtaining any registrations or approvals required for marketing our products, failure to receive these registrations or approvals, or future loss of previously obtained registrations or approvals would limit our ability to sell our products internationally. For example, international regulatory bodies have adopted various regulations governing product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, duties and tax requirements. These regulations vary from country to country. In order to sell our products in Europe, we must maintain ISO 13485:2003 certification and CE mark certification, which is an international symbol of quality and compliance with applicable European medical device directives. Failure to maintain ISO 13485:2003 certification or CE mark certification or other international regulatory approvals would prevent us from selling in some countries in the European Union.
In the United States, we are subject to regulation by the FDA, which could prevent our ability to sell our products domestically.
In order for us to market our products in the United States, we must obtain clearance or approval from the FDA. We cannot be sure that:
| · | we, or any collaborative partner, will make timely filings with the FDA; |
| · | the FDA will act favorably or quickly on these submissions; |
| · | we will not be required to submit additional information or perform additional clinical studies; or |
| · | we will not face other significant difficulties and costs necessary to obtain FDA clearance or approval. |
It can take several years from initial filing of a PMA application and require the submission of extensive supporting data and clinical information. The FDA may impose strict labeling or other requirements as a condition of its clearance or approval, any of which could limit our ability to market our products domestically. Further, if we wish to modify a product after FDA approval of a PMA application, including changes in indications or other modifications that could affect safety and efficacy, additional clearances or approvals will be required from the FDA. Any request by the FDA for additional data, or any requirement by the FDA that we conduct additional clinical studies, could result in a significant delay in bringing our products to market domestically and require substantial additional research and other expenditures. Similarly, any labeling or other conditions or restrictions imposed by the FDA could hinder our ability to effectively market our products domestically. Further, there may be new FDA policies or changes in FDA policies that could be adverse to us.
Even if we obtain clearance or approval to sell our products, we are subject to ongoing requirements and inspections that could lead to the restriction, suspension or revocation of our clearance.
We, as well as any potential collaborative partners, will be required to adhere to applicable regulations in the markets in which we operate and sell our products, regarding good manufacturing practice, which include testing, control, and documentation requirements. Ongoing compliance with good manufacturing practice and other applicable regulatory requirements will be strictly enforced applicable regulatory agencies. Failure to comply with these regulatory requirements could result in, among other things, warning letters, fines, injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, failure to obtain premarket clearance or premarket approval for devices, withdrawal of approvals previously obtained, and criminal prosecution. The restriction, suspension or revocation of regulatory approvals or any other failure to comply with regulatory requirements would limit our ability to operate and could increase our costs.
| 7 |
We depend on a limited number of customers and any reduction, delay or cancellation of an order from these customers or the loss of any of these customers could cause our revenue to decline.
Each year we have had one or a few customers that have accounted for substantially all of our limited revenues. As a result, the termination of a purchase order with any one of these customers may result in the loss of substantially all of our revenues. We are constantly working to develop new relationships with existing or new customers, but despite these efforts we may not be successful at generating new orders to maintain similar revenues as current purchase orders are filled. In addition, since a significant portion of our revenues is derived from a relatively few customers, any financial difficulties experienced by any one of these customers, or any delay in receiving payments from any one of these customers, could have a material adverse effect on our business, results of operations, financial condition and cash flows.
To successfully market and sell our products internationally, we must address many issues with which we have limited experience.
All of our sales of LuViva to date have been to customers outside of the United States. We expect that substantially all of our business will continue to come from sales in foreign markets, through increased penetration in countries where we currently sell LuViva, combined with expansion into new international markets. However, international sales are subject to a number of risks, including:
● difficulties in staffing and managing international operations;
● difficulties in penetrating markets in which our competitors’ products may be more established;
● reduced or no protection for intellectual property rights in some countries;
● export restrictions, trade regulations and foreign tax laws;
● fluctuating foreign currency exchange rates;
● foreign certification and regulatory clearance or approval requirements;
● difficulties in developing effective marketing campaigns for unfamiliar, foreign countries;
● customs clearance and shipping delays;
● political and economic instability; and
● preference for locally produced products.
If one or more of these risks were realized, it could require us to dedicate significant resources to remedy the situation, and even if we are able to find a solution, our revenues may still decline.
To market and sell LuViva internationally, we depend on distributors and they may not be successful.
We currently depend almost exclusively on third-party distributors to sell and service LuViva internationally and to train our international customers, and if these distributors terminate their relationships with us or under-perform, we may be unable to maintain or increase our level of international revenue. We will also need to engage additional international distributors to grow our business and expand the territories in which we sell LuViva. Distributors may not commit the necessary resources to market, sell and service LuViva to the level of our expectations. If current or future distributors do not perform adequately, or if we are unable to engage distributors in particular geographic areas, our revenue from international operations will be adversely affected
Our success largely depends on our ability to maintain and protect the proprietary information on which we base our products.
Our success depends in large part upon our ability to maintain and protect the proprietary nature of our technology through the patent process, as well as our ability to license from others patents and patent applications necessary to develop our products. If any of our patents are successfully challenged, invalidated or circumvented, or our right or ability to manufacture our products was to be limited, our ability to continue to manufacture and market our products could be adversely affected. In addition to patents, we rely on trade secrets and proprietary know-how, which we seek to protect, in part, through confidentiality and proprietary information agreements. The other parties to these agreements may breach these provisions, and we may not have adequate remedies for any breach. Additionally, our trade secrets could otherwise become known to or be independently developed by competitors.
| 8 |
As of March 31, 2016, we have been issued, or have rights to, 24 U.S. patents (including those under license). In addition, we have filed for, or have rights to, six U.S. patents (including those under license) that are still pending. There are additional international patents and pending applications. One or more of the patents we hold directly or license from third parties, including those for our cervical cancer detection products, may be successfully challenged, invalidated or circumvented, or we may otherwise be unable to rely on these patents. These risks are also present for the process we use or will use for manufacturing our products. In addition, our competitors, many of whom have substantial resources and have made substantial investments in competing technologies, may apply for and obtain patents that prevent, limit or interfere with our ability to make, use and sell our products, either in the United States or in international markets.
The medical device industry has been characterized by extensive litigation regarding patents and other intellectual property rights. In addition, the U.S. Patent and Trademark Office, or USPTO, may institute interference proceedings. The defense and prosecution of intellectual property suits, USPTO proceedings and related legal and administrative proceedings are both costly and time consuming. Moreover, we may need to litigate to enforce our patents, to protect our trade secrets or know-how, or to determine the enforceability, scope and validity of the proprietary rights of others. Any litigation or interference proceedings involving us may require us to incur substantial legal and other fees and expenses and may require some of our employees to devote all or a substantial portion of their time to the proceedings. An adverse determination in the proceedings could subject us to significant liabilities to third parties, require us to seek licenses from third parties or prevent us from selling our products in some or all markets. We may not be able to reach a satisfactory settlement of any dispute by licensing necessary patents or other intellectual property. Even if we reached a settlement, the settlement process may be expensive and time consuming, and the terms of the settlement may require us to pay substantial royalties. An adverse determination in a judicial or administrative proceeding or the failure to obtain a necessary license could prevent us from manufacturing and selling our products.
We may not be able to generate sufficient sales revenues to sustain our growth and strategy plans.
Our cervical cancer diagnostic activities have been financed to date through a combination of government grants, strategic partners and direct investment. Growing revenues for this product is the main focus of our business. In order to effectively market the cervical cancer detection product, additional capital will be needed.
Additional product lines involve the modification of the cervical cancer detection technology for use in other cancers. These product lines are only in the earliest stages of research and development and are currently not projected to reach market for several years. Our goal is to receive enough funding from government grants and contracts, as well as payments from strategic partners, to fund development of these product lines without diverting funds or other necessary resources from the cervical cancer program.
Because our products, which use different technology or apply technology in different ways than other medical devices, are or will be new to the market, we may not be successful in launching our products and our operations and growth would be adversely affected.
Our products are based on new methods of cancer detection. If our products do not achieve significant market acceptance, our sales will be limited and our financial condition may suffer. Physicians and individuals may not recommend or use our products unless they determine that these products are an attractive alternative to current tests that have a long history of safe and effective use. To date, our products have been used by only a limited number of people, and few independent studies regarding our products have been published. The lack of independent studies limits the ability of doctors or consumers to compare our products to conventional products.
If we are unable to compete effectively in the highly competitive medical device industry, our future growth and operating results will suffer.
The medical device industry in general and the markets in which we expect to offer products in particular, are intensely competitive. Many of our competitors have substantially greater financial, research, technical, manufacturing, marketing and distribution resources than we do and have greater name recognition and lengthier operating histories in the health care industry. We may not be able to effectively compete against these and other competitors. A number of competitors are currently marketing traditional laboratory-based tests for cervical cancer screening and diagnosis. These tests are widely accepted in the health care industry and have a long history of accurate and effective use. Further, if our products are not available at competitive prices, health care administrators who are subject to increasing pressures to reduce costs may not elect to purchase them. Also, a number of companies have announced that they are developing, or have introduced, products that permit non-invasive and less invasive cancer detection. Accordingly, competition in this area is expected to increase.
| 9 |
Furthermore, our competitors may succeed in developing, either before or after the development and commercialization of our products, devices and technologies that permit more efficient, less expensive non-invasive and less invasive cancer detection. It is also possible that one or more pharmaceutical or other health care companies will develop therapeutic drugs, treatments or other products that will substantially reduce the prevalence of cancers or otherwise render our products obsolete.
We have limited manufacturing experience, which could limit our growth.
We do not have manufacturing experience that would enable us to make products in the volumes that would be necessary for us to achieve significant commercial sales, and we rely upon our suppliers. In addition, we may not be able to establish and maintain reliable, efficient, full scale manufacturing at commercially reasonable costs in a timely fashion. Difficulties we encounter in manufacturing scale-up, or our failure to implement and maintain our manufacturing facilities in accordance with good manufacturing practice regulations, international quality standards or other regulatory requirements, could result in a delay or termination of production. In the past, we have had substantial difficulties in establishing and maintaining manufacturing for our products and those difficulties impacted our ability to increase sales. Companies often encounter difficulties in scaling up production, including problems involving production yield, quality control and assurance, and shortages of qualified personnel.
Since we rely on sole source suppliers for several of the components used in our products, any failure of those suppliers to perform would hurt our operations.
Several of the components used in our products or planned products, are available from only one supplier, and substitutes for these components could not be obtained easily or would require substantial modifications to our products. Any significant problem experienced by one of our sole source suppliers may result in a delay or interruption in the supply of components to us until that supplier cures the problem or an alternative source of the component is located and qualified. Any delay or interruption would likely lead to a delay or interruption in our manufacturing operations. For our products that require premarket approval, the inclusion of substitute components could require us to qualify the new supplier with the appropriate government regulatory authorities. Alternatively, for our products that qualify for premarket notification, the substitute components must meet our product specifications.
Because we operate in an industry with significant product liability risk, and we have not specifically insured against this risk, we may be subject to substantial claims against our products.
The development, manufacture and sale of medical products entail significant risks of product liability claims. We currently have no product liability insurance coverage beyond that provided by our general liability insurance. Accordingly, we may not be adequately protected from any liabilities, including any adverse judgments or settlements, we might incur in connection with the development, clinical testing, manufacture and sale of our products. A successful product liability claim or series of claims brought against us that result in an adverse judgment against or settlement by us in excess of any insurance coverage could seriously harm our financial condition or reputation. In addition, product liability insurance is expensive and may not be available to us on acceptable terms, if at all.
The availability of third party reimbursement for our products is uncertain, which may limit consumer use and the market for our products.
In the United States and elsewhere, sales of medical products are dependent, in part, on the ability of consumers of these products to obtain reimbursement for all or a portion of their cost from third-party payors, such as government and private insurance plans. Any inability of patients, hospitals, physicians and other users of our products to obtain sufficient reimbursement from third-party payors for our products, or adverse changes in relevant governmental policies or the policies of private third-party payors regarding reimbursement for these products, could limit our ability to sell our products on a competitive basis. We are unable to predict what changes will be made in the reimbursement methods used by third-party health care payors. Moreover, third-party payors are increasingly challenging the prices charged for medical products and services, and some health care providers are gradually adopting a managed care system in which the providers contract to provide comprehensive health care services for a fixed cost per person. Patients, hospitals and physicians may not be able to justify the use of our products by the attendant cost savings and clinical benefits that we believe will be derived from the use of our products, and therefore may not be able to obtain third-party reimbursement.
Reimbursement and health care payment systems in international markets vary significantly by country and include both government-sponsored health care and private insurance. We may not be able to obtain approvals for reimbursement from these international third-party payors in a timely manner, if at all. Any failure to receive international reimbursement approvals could have an adverse effect on market acceptance of our products in the international markets in which approvals are sought.
| 10 |
We have a substantial amount of indebtedness, which may adversely affect our cash flow and our ability to operate our business.
Our outstanding indebtedness, including ordinary course accounts payable and accrued payroll liabilities, was $4.0 million at March 31, 2016. We currently are required to make approximately $21,000 in monthly payments of interest and, beginning August 2016, $239,583 in quarterly payments of principal on our outstanding senior secured convertible note. The outstanding principal and accrued interest on our secured promissory note is due on August 31, 2016. In connection with the Shenghuo license agreement, we have agreed to issue Shenghuo a convertible note in principal amount of $240,000, due upon consummation of any capital raising transaction by us within 90 days and with net cash proceeds of at least $1.0 million. Absent such a transaction, the payment will increase to $300,000 and will be payable by December 31, 2016.
The terms of our indebtedness could have negative consequences to us, such as:
| · | we may be unable to obtain additional financing to fund working capital, operating losses, capital expenditures or acquisitions on terms acceptable to us, or at all; |
| · | the amount of our interest expense may increase if we are unable to make payments when due; |
| · | our assets might be subject to foreclosure if we default on our secured debt (see “—We have outstanding debt that is collateralized by a general security interest in all of our assets, including our intellectual property. If we were to fail to repay the debt when due, the holders would have the right to foreclose on these assets.”); |
| · | our vendors or employees may, and some have, instituted proceedings to collect on amounts owed them; |
| · | we have to use a substantial portion of our cash flows from operations to repay our indebtedness, including ordinary course accounts payable and accrued payroll liabilities, which reduces the amount of money we have for future operations, working capital, inventory, expansion, or general corporate or other business activities; and |
| · | we may be unable to refinance our indebtedness on terms acceptable to us, or at all. |
Our ability to meet our expenses and debt obligations will depend on our future performance, which will be affected by financial, business, economic, regulatory and other factors. We will be unable to control many of these factors, such as economic conditions. We cannot be certain that our earnings will be sufficient to allow us to pay the principal and interest on our debt and meet any other obligations. If we do not have enough money to service our debt, we may be required, but unable, to refinance all or part of our existing debt, sell assets, borrow money or raise equity on terms acceptable to us, if at all.
We have outstanding debt that is collateralized by a general security interest in all of our assets, including our intellectual property. If we were to fail to repay the debt when due, the holders would have the right to foreclose on these assets.
At June 15, 2016, we had notes outstanding that are collateralized by a security interest in our current and future inventory and accounts receivable. We also had a note outstanding that is collateralized by a security interest in all of our assets, including our intellectual property. When the debt is repaid, the holders’ security interests on our assets will be extinguished. However, if an event of default occurs under the notes prior to their repayment, the holders may exercise their rights to foreclose on these secured assets for the payment of these obligations. Under “cross-default” provisions in each of the notes, an event of default under one note is automatically an event of default under the other notes. Any such default and resulting foreclosure would have a material adverse effect on our business, financial condition and results of operations.
We are subject to restrictive covenants under the terms of our outstanding secured debt. If we were to default under the terms of these covenants, the holders would have the right to foreclose on the assets that secure the debt.
The instruments governing our outstanding secured debt contain restrictive covenants. For example, our senior secured convertible note prohibits us from incurring additional indebtedness for borrowed money, repurchasing any outstanding shares of our common stock, or paying any dividends on our capital stock, in each case without the note holder's prior written consent, If we were to breach any of these covenants, the holder could declare an event of default on the note, and exercise its rights to foreclose on the assets securing the note.
Our success depends on our ability to attract and retain scientific, technical, managerial and finance personnel.
Our ability to operate successfully and manage our future growth depends in significant part upon the continued service of key scientific, technical, managerial and finance personnel, as well as our ability to attract and retain additional highly qualified personnel in these fields. We may not be able to attract and retain key employees when necessary, which would limit our operations and growth. Only our Chief Executive Officer and our Senior Vice President of Engineering have employment contracts with us, and none of our employees are covered by key person or similar insurance. In addition, if we are able to successfully develop and commercialize our products, we will need to hire additional scientific, technical, marketing, managerial and finance personnel. We face intense competition for qualified personnel in these areas, many of whom are often subject to competing employment offers.
| 11 |
We are significantly influenced by our directors, executive officers and their affiliated entities.
Our directors, executive officers and entities affiliated with them controlled 15.31 %, of the voting power of our outstanding common stock as of June 15, 2016. These stockholders, acting together, would be able to exert significant influence on substantially all matters requiring approval by our stockholders, including the election of directors and the approval of mergers and other business combination transactions.
Certain provisions of our certificate of incorporation that authorize the issuance of additional shares of preferred stock may make it more difficult for a third party to effect a change in control.
Our certificate of incorporation authorizes our board of directors to issue up to 9.0 million shares of preferred stock. Our undesignated shares of preferred stock may be issued in one or more series, the terms of which may be determined by the board without further stockholder action. These terms may include, among other terms, voting rights, including the right to vote as a series on particular matters, preferences as to liquidation and dividends, repurchase rights, conversion rights, redemption rights and sinking fund provisions. The issuance of any preferred stock could diminish the rights of holders of our common stock, and therefore could reduce the value of our common stock. In addition, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with or sell assets to a third party. The ability of our board to issue preferred stock could make it more difficult, delay, discourage, prevent or make it more costly to acquire or effect a change in control, which in turn could prevent our stockholders from recognizing a gain in the event that a favorable offer is extended and could materially and negatively affect the market price of our common stock.
Risks Related to Our Common Stock
Our board of directors has asked our stockholders to approve a proposal to authorize our board to effect a reverse stock split of our common stock. There are risks associated with a reverse stock split, if it is effected.
On May 18, 2016, our board determined to recommend that at our 2016 annual stockholders’ meeting, our stockholders grant the board the authority, in its discretion, to effect a reverse stock split by a ratio of not less than 1:10 and not more than 1:400, with no change in the number of authorized shares of our common stock, and all fractional shares created by the reverse stock split to be rounded to the nearest whole share. On May 26, 2016, our largest stockholder, John Imhoff, who is also one of our directors, agreed to vote his shares of common stock in favor of the reverse stock split. As of the record date for the 2016 annual meeting, Dr. Imhoff held 10,361,179 shares, or 19.26%, of the outstanding shares of common stock.
We intend to effect the reverse stock split prior to the consummation of this offering; however, there are no assurances that the reverse stock split will be implemented. If the reverse stock split is effected, there are certain risks associated with the reverse stock split, including the following:
| · | We would have additional authorized shares of common stock that the board could issue in future without stockholder approval, and such additional shares could be issued, among other purposes, in financing transactions or to resist or frustrate a third-party transaction that is favored by a majority of the independent stockholders. This could have an anti-takeover effect, in that additional shares could be issued, within the limits imposed by applicable law, in one or more transactions that could make a change in control or takeover of us more difficult. |
| · | There can be no assurance that the reverse stock split, if completed, will achieve the benefits that we hope it will achieve. The total market capitalization of our common stock after the reverse stock split may be lower than the total market capitalization before the reverse stock split. |
The reverse stock split may decrease the liquidity of the shares of our common stock.
The liquidity of the shares of our common stock may be affected adversely by the reverse stock split given the reduced number of shares that will be outstanding following the reverse stock split, especially if the market price of our common stock does not increase as a result of the reverse stock split. In addition, the reverse stock split may increase the number of stockholders who own odd lots of our common stock, creating the potential for such stockholders to experience an increase in the cost of selling their shares and greater difficulty effecting such sales.
| 12 |
Following the reverse stock split, the resulting market price of our common stock may not attract new investors, including institutional investors, and may not satisfy the investing requirements of those investors. Consequently, the trading liquidity of our common stock may not improve.
Although we believe that a higher market price of our common stock may help generate greater or broader investor interest, there can be no assurance that the reverse stock split will result in a share price that will attract new investors, including institutional investors. In addition, there can be no assurance that the market price of our common stock will satisfy the investing requirements of those investors. As a result, the trading liquidity of our common stock may not necessarily improve.
The number of shares of our common stock issuable upon the conversion of our outstanding convertible debt and preferred stock or exercise of outstanding warrants and options (excluding the convertible stock or warrants in this offering) is substantial.
As of June 15, 2016, our outstanding convertible debt was convertible into an aggregate of 151,583,843 shares of our common stock, and the outstanding shares of our Series C and Series C1 preferred stock were convertible into an aggregate of 433,385,611 shares of common stock. Also, as of that date we had warrants outstanding that were exercisable for an aggregate of 233,615,051 shares, contractual obligations to issue 5,205,101 shares, and outstanding options to purchase 104,356 shares. The shares of common stock issuable upon conversion or exercise of these securities would have constituted approximately 91.8 % of the total number of shares of common stock then issued and outstanding.
Further, under the terms of our convertible debt and preferred stock, as well as certain of our outstanding warrants, the conversion price or exercise price, as the case may be, could be adjusted downward, causing substantial dilution. See “—Adjustments to the conversion price for our convertible debt and preferred stock, and the exercise price for certain of our warrants (including the convertible stock and warrants in this offering), will dilute the ownership interests of our existing stockholders.”
Adjustments to the conversion price for a portion of our convertible debt or our Series C preferred stock will dilute the ownership interests of our existing stockholders.
Under the terms of a portion of our convertible debt, the conversion price fluctuates with the market price of our common stock. Additionally, under the terms of our Series C preferred stock and the preferred stock part of this offering, any dividends we choose to pay in shares of our common stock will be calculated based on the then-current market price of our common stock. Accordingly, if the market price of our common stock decreases, the number of shares of our common stock issuable upon conversion of the convertible debt or upon payment of dividends on our outstanding preferred stock and preferred stock part of this offering will increase, and may result in the issuance of a significant number of additional shares of our common stock.
Under the terms of our preferred stock (including the preferred stock part of this offering) and certain of our convertible notes, the conversion price will be lowered if we issue common stock at a per share price below the then conversion price for those securities. Reductions in the conversion price would result in the issuance of a significant number of additional shares of our common stock upon conversion, which would result in dilution in the value of the shares of our outstanding common stock and the voting power represented thereby.
Our stock is thinly traded, so you may be unable to sell at or near ask prices or at all.
The shares of our common stock are dually quoted on the OTCBB and the OTCQB. Shares of our common stock are thinly traded, meaning that the number of persons interested in purchasing our common shares at or near ask prices at any given time may be relatively small or non-existent. This situation is attributable to a number of factors, including:
| · | we are a small company that is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume; and |
| · | stock analysts, stock brokers and institutional investors may be risk-averse and be reluctant to follow a company such as ours that faces substantial doubt about its ability to continue as a going concern or to purchase or recommend the purchase of our shares until such time as we became more viable. |
As a consequence, our stock price may not reflect an actual or perceived value. Also, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer that has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. A broader or more active public trading market for our common shares may not develop or if developed, may not be sustained. Due to these conditions, you may not be able to sell your shares at or near ask prices or at all if you need money or otherwise desire to liquidate your shares.
| 13 |
Trading in our common stock is subject to special sales practices and may be difficult to sell.
Our common stock is subject to the Securities and Exchange Commission’s “penny stock” rule, which imposes special sales practice requirements upon broker-dealers who sell such securities to persons other than established customers or accredited investors. Penny stocks are generally defined to be an equity security that has a market price of less than $5.00 per share. For transactions covered by the rule, the broker-dealer must make a special suitability determination for the purchaser and receive the purchaser’s written agreement to the transaction prior to the sale. Consequently, the rule may affect the ability of broker-dealers to sell our securities and also may affect the ability of our stockholders to sell their securities in any market that might develop.
Stockholders should be aware that, according to Securities and Exchange Commission, the market for penny stocks has suffered from patterns of fraud and abuse. Such patterns include:
| · | control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; |
| · | manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; |
| · | “boiler room” practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; |
| · | excessive and undisclosed bid-ask differentials and markups by selling broker-dealers; and |
| · | the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequent investor losses. |
Our management is aware of the abuses that have occurred historically in the penny stock market. Although we do not expect to be in a position to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive within the confines of practical limitations to prevent the described patterns from being established with respect to our common stock.
Our need to raise additional capital in the near future or to use our equity securities for payments could have a dilutive effect on your investment.
In order to continue operations, we will need to raise additional capital. We may attempt to raise capital through the public or private sale of our common stock or securities convertible into or exercisable for our common stock. In addition, from time to time we have issued our common stock or warrants in lieu of cash payments. If we sell additional shares of our common stock or other equity securities, or issue such securities in respect of other claims or indebtedness, such sales or issuances will further dilute the percentage of our equity that you own. Depending upon the price per share of securities that we sell or issue in the future, if any, your interest in us could be further diluted by any adjustments to the number of shares and the applicable exercise price required pursuant to the terms of the agreements under which we previously issued convertible securities.
| 14 |
USE OF PROCEEDS
We estimate that the net cash proceeds to us from the sale of the securities offered by this prospectus, excluding the proceeds, if any, from the exercise of the warrants included in the offering, will be approximately $4.26 million, assuming the sale by us of 5,000 shares of Series D preferred stock and warrants at an assumed public offering price of $1,000 per share and after deducting the estimated placement agent fees and estimated offering expenses payable by us. However, this is a best efforts offering with no minimum, and we may not sell all or any of the securities; as a result, we may receive significantly less in net cash proceeds, and the net cash proceeds received may not be sufficient to continue to operate our business. In addition, expected net proceeds include a non-cash benefit for the extinguishment of up to $175,000 in outstanding principal and interest on simple interest notes from certain of our investors.
We intend to apply any proceeds received in connection with the offering to increase inventory of LuViva to meet current demand for the product, and to support general working capital and operations. However, we will retain broad discretion over the use of the net proceeds and may use the money for other corporate purposes.
The following table summarizes our currently estimated and intended use of net cash proceeds if we receive the maximum amount of proceeds to be potentially obtained in this offering, which we expect would provide funding for our operations for approximately 24 months. The data in the table set forth below excludes any proceeds we could receive from the exercise of the warrants to be issued in this offering.
| Expected Use for Net Cash Proceeds | Expected Amount of Net Cash Proceeds ($ in 000s) |
Expected % of Net Cash Proceeds | ||
| Increase inventory of LuViva advanced cervical device | $1,750,000 | 41% | ||
| General working capital and operations | 2,510,000 | 59% | ||
| Total | $4,260,000 | 100% | ||
If the net cash proceeds of this offering are less than the maximum, we except to divide the proceeds for purposes listed above on a similar percentage basis.
If a warrant holder elects to exercise the warrants issued in this offering, we may also receive proceeds from the exercise of the warrants. We cannot predict when or if the warrants will be exercised. It is possible that the warrants may expire and may never be exercised.
DILUTION
Our net tangible book value deficiency as of March 31, 2016 was approximately $7.3 million, or $1.37 per share of common stock. “Net tangible book value” represents total tangible assets less total liabilities. “Net tangible book value per share” represents net tangible book value divided by the total number of shares of common stock outstanding.
After giving effect to the assumed sale of 5,000 shares of Series D preferred stock and accompanying warrants in this offering at a public offering price of $1,000 per share, and assuming the conversion of all the shares of Series D preferred stock sold in the offering at an assumed conversion price of $0.01, which was the last reported sale price of our common stock on June 15, 2016 (and excluding shares of common stock issuable upon exercise of warrants), and after deducting estimated placement agent fees and estimated offering expenses payable by us, our pro forma net tangible book value deficiency as of March 31, 2016 would have been approximately $5.8 million, or $0.01 per share of common stock. This represents an immediate increase in net tangible book value of $1.36 per share to our existing stockholders and an immediate dilution in net tangible book value of $(0.02) per share to investors participating in this offering. The following table illustrates this dilution per share to investors participating in this offering:
| Assumed Series D Conversion Price | $ | 0.01 | ||
| Net tangible book value per share as of March 31, 2016 | $ | (1.37 | ) | |
| Increase in net tangible book value per share attributable to this offering | $ | 1.36 | ||
| Pro forma net tangible book value per share after giving effect to the offering | $ | (0.01 | ) | |
| Dilution per share to new investors in this offering | $ | 0.02 |
The above discussion and table are based on 5,322,003 shares of common stock outstanding as of March 31, 2016, which does not include the following, all as of June 15, 2016:
| 15 |
| · | 104,356 shares of common stock reserved for future issuance under our 1995 Stock Plan; |
| · | 433,385,611 shares of common stock reserved for issuance upon conversion of, or issuance as dividends on, our Series C and Series C1 convertible preferred stock, which we assume will be exchanged for shares of Series D preferred stock and warrants as part of this offering; |
| · | 151,583,843 shares reserved for issuance upon conversion of our outstanding convertible debt; |
| · | 233,615,051 shares of common stock issuable upon the exercise of outstanding warrants or warrants to be exchanged for outstanding warrants |
| · | 5,205,101 shares reserved for issuance pursuant to contractual obligations; and |
| · | up to ______ shares of common stock issuable upon exercise of warrants sold as part of this offering, including up to ______ shares of common stock issuable upon exercise of warrants to be issued to the placement agent |
A $0.001 increase (decrease) in the assumed Series D preferred stock conversion price of $0.01 per share would not change the pro forma as adjusted net tangible book value deficiency resulting from the offering ($5.8 million), and our as adjusted net tangible book value deficiency per share by approximately $0.01, but would increase (decrease) the dilution per share to new investors by approximately $0.001, assuming the assumed number of shares of Series D preferred stock sold remains the same. A 10% increase (decrease) in the number of shares sold would would not change the pro forma as adjusted net tangible book value deficiency resulting from the offering ($5.8 million), and our as adjusted net tangible book value deficiency per share by approximately $0.01, but would increase (decrease) the dilution per share to new investors by approximately $0.001, assuming the assumed number of shares of Series D preferred stock sold remains the same.
Certain of our outstanding indebtedness and our outstanding warrants are subject to customary “downside price protection” provisions in the event we sell any common stock at a price lower than the then-conversion price of these securities. The Series D preferred stock offered pursuant to this prospectus will also be subject to similar adjustment. These provisions are further described under the captions “Description of Securities” and “Description of Securities We Are Offering.”
| 16 |
CAPITALIZATION
The following table shows our cash and cash equivalents and our capitalization as of March 31, 2016:
| · | on an actual basis; |
| · | as adjusted to give effect to transactions described in “Summary—Recent Developments”; and |
| · | as further adjusted to give effect to this offering. |
You should read this table together with the information under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our audited annual consolidated financial statements and the related notes and other financial information incorporated by reference into this prospectus from our annual report on Form 10-K for the fiscal year ended December 31, 2015 and our quarterly report on Form 10-Q for the quarterly period ended March 31, 2016.
| As of March 31, 2016 | ||||||||||||
| Actual | As Adjusted(a) | As Further Adjusted(b) | ||||||||||
| (in thousands) | ||||||||||||
| Cash and cash equivalents | $ | 56 | 431 | 4,691 | ||||||||
| Short-term debt | 259 | 434 | 434 | |||||||||
| Short-term convertible note | 591 | 677 | 86 | |||||||||
| Convertible notes payable – current portion | 479 | 479 | 479 | |||||||||
| Long-term convertible notes payable, net | 197 | 197 | 197 | |||||||||
| Outstanding warrants, at fair value | 1,588 | 1,310 | 185 | |||||||||
| Total liabilities | 3,114 | 3,097 | 1,381 | |||||||||
| Series C preferred stock | 1,817 | 1,116 | — | |||||||||
| Series C1 preferred stock | — | 701 | — | |||||||||
| Series D preferred stock | — | — | 6,668 | |||||||||
| Common stock | 297 | 329 | 329 | |||||||||
| Additional paid-in capital | 115,471 | 115,831 | 116,956 | |||||||||
| Treasury stock, at cost | (132 | ) | (132 | ) | (132 | ) | ||||||
| Accumulated deficit | (122,903 | ) | (122,903 | ) | (122,903 | ) | ||||||
| Total stockholders’ surplus (deficit) | (5,450 | ) | (5,058 | ) | 918 | |||||||
| Total capitalization | $ | (8,564 | ) | (8,155 | ) | (463 | ) | |||||
___________________________________
| (a) | Reflects the following, each further described under “Summary—Recent Developments”: |
| · | the April-May 2016 issuances of an aggregate 4,311 shares of Series C1 preferred stock and 18,396,800 shares common stock in exchange for 1,916 then-outstanding shares of Series C preferred stock; |
| · | the cancelation of warrants held by Aquarius Opportunity Fund that were exercisable for an aggregate of 7,148,813 shares of common stock; |
| · | the May 2016 cash advances of $175,000 by Aquarius Opportunity Fund and GPB Debt Holdings II LLC; |
| · | the June 2016 obligation to issue a convertible note and warrant in connection with the Shenghuo license agreement; and |
| · | the June 2016 issuances of an aggregate of 13,517,342 shares of common stock and rights, which includes 8,312,241 common stock shares that were issued and 5,205,101 rights, in exchange for warrants previously exercisable for 8,142,977 shares of common stock. |
| (b) | Assumes sale of all of the securities offered, except (i) shares of common stock that could be issued upon conversion of the Series D preferred stock or upon exercise of the warrants sold (or issued to the placement agent) as part of this offering. Reflects receipt of the net cash proceeds from this offering prior to the application thereof. Also reflects the following, each of which is contingent upon consummation of the offering, as further described under “Summary—Recent Developments”: |
| · | the extinguishment of indebtedness represented by the May 2016 short-term cash advances as a result of the participation by Aquarius Opportunity Fund and GPB Debt Holdings II LLC in this offering at least to the extent of the principal and interest on the cash advances; |
| · | the exchange, on a dollar-for-dollar basis, of all outstanding shares Series C and Series C1 preferred stock, as well as any remaining principal and accrued interest on our convertible promissory note held by Aquarius Opportunity Fund, for shares of Series D preferred stock and warrants; |
| · | the exchange of certain warrants, exercisable for 115,237,788 shares of common stock, for new warrants, exercisable for 214,189,622 shares of common stock. |
| 17 |
PLAN OF DISTRIBUTION
We are offering up to 5,000 shares of our Series D convertible preferred stock, together with warrants to purchase __________ shares of common stock, at a purchase price of $1,000 (and the shares issuable from time to time upon conversion of the Series D preferred stock and the exercise of the warrants) pursuant to this prospectus. The shares of Series D preferred stock and warrants are immediately separable and will be separately issued. There is no minimum offering amount required as a condition to closing and we may sell significantly fewer securities in the offering. The offering will terminate on ___________, unless the offering is fully subscribed before that date or we decide to terminate the offering prior to that date.
