Attached files
| file | filename |
|---|---|
| EX-10.91 - EXHIBIT 10.91 - Protea Biosciences Group, Inc. | v442685_ex10-91.htm |
| EX-23.1 - EXHIBIT 23.1 - Protea Biosciences Group, Inc. | v442685_ex23-1.htm |
| EX-14.1 - EXHIBIT 14.1 - Protea Biosciences Group, Inc. | v442685_ex14-1.htm |
| EX-10.92 - EXHIBIT 10.92 - Protea Biosciences Group, Inc. | v442685_ex10-92.htm |
| EX-4.19 - EXHIBIT 4.19 - Protea Biosciences Group, Inc. | v442685_ex4-19.htm |
| EX-4.18 - EXHIBIT 4.18 - Protea Biosciences Group, Inc. | v442685_ex4-18.htm |
| EX-3.2 - EXHIBIT 3.2 - Protea Biosciences Group, Inc. | v442685_ex3-2.htm |
As filed with the Securities and Exchange Commission on June 22, 2016
Registration No. 333-211674
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT 1 to
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933

PROTEA BIOSCIENCES GROUP, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 20-2903252 | ||
|
(State or Other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
1311 Pineview Drive, Suite 501
Morgantown, West Virginia 26505
1-304-292-2226
(Address, including zip code, and telephone number, including area code,
of registrant’s principal executive offices)
Stephen Turner
Chief Executive Officer
Protea Biosciences Group, Inc.
1311 Pineview Drive, Suite 501
Morgantown, West Virginia 26505
1-304-292-2226
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
Stephen A. Weiss, Esq. Barrett S. DiPaolo, Esq. CKR Law LLP 1330 Avenue of the Americas New York, New York 10019 Tel: (212) 259-7300 Fax: (212) 259-8200 |
Richard A. Friedman, Esq. Stephen A. Cohen, Esq. Sichenzia Ross Friedman Ference LLP 61 Broadway, 32nd Floor New York, New York 10006 Telephone: (212) 930-9700 Facsimile: (212) 930-9725 |
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ |
| Non-accelerated filer | ¨ | Smaller reporting company | x |
| Title of Each Class of Securities to be Registered | Amount to be Registered |
Proposed Maximum Aggregate Offering Price |
Amount of Registration Fee |
||||||||
| Common stock, par value $0.0001 per share | 3,565,000 shares | (1) | $ | 19,607,500 | (5) | $ | 1,974.48 | ||||
| Common stock offered for selling stockholders | 530,119 shares | (2) | 2,915,655 | (3) | 293.61 | ||||||
| Representative’s common stock purchase warrants | N/A | (4) | |||||||||
| Common stock issuable upon exercise of Representative’s purchase warrants | 155,000 shares | (4) | $ | 1,065,625 | (5) | $ | 107.31 | ||||
| Total | 4,250,119 shares | $ | 23,588,780 | $ | 2,375.40 | (3) | |||||
| (1) | Gives retroactive effect to a 1-for-25 reverse stock split to be effected prior to the effective date of this Registration Statement and assumes a public offering price of $5.50 per share. The proposed maximum aggregate offering price assumes an offering of 3,100,000 shares of common stock and the full exercise of the underwriters’ over-allotment option to purchase up to an additional 465,000 shares, and is estimated solely for the purpose of calculating the amount of the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended. Includes the offering price of additional shares that the underwriters have the option to purchase to cover over-allotments. |
| (2) | Consists (after giving retroactive effect to the proposed 1-for-25 reverse stock split) of (a) 318,889 outstanding shares of the registrant’s common stock and (b) 211,230 shares of common stock issuable upon exercise of outstanding common stock purchase warrants. Commencing 90 days after the date of the prospectus forming part of this Registration Statement, the selling stockholders may sell such shares. Pursuant to Rule 416 under the Securities Act of 1933, as amended, to the extent that such outstanding shares and warrants provide for an increase in amount issuable or exercisable to prevent dilution resulting from stock splits, stock dividends, or similar transactions, this registration statement shall be deemed to cover such additional shares of common stock issuable in connection with any such provision. |
| (3) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act of 1933, as amended, based on the average of the high and low bid prices of the registrant’s common stock as reported by OTC Markets on June 21, 2016. The shares offered hereunder may be sold by the selling stockholders from time to time in the open market, through privately negotiated transactions or a combination of these methods, at market prices prevailing at the time of sale or at negotiated prices. |
| (4) | We have agreed to issue, upon closing of this offering, compensation warrants exercisable commencing on a date which is six months after the effective date of the registration statement of which this prospectus forms a part and expiring five years following the effective date of the registration statement of which this prospectus forms a part representing 5% of the aggregate number of shares of common stock issued in the offering but not including the over-allotment option, or the “representative warrants,” to Laidlaw & Co. (UK) Ltd., as Representative of the underwriters. Resales of the representative warrants on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended (the “Securities Act”) are registered hereby. Resales of common stock issuable upon exercise of the representative warrants are also being similarly registered on a delayed or continuous basis hereby. See “Underwriting.” |
| (5) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(g) under the Securities Act, based on an estimated proposed maximum aggregate offering price of $5.50 for the common stock issuable upon exercise of the representative’s common stock purchase warrant. We have calculated the proposed maximum aggregate offering price of the common stock underlying the representative’s warrants by assuming that such warrants are exercisable to purchase shares of common stock at a price per share equal to 100% of the price per share sold in this offering. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
EXPLANATORY NOTE
This Registration Statement contains two forms of prospectuses: one to be used in connection with a public offering of up to 3,100,000 shares of our common stock (excluding 465,000 shares of common stock which may be sold upon exercise of the underwriters’ over-allotment option) through the underwriters named on the cover page of this prospectus (the “Prospectus”) and one to be used in connection with the potential resale by certain selling stockholders of an aggregate of 530,119 shares of our common stock (the “Selling Security holder Prospectus”), consisting of (i) 318,889 shares of our common stock and (ii) 211,230 shares of our common stock issuable upon exercise of outstanding warrants held by certain of the selling stockholders. The Prospectus and Selling Security holder Prospectus will be identical in all respects except for the alternate pages for the Selling Security holder Prospectus included herein which are labeled “Alternate Page for Selling Security holder Prospectus.”
The Selling Security holder Prospectus is substantively identical to the Prospectus, except for the following principal points:
| · | they contain different outside and inside front covers; |
| · | they contain different Offering sections in the Prospectus Summary section on page 3; |
| · | they contain different Use of Proceeds sections on page 22; |
| · | the Capitalization section on page 24 is deleted from the Selling Security holder Prospectus; |
| · | a Selling Security holder section is included in the Selling Security holder Prospectus beginning on page 81; |
| · | the Underwriting section from the Prospectus on page 70 is deleted from the Selling Security holder Prospectus and a Plan of Distribution is inserted in its place on page 84; and |
| · | the Legal Matters section in the Selling Security holder Prospectus on page 86 deletes the reference to counsel for the underwriters; |
The registrant has included in this Registration Statement, after the financial statements, a set of alternate pages to reflect the foregoing differences of the Selling Security holder Prospectus as compared to the Prospectus.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
Subject to Completion, dated May 27, 2016
PRELIMINARY PROSPECTUS

Protea Biosciences Group, Inc.
3,100,000 Shares of Common Stock
We are offering for sale a total of 3,100,000 shares of our common stock for the account of our company, the net proceeds of which will be used primarily for working capital and to expand our business.
We intend to list our common stock on the Nasdaq Capital Market under the symbol “PRGB.” No assurance can be given that our Nasdaq listing application will be approved. Our common stock is currently traded on the OTCQB marketplace of OTC Markets Group Inc. The last reported sale price of our common stock as reported on the OTCQB on June 21, 2016 was $0.17, which after giving effect to a 1 for 25 reverse stock split described below, equates to $4.25. However, in this prospectus, we assume that the price per share in this offering, on a post-split basis, will be in the range of $4.50 to $6.50, and we have used the midpoint of such range ($5.50) for the assumptions set forth herein.
We will effect a reverse stock split of our outstanding shares of common stock prior to or upon the effective date of the registration statement of which this prospectus forms a part. However, following the stock split, our common stock may not trade at a price consistent with such reverse split. We expect to affect a one-for-25 reverse split of our outstanding shares of our common stock, which reverse stock split was approved by our stockholders in December 2015.
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 10 of this prospectus for a discussion of information that should be considered in connection with an investment in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012, and we have elected to comply with certain reduced public company reporting requirements.
| Per Share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions (1) | ||||||||
| Proceeds to us, before expenses | $ | $ | ||||||
| (1) | Does not include a non-accountable expense allowance equal to 1% of the gross proceeds of this offering payable to Laidlaw & Company (UK) Ltd., the representative of the underwriters. See “Underwriting” for a complete description of compensation payable to the underwriters. |
We have granted a 45 day option to the underwriters to purchase up to 465,000 additional shares of common stock, at the public offering price less underwriting discounts and commissions, to cover over -allotments, if any. If the over-allotment option is exercised in full, the total proceeds to us, after deducting underwriting discounts and commissions and before expenses, will be approximately $__________.
The underwriters expect to deliver the shares against payment therefor on or about ________, 2016.
LAIDLAW & COMPANY (UK) LTD.
The date of this prospectus is _______, 2016
You should rely only on the information contained in this prospectus or any prospectus supplement or amendment. We have not authorized any other person to provide you with information that is different from, or adds to, that contained in this prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. The selling stockholders are offering to sell and seeking offers to buy our common stock only in jurisdictions where offers and sales are permitted. You should assume that the information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date. We are not making an offer of any securities in any jurisdiction.
TABLE OF CONTENTS
| ABOUT THIS PROSPECTUS | 1 |
| MARKET DATA | 1 |
| SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS | 1 |
| PROSPECTUS SUMMARY | 3 |
| RISK FACTORS | 10 |
| USE OF PROCEEDS | 22 |
| DIVIDEND POLICY | 23 |
| CAPITALIZATION | 24 |
| DILUTION | 25 |
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 27 |
| MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS | 34 |
| BUSINESS | 37 |
| MANAGEMENT | 53 |
| PRINCIPAL STOCKHOLDERS | 59 |
| CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS | 62 |
| DESCRIPTION OF SECURITIES | 63 |
| SHARES ELIGIBLE FOR FUTURE SALE | 68 |
| UNDERWRITING | 70 |
| SELLING STOCKHOLDERS’ PLAN OF DISTRIBUTION | |
| DISCLOSURE OF COMMISSION POSITION OF INDEMNIFICATION FOR SECURITIES ACT LIABILITIES | |
| EXPERTS | 77 |
| LEGAL MATTERS AND INTERESTS OF NAMED EXPERTS | 77 |
| WHERE YOU CAN FIND MORE INFORMATION | 78 |
‘No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus. You must not rely on any unauthorized information or representations. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is current only as of its date.
Throughout this prospectus, unless otherwise designated or the context suggests otherwise,
| · | all references to the “Company,” the “registrant,” “Protea,” “we,” “our,” or “us” in this prospectus mean Protea Biosciences Group, Inc. and its consolidated subsidiaries; |
| · | all shares, common stock, and per share data assumes and gives pro forma retroactive effect to a one-for-25 reverse stock split of Protea’s outstanding capital stock that will be consummated immediately prior to the effective date of the registration statement of which this prospectus is a part; |
| · | assumes a public offering price of our common stock (after giving effect to such reverse stock split) of $5.50 per share, the mid-range of the estimated $4.50 to $6.50 per share; |
| · | “year” or “fiscal year” mean the year ending December 31st |
| · | all dollar or $ references when used in this prospectus refer to United States dollars. |
Market data and certain industry data and forecasts used throughout this prospectus were obtained from internal company surveys, market research, consultant surveys, publicly available information, reports of governmental agencies and industry publications and surveys. Industry surveys, publications, consultant surveys and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable, but the accuracy and completeness of such information is not guaranteed. We have not independently verified any of the data from third party sources, nor have we ascertained the underlying economic assumptions relied upon therein. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based on our management’s knowledge of the industry, have not been independently verified. Forecasts are particularly likely to be inaccurate, especially over long periods of time. Statements as to our market position are based on the most currently available data. While we are not aware of any misstatements regarding the industry data presented in this prospectus, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” in this prospectus.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains “forward-looking statements.” Forward-looking statements reflect the current view about future events. When used in this prospectus, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward-looking statements. Such statements, include, but are not limited to, statements contained in this prospectus relating to our business strategy, our future operating results and liquidity and capital resources outlook. Forward-looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions. Because forward–looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward-looking statements. They are neither statements of historical fact nor guarantees of assurance of future performance. We caution you therefore against relying on any of these forward-looking statements. Important factors that could cause actual results to differ materially from those in the forward-looking statements include, without limitation:
| · | the results of clinical trials and the regulatory approval process; |
| · | our ability to raise capital to fund continuing operations; |
| · | market acceptance of any products that may be approved for commercialization; |
| · | our ability to protect our intellectual property rights; |
| · | the impact of any infringement actions or other litigation brought against us; |
| · | competition from other providers and products; our ability to develop and commercialize new and improved products and services; |
| · | changes in government regulation; |
| · | our ability to complete capital raising transactions; |
| · | and other factors (including the risks contained in the section of this prospectus entitled “Risk Factors”) relating to our industry, our operations and results of operations. |
Should one or more of these risks or uncertainties materialize, or should the underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned.
Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
2
This summary provides a brief overview of the key aspects of our business and our securities. The reader should read the entire prospectus carefully, especially the risks of investing in our common stock discussed under “Risk Factors.” Some of the statements contained in this prospectus, including statements under “Summary” and “Risk Factors” as well as those noted in the documents incorporated herein by reference, are forward-looking statements and may involve a number of risks and uncertainties. Our actual results and future events may differ significantly based upon a number of factors. The reader should not put undue reliance on the forward-looking statements in this document, which speak only as of the date on the cover of this prospectus.
Except as otherwise indicated, all share and per share amounts in this prospectus assumes and gives retroactive effect to a 1-for-25 reverse stock split which will occur prior to the effectiveness of the registration statement of which this prospectus is a part. We have estimated that the initial offering price of our common stock will range between $4.50 and $6.50 per share and have assumed an initial offering price of $5.50, representing the mid-point of such range. However, based on our current pre-offering closing market price at June 21, 2016 of $0.17 per share, in order to offer our shares of common stock within the price range referred to above and list our shares on the Nasdaq Capital Market or other national securities exchange approved by our underwriters, prior to the effective date of the registration statement of which this prospectus is a part, we may be required to obtain stockholder approval to increase such reverse stock split to an amount in excess of 1-for-25.
About Our Business
Protea is an emerging growth, molecular information company that has developed a revolutionary platform technology, which enables the direct analysis, mapping and display of biologically active molecules in living cells and tissue samples. The technology platform offers new, unprecedented capabilities useful to the pharmaceutical, diagnostic, clinical research, agricultural and life science industries.
“Molecular information” refers to the generation and bioinformatic processing of very large data sets, known as “big data,” obtained by applying the Company’s technology to identify and characterize the proteins, metabolites, lipids and other molecules which are the biologically active molecular products of all living cells and life forms.
Our technology is used to improve pharmaceutical development and life science research productivity and outcomes, and to extend and add value to other technologies that are used in research and development (“R&D”), such as three-dimensional tissue models, biomarker discovery, synthetic biologicals and mass spectrometry. In particular, the Company believes that its ability to rapidly provide comprehensive molecular image-based datasets addresses a universal need of the pharmaceutical, diagnostic and clinical research and life science industries.
Known as LAESI® (Laser Ablation Electrospray Ionization), our technology platform is exclusively licensed from The George Washington University (“GWU”). We have subsequently completed the development of the first fully automated LAESI instruments and requisite proprietary software suites, used for LAESI data analysis and for molecular imaging – the direct analysis and visualization of molecules in cells and tissue. LAESI technology is currently the subject of eleven issued patents and over 40 peer-reviewed publications.
LAESI is intended to meet the broad need of the biologist for the direct, unbiased identification and characterization of molecules in biological samples. By virtue of LAESI’s improved speed and the comprehensive datasets it generates, the Company is pursuing its vision of what it believes will be a new era of human molecular information, where the molecular networks of human disease will be clearly elucidated, with data more rapidly available, thereby accelerating pharmaceutical development and improving healthcare.
Our Business Strategy and Products and Services
Protea has applied its core technology to establish commercial leadership in the emerging field of mass spectrometry imaging (“MSI”) services. MSI is a revolutionary capability that for the first time enables the identification of all classes of biologically active molecules produced by cells and combines this with the ability to instantly spatially-display the molecules in tissue histology sections. MSI can be performed with no sample preparation and no labeling or antibody techniques, thereby integrating direct molecular identification with tissue pathology.
The Company intends to achieve its business objectives by leveraging its core MSI technology and associated bioanalytical mass spectrometry platforms to improve the availability, comprehensiveness and usefulness of molecular information to address the needs of pharmaceutical research, biomarker discovery, agriculture and other life science research markets.
The Company’s commercial development is centered in three business lines:
| · | Molecular Information Services – the Company believes that it is the commercial leader in providing multimodal MSI, combining LAESI, MALDI, and optical microscopy. Our proprietary services enable the identification and quantitation of both small molecules (e.g., lipids and metabolites) and large molecules (e.g. proteins) and our services portfolio, inclusive of MSI, proteomics, metabolomics, lipidomics and bioinformatics is unique in the industry. Our clients include major pharmaceutical, chemical and biotechnology companies; | |
3
| · | LAESI Instruments, Software and Consumables – we offer our proprietary LAESI DP-1000 instrument, software and bioanalytical consumables to corporate and academic research laboratories; and |
| · | Molecular Diagnostics and Clinical Research – we apply our multimodal MSI technologies and workflows in partnership with top-tier medical research institutions to co-develop new, molecular diagnostic tests that the company believes can be used to improve the diagnosis and prognosis of cancer and provide requisite information useful in predicting the outcome of cancer treatment. Our current test development programs target the differential diagnosis and prognosis of malignant melanoma and the molecular profiling of subpopulations of cells in lung adenocarcinoma. |
Molecular Information Services
We believe we are the commercial leader in the offering of MSI services. Our clients send their tissue and biofluid samples directly to the Company’s laboratory, where samples are analyzed by the Company’s state of the art mass spectrometry instrumentation, scientific staff and bioinformatics capabilities. We combine our proprietary LAESI platform with matrix-assisted laser desorption ionization (“MALDI”), and liquid chromatography mass spectrometry (LCMS) workflows. Our clients include major pharmaceutical, biotechnology, chemical and medical device and consumer products companies, and both academic and government institutions. The services unit is operated within a Quality by Design environment, which is necessary to meet the internal research and development standards of pharmaceutical and clinical research clients.
The Company’s MSI services represent a revolutionary capability that for the first time enables the identification of all classes of biologically-active molecules produced by cells, and combines this with the ability to instantly spatially-display the molecules (both two- and three-dimensional) in tissue histology sections. MSI can be performed without sample preparation, labeling or antibody techniques, thereby integrating direct molecular identification with tissue pathology for the first time. Since the sample is not touched, data is unbiased and more rapidly available. Providing molecular information that rapidly answers questions critical to the drug development process, such as, “is my drug actually getting inside the target cells,” “is my immune modulating agent changing the molecular output of the tumor cell,” or “how long did it take for the drug to enter the target cells” as well as questions of drug concentration and duration.
We have, in collaboration with industry leaders, developed advanced bioanalytical workflows for the characterization of protein biotherapeutics, such as monoclonal antibodies, to address regulatory requirements for safety, efficacy, and bioactivity in manufacturing and storage of these products. To this end, we have applied proteomics and metabolomics technologies along with LAESI direct analysis to aid in optimizing the expression systems used to produce monoclonal antibodies for drug discovery purposes. Key collaborations and partnerships have been formed to address the bioinformatics analysis required including statistical and pathway analysis.
In January 2014, the Company, as a subcontractor to GWU, was awarded a multi-year project with the Defense Advanced Research Projects Agency (“DARPA”). In addition to Protea, The Stanford Research Institute International and GE Global Research also collaborate on the project entitled, “New Tools for Comparative Systems Biology of Threat Agent Action Mechanisms.” A $15 million five year project, the goal of DARPA’s Rapid Threat Assessment (“RTA”) program is to develop new tools and methods to elucidate the mechanism of action of a threat agent, drug, biologic or chemical on living cells within 30 days from exposure. Uncovering the mechanism of action of such agents in 30 days, compared to the years currently required, could be key to the development of effective countermeasures. The molecular networks within living cells are vast and complex. Conventional approaches fail to capture the system-wide response of a living cell to a threat agent. The Company believes this technology will be applicable to preclinical drug research as well.
LAESI instruments, software and consumables
LAESI technology was invented in the laboratory of Professor Akos Vertes, Ph.D., Dept. of Chemistry, GWU, in 2007 and was exclusively-licensed to Protea in 2008. The LAESI DP-1000 instrument, the Company’s prototype embodiment of its proprietary LAESI technology, integrates with laboratory instruments known as mass spectrometers. LAESI employs a proprietary (patented) method that utilizes the water content in a sample (native or applied) to transition the sample into a gas state, where it can be analyzed by a mass spectrometer. LAESI accomplishes this without requiring the sample to be touched. By eliminating sample preparation, the biological sample can be analyzed without the possible contamination, bias or sample loss that occurs with current techniques, which require the introduction of chemicals or the destruction of the sample itself in order to enable analysis by mass spectrometry.
4
This technology enables the direct identification of proteins, lipids and metabolites in tissues, cells and biofluids such as serum and urine. Data is available in seconds to minutes, allowing rapid time to results and the capacity to analyze thousands of samples in a single work period. As an example, a researcher testing a new drug’s effects on living cells can analyze changes in the cells’ metabolism across a specific time course, thereby almost immediately obtaining data as to the activity of the new drug. The Company believes that LAESI technology has the potential to significantly improve the availability of molecular information in pharmaceutical research as well as many other fields including agriculture, pathology, biomarker discovery, biodefense and forensics.
LAESI instruments employ proprietary software developed by the Company that creates “molecular maps” - the ability to instantly display the distribution of molecules throughout tissue sample in both two- and three-dimensional imaging. The Company currently offers the LAESI Desktop Software and ProteaPlot software that facilitates operating the instrument and the storage and display of datasets in a user friendly, intuitive software environment. The ProteaPlot software includes tools for post-processing of LAESI-MS data, generation of molecular maps/images and mass spectral comparison for regions of interest. LAESI software displays the data obtained by mass spectrometry analysis combined with actual images of the tissue and cell samples. Thus, mass spectrometry data can be integrated with a sample’s tissue architecture.
We have developed and brought to market related, proprietary consumable products for use in molecular analysis including REDIchipsTM (Resonance-Enhanced Desorption Ionization - sample target plates with nanopost array surfaces for matrix-free laser desorption ionization of small molecules) and ProgentaTM (acid labile surfactants use to solubilize proteins for mass spectrometry analysis).
Molecular Diagnostics and Clinical Research
The Company is employing both its proprietary LAESI platform and MALDI methodologies to create comprehensive, tissue-based molecular profiles to improve the differential diagnosis of cancer. Our bioinformatics capability allows the integration of the Company’s MSI data files with related pathology, gene expression and demographic datasets, with the purpose of improving human disease state detection, assessment and management. The Company has entered into collaborative research partnerships with top tier academic centers to develop and validate new, proprietary, MSI–based cancer diagnostic tests.
We have established a collaborative research partnership with the Memorial Sloan-Kettering Cancer Center and the Dana-Farber Cancer Institute. The first target of the collaboration is early stage lung adenocarcinoma. The objectives of the collaboration are to demonstrate that different cancer cell sub-groups within a lung cancer will have different molecular profiles and will behave differently. The goal is to define these molecular differences and to identify the sub-group of cancer cells with the worst prognosis that are most likely to recur thereby enabling earlier treatment intervention, and to use these findings to achieve lung adenocarcinoma tumor cell “molecular profiling,” leading to more precise treatment selection and higher survivor rates.
We established a collaborative research initiative with The Yale University School of Medicine that employs our MSI technology to differentiate benign melanocytic nevi from malignant melanoma. We believe that our core MSI technology can identify unique protein expression profiles that can discriminate between benign melanocytic nevi and malignant melanoma.
In April 2016 we entered into an exclusive license agreement for technology with Yale University related to the differential diagnosis of melanoma, specifically designated as a "Method of Differentiating Benign Melanocytic Nevi from Malignant Melanoma." The technology was co-invented by Dr. Rossitza Lazova, of the Department of Dermatology at Yale School of Medicine, and Dr. Erin Seeley our Clinical Imaging Principal Investigator. Under the terms of the license agreement, we have been granted the exclusive worldwide rights to commercialize the technology. We are obligated to pay expenses for the preparation, filing and prosecution of future related patent applications governed by the license agreement and related license fees. Unless earlier terminated in accordance with its terms, the Yale license agreement expires upon the later of 20 years from the effective date or the end of the term of the last underlying patent to expire.
To support our MSI–based diagnostic development, we developed new software known as “Histology Guided Mass Spec Imaging (HG-MSI)” that enables pathologists to combine traditional microscopy and histology with high resolution mass spectrometry molecular imaging. Clinicians can share, annotate and direct the analysis of specific tissue morphologies and cell subpopulations by MSI. Molecular profiling data are collected from discrete locations within a tissue section using a histology stained section as a guide. The digital tissue scans are visually analyzed by pathologists by logging onto a secure website portal and they then annotate specific cellular areas for further analysis.
We intend to expand our collaborations with major medical research centers to develop additional molecular profiles for the improved differential diagnosis and prognosis of cancer.
Recent Developments
We entered into a Memorandum of Understanding (the “MOU”) with Agilent Technologies, Inc. (NYSE: A) (“Agilent”), to develop new bioanalytical workflows in order to meet the emerging needs of the growing biopharmaceutical industry. Under the terms of the MOU, Protea, using Agilent instrumentation combined with its expertise, will develop workflows to improve the characterization of protein therapeutics including monoclonal antibodies and new methods for the field of metabolomics. We believe, along with Agilent, that the field of biotherapeutics is advancing rapidly and is in need of new, innovative solutions that identify changes in the metabolic profiles of cells due to disease processes and drug interactions.
5
We have announced new workflows to support the bioanalytical needs of immuno-oncology, an emerging frontier of cancer treatment that utilizes the body’s own immune system to fight diseases. Our new workflows help provide both visual and analytical certainty of therapeutic efficacy and apply the Company’s core MSI technology to the analysis of drug target tissues and tumor microenvironments.
We have commercialized a consumable product out of the DARPA research grant that is currently being sold to research laboratories performing small molecule analysis. This product called REDIchip (Resonance-Enhanced Desorption Ionization - sample target plates with nanopost array surfaces for matrix-free laser desorption ionization of small molecules) enables researchers to rapidly profile small molecules and metabolites utilizing a MALDI platform. This product does not require the addition of a traditional chemical matrix, but is able to detect and quantitate small molecules due to the highly structured nanopost array. These products are being used with researchers investigating metabolites and small molecule drugs, and they have potential applications within clinical mass spectrometry pain panel management operations.
In April 2016 we entered into an exclusive license agreement for technology with Yale University related to the differential diagnosis of melanoma, specifically designated as a "Method of Differentiating Benign Melanocytic Nevi from Malignant Melanoma." The technology was co-invented by Dr. Rossitza Lazova, of the Department of Dermatology at Yale School of Medicine, and Dr. Erin Seeley our Clinical Imaging Principal Investigator. Under the terms of the license agreement, we have been granted the exclusive worldwide rights to commercialize the technology. We are obligated to pay expenses for the preparation, filing and prosecution of future related patent applications governed by the license agreement and related license fees. Unless earlier terminated in accordance with its terms, the Yale license agreement expires upon the later of 20 years from the effective date or the end of the term of the last underlying patent to expire.
Risk Factors
Our business is subject to a number of risks. You should be aware of these risks before making an investment decision. These risks are discussed more fully in the section of this prospectus titled “Risk Factors,” which begins page of this prospectus and includes:
| · | We have a history of losses; |
| · | We will be required to raise additional financing; |
| · | We have a significant amount of indebtedness; |
| · | We may be unable to protect our intellectual property; |
| · | Market acceptance of our products is still uncertain; |
| · | We face significant competition; and |
| · | Investors in this offering will incur substantial dilution . |
The Reverse Stock Split
In order to seek to qualify the listing of our shares of common stock on the Nasdaq Capital Market or another national securities exchange, on December 1, 2015, we obtained shareholder approval to seek discretionary authority to effect a reverse stock split of our issued and outstanding shares of common stock within a range of between (i) one-for-15 to (ii) one-for-25, as determined at the discretion of our board of directors to be in our best interests without further approval from our stockholders.
We intend to consummate a one-for-25 reverse stock split immediately prior to the effective date of the registration statement of which this prospectus is a part. No fractional shares of common stock were issued in connection with the reverse stock split, and all such fractional interests will be rounded down to the nearest whole number. Issued and outstanding stock options, convertible notes and warrants will be split on the same basis and exercise prices will be adjusted accordingly.
Unless otherwise indicated, all information presented in this prospectus gives retroactive effect to such 1-for-25 reverse stock split and all share price, per share, convertible note conversion prices and stock option and warrant exercise price data set forth in this prospectus has been adjusted to give effect to the one-for-25 reverse stock split.
As of May 26, 2016 and December 31, 2015 we had issued and outstanding a total of (i) 133,720,519 and 133,146,250 shares of our common stock, respectively, and (ii) warrants to purchase a total of 80,807,644 and 71,342,894 shares of our common stock, respectively, at certain exercise prices ranging from $0.25 to $2.25 per share.
As a result of the proposed 1-for-25 reverse stock split, (i) all outstanding 133,720,519 shares of our common stock, prior to the consummation of such reverse stock split, will be reduced to 5,348,820 shares and (ii) all shares of our common stock issuable upon exercise of outstanding 80,807,644 warrants, prior to the consummation of the reverse stock split, will be reduced to 3,232,305 shares with a corresponding increase in the exercise prices of such warrants to prices ranging from $6.25 to $56.25 per share.
Notwithstanding the above, based on our current pre-offering closing market price on the OTCQB at June 21, 2016 of $0.17 per share, in order to offer our shares of common stock within a price range of between $4.50 and $6.50 per share and meet the requirements to list our shares on the Nasdaq Capital Market or other national securities exchange approved by our underwriters, prior to the effective date of the registration statement of which this prospectus is a part, we may be required to obtain stockholder approval to increase such reverse stock split to an amount in excess of 1-for-25. There can be no assurance that such stockholder approval will be obtained. See “Risk Factors’ elsewhere in this prospectus.
Additional information regarding our issued and outstanding securities may be found in the section of this prospectus titled “Description of Securities.”
6
Related Party Indebtedness
As of the date of this prospectus, we are indebted to certain members of our board of directors or their affiliates for loans and advances made to our Company over the past five years in the aggregate amount of $2,676,500. We are currently in discussions with members of our Board of Directors, including Stephen Turner, our President and CEO, to repay (on a pro-rata basis), out of the net proceeds of this offering and on a date which shall be 30 days following the listing of our common stock on the Nasdaq Capital Market, an aggregate of $669,125, or 25% of the total of $2,676,500 of related party advances from such persons. See “Use of Proceeds”. The balance of such indebtedness to our directors and their affiliates would be evidenced by interest-bearing notes due as to principal and accrued interest over a one year period. There can be no assurance that we will be successful in completing the contemplated agreements or the terms and conditions of any such arrangements. Any such agreements may be subject to completion of this offering. In addition, we agreed to issue to certain of our directors, three year warrants to purchase up to 120,000 shares of common stock at an exercise price of $10.00 per share (after giving effect to the reverse stock split), in consideration of their extension of their personal guarantees of our bank line of credit. See “Certain Relationships and Related Transactions” elsewhere in this prospectus.
Organizational History
We were incorporated as SRKP 5, Inc., in Delaware on May 24, 2005. Prior to the Reverse Merger (as defined below) and split-off (as described below), our business was to provide software solutions to deliver geo-location targeted coupon advertising to mobile internet devices.
On September 2, 2011, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Protea Biosciences, Inc., a Delaware corporation (“PBI”), and our wholly-owned subsidiary formed to complete the merger. Under the terms of the Merger Agreement, our subsidiary merged with and into PBI, as a result of which PBI became our wholly owned subsidiary (the “Reverse Merger”). In the Reverse Merger, each share of PBI common stock was automatically converted into one share of our common stock, all shares of PBI common stock in treasury were canceled and we assumed all of PBI’s rights and obligations for outstanding convertible securities and warrants. Upon the Reverse Merger, we discontinued our prior business, and our business became the business of PBI and its subsidiaries.
7
Corporate Information
Our principal executive office is located 1311 Pineview Drive, Suite 501, Morgantown, West Virginia 26505. Our telephone number is 1-304-292-2226. Our web address is http://proteabio.com. Information included on our website is not part of this prospectus.
Implications of Being an Emerging Growth Company
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an emerging growth company until the earlier of (i) December 31, 2019, the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement under the Securities Act; (ii) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under applicable SEC rules. We expect that we will remain an emerging growth company for the foreseeable future, but cannot retain our emerging growth company status indefinitely and will no longer qualify as an emerging growth company on or before December 31, 2019. We refer to the Jumpstart Our Business Startups Act of 2012 herein as the "JOBS Act," and references herein to "emerging growth company" have the meaning associated with it in the JOBS Act. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from specified disclosure requirements that are applicable to other public companies that are not emerging growth companies.
These exemptions include:
| · | being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced "Management's Discussion and Analysis of Financial Condition and Results of Operations" disclosure; | |
| · | not being required to comply with the requirement of auditor attestation of our internal controls over financial reporting; |
| · | not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements; | |
| · | reduced disclosure obligations regarding executive compensation; and | |
| · | not being required to hold a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
For as long as we continue to be an emerging growth company, we expect that we will take advantage of the reduced disclosure obligations available to us as a result of that classification. We have taken advantage of certain of those reduced reporting burdens in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
An emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected to avail ourselves of this extended transition period and, as a result, we will not be required to adopt new or revised accounting standards on the dates on which adoption of such standards is required for other public reporting companies.
We are also a "smaller reporting company" as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and have elected to take advantage of certain of the scaled disclosure available for smaller reporting companies.
8
THE OFFERING
| Common stock outstanding prior to the offering | 5,348,820 shares (1) |
| Common stock offered by the Company | 3,100,000 shares | |
| Over-allotment option | The underwriters have an option for a period of 45 days to purchase up to 465,000 additional shares of our common stock to cover over-allotments, if any. | |
| Use of proceeds | We estimate that the net proceeds to us from this offering will be approximately $15,000,000 or approximately $17,352,900 if the underwriters exercise their option to purchase additional shares in full, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. We intend to use the net proceeds of this offering to reduce our indebtedness by approximately $1,324,125 (including, approximately $669,125 owed to certain of our stockholders and directors), to fund research and development of new products, to increase our sales and marketing efforts, to build infrastructure, including hiring of additional personnel, and for working capital and other general corporate purposes. For additional information, please refer to the section entitled “Use of Proceeds’ on page 22 of this prospectus. | |
| Proposed Nasdaq Stock Market symbol: | PRGB. Our common stock is currently quoted on the OTCQB under the symbol “PRGB.” | |
| Risk Factors | You should carefully consider the information set forth in this prospectus and, in particular, the specific factors set forth in the “Risk Factors” section beginning on page 10 of this prospectus before deciding whether or not to invest in shares of our common stock. |
| (1) | Represents shares of our common stock outstanding as of March 31, 2016. Does not include: |
| · | 394,203 shares of common stock issuable upon the exercise of outstanding stock options, at a weighted average exercise price of $17.25 per share; | |
| · | 3,141,890 shares of common stock issuable upon the exercise of outstanding warrants at a weighted average exercise price of $17.64 per share; | |
| · | 120,000 shares of common stock issuable upon the exercise of warrants we intend to issue to certain of our directors at an exercise price of $10.00 per share; | |
| · | 40,242 shares of common stock issuable upon conversion of $400,000 of convertible notes and 235,000 shares of common stock issuable upon conversion of $1,468.750 of convertible notes issued in May and June 2016; | |
| · | 90,415 shares of our common stock underlying warrants issued to the representative of the underwriters, in their capacity as placement agent in two private placements consummated in the fourth quarter of 2015, the first quarter of 2016, and May and June 2016, respectively, and | |
| · | shares of our common stock underlying the warrants to be issued to the representative of the underwriters in connection with this offering. |
Except as otherwise indicated, all information in this prospectus assumes and gives effect to:
| · | A 1-for-25 reverse split of our common stock, which will occur prior to the effectiveness of the registration statement of which this prospectus is a part. |
| · | no exercise by the underwriters of their option to purchase up to an additional 465,000 shares of our common stock. |
| · | no exercise of the of the representative’s warrants described above. |
9
Our business is subject to many risks and uncertainties, which may affect our future financial performance. If any of the events or circumstances described below occur, our business and financial performance could be adversely affected, our actual results could differ materially from our expectations, and the price of our stock could decline. The risks and uncertainties discussed below are not the only ones we face. There may be additional risks and uncertainties not currently known to us or that we currently do not believe are material that may adversely affect our business and financial performance. You should carefully consider the risks described below, together with all other information included in this prospectus including our financial statements and related notes, before making an investment decision. The statements contained in this prospectus that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and investors in our securities may lose all or part of their investment.
Risks Related to Our Business
We are an emerging growth company with a limited operating history and limited sales to date.
The Company is subject to all of the risks inherent in the establishment of an emerging growth company including the absence of an operating history and the risk that we may be unable to successfully develop, manufacture and sell our products. There can be no assurance that the Company will be able to execute its business plan, including without limitation the Company’s plans to develop, then manufacture, market and sell its technologies, products and services. The Company has engaged in limited manufacturing operations to date and although the Company believes that its plans to conduct manufacturing of its products internally will work, there is no assurance that this will be the case. The Company began to sell products and services in the fourth quarter of 2007 and sales to date are limited. There can be no assurance that the Company’s sales projections and marketing plans will be achieved as anticipated and planned. It is likely that losses will be incurred during the early stages of operations. The Company believes that its future success will depend on its ability to develop and introduce its instruments and services for mass spec molecular imaging, to meet a wide range of customer needs and achieve market acceptance. The Company cannot assure prospective investors that it will be able to successfully develop and market its products or that it will recover the initial investment that must be made to develop and market such products.
We have incurred net losses since inception.
We incurred a net loss of $1,085,496 for the quarter ended March 31, 2016, $9,574,434 for the year ended December 31, 2015 and $11,474,770 for the year ended December 31, 2014. As of March 31, 2016, the Company has an accumulated deficit of $80,683,152 since inception. Our independent registered public accountants issued an opinion on our audited financial statements as of and for the year ended December 31, 2015 that contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. We can provide no assurance as to when, or if, we will be profitable in the future. Even if the Company achieves profitability, it may not be able to sustain such profitability
We must raise additional working capital.
At the present time, our ability to continue as a going concern is dependent upon raising capital from financing transactions. To stay in business, we will need to raise additional working capital through public or private sales of our equity securities, debt financing or short-term bank loans, or a combination of the foregoing. We are currently in the process of seeking to obtain additional working capital through the private placement of additional bridge notes and warrants. Based on our current spending levels, management estimates that the Company will need to raise approximately $6,000,000 in additional working capital to maintain current operations through the next twelve calendar months.
There can be no assurance that we will be able to raise sufficient additional working capital financing to sustain our operations on acceptable terms, or at all. If such financing is not available on satisfactory terms or is not available at all, we may be required to delay, scale back or eliminate the development of business opportunities and our operations and our financial condition may be materially adversely affected. Debt financing, if obtained, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt and could increase our expenses and require that our assets be provided as a security for such debt. Debt financing would also be required to be repaid regardless of our operating results. Equity financing, if obtained, could result in dilution to our then existing stockholders.
10
Issuance of common stock to fund our operations or upon the conversion of convertible debt or equity securities or exercise of outstanding warrants and options may dilute your investment.
We have been operating at a loss since inception and our working capital requirements continue to be significant. We have been supporting our business through the sale of debt and equity since inception. We will need additional funding for developing products and services, increasing our sales and marketing capabilities, technologies and assets, as well as for working capital requirements and other operating and general corporate purposes. Our working capital requirements depend and will continue to depend on numerous factors including the timing of revenues, the expense involved in development of our products, and capital improvements. If we are unable to generate sufficient revenue and cash flow from operations, we will need to seek additional financing through the sale of additional shares of common stock, warrants and/or convertible debt securities to provide the capital required to maintain or expand our operations, which may have the effect of diluting our existing stockholders or restricting our ability to run our business.
As of the date of this prospectus, we have warrants to purchase an aggregate of 3,155,930 shares of common stock issued and outstanding, as adjusted for the reverse stock split. The Company also has reserved an aggregate of 166,000 shares of common stock for issuance under its 2002 Equity Incentive Plan (the “2002 Plan”) and 500,000 shares of common stock have been reserved for issuance under the Company’s 2013 Equity Incentive Plan (the “2013 Plan”). As of the date of this prospectus, options to purchase an aggregate of 394,203 shares of common stock have been granted and are outstanding under the 2002 Plan and the 2013 Plan, collectively.
We have significant short-term indebtedness.
As of June 20, 2016, we have total indebtedness of $10,282,400, which includes $5,766,965 that is due within twelve months. Of this short-term component, $2,676,500 is owed to certain members of the Company’s Board of Directors or their affiliates, $961,226 is represented by notes owed primarily to three governmental agencies of the State of West Virginia, $1,739,568 is represented by convertible notes issued in 2016, $389,671 is related to capitalized leases. We are currently in discussions with existing directors holding $2,676,500 of related party indebtedness to defer payment of an estimated $ 2,007,375 or 75% of the total amount owed to them. Although we have allocated an estimated $1,324,125 of the net proceeds of this offering to reduce our indebtedness (including approximately $669,125 owed to certain of our stockholders and directors) there can be no assurance that the maturity dates of the balance of our obligations owed to the existing directors and shareholders, and the governmental agencies of the State of West Virginia can or will be extended, if required, nor do we know the final terms and conditions of any extensions, if any. If such obligations are not extended, we will be required to apply additional net proceeds of this offering to further reduce our debt obligations. See “Use of Proceeds” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and “Certain Relationships and Related Transactions” elsewhere in this prospectus.
We depend on the pharmaceutical and biotechnology industries.
Over the past several years, some areas of our businesses have grown significantly as a result of an increase in the sales of our bioanalytical instrument platform known as “LAESI®” and the increase in pharmaceutical, academic and clinical research laboratory outsourcing of their clinical drug research support activities. We believe that due to the significant investment in facilities and personnel required to support drug development, pharmaceutical, academic and clinical research laboratories look to purchase our bioanalytical instrument platforms and solutions technology to meet and administer their drug research requirements. Our revenues depend greatly on the expenditures made by these pharmaceutical and academic/clinical research laboratory companies in research and development. In some instances, companies in these industries are reliant on their ability to raise capital in order to fund their research and development projects. Accordingly, economic factors and industry trends that affect our clients in these industries also affect our business. If companies in these industries were to reduce the number of research and development projects they conduct or outsource, our business could be materially adversely affected.
Changes in government regulation or in practices relating to the pharmaceutical industry could change the need for the services we provide.
Governmental agencies throughout the world, but particularly in the United States, strictly regulate the drug development process. Changes in regulation, such as regulatory submissions to meet the internal research and development standards of pharmaceutical research, a relaxation in existing regulatory requirements, the introduction of simplified drug approval procedures or an increase in regulatory requirements that we may have difficulty satisfying or that make our services less competitive, could substantially change the demand for our services. Also, if the government increases efforts to contain drug costs and pharmaceutical companies profits from new drugs, our customers may spend less, or reduce their growth in spending on research and development.
We may be affected by health care reform.
In March 2010, the United States Congress enacted the Patient Protection and Affordable Care Act (“PPACA”) which is intended over time to expand health insurance coverage and impose health industry cost containment measures. PPACA legislation and the accompanying regulations may significantly impact the pharmaceutical and biotechnology industries as it is implemented over the next several years. In addition, the U.S. Congress, various state legislatures and European and Asian governments may consider various types of health care reform in order to control growing health care costs. We are unable to predict what legislative proposals will be adopted in the future, if any.
Implementation of health care reform legislation may have certain benefits but also may contain costs that could limit the profits that can be made from the development of new drugs. This could adversely affect research and development expenditures by pharmaceutical and biotechnology companies, which could in turn decrease the business opportunities available to us both in the United States and abroad. In addition, new laws or regulations may create a risk of liability, increase our costs or limit our service offerings.
11
A reduction in research and development budgets at pharmaceutical companies and clinical research institutions may adversely affect our business.
Our customers include researchers at pharmaceutical companies and academic/clinical research laboratory institutions. Our ability to continue to grow and win new business is dependent in large part upon the ability and willingness of the pharmaceutical and biotechnology industries to continue to spend on research and development and to outsource their product equipment and service needs. Fluctuations in the research and development budgets of these researchers and their organizations could have a significant effect on the demand for our products and services. Research and development budgets fluctuate due to changes in available resources, mergers of pharmaceutical companies and spending priorities and institutional budgetary policies of academic/clinical research organizations. Our business could be adversely affected by any significant decrease in life sciences research and development expenditures by pharmaceutical and academic/clinical research companies. Similarly, economic factors and industry trends that affect our clients in these industries also affect our business.
We rely on a limited number of key customers, the importance of which may vary dramatically from year to year, and a loss of one or more of these key customers may adversely affect our operating results.
A small number of customers have accounted for a substantial portion of our revenues in 2015. Five customers accounted for approximately 52% of our gross revenue in fiscal 2015 and five customers accounted for approximately 42% of our gross revenues in fiscal 2014. One large pharmaceutical company accounted for 20% of our gross revenue in 2015, and this customer will continue to be a significant contributor to revenue in 2016. The loss of a significant amount of business from one of our major customers would materially and adversely affect our results of operations until such time, if ever, as we are able to replace the lost business. Significant clients or projects in any one period may not continue to be significant clients or projects in other periods. In any given year, there is a possibility that a single pharmaceutical or academic/ clinical research laboratory company may account for 5% or more of our gross revenue or that our business may be dependent on one or more large projects. To the extent that we are dependent on any single customer, we are subject to the risks faced by that customer to the extent that such risks impede the customer's ability to stay in business and make timely payments to us.
We may bear financial risk if we underprice our contracts or overrun cost estimates.
Since some of our contracts are structured as fixed price or fee-for-service, we bear the financial risk if we initially underprice our contracts or otherwise overrun our cost estimates. Such underpricing or significant cost overruns could have a material adverse effect on our business, results of operations, financial condition, and cash flows.
A default in our credit facility could materially and adversely affect our operating results and our financial condition.
The Company has an outstanding line of credit with United Bank. This credit facility requires us to adhere to certain contractual covenants. If there were an event of default under our credit facility that was not cured or waived, the lenders of the defaulted debt could cause all amounts outstanding with respect to that debt to be due and payable immediately. Although the outstanding balance of this line of credit is currently payable on demand, the amount due and the status of the payment terms have been unchanged since the first quarter of 2014. We cannot assure that our assets or cash flow would be sufficient to fully repay borrowings under the credit facility, either upon the bank’s demand or upon an event of default, or that we would be able to refinance or restructure the payments becoming due on the credit facility. Please see Note 3 to the Consolidated Financial Statements, “Bank Line of Credit,” for additional detail regarding our credit facility.
We might incur expenses to develop products that are never successfully commercialized.
We have incurred and expect to continue to incur research and development and other expenses in connection with our products business. The potential products to which we devote resources might never be successfully developed or commercialized by us for numerous reasons including:
| · | inability to develop products that address our customers’ needs; | |
| · | competitive products with superior performance; | |
| · | patent conflicts or unenforceable intellectual property rights; | |
| · | demand for the particular product; | |
| · | other factors that could make the product uneconomical; and | |
| · | termination of pre-existing license agreements. |
Incurring expenses for a potential product that is not successfully developed and/or commercialized could have a material adverse effect on our business, financial condition, prospects and stock price.
12
Our business uses biological and hazardous materials, which could injure people or violate laws, resulting in liability that could adversely impact our financial condition and business.
Our activities involve the controlled use of potentially harmful biological materials as well as hazardous materials and chemicals. We cannot completely eliminate the risk of accidental contamination or injury from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for damages that result and any liability could exceed our insurance coverage and ability to pay. Any contamination or injury could also damage our reputation, which is critical to obtaining new business. In addition, we are subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations is significant and if changes are made to impose additional requirements, these costs could increase and have an adverse impact on our financial condition and results of operations.
Hardware or software failures, delays in the operations of our computer and communications systems or the failure to implement system enhancements could harm our business.
Our success depends on the efficient and uninterrupted operation of our computer and communications systems. A failure of our network or data gathering procedures could impede the processing of data, delivery of databases and services, client orders and day-to-day management of our business and could result in the corruption or loss of data. While all of our operations have disaster recovery plans in place, they might not adequately protect us. Despite any precautions we take, damage from fire, floods, hurricanes, power loss, telecommunications failures, computer viruses, break-ins and similar events at our computer facilities could result in interruptions in the flow of data to our servers and from our servers to our clients. In addition, any failure by our computer environment to provide our required data communications capacity could result in interruptions in our service. In the event of a delay in the delivery of data, we could be required to transfer our data collection operations to an alternative provider of server hosting services. Such a transfer could result in delays in our ability to deliver our products and services to our clients. Additionally, significant delays in the planned delivery of system enhancements, improvements and inadequate performance of the systems once they are completed could damage our reputation and harm our business. Finally, long-term disruptions in the infrastructure caused by events such as natural disasters, the outbreak of war, the escalation of hostilities and acts of terrorism, particularly involving cities in which we have offices, could adversely affect our businesses. Although we carry property and business interruption insurance, our coverage might not be adequate to compensate us for all losses that may occur.
We rely on third parties for important services.
We depend on third parties to provide us with services critical to our business. The failure of any of these third parties to adequately provide the needed services including, without limitation, licensed intellectual property rights, could have a material adverse effect on our business.
We license a significant portion of our intellectual property from third parties; if the Company fails to remain in compliance with these agreements the Company’s business may be adversely affected.
The Company has entered into a number of technology license agreements with various universities for the exclusive use of a significant portion of the patent-based intellectual property that the Company uses. Generally, the license agreements imposes, and we expect that future license agreements will impose, various diligence, milestone payment, royalty and other obligations on us. If we fail to comply with our obligations under these agreements, or if we file for bankruptcy, we may be required to make certain payments to the licensor, we may lose the exclusivity of our license, or the licensor may have the right to terminate the license, in which event we would not be able to develop or market products covered by the license. Additionally, the milestone and other payments associated with these licenses could materially and adversely affect our business, financial condition and results of operations. While the Company is currently in compliance with the respective terms of these agreements, if there are one or more breaches thereunder, such as the failure to pay the applicable royalties, and one or more of these agreements are terminated, the Company will not be able to use such technology and the Company’s business may be adversely affected.
In some cases, patent prosecution of our licensed technology may be controlled solely by the licensor. If our licensors fail to obtain and maintain patent or other protection for the proprietary intellectual property we in-license from them, we could lose our rights to the intellectual property or our exclusivity with respect to those rights, and our competitors could market competing products using the intellectual property. In certain cases, we may control the prosecution of patents resulting from licensed technology. In the event we breach any of our obligations related to such prosecution, we may incur significant liability to our licensing partners. Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues. Disputes may arise regarding intellectual property subject to a licensing agreement, including, but not limited to:
| · | the scope of rights granted under the license agreement and other interpretation-related issues; |
| · | the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| · | the sublicensing of patent and other rights; |
| · | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
13
| · | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our collaborators; and |
| · | the priority of invention of patented technology. |
If disputes over intellectual property and other rights that we have in-licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected drug candidates. If we fail to comply with any such obligations to our licensor, such licensor may terminate their licenses to us, in which case we would not be able to market products covered by these licenses. The loss of any of our current licensing arrangements and potentially other licenses that we enter into in the future, would have a material adverse effect on our business.
We may be unable to obtain or maintain patent or other intellectual property protection for any products or processes that we may develop.
The Company faces risks and uncertainties related to intellectual property rights. The Company may be unable to obtain or maintain its patents or other intellectual property protection for any products or processes that it may develop; third parties may obtain patents covering the manufacture, use or sale of these products or processes which may prevent the Company from commercializing its technology; or any patents that the Company may obtain may not prevent other companies from competing with it by designing their products or conducting their activities so as to avoid the coverage of the Company’s patents.
Since patent applications in the U.S. are maintained in secrecy for at least portions of their pendency periods (published on U.S. patent issuance or, if earlier, 18 months from the earliest filing date for most applications) and since other publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we are the first to make the inventions to be covered by our patent applications. The patent position of biopharmaceutical firms generally is highly uncertain and involves complex legal and factual questions. The U.S. Patent and Trademark Office has not established a consistent policy regarding the breadth of claims that it will allow in biotechnology patents.
Proceedings to obtain, enforce or defend patents and to defend against charges of infringement are time consuming and expensive activities, and it is possible that the Company could become involved in such proceedings. Unfavorable outcomes in these proceedings could limit the Company’s activities and any patent rights that the Company may obtain, which could adversely affect its business or financial condition. Even if such proceedings ultimately are determined to be without merit, they can be expensive and distracting for the Company’s operations and personnel.
In addition, the Company’s success will depend in part on the ability of the Company to preserve its trade secrets. The Company cannot ensure investors that the obligations to maintain the confidentiality of trade secrets or proprietary information will not wrongfully be breached by employees, consultants, advisors or others or that the Company’s trade secrets or proprietary know how will not otherwise become known or be independently developed by competitors in such a manner that the Company has no legal recourse.
We may infringe the intellectual property rights of others, which may prevent or delay our development efforts and prevent us from commercializing or increase the costs of commercializing our products.
Our commercial success depends significantly on our ability to operate without infringing the patents and other intellectual property rights of third parties. For example, there could be issued patents of which we are not aware that our current or potential future product candidates infringe. There also could be patents that we believe we do not infringe, but that we may ultimately be found to infringe.
Moreover, patent applications are in some cases maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications were filed. Because patents can take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents that our drug candidates or potential products infringe. For example, pending applications may exist that claim or can be amended to claim subject matter that our product candidates or potential products infringe. Competitors may file continuing patent applications claiming priority to already issued patents in the form of continuation, divisional, or continuation-in-part applications, in order to maintain the pendency of a patent family and attempt to cover our product candidates.
Third parties may assert that we are employing their proprietary technology without authorization and may sue us for patent or other intellectual property infringement. These lawsuits are costly and could adversely affect our business, financial condition and results of operations and divert the attention of managerial and scientific personnel. If we are sued for patent infringement, we would need to demonstrate that our drug candidates, potential products or methods either do not infringe the claims of the relevant patent or that the patent claims are invalid, and we may not be able to do this. Proving invalidity is difficult. For example, in the U.S., proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in pursuing these proceedings, which could have a material adverse effect on us. In addition, we may not have sufficient resources to bring these actions to a successful conclusion. If a court holds that any third-party patents are valid, enforceable and cover our products or their use, the holders of any of these patents may be able to block our ability to commercialize our products unless we acquire or obtain a license under the applicable patents or until the patents expire.
