Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - MARINUS PHARMACEUTICALS INC | a16-14156_1ex99d1.htm |
| 8-K - 8-K - MARINUS PHARMACEUTICALS INC | a16-14156_18k.htm |
Exhibit 99.2
Mindful Innovation Exploratory Clinical Study Evaluating Ganaxolone for Behaviors in Children with Fragile X Syndrome June 28, 2016

Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding Marinus, they are forward-looking statements reflecting the current beliefs and expectations of management. Words such as “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Examples of forward looking statements contained in this presentation include, among others, statements regarding our ability to develop and commercialize ganaxolone; status, timing and results of preclinical studies and clinical trials; the potential benefits of ganaxolone; the timing of seeking regulatory approval of ganaxolone; our ability to obtain and maintain regulatory approval; our estimates of expenses and future revenue and profitability; our estimates regarding our capital requirements and our needs for additional financing; our plans to develop and market ganaxolone and the timing of our development programs; our estimates of the size of the potential markets for ganaxolone; our selection and licensing of ganaxolone; our ability to attract collaborators with acceptable development, regulatory and commercial expertise; the benefits to be derived from corporate collaborations, license agreements, and other collaborative or acquisition efforts, including those relating to the development and commercialization of ganaxolone; sources of revenue, including contributions from corporate collaborations, license agreements, and other collaborative efforts for the development and commercialization of products; our ability to create an effective sales and marketing infrastructure if we elect to market and sell