In determining the offering price of the securities offered hereby, we will consider a number of factors including, but not limited to, the current market price of our common stock, trading prices of our common stock over time, the illiquidity and volatility of our common stock, our current financial condition and the prospects for our future cash flows and earnings, and market and economic conditions at the time of the offering. Once the offering price is determined, the offering price for the securities will remain fixed for the duration of the offering.
Ladenburg Thalmann & Co. Inc. has agreed to act as our exclusive placement agent in connection with this offering, subject to the terms and conditions of the placement agent agreement dated _______________. Ladenburg is not purchasing or selling any securities offered by this prospectus, nor is it required to arrange the purchase or sale of any specific number or dollar amount of securities, but has agreed to use its best efforts to arrange for the sale of all of the shares of Series D preferred stock and warrants offered hereby. Therefore, we will enter into purchase agreements directly with investors in connection with this offering. Ladenburg may retain other brokers or dealers to act as sub-agents or selected-dealers on its behalf in connection with the offering. Offers will only be made to, and subscriptions accepted from, institutional investors within the meaning of state securities laws of the state in which such investor is domiciled.
All cash funds received in payment for securities sold in this offering will be required to be submitted by subscribers to a non-interest bearing escrow account, and will be held by the escrow agent for such account until we and the placement agent notify the escrow agent that the offering has closed. The closing will occur, as to all subscriptions duly received and accepted by us, in one closing; we do not intend to hold multiple closings in the offering. In the event we do not accept the subscriptions and do not close the offering, escrowed funds will be promptly returned to subscribers without interest or offset.
We have agreed to pay Ladenburg a placement agent’s fee of 9% of the aggregate cash purchase price of the securities sold in this offering. We will also reimburse Ladenburg for its reasonable out-of-pocket expenses, including, without limitation, fees and expenses of its counsel.
The following table shows the per share and total placement agent’s fees that we will pay to Ladenburg in connection with the sale of the securities offered pursuant to this prospectus, assuming the purchase of all of the shares of Series D preferred stock and accompanying warrants offered hereby.
| Per share placement agent’s fees . . . . . | $90 |
| Maximum offering total placement agent’s fees . . . . . | $450,000 |
Because there is no minimum amount required as a condition to the closing in this offering, the actual total offering commissions, if any, are not presently determinable and may be substantially less than the maximum amount set forth above.
We have also agreed to issue warrants to Ladenburg or it designees (as permitted by FINRA Rule 5110(g)) to purchase the number of shares of our common stock equivalent to 5% of the number of shares of common stock issuable upon conversion of the Series D preferred stock (subject to reduction if required by FINRA Rule 5110), at an exercise price equal to 125% of the conversion price per share at the offering. The placement agent warrants will contain a cashless exercise provision, and a piggyback registration rights provision for the life of the warrants, but shall not contain any price-based anti-dilution provisions. The placement agent warrants will have a term expiring five years from the effective date of the registration statement of which this prospectus forms a part. Pursuant to FINRA Rule 5110(g), the placement agent warrants and any shares issued upon exercise of the placement agent warrants may not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the date of effectiveness or commencement of sales of this offering, except the transfer of any security:
| · | by operation of law or by reason of our reorganization; |
| · | to any FINRA member firm participating in the offering and the officers or partners thereof, as long as all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period; |
| 18 |
| · | if the aggregate amount of our securities held by the holder of the placement agent warrants or related persons do not exceed 1% of the securities being offered; |
| · | that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund, and participating members in the aggregate do not own more than 10% of the equity in the fund; or |
| · | the exercise or conversion of any security, if all securities received remain subject to the lock-up restriction set forth above for the remainder of the time period. |
In addition, we have granted a right of first refusal to the placement agent pursuant to which it has the right to act as the lead manager or underwriter or agent, as applicable, if we or our subsidiary raise capital through a public or private offering of equity or debt securities at any time prior to eighteen months from the date of closing of this offering.
Our obligations to issue and sell securities to the purchasers is subject to the conditions set forth in the securities purchase agreement, which may be waived by us at our discretion. A purchaser’s obligation to purchase securities is subject to the conditions set forth in the securities purchase agreement as well, which may also be waived by the purchaser in its discretion.
We estimate the total offering expenses in this offering that will be payable by us, excluding the placement agent’s fees, will be approximately $290,000, which includes legal, accounting and printing costs, various other fees and reimbursement of the placement agent’s expenses.
We have also agreed to indemnify the placement agent for certain liabilities under the Securities Act.
The foregoing does not purport to be a complete statement of the terms and conditions of the placement agent agreement or the securities purchase agreement. A copy of the placement agent agreement and the form of securities purchase agreement with investors are included as exhibits to the registration statement of which this prospectus forms a part.
Ladenburg is an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. As an underwriter, Ladenburg is required to comply with the Securities Act and the Securities Exchange Act of 1934, including Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of our securities by Ladenburg acting as principal. Under these rules and regulations, Ladenburg:
| · | may not engage in any stabilization activity in connection with our securities; and |
| · | may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution. |
Pursuant to the purchase agreement to be entered into in connection with this offering, we will be prohibited from issuing, entering into any agreement to issue or announcing the issuance or proposed issuance of any shares of common stock or common stock equivalents (subject to certain exceptions) for a period of 90 days from the closing of this offering.
| 19 |
DESCRIPTION OF SECURITIES WE ARE OFFERING
We are offering up to 5,000 shares of our Series D convertible preferred stock, convertible into an aggregate of _________ shares of our common stock, and five-year warrants exercisable for an aggregate of ____________ shares of our common stock. Each share of Series D preferred stock we sell in the offering will be accompanied by a warrant to purchase _____ of a share of common stock, and will be sold at a combined price of $1,000.
The shares of Series D preferred stock and the warrants are immediately separable and will be issued separately. Subject to certain ownership limitations, the Series D preferred stock is convertible at any time at the holder’s option into shares of our common stock at an initial conversion price of $0.01 per share, and the warrants are immediately exercisable at an initial exercise price of $_____ per share. This prospectus also covers the shares of common stock issuable upon conversion of the Series D preferred stock and upon exercise of the warrants. The material terms and provisions of our common stock, which underlies the Series D preferred stock and the warrants, and each other class of securities which qualifies or limits the foregoing, are described under the caption “Description of Securities.”
Series D Preferred Stock
The material terms and provisions of the Series D preferred stock being offered pursuant to this prospectus are described below. The following description is qualified in its entirety by reference to the form of certificate of designation filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions of the form of the certificate of designation for a complete description of the terms and conditions of the Series D preferred stock.
Our Board has designated 5,000 shares of preferred stock as Series D Convertible Preferred Stock, par value $0.001 per share. As of June 15, 2016, there were no shares of Series D preferred stock outstanding.
Voting Rights. Except as required by law, holders of the Series D preferred stock do not have rights to vote on any matters, questions or proceedings, including the election of directors. However, as long as any shares of Series D preferred stock are outstanding, we will not, without the affirmative vote of the holders of a majority of the then-outstanding shares of the Series D preferred stock, (1) alter or change adversely the powers, preferences or rights given to the Series D preferred stock or alter or amend the certificate of designation, (2) authorize or create any class of stock ranking as to dividends, redemption or distribution of assets upon liquidation senior to, or otherwise pari passu with, the Series D preferred stock, (3) amend our certificate of incorporation or other charter documents in any manner that adversely affects any rights of the holders of Series D preferred stock, (4) increase the number of authorized shares of Series D preferred stock, or (5) enter into any agreement with respect to any of the foregoing.
Liquidation Preference. The Series D preferred stock ranks, with respect to rights upon liquidation, winding-up or dissolution, (1) senior to common stock, (2) senior to any series of preferred stock ranked junior to the Series D preferred stock, (3) junior to our Series C preferred stock, and (4) junior to all of our existing and future indebtedness.
Redemption. We have the right to redeem the Series D preferred stock for a cash payment equal to 120% of the stated value of the Series D preferred stock. Holders of Series D preferred stock will receive 20 trading days prior notice of any redemption and will have the ability to convert the Series D preferred stock into common stock during this notice period.
Conversion; Beneficial Ownership Limitation. Subject to certain ownership limitations as described below, the Series D preferred stock is convertible at any time at the option of the holder into shares of our common stock at a conversion ratio determined by dividing the $1,000 stated value of the Series D preferred stock by a conversion price of $____ per share. The conversion price is subject to adjustment in the case of stock splits, stock dividends, combinations of shares and similar recapitalization transactions. In addition, until our common stock is listed on a national securities exchange, if we sell or grant any right to purchase or sell any common stock or common stock equivalents entitling any person to acquire shares of common stock (subject to exceptions for certain exempt issuances, including issuances pursuant to equity compensation plans, certain issuances to consultants, issuances in connection with exercise or exchange of common stock equivalents already outstanding and issuances pursuant to acquisitions or strategic transactions or arrangements) at an effective price per share that is lower than the then-conversion price of the Series D preferred stock, then the conversion price will be further reduced to equal such lower price per share.
Subject to limited exceptions, a holder of shares of Series D preferred stock will not have the right to convert any portion of its Series D preferred stock if the holder, together with its affiliates, would beneficially own in excess of 4.99% of the number of shares of our common stock outstanding immediately after giving effect to its conversion; provided, however, that upon 61 days’ prior notice to us, the holder may increase such percentage to 9.99%. We have the right to force the conversion of the Series D preferred stock if our common stock meets certain minimum price and trading volume thresholds.
| 20 |
Dividends. The Series D preferred stock is entitled to receive dividends (on an “as converted to common stock” basis) upon and in the same form as dividends actually paid on shares of our common stock. No other dividends will be paid on shares of Series D preferred stock.
Liquidation. Upon our liquidation, dissolution or winding up after payment or provision for payment of our debts and other liabilities, but before any distribution or payment is made to the holders of any junior securities, the holders of Series D preferred stock will be entitled to be paid out of our assets available for distribution to our stockholders in an amount equal to $1,000 per share, after which any of our remaining assets will be distributed among the holders of junior classes of our capital stock in accordance with our certificate of incorporation.
Warrants
The material terms and provisions of the warrants being offered pursuant to this prospectus are described below. The following description is qualified in its entirety by reference to the form of warrant filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions of the form of the warrant for a complete description of the terms and conditions of the warrants.
Duration and Exercise Price. The warrants offered hereby (including those to be issued to the placement agent, which have substantially similar terms except as indicated below, but also have certain provisions required by FINRA and an exercise price of 125% of the combined public offering price per share and warrant) will entitle the holders thereof to purchase up to an aggregate of ____________ shares of our common stock at an exercise price of $____ per share (assuming we offer ____________ shares of Series D preferred stock at an assumed conversion price of $____ per share), commencing immediately on the issuance date and will expire five years following the issuance date. The warrants will be issued separately from the Series D preferred stock offered, and may be transferred separately immediately thereafter. No warrants exercisable for a fractional amount of shares of common stock will be issued. If an investor would otherwise be entitled to receive a fractional warrant, the number of shares issuable upon exercise of the warrant will be rounded up to the nearest whole warrant. The warrant holders must pay the exercise price in cash upon exercise of the warrants. After the close of business on the expiration date, unexercised warrants will become void.
Anti-Dilution Protection. The exercise price of the warrants is subject to appropriate adjustment in the event of stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock, and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Fundamental Transactions. In the event of any fundamental transaction, as described in the warrants and generally including any merger with another entity, the sale, transfer or other disposition of all or substantially all of our assets to another entity, or the acquisition by a person of more than 50% of our common stock, then, subject to the agreement of the counterparty to the fundamental transaction, the holders of the warrants will thereafter have the right to receive upon exercise of the warrants such shares of stock, securities or assets as would have been issuable or payable with respect to or in exchange for a number of shares of our common stock equal to the number of shares of our common stock issuable upon exercise of the warrants immediately prior to the fundamental transaction, had the fundamental transaction not taken place, and appropriate provision will be made so that the provisions of the warrants (including, for example, provisions relating to the adjustment of the exercise price) will thereafter be applicable, as nearly equivalent as may be practicable in relation to any share of stock, securities or assets deliverable upon the exercise of the warrants after the fundamental transaction.
Transferability. The warrants may be transferred at the option of the holder upon surrender of the warrants with the appropriate instruments of transfer.
Right as a Stockholder. Except by virtue of a holder’s ownership of shares of our common stock, the holders of the warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their warrants.
Exercisability. The warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise. Subject to limited exceptions, a holder of will not have the right to exercise any portion of its warrant if the holder, together with its affiliates, would beneficially own in excess of 4.99% of the number of shares of our common stock outstanding immediately after giving effect to its exercise; provided, however, that upon 61 days’ prior notice to us, the holder may increase such percentage to 9.99%.
| 21 |
Waivers and Amendments. Subject to certain exceptions, any term of the warrants may be amended or waived with our written consent and the written consent of each holder of the then-outstanding warrants.
DESCRIPTION OF SECURITIES
We are authorized to issue 1,005,000,000 shares of stock, in two classes: 1,000,000 shares of common stock, par value $.001 per share, and 5,000,000 million shares of preferred stock. As of June 15, 2016, there were 67,901,547 shares of common stock outstanding, which were held of record by 209 stockholders, and 6,679 shares of preferred stock outstanding, consisting of 2,368 shares of Series C preferred stock, which were held of record by 3 stockholders, and 4,311 shares of Series C1 preferred stock, which were held of record by 6 stockholders. Upon consummation of the offering, all outstanding shares of Series C and Series C1 preferred stock will be exchanged for shares of the Series D preferred stock and warrants as part of this offering.
Common Stock
The holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. Subject to preferences that may be applicable to any outstanding preferred stock, holders of common stock are entitled to receive ratably such dividends as may be declared by the board out of funds legally available therefor and in liquidation proceedings. Holders of common stock have no preemptive or subscription rights and there are no redemption rights with respect to such shares.
Preferred Stock
Our board is authorized, without further stockholder action, to issue preferred stock in one or more series and to fix the voting rights, liquidation preferences, dividend rights, repurchase rights, conversion rights, redemption rights and terms, including sinking fund provisions, and certain other rights and preferences, of the preferred stock. Our board designated 525,000 shares of preferred stock as redeemable convertible preferred stock, none of which remain outstanding; 3,000 shares of preferred stock as Series B Convertible Preferred Stock, none of which remain outstanding; 9,000 shares of preferred stock as Series C Convertible Preferred Stock, of which 2,368 were outstanding as of June 15, 2016; 20,250 shares of preferred stock as Series C1 Convertible Preferred Stock, of which 4,311 were outstanding as of June 15, 2016; and 5,000 shares of preferred stock as Series D Convertible Preferred Stock, none of which were outstanding as of June 15, 2016. The material terms and provisions of our Series D preferred stock are described under the caption “Description of Securities We Are Offering.”
Although there is no current intention to do so, our board may, without stockholder approval, issue additional shares of preferred stock with voting and conversion rights that could adversely affect the voting power or dividend rights of the holders of common stock and may have the effect of delaying, deferring or preventing a change in control.
Series C Convertible Preferred Stock
Dividends. From the original issue date until 42 months thereafter, the holders of Series C preferred stock are entitled to receive quarterly cumulative dividends at a rate per share (as a percentage of stated value per share) of 12% per year per share payable quarterly on each January 1, April 1, July 1 and October 1 during such period (beginning October 1, 2015) in shares of our common stock (subject to certain conditions) or, at our option, in cash. In addition, upon the conversion of the Series C preferred stock (other than a forced conversion, described below) during the such period, we must pay the converting holder a “make-whole payment” in cash or, at our option (subject to certain conditions), shares of our common stock with respect to the converted shares of Series C preferred stock in an amount equal to $420 per $1,000 of stated value, less the amount of any dividends already paid on such shares of Series C preferred stock. To the extent we choose to pay any dividends or make-whole payments in shares of our common stock, such shares will be valued at 80% of then-current market price (calculated as the average daily volume weighted average price of our common stock for the five consecutive trading days prior to payment). After the dividend payment period, holders of Series C preferred stock are only entitled to receive dividends on an as-if-converted basis) with holder of our common stock (other than dividends paid in additional shares of common stock).
Voting. Except as otherwise proved by law or in the Series C designations, the Series C preferred stock has no voting rights. We may not, without the consent of the holders of a majority of the shares of Series C preferred stock then outstanding, alter or change adversely the powers, preferences or rights given to the Series C preferred stock or alter or amend the Series C designations, create any class of stock with a liquidation preference equal or senior to the Series C preferred stock, amend our charter in any manner that adversely affects any rights of the holders of Series C preferred stock, increase the number of authorized shares of Series C preferred stock, or enter into any agreement with respect to any of the foregoing.
| 22 |
Liquidation. In the event of our liquidation, dissolution or winding-up, whether voluntary or involuntary, the holders of our Series C preferred stock will be entitled to receive out of our assets an amount equal to the stated value of their shares, plus any other fees, liquidated damages or dividends then due and owing on their shares, before any distribution or payment to the holders of any junior securities.
Conversion. The Series C preferred stock is convertible at any time, at the option of the holder. In addition, if the market price of our common stock for each trading day for 20 of any 30 consecutive trading-day period exceeds 250% of the highest conversion price in effect during such period, we may force each holder to convert all or part of such holder’s shares into shares of common stock.
Each share of Series C preferred stock is convertible into the number of shares of common stock equal to the quotient obtained by dividing (1) the sum of the stated value plus all accrued but unpaid dividends on such share, by (2) the per share conversion price. The initial per share conversion price was $0.095 (pre-stock split), but has been reset pursuant to the Series C designations to $0.017824 as of June 15, 2016. The conversion price will automatically adjust downward to 80% of the then-current market price of our common stock 15 trading days after any subsequent reverse stock split of the common stock, and 5 trading days after any conversions of our outstanding convertible debt. The conversion price is subject to further adjustment under certain circumstances to protect the holders of Series C preferred stock from dilution relative to certain issuances of common stock, or securities convertible into or exercisable for shares of common stock. Subject to certain exceptions, if we issue shares of common stock, or such other securities, at a price per share less than the then-effective conversion price, the conversion price will be adjusted to equal such lower per share consideration.
Series C1 Convertible Preferred Stock
The Series C1 preferred stock has terms that are substantially the same as the Series C preferred stock, except that the Series C1 preferred stock does not pay dividends (unless and to the extent declared on the common stock) or at-the-market “make-whole payments.”
Secured Promissory Note
As of June 15, 2016, we have issued and outstanding $507,614 in aggregate principal amount of secured promissory notes that, as amended, provide the holders the right to convert the outstanding balance into shares of our common stock at a conversion price per share equal to the lower of (1) $25.00 and (2) 75% of the lowest daily volume weighted average price per share of our common stock during the five business days prior to conversion. If the conversion price at the time of any conversion request would be lower than $15.00 per share, we have the option of delivering the conversion amount in cash in lieu of shares of our common stock. The note is secured by a lien on our inventory and accounts receivables.
Senior Secured Convertible Note
As of June 15, 2016, we have issued and outstanding $1,525,000 million in aggregate principal amount of a senior secured convertible note that is convertible at any time, in whole or in part, at the holder’s option, into shares of our common stock, at a conversion price equal to $0.014224 per share, subject to certain customary adjustments and anti-dilution provisions. The note is secured by a lien on all of our assets.
Convertible Note
As of June 15, 2016, we have issued and outstanding $240,000 in aggregate principal amount of a convertible note that is convertible at any time, in whole or in part, at the holder’s option, into shares of our common stock, at a conversion price equal to $0.017402 per share, subject to certain customary adjustments and anti-dilution provisions.
Warrants and Options
We have issued warrants to purchase our common stock from time to time in connection with certain financing arrangements. As of June 15, 2016, there are warrants exercisable for an aggregate of 191,815,878 shares of common stock outstanding, as follows:
| 23 |
|
Warrants |
Exercise Price Per Share |
Expiration Date | |
| 2,852 | (1) | $105.00 | November 20, 2016 |
| 18,581 | (2) | $10.46 | May 23, 2018 |
| 12,066,450 | (3) | $0.09375 | May 23, 2018 |
| 2,000 | (4) | $50.00 | April 23, 2019 |
| 5,618 | (5) | $45.00 | May 22, 2019 |
| 1,842 | (5) | $38.00 | September 10, 2019 |
| 3,255 | (6) | $46.081 | September 27, 2019 |
| 7,553 | (7) | $28.13 | December 2, 2019 |
| 83,927 | (8) | $9.00 | December 2, 2020 |
| 83,927 | (8) | $11.00 | December 2, 2020 |
| 20,000 | (9) | $25.50 | March 30, 2018 |
| 17,547 | (10) | $11.88 | June 29, 2020 |
| 120,000 | (11) | $1.03 | June 29, 2020 |
| 115,789 | (12) | $1.03 | September 4, 2020 |
| 131,842 | (13) | $1.03 | September 21, 2020 |
| 5,163 | (14) | $11.88 | September 4, 2020 |
| 157,895 | (15) | $1.03 | October 23, 2020 |
| 5,163 | (16) | $11.88 | October 23, 2020 |
| 202,123,172 | (17) | $0.01422 | June 6, 2021 |
| 4,850,956 | (18) | $0.01422 | February 12, 2021 |
| 13,791,518 | (19) | $0.017402 | June 6, 2021 |
| 233,615,051 | |||
| (1) | Issued as part of a November 2011 private placement. |
| (2) | Issued in June 2015 in exchange for warrants originally issued in a May 2013 private placement. |
| (3) | Issued in June 2015 in exchange for warrants originally issued in a May 2013 private placement. |
| (4) | Issued to a placement agent in conjunction with an April 2014 private placement. |
| (5) | Issued to a placement agent in conjunction with a September 2014 private placement. |
| (6) | Issued as part of a September 2014 Regulation S offering. |
| (7) | Issued to a placement agent in conjunction with a 2014 public offering. |
| (8) | Issued in June 2015 in exchange for warrants originally issued as part of a 2014 public offering. |
| (9) | Issued as part of a March 2015 private placement. |
| (10) | Issued to a placement agent in conjunction with a June 2015 private placement. |
| (11) | Issued as part of a June 2015 private placement. |
| (12) | Issued as part of a June 2015 private placement. |
| (13) | Issued as part of a June 2015 private placement. |
| (14) | Issued to a placement agent in conjunction with a June 2015 private placement. |
| (15) | Issued as part of a June 2015 private placement. |
| (16) | Issued to a placement agent in conjunction with a June 2015 private placement. |
| (17) | Issued as part of a February 2016 private placement. |
| (18) | Issued to a placement agent in conjunction with a February 2016 private placement. |
| (19) | Contractually obligated to be issued pursuant to a strategic license agreement. |
All outstanding warrant agreements provide for anti-dilution adjustments in the event of certain mergers, consolidations, reorganizations, recapitalizations, stock dividends, stock splits or other changes in our corporate structure. In addition, warrants subject to footnotes (3) and (11)-(19) in the table above are subject to “lower price issuance” anti-dilution provisions that automatically reduce the exercise price of the warrants (and, in the cases of warrants subject to footnote (3) in the table above and footnote (17), increase the number of shares of common stock issuable upon exercise), to the offering price in a subsequent issuance of our common stock, unless such subsequent issuance is exempt under the terms of the warrants.
The warrants subject to footnote (3) are subject to a mandatory exercise provision. This provision permits us, subject to certain limitations, to require exercise of such warrants at any time following (a) the date that is the 30th day after the later of our receipt of an approvable letter from the FDA for LuViva and the date on which the common stock achieves an average market price for 20 consecutive trading days of at least $1.30 with an average daily trading volume during such 20 consecutive trading days of at least 250 shares, or (b) the date on which the average market price of the common stock for 20 consecutive trading days immediately prior to the date we deliver a notice demanding exercise is at least $162.00 and the average daily trading volume of the common stock exceeds 250 shares for such 20 consecutive trading days. If these warrants are not timely exercised upon demand, they will expire. Upon the occurrence of certain events, we also may be required to repurchase these warrants, as well as the warrants subject to footnote (2) in the table above.
| 24 |
The warrants subject to footnote (6) in the table above are also subject to a mandatory exercise provision. This provision permits us, subject to certain limitations, to require the exercise of such warrants should the average trading price of our common stock over any 30 consecutive day trading period exceed $92.16.
The warrants subject to footnote (8) in the table above are also subject to a mandatory exercise provision. This provision permits us, subject to certain limitations, to require exercise of 50% of the then-outstanding warrants if the trading price of our common stock is at least two times the initial warrant exercise price for any 20-day trading period. Further, in the event that the trading price of our common stock is at least 2.5 times the initial warrant exercise price for any 20-day trading period, we will have the right to require the immediate exercise of 50% of the then-outstanding warrants. Any warrants not exercised within the prescribed time periods will be canceled to the extent of the number of shares subject to mandatory exercise.
The holders of the warrants subject to footnote (3) in the table above have agreed to surrender the warrants upon consummation of this offering for new warrants exercisable for 200% of the number of shares underlying the surrendered warrants, but without certain anti-dilution protections included with the surrendered warrants.
As of June 15, 2016, we have issued options to purchase a total of 104,356 shares of our common stock pursuant to our equity incentive plan, at a weighted average exercise price of $45.46 per share. Recommendations for option grants under our equity incentive plans are made by the compensation committee of our board, subject to ratification by the full board. The compensation committee may issue options with varying vesting schedules, but all options granted pursuant to our equity incentive plans must be exercised within ten years from the date of grant.
| 25 |
OUR BUSINESS
We are a medical technology company focused on developing innovative medical devices that have the potential to improve healthcare. Our primary focus is the sales and marketing of our LuViva® Advanced Cervical Scan non-invasive cervical cancer detection device. The underlying technology of LuViva primarily relates to the use of biophotonics for the non-invasive detection of cancers. LuViva is designed to identify cervical cancers and precancers painlessly, non-invasively and at the point of care by scanning the cervix with light, then analyzing the reflected and fluorescent light.

LuViva provides a less invasive and painless alternative to conventional tests for cervical cancer screening and detection. Additionally, LuViva improves patient well-being not only because it eliminates pain, but also because it is convenient to use and provides rapid results at the point of care. We focus on two primary applications for LuViva: first, as a cancer screening tool in the developing world, where infrastructure to support traditional cancer-screening methods is limited or non-existent, and second, as a triage following traditional screening in the developed world, where a high number of false positive results cause a high rate of unnecessary and ultimately costly follow-up tests.
Screening for cervical cancer represents one of the most significant demands on the practice of diagnostic medicine. As cervical cancer is linked to a sexually transmitted disease—the human pampillomavirus (HPV)—every woman essentially becomes “at risk” for cervical cancer simply after becoming sexually active. In the developing world there are approximately 2.0 billion women aged 15 and older who are potentially eligible for screening with LuViva. Guidelines for screening intervals vary across the world, but U.S. guidelines call for screening every three years. Traditionally, the Pap smear screening test, or Pap test, is the primary cervical cancer screening methodology in the developed world. However, in developing countries, cancer screening using Pap tests is expensive and requires infrastructure and skill not currently existing, and not likely to be developed in the near future, in these countries.
We believe LuViva is the answer to the developing world’s cervical cancer screening needs. Screening for cervical cancer in the developing world often requires working directly with foreign governments or non-governmental agencies (NGOs). By partnering with governments or NGOs, we are able to provide immediate access to cervical cancer detection to large segments of a nation’s population as part of national or regional governmental healthcare programs, eliminating the need to develop expensive and resource-intensive infrastructures.
| 26 |
Manufacturing, Sales Marketing and Distribution
We manufacture LuViva at our Norcross, Georgia facility. Most of the components of LuViva are custom made for us by third-party manufacturers. We adhere to ISO 13485:2003 quality standards in our manufacturing processes.
Research, Development and Engineering
We have been engaged primarily in the research, development and testing of our LuViva non-invasive cervical cancer detection product and our core biophotonic technology. Since 2013, we have incurred about $7.0 million in research and development expenses, net of about $927,000 reimbursed through collaborative arrangements and government grants. Research and development costs were approximately $1.5 million and $2.8 million in 2015 and 2014, respectively
Patents
We have pursued a course of developing and acquiring patents and patent rights and licensing technology. Our success depends in large part on our ability to establish and maintain the proprietary nature of our technology through the patent process and to license from others patents and patent applications necessary to develop our products. As of December 31, 2015, we have 24 granted U.S. patents relating to our biophotonic cancer detection technology and six pending U.S. patent applications. We also have three granted patents that apply to our interstitial fluid analysis system.
The Need for LuViva
In the developed world, we believe LuViva offers a more accurate and ultimately cost-effective triage medical device, to be used once a traditional Pap test indicates the possibility of cervical cancer. Due to the high number of false positive results from Pap tests, traditional follow-on tests entail increased medical treatment costs. We believe these costs can be minimized by utilizing LuViva as a triage to determine whether follow-on tests are actually warranted.
We believe our non-invasive
cervical cancer detection technology can be applied to the early detection of other cancers as well. In 2013, we announced a license
agreement with Konica Minolta, Inc. allowing us to manufacture and develop a non-invasive esophageal cancer detection product from
Konica Minolta based on our biophotonic technology platform. Early market analyses of our biophotonic technology indicated that
skin cancer detection was also promising, but currently we are focused primarily on the large-scale commercialization of LuViva.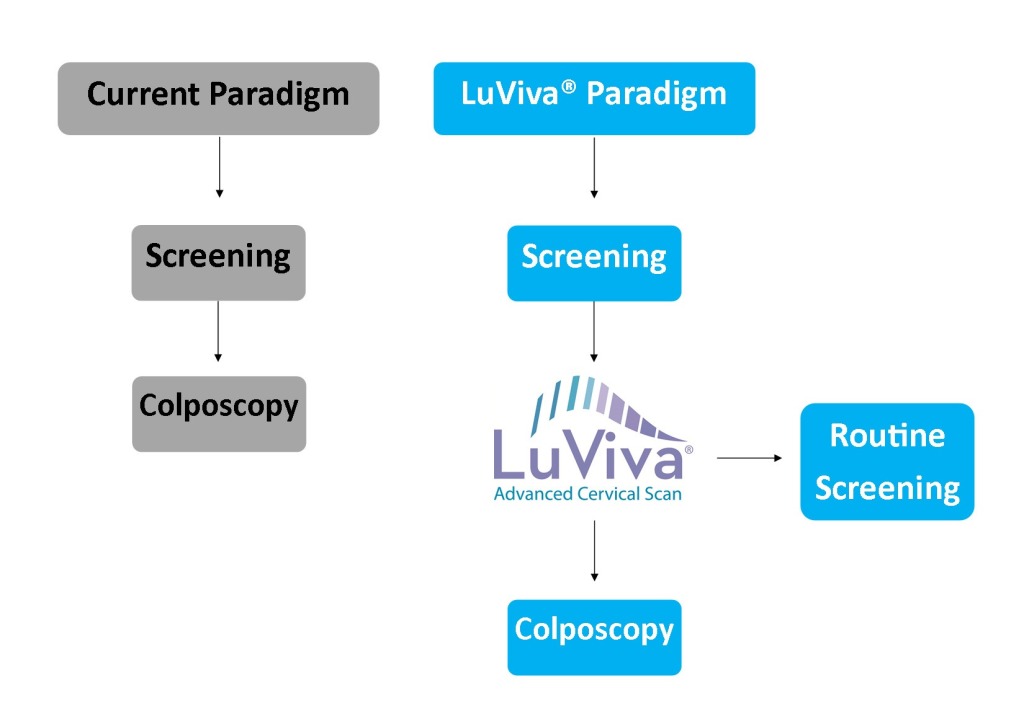
| 27 |
Cancer
Cancer is a group of many related diseases. All forms of cancer involve the out-of-control growth and spread of abnormal cells. Normal cells grow, divide, and die in an orderly fashion. Cancer cells, however, continue to grow and divide and can spread to other parts of the body. In America, half of all men and one-third of all women will develop some form of cancer during their lifetimes. According to the American Cancer Society, the sooner a cancer is found and treatment begins, the better a patient’s chances are of being cured. We began investigating the applications of our biophotonic technology to cancer detection before 1997, when we initiated a preliminary market analysis. We concluded that our biophotonic technology had applications for the detection of a variety of cancers through the exposure of tissue to light. We selected detection of cervical cancer and skin cancer from a list of the ten most promising applications to pursue initially, and ultimately focused primarily on our LuViva cervical cancer detection device.
Cervical cancer is a cancer that begins in the lining of the cervix (which is located in the lower part of the uterus). Cervical cancer forms over time and may spread to other parts of the body if left untreated. There is generally a gradual change from a normal cervix to a cervix with precancerous cells to cervical cancer. For some women, precancerous changes may go away without any treatment. While the majority of precancerous changes in the cervix do not advance to cancer, if precancers are treated, the risk that they will become cancers can be greatly reduced.