14
We may not be able to enter into licensing arrangements or make other arrangements at a reasonable cost or on reasonable terms. Any inability to secure licenses or alternative technology could result in delays in the introduction of our products or lead to prohibition of the manufacture or sale of products by us. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, in any such proceeding or litigation, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our drug candidates or force us to cease some of our business operations, which could materially and adversely affect our business, financial condition and results of operations. Any claims by third parties that we have misappropriated their confidential information or trade secrets could have a similar material and adverse effect on our business, financial condition and results of operations. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
Any claims or lawsuits relating to infringement of intellectual property rights brought by or against us will be costly and time consuming and may adversely affect our business, financial condition and results of operations.
We may be required to initiate litigation to enforce or defend our licensed and owned intellectual property. These lawsuits can be very time consuming and costly. There is a substantial amount of litigation involving patent and other intellectual property rights in the biopharmaceutical industry generally. Such litigation or proceedings could substantially increase our operating expenses and reduce the resources available for development activities or any future sales, marketing or distribution activities.
In any infringement litigation, any award of monetary damages we receive may not be commercially valuable. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during litigation. Moreover, there can be no assurance that we will have sufficient financial or other resources to file and pursue such infringement claims, which typically last for years before they are resolved. Further, any claims we assert against a perceived infringer could provoke these parties to assert counterclaims against us alleging that we have infringed their patents. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
In addition, our licensed patents and patent applications, and patents and patent applications that we may own or license in the future, could face other challenges, such as interference proceedings, opposition proceedings, re-examination proceedings and other forms of post-grant review. Any of these challenges, if successful, could result in the invalidation of, or in a narrowing of the scope of, any of our licensed patents and patent applications and patents and patent applications that we may own or license in the future subject to challenge. Any of these challenges, regardless of their success, would likely be time consuming and expensive to defend and resolve and would divert our management and scientific personnel’s time and attention.
In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the market price of our common stock.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on drug candidates throughout the world would be prohibitively expensive. Competitors may use our licensed and owned technologies in jurisdictions where we have not licensed or obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we may obtain or license patent protection, but where patent enforcement is not as strong as that in the U.S. These products may compete with our products in jurisdictions where we do not have any issued or licensed patents and any future patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our licensed patents and future patents we may own, or marketing of competing products in violation of our proprietary rights generally. Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the U.S. As a result, we may encounter significant problems in protecting and defending our licensed and owned intellectual property both in the U.S. and abroad. Proceedings to enforce our future patent rights, if any, in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
15
We are in a highly competitive market.
The Company is engaged in the highly competitive field of biotechnology. Competition from numerous existing biotechnology companies and others entering the proteomics field is intense and expected to increase. Many of these companies are larger, more established and recognized in the marketplace, and/or have substantially greater financial and business resources than the Company. Moreover, competitors who are able to develop and to commence commercial sales of their products before the Company may enjoy a significant competitive advantage. Likewise, innovations by competitors could cause the Company’s products or services to become obsolete or less attractive in the marketplace, adversely affecting sales and/or sales projections. The Company cannot assure investors that its technology will enable it to compete successfully in the future.
We may expand our business through acquisitions.
We occasionally review acquisition candidates. Factors which may affect our ability to grow successfully through acquisitions include:
| · | inability to obtain financing; |
| · | difficulties and expenses in connection with integrating the acquired companies and achieving the expected benefits; |
| · | diversion of management’s attention from current operations; |
| · | the possibility that we may be adversely affected by risk factors facing the acquired companies; |
| · | acquisitions could be dilutive to earnings, or in the event of acquisitions made through the issuance of our common stock to the shareholders of the acquired company, dilutive to the percentage of ownership of our existing stockholders; |
| · | potential losses resulting from undiscovered liabilities of acquired companies not covered by the indemnification we may obtain from the seller; and |
| · | loss of key employees of the acquired companies. |
We are dependent on certain key personnel.
The success of the Company is dependent to a significant degree upon the skill and experience of its founders and other key personnel including Stephen Turner, Matthew Powell, Greg Kilby and others. The loss of the services of any of these individuals would adversely affect the Company’s business. Although the Company has obtained key man life insurance policies on Mr. Turner, its President and CEO, there is no assurance that policy proceeds would cover all potential costs or operational challenges that would result from the loss of services from Mr. Turner and in any event, such policy would not cover the lives or loss of these other individuals. The Company cannot assure prospective investors that it would be able to find adequate replacements for these key individuals. In addition, the Company believes that its future success will depend in large part upon its continued ability to attract and retain highly skilled employees, who are in great demand.
We do not currently have a full-time Chief Financial Officer.
At the present time, the function of chief financial officer is being performed in an interim basis by Paul Kane, as a consultant. Although we intend to offer Mr. Kane the opportunity to become our full-time chief financial officer following completion of this offering, to date, Mr. Kane and not agreed to accept such position nor have we agreed upon final terms and conditions of his employment. Mr. Kane has, however, agreed to continue to perform the services of our chief financial officer for a period of up to 90 days following completion of this offering pending our obtaining the services of another qualified senior executive to fill such function. Although we intend to diligently undertake a search for another qualified person in the absence of Mr. Kane’s acceptance of the position, there can be no assurance that we will be able to find an acceptable qualified person to serve as our permanent chief financial officer.
We are developing products in a rapidly evolving field and there are no assurances that the results of our research and development efforts will not be rendered obsolete by the research efforts and technological activities of others.
The bioanalytics field in which the Company is developing products is rapidly evolving. The Company cannot assure prospective investors that any results of the Company’s research and development efforts will not be rendered obsolete by the research efforts and technological activities of others, including the efforts and activities of governments, major research facilities and large multinational corporations. While the Company believes that its initial efforts to develop its bioanalytics technology platform have been successful thus far, there can be no assurance that the Company will be able to successfully expand its operations in the future, to commercialize, market and sell products and services at projected levels, or to fully develop the technology in a timely and successful manner.
There is no assurance that the Company’s manufacturing plans will be successful.
The Company employs internal and contract manufacturing. There is no assurance that the Company’s manufacturing plans will be successful. While the Company has a quality assurance program for its products, there nonetheless is inherent in any manufacturing process the risk of product defects or manufacturing problems that could result in potential liability for product liability risks.
Sale of European Subsidiary
As described in greater detail in the “Business” and “Certain Relationships and Related Transactions” sections below, on December 12, 2014 the Company completed the sale of 100% of the issued and outstanding capital stock of ProteaBio Europe SAS (“Protea Europe”), a wholly-owned subsidiary of Protea Biosciences, Inc., the Company’s wholly owned subsidiary, to AzurRx BioPharma, Inc. (“AzurRx”) pursuant to the terms and conditions of a stock purchase and sale agreement, dated as of May 21, 2014. Effective upon the closing of the Protea Europe sale, Thijs Spoor, a former director of the Company, was appointed to serve as a director and the Executive Chairman of AzurRx. While the Board did not establish a special committee to evaluate the fairness of this related party transaction or obtain a fairness opinion, our Board nevertheless believes that the terms of the transaction are no less favorable to the Company and its shareholders than such terms that would have been obtained from an unaffiliated third party.
In connection with the consummation of the Protea Europe sales, we received (i) $300,000, inclusive of the forgiveness of a $100,000 Company note, (ii) 100 shares of Series A Preferred Stock of AzurRx that is convertible into 33% of the issued and outstanding common stock of AzurRx and the right to receive certain other contingent consideration to be paid upon the satisfaction of certain events. While the Protea Europe sale agreement contemplates certain contingent payments to us, there can be no assurance that we will ever recoup our $4.0 million investment in Protea Europe.
16
In November 2015, we converted 29 shares of Series A Preferred Stock of AzurRx into 707,416 shares of common stock of AzurRx. Subsequently, we sold such shares of common stock of AzurRx and received gross proceeds of $910,000 as of December 31, 2015.
In the first quarter of 2016, the Company notified AzurRx of its intent to convert 71 of its Series A convertible Preferred Stock, $0.0001 par value per share, into shares of AzurRx common stock, $0.0001 par value per share, pursuant to the terms and conditions of the Certificate of Designations. The conversion rate was 24,393 shares of Common Stock per share of Series A Preferred Stock. The Company received 1,731,949 shares of AzurRx common stock upon conversion.
In addition, in the first quarter of 2016, the Company sold 1,016,941 shares of its AzurRx Common Stock and received aggregate proceeds of $637,100 from the sale.
In the second quarter of 2016, the Company sold 550,000 shares of AzurRx Common Stock generating net proceeds of $675,000. After this sale, the Company held 265,757 shares AzurRx Common Stock representing a 3.60% ownership interest in AzurRx, on a fully-diluted basis.
Unfavorable general economic conditions may materially adversely affect our business.
While it is difficult for us to predict the impact of general economic conditions on our business, these conditions could reduce customer demand for some of our products or services which could cause our revenue to decline. Also, our customers that are especially reliant on the credit and capital markets may not be able to obtain adequate access to credit or equity funding, which could affect their ability to make timely payments to us. Moreover, we rely on obtaining additional capital and/or additional funding to provide working capital to support our operations. We regularly evaluate alternative financing sources. Further changes in the commercial capital markets or in the financial stability of our investors and creditors may impact the ability of our investors and creditors to provide additional financing. In addition, the financial condition of our credit facility providers, which is beyond our control, may adversely change. Any decrease in our access to borrowings under our credit facility, tightening of lending standards and other changes to our sources of liquidity could adversely impact our ability to obtain the financing we need to continue operating the business in our current manner. For these reasons, among others, if the economic conditions stagnate or decline, our operating results and financial condition could be adversely affected.
Risks Relating to Ownership of Our Securities
There is no active public trading market for our common stock and we cannot assure you that an active trading market will develop in the near future.
Our common stock is quoted under the symbol “PRGB” in the over-the-counter markets, including the OTCQB tier of the OTC Markets Group, Inc.; however, it is not listed on any stock exchange and there is currently very limited trading in our securities. Although we intend upon completion of this offering to list our common stock for trading on The Nasdaq Stock Market, we cannot assure you that an active trading market for our common stock will develop in the future due to a number of factors, including the fact that we are a small company that is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. There may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales. We cannot give you any assurance that an active public trading market for our common stock will develop or be sustained. You may not be able to liquidate your shares quickly or at the market price if trading in our common stock is not active.
Our share price could be volatile and our trading volume may fluctuate substantially.
The market price of our common stock may experience volatility. Many factors could have a significant impact on the future price of our common stock including:
| · | our failure to successfully implement our business objectives; |
| · | compliance with ongoing regulatory requirements; |
| · | market acceptance of our products; |
| · | technological innovations, new commercial products or drug discovery efforts and clinical activities by us or our competitors; |
| · | changes in government regulations; |
| · | general economic conditions and other external factors; |
| · | actual or anticipated fluctuations in our quarterly financial and operating results; |
| · | the degree of trading liquidity in our common stock; and |
| · | our ability to meet the minimum standards required for remaining listed on the OTC Markets. |
17
These factors also include ones beyond our control such as market conditions within our industry and changes in the pharmaceutical and biotechnology industries. In addition, in recent years, the stock market has experienced significant price and volume fluctuations. The stock market, and in particular the market for pharmaceutical and biotechnology company stocks, has also experienced significant decreases in value in the past. This volatility and valuation decline has affected the market prices of securities issued by many companies, often for reasons unrelated to their operating performance, and might adversely affect the price of our common stock.
We have established Preferred Stock which can be designated by the Company’s Board of Directors without shareholder approval.
The Company has authorized 10,000,000 shares of Preferred Stock, of which none are issued and outstanding. The shares of Preferred Stock of the Company may be issued from time to time in one or more series, each of which shall have a distinctive designation or title as shall be determined by the Board of Directors of the Company prior to the issuance of any shares thereof. The Preferred Stock shall have such voting powers, full or limited, or no voting powers, and such preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof as adopted by the Board of Directors. In each such case, we will not need any further action or vote by our stockholders. One of the effects of undesignated Preferred Stock may be to enable the Board of Directors to render more difficult or to discourage an attempt to obtain control of us by means of a tender offer, proxy contest, merger or otherwise, and thereby to protect the continuity of our management. The issuance of shares of Preferred Stock pursuant to the Board of Director's authority described above may adversely affect the rights of holders of common stock. For example, Preferred Stock issued by us may rank senior to the common stock as to dividend rights, liquidation preference or both, may have full or limited voting rights and may be convertible into shares of common stock. Accordingly, the issuance of shares of Preferred Stock may discourage bids for the common stock at a premium or may otherwise adversely affect the market price of the common stock.
Stockholders holding aggregate of 1,074,839 shares of our Common Stock, including our directors, officers and certain key employees, are subject to a market standoff agreement which provides that the purchaser will not sell, assign or otherwise transfer or dispose of any common stock, warrants or other securities of the Company if so requested by an underwriter for a period not to exceed 180 days. If our securities are locked up at the request of any underwriter, the price of our common stock could decline if there are substantial sales of our common stock following the “lock-up” period, particularly sales by our directors, executive officers and significant stockholders, or if there is a large number of shares of our common stock available for sale.
Our Certificate of Incorporation provides our directors with limited liability.
Our Certificate of Incorporation states that our directors shall not be personally liable to us or any stockholder for monetary damages for breach of fiduciary duty as a director, except for any matter in respect of which such director shall be liable under Section 174 of the Delaware General Corporation Law (the “DGCL”) or shall be liable because the director (1) shall have breached his duty of loyalty to us or our stockholders, (2) shall have acted not in good faith or in a manner involving intentional misconduct or a knowing violation of law or, in failing to act, shall have acted not in good faith or in a manner involving intentional misconduct or a knowing violation of law, or (3) shall have derived an improper personal benefit. Article Seven further states that the liability of our directors shall be eliminated or limited to the fullest extent permitted by the DGCL, as it may be amended. These provisions may discourage stockholders from bringing suit against a director for breach of fiduciary duty and may reduce the likelihood of derivative litigation brought by stockholders on our behalf against a director.
Certain provisions of our Certificate of Incorporation and Delaware law make it more difficult for a third party to acquire us and make a takeover more difficult to complete, even if such a transaction were in the stockholders’ interest.
Our Certificate of Incorporation and the DGCL contain provisions that may have the effect of making it more difficult or delaying attempts by others to obtain control of the Company, even when these attempts may be in the best interests of our stockholders.
We also are subject to the anti-takeover provisions of the DGCL, which prohibit us from engaging in a “business combination” with an “interested stockholder” unless the business combination is approved in a prescribed manner and prohibit the voting of shares held by persons acquiring certain numbers of shares without obtaining requisite approval. This statute has the effect of making it more difficult to effect a change in control of a Delaware company.
Our financial controls and procedures may not be sufficient to ensure timely and reliable reporting of financial information, which, as a public company, could materially harm our stock price.
As a public reporting company, we require significant financial resources to maintain our public reporting status. We cannot assure you we will be able to maintain adequate resources to ensure that we will not have any future material weakness in our system of internal controls. The effectiveness of our controls and procedures may in the future be limited by a variety of factors including:
| · | faulty human judgment and simple errors, omissions or mistakes; |
| · | fraudulent action of an individual or collusion of two or more people; |
| · | inappropriate management override of procedures; and |
| · | the possibility that any enhancements to controls and procedures may still not be adequate to assure timely and accurate financial information. |
18
Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Our internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Despite these controls, because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance of achieving their control objectives. Furthermore, smaller reporting companies like us face additional limitations. Smaller reporting companies employ fewer individuals and can find it difficult to employ resources for complicated transactions and effective risk management. Additionally, smaller reporting companies tend to utilize general accounting software packages that lack a rigorous set of software controls.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2015 based on the criteria established in “Internal Control - Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management concluded as a result of material weaknesses in our internal control over financial reporting, our disclosure controls and procedures were not effective as of December 31, 2015. The ineffectiveness of our disclosure controls and procedures was due to the following material weaknesses our internal control over financial reporting, which are common to many small companies: (1) lack of sufficient personnel commensurate with the Company’s reporting requirements; (2) the Company did not consistently establish appropriate authorities and responsibilities in pursuit of the Company’s financial reporting objectives; and (3) insufficient written documentation or training of internal control policies and procedures which provide staff with guidance or framework for accounting and disclosing financial transactions.
If we fail to have effective controls and procedures for financial reporting in place, we could be unable to provide timely and accurate financial information and be subject to investigation by the Securities and Exchange Commission (the “SEC”) and civil or criminal sanctions.
We must implement additional and expensive procedures and controls in order to grow our business and organization and to satisfy new reporting requirements, which will increase our costs and require additional management resources.
As a public reporting company, we are required to comply with the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”) and the related rules and regulations of the SEC, including the requirements that we maintain disclosure controls and procedures and adequate internal control over financial reporting. In the future, if our securities are listed on a national exchange, we may also be required to comply with marketplace rules and heightened corporate governance standards. Compliance with the Sarbanes-Oxley Act and other SEC and national exchange requirements will increase our costs and require additional management resources. We recently have begun upgrading our procedures and controls and will need to continue to implement additional procedures and controls as we grow our business and organization and to satisfy new reporting requirements. If we are unable to complete the required assessment as to the adequacy of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act or if we fail to maintain internal control over financial reporting, our ability to produce timely, accurate and reliable periodic financial statements could be impaired.
If we do not maintain adequate internal control over financial reporting, investors could lose confidence in the accuracy of our periodic reports filed under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Additionally, our ability to obtain additional financing could be impaired or a lack of investor confidence in the reliability and accuracy of our public reporting could cause our stock price to decline.
Our directors, officers and principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
Our officers, directors and principal stockholders collectively beneficially own approximately 20% of our outstanding common stock. As a result, these stockholders, if they act together, will be able to control the management and affairs of our company and most matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control and might adversely affect the market price of our common stock. This concentration of ownership may not be in the best interests of our other stockholders.
19
We have not paid dividends in the past and do not expect to pay dividends in the future, and any return on investment may be limited to the value of our stock.
We have never paid cash dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable future. We currently intend to retain any future earnings to support the development of our business and do not anticipate paying cash dividends in the foreseeable future. Our payment of any future dividends will be at the discretion of our Board of Directors after taking into account various factors, including, but not limited to, our financial condition, operating results, cash needs, growth plans and the terms of any credit agreements that we may be a party to at the time. In addition, our ability to pay dividends on our common stock may be limited by Delaware state law. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize a return on their investment. Investors seeking cash dividends should not purchase our common stock.
Risks Related to Our Reverse Stock Split
The recent decline in the market price for our common stock may adversely impact our proposed 1-for-25 reverse stock split and the offering of our common stock.
Between April 2014 and March 31, 2016, the market price of our common stock has declined from a high of $2.10 per share to as low as $0.11 per share and closed on June 21, 2016 at $0.17 per share. In order to qualify to list our shares on the Nasdaq Capital Market we will need to maintain a minimum $4.00 per share market price. Accordingly, in order to offer our shares of common stock for sale within the anticipated price range of between $4.50 and $6.50 per share, unless the current market price of our common stock increases prior to the effective date of the registration statement of which this prospectus is a part, we may be required to obtain stockholder approval to increase such reverse stock split to an amount in excess of 1-for-25, which increase could be significant. There can be no assurance that such stockholder approval will be obtained. If we do not obtain such stockholder approval, if required, we will not be able to list our common stock on the Nasdaq Capital market or other national securities exchange acceptable to our underwrites and therefore may be required to abandon this offering.
Even if the reverse stock split achieves the requisite increase in the market price of our common stock, we cannot assure you that we will be able to continue to comply with the minimum bid price requirement of the Nasdaq Capital Market.
Even if the reverse stock split achieves the requisite increase in the market price of our common stock to be in compliance with the minimum bid price of the Nasdaq Capital Market, there can be no assurance that the market price of our common stock following the reverse stock split will remain at the level required for continuing compliance with that requirement. It is not uncommon for the market price of a company's common stock to decline in the period following a reverse stock split. If the market price of our common stock declines following the effectuation of a reverse stock split, the percentage decline may be greater than would occur in the absence of a reverse stock split. In any event, other factors unrelated to the number of shares of our common stock outstanding, such as negative financial or operational results, could adversely affect the market price of our common stock and jeopardize our ability to meet or maintain the Nasdaq Capital Market's minimum bid price requirement. In addition to specific listing and maintenance standards, the Nasdaq Capital Market has broad discretionary authority over the initial and continued listing of securities, which it could exercise with respect to the listing of our common stock.
Even if the reverse stock split increases the market price of our common stock, there can be no assurance that we will be able to comply with other continued listing standards of the Nasdaq Capital Market.
Even if the market price of our common stock increases sufficiently so that we comply with the minimum bid price requirement, we cannot assure you that we will be able to comply with the other standards that we are required to meet in order to maintain a listing of our common stock on the Nasdaq Capital Market. Our failure to meet these requirements may result in our common stock being delisted from the Nasdaq Capital Market, irrespective of our compliance with the minimum bid price requirement.
The reverse stock split may decrease the liquidity of the shares of our common stock.
The liquidity of the shares of our common stock may be affected adversely by the reverse stock split given the reduced number of shares that will be outstanding following the reverse stock split, especially if the market price of our common stock does not increase as a result of the reverse stock split. In addition, the reverse stock split may increase the number of stockholders who own odd lots (less than 100 shares) of our common stock, creating the potential for such stockholders to experience an increase in the cost of selling their shares and greater difficulty effecting such sales.
Following the reverse stock split, the resulting market price of our common stock may not attract new investors, including institutional investors, and may not satisfy the investing requirements of those investors. Consequently, the trading liquidity of our common stock may not improve.
Although we believe that a higher market price of our common stock may help generate greater or broader investor interest, there can be no assurance that the reverse stock split will result in a share price that will attract new investors, including institutional investors. In addition, there can be no assurance that the market price of our common stock will satisfy the investing requirements of those investors. As a result, the trading liquidity of our common stock may not necessarily improve.
20
Risks Relating to this Offering
We have significant discretion over the use of the gross proceeds.
The maximum gross proceeds to us from the sale of our common stock will be $17,000,000 ($19,352,900 if the over-allotment is exercised). Substantially all of the remaining net proceeds of this Offering will be used for working capital and general corporate purposes. The proceeds shall be used to repay indebtedness, carry out our business plan, pay salaries to our employees and satisfy our expenses, foreseeable and unforeseeable. As is the case with any business, it should be expected that certain expenses unforeseeable to management at this juncture will arise in the future. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us. The failure of our management to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flow. Investors are urged to consult with their attorneys, accountants and personal investment advisors prior to making any decision to invest in the Company.
Purchasers in this offering will likely experience immediate and substantial dilution in the book value of their investment.
Because the prices per share at which shares of our common stock are sold in this offering may be substantially higher than the book value per share of our common stock, you may suffer immediate and substantial dilution in the net tangible book value of the common stock you purchase in this offering. After giving effect to the sale of 3,100,000 shares of common stock in this offering at the anticipated offering price of $5.50 per share, and after deducting estimated offering expenses payable by us, our net tangible book value as of March 31, 2016 would have been $5,807,007, or approximately $0.69 per share of our common stock. This represents an immediate increase in as adjusted pro forma, net tangible book value per share of $2.41 to the existing stockholders and an immediate dilution in as adjusted pro forma net tangible book value per share of $4.81 to new investors who purchase our common stock in the offering. See the section entitled “Dilution” for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering.
A substantial number of shares of our common stock may be sold in this offering, which could cause the price of our common stock to decline.
In this offering we will sell 3,100,000 shares, or approximately 39% of the total number of shares of our common stock to be outstanding stock after giving effect to completion of this offering. In addition, as many as an additional 3,816,750 shares may be issued upon exercise of our outstanding warrants and options and conversion of our outstanding convertible notes. The sale in this offering and any future sales of a substantial number of shares of our common stock in the public market (in, or the perception that such sales may occur, could adversely affect the price of our common stock. We cannot predict the effect, if any, that market sales of those shares of common stock or the availability of those shares of common stock for sale will have on the market price of our common stock.
If our stockholders sell, or the market perceives that our stockholders intend to sell for various reasons, including the ending of restriction on resale or the expiration of lock-up agreements such as those entered into in connection with this offering, substantial amounts of our common stock in the public market, including shares issued upon the exercise of outstanding options or warrants, the market price of our common stock could fall. Sales of a substantial number of shares of our common stock may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate. We may become involved in securities class action litigation that could divert management's attention and harm our business.
The stock markets have from time to time experienced significant price and volume fluctuations that have affected the market prices for the common stock of biotechnology and biopharmaceutical companies. These broad market fluctuations may cause the market price of our common stock to decline. In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and biopharmaceutical companies have experienced significant stock price volatility in recent years. We may become involved in this type of litigation in the future. Litigation often is expensive and diverts management's attention and resources, which could adversely affect our business.
An investment in the Company’s common stock is speculative and there can be no assurance of any return on any such investment.
An investment in the Company’s common stock is speculative and there is no assurance that investors will obtain any return on their investment. Investors will be subject to substantial risks involved in an investment in the Company, including the risk of losing their entire investment.
If securities or industry analysts do not publish research or reports about our business, or publish negative reports about our business, our share price and trading volume could decline.
The trading market for our common stock will, to some extent, depend on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. If one or more of the analysts who cover us downgrade our shares or change their opinion of our shares, our share price would likely decline. If one or more of these analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
IN ADDITION TO THE ABOVE RISKS, BUSINESSES ARE OFTEN SUBJECT TO RISKS NOT FORESEEN OR FULLY APPRECIATED BY MANAGEMENT. IN REVIEWING THIS PROSPECTUS, POTENTIAL INVESTORS SHOULD KEEP IN MIND THAT OTHER POSSIBLE RISKS MAY ADVERSELY IMPACT THE COMPANY’S BUSINESS OPERATIONS AND THE VALUE OF THE COMPANY’S SECURITIES.
21
EXPLANATORY NOTE REGARDING REVERSE STOCK SPLIT
In order to seek to qualify the listing of our shares of common stock on The Nasdaq Capital Market or another national securities exchange, on December 1, 2015, we obtained shareholder approval to seek discretionary authority to effect a reverse stock split of our issued and outstanding shares of common stock within a range of between (i) one-for-15 to (ii) one-for-25, as determined at the discretion of our board of directors to be in our best interests without further approval from our stockholders.
We intend to consummate a one-for-25 reverse stock split immediately prior to the effective date of the registration statement of which this prospectus is a part. No fractional shares of common stock were issued in connection with the reverse stock split, and all such fractional interests will be rounded down to the nearest whole number. Issued and outstanding stock options, convertible notes and warrants will be split on the same basis and exercise prices will be adjusted accordingly.
Unless otherwise indicated, all information presented in this prospectus gives retroactive effect to such 1-for-25 reverse stock split and all share price, per share, convertible note conversion prices and stock option and warrant exercise price data set forth in this prospectus has been adjusted to give effect to the one-for-25 reverse stock split.
As of June 21, 2016 and December 31, 2015 we had issued and outstanding a total of (i) 133,720,519 and 133,146,250 shares of our common stock, respectively, and (ii) warrants to purchase a total of 80,807,644 and 71,342,894 shares of our common stock, respectively, at certain exercise prices ranging from $0.25 to $2.25 per share.
As a result of the anticipated 1-for-25 reverse stock split, (i) all outstanding 133,720,519 shares of our common stock, prior to the consummation of such reverse stock split, will be reduced to 5,348,820 shares and (ii) all shares of our common stock issuable upon exercise of outstanding 80,807,644 warrants, prior to the consummation of the reverse stock split, will be reduced to 3,232,305 shares with a corresponding increase in the exercise prices of such warrants to prices ranging from $6.25 to $56.25 per share.
On June 21, 2016 the market price for our common stock was $0.17 per share. In order to qualify to list our shares on the Nasdaq Capital Market we will need to maintain a minimum $4.00 per share market price. Accordingly, in order to offer our shares of common stock for sale within the anticipated price range of between $4.50 and $6.50 per share, unless the current market price of our common stock increases, prior to the effective date of the registration statement of which this prospectus is a part, we may be required to obtain stockholder approval to increase such reverse stock split to an amount in excess of 1-for-25, which increase could be significant. There can be no assurance that such stockholder approval will be obtained. Additional information regarding our issued and outstanding securities may be found in the section of this prospectus titled “Description of Securities.”
The net proceeds from the sale of our common stock, after deducting the underwriters’ commissions and estimated offering expenses payable by us, will be approximately $15,000,000 or approximately $17,352,900 if the underwriters’ over-allotment option is exercised in full, based on an assumed public offering price of $5.50 per share (after giving effect to the proposed reverse split), such net proceeds will be applied, approximately, as follows:
| · | approximately $655,000 will be used to retire a notes issued in March 2016 (1); |
| · | approximately $669,125 will be used to reduce other outstanding indebtedness to affiliates (2) |
| · | approximately, $3,200,000 will be used to increase our sales and marketing efforts; |
| · | approximately $3,400,000 will be used for research and development of new products; |
| · | approximately $2,900,000 to build infrastructure, including hiring of additional personnel; and |
| · | the balance of approximately $4,175,875 for working capital and other general corporate purposes. |
| (1) | Does not include approximately $1,468,750 face amount of six month convertible notes issued in May and June 2016 that, unless converted into our common stock, mature on various dates between November 20, 2016 and December 10, 2016. The notes are convertible at any time by the holders at a price of $6.25 per share. To the extent that the holders of these notes do not elect to convert them prior to their maturity dates, we will pay such notes out of our available working capital. | |
| (2) | Consists of an estimated 25% reduction in the total of $2,676,500 of debt obligations and advances to certain members of our board of directors and their affiliates. At present, such indebtedness is not evidenced by notes, but is due on demand. We anticipate paying such $669,125 amount commencing 30 days after our common stock begins trading on the Nasdaq Capital Market. We are currently in discussions with these board members regarding the repayment of the $2,007,375 balance owed to such persons through the establishment of six percent (6%) interest-bearing notes due as to principal and accrued interest on a date which shall be one year following the date of this prospectus. There can be no assurance that we will be successful in completing the contemplated agreements or the terms and conditions of any such arrangements. Any such agreements may be subject to completion of this offering. See “Certain Relationships and Related Transactions” elsewhere in this prospectus. |
Our expected use of the net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot predict with complete certainty all of the particular uses for the net proceeds to be received upon the completion of this offering or the actual amounts that we will spend on the uses set forth above. The costs and timing of development activities are highly uncertain, subject to substantial risks and can often change. Due to the many variables inherent to the development of our drug candidates, we cannot currently predict the stage of development we expect the net proceeds of this offering to achieve for our clinical trials, preclinical studies and drug candidates.
22
Our management will have broad discretion in the application of the net proceeds in the category of other working capital and general corporate purposes. For example, if we identify opportunities that we believe are in the best interests of our stockholders, we may use a portion of the net proceeds from this offering to acquire, invest in or license complementary products, technologies or businesses, although we have no current understandings, agreements or commitments to do so. In addition, the amounts and timing of our actual expenditures will depend upon numerous factors, including the results of our research and development efforts, the timing and success of preclinical studies, our ongoing clinical trials or clinical trials we may commence in the future and the timing of regulatory submissions. Depending on the outcome of these activities and other unforeseen events, our plans and priorities may change and we may apply the net proceeds of this offering toward different uses and in different proportions than we currently anticipate.
Pending use of the proceeds from this offering as described above, we intend to invest the net proceeds of this offering in short-term, interest-bearing, investment-grade securities or certificates of deposit.
We have never declared or paid any cash dividends on our common stock and we currently do not anticipate paying any cash dividends for the foreseeable future. Instead, we anticipate that all of our earnings on our common stock will be used to provide working capital, to support our operations, and to finance the growth and development of our business, or investment in, businesses, technologies or products that complement our existing business. Any future determination relating to dividend policy will be made at the discretion of our Board and will depend on a number of factors, including, but not limited to, our future earnings, capital requirements, financial condition, future prospects, applicable Delaware law, which provides that dividends are only payable out of surplus or current net profits and other factors our Board might deem relevant.
23
The following table sets forth our cash and cash equivalents and capitalization as of March 31, 2016, on:
| · | an actual basis, after giving effect to our one-for-25 reverse stock split; and |
| · | on a pro forma as adjusted basis giving effect to the foregoing and the sale of 3,100,000 shares of common stock by us in this offering at the estimated initial public offering price of $5.50 per share after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. |
| March 31, 2016 | ||||||||
| Actual | As Adjusted | |||||||
| Cash and cash equivalents* | $ | — | $ | 13,652,975 | ||||
| Indebtedness due within one year (2) | $ | 4,702,542 | $ | 4,589,306 | ||||
| Total long term debt - net of current portion (1) | $ | 4,613,420 | $ | 4,613,420 | ||||
| Stockholders’ equity: | ||||||||
| Common stock, $0.0001 par value, 250,000,000 shares authorized, 5,348,820 actual shares and 8,448,820 as adjusted shares outstanding (1) | 534 | 773 | ||||||
| Preferred stock, $0.0001 par value, 10,000,000 shares authorized; 0 shares outstanding | - | - | ||||||
| Additional paid-in capital | 71,502,299 | 86,489,199 | ||||||
| Accumulated deficit | (80,683,152 | ) | (80,683,152 | ) | ||||
| Accumulated other comprehensive (loss) income | 187 | 187 | ||||||
| Total stockholders' equity (deficit) | (9,180,132 | ) | 5,807,007 | |||||
| Total capitalization | $ | 135,830 | $ | 15,009,733 | ||||
| (1) | On June 20 2016, the Company and United Bank entered into a Letter Agreement that extended to July 2018 the maturity date of a $3,000,000 line of credit facility between the Company and the bank that was previously payable on demand. As at March 31, 2016, all $3,000,000 had been drawn under the line of credit. In addition, in April 2016 related parties contributed an additional $50,000 to the Company, and in May and June 2016 the Company issued convertible promissory notes that had a total outstanding balance of $1,210,889 as of June 20, 2016. |
| (2) | As adjusted, cash increased based on the anticipated net proceeds of this offering, after and payment of related parties debt of $669,125 and a third party note of $655,000. Indebtedness also decreased by $1,324,125 as a result of such payments. |
*Excludes a bank overdraft of $264,661 that is presented as a current liability on the Company’s consolidated balance sheet.
(A) $1.00 increase in the assumed public offering price of $5.50 per share would decrease the as adjusted number of shares of common stock issued and outstanding by approximately 464,615 and a $1.00 decrease in the assumed public offering price of $5.50 per common share, which was the minimum anticipated offering price based on an assumed share price of $0.22 pre-split, would increase the adjusted number of shares issued and outstanding by approximately 677,778. A 100,000 share increase (decrease) in the number of shares offered by us at the assumed public offering price of $5.50 per common share would increase (decrease) the as adjusted amount of each of cash, cash equivalents and short-term investments, additional paid-in capital, total stockholders' equity and total capitalization by approximately $485,000, after deducting estimated placement agent's fees and estimated offering expenses payable by us. The as adjusted information discussed above is illustrative only and will adjust based on the actual public offering price and other terms of this offering determined at pricing.
(B) The number of shares of our common stock prior to and to be outstanding immediately after this offering is based on 5,348,820 shares of our common stock outstanding as of June 21, 2016.
The actual and as adjusted number of shares of our common stock outstanding, above excludes:
| · | 666,000 shares of common stock issuable upon exercise of options granted under the 2002 Equity incentive plan and the 2013 Equity Incentive Plan of which 394,203 shares have vested to date, and 271,796 additional shares we reserved for issuance under such plans. |
| · | 3,261,890 shares of common stock issuable upon exercise of outstanding warrants with an exercise prices ranging from $6.25 to $56.25 per share. |
| · | 90,415 shares of common stock issuable to Laidlaw & Co. (UK) Ltd. or its assigns in connection with acting as placement agent in private placements of our securities consummated in 2015, the first quarter of 2016 and in May and June 2016. |
| · | 40,242 shares of common stock issuable upon conversion of $400,000 of convertible notes as at March 31, 2016 and 235,000 shares of common stock issuable upon conversion of $1,468,750 of convertible notes issued in May and June 2016.. |
| · | 465,000 shares of common stock issuable upon the full exercise of the underwriters’ over-allotment option. |
| · | up to 155,000 shares of common stock issuable upon exercise of the warrants to be issued to the representative of the underwriters in connection with this offering. |
24
Purchasers of our common stock in this offering will experience an immediate and substantial dilution in the as adjusted net tangible book value of their shares of common stock. Dilution in as adjusted net tangible book value represents the difference between the public offering price per share and the as adjusted net tangible book value per share of our common stock immediately after the offering.
The historical net tangible book value of our common stock as of March 31, 2016 was $(9,180,132) or $(1.72) per share. Historical net tangible book value per share of our common stock represents our total tangible assets (total assets less intangible assets) less total liabilities divided by the number of shares of common stock outstanding as of that date. On a pro forma basis, after giving effect to the sale of 3,100,000 shares in this offering at an assumed initial public offering price of $5.50 per share for net proceeds of $15,000,000, as if such offering had occurred at the end of the March 31, 2016, our pro forma net tangible book value as of March 31, 2016 would have been approximately $5,807,007, or approximately $0.69 per share of our common stock. This represents an immediate increase in as adjusted pro forma, net tangible book value per share of $2.41 to the existing stockholders and an immediate dilution in as adjusted pro forma net tangible book value per share of $4.81 to new investors who purchase shares in the offering. The following table illustrates this per share dilution to new investors:
| Assumed public offering price per share | $ | 5.50 | ||||||
| Historical net tangible book value per share as of March 31, 2016 | $ | (1.72 | ) | |||||
| Pro forma net tangible book value (deficit) per share as of March 31, 2016 | 0.69 | |||||||
| Increase in as adjusted pro forma net tangible book value per share attributable to the offering | 2.41 | |||||||
| Dilution in net tangible book value per share to new investors | $ | 4.81 |
If the underwriters exercise their option to purchase additional shares in full, the as adjusted net tangible book value per share after giving effect to the offering would be $0.92 per share. This represents an increase in as adjusted net tangible book value of $2.64 per share to existing stockholders and dilution in as adjusted net tangible book value of $4.58 per share to new investors.
A $1.00 increase in the assumed public offering price of $5.50 per share of common stock would increase our as adjusted net tangible book value by $3,100,000 to approximately $1.05 per share, and dilution per share to new investors to approximately $4.45 per share, assuming that the number of shares of common stock offered by us remains the same. A $1.00 decrease in in the assumed public offering price of $5.50 per share of common stock would decrease our as adjusted net tangible book value by $3,100,000 to approximately $0.32 per share and dilution per share to new investors by approximately $5.18.
Sales of 530,119 shares of common stock by the selling stockholders in the offering covered by a separate prospectus will reduce the number of shares of common stock held by existing stockholders to 4,842,010, or approximately 61% of the total shares of common stock outstanding after this offering, and will increase the 3,100,000 shares held by new investors to approximately 39% of the total shares of common stock outstanding after this offering.
If the underwriters’ option to purchase additional shares to cover over-allotments is exercised in full, our existing stockholders would own approximately 60% and our new investors would own approximately 40% of the total number of shares of our common stock outstanding after this offering, and before sales of any of the 530,119 shares by the selling stockholders.
The above table and discussion exclude:
| · | 666,000 shares of common stock issuable upon exercise of options granted under the 2002 Equity incentive plan and the 2013 Equity Incentive Plan of which 394,203 shares have vested to date, and 271,796 additional shares reserved for issuance under such plans. |
| · | 3,261,890 shares of common stock issuable upon exercise of outstanding warrants with an exercise prices ranging from $6.25 to $56.25 per share. |
| · | 90,415 shares of common stock issuable to Laidlaw & Co. (UK) Ltd. or its assigns in connection with acting as placement agent in private placements of our securities consummated in 2015, the first quarter of 2016 and in May and June 2016. |
| · | 40,242 shares of common stock issuable upon conversion of $400,000 of convertible notes as at March 31, 2016 and 235,000 shares of common stock issuable upon conversion of $1,468,750 of convertible notes issued in May and June 2016.. |
| · | 465,000 shares of common stock issuable upon the full exercise of the underwriters’ over-allotment option. |
| · | up to 155,000 shares of common stock issuable upon exercise of the warrants to be issued to the representative of the underwriters in connection with this offering. |
To the extent that outstanding options or warrants are exercised and outstanding convertible notes are converted into common stock, you will experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities may result in further dilution to our stockholders.
25
The following table summarizes, on an as adjusted basis as of March 31, 2016 the differences between the number of shares of common stock purchased from us, the total consideration and the average price per share paid by existing stockholders and by investors participating in this offering, after deducting estimated underwriting discounts and commissions and estimated offering expenses, at an assumed public offering price of $5.50 as shown on the cover page of this prospectus.
| Shares Purchased | Total Consideration | Average Price | ||||||||||||||||||
| Number | % | Amount | % | Per Share | ||||||||||||||||
| Existing stockholders | 5,348,820 | 63.3 | $ | 71,324,887 | 80.7 | % | $ | 13.33 | ||||||||||||
| New investors from this offering | 3,100,000 | 36.7 | 17,050,000 | 19.3 | % | 5.50 | ||||||||||||||
| Total | 8,448,820 | 100.0 | $ | 88,374,887 | 100.0 | % | ||||||||||||||
26
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Forward-Looking Statement Notice
Certain statements made in this prospectus are “forward-looking statements” regarding the plans and objectives of management for future operations. Such statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance or achievements of Protea Biosciences Group, Inc. (“we,” “us,” “our,” “Protea” or the “Company”) to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. The forward-looking statements included herein are based on current expectations that involve numerous risks and uncertainties. The Company’s plans and objectives are based, in part, on assumptions involving the continued expansion of business. Assumptions relating to the foregoing involve judgments with respect to, among other things, future economic, competitive and market conditions and future business decisions, all of which are difficult or impossible to predict accurately and many of which are beyond the control of the Company. Although the Company believes its assumptions underlying the forward-looking statements are reasonable, any of the assumptions could prove inaccurate and therefore, there can be no assurance the forward-looking statements included in this prospectus will prove to be accurate. In light of the significant uncertainties inherent in the forward-looking statements included herein, the inclusion of such information should not be regarded as a representation by the Company or any other person that the objectives and plans of the Company will be achieved.
Protea is an emerging growth, molecular information company that has developed a revolutionary platform technology, which enables the direct analysis, mapping and display of biologically active molecules in living cells and tissue samples. The technology platform offers new, unprecedented capabilities useful to the pharmaceutical, diagnostic, clinical research, agricultural and life science industries.
“Molecular information” refers to the generation and bioinformatic processing of very large data sets, known as “big data,” obtained by applying the Company’s technology to identify and characterize the proteins, metabolites, lipids and other molecules which are the biologically active molecular products of all living cells and life forms.
Our technology is used to improve pharmaceutical development and life science research productivity and outcomes, and to extend and add value to other technologies that are used in research and development (“R&D”), such as three-dimensional tissue models, biomarker discovery, synthetic biologicals and mass spectrometry. In particular, the Company believes that its ability to rapidly provide comprehensive molecular image-based datasets addresses a universal need of the pharmaceutical, diagnostic and clinical research and life science industries.
Known as LAESI® (Laser Ablation Electrospray Ionization), our technology platform is exclusively licensed from The George Washington University (“GWU”). We have subsequently completed the development of the first fully automated LAESI instruments and requisite proprietary software suites, used for LAESI data analysis and for molecular imaging – the direct analysis and visualization of molecules in cells and tissue. LAESI technology is currently the subject of eleven issued patents and over 40 peer-reviewed publications.
LAESI is intended to meet the broad need of the biologist for the direct, unbiased identification and characterization of molecules in biological samples. By virtue of LAESI’s improved speed and the comprehensive datasets it generates, the Company is pursuing its vision of what it believes will be a new era of human molecular information, where the molecular networks of human disease will be clearly elucidated, with data more rapidly available, thereby accelerating pharmaceutical development and improving healthcare.
Our Business Strategy and Products and Services
Protea has applied its core technology to establish commercial leadership in the emerging field of mass spectrometry imaging (“MSI”) services. MSI is a revolutionary capability that for the first time enables the identification of all classes of biologically-active molecules produced by cells, and combines this with the ability to instantly spatially-display the molecules in tissue histology sections. MSI can be performed with no sample preparation, and no labeling or antibody techniques, thereby integrating direct molecular identification with tissue pathology.
The Company intends to achieve its business objectives by leveraging its core MSI technology and associated bioanalytical mass spectrometry platforms to improve the availability, comprehensiveness and usefulness of molecular information to address the needs of pharmaceutical research, biomarker discovery, agriculture and other life science research markets.
The Company’s commercial development is centered in three business lines:
| · | Molecular Information Services – the Company believes that it is the commercial leader in providing multimodal MSI services, combining LAESI, MALDI, and optical microscopy. Our proprietary services enable the identification and quantitation of both small molecules (e.g., lipids and metabolites) and large molecules (e.g. proteins) and our services portfolio, inclusive of MSI, proteomics, metabolomics, lipidomics and bioinformatics is unique in the industry. Our clients include major pharmaceutical chemical and biotechnology companies; |
27
| · | LAESI Instruments, Software and Consumables – we offer our proprietary LAESI DP-1000 instrument, software and bioanalytical consumables to corporate and academic research laboratories; and |
| · | Molecular Diagnostics and Clinical Research – we apply our multimodal MSI technologies and workflows in partnership with top-tier medical research institutions to co-develop new molecular diagnostic tests that the company believes can be used to improve the diagnosis and prognosis of cancer and provide requisite information useful in predicting the outcome of cancer treatment. Our current test development programs target the differential diagnosis and prognosis of malignant melanoma and the molecular profiling of subpopulations of cells in lung adenocarcinoma. |
Molecular Information Services
We believe we are the commercial leader in the offering of MSI services. Our clients send their tissue and biofluid samples directly to the Company’s laboratory where samples are analyzed by the Company’s state of the art mass spectrometry instrumentation, scientific staff and bioinformatics capabilities. We combine our proprietary LAESI platform with matrix-assisted laser desorption ionization (“MALDI”) workflows. Our clients include major pharmaceutical, biotechnology, chemical and medical device and consumer products companies. The services unit is operated within a “Quality by Design” environment, which is necessary to meet the internal research and development standards of pharmaceutical and clinical research clients.
The Company’s MSI services represent a revolutionary capability that for the first time enables the identification of all classes of biologically-active molecules produced by cells and combines this with the ability to instantly spatially-display the molecules (both two- and three-dimensional) in tissue histology sections. MSI can be performed without sample preparation, labeling or antibody techniques, thereby integrating direct molecular identification with tissue pathology for the first time. Since the sample is not touched, data is unbiased and more rapidly available, providing molecular information that rapidly answers questions critical to the drug development process, such as, “is my drug actually getting inside the target cells,” “is my immune modulating agent changing the molecular output of the tumor cell,” or “how long did it take for the drug to enter the target cells” as well as questions of drug concentration and duration.
We have developed workflows specifically for the characterization of therapeutic antibodies to provide both visual and analytical certainty of therapeutic efficacy and validation of therapeutic design. Once target tissues are imaged, data analysis can include quantitation of the dosed therapeutic, distribution analysis and other bioinformatics workflows, including statistical and pathway analysis.
In January 2014, the Company, as a subcontractor to GWU, was awarded a multi-year project with the Defense Advanced Research Projects Agency (“DARPA”). In addition to Protea, The Stanford Research Institute International and GE Global Research also collaborate on the project entitled, “New Tools for Comparative Systems Biology of Threat Agent Action Mechanisms.” A $15 million five year project, the goal of DARPA’s Rapid Threat Assessment (“RTA”) program is to develop new tools and methods to elucidate the mechanism of action of a threat agent, drug, biologic or chemical on living cells within 30 days from exposure. Uncovering the mechanism of action of such agents in 30 days, compared to the years currently required, could be key to the development of effective countermeasures. The molecular networks within living cells are vast and complex. Conventional approaches fail to capture the system-wide response of a living cell to a threat agent. The Company believes this technology will be applicable to preclinical drug research as well.
LAESI instruments, software and consumables
LAESI technology was invented in the laboratory of Professor Akos Vertes, Ph.D., Dept. of Chemistry, GWU, in 2007 and was exclusively licensed to Protea in 2008. The LAESI DP-1000 instrument, the Company’s prototype embodiment of its proprietary LAESI technology, integrates with laboratory instruments known as mass spectrometers. LAESI employs a proprietary (patented) method that utilizes the water content in a sample (native or applied) to transition the sample into a gas state, where it can be analyzed by a mass spectrometer. LAESI accomplishes this without requiring the sample to be touched. By eliminating sample preparation, the biological sample can be analyzed without the possible contamination, bias or sample loss that occurs with current techniques, which require the introduction of chemicals, or the destruction of the sample itself, in order to enable analysis by mass spectrometry.
This technology enables the direct identification of proteins, lipids and metabolites in tissues, cells and biofluids such as serum and urine. Data is available in seconds to minutes, allowing rapid time to results and the capacity to analyze thousands of samples in a single work period. As an example, a researcher testing a new drug’s effects on living cells can analyze changes in the cells’ metabolism across a specific time course, thereby almost immediately obtaining data as to the activity of the new drug. The Company believes that LAESI technology has the potential to significantly improve the availability of molecular information in pharmaceutical research as well as many other fields including agriculture, pathology, biomarker discovery, biodefense and forensics.
LAESI instruments employ proprietary software developed by the Company that creates “molecular maps” - the ability to instantly display the distribution of molecules throughout tissue sample in both two- and three-dimensional imaging. The Company currently offers the LAESI Desktop Software and ProteaPlot software that facilitates operating the instrument and the storage and display of datasets in a user friendly, intuitive software environment. The LAESI and ProteaPlot software includes tools for post-processing of LAESI-MS data, generation of molecular maps/images and mass spectral comparison for regions of interest. LAESI software display the data obtained by mass spectrometry analysis combined with actual images of the tissue and cell samples. Thus, mass spectrometry data can be integrated with a sample’s tissue architecture.
28
We have developed and brought to market related, proprietary consumable products for use in molecular analysis including REDIchipsTM (Resonance-Enhanced Desorption Ionization - sample target plates with nanopost array surfaces for matrix-free laser desorption ionization of small molecules) and ProgentaTM (acid labile surfactants use to solubilize proteins for mass spectrometry analysis).
Molecular Diagnostics and Clinical Research
The Company is employing both its proprietary LAESI platform and MALDI methodologies to create comprehensive, tissue-based molecular profiles to improve the differential diagnosis of cancer. Our bioinformatics capability allows the integration of the Company’s MSI data files with related pathology, gene expression and demographic datasets with the purpose of improving human disease state detection, assessment and management. The Company has entered into collaborative research partnerships with top tier academic centers to develop and validate new, proprietary, MSI-based cancer diagnostic tests.
We have established a collaborative research partnership with the Memorial Sloan-Kettering Cancer Center and the Dana-Farber Cancer Institute. The first target of the collaboration is early stage lung adenocarcinoma. The objectives of the collaboration are to demonstrate that different cancer cell sub-groups within a lung cancer will have different molecular profiles and will behave differently. The goal is to define these molecular differences and to identify the sub-group of cancer cells with the worst prognosis that are most likely to recur thereby enabling earlier treatment intervention, and to use these findings to achieve lung adenocarcinoma tumor cell “molecular profiling,” leading to more precise treatment selection and higher survivor rates.
We have established a collaborative research initiative with The Yale University School of Medicine, the laboratory of Rozzitsa Lasova, MD, Associate Professor of Dermatology and Pathology, that employs the company’s MSI technology to differentiate melanoma from spitz nevi cells. The Company believes that its core MSI technology can identify unique protein expression profiles that can discriminate between benign melanocytic nevi and malignant melanoma.
To support our MSI–based diagnostic development, we developed new software known as “Histology Guided Mass Spec Imaging (HG-MSI)” that enables pathologists to combine traditional microscopy and histology with high resolution mass spectrometry molecular imaging. Clinicians can share, annotate and direct the analysis of specific tissue morphologies and cell subpopulations by MSI. Molecular profiling data are collected from discrete locations within a tissue section using a histology stained section as a guide. The digital tissue scans are visually analyzed by pathologists by logging onto a secure website portal and they then annotate specific cellular areas for further analysis.
We intend to expand our collaborations with major medical research centers to develop additional molecular profiles for the improved differential diagnosis and prognosis of cancer.
Recent Developments
We entered into a Memorandum of Understanding (the “MOU”) with Agilent Technologies, Inc. (NYSE: A) (“Agilent”), to develop new bioanalytical workflows in order to meet the emerging needs of the growing biopharmaceutical industry. Under the terms of the MOU, Protea, using Agilent instrumentation combined with its expertise, will develop workflows to improve the characterization of protein therapeutics including monoclonal antibodies, and new methods for the field of metabolomics. We believe, along with Agilent, that the field of biotherapeutics is advancing rapidly and is in need of new, innovative solutions that identify changes in the metabolic profiles of cells due to disease processes and drug interactions.
We have announced new workflows to support the bioanalytical needs of immuno-oncology, an emerging frontier of cancer treatment that utilizes the body’s own immune system to fight diseases. Our new workflows help provide both visual and analytical certainty of therapeutic efficacy and apply the Company’s core MSI technology to the analysis of drug target tissues and tumor microenvironments.
We intend prior to completion of this offering to consummate a one-for-25 reverse stock split. No fractional shares of common stock were issued in connection with the reverse stock split, and all such fractional interests will be rounded down to the nearest whole number. Issued and outstanding stock options, convertible notes and warrants will be split on the same basis and exercise prices will be adjusted accordingly.
As of June 21, 2016 and December 31, 2015 we had issued and outstanding a total of (i) 133,720,519 and 133,146,250 shares of our common stock, respectively, and (ii) warrants to purchase a total of 80,807,644 and 71,342,894 shares of our common stock, respectively, at certain exercise prices ranging from $0.25 to $2.25 per share.
29
Reverse Stock Split
In an attempt to implement our strategy to list our common stock of the Nasdaq Capital Market, on December 1, 2015, our stockholders approved and authorized our board of directors to consummate a reverse stock split of our issued and outstanding common stock at a ratio of not less than 1-for-15 and not more than 1-for-25, with the exact reverse stock split ratio to be determined by the board in its sole discretion based upon the market price of the Company’s common stock on the date of such determination and with such reverse stock split to be effective at such time and date, if at all, as determined by the board in its sole discretion. Prior to the effective date of this offering, the board of directors intends to effect a 1-for-25 reverse stock split. As a result of our one-for-25 reverse stock split, (i) all outstanding 133,720,519 shares of our common stock, prior to the consummation of the reverse stock split, will be reduced to 5,348,820 shares and (ii) all shares of our common stock issuable upon exercise of outstanding 80,807,644 warrants, prior to the consummation of the reverse stock split, will be reduced to 3,232,305 shares with a corresponding increase in the exercise prices of such warrants to prices ranging from $6.25 to $56.25 per share.
Between April 2014 and March 31, 2016, the market price of our common stock has declined from a high of $2.10 per share to as low as $0.11 per share and closed on June 21, 2016 at $0.17 per share. In order to qualify to list our shares on the Nasdaq Capital Market we will need to maintain a minimum $4.00 per share market price. Accordingly, in order to offer our shares of common stock for sale within the anticipated price range of between $4.50 and $6.50 per share, unless the current market price of our common stock increases, prior to the effective date of the registration statement of which this prospectus is a part, we may be required to obtain stockholder approval to increase such reverse stock split to an amount in excess of 1-for-25, which increase could be significant.