ganaxolone directly; the rate and degree of market acceptance of ganaxolone; the timing and amount or reimbursement for ganaxolone; the success of other competing therapies that may become available; the manufacturing capacity for ganaxolone; our intellectual property position; our ability to maintain and protect our intellectual property rights; our results of operations, financial condition, liquidity, prospects, and growth strategies; our spending of the proceeds from this offering; the industry in which we operate; and the trends that may affect the industry or us. We undertake no obligation to update or revise any forward-looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the company in general, see Marinus's 10-K dated March 7, 2016 and other filings by the company with the U.S. Securities and Exchange Commission. You may access these documents for free by visiting EDGAR on the SEC web site at www.sec.gov. 1

Agenda Introduction – Christopher M. Cashman Rationale for GNX in FXS – Dr. Frank Kooy Clinical trial design & results – Dr. Julia Tsai Case summaries – Dr. Anke VanDijck Closing remarks – Christopher M. Cashman 2

3 Ganaxolone: New MOA, Demonstrated Efficacy, Good Safety Data, Convenient Goal: Improve the lives of patients with epilepsy & anxiety disorders Designed with selectivity and safety in mind New MOA Anti-seizure & anti-anxiety activity CNS-selective GABAA modulator Safety, Tolerability & Efficacy Designed for safe, chronic administration Dosed >1300 adults & children Demonstrated efficacy Patient Access Drug resistant seizures IV, Capsule, Liquid Hospital / Out-patient
Rationale for Ganaxolone Dr. Frank Kooy