The Developing World
According to the most recent data published by the World Health Organization (WHO), cervical cancer is the fourth most frequent cancer in women worldwide, with an estimated 530,000 new cases in 2012. For women living in less developed regions, however, cervical cancer is the second most common cancer, with an estimated 445,000 new cases in 2012 (84% of the new cases worldwide). In 2012, approximately 270 000 women died from cervical cancer; more than 85% of these deaths occurring in low- and middle-income countries.
| 28 |
As noted by the WHO, in developed countries, programs are in places that enable women to get screened, making most pre-cancerous lesions identifiable at stages when they can easily be treated. Early treatment prevents up to 80% of cervical cancers in these countries. In developing countries, however, limited access to effective screening means that the disease is often not identified until it is further advanced and symptoms develop. In addition, prospects for treatment of such late-stage disease may be poor, resulting in a higher rate of death from cervical cancer in these countries.
We believe that the greatest need and market opportunity for LuViva lies in screening for cervical cancer in developing countries where the infrastructure for traditional screening may be limited or non-existent.
We are actively working with distributors in the following countries to implement government-sponsored screening programs: Turkey, Bangladesh, Indonesia, Kenya and Nigeria. The number of screening candidates in those countries is approximately 246 million and represents 3 of the 10 most populous countries in the world.

The Developed World
The Pap test, which involves a sample of cervical tissue being placed on a slide and observed in a laboratory, is currently the most common form of cervical cancer screening. Since the introduction of screening and diagnostic methods, the number of cervical cancer deaths in the developed world has declined dramatically, due mainly to the increased use of the Pap test. However, the Pap test has a wide variation in sensitivity, which is the ability to detect the disease, and specificity, which is the ability to exclude false positives. A study by Duke University for the U.S. Agency for Health Care Policy and Research published in 1999 showed Pap test performance ranging from a 22%-95% sensitivity and 78%-10% specificity, although new technologies improving the sensitivity and specificity of the Pap test have recently been introduced and are finding acceptance in the marketplace. About 60 million Pap tests are given annually in the United States, at an average price of approximately $26 per test.
| 29 |
After a Pap test returns a positive result for cervical cancer, accepted protocol calls for a visual examination of the cervix using a colposcope, usually followed by a biopsy, or tissue sampling, at one or more locations in the cervix. This method looks for visual changes attributable to cancer. There are about two million colposcope examinations annually in the United States and Europe. In 2003, the average cost of a stand-alone colposcope examination in the United States was $185 and the average cost of a colposcopy with biopsy was $277.
Given this landscape, we believe that there is a material need and market opportunity for LuViva as a triage device in the developed world where LuViva represents a more cost-effective method of verifying a positive Pap test than the alternatives.
The LuViva Advanced Cervical Scan
LuViva is designed to identify cervical cancers and precancers painlessly, non-invasively and at the point of care by scanning the cervix with light, then analyzing the light reflected from the cervix. The information presented by the light would be used to indicate the likelihood of cervical cancer or precancers. Our product, in addition to detecting the structural changes attributed to cervical cancer, is also designed to detect the biochemical changes that precede the development of visual lesions. In this way, cervical cancer may be detected earlier in its development, which should increase the chances of effective treatment. In addition to the device itself, operation of LuViva requires employment of our single-use, disposable calibration and alignment cervical guide.
To date, thousands of women in multiple international clinical settings have been tested with LuViva. As a result, more than 25 papers and presentations have been published regarding LuViva in a clinical setting, the most recent being presented at the International Federation of Gynaecology and Obstetrics Congress in 2015.
Internationally, we contract with country-specific or regional distributors. We believe that the international market will be significantly larger than the U.S. market due to the international demand for cervical cancer screening. We have formal distribution agreements in place covering 54 countries and plan on adding additional countries in 2016.
We have regulatory approval to sell LuViva in Europe under our Edition 3 CE Mark. Additionally, LuViva has marketing approval from Health Canada, COFEPRIS in Mexico, Ministry of Health in Kenya and the Singapore Health Sciences Authority. We currently are seeking regulatory approval to market LuViva in the United States, but have not yet received approval from the U.S. Food and Drug Administration (FDA). As of March 7, 2016, we have sold 93 LuViva devices and approximately 35,000 single-use-disposable cervical guides to international distributors.
We believe our non-invasive cervical cancer detection technology can be applied to the early detection of other cancers as well. From 2008 to early 2013, we worked with Konica Minolta to explore the feasibility of adapting our microporation and biophotonic cancer detection technology to other areas of medicine and to determine potential markets for these products in anticipation of a development agreement. In February 2013, we replaced our existing agreements with Konica Minolta with a new agreement, pursuant to which, subject to the payment of a nominal license fee due upon FDA approval, Konica Minolta has granted us a five-year, world-wide, non-transferable and non-exclusive right and license to manufacture and to develop a non-invasive esophageal cancer detection product from Konica Minolta and based on our biophotonic technology platform. The license permits us to use certain related intellectual property of Konica Minolta. In return for the license, we have agreed to pay Konica Minolta a royalty for each licensed product we sell. We continue to seek new collaborative partners to further develop our biophotonic technology.
Manufacturing, Sales Marketing and Distribution
We manufacture LuViva at our Norcross, Georgia facility. Most of the components of LuViva are custom made for us by third-party manufacturers. We adhere to ISO 13485:2003 quality standards in our manufacturing processes. Our single-use cervical guides are manufactured by a vendor that specializes in injection molding of plastic medical products.
We rely on distributors to sell our products. Distributors can be country exclusive or cover multiple countries in a region. We manage these distributors, provide them marketing materials and train them to demonstrate and operate LuViva. We seek distributors that have experience in gynecology and in introducing new technology into their assigned territories.
We have only limited experience in the production planning, quality system management, facility development, and production scaling that will be needed to bring production to increased sustained commercial levels. We will likely need to develop additional expertise in order to successfully manufacture, market, and distribute any future products.
| 30 |
Research, Development and Engineering
We have been engaged primarily in the research, development and testing of our LuViva non-invasive cervical cancer detection product and our core biophotonic technology. Since 2013, we have incurred about $7.0 million in research and development expenses, net of about $927,000 reimbursed through collaborative arrangements and government grants. Research and development costs were approximately $1.5 million and $2.8 million in 2015 and 2014, respectively.
Since 2013, we have focused our research and development and our engineering resources almost exclusively on development of our biophotonic technology, with only limited support of other programs funded through government contracts or third party funding. Because our research and clinical development programs for other cancers are at a very early stage, substantial additional research and development and clinical trials will be necessary before we can produce commercial prototypes of other cancer detection products.
Several of the components used in LuViva are available from only one supplier, and substitutes for these components could not be obtained easily or would require substantial modifications to our products.
Patents
We have pursued a course of developing and acquiring patents and patent rights and licensing technology. Our success depends in large part on our ability to establish and maintain the proprietary nature of our technology through the patent process and to license from others patents and patent applications necessary to develop our products. As of December 31, 2015, we have 24 granted U.S. patents relating to our biophotonic cancer detection technology and six pending U.S. patent applications. We also have three granted patents that apply to our interstitial fluid analysis system.
Competition
The medical device industry in general and the markets for cervical cancer detection in particular, are intensely competitive. If successful in our product development, we will compete with other providers of cervical cancer detection and prevention products.
Current cervical cancer screening and diagnostic tests, primarily the Pap test, HPV test, and colposcopy, are well established and pervasive. Improvements and new technologies for cervical cancer detection and prevention, such as Thin-Prep from Hologic and HPV testing from Qiagen, have led to other new competitors. In addition, there are other companies attempting to develop products using forms of biophotonic technologies in cervical cancer detection, such as Spectrascience, which has a very limited FDA approval to market its device for detection of cervical cancers, but has not yet entered the market. The approval limits use of the Spectrascience device only after a colposcopy, as an adjunct. In addition to the Spectrascience device, there are other technologies that are seeking to enter the market as adjuncts to colposcopy, including devices from Dysis and Zedco. While these technologies are not direct competitors to LuViva, modifications to them or other new technologies will require us to develop devices that are more accurate, easier to use or less costly to administer so that our products have a competitive advantage.
In April 2014, the FDA approved the use of the Roche cobas HPV test as a primary screener for cervical cancer. Using a sample of cervical cells, the cobas HPV test detects DNA from 14 high-risk HPV types. The test specifically identifies HPV 16 and HPV 18, while concurrently detecting 12 other types of high-risk HPVs. This could make HPV testing a competitor to the Pap test. However, due to its lower specificity, we believe that screening with HPV will increase the number of false positive results if widely adopted.
In June 2006, the FDA approved the HPV vaccine Gardasil from drug maker Merck. Gardasil is a prophylactic HPV vaccine, meaning that it is designed to prevent the initial establishment of HPV infections. For maximum efficacy, it is recommended that girls receive the vaccine prior to becoming sexually active. Since Gardasil will not block infection with all of the HPV types that can cause cervical cancer, the vaccine should not be considered a substitute for routine Pap tests. On October 16, 2009, GlaxoSmithKline PLC was granted approval in the United States for a similar preventive HPV vaccine, known as Cervarix. Due to the limited availability and lack of 100% protection against all potentially cancer-causing strains of HPV, we believe that the vaccines will have a limited impact on the cervical cancer screening and diagnostic market for many years.
Government Regulation
The medical devices that we manufacture are subject to regulation by numerous regulatory bodies, including the FDA and comparable international regulatory agencies. These agencies require manufacturers of medical devices to comply with applicable laws and regulations governing the development, testing, manufacturing, labeling, marketing and distribution of medical devices. Devices are generally subject to varying levels of regulatory control, the most comprehensive of which requires that a clinical evaluation program be conducted before a device receives approval for commercial distribution.
| 31 |
In the European Union, medical devices are required to comply with the Medical Devices Directive and obtain CE Mark certification in order to market medical devices. The CE Mark certification, granted following approval from an independent “Notified Body,” is an international symbol of adherence to quality assurance standards and compliance with applicable European Medical Devices Directives. We maintain ISO 13485:2003 certification, which since 2014 has allowed us to issue our Edition 3 CE Mark and sell LuViva in the European Union and other markets.
Distributors of medical devices may also be required to comply with other foreign regulatory agencies, and we or our distributors currently have marketing approval for LuViva from Health Canada, COFEPRIS in Mexico, the Ministry of Health in Kenya, and the Singapore Health Sciences Authority. The time required to obtain these foreign approvals to market our products may be longer or shorter than that required in the United States, and requirements for those approvals may differ from those required by the FDA.
In the United States, permission to distribute a new device generally can be met in one of two ways. The first process requires that a pre-market notification (510(k) Submission) be made to the FDA to demonstrate that the device is as safe and effective as, or substantially equivalent to, a legally marketed device that is not subject to premarket approval (PMA). A legally marketed device is a device that (1) was legally marketed prior to May 28, 1976, (2) has been reclassified from Class III to Class II or I, or (3) has been found to be substantially equivalent to another legally marketed device following a 510(k) Submission. The legally marketed device to which equivalence is drawn is known as the “predicate” device. Applicants must submit descriptive data and, when necessary, performance data to establish that the device is substantially equivalent to a predicate device. In some instances, data from human clinical studies must also be submitted in support of a 510(k) Submission. If so, these data must be collected in a manner that conforms with specific requirements in accordance with federal regulations. The FDA must issue an order finding substantial equivalence before commercial distribution can occur. Changes to existing devices covered by a 510(k) Submission which do not significantly affect safety or effectiveness can generally be made by us without additional 510(k) Submissions.
The second process requires that an application for premarket approval (PMA) be made to the FDA to demonstrate that the device is safe and effective for its intended use as manufactured. This approval process applies to most Class III devices, including LuViva. In this case, two steps of FDA approval are generally required before marketing in the United States can begin. First, investigational device exemption (IDE) regulations must be complied with in connection with any human clinical investigation of the device in the United States. Second, the FDA must review the PMA application, which contains, among other things, clinical information acquired under the IDE. The FDA will approve the PMA application if it finds that there is a reasonable assurance that the device is safe and effective for its intended purpose.
We completed enrollment in our FDA pivotal trial of LuViva in 2008 and on November 18, 2010, the FDA accepted our completed PMA application, effective September 23, 2010, for substantive review. On March 7, 2011, we announced that the FDA had inspected two clinical trial sites as part of its review process and raised no formal compliance issues. On January 20, 2012, we announced our intent to seek an independent panel review of our PMA application after receiving a “not-approvable” letter from the FDA. On November 14, 2012 we filed an amended PMA with the FDA. On September 6, 2013, we received a letter from the FDA with additional questions and met with the FDA on May 8, 2014 to discuss our response. On July 25, 2014, we announced that we had responded to the FDA’s most recent questions
We received a “not-approvable” letter from the FDA on May 15, 2015. We had a follow up meeting with the FDA to discuss a path forward on November 30, 2015, at which we agreed to submit a detailed clinical protocol for FDA review so that additional studies can be completed. These studies will not be completed in 2016. We remain committed to obtaining FDA approval, but we are focused on international sales growth, where we believe the commercial opportunities are larger and the clinical need is more significant.
The process of obtaining clearance to market products is costly and time-consuming in virtually all of the major markets in which we sell, or expect to sell, our products and may delay the marketing and sale of our products. Countries around the world have recently adopted more stringent regulatory requirements, which are expected to add to the delays and uncertainties associated with new product releases, as well as the clinical and regulatory costs of supporting those releases. No assurance can be given that our products will be approved on a timely basis in any particular jurisdiction, if at all. In addition, regulations regarding the development, manufacture and sale of medical devices are subject to future change. We cannot predict what impact, if any, those changes might have on our business. Failure to comply with regulatory requirements could have a material adverse effect on our business, financial condition and results of operations.
| 32 |
Noncompliance with applicable requirements can result in import detentions, fines, civil penalties, injunctions, suspensions or losses of regulatory approvals or clearances, recall or seizure of products, operating restrictions, denial of export applications, governmental prohibitions on entering into supply contracts, and criminal prosecution. Failure to obtain regulatory approvals or the restriction, suspension or revocation of regulatory approvals or clearances, as well as any other failure to comply with regulatory requirements, would have a material adverse effect on our business, financial condition and results of operations.
Regulatory approvals and clearances, if granted, may include significant labeling limitations and limitations on the indicated uses for which the product may be marketed. In addition, to obtain regulatory approvals and clearances, the FDA and some foreign regulatory authorities impose numerous other requirements with which medical device manufacturers must comply. FDA enforcement policy strictly prohibits the marketing of approved medical devices for unapproved uses. Any products we manufacture or distribute under FDA clearances or approvals are subject to pervasive and continuing regulation by the FDA. The FDA also requires us to provide it with information on death and serious injuries alleged to have been associated with the use of our products, as well as any malfunctions that would likely cause or contribute to death or serious injury.
The FDA requires us to register as a medical device manufacturer and list our products. We are also subject to inspections by the FDA and state agencies acting under contract with the FDA to confirm compliance with good manufacturing practice. These regulations require that we manufacture our products and maintain documents in a prescribed manner with respect to manufacturing, testing, quality assurance and quality control activities. The FDA also has promulgated final regulatory changes to these regulations that require, among other things, design controls and maintenance of service records. These changes will increase the cost of complying with good manufacturing practice requirements.
We are also subject to a variety of other controls that affect our business. Labeling and promotional activities are subject to scrutiny by the FDA and, in some instances, by the U.S. Federal Trade Commission. The FDA actively enforces regulations prohibiting marketing of products for unapproved users. We are also subject, as are our products, to a variety of state and local laws and regulations in those states and localities where our products are or will be marketed. Any applicable state or local regulations may hinder our ability to market our products in those regions. Manufacturers are also subject to numerous federal, state and local laws relating to matters such as safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances. We may be required to incur significant costs to comply with these laws and regulations now or in the future. These laws or regulations may have a material adverse effect on our ability to do business.
We will be responsible for obtaining and maintaining regulatory approvals for our products. The inability or failure to comply with the varying regulations or the imposition of new regulations would materially adversely affect our business, financial condition and results of operations.
Employees and Consultants
As of March 31, 2016, we had ten regular employees and two consultants to provide services to us on a full- or part-time basis. Of the 12 people employed or engaged by us, 3 are engaged in engineering, manufacturing and development, 3 are engaged in sales and marketing activities, 1 is engaged in clinical testing and regulatory affairs, 1 are engaged in research and development activities, and 4 are engaged in administration and accounting. No employees are covered by collective bargaining agreements, and we believe we maintain good relations with our employees.
Our ability to operate successfully and manage our potential future growth depends in significant part upon the continued service of key scientific, technical, managerial and finance personnel, and our ability to attract and retain additional highly qualified personnel in these fields. Two of these key employees have an employment contract with us; none are covered by key person or similar insurance. In addition, if we are able to successfully develop and commercialize our products, we likely will need to hire additional scientific, technical, marketing, managerial and finance personnel. We face intense competition for qualified personnel in these areas, many of whom are often subject to competing employment offers. The loss of key personnel or our inability to hire and retain additional qualified personnel in the future could have a material adverse effect on our business, financial condition and results of operations.
Corporate History
We are a Delaware corporation, originally incorporated in 1992 under the name “SpectRx, Inc.,” and, on February 22, 2008, changed our name to Guided Therapeutics, Inc. At the same time, we renamed our wholly owned subsidiary, InterScan, which originally had been incorporated as “Guided Therapeutics.”
| 33 |
PROPERTIES
Our corporate offices, which also comprise our administrative, research and development, marketing and production facilities, are located at 5835 Peachtree Corners East, Suite D, Norcross, Georgia 30092, where we lease approximately 23,000 square feet under a lease that expires in June 2017.
LEGAL PROCEEDINGS
We are subject to claims and legal actions that arise in the ordinary course of business. However, we are not currently subject to any claims or actions that we believe would have a material adverse effect on our financial position or results of operations.
MARKET FOR OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Our common stock is dually quoted on the OTC Bulletin Board (OTCBB) and the OTCQB quotation systems under the ticker symbol “GTHP.” The number of record holders of our common stock at June 15, 2016 was 209. On February 24, 2016, we implemented a 1:100 reverse stock split of all of our issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock was converted into 1 share of common stock. All fractional shares created by the reverse stock split were rounded to the nearest whole share. The number of authorized shares of common stock did not change.
The high and low sales prices for the first and second quarter of 2016 and calendar years 2015 and 2014, as reported by the OTCBB, are as set forth in the following table. All share prices reflect the 1:100 reverse stock split of our common stock.
| 2016 | 2015 | 2014 | |||||||||||||
| High | Low | High | Low | High | Low | ||||||||||
| First Quarter | $ 1.690 | $ 0.107 | $ | 23.00 | $ | 14.00 | $ | 60.00 | $ | 46.00 | |||||
| Second Quarter* | $ 0.175 | $ 0.010 | $ | 25.00 | $ | 8.00 | $ | 60.00 | $ | 40.00 | |||||
| Third Quarter | $ | 12.00 | $ | 5.00 | $ | 50.00 | $ | 31.00 | |||||||
| Fourth Quarter | $ | 6.00 | $ | 1.00 | $ | 34.00 | $ | 21.00 | |||||||
*Through June 15, 2016.
Dividend Policy
We have not paid any dividends since our inception and do not intend to pay any dividends in the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation Plans
All the securities we have provided our employees, directors and consultants have been issued under our stock option plans, which are approved by our stockholders. We have issued common stock to other individuals that are not employees or directors, in lieu of cash payments, that are not part of any plan approved by our stockholders.
Securities authorized for issuance under equity compensation plans, as of June 15, 2016:
|
Plan category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||
| (a) | (b) | (c) | ||||
| Equity compensation plans approved by security holders |
104,356 |
$45.00 |
26,616 | |||
| Equity compensation plans not approved by security holders |
- |
- |
- | |||
| TOTAL | 104,356 | $45.00 | 26,616 |
| 34 |
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with our financial statements and notes thereto accompanying this prospectus.
Overview
We are a medical technology company focused on developing innovative medical devices that have the potential to improve healthcare. Our primary focus is the sales and marketing of our LuViva® Advanced Cervical Scan non-invasive cervical cancer detection device. The underlying technology of LuViva primarily relates to the use of biophotonics for the non-invasive detection of cancers. LuViva is designed to identify cervical cancers and precancers painlessly, non-invasively and at the point of care by scanning the cervix with light, then analyzing the reflected and fluorescent light.
LuViva provides a less invasive and painless alternative to conventional tests for cervical cancer screening and detection. Additionally, LuViva improves patient well-being not only because it eliminates pain, but also because it is convenient to use and provides rapid results at the point of care. We focus on two primary applications for LuViva: first, as a cancer screening tool in the developing world, where infrastructure to support traditional cancer-screening methods is limited or non-existent, and second, as a triage following traditional screening in the developed world, where a high number of false positive results cause a high rate of unnecessary and ultimately costly follow-up tests.
We are a Delaware corporation, originally incorporated in 1992 under the name “SpectRx, Inc.,” and, on February 22, 2008, changed our name to Guided Therapeutics, Inc. At the same time, we renamed our wholly owned subsidiary, InterScan, which originally had been incorporated as “Guided Therapeutics.”
Since our inception, we have raised capital through the public and private sale of debt and equity, funding from collaborative arrangements, and grants.
Our prospects must be considered in light of the substantial risks, expenses and difficulties encountered by entrants into the medical device industry. This industry is characterized by an increasing number of participants, intense competition and a high failure rate. We have experienced operating losses since our inception and, as of March 31, 2016 we have an accumulated deficit of about $122.9 million. To date, we have engaged primarily in research and development efforts and the early stages of marketing our products. We do not have significant experience in manufacturing, marketing or selling our products. We may not be successful in growing sales for our products. Moreover, required regulatory clearances or approvals may not be obtained in a timely manner, or at all. Our products may not ever gain market acceptance and we may not ever generate significant revenues or achieve profitability. The development and commercialization of our products requires substantial development, regulatory, sales and marketing, manufacturing and other expenditures. We expect our operating losses to continue through at least the end of 2016 as we continue to expend substantial resources to complete commercialization of our products, obtain regulatory clearances or approvals, build our marketing, sales, manufacturing and finance capabilities, and conduct further research and development.
Our product revenues to date have been limited. In 2015, the majority of our revenues were from the sale of LuViva devices and disposables, as well as some revenue from grants from the NIH and licensing agreement fees received. We expect that the majority of our revenue in 2016 will be derived from revenue from the sale of LuViva devices and disposables.
Recent Developments
Reverse Stock Split. On May 18, 2016, our board determined to recommend that at our 2016 annual stockholders’ meeting, our stockholders grant the board the authority, in its discretion, to effect a reverse stock split by a ratio of not less than 1:10 and not more than 1:400, with no change in the number of authorized shares of our common stock, and all fractional shares created by the reverse stock split to be rounded to the nearest whole share. On May 26, 2016, our largest stockholder, John Imhoff, who is also one of our directors, agreed to vote his shares of common stock in favor of the reverse stock split. As of the record date for the 2016 annual meeting, Dr. Imhoff held 19.26% of the outstanding shares of common stock. The reverse stock split will not be effective unless and until the board files an amendment to our certificate of incorporation, which must occur prior to the first anniversary of the 2016 annual meeting. It is our intent to effect the reverse stock split prior to the closing of this offering.
| 35 |
Warrant Restructuring. Between June 13, 2016 and June 14, 2016, we entered into various agreements with holders of certain warrants (including John Imhoff, the chairman of our board of directors) originally issued in May 2013, and with GPB Debt Holdings II LLC, holder of a warrant issued February 12, 2016, pursuant to which each holder separately agreed to exchange warrants for either (1) shares of common stock equal to 166% of the number of shares of common stock underlying the surrendered warrants, or (2) new warrants exercisable for 200% of the number of shares underlying the surrendered warrants, but without certain anti-dilution protections included with the surrendered warrants. As a result of the exchanges, we effectively eliminate any potential exponential increase in the number of underlying shares issuable upon exercise of our outstanding warrants. In total, for surrendered warrants then-exercisable for an aggregate of 115,237,788 shares of common stock (but subject to exponential increase upon operation of certain anti-dilution provisions), we issued or are obligated to issue 13,517,342 shares of common stock and new warrants that, if exercised as of June 14, 2016, would be exercisable for an aggregate of 214,189,622 shares of common stock.
In certain circumstances, in lieu of presently issuing all of the shares (for each holder that opted for shares of common stock), we and the holder further agreed that we will, subject to the terms and conditions set forth in the applicable warrant exchange agreement, from time to time, be obligated to issue the remaining shares to the holder. No additional consideration will be payable in connection with the issuance of the remaining shares.
The holders that elected to receive shares for their surrendered warrants have agreed that they will not sell shares on any trading day in an amount, in the aggregate, exceeding 20% of the composite aggregate trading volume of the common stock for that trading day.
The holders that elected to receive new warrants will be required to surrender their old warrants upon consummation of this offering. The new warrants will have an initial exercise price equal to the exercise price of the surrendered warrants as of immediately prior to consummation of this offering, subject to customary “downside price protection” for as long as our common stock is not listed on a national securities exchange, and will expire five years from the date of issuance.
Shenghuo License Agreement. On June 5, 2016, we entered into a license agreement with Shenghuo Medical, LLC pursuant to which we granted Shenghuo an exclusive license to manufacture, sell and distribute LuViva in Taiwan, Brunei Darussalam, Cambodia, Laos, Myanmar, Philippines, Singapore, Thailand, and Vietnam. Shenghuo was already our exclusive distributor in China, Macau and Hong Kong, and the license extends to manufacturing in those countries as well. Under the terms of the license agreement, once Shenghuo is capable of manufacturing LuViva in accordance with ISO 13485 for medical devices, Shenghuo will pay us a royalty equal to $2.00 or 20% of the distributor price (subject to a discount under certain circumstances), whichever is higher, per disposable distributed within Shenghuo’s exclusive territories.
In connection with the license grant, Shenghuo will underwrite the cost of securing approval of LuViva with Chinese Food and Drug Administration. At its option, Shenghuo also will provide up to $1.0 million in furtherance of our efforts to secure regulatory approval for LuViva from the U.S. Food and Drug Administration, in exchange for the right to receive payments equal to 2% of our future sales in the United States, up to an aggregate of $4.0 million.
Pursuant to the license agreement, Shenghuo has the option, after our next annual meeting of stockholders, to have a designee appointed to our board of directors.
As partial consideration for, and as a condition to, the license, and to further align the strategic interests of the parties, we have agreed to issue a convertible note to Shenghuo, in exchange for an aggregate cash investment of $200,000. The note will provide for a payment to Shenghuo of $240,000, due upon consummation of any capital raising transaction by us within 90 days and with net cash proceeds of at least $1.0 million. Absent such a transaction, the payment will increase to $300,000 and will be payable by December 31, 2016. The note will accrue interest at 20% per year on any unpaid amounts due after that date. The note will be convertible into shares of our common stock at a conversion price per share of $0.017402, subject to customary anti-dilution adjustment. The note will be unsecured, and is expected to provide for customary events of default. We will also issue Shenghuo a five-year warrant exercisable immediately for approximately 13.8 million shares of common stock at an exercise price equal to the conversion price of the note, subject to customary anti-dilution adjustment.
Short Term Promissory Notes. On May 4, 2016 and May 26, 2016, Aquarius Opportunity Fund advanced us a total of $87,500 for 2% simple interest notes due the earlier of December 31, 2016 or consummation of this offering. Also on May 26, 2016, GPB Debt Holdings II LLC, holder of our outstanding senior secured convertible note, advanced as an additional $87,500, on the same terms as the note. We intend to offer each of them the opportunity to participate in the offering at least up to the extent of the outstanding principal and interest on these cash advances, by extinguishing all or a portion of the debt on a dollar-for-dollar basis.
Series C Exchanges. Series C Exchanges. Between April 27, 2016 and May 3, 2016, we entered into various agreements with certain holders of our Series C preferred stock, including John Imhoff, one of our directors, pursuant to which those holders separately agreed to exchange each share of Series C preferred stock held for 2.25 shares of our newly created Series C1 preferred stock and 9,600 shares of our common stock. In connection with these exchanges, each holder also agreed to exchange the $1,000 stated value per share of the holder’s shares of Series C1 preferred stock for new securities that we issue in the next qualifying financing we undertake on a dollar-for-dollar basis. We expect this offering will be a qualifying financing. Finally, each holder agreed, except in the event of an additional $50,000 cash investment in the financing by the holder, to execute a customary “lockup” agreement in connection with the qualifying financing. In total, for 1,916 shares of Series C preferred stock surrendered, we issued 4,311 shares of Series C1 preferred stock and 18,396,800 shares of common stock.
| 36 |
The Series C1 preferred stock has terms that are substantially the same as the Series C preferred stock, except that the Series C1 preferred stock does not pay dividends (unless and to the extent declared on the common stock) or at-the-market “make-whole payments.” See “Description of Securities” for a description of the material terms of the Series C1 preferred stock.
Separately, on April 27, 2016, we entered into a rollover and amendment agreement with another holder of Series C preferred stock, Aquarius Opportunity Fund, pursuant to which Aquarius agreed to exchange any shares of Series C preferred stock (stated value plus make-whole dividend), as well as any remaining principal and accrued interest on our convertible promissory note Aquarius holds, for new securities that we issue in our next financing, all on a dollar-for-dollar basis, as long as the next financing involves at least $1 million in cash from investors unaffiliated with Aquarius. We expect this offering will be a qualifying financing. Aquarius also agreed to return to us for cancelation warrants exercisable for 7,148,813 shares of our common stock that it held. Except in the event of an additional $50,000 cash investment by Aquarius in the qualifying financing, Aquarius has agreed to execute a customary “lockup” agreement in connection with the financing. Finally, Aquarius, as the holder of a majority of the outstanding Series C preferred stock, agreed to amend the Series C stock purchase agreement to eliminate any participation rights held by the Series C shareholders and to waive operation of certain anti-dilution provisions of the Series C that would otherwise be triggered by the transactions described in “—Recent Developments.”
Critical Accounting Policies
Our material accounting policies, which we believe are the most critical to an investors understanding of our financial results and condition, are discussed below. Because we are still early in our enterprise development, the number of these policies requiring explanation is limited. As we begin to generate increased revenue from different sources, we expect that the number of applicable policies and complexity of the judgments required will increase.
Revenue Recognition: We recognize revenue from contracts on a straight line basis, over the terms of the contract. We recognize revenue from grants based on the grant agreement, at the time the expenses are incurred. Revenue from the sale of the Company’s products is recognized upon shipment of such products to its customers.
Valuation of Deferred Taxes: We account for income taxes in accordance with the liability method. Under the liability method, we recognize deferred assets and liabilities based upon anticipated future tax consequences attributable to differences between financial statement carrying amounts of assets and liabilities and their respective tax bases. We establish a valuation allowance to the extent that it is more likely than not that deferred tax assets will not be utilized against future taxable income.
Valuation of Equity Instruments Granted to Employee, Service Providers and Investors: On the date of issuance, the instruments are recorded at their fair value as determined using either the Black-Scholes valuation model or Monte Carlo Simulation model. See Note 4 to the consolidated financial statements accompanying this report for the assumptions used in the Black-Scholes valuation.
Allowance for Accounts Receivable: We estimate losses from the inability of our customers to make required payments and periodically review the payment history of each of our customers, as well as their financial condition, and revise our reserves as a result.
Inventory Valuation: All inventories are stated at lower of cost or market, with cost determined substantially on a “first-in, first-out” basis. Selling, general, and administrative expenses are not inventoried, but are charged to expense when purchased.
Reverse Stock Split: On February 24, 2016, we implemented a 1:100 reverse stock split of all of our issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock was converted into 1 share of common stock. All fractional shares created by the reverse stock split were rounded to the nearest whole share. The number of authorized shares of common stock did not change.
| 37 |
Results of Operations (except where indicated, all per share amounts reported on a post-February 2016 reverse stock split basis, but on a pre-__________ 2016 reverse stock split basis)
Comparison of the Three Months Ended March 31, 2016 and 2015
Sales Revenue, Cost of Sales and Gross Loss from Devices and Disposables: Sales revenue from the sale of LuViva devices and disposables for the three months ended March 31, 2016, was $262,000, a 106% increase compared to the same period in 2015. Related costs of sales and net realizable value expenses were approximately $68,000, which resulted in a gross profit of approximately $194,000. Sales revenue for the three months ended March 31, 2015, was approximately $127,000. Related costs of sales were approximately $107,000, which resulted in a gross loss on the device and disposables of approximately $20,000. The increase was related to additional sales of devices and disposables with the Company’s primary distributor, which carry a higher profit margin than unit sales.
Research and Development Expenses: Research and development expenses decreased to approximately $290,000 for the three months ended March 31, 2016, compared to $373,000 for the same period in 2015. The decrease, of approximately $83,000, was primarily due to a slight decrease in payroll expenses.
Sales and Marketing Expenses: Sales and marketing expenses were approximately $117,000 during the three months ended March 31, 2016, compared to $172,000 for the same period in 2015. The decrease was primarily due to the Company-wide expense reduction and cost savings efforts.
General and Administrative Expenses: General and administrative expenses decreased to approximately $917,000 during the three months ended March 31, 2016, compared to approximately $963,000 for the same period in 2015. The decrease of approximately $46,000, or 5.0%, is primarily related to lower compensation and option expenses incurred during the same period.
Other Income: Other income for the three months ended March 31, 2016, was approximately $23,000, compared to other income of approximately $21,000 for the three months ended March 31, 2015.
Interest Expense: Interest expense decreased to approximately $158,000 for the three months ended March 31, 2016, as compared to approximately $492,000 for the same period in 2015, primarily due to amortization of debt discount and debt issuance costs that were higher for the same period in 2015.
Fair Value of Warrants Expense: Fair value of warrants expense recovery was approximately $1,395,000 for the three months ended March 31, 2016, as compared to approximately $714,000 for the same period in 2015.
Net income was approximately $130,000 during the three months ended March 31, 2016, compared to a net loss of $1,245,000 for the same period in 2015, for the reasons outlined above.
Comparison of 2015 and 2014
General: Net loss attributable to common stockholders deceased to approximately $9.5 million, or $7.42 per share, in 2015, from $10.0 million, or $13.02 per share, in 2014.