Additional information regarding our issued and outstanding securities may be found in the section of this prospectus titled “Description of Securities.”
Recent Financings
In May 2015, the Company received an aggregate of $2,000,000 in gross cash proceeds from one accredited investor in connection with the sale of original issue discount unsecured short-term convertible debenture due November 22, 2015. The Company issued (a) the notes with a total principal amount of $2,500,000 that bears interest at a rate of 10% per annum, and (b) a three-year warrant to purchase up to 300,000 shares of common stock of the Company at an exercise price of $8.13 per share. As of December 30, 2015, the debenture was converted in whole, into 424,222 shares of common stock at a conversion price of $6.25 per share. The Company received net proceeds of $1,690,655 from the sale of the notes and warrants. Prior to conversion, the outstanding balance was $2,500,000.
In August 2015, the Company commenced a private placement offering of up to $4,500,000 (including a $500,000 over-allotment option) of its equity securities (the “Fall 2015 Offering”). The Fall 2015 Offering consisted of up to 45 units of its equity securities; each unit consisting of 16,000 shares of common stock at a purchase price of $6.25 per share, and 8,000 three-year warrants to purchase common stock at an exercise price of $9.38 per share. As of December 31, 2015, a total of 14.23 units were sold and the Company received gross proceeds of $1,422,500. The Fall 2015 Offering of such units was concluded on December 22, 2015.
Laidlaw & Company (UK) Ltd, also served as placement agent for the Company in connection with the May 2015 and Fall 2015 private placements, and received compensation aggregating $155,700 plus warrants to purchase 29,640 shares of common stock of the Company at the same exercise prices as the warrants sold in each of the above private offerings.
In March 2016, we sold $655,000 principal amount of original issue discount unsecured notes due six months from funding and providing an estimated 23% yield to maturity (which we refer to as the “March 2016 Bridge Note”) to one accredited investor, pursuant to which we received an aggregate gross proceeds of $500,000. The March 2016 Bridge Note does not accrue additional interest and includes legal fees of $5,000, which was added to the principal amount. We also issued to the investor an aggregate of 4,347 shares of our common stock and five year warrants to purchase up to 65,500 additional shares of common stock at an exercise price of $18.75, subject to adjustment in certain events as provided therein.
If we fail to pay the March 2016 Bridge Note on or before its maturity date or otherwise commit an event of default, as defined in the March 2016 Bridge Note, the holder may convert the outstanding principal amount of the March 2016 Bridge Note into our common stock at a conversion price initially equal to 70% of the lowest closing bid price for the common stock in the twenty trading days immediately preceding the conversion, subject to adjustment in certain events as provided therein.
In connection with our sale of the March 2016 Bridge Note, we paid to Laidlaw & Company (UK) Ltd., who acted as the placement agent, an aggregate of approximately $60,000 in cash compensation, representing fees and an expense allowance. In addition, we issued to the placement agent (or its designees) a five-year warrant to purchase an aggregate of approximately 19,650 shares of our common stock at an exercise price of $6.25 per share.
In order to provide us with sufficient interim working capital to sustain our operations pending completion of this offering, the Company has engaged Laidlaw & Company (UK) Ltd. to assist us in the private placement and sale of additional bridge notes and warrants. There can be no assurance that the Company will be able to obtain such interim financing.
30
In May and June 2016, the Company received an aggregate of $1,175,000 in gross cash proceeds from 22 accredited investors in connection with the sale of original issue discount unsecured short-term convertible notes due November 20, 2016, November 30, 2016 and December 10, 2016, being six months following the dates of issuance of each of the notes. The Company notes (a) were issued with a total principal amount of $1,468,750 (b) bears interest at a rate of 10% per annum, and (c) are convertible at any time by the holders into our common stock at a conversion price of $6.25. In addition, we issued to the note holders three-year warrants to purchase up to 176,260 shares of common stock of the Company at an exercise price of $8.13 per share.
In connection with our sale of the May and June 2016 Bridge Notes, we paid to Laidlaw & Company (UK) Ltd., who acted as the placement agent, an aggregate of approximately $141,000 in cash compensation, representing fees and an expense allowance. In addition, we issued to the placement agent (or its designees) a five-year warrant to purchase an aggregate of approximately 41,125 shares of our common stock at an exercise price of $0.325 per share.
In May 2016, the Company entered into an addendum with WVJITB to extend the maturity date for two outstanding convertible promissory notes to September 30, 2016 from March 31, 2016. This addendum, effective March 31, 2016, includes the Company to grant WVJITB a five-year warrant for the purchase of 23,600 shares of the Company’s Common Stock at an exercise price of to be determined by the Board of Directors.
Results of Operations
Three Months Ended March 31, 2016 Compared to the Three Months Ended March 31, 2015
The Company recorded a net loss for the three months ended March 31, 2016 of $1,085,544. This result marked a substantial improvement over the net loss of $2,967,498 recorded for the three months ended March 31, 2015. The change reflects an increase in gross revenue, a related increase in cost of revenue, decreases in sales, general and administrative (“SG&A”) and research and development (“R&D”) expenses, and an increase in other income.
Gross revenue for the three months ended March 31, 2016 increased by 22% over the gross revenue recognized for the three months ended March 31, 2015, including increases in all three revenue components. Revenue from molecular information services increased by 27%. The increase reflects increases in both the number of customers for whom we performed services and the number of services projects completed in the quarter. Molecular information service customers include pharmaceutical, biotechnology, chemical and medical device companies.
We recorded revenue from the sale of one LAESI® device in both the three months ended March 31, 2016 and 2015. However, revenue for the three months ended March 31, 2016 was 17% higher than the comparable period in 2015 because the unit sold in the first quarter of 2016 was at an incrementally higher unit price and also included the sale of ancillary equipment. Customers for our LAESI® instrument platform mirror those for molecular information services. Revenue from research products increased by 18%. This increase primarily reflects an increase in revenue from sales of the Company’s REDIchip™ product.
For the three months ended March 31, 2016 versus the three months ended March 31, 2015, cost of revenue increased by 41% primarily related to an increase in personal costs and consumables attributable to molecular information services and an increase in costs associated with the LAESI® device, including component parts, royalty expenses, and warranty expense (associated with the sale of an extended warranty for the unit sold in the first quarter of 2016; the related revenue is recognized over the warranty period whereas the estimated warranty expense is recognized at the time of sale). The period-over-period increase in gross profit reflects the increase in sales and a partially offsetting increase in cost of revenue.
Selling, general and administrative (“SG&A”) expenses recognized during the three months ended March 31, 2016 were $1,604,404 or 20% less than the SG&A expenses recorded during the comparable period in 2015. The change reflects the Company’s efforts to reduce personnel costs, outside services, consulting expenses, and other activity recorded as SG&A expenses, in particular during the first nine months of 2015.
For the three months ended March 31, 2016 compared to the three months ended March 31, 2015, research and development expenses decreased 39% primarily due to a decrease in costs associated with the development of the LAESI® instrument platform and Nanopost Array (“NAPA”) technologies (the latter includes the Company’s REDIchip™ product). Both of these products are now considered commercial products. In addition, funding for certain expenses incurred by the Company in support of its ongoing participation in the Defense Advanced Research Projects Agency (“DARPA”) program referred to as the Rapid Threat Assessment program is recorded in this line item. The funding was $40,678 for the three months ended March 31, 2016 versus $18,344 for the three months ended March 31, 2015. The amount and timing of future expenses and expense recovery related to our participation in this program are difficult to forecast considering the research and development nature of the collaboration.
The gain on sale of AzurRx Common Stock of $637,100 recorded during the first quarter of 2016 reflects the sale of 1,016,941 shares of AzurRx BioPharma, Inc. (“AzurRx”) Common Stock. The gain reflects proceeds resulting from the sale of the shares less transaction costs as the basis for the Company’s AzurRx investment had previously been reduced to $0. The Company sold an additional 550,000 shares of AzurRx Common Stock in the second quarter of 2016 resulting in net proceeds of $675,000. See also Note 3 Gain on Sale of Investment and Note 19 Evaluation of Subsequent Events in Notes to Consolidated Financial Statements included in Part I, Item 1 of this report for additional information regarding the Company’s investment in AzurRx.
For the three months ended March 31, 2016, interest expense decreased 8%. As of March 31, 2016, interest-bearing indebtedness was $7,736,317 versus $7,205,047 as of March 31, 2015. The March 31, 2016 balance includes $655,000 related to a convertible debenture issued in March 2016. As of December 31, 2015, interest-bearing indebtedness was $7,166,752. There were no debt conversion inducement costs recognized in the first three months of 2016. The $60,419 recorded in the comparable period in 2015 reflects the fair value of warrants issued to Summit Resources, Inc. (“Summit”) related to the conversion of certain amounts due to Summit. See also Note 11 Bank Line of Credit, Note 12 Loans Payable to Stockholders, Note 14 Debt, and Note 19 Evaluation of Subsequent Events in Notes to Consolidated Financial Statements included in Part I, Item 1 of this report for additional information regarding the Company’s short and long-term debt.
31
The Company recognized a minimal change in the fair value of derivative liabilities during the three months ended March 31, 2016 as there were no changes in the amount of derivative instruments outstanding versus December 31, 2015 and as compared with December 31, 2015, no changes in the assumptions underlying the calculation of estimated fair value of the derivative instruments. The amount recorded in this line item for the three months ended March 31, 2015 was primarily related to an increase in the estimated fair value during that quarter of the liabilities for warrants and anti-dilution arrangements associated with certain outstanding Common Stock. Detailed information regarding the fair value of the Company’s financial assets and liabilities, including the assumptions used to estimate the fair value of warrants and anti-dilution arrangements can be found in Note 5 Fair Value of Financial Assets and Liabilities – Derivative Financial Instruments in Notes to Consolidated Financial Statements in Part I, Item 1 of this report.
The Company did not recognize a provision for income taxes for the three months ended March 31, 2016 or the comparable period in 2015. The Company has evaluated its income tax position in accordance with the Financial Accounting Standards Board’s Accounting Standards Codification Topic 740, Income Taxes, and determined that a full valuation allowance against its deferred tax asset was appropriate for both balance sheet dates. As of March 31, 2016, the Company had a deferred tax asset of approximately $28.2 million with a full, offsetting valuation allowance. Net operating loss (“NOL”) carryforwards totaled approximately $69.4 million as of March 31, 2016. These NOLs begin to expire in 2021 for both federal and state tax purposes.
Going Concern
The accompanying Consolidated Financial Statements have been prepared on the assumption that the Company will continue as a going concern. As detailed below, the Company will require additional financial resources to continue its operations. If we cannot obtain additional financial resources through additional funding or the sale of assets, we may be forced to further curtail our operations or consider other strategic alternatives. Even if we are successful in raising additional financial resources, there can be no assurance regarding the terms of any asset sales or additional investment–any such investment or other strategic alternative would likely substantially dilute our current stockholders.
Fiscal Year Ended December 31, 2015 Compared to the Fiscal Year Ended December 31, 2014
We earned revenue of $2,003,286 for the fiscal year ended December 31, 2015 as compared to revenue of $1,768,312 for the year ended December 31, 2014, an increase of $234,974, or approximately 13%. During the year ended December 31, 2015, the Company realized service revenues of $929,076 compared to $504,750 for the year ended December 31, 2014, an increase of $424,326 or approximately 84%. Service clients include major pharmaceutical, biotechnology, chemical and medical device companies. In addition, the Company received grant revenues associated with the DARPA grant totaling $141,484 for the year ended December 31, 2015 compared to $120,171 for the year ended December 31, 2014. The Company also earned LAESI instrument revenue of $640,060 and research product revenue of $292,666 for the year ended December 31, 2015.
Cost of revenue totaled $721,656 for the year ended December 31, 2015 compared to cost of revenue of $871,904 for the year ended December 31, 2014, a decrease of $150,248 or 17%. During 2014, the Company engaged a new contract manufacture of the LAESI unit, which substantially lowered the cost of each unit sold. The Company realized gross profits of $1,281,630 for the year ended December 31, 2015 compared to $896,408 for the year ended December 31, 2014.
Selling, general and administrative expenses totaled $6,942,406 for the year ended December 31, 2015, compared to $8,736,478 for the fiscal year ended December 31, 2014, a decrease of $1,794,072 or approximately 21%. The decrease relates to a reduction in personnel costs, outside services, consulting expenses, and other items as the Company has focused efforts on reducing expenses.
Research and development expense totaled $1,479,367 for the fiscal year ended December 31, 2015, compared to research and development expense of $2,853,078 for the fiscal year ended December 31, 2014, a decrease of $1,373,711 or approximately 48%. The reduction in expenses relates largely to the disposition of the Company’s European subsidiary effective at the end of 2014. No expenses were incurred for the year ended December 31, 2015 related to the disposed subsidiary. For the year ended December 31, 2015, research and development costs included costs associated with the development of its LAESI and NAPA technology, known as REDIchips. In connection with the research and development of the Company's LAESI technology, the Company recorded research and development costs of $1,239,350 and $1,451,000 for the fiscal years 2015 and 2014, respectively. In connection with the research and development of the Company's REDIchip technology, the Company recorded research and development costs of $240,017 for the year ended December 31, 2015 compared to $221,477 for the year ended December 31, 2014.
As a result, loss from operations for the fiscal year ended December 31, 2015 was $7,140,143, compared to a loss from operations of $10,693,148 for the fiscal year ended December 31, 2014. This is a decrease of $3,553,005 or 33% from the prior year.
During the year ended December 31, 2015, we had other expense of $2,434,291 as compared to other expense for the year ended December 31, 2014 of $781,622. Other expense increased as a result of the change in fair market value on derivatives related to anti-dilution protection on certain securities and interest expense incurred on new debt originated in 2015. This was partially off-set by a gain of $910,000 on the sale of equity method investment.
After foreign currency translation adjustments of $429 and $(23,455), respectively, we had a total comprehensive loss of $9,574,005, or $0.09 per share, for the fiscal year ended December 31, 2015 as compared to a total comprehensive loss of $11,498,225, or $0.17 per share, for the fiscal year ended December 31, 2014.
32
Liquidity and Capital Resources
As of March 31, 2016, the Company’s current assets totaled $464,401, current liabilities $10,486,033, and working capital was a deficit of $10,021,632. As of December 31, 2015, current assets totaled $411,591, current liabilities $9,590,170, and working capital was a deficit of $9,178,579. The increase in current assets reflects an increase in trade accounts receivable due primarily to an increase in sales for March 2016 as compared to December 2015.
Regarding current liabilities, the amounts categorized as accounts payable and other payables and accrued expenses totaled $1,813,622 and $1,729,675 as of March 31, 2016 and December 31, 2015, respectively, or an incremental increase of 5%. Other payables and accrued expenses included incremental increases in the items recorded in this line item, as listed in Note 13 in Notes to Consolidated Financial Statements included in Part I, Item 1 of this report.
As at March 31, 2016, the Company and its wholly-owned subsidiary owes $3,000,000 under a senior secured line of credit with United Bank West Virginia, which was previously due on demand. The interest rate is variable–we currently pay interest at a rate of 5.87% per annum. The line of credit is secured by our equipment and the personal guarantees of three of our directors and the estate of a former director. The line of credit has had an outstanding of $3,000,000 since the first quarter of 2014. On June 20, 2016, the bank agreed to extend the maturity date of the line of credit and the Company note issued under the line of credit to July 12, 2018. Consequently, we have not included any proceeds from this offering as being used to reduce or repay the outstanding balance of this line of credit.
The Company had a bank overdraft of $264,661 as of March 31, 2016 that represents payments by our bank of checks we issued in an amount that exceeded our cash balance with the bank as of that date. As detailed in Note 11 Bank Line of Credit, Note 12 Loans Payable to Stockholders, and Note 14 Debt in Notes to Consolidated Financial Statements included in Part I, Item 1 of this report, the Company continues to have a substantial amount of indebtedness outstanding that is payable within twelve months, including amounts due to both third-parties and stockholder.
Net cash used in operating activities for the three months ended March 31, 2016 totaled $1,296,505, which was a decrease of $140,683 or 10% from the net cash used in operating activities of $1,437,188 for the three months ended March 31, 2015. The largest factors for the improvement include a lower level of disbursements in the first quarter of 2016 for payables and inventory partially offset by less cash collected on trade receivables in the first quarter of 2016.
Net cash provided by investing activities for the three months ended March 31, 2016 totaled $637,125, which is the net proceeds from the sale of AzurRx Common Stock received during the period and proceeds from sale of equipment. Net cash used by investing activities for the three months ended March 31, 2015 was $7,413, net, that resulting from the purchase and sale of certain equipment.
Net cash provided by financing activities for the three months ended March 31, 2016 was $652,882 which was a decrease of $549,564 or 46% from the net cash provided by financing activities of $1,202,446 for the three months ended March 31, 2015. The first quarter of 2016 included proceeds from the issuance of a convertible debenture to an accredited investor of $500,000, payments of principal on short-term debt and for capital leases totaling $103,779 and payment of stockholder loans of $57,500. The first quarter of 2015 included proceeds from the sale of the Company’s Preferred Stock of $571,323 and proceeds from the issuance of a promissory note to a stockholder in the period of $678,000. As discussed below, the Company requires additional capital resources to fund future operations and to continue as a going concern.
Cash Requirements
The Company’s consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has funded its activities to date almost exclusively from debt and equity financings as well as sales of certain assets. Substantially all the Company’s property and equipment are security for outstanding indebtedness. We will continue to require substantial funds to support our molecular information services business and advance global commercialization of our LAESI® instrument platform.
The Company has experienced negative cash flows from operations since inception. Since inception, our operations have been funded primarily through proceeds received from the issuance of debt and sale of equity securities in private placement offerings and, from time-to-time sales of certain assets. Management intends to continue to meet the Company’s operating cash flow requirements by raising additional funds from the sale of equity or debt securities and possibly developing corporate development partnerships to advance our molecular information technology development activities by sharing the costs of development and commercialization. We have also worked closely with various parties that financed our short- and long-term debt to obtain extensions and modifications that deferred or reduced amounts due under these obligations. Such extensions and modifications have included, in certain cases, the issuance of warrants. In addition, three members of the Company’s Board of Directors and the estate of a former board member guarantee payment of the Company’s outstanding bank line of credit. Such extensions, modifications and guarantees have been an important part of the Company’s ability to manage its liquidity and short-term capital resources. In addition, as of March 31, 2016, the Company was in an overdraft position with its bank. The amount of the overdraft was $264,661. There can be no assurances that the Company will be able to continue to obtain such extensions and modifications to outstanding debt or use other methods such as guarantees by stockholders, when and if necessary, to ensure the Company has the liquidity and capital resources necessary to fund future operations and to continue as a going concern.
33
We may also consider the sale of additional assets or entering into a transaction such as a merger with a business complimentary to ours. As discussed in Note 3 Sale of Equity Method Investment, we raised proceeds of $637,100 during the three months ended March 31, 2016 through sales of a portion of our ownership interest in AzurRx BioPharma, Inc. (“AzurRx”). Also, as discussed in Note 19 Evaluation of Subsequent Events, in the second quarter of 2016 we sold an additional ownership interest in AzurRx generating net proceeds of $675,000.
While we have been successful in raising funds and selling certain assets to fund our operations since inception and we believe that we will be successful in obtaining the necessary capital resources to fund our operations going forward, there are no assurances that we will be able to secure any additional funding or capital resources or the terms of such arrangements. These factors raise substantial doubt about our ability to continue as a going-concern.
The accompanying consolidated financial statements do not include any adjustment that might be necessary if the Company is unable to continue as a going concern.
The Company is in immediate need of additional working capital in order to sustain its current level of operations. Based on our current spending levels, management estimates that the Company will need approximately $6,000,000 in additional working capital, net of cash flows from revenue, to maintain current operations for the next twelve calendar months. With a goal of raising additional capital in the third quarter of 2016 to at least meet our working capital needs through the end of 2016, we are working closely with our financial advisors to determine our tactical approach to the equity markets, with particular emphasis on identifying the best deal structure to attract and retain meaningful capital sponsorship from retail and/or institutional investing communities. However, there can be no assurance that we will be successful in raising additional capital on terms acceptable to us or at all.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet guarantees, interest rate swap transactions or foreign currency contracts. We do not engage in trading activities involving non-exchange traded contracts.
Critical Accounting Policies
The discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses for each period. For further discussion of critical accounting policies, please see Note 2 to our financial statements included in this prospectus.
Recently Issued Accounting Pronouncements
For a discussion of recently issued accounting pronouncements, please see Note 2 to our financial statements included in this prospectus.
Our common stock is currently quoted on OTCQB under the symbol “PRGB,” however it is not listed on any stock exchange, and there is currently limited trading in our securities. Subject to meeting all of the Nasdaq listing standards, including the completion of this offering, our common stock will be approved for listing on The Nasdaq Stock Market under the symbol “PRGB.”
The quotation of our common stock began on or about April 15, 2014. On June 21, 2016 the last reported sales price of our common stock was $0.17 per share.
We intend to complete a one-for-25 reverse split of our outstanding common stock prior to the completion of the offering described in this prospectus. The information below has not been adjusted to give retroactive effect to such contemplated 1-for-25 reverse stock split.
As of June 21, 2016, we had 133,720,519 shares of our common stock issued and outstanding held by approximately 526 stockholders of record.
As of June 21, 2016 we also have outstanding:
| · | warrants to purchase up to 80,807,644 shares of our common stock at exercise prices ranging between $0.25 and $2.25 per share, subject to adjustment in certain circumstances as provided therein; and |
34
| · | options to purchase up to 16,650,000 shares of our common stock at a weighted average exercise price of $0.69 per share, subject to adjustment in certain circumstances as provided therein; |
The following table sets forth the high and low closing bid prices for our common stock for the fiscal quarter indicated as reported on the OTCQB. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions. Our common stock is very thinly traded and, thus, pricing of our common stock on the OTC Markets does not necessarily represent its fair market value.
| Period | High | Low | ||||||
| Quarter ending June 30, 2014 (from April 11, 2014) | $ | 2.10 | $ | 0.70 | ||||
| Quarter ending September 30, 2014 | 0.88 | 0.51 | ||||||
| Quarter ending December 31, 2014 | 0.55 | 0.20 | ||||||
| Quarter ending March 31, 2015 | 0.60 | 0.12 | ||||||
| Quarter ending June 30, 2015 | 0.20 | |||||||
| Quarter ending September 30, 2015 | 0.47 | 0.16 | ||||||
| Quarter ending December 31, 2015 | 0.25 | 0.10 | ||||||
| Quarter Ending March 31, 2016 | 0.35 | 0.11 | ||||||
| Quarter Ending June 30, 2016 (through May 23, 2016) | 0.35 | 0.11 | ||||||
Dividends
We have not declared any cash dividends since inception and we do not anticipate paying any dividends in the foreseeable future. The payment of dividends is within the discretion of the Board of Directors and will depend on our earnings, capital requirements, financial condition, and other relevant factors. There are no restrictions that currently limit our ability to pay dividends on our common stock other than those generally imposed by applicable state law.
Securities Authorized for Issuance under Equity Compensation Plans
In 2002, the Board of Directors of PBI adopted, and our stockholders subsequently approved, the 2002 Equity Incentive Plan (the “2002 Plan”) which governed equity awards to employees, directors and consultants of the Company. There were 166,000 shares of common stock reserved for issuance under the Plan. Following the Reverse Merger, and in accordance with the 2002 Plan, the Company’s Board of Directors approved the substitution of the shares of PBI’s common stock underlying the options granted under the 2002 Plan with shares of common stock of the Company, subject to any further approvals or actions as may be required to ensure that the implementation of the substitution is in accordance with all state and federal rules and regulations that may be applicable.
The 2002 Plan had a term of ten years and expired in July 2012. The types of awards permitted under the 2002 Plan include qualified incentive stock options and non-qualified stock options, and restricted stock. Each option shall be exercisable at such times and subject to such terms and conditions as the Board may specify. Stock options generally vest over four years and expire no later than ten years from the date of grant.
On February 8, 2013, the Board of Directors of the Company adopted, and the Company’s stockholders subsequently approved, the 2013 Equity Incentive Plan (the “2013 Plan”), which governs equity awards to employees, directors and consultants of the Company. The 2013 Plan has a term of ten years and permits the grant of qualified incentive stock options, non-qualified stock options, restricted stock awards as well as performance based cash compensation awards. Under the 2013 Equity Incentive Plan, an additional 200,000 shares of common stock are reserved for issuance.
As of December 1, 2014, our stockholders approved an amendment to the 2013 Plan to permit the Board to increase the number of shares of common stock issuable under the 2013 Plan on January 1 of each year in an amount equal to the lesser of: (i) 500,000 shares of common stock or the equivalent of such number of shares after the Administrator (as defined in the 2013 Plan), in its sole discretion, has interpreted the effect of any stock split, stock dividend, combination, recapitalization or similar transaction in accordance with Section 11(a) of the 2013 Plan; (ii) 15% of the shares of common stock issued and outstanding as of the last day of the prior year; or (iii) an amount determined by the Board. As a result, as of January 1, 2016, the total number of shares of common stock issuable under the 2013 Plan was increased to 666,000.
The Board of Directors has the power to amend, suspend or terminate the 2013 Plan without stockholder approval or ratification at any time or from time to time. No change may be made that increases the total number of shares of our common stock reserved for issuance pursuant to incentive awards or reduces the minimum exercise price for options or exchange of options for other incentive awards, unless such change is authorized by our stockholders within one year. Unless sooner terminated, the 2013 Plan will terminate ten years after it was adopted.
35
Equity Compensation Plan Information
The following table summarizes information about stock options at March 31, 2016:
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders - 2013 Plan | 289,013 | $ | 11.00 | 210,986 | ||||||||
| Equity compensation plans not approved by security holders – 2002 | 105,190 | $ | 35.62 | - | ||||||||
| Total | 394,203 | $ | 17.50 | 210,986 | ||||||||
The following table summarizes information about stock options at March 31, 2016:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||
| Exercise Price | Outstanding | Weighted Average Remaining contractual life (in years) | Weighted Average Exercise Price | Exercisable | Weighted Average Exercise Price | |||||||||||||||||
| $ | 6.25 | 106,133 | 9,259 | |||||||||||||||||||
| $ | 12.00 | 6,000 | 750 | |||||||||||||||||||
| $ | 12.50 | 3,160 | 3,160 | |||||||||||||||||||
| $ | 13.25 | 23,800 | 4,463 | |||||||||||||||||||
| $ | 13.75 | 150,080 | 97,378 | |||||||||||||||||||
| $ | 20.00 | 12,800 | 12,800 | |||||||||||||||||||
| $ | 31.25 | 12,400 | 12,400 | |||||||||||||||||||
| $ | 37.50 | 71,240 | 71,240 | |||||||||||||||||||
| $ | 50.00 | 8,590 | 8,590 | |||||||||||||||||||
| $ | 6.25 - $50.00 | 394,203 | 7.26 | $ | 17.50 | 220,040 | $ | 23.75 | ||||||||||||||
36
Company Overview
Protea is an emerging growth, molecular information company that has developed a revolutionary platform technology, which enables the direct analysis, mapping and display of biologically active molecules in living cells and tissue samples. The technology platform offers new, unprecedented capabilities useful to the pharmaceutical, diagnostic, clinical research, agricultural and life science industries.
“Molecular information” refers to the generation and bioinformatic processing of very large data sets, known as “big data,” obtained by applying the Company’s technology to identify and characterize the proteins, metabolites, lipids and other molecules which are the biologically active molecular products of all living cells and life forms.
Our technology is used to improve pharmaceutical development and life science research productivity and outcomes and to extend and add value to other technologies that are used in research and development (“R&D”), such as three-dimensional tissue models, biomarker discovery, synthetic biologicals and mass spectrometry. In particular, the Company believes that its ability to rapidly provide comprehensive molecular image-based datasets addresses a universal need of the pharmaceutical, diagnostic and clinical research and life science industries. To date, we have established a number of collaborative research programs with major medical facilities. See “Strategic Relationships” below.
Known as LAESI® (Laser Ablation Electrospray Ionization), our technology platform is exclusively licensed from The George Washington University (“GWU”). We have subsequently completed the development of the first fully automated LAESI instruments and requisite proprietary software suites, used for LAESI data analysis and for molecular imaging – the direct analysis and visualization of molecules in cells and tissue. LAESI technology is currently the subject of eleven issued patents and over 40 peer-reviewed publications.
LAESI is intended to meet the broad need of the biologist for the direct, unbiased identification and characterization of molecules in biological samples. By virtue of LAESI’s improved speed and the comprehensive datasets it generates, the Company is pursuing its vision of what it believes will be a new era of human molecular information, where the molecular networks of human disease will be clearly elucidated, with data more rapidly available, thereby accelerating pharmaceutical development and improving healthcare.
Our Business Strategy and Products and Services
Protea has applied its core technology to establish commercial leadership in the emerging field of mass spectrometry imaging (“MSI”) services. MSI is a revolutionary capability that for the first time enables the identification of all classes of biologically-active molecules produced by cells, and combines this with the ability to instantly spatially-display the molecules in tissue histology sections. MSI can be performed with no sample preparation, and no labeling or antibody techniques, thereby integrating direct molecular identification with tissue pathology.
The Company intends to achieve its business objectives by leveraging its core MSI technology and associated bioanalytical mass spectrometry platforms to improve the availability, comprehensiveness and usefulness of molecular information to address the needs of pharmaceutical research, biomarker discovery, agriculture and other life science research markets.
The Company’s commercial development is centered in three business lines:
| · | Molecular Information Services – the Company believes that we are the commercial leader in providing multimodal MSI services, combining LAESI, MALDI, and optical microscopy. Our proprietary services enable the identification and quantitation of both small molecules (e.g. lipids and metabolites) and large molecules (e.g. proteins) and our services portfolio, inclusive of MSI, proteomics, metabolomics, lipidomics and bioinformatics is unique in the industry. Our clients include major pharmaceutical, chemical and biotechnology companies; |
| · | LAESI Instruments, Software and Consumables – we offer our proprietary LAESI DP-1000 instrument, software and bioanalytical consumables to corporate and academic research laboratories; and |
| · | Molecular Diagnostics and Clinical Research – we apply our multimodal MSI technologies and workflows in partnership with top-tier medical research institutions to co-develop new, molecular diagnostic tests that the Company believes can be used to improve the diagnosis and prognosis of cancer and provide requisite information useful in predicting the outcome of cancer treatment. Our current test development programs target the differential diagnosis and prognosis of malignant melanoma and the molecular profiling of subpopulations of cells in lung adenocarcinoma. |
37
Industry and Market Overview
We believe there is a large and broad-based market opportunity for molecular information. The market opportunity can be viewed in terms of the primary markets for, and the platform technologies that generate, molecular information.
Molecular Information Markets: The Preclinical Pharmaceutical Research and Biomarker Discovery Markets
Preclinical pharmaceutical research refers to the discovery and testing of new therapeutic compound candidates prior to their use in human clinical trials. For every 5,000-10,000 preclinical drug candidates, only five enter human clinical trials. There is a substantial failure rate, estimated that, even after completing all preclinical toxicology and efficacy testing, 90% of drug candidates fail after entering human testing (Nature Reviews 2004).
There is a great need to obtain higher quality datasets more rapidly to better identify the most promising drug candidates. The Pharmaceutical Research and Manufacturers of America (“PhRMA”), an industry group comprised of 45 United States-based biopharmaceutical companies had a total of $49.58 billion in research and development expenditures in 2012. Of this, $11.82 billion was spent on prehuman/preclinical functions, which represented 23.8% of all R&D expenditures. This figure, which is only comprised of R&D by PhRMA member companies, does not include R&D expenditures by government agencies such as the National Institute of Health (NIH) and National Institute of Cancer (NIC) or domestic and international biopharmaceutical companies that are not members of PhRMA.
Biomarker discovery is the process of finding specific molecules, or panels of molecules, that have been found to be regulated in conjunction with specific disease states or drug treatments relevant to human health and disease. The global market for biomarkers in 2011 was $13.8 billion and expected to reach $37.68 billion in 2022. The global market for biomarkers includes biomarker development and technologies, biomarker diagnostics and biomarker services. Biomarker development and technologies accounted for 53.1% of the global market in 2011 and is expected to account for 43.3% of the 2022 market. In 2011, biomarker diagnostics and biomarker services accounted for 40.2% and 6.7% of the market and are expected to account for 49.8% and 6.9% of the market in 2022, respectively (Visiongain 2012. Biomarkers: Technological and Commercial Outlook 2012 - 2022).
Biomarker discovery is the essential starting point for the development of new prognostic and diagnostic tests, as well as for the emerging field of predictive medicine, where biomarkers can identify those patients who will respond well to specific therapies. The human disease biomarker sector seeks to identify and validate biomolecules that are associated with the onset and progression of a specific disease, and thus can become new diagnostics, or biomarkers, to be used for personalized medicine, as well as companion diagnostics to guide new pharmaceutical development for specific patient subgroups.
Biomarker discovery can be classified into four major sub segments: genomics, proteomics, bioinformatics, metabolomics and other, which is based on the technology used and class of molecule that is of interest. Genomics is focused on the analysis of DNA through the use of polymerase chain reaction or DNA sequencing to extract genetic information from biological samples. The Human Genome Project, which was completed in 2003, marked the sequencing of an entire human genome that led to the discovery that there are 20,000 - 25,000 human genes. Proteomics is the study of proteins, which are synthesized by DNA and are biologically active. It is estimated that there are up to 400,000 distinct proteins in the human body. Bioinformatics is comprised of tools and technologies such as software programs that are used to analyze large sets of data.
Additional molecular information markets addressed by Protea include agriculture, microbiology, consumer products and medical devices.
Molecular Information Platform Technologies
The two primary technologies for identifying and characterizing the biologically active molecules that are produced by cells are mass spectrometry and indirect molecular imaging.
Mass Spectrometry
Mass spectrometry is a 50 year old sector and represents the backbone of the bioanalytics industry. Mass spectrometers are used in a wide range of research applications in academic, pharmaceutical and chemical/industrial based laboratories worldwide, including their use to identify molecules in biological samples based on their “mass to charge” ratios.
According to TechNavio’s 2011 - 2015 Global Mass Spectrometry Market report, the global mass spectrometry market was valued at U.S. $3.9 billion in 2013 and is expected to reach US $5.9 billion by 2018, growing at a compound annual growth rate (“CAGR”) of 8.7% from 2013 to 2018. In 2013, it was estimated that there was a total installed base of 6,200 mass spectrometers in the large pharmaceutical companies sub-segment alone. The CAGR for the installed base for this sub-segment is expected to be 3.7% during the period 2011-2015. In 2013, a total of 715 mass spectrometers were expected to be installed, which includes both new mass spectrometer systems and replaced mass spectrometer systems in this sub-segment. Mass spectrometers are used extensively in the biopharmaceutical, industrial, chemical, materials and forensics industries as well as in academic research institutions.
38
According to the SDI Report, the mass spectrometry instrument market can be segmented into three major sub segments: Biopharmaceuticals, Academia and Industrial which represented 40%, 29% and 31% of the market, respectively, as cited in Strategic Directions International, Inc. and Global Assessment Report 12th Edition, The Laboratory Analytical & Life Science Instrumentation Industry Market Forecast 2012 - 2016, October 2012.
Protea estimates that the current LAESI instruments are compatible with approximately 6,000 mass spectrometers worldwide that are produced by Waters Corp. (NYSE:WAT) and Thermo Fisher Scientific (NYSE:TMO). Protea’s LAESI platform development roadmap includes enabling LAESI instrument compatibility with mass spectrometers from all major manufacturers.
The global mass spectrometry market is dominated by five key players: Agilent Technologies Inc., Bruker Corp., Danaher Corp., Thermo Fisher Scientific Inc. and Waters Corp.
| Company | Market Cap USD ($B) | Annual Sales USD ($M) | ||||||
| Agilent (NYSE:A) | $ | 18.69 | B | $ | 580 | M | ||
| Bruker (Nasdaq:BRKR) | $ | 3.82 | B | $ | 290 | M | ||
| Danaher (NYSE:DHR) | $ | 51.77 | B | $ | 640 | M | ||
| Thermo Fisher (NYSE:TMO) | $ | 48.47 | B | $ | 550 | M | ||
| Waters Corp. (NYSE:WAT) | $ | 8.77 | B | $ | 440 | M | ||
Source: TechNavio Analysis, Global Mass Spectrometry Market 2011 - 2015. Capital IQ as of July 31, 2014.
Quantitative Whole Body Autoradiography (“QWBA”)
Autoradiography is defined as an image recorded on a photographic film or plate produced by the radiation emitted from a specimen, such as a section of tissue that has been treated or injected with a radioactively labeled isotope.
Traditional bioanalytical measurements determine concentrations of drug and metabolites in plasma; however, most drugs exert their effects in defined target tissues. As there is no clear relation between concentrations in plasma and those in tissue, alternative methods must be employed to study the absorption, distribution, metabolism and excretion properties of new therapeutic agents. The FDA requires distribution data in most new NDA applications prior to Phase II study initiation. Quantitative whole-body autoradiography is currently used in the drug development process to determine the distribution and concentrations of radiolabeled test compounds in laboratory animals. Quantitative whole-body autoradiography can provide information on tissue PKs, penetration, accumulation and retention. Although the technique is considered the industry standard for performing preclinical tissue distribution studies, It's disadvantages include the high cost and the radiolabeling of the molecule of interest and radiolabeling can take 90 days longer depending on the complexity (synthesis process and label required). Another shortcoming of the technique is that QWBA can take weeks to perform and only indicates the drug associated radioactivity. Additional studies then need to be performed to obtain the drug profile in tissues.
Mass spec imaging eliminates the need for a radiolabel, takes days instead of weeks, looks at the molecule and its metabolites directly eliminating the need for additional profiling studies, and is significantly less expensive than QWBA.
The Total Global indirect molecular imaging market is estimated at $7.3 billion according to a 2009 report by Scienta Advisors /Kolorama Information. The overall pre-clinical imaging (in vivo) market was valued at $790 million in 2012, and is estimated to grow at an annual growth rate of 14.5% over the next five years per a January 2013 Pre-Clinical Molecular Imaging Market report by marketsandmarkets.com. The global market for molecular imaging devices was valued at $2 billion in 2012. This market is expected to reach $2.2 billion in 2013 and nearly $3 billion by 2018, with a CAGR of 6.2% for the period of 2013 to 2018 according to a November 2013 report by BCC-Global Markets for Molecular Imaging Technology and Devices.
Limitations of Existing Technologies
There are a number of limitations to the existing platforms, as described below:
Mass Spectrometry
| · | Sample Preparation is Required: Mass spectrometry requires sample preparation that is tedious, time consuming and results in operating complexity that requires experienced and highly skilled users. |
| · | Loss of Spatial Distribution Information: Traditional mass spectrometry requires that a sample be homogenized, sometimes purified, or have additional chemicals introduced to it in order to be analyzed by a mass spectrometer. These processes destroy the tissue structure, which eliminates the ability to show where a specific molecule is located within a given tissue region. Understanding where a specific compound is localized could provide insight into a given disease or treatment action. |
39
| · | Limited Type and Number of Compatible Samples: Current mass spectrometry analysis occurs under vacuum conditions and therefore cannot analyze living cell samples such as bacterial colonies or cell cultures because the sample will be immediately destroyed as a result of the vacuum conditions. These are sample types used extensively in preclinical pharmaceutical research and biomarker discovery. |
| · | Limited Imaging Capability: Mass spectra can be very complex data sets that are difficult to interpret and can have limited relevance to biology-centric applications. Additionally, traditional mass spectrometry analyses do not provide direct, spatial relationships with the sample. Normally, bulk sample is processed and analyzed, which causes loss of the spatial association and value. |
Quantitative Whole Body Autoradiography:
| · | require radioactive labels that must be incorporated or synthesized in the drug to generate the images. As a result, they have half-lives, which can be as short as a few minutes in duration. The synthesis of these compounds takes time and requires highly trained technicians. |
| · | are only able to analyze a few molecules in a single analysis. Typically, only the labeled molecules can be visualized and if the label does not become a part of any metabolites, then they remain invisible to the process. |
| · | have resolutions that only reach the millimeter-scale level. While millimeter resolution enables the visualization of large anatomical components such as bones and organs, it is not adequate for tissue or cell-specific molecular analysis. |
| · | can only detect the drug associated radioactivity and not the distribution of the molecule and its metabolites. Further investigation and follow on studies are required to identify the actual distribution profile. |
| · | require special licensing for handling radioactive samples. |
Advantages of LAESI Technology Platform
We believe that our proprietary LAESITM platform is disruptive technology that provides us with certain competitive advances over existing technologies and which we believe will become a new industry standard.
The LAESI platform eliminates two of the major drawbacks of traditional mass spectrometry - sample preparation and loss of spatial information. LAESI can analyze a wide range of sample types with no sample preparation required. In addition, samples such as a tissue section or cells can be analyzed “as is,” even live cells and bacterial colonies. LAESI technology generates big data molecular profiles of tissue sections, biofluids (blood, urine and serum) and many other sample types, including horticulture specimens, cell lines & pellets, bacterial colonies, hair fibers, contact lenses and hydrogels.
Enables Mass Spectrometry Imaging: Protea's LAESI technology directly analyzes biological samples without the need to apply chemicals or introduce tags or tracers. Proprietary software developed at the company enables two- and three-dimensional direct molecular imaging, displaying the distribution of molecules in biological samples.
Numerous Molecules per Analysis: It is not unusual to detect over 1,000 individual molecules in a single experiment. Larger databases aid the “molecular eyesight” of a researcher, improving their prospects to find new biomarkers or molecular data that will provide new research insight.
No Sample Preparation Required: Protea’s LAESI technology analyzes samples directly without the need for sample preparation other than standard tissue sectioning. Virtually all sample types can be inserted directly into the LAESI instrument where they are ablated (put into gas phase), ionized (charged) and sent into the mass spectrometer, with this process taking a fraction of a second. LAESI instrumentation employs user-friendly software that eliminates the operation complexity of traditional mass spectrometry. Molecular information is acquired on samples in their native state; such data can be more useful, particularly to biologists.
Minimal Sample Destruction: Because of the small spot size, the sample is only locally destroyed by the laser ablation pulse. With the use of positional stages, LAESI allows, the spatial localization of biomolecules within a sample, thereby enabling two- and three-dimensional direct molecular imaging.
High Throughput Sample Profiling: The LAESI platform also functions as a high throughput processing system for well plate and bacterial analysis. With the ability to analyze samples in seconds, researchers are able to quickly screen libraries of compounds, strains, and other assays very rapidly.
Improvements in Resolution: Protea's LAESI technology has the ability to produce images that have micron-scale resolution, enabling imaging at the cellular level. This gives Protea the ability to image a select number of cells of interest in a given biological sample.
40
Improvement in Analysis Speed: Due to the elimination of sample preparation and the rapid speed of LAESI, data can be made available in seconds to minutes. This factor makes LAESI attractive to users that have high-throughput molecular information applications (i.e., a large number of samples must be analyzed a limited number of times).
A LAESI Mass Spec Imaging Data File
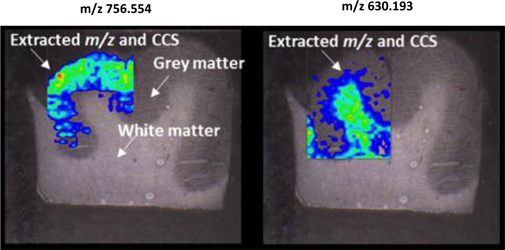
LAESI image of human brain tissue showing marked localization in the gray matter (left) for the lipid at 756 m/z, and white matter (right) of a lipid (m/z 630). This dataset shows the power of LAESI to provide distinct spatial localization visualized for these two lipids, which would be lost with a traditional approach. (Anal Chem. 2015 Jan 20; 87(2): 1137–1144. Source: The Company.)
Our Technology
LAESI Mass Spectrometry
Protea exclusively licensed the technology known as Laser Ablation Electrospray Ionization (“LAESI”) from GWU in 2008, invented in the laboratory of Professor Akos Vertes, Ph.D. Protea has completed the development of a novel bioanalytical instrument platform known as the LAESI DP-1000 Direct Ionization System. This technology enables the direct identification of proteins, lipids and metabolites in tissue, cells and biofluids, such as serum and urine, without any sample preparation prior to analysis. By eliminating sample preparation, the biological sample can be analyzed without the possible contamination, bias or sample loss that occurs with the current techniques, which require the introduction of chemicals, or the destruction of the sample itself, in order to enable analysis by mass spectrometry. The ProteaPlot software then can display the data obtained by mass spectrometry analysis combined with actual images of the tissue and cell samples. Thus, mass spectrometry data can be evaluated in the context of the biology of the sample; this allows the integration of mass spectrometry data with current pathology and microscopic imaging techniques. Data is available in seconds to minutes, allowing rapid time to results and the capacity to analyze thousands of samples in a single work period. As an example, a researcher testing a new drug’s effects on living cells can analyze changes in the cells’ metabolism across a specific time course, thereby almost immediately obtaining data as to the activity of the new drug. The Company believes that LAESI technology has the potential to significantly improve the availability of molecular information in pharmaceutical research, as well as many other fields, including agriculture, pathology, biomarker discovery, biodefense and forensics.
LAESI Illustration
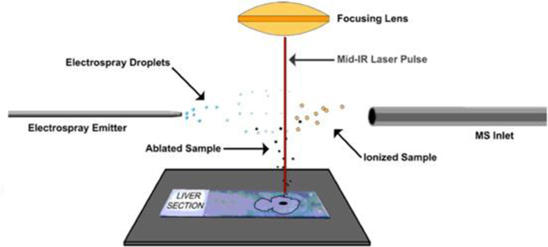
41
As depicted above, LAESI utilizes a mid-range infrared laser pulse that rapidly (microseconds) boils the water in a given sample creating an ablation event that results in the biomolecules from that particular area being ejected vertically where they meet a stream of electrospray droplets and become charged biomolecules or ions. The ionized sample then moves through an inlet tube and into the mass spectrometer where the mass analyzer determines the mass-to-charge ratio of the representative biomolecules and their relative abundance is determined by the detector.
Key LAESI Components
ProteaPlotTM Direct Molecular Mass Spec Imaging Software
Using its own internal software development team, Protea developed the software platform ProteaPlot to address two other limitations of traditional mass spectrometry: the inability to determine the location of a molecule or ion in a sample and the difficulty a non-analytical chemist has in interpreting mass spectrometry data. ProteaPlot is designed to create images known as ion maps or “molecular maps,” from traditional mass spectrometry data (mass spectrum). These molecular maps depict the distribution of a specific molecule in a given sample and the relative intensity (abundance) of that molecule.
The image of a rat brain below is a screen shot from the ProteaPlot software program. A traditional mass spectrum can be seen on the right side of the image. The image to the left is an ion map of clozapine, an anti-psychotic drug prescribed for the treatment of schizophrenia, with a mass-to-charge ratio (m/z) of 270 Da. The colorimetric scale represents the relative intensity of this ion with blue representing the regions with the lowest relative intensity, the areas in red representing the regions with the highest relative intensity and the areas in black representing regions where that molecule is not present at all.
42
ProteaPlot Software Screen showing molecular (ion) distribution image.
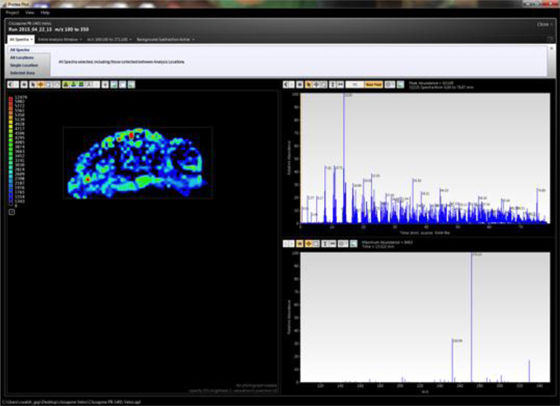
Molecular (ion) Map of a dosed pharmaceutical, clozapine, using ProteaPlot Software. The histologically stained section is highlighted below to show the correlation of the dosed drug to its target in the cerebral cortex and other regions in the brain.
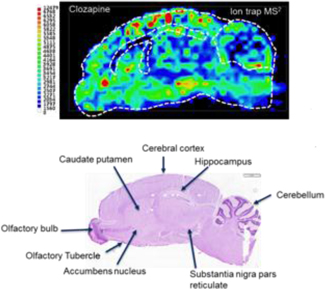
The ability to display molecular information obtained from LAESI as direct molecular images is of great advantage to pathologists who can analyze samples of choice with no sample preparation, and can rapidly obtain molecular information along with traditional microscopic analysis. Also, the remaining sample is not destroyed so ion location information can be obtained and the ion maps can be visualized over the sample of origin for integration with more traditional biological analyses such as histology.
Proprietary MSI Software
In January 2015, the Company announced it had completed the in-house development of a novel workflow and software suite. Known as “Histology Guided Mass Spec Imaging (HG-MSI),” the workflow and software enables pathologists to combine traditional microscopy and histology with high resolution mass spectrometry molecular imaging, allowing researchers to share, annotate and direct the analysis of specific tissue morphologies and cell subpopulations by mass spec imaging. With HG-MSI, molecular profiling data are collected from discrete locations within a tissue section using a histology stained section as a guide. The digital tissue scans are visually analyzed by pathologists, who then annotate specific areas for further analysis. The annotated areas are then targeted by mass spectrometry to acquire a chemical fingerprint of the representative area. This chemical information can then be used to identify specific molecules of interest and map the biomolecules present in a visualized morphology. This workflow is applicable to all classes of biomolecules (e.g., proteins, peptides, lipids, metabolites) and can be carried out on both fresh frozen and formalin fixed, paraffin embedded (FFPE) tissue specimens.
43
This web interface can be accessed via any device (e.g., laptop, tablet, cell phone, etc.) allowing the end user to visualize, annotate and download microscopy images from anywhere in the world. The development of the ProteaScope portal and HG-MSI workflow and service enables researchers to obtain molecular information from specific regions of tissues, combining optical morphology with spatial biomolecular information Clinicians can share, annotate and direct the analysis of specific tissue morphologies and cell subpopulations by MSI. Understanding the molecular differences between select cell sub-populations supports the identification and characterization of biomarkers within these regions that improves the differential diagnosis of cancer.
Our Business Strategy
Protea intends to achieve its business objectives by leveraging its knowhow and technology to improve the availability, comprehensiveness and usefulness of molecular information to address the needs of the preclinical pharmaceutical research, biomarker discovery and other life science markets. Key strategic elements are the following:
| · | Maintain and advance our commercial leadership in developing and providing mass spectrometry imaging services, combined with existing bioanalytical workflows, to improve the availability, comprehensiveness, and usefulness of molecular information to address the needs of our pharmaceutical research, biomarker discovery, agriculture and other life science research clients. |
| · | Collaborate with platform extending companies including three-dimensional tissue suppliers, biomarker discovery and other commercial technology platforms where the Company’s technology can advance and add value to their capabilities. |
| · | Advance the Company’s market by developing and validating an expanding portfolio of LAESI capabilities and continuously expand the commercial applications for the technology. Our customers continuously provide guidance for new applications and workflows which we can address with the LAESI platform. |
| · | Continuously advance and upgrade our software offering, and introduce new software-driven data bioinformatics capabilities. Protea is focused on creating software and analytical tools that enhance the value of the data, including bioinformatics datamining tools. In January 2015, we announced our development of new software, known as ”Histology Guided Mass Spec Imaging (HG-MSI).” The software enables pathologists to combine traditional microscopy and histology with high resolution mass spectrometry molecular imaging, allowing researchers to share, annotate, and direct the analysis of specific tissue morphologies and cell subpopulations by mass spec imaging. |
·
| Partner with first tier medical research laboratories to apply our core MSI technology and workflows to create new, molecular diagnostic tests that can be used to improve the diagnosis and prognosis of cancer. Our current programs target the differential diagnosis and prognosis of malignant melanoma and the characterization of subpopulations of cells in lung adenocarcinoma |
Our Commercial Offering
Molecular Information Services
We believe we are the commercial leader in the offering of MSI services. Our clients send their tissue and biofluid samples directly to the Company’s laboratory, where samples are analyzed utilizing the Company’s state of the art mass spectrometry instrumentation, scientific expertise and bioinformatics capabilities. We combine our proprietary LAESI platform with MALDI workflows. Our clients include major pharmaceutical, biotechnology, chemical and medical device and consumer products companies. The services unit is operated within a “Quality by Design” environment, which is necessary to meet the internal research and development standards of pharmaceutical and clinical research clients.
The Company’s MSI services represent a revolutionary capability that for the first time enables the identification of all classes of biologically-active molecules produced by cells, and combines this with the ability to instantly spatially-display the molecules (both two- and three-dimensional) in tissue histology sections. MSI can be performed without sample preparation, labeling or antibody techniques, thereby for the first time integrating direct molecular identification with tissue pathology. Since the sample is not touched, data is unbiased and more rapidly available, providing molecular information that rapidly answers questions critical to the drug development process, such as, “is my drug actually getting inside the target cells,” “is my immune modulating agent changing the molecular output of the tumor cell,” or “how long did it take for the drug to enter the target cells” as well as questions of drug concentration and duration.
We have developed workflows specifically for the characterization of therapeutic antibodies, to provide both visual and analytical certainty of therapeutic efficacy, and validation of therapeutic design. Once target tissues are imaged, data analysis can include quantitation of the dosed therapeutic, distribution analysis, and other bioinformatics workflows, including statistical and pathway analysis.
44
In January 2014, the Company, as a subcontractor to GWU, was awarded a multi-year project with the Defense Advanced Research Projects Agency (DARPA). In addition to Protea, The Stanford Research Institute International and GE Global Research also collaborate on the project entitled, “New Tools for Comparative Systems Biology of Threat Agent Action Mechanisms.” A $14.6 million five year project, the goal of DARPA’s Rapid Threat Assessment (“RTA”) program is to develop new tools and methods to elucidate the mechanism of action of a threat agent, drug, biologic or chemical on living cells within 30 days from exposure. Uncovering the mechanism of action of such agents in 30 days, compared to the years currently required, could be key to the development of effective countermeasures. The molecular networks within living cells are vast and complex. Conventional approaches fail to capture the system-wide response of a living cell to a threat agent.
LAESI Instrument, Software and Consumables
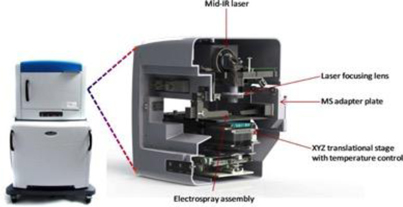
LAESI technology was invented in the laboratory of Professor Akos Vertes, Ph.D., Dept. of Chemistry, GWU, in 2007 and was exclusively-licensed to Protea in 2008. The LAESI DP-1000 instrument, the Company’s prototype embodiment of its proprietary LAESI technology, integrates with laboratory instruments known as mass spectrometers.