Fragile X Syndrome – Disease Overview Rare genetic condition Caused by mutation of the FMR1 gene on the X chromosome Most common known inherited form of intellectual disability Associated with spectrum of learning disabilities, neurological problems (seizures) and behavior difficulties (anxiety, attention deficit, hyperactivity & autism spectrum disorder) Prevalence: ~100,000 children and adults in the U.S.1 Occurs in ~ 1 in 3600 males and 1 in 6000 females1 No cure or approved therapies 5 1https://fragilex.org/fragile-x/prevalence/
Ganaxolone in FXS Knock-Out Mouse Model Knock-out mouse model is clinically translatable Mice exhibit increased seizure susceptibility, increased anxiety and cognitive deficits1 Phenotype is very similar to that of human fragile X FMR1 mice have deficits in GABA, causing a reduction in2: GABAA receptors GABAergic signaling GABA concentrations GABAergic neuronal number Restoration of GABAergic function may affect one or more symptoms of Fragile X3 Preclinical data shows that ganaxolone reverses seizures and neurosteroids reverse anxiety in Fragile X mice4 6 1 Lozano 2014, Braat and Kooy, 2014, Heulens et al., 2012 2 Braat S1, Kooy RF. 2015 Jan;88:48-54. doi: 10.1016/j.neuropharm.2014.06.028. Epub 2014 Jul 10. 3 Lozano, 2014; 10: 1769–1779.Published online 2014 Sep 16. doi: 10.2147/NDT.S42919 4 Heulens et al., 2012
Rationale for Selecting Ganaxolone Anxiety and attention difficulties are seen in children with FXS Ganaxolone has been found to improve symptoms in mouse models of FXS1 and in humans Ganaxolone should increase and normalize GABAA mediated signaling by boosting the signaling capacity of existing receptors 7 1 Heulens et al., 2012
Expectations for Study Primary Objective: Assess safety, tolerability and efficacy of ganaxolone for treatment of anxiety and attention in patients with FXS Secondary Objective: Assess changes correlated with domains of attention, social behavior and inhibitory control using objective laboratory tests of CNS function All-comers – Patients were not screened for specific symptomatology for acceptance into the study Exploratory signal finding Expected anxiety and attention benefits Evaluated other FXS-related symptoms where benefit would be unexpected Evaluate multiple rating scales for sensitivity and suitability for use in more advanced trials 8