Sales Revenue, Cost of Sales and Gross Loss from Devices and Disposables: Revenues from the sale of LuViva devices for 2015 and 2014 were approximately $564,000 and $758,000, respectively. Related costs of sales and valuation allowances on the net realizable values were approximately $537,000 and $891,000 in 2015 and 2014, respectively, which resulted in a gross profit of approximately $27,000 on the sales of devices and disposables for 2015 and a gross loss of approximately $133,000 for 2014.
Revenue from Grants and other Agreements: Revenue from grants and other agreements decreased to approximately $42,000 in 2015, from $65,000 in 2014, primarily due to fewer royalty receipts from certain agreements in 2015. There were no costs of sales associated with this revenue in 2015 and 2014.
Research and Development Expenses: Research and development expenses decreased to approximately $1.5 million for 2015, from approximately $2.8 million in 2014. The decrease was primarily due to reduction in research and development expenses due to product launch.
Sales and Marketing Expenses: Sales and marketing expenses decreased to approximately $718,000 in 2015, compared to approximately $1.2 million in 2014, due to an ongoing expense reduction program.
| 38 |
General and Administrative Expense: General and administrative expense decreased to approximately $4.1 million in 2015, from about $4.6 million in 2014. The decrease, of approximately 548,000 or 11.8%, was primarily due to an overall reduction in operating expenses in 2015.
Other Income: Other income was approximately $74,000 in 2015, compared to $25,000 in 2014. The increase was primarily related to income from a distribution agreement entered into in December 31, 2015.
Interest Expense: Interest expense increased to approximately $1.3 million for 2015, as compared to approximately $979,000 for 2014. The increase was primarily related to amortization expense of debt issuance cost and amortization of debt discount, in conjunction with our financing efforts in 2015.
Loss on Extinguishment of Debt: Loss on extinguishment of debt was zero for 2015, compared to approximately $325,000 for 2014. There was no debt extinguished in the year ended December 31, 2015.
Fair Value of Warrants Expense: Fair value of warrants recovery was approximately $568,000 for 2015, as compared to recovery of $65,000 for 2014. The change in fair value of warrants was primarily due to the significant changes in warrant conversion prices, in the fiscal year ended December 31, 2015.
There was no income tax benefit recorded for 2015 or 2014, due to recurring net operating losses.
Liquidity and Capital Resources
Since our inception, we have raised capital through the private sale of preferred stock and debt securities, public and private sales of common stock, funding from collaborative arrangements, and grants. At March 31, 2016, we had cash of approximately $56,000 and working capital deficit of approximately $4.0 million.
Our major cash flows in the quarter ended March 31, 2016, consisted of cash out-flows of approximately $955,000 from operations, no cash inflow nor outflow from investing activities and a net change from financing activities of $976,000, which primarily represents the proceeds received from the issuance of $1,437,500 in principal amount of a 17.0% senior secured convertible note, which resulted in net cash proceeds to the Company of $1,029,000.
Our major cash flows for the year ended December 31, 2015 consisted of cash out-flows of $4.0 million from operations, including approximately $6.9 million of net loss, cash outflows of $8,000 from investing activities and a net change from financing activities of $3.9 million, which primarily represented the proceeds received from issuance of common stock and warrants, proceeds from debt financing, as well as exercise of outstanding warrants and options.
On February 20, 2014, our President and CEO, Gene Cartwright, advanced us $50,000, in cash, for a 10% simple interest note. On October 23, 2014, August 4, 2014, and May 21, 2014, he advanced us $30,000, $200,000, and $100,000, respectively, in cash for 5% simple interest notes. On December 30, 2015, he advanced us $10,000 in cash for a 6% simple interest note. On October 24, 2014, October 7, 2014, and August 26, 2014, our Senior Vice President of Engineering, Richard Fowler, advanced us $6,100, $20,000 and $75,000, respectively, in cash for 6% simple interest notes. On October 7, 2014, our Director of Marketing advanced us $10,000 in cash for a 6% simple interest note. On November 4, 2014, Richard Blumberg, one of our stockholders, advanced us $100,000 in cash for a note for $106,500 in aggregate principal and interest due November 30, 2014. On February 20, 2014, directors Michael James, Linda Rosenstock, and John Imhoff advanced us $50,000, $50,000, $50,000, and $25,000 in cash, respectively, for 10% simple interest notes.
On April 23, 2014, we entered into a securities purchase agreement with an accredited investor for the issuance and sale of a 6% senior convertible note with an initial principal amount of $1.5 million and an 18-month term, for a purchase price of $1.0 million (an approximately 33.3% original issue discount). Additionally, pursuant to the purchase agreement, the investor purchased on May 23, 2014 an additional 6% senior convertible note with a principal amount of $2.0 million and an 18-month term, for a fixed purchase price of $2.0 million. On July 1, 2015, we repaid the entire outstanding balance.
On September 2, 2014, we entered into a subscription agreement with a Turkish corporation, pursuant to which, on September 27, 2014, we sold 1,651,042 shares of our common stock (pre-stock split) and a warrant to purchase an additional 325,521 shares (pre-stock split), for an aggregate purchase price of $200,000. The warrant is immediately exercisable, has an exercise price per share of $0.4608 (pre-stock split), and expires five years from the date of issuance.
| 39 |
On September 10, 2014, we entered into a note purchase agreement with an accredited investor pursuant to which we sold a secured promissory note with an initial principal amount of $1,275,000, for a purchase price of $700,000 (an original issue discount of $560,000). We may prepay the note at any time. The note is secured by our current and future accounts receivable and inventory, pursuant to a security agreement entered into in connection with the note purchase agreement. As a result of a series of amendments to the note (since assigned to two accredited investors), the maturity date currently is August 31, 2016, and has been accruing interest at a rate of the lesser of 18% per year or the maximum rate permitted by applicable law. Pursuant to the amendments, the holders may convert the outstanding balance into shares of our common stock, at a conversion price per share equal to the lower of (1) $0.25 (pre-stock split) and (2) 75% of the lowest daily volume weighted average price per share of our common stock during the five business days prior to conversion. If the conversion price at the time of any conversion request would be lower than $0.15(pre-stock split) per share, we have the option of delivering the conversion amount in cash in lieu of shares of our common stock.
On November 26, 2014, we entered into a securities purchase agreement with certain accredited investors providing for the issuance and sale in a public offering of an aggregate of 16,785,415 shares of our common stock (pre-stock split) and five-year warrants to purchase an aggregate of 8,392,708 additional shares (pre-stock split) at a purchase price of $0.225 per share (pre-stock split), for aggregate gross proceeds (including non-cash extinguishment of debt) of approximately $3.8 million. We consummated the public offering on December 2, 2014.
On March 16, 2015 and March 19, 2015, we entered into subscription agreements with certain accredited investors, pursuant to which we agreed to sell an aggregate of 4.0 million shares of our common stock (pre-stock split) and three-year warrants to purchase an additional 2.0 million shares (pre-stock split) with an exercise price per share of $0.255 (pre-stock split), for an aggregate purchase price of $720,000.
On June 29, 2015, we entered into a securities purchase agreement with certain accredited investors for the issuance and sale of an aggregate of 6,737 shares of our Series C preferred stock, at a purchase price of $750 per share and an initial conversion price of $0.095 per share (pre-stock split), and five-year warrants exercisable to purchase an aggregate of approximately 106.4 million shares of our common stock (pre-stock split) at an initial exercise price of $0.095 per share (pre-stock split), subject to certain customary adjustments and anti-dilution provisions. On September 3, 2015 we entered into an interim agreement amending the securities purchase agreement to provide for certain of the investors to purchase an additional aggregate of $550,000 in shares of Series C preferred stock and warrants on the same terms as set forth in the original agreement.
On February 24, 2016, we implemented a 1:100 reverse stock split of all of our issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock was converted into 1 share of common stock. All fractional shares created by the reverse stock split were rounded to the nearest whole share. The number of authorized shares of common stock did not change.
On February 11, 2016, we consented to an assignment of our outstanding secured promissory note to two accredited investors. In connection with the assignment, the holders waived an ongoing event of default under the notes related to our minimum market capitalization, and agreed to eliminate the requirement going forward. On March 7, 2016, we further amended the notes to eliminate the volume limitations on sales of common stock issued or issuable upon conversion of the notes.
On February 11, 2016, we entered into a securities purchase agreement with an accredited investor for the issuance and sale on February 12, 2016 of approximately $1.4 million in aggregate principal amount of a senior secured convertible note for an aggregate purchase price of $1.15 million (a 20% original issue discount). The note matures on the second anniversary of issuance and, in addition to the 20% original issue discount, accrues interest at a rate of 17% per year. Subject to certain restrictions, it is convertible at any time, in whole or in part, at the holder’s option, into shares of our common stock, at a conversion price equal to $0.80 per share, subject to certain customary adjustments and anti-dilution provisions. In addition, the investor received a five-year warrant exercisable to purchase an aggregate of approximately 1.79 million shares of our common stock with an exercise price of $0.80 per share, subject to certain customary adjustments and anti-dilution provisions.
In connection with the transaction, on February 12, 2016, we and the investor entered into a four-year consulting agreement, pursuant to which the investor will provide management consulting services to us in exchange for a royalty payment, payable quarterly, equal to 3.5% of our revenues from the sale of products.
See “—Recent Developments” for information regarding capital-raising activities since March 31, 2016.
We will be required to raise additional funds through public or private financing, additional collaborative relationships or other arrangements, as soon as possible. We cannot be certain that our existing and available capital resources will be sufficient to satisfy our funding requirements through the second quarter of 2016. We are evaluating various options to further reduce our cash requirements to operate at a reduced rate, as well as options to raise additional funds, including loans.
| 40 |
Substantial capital will be required to develop our products, including completing product testing and clinical trials, obtaining all required U.S. and foreign regulatory approvals and clearances, and commencing and scaling up manufacturing and marketing our products. Any failure to obtain capital would have a material adverse effect on our business, financial condition and results of operations.
Our financial statements have been prepared and presented on a basis assuming we will continue as a going concern. The above factors raise substantial doubt about our ability to continue as a going concern, as more fully discussed in Note 1 to the consolidated financial statements contained herein and in the report of our independent registered public accounting firm accompanying our financial statements.
Off-Balance Sheet Arrangements
We have no material off-balance sheet arrangements, no special purpose entities, and no activities that include non-exchange-traded contracts accounted for at fair value.
| 41 |
DIRECTORS AND EXECUTIVE OFFICERS
Our executive officers are elected by and serve at the discretion of our board of directors. The following table lists information about our directors and executive officers as of June 15, 2016:
| Name | Age | Position with Guided Therapeutics |
| Gene S. Cartwright, Ph.D. | 62 | Chief Execute Officer, President, Acting Chief Financial Officer and Director |
| Richard L. Fowler | 59 | Senior Vice President of Engineering |
| John E. Imhoff, M.D. | 67 | Director |
| Michael C. James | 57 | Chairman and Director |
| Jonathan M. Niloff, M.D. | 62 | Director |
| Linda Rosenstock, M.D. | 65 | Director |
Except as set forth below, all of the executive officers have been associated with us in their present or other capacities for more than the past five years. Officers are elected annually by the board of directors and serve at the discretion of the board. There are no family relationships among any of our executive officers and directors. On May 5, 2016 and May 9, 2016 respectively, Drs. Niloff and Rosenstock formally announced their decisions not to stand for reelection to our board of directors at our 2016 annual meeting of stockholders. Both of their decisions to resign were for personal reasons and were not a result of any disagreement with the Company on any matter relating to our operations, policies or practices.
Gene S. Cartwright, Ph.D. joined us in January 2014 as the President, Chief Executive Officer and Acting Chief Financial Officer. He was elected as a director on January 31, 2014. His most recent position was with Omnyx, LLC, a Joint Venture between GE Healthcare and the University of Pittsburgh Medical Center, where, as CEO for over four years he founded and managed the successful development of products for the field of Digital Pathology. Prior to his work with Omnyx, LLC, he was President of Molecular Diagnostics for GE Healthcare. Prior to GE, Dr. Cartwright was Divisional Vice President/General Manager for Abbott Diagnostics’ Molecular Diagnostics business. In his 24 year career at Abbott, he also served as Divisional Vice President for U.S. Marketing for five years. He received a Masters of Management degree from Northwestern’s Kellogg School of Management and also holds a Ph.D. in chemistry from Stanford University and an AB from Dartmouth College.
Dr. Cartwright brings over 30 years of experience working in the IVD diagnostics industry. He has great experience in the diagnostics market both in the development and introduction of new diagnostics technologies, as well as extensive successful commercial experience with global businesses. With his background and experience, Dr. Cartwright, as President and Chief Executive Officer, as well as Acting Chief Financial Officer, works with and advises the board as to how we can successfully market and build LuViva international sales.
Rick Fowler, Mr. Fowler, Senior Vice President of Engineering is an accomplished Executive with significant experience in the management of businesses that sell, market, produce and develop sophisticated medical devices and instrumentation. Mr. Fowler’s 25 plus years of experience includes assembling and managing teams, leading businesses and negotiating contracts, conducting litigation, and developing ISO, CE, FDA QSR, GMP and GCP compliant processes and products. He is adept at providing product life cycle management through effective process definition and communication - from requirements gathering, R&D feasibility, product development, product launch, production startup and support. Mr. Fowler combines outstanding analytical, out-of-the-box, and strategic thinking with strong leadership, technical, and communication skills and he excels in dynamic, demanding environments while remaining pragmatic and focused. He is able to deliver high risk projects on time and under budget as well as enhance operational effectiveness through outstanding cross-functional team leadership (R&D, marketing, product development, operations, quality assurance, sales, service, and finance). In addition, Mr. Fowler is well versed in global medical device regulatory and product compliance requirements.
John E. Imhoff, M.D. has served as a member of our Board of Directors since April 2006. Dr. Imhoff is an ophthalmic surgeon who specializes in cataract and refractive surgery. He is one of our principal stockholders and invests in many other private and public companies. He has a B.S. in Industrial Engineering from Oklahoma State University, an M.D. from the University of Oklahoma and completed his ophthalmic residency at the Dean A. McGee Eye Institute. He has worked as an ophthalmic surgeon and owner of Southeast Eye Center since 1983.
Dr. Imhoff has experience in clinical trials and in other technical aspects of a medical device company. His background in industrial engineering is especially helpful to us, especially as Dr. Imhoff can combine this knowledge with clinical applications. His experience in the investment community is invaluable to a public company often undertaking capital raising efforts.
| 42 |
Michael C. James has served as a member of our Board of Directors since March 2007 and as Chairman of the Board since October 15, 2013. Mr. James is also the Managing Partner of Kuekenhof Capital Management, LLC, a private investment management company, Chief Executive Officer and the Chief Financial Officer of Inergetics, Inc., a nutraceutical supplements company and also the Chief Financial Officer of Terra Tech Corporation, which is a hydroponic and agricultural company. He also holds the position of Managing Director of Kuekenhof Equity Fund, L.P. and Kuekenhof Partners, L.P. Mr. James currently sits on the Board of Directors of Inergetics; Inc. Mr. James was Chief Executive Officer of Nestor, Inc. from January 2009 to September 2009 and served on their Board of Directors from July 2006 to June 2009. He was employed by Moore Capital Management, Inc., a private investment management company from 1995 to 1999 and held position of Partner. He was employed by Buffalo Partners, L.P., a private investment management company from 1991 to 1994 and held the position of Chief Financial and Administrative Officer. He began his career in 1980 as a staff accountant with Eisner LLP. Mr. James received a B.S. degree in Accounting from Farleigh Dickinson University in 1980.
Mr. James has experience both in the areas of company finance and accounting, which is invaluable to us during financial audits and offerings. Mr. James has extensive experience in the management of both small and large companies and his entrepreneurial background is relevant as we develop as a company.
Jonathan M. Niloff, M.D. was elected as a director in April 2010. Dr. Niloff was Vice President and Chief Medical Officer at McKesson Technology Solutions, a medical software company until May 6, 2016. Prior to that, Dr. Niloff was the Founder, Chairman of the Board and Chief Medical Officer of MedVentive Inc. Prior to joining MedVentive, Dr. Niloff served as President of the Beth Israel Deaconess Physicians Organization, Medical Director for Obstetrics and Gynecology for its Affiliated Physicians Group, and Chief of Gynecology at New England Deaconess Hospital. He served as an Associate Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School. He has deep expertise in all aspects of medical cost and quality improvement, and has published extensively on the topic of gynecologic oncology including the development of the CA125 test for ovarian cancer. Dr. Niloff received his undergraduate education at The Johns Hopkins University, an M.D. degree from McGill University, and an MBA degree from Boston University.
Dr. Niloff is uniquely qualified to assist the Board and management because he combines his clinical background as a Harvard Ob-Gyn with his business acumen developed through an MBA degree and as CMO of MedVentive. Dr. Niloff has specific experience in evaluating new medical technology (e.g., CA125) and its implications to cost containment and reimbursement. Furthermore, Dr. Niloff has numerous professional contacts in the Ob-Gyn community that can aid in our development and marketing of our cervical cancer detection technology.
Linda Rosenstock, M.D. was appointed to the Board in April 2012. Dr. Linda Rosenstock is Dean Emeritus (served as Dean from 2000 - 2012) of the University of California, Los Angeles (UCLA) Fielding School of Public Health. She holds appointments at UCLA as Professor of Health Policy and Management, Medicine and Environmental Health Sciences and is a recognized authority in broad areas of public health and science policy. Internationally, Dr. Rosenstock has been active in teaching and research in many developing countries and has served as an advisor to the World Health Organization. Dr. Rosenstock also chaired the United Auto Workers/General Motors Occupational Health Advisory Board. She is an Honorary Fellow of the Royal College of Physicians and an elected member of the National Academy of Sciences' Institute of Medicine where she has served as a member of their Board on Health Sciences Policy and Chair of the Committee for Preventive Services for Women. In January 2011, she was appointed by President Obama to the Advisory Group on Prevention, Health Promotion and Integrative and Public Health. She has served on the Board of Directors for Skilled Health Care since 2009.
Before coming to UCLA in 2000, Dr. Rosenstock served as Director of the National Institute for Occupational Safety and Health (NIOSH) for nearly seven years. As Director of NIOSH, Dr. Rosenstock led the only federal agency with a mandate to undertake research and prevention activities in occupational safety and health. During her tenure, she was instrumental in creating the National Occupational Research Agenda, a framework for guiding occupational safety and health research, and in expanding the agency's responsibilities. In recognition of her efforts, Dr. Rosenstock received the Presidential Distinguished Executive Rank Award, the highest executive service award in the government and was also the James P. Keogh Award Winner for 2011 in appreciation of a lifetime of extraordinary leadership in occupational health and safety. Dr. Rosenstock received her M.D. and M.P.H. from The Johns Hopkins University. She conducted her advanced training at the University of Washington, where she was Chief Resident in Primary Care Internal Medicine and a Robert Wood Johnson Clinical Scholar.
Dr. Rosenstock is uniquely qualified as a Board Member for Guided Therapeutics. First, as a trained physician who has also chaired the Preventive Services for Women Committee of the National Academies’ Institute of Medicine, she has been directly involved in setting institutional and government policy for breast and cervical cancer screening, which is directly relevant to our LuViva cervical cancer detection device. Secondly, she brings a wealth of international experience in developing countries, which is a focus of our product distribution effort in cancer detection. Thirdly, she has demonstrated a lifetime of extraordinary leadership and her international recognition as an expert in health policy will provide outstanding credibility to Guided Therapeutics as a leading innovator in women’s healthcare.
| 43 |
CORPORATE GOVERNANCE
Board Meetings and Committees
Our board of directors held fourteen meetings in 2015. No director attended fewer than 75% of the meetings of the board of directors or the committees on which he or she served during 2015. We encourage our directors to attend the annual meeting of stockholders. In 2015, all of our directors attended our annual meeting. All of the members of the board of directors, with the exception of our Chief Executive Officer, serve on the audit, compensation and nomination committees. Although we are not subject to the listing standards of any national securities exchange or inter-dealer quotation system, based on the definition of independence in the NASDAQ listing standards, Dr. Imhoff, Mr. James, Dr. Niloff and Dr. Rosenstock are independent directors. The board works with its members and management to identify new board members, and will consider nominees recommended by stockholders. Any recommendation should be addressed in writing to the Board of Directors, c/o Corporate Secretary, 5835 Peachtree Corners East, Suite D, Norcross, Georgia 30092.
The audit committee selects and engages the independent registered public accounting firm to audit our annual financial statements and pre-approves all allowable audit services and any special assignments given to the accountants. The audit committee also determines the planned scope of the annual audit, any changes in accounting principles, the effectiveness and efficiency of our internal accounting staff and the independence of our external auditors. The audit committee met fourteen times in 2015. The audit committee currently consists of Dr. Imhoff, Mr. James and Drs. Niloff, and Rosenstock. Each member of the audit committee is independent in accordance with the NASDAQ listing standards for audit committee independence and applicable SEC regulations. None of the members of the audit committee has participated in the preparation of our financial statements at any time during the past three years. The board has also determined that Mr. James and Drs. Niloff, Imhoff, and Rosenstock meet the criteria specified under applicable SEC regulations for an “audit committee financial expert” and that the committee members are financially sophisticated.
The board of directors, in consultation with our Chief Executive Officer, sets the compensation for our officers, reviews management organization and development, reviews significant employee benefit programs and establishes and administers executive compensation programs. The compensation committee currently consists of Dr. Imhoff, Mr. James, Drs. Niloff and Rosenstock, each of whom is independent under NASDAQ listing standards. The compensation committee met fourteen times in 2015.
The board of directors, in consultation with our Chief Executive Officer, reviews and recommends individuals to be nominated as directors. Our board has historically evaluated all candidates based upon, among other factors, a candidate’s financial literacy, knowledge of our industry or other background relevant to our needs, status as a stakeholder, independence, and willingness, ability and availability for service. Other than the foregoing, there have been no stated minimum criteria for director nominees, although our board has considered such other factors as it has deemed to be in the best interests of us and our stockholders. The board has considered diversity as it has deemed appropriate in this context (without having a formal diversity policy), given current needs and the current needs of the board to maintain a balance of knowledge, experience and capability. When considering diversity, the board has considered diversity as one factor, of no greater or lesser importance than other factors and has considered diversity in a broad context of race, gender, age, business experience, skills, international experience, education, other board experience and other relevant factors.
The audit committee and the compensation committee have each adopted charters, which are available on our web site, at www.guidedinc.com/Investors.htm. The nomination committee currently operates without a charter.
Board Leadership Structure and Role in Risk Oversight
Dr. Cartwright, our President and Chief Executive Officer, also serves as a director; our board is led by the Chairman, Mr. James, one of our independent directors. Our board, as a whole, has responsibility for risk oversight, with reviews of certain areas being conducted by the relevant board committees that report on their deliberations to the full board, as further described below. In addition, our management regularly communicates with the board to discuss important risks for their review and oversight, including regulatory risk and risks stemming from periodic litigation or other legal matters in which we are involved. Given the small size of the board, the board feels that this structure for risk oversight is appropriate (except for those risks that require risk oversight by independent directors only).
| 44 |
The board of directors is specifically charged with discussing risk management (primarily financial and internal control risk), and receives regular reports from management, independent auditors, internal audit and outside legal counsel on risks related to, among others, our financial controls and reporting. The board of directors reviews risks related to compensation and makes recommendations to the board with respect to whether our compensation policies are properly aligned to discourage inappropriate risk-taking, and is regularly advised by management and, as deemed appropriate, outside legal counsel.
Communication with Directors
Any stockholder is welcome to communicate with any director or the board of directors by writing to a director or the board as a whole, c/o Corporate Secretary, 5835 Peachtree Corners East, Suite D, Norcross, Georgia 30092.
Director Compensation
None of our directors received any compensation or reimbursement in cash in 2015; however, they did receive stock options or shares, in lieu of cash, for 2015 and 2014, respectively, in connection with their services as members of the board of directors and their service on board committees.
Director Compensation Table, for year ended December 31, 2015
| Name and Principal Position |
Stock Option Awards (#) |
|||
| Michael C. James, Chairman and Director | 6,000 | |||
| John E. Imhoff, M.D., Director | 6,000 | |||
| Jonathan M. Niloff, M.D., Director | 6,000 | |||
| Linda Rosenstock, M.D., Director | 6,000 |
EXECUTIVE COMPENSATION
The purpose of our compensation philosophy, policies and practices is to attract and retain experienced, highly qualified executives critical to our long-term success and enhancement of stockholder value. We rely on a combination of base salary and customary benefits along with long-term equity incentive compensation to achieve these objectives. In establishing compensation packages for our executive officers, the compensation committee believes that we provide our executive officers with the opportunity to earn a competitive annual base salary, and that options grants reward our executive officers for meaningful performance that contributes to enhanced long-term stockholder value and our general long-term financial health, and helps to align the interests of our executive officers with those of our stockholders.
Summary Compensation Table
The following table lists specified compensation we paid or accrued during each of the fiscal years ended December 31, 2015 and 2014 to the Chief Executive Officer and our two other most highly compensated executive officers, collectively referred to as the “named executive officers,” in 2015:
2015 and 2014 Summary Compensation Table
|
Name and Principal Position |
Year |
Salary ($) |
Bonus ($) |
Option Awards ($)(1) |
Total ($) |
|
Gene S. Cartwright, Ph.D. President, CEO, Acting CFO and Director (2) |
2015 | 300,000 | 150,000 | - | $450,000 |
| 2014 | 300,000 | 150,000 | $731,000 | $1,046,000 | |
|
Mark Faupel, Ph.D. Former President, CEO and Acting CFO(3) |
2015 | 198,073 | - | $30,400 | 228,473 |
| 2014 | 264,097 | 13,600 | 17,000 | 294,697 | |
|
Richard Fowler, Senior Vice President of Engineering |
2015 2014 |
243,000 203,000 |
- - |
$30,880 17,000 |
273,880 220,000 |
| (1) | See Note 4 to the audited consolidated financial statements that accompany this prospectus. |
| (2) | All amounts reported as accrued. Dr. Cartwright has elected to get paid partial salary, due to the Company’s cash position. |
| (3) | Dr. Faupel is no longer employed by the Company, but instead provides consulting services to the Company. |
| 45 |
Dr. Cartwright’s 2015 and 2014 compensation consisted of a base salary of $300,000, with $150,000 performance condition related bonus, and the usual customary company benefits. He also received 20,000 both market and performance condition restricted shares of common stock (10,000 shares if the stock price closes at/above $1.50 for 30 consecutive trading days (the “Tier 1 Vesting Date”); subject to the Executive’s continuous employment with the Company through the applicable vesting date; (i) 5,000 shares will vest on the Tier 1 Vesting Date; and (ii) 5,000 shares will vest on the first anniversary of the Tier 1 Vesting Date. 10,000 GT stock price closes at/above $250.00 for 30 consecutive trading days (the “Tier 2 Vesting Date”); subject to the Executive’s continuous employment with the Company through the applicable vesting date: (i) 5,000 shares will vest on the Tier 2 Vesting Date; and (ii) 5,000 shares will vest on the first anniversary of the Tier 2 Vesting Date). Dr. Cartwright was also issued 350 and 2,500 of stock options that vest over 48 months in 2014. As of December 31, 2015, Dr. Cartwright’s deferred salary plus interest was $282,734 and his deferred bonus was $300,000.
Dr. Faupel’s 2015 and 2014 compensation consisted of a base salary of $198,073 and $264,097, respectively, and usual and customary company benefits. He received no bonus in the years ended December 31, 2015 and 2014. He received 1,900 and 350 shares of stock options, which vest over 48 months in 20154 and 2014, respectively. As of December 31, 2015, Dr. Faupel’s remaining deferred salary plus interest and bonus was $53,433. He also holds a promissory note of $250,512 for past un-paid salary.
Mr. Fowler’s 2015 and 2014 compensation consisted of a base salary of $243,000 and $203,000, respectively, and usual and customary company benefits. He received no bonus in the years ended December 31, 2015 and 2014. He received 1,930 and 350 shares of stock options, which vest over 48 months in 2015 and 2014, respectively. As of December 31, 2015, Mr. Fowler’s total deferred salary plus interest was approximately $247,469.
Outstanding Equity Awards to Officers at December 31, 2015
| Option Awards | |||||
|
Name and Principal Position |
Number of Securities Underlying Options Exercisable (#)(1) |
Number of Securities Underlying Options Un-exercisable (#) |
Equity Incentive Plan Awards: Number of Securities Under- lying Unexercised Unearned Options (#) |
Option Exercise Price ($)(2) |
Option Expiration Date |
|
Gene S. Cartwright, Ph.D. President, CEO, Acting CFO and Director |
756 | - | 2,094 | 27.00 | 12/31/2024 |
|
Mark Faupel, Ph.D. Former President, CEO & Acting CFO |
21,058 | - | 1,842 | 72.00 | 12/31/2024 |
|
Richard Fowler Senior Vice President of Engineering |
6,173 | - | 1,977 | 59.00 | 12/31/2024 |
| (1) | Represents fully vested options. |
| (2) | Based on all outstanding options. |
Outstanding Equity Awards to Directors at December 31, 2015
| Option Awards | ||
| Name and Principal Position |
Option Awards (#) |
Exercise Price ($) |
| Ronald W. Hart, Ph.D., Director (resigned as of December 11, 2015) | 10,925 | 22.00 |
| John E. Imhoff, M.D., Director | 9,038 | 33.00 |
| Michael C. James, Chairman and Director | 7,075 | 20.00 |
| Jonathan Niloff, M.D., Director | 7,429 | 22.00 |
| Linda Rosenstock, M.D., Director | 7,250 | 21.00 |
| 46 |
SHARE OWNERSHIP OF DIRECTORS, OFFICERS AND CERTAIN BENEFICIAL OWNERS
The following table lists information regarding the beneficial ownership of our common stock as of June 15, 2016 by (1) each person whom we know to beneficially own more than 5% of the outstanding shares of our common stock, (2) each director, (3) each officer named in the summary compensation table above, and (4) all directors and executive officers as a group. Unless otherwise indicated, the address of each officer and director is 5835 Peachtree Corners East, Suite D. Norcross, Georgia 30092.
|
Name and Address of Beneficial Owner |
Amount and Nature of Beneficial Ownership (1) |
Percent of Class (2) | |||
| John E. Imhoff (3) | 186,727,185 | 76.44 | % | ||
| Michael C. James / Kuekenhof Equity Fund, LLP (4) | 16,884 | * | |||
| Gene Cartwright (5) | 26,379 | * | |||
| Richard L. Fowler (6) | 7,224 | * | |||
| Linda Rosenstock (7) | 10,123 | * | |||
| Jonathan Niloff (8) | 10,828 | * | |||
| All directors and executive officers as a group (6 persons) (9) | 186,798,623 | 76.46 | % | ||
| (*) | Less than 1%. |
| (1) | Except as otherwise indicated in the footnotes to this table and pursuant to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock. |
| (2) | Percentage ownership is based on 67,901,547 shares of common stock outstanding as of the record date. Beneficial ownership is determined in accordance with the rules of the SEC, based on factors that include voting and investment power with respect to shares. Shares of common stock subject to convertible securities convertible or exercisable within 60 days after the record date, are deemed outstanding for purposes of computing the percentage ownership of the person holding those securities, but are not deemed outstanding for purposes of computing the percentage ownership of any other person. |
| (3) | Consists of 10,361,179 shares of common stock, 168,781,637 shares issuable upon conversion of 2,400.75 shares of Series C1 preferred stock, 7,575,331 shares issuable upon exercise of warrants, and 9,038 shares subject to options. As of the record date, Dr. Imhoff is on the board of directors. |
| (4) | Consists of 7,451 shares of common stock, 2,357 shares issuable upon exercise of warrants, and 7,076 shares subject to options. As of the record date, Mr. James is on the board of directors. |
| (5) | Consists of 22,732 shares of common stock, 2,357 shares issuable upon exercise of warrants, and 1,290 shares subject to options. As of the record date, Mr. Cartwright is the CEO and on the board of directors. |
| (6) | Consists of 981 shares of common stock and 6,243 shares subject to options. |
| (7) | Consists of 2,872 shares of common stock and 7,251 shares subject to options. As of the record date, Dr. Rosenstock is on the board of directors. |
| (8) | Consists of 3,399 shares of common stock and 7,429 shares subject to options. As of the record date, Dr. Niloff is on the board of directors. |
| (9) | Consists of 10,398,614 shares of common stock, 168,781,637 shares issuable upon conversion of 2,400.75 shares of Series C1 preferred stock, 7,580,046 shares issuable upon exercise of warrants, and 38,327 shares subject to options. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
AND DIRECTOR INDEPENDENCE
Our board recognizes that related person transactions present a heightened risk of conflicts of interest. The audit committee has the authority to review and approve all related party transactions involving our directors or executive officers.
Under the policy, when management becomes aware of a related person transaction, management reports the transaction to the audit committee and requests approval or ratification of the transaction. Generally, the audit committee will approve only related party transactions that are on terms comparable to those that could be obtained in arm’s length dealings with an unrelated third person. The audit committee will report to the full board all related person transactions presented to it.
Based on the definition of independence of the NASDAQ Stock Market, the board has determined that Mr. James, and Drs. Niloff, Imhoff and Rosenstock are independent directors.
In September 2015, Dr. Imhoff participated in our Series C preferred stock issuance by exchanging his shares of Series B preferred stock and investing $300,000 in cash, for a total of 1,067 shares of Series C preferred stock and warrants to purchase 16,847,368 shares of common stock. In April 2016, Dr. Imhoff exchanged his shares of Series C preferred stock for a total of 2400.75 shares of Series C1 preferred stock and 10,243,200 shares of common stock. See “Summary—Recent Developments.”
| 47 |
LEGAL MATTERS
Jones Day, Atlanta, Georgia, passed upon the validity of the shares of common stock that may be offered by this prospectus. Ellenoff Grossman & Schole LLP, New York, New York, acted as counsel to the placement agent.