This technology enables the direct identification of proteins, lipids and metabolites in tissues, cells and biofluids, such as serum and urine. Data is available in seconds to minutes, allowing rapid time to results and the capacity to analyze thousands of samples in a single work period. As an example, a researcher testing a new drug’s effects on living cells can analyze changes in the cells’ metabolism across a specific time course, thereby almost immediately obtaining data as to the activity of the new drug. The Company believes that LAESI technology has the potential to significantly improve the availability of molecular information in pharmaceutical research, as well as many other fields, including agriculture, pathology, biomarker discovery, biodefense and forensics.
LAESI instruments employ proprietary software developed by the Company that creates “molecular maps” - the ability to instantly display the distribution of molecules throughout tissue sample in both two- and three-dimensional imaging. The Company currently offers the LAESI Desktop Software and ProteaPlot software that facilitates operating the instrument, and the storage and display of datasets in a user friendly, intuitive software environment. The LAESI and ProteaPlot software includes tools for post-processing of LAESI-MS data, generation of molecular maps/images, and mass spectral comparison for regions of interest. LAESI software display the data obtained by mass spectrometry analysis combined with actual images of the tissue and cell samples. Thus, mass spectrometry data can be integrated with a sample’s tissue architecture.
We have developed and brought to market related, proprietary consumable products for use in molecular analysis, including REDIchipsTM (Resonance-Enhanced Desorption Ionization - sample target plates with nanopost array surfaces for matrix-free laser desorption ionization of small molecules); and, ProgentaTM a(acid labile surfactants use to solubilize proteins for mass spectrometry analysis).
LAESI Applications
Direct Molecular Imaging of Tissues and Cell samples
The LAESI is capable of rapidly analyzing and mapping the spatial distribution of biomolecules in tissue. The LAESI DP-1000 currently has a resolution of 200 (micron) that allows it to analyze a small number of cells per ablation, which occurs in a matter of seconds. The LAESI DP-1000 can analyze and image both human and animal tissue. It is not unusual for over a thousand individual molecular entities to be identified in a single experiment. Below is a LAESI mass spec image of two molecules shown simultaneously in a mouse pup section.
45
LAESI 2 dimensional map image of four molecules shown simultaneously in a mouse pup brain section
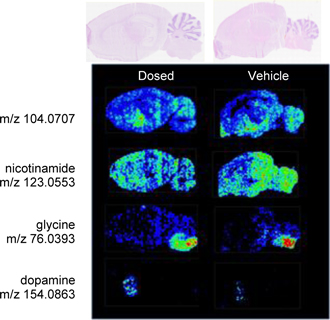
Rapid Profiling of Biofluids and Assays
LAESI is capable of rapidly analyzing micro titer well plates filled with biofluids such as blood, urine or serum. The LAESI Desktop Software makes the automation of this type of analysis very easy for the user. A grid pattern can be quickly drawn for 96, 384 or 1536 sample well plates and the number of ablations per well can be set. Once the user’s settings have been entered the analysis will assess each well systematically and takes less than ten seconds to complete per well. This profiling speed is incredibly useful for researchers with a large number of samples interested in a rapid “snapshot” of the molecular content in a well. This screening application can aid in assay monitoring and the detection of specific compound presence or production.
In Vivo Direct Molecular Imaging
Because LAESI operates at ambient pressure (non-vacuum) it is the only laser based mass spectrometry imaging technology that can analyze vacuum incompatible samples such as living cell cultures and bacterial colonies. Due to this unique capability, LAESI can be used to analyze a bacterial colony and then that colony can be re-incubated and analyzed at a later time, enabling biodynamics studies on living samples for the first time. Below is a LAESI mass spec image of a bacterial colony. This application can be used to rapidly screen and discover potential new pharmaceuticals produced from bacteria and fungi. In addition, LAESI can be used to monitor the production of a specific compound being overexpressed for increased production and yield in bioprocessing workflows.
LAESI 3 dimensional ion molecular map image of a live bacterial colony showing the spatial distribution of an antibiotic molecule (green) and a biomolecule produced by the bacterial cells (orange)

Three-Dimensional LAESI Mass Spectrometry Imaging
LAESI is the only laser based mass spectrometry imaging technology that performs a volumetric sampling. All other mass spectrometry imaging technologies perform surface analysis or desorption whereby they are only extracting small amounts of molecular information. This is a key, unique advantage, as the LAESI DP-1000 mid-IR laser enables it to penetrate samples in the Z-plane, which enables 3 dimensional analysis and imaging. The ProteaPlot software then is able to recompile the molecular data into planes representing each layer of sample analyzed by the LAESI system. Below is a three-dimensional LAESI mass spectrometry image that assessed the distribution of a molecule in a contact lens.
46
LAESI 2 Dimensional (left) and 3 Dimensional (right) Ion Molecular Map Image of a Contact Lens, displaying the presence, location and intensity of a molecule
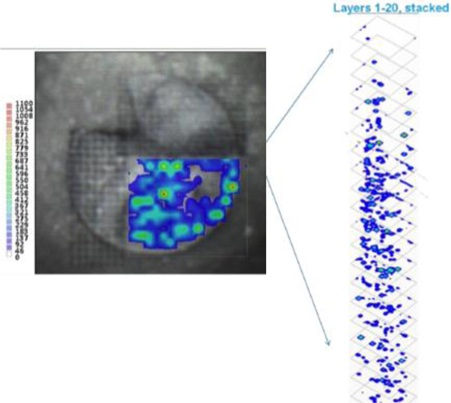
Source: The Company
Molecular Diagnostics and Clinical Research
The Company is employing both its proprietary LAESI platform and MALDI methodologies to create comprehensive, tissue-based molecular profiles to improve the differential diagnosis of cancer. Our bioinformatics capability allows the integration of the Company’s MSI data files with related pathology, gene expression and demographic datasets, with the purpose of improving human disease state detection, assessment and management. The Company has entered into collaborative research partnerships with top tier academic centers to develop and validate new, proprietary, mass spec imaging - based cancer diagnostic tests.
We have established a collaborative research partnership with the Memorial Sloan-Kettering Cancer Center and the Dana-Farber Cancer Institute. The first target of the collaboration is early stage lung adenocarcinoma. The objectives of the collaboration are to demonstrate that different cancer cell sub-groups within a lung cancer will have different molecular profiles and will behave differently. The goal is to define these molecular differences, to identify the sub-group of cancer cells with the worst prognosis that are most likely to recur, thereby enabling earlier treatment intervention, and to use these findings to achieve lung adenocarcinoma tumor cell “molecular profiling,” leading to more precise treatment selection and higher survivor rates.
The market for analysis of indeterminate or borderline analysis is $720 million annually, based upon current pricing. Moreover, 41% of metastatic melanomas were missed by experts in a recent study of borderline melanocytic lesions (need reference). About 500,000 skin biopsies are labeled as indeterminate or borderline every year.
We are advancing our MSI melanoma test with further clinical studies.
47
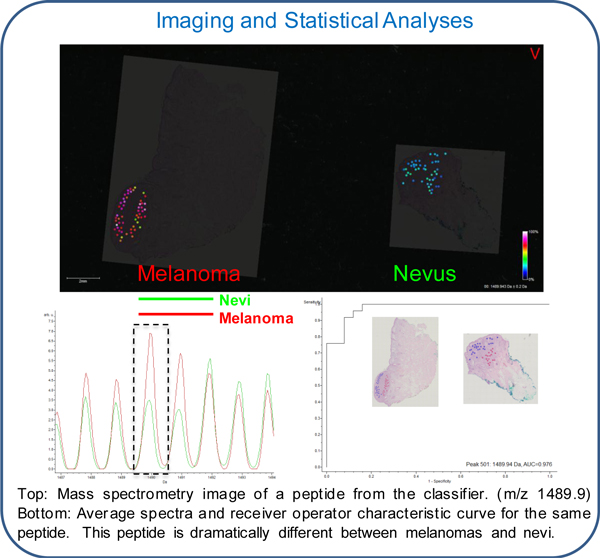
(1) Source: “Mass Spectrometry Imaging - an objective and reliable method to differentiate between benign melanocytic nevi and malignant melanomas”, Rossitza Lazova, MD1 and Erin H. Seeley, PhD2; 1Department of Dermatology, Yale University School of Medicine, New Haven, CT. 2Protea Biosciences, Inc., Morgantown, WV; October, 2015 Annual meeting of the American Society of Dermatopathology, San Francisco, CA.
Intellectual Property
Protea currently owns eight patents (with additional pending applications) and has an exclusive license to fourteen additional patents and other pending applications owned by GWU. The subjects of the patent applications include: 1) Laser Ablation Electrospray Ionization (“LAESI”) for high throughput and imaging mass spectrometry (two- and three-dimensional biomolecular imaging); 2) nanopost arrays (“NAPA”) for high sensitivity and matrix-free analysis of biological samples in MALDI mass spectrometers; 3) novel acid-cleavable chemical surfactants; and 4) protein microscope.
Research and Development
The Company’s primary research focus is advancing the capabilities of its proprietary LAESI technology platform, related technologies and software. New advances and applications for the LAESI technology platform and software include increasing spatial resolution, improving sensitivity, expanding applications for LAESI direct molecular imaging services, developing new software database structures and next generation instrumentation.
In addition, through the development of diagnostics, the Company is developing intellectual property and knowledge relating to the presence or absence of specific molecules in normal and disease-specific tissues, to be used to validate a new tissue analytics-based class of tests for the differential diagnosis of cancer.
The Company devotes most of its R&D resources to advancing its core LAESI technology platform. New advances and applications for the LAESI technology platform are in development, including the integration of the LAESI-DP 1000 system with expanded mass spectrometer vendor coverage and advancing the spatial resolution of the LAESI technology.
In 2014, we teamed with GWU, Stanford Research International (SRI) and GE Global Research and were awarded up to $14.6 million for a cooperative agreement from DARPA’s Rapid Threat Assessment program.
48
The goal of DARPA’s Rapid Threat Assessment program is to develop new tools and methods to rapidly define the mechanism of action of a threat agent, drug, biologic or chemical on living cells - within 30 days from the time of exposure. Uncovering the mechanism of action of such agents in 30 days, compared to the years currently required, will aid the development of threat mitigations and countermeasures. Such a breakthrough capability could also reduce the strategic advantage associated with rapidly emerging chemical or biological threats in a national security context. Current methods fail to capture the system-wide response of living cells to a threat agent. To accomplish this objective, Protea has developed a novel silicon wafer known as a “REDIchip” (resonance enhanced desorption ionization), exclusively licensed to Protea by GWU that will help to achieve the goal of real time threat identification on the battlefield.
The company has successfully commercialized the first generation REDIchip product in late 2015. REDIchip can be used on commercially available MALDI mass spectrometers and has numerous applications outside of rapid threat assessment such as biomarker discover, clinical research, polymer science, food safety and others. The Company believes that REDIchip has advantages over existing MALDI mass spectrometry due to the ability to analyze samples without the addition of a chemical matrix, which enables a more sensitive, rapid, and quantitative workflow. The REDIchip is easily integrated into existing MALDI-MS instrumentation and can improve throughput, reduces background signal, and enables reproducible small molecule characterization and quantitation results.
Sales and Marketing
The Company markets its products and services worldwide, utilizing a combination of field sales, distributors, in-house sales support and web-based marketing. The Company attends exhibitions in the U.S. and Europe to present its products, and in the last 12 months has exhibited at over eight major industry exhibitions and over ten regional conferences. Protea's sales and marketing staff consists of three field sales professionals, two inside sales representatives and two marketing professionals. In addition, Protea established a global co-marketing agreement with Waters Corp. (NYSE:WAT), a major mass spectrometry focused company headquartered in Milford, Massachusetts in 2012.
In 2015, Protea announced a joint collaboration with Agilent Technologies, Inc. (NYSE: A) (“Agilent”), to develop new bioanalytical workflows in order to meet the emerging needs of the growing biopharmaceutical industry. Under the terms of the Memorandum of Understanding, Protea, using Agilent instrumentation combined with its technology, will develop workflows that focus on developing new methods for the field of biotherapeutics.
In March 2016, the Company entered into an agreement with an advertising firm for certain services to be rendered to the Company over an estimated ninety (90) day period
Protea also engages distributor partners globally including VWR International and Fisher Scientific.
Competition
The Company believes that its technology differs substantially from what is currently on the market. Bioanalytics is a major sector of the biotechnology industry, and competition is expected to be broad-based. However, the Company also believes that the mass spec industry affords opportunities for commercial partnerships and that industry participants can also be potential partners. The following list of competitors is not intended to be exhaustive, and there are other existing competitors and there likely will be new potential competitors in the future. Many competitors, including most of the companies identified below, are much larger than Protea in terms of capital, employees and other measures of size and have been in business longer than Protea and thus may have greater market acceptance or brand recognition:
| · | Imabiotech (Loos, France, http://www.imabiotech.com/?lang=en). Imabiotech develops software that enables quantitative MALDI mass spectrometry imaging and offers only MALDI based mass spectrometry services to pharmaceutical and biotechnology companies. |
| · | Kinemed (Emeryville, CA, http://www.kinemed.com). Kinemed works with major pharmaceutical and biotechnology companies to making predictions of efficacy and toxicity to accelerate drug development and reduce the overall cost. |
| · | Prosolia (Indianapolis, IN, www.prosolia.com). Prosolia develops and markets DESI, a mass spectrometry imaging technology and provides DESI based services to pharmaceutical and biotechnology companies. |
| · | Molecular Imaging, Inc. (Ann Arbor, MI, http://www.molecularimaging.com). Molecular Imaging is a CRO that provides in vivo pharmacology and small animal in vivo imaging services to pharmaceutical and biotechnology companies. |
49
Strategic Relationships
Over the past eight years we have established collaborative research initiatives with certain major universities and teaching medical facilities. These include:
Agreement with The George Washington University (“GWU”)
In December 2008, the Company entered into an Exclusive License Agreement, as amended on February 22, 2010 and from time to time thereafter with GWU (Washington, D.C.) for the LAESI technology developed in the laboratory of Akos Vertes Ph.D., Professor of Chemistry, Professor of Biochemistry & Molecular Biology, Founder and Co-Director of the W.M. Keck Institute for Proteomics Technology and Applications, the Department of Chemistry, who is a science advisor to the Company. Under the terms of the license agreement, the Company has the exclusive, worldwide rights to commercialize the technology. The Company is obligated to pay expenses for the preparation, filing and prosecution of future related patent applications governed by the license agreement and related license fees, annual royalties equal to 5% of the net sales of products and processes sold by the Company, or an affiliate that utilizes the subject technology, after taking into account the annual minimum royalty fees described below, and 50% of payments received by the Company in connection with any sublicense of the technology under the agreement. On the first anniversary of either the date on which the Company first sells a product or service utilizing the technology underlying the agreement or the date on which the Company enters into its first sublicense agreement, whichever occurs first (the “First Sale Date”), the Company is required to pay GWU a non-refundable minimum royalty payment equal to $5,000. The university is also entitled to the following non-refundable minimum royalty payments on each subsequent anniversary of the First Sale Date: second anniversary: $10,000; third anniversary: $15,000; fourth anniversary and continuing annually through the expiration or termination of the agreement: $20,000.
Unless earlier terminated in accordance with its terms, the agreement expires upon the later of 20 years from the effective date or the end of the term of the last underlying patent to expire. Currently, the LAESI patent (US 7,964,843) is the underlying patent and will expire on May 21, 2026. The Company has made all the payments required under the agreement, and is otherwise in full compliance with the terms of the agreement.
In November 2012, the Company entered into a Patent License Agreement with GWU for Laser Desorption/Ionization and Peptide Sequencing on Laser-Induced Silicon Microcolumn Arrays (Matrix) and Nanophotonic Production, Modulation and Switching of Ions by Silicon Microcolumn Arrays technology developed in the laboratory of Akos Vertes Ph.D. Under the terms of the license agreement, the Company has an exclusive, worldwide license to make, have made, use, import, offer for sale and sell the licensed products. The Company paid a license initiation fee of $25,000 and a license diligence resource fee of $12,500 in 2013. In addition, the Company is required to pay $20,000 each year thereafter to develop and commercialize the products, $30,000 milestone payment upon the first sale of a licensed product, and royalties will be 7% of the net sales of licensed products and 5% of the net sales of combination products for combined cumulative net sales up to $50 million. Thereafter, royalties reduce to 6% and 4%, respectively, after taking into account quarterly minimum royalties of $1,500 for the first four quarters after the first sale of a licensed product, $2,500 for the next four quarters, $3,500 for the next four quarters and $6,000 for each succeeding quarter. The Company has made all the payments required under the Patent License Agreement, and the Company is otherwise in full compliance with the terms of the Patent License Agreement.
Agreement with West Virginia University (“WVU”)
On December 21, 2005, the Company entered into an Exclusive License Agreement (the “WVU Agreement”) with the West Virginia University Research Corporation, a nonprofit West Virginia corporation (“WVURC”) acting for and on behalf of West Virginia University. Under the terms of the WVU Agreement, the Company is required to pay (i) a license fee equal to $25,000 due within 90 days of the date on which the first notice of allowance is issued by the United States Patent and Trademark Office or a similar foreign country with respect to the technology underlying the WVU Agreement; (ii) annual royalties equal to 4% of the gross sales of products and services that utilize the subject technology which are payable semi-annually within 30 days of June 30 and December 31 of each calendar year covering gross sales received during the preceding year; and (iii) expenses for the preparation, filing and prosecution of related patent applications. In the event the Company is required to license any intellectual property from third parties in order to practice or commercialize the technology underlying the WVU Agreement, the royalty payments will be reduced by the lesser of (a) 50% or (b) the royalty and licensing fees actually incurred by the Company to license the intellectual property rights from such third party. If such a reduction is applicable, WVURC is entitled to earned royalties of at least 2% on gross sales of products and services that utilize the WVU subject technology. For any sublicense granted to sub-licensees, WVURC is entitled to 10% of any license fee and other payments or fees received from the sublicensee, which is due and payable within ten days of receipt by the Company from the sublicensee. At present, the Company sponsors collaborative research in the WVU School of Medicine Department of Pathology and the Mary Babb Randolph Cancer Center (MBRCC).
Unless earlier terminated in accordance with its terms, the WVU Agreement automatically terminates upon the later of: (i) the expiration of the last patent to expire issued in respect of the licensed technology, or (ii) 20 years from the first commercial sale by the Company of the last licensed product included in the subject technology by amendment to the WVU Agreement. At present, there are no patent applications being pursued by WVU in respect of the technology licensed to the Company under the WVU Agreement. The Company has made all the payments required under the WVU Agreement, and the Company is otherwise in full compliance with the terms of the WVU Agreement.
50
Memorial Sloan-Kettering Cancer Center and the Dana-Farber Cancer Institute
We have established a collaborative research partnership with the Memorial Sloan-Kettering Cancer Center and the Dana-Farber Cancer Institute. The first target of the collaboration is early stage lung adenocarcinoma. The objectives of the collaboration are to demonstrate that different cancer cell sub-groups within a lung cancer will have different molecular profiles and will behave differently. The goal is to define these molecular differences and to identify the sub-group of cancer cells with the worst prognosis that are most likely to recur thereby enabling earlier treatment intervention, and to use these findings to achieve lung adenocarcinoma tumor cell “molecular profiling,” leading to more precise treatment selection and higher survivor rates.
Yale License Agreement
We established a collaborative research initiative with The Yale University School of Medicine that employs the Company’s MSI technology to differentiate melanoma from benign spitz nevi cells. In a recent publication, we showed that MSI can differentiate between benign melanocytic nevi and malignant melanomas in formalin-fixed, paraffin-embedded tissue sections based on proteomic differences, that MSI is an objective and reliable method that may be helpful in difficult cases, in which rendering a firm diagnosis of either benign nevus or malignant melanoma may be very difficult.
In April 2016 we entered into an exclusive license agreement for technology with Yale University related to the differential diagnosis of melanoma, specifically designated as a "Method of Differentiating Benign Melanocytic Nevi from Malignant Melanoma." The technology was co-invented by Dr. Rossitza Lazova, of the Department of Dermatology at Yale School of Medicine, and Dr. Erin Seeley our Clinical Imaging Principal Investigator. Under the terms of the license agreement, we have been granted the exclusive worldwide rights to commercialize the technology. We are obligated to pay expenses for the preparation, filing and prosecution of future related patent applications governed by the license agreement and related license fees. We are obligated to pay a non-refundable royalty of $5,000 payable to Yale University in May 2016, and an annual license maintenance royalty of $2,500 in April 2017 and an annual license maintenance royalty of $5,000 in each anniversary year thereafter. We also pay a non-refundable royalty of $5,000 upon our making our first sale of a licensed product, $7,500 when we file for an approval for commercial sale with a government regulatory agency and $10,000 upon our “first sale” of a licensed product that is approved by such governmental agency. In addition, we will pay Yale an earned royalty of 2.5% on worldwide cumulative net sales of licensed products by our company or under any sub-license or affiliate arrangements, to accrue within thirty (30) days from the end of each calendar quarter, subject to the payment of minimum annual royalties of $10,000, payable on the first day of January in the year following our first sale of licensed products (the “Minimum Royalty Effective Date”), and increasing to $15,000 on the first anniversary of the Minimum Royalty Effective Date, $20,000 on the second anniversary of the Minimum Royalty Effective Date, $30,000 on the third anniversary of the Minimum Royalty Effective Date, $40,000 on the fourth anniversary of the Minimum Royalty Effective Date and $50,000 on the fifth anniversary of the Minimum Royalty Effective Date and each subsequent anniversary year thereafter.
Unless earlier terminated in accordance with its terms, the Yale license agreement expires upon the later of 20 years from the effective date or the end of the term of the last underlying patent to expire.
Sale of European Subsidiary
In December 2014, Protea Biosciences, Inc., our wholly-owned subsidiary, completed the sale of 100% of the issued and outstanding capital stock of ProteaBio Europe SAS, to AzurRx BioPharma, Inc. (“AzurRx”). Pursuant to the terms of the Stock Purchase and Sale Agreement (the “SPA”), we received the following consideration in exchange for the subsidiary:
| (i) | an aggregate amount of $300,000 including $200,000 in cash and $100,000 from the forgiveness of outstanding indebtedness of the Company owed to AzurRx; | |
| (ii) | 100 shares of Series A Preferred Stock of AzurRx (the “Preferred Stock”) that is convertible into 33% of the issued and outstanding common stock of AzurRx; and | |
| (iii) | the right to receive certain other contingent consideration to be paid upon the satisfaction of certain events, including (a) a one-time milestone payment of $2,000,000 due within (10) days of receipt of the first approval by the FDA of a New Drug Application or Biologics License Application for a Business Product (as such term is defined in the SPA); (b) royalty payments equal to 2.5% of net sales of Business Product up to $100,000,000 and 1.5% of net sales of Business Product in excess of $100,000,000 and (c) ten percent (10%) of the Transaction Value (as defined in the SPA) received in connection with a sale or transfer of the pharmaceutical development business of Protea Europe. |
Effective upon the closing of the Sale, Thijs Spoor, a former director of the Company, was appointed as the sole director and Executive Chairman of AzurRx. While Mr. Spoor was a director of the Company, he did not receive any compensation for his services rendered as an executive officer or director of AzurRx. Pursuant to the SPA, so long as the Company owns such number of shares of Preferred Stock that are convertible into twenty percent (20%) or more of the issued and outstanding common stock of AzurRx, the Company will have the right to designate at least one member of AzurRx's board of directors.
51
In November 2015, the Company converted 29 shares of Series A Preferred Stock of AzurRx into 707,416 shares of common stock of AzurRx. Subsequently, the Company entered into various stock purchase agreements for the sale of such shares of common stock of AzurRx and received gross proceeds of $910,000 as of December 31, 2015.
In the first quarter of 2016, the Company notified AzurRx of its intent to convert 71 of its Series A convertible Preferred Stock, $0.0001 par value per share, into shares of AzurRx common stock, $0.0001 par value per share, pursuant to the terms and conditions of the Certificate of Designations. The conversion rate was 24,393 shares of Common Stock per share of Series A Preferred Stock. The Company received 1,731,949 shares of AzurRx common stock upon conversion.
In addition, the Company entered into various Stock Purchase Agreements to sell shares of its AzurRx Common Stock and received aggregate proceeds of $1,262,100 from the sale.
In the second quarter of 2016, the Company sold 550,000 shares of AzurRx Common Stock generating net proceeds of $675,000. After this sale, the Company held 265,757 shares AzurRx Common Stock representing a 3.60% ownership interest in AzurRx, on a fully-diluted basis.
Government Regulation
The Company’s products and services are sold for research use only and are not subject to U.S. Food and Drug Administration or other government agency approval.
Sources and Availability of Raw Materials
The Company does not believe that it has any critical issues of availability of raw materials or vendors where there are not multiple sources for the raw materials or vendor support that its business requires.
Environmental Matters
We are subject to various laws and governmental regulations concerning environmental matters and employee safety and health in the United States and other countries. U.S. federal environmental legislation that affects us includes the Toxic Substances Control Act, the Resource Conservation and Recovery Act, the Clean Air Act, the Clean Water Act, the Safe Drinking Water Act and the Comprehensive Environmental Response Compensation and Liability Act (CERCLA). We are also subject to regulation by the Occupational Safety and Health Administration (OSHA) concerning employee safety and health matters. The United States Environmental Protection Agency (EPA), OSHA, and other federal agencies have the authority to promulgate regulations that have an effect on Protea’s operations. As of the date of this prospectus, we did not have any accrued liabilities related to environmental matters.
Employees
As of the date of this prospectus, the Company currently has 35 full-time and 1 part-time employee, consisting of 17 technicians and scientists (6 PhD level), 8 management/administrative (2 PhD level), 3 IT/software development, and 7 sales and marketing (1 PhD level). None of our employees are represented by a union.
Facilities
The Company leases laboratory and office space of approximately 10,412 square feet located at White Birch Towers II, 1311 Pineview Drive, Suite 501, Morgantown, WV 26505. The lease has an initial five-year term beginning on April 1, 2012, (the “Initial Term”). The rent during the Initial Term is equal to $16.10 per square foot per year or $13,969 per month. The Company has the exclusive option to renew the term of the lease for an additional five years following the expiration of the Initial Term, (the “Renewal Option”). The Renewal Option must be exercised at least 120 days prior to the end of the Initial Term. If the Renewal Option is exercised, the rent payable during the Renewal Period shall be equal to $17.75 per square foot per year. We have the option to terminate the lease after reaching the thirty-seventh month of the then existing term upon 90 days advance written notice to the lessor and the payment of an amount equal to two months’ rent.
Legal Proceedings
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business.
There are currently no pending legal proceedings to which the Company or any of its subsidiaries is a party or of which any of their property is the subject that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating results. As far as we are aware, no governmental authority is contemplating any such proceeding.
52
The names, positions and ages of our directors and executive officers as of the date of this prospectus, are as follows:
| Name | Age | Position | Officer/Director Since | ||||
| Stephen Turner | 70 | Chief Executive Officer and Chairman of the Board | 2001 | * | |||
| Greg Kilby | 47 | Vice President and Chief Operating Officer | 2014 | ||||
| Matthew Powell | 40 | Vice President, Research & Development | 2006 | * | |||
| Paul Kane (1) | __ | Interim Chief Financial Officer (1) | 2016 | ||||
| Stanley Hostler | 87 | Vice President, Secretary and Director | 2006 | * | |||
| Steven Antoline | 59 | Director | 2010 | * | |||
| Leonard Harris | 79 | Director | 2003 | * | |||
| Ed Roberson | 70 | Director | 2009 | * | |||
| Scott Segal | 60 | Director | 2008 | * | |||
| Josiah T. Austin | 68 | Director | 2013 | * | |||
| Patrick Gallagher | 51 | Director | 2015 | ||||
| Maged Shenouda | 51 | Director | 2015 |
* Represents the date on which such person was appointed to the referenced office of Protea Biosciences, Inc..
(1) Mr. Kane is currently serving in a consulting capacity to our Company and has assumed the duties of Interim Chief Financial Officer. In such capacity, he is performing all functions of a Chief Financial Officer of a publicly traded company. Subject to completion of this offering, we intend to offer Mr. Kane the position of Chief Financial Officer on terms to be mutually agreed upon. Although Mr. Kane has not committed at this time to accept such position if offered, he has agreed to stay on as a full-time interim Chief Financial Officer for up to 90 days following completion of this offering pending our hiring of another qualified person to fill the position of Chief Financial Officer. Management and the board of directors are committed to having a qualified Chief Financial Officer in place as soon as practicable following completion of this offering.
There are no family relationships among any of our executive officers and directors. None of our directors has, during the past ten years, been involved in any legal proceedings described in subparagraph (f) of Item 401 of Regulation S-K.
Business Experience
The following is a brief summary of the background of each of our directors and executive officers.
Stephen Turner is Chief Executive Officer and Chairman of the Board, positions he has held since founding the Company in July, 2001. From 1999 to 2001 he served as President and CEO of Quorum Sciences, Inc. From 1984 to 1997 he was President and CEO of Oncor, Inc. He founded Bethesda Research Laboratories, Inc. in 1975 and served as its Chairman and CEO from 1975 to 1983, at which time BRL became the molecular biology division of Life Technologies, Inc. Prior to commencing his career in biotechnology, Mr. Turner held the position of Director of Marketing for the Clinical Microbiology Division of Becton, Dickinson & Co. He received his B.A. from Stanford University in 1967. In 1994 he received the Ernst & Young Entrepreneur of the Year Award in Life Sciences for the Washington D.C. region. Mr. Turner was appointed to serve as a director of the Company because he is the founder of Protea and his deep knowledge of our products and market opportunity led the Board to determine that he should serve as a director.
Greg W. Kilby, Ph.D. is Vice President, Operations, Director. Dr. Kilby has over 18 years of experience in applying advanced biological mass spectrometry to areas of research including structural biology, protein characterization, and proteomics to support drug discovery and development and to support the sales of liquid chromatography mass spectrometry (LCMS) analytical equipment into the life sciences, government, academic, and applied markets in the Americas. Prior to joining Protea, Dr. Kilby held a position in Thermo Fisher Scientific of Director, North America Life Sciences Mass Spectrometry Application and Demonstration Laboratories, leading a team responsible for providing product demonstrations and application services to support quota performance and business growth of the Thermo Scientific life sciences mass spectrometry portfolio in North America. Before joining Thermo Fisher Scientific in 2012, Dr. Kilby held several positions in Agilent Technologies, starting as a senior Proteomics and BioPharma applications scientist as well as being responsible for developing and implementing two state of the art Demonstration Centers of Excellences (COE) in Wilmington DE and Santa Clara CA, showcasing Agilent’s entire breadth of analytical technologies portfolio. In 2007, Dr. Kilby moved to a management position within Agilent responsible for managing the two COE facilities and two satellite laboratories across North America and the respective mass spectrometry applications scientist, administrative and logistics staff. Prior to his work at Agilent, Dr. Kilby held, from 1998 to 2004, several senior positions in the Discovery Technologies Department with Pfizer Global Research & Development, culminating in Research Associate, responsible for leading a team of scientists to provide advanced mass spectrometry support for structural biology and therapeutic area projects and as part of Pfizer's global proteomics center of emphasis (COE). Dr. Kilby received his Ph.D. in Analytical Chemistry from the University of Wollongong, Australia in 1996.
Matthew Powell, Ph.D. is Vice President, Research & Development and Chief Scientific Officer since 2006. He received his Ph.D. in Analytical Chemistry from West Virginia University in 2005. Dr. Powell is considered by the Company to be an expert in the field of biological mass spectrometry and is an inventor of several proprietary bioanalytical technologies in development at the Company.
53
Stanley Hostler is Vice President, Secretary and Director. He has been a Director of the Company since January 2006 and Vice President and Secretary since June 2006. Mr. Hostler is an attorney with a career practice in the field of labor and employment law. From 2000 to 2010 he served as Special Assistant to the Governor of the State of West Virginia. From 2002 to 2004 he served as Counsel to the Prim Law Firm. From 2000 to 2010 he served on the West Virginia University Foundation Board of Directors, and from 1995 to 2010 on the Advisory Committee of the WVU School of Medicine. He is a graduate of the West Virginia University School of Law (1965). Mr. Hostler’s legal experience and business contacts and relationships with West Virginia University and the State of West Virginia have been an asset to the Company and led the Board to determine that he should serve as a director of the Company.
Steven Antoline joined the Board of Directors in April 2010. He is a successful owner, developer and manager of coal and natural resource properties and inventor of new equipment for coal mining. From 1996 to 2006, he was President and owner of Superior Highwall Mining, Inc., which was sold to a partnership comprised of Lehman Bros. (60%) and Tennessee Valley Ventures (40%). He received his Bachelor of Arts from West Virginia University. Mr. Antoline was appointed to serve as a director of the Company because of his prior experience in the development and sale of companies, and in working with investment bankers.
Leonard Harris joined the Board of Directors in April 2003. Since 1977, he is the founder and Chief Executive Officer of Southern Computer Consultants, Inc. located in Frederick, Maryland, a company which provides products and services to the United States government and Fortune 500 corporations. Mr. Harris’ extensive experience in technology-based corporate development, which provides support and guidance to the Company’s LAESI instrument platform, led the Board to determine that he should serve as a director of the Company.
Ed Roberson joined the Board of Directors in September 2009. From July 2006 to June 2010 he served as Chairman of the Board of the Methodist Healthcare System. He received his MBA in accounting in 1972 from the University of Georgia. From 2006-2011 he was President of Beacon Financial in Memphis, Tennessee, and from 2006-2007 President of Conwood LLC. He was a director of the Paragon National Bank from 2004 to 2016. He is currently a director of Consol Energy. From 1972 to 1992, Mr. Roberson was employed by KPMG, most recently as partner. Mr. Roberson’s experience, both as a Partner with KPMG and subsequently as a CEO, led the Board to determine that he should serve as a director of the Company.
Scott Segal joined the Board of Directors in February 2008. He is a practicing attorney, specializing in the fields of personal injury, product liability and related matters, and is the President of the Segal Law Firm, Charleston, West Virginia. He received his JD from the West Virginia University School of Law in 1981, and has been a member of the American Bar Association from 1981 to present. Mr. Segal has extensive relationships within the State of West Virginia and is considered by the Company to be an expert in several areas which may have use for the Company’s technology, including forensics and occupational health, which led the Board to determine that he should serve as a director of the Company.
Josiah T. Austin joined the Board of Directors in January 2013. Mr. Austin serves as the managing member of El Coronado Holdings, L.L.C., a privately owned investment holding company which invests in public and private companies. He and his family own and operate agricultural properties in the states of Arizona, Montana, and northern Sonora, Mexico through El Coronado Ranch & Cattle Company, L.L.C. and other entities. Mr. Austin previously served on the Board of Directors of Monterey Bay Bancorp of Watsonville, California, and is a prior board member of New York Bancorp, Inc., and North Fork Bancorporation. He has served as a director of Goodrich Petroleum, Inc. since April 2002 and was named to the Board of Directors of Novogen Limited in September 2010. Mr. Austin also serves as a trustee of the Cuenca Los Ojos Foundation Trust, a non-profit organization working to preserve and restore the biodiversity of the borderland region between the United States and Mexico through land protection, habitat restoration and wildlife reintroduction. Mr. Austin graduated from the University of Denver with a Bachelor of Science in Finance in 1971.
Patrick Gallagher joined the Board of Directors in April 2015. Mr. Gallagher is a CFA and an accomplished Capital Markets executive, advisor and investor with a distinguished record of success in both the public and private markets with a focus on healthcare, agriculture and industrials. He has over two decades of experience on Wall Street and extensive experience in alternative investments, research and marketing. He is a Managing Director and Head of Institutional Sales for Laidlaw & Company (UK) Ltd., a healthcare focused investment bank. Mr. Gallagher co-founded Black Diamond Research, LLC (“BDR”), an independent sell-side research firm specializing in healthcare and industrial investing, financing and operations, serving the institutional investing community at large. As CEO of BDR, Mr. Gallagher oversaw institutional research and sales. Prior, he held various sales positions at Kidder Peabody, PaineWebber, New Vernon Associates and Concept Capital. Mr. Gallagher served as VP of Business Development and Investor Relations as well as a strategic consultant for Kinex Pharmaceuticals, a biotechnology firm focused on next-generation therapies in oncology and immunology. He also serves as an advisor to CHD Biosciences, a novel antimicrobial company. Mr. Gallagher sits on the board of directors for BioSig Technologies, Inc., a medical technology company that is developing a proprietary platform in the electrophysiology space. Mr. Gallagher received his B.S. from the University of Vermont, his M.B.A. from Pennsylvania State University and is a CFA charter holder.
Maged Shenouda joined the Board of Directors in April 2015. Mr. Shenouda has over 25 years of experience in the pharmaceutical and securities industries. Most recently, Mr. Shenouda was the Head of Business Development and Licensing at Retrophin, Inc. Prior to that, he served as the Head of East Coast Operations at Blueprint Life Science Group, a strategic investor relations consultancy. Mr. Shenouda spent the bulk of his career as an equity analyst with senior level positions at Stifel Nicolaus, UBS and JP Morgan, covering a broad range of small and large cap biotechnology companies. Mr. Shenouda started his sell-side career with Citigroup and Bear Stearns where his coverage universe focused on U.S. and European pharmaceutical companies. Before entering Wall Street, Mr. Shenouda was a management consultant with PricewaterhouseCoopers’ Pharmaceutical Consulting practice and also spent time in pharmaceutical sales, having worked as a hospital representative and managed care specialist for Abbott Laboratories’ Pharmaceutical Products Division. Mr. Shenouda earned a B.S. in pharmacy from St. John's University and is a registered pharmacist in New Jersey and California. He also received an M.B.A. from Rutgers University Graduate School of Management.
Paul Kane joined Protea in April 2016 through an arrangement with Resources Global Professionals, where he is currently employed as a consultant. Mr. Kane was previously Vice President and Chief Financial Officer of AMPAC Fine Chemicals, Inc., a California-based active pharmaceutical ingredient manufacturer and wholly-owned subsidiary of American Pacific Corporation, which was acquired by HIG Capital in 2015. Mr. Kane had been Vice President and Chief Financial Officer of Aerojet Fine Chemicals, Inc., which was acquired by American Pacific Corporation in 2005 (and renamed AMPAC Fine Chemicals, Inc.). Mr. Kane also held an executive finance position with GenCorp, Inc. and was controller of OTG Software, Inc. and Best Software, Inc. Mr. Kane also served in a number of positions of increasing responsibility in the accounting function at US Airways. He started his career at KPMG LLP in Washington, DC. Mr. Kane is a certified public accountant and holds a bachelor’s degree in accounting from The Pennsylvania State University.
54
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Exchange Act requires executive officers, directors and persons who own more than 10% of a registered class of our equity securities to file reports of ownership with the SEC. Executive officers, directors and more than 10% shareholders are required by regulations to furnish us with copies of all Section 16(a) reports they file.
Based solely on the Company’s review of the copies of the forms received by it during the fiscal year ended December 31, 2015 and written representations that no other reports were required, the Company believes that the following person(s) who, at any time during such fiscal year, was a director, officer or beneficial owner of more than 10% of the Company’s common stock failed to comply with all Section 16(a) filing requirements during such fiscal years:
| Name | Number of Late Reports |
Number of Transactions not Reported on a Timely Basis |
Failure to File a Required Form | |||
| Steve Turner | None | None | None | |||
| Greg Kilby | 2 | 2 | None | |||
| Matthew Powell | None | None | None | |||
| Stanley Hostler | 2 | 3 | None | |||
| Scott Segal | 2 | 4 | None | |||
| Leonard Harris | 1 | 2 | None | |||
| Ed Roberson | 2 | 4 | None | |||
| Summit Resources, Inc. | 5 | 6 | None | |||
| Josiah T. Austin | 6 | 8 | None | |||
| Maged Shenouda | 2 | 3 | None | |||
| Patrick Gallagher | 2 | 3 | None |
Code of Ethics
On June 22, 2016, we amended our formal Code of Business Conducts and Ethics (the “Code of Ethics”) for our employees, officers and directors, which was initially adopted on December 20, 2007 (a form of which has been filed as an exhibit to the Company’s Form 10-KSB filed with the SEC on March 7, 2008), to reflect, among others things, the Company’s current information. The current Code of Ethics is available free of charge on our website at http://proteabio.com and is filed as an exhibit to this report. The Code of Ethics complies with the SEC regulations and is designed to deter wrongdoing and to promote ethical conduct and full, fair, accurate, timely and understandable reports that the Company files or submits to the SEC and others. Requests for copies of the Code of Ethics should be sent in writing to Protea Biosciences Group, Inc., Attention: Chief Executive Officer, 1311 Pineview Drive, Suite 501, Morgantown, West Virginia 26505.
Corporate Governance
Our Board of Directors adopted board charters and polices and established Audit and Compensation Committees in accordance with the rules of the SEC and Nasdaq Stock Market. Our Board of Directors also established procedures and adopted resolutions that address the nomination process of directors and independent director oversight in accordance with the rules of the Nasdaq Stock Market, which requires that director nominees be selected or recommended for the selection by a majority of the "Independent Directors,” as defined under the rules of the Nasdaq Capital Market, in a vote in which only independent directors participate.
Audit Committee
Ed Roberson, Leonard Harris and Maged Shenouda are each members of our Audit Committee. The Audit Committee’s function is to oversee our accounting and financial reporting processes, internal systems of control, independent auditor relationships and the audit of our financial statements. Ed Roberson is currently serving as audit committee chairman and financial expert. All members of the Audit Committee are independent under the applicable definition contained in the rules of the Nasdaq Stock Market and Rule 10A-3(b)(1) under the Exchange Act. The Audit Committee is governed by a written charter approved by the Board of Directors.
A current copy of the Audit Committee's charter is available on the Company's website at http://proteabio.com.
Compensation Committee
Steve Antoline, Leonard Harris, Josiah Austin and Maged Shenouda are each members of the Compensation Committee, with the latter being Chairman. The purpose of the Compensation Committee is to aid the Board of Directors in meeting its responsibilities with regard to oversight and determination of executive compensation. Among other things, the Compensation Committee reviews, recommends and approves salaries and other compensation of the Company’s executive officers, and will administer the Company’s equity incentive plans (including reviewing, recommending and approving stock option and other equity incentive grants to executive officers).
A current copy of the Compensation Committee's charter is available on the Company's website at http://proteabio.com.
55
Family Relationships
There are no family relationships among our directors and executive officers.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers has, during the past ten years, been involved in any legal proceedings described in subparagraph (f) of Item 401 of Regulation S-K.
Insider Participation in Meetings of Directors
Our determination of the independence of directors is made using the definition of “independent” contained in Rule 5605(a)(2) of The Nasdaq Stock Market. On the basis of information solicited from each director, the Board has determined that each of our directors with the exception of Mr. Turner and Mr. Hostler is independent within the meaning of such rule.
Other than Mr. Turner, our Chief Executive Officer and President and Mr. Hostler, our Vice-President, all of our remaining directors are independent directors. All matters to be acted upon where potential conflicts exist between our executive officers and the Company (for example, the terms of employment agreements, option grants, bonus awards, etc.) are discussed and approved by the non-interested directors. During the years ended December 31, 2015 and 2014, Mr. Turner did not participate in deliberations of the Company's Board of Directors concerning his compensation.
Executive Compensation
The following table illustrates the compensation paid by Protea to its Chief Executive Officer, its three most highly compensated executive officers other than the Chief Executive Officer who were serving as executive officers at the end of the last completed fiscal year and who earned in excess of $100,000, and up to one additional individual for whom disclosure would have been provided but for the fact that the individual was not serving as an executive officer of the Company at the end of the last completed fiscal year. We refer to these individuals as the “Named Executive Officers”. The disclosure is provided for the years ended December 31, 2015 and 2014.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Other Benefits ($) (1) | Option Award ($) (2) | Total ($) | ||||||||||||||||||
| Stephen Turner, Chief Executive Officer, President | 2015 | 240,000 | - | 19,336 | - | 259,336 | ||||||||||||||||||
| 2014 | 240,000 | - | 18,819 | 130,600 | 389,419 | |||||||||||||||||||
| Matthew Powell, Vice President, R&D, CSO | 2015 | 180,000 | 6,009 | 18,049 | 4,412 | 208,469 | ||||||||||||||||||
| 2014 | 151,357 | 2,500 | 17,901 | 17,647 | 189,405 | |||||||||||||||||||
| Gregory Kilby, Vice President, Operations | 2015 | 210,000 | - | 9,676 | 13,151 | 232,827 | ||||||||||||||||||
| 2014 | 210,000 | - | 18,313 | 27,961 | 256,274 | |||||||||||||||||||
| (1) | Other benefits include living allowances, insurance benefits paid by company and cell phone reimbursement. |
| (2) | The amount included in the “Option Awards” column does not reflect compensation actually received by the Named Executive Officer but represents the compensation cost that we recognized in each year presented, determined in accordance with FASB ASC 718. The valuation assumptions used in determining such amounts are described in Note 8 of our financial statements for the fiscal year ended December 31, 2015. |
We do not currently have employment agreements with our Named Executive Officers, although we may enter into such agreements in the future.
56
The following table provides information about equity awards granted to our Named Executive Officers that were outstanding on December 31, 2015.
| Name | Number of
securities underlying unexercised options (#) exercisable | Number of
securities underlying unexercised options (#) unexercisable | Equity
incentive plan awards: Number of securities underlying unexercised unearned options (#) | Options
Exercise Price ($) | Options Expiration Date | Number
of shares or units of stock that have not vested (#) | Market
value of shares of units of stock that have not vested ($) | Equity
incentive plan awards: Number of unearned shares, units or other rights that have not vested (#) | Equity
incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested ($) | |||||||||||||||||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | |||||||||||||||||||||||||||||||
| Stephen Turner | 4,000 | - | - | $ | 20.00 | 6/07/2016 | - | - | - | - | ||||||||||||||||||||||||||||||
| 10,000 | - | - | $ | 37.50 | 4/23/2020 | - | - | - | - | |||||||||||||||||||||||||||||||
| - | 28,000 | - | $ | 13.75 | 2/27/2024 | - | - | - | - | |||||||||||||||||||||||||||||||
| Matthew Powell | 4,000 | - | - | $ | 31.25 | 1/19/2017 | - | - | - | - | ||||||||||||||||||||||||||||||
| 1,600 | - | - | $ | 37.50 | 12/31/2019 | - | - | - | - | |||||||||||||||||||||||||||||||
| 1,200 | (1 | ) | 2,000 | - | $ | 13.75 | 4/1/2024 | - | - | - | - | |||||||||||||||||||||||||||||
| Gregory Kilby | 2,625 | (1 | ) | 3,375 | - | $ | 13.75 | 2/3/2024 | - | - | - | - | ||||||||||||||||||||||||||||
| 750 | (1 | ) | 5,250 | $ | 13.25 | 4/1/2025 | - | - | - | - | ||||||||||||||||||||||||||||||
| - | (1 | ) | 18,000 | $ | 6.25 | 12/18/2025 | - | - | - | - | ||||||||||||||||||||||||||||||
| - | (2 | ) | 22,000 | $ | 6.25 | 12/18/2025 | - | - | - | - | ||||||||||||||||||||||||||||||
| (1) | Options vest in 25% increments over a four-year vesting schedule. Grant dates are ten years prior to expiration date. |
| (2) | Performance award will vest upon realization of certain measures as of 12/31/2016. |
Stephen Turner Employment Agreement
On April 1, 2015, the Company entered into an employment agreement (the “Employment Agreement”) with Stephen Turner as Chief Executive Officer and Chairman of the Board of Directors (the “Board”). The Employment Agreement provides for (i) a term of employment of three years from April 1, 2015, unless terminated by the Company or Mr. Turner, (ii) a base salary of $240,000, (iii) the eligibility of Mr. Turner to receive an annual bonus of at least 20% of his annual base salary (the “Performance Bonus”) provided that he has achieved the metrics established for such calendar year by the Compensation Committee of the Board, (iv) the eligibility of Mr. Turner to receive an additional discretionary bonus as determined by the Board, (v) the eligibility of Mr. Turner to receive a financing bonus in the amount of one $100,000 (the “Financing Bonus”) within thirty (30) days of the completion of the Company’s next round of financing, (vi) the eligibility of Mr. Turner to participate in the Company’s family health insurance benefits and to be covered under the Company’s key man and other life insurance policies and (vii) Mr. Turner’s entitlement to reimbursement for all reasonable out-of-pocket business, entertainment and travel expenses incurred in connection with the performance of his duties under the Employment Agreement. In the discretion of the Board, Mr. Turner may also be eligible for additional Financing Bonuses to the extent the Company raises additional funds. The Company will continue to provide Mr. Turner with coverage under, or equivalent to, its professional liability, directors and officers, and other similar policies for a period of three years following termination of his employment for any reason.
The Employment Agreement may be terminated by either the Company or Mr. Turner at any time and for any reason upon a minimum of 30 days’ advice written notice. In the event that Mr. Turner is terminated by the Company for Cause (as defined in the Employment Agreement), Mr. Turner would be entitled to (x) any accrued but unpaid Base Salary through the date of termination, (y) any unused vacation time, and (z) reimbursement for any unreimbursed business expenses properly incurred by Mr. Turner. In the event that Mr. Turner is terminated by the Company without Cause, Mr. Turner would be entitled to the amounts specified in (x), (y) and (z) above as well as any unpaid Performance Bonus and unpaid Financing Bonus (all such amounts, the “Accrued Amounts”), and a lump sum payment of 18 months’ of his base salary; the Company will pay the premiums for his family health insurance coverage for a period of 18 months; and all stock, restricted stock, options, stock appreciation rights, performance compensation, and any other equity or phantom equity awarded to him that is unvested will vest as of the termination date. In the event the employment term is terminated due to the acquisition of the Company by another company, Mr. Turner will be entitled to receive the Accrued Amounts, and he will be entitled to receive continued base salary for the remainder of the original employment term. In the event of Mr. Turner’s death or disability, the Company will pay him (or his estate and/or beneficiaries, as the case may be) the Accrued Amounts and a lump sum payment of 18 months’ of his base salary. In the event Mr. Turner’s employment terminates due to disability, the Company will also pay the premiums for his family health insurance coverage for a period of 18 months.
The Company agrees to indemnify Mr. Turner for any liabilities, damages, costs, and expenses (including attorneys’ fees) relating, directly or indirectly, to any claims, charges or proceedings of any nature that arise out of or concern his employment with or service to the Company and any of its affiliates, subsidiaries, or related entities, including his employment with or services to the Company that preceded the execution of the Employment Agreement.
57
The Employment Agreement is filed as an Exhibit to this Registration Statement on Form S-1. The foregoing summary of the terms of the Employment Agreement is subject to, and qualified in its entirety by, the full text of such document, which is incorporated herein by reference. The Company does not have any employment agreements with any of its other executive officers.
Board Compensation
During the fiscal year ended December 31, 2015, each non-employee member of the Board of Directors was entitled to receive cash compensation for his services. In lieu of such cash compensation, each non-employee Director agreed to accept Common Stock at $0.25 per share. The following table provides information about such shares of the Company’s Common Stock issued to the Directors.
| Name | Fees earned and paid in Common Stock (1) | Option Award | Other Compensation | Total | ||||||||||||
| Stanley Hostler | $ | 25,000 | - | - | $ | 25,000 | ||||||||||
| Steven Antoline | $ | 25,000 | - | - | $ | 25,000 | ||||||||||
| Leo Harris | $ | 25,000 | - | - | $ | 25,000 | ||||||||||
| Ed Roberson | $ | 30,000 | - | - | $ | 30,000 | ||||||||||
| Scott Segal | $ | 20,000 | - | - | $ | 20,000 | ||||||||||
| Josiah Austin | $ | 25,000 | - | - | $ | 25,000 | ||||||||||
| Maged Shenouda | $ | 25,000 | - | - | $ | 25,000 | ||||||||||
| Patrick Gallagher | $ | 20,000 | - | - | $ | 20,000 | ||||||||||
(1) Board compensation paid in Common Stock at $0.25 per share
(2) The amount included in the “Option Awards” column does not reflect compensation actually received by the Named Executive Officer but represents the compensation cost that we recognized in each year presented, determined in accordance with FASB ASC 718. The valuation assumptions used in determining such amounts are described in Note 8 of our financial statements for the fiscal year ended December 31, 2015.
The following table provides information about equity awards granted to members of our Board of Directors that were outstanding on December 31, 2015.
| Number of Securities Underlying Unexercised Options | Options | |||||||||||||
| Not | Exercise | Options Expiration | ||||||||||||
| Name | Exercisable | Exercisable | Price ($) | Date | ||||||||||
| Stanley Hostler | 4,000 | - | $ | 20.00 | 6/07/2016 | |||||||||
| 4,000 | - | $ | 37.50 | 9/17/2020 | ||||||||||
| 10,000 | - | $ | 13.75 | 3/22/2023 | ||||||||||
| 640 | - | $ | 37.50 | 12/31/2017 | ||||||||||
| 1,920 | - | $ | 37.50 | 12/31/2018 | ||||||||||
| 1,920 | - | $ | 37.50 | 12/31/2019 | ||||||||||
| 1,920 | - | $ | 37.50 | 12/31/2020 | ||||||||||
| 1,440 | - | $ | 37.50 | 12/31/2021 | ||||||||||
| 480 | - | $ | 37.50 | 12/31/2021 | ||||||||||
| 1,760 | - | $ | 50.00 | 11/30/2022 | ||||||||||
| 160 | - | $ | 12.50 | 12/31/2022 | ||||||||||
| 1,920 | - | $ | 13.75 | 12/31/2023 | ||||||||||
| 480 | - | $ | 13.75 | 3/31/2024 | ||||||||||
| - | (2) | 2,667 | $ | 6.25 | 12/1/2025 | |||||||||
| Steven Antoline | 4,000 | - | $ | 37.50 | 4/23/2020 | |||||||||
| - | (2) | 2,667 | $ | 6.25 | 12/1/2025 | |||||||||
| Leo Harris | 4,000 | - | $ | 20.00 | 6/07/2016 | |||||||||
| 4,000 | - | $ | 37.50 | 9/17/2020 | ||||||||||
| 10,000 | - | $ | 13.75 | 3/22/2023 | ||||||||||
| - | (2) | 2,667 | $ | 6.25 | 12/1/2025 | |||||||||
| Ed Roberson | 4,000 | - | $ | 37.50 | 4/23/2020 | |||||||||
| 2,310 | (1) | 1,690 | $ | 13.75 | 1/1/2024 | |||||||||
| - | (2) | 2,667 | $ | 6.25 | 12/1/2025 | |||||||||
| Scott Segal | 4,000 | - | $ | 37.50 | 5/30/2018 | |||||||||
| 10,000 | - | $ | 13.75 | 3/22/2023 | ||||||||||
| - | (2) | 2,667 | $ | 6.25 | 12/1/2025 | |||||||||
| Josiah T. Austin | 5,783 | (1) | 217 | $ | 13.75 | 1/28/2023 | ||||||||
| - | (2) | 2,667 | $ | 6.25 | 12/1/2025 | |||||||||
| Maged Shenouda | - | (2) | 2,667 | $ | 6.25 | 12/1/2025 | ||||||||
| Patrick Gallagher | - | (2) | 2,667 | $ | 6.25 | 12/1/2025 | ||||||||
(1) Options vest in 33% increments over a three-year vesting schedule. Grant dates are ten years prior to expiration date.