Proof-of-Concept Clinical Study Ganaxolone for Behaviors in Fragile X Syndrome Dr. Julia Tsai

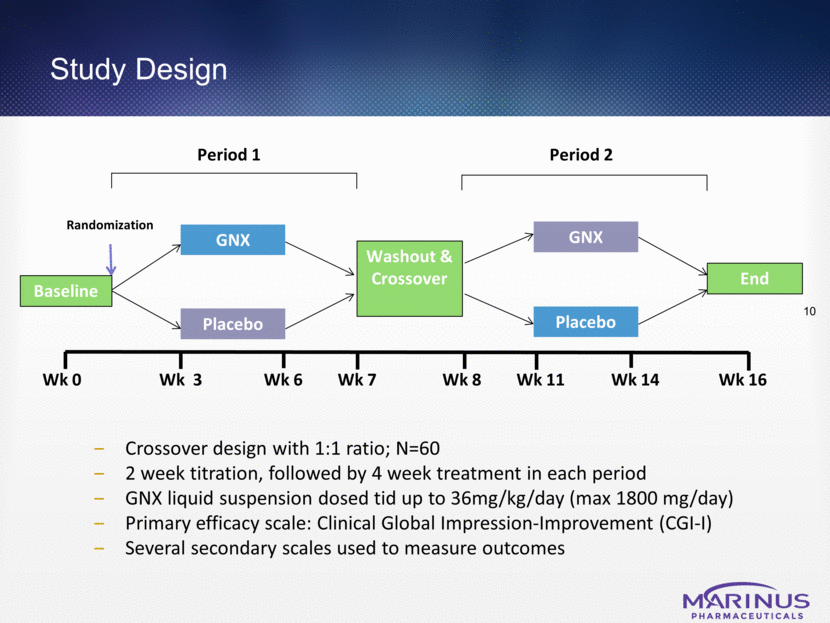
Study Design 10 Crossover design with 1:1 ratio; N=60 2 week titration, followed by 4 week treatment in each period GNX liquid suspension dosed tid up to 36mg/kg/day (max 1800 mg/day) Primary efficacy scale: Clinical Global Impression-Improvement (CGI-I) Several secondary scales used to measure outcomes Randomization Wk 0 Wk 3 Wk 7 Wk 16 Baseline Washout & Crossover GNX Placebo End Placebo GNX Period 1 Period 2 Wk 8 Wk 11 Wk 6 Wk 14
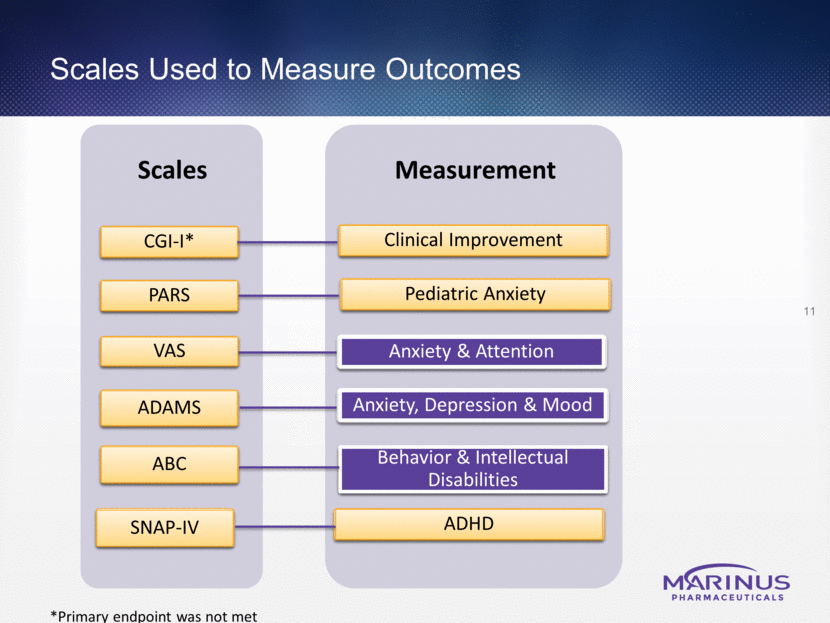
Measurement Scales Scales Used to Measure Outcomes 11 CGI-I* PARS VAS ADAMS ABC SNAP-IV Anxiety & Attention Anxiety, Depression & Mood Clinical Improvement Behavior & Intellectual Disabilities ADHD Pediatric Anxiety *Primary endpoint was not met
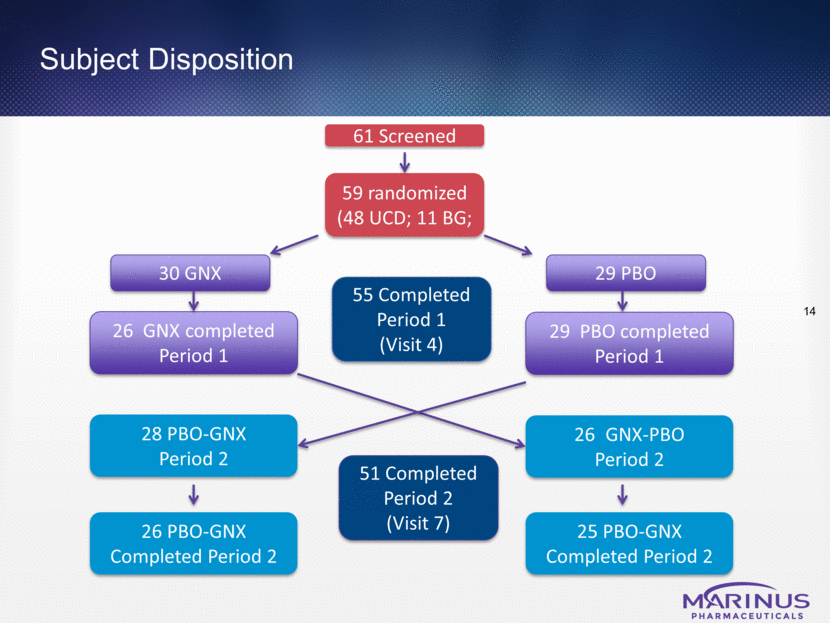
Subject Disposition 61 Screened 59 randomized (48 UCD; 11 BG; 30 GNX 26 GNX completed Period 1 29 PBO 29 PBO completed Period 1 GNX-PBO Period 2 28 PBO-GNX Period 2 55 Completed Period 1 (Visit 4) 26 PBO-GNX Completed Period 2 25 PBO-GNX Completed Period 2 51 Completed Period 2 (Visit 7) 14
Anxious Patient Population N=29 PARS >13 PARS – clinician rated scale to assess severity of anxiety Scales designed to detect changes in anxiety, attention and hyperactivity: VAS ADAMS ABC 13
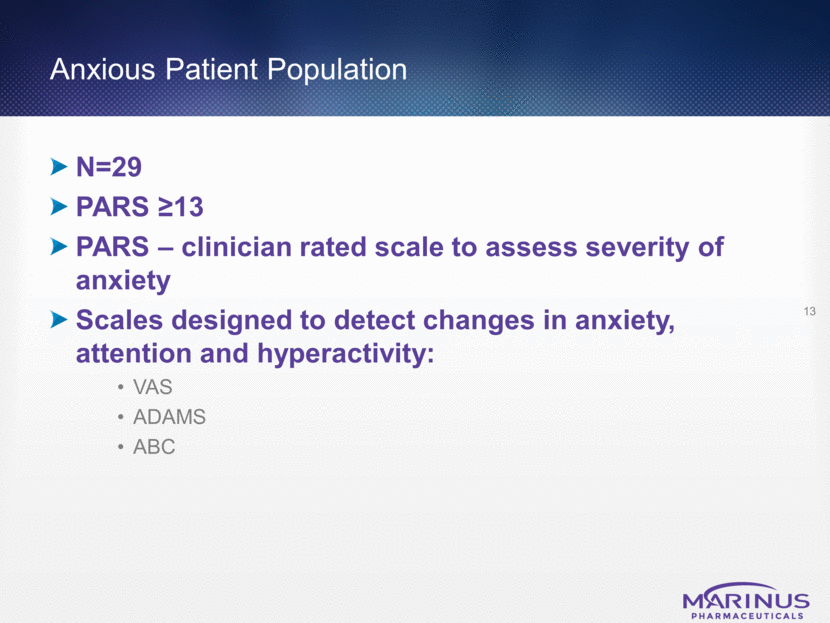
Mean % Improvement in VAS Scale 14 Ganaxolone vs. Placebo in Patients (with PARS >13)
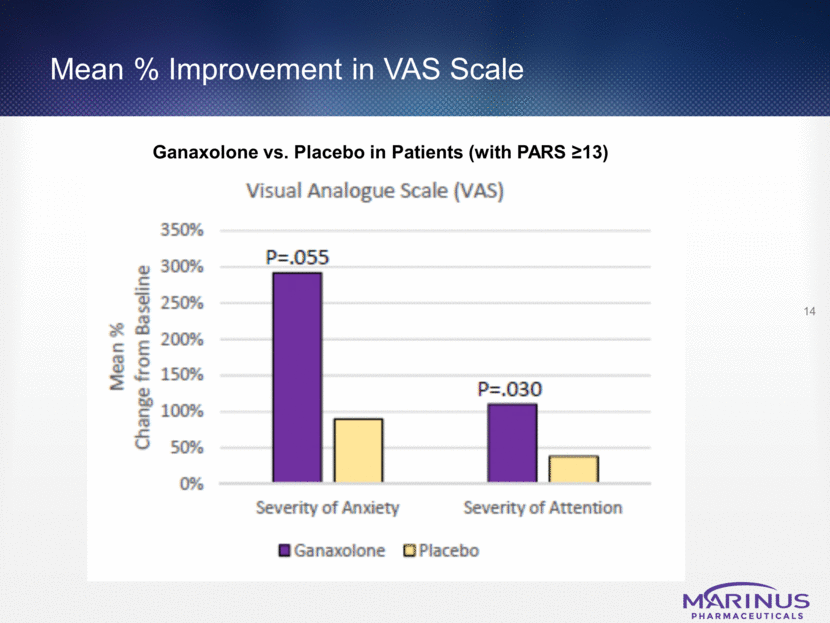
Mean % Improvement in ADAMS Scale 15 Ganaxolone vs. Placebo in Patients (with PARS >13)