EXPERTS
Our consolidated financial statements as of December 31, 2015 and 2014, and for the years then ended have been audited by UHY LLP, an independent registered public accounting firm, as set forth in its report, included in this prospectus. Our financial statements and the related independent registered public accounting firm report thereon have been included herein in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN GET MORE INFORMATION
We have filed with the SEC under the Securities Act a registration statement on Form S-1 of which this prospectus forms a part. This prospectus does not contain all of the information contained in the registration statement and its exhibits. We strongly encourage you to read carefully the registration statement and its exhibits.
Any statement made in this prospectus concerning the contents of any contract, agreement or other document is only a summary of the actual contract, agreement or other document. If we have filed any contract, agreement or other document as an exhibit to the registration statement, you should read the exhibit for a more complete understanding of the document or matter involved.
We file annual, quarterly and current reports; proxy statements and other information with the SEC. You may read and copy any of this information at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for information on the operation of the Public Reference Room. The SEC also maintains an Internet website that contains reports, proxy statements and other information regarding issuers, including us, who file electronically with the SEC. The address of that site is http://www.sec.gov. The information contained on the SEC’s website is expressly not incorporated by reference into this prospectus.
| 48 |
INDEX TO FINANCIAL STATEMENTS
| Annual Consolidated Financial Statements | |
| Report of Independent Registered Public Accounting Firm | F-2 |
| Consolidated Balance Sheets as of December 31, 2015 and 2014 | F-3 |
| Consolidated Statements of Operations for the Years Ended December 31, 2015 and 2014 | F-4 |
| Consolidated Statements of Stockholders’ Deficit for the Years Ended December 31, 2015 and 2014 | F-5 |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2015 and 2014 | F-6 |
| Notes to Consolidated Financial Statements | F-8 |
| Unaudited Condensed Consolidated Financial Statements | |
| Condensed Consolidated Balance Sheets as of March 31, 2016 (unaudited) and December 31, 2015 | F-3 |
| Condensed Consolidated Statements of Operations (unaudited) for the Three Months Ended March 31, 2016 and 2015 | F-4 |
| Condensed Consolidated Statements of Cash Flows (unaudited) for the Three Months Ended March 31, 2016 and 2015 | F-6 |
| Notes to Condensed Consolidated Financial Statements | F-8 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and
Stockholders of Guided Therapeutics, Inc.
We have audited the accompanying consolidated balance sheets of Guided Therapeutics, Inc. and Subsidiary (the “Company”) as of December 31, 2015 and 2014, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for the years then ended. The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Guided Therapeutics, Inc. and Subsidiary as of December 31, 2015 and 2014, and the results of their operations and their cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
As described in Note 1 to the consolidated financial statements, the accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. The Company’s recurring losses from operations and accumulated deficit raise substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters are also discussed in Note 1 to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ UHY LLP
UHY LLP
Sterling Heights, Michigan
March 15, 2016
F-2
| GUIDED THERAPEUTICS, INC. AND SUBSIDIARY | ||||||||
| CONSOLIDATED BALANCE SHEETS | ||||||||
| AS OF DECEMBER 31, 2015 AND 2014 | ||||||||
| (In Thousands) | ||||||||
| ASSETS | 2015 | 2014 | ||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 35 | $ | 162 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $95 and $76 at December 31, 2015 and 2014, respectively | 190 | 338 | ||||||
| Inventory, net of reserves of $118 and $144 at December 31, 2015 and 2014, respectively | 1,119 | 1,180 | ||||||
| Other current assets | 780 | 99 | ||||||
| Total current assets | 2,124 | 1,779 | ||||||
| Property and equipment, net | 318 | 587 | ||||||
| Debt issuance costs | 48 | 564 | ||||||
| Other assets | 73 | 101 | ||||||
| Total noncurrent assets | 806 | 1,252 | ||||||
| TOTAL ASSETS | 2,563 | $ | 3,031 | |||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Short-term notes payable, including related parties current portion of long term debt | 752 | 646 | ||||||
| Note payable, in default | 133 | 123 | ||||||
| Short-term convertible notes payable, net of discount | 686 | 1,062 | ||||||
| Accounts payable | 1,824 | 1,733 | ||||||
| Accrued liabilities | 1,907 | 1,015 | ||||||
| Deferred revenue | 217 | 24 | ||||||
| Total current liabilities | 5,519 | 4,603 | ||||||
| Warrants, at fair value | 2,606 | 2,070 | ||||||
| Long-term debt, net | — | 40 | ||||||
| Convertible note, net of discount | — | 783 | ||||||
| Total long-term liabilities | 2,606 | 2,893 | ||||||
| TOTAL LIABILITIES | 8,125 | 7,496 | ||||||
| COMMITMENTS & CONTINGENCIES (Note 6) | ||||||||
| STOCKHOLDERS’ DEFICIT: | ||||||||
| Series B convertible preferred stock, $.001 par value; 3 shares authorized, zero and 1.2 shares issued and outstanding as of December 31, 2015 and 2014, respectively (liquidation preference of none in December 31, 2015 and $1,200 at December 31, 2014, respectively) | — | 678 | ||||||
| Series C convertible preferred stock, $.001 par value; 9.0 shares authorized, 5.6 shares issued and outstanding as of December 31, 2015 and none authorized or issued and outstanding at December 31, 2014. (Liquidation preference of $5,555 at December 31, 2015 and none at December 31, 2014). | 2,052 | — | ||||||
| Common stock, $.001 par value; 1,000,000 and 195,000 shares authorized, 2,371 and 969 shares issued and outstanding as of December 31, 2015 and 2014, respectively | 236 | 97 | ||||||
| Additional paid-in capital | 114,845 | 107,952 | ||||||
| Treasury stock, at cost | (132 | ) | (132 | ) | ||||
| Accumulated deficit | (122,563 | ) | (113,060 | ) | ||||
| TOTAL GUIDED THERAPEUTICS STOCKHOLDERS’ DEFICIT | (5,562 | ) | (4,465 | ) | ||||
| TOTAL STOCKHOLDERS’ DEFICIT | (5,562 | ) | (4,465 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 2,563 | $ | 3,031 | ||||
| The accompanying notes are an integral part of these consolidated statements. | ||||||||
| F-3 |
| GUIDED THERAPEUTICS, INC. AND SUBSIDIARY | ||||||||
| CONSOLIDATED STATEMENTS OF OPERATIONS | ||||||||
| FOR THE YEARS ENDED DECEMBER 31, 2015 AND 2014 | ||||||||
| (In Thousands ) | ||||||||
| 2015 | 2014 | |||||||
| REVENUE: | ||||||||
| Sales – devices and disposables | $ | 564 | $ | 758 | ||||
| Cost of goods sold | 537 | 891 | ||||||
| Gross profit (loss) | 27 | (133 | ) | |||||
| Contract and grant revenue | 42 | 65 | ||||||
| OPERATING EXPENSES: | ||||||||
| Research and development | 1,477 | 2,788 | ||||||
| Sales and marketing | 718 | 1,164 | ||||||
| General and administrative | 4,101 | 4,649 | ||||||
| Total operating expenses | 6,296 | 8,601 | ||||||
| Operating loss | (6,227 | ) | (8,669 | ) | ||||
| OTHER INCOME (EXPENSES): | ||||||||
| Other income | 74 | 25 | ||||||
| Interest expense | (1,317 | ) | (979 | ) | ||||
| Loss on extinguishment of debt | — | (325 | ) | |||||
| Change in fair value of warrants | 568 | 65 | ||||||
| Total other expenses | (675 | ) | (1,214 | ) | ||||
| LOSS FROM OPERATIONS | (6,902 | ) | (9,883 | ) | ||||
| PROVISION FOR INCOME TAXES | — | — | ||||||
| NET LOSS | (6,902 | ) | (9,883 | ) | ||||
| DEEMED DIVIDENDS | (1,263 | ) | — | |||||
| PREFERRED STOCK DIVIDENDS | (1,338 | ) | (152 | ) | ||||
| NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS | $ | (9,503 | ) | $ | (10,035 | ) | ||
BASIC AND DILUTED NET LOSS PER SHARE ATTRIBUTABLE TO COMMON STOCKHOLDERS (post 1:100 reverse stock split) | $ | (7.42 | ) | $ | (13.02 | ) | ||
| WEIGHTED AVERAGE SHARES OUTSTANDING | 1,280 | 771 | ||||||
| The accompanying notes are an integral part of these consolidated statements. | ||||||||
| F-4 |
GUIDED THERAPEUTICS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
FOR THE YEARS ENDED DECEMBER 31, 2015 AND 2014 (In Thousands)
Preferred Stock Series B | Preferred Stock Series C |
Common Stock | Additional Paid-In |
Treasury |
Accumulated | |||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Stock | Deficit | TOTAL | |||||||||||||||||||||||||||||||
| BALANCE, January 1, 2014 | 2 | $ | 1,139 | — | $ | — | 705 | $ | — | $ | 101,840 | $ | (132 | ) | $ | (103,025 | ) | $ | (107 | ) | ||||||||||||||||||||
| Preferred dividends | — | — | — | — | — | — | — | — | (152 | ) | (152 | ) | ||||||||||||||||||||||||||||
| Conversion of preferred stock | (1 | ) | (461 | ) | — | — | 23 | 2 | 459 | — | — | — | ||||||||||||||||||||||||||||
| Issuance of common stock and warrants | — | — | — | — | 209 | 21 | 3,348 | — | — | 3,369 | ||||||||||||||||||||||||||||||
| Exercise of warrants and options into common stock | — | — | — | — | 4 | — | 96 | — | — | 96 | ||||||||||||||||||||||||||||||
| Conversion of debt into common stock | — | — | — | — | 28 | 3 | 890 | — | — | 893 | ||||||||||||||||||||||||||||||
| Issuance of warrants and options | — | — | — | — | — | — | 433 | — | — | 433 | ||||||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | — | — | 886 | — | — | 886 | ||||||||||||||||||||||||||||||
| Net Loss | — | — | — | — | — | — | — | — | (9,883 | ) | (9,883 | ) | ||||||||||||||||||||||||||||
| BALANCE, December 31, 2014 | 1 | $ | 678 | — | — | 969 | 97 | 107,952 | (132 | ) | (113,060 | ) | (4,465 | ) | ||||||||||||||||||||||||||
Preferred Stock Series B | Preferred Stock Series C |
Common Stock | Additional Paid-In |
Treasury |
Accumulated | |||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Stock | Deficit | TOTAL | |||||||||||||||||||||||||||||||
| BALANCE, January 1, 2015 | 1 | $ | 678 | — | $ | — | 969 | $ | 97 | $ | 107,952 | $ | (132 | ) | $ | (113,060 | ) | $ | (4,465 | ) | ||||||||||||||||||||
| Preferred dividends | — | — | — | — | — | — | — | — | (352 | ) | (352 | ) | ||||||||||||||||||||||||||||
| Conversion of Series C preferred stock to common stock | — | — | (2 | ) | (840 | ) | 990 | 99 | 1,727 | — | (986 | ) | — | |||||||||||||||||||||||||||
| Issuance of common stock and warrants | — | — | — | — | 106 | 11 | 1,327 | — | — | 1,338 | ||||||||||||||||||||||||||||||
| Exercise of warrants and options for common stock | — | — | — | — | 111 | 11 | 132 | — | — | 143 | ||||||||||||||||||||||||||||||
| Conversion of debt into common stock | — | — | — | — | 168 | 15 | 999 | — | — | 1,014 | ||||||||||||||||||||||||||||||
| December 2014 public offering warrants exchange and common shares issuance | — | — | — | — | 27 | 3 | 1,368 | — | (1,049 | ) | 322 | |||||||||||||||||||||||||||||
| Series B, Tranche A, warrant price adjustment | — | — | — | — | — | — | 64 | — | (64 | ) | — | |||||||||||||||||||||||||||||
| Series C preferred stock and warrant issuance | (1 | ) | (678 | ) | 8 | 2,892 | — | — | 268 | — | (150 | ) | 2,332 | |||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | 1,008 | — | — | 1,008 | ||||||||||||||||||||||||||||||||
| Net Loss | — | — | — | — | — | — | (6,902 | ) | (6,902 | ) | ||||||||||||||||||||||||||||||
| BALANCE, December 31, 2015 | — | $ | — | 6 | $ | 2,052 | 2,371 | $ | 236 | $ | 114,845 | $ | (132 | ) | $ | (122,563 | ) | $ | (5,562 | ) | ||||||||||||||||||||
The accompanying notes are an integral part of these consolidated statements.
F-5
| GUIDED THERAPEUTICS, INC. AND SUBSIDIARY | ||||||||
| CONSOLIDATED STATEMENTS OF CASH FLOWS | ||||||||
| FOR THE YEARS ENDED DECEMBER 31, 2015 AND 2014 | ||||||||
| (In Thousands) | ||||||||
| 2015 | 2014 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (6,902 | ) | $ | (9,883 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Bad debt expense | 19 | 63 | ||||||
| Depreciation and Amortization | 1,054 | 1,240 | ||||||
| Stock-based compensation | 1,008 | 886 | ||||||
| Non-employee stock based compensation | 400 | — | ||||||
| Change in fair value of warrants | (568 | ) | (65 | ) | ||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | 129 | (267 | ) | |||||
| Inventory | 61 | 14 | ||||||
| Other current assets | (681 | ) | 2 | |||||
| Other assets | 28 | 254 | ||||||
| Accounts payable | 91 | 842 | ||||||
| Deferred revenue | 193 | 10 | ||||||
| Accrued liabilities | 1,125 | 296 | ||||||
| Total adjustments | 2,859 | 3,600 | ||||||
| Net cash used in operating activities | (4,043 | ) | (6,283 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Additions to fixed assets | (8 | ) | (150 | ) | ||||
| Net cash used in investing activities | (8 | ) | (150 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Net proceeds from issuance of preferred stock and warrants, net | 3,698 | — | ||||||
| Net proceed from issuance of common stock and warrants | 720 | 1,730 | ||||||
| Proceeds from debt financing, net of discount | 425 | 5,263 | ||||||
| Payment for debt issuance costs | (48 | ) | (452 | ) | ||||
| Payments on notes | (1,014 | ) | (656 | ) | ||||
| Proceeds from options and warrants exercised | 143 | 97 | ||||||
| Net cash provided by financing activities | 3,924 | 5,982 | ||||||
| NET CHANGE IN CASH AND CASH EQUIVALENTS | (127 | ) | (451 | ) | ||||
| CASH AND CASH EQUIVALENTS, beginning of year | 162 | 613 | ||||||
| CASH AND CASH EQUIVALENTS, end of year | $ | 35 | $ | 162 | ||||
| SUPPLEMENTAL SCHEDULE OF: | ||||||||
| Cash paid for: | ||||||||
| Interest | $ | 76 | $ | 33 | ||||
| NONCASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Deemed dividend on beneficial conversion features of Series C Preferred stock | $ | 150 | $ | — | ||||
| Deemed dividend on price changes for Series B preferred stock warrants | $ | 64 | $ | — | ||||
| Deemed dividend on December 2014 public offering warrants | $ | 1,049 | $ | — | ||||
| Term changes on Series B preferred stock and December 2014 public offering warrants resulting in transfer to equity | $ | 324 | $ | — | ||||
| Issuance of common stock as debt repayment | $ | 1,014 | $ | — | ||||
| Repayment of deferred compensation via issuance of preferred stock | $ | 100 | $ | — | ||||
| Dividends on preferred stock | $ | 1,338 | $ | 152 | ||||
| Conversion of accrued expenses into common stock | $ | — | $ | 207 | ||||
| Payment of debt issuance costs via warrants and common stock | $ | — | $ | 522 | ||||
| Conversion of convertible debt into common stock | $ | — | $ | 893 | ||||
| Repayment of debt via issuance of common stock and warrants | $ | — | $ | 1,697 | ||||
| Issuance of common stock as board compensation | $ | — | $ | 355 | ||||
| The accompanying notes are an integral part of these consolidated statements. | ||||||||
| F-6 |
GUIDED THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2015 AND 2014
1. Organization, Background, and Basis of Presentation
Guided Therapeutics, Inc. (formerly SpectRx, Inc.), together with its wholly owned subsidiary, InterScan, Inc. (formerly Guided Therapeutics, Inc.), collectively referred to herein as the “Company”, is a medical technology company focused on developing innovative medical devices that have the potential to improve healthcare. The Company’s primary focus is the continued commercialization of its LuViva non-invasive cervical cancer detection device and extension of its cancer detection technology into other cancers, including esophageal. The Company’s technology, including products in research and development, primarily relates to biophotonics technology for the non-invasive detection of cancers.
Basis of Presentation
All information and footnote disclosures included in the consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States.
The Company’s prospects must be considered in light of the substantial risks, expenses and difficulties encountered by entrants into the medical device industry. This industry is characterized by an increasing number of participants, intense competition and a high failure rate. The Company has experienced net losses since its inception and, as of December 31, 2015, it had an accumulated deficit of approximately $122.6 million. Through December 31, 2015, the Company has devoted substantial resources to research and development efforts. The Company first generated revenue from product sales in 1998, but does not have significant experience in manufacturing, marketing or selling its products. The Company’s development efforts may not result in commercially viable products and it may not be successful in growing sales for its products. Moreover, required regulatory clearances or approvals may not be obtained. The Company’s products may not ever gain market acceptance and the Company may not ever achieve levels of revenue to sustain further development costs and support ongoing operations or achieve profitability. The development and continued commercialization of the Company’s products will require substantial development, regulatory, sales and marketing, manufacturing and other expenditures. The Company expects operating losses to continue through the foreseeable future as it continues to expend substantial resources to complete development of its products, obtain regulatory clearances or approvals and conduct further research and development.
On February 24, 2016, the Company implemented a 1:100 reverse stock split of its issued and outstanding common stock. The reverse stock split decreased the Company’s issued and outstanding shares of common stock from 237,101,702 shares of Common Stock to 2,371,007 shares as of that date. See Note 12, Subsequent Events. Unless otherwise specified, all per share amounts are reported on a post-stock split basis, as of December 31, 2015.
Going Concern
The Company’s consolidated financial statements have been prepared and presented on a basis assuming it will continue as a going concern. The factors below raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might be necessary from the outcome of this uncertainty.
At December 31, 2015, the Company had a negative working capital of approximately $3.4 million, accumulated deficit of $122.6 million, and incurred a net loss of $6.9 million for the year then ended. Stockholders’ deficit totaled approximately $5.6 million at December 31, 2015, primarily due to recurring net losses from operations, deemed dividends on warrants and preferred stock, offset by proceeds from the exercise of options and warrants and proceeds from sales of stock.
The Company’s capital-raising efforts are ongoing. If sufficient capital cannot be raised during the second quarter of 2016, the Company has plans to curtail operations by reducing discretionary spending and staffing levels, and attempting to operate by only pursuing activities for which it has external financial support. However, there can be no assurance that such external financial support will be sufficient to maintain even limited operations or that the Company will be able to raise additional funds on acceptable terms, or at all. In such a case, the Company might be required to enter into unfavorable agreements or, if that is not possible, be unable to continue operations, and to the extent practicable, liquidate and/or file for bankruptcy protection.
The Company had warrants exercisable for approximately 2.8 million shares of its common stock outstanding at December 31, 2015, with exercise prices ranging between $1.03 and $105 per share. Exercises of these warrants would generate a total of approximately $6.7 million in cash, assuming full exercise, although the Company cannot be assured that holders will exercise any warrants. Management may obtain additional funds through the public or private sale of debt or equity, and grants, if available.
| F-7 |
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant areas where estimates are used include the allowance for doubtful accounts, inventory valuation and input variables for Black-Scholes, Monte Carlo simulations and binomial calculations.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Guided Therapeutics, Inc. and its wholly owned subsidiary.
Accounting Standard Updates
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers,” (“ASU 2014-09”). ASU 2014-09 outlines a new, single comprehensive model for entities to use in accounting for revenue arising from contracts with customers and supersedes most current revenue recognition guidance, including industry-specific guidance. This new revenue recognition model provides a five step analysis in determining when and how revenue is recognized. The new model will require revenue recognition to depict the transfer of promised goods or services to customers in an amount that reflects the consideration a company expects to receive in exchange for those goods or services. ASU 2014-09 is effective for reporting periods beginning January 2018 with early adoption permitted in the first quarter of fiscal year 2017. The Company is evaluating the impact that adoption of this guidance will have on the determination or reporting of its financial results.
In June 2014, the FASB issued ASU 2014-12, “Accounting for Share-Based Payments When the Terms of an Award Provide that a Performance Target Could be Achieved after the Requisite Service Period,” (“ASU 2014-12”). ASU 2014-12 requires that a performance target that affects vesting, and that could be achieved after the requisite service period, be treated as a performance condition. As such, the performance target should not be reflected in estimating the grant date fair value of the award. ASU 2014-12 is effective for the reporting periods beginning after December 15, 2015. Early adoption is permitted. The Company is evaluating the impact that adoption of this guidance will have on the determination or reporting of its financial results.
In August 2014, the FASB issued ASU 2014-15, “Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” (“ASU 2014-15”). ASU 2014-15 requires management to perform interim and annual assessments of an entity’s ability to continue as a going concern for a one year period subsequent to the date of the financial statements, as entity must provide certain disclosures if conditions or events raise substantial doubt about the entity’s ability to continue as a going concern. The guidance is effective for all entities for the first annual period ending after December 15, 2016 and interim periods thereafter, with early adoption permitted. The Company is evaluating the impact that adoption of this guidance will have on the determination or reporting of its financial results.
In April 2015, the FASB issued ASU 2015-03, “Imputation of Interest: Simplifying the Presentation of Debt Issuance Costs,” (“ASU 2015-03”). ASU 2015-03 requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The guidance is effective for reporting periods beginning after December 15, 2015 and interim periods within those fiscal years with early adoption permitted. ASU 2015-03 should be applied on a retrospective basis, wherein the balance sheet of each period presented should be adjusted to reflect the effects of adoption. The Company is evaluating the impact that adoption of this guidance will have on the determination or reporting of its financial results.
In July 2015, the FASB issued ASU 2015-11, “Simplifying the Measurement of Inventory,” (“ASU 2015-11”). ASU 2015-11 requires inventory be measured at the lower of cost and net realizable value and options that currently exist for market value be eliminated. ASU 2015-11 defines net realizable value as estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The guidance is effective for reporting periods beginning after December 15, 2016 and interim periods within those fiscal years with early adoption permitted. ASU 2015-11 should be applied prospectively. The Company is evaluating the impact adoption of this guidance will have on determination or reporting of its financial results.
| F-8 |
In August 2015, the FASB issued ASU 2015-15, “Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements,” which amends ASC 835-30, “Interest - Imputation of Interest”. The ASU clarifies the presentation and subsequent measurement of debt issuance costs associated with lines of credit. These costs may be presented as an asset and amortized ratably over the term of the line of credit arrangement, regardless of whether there are outstanding borrowings on the arrangement. The effective date will be the first quarter of fiscal year 2016 and will be applied retrospectively. The Company is evaluating the impact adoption of this guidance will have on determination or reporting of its financial results.
In September 2015, the FASB issued ASU 2015-16, “Business Combinations: Simplifying the Accounting for Measurement-Period Adjustments.” This ASU requires that an acquirer recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. The effective date will be the first quarter of fiscal year 2016. The Company is evaluating the impact adoption of this guidance will have on determination or reporting of its financial results.
In November 2015, the FASB issued ASU 2015-17, “Income Taxes (Topic 740) Balance Sheet Classification of Deferred Taxes.” The amendments in ASU 2015-17 seek to simplify the presentation of deferred income taxes and require that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. ASU 2015-17 is effective for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods, with early application permitted for all entities as of the beginning of an interim or annual reporting period. The Company is evaluating the impact adoption of this guidance will have on determination or reporting of its financial results.
In January 2016, the FASB issued ASU 2016-01, "Financial Instruments-Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities." The amendments in ASU 2016-01, among other things, require equity investments (except those accounted for under the equity method of accounting, or those that result in consolidation of the investee) to be measured at fair value with changes in fair value recognized in net income; requires public business entities to use the exit price notion when measuring fair value of financial instruments for disclosure purposes; requires separate presentation of financial assets and financial liabilities by measurement category and form of financial asset (i.e., securities or loans and receivables); and eliminates the requirement for public business entities to disclose the method(s) and significant assumptions used to estimate fair value that is required to be disclosed for financial instruments measured at amortized cost. The effective date will be the first quarter of fiscal year 2018. The Company is evaluating the impact the adoption of this new standard will have on its consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02, “Leases (Topic 842)” that requires lessees to recognize on the balance sheet the assets and liabilities associated with the rights and obligations created by those leases. Under the new guidance, a lessee will be required to recognize assets and liabilities for leases with lease terms of more than 12 months. Consistent with current U.S. GAAP, the recognition, measurement, and presentation of expenses and cash flows arising from a lease by a lessee primarily will depend on its classification as finance or operating lease. The update is effective for reporting periods beginning after December 15, 2018. Early adoption is permitted. The Company is evaluating the impact adoption of this guidance will have on determination or reporting of its financial results.
Except as noted above, the guidance issued by the FASB during the current year is not expected to have a material effect on the Company’s consolidated financial statements.
Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less when purchased to be a cash equivalent.
Accounts Receivable
The Company performs periodic credit evaluations of its customers’ financial conditions and generally does not require collateral. The Company reviews all outstanding accounts receivable for collectability on a quarterly basis. An allowance for doubtful accounts is recorded for any amounts deemed uncollectable. The Company does not accrue interest receivable on past due accounts receivable.
| F-9 |
Concentrations of Credit Risk
The Company, from time to time during the years covered by these consolidated financial statements, may have bank balances in excess of its insured limits. Management has deemed this a normal business risk.
The Company performs periodic credit evaluations of its customers’ financial conditions and generally does not require collateral. The Company reviews all outstanding accounts receivable for collectability on a quarterly basis. An allowance for doubtful accounts is recorded for any amounts deemed uncollectable. The Company does not accrue interest receivable on past due accounts receivable.
Inventory Valuation
All inventories are stated at lower of cost or market, with cost determined substantially on a “first-in, first-out” basis. Selling, general, and administrative expenses are not inventoried, but are charged to expense when purchased. At December 31, 2015 and December 31, 2014, our inventories were as follows (in thousands):
| Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Raw materials | $ | 686 | $ | 884 | ||||
| Work in process | 186 | 304 | ||||||
| Finished goods | 365 | 136 | ||||||
| Inventory reserve | (118 | ) | (144 | ) | ||||
| Total | $ | 1,119 | $ | 1,180 | ||||
Property and Equipment
Property and equipment are recorded at cost. Depreciation is computed using the straight-line method over estimated useful lives of three to seven years. Leasehold improvements are depreciated at the shorter of the useful life of the asset or the remaining lease term. Depreciation expense is included in general and administrative expense on the statement of operations. Expenditures for repairs and maintenance are expensed as incurred. Property and equipment are summarized as follows at December 31, 2015 and 2014 (in thousands):
| Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Equipment | $ | 1,377 | $ | 1,391 | ||||
| Software | 740 | 737 | ||||||
| Furniture and fixtures | 124 | 124 | ||||||
| Leasehold Improvement | 199 | 180 | ||||||
| 2,440 | 2,432 | |||||||
| Less accumulated depreciation | (2,122 | ) | (1,845 | ) | ||||
| Total | $ | 318 | $ | 587 | ||||
Debt Issuance Costs
Debt issuance costs incurred in securing the Company’s financing arrangements are capitalized and amortized over the term of the debt. Deferred financing costs are included in other long term assets.
Other Assets
Other assets primarily consist of short and long-term deposits for various tooling inventory that are being constructed for the Company. At December 31, 2015 and 2014, such balances were approximately $413,000 and $72,000, respectively.
Patent Costs (Principally Legal Fees)
Costs incurred in filing, prosecuting, and maintaining patents are recurring, and expensed as incurred. Maintaining patents are expensed as incurred as the Company has not yet received FDA approval and recovery of these costs is uncertain. Such costs aggregated approximately $47,000 and $50,000 in 2015 and 2014, respectively.
| F-10 |
Accrued Liabilities
Accrued liabilities are summarized as follows at December 31, 2015 and 2014 (in thousands):
As of December 31, | ||||||||
| 2015 | 2014 | |||||||
| Accrued compensation | $ | 1,235 | $ | 447 | ||||
| Accrued professional fees | 154 | 203 | ||||||
| Deferred rent | 36 | 54 | ||||||
| Accrued warranty | 82 | 119 | ||||||
| Accrued vacation | 177 | 144 | ||||||
| Other accrued expenses | 223 | 48 | ||||||
| Total | $ | 1,907 | $ | 1,015 | ||||
Revenue Recognition
Revenue from the sale of the Company’s products is recognized upon shipment of such products to its customers. The Company recognizes revenue from contracts on a straight line basis, over the terms of the contracts. The Company recognizes revenue from grants based on the grant agreements, at the time the expenses are incurred.
Significant Customers
In 2015 and 2014, the majority of the Company’s revenues were from four and two customers, respectively. Revenue from these customers totaled approximately $280,000 or 73% and approximately $414,000 or 50% of total revenue for the year ended December 31, 2015 and 2014, respectively. Accounts receivable due from those customers represents 62% and 17% as of December 31, 2015 and 2014, respectively.
Deferred Revenue
The Company defers payments received as revenue until earned based on the related contracts on a straight line basis, over the terms of the contract.
Research and Development
Research and development expenses consist of expenditures for research conducted by the Company and payments made under contracts with consultants or other outside parties and costs associated with internal and contracted clinical trials. All research and development costs are expensed as incurred.
Income Taxes
The Company uses the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Management provides valuation allowances against the deferred tax assets for amounts that are not considered more likely than not to be realized.
Uncertain Tax Positions
Effective January 1, 2007 the Company adopted ASC guidance regarding accounting for uncertainty in income taxes. This guidance clarifies the accounting for income taxes by prescribing the minimum recognition threshold an income tax position is required to meet before being recognized in the financial statements and applies to all income tax positions. Each income tax position is assessed using a two-step process. A determination is first made as to whether it is more likely than not that the income tax position will be sustained, based upon technical merits, upon examination by the taxing authorities. If the income tax position is expected to meet the more likely than not criteria, the benefit recorded in the financial statements equals the largest amount that is greater than 50% likely to be realized upon its ultimate settlement. At December 31, 2015 and 2014, there were no uncertain tax positions.
| F-11 |
The Company is current with its federal and applicable state tax returns filings. Although we have been experiencing recurring losses, we are obligated to file tax returns for compliance with Internal Revenue Service (“IRS”) regulations and that of applicable state jurisdictions. As of December 31, 2015, the Company has approximately $27.8 million of net operating loss eligible to be carried forward for tax purposes at federal and applicable states level.
None of the Company’s federal or state income tax returns are currently under examination by the IRS or state authorities.
Warrants
The Company has issued warrants, which allow the warrant holder to purchase one share of stock at a specified price for a specified period of time. The Company records equity instruments including warrants issued to non-employees based on the fair value at the date of issue. The fair value of warrants classified as equity instruments at the date of issuance is estimated using the Black-Scholes Model. The fair value of warrants classified as liabilities at the date of issuance is estimated using the Monte Carlo Simulation or Binomial model.
Stock Based Compensation
The Company records compensation expense related to options granted to non-employees based on the fair value of the award.
Compensation cost is recorded as earned for all unvested stock options outstanding at the beginning of the first year based upon the grant date fair value estimates, and for compensation cost for all share-based payments granted or modified subsequently based on fair value estimates.
For the years ended December 31, 2015 and 2014, share-based compensation for options attributable to employees, officers and Board members were approximately $1,008,000 and $886,000, respectively. These amounts have been included in the Company’s statements of operations. Compensation costs for stock options which vest over time are recognized over the vesting period. As of December 31, 2015, the Company had no unrecognized compensation costs related to granted stock options to be recognized over the remaining vesting period of approximately three years.
3. FAIR VALUE OF FINANCIAL INSTRUMENTS
The guidance for fair value measurements, ASC820, Fair Value Measurements and Disclosures, establishes the authoritative definition of fair value, sets out a framework for measuring fair value, and outlines the required disclosures regarding fair value measurements. Fair value is the price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. The Company uses a three-tier fair value hierarchy based upon observable and non-observable inputs as follow:
| · | Level 1 – Quoted market prices in active markets for identical assets and liabilities; |
| · | Level 2 – Inputs, other than level 1 inputs, either directly or indirectly observable; and |
| · | Level 3 – Unobservable inputs developed using internal estimates and assumptions (there is little or no market date) which reflect those that market participants would use. |
The Company records its derivative activities at fair value, which consisted of warrants as of December 31, 2015. The fair value of the warrants was estimated using the Monte Carlo Simulation model. Gains and losses from derivative contracts are included in net gain (loss) from derivative contracts in the statement of operations. The fair value of the Company’s derivative warrants is classified as a Level 3 measurement, since unobservable inputs are used in the valuation.