(2) Options vest incrementally over a one-year vesting schedule. Grant dates are ten years prior to expiration date.
58
The following table sets forth certain information, as of June 21, 2016, with respect to the holdings of (1) each person who is the beneficial owner of more than 5% of our Common Stock, (2) each of our directors, (3) each executive officer, and (4) all of our current directors and executive officers as a group.
All shares, Common Stock, and per share data gives pro forma effect to the reverse stock split of our outstanding capital stock that was consummated on __________ ____, 2016.
Beneficial ownership before offering of the common stock is determined in accordance with the rules of the Securities and Exchange Commission and includes any shares of common stock over which a person exercises sole or shared voting or investment power, or of which a person has a right to acquire ownership at any time within 60 days of the date of this prospectus. Except as otherwise indicated, we believe that the persons named in this table have sole voting and investment power with respect to all shares of common stock held by them. Applicable percentage ownership in the following table is based on 5,348,820 shares of common stock plus, for each individual, any securities that individual has the right to acquire within 60 days of June 21, 2016.
To the best of our knowledge, except as otherwise indicated, each of the persons named in the table has sole voting and investment power with respect to the shares of our common stock beneficially owned by such person, except to the extent such power may be shared with a spouse. To our knowledge, none of the shares listed below are held under a voting trust or similar agreement, except as noted. To our knowledge, there is no arrangement, including any pledge by any person of securities of the Company, the operation of which may at a subsequent date result in a change in control of the Company.
Title of Class: Common Stock – Before Offering
| Name and Address of Beneficial Owner | Title | Beneficially Owned* | Percent of Class** | |||||||
| Officers and Directors | ||||||||||
| Stephen Turner | Chief Executive Officer and Chairman of the Board | 106,861 | (1) | 1.99 | % | |||||
| Stanley Hostler | Secretary and Director | 370,876 | (2) | 6.78 | % | |||||
| Greg Kilby | Vice President and Chief Operating Officer | 5,250 | (3) | - | * | |||||
| Matthew Powell | Vice President, Research & Development | 7,000 | (4) | - | * | |||||
| Steve Antoline | Director | 713,307 | (5) | 12.42 | % | |||||
| Leonard Harris | Director | 165,465 | (6) | 3.07 | %* | |||||
| Ed Roberson | Director | 25,815 | (7) | - | * | |||||
| Scott Segal | Director | 134,326 | (8) | 2.50 | %* | |||||
| Josiah T. Austin | Director | 385,241 | (9) | 6.96 | %* | |||||
| Patrick Gallagher | Director | 3,867 | (10) | - | * | |||||
| Maged Shenouda | Director | 4,667 | (11) | - | * | |||||
| Officers and Directors as a Group (total of 13 persons) | 1,922,675 | 31.06 | % | |||||||
| 5% Stockholders | ||||||||||
| El Coronado Holdings, LLC | 580,406 | (12) | 10.55 | % | ||||||
| Summit Resources, Inc. | - | 617,278 | (13) | 10.82 | % | |||||
| Andreas Wawrla | 724,222 | (14) | 12.83 | % | ||||||
| * | Represents ownership under 1%. |
| (1) | Includes 86,135 shares of Common Stock, 6,726 shares of Common Stock to be acquired upon the exercise of warrants and 14,000 shares of Common Stock to be acquired upon the exercise of stock options. |
| (2) | Includes 142,176 shares of common stock, 55,608 shares of common stock to be acquired upon the exercise of warrants and 31,307 shares of common stock to be acquired upon the exercise of stock options. Also includes 95,267 shares of common stock held by Mr. Hostler’s wife, Virginia Child and 36,137 shares of common stock to be acquired upon the exercise of warrants held by Mr. Hostler’s wife, Virginia Child. Also includes 5,932 shares of common stock and 4,449 shares of common stock to be acquired upon the exercise of warrants jointly held by Stanley Hostler and Virginia Child. |
| (3) | Includes 5,250 shares of common stock to be acquired upon the exercise of stock options. |
59
| (4) | Includes 7,000 shares of common stock to be acquired upon the exercise of stock options. |
| (5) | Includes 60,562 shares of common stock and 30,800 shares of common stock to be acquired upon the exercise of warrants owned of record by the Steve A. Antoline 2006 Irrevocable Trust (the “Antoline Trust”). Also includes 252,777 shares of common stock and 364,501 shares of common stock to be acquired upon the exercise of warrants owned of record by Summit Resources, Inc. As the trustee of the Antoline Trust and president of Summit Resources, Inc., Mr. Antoline has voting and dispositive control over any securities owned of record by the Antoline Trust and Summit Resources, Inc. Therefore, he may be deemed to beneficially own the shares of common stock and the shares of common stock to be acquired upon the exercise of warrants held of record by the Antoline Trust and Summit Resources, Inc. Includes 4,667 shares of common stock to be acquired upon the exercise of stock options. |
| (6) | Includes 118,269 shares of common stock, 28,529 shares of common stock to be acquired upon the exercise of warrants and 18,667 shares of common stock to be acquired upon the exercise of stock options. |
| (7) | Includes 11,504 shares of common stock, 2,290 shares of common stock to be acquired upon the exercise of warrants and 7,307 shares of common stock to be acquired upon the exercise of stock options. Also includes 2,714 shares of common stock and warrants to purchase 2,000 shares of common stock owned of record by Morgan Keegan & Co, Inc., an IRA account of Ed Roberson. |
| (8) | Includes 98,647 shares of common stock, 21,012 shares of common stock to be acquired upon the exercise of warrants and 14,667 shares of common stock to be acquired upon the exercise of stock options. |
| (9) | Includes 193,656 shares of common stock, 6,667 shares of common stock to be acquired upon the exercise of stock options, 160,676 shares to be acquired upon the exercise of warrants, and 24,242 shares of common stock upon the conversion of a convertible debenture. |
| (10) | Includes 3,200 shares of common stock and 667 shares of common stock to be acquired upon the exercise of stock options. |
| (11) | Includes 4,000 shares of common stock and 667 shares of common stock to be acquired upon the exercise of stock options. |
| (12) | Includes 419,730 shares of common stock and 160,676 shares of common stock to be acquired upon the exercise of warrants. |
| (13) | Includes 252,777 shares of common stock and 364,501 shares of common stock to be acquired upon the exercise of warrants. As president of Summit Resources, Inc., Mr. Antoline has voting and dispositive control over any securities owned of record by Summit Resources, Inc. Therefore, he may be deemed to beneficially own the shares of common stock and the shares of common stock to be acquired upon the exercise of warrants held of record by Summit Resources, Inc. |
| (14) | Includes 424,222 shares of common stock and 300,000 shares of common stock to be acquired upon the exercise of warrants. |
Beneficial ownership post offering of the Common Stock is determined in accordance with the rules of the Securities and Exchange Commission and includes any shares of Common Stock over which a person exercises sole or shared voting or investment power, or of which a person has a right to acquire ownership at any time within 60 days of March 31, 2016. Except as otherwise indicated, we believe that the persons named in this table have sole voting and investment power with respect to all shares of Common Stock held by them. Applicable percentage ownership in the following table is based on 5,348,820 shares of common stock outstanding as of March 31, 2016 plus, for each individual, any securities that individual has the right to acquire within 60 days of March 31, 2016.
All shares, Common Stock, and per share data gives pro forma effect to the 1-for-25 reverse stock split of our outstanding capital stock to be consummated prior to the effective date of the registration statement of which this prospectus is a part.
60
Title of Class: Common Stock – Post Offering
| Name and Address of Beneficial Owner | Title | Beneficially Owned* | Percent of Class** | |||||||
| Officers and Directors | ||||||||||
| Stephen Turner | Chief Executive Officer and Chairman of the Board | 106,861 | (1) | 1.3 | % | |||||
| Stanley Hostler | Secretary and Director | 370,876 | (2) | 4.4 | % | |||||
| Greg Kilby | Vice President and Chief Operating Officer | 5,250 | (3) | - | * | |||||
| Matthew Powell | Vice President, Research & Development | 7,000 | (4) | - | * | |||||
| Steve Antoline | Director | 713,307 | (5) | 8.4 | % | |||||
| Leonard Harris | Director | 165,465 | (6) | 2 | % | |||||
| Ed Roberson | Director | 25,815 | (7) | - | * | |||||
| Scott Segal | Director | 134,326 | (8) | 1.6 | % | |||||
| Josiah T. Austin | Director | 385,241 | (9) | 4.6 | % | |||||
| Patrick Gallagher | Director | 3,867 | (10) | - | * | |||||
| Maged Shenouda | Director | 4,667 | (11) | - | * | |||||
Officers and Directors as a Group (total of 13 persons) | 1,922,675 | 23 | % | |||||||
| 5% Stockholders | ||||||||||
| El Coronado Holdings, LLC | 580,406 | (12) | 6.9 | % | ||||||
| Summit Resources, Inc. | - | 617,278 | (13) | 7.3 | % | |||||
| Andreas Wawrla | 724,222 | (14) | 8.6 | % | ||||||
* Represents ownership under 1%.
| (1) | Includes 86,135 shares of common stock, 6,726 shares of common stock to be acquired upon the exercise of warrants and 14,000 shares of common stock to be acquired upon the exercise of stock options. |
| (2) | Includes 142,176 shares of common stock, 55,608 shares of common stock to be acquired upon the exercise of warrants and 31,307 shares of common stock to be acquired upon the exercise of stock options. Also includes 95,267 shares of common stock held by Mr. Hostler’s wife, Virginia Child and 36,137 shares of common stock to be acquired upon the exercise of warrants held by Mr. Hostler’s wife, Virginia Child. Also includes 5,932 shares of common stock and 4,449 shares of common stock to be acquired upon the exercise of warrants jointly held by Stanley Hostler and Virginia Child. |
| (3) | Includes 5,250 shares of common stock to be acquired upon the exercise of stock options. |
| (4) | Includes 7,000 shares of common stock to be acquired upon the exercise of stock options. |
| (5) | Includes 60,562 shares of common stock and 30,800 shares of common stock to be acquired upon the exercise of warrants owned of record by the Steve A. Antoline 2006 Irrevocable Trust (the “Antoline Trust”). Also includes 252,777 shares of common stock and 364,501 shares of common stock to be acquired upon the exercise of warrants owned of record by Summit Resources, Inc. As the trustee of the Antoline Trust and president of Summit Resources, Inc., Mr. Antoline has voting and dispositive control over any securities owned of record by the Antoline Trust and Summit Resources, Inc. Therefore, he may be deemed to beneficially own the shares of common stock and the shares of common stock to be acquired upon the exercise of warrants held of record by the Antoline Trust and Summit Resources, Inc. Includes 4,667 shares of common stock to be acquired upon the exercise of stock options. |
| (6) | Includes 118,269 shares of common stock, 28,529 shares of common stock to be acquired upon the exercise of warrants and 18,667 shares of common stock to be acquired upon the exercise of stock options. |
| (7) | Includes 11,504 shares of common stock, 2,290 shares of common stock to be acquired upon the exercise of warrants and 7,307 shares of common stock to be acquired upon the exercise of stock options. Also includes 2,714 shares of common stock and warrants to purchase 2,000 shares of common stock owned of record by Morgan Keegan & Co, Inc., an IRA account of Ed Roberson. |
| (8) | Includes 98,647 shares of common stock, 21,012 shares of common stock to be acquired upon the exercise of warrants and 14,667 shares of common stock to be acquired upon the exercise of stock options. |
| (9) | Includes 193,656 shares of common stock, 6,667 shares of common stock to be acquired upon the exercise of stock options, 160,676 shares to be acquired upon the exercise of warrants, and 24,242 shares of common stock upon the conversion of a convertible debenture |
| (10) | Includes 3,200 shares of common stock and 667 shares of common stock to be acquired upon the exercise of stock options. |
| (11) | Includes 4,000 shares of common stock and 667 shares of common stock to be acquired upon the exercise of stock options. |
| (12) | Includes 419,730 shares of common stock and 160,676 shares of common stock to be acquired upon the exercise of warrants. |
61
| (13) | Includes 252,777 shares of common stock and 364,501 shares of common stock to be acquired upon the exercise of warrants. As president of Summit Resources, Inc., Mr. Antoline has voting and dispositive control over any securities owned of record by Summit Resources, Inc. Therefore, he may be deemed to beneficially own the shares of common stock and the shares of common stock to be acquired upon the exercise of warrants held of record by Summit Resources, Inc. |
| (14) | Includes 424,222 shares of common stock and 300,000 shares of common stock to be acquired upon the exercise of warrants. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Described below are transactions or series of transactions that occurred from January 1, 2015 through the date of this prospectus (the “Period Reported”) between us and our executive officers, directors or the beneficial owners of 5% or more of our Common Stock, and certain persons affiliated with or related to these persons, including family members, in which they had or will have a direct or indirect material interest in an amount that exceeds the lesser of $120,000 or 1% of the average of our total assets as of year-end for the last two completed fiscal years, other than compensation arrangements that are otherwise described under “Executive Compensation,” above, which information is incorporated herein by reference. See “Principal Stockholders,” above for information relating to outstanding options and warrants to purchase our Common Stock held by such persons, which information is incorporated herein by reference.
During 2014, the Company received advances equal to an aggregate of $265,000 from Stanley Hostler, a director of the Company. The Company has repaid $125,000 to Stanley Hostler. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this report.
On May 22, 2014 and October 10, 2014, the Company received advances equal to $30,000 from Stephen and Nancy Turner, our Chief Executive Officer and director of the Company and his spouse. The Company has repaid $20,000 to Mr. Turner. No terms of repayment have been specified on the remaining aforementioned advance as of the date of this report.
During 2014, the Company received advances equal to an aggregate of $1,415,000 from Summit. In exchange for a portion of the advances received, the Company entered into Note and Warrant Purchase Agreements and issued (a) one-year promissory notes bearing simple interest at the rate of 10% per annum to Summit in an aggregate principal amount of $1,415,000 and (b) five-year warrants to purchase up to 1,415,000 shares of Common Stock at an exercise price of $0.80 per share. On June 3, 2014, the Board approved an increase in the total offering amount of the promissory notes issuable to $1,500,000 from $900,000. The fair value of these warrants was estimated to be $101,177, which was recorded as a discount to the promissory note, and will be accreted based on the repayment of the obligation. The Company repaid $250,000 as of December 31, 2014. Accretion expense of $10,950 was recognized during the year ended December 31, 2014. The outstanding balance, net of discount, was $1,074,773 as of December 31, 2014. On March 20, 2015, the Company converted the $165,000 of outstanding principal and unpaid accrued interest of $105,078, and issued Summit 1,080,312 shares of Common Stock at a conversion price of $0.25 per share and as an inducement to convert, warrants in aggregate of 540,156 to purchase shares of Common Stock at an exercise price of $0.50 per share. The Company recognized accretion expense of $7,227 during the three months ended March 31, 2015. In addition, the Company recognized debt conversion inducement cost related to the fair value of the warrants issued to Summit of $31,455 during the three months ended March 31, 2015. As of December 31, 2015, the outstanding balance was $1,000,000 or $917,000, net of discount.
On March 20, 2015, the Company issued Common Stock in connection with the conversion of $550,262 outstanding principal and accrued unpaid interest of certain convertible promissory notes (the "Notes") to Summit Resources, Inc. The Notes were converted into 88,041 shares of Common Stock determined by dividing the Notes by $6.25 per share. In connection with the conversion of the Notes, the Summit Resources, Inc. also received a 5 year warrant to purchase 44,020 shares of the Company’s Common Stock, exercisable at $12.50 per share.
On July 1, 2015, the Company received aggregate gross proceeds of $200,000 from one director and issued a 10% Convertible Promissory Note due on December 31, 2015. The note is convertible into Common Stock at a conversion price of $8.25 per share. The Company also issued 3,636 shares of Common Stock as commitment fee to the director with a price per share of $ 8,25.
In 2015, the Company received advances equal to an aggregate of $193,000 from Leo Harris a director of the Company. No terms of repayment have been specified on the aforementioned advances as of the date of this report.
In 2015, the Company received advances equal to an aggregate of $725,000 from Stanley Hostler a director of the Company. The Company repaid $25,000 to Stanley Hostler. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this report.
On January 30, 2015, the Company received advances equal to an aggregate of $57,500 from Steve and Nancy Turner, our Chief Executive Officer and director of the Company and his spouse. No terms of repayment have been specified on the aforementioned advances as of the date of this report.
62
In 2015, the Company received advances equal to an aggregate of $255,000 from Steve Antoline, a director of the Company. The Company repaid $90,000 to Steve Antoline. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this report.
In 2015, the Company received advances equal to an aggregate of $72,000 from Josiah Austin, a director of the Company. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this report.
In 2015, the Company received an advance equal to $25,000 from Scott Segal, a director of the Company. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this report.
In 2015, the Company received advances equal to an aggregate of $30,000 from Ed Roberson, a director of the Company. No terms of repayment have been specified on the remaining aforementioned advances as of the date of this report.
Patrick Gallagher, one of our Directors, is a Managing Director and Head of Institutional Sales of Laidlaw & Co., an affiliate of the entity that acted as placement agent for the Company’s winter 2013, winter 2014 and fall 2015 private placements, for which the placement agent received cash fees aggregating $1,689,078 and warrants to purchase an aggregate of 285,919 shares of Common Stock at exercise prices between $6.25 and $12.50, and was reimbursed for approximately $187,500 expenses. The placement agent and its affiliates beneficially own shares of our Common Stock. See “Security Ownership of Certain Beneficial Owners and Management” and “Description of Securities—Warrants.” The placement agent and its affiliates may provide investment banking and other services to us in the future.
For the four months ended April 30, 2016, the Company’s Board of Directors authorized the conversion of an aggregate of $33,333 of accrued interest on promissory notes issued by the Company to Summit Resources, Inc. (“Summit”), an affiliate of Steven Antoline, who is a director of the Company, and $8,060 of accounts payable by the Company to Summit, into 6,623 shares of Common Stock at the rate of $6.25.
For the four months ended April 30, 2016, the Company issued warrants to purchase 18,000 shares of Common Stock to Summit as consideration for advances. The warrants are exercisable at an exercise price of $10.00 per share for a five year term.
For the four months ended April 30, 2016, the Company received advances equal to an aggregate of $174,000 from certain current directors and related parties. Repayment of $25,000 has been made on the aforementioned advances and repayment of $47,000 on prior advances. No terms of repayment have been specified on the balance of the aforementioned advances.
Our $3,000,000 line of credit with United Bank is unconditionally guaranteed by three of our directors and the estate of a former board member, to the maximum extent of $750,000 by each of such individuals. In February 2016, and in consideration for their agreement to extend their personal guarantees of our $3,000,000 line of credit, we agreed to issue, on a pro-rata basis, to such directors and the estate of our former board member, three year warrants to purchase 120,000 shares of our common stock at an exercise price $10.00 per share.
As of the date of this prospectus, we are indebted to certain members of our board of directors and the estate of a former director for loans and advances made to our Company over the past five years in the aggregate amount of $2,676,500. We anticipate paying (on a pro rata basis) out of the net proceeds of this offering $669,125, or 25% of the total $2,676,500 amount owed, commencing 30 days after our common stock begins trading on the Nasdaq Capital Market. See “Use of Proceeds”. The 75% balance of such indebtedness to our directors and their affiliates (approximately $2,007,375) would be evidenced by 6% notes due as to principal and accrued interest on a date which shall be one year from the date of this prospectus. There can be no assurance that we will be successful in completing the contemplated agreements or the terms and conditions of any such arrangements. Any such agreements may be subject to completion of this offering.
The following description of our securities is only a summary, and is qualified in its entirety by reference to the actual terms and provisions of the capital stock contained in our articles of incorporation and our bylaws.
Reverse Stock Split
In an attempt to implement our strategy to list our common stock of the Nasdaq Capital Market, in December 2015, our stockholders approved and authorized our board of directors to consummate a reverse split of our issued and outstanding common stock at a ratio of not less than 1-for-15 and not more than 1-for-25, with the exact reverse stock split ratio to be determined by the board in its sole discretion based upon the market price of the Company’s common stock on the date of such determination and with such reverse stock split to be effective at such time and date, if at all, as determined by the board in its sole discretion. Prior to the effective date of this offering, the board of directors intends to effect a 1-for-25 reverse stock split.
As a result of our one-for-25 reverse stock split, (i) all outstanding 133,720,519 shares of our common stock, prior to the consummation of the reverse stock split, will be reduced to 5,348,820 shares and (ii) all shares of our common stock issuable upon exercise of outstanding 80,807,644 warrants, prior to the consummation of the reverse stock split, will be reduced to 3,232,305 shares with a corresponding increase in the exercise prices of such warrants to prices ranging from $6.25 to $56.25 per share. Our common stock is held of record by approximately 526 stockholders.
63
Our authorized capital stock consists of 260,000,000 shares, of which 250,000,000 are designated common stock, par value $0.0001 per share and 10,000,000 are designated preferred stock, par value $0.0001 per share, all of which shares of preferred stock, subject to the next two sentences, shall remain undesignated until such time as the Board of Directors, by resolution or resolutions and the filing of a certificate pursuant to applicable laws of the State of Delaware establishes from time to time the number of shares to be included in each such series, and fixes the designation, powers, preferences and rights of the shares of each such series and the qualifications, limitations or restrictions thereof.
Common Stock
The holders of our common stock are entitled to the following rights:
Voting Rights
Each share of our common stock entitles its holder to one vote per share on all matters to be voted or consented upon by the stockholders. Holders of our common stock are not entitled to cumulative voting rights with respect to the election of directors.
Dividend Rights
Subject to limitations under Delaware law and preferences that may apply to any shares of preferred stock that we may decide to issue in the future, holders of our common stock are entitled to receive ratably such dividends or other distributions, if any, as may be declared by our Board out of funds legally available therefor
Liquidation Rights
In the event of the liquidation, dissolution or winding up of our business, the holders of our common stock are entitled to share ratably in the assets available for distribution after the payment of all of our debts and other liabilities, subject to the prior rights of the holders of our preferred stock.
Other Matters
The holders of our common stock have no subscription, redemption or conversion privileges. Our common stock does not entitle its holders to preemptive rights. All of the outstanding shares of our common stock are fully paid and non-assessable. The rights, preferences and privileges of the holders of our common stock are subject to the rights of the holders of shares of any series of preferred stock which we may issue in the future.
Preferred Stock
Our authorized preferred stock consists of 10,000,000 shares of preferred stock, par value $0.0001 per share. As of the date of this prospectus, no shares of preferred stock are outstanding. Our Board has the authority to issue preferred stock in one or more classes or series and to fix the designations, powers, preferences, and rights, and the qualifications, limitations or restrictions thereof including dividend rights, dividend rates, conversion rights, voting rights, terms of redemption, redemption prices, liquidation preferences and the number of shares constituting any class or series, without further vote or action by the stockholders.
While we do not currently have any plans for the issuance of any preferred stock, the issuance of preferred stock could adversely affect the rights of the holders of common stock and, therefore, reduce the value of the common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of the common stock until the Board of Directors determines the specific rights of the holders of the preferred stock; however, these effects may include:
| · | Restricting dividends on the common stock; |
| · | Diluting the voting power of the common stock; |
| · | Impairing the liquidation rights of the common stock; or |
| · | Delaying or preventing a change in control of the Company without further action by the stockholders. |
Warrants
As of the date of this prospectus, there are warrants to purchase a total of 3,155,930 shares of Common Stock outstanding at the exercise prices set forth below:
| (i) | 186,166 shares of common stock at an exercise price of $6.25 per share. |
| (ii) | 441,000 shares of common stock at an exercise price of $8.13 per share. |
64
| (iii) | 736,684 shares of common stock at an exercise price of $9.38 per share. |
| (iv) | 41,600 shares of common stock at an exercise price of $10.00 per share. |
| (v) | 69,200 shares of common stock at an exercise price of $11.25 per share. |
| (vi) | 90,020 shares of common stock at an exercise price of $12.50 per share. |
| (vii) | 310,492 shares of common stock at an exercise price of $15.60 per share. |
| (viii) | 217,263 shares of common stock at an exercise price of $18.75 per share. |
| (ix) | 56,600 shares of common stock at an exercise price of $20.00 per share. |
| (x) | 762,174 shares of common stock at an exercise price of $27.50 per share. |
| (xi) | 10,550 shares of Common Stock at an exercise price of $28.00 per share. |
| (xii) | 154,792 shares of common stock at an exercise price of $50.00 per share. |
| (xiii) | 3,933 shares of common stock at an exercise price of $55.00 per share. |
| (xiv) | 51,856 shares of common stock at an exercise price of $56.25 per share. |
| (xv) | 23,600 shares of common stock at an exercise price to be determined by the Board of Directors. |
The foregoing warrants do not include three year warrants we intend to issue prior to completion of this offering to three of our directors and the estate of a former director entitling such recipients to purchase up to 120,000 shares of common stock at an exercise price of $10.00 per share. See “Certain Relationships and Related Transactions” elsewhere in this prospectus.
Each $56.25 warrant is exercisable at an exercise price of $56.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of Common Stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof.
Each $27.50 warrant is exercisable for a term of five years from the issue date issuance at an exercise price of $27.50 per share.
2013 Warrants
In connection with a $7,762,321 private placement of 620,986 shares of common stock and five year warrants to purchase 310,493 shares of our common stock, we issue warrants to purchase 310,493 shares of common stock (the “B Warrants”). Each B Warrant entitles the holder (to purchase from time to time the number of shares of common stock equal to 50% of the number of shares purchased by the holder in the 2013 offering, at an exercise price of $18.75 per share. The B Warrant contains a cashless exercise provision.
In the event that prior to the expiration of such purchase rights by exercise or by the terms of the B Warrant, (i) the Company effects any merger or consolidation of the Company with or into another person, (ii) the Company effects any sale of all or substantially all of its assets in one or a series of related transactions, (iii) any tender offer or exchange offer (whether by the Company or another person) is completed pursuant to which holders of common stock are permitted to tender or exchange their shares for other securities, cash or property or (iv) the Company effects any reclassification of the common stock or any compulsory share exchange pursuant to which the common stock is effectively converted into or exchanged for other securities, cash or property (each a “Fundamental Transaction”), then, upon any subsequent exercise of the B Warrant, the B Warrant Holder shall have the right to receive, for each B Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, the number of shares of common stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such merger, consolidation or disposition of assets by a holder of the number of shares of common stock for which the B Warrant is exercisable immediately prior to such event. For purposes of any such exercise, the determination of the B Warrant exercise price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of common stock in such Fundamental Transaction, and the Company shall apportion the B Warrant exercise price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration.
65
2013 Placement Agent Warrants
In 2013, in connection with the 2013 winter offering, the Company agreed to issue to Laidlaw & Company (UK) Ltd., as the Placement Agent, and or its designee(s) warrants (the “2013 Placement Agent Warrants”) equal to 10% (ten percent) of the shares of common stock and shares underlying the Investor Warrants sold in the 2013 Offering. The 2013 Placement Agent Warrants shall have an exercise price equal to the lowest price per share of the shares or Investor Warrants. The 2013 Placement Agent Warrants will otherwise contain the same terms as the Investor Warrants including that the 2013 Placement Agent Warrants shall not be callable for five years, shall contain a cashless exercise provision and registration rights in respect of the shares of common stock underlying the 2013 Placement Agent Warrants, and shall have a strike price of $6.25 per share.
2014 Private Placement and Warrants
During the fourth quarter of 2014 and the first quarter of 2015, the Company raised $7,415,552 from the sale of approximately 74.151 units of its securities in a private placement offering that included three-year warrants to purchase 8,000 shares of our common stock at an exercise price of $9.50 per share. Commencing at any time after the date on which closing bid price of our common stock remains at or over $25.00 per share for at least twenty consecutive trading days, the Company will have the right, upon 45 days’ notice to the warrant holder given not later than 45 trading days after the date on which the price condition is satisfied, to redeem the number warrant shares at a price of $6.25 per share, but in no event earlier than 45 days following the date of the receipt by the holder of the redemption notice. Any portion of a warrant that is subject to the redemption that is not exercised by the redemption date will no longer be exercisable.
The Company also issued a warrant to Laidlaw & Company (UK) Ltd., as placement agent in this offering to purchase an aggregate of 124,166 shares of Common Stock at exercise price equal to $6.25 per share.
2015 Short-term Convertible Debenture and Placement Agent Warrants
On May 22, 2015, the Company entered into a Subscription Agreement with one accredited investor, pursuant to which the Company received an aggregate gross proceeds of $2,000,000 for the sale of an 20% original issue discount unsecured convertible debenture (the “Debenture”) due November 22, 2015. The Company issued (a) the Debenture with a principal amount of $2,500,000 that bears interest at a rate of 10% per annum, and (b) a three-year warrant to purchase up to 300,000 shares of Common Stock at an exercise price of $8.25.
In connection with the Debenture, the Company paid to a FINRA registered broker dealer that acted as the placement agent an aggregate of approximately $440,000 in cash compensation, representing fees and an expense allowance. In addition, the Company issued a warrant to purchase an aggregate of 62,000 shares of Common Stock to the placement agent at exercise price equal to $6.25 per share.
2015 Private Placement Warrants
Between August 27, 2015 and December 22, 2015, the Company completed a private placement offering (the “Fall 2015 Offering”) of units of common stock and three year warrants to purchase common stock of the Company. A total of $1,422,500 of gross proceeds was raised in the Fall 2015 Offering, consisting of the sale of 227,600 shares of common stock at a purchase price of $6.25 per share and warrants to purchase 113,800 shares of common stock at an exercise price of $9.38 per share. Laidlaw & Company (UK) Ltd., as placement agent in the Fall 2015 Offering, received a warrant to purchase an aggregate of 29,640 shares of common stock at an exercise price of $9.38 per share.
2016 Bridge Notes
In March 2016, the Company sold $655,000 principal amount of original issue discount unsecured note due six months from funding and providing an estimated 23% yield to maturity (the “March 2016 Bridge Note”) to one accredited investor, pursuant to which the Company received an aggregate gross proceeds of $500,000. The March 2016 Bridge Note does not accrue additional interest and included legal fees of $5,000, which was added to the principal amount. The Company also issued to the investor an aggregate of 4,347 shares of our common stock and five year warrants to purchase up to 65,500 additional shares of common stock at an exercise price of $18.75, subject to adjustment in certain events as provided therein.
If we fail to pay the March 2016 Bridge Note on or before their maturity date or otherwise commit an event of default, as defined in the Bridge Note, the holders may convert the outstanding principal amount of the March 2016 Bridge Notes into our common stock at a conversion price initially equal to 70% of the lowest closing bid price for the common stock in the twenty trading days immediately preceding the conversion, subject to adjustment in certain events as provided therein.
In connection with our sale of the March 2016 Bridge Note, the Company paid to Laidlaw & Company (UK) Ltd., who acted as the placement agent, an aggregate of approximately $60,000 in cash compensation, representing fees and an expense allowance. In addition, we issued to the placement agent (or its designees) a five-year warrant to purchase an aggregate of approximately 19,650 shares of our common stock at an exercise price of $6.25 per share.
66
In May and June 2016, the Company received an aggregate of $1,175,000 in gross cash proceeds from 22 accredited investors in connection with the sale of original issue discount unsecured short-term convertible notes due November 20, 2016, November 30, 2016 and December 10, 2016, being six months following the dates of issuance of each of the notes. The Company notes (a) were issued with a total principal amount of $1,468,750 (b) bears interest at a rate of 10% per annum, and (c) are convertible at any time by the holders into our common stock at a conversion price of $6.25. In addition, we issued to the note holders three-year warrants to purchase up to 176,260 shares of common stock of the Company at an exercise price of $8.13 per share.
In connection with our sale of the May and June 2016 Bridge Notes, we paid to Laidlaw & Company (UK) Ltd., who acted as the placement agent, an aggregate of approximately $141,000 in cash compensation, representing fees and an expense allowance. In addition, we issued to the placement agent (or its designees) a five-year warrant to purchase an aggregate of approximately 41,125 shares of our common stock at an exercise price of $0.325 per share.
Convertible Debt
As of the date of this prospectus, the Company has $400,000 in outstanding convertible debt, subject to conversion into approximately 40,242 shares of Common Stock and comprised of the following:
| (i) | $200,000 in convertible debt issued in April 2012 to the West Virginia Jobs Investment Trust Board as part of an original 3-month note in the amount of $400,000, as amended. The note bears interest at 10% providing for monthly interest-only payments starting May 2012 through June 2012, then final interest and principal payments due July 2012. The note includes a stock warrant for 3,555 shares. On June 18, 2012, the West Virginia Jobs Investment Trust Board signed an addendum to the note agreeing to extend the maturity date by 90 days to October 15, 2012. On March 11, 2013, the West Virginia Jobs Investment Trust Board approved an additional deferral of principal and interest until May 15, 2013 and reduced the price of converting into common shares to $12.50 per share. Subsequently the West Virginia Jobs Investment Trust Board further extended the maturity date until October 15, 2013. In November 2013 the West Virginia Jobs Investment Trust Board was paid $100,000 and the final $100,000 of the principal amount had its maturity date extended to January 15, 2014. On March 31, 2016 the West Virginia Jobs Investment Trust Board and the Company entered into a Loan Modification and Warrant Issuance Agreement, providing for certain amendments and modifications to the original April 2012 debenture, including but not limited to, the following: (i) an increase in the amount of convertible debt from $100,000 in principal under the original note to $200,000 in principal to be subjected to optional conversion into an aggregate of 16,000 shares of our Common Stock, (ii) an amendment to the notes maturity date and payment of the final $100,000 cash payment of the principal amount to be extended from March 31, 2016 to September 30, 2016. |
| (ii) | $200,000, plus accrued and unpaid interest, in convertible debt issued on July 1, 2015 to Josiah Austin, a director of the Company. The Company received aggregate gross proceeds of $200,000 from Mr. Austin and issued a 10% Convertible Promissory Note due on December 31, 2015. The $200,000 principal indebtedness, plus accrued and unpaid interest, is convertible into Common Stock at a conversion price of $8.25 per share. Prior to the reverse split, the Company also issued 4,546 shares of Common Stock as commitment fee to the director with a price per share of $8.25. On February 26, 2015, the Company and Mr. Austin entered into an agreement to extend the maturity date of the convertible note from January 31, 2016 to March 31, 2016. |
Related Party Debt
As of the date of this prospectus, we are indebted to certain members of our board of directors and the estate of a former director for loans and advances made to our Company over the past five years in the aggregate amount of $2,676,500. We intend to repay (on a pro-rata basis) out of the net proceeds of this offering $ 669,125, or 25% of the total of $2,676,500 of related party advances from such persons, which payment shall be made 30 days after our common stock begins trading on the Nasdaq Capital Market. See “Use of Proceeds”. We are currently in discussions with such persons or their representatives to pay the $2,007,375 balance of such indebtedness by the issuance of 6% interest-bearing notes due as to principal and accrued interest on a date which shall be one year following the date of this prospectus. There can be no assurance that we will be successful in completing the contemplated agreements or the terms and conditions of any such arrangements. Any such agreements may be subject to completion of this offering.
Pre-Emptive Rights
| (i) | An aggregate of 21,100 shares of the Company’s common stock are subject to full ratchet anti-dilution rights in the event the Company issues any shares of common stock less than $50.00 per share until October, 2015 at the latest requiring the Company to issue an additional number of shares to such holders as may be required so that the purchase price paid for the shares shall be equal to the subsequent lower purchase price at which shares were issued by the Company; provided however, that shares shall not be issued at a purchase price that is less than $1.00 per share. As of the date hereof, the Company has issued 21,100 additional shares of common stock to such holders in accordance with this provision at the $25.00 floor price and is therefore not obligated to issue any additional shares of common stock to such holders. |
An additional 10,550 shares of common stock underlying outstanding warrants are also subject to full ratchet price protection, expiring October, 2015 which provide that if the Company issues or sells additional shares of common stock, for consideration that is less than $50.00, on the date of and immediately prior to the issuance or sale, the exercise price, then in effect, will be reduced concurrently with the issuance or sale to a price equal to the lowest price per share at which any additional share of common stock is issued or sold; provided, however, that in no event shall the exercise price be reduced to a price that is less than $28.00 per share.
| (ii) | Pursuant to the terms of the 2013 Offering, an aggregate of 620,986 shares of the Company’s common stock are subject to full ratchet anti-dilution rights until the earlier of (1) five years from the closing date on which the purchaser received the securities or (2) the date on which any purchaser no longer owns shares of common stock sold in the 2013 Offering, if the Company issues or sells any shares of common stock or any Common Stock Equivalent pursuant to which shares may be acquired at a price less than $12.50 per share (the “Base Price”) (subject to appropriate adjustments for any stock dividend, stock split, stock combination, reclassification or similar transaction) (each a “Dilutive Issuance”), the Company shall be required to issue additional shares of common stock to each purchaser, for no additional consideration, in an amount sufficient such that the pro rata portion of the Purchase Price paid by such purchaser for the shares of common stock then held, when divided by the total number of shares of common stock then held by such purchaser plus those shares of common stock issued as a result of the Dilutive Issuance will equal the Base Price. As a result of the 2013 Offering, the Company issued 620,986 additional shares of common stock. |
67
Additionally, the exercise price of the B Warrants issued in the 2013 Offering will be reduced on a weighted average basis if common stock may be acquired at a price less than the Base Price, until December 2014 and December 2018, respectively. As a result, the Company reduced the exercise price to $6.25, respectively, assuming the Maximum Offering Amount plus the overallotment option exercised in full.
| (iii) | In connection with the 2014 Offering, the Company has agreed to adjust the exercise price of the warrant to purchase 132,113 shares of common stock issued to the 2014 Placement Agent in connection with the 2013 Offering to an exercise price of $6.25 per share. |
Governing Documents that May Have an Antitakeover Effect
Certain provisions of our Amended Certificate of Incorporation and our Bylaws, which are discussed below could discourage or make it more difficult to accomplish a proxy contest, change in our management or the acquisition of control by a holder of a substantial amount of our voting stock.
Amended Certificate of Incorporation provide that our Board has the authority to issue preferred stock in one or more classes or series and fix such designations, powers, preferences and rights and the qualifications thereof without further vote by our stockholders. The issuance of preferred stock may have the effect of delaying, deferring or preventing a change in control of our company without further action by the stockholders and may adversely affect the voting and other rights of the holders of our common stock.
Our By-laws limit the ability to call special meetings of the stockholders to the Chairman of the Board, or the Chief Executive Officer, or, if there is no Chairman or Chief Executive Officer, then by the president. The stockholders have no right to request or call a special meeting and cannot take action by written consent.
Our By-laws provide that the removal of a director from the Board, with cause, must be by affirmative vote of not less than a majority of the voting power of our issued and outstanding stock entitled to vote generally in the election of directors (voting as a single class), excluding stock entitled to vote only upon the happening of a fact or event unless such fact or event shall have occurred, is required to remove a director from the Board with or without cause.
Representative’s Warrant
We have agreed to issue to the representative of the underwriters in this offering a warrant to purchase an estimated 155,000 shares of our common stock at a per share exercise price equal to 125% of the per share public offering price. A complete description of the warrant is included in the “Underwriting – Representative’s Warrant” section of this prospectus.
Transfer Agent and Registrar
Our transfer agent and registrar is Island Stock Transfer, located at 15500 Roosevelt Blvd, Clearwater, FL 33760. Its telephone number is 1-727-289-0010.
Listing
We intend to apply to list our common stock on the Nasdaq Capital Market under the symbol “PRGB.” There can be no assurance that our application to list our shares will be approved by the Nasdaq Capital Market.
SHARES ELIGIBLE FOR FUTURE SALE
There is not currently an established U.S. trading market for our common stock. We cannot predict the effect, if any, that market sales of shares of our common stock or the availability of shares of our common stock for sale will have on the market price of our common stock prevailing from time to time. Sales of substantial amounts of our common stock, including shares issued upon exercise of outstanding warrants, in the public market after this offering, could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through the sale of our equity securities.
All of the shares of common stock and shares of common stock issuable upon exercise of warrants, when sold pursuant to this prospectus, will be freely tradable, except that any shares acquired by our affiliates, as that term is defined in Rule 144 under the Securities Act, may only be sold in compliance with the limitations described below. As of March 31, 2016, our directors and executive officers held a total of approximately 1,922,675 shares or approximately 31% of the common stock outstanding as of that date.
Selling Stockholder Prospectus
As explained in the Explanatory Note to the registration statement of which this prospectus forms a part, the registration statement of which this prospectus forms a part also contains a prospectus (the “Selling Stockholders Prospectus”) to be used in connection with the potential resale by certain selling stockholders of up to an aggregate of 530,119 additional shares of our common stock, which shares (including 211,230 shares issuable upon exercise of outstanding warrants) may not be resold by such selling stockholders until at least 90 days following the date of this prospectus. We will not receive any of the net proceeds from the sale of shares by any of the selling stockholders. The shares of common stock being registered under the Selling Stockholder Prospectus permit public resales of such shares, and the selling stockholders may offer the shares for resale from time to time pursuant to the Selling Stockholder Prospectus. The selling stockholders may also sell, transfer or otherwise dispose of all or a portion of their shares in transactions exempt from the registration requirements of the Securities Act of 1933, as amended, or pursuant to another effective registration statement covering those shares.
68
________ shares of our outstanding common stock that are not registered under the registration statement of which this prospectus is a part and have not been registered under another registration statement will be deemed restricted securities as defined under Rule 144. Restricted shares may be sold in the public market only if registered or if they qualify for an exemption from registration promulgated under the Securities Act. Subject to the provisions of Rule 144, all of the outstanding shares of common stock that are currently restricted are available for sale in the public market under Rule 144.
For information about shares of common stock issuable upon the exercise of options and warrants, see “Description of Securities.”
In general, under Rule 144 as currently in effect, a person, or group of persons whose shares are required to be aggregated, who is deemed to have been an affiliate at any time during the three months preceding a sale, who has beneficially owned shares that are restricted securities as defined in Rule 144 for at least six months is entitled to sell, within any three-month period commencing 90 days after the date of this prospectus, a number of shares that does not exceed 1% of the then outstanding shares of our common stock.
Sale under Rule 144 by affiliates, whether of restricted or non-restricted shares, include requirements for current public information about the Company; selling the shares pursuant to broker transactions; and limitations on the number of shares sold within a three-month period.
In addition, a person who is not deemed to have been one of our affiliates at any time during the 90 days preceding a sale and who has beneficially owned shares of our common stock for at least six months, including the holding period of any prior owner, except if the prior owner was one of our affiliates, would be entitled to sell all of their shares, provided the availability of current public information about our company. To the extent that shares were acquired from one of our affiliates, a person’s holding period for the purpose of effecting a sale under Rule 144 would commence on the date the shares were acquired from the affiliate.
69
Laidlaw & Company (UK) Ltd. is acting as the book running manager of the offering and as the representative of the underwriters named below. Subject to the terms and conditions set forth in an underwriting agreement among us and the underwriters, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the number of shares of common stock set forth opposite its name below.
| Underwriters | Number of Shares | |||
| Laidlaw & Company (UK) Ltd. | ||||
| Total | ||||
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the shares sold under the underwriting agreement if any of these shares are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated. The underwriters are not obligated to purchase the shares of common stock covered by the underwriters’ over-allotment option described below.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officer’s certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts
The representative has advised us that the underwriters propose to offer directly to the public the 3,100,000 shares purchased pursuant to the underwriting agreement at the initial public offering price set forth on the cover page of this prospectus and to certain securities dealers at the initial public offering price less a concession not in excess of $ per share. After the offering, the representative may change the offering price and other selling terms.
The following table shows the per share and total underwriting discount to be paid to the underwriters by us at $5.50, the midpoint of the price range set forth on the cover page of this prospectus. Such amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Per Share |
Total Without Over-Allotment Option |
Total With Over-Allotment Option |
||||||||||
| Public offering price | ||||||||||||
| Underwriting discounts and commissions | ||||||||||||
| Non-accountable expense allowance | ||||||||||||
| Proceeds, before expenses, to us | ||||||||||||
We have agreed to pay a non-accountable expense allowance to the representative of the underwriters equal to 1% of the gross proceeds received in this offering; however, an allowance shall not be paid in connection with the over-allotment option if the over-allotment option is exercised. We have paid an expense deposit of $25,000 to the representative of the underwriters, which will be applied against accountable expenses that will be paid by us to the representative in connection with this offering, which advance will be refunded to us to the extent not actually incurred by the representative in the event this offering is terminated. We have also agreed to pay the representative’s expenses relating to the offering, including, but not limited to, (1) all actual filing fees incurred in connection with the review of this offering by the Financial Industry Regulatory Authority, Inc., or FINRA, (2) all fees and expenses relating to the listing of our shares of common stock on the Nasdaq Capital Market, (3) all actual fees, expenses and disbursements relating to background checks of our officers and directors in an amount not to exceed $5,000in the aggregate, (4) all actual fees, expenses and disbursements relating to the registration, qualification or exemption of the shares of common stock being offered by this prospectus under state securities laws, or “blue sky” laws, or under the securities laws of foreign jurisdictions designated by the representative, (5) all actual fees, expenses and disbursements relating to the registration, qualification or exemption of our shares of common stock under the securities laws of such foreign jurisdictions as the representative may reasonably designate, (6) the costs of all mailing and printing of the underwriting documents as the representative may reasonably deem necessary, (7) all fees and expenses of the Representative of the underwriters’ including the fees and expenses of the representative’s legal counsel; provided, that all such fees and expenses (including background checks of the Company’s officers and directors) shall not exceed $130,000, (8) the fees and expenses of the Company’s accountants, and (9) the legal fees and expenses and fees and expenses of any other agents and representatives of the Company incurred as a result of the offering.
70
The expenses of the offering, not including the underwriting discount, are estimated at approximately $856,500 and are payable by us.
Option to Purchase Additional Shares
We have granted an option to the underwriters, exercisable for 45 days after the date of this prospectus, to purchase up to 465,000 additional shares at the public offering price, less the underwriting discount. If the underwriters exercise this option, each underwriter will be obligated, subject to conditions contained in the underwriting agreement, to purchase a number of additional shares proportionate to that underwriter’s initial amount reflected in the above table.
Representative’s Warrant
We have agreed to issue to the representative of the underwriters as additional compensation a warrant to purchase the aggregate of % of the shares sold in this offering, excluding the over-allotment option. The shares issuable upon exercise of the warrant are identical to those offered by this prospectus. The warrant is exercisable for cash or on a cashless basis at a per share exercise price equal to $__ or 100% of the public offering price per share in this offering commencing on a date which is one year from the effective date of the registration statement of which this prospectus forms a part and expiring on the date which is five years after the effective date of the registration statement of which this prospectus forms a part in compliance with FINRA Rule 5110(f)(2)(G). The warrant and the shares of common stock underlying the warrant have been deemed compensation by FINRA and are, therefore, subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The representative (or permitted assignees under FINRA Rule 5110(g)(1)) will not sell, transfer, assign, pledge or hypothecate the warrant or the shares of common stock underlying the warrant, nor will it engage in any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the warrant or the underlying shares of common stock for a period of 180 days after the effective date of the registration statement of which this prospectus forms a part. In addition, the warrant provides for registration rights upon request, under certain circumstances. The demand registration right provided in connection with the warrant commences on the one year anniversary of the effective date of the registration statement of which this prospectus forms a part and terminates on the fifth anniversary of the effective date of the registration statement of which this prospectus forms a part. The piggyback registration right provided in connection with the warrant terminates seven years after the effective date of the registration statement of which this prospectus forms a part in compliance with FINRA Rule 5110(f)(2)(G). We will bear all fees and expenses attendant to registering the shares of common stock issuable upon exercise of the warrant, other than underwriting commissions incurred and payable by the holder of the warrant and the expenses of any legal counsel selected by the holder to represent it in connection with the sale of the shares of common stock underlying the warrant. The exercise price and number of shares issuable upon exercise of the warrant may be adjusted in certain circumstances, including in the event of a stock dividend or our reclassification, reorganization, combination or consolidation. However, the warrant exercise price or underlying shares will not be adjusted for issuances of common stock at a price below the warrant exercise price.
Lock-Up Agreements
In addition, we, and all of our directors and executive officers, and holders of 5% or more of our outstanding securities have agreed that, for a period of 180 days after the date of this prospectus, subject to certain limited exceptions described below, we and they will not, directly or indirectly, without the prior written consent of the representative of the underwriters, (1) offer, sell, pledge or otherwise transfer or dispose of any shares of common stock (including, without limitation, shares of common stock that may be deemed to be beneficially owned by us or them in accordance with the rules and regulations of the SEC and shares of common stock that may be issued upon exercise of any options or warrants) or securities convertible into or exercisable or exchangeable for common stock, (2) enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of common stock, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of common stock or other securities, in cash or otherwise, or publicly announce an intention to do either of the foregoing, (3) engage in any short selling of any shares of common stock, or (4) make any demand for or exercise any right with respect to the registration of any shares of common stock or securities convertible into or exercisable or exchangeable for common stock or any of our other securities.
71
Right of First Refusal
These lock-up restrictions will not apply to: (1) bona fide gifts, as long as such donee agrees to be bound by the terms of the lock-up agreement, (2) transfers pursuant to a valid domestic order or divorce decree or settlement or by will, other testamentary document or intestate succession upon the death of the holder, as long as such transferee agrees to be bound by the terms of the lock-up agreement, (3) transfers to any family member or any trust for the direct or indirect benefit of the holder or the immediate family of the holder, so long as such transferee agrees to be bound by the terms of the lock-up agreement and so long as any such transfer does not involve a disposition for value, (4) transfers as part of a transfer or distribution by the holder to its stockholders, members, partners, beneficiaries or other equity holders, so long as the holder is a corporation, limited liability company, partnership, trust or other business, and so long as such transferee agrees to be bound by the terms of the lock-up agreement and any such transfer or distribution does not involve a disposition for value, (5) transfers to us pursuant to the vesting of or exercise by the holder of any equity incentive awards issued pursuant to our stock option or incentive plans as disclosed in this prospectus, on a “cashless” or “net exercise” basis, so long as the shares of our common stock received upon such exercise will remain subject to the restrictions set forth in the lock-up agreement, (6) transfers to us pursuant to any contractual arrangement that provides for the repurchase of the holder’s shares of our common stock or such other securities by us or in connection with the termination of the holder’s employment or other service relationship with us, or (7) transfers pursuant to a bona fide third party tender offer, merger, consolidation or other similar transaction made to all holders of our capital stock involving a change in control of our company.
The representative of the underwriters may release the common stock and other securities subject to the lock-up agreements described above in whole or in part at any time. When determining whether or not to release common stock and other securities from lock-up agreements, the representative of the underwriters will consider, among other factors, the holder’s reasons for requesting the release, the number of shares of common stock and other securities for which the release is being requested and market conditions at the time.
At least three business days before the effectiveness of any release or waiver of any of the restrictions described above with respect to an officer or director of the Company, the representative of the underwriters will notify us of the impending release or waiver and we have agreed to announce the impending release or waiver by press release through a major news service at least two business days before the effective date of the release or waiver.
Laidlaw & Company (UK) Ltd. shall have a right of first refusal, for a period of twelve (12) months after the date this offering is completed, to act as underwriter, placement agent, financial advisor or in any other similar capacity, on the customary terms and conditions of Laidlaw & Company (UK) Ltd., in the event we retain or otherwise use (or seek to retain or use) the services of an investment bank to pursue an offering of our securities (in addition to this offering) or engage in a merger, acquisition, or equivalent transaction (each, a “Subject Transaction”).
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
Stabilization, Short Positions and Penalty Bids
The representative may engage in stabilizing transactions, short sales and purchases to cover positions created by short sales, and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of the common stock, in accordance with Regulation M under the Exchange Act:
| · | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. |
| · | A short position involves a sale by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase in the offering, which creates the syndicate short position. This short position may be either a covered short position or a naked short position. In a covered short position, the number of shares involved in the sales made by the underwriters in excess of the number of shares they are obligated to purchase is not greater than the number of shares that they may purchase by exercising their option to purchase additional shares. In a naked short position, the number of shares involved is greater than the number of shares in their option to purchase additional shares. The underwriters may close out any short position by either exercising their option to purchase additional shares and/or purchasing shares in the open market. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through their option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. |
| · | Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. |
| · | Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of the common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on the Nasdaq Capital Market or otherwise and, if commenced, may be discontinued at any time.
72
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor any of the underwriters make any representation that the representative will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Distribution
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares of common stock to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representative to underwriters and selling group members that may make Internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ websites and any information contained in any other website maintained by the underwriters is not part of this prospectus or the registration statement of which this prospectus forms a part.
Other Relationships
From time to time, certain of the underwriters and their affiliates have provided, and may provide in the future, various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions. However, except as disclosed in this prospectus, we have no present arrangements with any of the underwriters for any further services.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive, or the Relevant Member States, with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, or the Relevant Implementation Date, our securities will not be offered to the public in that Relevant Member State prior to the publication of a prospectus in relation to the securities that has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that, with effect from and including the Relevant Implementation Date, an offer of securities may be made to the public in that Relevant Member State at any time:
| · | to any legal entity that is a qualified investor as defined in the Prospectus Directive; |
| · | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the manager for any such offer; or |
| · | in any other circumstances which do not require the publication by the issuer of a prospectus pursuant to Article 3(2) of the Prospectus Directive. |
For the purposes of this provision, the expression an “offer of common shares to the public” in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the common shares to be offered so as to enable an investor to decide to purchase or subscribe the common shares, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State. The expression “Prospectus Directive” means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in each Relevant Member State and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
We have not authorized, and do not authorize the making of, any offer of shares through any financial intermediary on our behalf, other than offers made by the underwriters with a view to the final placement of the shares as contemplated by this prospectus. Accordingly, no purchaser of the securities, other than the underwriters, is authorized to make any further offer of the shares on our or the underwriters’ behalf.
73
United Kingdom
Our securities may not be offered or sold and will not be offered or sold to any persons in the United Kingdom other than persons whose ordinary activities involve acquiring, holding, managing or disposing of investments (as principal or as agent) for the purposes of their businesses and in compliance with all applicable provisions of the Financial Services and Markets Act 2000, or FSMA, with respect to anything done in relation to our securities in, from or otherwise involving the United Kingdom.
In addition, each underwriter:
| · | has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) to persons who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act of 2000 (Financial Promotion) Order 2005 or in circumstances in which section 21 of FSMA does not apply to us; and |
| · | has complied with, and will comply with all applicable provisions of FSMA with respect to anything done by it in relation to the securities in, from or otherwise involving the United Kingdom. |
Australia
No prospectus or other disclosure document (as defined in the Corporations Act 2001 (Cth) of Australia, or the Corporations Act) in relation to the securities has been or will be lodged with the Australian Securities & Investments Commission, or the ASIC. This document has not been lodged with ASIC and is only directed to certain categories of exempt persons. Accordingly, if you receive this document in Australia:
(1) you confirm and warrant that you are either:
(a) a “sophisticated investor” under section 708(8)(a) or (b) of the Corporations Act;
(b) a “sophisticated investor” under section 708(8)(c) or (d) of the Corporations Act and that you have provided an accountant’s certificate to us which complies with the requirements of section 708(8)(c)(i) or (ii) of the Corporations Act and related regulations before the offer has been made;
(c) a person associated with us under section 708(12) of the Corporations Act; or
(d) a “professional investor” within the meaning of section 708(11)(a) or (b) of the Corporations Act, and to the extent that you are unable to confirm or warrant that you are an exempt sophisticated investor, associated person or professional investor under the Corporations Act, any offer made to you under this document is void and incapable of acceptance; and
(2) you warrant and agree that you will not offer any of the securities for resale in Australia within 12 months of those securities being issued unless any such resale offer is exempt from the requirement to issue a disclosure document under section 708 of the Corporations Act.