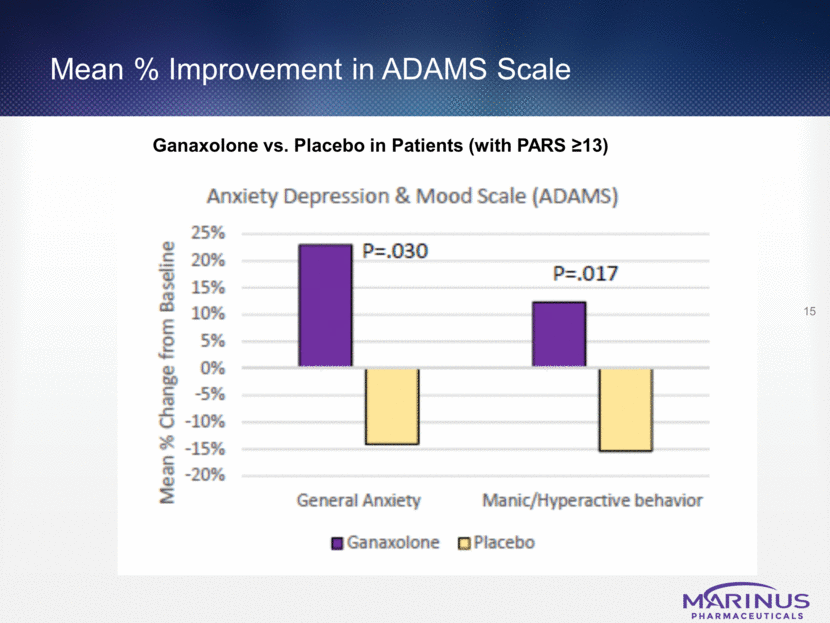
Mean % Improvement in ABC Scale 16 Ganaxolone vs. Placebo in Patients (with PARS >13)
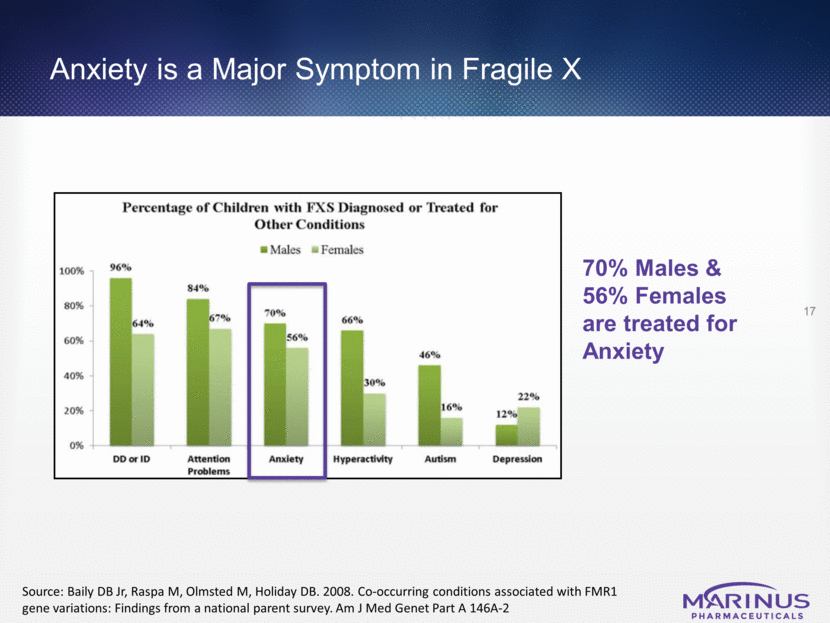
Anxiety is a Major Symptom in Fragile X 17 70% Males & 56% Females are treated for Anxiety Source: Baily DB Jr, Raspa M, Olmsted M, Holiday DB. 2008. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. Am J Med Genet Part A 146A-2
Ganaxolone Safe and Well Tolerated in FXS Generally safe and well tolerated AEs consistent with other GNX studies and drug mechanism No SAEs AEs more frequent in ganaxolone than placebo: fatigue, somnolence, diarrhea, decreased appetite, rash Mild to moderate in severity, recovered without action taken with study drug 5 discontinuations due to AE 4 GNX: aggression/agitation, incoordination/fatigue/rash, rash/itchiness, rash 1 PBO: fever/upper respiratory infection 18

Summary of Findings and Next Steps Ganaxolone benefit on key anxiety scales for anxious FXS patients Ganaxolone was safe and generally well-tolerated in patients with FXS Results support further study of ganaxolone in FXS patients Next Steps Engage Advisors to finalize clinical and regulatory plans in FXS Apply for Orphan Drug Designation for ganaxolone in FXS 19

Case summaries Dr. Anke Van Dijck, Sub-PI, Antwerp University Hospital, Belgium

Why Ganaxolone in Fragile X Syndrome? 21 Caused by mutation of FMR1 gene Genetic testing available Biomarker Compromised GABAA system Ganaxolone should increase and normalize GABAA mediated signaling by boosting the signaling capacity of existing receptors Mechanistic Rationale Rare genetic condition affecting 100,000 children & adolescents Quick & efficient regulatory path to approval Orphan Disease Seizures - ~23% of patients Anxiety - >50% of patients Comorbidities No approved therapies Treatments focus on addressing symptoms Unmet Medical Need
Thank you