The following table presents the fair value for those liabilities measured on a recurring basis as of December 31, 2015 and 2014:
FAIR VALUE MEASUREMENTS ( In Thousands)
| Description | Level 1 | Level 2 | Level 3 | Total | Date | |||||||||||||||
| Warrants issued in connection with the issuance of Series C preferred stock | $ | — | $ | — | $ | (1,145 | ) | $ | (1,145 | ) |
December 31, 2015 | |||||||||
| Warrants issued in connection with the issuance of Series B preferred stock | $ | (1,461 | ) | $ | (1,461 | ) | December 31, 2015 | |||||||||||||
| Total long-term liabilities at fair value | $ | — | $ | — | $ | (2,606 | ) | $ | (2,606 | ) | December 31, 2015 |
| F-12 |
| Description | Level 1 | Level 2 | Level 3 | Total | Date | |||||||||||||||
| Warrants issued in connection with the December 2014 public offering of common stock | $ | — | $ | — | $ | (587 | ) | $ | (587 | ) |
December 31, 2014 | |||||||||
| Warrants issued in connection with the issuance of Series B preferred stock | $ | — | $ | — | $ | (1,483 | ) | $ | (1,483 | ) |
December 31, 2014 | |||||||||
| Total long-term liabilities at fair value | $ | — | $ | — | $ | (2,070 | ) | $ | (2,070 | ) | ||||||||||
Fair Value Measurements Using Significant Unobservable Inputs (Level 3) | ||||||||||||||||||||
December 2014 Public Offering Exchanged Warrants | Series B Warrants | Series C Warrants | Total | |||||||||||||||||
| Balance, December 31, 2014 | $ | (587 | ) | $ | (1,483 | ) | $ | — | $ | (2,070 | ) | |||||||||
| Warrants issued during the period | — | — | (1,428 | ) | (1,428 | ) | ||||||||||||||
| Change in fair value during the period | 263 | 22 | 283 | 568 | ||||||||||||||||
| Transfer to equity as a result of changes to provisions | 324 | — | — | 324 | ||||||||||||||||
| Balance, December 31, 2015 | $ | — | $ | (1,461 | ) | $ | (1,145 | ) | $ | (2,606 | ) | |||||||||
4. Stockholders’ Equity
Common Stock
The Company has authorized 1,000,000,000 shares of common stock with $0.001 par value, of which 2,371,017 were issued and outstanding as of December 31, 2015. For the year ended December 31, 2014, there were 195,000,000 authorized shares of common stock, of which 96,889 were issued and outstanding.
For the year ended December 31, 2015, the Company issued 1,402,128 shares of common stock as listed below:
| Sales of unregistered securities - Issuance - For Cash | 40,000 | |
| Issuance - For Debt Repayment | 152,117 | |
| Series C Conversion | 1,006,777 | |
| Series C Dividends | 18,562 | |
| Series B Dividends | 11,086 | |
| Option Exercised | 1,786 | |
| Warrant Exercised - Cashless | 95,413 | |
| Warrant Exercised – For Cash | 13,500 | |
| Issued for December 2014 public offering warrant exchange | 26,190 | |
| Issued for Consulting Services | 36,697 | |
| Total | 1,402,128 | |
Preferred Stock
The Company has authorized 9,000,000 shares of preferred stock with a $.001 par value. The board of directors has the authority to issue these shares and to set dividends, voting and conversion rights, redemption provisions, liquidation preferences, and other rights and restrictions. The board of directors designated 525,000 shares of preferred stock as redeemable convertible preferred stock, none of which remain outstanding; 3,000 shares of preferred stock as Series B Convertible Preferred Stock, of which none and 1,277 shares were issued and outstanding at December 31, 2015 and December 31, 2014, respectively; and 9,000 shares of preferred stock as Series C Convertible Preferred Stock, of which 5,555 and 0 shares were issued and outstanding at December 31, 2015 and December 31, 2014, respectively.
| F-13 |
Series B Convertible Preferred Stock
Pursuant to the terms of the Series B Preferred Stock set forth in the Series B designations, shares of Series B Preferred Stock were convertible into common stock by their holder at any time, and were mandatorily convertible upon the achievement of certain conditions, including the receipt of certain approvals from the U.S. Food and Drug Administration and the achievement by the Company of specified average trading prices and volumes for the common stock.
Holders of the Series B Preferred Stock were entitled to quarterly dividends at an annual rate of 10.0%, payable in cash or, subject to certain conditions, common stock, at the Company’s option. Preferred dividends totaled approximately $352,000 and $152,000 for 2015 and 2014, respectively. Dividends were paid via issuance of common stock.
The Series B Preferred Stock was originally issued with Tranche A warrants to purchase 18,581 shares of common stock and Tranche B warrants purchasing 18,581 shares of common stock, both at an exercise price of $108.00 per share.
On June 30, 2015, as consideration for obtaining consents to an amendment to the Series B designations, the Company reduced the exercise price of the Tranche A warrants from $108.00 to $104.55 per share, and the exercise price of the Tranche B warrants from $10.455 to $9.00 per share. The change in exercise price of these warrants resulted in a deemed dividend totaling $64,000 that has been recorded as an increase to additional paid-in capital with an offsetting charge to retained earnings. The deemed dividend has been subtracted from income (added to the loss) in computing loss per common stockholder.
At December 31, 2015, as a result of the operation of certain anti-dilution provisions, the Tranche B warrants were convertible into 1,292,819 shares of common stock. These warrants are re-measured based upon their fair value each reporting period and classified as a liability on the Balance Sheet.
Series C Convertible Preferred Stock
On June 29, 2015, the Company entered into a securities purchase agreement with certain accredited investors for the issuance and sale of an aggregate of 6,737 shares of Series C convertible preferred stock, at a purchase price of $750 per share and a stated value of $1,000 per share. On September 3, 2015 the Company entered into an interim agreement amending the securities purchase agreement to provide for certain of the investors to purchase an additional aggregate of 1,166 shares. Total cash and non-cash expenses were valued at $853,000, resulting in net proceeds of $3,698,000. In connection with the transaction, the Company issued five-year warrants exercisable for an aggregate of 1,247,737 shares of common stock at an exercise price of $9.50 per share.
Pursuant to the Series C certificate of designations, shares of Series C preferred stock are convertible into common stock by their holder at any time, and may be mandatorily convertible upon the achievement of specified average trading prices for the Company’s common stock. At December 31, 2015, there were 5,555 shares outstanding with a conversion price of $1.03 per share, such that each share of Series C preferred stock would convert into approximately 97,324 shares of the Company’s common stock, subject to customary adjustments, including for any accrued but unpaid dividends and pursuant to certain anti-dilution provisions, as set forth in the Series C certificate of designations. The conversion price will automatically adjust downward to 80% of the then-current market price of the Company’s common stock 15 trading days after any reverse stock split of the Company’s common stock, and 5 trading days after any conversions of the Company’s outstanding convertible debt.
Holders of the Series C preferred stock are entitled to quarterly cumulative dividends at an annual rate of 12.0% until 42 months after the original issuance date (the “Dividend End Date”), payable in cash or, subject to certain conditions, the Company’s common stock. In addition, upon conversion of the Series C Preferred Stock prior to the Dividend End Date, the Company will also pay to the converting holder a “make-whole payment” equal to the amount of unpaid dividends through the Dividend End Date on the converted shares. The Series C preferred stock generally has no voting rights except as required by Delaware law. Upon the Company’s liquidation or sale to or merger with another corporation, each share will be entitled to a liquidation preference of $1,000, plus any accrued but unpaid dividends.
The Company has provisionally allocated net proceeds totaling $935,200 to the fair value of the Series C preferred stock. The effective conversion price of $935,000 allocated to the Series C preferred stock resulted in an associated beneficial conversion feature totaling $148,000 that has been recorded as an increase to additional paid-in capital with an offsetting charge to retained earnings representing a deemed dividend. The deemed dividend has been subtracted from income (added to the loss) in computing loss per common stockholder.
| F-14 |
In addition, the purchasers of the Series C preferred stock received, on a pro rata basis, warrants exercisable to purchase an aggregate of approximately 106.4 million shares of Company’s common stock. The warrants contain anti-dilution adjustments in the event that the Company issues shares of common stock, or securities exercisable for or convertible into shares of common stock, at prices below the exercise price of such warrants. As a result of the anti-dilution protection, the Company is required to account for the warrants as a liability recorded at fair value each reporting period. The Company has valued the warrants using a Binomial model and allocated $1,349,000 to the fair value of the warrants. At December 31, 2015, the exercise price per share was $1.03.
Stock Options
Under the Company’s 1995 Stock Plan (the “Plan”), a total of 26,616 shares remained available at December 31, 2015 and 105,936 shares were subject to stock options outstanding as of that date, bringing the total number of shares subject to stock options outstanding and those remaining available for issue to 132,552 shares of common stock as of December 31, 2014. The Plan allows the issuance of incentive stock options, nonqualified stock options, and stock purchase rights. The exercise price of options is determined by the Company’s board of directors, but incentive stock options must be granted at an exercise price equal to the fair market value of the Company’s common stock as of the grant date. Options historically granted have generally become exercisable over four years and expire ten years from the date of grant.
The fair value of stock options granted in 2015 and 2014 were estimated using the Black-Scholes option pricing model. A summary of the assumptions used in determining the fair value of options follows:
| 2015 | 2014 | |||||||
| Expected volatility | 152.32 | % | 157.70 | % | ||||
| Expected option life in years | 9.98 | 9.98 | ||||||
| Expected dividend yield | 0.00 | % | 0.00 | % | ||||
| Risk-free interest rate | 1.33 | % | 2.55 | % | ||||
| Weighted average fair value per option at grant date | $ | 0.49 | $ | 0.40 | ||||
Application of the Black-Scholes option pricing model involves assumptions that are judgmental and affect compensation expense. Historical information is the primary basis for the selection of expected volatility, expected option life and expected dividend yield. Expected volatility is based on the most recent historical period equal to the expected life of the option. The risk-free interest rate is based on yields of U.S. Treasury zero-coupon issues with a term equal to the expected life of the option on the date the stock options were granted.
Stock option activity for each of the two years ended December 31, 2015, as follows:
| 2015 | 2014 | |||||||||||||||
Weighted Average Exercise | Weighted Average Exercise | |||||||||||||||
| Shares | Price | Shares | Price | |||||||||||||
| Outstanding at beginning of year | 69,404 | $ | 66.00 | 65,312 | $ | 68.00 | ||||||||||
| Options granted | 42,140 | $ | 12.00 | 7,548 | $ | 40.00 | ||||||||||
| Options exercised | (1,786 | ) | $ | 48.00 | (2,424 | ) | $ | 32.00 | ||||||||
| Options expired/forfeited | (3,822 | ) | $ | 43.00 | (1,031 | ) | $ | 68.00 | ||||||||
| Outstanding at end of year | 105,936 | $ | 45.00 | 69,404 | $ | 66.00 | ||||||||||
| Options vested and exercisable at year-end | 90,411 | $ | 49.00 | 59,881 | $ | 66.00 | ||||||||||
| Options available for grant at year-end | 26,616 | 63,148 | ||||||||||||||
| Aggregate intrinsic value – options exercised | $ | — | $ | 497 | ||||||||||||
| Aggregate intrinsic value – options outstanding | $ | — | $ | 4,941 | ||||||||||||
| Aggregate intrinsic value – options vested and exercisable | $ | — | $ | 6,129 | ||||||||||||
| Options unvested, balance at beginning of year (1) | 9,523 | 10,672 | $ | 112.00 | ||||||||||||
| Options granted (1) | 42,140 | $ | 40.00 | 7,548 | $ | 40.00 | ||||||||||
| Vested (1) | (34,383 | ) | $ | 66.00 | (7,666 | ) | $ | 66.00 | ||||||||
| Cancelled/Forfeited | (2,455 | ) | $ | 68.00 | (1,031 | $ | 68.00 | |||||||||
| Balance, end of period (1) | 14,825 | 9,523 | — | |||||||||||||
| | ||||||||||||||||
| (1) Includes awards not captured in valuation fragments | ||||||||||||||||
| F-15 |
The Company estimates the fair value of stock options using a Black-Scholes valuation model. Key input assumptions used to estimate the fair value of stock options include the expected term, expected volatility of the Company’s common stock, the risk free interest rate, option forfeiture rates, and dividends, if any. The expected term of the options is based upon the historical term until exercise or expiration of all granted options. The expected volatility is derived from the historical volatility of the Company’s stock on the OTCBB market for a period that matches the expected term of the option. The risk-free interest rate is the constant maturity rate published by the U.S. Federal Reserve Board that corresponds to the expected term of the option.
Warrants
The following table summarizes transactions involving the Company’s outstanding warrants to purchase common stock for the year ended December 31, 2015:
Warrants (Underlying Shares) | ||||
| Outstanding, January 1, 2015 | 297,961 | |||
| Issuances | 2,751,872 | |||
| Canceled / Expired | (35,973 | ) | ||
| Exercised | (211,476 | ) | ||
| Outstanding, December 31, 2015 | 2,802,384 | |||
The Company had the following shares reserved for the warrants as of December 31, 2015:
|
Warrants (Underlying Shares) |
Exercise Price Per Share |
Expiration Date | |
| 4,399 | (1) | $68.00 | March 31, 2016 |
| 2,852 | (2) | $105.00 | November 20, 2016 |
| 18,581 | (3) | $10.46 | May 23, 2018 |
| 1,292,820 | (3) | $1.03 | May 23, 2018 |
| 2,000 | (4) | $50.00 | April 23, 2019 |
| 5,618 | (4) | $45.00 | May 22, 2019 |
| 1,842 | (5) | 38.00 | September 10, 2019 |
| 3,255 | (6) | $46.08 | September 27, 2019 |
| 7,553 | (7) | $28.13 | December 2, 2019 |
| 83,927 | (8) | $9.00 | December 2, 2020 |
| 83,927 | (8) | $11.00 | December 2, 2020 |
| 20,000 | (9) | $25.50 | March 30, 2018 |
| 17,547 | (10) | $11.88 | June 29, 2020 |
| 526,421 | (11) | $1.03 | June 29, 2020 |
| 273,684 | (12) | $1.03 | September 4, 2020 |
| 289,737 | (13) | $1.03 | September 21, 2020 |
| 5,163 | (14) | $11.88 | September 4,2020 |
| 157,895 | (15) | $1.03 | October 23, 2020 |
| 5,163 | (16) | $11.88 | October 23,2020 |
| 2,802,384 | |||
| (1) | Issued in February 2014 as part of a buy-back of a minority interest in Interscan in December 2012. |
| (2) | Issued as part of a November 2011 private placement. |
| (3) | Issued in June 2015 in exchange for warrants originally issued as part of a May 2013 private placement. |
| (4) | Issued to a placement agent in conjunction with an April 2014 private placement. |
| (5) | Issued to a placement agent in conjunction with a September 2014 private placement. |
| (6) | Issued as part of a September 2014 Regulation S offering. |
| (7) | Issued to a placement agent in conjunction with a 2014 public offering. |
| (8) | Issued in June 2015 in exchange for warrants originally issued as part of a 2014 public offering. |
| (9) | Issued as part of a March 2015 private placement. |
| (10) | Issued to a placement agent in conjunction with a June 2015 private placement. |
| (11) | Issued as part of a June 2015 private placement. |
| (12) | Issued as part of a June 2015 private placement. |
| (13) | Issued as part of a June 2015 private placement. |
| (14) | Issued to a placement agent in conjunction with a June 2015 private placement. |
| (15) | Issued as part of a June 2015 private placement. |
| (16) | Issued to a placement agent in conjunction with a June 2015 private placement. |
| F-16 |
5. Income Taxes
The Company has incurred net operating losses (“NOLs”) since inception. As of December 31, 2015, the Company had NOL carryforwards available through 2034 of approximately $73.0 million to offset its future income tax liability. The NOL carryforwards began to expire in 2008. The Company has recorded a valuation allowance for all deferred tax assets related to the NOLs. Utilization of existing NOL carry forwards may be limited in future years based on significant ownership changes. The Company is in the process of analyzing its NOLs and has not determined if it is subject to any restrictions in the Internal Revenue Code that could limit the future use of NOL.
Components of deferred taxes are as follows at December 31 (in thousands):
| 2015 | 2014 | |||||||
| Deferred tax assets | $ | 626 | $ | 466 | ||||
| Net operating loss carry forwards | 27,770 | 25,994 | ||||||
| Deferred tax liabilities | — | — | ||||||
| 28,396 | 26,460 | |||||||
| Valuation allowance | (28,396 | ) | (26,460 | ) | ||||
| $ | 0 | $ | 0 | |||||
The following is a summary of the items that caused recorded income taxes to differ from taxes computed using the statutory federal income tax rate for the years ended December 31:
| 2015 | 2014 | |||||||
| Statutory federal tax rate | 34 | % | 34 | % | ||||
| State taxes, net of federal benefit | 4 | 4 | ||||||
| Nondeductible expenses | — | — | ||||||
| Valuation allowance | (38 | ) | (38 | ) | ||||
| 0 | % | 0 | % | |||||
6. Commitments and Contingencies
Operating Leases
In December 2009, the Company moved its offices, which comprise its administrative, research and development, marketing and production facilities to 5835 Peachtree Corners East, Suite D, Norcross, Georgia 30092. The Company leases approximately 23,000 square feet under a lease that expires in June 2017. The fixed monthly lease expense is approximately $15,000 plus common charges. The Company also leases office and equipment under operating lease agreements with monthly payments of approximately $2,000. These leases expire at various dates through April 2016. Future minimum rental payments at December 31, 2015 under non-cancellable operating leases for office space and equipment are as follows (in thousands):
| Year | Amount | |||||
| 2016 | $ | 201 | ||||
| 2017 | 98 | |||||
| Total | $ | 299 |
Rental expense was approximately $170, 000 in 2015 and 2014.
Litigation and Claims
From time to time, the Company may be involved in various legal proceedings and claims arising in the ordinary course of business. Management believes that the dispositions of these matters, individually or in the aggregate, are not expected to have a material adverse effect on the Company’s financial condition. However, depending on the amount and timing of such disposition, an unfavorable resolution of some or all of these matters could materially affect the future results of operations or cash flows in a particular period.
As of December 31, 2015 and December 31, 2014, there was no accrual recorded for any potential losses related to pending litigation.
| F-17 |
Contracts
At December 31, 2015 and 2014, the Company had a royalty agreement with Freedom Meditech, entered into on August 26, 2008, in which the Company receives quarterly royalty payments on Freedom Meditech’s sales of products using technology licensed from the Company. The royalty payment equals 3% of net sales of licensed products covered by a valid claim. The aggregate royalties payable are capped at $4,000,000. Freedom Meditech’s obligation to pay royalties with respect to sales in a particular country until the earlier of the date of expiration of the last valid claim in such country or the aggregate royalty cap is reached. For the years ended December 31, 2015 and 2014, the Company received approximately $36,000 and 40,000 in royalties, respectively.
7. License and Technology Agreements
As part of the Company’s efforts to conduct research and development activities and to commercialize potential products, the Company, from time to time, enters into agreements with certain organizations and individuals that further those efforts but also obligate the Company to make future minimum payments or to remit royalties ranging from 1% to 3% of revenue from the sale of commercial products developed from the research. The Company generally is required to make minimum royalty payments for the exclusive license to develop certain technology.
8. Notes Payable
Short Term Notes Payable
At December 31, 2015 and 2014, the Company maintained notes payable to Premium Assignment Corporation, an insurance premium financing company, of approximately $172,000 and $100,000, respectively. These notes are 10 month straight-line amortizing loan dated June 24, 2015 and June 24, 2014. The notes carry annual interest of 5.2% and 4.6%, respectively. The balance due to on the Premium Assignment note was approximately $70,000 and $37,000 at December 31, 2015 and December 31, 2014, respectively.
Note Payable in Default
At December 31, 2015, the Company maintained a note payable totaling approximately $133,000 of principal and accrued interest. The note accrues interest at 9% with a 16% default rate and requires monthly payments of $10,000. As of December 31, 2015 the note is accruing interest at the default rate and is past due. As of December 31, 2014, the balance was $163,000 and was not in default.
Short Term Convertible Note Payable
On September 10, 2014, the Company sold a secured promissory note an accredited investor with an initial principal amount of $1,275,000, for a purchase price of $700,000 (an original issue discount of $560,000). The Company may prepay the note at any time. The note is secured by the Company’s current and future accounts receivable and inventory, pursuant to a security agreement entered into in connection with the sale. On March 10, 2015, May 4, 2015, June 1, 2015, June 16, 2015, June 29, 2015, January 21, January 29 and February 12, 2016 the Company amended the terms of the note to extend the maturity ultimately until August 31, 2016. During the extension, interest accrues on the note at a rate of the lesser of 18% per year or the maximum rate permitted by applicable law. Pursuant to the terms of the amended note, the holder may convert the outstanding balance into shares of common stock at a conversion price per share equal to the lower of (1) $25.0 or (2) 75% of the lowest daily volume weighted average price of the common stock during the five days prior to conversion. If the conversion price at the time of any conversion is lower than $15.00, the Company has the option of delivering the conversion amount in cash in lieu of shares of common stock. As of December 31, 2015, the holder had converted $5,395 in outstanding principal and accrued interest into 90,240 shares of common stock. The Company paid a total of $65,000 in loan modification fees. At December 31, 2015 and 2014, the balance on the note was approximately $686,000 and $1,062,000, respectively. The original issue discount of $560,000 was fully amortized as of June 30, 2015.
Convertible Debt
On April 23, 2014, the Company entered into a securities purchase agreement with an accredited investor, pursuant to which the Company sold a 6% senior convertible note with an initial principal amount of $1.5 million and an 18-month term, for a purchase price of $1.0 million (an approximately 33.3% original issue discount). Additionally, pursuant to the purchase agreement, on May 23, 2014 the investor purchased an additional senior convertible note with an initial principal amount of $2.0 million and an 18-month term, for a fixed purchase price of $2.0 million. As of July 1, 2015, the notes were repaid in full. The balance at December 31, 2014 was $783,000.
| F-18 |
The Company paid the investor a commitment fee for entering into the purchase agreement in the form of 3,218 shares of common stock. The Company also paid $50,000 of attorneys’ fees and expenses incurred by Magna in connection with the transaction. Total debt issuance costs incurred on the senior convertible note was approximately $844,000. This amount was fully amortized at December 31, 2015. Total amortization expense for the year ended December 31, 2015 and 2014 was $516,000 and $328,000, respectively.
In connection with the sale of the convertible notes, the Company issued its placement agent warrants exercisable for 2,000 shares of common stock at $50.00 per share with an expiration date of April 23, 2019, and warrants exercisable for 5,618 shares of common stock at $45.00 per share with an expiration date of May 22, 2019.
Prior to repayment in full, the Company had issued a total of 252,804 shares of common stock in conjunction with conversions of the senior convertible notes.
Loss on Extinguishment of Debt
As part of the Company’s December 2014 public offering of common stock, the Company repaid approximately $1.4 million of debt via the issuance of 77,005 shares of common stock and five-year warrants to purchase an additional 3,850,252 shares at an exercise price of $22.50 per share. Pursuant to the senior convertible note purchase agreement, the Company had to pay a prepayment penalty of 25% of the amount prepaid. The penalty on the transaction was approximately $325,000 and was charged to loss on extinguishment of debt on the statement of operations for the year ended December 31, 2014. There was no loss on extinguishment of debt in the year ended December 31, 2015.
9. Related Party Transactions
As of December 31, 2015 and 2014, the Company maintained notes payable and accrued interest to related parties totaling approximately $682,000 and $609,000, respectively. These notes are short term, straight-line amortizing notes. The notes carry an annual interest rate of between 5% and 10%. Included in the short-term notes payable, related parties, on the accompanying Balance Sheet.
Certain of the Company’s directors and officers invested a total of $182,603 in the December 2014 public offering and received 8,116 shares of common stock, as well as five-year warrants to purchase an additional 4,058 shares at $22.50 per share.
Directors and Officers holding the Company’s Series B preferred stock participated in the issuance of the Company’s Series C preferred stock in the second half of 2015 by exchanging a total of $525,000 in liquidation value of Series B preferred stock and $400,000 in cash, and received 1,233 shares of Series C preferred stock and five-year warrants to purchase an additional 131,526 shares.
10. Valuation and Qualifying Accounts
Allowance for Doubtful Accounts
The Company has the following allowances for doubtful accounts (in thousands):
Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Beginning balance | $ | 76 | $ | 18 | ||||
| Additions / (Adjustments) | 19 | 58 | ||||||
| Balance | $ | 95 | $ | 76 | ||||
| F-19 |
Inventory Reserves
The Company has the following reserves for inventory balance (in thousands):
Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Beginning balance | $ | 144 | $ | 184 | ||||
| Additions / (Adjustments) | (26 | ) | (40 | ) | ||||
| Balance | $ | 118 | $ | 144 | ||||
11. Loss Per Common Share
Basic net loss per share attributable to common stockholders amounts are computed by dividing the net loss plus preferred stock dividends and deemed dividends on preferred stock by the weighted average number of shares outstanding during the period.
12. Subsequent Events
On February 11, 2016, the Company entered into a securities purchase agreement with an accredited investor for the issuance and sale on February 12, 2016 of $1.4375 million in aggregate principal amount of a senior secured convertible note for an aggregate purchase price of $1.15 million (a 20% original issue discount). In addition, the investor received a warrant exercisable to purchase an aggregate of approximately 179.7 million shares of the Company’s common stock. The convertible note matures on the second anniversary of issuance and, in addition to the 20% original issue discount, accrues interest at a rate of 17% per year. The Company will pay monthly interest coupons and, beginning six months after issuance, will pay amortized quarterly principal payments. If the Company does not receive, on or before the first anniversary after issuance, an aggregate of at least $3.0 million from future equity or debt financings or non-dilutive grants, then the holder will have the option of accelerating the maturity date to the first anniversary of issuance. The Company may prepay the convertible note, in whole or in part, without penalty, upon 20 days’ prior written notice. Subject to resale restrictions under Federal securities laws and the availability of sufficient authorized but unissued shares of the Company’s common stock, the convertible note is convertible at any time, in whole or in part, at the holder’s option, into shares of the Company’s common stock, at a conversion price equal to the lesser of $0.008 per share (pre-stock split) or 70% of the average closing price per share for the five trading days prior to issuance, subject to certain customary adjustments and anti-dilution provisions contained in the convertible note.
The convertible note includes customary event of default provisions and a default interest rate of the lesser of 19% or the maximum amount permitted by law. Upon the occurrence of an event of default, the holder may require the Company to redeem the convertible note at 120% of the outstanding principal balance. The convertible note is secured by a lien on all of the Company’s assets, including its intellectual property, pursuant to a security agreement entered into by the Company and GPB in connection with the transaction.
The warrant is exercisable at any time, pending availability of sufficient authorized but unissued shares of the Company’s common stock, at an exercise price per share equal to the conversion price of the convertible note, subject to certain customary adjustments and anti-dilution provisions contained in the warrant. The warrant has a five-year term.
The Company used a placement agent in connection with the transaction. For its services, the placement agent received a cash placement fee equal to 4% of the aggregate gross proceeds from the transaction and a warrant to purchase shares of common stock equal to an aggregate of 6% of the total number of shares underlying the securities sold in the transaction, at an exercise price equal to, and terms otherwise identical to, the warrant issued to the investor. Finally, the Company agreed to reimburse the placement agent for its reasonable out-of-pocket expenses.
In connection with the transaction, on February 12, 2016, the Company and the investor entered into a four-year consulting agreement, pursuant to which the investor will provide management consulting services to the Company in exchange for a royalty payment, payable quarterly, equal to 3.5% of the Company’s revenues from the sale of products.
On February 24, 2016, the Company implemented a 1:100 reverse stock split of all of its issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock of the Company were converted into 1 share. All fractional shares created by the reverse stock split were rounded to the nearest whole share. The reverse stock split decreased the Company’s issued and outstanding shares of common stock from 287,729,113 shares to 2,877,291 shares. The number of the authorized shares did not change.
| F-20 |
| GUIDED THERAPEUTICS, INC. AND SUBSIDIARY | ||||||||
| CONDENSED CONSOLIDATED BALANCE SHEETS Unaudited (in Thousands) | ||||||||
| ASSETS | March 31, 2016 | December 31, 2015 | ||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 56 | $ | 35 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $75 and $95 at March 31, 2016 and December 31, 2015, respectively | 230 | 190 | ||||||
| Inventory, net of reserves of $156 and $118, at March 31, 2016 and December 31, 2015, respectively | 1,253 | 1,119 | ||||||
| Prepaid expenses and deposits | 509 | 780 | ||||||
| Total current assets | 2,048 | 2,124 | ||||||
| Property and equipment, net | 264 | 318 | ||||||
| Other assets | 54 | 121 | ||||||
| Total noncurrent assets | 318 | 439 | ||||||
| TOTAL ASSETS | $ | 2,366 | $ | 2,563 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Short-term notes payable, including related parties | $ | 259 | $ | 752 | ||||
| Note payable in default, including related parties | 590 | 133 | ||||||
| Convertible note – current portion | 479 | — | ||||||
| Short-term convertible notes payable, net | 591 | 686 | ||||||
| Accounts payable | 1,977 | 1,824 | ||||||
| Accrued liabilities | 2,069 | 1,907 | ||||||
| Deferred revenue | 66 | 217 | ||||||
| Total current liabilities | 6,031 | 5,519 | ||||||
| Warrants, at fair value | 1,588 | 2,606 | ||||||
| Long-term convertible notes payable, net | 197 | — | ||||||
| Total long-term liabilities | 1,785 | 2,606 | ||||||
| TOTAL LIABILITIES | 7,816 | 8,125 | ||||||
| STOCKHOLDERS’ DEFICIT: | ||||||||
| Series C convertible preferred stock, $.001 par value; 9.0 shares authorized, 5.0 and 5.6 shares issued and outstanding as of March 31, 2016 and December 31, 2015, (Liquidation preference of $4,966 and $5,555 at March 31, 2016 and December 31, 2015) | 1,817 | 2,052 | ||||||
| Common stock, $.001 Par value; 1,000,000 shares authorized, 5,322 and 2,371 shares issued and outstanding as of March, 31 2016 and December 31, 2015 | 297 | 236 | ||||||
| Additional paid-in capital | 115,471 | 114,845 | ||||||
| Treasury stock, at cost | (132 | ) | (132 | ) | ||||
| Accumulated deficit | (122,903 | ) | (122,563 | ) | ||||
| TOTAL STOCKHOLDERS’ DEFICIT | (5,450 | ) | (5,562 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 2,366 | $ | 2,563 | ||||
| The accompanying notes are an integral part of these condensed consolidated financial statements. | ||||||||
| F-21 |
GUIDED THERAPEUTICS INC. AND SUBSIDIARY | ||||||||
| CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS | ||||||||
| (Unaudited, in Thousands) | ||||||||
FOR THE THREE MONTHS ENDED MARCH 31, | ||||||||
| 2016 | 2015 | |||||||
| REVENUE: | ||||||||
| Sales – devices and disposables | $ | 262 | $ | 127 | ||||
| Cost of goods sold | 68 | 107 | ||||||
| Gross profit | 194 | 20 | ||||||
| OPERATING EXPENSES: | ||||||||
| Research and development | 290 | 373 | ||||||
| Sales and marketing | 117 | 172 | ||||||
| General and administrative | 917 | 963 | ||||||
| Total operating expenses | 1,324 | 1,508 | ||||||
| Operating loss | (1,130 | ) | (1,488 | ) | ||||
| OTHER INCOME (EXPENSES): | ||||||||
| Other income | 23 | 21 | ||||||
| Interest expense | (158 | ) | (492 | ) | ||||
| Change in fair value of warrants | 1,395 | 714 | ||||||
| Total other income | 1,260 | 243 | ||||||
| INCOME (LOSS) FROM OPERATIONS | 130 | (1,245 | ) | |||||
| PROVISION FOR INCOME TAXES | — | — | ||||||
| NET INCOME (LOSS) | $ | 130 | $ | (1,245 | ) | |||
PREFERRED STOCK DIVIDENDS | (470 | ) | (31 | ) | ||||
NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS | $ | (340 | ) | $ | (1,276 | ) | ||
| NET LOSS PER SHARE ATTRIBUTABLE TO COMMON STOCKHOLDERS | ||||||||
| BASIC | $ | (0.11 | ) | $ | (1.31 | ) | ||
| DILUTED | $ | (0.00 | ) | $ | (1.31 | ) | ||
| WEIGHTED AVERAGE SHARES OUTSTANDING | ||||||||
| BASIC | 3,084 | 973 | ||||||
| DILUTED | 109,899 | 973 | ||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| F-22 |
GUIDED THERAPEUTICS INC. AND SUBSIDIARY | ||||||||
| CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS | ||||||||
| (Unaudited, in Thousands) | ||||||||
FOR THE THREE MONTHS ENDED MARCH 31, | ||||||||
| 2016 | 2015 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net income (loss) | $ | 130 | $ | (1,245 | ) | |||
| Adjustments to reconcile net income (loss) to net cash used in operating activities: | ||||||||
| Bad debt expense | 17 | — | ||||||
| Depreciation | 54 | 72 | ||||||
| Amortization | 54 | 402 | ||||||
| Stock based compensation | 30 | 192 | ||||||
| Change in fair value of warrants | (1,395 | ) | (714 | ) | ||||
| Changes in operating assets and liabilities: | ||||||||
| Inventory | (135 | ) | (20 | ) | ||||
| Accounts receivable | (56 | ) | 31 | |||||
| Other current assets | 271 | 47 | ||||||
| Other assets | 18 | 8 | ||||||
| Accounts payable | 153 | (169 | ) | |||||
| Deferred revenue | (151 | ) | — | |||||
| Accrued liabilities | 55 | 508 | ||||||
| Other long-term liabilities | — | 262 | ||||||
| Total adjustments | (1,085 | ) | 619 | |||||
| Net cash used in operating activities | (955 | ) | (626 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Net proceeds from issuance of common stock and warrants, net | — | 451 | ||||||
| Proceeds from debt financing, net of discounts and debt issuance costs | 1,029 | 62 | ||||||
| Payments made on notes payable | (53 | ) | (31 | ) | ||||
| Net cash provided by financing activities | 976 | 482 | ||||||
| NET CHANGE IN CASH AND CASH EQUIVALENTS | 21 | (144 | ) | |||||
| CASH AND CASH EQUIVALENTS, beginning of year | 35 | 162 | ||||||
| CASH AND CASH EQUIVALENTS, end of period | $ | 56 | $ | 18 | ||||
| SUPPLEMENTAL SCHEDULE OF: | ||||||||
| Cash paid for: | ||||||||
| Interest | $ | 1 | $ | 22 | ||||
| NONCASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Issuance of common stock as debt repayment | $ | 125 | $ | 50 | ||||
| Dividends on preferred stock | $ | 470 | $ | 31 | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| F-23 |
GUIDED THERAPEUTICS, INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
1. BASIS OF PRESENTATION
The accompanying unaudited condensed consolidated financial statements included herein have been prepared in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”) for interim financial reporting and with the instructions to Form 10-Q and Article 10 of Regulation S-X by Guided Therapeutics, Inc. (formerly SpectRx, Inc.), together with its wholly owned subsidiary InterScan, Inc., (“Interscan”) (formerly Guided Therapeutics, Inc.), collectively referred to herein as the “Company”. Accordingly, they do not include all information and footnotes required by GAAP for complete financial statements. These statements reflect adjustments, all of which are of a normal, recurring nature, and which are, in the opinion of management, necessary to present fairly the Company’s financial position as of March 31, 2016, results of operations for the three months ended March 31, 2016 and 2015, and cash flows for the three months ended March 31, 2016 and 2015. The results of operations for the three months ended March 31, 2016 are not necessarily indicative of the results for a full fiscal year. Preparing financial statements requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses and disclosure of contingent assets and liabilities. Actual results could differ from those estimates. These financial statements should be read in conjunction with the financial statements and notes thereto included in the Company’s annual report on Form 10-K for the year ended December 31, 2015.