Hong Kong
The securities may not be offered or sold in Hong Kong by means of any document other than (1) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap. 32, Laws of Hong Kong), (2) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder, or (3) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap. 32, Laws of Hong Kong) and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to securities which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Japan
The securities offered in this prospectus have not been and will not be registered under the Financial Instruments and Exchange Law of Japan. The securities have not been offered or sold and will not be offered or sold, directly or indirectly, in Japan or to or for the account of any resident of Japan (including any corporation or other entity organized under the laws of Japan), except (i) pursuant to an exemption from the registration requirements of the Financial Instruments and Exchange Law and (ii) in compliance with any other applicable requirements of Japanese law.
74
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the securities may not be circulated or distributed, nor may the securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (1) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (2) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275 of the SFA, or (3) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to compliance with conditions set forth in the SFA.
Where the securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| · | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| · | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, shares, notes and units of shares and notes of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares pursuant to an offer made under Section 275 of the SFA except: |
| – | to an institutional investor (for corporations, under Section 274 of the SFA) or to a relevant person defined in Section 275(2) of the SFA, or to any person pursuant to an offer that is made on terms that such shares, notes and units of shares and notes of that corporation or such rights and interest in that trust are acquired at a consideration of not less than $200,000 (or its equivalent in a foreign currency) for each transaction, whether such amount is to be paid for in cash or by exchange of securities or other assets, and further for corporations, in accordance with the conditions specified in Section 275 of the SFA; |
| – | where no consideration is or will be given for the transfer; or |
| – | where the transfer is by operation of law. |
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or the SIX, or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the securities or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, us, or the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of the shares.
Canada
Resale Restrictions
The distribution of our securities in Canada is being made only in the provinces of Ontario, Quebec, Alberta, British Columbia and Manitoba on a private placement basis exempt from the requirement that we prepare and file a prospectus with the securities regulatory authorities in each province where trades of common stock are made. Any resale of the common stock in Canada must be made under applicable securities laws which may vary depending on the relevant jurisdiction, and which may require resales to be made under available statutory exemptions or under a discretionary exemption granted by the applicable Canadian securities regulatory authority. Purchasers are advised to seek legal advice prior to any resale of the common stock.
Representations of Purchasers
By purchasing securities in Canada and accepting delivery of a purchase confirmation, a purchaser is representing to us and the dealer from whom the purchase confirmation is received that:
| · | the purchaser is entitled under applicable provincial securities laws to purchase the securities without the benefit of a prospectus qualified under those securities laws as it is an “accredited investor” as defined under National Instrument 45-106—Prospectus and Registration Exemptions; |
| · | the purchaser is a “Canadian permitted client” as defined in National Instrument 31-103—Registration Requirements and Exemptions, or as otherwise interpreted and applied by the Canadian Securities Administrators; |
75
| · | where required by law, the purchaser is purchasing as principal and not as agent; |
| · | the purchaser has reviewed the text above under “—Resale Restrictions”; and |
| · | the purchaser acknowledges and consents to the provision of specified information concerning the purchase of the securities to the regulatory authority that by law is entitled to collect the information, including certain personal information. For purchasers in Ontario, questions about such indirect collection of personal information should be directed to Administrative Support Clerk, Ontario Securities Commission, Suite 1903, Box 55, 20 Queen Street West, Toronto, Ontario M5H 3S8 or on (416) 593-3684. |
Rights of Action—Ontario Purchasers
Under Ontario securities legislation, certain purchasers who purchase any securities offered by this prospectus during the period of distribution will have a statutory right of action for damages, or while still the owner of the securities, for rescission against us in the event that this prospectus contain a misrepresentation without regard to whether the purchaser relied on the misrepresentation. The right of action for damages is exercisable not later than the earlier of 180 days from the date the purchaser first had knowledge of the facts giving rise to the cause of action and three years from the date on which payment is made for the securities. The right of action for rescission is exercisable not later than 180 days from the date on which payment is made for the securities. If a purchaser elects to exercise the right of action for rescission, the purchaser will have no right of action for damages against us. In no case will the amount recoverable in any action exceed the price at which the securities were offered to the purchaser and if the purchaser is shown to have purchased the securities with knowledge of the misrepresentation, we will have no liability. In the case of an action for damages, we will not be liable for all or any portion of the damages that are proven to not represent the depreciation in value of the common stock as a result of the misrepresentation relied upon. These rights are in addition to, and without derogation from, any other rights or remedies available at law to an Ontario purchaser. The foregoing is a summary of the rights available to an Ontario purchaser. Ontario purchasers should refer to the complete text of the relevant statutory provisions.
76
Schneider Downs & Co., Inc., an independent registered public accounting firm, audited our financial statements for the years ended December 31, 2015 and 2014, as set forth in report appearing herein. We have included our financial statements in this prospectus and elsewhere in the registration statement in reliance on the reports of Schneider Downs & Co., Inc., given on their authority as experts in accounting and auditing.
LEGAL MATTERS AND INTERESTS OF NAMED EXPERTS
The validity of the securities being offered by this prospectus will be passed upon for us by CKR Law LLP, New York, New York. Certain legal matters in connection with this offering will be passed upon for the underwriters by Sichenzia Ross Friedman Ference LLP, New York, New York.
77
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1, together with any amendments and related exhibits, under the Securities Act, with respect to our shares of common stock offered by this prospectus. The registration statement contains additional information about us and our shares of common stock that we are offering in this prospectus. We are subject to the informational requirements of the Exchange Act and file annual, quarterly and current reports and other information with the SEC. You can read our filings over the Internet at the SEC’s website at www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facility at 100 F Street, N.E., Washington, D.C., 20549, on official business days during the hours of 10 a.m. to 3 p.m. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C., 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facility. In addition, you can find more information about us on our website at https://proteabio.com.
78
PREFACE STATEMENT TO THE CONSOLIDATED FINANCIAL STATEMENTS
The following consolidated financial statements of Protea Biosciences Group, Inc. and subsidiaries are presented in draft form. We expect to be in a position to issue the following draft report, in the form presented, at effectiveness pending Protea’s Board of Directors approval of the reverse-stock split as presented in Note 16 to the consolidated financial statements and subject to normal subsequent event procedures in accordance with AU Section 560 “Subsequent Events.”
June 21, 2016
/s/ SCHNEIDER DOWNS & CO., INC.
Pittsburgh, PA
79
PROTEA BIOSCIENCES GROUP, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Protea Biosciences Group, Inc.
We have audited the accompanying consolidated balance sheets of Protea Biosciences Group, Inc. and subsidiaries (the Company) as of December 31, 2015 and 2014, and the related consolidated statements of operations and comprehensive loss, cash flows and shareholders’ equity for each of the years in the two-year period ended December 31, 2015. The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audit in accordance with the Standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purposes of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Protea Biosciences Group, Inc. as of December 31, 2015 and 2014, and the consolidated results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company’s products are being developed and have not generated significant revenues. As a result, the Company has suffered recurring losses and its liabilities exceed its assets. This raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Pittsburgh, Pennsylvania
March 16, 2016 (except for the effect of the reverse stock split discussed in Note 16 to the financial statements, as to which the date is _______ __, 2016)
F-2
PROTEA BIOSCIENCES GROUP, INC.
Consolidated Balance Sheets
See Accompanying Notes to Financial Statements
| December 31, 2015 | December 31, 2014 | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | 6,450 | $ | 322,877 | ||||
| Trade accounts receivable, net | 195,823 | 273,914 | ||||||
| Other receivables | - | 119,230 | ||||||
| Inventory | 111,087 | 161,301 | ||||||
| Prepaid expenses | 98,231 | 54,022 | ||||||
| Total current assets | 411,591 | 931,344 | ||||||
| Property and equipment, net | 2,626,907 | 2,960,090 | ||||||
| Other noncurrent assets | 5,248 | 136,693 | ||||||
| Total Assets | $ | 3,043,746 | $ | 4,028,127 | ||||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| Current Liabilities: | ||||||||
| Current maturities on short and long-term debt, net of discount | $ | 1,323,594 | $ | 1,457,800 | ||||
| Accounts payable | 1,338,367 | 1,253,385 | ||||||
| Bank line of credit | 3,000,000 | 3,000,000 | ||||||
| Loans payable to stockholders, net of discount | 2,574,500 | 1,381,498 | ||||||
| Derivative liabilities | 962,401 | 154,058 | ||||||
| Other payables and accrued expenses | 391,308 | 518,821 | ||||||
| Total current liabilities | $ | 9,590,170 | 7,765,562 | |||||
| Long-term debt - net of current portion | 1,726,158 | 1,817,237 | ||||||
| Stockholders' Equity: | ||||||||
| Preferred stock ($.0001 par value; 10,000,000 shares authorized; none and 3,337,725 issued or outstanding at December 31, 2015 and December 31, 2014) | - | 334 | ||||||
| Common stock ($.0001 par value; 250,000,000 shares authorized; 5,325,850 and 2,663,544 shares issued and outstanding at December 31, 2015 and December 31, 2014) | 533 | 266 | ||||||
| Additional paid in capital | 71,324,354 | 64,312,136 | ||||||
| Accumulated deficit | (79,597,608 | ) | (69,867,118 | ) | ||||
| Accumulated other comprehensive income (loss) | 139 | (290 | ) | |||||
| Total Stockholders' Deficit | (8,272,582 | ) | (5,554,672 | ) | ||||
| Total Liabilities and Stockholders' Deficit | $ | 3,043,746 | $ | 4,028,127 | ||||
F-3
PROTEA BIOSCIENCES GROUP, INC.
Consolidated Statements of Operations and Total Comprehensive Loss
See Accompanying Notes to Financial Statements
For the Year Ended December 31, | For the Year Ended December 31, | |||||||
| 2015 | 2014 | |||||||
| Gross revenue | $ | 2,003,286 | $ | 1,768,312 | ||||
| Cost of revenue | (721,656 | ) | (871,904 | ) | ||||
| Gross profit | 1,281,630 | 896,408 | ||||||
| Selling, general, administrative expenses | (6,942,406 | ) | (8,736,478 | ) | ||||
| Research and development expense | (1,479,367 | ) | (2,853,078 | ) | ||||
| Loss from operations | (7,140,143 | ) | (10,693,148 | ) | ||||
| Other income (expense): | ||||||||
| Gain on sale of equity method investment | 910,000 | - | ||||||
| Other income and currency exchange income (expense) | 7,248 | 27,072 | ||||||
| Interest expense | (1,617,028 | ) | (745,277 | ) | ||||
| Debt conversion inducement cost | (60,419 | ) | - | |||||
| Loss on asset disposal | (42,506 | ) | (19,144 | ) | ||||
| Gain on discontinued operations | - | 1,245,712 | ||||||
| Change in fair value of derivative | (1,631,586 | ) | (1,289,985 | ) | ||||
| Total other income (expense) | (2,434,291 | ) | (781,622 | ) | ||||
| Loss before income taxes | (9,574,434 | ) | (11,474,770 | ) | ||||
| Income taxes | - | - | ||||||
| Net loss | (9,574,434 | ) | (11,474,770 | ) | ||||
| Foreign currency translation adjustment | 429 | (23,455 | ) | |||||
| Total comprehensive loss | $ | (9,574,005 | ) | $ | (11,498,225 | ) | ||
| Net loss per share - basic and diluted | $ | (2.18 | ) | $ | (4.24 | ) | ||
| Weighted average number of shares outstanding - basic and diluted | 4,387,961 | 2,710,505 | ||||||
F-4
PROTEA BIOSCIENCES GROUP, INC.
Consolidated Statements of Stockholders' Equity (Deficit)
See Accompanying Notes to Financial Statements
| Stock Par Value Preferred $0.0001 | Stock Par Value Common
$0.0001 | Additional Paid in | Accumulated | Accumulated Other Comprehensive Income | Total Stockholders’ Equity | |||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | (Loss) | (Deficit) | |||||||||||||||||||||||||
| December 31, 2013 | - | $ | - | 2,617,709 | $ | 262 | $ | 55,357,896 | $ | (58,392,348 | ) | $ | 23,165 | $ | (3,011,025 | ) | ||||||||||||||||
| Issuance of preferred stock for cash (net of issuance cost of $814,604) | 2,264,238 | 227 | - | - | 3,713,645 | - | - | 3,713,872 | ||||||||||||||||||||||||
| Issuance of preferred stock upon conversion of convertible notes | 1,073,487 | 107 | - | - | 2,146,868 | - | - | 2,146,975 | ||||||||||||||||||||||||
| Issuance of stock for services (net of issuance cost of $105,168) | - | - | 44,494 | 4 | 880,369 | - | - | 880,373 | ||||||||||||||||||||||||
| Stock options exercised | - | - | 1,000 | - | 19,265 | - | - | 19,265 | ||||||||||||||||||||||||
| Stock warrants exercised | - | - | 341 | - | - | - | - | - | ||||||||||||||||||||||||
| Stock-based compensation expense | - | - | - | - | 283,481 | - | - | 283,481 | ||||||||||||||||||||||||
| Stock warrants issued for services and debt | - | - | - | - | 150,409 | - | - | 150,409 | ||||||||||||||||||||||||
| Stock warrants issued to placement Agent | - | - | - | - | (1,861 | ) | - | - | (1,861 | ) | ||||||||||||||||||||||
| Recognition of derivative liabilities | - | - | - | - | (9,298 | ) | - | - | (9,298 | ) | ||||||||||||||||||||||
| Anti-dilution shares to be issued | - | - | - | - | 1,771,362 | - | - | 1,771,362 | ||||||||||||||||||||||||
| Net loss | - | - | - | - | - | (11,474,770 | ) | (11,474,770 | ) | |||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | - | (23,455 | ) | (23,455 | ) | ||||||||||||||||||||||
| December 31, 2014 | 3,337,725 | $ | 334 | 2,663,544 | $ | 266 | $ | 64,312,136 | $ | (69,867,118 | ) | $ | (290 | ) | $ | (5,554,672 | ) | |||||||||||||||
F-5
PROTEA BIOSCIENCES GROUP, INC.
Consolidated Statements of Stockholders' Equity (Deficit) (Unaudited)
See Accompanying Notes to Financial Statements
| Accumulated | Total | |||||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Additional | Other | Stockholders' | ||||||||||||||||||||||||||||
| Par Value $.0001 | Par Value $.0001 | Paid in Capital | Accumulated | Comprehensive | Equity | |||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Common Stock | Deficit | Income (Loss) | (Deficit) | |||||||||||||||||||||||||
| December 31, 2014 | 3,337,725 | $ | 334 | 2,663,544 | $ | 266 | $ | 64,312,136 | $ | (69,867,118 | ) | $ | (290 | ) | $ | (5,554,672 | ) | |||||||||||||||
| Issuance of preferred stock, net issuance costs of $345,143 | 370,050 | 37 | - | - | 394,920 | - | - | 394,957 | ||||||||||||||||||||||||
| Issuance of common stock, net issuance costs of $310,197 | - | - | 227,600 | 23 | 1,112,280 | - | - | 1,112,303 | ||||||||||||||||||||||||
| Stock dividend declared on preferred stock | 78,040 | 8 | - | - | 156,048 | (156,056 | ) | - | ||||||||||||||||||||||||
| Issuance of stock upon conversion of convertible notes, net of issuance costs of $309,345 | - | - | 522,042 | 52 | 2,953,368 | - | - | 2,953,420 | ||||||||||||||||||||||||
| Issuance of stock for services | - | - | 80,217 | 8 | 544,622 | - | - | 544,630 | ||||||||||||||||||||||||
| Issuance of stock under anti-dilution provision | - | - | 620,986 | 63 | 945,388 | - | - | 945,451 | ||||||||||||||||||||||||
| Conversion of preferred stock to common stock | (3,785,815 | ) | (379 | ) | 1,211,461 | 121 | 258 | - | - | - | ||||||||||||||||||||||
| Stock-based compensation expense | - | - | - | - | 267,638 | - | - | 267,638 | ||||||||||||||||||||||||
| Stock warrants issued for services and debt | - | - | - | - | 517,843 | - | - | 517,843 | ||||||||||||||||||||||||
| Stock warrants issued as part of debt conversion inducement cost | - | - | - | - | 60,419 | - | - | 60,419 | ||||||||||||||||||||||||
| Stock warrants issued to placement agent | - | - | - | - | 175,694 | - | - | 175,694 | ||||||||||||||||||||||||
| Recognition of derivative liability | - | - | - | - | (116,260 | ) | - | - | (116,260 | ) | ||||||||||||||||||||||
| Net loss | - | - | - | - | - | (9,574,434 | ) | - | (9,574,434 | ) | ||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | - | 429 | 429 | ||||||||||||||||||||||||
| December 31, 2015 | - | $ | - | 5,325,850 | $ | 533 | $ | 71,324,354 | $ | (79,597,608 | ) | $ | 139 | $ | (8,272,582 | ) | ||||||||||||||||
F-6
| PROTEA BIOSCIENCES GROUP, INC. |
| Consolidated Statements of Cash Flows (Unaudited) |
| See Accompanying Notes to Financial Statements |
| For the Year Ended December 31, | For the Year Ended December 31, | |||||||
| 2015 | 2014 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (9,574,434 | ) | $ | (11,474,770 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 686,820 | 825,780 | ||||||
| Non-cash compensation | 267,638 | 283,481 | ||||||
| Issuance of common stock and warrants for services | 610,659 | 1,035,462 | ||||||
| Issuance of preferred and common stock for accrued interest | 394,174 | 70,976 | ||||||
| Accretion of discount on short-term convertible debenture | 500,000 | - | ||||||
| Accretion of convertible debenture discount | 463,827 | 192,421 | ||||||
| Debt conversion inducement cost | 60,419 | - | ||||||
| Loss on disposal of assets | 42,506 | 19,144 | ||||||
| Gain on subsidiary sale | - | (1,245,712 | ) | |||||
| Gain on sale of equity method investment | (910,000 | ) | - | |||||
| Bad debt expense | 119,230 | 3,000 | ||||||
| Loss from change in fair value of derivative | 1,631,586 | 1,289,985 | ||||||
| Net change in assets: Decrease (increase) | ||||||||
| Trade accounts receivable | 78,091 | (60,050 | ) | |||||
| Prepaid expenses | (44,209 | ) | 235,877 | |||||
| Other receivables | - | (198,081 | ) | |||||
| Inventory | 146,550 | 304,033 | ||||||
| Other noncurrent assets | (93,013 | ) | - | |||||
| Net change in liabilities: Increase (decrease) | ||||||||
| Trade accounts payable | 148,619 | 810,598 | ||||||
| Other payables and accrued expenses | (127,513 | ) | 575,043 | |||||
| Net cash used in operating activities | (5,599,050 | ) | (7,332,813 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchase of and deposits on equipment | (119,254 | ) | (413,806 | ) | ||||
| Proceeds from sale of equipment | 243,606 | 6,000 | ||||||
| Proceeds from subsidiary sale - Option Fee | - | 586,638 | ||||||
| Proceeds from sale of equity method investment | 910,000 | - | ||||||
| Cash divested from subsidiary sale | - | (63,185 | ) | |||||
| Net cash provided by investing activities | 1,034,352 | 115,647 | ||||||
| Cash flows from financing activities: | ||||||||
| Net advances on bank line of credit | - | 275,000 | ||||||
| Proceeds from sale of preferred stock, net | 575,042 | 3,713,872 | ||||||
| Proceeds from sale of common stock, net | 1,112,304 | 13,750 | ||||||
| Proceeds from short-term debt | 2,000,000 | 536,000 | ||||||
| Payment of issuance cost from short-term debt | (309,345 | ) | - | |||||
| Proceeds from stockholder debt | 1,602,500 | 3,595,000 | ||||||
| Repayment of stockholder debt | (115,000 | ) | (1,149,694 | ) | ||||
| Repayment of short and long-term debt | (617,659 | ) | (270,657 | ) | ||||
| Repayment of Obligation related to the Letter of Credit | - | (238,695 | ) | |||||
| Net cash provided by financing activities | 4,247,842 | 6,474,576 | ||||||
| Effect of exchange rate changes on cash | 429 | (20,863 | ) | |||||
| Net (decrease) in cash | (316,427 | ) | (763,453 | ) | ||||
| Cash, beginning of period | 322,877 | 1,086,330 | ||||||
| Cash, end of period | $ | 6,450 | $ | 322,877 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Cash paid during the period for interest | $ | 352,140 | $ | 319,947 | ||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||
| Financed equipment | $ | 516,832 | $ | 501,917 | ||||
| Debt converted to common stock | $ | 2,953,420 | $ | 2,146,976 | ||||
| Dividends paid in preferred stock | $ | 156,056 | $ | - | ||||
| Stock warrants issued to placement agent | $ | 175,694 | $ | - | ||||
| Common stock shares issuable | $ | - | $ | 1,771,362 | ||||
F-7
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
1. Description of Company and Nature of Business
Protea Biosciences Group, Inc. (referred to as “Protea,” “the Company,” “we,” “us” and “our”) is an emerging growth molecular information company that has developed a revolutionary platform technology, which enables the direct analysis, mapping and display of biologically active molecules in living cells and tissue samples. The technology platform offers new, unprecedented capabilities useful to the pharmaceutical, diagnostic, clinical research, agricultural and life science industries.
“Molecular information” refers to the generation and bioinformatic processing of very large data sets, known as “big data,” obtained by applying the Company’s technology to identify and characterize the proteins, metabolites, lipids and other molecules which are the biologically active molecular products of all living cells and life forms.
Our technology is used to improve pharmaceutical development and life science research productivity and outcomes, and to extend and add value to other technologies that are used in research and development (“R&D”), such as three-dimensional tissue models, biomarker discovery, synthetic biologicals and mass spectrometry. In particular, the Company believes that its ability to rapidly provide comprehensive molecular image-based datasets addresses a universal need of the pharmaceutical, diagnostic and clinical research and life science industries.
Reverse Stock-Split
On ________, 2016, the Company effected a 1-for-25 reverse stock split of its issued common stock. All applicable share data, per share amounts and related information in the consolidated financial statements and notes thereto have been adjusted retroactively to give effect to the 1-for-25 reverse stock split. See Note 16.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Protea Biosciences Group, Inc. and its wholly-owned subsidiary, Protea Biosciences, Inc. All material accounts and transactions have been eliminated in consolidation. During 2014, the Company sold Proteabio Europe but retained continued involvement through an equity method investment in AzurRx BioPharma, Inc., the buyer of Proteabio Europe. As the sale was not fully consummated due to an outstanding contingency, the transaction was not recognized until December 12, 2014. As such, the results of Proteabio Europe were deconsolidated on December 12, 2014.
Going Concern
The Company’s financial statements have been prepared on a going-concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has funded its activities to date almost exclusively from debt and equity financings. The Company will continue to require substantial funds to advance the research and development of its core technologies and to develop new products and services based upon its proprietary protein recovery and identification technologies.
Management intends to meet its operating cash flow requirements primarily from the sale of equity and debt securities. The Company seeks additional capital through sales of equity securities or convertible debt and, if appropriate, to pursue partnerships to advance various research and development activities. The Company may also consider the sale of certain assets, or entering into a transaction such as a merger with a business complimentary to theirs.
While the Company believes that it will be successful in obtaining the necessary financing to fund its operations, they have no committed sources of funding and are not assured that additional funding will be available to them. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying statements do not include any adjustment that might be necessary if the Company is unable to continue as a going concern.
Estimates and Assumptions
The presentation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
F-8
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
2. Summary of Significant Accounting Policies (continued)
Cash and Cash Equivalents
The Company considers all highly liquid debt instruments purchased with a maturity date of three months or less to be cash equivalents. The Company has cash on deposit at banks that may exceed the federally-insured limits at times.
Trade Accounts Receivable
Trade accounts receivable are reported at the amount management expects to collect from outstanding balances. Differences between the amount due and the amount management expects to collect are reported in the statement of operations of the year in which those differences are determined, with an offsetting entry to a valuation allowance for trade accounts receivable. Balances that are still outstanding after management has used reasonable collection efforts are written off through a charge to the valuation allowance and a credit to trade accounts receivable. The Company performs ongoing credit evaluations of its customers and generally has not required collateral.
The Company maintains allowances for doubtful accounts based on management’s analysis of historical losses from uncollectible accounts and risks identified for specific customers who may not be able to make required payments. If the financial condition of customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required. An allowance of $3,000 was deemed necessary as of December 31, 2015 and December 31, 2014, respectively.
Equity Method Investments
As a result of the sale of its subsidiary in 2014, the Company received 100 shares of Series A Preferred Stock, a 33% interest (on a fully diluted basis) in AzurRx BioPharma, Inc. (“AzurRx”) that is accounted for on the equity method. AzurRx is a private biotechnology company formed to focus on the development of the early stage pharmaceutical assets of ProteaBio Europe. Investee companies that are not consolidated, but over which the Company exercises significant influence, are accounted for under the equity method of accounting. Whether or not the Company exercises significant influence with respect to an investee depends on an evaluation of several factors including, among others, representation on the investee company’s board of directors and ownership level, which is generally a 20% to 50% interest in the voting securities of the investee company. Under the equity method of accounting, an investee company’s accounts are not reflected within the Company’s Consolidated Balance Sheets and Consolidated Statements of Income; however, the Company’s share of the earnings or losses of the investee company is reflected in the caption “Income from equity method investments” in the Consolidated Statements of Income. The Company’s carrying value in an equity method investee company would be reflected in the caption “Equity method investments” in the Company’s Consolidated Balance Sheets.
AzurRx is still in the early stages of organizing its business activities, has not reached commercialization, and will continue realizing substantial research and development expenses. Thus, the Company has suspended the equity method of accounting as the losses have reduced the Company’s basis in AzurRx below zero. The value of this investment as presented on our consolidated balance sheet was $0 at December 31, 2015 and 2014.
In November 2015, the Company converted 29 shares of Series A Preferred Stock of AzurRx into 707,416 shares of Common Stock of AzurRx. Subsequently, the Company entered into various stock purchase agreements for the sale of such shares of Common Stock of AzurRx and received gross proceeds of $910,000 as of December 31, 2015. As a result these sales, the Company’s interest was reduced to 25% as of December 31, 2015.
F-9
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
2. Summary of Significant Accounting Policies (continued)
Other Receivables and Other Noncurrent assets
Other receivables, which reflect amounts from non-trade activity and other noncurrent assets, consist of the following at:
| December 31, 2015 | December 31, 2014 | |||||||
| Other receivables | - | 119,230 | ||||||
| Other receivables - current | $ | - | $ | 119,230 | ||||
| Deposits | $ | 5,248 | $ | 136,693 | ||||
| Other noncurrent assets | $ | 5,248 | $ | 136,693 | ||||
Inventory
Inventory represents finished goods and work in progress. Finished goods and work in progress consist primarily of specifically identifiable items that are valued at the lower of cost or fair market value using the first-in, first-out (FIFO) method. Inventory consists of the following at:
| December 31, 2015 | December 31, 2014 | |||||||
| Finished goods | $ | 12,650 | $ | 18,739 | ||||
| Work in progress | 98,437 | 142,562 | ||||||
| Total Inventory | $ | 111,087 | $ | 161,301 | ||||
Property and Equipment
Expenditures for maintenance and repairs are charged to expense and the costs of significant improvements that extend the life of underlying assets are capitalized.
Property and equipment and leasehold improvements are capitalized at cost and depreciated using the straight-line method (the Company used the double-declining balance method for property and equipment placed in service prior to January 2011) over estimated lives as follows:
| Laboratory equipment | 5 - 10 years |
| Computers | 5 years |
| Leasehold improvements | Life of lease |
| Software | 3 years |
Property and equipment consists of the following at:
| December 31, 2015 | December 31, 2014 | |||||||
| Lab equipment | $ | 6,617,038 | $ | 7,313,979 | ||||
| Computer equipment | 540,212 | 540,814 | ||||||
| Office equipment | 191,248 | 229,785 | ||||||
| Leasehold improvements | 212,730 | 434,304 | ||||||
| 7,561,228 | 8,518,882 | |||||||
| Accumulated depreciation | (4,934,321 | ) | (5,558,792 | ) | ||||
| Property and equipment, net | $ | 2,626,907 | $ | 2,960,090 | ||||
Depreciation expense is charged to either research and development or selling, general and administrative expenses and totals $686,820 in 2015 and $825,780 in 2014.
The Company evaluates the potential impairment of property and equipment whenever events or changes in circumstances indicate that the carrying value of a group of assets may not be recoverable. An impairment loss would be recognized when the carrying amount of the asset group exceeds the estimated undiscounted future cash flows expected to be generated from the use of the asset group and its eventual disposition. The amount of impairment loss to be recorded is measured as the excess of the carrying value of the asset group over its fair value. Fair value is generally determined using a discounted cash flow analysis or market prices for similar assets.
F-10
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
2. Summary of Significant Accounting Policies (continued)
Revenue Recognition
We follow the provisions of FASB ASC 605, “Revenue Recognition.” We recognize revenue of products when persuasive evidence of a sale arrangement exists, the price to the buyer is fixed or determinable, delivery has occurred /title has passed, and collectability of the sales price is reasonably assured. The Company recognizes revenue from the sale of its ProteaPlot software when bundled with the LAESI platform, which facilitates operating the instrument and storage and display of datasets. The Company also recognizes revenue of standalone sales of ProteaPlot, which generally consists of additional user licenses. Revenue is recognized once the title is passed to the customer.
We account for shipping and handling fees and costs in accordance with the provisions of FASB ASC 605-45-45, “Revenue Recognition - Principal Agent Considerations,” which requires all amounts charged to customers for shipping and handling to be classified as revenues. Shipping and handling costs charged to customers are recorded in the period the related product sales revenue is recognized.
Regarding short-term service contracts, the majority of these service contracts involve the processing of imaging and bioanalytical samples for pharmaceutical and academic or clinical research laboratories. These contracts generally provide for a fixed fee for each method developed or sample processed and revenue is recognized when the analysis is complete and a report is delivered.
For longer-term contracts involving multiple elements, the items included in the arrangement (deliverables) are evaluated to determine whether they represent separate units of accounting. We perform this evaluation at the inception of an arrangement and as each item in the arrangement is delivered. Generally, we account for a deliverable (or a group of deliverables) separately if: (i) the delivered item(s) have standalone value to the customer, and the delivery or performance of the service(s) is probable and substantially in our control. Revenue on multiple revenue arrangements is recognized using a proportional method for each separately identified element. All revenue from contracts determined not to have separate units of accounting is recognized based on consideration of the most substantive delivery factor of all the elements in the contract or, if there is no predominant deliverable, upon delivery of the final element of the arrangement.
Revenues from grants are based upon internal costs that are specifically covered by the grants, and where applicable, an additional facilities and administrative rate that provides funding for overhead expenses. These revenues are recognized when expenses have been incurred by the Company that is related to the grants. The Company has revenue from four major components: molecular information services, LAESI instrument platform, research products, and grants and other collaboration revenues.
Revenue by component was as follows:
| Year Ended | ||||||||
| December 31, 2015 | December 31, 2014 | |||||||
| Molecular information services | $ | 929,076 | $ | 504,750 | ||||
| LAESI instrument platform | 640,060 | 743,250 | ||||||
| Research products | 292,666 | 400,141 | ||||||
| Grants and other collaborations | 141,484 | 120,171 | ||||||
| Gross revenue | $ | 2,003,286 | $ | 1,768,312 | ||||
A small number of customers have accounted for a substantial portion of our revenues in 2015. Five customers represented 52% of gross revenues for the year ended December 31, 2015. One large pharmaceutical company accounted for 20% of our gross revenue in 2015.
F-11
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
2. Summary of Significant Accounting Policies (continued)
Other Payables and Accrued Expenses
Other payables and accrued expenses, which reflect amounts due from non-trade activity, consist of the following at:
| December 31, 2015 | December 31, 2014 | |||||||
| Accrued expenses | $ | 28,112 | $ | 31,104 | ||||
| Accrued interest | 108,731 | 201,844 | ||||||
| Accrued warranties | 50,000 | 50,000 | ||||||
| Accrued payroll and benefits | 124,183 | 219,973 | ||||||
| Accrued sales tax | 616 | - | ||||||
| Unearned revenue | 79,666 | 15,900 | ||||||
| Other payables and accrued expenses | $ | 391,308 | $ | 518,821 | ||||
Warranty Costs
The Company provides for a one-year warranty with the sale of its LAESI instrument. Additionally, the Company sells extended warranties for an additional cost. During 2015, the Company sold one three-year extended LAESI warranty for $45,000, which is recorded in unearned revenue. The extended warranty will be amortized and revenue will be recognized proportionally over the life. All other product warranties are 90 days from the date of delivery of the goods. As the Company does not currently have sufficient historical data on warranty claims, the Company's estimates of anticipated rates of warranty claims and costs are primarily based on comparable industry metrics. The Company engages in extensive product quality programs and processes, including actively monitoring and evaluating the quality of its product. During the year ended December 31, 2015 and 2014, the Company recorded accrued warranty expense of $50,000, respectively.
Foreign Currency
The Company records foreign currency adjustments resulting from international sales of its products and services are reflected in accumulated other comprehensive income (loss).
Advertising
The Company follows the policy of charging the costs of advertising to expense as incurred.
Research and Development Credit
The Company follows the policy of charging the costs of research and development to expense as incurred. During 2014, the Company’s then subsidiary received a research credit from the French Government. Research and Development expense disclosed on the Consolidated Statement of Operations was net of $379,737 or the value of the French Government research credit received in 2014. No research credit was received in 2015.
Net Loss per Share
Basic and diluted loss per common share is computed based on the weighted average number of common shares outstanding. Common share equivalents (which may consist of convertible preferred stock and dividends, options, warrants and convertible debt) are excluded from the computation of diluted loss per share since the effect would be anti-dilutive. Common share equivalents which could potentially dilute basic earnings per share in the future, and which were excluded from the computation of diluted loss per share, totaled approximately 3,092,680 and 3,393,360 at December 31, 2015 and 2014, respectively.
F-12
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
2. Summary of Significant Accounting Policies (continued)
Income Taxes
Deferred income taxes are reported using the liability method. Deferred tax assets are recognized for deductible temporary differences and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax bases. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment.
Stock-Based Compensation
The Company follows the provisions of FASB ASC 718, “Stock-Based Compensation”. Stock-Based compensation expense is estimated as of the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which generally represents the vesting period. Fair value of Company stock options is estimated using the Black-Scholes option-pricing model. The associated compensation expense is recognized on a straight-line basis over the requisite service period, net of estimated forfeitures.
Estimating the fair value for stock options for each grant requires judgment, including estimating stock-price volatility, expected term, expected dividends and risk-free interest rates. The expected volatility rates are estimated based on the volatility of similar entities whose share information is publicly available. The expected term represents the average time that options are expected to be outstanding and is estimated based on the average of the contractual term and the vesting period of the options as provided in SEC Staff Accounting Bulletin #110 as the “simplified” method. The risk-free interest rate for periods approximating the expected term of the options is based on the U.S. Treasury yield curve in effect at the time of grant. Expected dividends are zero as the Company has no plans to issue dividends on Common Stock.
Due to a lack of trading history, the Company utilizes a peer group to estimate its expected volatility assumptions used in the Black-Scholes option-pricing model. The Company completed an analysis and identified four similar companies considering their industry, stage of life cycle, size, and financial leverage. (See Note 8, Stock Options and Stock-Compensation).
Derivative Liabilities
The Company does not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks; however, the Company has certain financial instruments that are embedded derivatives associated with capital raises and common stock purchase warrants. The Company evaluates all its financial instruments to determine if those contracts or any potential embedded components of those contracts qualify as derivatives to be separately accounted for in accordance with FASB ASC 810-10-05-4 and 815-40. This accounting treatment requires that the carrying amount of any embedded derivatives be recorded at fair value at issuance and marked-to-market at each balance sheet date. In the event that the fair value is recorded as a liability, as is the case with the Company, the change in the fair value during the period is recorded as either income or expense. Upon conversion or exercise, the derivative liability is marked to fair value at the conversion date and then the related fair value is reclassified to equity.
Fair value of financial assets and liabilities - Derivative Instruments
We measure the fair value of financial assets and liabilities in accordance with GAAP, which defines fair value, establishes a framework for measuring fair value, and requires certain disclosures about fair value measurements.
GAAP defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. GAAP also establishes the fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
F-13
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
2. Summary of Significant Accounting Policies (continued)
The three levels of inputs used to measure fair value:
Level 1 - quoted prices in active markets for identical assets or liabilities.
Level 2 - quoted prices for similar assets and liabilities in active markets or inputs that are observable.
Level 3 - inputs that are unobservable (for example, the probability of a capital raise in a “binomial” methodology for valuation of a derivative liability directly related to the issuance of common stock warrants).
The Company has entered into certain financial instruments and contracts such as, equity financing arrangements for the issuance of common stock, which include anti-dilution arrangements and detachable stock warrants that are (i) not afforded equity classification, (ii) embody risks not clearly and closely related to host contracts, or (iii) may be net-cash settled by the counterparty. These instruments are recorded as derivative liabilities at fair value at the issuance date. Subsequent changes in fair value are recorded through the statement of operations.
The Company’s derivative liabilities are related to common stock issuances, detachable common stock purchase warrants (“warrants”) issued in conjunction with debt and common stock, or warrants issued to the placement agents for financial instrument issuances. The Company estimates fair values of the warrants that do contain “Down Round Protections” utilizing valuation models and techniques that have been developed and are widely accepted that take into account the additional value inherent in “Down Round Protection.” These widely accepted techniques include “Modified Binomial,” “Monte Carlo Simulation” and the “Lattice Model.” The “core” assumptions and inputs to the “Binomial” model are the same as for “Black-Scholes,” such as trading volatility, remaining term to maturity, market price, strike price, and risk free rates; all Level 2 inputs. Fair value measurements are classified according to the lowest level input or value-driver that is significant to the valuation. A measurement may therefore be classified within Level 3 even though there may be significant inputs that are readily observable. However, a key input to a “Binomial” model (in our case, the “Monte Carlo Simulation” for which we engaged an independent valuation firm to perform) is the probability of a future capital raise. By definition, this input assumption does not meet the requirements for Level 1 or Level 2 outlined above; therefore, the entire fair value calculation is deemed to be Level 3 under accounting requirements due to this single Level 3 assumption. This input to the Monte Carlo Simulation model was developed with significant input from management based on its knowledge of the business, current financial position and the strategic business plan with its best efforts.
As discussed above, financial liabilities are considered Level 3 when their fair values are determined using pricing models or similar techniques and at least one significant model assumption or input is unobservable. For the Company, the Level 3 financial liability is the derivative liability related to the common stock and warrants that include “Down Round Protection” and they were valued using the “Monte Carlo Simulation” technique. This technique, while the majority of inputs are Level 2, necessarily incorporates a Capital Raise Assumption which is unobservable and, therefore, a Level 3 input.
A range of key quantitative assumptions related to the common stock and warrants issued during 2015 and 2014 are as follows:
| December 31, 2015 | ||||||||||||
| Expected Life (Years) | Risk Free Rate | Volatility | Probability of a Capital Raise | |||||||||
| Derivative liabilities | 2-3.33 | 0.16% and 1.31% | 75.54 | % | 100 | % | ||||||
| December 31, 2014 | ||||||||||||||
| Expected Life (Years) | Risk Free Rate | Volatility | Probability of a Capital Raise | |||||||||||
| Derivative liabilities | 1-5 | 1.38 | % | 77.86 | % | 100 | % | |||||||
F-14
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
2. Summary of Significant Accounting Policies (continued)
Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis
The Company’s derivative liabilities are related to common stock issuances, detachable warrants issued in conjunction with debt and common stock, or warrants issued to the placement agents for financial instrument issuances. The derivative liabilities measured at fair value on a recurring basis are summarized below:
| Fair Value Measurements at December 31, 2015 | ||||||||||||||||
| Carrying Value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Derivative liabilities - Common Stock | $ | 777,002 | - | - | $ | 777,002 | ||||||||||
| Derivative liabilities - warrants | 185,399 | - | - | 185,399 | ||||||||||||
| Total | $ | 962,401 | $ | - | $ | - | $ | 962,401 | ||||||||
For the year ended December 31, 2015, the Company revised its assessment of the probability of future down-rounds, due to a probability of closing on subsequent capital raises at levels that are unlikely, but could possibly result in anti-dilution triggers, currently at $6.25 per share. This is based on the Company’s best estimate of its future capital raise activities. The assumptions used for estimating future capital raises could be materially different from the actual results. These differences could materially impact the derivative liability and have a materially adverse effect on the Company’s results of operations and financial condition in the near term.
| Fair Value Measurements at December 31, 2014 | ||||||||||||||||
| Carrying Value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Derivative liabilities - Common Stock | $ | 88,871 | - | - | $ | 88,871 | ||||||||||
| Derivative liabilities - warrants | 65,187 | - | - | 65,187 | ||||||||||||
| Total | $ | 154,058 | - | - | $ | 154,058 | ||||||||||
As a result of the 2014 winter offering, the Company issued 620,986 shares of Common Stock related to anti-dilution protection rights to various stockholders during the three months ended June 30, 2015. As of December 31, 2014, the Company believed that only 404,883 shares of Common Stock were required under these rights. Additionally, the Company had not issued these shares at December 31, 2014, the fair value of which was $1,771,362, was reduced from the derivative liability, and recorded as Common Stock to be issued in additional paid in capital as of December 31, 2014. These shares were issued in April 2015. In May 2015, the Company determined that an additional 216,103 shares of Common Stock were required to be issued under these same anti-dilution rights. The Company issued the remaining shares in June 2015. The fair value of the additional 216,103 shares was $945,451 upon issuing the anti-dilution shares, and the related loss was recognized in the change in fair market value of the derivative liability and recorded as additional paid in capital.
The table below provides a summary of the changes in fair value of the derivative liabilities measured at fair value on a recurring basis:
| Year Ended December 31, 2015 | ||||||||||||
| Derivative liabilities - Common Stock | Derivative liabilities - Warrants | Total Fair Value Measurements Using Level 3 Inputs | ||||||||||
| Beginning balance | $ | 88,871 | $ | 65,187 | $ | 154,058 | ||||||
| Issuance of warrants | - | 5,948 | 5,948 | |||||||||
| Anti-dilution shares issued | (945,451 | ) | - | (945,451 | ) | |||||||
| Unrealized (gain) loss on derivative liabilities | 1,551,703 | 79,883 | 1,631,586 | |||||||||
| Recognition of derivative liabilities | 81,879 | 34,381 | 116,260 | |||||||||
| Ending balance | $ | 777,002 | $ | 185,399 | $ | 962,401 | ||||||
F-15
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
2. Summary of Significant Accounting Policies (continued)
| Year Ended December 31, 2014 | ||||||||||||
| Derivative liabilities - Common Stock | Derivative liabilities - Warrants | Total Fair Value Measurements Using Level 3 Inputs | ||||||||||
| Beginning balance | $ | 558,799 | $ | 64,788 | $ | 623,587 | ||||||
| Issuance of warrants | - | 2,550 | 2,550 | |||||||||
| Anti-dilution shares to be issued | (1,771,362 | ) | - | (1,771,362 | ) | |||||||
| Unrealized (gain) loss on derivative liabilities | 1,301,434 | (11,449 | ) | 1,289,985 | ||||||||
| Recognition of derivative liabilities | - | 9,298 | 9,298 | |||||||||
| Ending balance | $ | 88,871 | $ | 65,187 | $ | 154,058 | ||||||
RECENTLY ISSUED ACCOUNTING PRONOUNCEMENTS
In May 2014, the FASB issued a standard on revenue recognition that provides a single, comprehensive revenue recognition model for all contracts with customers. The standard is principle-based and provides a five-step model to determine the measurement of revenue and timing of when it is recognized. The core principle is that a company will recognize revenue to reflect the transfer of goods or services to customers at an amount that the Company expects to be entitled to in exchange for those goods or services. This standard is to be applied retrospectively and is effective for fiscal years beginning after December 15, 2017 as deferred by the FASB in July 2015. The Company is evaluating the impact that this standard will have on its consolidated financial statements.
In June 2014, the FASB issued authoritative guidance on stock compensation, which requires performance targets that affect vesting and can be achieved after the requisite service period, to be treated as a performance condition. As such, the performance target should not be reflected in estimating the grant-date fair value of the award. Compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. If achievement of the performance target becomes probable before the end of the requisite service period, the remaining unrecognized compensation cost should be recognized prospectively over the remaining requisite service period. The amendments are effective for fiscal years beginning after December 15, 2015. The Company is evaluating the impact that this standard will have on its consolidated financial statements.
In August 2014, the FASB issued authoritative guidance on going concern disclosures and financial statement presentation, which is intended to define management’s responsibility to evaluate whether there is substantial doubt about an organization’s ability to continue as a going concern and to provide related footnote disclosures. Substantial doubt about an entity’s ability to continue as a going concern exists when relevant conditions and events, considered in the aggregate, indicate that it is probable that the entity will be unable to meet its obligations as they become due within one year after the date that the financial statements are issued (or available to be issued). This standard provides guidance to an organization’s management, with principles and definitions that are intended to reduce diversity in the timing and content of disclosures that are commonly provided by organizations in the financial statement footnotes. This standard is effective for annual reporting periods ending after December 15, 2016, and to annual and interim periods thereafter. Early adoption is permitted. The Company is currently in the process of evaluating the impact of adoption of this standard on our consolidated financial statements and related disclosures.
In November, 2014, the FASB issued ASU 2014-16, "Derivatives and Hedging (Topic 815): Determining Whether the Host Contract in a Hybrid Financial Instrument Issued in the Form of a Share Is More Akin to Debt or to Equity (a consensus of the FASB Emerging Issues Task Force)." The ASU clarifies how current guidance should be interpreted in evaluating the economic characteristics and risks of a host contract in a hybrid financial instrument that is issued in the form of a share. Specifically, the amendments clarify that an entity should consider all relevant terms and features, including the embedded derivative feature being evaluated for bifurcation, in evaluating the nature of a host contract. The ASU is effective for fiscal years and interim periods beginning after December 15, 2015. The Company is currently in the process of evaluating the impact of adoption of this standard on our consolidated financial statements.
F-16
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
RECENTLY ISSUED ACCOUNTING PRONOUNCEMENTS (continued)
In April 2015, the FASB issued ASU 2015-03, “Simplifying the Presentation of Debt Issuance Costs.” This standard update requires an entity to present debt issuance costs on the balance sheet as a direct deduction from the related debt liability as opposed to an asset. Amortization of the costs will continue to be reported as interest expense. The update is effective for annual reporting periods (including interim reporting periods within those periods) beginning after December 15, 2015. Early adoption is permitted for financial statements that have not been previously issued, and the new guidance would be applied retrospectively to all prior periods presented. The Company is currently in the process of evaluating the impact of adoption of this standard on our consolidated financial statements and related disclosures.
In July 2015, the FASB issued ASU 2015-11, “Simplifying the Measurement of Inventory.” This standard requires that inventory be valued at the lower of cost or net realizable value, which is defined as the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The update is effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. The Company is currently evaluating the impact that this standard will have on its consolidated financial statements and related disclosures.
In November 2015, the FASB issued ASU 2015-17, "Balance Sheet Classification of Deferred Taxes," which simplifies the presentation of deferred income taxes. This ASU requires that deferred tax assets and liabilities be classified as non-current in a statement of financial position. The Company is currently in the process of evaluating the effect this guidance will have on its consolidated financial statements and related disclosures.
In February 2016, the FASB issued ASU 2016-02, “Leases” (Topic 842), which changes current lease accounting standard by requiring the recognition of lease assets and lease liabilities for all leases, including those currently classified as operating leases. This new guidance is to be applied under a modified retrospective application to the earliest reporting period presented for reporting periods beginning after December 15, 2018. Early adoption is permitted. While the Company is evaluating the comprehensive impact of this guidance, this new guidance would require us to capitalize, at the appropriate discount rate, our operating lease commitments as disclosed in Note 12 Lease Commitments.
3. Bank Line of Credit
The Company has a line of credit that is authorized to $3,000,000 and payable on demand. The interest rate is variable and is .75% plus prime with a minimum rate of 5.87%. As of December 31, 2015 and 2014, the balance was $3,000,000, respectively. During both periods, the interest rate payable was 5.87% and all covenants had been met. Borrowings under the line are secured by the personal guarantee of three board members and the estate of a former board member.
4. Loans Payable to Stockholders
On August 6, 2013, the Company entered into the Note and Warrant Purchase Agreement (the “Summit Agreement”) with Summit Resources, Inc. (“Summit”), an affiliate of Steve Antoline, a director of the Company, pursuant to which Summit acquired (a) a promissory note (the “Summit Note”) bearing simple interest at a rate of 10% per annum, in an aggregate principal amount of $600,000 and (b) a five year warrant to purchase 40,000 shares of Common Stock at an exercise price of $27.50 per share. The fair value of these warrants was estimated to be $134,117, which was recorded as a discount to the promissory note, and will be accreted based on the repayment of the obligation. In accordance with the terms of the Summit Agreement, up to $150,000 of the gross proceeds received by the Company from the sale of each LAESI instrument shall be used to repay the principal amounts due under the Summit Note. The Company repaid $423,915 as of December 31, 2014. Accretion expense of $94,757 was recognized during the year ended December 31, 2014. On March 20, 2015, the Company converted the remaining principal balance of $176,085 and unpaid accrued interest of $87,707, and issued Summit 42,207 shares of Common Stock at a conversion price of $6.25 per share and as an inducement to convert, warrants in aggregate of 21,103 to purchase shares of Common Stock at an exercise price of $12.50 per share. The Company recognized accretion expense of $39,360 during the three months ended March 31, 2015. In addition, the Company recognized debt conversion inducement cost related to the fair value of the warrants issued to Summit of $28,964 during the three months ended March 31, 2015. As of March 31, 2015, the Note was considered fully satisfied.
F-17
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
4. Loans Payable to Stockholders (continued)
During 2014, the Company received advances equal to an aggregate of $1,415,000 from Summit. In exchange for a portion of the advances received, the Company entered into Note and Warrant Purchase Agreements and issued (a) one-year promissory notes bearing simple interest at the rate of 10% per annum to Summit in an aggregate principal amount of $1,415,000 and (b) five-year warrants to purchase up to 56,600 shares of Common Stock at an exercise price of $20.00 per share. On June 3, 2014, the Board approved an increase in the total offering amount of the promissory notes issuable to $1,500,000 from $900,000. The fair value of these warrants was estimated to be $101,177, which was recorded as a discount to the promissory note, and will be accreted based on the repayment of the obligation. The Company repaid $250,000 as of December 31, 2014. Accretion expense of $10,950 was recognized during the year ended December 31, 2014. The outstanding balance, net of discount, was $1,074,773 as of December 31, 2014. On March 20, 2015, the Company converted the $165,000 of outstanding principal and unpaid accrued interest of $105,078, and issued Summit 43,212 shares of Common Stock at a conversion price of $6.25 per share and as an inducement to convert, warrants in aggregate of 21,606 to purchase shares of Common Stock at an exercise price of $12.50 per share. The Company recognized accretion expense of $7,227 during the three months ended March 31, 2015. In addition, the Company recognized debt conversion inducement cost related to the fair value of the warrants issued to Summit of $31,455 during the three months ended March 31, 2015. As of December 31, 2015, the outstanding balance was $1,000,000 or $917,000, net of discount.
During 2015, the Company received aggregate gross proceeds of $200,000 from one director and issued a 10% Convertible Promissory Note due on December 31, 2015. This note has been extended until January 31, 2016. Subsequently, the note was further extended with $25,000 due on March 31, 2016 and the remaining balance due on May 31, 2016. The note is convertible into Common Stock at a conversion price of $8.25 per share. The Company also issued 3,636 shares of Common Stock as commitment fee to the director.
During 2014, the Company received advances equal to an aggregate of $540,000 from various directors and current stockholders of the Company. The Company repaid $370,000 to these related parties. No terms of repayment have been specified on the remaining $170,000 aforementioned advances as of the filing date.
During 2015, the Company received advances equal to an aggregate of $1,402,500 from various directors and current stockholders of the Company. The Company repaid $115,000 to these related parties in 2015. No terms of repayment have been specified on the remaining $1,287,500 aforementioned advances as of the filing date.
5. Long-term Debt
| 1) | Note Payable to the West Virginia Development Office (“WVDO”) |
In March 2007, the Company obtained an 8-year loan in the amount of $685,000 from the WVDO. The note bears interest at 3% providing for 96 monthly principal and interest payments of $8,035 through April 2015, at which time the note was due and payable. The note was secured by equipment costing $1,057,167. On February 4, 2015, the repayment terms were modified, whereby the WVDO approved a deferral of principal and interest until January 31, 2016 with the final payment due January 2017.
| 2) | Note Payable to the West Virginia Economic Development Authority (“WVEDA”) |
In August 2009, the Company obtained a 10-year loan in the amount of $242,631 from the WVEDA. The note bears interest at 4% providing for 120 monthly principal and interest payments of $2,457 through August 2019, at which time the note was due and payable. The note was secured by 50% of equipment costing $531,522. Effective May 1, 2015, the Company and WVEDA entered into the First Amendment to Promissory Note; whereby, the original note was modified so that the remaining principal balance is payable in five annual installments; the next payment of $33,880 will be due on December 31, 2015 with final payment due August 3, 2019. During 2015, the Company repaid $1,832 on the note. On December 17, 2015, the Company elected to defer the remaining 2015 payment until December 2016. If the Company fails to make any of the five remaining payments, the WVEDA shall increase the interest rate by 1.5% on each successive payment failure with all principal and unpaid interest due on August 3, 2019.
F-18
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
5. Long-term Debt (continued)
| 3) | Note Payable to the West Virginia Infrastructure and Jobs Development Council (“WVIJDC”) |
In August 2009, the Company obtained a 10-year loan in the amount of $242,630 from the WVIJDC. The note bears interest at 3.25% providing for 120 monthly principal and interest payments of $2,371 through August 2019, at which time the note was due and payable. The note was secured by 50% of equipment costing $531,522. On February 4, 2015, the repayment terms were modified whereby, the WVIJDC approved a deferral of principal and interest until December 31, 2015 with monthly payments of $2,404 beginning in January 2016 and final payment due April 2021. During 2015, the Company repaid $1,832 of principal on the note. On January 6, 2016, the WVIJDC approved another deferral of principal and interest until January 2017.
| 4) | Note Payable to the West Virginia Economic Development Authority (“WVEDA”) |
In October 2010, the Company issued a 10-year note in the amount of $900,000 from the WVEDA. The note bears interest at 3.26% providing for 120 monthly principal and interest payments of $8,802 through October 2020, at which time the note was due and payable. The note was secured by equipment costing $477,095. Effective May 1, 2015, the Company and WVEDA entered into the First Amendment to Promissory Note; whereby, the original note was modified so that the remaining principal balance is payable in six annual installments; the next payment of $125,990 will be due on December 31, 2015 with final payment due October 21, 2020. During 2015, the Company repaid $57,513 on the note. On December 17, 2015, the Company elected to defer the remaining 2015 payment until December 2016. If the Company fails to make any of the remaining payments, the WVEDA shall increase the interest rate by 1.5% on each successive payment failure with all principal and unpaid interest due on October 21, 2020.
| 5) | Note Payable to the West Virginia Infrastructure & Jobs Development Council (“WVIJDC”) |
In December 2010, the Company issued a 10-year note in the amount of $900,000 from the WVIJDC. The note bears interest at 3.25% providing for 120 monthly principal and interest payments of $8,781 through December 2020, at which time the note was due and payable. The note was secured by equipment costing $1,098,249. On February 4, 2015, the repayment terms were modified whereby, the WVIJDC approved a deferral of principal and interest until December 31, 2015 with monthly payments of $8,902 beginning in January 2016 and final payment due August 2022. During 2015, the Company repaid $57,513 on the note. On January 6, 2016, the WVIJDC approved another deferral of principal and interest until January 2017.
| 6) | Convertible Promissory Note Payable to the West Virginia Jobs Investment Trust Board (“WVJITB”) |
In March 2012, the Company issued an 18-month note in the amount of $290,000 from the WVJITB. The note bears interest at 6% providing for monthly interest-only payments starting April 2012 through August 2013, then final interest and principal payments due September 2013. The note has an adjustable conversion price, initially $50.00 per share, and includes a stock warrant for 2,900 shares. On December 13, 2013, the Company and the WVJITB entered into a Loan Modification Agreement whereby the maturity date changed from September 14, 2013 to $100,000 due on March 15, 2014 and the remaining $190,000 due on June 15, 2014. The WVJITB and the Company signed three addendums to the note extending the maturity date and deferring interest and principal payments until March 2, 2015. During 2014, the Company made interest only payments through October 31, 2014. On February 2, 2015, the Company and WVJITB entered into a Loan Modification Agreement whereby the interest payable for the periods of October 31, 2014 through June 30, 2015 would be due on June 30, 2015. On December 30, 2015, the Company and WVJITB entered into another Loan Modification Agreement to extend the maturity date and unpaid accrued interest payment until March 31, 2016.
F-19
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
5. Long-term Debt (continued)
| 7) | Convertible Promissory Note Payable to the West Virginia Jobs Investment Trust Board (“WVJITB”) |
In April 2012, the Company issued a 3-month note in the amount of $400,000 from the WVJITB. The note bears interest at 10% providing for monthly interest-only payments starting May 2012 through June 2012, then final interest and principal payments due July 2012. The note includes a stock warrant for 3,556 shares. The WVJITB and the Company have signed several addendums to the note extending the maturity date and reducing the price of converting into common shares to $12.50 per share. The WVJITB further extended the maturity date until November 29, 2013, with a $100,000 principal payment due on or before November 15, 2013. The Company repaid the $100,000 as agreed, which reduced the principal outstanding balance to $300,000. The WVJITB and the Company signed four addendums to the note extending the maturity date and deferring interest and principal payments until March 2, 2015. During 2014, the Company made interest only payments through October 31, 2014. On February 2, 2015, the Company and WVJITB entered into a Loan Modification Agreement whereby the interest payable for the periods of October 31, 2014 through June 30, 2015 would be due on June 30, 2015. The maturity date of the loan was also modified and extended to December 31, 2015. On December 30, 2015, the Company and WVJITB entered into another Loan Modification Agreement to extend the maturity date and unpaid accrued interest payment to March 31, 2016.
| 8) | Convertible Promissory Note Payable to the West Virginia High Technology Consortium Foundation (“WVHTCF”) |
In May 2012, the Company issued a 30-month note in the amount of $200,000 from the WVHTCF. The note bears interest at 8% providing for 25 monthly principal and interest payments of $9,001 starting December 2012 through November 2014, then final interest and principal payments due December 2014. The note was secured by 50% of equipment costing $447,320. On April 1, 2015, the Company and WVHTCF entered into the First Amendment to Promissory Note whereby the original note was modified so that the principal balance of $74,726 is payable in nine monthly installments of $8,789 with final payment due December 15, 2015. On December 16, 2015 the note was paid in full.
| 9) | Note Payable to the West Virginia Economic Development Authority (“WVEDA”) |
In June 2012, the Company issued a 10-year note in the amount of $200,000 from the WVEDA. The note bears interest at 2% providing for 120 monthly principal and interest payments of $1,840 through June 2022, at which time the note was due and payable. The note was secured by 50% of equipment costing $447,320. On May 1, 2015, the Company and WVEDA entered into the First Amendment to Promissory Note; whereby, the original note was modified so that the remaining principal balance is payable in eight annual installments; the next payment of $26,673 will be due on December 31, 2015 with final payment due June 11, 2022. On December 17, 2015, the Company elected to defer the 2015 payment until December 2016. If the Company fails to make any of remaining payments, the WVEDA shall increase the interest rate by 1.5% on each successive payment failure with all principal and unpaid interest due on June 11, 2022.
| 10) | Short-term Convertible Debenture |
In May 2015, the Company received an aggregate of $2,000,000 in gross cash proceeds, less transaction costs such as commission and legal fees of $309,344, from one accredited investor in connection with the sale of a 20% original issue discount unsecured convertible debenture (the “Debenture”) due November 22, 2015, pursuant to the terms and condition of a Subscription Agreement. The Company issued (a) the Debenture with a principal amount of $2,500,000 that bears interest at a rate of 10% per annum, and (b) a three-year warrant to purchase up to 300,000 shares of Common Stock of the Company par value $0.001 per share at an exercise price of $8.13 per share. The related fair value of these warrants was estimated to be $411,750, which was recorded as a discount to the promissory note, and will be accreted over the life of the loan. The Debenture may be converted in whole or in part, into shares of Common Stock at a conversion price of $6.25 per share. The Company recognized accretion expense of $411,750 related to the fair value of the warrants and interest expense of $500,000 related to the original issue discount during the year ended December 31, 2015. On December 30, 2015, the Convertible Debenture and accrued interest were converted to 424,222 shares of stock at an exercise price of $6.25 per share.
| 11) | Capital Leases |
From time to time, in the normal course of business, the Company enters into capital leases to finance equipment. As of December 31, 2015, the Company had five capital lease obligations outstanding with imputed interest rates ranging from 5.86% to 6.00%. The leases require 24-60 monthly payments and begin to expire in December 2016 through October 2019. These leases are secured by equipment with an aggregate cost of $1,507,623. As of December 31, 2015, the Company was current on all lease payments.
F-20
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
5. Long-term Debt (continued)
Total debts outstanding are as follows:
| December 31, 2015 | December 31, 2014 | |||||||
| 1) Note Payable to the WVDO | $ | 100,656 | $ | 99,365 | ||||
| 2) Note Payable to the WVEDA | 143,312 | 141,369 | ||||||
| 3) Note Payable to the WVIJDC | 139,229 | 139,128 | ||||||
| 4) Note Payable to the WVEDA | 572,148 | 617,767 | ||||||
| 5) Note Payable to the WVIJDC | 581,987 | 630,738 | ||||||
| 6) Note Payable to the WVJITB | 290,000 | 290,000 | ||||||
| 7) Note Payable to the WVJITB | 300,000 | 300,000 | ||||||
| 8) Note Payable to the WVHTCF | - | 69,888 | ||||||
| 9) Note Payable to the WVEDA | 168,362 | 166,258 | ||||||
| 10) Convertible Debenture | - | - | ||||||
| 11) Capital leases | 754,058 | 820,524 | ||||||
| Total | 3,049,752 | 3,275,037 | ||||||
| Less: current portion | (1,323,594 | ) | (1,457,800 | ) | ||||
| Long-term portion | $ | 1,726,158 | $ | 1,817,237 | ||||
Future required minimum principal repayments over the next five years are as follows:
| Year ending December 31: | Future required minimum principal repayments | |||
| 2016 | 1,323,594 | |||
| 2017 | 577,102 | |||
| 2018 | 371,716 | |||
| 2019 | 299,132 | |||
| 2020 & Thereafter | 478,208 | |||
| Total | $ | 3,049,752 | ||
F-21
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
6. Common Stock
The Company is authorized to issue a total of 260,000,000 of shares of stock, of which 250,000,000 shares are designated Common Stock and 10,000,000 shares are designated Preferred Stock.
Common Stock - par value of $.0001 per share with one vote in respect of each share held. Holders of Common Stock do not have cumulative voting rights. The members of the Board are elected by the affirmative vote of the holders of a majority of the Company’s outstanding Common Stock.
Common Stock issues during 2015 are as follows:
| Common Stock | # Shares Issued | Par Value | Price Per Share | Gross Proceeds | Value of Services Obtained | Par Value | Additional Paid in Capital (1) | |||||||||||||||||||||
| Balance at December 31, 2014 | 2,663,544 | $ | .0001 | Various | $ | 53,708,521 | $ | 1,814,673 | $ | 266 | $ | 55,522,928 | ||||||||||||||||
| Issuance of stock (2) | 522,042 | .0001 | $ | 6.25 | 3,262,765 | - | 52 | 3,262,713 | ||||||||||||||||||||
| Issuance of stock (3) | 1,211,461 | .0001 | $ | 6.25 | - | - | 121 | (121 | ) | |||||||||||||||||||
| Issuance of stock (4) | 620,986 | .0001 | $ | 6.25 | - | - | 63 | (63 | ) | |||||||||||||||||||
| Issuance of stock (5) | 45,381 | .0001 | Various | - | $ | 319,630 | 5 | 319,521 | ||||||||||||||||||||
| Issuance of stock (6) | 3,636 | .0001 | $ | 8.25 | - | $ | 30,000 | - | 30,000 | |||||||||||||||||||
| Issuance of stock (7) | 31,200 | .0001 | $ | 6.25 | - | $ | 195,000 | 3 | 194,997 | |||||||||||||||||||
| Issuance of stock (8) | 227,600 | .0001 | $ | 6.25 | 1,422,500 | - | 23 | 1,422,477 | ||||||||||||||||||||
| Balance at December 31, 2015 | 5,325,850 | $ | 58,393,786 | $ | 2,359,303 | $ | 533 | $ | 60,752,561 | |||||||||||||||||||
| (1) | Balance does not include issuance costs, deferred financing, warrants portion of convertible debentures and stock options. |
| (2) | Shares issued for conversion of an aggregate of $2,841,085 principal amount and $394,173 of accrued interest on promissory notes/convertible notes and $27,506 of accounts payable. Shares do not contain an anti-dilution provision. |
| (3) | Conversion of preferred stock and accrued dividends into shares of Common Stock and contain an anti-dilution provision. |
| (4) | Shares issued under certain anti-dilution privileges. |
| (5) | Shares issued to consultants for services rendered. Shares do not contain anti-dilution provision. |
| (6) | Shares issued to a director as a commitment fee as part of Promissory Note. Shares do not contain anti-dilution provision. |
| (7) | Shares issued to directors for compensation for their service to the Company. Share do not contain anti-dilution provision. |
| (8) | Shares issued contain an anti-dilution provision that expire upon the earlier of 1) three years from date of issuance or 2) uplisting to a senior stock exchange. |
Common Stock issues during 2014 are as follows:
| Common Stock | # Shares Issued | Par Value | Price Per Share | Gross Proceeds | Value of Services Obtained | Par Value | Additional Paid in Capital (1) | |||||||||||||||||||||
| December 31, 2013 Balance | 2,617,709 | .0001 | Various | $ | 53,694,771 | $ | 829,132 | $ | 262 | $ | 54,523,641 | |||||||||||||||||
| Issuance of stock (2) | 44,494 | .0001 | Various | - | 985,541 | 4 | 985,537 | |||||||||||||||||||||
| Stock options exercised (3) | 1,000 | .0001 | $ | 0.55 | 13,750 | - | - | 13,750 | ||||||||||||||||||||
| Stock warrants exercised (4) | 341 | .0001 | $ | 1.77 | - | - | - | - | ||||||||||||||||||||
| Total December 31, 2014 | 2,623,544 | $ | 53,708,521 | $ | 1,814,673 | $ | 266 | $ | 55,522,928 | |||||||||||||||||||
| (1) Activity does not include issuance costs, deferred financing, warrants portion of convertible notes and stock options. |
| (2) Shares issued for services performed and does not contain an anti-dilution provision |
| (3) Shares issued for exercised stock options and does not contain an anti-dilution provision |
| (4) Shares issued under cashless stock warrant exercise and does not contain an anti-dilution provision |
F-22
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
7. Preferred Stock
Preferred Stock - par value of $.0001 per share with one vote in respect of each share held. The Company is authorized to issue Preferred Stock in one or more series and containing such rights, privileges and limitations, including voting rights, dividend rates, conversion privileges, redemption rights and terms, redemption prices and liquidation preferences, as the Board may, from time to time, determine. No shares of the Preferred Stock were issued prior to 2014.
Preferred Stock issues during 2015 are as follows:
| Preferred Stock | # Shares Issued | Par Value | Price Per Share | Gross Proceeds | Par Value | Additional Paid in Capital (1) | ||||||||||||||||||
| Balance at December 31, 2014 | 3,337,725 | $ | .0001 | $ | 2.00 | $ | 6,675,452 | $ | 334 | $ | 6,675,118 | |||||||||||||
| Issuance of stock (2) | 370,050 | .0001 | $ | 2.00 | 740,100 | 37 | 740,063 | |||||||||||||||||
| Stock dividend | 78,040 | .0001 | $ | 2.00 | (156,056 | ) | 8 | 156,048 | ||||||||||||||||
| Conversion of preferred stock | (3,785,815 | ) | .0001 | $ | 2.00 | (7,259,496 | ) | (379 | ) | (7,571,229 | ) | |||||||||||||
| Total December 31, 2015 | - | $ | - | $ | - | $ | - | |||||||||||||||||
(1) Activity does not include issuance costs, deferred financing, warrants portion of convertible notes and stock options.
(2) Shares issued contain an anti-dilution provision expiring upon the conversion to common stock.
Preferred Stock issues during 2014 are as follows:
| Preferred Stock | # Shares Issued | Par Value | Price Per Share | Gross Proceeds | Par Value | Additional Paid in Capital (1) | ||||||||||||||||||
| December 31, 2013 Balance | - | - | - | $ | - | $ | - | $ | - | |||||||||||||||
| Issuance of stock (2) | 2,264,238 | .0001 | $ | 2.00 | 4,528,476 | 227 | 4,528,249 | |||||||||||||||||
| Conversion of convertible debt (2) | 1,073,487 | .0001 | $ | 2.00 | 2,146,976 | 107 | 2,146,869 | |||||||||||||||||
| Total December 31, 2014 | 3,337,725 | $ | 6,675,452 | $ | 334 | $ | 6,675,118 | |||||||||||||||||
(1) Activity does not include issuance costs, deferred financing, warrants portion of convertible notes and stock options.
(2) Shares issued contain an anti-dilution provision expiring upon the conversion to common stock.
During 2014 and 2015, the Company sold units of Preferred Stock consisting of 50,000 shares of Series A convertible Preferred Stock and a three year warrant to purchase 8,000 shares of Common Stock at an exercise price of $9.38 per share. Each share of Preferred Stock has a stated value equal to $2.00. The Preferred Stock automatically converted into shares of Common Stock determined by dividing the stated value by $0.25 per share on March 31, 2015. Under certain circumstances, the holders of the Preferred Stock had voluntary conversion rights, were entitled to receive stock dividends at the rate of 6.0% per annum and are entitled to certain anti-dilution protections.
8. Stock Options and Stock-based Compensation
In 2002, the Board adopted the 2002 Equity Incentive Plan (“the 2002 Plan”) that governed equity awards to employees, directors and consultants of the Company. Under the Plan, 18,000 shares of Common Stock were reserved for issuance. From 2006 through 2012, the 2002 Plan was amended several times to increase the total number of shares authorized under the 2002 Plan to 166,000 shares. During the first quarter 2013, the Board adopted the 2013 Equity Incentive Plan (the “2013 Plan”) and together with the 2002 Plan (the “Plans”) governs the equity awards to employees, directors and consultants of the Company. Under the 2013 Plan, an additional 200,000 shares of Common Stock has been reserved for issuance. On June 18, 2013, the 2013 Plan was approved by holders of a majority of the issued and outstanding shares of Common Stock of the Company. On December 18, 2015, the Board approved an increase of 300,000 shares of Common Stock to be reserved for issuance on January 1, 2016; thus, increasing the total reserved shares to 500,000.
The types of awards permitted under the Plan include qualified incentive stock options (ISO) and non-qualified stock options (NQO), and restricted stock. Each option shall be exercisable at such times and subject to such terms and conditions as the Board may specify. Stock options generally vest over four years and expire no later than ten years from the date of grant.
F-23
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
8. Stock Options and Stock-based Compensation (Continued)
A summary of stock option activity is as follows:
| Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (in years) | ||||||||||
| Outstanding at December 31, 2013 | 203,310 | $ | 27.75 | 7.02 | ||||||||
| Granted | 96,180 | $ | 13.75 | |||||||||
| Exercised | (1,000 | ) | $ | 13.75 | ||||||||
| Cancelled or expired | (17,700 | ) | $ | 27.75 | ||||||||
| Outstanding at December 31, 2014 | 280,790 | $ | 23.00 | 6.69 | ||||||||
| Granted | 158,313 | $ | 8.25 | |||||||||
| Exercised | - | |||||||||||
| Cancelled or expired | (37,100 | ) | $ | 22.50 | ||||||||
| Outstanding at December 31, 2015 | 402,003 | $ | 17.25 | 7.26 | ||||||||
| Exercisable at December 31, 2014 | 183,859 | $ | 27.75 | 6.52 | ||||||||
| Exercisable at December 31, 2015 | 197,547 | $ | 25.25 | 5.59 | ||||||||
The following table summarizes information about stock options at December 31, 2015:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||
| Exercise Price | Outstanding | Weighted Average Remaining contractual life (in years) | Weighted Average Exercise Price | Exercisable | Weighted Average Exercise Price | |||||||||||||||||
| $ | 6.25 | 112,133 | - | |||||||||||||||||||
| $ | 12.00 | 6,000 | 375 | |||||||||||||||||||
| $ | 12.50 | 3,160 | 3,160 | |||||||||||||||||||
| $ | 13.25 | 24,000 | 3,175 | |||||||||||||||||||
| $ | 13.75 | 151,680 | 85,807 | |||||||||||||||||||
| $ | 20.00 | 12,800 | 12,800 | |||||||||||||||||||
| $ | 31.25 | 12,400 | 12,400 | |||||||||||||||||||
| $ | 37.50 | 71,240 | 71,240 | |||||||||||||||||||
| $ | 50.00 | 8,590 | 8,590 | |||||||||||||||||||
| $6.25 - $50.00 | 402,003 | 7.26 | $ | 17.25 | 197,547 | $ | 25.25 | |||||||||||||||
At December 31, 2015, the total aggregate intrinsic value for options currently exercisable and options outstanding was $0. These values represent the total pre-tax intrinsic value based on the estimated fair value of the Company’s stock price of $0.20 as of December 31, 2015. During the year ended December 31, 2015 no shares were exercised, whereas 25,000 shares were exercised during the year ended December 31, 2014.
The following table summarizes the activity of the Company’s stock options that have not vested for the year ended December 31, 2015:
| Shares | Weighted Average Grant-date Fair Value | |||||||
| Nonvested at December 31, 2013 | 43,087 | $ | 0.467 | |||||
| Granted | 79,446 | $ | 0.207 | |||||
| Forfeited | (10,362 | ) | $ | 0.513 | ||||
| Vested | (15,240 | ) | $ | 0.734 | ||||
| Nonvested at December 31, 2014 | 96,931 | $ | 0.258 | |||||
| Granted | 158,313 | $ | 0.155 | |||||
| Forfeited | (37,100 | ) | $ | 0.386 | ||||
| Vested | (13,688 | ) | $ | 0.283 | ||||
| Nonvested at December 31, 2015 | 204,456 | $ | 0.153 | |||||
F-24
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
8. Stock Options and Stock-based Compensation (continued)
The fair value of non-vested options to be recognized in future periods is $722,521, which is expected to be recognized over a weighted average period of 2.3 years. The total fair value of options vested during the year ended December 31, 2015 was $267,638 compared to $288,995 for the year ended December 31, 2014.
Stock-based compensation expense is as follows:
| Year ended | ||||||||
| December 31, 2015 | December 31, 2014 | |||||||
| Selling, general, and administrative expense | $ | 230,016 | $ | 251,101 | ||||
| Research and development expense | 37,622 | 37,894 | ||||||
| Total stock-based compensation expense | $ | 267,638 | $ | 288,995 | ||||
The weighted average grant-date fair value of options granted during the year ended December 31, 2015 was $0.155 and for the year ended December 31, 2014 was $0.207 per option.
The fair value of the option grants was estimated using the Black-Scholes option-pricing model with the following weighted average assumptions:
| Year ended | ||||||||
| December 31, 2015 | December 31, 2014 | |||||||
| Risk-free interest rate | 1.82 | % | 1.91 | % | ||||
| Volatility factor | 65.78 | % | 70.03 | % | ||||
| Weighted average expected life (in years) | 7 | 7 | ||||||
| Dividend rate | 0.0 | % | 0.0 | % | ||||
The Company utilizes a peer group to estimate its expected volatility assumptions used in the Black-Scholes option-pricing model. The Company completed an analysis and identified four similar companies considering their industry, stage of life cycle, size, and financial leverage. Given the Company’s limited history with stock options, the Company’s expected term is based on an average of the contractual term and the vesting period of the options (the SAB 110 “Simplified” method).
9. Stock Warrants
From 2008 through 2015, the Company issued warrants to purchase shares of Common Stock. The warrants are exercisable for three to five years from date of issuance and are exercisable at exercise prices that range from $6.25 to $56.25 per share.
As of December 31, 2015, warrants to purchase 2,853,716 shares of Common Stock were outstanding and exercisable. During the year ended December 31, 2015, the Company recognized a total of $463,827 in interest expense from the accretion of warrants earned, $60,419 in debt conversion costs related to warrants issued as part of debt conversion consideration, $100,603 in consulting services, and $175,694 in placement agent services as a result of an aggregate of 215,806 warrants earned.
| Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (in years) | ||||||||||
| Outstanding at December 31, 2014 | 2,124,488 | $ | 24.50 | 3.27 | ||||||||
| Granted | 825,095 | $ | 8.86 | 2.81 | ||||||||
| Exercised | - | - | ||||||||||
| Cancelled or expired | (95,867 | ) | $ | 50.00 | ||||||||
| Outstanding at December 31, 2015 | 2,853,716 | $ | 19.14 | 2.37 | ||||||||
F-25
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
9. Stock Warrants (continued)
The following table summarizes information about stock warrants at December 31, 2015:
| Warrants Outstanding | ||||||||||||||
| Exercise Price | Outstanding | Weighted Average Remaining Contractual Life (in years) | Weighted Average Exercise Price | |||||||||||
| $ | 6.25 | 186,166 | ||||||||||||
| $ | 8.25 | 300,000 | ||||||||||||
| $ | 9.50 | 707,044 | ||||||||||||
| $ | 11.25 | 69,200 | ||||||||||||
| $ | 12.50 | 90,020 | ||||||||||||
| $ | 18.75 | 442,606 | ||||||||||||
| $ | 20.00 | 56,600 | ||||||||||||
| $ | 27.50 | 762,174 | ||||||||||||
| $ | 28.00 | 10,550 | ||||||||||||
| $ | 50.00 | 173,567 | ||||||||||||
| $ | 55.00 | 3,933 | ||||||||||||
| $ | 56.25 | 51,856 | ||||||||||||
| $6.25 - $56.25 | 2,853,716 | 2.37 | $ | 19.25 | ||||||||||
10. Treasury Stock
Treasury stock is accounted for using the par value method and is constructively cancelled when received.
11. Income Taxes
The provision for income taxes, if any, is comprised of current and deferred components. The current component, if any, presents the amount of federal and state income taxes that are currently reportable to the respective tax authorities and is measured by applying statutory rates to the Company’s taxable income as reported in its income tax returns. The Company has evaluated its income tax positions in accordance with FASB ASC 740. There were no changes to unrecognized tax benefits during 2014. The tax years 2011 through 2014 remain open to review by various taxing authorities.
ASC Topic 740, Income Taxes defines the confidence level that a tax position must meet in order to be recognized in the financial statements. Accordingly, we have assessed uncertain tax positions in each of the tax jurisdictions in which we have operations and account for the related financial statement implications. We recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities based on the technical merits of the position. The tax benefits recognized in the financial statements on a particular tax position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon settlement. The amount of unrecognized tax benefits is adjusted as appropriate for changes in facts and circumstances, such as significant amendments to existing tax law, new regulations or interpretations by the taxing authorities, new information obtained during a tax examination, or resolution of an examination. We recognize both accrued interest and penalties, where appropriate, related to unrecognized tax benefits in income tax expense.
Significant management judgment is required in determining the provision for income taxes, deferred tax assets and liabilities, and any valuation allowance recorded in connection with the deferred tax assets.
The Company provides for a full valuation allowance against the deferred tax asset. Net operating loss carryforwards start to expire beginning 2021 for both federal and state purposes. The net operating tax loss carryforward totals approximately $68,600,000 and $59,800,000 at December 31, 2015 and December 31, 2014, respectively.
We have recorded a full valuation allowance of $27,916,317 and $24,352,000 as of December 31, 2015 and 2014, respectively, due to uncertainties related to our ability to utilize the Company’s deferred tax assets, primarily consisting of certain net operating losses carried forward.
F-26
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
11. Income Taxes (continued)
The valuation allowance is based on management’s current estimates of taxable income for the jurisdictions in which we operate and the period over which the deferred tax assets will be recoverable.
| December 31, 2015 | December 31, 2014 | |||||||
| Current deferred income tax asset: | ||||||||
| Tax net operating loss carry forward | $ | 26,751,533 | $ | 23,305,265 | ||||
| Tax-deferred stock option expense | 1,164,784 | 1,047,309 | ||||||
| Research and development expense | - | - | ||||||
| Total current deferred income tax asset | 27,916,317 | 24,352,574 | ||||||
| Valuation Allowance | (27,916,317 | ) | (24,352,574 | ) | ||||
| Net deferred income tax asset | $ | - | $ | - | ||||
In the event that actual results differ from these estimates, or these estimates are adjusted in future periods, and realization of these deferred tax assets for which the valuation allowance has been provided occur, the provision for income taxes may decrease, raising income and positively impacting the Company’s financial position.
12. Lease Commitments
The Company leases its facility under an operating lease beginning April 2012 through March 2017. The Company also has one equipment operating lease with a term of five years.
Future minimum rental payments are as follows for the year ending December 31, 2015:
| Year ending | Future minimum rental payments | |||||
| 2015 (payment in arrears) | $ | 27,938 | ||||
| 2016 | 172,428 | |||||
| 2017 | 46,707 | |||||
| 2018 | 4,800 | |||||
| 2019 & Thereafter | 4,000 | |||||
Rent expense totals $274,580 for the year ended December 2015 and $371,198 for 2014. As of September 30, 2015, the Company consolidated into one facility. As a result of the consolidation, rent expense declined from 2014 to 2015.
13. Retirement Plan
The Company provides a 401(k) Profit Sharing Plan for elective deferrals whereby participants can defer up to 100% of their wages not to exceed a maximum dollar amount determined by the Federal Government each year. The Company, at its discretion, can make matching contributions to the Plan. The Company may also make qualified non-elective contributions to participants who are not highly compensated employees. All employees meeting age and hours of service requirements are eligible to participate in the Plan after completing one year of service. Participants become vested in employer contributions on a graduated scale with full vesting after five years. No Company contributions have yet been made.
14. Commitments and Contingencies
Legal Proceedings
The Company currently is not a party to any material legal proceeding and has no knowledge of any material legal proceeding contemplated by any governmental authority or third party. The Company may be subject to a number of claims and legal proceedings which, in the opinion of our management, are incidental to normal business operations. In managements’ opinion, although final settlement of these claims and suits may impact the financial statements in a particular period, they will not, in the aggregate, have a material adverse effect on the Company’s financial position, cash flows or results of operations.
| F-27 |
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
14. Commitments and Contingencies (continued)
Indemnity of Directors and Officers
As permitted under Delaware law and required by corporate by-laws, the Company indemnifies and holds harmless its directors and officers for certain events or occurrences while the director or officer is or was serving in such capacity. The maximum potential amount of future payments that could be required under these indemnification obligations is unlimited; however, the Company maintains a Directors and Officers liability insurance policy that enables it to recover a portion of any future amounts paid with a limit of liability of $5,000,000. The Company may incur an additional liability if indemnity for more than the limit of liability occurred, and such liability may have a material adverse effect on our financial position, cash flows and results of operations. As there were no known or pending claims, the Company has not accrued a liability for such claims as of December 31, 2015.
Warranty Costs
The Company provides for a one-year warranty with the sale of its LAESI instrument. Additionally, the Company sells extended warranties for an additional cost. During 2015, the Company sold one three-year extended LAESI warranty for $45,000, which is recorded in unearned revenue. The extended warranty will be amortized and revenue will be recognized proportionally over the life. All other product warranties are 90 days from the date of delivery of the goods. As the Company does not currently have sufficient historical data on warranty claims, the Company's estimates of anticipated rates of warranty claims and costs are primarily based on comparable industry metrics. The Company engages in extensive product quality programs and processes, including actively monitoring and evaluating the quality of its product. During the year ended December 31, 2015 and 2014, the Company recorded accrued warranty expense of $50,000, respectively.
University License Agreements
The Company has agreements with universities related to in-licensed technologies as follows:
AGREEMENT WITH WEST VIRGINIA UNIVERSITY (WVU)
The Company has entered into a License and Exclusive Option to License Agreement with the West Virginia University Research Corporation, a nonprofit West Virginia corporation (“WVURC”) acting for and on behalf of WVU. Under the terms of this Agreement, the WVURC has granted the Company an exclusive option to license technology from the laboratories of certain WVU principal investigators in the field of protein discovery for therapeutic, diagnostic and all other commercial fields worldwide.
Under the terms of this Agreement, the Company pays expenses for the preparation, filing and prosecution of related patent applications, and the Company will pay royalties on the net revenue resulting from the sale of products and services that utilize the WVU subject technology.
AGREEMENT WITH GEORGE WASHINGTON UNIVERSITY (GWU)
In December 2008, the Company entered into an Exclusive License Agreement with George Washington University (Washington D.C.) for technology developed in the laboratory of Dr. Akos Vertes Ph.D., Professor of Chemistry, Professor of Biochemistry & Molecular Biology, Founder and Co-Director of the W.M. Keck Institute for Proteomics Technology and Applications, the Department of Chemistry, who is a science advisor to the Company. The technology field is LAESI - laser ablation electrospray ionization, a new method of bioanalytical analysis that enables high throughput biomolecule characterization.
Under the terms of the license agreement, the Company has the exclusive, worldwide rights to commercialize the technology. The Company is obligated to pay expenses for the preparation, filing and prosecution of related patent applications, and the Company will pay royalties on the net revenues resulting from the sale of products and services that utilize the GWU subject technology. During the year ended December 31, 2015, the Company recorded royalty expenses of approximately $41,505 compared to $45,500 as of December 31, 2014. As of December 31, 2015, the Company’s accounts payable balance includes approximately $64,912 to GWU, of which approximately $54,693 was in arrears, for royalties on LAESI sales compared to $29,300 as of December 31, 2014.
| F-28 |
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
14. Commitments and Contingencies (continued)
In November 2012, the Company entered into a Patent License Agreement with George Washington University (Washington D.C) for technology developed in the laboratory of Akos Vertes Ph.D. The technology field is Laser Desorption/Ionization and Peptide Sequencing on Laser-Induced Silicon Microcolumn Arrays (Matrix) and Nanophotonic Production, Modulation and Switching of Ions by Silicon Microcolumn Arrays.
Under the terms of the license agreement, the Company has an exclusive, worldwide license to make, have made, use, import, offer for sale and sell the licensed products. The Company is obligated to pay a license initiation fee of $25,000 and a minimum license diligence resources of $12,500 in year two and $20,000 each year thereafter to develop and commercialize the products, $30,000 milestone payment upon the first sale of a licensed product, and royalties will be 7% of the net sales of licensed products and 5% of the net sales of combination products for combined cumulative net sales up to $50 million. Thereafter, royalties reduce to 6% and 4%, respectively, after taking into account quarterly minimum royalties of $1,500 for the first four quarters after the first sale of a licensed product, $2,500 for the next four quarters, $3,500 for the next four quarters and $6,000 for each succeeding quarter. During the third quarter of 2015, the Company reached the $30,000 milestone payment under this agreement.
In January 2014, the Company became a subcontractor to GWU in a multi-year project with the Defense Advanced Research Projects Agency (“DARPA”) to develop new tools and methods to elucidate the mechanism of action of a threat agent, drug, biologic or chemical on living cells within 30 days of exposure. During the year ended December 31, 2015, the Company recorded royalty expenses of approximately $3,000 on REDIchip sales. As of December 31, 2015, the Company’s accounts payable balance includes approximately $33,000 to GWU, which includes the milestone commitment.
AGREEMENT WITH OMICS2IMAGE
In 2014, the Company entered into an agreement with Omics2Image related to the “Next Generation Ambient Imaging Mass Spectrometry for (Bio)polymers and Smart Materials” (“COAST Project”). The Company has committed to fund €60,000 or $76,000 related to the COAST Project over the next twelve month period. Omics2Image agrees to evenly share the value gained from the efforts outlined within the scope of the COAST Project, which may include new intellectual property and the development of a commercial, integrated instrument system. As of December 31, 2015, the Company had made two payment of €40,000 or $46,600 to Omics2Image.
Engineering and Design Services
The Company has engaged Dynamic Manufacturing LLC, to produce ten LAESI units and of December 31, 2015, the Company has received eight LAESI units. As of December 31, 2015, the Company has approximately $53,063 in outstanding commitments with Dynamic.
The Company had approximately $0 in outstanding commitments with Pantec for lasers related to the LAESI technology. As of December 31, 2015, the Company owed approximately $22,000 in payables due to Pantec.
15. Contingent Acquisitions
On March 31, 2015, the Company entered into a share purchase agreement, as amended on June 4, 2015 (the “Purchase Agreement”) with the shareholders of vivoPharm Pty. Ltd., a corporation organized under the laws of Australia (“vivoPharm”) to acquire 100% of the capital stock of vivoPharm and its subsidiaries (the “Acquisition”). The purchase price for the vivoPharm shares is $12,063,084 (the “Purchase Price”), of which (i) $100,000.00 was payable by way of deposit prior to the closing of the Acquisition (the “Deposit”), to be credited as partial payment towards the Purchase Price, (ii) $5,690,280 is payable in cash at the closing of the Acquisition (the “Closing Cash Consideration”, together with the Deposit, the “Cash Consideration”) and (iii) the balance of $6,272,804 is payable in the form of 627,280.4 shares of the new Company’s 4% Series A Convertible Preferred Stock, with a liquidation value of $10.00 per share, to be issued to vivoPharm on the closing date of the Acquisition (“Series A Convertible Preferred Stock”). On August 7, 2015 the Company and vivoPharm agreed to extend the closing date to Janaury 31, 2016.
As of December 31, 2015, the Company has not raised any funds for the Acquisition and the Company wrote off the $100,000 deposit. The agreement terminated on January 31, 2016.
| F-29 |
| PROTEA BIOSCIENCES GROUP, INC. |
| Notes to Consolidated Financial Statements |
16. Evaluation of Subsequent Events
Sale of AzurRx Common Stock
Subsequent to December 31, 2015, the Company notified AzurRx of its intent to convert 30 of its Series A convertible Preferred Stock, $0.0001 par value per share, into shares of AzurRx common stock, $0.0001 par value per share, pursuant to the terms and conditions of the Certificate of Designations. The conversion rate was 24,393 shares of Common Stock per share of Series A Preferred Stock. The Company received 731,810 shares of AzurRx common stock upon conversion.
In addition, the Company entered into various Stock Purchase Agreements to sell shares of its AzurRx Common Stock and received aggregate proceeds of $587,100 from the sale.
Conversion of Accrued Interest and Accounts Payable
Subsequent to December 31, 2015, the Company’s Board of Directors authorized the conversion of an aggregate of $33,333 of accrued interest on promissory notes issued by the Company to Summit Resources, Inc. (“Summit”), an affiliate of Steven Antoline, who is a director of the Company, and $8,060 of accounts payable by the Company to Summit, into 6,623 shares of Common Stock at the rate of $6.25.
Stock Issuance
Subsequent to December 31, 2015 the Company issued aggregate of 6,000 shares of Common Stock for services rendered.
Stock Warrants
Subsequent to December 31, 2015, the Company issued warrants to purchase 18,000 shares of Common Stock to Summit as consideration for advances. The warrants are exercisable at an exercise price of $10.00 per share for a five year term.
Subsequent to December 31, 2015, the Company issued warrants to purchase 23,600 shares of Common Stock to West Virginia Jobs Investment Trust Board as consideration for modifying the terms of the loan agreement. The warrants are exercisable at an exercise price of $10.00 per share for a five year term.
Advances from Stockholders
Subsequent to December 31, 2015, the Company received advances equal to an aggregate of $95,000 from certain current directors and related parties. Repayment of $25,000 has been made on the aforementioned advances and repayment of $47,000 on prior advances. No terms of repayment have been specified on the balance of the aforementioned advances as of the filing date.
Convertible Note and Warrant
Subsequent to December 31, 2015, the Company entered into a Subscription Agreement with 1 accredited investor, pursuant to which the Company received an aggregate gross proceeds of $500,000 for the sale of an 23% original issue discount unsecured convertible note (the “Note”) due six months from funding. The Note includes legal fees of $5,000, which was added to the balance of the Note. The Note shall not accrue additional interest. The Company issued to the investor (a) the Note with a principal amount of $655,000, (b) 4,348 shares of the Company’s Common Stock and (c) a five-year warrant to purchase up to 65,500 shares of Common Stock at an exercise price of $18.75, subject to adjustment in certain events as provided therein.
Upon an event of default as defined in the Debenture, the outstanding balance of the Note is convertible at the holder’s option into Common Stock at a conversion price equal to (initially) 70% of the lowest closing bid price for the Common stock in the twenty trading days immediately preceding the conversion, subject to adjustment in certain events as provided therein.
In connection with the Note, the Company paid to a FINRA registered broker dealer that acted as the placement agent an aggregate of approximately $60,000 in cash compensation, representing fees and an expense allowance. In addition, the Company agreed to issue a warrant to purchase an aggregate of approximately 19,650 shares of Common Stock to the placement agent (or its designees) with an exercise price of $6.25 per share and term of five years.
Reverse Stock-Split
On ________, 2016, the Company effected a 1-for-25 reverse stock split of its issued common stock. The reverse stock split did not cause an adjustment to the par value of the authorized shares of common stock. As a result of the reverse stock split, the Company also adjusted the share and per-share amounts under its incentive stock plan and common stock warrant agreements with third parties. No fractional shares were issued in connection with the reverse stock split. All disclosure of common shares and per share common per-share data in the accompanying consolidated financial statements and related notes have been adjusted retroactively to reflect the reverse stock split for all periods presented.
| F-30 |
PROTEA BIOSCIENCES GROUP, INC.
Consolidated Balance Sheets
| March 31, 2016 | December 31, 2015 | |||||||
| (unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | – | $ | 6,450 | ||||
| Trade accounts receivable, net | 318,462 | 195,823 | ||||||
| Inventory | 102,819 | 111,087 | ||||||
| Prepaid expenses | 43,120 | 98,231 | ||||||
| Total current assets | 464,401 | 411,591 | ||||||
| Property and equipment, net | 2,449,672 | 2,626,907 | ||||||
| Other noncurrent assets | 5,248 | 5,248 | ||||||
| Total Assets | $ | 2,919,321 | $ | 3,043,746 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities | ||||||||
| Current maturities on short and | ||||||||
| long-term debt, net of discount | $ | 1,811,381 | $ | 1,323,594 | ||||
| Accounts payable | 1,278,506 | 1,338,367 | ||||||
| Bank overdraft | 264,661 | – | ||||||
| Bank line of credit | 3,000,000 | 3,000,000 | ||||||
| Loans payable to stockholders, net of discount | 2,626,500 | 2,574,500 | ||||||
| Derivative liabilities | 969,869 | 962,401 | ||||||
| Other payables and accrued expenses | 535,116 | 391,308 | ||||||
| Total current liabilities | 10,486,033 | $ | 9,590,170 | |||||
| Long-term debt, net of current maturities | 1,613,420 | 1,726,158 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ Deficit | ||||||||
| Preferred stock ($0.0001 par value; 10,000,000 shares authorized; none issued or outstanding as of March 31, 2016 and December 31, 2015) | – | – | ||||||
| Common stock ($0.0001 par value; 250,000,000 shares authorized; 5,342,820 and 5,325,850 shares issued and outstanding as of March 31, 2016 and December 31, 2015, respectively) | 534 | 533 | ||||||
| Additional paid-in capital | 71,502,299 | 71,324,354 | ||||||
| Accumulated deficit | (80,683,152 | ) | (79,597,608 | ) | ||||
| Accumulated other comprehensive income | 187 | 139 | ||||||
| Total Stockholders’ Deficit | (9,180,132 | ) | (8,272,582 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 2,919,321 | $ | 3,043,746 | ||||
See Accompanying Notes to Consolidated Financial Statements
F-31
PROTEA BIOSCIENCES GROUP, INC.
Consolidated Statements of Comprehensive Loss
(Unaudited)
| THREE MONTHS ENDED MARCH 31, | ||||||||
| 2016 | 2015 | |||||||
| Gross revenue | $ | 475,338 | $ | 390,529 | ||||
| Cost of revenue | (204,375 | ) | (144,566 | ) | ||||
| Gross profit | 270,963 | 245,963 | ||||||
| Selling, general and administrative expenses | (1,604,404 | ) | (2,007,741 | ) | ||||
| Research and development expense | (232,610 | ) | (382,750 | ) | ||||
| Loss from operations | (1,566,051 | ) | (2,144,528 | ) | ||||
| Other income (expense) | ||||||||
| Gain on sale of investment | 637,100 | – | ||||||
| Other income and currency exchange income | 1,386 | 1,541 | ||||||
| Interest expense | (150,329 | ) | (164,050 | ) | ||||
| Debt conversion inducement cost | – | (60,419 | ) | |||||
| Loss on disposal of assets | (182 | ) | (874 | ) | ||||
| Change in fair value of derivative liabilities | (7,468 | ) | (599,168 | ) | ||||
| Total other income (expense) | 480,507 | (822,970 | ) | |||||
| Loss before income taxes | (1,085,544 | ) | (2,967,498 | ) | ||||
| Provision for income taxes | – | – | ||||||
| Net loss | (1,085,544 | ) | (2,967,498 | ) | ||||
| Foreign currency translation adjustment | 48 | (392 | ) | |||||
| Total comprehensive loss | $ | (1,085,496 | ) | $ | (2,967,890 | ) | ||
Weighted average number of shares outstanding – basic and diluted | 5,331,979 | 3,770,589 | ||||||
| Net loss per share – basic and diluted | $ | (0.25 | ) | $ | (0.75 | ) | ||
See Accompanying Notes to Consolidated Financial Statements
(this space intentionally left blank)
F-32
PROTEA BIOSCIENCES GROUP, INC.
Consolidated Statements of Stockholders’ Equity (Deficit)
(Unaudited)
| Accumulated | Total | |||||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Additional | Other | Stockholders’ | ||||||||||||||||||||||||||||
| Par Value $0.0001 | Par Value $0.0001 | Paid-in Capital | Accumulated | Comprehensive | Equity | |||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Common Stock | Deficit | Income | (Deficit) | |||||||||||||||||||||||||
| December 31, 2015 | – | $ | – | 5,325,850 | $ | 533 | $ | 71,324,354 | $ | (79,597,608 | ) | $ | 139 | $ | (8,272,582 | ) | ||||||||||||||||
| Issuance of common stock upon conversion of accrued interest | – | – | 6,623 | – | 41,393 | – | – | 41,393 | ||||||||||||||||||||||||
| Issuance of common stock for services | – | – | 6,000 | 1 | 37,499 | – | – | 37,500 | ||||||||||||||||||||||||
| Issuance of common stock with convertible debenture | – | – | 4,348 | – | 27,174 | – | – | 27,174 | ||||||||||||||||||||||||
| Issuance cost of convertible debt | – | – | – | – | – | – | – | – | ||||||||||||||||||||||||
| Stock-based compensation | – | – | – | – | 63,800 | – | – | 63,800 | ||||||||||||||||||||||||
| Stock warrants issued to debtholders | – | – | – | – | 5,623 | – | – | 5,623 | ||||||||||||||||||||||||
| Stock warrants to be issued to placement agent | – | – | – | – | 2,456 | – | – | 2,456 | ||||||||||||||||||||||||
| Net loss | – | (1,085,544 | ) | – | (1,085,544 | ) | ||||||||||||||||||||||||||
| Foreign currency translation adj. | – | – | – | – | – | – | 48 | 48 | ||||||||||||||||||||||||
| March 31, 2016 | – | $ | – | 5,342,821 | $ | 534 | $ | 71,502,299 | $ | (80,683,152 | ) | $ | 187 | $ | (9,180,132 | ) | ||||||||||||||||
See Accompanying Notes to Consolidated Financial Statements
(this space intentionally left blank)
F-33
PROTEA BIOSCIENCES GROUP, INC.
Consolidated Statements of Cash Flows
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2016 | 2015 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (1,085,544 | ) | $ | (2,967,498 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 158,993 | 174,721 | ||||||
| Stock-based compensation | 63,800 | 88,435 | ||||||
| Issuance of common stock and warrants for services | 38,445 | 9,196 | ||||||
| Accretion of discount on debt | 27,618 | 46,587 | ||||||
| Debt conversion inducement cost | – | 60,419 | ||||||
| Capitalized interest on debt modification | – | – | ||||||
| Issuance of common stock with convertible debt | 27,174 | – | ||||||
| Loss on disposal of assets | 182 | 874 | ||||||
| Gain on sale of investment | (637,100 | ) | – | |||||
| Bad debt expense | – | 119,230 | ||||||
| Change in fair value of derivatives | 7,468 | 599,168 | ||||||
| Net changes in assets and liabilities: | ||||||||
| Trade accounts receivable | (122,639 | ) | 23,190 | |||||
| Prepaid expenses | 55,111 | (1,011 | ) | |||||
| Other receivables | – | (73,078 | ) | |||||
| Inventory | 44,648 | 77,405 | ||||||
| Trade accounts payable | (59,862 | ) | 241,031 | |||||
| Other payables and accrued expenses | 185,201 | 164,143 | ||||||
| Net cash used in operating activities | (1,296,505 | ) | (1,437,188 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchase of and deposits on equipment | – | (16,168 | ) | |||||
| Proceeds from sale of equipment | 25 | 8,755 | ||||||
| Proceeds from sale of investment | 637,100 | – | ||||||
| Net cash provided by (used in) investing activities | 637,125 | (7,413 | ) | |||||
| Cash flows from financing activities: | ||||||||
| Proceeds from sale of preferred stock, net | – | 571,323 | ||||||
| Proceeds from short-term debt | 500,000 | – | ||||||
| Issuance costs for short-term debt | (60,000 | ) | – | |||||
| Bank overdraft | 264,661 | – | ||||||
| Proceeds from shareholder debt | 109,500 | 678,000 | ||||||
| Repayment of shareholder debt | (57,500 | ) | (63,700 | ) | ||||
| Repayment of short and long-term debt | (7,783 | ) | – | |||||
| Repayment of long-term leases | (95,996 | ) | – | |||||
| Capitalized interest on debt modification | – | 16,823 | ||||||
| Net cash provided by financing activities | 652,882 | 1,202,446 | ||||||
| Effect of exchange rate changes on cash | 48 | (392 | ) | |||||
| Net decrease in cash | (6,450 | ) | (242,547 | ) | ||||
| Cash, beginning of period | 6,450 | 322,877 | ||||||
| Cash, end of period | $ | – | $ | 80,330 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Cash paid during the period for interest | $ | 47,459 | $ | 41,160 | ||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||
| Financed equipment | $ | 18,344 | $ | 85,974 | ||||
| Debt converted to preferred stock | – | 550,261 | ||||||
| Accrued interest converted to common stock | 41,393 | 156,056 | ||||||
| Issuance costs for short-term debt | 5,000 | – | ||||||
| Fair value of warrant committed to placement agent | 2,456 | 170,418 | ||||||
See Accompanying Notes to Consolidated Financial Statements
F-34
Notes to Consolidated Financial Statements (Unaudited)
| 1. | Description of Business and Basis of Presentation |
The Company
Protea Biosciences Group, Inc. (referred to herein as “Protea,” “the Company,” “we,” “us” and “our”) is an emerging growth, molecular information company that has developed a revolutionary platform technology that enables the direct analysis, mapping, and display of biologically active molecules in living cells and tissue samples. The technology platform offers new, unprecedented capabilities useful to the pharmaceutical, diagnostic, clinical research, agricultural and life science industries. “Molecular information” refers to the generation and bioinformatic processing of very large data sets, known as “big data”, obtained by applying the Company’s technology to identify and characterize the proteins, metabolites, lipids and other molecules which are the biologically active molecular products of all living cells and life forms.
Our technology is used to improve pharmaceutical development and life science research productivity and outcomes, and to extend and add value to other technologies that are used in research and development, such as three-dimensional tissue models, biomarker discovery, synthetic biologicals, and mass spectrometry. In particular, the Company believes that its ability to rapidly provide comprehensive molecular image-based datasets addresses a universal need of the pharmaceutical, diagnostic and clinical research and life science industries.
The Company’s commercial development is centered on three lines of business:
| · | Molecular information services – We believe that we are the commercial leader in providing multi-modal Mass Spectrometry Imaging (“MSI”), combining Laser Ablation Electrospray Ionization (“LAESI®”), Matrix Assisted Laser Desorption Ionization (“MALDI”) and optical microscopy. Our proprietary services enable the identification and quantitation of both small molecules (e.g., lipids and metabolites) and large molecules (e.g., proteins) and our services portfolio, inclusive of MSI, proteomics, metabolomics, lipidomics and bioinformatics is unique in the industry. Our clients include major pharmaceutical, chemical and biotechnology companies; |
| · | LAESI® Instruments, Software and Consumables – we offer our proprietary LAESI® DP-1000 instrument, software and bioanalytical consumables to corporate and academic research laboratories, and; |
| · | Molecular diagnostics and clinical research – we apply our multi-modal MSI technologies and workflows in partnership with top-tier medical research institutions to co-develop new molecular diagnostic tests that the company believes can be used to improve the diagnosis and prognosis of cancer and provide requisite information useful in predicting the outcome of cancer treatment. Our current test development programs target the differential diagnosis and prognosis of malignant melanoma and the molecular profiling of subpopulations of cells in lung adenocarcinoma. |
Basis of Presentation
The unaudited consolidated financial statements include the accounts of Protea Biosciences Group, Inc. and its wholly-owned subsidiary, Protea Biosciences, Inc. In the opinion of management, these statements have been prepared on a basis consistent with the December 31, 2015 audited consolidated financial statements and include all material adjustments, consisting of normal recurring adjustments and the elimination of material intercompany transactions and balances, necessary for a fair presentation of the Company’s consolidated financial position and results of operations for the interim periods presented. These unaudited, interim, consolidated financial statements have been prepared pursuant to the rules and regulations of the U.S. Securities and Exchange Commission (“SEC”). Accordingly, they do not include certain information and footnotes normally required by accounting principles generally accepted in the United States of America (“GAAP”) for complete consolidated financial statements.
The consolidated results of operations for the three months ended March 31, 2016 are not necessarily indicative of the results of operations for the full fiscal year. These consolidated financial statements and related footnotes should be read in conjunction with the financial statements and footnotes thereto included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2015 filed with the SEC on March 16, 2016 (“2015 Form 10-K”).
Certain amounts included in the Company’s consolidated statement of comprehensive loss for the three months ended March 31, 2015 have been reclassified to conform to 2016 classifications.
F-35
Emerging Growth Company
The Company is an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”) and may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act, and exemptions from the requirements of Sections 14A(a) and (b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), to hold a nonbinding advisory vote of shareholders on executive compensation and any golden parachute payments not previously approved.
The Company has elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1) of the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As a result of this election, our consolidated financial statements may not be comparable to companies that comply with public company effective dates.
We will remain an emerging growth company for up to five years following our initial public offering, that is, until February 2019, although we will lose that status sooner if our revenues exceed $1 billion, if we issue more than $1 billion in non-convertible debt in a three-year period, or if the market value of our common stock that is held by non-affiliates exceeds $700 million as of any June 30.
To the extent that we continue to qualify as a “smaller reporting company”, as such term is defined in Rule 12b-2 under the Exchange Act, after we cease to qualify as an emerging growth company, certain of the exemptions available to us as an emerging growth company may continue to be available to us as a smaller reporting company, including: (1) not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes Oxley Act; (2) scaled executive compensation disclosures; and (3) the requirement to provide only two years of audited financial statements instead of three years.
Critical Accounting Policies
Information with respect to the Company’s critical accounting policies which management believes are the most important to the portrayal of the Company’s reported results of operations and financial condition and that require management’s most difficult, subjective or complex judgements, often as the result of the need to make estimates about the effects of matters that are inherently uncertain, is contained in Part II, Item 7 of our 2015 Form 10-K.
Recently Issued Accounting Pronouncements
There were no changes to the recently issued accounting pronouncements as described in the consolidated financial statements included in our 2015 Form 10-K, except as related to the Financial Accounting Standards Board’s (“FASB”) Accounting Standard Update 2015-03, “Interest–Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs” (“ASU 2015-03”). This standard update, which was issued in April 2015, requires an entity to present debt issuance costs on the balance sheet as a direct deduction from the related debt liability as opposed to an asset. Amortization of the costs will continue to be reported as interest expense. The update is effective for annual reporting periods (including interim reporting periods within those periods) beginning after December 15, 2015. Accordingly, the Company adopted ASU 2015-03 as of January 1, 2016; the adoption of this standard update did not have a material impact on the Company’s consolidated financial statements.
As mentioned above, as permitted under the JOBS Act, the Company has elected to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies.
Going Concern
The Company’s consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has funded its activities to date almost exclusively from debt and equity financings as well as sales of certain assets. Substantially all the Company’s property and equipment are security for outstanding indebtedness. We will continue to require substantial funds to support our molecular information services business and advance global commercialization of our LAESI® instrument platform.