On February 24, 2016, the Company implemented a 1:100 reverse stock split of its issued and outstanding common stock. The reverse stock split decreased the Company’s issued and outstanding shares of common stock from 237,101,702 shares of Common Stock to 2,371,007 shares as of that date. See Note 4, Stockholders’ Deficit. Unless otherwise specified, all per share amounts are reported on a post-stock split basis, as of March 31, 2016.
The Company's prospects must be considered in light of the substantial risks, expenses and difficulties encountered by entrants into the medical device industry. This industry is characterized by an increasing number of participants, intense competition and a high failure rate. The Company has experienced net losses since its inception and, as of March 31, 2016, it had an accumulated deficit of approximately $122.9 million. Through March 31, 2016, the Company has devoted substantial resources to research and development efforts. The Company does not have significant experience in manufacturing, marketing or selling its products. The Company's development efforts may not result in commercially viable products and it may not be successful in introducing its products. Moreover, required regulatory clearances or approvals may not be obtained. The Company's products may not ever gain market acceptance and the Company may not ever achieve levels of revenue to sustain further development costs and support ongoing operations or achieve profitability. The development and commercialization of the Company's products will require substantial development, regulatory, sales and marketing, manufacturing and other expenditures. The Company expects operating losses to continue through the foreseeable future as it continues to expend substantial resources to complete development of its products, obtain regulatory clearances or approvals and conduct further research and development.
Going Concern
The Company’s consolidated financial statements have been prepared and presented on a basis assuming it will continue as a going concern. The factors below raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might be necessary from the outcome of this uncertainty. If the Company is unable to continue operations, it may have to the extent practicable, liquidate and/or file for bankruptcy protection.
Liquidity
At March 31, 2016, the Company had a working capital deficit of approximately $4.0 million and the stockholders’ deficit was approximately $5.5 million, primarily due to recurring net losses from operations and dividends on preferred stock, offset by proceeds from the exercise of options and warrants and proceeds from the sales of stock.
The Company’s plans with respect to its liquidity management include the following:
| • | The Company has curtailed operations and reduced discretionary spending and staffing levels. |
| • | The Company is only pursuing activities where it has financial support. However, there can be no assurance that such external financial support will be sufficient to maintain even limited operations. |
| • | The Company is seeking additional capital in the private and/or public equity markets to continue operations and build sales, marketing, and distribution. The Company is currently evaluating additional equity and debt financing opportunities and may execute them when appropriate. However, there can be no assurances that the Company can consummate such a transaction, or consummate a transaction at favorable pricing. |
| F-24 |
| • | The Company is seeking additional sources of cash flow with strategic businesses. |
| • | The Company had warrants exercisable for approximately 31.8 million shares of its common stock outstanding at March 31, 2016, with exercise prices of $0.09375 to $105.00 per share. Exercises of these warrants would generate a total of approximately $7.1 million in cash, assuming full exercise, although the Company cannot be assured that holders will exercise any warrants |
2. SIGNIFICANT ACCOUNTING POLICIES
The Company’s significant accounting policies were set forth in the audited financial statements and notes thereto for the year ended December 31, 2015 included in its annual report on Form 10-K, filed with the Securities and Exchange Commission (“SEC”).
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant areas where estimates are used include the allowance for doubtful accounts, inventory valuation and input variables for Black-Scholes, Monte Carlo simulations and binomial calculations.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Guided Therapeutics, Inc. and its wholly owned subsidiary.
Accounting Standard Updates
In April 2015, the FASB issued Accounting Standards Update No. 2015-03, Interest - Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs, or ASU 2015-03. ASU 2015-03 amends current presentation guidance by requiring that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. Prior to the issuance of ASU 2015-03 debt issuance costs were required to be presented as an asset in the balance sheet. On January 1, 2016, the Company adopted the provisions of ASU 2015-03 and prior period amounts have been reclassified to conform to the current period presentation
Accounts Receivable
The Company performs periodic credit evaluations of its customers’ financial conditions and generally does not require collateral. The Company reviews all outstanding accounts receivable for collectability on a quarterly basis. An allowance for doubtful accounts is recorded for any amounts deemed uncollectable. The Company does not accrue interest receivable on past due accounts receivable.
Revenue Recognition
Revenue from the sale of the Company’s products is recognized upon shipment of such products to its customers. The Company recognizes revenue from contracts on a straight line basis, over the terms of the contract. The Company recognizes revenue from grants based on the grant agreement, at the time the expenses are incurred.
During the three months ended March 31, 2016, the majority of the Company’s revenues were from four customers. Revenue from these customers totaled approximately $262,000 or 100% for the period ended March 31, 2016 and approximately $127,000 or 73% of total revenue for the period ended March 31, 2015. Accounts receivable due from those customers represents 59% for the period ended March 31, 2016.
Deferred Revenue
The Company defers payments received as revenue until earned based on the related contracts on a straight line basis, over the terms of the contract.
| F-25 |
Inventory Valuation
All inventories are stated at lower of cost or market, with cost determined substantially on a “first-in, first-out” basis. Selling, general, and administrative expenses are not inventoried, but are charged to expense when purchased. At March 31, 2016 and December 31, 2015, our inventories were as follows (in thousands):
| March 31, | December 31, | |||||||
| 2016 | 2015 | |||||||
| Raw materials | $ | 827 | $ | 686 | ||||
| Work in process | 256 | 186 | ||||||
| Finished goods | 326 | 365 | ||||||
| Inventory reserve | (156 | ) | (118 | ) | ||||
| Total | $ | 1,253 | $ | 1,119 | ||||
Property and Equipment
Property and equipment are recorded at cost. Depreciation is computed using the straight-line method over estimated useful lives of three to seven years. Leasehold improvements are depreciated at the shorter of the useful life of the asset or the remaining lease term. Depreciation expense is included in general and administrative expense on the statement of operations. Expenditures for repairs and maintenance are expensed as incurred. Property and equipment are summarized as follows at March 31, 2016 and December 31, 2015 (in thousands):
| March 31, | December 31, | |||||||
| 2016 | 2015 | |||||||
| Equipment | $ | 1,377 | $ | 1,377 | ||||
| Software | 740 | 740 | ||||||
| Furniture and fixtures | 124 | 124 | ||||||
| Leasehold Improvement | 199 | 199 | ||||||
| 2,440 | 2,440 | |||||||
| Less accumulated depreciation | (2,174 | ) | (2,122 | ) | ||||
| Total | $ | 264 | $ | 318 | ||||
Accrued Liabilities
Accrued liabilities are summarized as follows (in thousands):
March 31, 2016 | December 31, 2014 | |||||||
| Accrued compensation | $ | 1,249 | $ | 1,235 | ||||
| Accrued professional fees | 71 | 154 | ||||||
| Deferred rent | 31 | 36 | ||||||
| Accrued warranty | 70 | 82 | ||||||
| Accrued vacation | 190 | 177 | ||||||
| Accrued interest | 33 | — | ||||||
| Accrued dividends | 332 | 167 | ||||||
| Other accrued expenses | 93 | 56 | ||||||
| Total | $ | 2,069 | $ | 1,907 | ||||
| F-26 |
Significant Customers
Research and Development
Research and development expenses consist of expenditures for research conducted by the Company and payments made under contracts with consultants or other outside parties and costs associated with internal and contracted clinical trials. All research and development costs are expensed as incurred.
Income Taxes
The Company uses the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Management provides valuation allowances against the deferred tax assets for amounts that are not considered more likely than not to be realized.
The Company is current with its federal and applicable state tax returns filings. Although we have been experiencing recurring losses, we are obligated to file tax returns for compliance with Internal Revenue Service (“IRS”) regulations and that of applicable state jurisdictions. As December 31, 2015, the Company has approximately $28.0 million of net operating loss eligible to be carried forward for tax purposes at federal and applicable states level. A full valuation allowance has been recorded related the deferred tax assets generated from the net operating losses.
None of the Company’s federal or state income tax returns are currently under examination by the IRS or state authorities.
Uncertain Tax Positions
Effective January 1, 2007 the Company adopted ASC guidance regarding accounting for uncertainty in income taxes. This guidance clarifies the accounting for income taxes by prescribing the minimum recognition threshold an income tax position is required to meet before being recognized in the financial statements and applies to all income tax positions. Each income tax position is assessed using a two-step process. A determination is first made as to whether it is more likely than not that the income tax position will be sustained, based upon technical merits, upon examination by the taxing authorities. If the income tax position is expected to meet the more likely than not criteria, the benefit recorded in the financial statements equals the largest amount that is greater than 50% likely to be realized upon its ultimate settlement. At March 31, 2016 and December 31, 2015 there were no uncertain tax positions.
Warrants
The Company has issued warrants, which allow the warrant holder to purchase one share of stock at a specified price for a specified period of time. The Company records equity instruments including warrants issued to non-employees based on the fair value at the date of issue. The fair value of warrants classified as equity instruments at the date of issuance is estimated using the Black-Scholes Model. The fair value of warrants classified as liabilities at the date of issuance is estimated using the Monte Carlo Simulation or Binomial model.
Stock Based Compensation
The Company records compensation expense related to options granted to non-employees based on the fair value of the award.
Compensation cost is recorded as earned for all unvested stock options outstanding at the beginning of the first year based upon the grant date fair value estimates, and for compensation cost for all share-based payments granted or modified subsequently based on fair value estimates.
For the three ended March 31, 2016 and 2015 share-based compensation for options attributable to employees, officers and Board members were approximately $30,053 and $191,570. These amounts have been included in the Company’s statements of operations. Compensation costs for stock options which vest over time are recognized over the vesting period. As of March 31, 2016, the Company had approximately $202,000 of unrecognized compensation costs related to granted stock options that will be recognized over the remaining vesting period of approximately three years.
3. FAIR VALUE OF FINANCIAL INSTRUMENTS
The guidance for fair value measurements, ASC820, Fair Value Measurements and Disclosures, establishes the authoritative definition of fair value, sets out a framework for measuring fair value, and outlines the required disclosures regarding fair value measurements. Fair value is the price that would be received to sell an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. The Company uses a three-tier fair value hierarchy based upon observable and non-observable inputs as follow:
| F-27 |
| · | Level 1 – Quoted market prices in active markets for identical assets and liabilities; |
| · | Level 2 – Inputs, other than level 1 inputs, either directly or indirectly observable; and |
| · | Level 3 – Unobservable inputs developed using internal estimates and assumptions (there is little or no market date) which reflect those that market participants would use. | |
The Company records its derivative activities at fair value, which consisted of warrants as of March 31, 2016. The fair value of the warrants was estimated using the Binomial Simulation model. Gains and losses from derivative contracts are included in net gain (loss) from derivative contracts in the statement of operations. The fair value of the Company’s derivative warrants is classified as a Level 3 measurement, since unobservable inputs are used in the valuation.
The following table presents the fair value for those liabilities measured on a recurring basis as of March 31, 2016 and December 31, 2015:
FAIR VALUE MEASUREMENTS (In Thousands)
The following is summary of items that the Company measures at fair value on a recurring basis:
| Fair Value at March 31, 2016 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Warrants issued in connection with the issuance of Series C preferred stock | $ | - | $ | - | $ | (70 | ) | $ | (70 | ) | ||||||
| Warrants issued in connection with the issuance of Series B preferred stock | - | - | (612 | ) | (612 | ) | ||||||||||
| Warrants issued in connection with Senior Secured Debt | - | - | (906 | ) | (906 | ) | ||||||||||
| Total long-term liabilities at fair value | $ | - | $ | - | $ | (1,588 | ) | $ | (1,588 | ) | ||||||
|
Fair Value at December 31, 2015 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Warrants issued in connection with the issuance of Series C preferred stock | $ | — | $ | — | $ | (1,145 | ) | $ | (1,145 | ) | ||||||
| Warrants issued in connection with the issuance of Series B preferred stock | — | — | (1,461 | ) | (1,461 | ) | ||||||||||
| Total long-term liabilities at fair value | $ | — | $ | — | $ | (2,606 | ) | $ | (2,606 | ) | ||||||
The following is a summary of changes to Level 3 instruments during the three months ended March 31, 2016:
Fair Value Measurements Using Significant Unobservable Inputs (Level 3) | ||||||||||||||||||||||
| Senior Secured Debt | Series B Warrants | Series C Warrants | Total | |||||||||||||||||||
| Balance, December 31, 2015 | $ | — | $ | (1,461 | ) | $ | (1,145 | ) | $ | (2,606 | ) | |||||||||||
| Warrants issued during the period | (377 | ) | — | — | (377 | ) | ||||||||||||||||
| Change in fair value during the period | (529 | 849 | 1,075 | 1,395 | ||||||||||||||||||
| Balance, March 31, 2016 | $ | (906 | $ | (612 | ) | $ | (70 | ) | $ | (1,588 | ) | |||||||||||
| F-28 |
As of March 31, 2016, the fair value of warrants was approximately $1.6 million. A net change of approximately $1.4 million has been recorded to the accompanying statement of operations for the three months ended.
4. STOCKHOLDERS’ DEFICIT
Common Stock
The Company has authorized 1,000,000,000 shares of common stock with $0.001 par value, of which 5,322,003 were issued and outstanding as of March 31, 2016. For the year ended December 31, 2015, there were 1,000,000,000 authorized shares of common stock, of which 2,371,017 were issued and outstanding.
On February 24, 2016, the Company implemented a 1:100 reverse stock split of all of its issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock of the Company were converted into 1 share. All fractional shares created by the reverse stock split were rounded to the nearest whole share. The number of the authorized shares did not change.
For the three months ended March 31, 2016, the Company issued 2,950,986 shares of common stock as listed below:
| Series C Preferred Stock Conversions | 1,511,350 | |
| Series C Preferred Stock Dividends | 631,047 | |
| Convertible Debt Conversions | 808,589 | |
| Total | 2,950,986 |
Preferred Stock
The Company has authorized 5,000,000 shares of preferred stock with a $.001 par value. The board of directors has the authority to issue these shares and to set dividends, voting and conversion rights, redemption provisions, liquidation preferences, and other rights and restrictions. The board of directors designated 525,000 shares of preferred stock redeemable convertible preferred stock, none of which remain outstanding, 3,000 shares of preferred stock as Series B Preferred Stock, of which zero shares were issued and outstanding at both March 31, 2016 and December 31, 2015 and 9,000 shares of preferred stock as Series C Convertible Preferred Stock, of which 4,966 and 5,555 were issued and outstanding at March 31, 2016 and December 31, 2015, respectively.
On May 3, 2016, the board of directors designated 20,250 shares of preferred stock as Series C1 Convertible Preferred Stock. See Note 10, Subsequent Events.
Series C Convertible Preferred Stock
On June 29, 2015, the Company entered into a securities purchase agreement with certain accredited investors for the issuance and sale of an aggregate of 6,737 shares of Series C convertible preferred stock, at a purchase price of $750 per share and a stated value of $1,000 per share. On September 3, 2015 the Company entered into an interim agreement amending the securities purchase agreement to provide for certain of the investors to purchase an additional aggregate of 1,166 shares. Total cash and non-cash expenses were valued at $853,000, resulting in net proceeds of $3,698,000.
Pursuant to the Series C certificate of designations, shares of Series C preferred stock are convertible into common stock by their holder at any time, and may be mandatorily convertible upon the achievement of specified average trading prices for the Company’s common stock. At March 31, 2016, there were 4,966 shares outstanding with a conversion price of $0.09375 per share, such that each share of Series C preferred stock would convert into approximately 52,977,774 shares of the Company’s common stock, subject to customary adjustments, including for any accrued but unpaid dividends and pursuant to certain anti-dilution provisions, as set forth in the Series C certificate of designations. The conversion price will automatically adjust downward to 80% of the then-current market price of the Company’s common stock 15 trading days after any reverse stock split of the Company’s common stock, and 5 trading days after any conversions of the Company’s outstanding convertible debt.
| F-29 |
Holders of the Series C preferred stock are entitled to quarterly cumulative dividends at an annual rate of 12.0% until 42 months after the original issuance date (the “Dividend End Date”), payable in cash or, subject to certain conditions, the Company’s common stock. In addition, upon conversion of the Series C Preferred Stock prior to the Dividend End Date, the Company will also pay to the converting holder a “make-whole payment” equal to the amount of unpaid dividends through the Dividend End Date on the converted shares. The Series C preferred stock generally has no voting rights except as required by Delaware law. Upon the Company’s liquidation or sale to or merger with another corporation, each share will be entitled to a liquidation preference of $1,000, plus any accrued but unpaid dividends.
In addition, the purchasers of the Series C preferred stock received, on a pro rata basis, warrants exercisable to purchase an aggregate of approximately 1,247,737 shares of Company’s common stock. The warrants contain anti-dilution adjustments in the event that the Company issues shares of common stock, or securities exercisable or convertible into shares of common stock, at prices below the exercise price of such warrants. As a result of the anti-dilution protection, the Company is required to account for the warrants as a liability recorded at fair value each reporting period. At March 31, 2016, the exercise price per share was $0.09375.
Warrants
The following table summarizes transactions involving the Company’s outstanding warrants to purchase common stock for the quarter ended March 31, 2016:
Warrants (Underlying Shares) | ||||
| Outstanding, January 1, 2016 | 2,802,384 | |||
| Issuances | 28,952,716 | |||
| Canceled / Expired | — | |||
| Exercised | — | |||
| Outstanding, March 31, 2016 | 31,755,100 | |||
The Company had the following shares reserved for the warrants as of March 31, 2016:
|
Warrants |
Exercise Price |
Expiration Date | |
| 4,399 | (1) | $68.00 per share | April 1, 2016 |
| 2,852 | (2) | $105.00 per share | November 20, 2016 |
| 18,581 | (3) | $10.46 per share | May 23, 2018 |
| 14,176,202 | (3) | $0.09375 per share | May 23, 2018 |
| 2,000 | (4) | $50.00 per share | April 23, 2019 |
| 5,618 | (4) | $45.00 per share | May 22, 2019 |
| 1,842 | (5) | $38.00 per share | September 10, 2019 |
| 3,255 | (6) | $46.081 per share | September 27, 2019 |
| 7,553 | (7) | $28.13 per share | December 2, 2019 |
| 83,927 | (8) | $9.00 per share | December 2, 2020 |
| 83,927 | (8) | $11.00 per share | December 2, 2020 |
| 20,000 | (9) | $25.50 per share | March 30, 2018 |
| 17,547 | (10) | $11.88 per share | June 29, 2020 |
| 526,421 | (11) | $1.03 per share | June 29, 2020 |
| 273,684 | (12) | $1.03 per share | September 4, 2020 |
| 289,737 | (13) | $1.03 per share | September 21, 2020 |
| 5,163 | (14) | $11.88 per share | September 4, 2020 |
| 157,895 | (15) | $1.03 per share | October 23, 2020 |
| 5,163 | (16) | $11.88 per share | October 23, 2020 |
| 16,069,333 | (17) | $0.09375 per share | February 12, 2021 |
| 31,755,100 |
| (1) | Issued in February 2014 as part of a buy-back of a minority interest in Interscan in December 2012. |
| (2) | Issued as part of a November 2011 private placement. |
| F-30 |
| (3) |
Issued in June 2015 in exchange for warrants originally issued as part of a May 2013 private placement. These warrants have anti-dilution and price protection and adjust periodically as described later in the notes. |
| (4) | Issued to a placement agent in conjunction with an April 2014 private placement. |
| (5) | Issued to a placement agent in conjunction with a September 2014 private placement. |
| (6) | Issued as part of a September 2014 Regulation S offering. |
| (7) | Issued to a placement agent in conjunction with a 2014 public offering. |
| (8) | Issued in June 2015 in exchange for warrants originally issued as part of a 2014 public offering. |
| (9) | Issued as part of a March 2015 private placement. |
| (10) | Issued to a placement agent in conjunction with a June 2015 private placement. |
| (11) | Issued as part of a June 2015 private placement. |
| (12) | Issued as part of a June 2015 private placement. |
| (13) | Issued as part of a June 2015 private placement. |
| (14) | Issued to a placement agent in conjunction with a June 2015 private placement. |
| (15) | Issued as part of a June 2015 private placement. |
| (16) | Issued to a placement agent in conjunction with a June 2015 private placement. |
| (17) |
Issued to a placement agent and a senior secured convertible note holder. These warrants have anti-dilution and price protection and adjust periodically as described later in the notes. |
5. STOCK OPTIONS
Under the Company’s 1995 Stock Plan (the “Plan”), a total of 26,616 shares remained available at March 31, 2016 and 98,451 shares were subject to stock options outstanding as of that date, bringing the total number of shares subject to stock options outstanding and those remaining available for issue to 132,552 shares of common stock as of March 31, 2016. The Plan allows the issuance of incentive stock options, nonqualified stock options, and stock purchase rights. The exercise price of options is determined by the Company’s board of directors, but incentive stock options must be granted at an exercise price equal to the fair market value of the Company’s common stock as of the grant date. Options historically granted have generally become exercisable over four years and expire ten years from the date of grant.
The fair value of stock options granted in the period ended March 31, 2015 were estimated using the Black-Scholes option pricing model. No options were issued during the period ended March 31, 2016.
Stock option activity for March 31, 2016 as follows:
| 2016 | |||||||
Weighted Average Exercise | |||||||
| Shares | Price | ||||||
| Outstanding at beginning of year | 105,936 | $ | 45.00 | ||||
| Options granted | — | $ | — | ||||
| Options exercised | — | $ | — | ||||
| Options expired/forfeited | (7,485 | ) | $ | 43.00 | |||
| Outstanding at end of year | 98,451 | $ | 45.00 | ||||
6. LITIGATION AND CLAIMS
From time to time, the Company may be involved in various legal proceedings and claims arising in the ordinary course of business. Management believes that the dispositions of these matters, individually or in the aggregate, are not expected to have a material adverse effect on the Company’s financial condition. However, depending on the amount and timing of such disposition, an unfavorable resolution of some or all of these matters could materially affect the future results of operations or cash flows in a particular period.
As of March 31, 2016 and December 31, 2015, there was no accrual recorded for any potential losses related to pending litigation.
7. NOTES PAYABLE
| F-31 |
Short Term Notes Payable
At March 31, 2016 and December 31, 2015, the Company maintained notes payable and accrued interest to related parties totaling $241,000 and $682,000, respectively. These notes are short term, straight-line amortizing notes. The notes carry an annual interest rate of between 5% and 10%.
At March 31, 2016, the Company maintained a note payable to Premium Assignment Corporation, an insurance premium financing company, of approximately $17,500. This note is 10 month straight-line amortizing loan dated June 24, 2015. The note carries annual interest of 5.2%. The balance due to on the Premium Assignment note was approximately $17,500 and $70,000 at March 31, 2016 and December 31, 2015, respectively.
Notes Payable in Default
At March 31, 2016, and December 31, 2015 the Company maintained notes payable and accrued interest to related and unrelated parties totaling $590,000 and $133,000, respectively. The notes carry interest at 5% to 10% and have default rates as high a 16.5%. As of March 31, 2016 the notes are accruing interest at the default rate.
8. CONVERTIBLE DEBT
On September 10, 2014, the Company sold a secured promissory note to an accredited investor with an initial principal amount of $1,275,000, for a purchase price of $700,000 (an original issue discount of $560,000). The Company may prepay the note at any time. The note is secured by the Company’s current and future accounts receivable and inventory, pursuant to a security agreement entered into in connection with the sale. On March 10, 2015, May 4, 2015, June 1, 2015, June 16, 2015, June 29, 2015, January 21, January 29 and February 12, 2016 the Company amended the terms of the note to extend the maturity ultimately until August 31, 2016. During the extension, interest accrues on the note at a rate of the lesser of 18% per year or the maximum rate permitted by applicable law. On February 11, 2016, the Company consented to an assignment of the note to two accredited investors. In connection with the assignment, the holders waived an ongoing event of default under the notes related to the Company’s minimum market capitalization, and agreed to eliminate the requirement going forward. Pursuant to the terms of the amended note, the holder may convert the outstanding balance into shares of common stock at a conversion price per share equal to the lower of (1) $25.0 or (2) 75% of the lowest daily volume weighted average price of the common stock during the five days prior to conversion. If the conversion price at the time of any conversion is lower than $15.00, the Company has the option of delivering the conversion amount in cash in lieu of shares of common stock. On March 7, 2016, the Company further amended the notes to eliminate the volume limitations on sales of common stock issued or issuable upon conversion of the notes.
The balance due related to this note was $591,244 and $685,864 at March 31, 2016 and December 31, 2015, respectively.
Total debt issuance costs originally capitalized was approximately $130,000. This amount was being amortized over six months and is fully amortized as of March 31, 2015. Total amortized expense for the three months ended March 31, 2015 was approximately $49,000. For the three months ended March 31, 2015, the Company recorded amortization of approximately $213,000 on the discount. The original issue discount of $560,000 was fully amortized as of March 31, 2015.
On February 11, 2016, the Company entered into a securities purchase agreement with an accredited investor for the issuance and sale on February 12, 2016 of $1.4375 million in aggregate principal amount of a senior secured convertible note for an aggregate purchase price of $1.15 million (a 20% original issue discount of $287,500) and a discount for debt issuance costs paid at closing of $121,000 for a total of $408,500. In addition, the investor received a warrant exercisable to purchase an aggregate of approximately 1,796,875 million shares of the Company’s common stock. The Company allocated proceeds totaling 359,555 to the fair value of the warrants at issuance. This was recorded as an additional discount on the debt. The convertible note matures on the second anniversary of issuance and, in addition to the 20% original issue discount, accrues interest at a rate of 17% per year. The Company will pay monthly interest coupons and, beginning six months after issuance, will pay amortized quarterly principal payments. If the Company does not receive, on or before the first anniversary after issuance, an aggregate of at least $3.0 million from future equity or debt financings or non-dilutive grants, then the holder will have the option of accelerating the maturity date to the first anniversary of issuance. The Company may prepay the convertible note, in whole or in part, without penalty, upon 20 days’ prior written notice. Subject to resale restrictions under Federal securities laws and the availability of sufficient authorized but unissued shares of the Company’s common stock, the convertible note is convertible at any time, in whole or in part, at the holder’s option, into shares of the Company’s common stock, at a conversion price equal to the lesser of $0.80 per share or 70% of the average closing price per share for the five trading days prior to issuance, subject to certain customary adjustments and anti-dilution provisions contained in the convertible note. As of March 31, 2016, the balance on the convertible debt was $676,110 (net of debt issuance costs of $44,540 and debt discount of $716,850).
| F-32 |
The convertible note includes customary event of default provisions and a default interest rate of the lesser of 22% or the maximum amount permitted by law. Upon the occurrence of an event of default, the holder may require the Company to redeem the convertible note at 120% of the outstanding principal balance. The convertible note is secured by a lien on all of the Company’s assets, including its intellectual property, pursuant to a security agreement entered into by the Company and GPB in connection with the transaction.
The warrant is exercisable at any time, pending availability of sufficient authorized but unissued shares of the Company’s common stock, at an exercise price per share equal to the conversion price of the convertible note, subject to certain customary adjustments and anti-dilution provisions contained in the warrant. The warrant has a five-year term. As of March 31, 2016, the exercise price had been adjusted to $0.09375 and the number of common stock shares exchangeable for was 15,333,333. As of March 31, 2016, the effective interest rate considering debt costs was 29%.
The Company used a placement agent in connection with the transaction. For its services, the placement agent received a cash placement fee equal to 4% of the aggregate gross proceeds from the transaction and a warrant to purchase shares of common stock equal to an aggregate of 6% of the total number of shares underlying the securities sold in the transaction, at an exercise price equal to, and terms otherwise identical to, the warrant issued to the investor. Finally, the Company agreed to reimburse the placement agent for its reasonable out-of-pocket expenses.
In connection with the transaction, on February 12, 2016, the Company and the investor entered into a four-year consulting agreement, pursuant to which the investor will provide management consulting services to the Company in exchange for a royalty payment, payable quarterly, equal to 3.5% of the Company’s revenues from the sale of products. As of March 31, 2016 the investor had earned approximately $3,000 of royalties.
9. INCOME (LOSS) PER COMMON SHARE
Basic net income (loss) per share attributable to common stockholders amounts are computed by dividing the net income (loss) plus preferred stock dividends and deemed dividends on preferred stock by the weighted average number of shares outstanding during the period.
Diluted net income (loss) per share attributable to common stockholders amounts are computed by dividing the net income (loss) plus preferred stock dividends, deemed dividends on preferred stock, after-tax interest on convertible debt and convertible dividends by the weighted average number of shares outstanding during the period, plus Series C convertible preferred stock, convertible debt, convertible preferred dividends and warrants convertible into common stock shares.
10. SUBSEQUENT EVENTS
Between April 27, 2016 and May 3, 2016, the Company entered into various agreements with certain holders of Series C preferred stock, including John Imhoff, the chairman of the Company’s board of directors, pursuant to which those holders separately agreed to exchange each share of Series C preferred stock held for 2.25 shares of the Company’s newly created Series C1 preferred stock and 9,600 shares of the Company’s common stock (the “Series C Exchanges”). In connection with the Series C Exchanges, each holder also agreed to roll over the $1,000 stated value per share of the holder’s shares of Series C1 preferred stock into the next qualifying financing undertaken by the Company on a dollar-for-dollar basis and, except in the event of an additional $50,000 cash investment in the Company by the holder, to execute a customary “lockup” agreement in connection with the financing. In total, for 1,916 shares of Series C preferred stock surrendered, the Company issued 4,312 shares of Series C1 preferred stock and 18,396,800 shares of common stock.
The Series C1 preferred stock has terms that are substantially the same as the Series C preferred stock, except that the Series C1 preferred stock does not pay dividends (unless and to the extent declared on the common stock) or at-the-market “make-whole payments.” Separately, on April 27, 2016, the Company entered into a Rollover and Amendment Agreement with another holder of Series C preferred stock pursuant to which the holder agreed to roll over any shares of Series C preferred stock (stated value plus make-whole dividend), as well as any remaining principal and accrued interest on the Company’s convertible promissory note the holder holds, into the Company’s next financing, all on a dollar-for-dollar basis, as long as the next financing involves at least $1 million in cash from investors unaffiliated with the holder. The holder also agreed to return to the Company for cancelation warrants exercisable for 7,148,813 shares of the Company’s common stock that it holds. Except in the event of an additional $50,000 cash investment by the holder in the Company’s next financing, the holder has agreed to execute a customary “lockup” agreement in connection with the financing. Finally, the holder, as the holder of a majority of the outstanding Series C preferred stock, agreed to amend the Series C stock purchase agreement to eliminate any participation rights held by the Series C shareholders and to waive operation of certain anti-dilution provisions of the Series C that would otherwise be triggered by the transactions.
| F-33 |
On May 5, 2016 and May 9, 2016 respectively, Jonathan Niloff, age 62, and Linda Rosenstock, age 65, formally announced their decisions not to stand for reelection to the Company’s board of directors at the Company’s 2016 annual meeting of stockholders. Dr. Niloff was appointed to the board in 2010 and Dr. Rosenstock was appointed in 2012. Both of their decisions to resign were for personal reasons and were not a result of any disagreement with the Company on any matter relating to the Company’s operations, policies or practices.
| F-34 |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following table sets forth the various expenses and costs incurred or to be incurred by us in connection with the sale of the shares of common stock offered hereby, other than selling commissions, which will be borne by the selling stockholders. All the amounts shown are estimated except the SEC registration fee.
| Expense | Dollar Amount | |||
| SEC filing fee | $ | 504 | ||
| Legal fees and expenses | 250,000 | |||
| Accounting fees and expenses | 25,000 | |||
| Blue sky and related expenses | 5,800 | |||
| Miscellaneous | 8,696 | |||
| Total | $ | 290,000 | ||
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS
Section 145 of the Delaware General Corporation Law permits a corporation to include in its charter documents, and in agreements between the corporation and its directors and officers, provisions expanding the scope of indemnification beyond that specifically provided by the current law. Article VII of our certificate of incorporation provides for the indemnification of directors to the fullest extent permissible under Delaware law. Article VII of our bylaws provides for the indemnification of officers, directors and third parties acting on our behalf if such person acted in good faith and in a manner reasonably believed to be in and not opposed to our best interest and, with respect to any criminal action or proceeding, if the indemnified party had no reason to believe his conduct was unlawful.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES
On February 24, 2016, we implemented a 1:100 reverse stock split of all of our issued and outstanding common stock. As a result of the reverse stock split, every 100 shares of issued and outstanding common stock was converted into 1 share of common stock. In this Item 15, all share and per share amounts for transactions occurring prior to this date are reported on a pre-stock split basis.
November 2013 Warrant Exchange Program. In November 2013, we completed a warrant exchange program pursuant to which we exchanged warrants exercisable for a total of 3,573,691 shares of common stock, or 99.5% of the warrants eligible to participate, for new warrants exercisable for the same number of shares of common stock, but with a reduced exercise price of $0.40 per share and a shortened exercise period ending on November 27, 2013.
Simple Interest Notes. On February 20, 2014, our President and CEO, Gene Cartwright, advanced us $50,000, in cash, for a 10% simple interest note. On October 23, 2014, August 4, 2014, and May 21, 2014, he advanced us $30,000, $200,000, and $100,000, respectively, in cash for 5% simple interest notes. On December 30, 2015, he advanced us $10,000 in cash for a 6% simple interest note. On October 24, 2014, October 7, 2014, and August 26, 2014, our Senior Vice President of Engineering, Richard Fowler, advanced us $6,100, $20,000 and $75,000, respectively, in cash for 6% simple interest notes. On October 7, 2014, our Director of Marketing advanced us $10,000 in cash for a 6% simple interest note. On November 4, 2014, Richard Blumberg, one of our stockholders, advanced us $100,000 in cash for a note for $106,500 in aggregate principal and interest due November 30, 2014. On February 20, 2014, directors Michael James, Linda Rosenstock, and John Imhoff advanced us $50,000, $50,000, $50,000, and $25,000 in cash, respectively, for 10% simple interest notes.
April 2014 Senior Convertible Note Financing. On April 23, 2014, we entered into a securities purchase agreement with Magna Equities II, LLC (formerly known as Hanover Holdings I, LLC). Pursuant to the purchase agreement, we sold Magna a senior convertible note with a principal amount of $1.5 million, for a purchase price of $1.0 million (an approximately 33.3% original issue discount). As part of the sale, we also issued Magna 321,820 shares of common stock. Additionally, pursuant to the purchase agreement, Magna purchased on May 23, 2014 an additional senior convertible note with a principal amount of $2.0 million and an 18-month term, for a fixed purchase price of $2.0 million. We had the right at any time to redeem all or a portion of the total outstanding amount then remaining on the notes, at a 25% premium. As of June 30, 2015, the notes are no longer outstanding. The notes accrued interest at an annual rate of 6.0% and were convertible into shares of our common stock, at a conversion price equal to the lesser of $0.55 per share and a discount from the lowest daily volume-weighted average price of our common stock in the five trading days prior to conversion.
| II-1 |
Regulation S Offering. On September 2, 2014, we accepted a subscription agreement with ITEM Medikal Teknolojileri LTD STI, a Turkish corporation, referred to as ITEM, pursuant to we sold 651,042 shares of our common stock and a warrant to purchase an additional 325,521 shares, for an aggregate purchase price of $200,000. The warrant is immediately exercisable, has an exercise price per share of $0.4608, and expires five years from the date of issuance.
Secured Note Offering. On September 10, 2014, we entered into a note purchase agreement with Tonaquint, Inc., pursuant to which we sold a secured promissory note with an initial principal amount of $1,275,000, for a purchase price of $700,000 (an original issue discount of $560,000). We may prepay the note at any time. The note is secured by our current and future accounts receivable and inventory, pursuant to a security agreement entered into in connection with the note purchase agreement. As a result of a series of amendments to the note (since assigned to two accredited investors), the maturity date currently is August 31, 2016, and has been accruing interest at a rate of the lesser of 18% per year or the maximum rate permitted by applicable law. Pursuant to the amendments, the holders may convert the outstanding balance into shares of our common stock, at a conversion price per share equal to the lower of (1) $0.25 and (2) 75% of the lowest daily volume weighted average price per share of our common stock during the five business days prior to conversion. If the conversion price at the time of any conversion request would be lower than $0.15 per share, we have the option of delivering the conversion amount in cash in lieu of shares of our common stock.
March 2015 Private Placement. In March 2015, we entered into subscription agreements with certain accredited investors, pursuant to which we sold an aggregate of 4.0 million shares of our common stock and warrants to purchase an additional 2.0 million shares, for an aggregate purchase price of $720,000.
2015 Series B Warrant Exchanges. In order to secure the consent to an amendment to the terms of our Series B preferred stock, effective June 19, 2015 we agreed with each Series B holder to reduce the exercise price on certain “Tranche A” and “Tranche B” warrants, originally issued to the Series B holders on May 21, 2013. We reduced the “Tranche A” warrant exercise price per share from $1.08 to $0.10455, and the “Tranche B” warrant exercise price per share from $0.10455 to $0.09. We further agreed to grant the Series B holders the right to participate in our next capital-raising transaction by exchanging their shares of Series B preferred stock for the securities to be offered in any such transaction. Separately, we informed all other holders of outstanding “Tranche A” and “Tranche B” warrants that we had lowered the exercise prices per share for those warrants to $0.10455 and $0.09, respectively.
Public Offering Warrant Exchanges. On June 25 and 26, 2015, we entered into various agreements with holders of the warrants originally issued in December 2014, pursuant to which each holder separately agreed to exchange its warrant for two new warrants that, unlike the original warrant, do not contain any price- or share-reset provisions. Each new warrant is exercisable for the same number of shares of our common stock as the original warrant, at any time beginning December 2, 2015 and ending December 2, 2020. The exercise price of the first new warrant is $0.09 per share and the second new warrant is $0.11 per share but, aside from the exercise price, the new warrants are identical in terms to each other. As additional consideration, we agreed to issue an aggregate of approximately 3.1 million shares of our common stock to the holders, pro rata based on the amount of shares underlying their original warrants. The holders further agreed to amend to the securities purchase agreement, dated December 2, 2014 that governed (among other things) the issuance of the original warrants.
In connection with the agreement with one of the holders, Magna Equities II, LLC, on June 25, 2015 we also agreed to exchange an outstanding senior convertible note held by Magna for a new note, in stated principal amount equal to the then-outstanding principal on the original note. The new note had the same terms as the original note, except that, among other things, Magna was temporarily prohibited from converting any of the balance into shares of our common stock. On June 30, 2015, we paid in full the entire balance of the new note.
Series C Financing. On June 29, 2015, we entered into a securities purchase agreement with certain accredited investors, amended on September 3, 2015, for the issuance and sale of shares of an aggregate of 7,903 shares of our Series C preferred stock, at a purchase price of $750 per share and an initial conversion price of $0.095 per share, and five-year warrants exercisable to purchase an aggregate of approximately 124.8 million shares of our common stock at an initial exercise price of $0.095 per share, subject to certain customary adjustments and anti-dilution provisions.
| II-2 |
Senior Secured Convertible Note. On February 11, 2016, we entered into a securities purchase agreement with GPB Debt Holdings II LLC for the issuance and sale on February 12, 2016 of approximately $1.4 million in aggregate principal amount of a senior secured convertible note for an aggregate purchase price of $1.15 million (a 20% original issue discount). The note matures on the second anniversary of issuance and, in addition to the 20% original issue discount, accrues interest at a rate of 17% per year. Subject to certain restrictions, it is convertible at any time, in whole or in part, at the holder’s option, into shares of our common stock, at a conversion price equal to $0.80 per share, subject to certain customary adjustments and anti-dilution provisions. In addition, the investor received a five-year warrant exercisable to purchase an aggregate of approximately 1.79 million shares of our common stock with an exercise price of $0.80 per share, subject to certain customary adjustments and anti-dilution provisions. On March 17, 2016, our placement agent for the transaction received a warrant with similar terms exercisable for 86,250 shares. In connection with the transaction, on February 12, 2016, we and the investor entered into a four-year consulting agreement, pursuant to which the investor will provide management consulting services to us in exchange for a royalty payment, payable quarterly, equal to 3.5% of our revenues from the sale of products.
Series C Exchanges. Between April 27, 2016 and May 3, 2016, we entered into various agreements with certain holders of our Series C preferred stock, including John Imhoff, one of our directors, pursuant to which those holders separately agreed to exchange each share of Series C preferred stock held for 2.25 shares of our newly created Series C1 preferred stock and 9,600 shares of our common stock. In connection with these exchanges, each holder also agreed to exchange the $1,000 stated value per share of the holder’s shares of Series C1 preferred stock for new securities that we issue in the next qualifying financing we undertake on a dollar-for-dollar basis. We expect this offering will be a qualifying financing. Finally, each holder agreed, except in the event of an additional $50,000 cash investment in the financing by the holder, to execute a customary “lockup” agreement in connection with the qualifying financing. In total, for 1,916 shares of Series C preferred stock surrendered, we issued 4,311 shares of Series C1 preferred stock and 18,396,800 shares of common stock. The Series C1 preferred stock has terms that are substantially the same as the Series C preferred stock, except that the Series C1 preferred stock does not pay dividends (unless and to the extent declared on the common stock) or at-the-market “make-whole payments.”
Separately, on April 27, 2016, we entered into a rollover and amendment agreement with another holder of Series C preferred stock, Aquarius Opportunity Fund, pursuant to which Aquarius agreed to exchange any shares of Series C preferred stock (stated value plus make-whole dividend), as well as any remaining principal and accrued interest on our convertible promissory note Aquarius holds, for new securities that we issue in our next financing, all on a dollar-for-dollar basis, as long as the next financing involves at least $1 million in cash from investors unaffiliated with Aquarius. We expect this offering will be a qualifying financing. Aquarius also agreed to return to us for cancelation warrants exercisable for 7,148,813 shares of our common stock that it held. Except in the event of an additional $50,000 cash investment by Aquarius in the qualifying financing, Aquarius has agreed to execute a customary “lockup” agreement in connection with the financing. Finally, Aquarius, as the holder of a majority of the outstanding Series C preferred stock, agreed to amend the Series C stock purchase agreement to eliminate any participation rights held by the Series C shareholders and to waive operation of certain anti-dilution provisions of the Series C that would otherwise be triggered by the transactions described above.
Shenghuo License Agreement. On June 5, 2016, we entered into a license agreement with Shenghuo Medical, LLC pursuant to which we granted Shenghuo an exclusive license to manufacture, sell and distribute LuViva in Taiwan, Brunei Darussalam, Cambodia, Laos, Myanmar, Philippines, Singapore, Thailand, and Vietnam. Shenghuo was already our exclusive distributor in China, Macau and Hong Kong, and the license extends to manufacturing in those countries as well. As partial consideration for, and as a condition to, the license, and to further align the strategic interests of the parties, we have agreed to issue a convertible note to Shenghuo, in exchange for an aggregate cash investment of $200,000. The note will provide for a payment to Shenghuo of $240,000, due upon consummation of any capital raising transaction by us within 90 days and with net cash proceeds of at least $1.0 million. Absent such a transaction, the payment will increase to $300,000 and will be payable by December 31, 2016. The note will accrue interest at 20% per year on any unpaid amounts due after that date. The note will be convertible into shares of our common stock at a conversion price per share of $0.017402, subject to customary anti-dilution adjustment. The note will be unsecured, and is expected to provide for customary events of default. We will also issue Shenghuo a five-year warrant exercisable immediately for approximately 13.7 million shares of common stock at an exercise price equal to the conversion price of the note, subject to customary anti-dilution adjustment.
Warrant Restructuring. Between June 13, 2016 and June 14, 2016, we entered into various agreements with holders of certain warrants (including John Imhoff, the chairman of our board of directors) originally issued in May 2013, and with GPB Debt Holdings II LLC, holder of a warrant issued February 12, 2016, pursuant to which each holder separately agreed to exchange warrants for either (1) shares of common stock equal to 166% of the number of shares of common stock underlying the surrendered warrants, or (2) new warrants exercisable for 200% of the number of shares underlying the surrendered warrants, but without certain anti-dilution protections included with the surrendered warrants. As a result of the exchanges, we effectively eliminate any potential exponential increase in the number of underlying shares issuable upon exercise of our outstanding warrants. In total, for surrendered warrants then-exercisable for an aggregate of 94,828,888 shares of common stock (but subject to exponential increase upon operation of certain anti-dilution provisions), we issued or are obligated to issue 13,517,342 shares of common stock and new warrants that, if exercised as of June 14, 2016, would be exercisable for an aggregate of 214,189,622 shares of common stock.
| II-3 |
In certain circumstances, in lieu of presently issuing all of the shares (for each holder that opted for shares of common stock), we and the holder further agreed that we will, subject to the terms and conditions set forth in the applicable warrant exchange agreement, from time to time, be obligated to issue the remaining shares to the holder. No additional consideration will be payable in connection with the issuance of the remaining shares.
The holders that elected to receive shares for their surrendered warrants have agreed that they will not sell shares on any trading day in an amount, in the aggregate, exceeding 20% of the composite aggregate trading volume of the common stock for that trading day.
The holders that elected to receive new warrants will be required to surrender their old warrants upon consummation of this offering. The new warrants will have an initial exercise price equal to the exercise price of the surrendered warrants as of immediately prior to consummation of this offering, subject to customary “downside price protection” for as long as our common stock is not listed on a national securities exchange, and will expire five years from the date of issuance.
The sale of securities to ITEM was made to a non-U.S. person in an offshore offering in accordance with Regulation S under the Securities Act. The issuances of securities pursuant to the November 2013 warrant exchange program, the Series B warrant exchanges, the public offering warrant exchanges, the Series C exchanges and the warrant restructuring described above were exempt from registration under the Securities Act in reliance upon Section 3(a)(9) of the Securities Act as exchanges with existing securities holders exclusively where no commission or other remuneration was paid or given directly or indirectly for soliciting such exchanges. The remaining issuances of securities described above (as well as the issuances of common stock upon exercise of warrants received in the warrant exchange programs) were pursuant to private placements to accredited investors, and therefore were exempt from registration under the Securities Act in reliance upon Section 4(2) of the Securities Act as transactions by an issuer not involving a public offering. The securities described above are restricted securities for the purpose of the Securities Act. Any certificates representing the securities bear a restrictive legend providing that the securities have not been registered under the Securities Act and cannot be sold or otherwise transferred without an effective registration or an exemption therefrom. All cash proceeds from these issuances were used in product development, working capital and other general corporate purposes.
ITEM 16. EXHIBITS
(a) Exhibits
|
EXHIBIT NO. |
DESCRIPTION |
| 3.1 | Restated Certificate of Incorporation, as amended through May 3, 2016 (incorporated by reference to Exhibit 3.1 to the quarterly report on Form 10-Q for the quarter ended March 31, 2016, filed May 19, 2016) |
| 3.2 | Amended and Restated Bylaws (incorporated by reference to Exhibit 3.1 to the current report on Form 8-K, filed March 23, 2012) |
| 3.3* | Form of Certificate of Designations (Series D) |
| 4.1 | Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.1 to the amended registration statement on Form S-1/A (No. 333-22429) filed April 24, 1997) |
| 4.2 | Secured Promissory Note, dated September 10, 2014 (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed September 10, 2014) |
| 4.3 | Amendment #1 to Secured Promissory Note, dated March 10, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed March 19, 2015) |
| 4.4 | Amendment #2 to Secured Promissory Note, dated May 4, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed May 7, 2015) |
| 4.5 | Amendment #3 to Secured Promissory Note, dated June 1, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 5, 2015) |
| 4.6 | Amendment #4 to Secured Promissory Note, dated June 16, 2015 (incorporated by reference to Exhibit 10.4 to the current report on Form 8-K filed June 30, 2015) |
| 4.7 | Amendment #5 to Secured Promissory Note, dated June 29, 2015 (incorporated by reference to Exhibit 10.5 to the current report on Form 8-K filed June 30, 2015) |
| 4.8 | Amendment #6 to Secured Promissory Note, dated January 20, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed February 16, 2016) |
| 4.9 | Amendment #7 to Secured Promissory Note, dated February 11, 2016 (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed February 16, 2016) |
| 4.10 | Amendment #8 to Secured Promissory Note, dated March 7, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed March 7, 2016) |
| II-4 |
| 4.11 | Senior Secured Convertible Note, dated February 12, 2016 (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed February 12, 2016) |
| 4.12 | Form of Warrant (Standard Form) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K, filed September 14, 2010) |
| 4.13 | Form of Warrant (InterScan) (incorporated by reference to Exhibit 4.13 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014) |
| 4.14 | Form of Warrant (November 2011 Private Placement) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K/A, filed November 28, 2011) |
| 4.15 | Form of Warrant (Series B-Tranche A) (incorporated by reference to Exhibit 10.2 to amendment no. 1 to the current report on Form 8-K, filed May 23, 2013) |
| 4.16 | Form of Warrant (Series B-Tranche B) (incorporated by reference to Exhibit 10.3 to amendment no. 1 to the current report on Form 8-K, filed May 23, 2013) |
| 4.17 | Form of Warrant (Regulation S) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K, filed September 8, 2014) |
| 4.18 | Form of Warrant (2014 Public Offering Placement Agent) (incorporated by reference to Exhibit 4.2 to the current report on Form 8-K filed December 4, 2014) |
| 4.19 | Form of Warrant (2014 Public Offering Warrant Exchanges) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed June 30, 2015) |
| 4.20 | Form of Warrant (Series C) (incorporated by reference to Exhibit 4.3 to the current report on Form 8-K filed June 30, 2015) |
| 4.21 | Form of Warrant (Senior Secured Convertible Note) (incorporated by reference to Exhibit 10.5 to the current report on Form 8-K filed February 12, 2016) |
| 4.22 | Form of Warrant (Series B-Tranche B Exchanges; GPB Exchange) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed June 14, 2016) |
| 4.25* | Form of Warrant (Series D) |
| 5.1** | Opinion of Jones Day regarding validity |
| 10.1 | 1995 Stock Plan and form of Stock Option Agreement (Incorporated by reference to Exhibit 10.2 to the registration statement on Form S-1 (No. 333-22429 filed February 27, 1997) |
| 10.2 | 2005 Amendment to 1995 Stock Plan (incorporated by reference to Appendix 1 to the proxy statement on Schedule 14A, filed May 10, 2005) |
| 10.3 | 2010 Amendment to 1995 Stock Plan (incorporated by reference to Exhibit 10.3 to the registration statement on Form S-8 (File No. 333-178261), filed December 1, 2011) |
| 10.4 | 2012 Amendment to 1995 Stock Plan (incorporated by reference to Annex 1 to the proxy statement on Schedule 14A, filed April 30, 2012) |
| 10.5 | Agreement and Release, dated August 30, 2011 (incorporated by reference to 10.2 to the current report on Form 8-K, filed September 2, 2011) |
| 10.6 | Employment Agreement between the Company and Mark Faupel dated March 24, 2013 (incorporated by reference to Exhibit 10.10 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014) |
| 10.7 | Employment Agreement between the Company and Gene Cartwright, dated January 6, 2014 (incorporated by reference to Exhibit 10.11 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014). |
| 10.8 | Employment Agreement between the Company and Rick L. Fowler, automatically renewed on May 9, 2013 (incorporated by reference to Exhibit 10.12 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014) |
| 10.9 | Consulting Agreement between the Company and GPB Debt Holdings II LLC, dated February 12, 2016 (incorporated by reference to Exhibit 10.6 to the current report on Form 8-K filed February 12, 2016) |
| 10.10 | Securities Purchase Agreement (Magna Note), dated April 23, 2014, by and between the Company and Hanover Holdings I, LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed April 24, 2014). |
| 10.11 | Registration Rights Agreement (Magna Note), dated April 23, 2014, by and between the Company and Hanover Holdings I, LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed April 24, 2014) |
| 10.12 | Standstill Agreement (Magna Note), dated as of November 6, 2014, by and between the Company and Magna Equities II, LLC (incorporated by reference to Exhibit 19 to the registration statement on Form S-1 (No. 333-198733) filed November 10, 2014) |
| 10.13 | Exchange Agreement (Magna Note), dated as of June 25, 2015 (incorporated by reference to Exhibit 10.3 to the current report on Form 8-K filed June 30, 2015) |
| 10.14 | Subscription Agreement (Regulation S), accepted September 2, 2014 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed September 8, 2014) |
| II-5 |
| 10.15 | Form of Registration Rights Agreement (Regulation S), dated September 8, 2014 by and between the Company and the investor party thereto (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K, filed September 8, 2014) |
| 10.16 | Note Purchase Agreement (Secured Promissory Note), dated as of September 10, 2014, by and between the Company and Tonaquint, Inc. (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed September 10, 2014) |
| 10.17 | Security Agreement (Secured Promissory Note), dated as of September 10, 2014, by the Company and Tonaquint, Inc. (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K, filed September 10, 2014) |
| 10.18 | Form of Securities Purchase Agreement (2014 Public Offering) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed December 4, 2014) |
| 10.19 | Placement Agent Agreement (2014 Public Offering), by and between the Company and Olympus Securities, LLC (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed December 4, 2014) |
| 10.20 | Amendment to Securities Purchase Agreement (2014 Public Offering), dated as of June 26, 2015 (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed June 30, 2015) |
| 10.21 | Form of Letter Agreement (2014 Public Offering Warrant Exchanges) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 30, 2015) |
| 10.22 | Securities Purchase Agreement (Series C), dated June 29, 2015 (incorporated by reference to Exhibit 10.6 to the current report on Form 8-K filed June 30, 2015) |
| 10.23 | Registration Rights Agreement (Series C), dated June 29, 2015 (incorporated by reference to Exhibit 10.7 to the current report on Form 8-K filed June 30, 2015) |
| 10.24 | Form of Joinder Agreement (Series C) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed July 13, 2015) |
| 10.25 | Interim Securities Purchase Agreement (Series C), dated September 3, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed September 3, 2015) |
| 10.26 | Securities Purchase Agreement (Senior Secured Convertible Note), dated February 11, 2016 (incorporated by reference to Exhibit 10.3 to the current report on Form 8-K filed February 12, 2016) |
| 10.27 | Security Agreement (Senior Secured Convertible Note), dated February 11, 2016 (incorporated by reference to Exhibit 10.4 to the current report on Form 8-K filed February 12, 2016) |
| 10.28 | Rollover and Amendment Agreement, dated April 27, 2016, by and between the Company and Aquarius Opportunity Fund (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed May 3, 2016) |
| 10.29 | Form of Letter Agreement (Series C Exchanges) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed May 3, 2016) |
| 10.30 | License Agreement, dated June 5, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 8, 2016) |
| 10.31 | Form of Warrant Exchange Agreement (Warrant-for-Shares) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 14, 2016) |
| 10.32 | Form of Warrant Exchange Agreement (Warrant-for-Warrant) (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed June 14, 2016) |
| 10.33** | Form of Securities Purchase Agreement |
| 10.34** | Placement Agent Agreement, by and between the Company and Ladenburg Thalmann & Co. |
| 21.1 | Subsidiaries (incorporated by reference to Exhibit 21.1 to the registration statement on Form S-1 (No. 333-169755) filed October 5, 2010) |
| 23.1* | Consent of UHY LLP. |
| 23.2** | Consent of Jones Day (included in Exhibit 5.1). |
| 24.1 | Powers of Attorney (included at signature page). |
| 101.1* | Interactive Data File |
| *Filed herewith | |
| **To be filed via pre-effective amendment | |
| II-6 |
ITEM 17. UNDERTAKINGS
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
| II-7 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Norcross, State of Georgia, on June 28, 2016.
| GUIDED THERAPEUTICS, INC. | ||
| By: | /s/ Gene S. Cartwright | |
|
President, Chief Executive Officer and Acting Chief Financial Officer | ||
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Gene S. Cartwright with full power of substitution and resubstitution, as attorney-in-fact of the undersigned, for him and in his name, place and stead, to execute and file with the Securities and Exchange Commission (the “Commission”) under the Securities Act of 1933 any and all amendments, supplements and exhibits to this Registration Statement (including pre-effective and post-effective amendments and supplements), to execute and file any and all other applications or other documents to be filed with the Commission, such attorney to have full power to act with or without the others, and to have full power and authority to do and perform, in the name and on behalf of the undersigned, every act whatsoever necessary, advisable or appropriate to be done in the premises as fully and to all intents and purposes as the undersigned might or could do in person, hereby ratifying and approving the act of said attorney and any such substitute.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
| DATE | SIGNATURE | TITLE | ||
| June 28, 2016 | /s/ Gene S. Cartwright | President, Chief Executive Officer, Acting Chief Financial Officer (Principal Executive Officer and Principal Financial and Accounting Officer) | ||
| Gene S. Cartwright | ||||
|
June 28, 2016 |
/s/ Michael C. James |
Chairman of the Board and Director | ||
| Michael C. James | ||||
|
June 28, 2016 |
/s/ John E. Imhoff |
Director | ||
| John E. Imhoff | ||||
|
June 28, 2016 |
/s/ Jonathan M. Niloff |
Director | ||
| Jonathan M. Niloff | ||||
|
June 28, 2016 |
/s/ Linda Rosenstock |
Director | ||
| Linda Rosenstock |
| II-8 |
EXHIBIT INDEX
|
EXHIBIT NO. |
DESCRIPTION | |
| 3.1 | Restated Certificate of Incorporation, as amended through May 3, 2016 (incorporated by reference to Exhibit 3.1 to the quarterly report on Form 10-Q for the quarter ended March 31, 2016, filed May 19, 2016) | |
| 3.2 | Amended and Restated Bylaws (incorporated by reference to Exhibit 3.1 to the current report on Form 8-K, filed March 23, 2012) | |
| 3.3* | Form of Certificate of Designations (Series D) | |
| 4.1 | Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.1 to the amended registration statement on Form S-1/A (No. 333-22429) filed April 24, 1997) | |
| 4.2 | Secured Promissory Note, dated September 10, 2014 (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed September 10, 2014) | |
| 4.3 | Amendment #1 to Secured Promissory Note, dated March 10, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed March 19, 2015) | |
| 4.4 | Amendment #2 to Secured Promissory Note, dated May 4, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed May 7, 2015) | |
| 4.5 | Amendment #3 to Secured Promissory Note, dated June 1, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 5, 2015) | |
| 4.6 | Amendment #4 to Secured Promissory Note, dated June 16, 2015 (incorporated by reference to Exhibit 10.4 to the current report on Form 8-K filed June 30, 2015) | |
| 4.7 | Amendment #5 to Secured Promissory Note, dated June 29, 2015 (incorporated by reference to Exhibit 10.5 to the current report on Form 8-K filed June 30, 2015) | |
| 4.8 | Amendment #6 to Secured Promissory Note, dated January 20, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed February 16, 2016) | |
| 4.9 | Amendment #7 to Secured Promissory Note, dated February 11, 2016 (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed February 16, 2016) | |
| 4.10 | Amendment #8 to Secured Promissory Note, dated March 7, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed March 7, 2016) | |
| 4.11 | Senior Secured Convertible Note, dated February 12, 2016 (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed February 12, 2016) | |
| 4.12 | Form of Warrant (Standard Form) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K, filed September 14, 2010) | |
| 4.13 | Form of Warrant (InterScan) (incorporated by reference to Exhibit 4.13 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014) | |
| 4.14 | Form of Warrant (November 2011 Private Placement) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K/A, filed November 28, 2011) | |
| 4.15 | Form of Warrant (Series B-Tranche A) (incorporated by reference to Exhibit 10.2 to amendment no. 1 to the current report on Form 8-K, filed May 23, 2013) | |
| 4.16 | Form of Warrant (Series B-Tranche B) (incorporated by reference to Exhibit 10.3 to amendment no. 1 to the current report on Form 8-K, filed May 23, 2013) | |
| 4.17 | Form of Warrant (Regulation S) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K, filed September 8, 2014) | |
| 4.18 | Form of Warrant (2014 Public Offering Placement Agent) (incorporated by reference to Exhibit 4.2 to the current report on Form 8-K filed December 4, 2014) | |
| 4.19 | Form of Warrant (2014 Public Offering Warrant Exchanges) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed June 30, 2015) | |
| 4.20 | Form of Warrant (Series C) (incorporated by reference to Exhibit 4.3 to the current report on Form 8-K filed June 30, 2015) | |
| 4.21 | Form of Warrant (Senior Secured Convertible Note) (incorporated by reference to Exhibit 10.5 to the current report on Form 8-K filed February 12, 2016) | |
| 4.22 | Form of Warrant (Series B-Tranche B Exchanges; GPB Exchange) (incorporated by reference to Exhibit 4.1 to the current report on Form 8-K filed June 14, 2016) | |
| 4.25* | Form of Warrant (Series D) | |
| 5.1** | Opinion of Jones Day regarding validity | |
| 10.1 | 1995 Stock Plan and form of Stock Option Agreement (incorporated by reference to Exhibit 10.2 to the registration statement on Form S-1 (No. 333-22429) filed February 27, 1997) | |
| 10.2 | 2005 Amendment to 1995 Stock Plan (incorporated by reference to Appendix 1 to the proxy statement on Schedule 14A, filed May 10, 2005) | |
| 10.3 | 2010 Amendment to 1995 Stock Plan (incorporated by reference to Exhibit 10.3 to the registration statement on Form S-8 (File No. 333-178261), filed December 1, 2011) | |
| 10.4 | 2012 Amendment to 1995 Stock Plan (incorporated by reference to Annex 1 to the proxy statement on Schedule 14A, filed April 30, 2012) | |
| 10.5 | Agreement and Release, dated August 30, 2011 (incorporated by reference to 10.2 to the current report on Form 8-K, filed September 2, 2011) | |
| 10.6 | Employment Agreement between the Company and Mark Faupel dated March 24, 2013 (incorporated by reference to Exhibit 10.10 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014) | |
| 10.7 | Employment Agreement between the Company and Gene Cartwright, dated January 6, 2014 (incorporated by reference to Exhibit 10.11 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014). | |
| 10.8 | Employment Agreement between the Company and Rick L. Fowler, automatically renewed on May 9, 2013 (incorporated by reference to Exhibit 10.12 to the annual report on Form 10-K for the year ended December 31, 2013, filed March 27, 2014) | |
| 10.9 | Consulting Agreement between the Company and GPB Debt Holdings II LLC, dated February 12, 2016 (incorporated by reference to Exhibit 10.6 to the current report on Form 8-K filed February 12, 2016) | |
| 10.10 | Securities Purchase Agreement (Magna Note), dated April 23, 2014, by and between the Company and Hanover Holdings I, LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed April 24, 2014). | |
| 10.11 | Registration Rights Agreement (Magna Note), dated April 23, 2014, by and between the Company and Hanover Holdings I, LLC (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed April 24, 2014) | |
| 10.12 | Standstill Agreement (Magna Note), dated as of November 6, 2014, by and between the Company and Magna Equities II, LLC (incorporated by reference to Exhibit 19 to the registration statement on Form S-1 (No. 333-198733) filed November 10, 2014) | |
| 10.13 | Exchange Agreement (Magna Note), dated as of June 25, 2015 (incorporated by reference to Exhibit 10.3 to the current report on Form 8-K filed June 30, 2015) | |
| 10.14 | Subscription Agreement (Regulation S), accepted September 2, 2014 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed September 8, 2014) | |
| 10.15 | Form of Registration Rights Agreement (Regulation S), dated September 8, 2014 by and between the Company and the investor party thereto (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K, filed September 8, 2014) | |
| 10.16 | Note Purchase Agreement (Secured Promissory Note), dated as of September 10, 2014, by and between the Company and Tonaquint, Inc. (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K, filed September 10, 2014) | |
| 10.17 | Security Agreement (Secured Promissory Note), dated as of September 10, 2014, by the Company and Tonaquint, Inc. (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K, filed September 10, 2014) | |
| 10.18 | Form of Securities Purchase Agreement (2014 Public Offering) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed December 4, 2014) | |
| 10.19 | Placement Agent Agreement (2014 Public Offering), by and between the Company and Olympus Securities, LLC (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed December 4, 2014) | |
| 10.20 | Amendment to Securities Purchase Agreement (2014 Public Offering), dated as of June 26, 2015 (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed June 30, 2015) | |
| 10.21 | Form of Letter Agreement (2014 Public Offering Warrant Exchanges) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 30, 2015) | |
| 10.22 | Securities Purchase Agreement (Series C), dated June 29, 2015 (incorporated by reference to Exhibit 10.6 to the current report on Form 8-K filed June 30, 2015) | |
| 10.23 | Registration Rights Agreement (Series C), dated June 29, 2015 (incorporated by reference to Exhibit 10.7 to the current report on Form 8-K filed June 30, 2015) | |
| 10.24 | Form of Joinder Agreement (Series C) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed July 13, 2015) | |
| 10.25 | Interim Securities Purchase Agreement (Series C), dated September 3, 2015 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed September 3, 2015) | |
| 10.26 | Securities Purchase Agreement (Senior Secured Convertible Note), dated February 11, 2016 (incorporated by reference to Exhibit 10.3 to the current report on Form 8-K filed February 12, 2016) | |
| 10.27 | Security Agreement (Senior Secured Convertible Note), dated February 11, 2016 (incorporated by reference to Exhibit 10.4 to the current report on Form 8-K filed February 12, 2016) | |
| 10.28 | Rollover and Amendment Agreement, dated April 27, 2016, by and between the Company and Aquarius Opportunity Fund (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed May 3, 2016) | |
| 10.29 | Form of Letter Agreement (Series C Exchanges) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed May 3, 2016) | |
| 10.30 | License Agreement, dated June 5, 2016 (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 8, 2016) | |
| 10.31 | Form of Warrant Exchange Agreement (Warrant-for-Shares) (incorporated by reference to Exhibit 10.1 to the current report on Form 8-K filed June 14, 2016) | |
| 10.32 | Form of Warrant Exchange Agreement (Warrant-for-Warrant) (incorporated by reference to Exhibit 10.2 to the current report on Form 8-K filed June 14, 2016) | |
| 10.33** | Form of Securities Purchase Agreement | |
| 10.34** | Placement Agent Agreement, by and between the Company and Ladenburg Thalmann & Co. | |
| 21.1 | Subsidiaries (incorporated by reference to Exhibit 21.1 to the registration statement on Form S-1 (No. 333-169755) filed October 5, 2010) | |
| 23.1* | Consent of UHY LLP. | |
| 23.2** | Consent of Jones Day (included in Exhibit 5.1). | |
| 24.1 | Powers of Attorney (included at signature page). | |
| 101.1* | Interactive Data File | |
| *Filed herewith | ||
| **To be filed via pre-effective amendment | ||