F-36
The Company has experienced negative cash flows from operations since inception. Since inception, our operations have been funded primarily through proceeds received from the issuance of debt and sale of equity securities in private placement offerings and, from time-to-time, sales of certain assets. Management intends to continue to meet the Company’s operating cash flow requirements by raising additional funds from the sale of equity or debt securities and possibly developing corporate development partnerships to advance our molecular information technology development activities by sharing the costs of development and commercialization. We have also worked closely with various parties that financed our short- and long-term debt to obtain extensions and modifications that deferred or reduced amounts due under these obligations. Such extensions and modifications have included, in certain cases, the issuance of warrants. In addition, three members of the Company’s Board of Directors and the estate of a former board member guarantee payment of the Company’s outstanding bank line of credit. Such extensions, modifications and guarantees have been an important part of the Company’s ability to manage its liquidity and short-term capital resources. In addition, as of March 31, 2016, the Company was in an overdraft position with its bank. The amount of the overdraft was $264,661. There can be no assurances that the Company will be able to continue to obtain such extensions and modifications to outstanding debt or use other methods such as guarantees by stockholders, if and when necessary to ensure the Company has the liquidity and capital resources necessary to fund future operations and to continue as a going concern.
We may also consider the sale of additional assets or entering into a transaction such as a merger with a business complimentary to ours. As discussed in Note 3 Sale of Equity Method Investment, we raised net proceeds of $637,100 during the three months ended March 31, 2016 through sales of a portion of our ownership interest in AzurRx BioPharma, Inc. (“AzurRx”). Also, as discussed in Note 19 Evaluation of Subsequent Events, in April 2016 we sold an additional stake in AzurRx generating net proceeds of $625,000.
While we have been successful in raising funds and selling certain assets to fund our operations since inception and we believe that we will be successful in obtaining the necessary capital resources to fund our operations going forward, there are no assurances that we will be able to secure any additional funding or capital resources or the terms of such arrangements. These factors raise substantial doubt about our ability to continue as a going-concern.
The accompanying consolidated financial statements do not include any adjustment that might be necessary if the Company is unable to continue as a going concern.
| 2. | Gross Revenue |
The Company recognizes revenue from three major components: molecular information services, LAESI® instrument platform, and research products. Gross revenue by component was as follows:
| Three Months Ended March 31, | ||||||||
| 2016 | 2015 | |||||||
| Molecular information services | $ | 217,052 | $ | 170,645 | ||||
| LAESI® instrument platform | 179,795 | 153,362 | ||||||
| Research products | 78,491 | 66,522 | ||||||
| Gross revenue | $ | 475,338 | $ | 390,529 | ||||
| 3. | Gain on Sale of Investment |
In the first quarter of 2016, the Company notified AzurRx of its intent to convert 71 shares of its Series A convertible preferred stock, $0.0001 par value per share (“AzurRx Preferred Stock”), the remaining shares of AzurRx Preferred Stock held by the Company, into shares of AzurRx common stock, $0.0001 par value per share (“AzurRx Common Stock”), pursuant to the terms and conditions of the Certificate of Designations. The conversion rate was 24,393.65 shares of AzurRx Common Stock per share of AzurRx Preferred Stock. The Company received 1,731,949 shares of AzurRx Common Stock upon conversion. The Company sold 1,016,941 shares of AzurRx Common Stock during the first quarter of 2016 receiving net proceeds of $637,100, all which had been collected by the end of March 2016.
As of March 31, 2016, the Company’s ownership interest in AzurRx included 815,757 shares of AzurRx Common Stock representing an ownership stake in AzurRx of 11% (on a fully diluted basis). The Company previously accounted for its investment in AzurRx using the “equity method.” However, the Company suspended the use of the equity method of accounting, for this investment in 2014 once the carrying amount of the investment had been reduced to $0. Considering the reduction in the Company’s interest in AzurRx during the first three months of 2016, the Company has discontinued the use of the “equity method” of accounting for this investment, and is now accounting for the investment under the cost method as fair market value is not readily determinable.
See also Note 19 Evaluation of Subsequent Events regarding the Company’s sale of additional AzurRx Common Stock in April 2016.
F-37
| 4. | Derivative Liabilities |
The Company does not use derivative instruments to hedge exposures to cash flow, market or foreign currency risks. However, the Company has certain financial instruments that are embedded derivatives associated with capital raises and common stock purchase warrants. The Company evaluates all of its financial instruments to determine if those contracts or any potential embedded components of those contracts qualify as derivatives to be separately accounted for in accordance with FASB Accounting Standards Codification 815-10-05-4 and 815-40. This accounting treatment requires that the carrying amount of any embedded derivatives be recorded at fair value at issuance and marked-to-market at each balance sheet date. In the event that the fair value is recorded as a liability, as is the case with the Company, the change in the fair value during the period is recorded as either income or expense. Upon conversion or exercise, the derivative liability is marked to fair value at the conversion date and then the related fair value is reclassified to equity.
| 5. | Fair Value of Financial Assets and Liabilities – Derivative Financial Instruments |
We measure the fair value of financial assets and liabilities in accordance with GAAP, which defines fair value, establishes a framework for measuring fair value and requires certain disclosures about fair value measurements.
GAAP defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. GAAP also establishes the fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
The three levels of inputs used to measure fair value:
Level 1 – quoted prices in active markets for identical assets or liabilities
Level 2 – quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 – inputs that are unobservable (for example, the probability of a capital raise in a “binomial” methodology for valuation of a derivative liability directly related to the issuance of common stock warrants)
The Company has entered into certain financial instruments and contracts such as equity financing arrangements for the issuance of Common Stock, which include anti-dilution arrangements (“Down Round Protection”) and detachable warrants that are: (i) not afforded equity classification; (ii) embody risks not clearly and closely related to host contracts, or; (iii) may be net-cash settled by the counterparty. These instruments are recorded as derivative liabilities at fair value at the issuance date. Subsequent changes in fair value are recorded through the consolidated statement of comprehensive loss. For the Company, the Level 3 financial liability is the derivative liability related to the common stock and warrants that include “Down Round Protection,” that are valued using the “Monte Carlo Simulation” technique. This technique, while the majority of inputs are Level 2, necessarily incorporates a capital raise assumption which is unobservable and, therefore, a Level 3 input.
As of March 31, 2016, a range of key quantitative assumptions related to the common stock and warrants that include “Down Round Protection” are as follows:
| Expected life (years) | Risk-free rate | Volatility | Probability of a capital raise | |||||||||
| Derivative liabilities | 1.62 – 3.08 | 0.21% and 0.73% | 77.07 | % | 100 | % | ||||||
Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis
The Company’s derivative liabilities are related to issuances of Common Stock, detachable warrants issued in conjunction with debt and Common Stock or warrants issued to the placement agents for financial instrument issuances. The derivative liabilities measured at fair value on a recurring basis are summarized below:
| Fair value measurements as of March 31, 2016 | ||||||||||||||||
| Carrying value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Derivative liabilities – common stock | $ | 792,135 | $ | – | $ | – | $ | 792,135 | ||||||||
| Derivative liabilities – warrants | 177,734 | – | – | 177,734 | ||||||||||||
| Total | $ | 969,869 | $ | – | $ | – | $ | 969,869 | ||||||||
F-38
| Fair value measurements as of December 31, 2015 | ||||||||||||||||
| Carrying value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Derivative liabilities – common stock | $ | 777,002 | $ | – | $ | – | $ | 777,002 | ||||||||
| Derivative liabilities – warrants | 185,399 | – | – | 185,399 | ||||||||||||
| Total | $ | 962,401 | $ | – | $ | – | $ | 962,401 | ||||||||
The Company’s assessment of the probability of future capital raises is considered to be high. Such capital raise activities are estimated to take place at levels that are unlikely, but could possibly result in anti-dilution triggers (currently at $6.25 per share). The assumptions used for estimating future capital raises could be materially different from the actual results. Any such differences could materially impact the derivative liabilities and have a materially adverse effect on the Company’s results of operations and financial condition in the near term. The table below provides a summary of the changes in fair value of the derivative liabilities measured at fair value on a recurring basis:
| Three Months Ended March 31, 2016 | ||||||||||||
| Total fair value | ||||||||||||
| Derivative | Derivative | measurements | ||||||||||
| liabilities – | liabilities – | using Level 3 | ||||||||||
| common stock | warrants | inputs | ||||||||||
| Balance as of January 1, 2016 | $ | 777,002 | $ | 185,399 | $ | 962,401 | ||||||
| Warrant committed to placement agent (a) | – | 2,456 | 2,456 | |||||||||
| Anti-dilution shares issued | – | – | – | |||||||||
| Unrealized loss (income) on derivative liabilities | 15,133 | (10,121 | ) | 5,012 | ||||||||
| Recognition of derivative liabilities | – | – | – | |||||||||
| Balance as of March 31, 2016 | $ | 792,135 | $ | 177,734 | $ | 969,869 | ||||||
(a) See Note 14 Debt for additional information regarding this warrant
| 6. | Net Loss per Share |
Basic and diluted loss per common share is computed based on the weighted average number of shares of Common Stock outstanding, or 5,331,979 and 3,770,589 for the three months ended March 31, 2016 and March 31, 2015, respectively. Common share equivalents (which may consist of stock options, warrants and convertible debt) are excluded from the computation of diluted loss per share since the effect would be anti-dilutive. Common share equivalents which could potentially dilute basic earnings per share in the future, and which were excluded from the computation of diluted loss per share, totaled approximately 3,204,101 and 2,962,400 shares as of March 31, 2016 and March 31, 2015, respectively.
| 7. | Inventory |
Inventory represents finished goods and work in progress. Finished goods and work in progress consist primarily of specifically identifiable items that are valued at the lower of cost or fair market value using the first-in, first-out (FIFO) method. Inventory consists of the following at:
| March 31, 2016 | December 31, 2015 | |||||||
| Work-in-progress | $ | 91,144 | $ | 98,437 | ||||
| Finished goods | 11,675 | 12,650 | ||||||
| Total inventory | $ | 102,819 | $ | 111,087 | ||||
| 8. | Property and Equipment |
Property and equipment and leasehold improvements are capitalized at cost and depreciated using the straight-line method (the Company used the double-declining balance method for property and equipment placed in service prior to January 2011) over estimated lives as follows:
| Equipment | 5 –10 years | |||
| Vehicle | 5 years | |||
| Leasehold improvements | Life of lease | |||
| Software | 3 years |
F-39
Property and equipment consists of the following:
| March 31, 2016 | December 31, 2015 | |||||||
| Lab equipment | $ | 6,571,438 | $ | 6,617,038 | ||||
| Computer equipment | 557,010 | 540,212 | ||||||
| Office equipment | 191,248 | 191,248 | ||||||
| Leasehold improvements | 212,730 | 212,730 | ||||||
| Accumulated depreciation | (5,082,754 | ) | (4,934,321 | ) | ||||
| Property and equipment, net | $ | 2,449,672 | $ | 2,626,907 | ||||
For the three months ended March 31, 2016 and March 31, 2015, depreciation expense was $158,993 and $174,721, respectively.
| 9. | Trade Accounts Receivable and Other Noncurrent Assets |
Trade accounts receivable, net includes a bad debt allowance of $3,900 and $3,000 for March 31, 2016 and December 31, 2015, respectively.
Other noncurrent assets consisted of deposits totaling $5,248 as of March 31, 2016 and 2015.
| 10. | Bank Overdraft |
As of March 31, 2016, the company held a bank overdraft of $264,661.
| 11. | Bank Line of Credit |
A subsidiary of the Company has a line of credit with a bank that is authorized to $3,000,000 and payable on demand. The interest rate is variable and is 0.75% plus prime with a minimum rate of 5.87% per annum. The line of credit is subject to an annual review and certain covenants. As of March 31, 2016 and December 31, 2015, the outstanding balance was $3,000,000 with interest payable at 5.87% per annum. Borrowings under the line are secured by the personal guarantee of three board members and the estate of a former board member. As of the date of this report, the Company was one month in arrears on scheduled monthly interest payments on the line of credit.
| 12. | Loans Payable to Stockholders |
During the three months ended March 31, 2016, the Company received advances equal to an aggregate of $109,500 from various directors and current stockholders of the Company. The Company repaid $57,500 to three directors during this period, which included payment of $15,000 of the $2,547,500 in aggregate advances and other loans payable to stockholders outstanding as of December 31, 2015. As of March 31, 2016, the outstanding balance of advances and other loans payable was $2,626,500, which included two interest-bearing promissory notes with a combined outstanding balance of $1,117,000 with two stockholders and $1,509,000 in outstanding advances from stockholders that have no terms of repayment and do not bear interest. See also Note 19 Evaluation of Subsequent Events related to additional advances from stockholders subsequent to March 31, 2016.
See Note 15 Common Stock related to the conversion of accrued interest on promissory notes issued by the Company to Summit Resources, Inc. (“Summit”), an affiliate of Steve Antoline, a member of the Company’s Board of Directors, and account payable by the Company to Summit, into shares of Common Stock.
| 13. | Other Payables and Accrued Expenses |
Other payables and accrued expenses, which reflect amounts due from non-trade activity, consist of the following at:
| March 31, 2016 | December 31, 2015 | |||||||
| Accrued expenses | $ | 87,376 | $ | 28,728 | ||||
| Accrued interest | 126,953 | 108,731 | ||||||
| Accrued warranty expense | 65,000 | 50,000 | ||||||
| Accrued payroll and benefits | 155,304 | 124,183 | ||||||
| Unearned revenue | 100,483 | 79,666 | ||||||
| Other payables and accrued expenses | $ | 535,116 | $ | 391,308 | ||||
F-40
| 14. | Debt |
Short-Term Convertible Note
On March 4, 2016, the Company issued to St. George Investments LLC (“St. George Investments”), an accredited investor (as defined in Rule 501(a) under the Securities Act of 1933, as amended) a 23% original issue discount unsecured convertible note (the “March 2016 Convertible Note”) due September 4, 2016, with a principal amount of $655,000, for aggregate gross cash proceeds of $500,000, pursuant to the terms and conditions of a Securities Purchase Agreement dated March 4, 2016 (the “SPA”) between the Company and St. George Investments. In addition to the original issue discount of $150,000, the March 2016 Convertible Note amount includes legal fees of St. George Investments of $5,000. The March 2016 Convertible Note will not accrue additional interest. In connection with the issuance of the March 2016 Convertible Note, the Company issued to St. George Investments: (a) 4,348 shares of Company’s Common Stock (with a value of $27,174), and (b) a five-year warrant to purchase up to 65,500 shares of Common Stock at an exercise price of $18.75 per share, subject to adjustment in certain events as provided therein. Upon an event of default as defined in the March 2016 Convertible Note, the outstanding balance is convertible at the holder’s option into Common Stock at a conversion price equal to (initially) 70% of the lowest closing bid price for the Common Stock in the twenty (20) trading days immediately preceding the conversion, subject to adjustment in certain events as provided therein.
The relative fair value of the warrants were estimated to be $3,439, which was recorded as a discount to the March 2016 Convertible Note and will be accreted over the life of the loan. The Company recognized accretion expense of $536 related to the fair value of these warrants and interest expense of $23,387 related to the original issue discount during the three months ended March 31, 2016. As of March 31, 2016, the outstanding balance of the March 2016 Convertible Note was $655,000, which excludes unamortized discount and transaction costs of $129,516 and $65,000, respectively.
In connection with the March 2016 Convertible Note, the Company paid to a broker dealer registered with the Financial Industry Regulatory Authority that acted as the placement agent (“Placement Agent”) an aggregate of approximately $60,000 in cash compensation, representing fees and an expense allowance. In addition, the Company agreed to issue warrants to purchase an aggregate of approximately 19,650 shares of Common Stock to the Placement Agent (or its designees) with an exercise price of $6.25 per share and a term of five (5) years. As of the date of this report, the warrants had not been issued the Placement Agent. However, the relative fair value of these warrants was estimated to be $2,456, which was recorded as additional paid-in capital.
Convertible Promissory Notes with WVJITB
The Company has outstanding two convertible promissory notes with the West Virginia Jobs Investment Trust Board (“WVJITB”). One is an 18-month note issued in March 2012 with a principal amount of $290,000 (“March 2012 Promissory Note”) and the second a 3-month note issued in April 2012 with principal amount of $400,000 (“April 2012 Promissory Note”). As of March 31, 2016, the principal balance of the March 2012 Promissory Note was $290,000 with the principal balance of the April 2012 Promissory Note having been reduced to $300,000 as the result of a payment made by the Company in 2013. Since the issuance of these notes, the Company and WVJITB have enacted several addendums to both notes, including extending the maturity dates and reducing the price of converting the notes into shares of Common Stock. Note 5 Long-term Debt in Notes to Consolidated Financial Statements included in Part II, Item 8 of the Company’s 2015 Form 10-K provides details regarding these addendums.
On December 31, 2015, the Company and WVJITB entered into another addendum whereby the maturity dates of both promissory notes were extended to March 31, 2016. In return for the extension, in February 2016, the Company granted WVJITB a five-year warrant to purchase 23,600 shares of the Company’s Common Stock at an exercise price of $10.00 per share. Effective as of March 31, 2016, the Company and WVJITB entered into another addendum whereby the maturity dates of both promissory notes were extended to September 30, 2016. In return for the extension, the Company will grant WVJITB another five-year warrant to purchase 23,600 shares of the Company’s Common Stock at an exercise price to be determined by the Board of Directors. This addendum also granted WVJITB the option to convert $200,000 of the $300,000 outstanding principal balance of the April 2012 Promissory Notes to Common Stock at $12.50 per share at or prior to the revised maturity date. The Company would be required to pay WVJITB the remaining (unconverted) principal balance and all accrued and unpaid interest in cash on September 30, 2016. The warrants granted to WVJITB (the warrant granted in February 2016 and the one granted in May 2016) expire upon the Company completing an underwritten offering that results in net cash proceeds to the Company of at least $10.0 million, if such a transaction is completed before the end of the 5-year term.
F-41
Regarding the warrant granted to WVJITB in February 2016, the relative fair value at the issuance date, and the corresponding liability recorded for the warrant, was estimated at $1,239. The warrant will be marked-to-market at each balance sheet date with any change in fair value for a period recorded in other comprehensive income (loss) as either income or expense.
Capital Leases
From time to time, in the normal course of business, the Company enters into capital leases to finance equipment. As of March 31, 2016, the Company had six capital lease obligations outstanding with imputed interest rates ranging from 5.04% to 6.00%. The leases require 24 – 60 monthly payments and begin to expire in December 2016 through October 2019. These leases are secured by equipment with an aggregate cost of $1,507,623. As of March 31, 2016, the Company was current on all lease payments.
Total debts outstanding are as follows (table excludes the bank line of credit as discussed in Note 11 Bank Line of Credit and advances and other loans from stockholders as discussed in Note 12 Loans Payable to Stockholders):
| March 31, 2016 | December 31, 2015 | |||||||
| Note Payable to the WVDO (a) | $ | 92,873 | $ | 100,656 | ||||
| Note Payable to the WVEDA (b) | 143,312 | 143,312 | ||||||
| Note Payable to the WVIJDC (c) | 139,229 | 139,229 | ||||||
| Note Payable to the WVEDA (b) | 572,148 | 572,148 | ||||||
| Note Payable to the WVIJDC (c) | 581,987 | 581,987 | ||||||
| Note Payable to the WVJITB | 290,000 | 290,000 | ||||||
| Note Payable to the WVJITB | 300,000 | 300,000 | ||||||
| Note Payable to the WVEDA (b) | 168,362 | 168,362 | ||||||
| Convertible Debentures | 655,000 | – | ||||||
| Capital leases | 676,406 | 754,058 | ||||||
| Total | 3,619,317 | 3,049,752 | ||||||
| Less: current portion | (1,811,381 | ) | (1,323,594 | ) | ||||
| Less: unamortized discount | (129,516 | ) | – | |||||
| Less: debt issuance costs | (65,000 | ) | – | |||||
| Long-term portion | $ | 1,613,420 | $ | 1,726,158 | ||||
| (a) | West Virginia Development Office |
| (b) | West Virginia Economic Development Authority |
| (c) | West Virginia Infrastructure and Jobs Development Council |
Future required minimum principal repayments over the next five years are as follows (table excludes the bank line of credit as discussed in Note 11 Bank Line of Credit and advances and other loans from stockholders as discussed in Note 12 Loans Payable to Stockholders):
| Future Required Minimum | ||||||
| Year Ended December 31, 2015 | Principal Payments | |||||
| 2016 | $ | 1,879,047 | ||||
| 2017 | 584,815 | |||||
| 2018 | 378,115 | |||||
| 2019 | 299,132 | |||||
| 2020 and thereafter | 478,208 | |||||
| Total | $ | 3,619,317 | ||||
| 15. | Common Stock |
The Company is authorized to issue a total of 260,000,000 shares of stock, of which 250,000,000 shares are designated common stock, par value of $0.0001 per share with one vote in respect of each share held and no cumulative voting rights (“Common Stock”), and 10,000,000 are designated preferred stock, par value of $0.0001 per share with one vote in respect of each share held (“Preferred Stock”).There were no shares of Preferred Stock issued or outstanding as of December 31, 2015 and no such shares were issued during the three months ended March 31, 2016.
F-42
Conversion of Accrued Interest and Accounts Payable
In February 2016, the Company’s Board of Directors authorized the conversion of an aggregate of $33,333 of accrued interest on promissory notes issued by the Company to Summit and $8,060 of account payable (or a total of $41,393) by the Company to Summit, into 6,623 shares of Common Stock at the rate of $6.25 per share. The transaction also included the issuance of warrants to purchase 18,000 shares of Common Stock. The warrants have an exercise price of $10.00 per share and a five-year term. The relative fair value of these warrants at the issuance date, and the corresponding liability recorded for these warrants at that date, was estimated at $945. These warrants will be marked-to-market at each balance sheet date with the change in fair value recorded in other comprehensive loss as either income or expense.
Advertising Services Agreement
In March 2016, the Company entered into an agreement with an advertising firm for certain services to be rendered to the Company over an estimated ninety (90) day period. Besides cash compensation, the agreement required the Company to issue 6,000 shares of Common Stock to the advertising firm as of the execution date of the agreement and an additional 6,000 shares to be issued to the advertising firm thirty (30) days from the execution date of the agreement, assuming the advertising firm is providing the services contemplated and the agreement has not otherwise been terminated. The value of the shares issued in March 2016 was $37,500, which was recorded as a consulting expense.
Common Stock issued are as follows:
| Price | Value of | |||||||||||||||||||||||
| Shares | per | Gross | services | Par | Additional | |||||||||||||||||||
| Issued | share | proceeds | obtained | value | paid-in capital (a) | |||||||||||||||||||
| Balance as of December 31, 2015 | 5,325,850 | Various | $ | 58,393,786 | $ | 2,359,303 | $ | 533 | $ | 60,740,153 | ||||||||||||||
| Issuance (a) | 4,348 | $ | 6.25 | – | – | – | – | |||||||||||||||||
| Issuance (b) | 6,623 | $ | 6.25 | – | 41,393 | – | 41,393 | |||||||||||||||||
| Issuance (c) | 6,000 | $ | 6.25 | – | 37,499 | 1 | 37,500 | |||||||||||||||||
| Balance as of March 31, 2016 | 5,342,821 | $ | 58,393,786 | $ | 2,438,195 | $ | 534 | $ | 60,819,046 | |||||||||||||||
| (a) | Does not include issuance costs, deferred financing, warrants portion of convertible notes and stock options | |
| (b) | Issued for conversion of an aggregate of $33,333 of accrued interest on promissory notes and $8,060 of accounts payable; shares do not contain an anti-dilution provision | |
| (c) | Issued to a consultant for services rendered; shares do not contain an anti-dilution provision |
On December 1, 2015, the Company’s stockholders approved a Certificate of Amendment to the Company’s Certificate of Incorporation to effect a reverse stock split of Common Stock, within a range of no less than one-for-fifteen (1:15) and no more than one-for-twenty five (1:25), with such ratio to be determined by the Board of Directors, in its sole discretion (the “Reverse Split”), and with such Reverse Split to be effective at such time and date within one year after stockholder approval. As of the date of this report, the Company’s Board of Directors had not enacted the Reverse Split and, therefore, the information presented in this report does not include the effects of any such transaction.
| 16. | Stock Options and Stock-Based Compensation |
In 2002, the Board adopted the 2002 Equity Incentive Plan (the “2002 Plan”) that governed equity awards to employees, members of the Board of Directors, and consultants of the Company. Under the 2002 Plan, 18,000 shares of Common Stock were reserved for issuance. From 2006 through 2012, the 2002 Plan was amended several times to increase the total number of shares authorized under this plan to 166,000 shares. In 2013, the Board of Directors adopted the 2013 Equity Incentive Plan (the “2013 Plan”) and, together with the 2002 Plan (the “Plans”), governs the equity awards to employees, members of the Board of Directors, and consultants of the Company. Under the 2013 Plan, an additional 500,000 shares of Common Stock was reserved for issuance.
F-43
The types of awards permitted under the Plans include qualified incentive stock options (“ISO”), non-qualified stock options (“NQO”), and restricted stock. Each stock option shall be exercisable at such times and subject to such terms and conditions as the Board of Directors may specify. Stock options generally vest over four (4) years and expire no later than ten (10) years from the date of grant.
A summary of stock option activity is as follows:
| Weighted | Weighted | |||||||||||
| average | average remaining | |||||||||||
| exercise | contractual life | |||||||||||
| Stock options | price | (in years) | ||||||||||
| Outstanding as of December 31, 2015 | 402,003 | $ | 71.25 | 7.26 | ||||||||
| Granted | – | $ | – | |||||||||
| Exercised | – | $ | – | |||||||||
| Cancelled or expired | (7,800 | ) | $ | 8.00 | ||||||||
| Outstanding as of March 31, 2016 | 394,203 | $ | 17.50 | 6.98 | ||||||||
| Excercisable as of December 31, 2015 | 197,547 | $ | 25.25 | 5.59 | ||||||||
| Excercisable as of March 31, 2016 | 220,040 | $ | 23.75 | 5.38 | ||||||||
The following table summarizes information about stock options at March 31, 2016:
| Stock options outstanding | Stock options exercisable | |||||||||||||||||||||
| Weighted | Weighted | |||||||||||||||||||||
| Weighted average | average | average | ||||||||||||||||||||
| Remaining contractual | exercise | exercise | ||||||||||||||||||||
| Exercise price | Outstanding | Life (in years) | price | Exercisable | price | |||||||||||||||||
| $ | 6.25 | 106,133 | 9,258 | |||||||||||||||||||
| $ | 12.00 | 6,000 | 750 | |||||||||||||||||||
| $ | 12.50 | 3,160 | 3,160 | |||||||||||||||||||
| $ | 13.25 | 23,800 | 4,463 | |||||||||||||||||||
| $ | 13.75 | 150,080 | 97,378 | |||||||||||||||||||
| $ | 20.00 | 12,800 | 12,800 | |||||||||||||||||||
| $ | 31.25 | 12,400 | 12,400 | |||||||||||||||||||
| $ | 37.50 | 71,240 | 71,240 | |||||||||||||||||||
| $ | 50.00 | 8,590 | 8,590 | |||||||||||||||||||
| $ | 6.25 – $50.00 | 394,203 | 6.98 | $ | 17.50 | 220,039 | $ | 23,75 | ||||||||||||||
As of March 31, 2016, the total aggregate intrinsic value for stock options currently exercisable and stock options outstanding was $0. These values represent the total pre-tax, intrinsic value based on the estimated fair value of the Company’s stock price of $3.50 as of March 31, 2016. No stock options were exercised in the three months ended March 31, 2016 or March 31, 2015.
The following table summarizes the activity of the Company’s stock options that have not yet vested:
| Weighted average | ||||||||
| grant-date fair | ||||||||
| Stock options | value (per option) | |||||||
| Non-vested as of December 31, 2015 | 204,457 | $ | 3.814 | |||||
| Granted | – | – | ||||||
| Forfeited | (7,800 | ) | $ | 1.046 | ||||
| Vested | (13,853 | ) | $ | 4.492 | ||||
| Non-vested as of March 31, 2016 | 182,804 | $ | 3.883 | |||||
The fair value of non-vested stock options to be recognized in future periods is $638,004, which is expected to be recognized over a weighted average period of 2.19 years. The total fair value of stock options vested during the three months ended March 31, 2016 was $63,800 compared to $88,435 for the three months ended March 31, 2015.
F-44
Stock-based compensation expense is as follows:
| Three Months Ended March 31, | ||||||||
| 2016 | 2015 | |||||||
| Selling, general, and administrative expense | $ | 58,018 | $ | 77,705 | ||||
| Research and development expense | 5,782 | 10,730 | ||||||
| Total stock-based compensation | $ | 63,800 | $ | 88,435 | ||||
There were no stock options granted during the three months period ended March 31, 2016. The weighted average grant-date fair value of stock options granted during the three months ended March 31, 2015 was $5.75 per stock option.
The fair value of the stock option grants was estimated using the Black-Scholes option-pricing model with the following weighted average assumptions:
| Three Months Ended March 31, | ||||||||
| 2016 | 2015 | |||||||
| Weighted average risk-free interest rate | N/A | 1.93 | % | |||||
| Volatility factor | N/A | 63.90 | % | |||||
| Weighted average expected life (in years) | N/A | 10 | ||||||
| Dividend rate | N/A | 0 | % | |||||
The Company utilizes a peer group to estimate its expected volatility assumptions used in the Black-Scholes option-pricing model. The Company completed an analysis and identified four similar public companies considering their industry, stage of life cycle, size, and financial leverage. Given the Company’s limited history with stock options, the Company’s expected term is based on an average of the contractual term and the vesting period of the options (the SEC Staff Accounting Bulletin No. 110, “Simplified” method).
| 17. | Warrants |
See Note 12 Loans Payable to Stockholders and Note 14 Debt for information related to the issuance of warrants to Summit, WVJITB, and St. George’s Investments for the purchase of 18,000, 23,600, and 65,500 shares of Common Stock, respectively (a total of 107,100 shares), during the three months ended March 31, 2016. See also Note 19 Evaluation of Subsequent Events for information related to warrants issued after March 31, 2016.
As of March 31, 2016, warrants to purchase 2,942,040 shares of Common Stock were outstanding and exercisable. During the three months ended March 31, 2016, the Company recognized a total of $4,678 in interest expense from the accretion of discount related to warrants issued, $945 in debt conversion costs related to warrants issued as part of debt conversion consideration and $2,456 in placement agent fees as a result of an aggregate of 19,650 warrants earned. During the three months ended March 31, 2015, the Company recognized a total of $46,587 in interest expense, $60,419 in debt conversion costs and $9,196 in services related to the issuance of warrants.
| Weighted | Weighted | |||||||||||
| average | average remaining | |||||||||||
| exercise | contractual life | |||||||||||
| Warrants | price | (in years) | ||||||||||
| Outstanding as of December 31, 2015 | 2,853,716 | $ | 19.14 | 2.37 | ||||||||
| Granted | 107,100 | $ | 6.94 | 4.94 | ||||||||
| Exercised | – | $ | – | |||||||||
| Cancelled or expired | (18,775 | ) | $ | 50.00 | ||||||||
| Outstanding as of March 31, 2016 | 2,942,041 | $ | 18.50 | 2.24 | ||||||||
F-45
The following table summarizes information about stock warrants as of March 31, 2016:
| Warrants outstanding | ||||||||||||||
| Weighted | ||||||||||||||
| Weighted average | average | |||||||||||||
| Remaining contractual | exercise | |||||||||||||
| Exercise price | Outstanding | Life (in years) | price | |||||||||||
| $ | 5.00 | 65,500 | ||||||||||||
| $ | 6.25 | 186,166 | ||||||||||||
| $ | 8.25 | 300,000 | ||||||||||||
| $ | 9.50 | 707,044 | ||||||||||||
| $ | 10.00 | 41,600 | ||||||||||||
| $ | 11.25 | 69,200 | ||||||||||||
| $ | 12.50 | 90,021 | ||||||||||||
| $ | 18.75 | 442,606 | ||||||||||||
| $ | 20.00 | 56,600 | ||||||||||||
| $ | 27.50 | 762,174 | ||||||||||||
| $ | 28.00 | 10,550 | ||||||||||||
| $ | 50.00 | 154,792 | ||||||||||||
| $ | 55.00 | 3,933 | ||||||||||||
| $ | 56.25 | 51,856 | ||||||||||||
| $ | 5.00 – $56.25 | 2,942,041 | 2.24 | $ | 18.50 | |||||||||
| 18. | Commitments and Contingencies |
Legal Proceedings
The Company currently is not a party to any material legal proceeding and has no knowledge of any material legal proceeding contemplated by any governmental authority or third party. The Company may be subject to a number of claims and legal proceedings which, in the opinion of our management, are incidental to normal business operations. In management’s opinion, although final settlement of these claims and suits may impact the financial statements in a particular period, they will not, in the aggregate, have a material adverse effect on the Company’s financial position, cash flows or results of operations.
Warranty Expenses
The Company provides for a one-year warranty with the sale of its LAESI® instrument. All other product warranties are ninety (90) days from the date of delivery of the goods. As the Company does not currently have sufficient historical data on warranty claims, the Company’s estimates of anticipated rates of warranty claims and costs are primarily based on comparable industry metrics. The Company engages in extensive product quality programs and processes, including actively monitoring and evaluating the quality of its products. As of March 31, 2016 and December 31, 2015, the Company has accrued warranty expense of $65,000 and $50,000, respectively.
University License Agreements
The Company has agreements with West Virginia University (“WVU”) and George Washington University (GWU) related to in-licensed technologies as follows:
| WVU | The Company has entered into a License and Exclusive Option to License Agreement with the West Virginia University Research Corporation (“WVURC”), a nonprofit West Virginia corporation acting for and on behalf of WVU. Under the terms of this agreement, the WVURC has granted the Company an exclusive option to license technology from the laboratories of certain WVU principal investigators in the field of protein discovery, for therapeutic, diagnostic and all other commercial fields worldwide. Under the terms of this Agreement, the Company pays expenses for the preparation, filing and prosecution of related patent applications, and the Company will pay royalties on the net revenue resulting from the sale of products and services that utilize the WVU subject technology. |
F-46
| GWU | In March 2009, the Company entered into an Exclusive License Agreement with GWU for technology developed in the laboratory of Dr. Akos Vertes Ph.D., Professor of Chemistry, Professor of Biochemistry & Molecular Biology, Founder and Co-Director of the W.M. Keck Institute for Proteomics Technology and Applications, the Department of Chemistry, who is a science advisor to the Company. The technology field is laser ablation electrospray ionization, or LAESI, a new method of bioanalytical analysis that enables high throughput biomolecule characterization. Under the terms of this agreement, the Company has the exclusive, worldwide rights to commercialize the technology. The Company has obtained a registered trademark for “LAESI®”. The Company is obligated to pay expenses for the preparation, filing and prosecution of related patent applications, and the Company pays royalties on the net revenues resulting from the sale of products and services that utilize the GWU subject technology. During the three months ended March 31, 2016 and March 31, 2015, the Company recorded royalty expenses of $9,053 and $11,264, respectively. As of March 31 2016, the Company’s accounts payable balance included $100,596 to GWU, of which approximately $64,356 was in arrears, for royalties on LAESI® sales. |
In November 2012, the Company entered into a Patent License Agreement with GWU for technology developed in the laboratory of Akos Vertes Ph.D. The technology field is Laser Desorption/Ionization and Peptide Sequencing on Laser-Induced Silicon Microcolumn Arrays (“LISMA”) and Nanophotonic Production, Modulation and Switching of Ions by Silicon Microcolumn Arrays (Nanophotonic arrays, or “NAPA”). Under the terms of this agreement, the Company has an exclusive, worldwide license to make, have made, use, import, offer for sale and sell the licensed products. The Company is obligated to pay a license initiation fee of $25,000 and a minimum license diligence resources of $12,500 in year two and $20,000 each year thereafter to develop and commercialize the products, $30,000 milestone payment upon the first sale of a licensed product, and royalties will be 7% of the net sales of licensed products and 5% of the net sales of combination products for combined cumulative net sales up to $50 million. Thereafter, royalties reduce to 6% and 4%, respectively, after taking into account quarterly minimum royalties of $1,500 for the first four quarters after the first sale of a licensed product, $2,500 for the next four quarters, $3,500 for the next four quarters and $6,000 for each succeeding quarter. During the three months ended March 31, 2016, the Company recorded royalty expenses of $1,500 related to sales of REDIchip™. As of March 31, 2016, the Company’s accounts payable balance includes $4,500 payable to GWU related to this agreement.
In January 2014, the Company became a subcontractor to GWU in a multi-year project with the Defense Advanced Research Projects Agency (“DARPA”) to develop new tools and methods to elucidate the mechanism of action of a threat agent, drug, biologic or chemical on living cells within thirty (30) days of exposure. We have commercialized a NAPA-based product out of this arrangement that is currently being sold to research laboratories performing small molecule analysis. This product is called REDIChip™ (an acronym for Resonance-Enhanced Desorption Ionization).
COAST Project
In 2014, the Company entered into an agreement with Omics2Image B.V. (“O2I”) related to the “Next Generation Ambient Imaging Mass Spectrometry for (Bio)polymers and Smart Materials” (the “COAST Project”). As part of this arrangement, the Company agreed to commit €60,000 (approximately $76,000 at that time) to the COAST Project and O2I agreed to evenly share the value gained from the efforts as outlined within the scope of the project, which may include new intellectual property and the development of a commercial, integrated instrument system. As of March 31, 2016, the Company’s accounts payable balance included $22,380 payable to O2I related to this arrangement.
| 19. | Evaluation of Subsequent Events |
Gain on Sale of Investment
In April 2016 the Company sold 500,000 shares of AzurRx Common Stock generating net proceeds of $625,000. After this sale, the Company held 315,757 shares AzurRx Common Stock representing a 4.27% ownership interest in AzurRx, on a fully-diluted basis. See also Note 3 Sale of Equity Method Investment.
Advances from Stockholders
Subsequent to March 31, 2016, the Company received aggregate advances of $64,500 from certain current directors and related parties. As of the date of this report, such outstanding balance of such advances was $2,676,500. No terms of repayment have been specified on the aforementioned advances. See also Note 12 Loans Payable to Stockholders.
| F-47 |
Debt and Issuance of Warrants
As detailed in Note 14 Debt above, the Company entered into an addendum with WVJITB to extend the maturity date for two outstanding convertible promissory notes to September 30, 2016 from March 31, 2016. This addendum, effective March 31, 2016, includes the Company to grant WVJITB a five-year warrant for the purchase of 23,600 shares of the Company’s Common Stock at an exercise price of to be determined by the Board of Directors.
Issuance of Common Stock for Advertising Services
In April 2016 the Company issued 6,000 shares of Common Stock to an advertising firm for services rendered. The issuance was related to an agreement by which the advertising form was to provide certain advertising and marketing services in return for 12,000 shares of the Company’s Common Stock (payable in two installments) as well as cash payments, as stated in the agreement. See also Note 15 Common Stock.
(this space intentionally left blank)
| F-48 |
3,100,000 Shares

Common Stock
PROSPECTUS
Laidlaw & Company (UK) Ltd.
, 2016
ALTERNATE PAGES FOR SELLING STOCKHOLDER PROSPECTUS
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
Subject to Completion, dated ________, 2016
PROSPECTUS

Protea Biosciences Group, Inc.
530,119 Shares of Common Stock
This prospectus covers the resale by the selling stockholders named on page 87 of up to 530,119 shares of our common stock which include up to:
| · | 318,889 shares of common stock; |
| · | 96,940 shares of common stock issuable upon exercise of warrants at an exercise price of $9.38 per share; |
| · | 65,000 shares of our Common Stock issuable upon exercise of warrants at an exercise price of $18.85 per share issued in connection with the Bridge Offering (as defined below); and |
| · | 49,290 shares of our common stock issuable upon exercise of warrants held by Laidlaw & Co. (UK) Ltd., as the placement agent in two private placements consummated in 2015 and the first quarter of 2016 at an exercise price of $6.25 per share. |
The shares offered by this prospectus may be sold by the selling stockholders from time to time in the open market, through privately negotiated transactions or a combination of these methods, at market prices prevailing at the time of sale or at negotiated prices. By separate prospectus, we have registered an aggregate of 3,100,000 shares of our common stock which we are offering for sale to the public through Laidlaw & Co. (UK) Ltd., as representative of the underwriters, which we refer to herein as the Company Offering.
Our common stock is currently traded on the OTCQB marketplace of OTC Markets Group Inc. (“OTCQB”). The last reported sale price of our common stock as reported on the OTCQB on May ___, 2016 was $_____. We intend to list our common stock on The Nasdaq Capital Market under the symbol “PRGB.” In order to comply with certain of the initial listing requirements of The Nasdaq Capital Market, prior to completion of this offering, we intend to consummate a one-for-25 reverse split of the 133,720,519 currently outstanding shares of our common stock, which reverse stock split has been approved by our stockholders. If our common stock is not approved for listing on The Nasdaq Stock Market, we will not consummate the Company Offering.
The distribution of the shares by the selling stockholders is not subject to any underwriting agreement. We will not receive any proceeds from the sale of the shares by the selling stockholders. We will bear all expenses of registration incurred in connection with this offering, but all selling and other expenses incurred by the selling stockholders will be borne by them.
We are an “emerging growth company” under the federal securities laws and will be subject to reduced public company reporting requirements. An investment in our common stock may be considered speculative and involves a high degree of risk, including the risk of a substantial loss of your investment. See “Risk Factors” beginning on page 10 to read about the risks you should consider before buying shares of our common stock. An investment in our common stock is not suitable for all investors. See “Risk Factors—Risks Relating to Ownership of Our Securities.”
You should rely only on the information contained in this prospectus and any prospectus supplement or amendment. We have not authorized anyone to provide you with different information. This prospectus may only be used where it is legal to sell these securities. The information in this prospectus is only accurate on the date of this prospectus, regardless of the time of any sale of securities.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is _______, 2016
| 80 |
SELLING STOCKHOLDERS
This prospectus covers the resale from time to time by the selling stockholders identified in the table below of up to 530,119 shares of our common stock.
The selling stockholders identified in the table below may, from time to time, offer and sell under this prospectus any or all of the shares of our common stock described under the columns “Shares of Common Stock owned Prior to this Offering and Registered hereby” and “Shares Issuable Upon Exercise of Warrants owned Prior to this Offering and Registered hereby” in the table below.
Certain selling stockholders may be deemed to be “underwriters” as defined in the Securities Act. Any profits realized by such selling stockholder may be deemed to be underwriting commissions.
The table below has been prepared based upon information furnished to us. The selling stockholders identified below may have sold, transferred or otherwise disposed of some or all of their shares since the date on which the information in the following table is presented in transactions exempt from or not subject to the registration requirements of the Securities Act. Information concerning the selling stockholders may change from time to time and, if necessary, we will amend or supplement this prospectus accordingly. We cannot give an estimate as to the number of shares of our common stock that will actually be held by the selling stockholders upon termination of this offering because the selling stockholders may offer some or all of their common stock under the offering contemplated by this prospectus or acquire additional shares of common stock. The total number of shares that may be sold hereunder will not exceed the number of shares offered hereby. Please read the section entitled “Plan of Distribution” in this prospectus.
The following table sets forth the name of each selling stockholder, the number of shares of our common stock beneficially owned by such stockholder before this offering, the number of shares to be offered for such stockholder’s account and the number and (if one percent or more) the percentage of the class to be beneficially owned by such stockholder after completion of the offering. The number of shares owned are those beneficially owned, as determined under the rules of the SEC, and such information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares of our common stock as to which a person has sole or shared voting power or investment power and any shares of common stock which the person has the right to acquire within 60 days of __________ ___, 2016 (the “Determination Date”) through the exercise of any option, warrant or right, through conversion of any security or pursuant to the automatic termination of a power of attorney or revocation of a trust, discretionary account or similar arrangement, and such shares are deemed to be beneficially owned and outstanding for computing the share ownership and percentage of the person holding such options, warrants or other rights, but are not deemed outstanding for computing the percentage of any other person. Beneficial ownership percentages are calculated based on 5,348,821 shares of our common stock outstanding as of the Determination Date.
Unless otherwise set forth below, based upon the information furnished to us, (a) the persons and entities named in the table have sole voting and sole investment power with respect to the shares set forth opposite the selling stockholder’s name, subject to community property laws, where applicable, (b) no selling stockholder had any position, office or other material relationship within the past three years, with us or with any of our predecessors or affiliates, and (c) no selling stockholder is a broker-dealer or an affiliate of a broker-dealer. Selling stockholders who are broker-dealers or affiliates of broker-dealers are indicated by footnote. We have been advised that these broker-dealers and affiliates of broker-dealers purchased our common stock in the ordinary course of business, not for resale, and, at the time of purchase, did not have any agreements or understandings, directly or indirectly, with any person to distribute such common stock. The number of shares of our common stock shown as beneficially owned before the offering is based on information furnished to us or otherwise based on information available to us at the timing of the filing of the registration statement of which this prospectus forms a part.
| 81 |
| elling Stockholder | Shares of Common Stock Beneficially Owned Prior to the Offering | Shares of Common Stock owned Prior to this Offering and Registered Hereby | Shares Issuable Upon Exercise of Warrants owned Prior to this Offering and Registered Hereby1 | Shares of Completion of | Percentage of Common Stock Beneficially Owned Upon Completion of the Offering3 | |||||||||||||||
| Ashdale Associates, LLC4 | 15,400 | 10,400 | 5,000 | 0 | * | |||||||||||||||
| Benjamin Hasty | 19,200 | 9,120 | 10,080 | 0 | * | |||||||||||||||
| Bruce C. Ghrist | 2,400 | 1,600 | 800 | 0 | * | |||||||||||||||
| Carl F. Muckenhirn | 6,000 | 4,000 | 2,000 | 0 | * | |||||||||||||||
| Chad Elms | 11,000 | 8,000 | 3,000 | 0 | * | |||||||||||||||
| David L. Yaussy | 4,800 | 3,200 | 1,600 | 0 | * | |||||||||||||||
| David W. Frost | 96,824 | 64,824 | 32,000 | 0 | 1.14 | % | ||||||||||||||
| Douglas B. Pence | 6,000 | 4,000 | 2,000 | 0 | * | |||||||||||||||
| Evans Investments, LLC5 | 12,000 | 8,000 | 4,000 | 0 | * | |||||||||||||||
| Frank Scott Rotruck | 4,800 | 3,200 | 1,600 | 0 | * | |||||||||||||||
| James Ahern | 12,012 | 8,012 | 4,000 | 0 | * | |||||||||||||||
| James Glenn Schutte | 2,400 | 1,600 | 800 | 0 | * | |||||||||||||||
| Jeff L. Stevens | 24,000 | 16,000 | 8,000 | 0 | * | |||||||||||||||
| John D. Scudiere | 2,400 | 1,600 | 800 | 0 | * | |||||||||||||||
| John J. Nulty | 3,000 | 2,000 | 1,000 | 0 | * | |||||||||||||||
| John W. Pyles and Charlotte R. Pyles, JTWROS6 | 4,850 | 3,400 | 1,450 | 0 | * | |||||||||||||||
| Joseph P. Acquavella | 7,834 | 5,234 | 2,600 | 0 | * | |||||||||||||||
| Kenneth D. Dykstra | 3,600 | 2,400 | 1,200 | 0 | * | |||||||||||||||
| Laidlaw & Co. (UK) Ltd.7 | 49,290 | 0 | 49,290 | 0 | * | |||||||||||||||
| Mark Evans as Trustee and His Successor as Trustee of the Mark I. Evans Trust dtd 3/13/20018 | 12,000 | 8,000 | 4,000 | 0 | * | |||||||||||||||
| Matthew D. Eitner | 12,012 | 8,012 | 4,000 | 0 | * | |||||||||||||||
| Michael A. Parimucha | 24,000 | 16,000 | 8,000 | 0 | * | |||||||||||||||
| Michael B. Carroll and Sheila J. Carroll, JTWROS9 | 24,068 | 16,068 | 8,000 | 0 | * | |||||||||||||||
| Michael Watson | 9,600 | 6,400 | 3,200 | 0 | * | |||||||||||||||
| Paul David Crain | 6,240 | 4,160 | 2,080 | 0 | * | |||||||||||||||
| Randy O. Frost | 2,400 | 1,600 | 800 | 0 | * | |||||||||||||||
| Rippee Mineral Management, LLC10 | 6,000 | 4,000 | 2,000 | 0 | * | |||||||||||||||
| Stephen Hart | 3,400 | 2,400 | 1,000 | 0 | * | |||||||||||||||
| Stephen J. Hubner | 3,000 | 2,000 | 1,000 | 0 | * | |||||||||||||||
| Sterne Agee & Leach Inc., C/F David W. Frost IRA 11 | 29,020 | 19,419 | 9,600 | 0 | * | |||||||||||||||
| Sterne Agee & Leach Inc., C/F Donald Goodin R/O IRA 12 | 18,000 | 12,000 | 6,000 | 0 | * | |||||||||||||||
| Sterne Agee & Leach Inc., C/F Donald William Moore IRA13 | 4,800 | 3,200 | 1,600 | 0 | * | |||||||||||||||
| Sterne Agee & Leach Inc., C/F James Edward Nileshwar R/O IRA14 | 3,600 | 2,400 | 1,200 | 0 | * | |||||||||||||||
| Sterne Agee & Leach Inc., C/F Kenneth J. Broadbent R/O IRA15 | 5,760 | 3,840 | 1,920 | 0 |
* | |||||||||||||||
| Sterne Agee & Leach Inc., C/F Michael A. Luzzi SEP IRA16 | 3,120 | 2,080 | 1,040 | 0 | * | |||||||||||||||
| Sterne Agee & Leach Inc., C/F Joseph Acquavella R/O IRA 17 | 3,660 | 2,460 | 1,200 | 0 | * | |||||||||||||||
| Thomas E. Taliaferro Jr. | 9,010 | 6,380 | 2,630 | 0 | * | |||||||||||||||
| Timothy L. Dewey | 60,000 | 40,000 | 20,000 | 0 | * | |||||||||||||||
| William A. Neal | 2,620 | 1,880 | 740 | 0 | * | |||||||||||||||
| 318,889 | 211,230 | |||||||||||||||||||
*less than one percent.
| 82 |
| 1 | An aggregate of 211,230 of the shares of common stock being offered by the selling security holders are issuable upon exercise of common stock purchase warrants. |
| 2 | Assumes all of the shares of common stock to be registered on the registration statement of which this prospectus is a part, including all shares of common stock underlying common stock purchase warrants held by the selling stockholders, are sold in the offering and that shares of common stock beneficially owned by such selling stockholder but not being registered by this prospectus (if any) are not sold. |
| 3 | Percentages are based on the 8,448,820 shares of common stock issued and outstanding as of the Determination Date. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of common stock underlying shares of preferred stock, options or warrants currently exercisable or convertible, or exercisable or convertible within 60 days after the Determination Date are deemed outstanding for computing the percentage of the person holding such shares of preferred stock, options or warrants but are not deemed outstanding for computing the percentage of any other person. |
| 4 | Ronald G. Stovash is the managing member of Ashdale Associates, LLC, and shares joint voting and investment power over the shares owned by Ashdale Associates, LLC, with Robert V. Stovash. Includes warrants covering 5,000 shares of common stock held by Ashdale Associates, LLC. |
| 5 | Mark I. Evans is the managing partner of Evans Investments, LLC, and has sole voting and investment power over the shares owned thereby. Includes warrants covering 4,000 shares of common stock held by Evans Investments, LLC. |
| 6 | John W. Pyles and Charlotte R. Pyles are joint tenants with the right of survivorship and have equal voting and investment power over the shares owned thereby. Includes warrants covering 1,450 shares of common stock held by John W. Pyles and Charlotte R. Pyles, JTWROS. |
| 7 | Matthew D. Eitner is the chief executive officer of Laidlaw & Company (UK) Ltd., and has dispositive power over warrants covering 49,290 shares of common stock held by Laidlaw & Company (UK) Ltd. |
| 8 | Mark Evans is the trustee of the Mark I. Evans Trust dtd 3/13/2001 and has sole voting and investment power over the shares owned thereby. Includes warrants covering 4,000 shares of common stock held by the Mark I. Evans Trust dtd 3/13/2001. |
| 9 | Michael B. Carroll and Sheila J. Carroll are joint tenants with the right of survivorship and have equal voting and investment power over the shares owned thereby. Includes warrants covering 8,000 shares of common stock held by Michael B. Carroll and Sheila J. Carroll, JTWROS. |
| 10 | David Scott Rippee is the manager of Rippee Mineral Management, LLC, and has sole voting and investment power over the shares owned thereby. Includes warrants covering 2,000 shares of common stock held by Rippee Mineral Management, LLC. |
| 11 | David W. Frost is the custodian of Sterne Agee & Leach, Inc., C/F David W. Frost IRA and has sole voting and investment power over the shares owned thereby. Includes warrants covering 9,600 shares of common stock held by Sterne Agee & Leach Inc., C/F David W. Frost IRA. |
| 12 | Donald Goodin is the custodian of Sterne Agee & Leach, Inc., C/F Donald Edward Goodin R/O IRA and has sole voting and investment power over the shares owned thereby. Includes warrants covering 6,000 shares of common stock held by Sterne Agee & Leach, Inc. C/F Donald Edward Goodin R/O IRA. |
| 13 | Donald William Moore is the custodian of Sterne Agee & Leach Inc., C/F Donald William Moore IRA and has sole voting and investment power over the shares owned thereby. Includes warrants covering 1,600 shares of common stock held by Sterne Agee & Leach Inc., C/F Donald William Moore IRA. |
| 14 | James Edward Nileshwar is the custodian of Sterne Agee & Leach, Inc., C/F James Edward Nileshwar R/O IRA and has sole voting and investment power over the shares owned thereby. Includes warrants covering 1,920 shares of common stock held by Sterne Agee & Leach, Inc., C/F James Edward Nileshwar R/O IRA. |
| 15 | Kenneth J. Broadbent is the custodian of Sterne Agee & Leach Inc., C/F Kenneth J. Broadbent R/O IRA and has sole voting and investment power over the shares owned thereby. Includes warrants covering 1,920 shares of common stock held by Sterne Agee & Leach Inc., C/F Kenneth J. Broadbent R/O IRA. |
| 16 | Michael A. Luzzi is the custodian of Sterne Agee & Leach Inc., C/F Michael A. Luzzi SEP IRA and has sole voting and investment power over the shares owned thereby. Includes warrants covering 1,040 shares of common stock held by Sterne Agee & Leach Inc., C/F Michael A. Luzzi SEP IRA. |
| 83 |
| 17 | Joseph Acquavella is the custodian of Sterne Agee & Leach, Inc., C/F Joseph Acquavella R/O IRA and has sole voting and investment power over the shares owned thereby. Includes warrants covering 1,200 shares of common stock held by Sterne Agee & Leach Inc., C/F Joseph Acquavella R/O IRA. |
PLAN OF DISTRIBUTION
The selling stockholders may, from time to time, sell any or all of their shares of our common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. If the shares of our common stock are sold through underwriters, the selling stockholders will be responsible for underwriting discounts or commissions or agent’s commissions. All selling stockholders who are broker-dealers are deemed to be underwriters. These sales may be at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale or at negotiated prices. The selling stockholders may use any one or more of the following methods when selling shares:
| · | any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale; | |
| · | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; | |
| · | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; | |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; | |
| · | transactions other than on these exchanges or systems or in the over-the-counter market; | |
| · | through the writing of options, whether such options are listed on an options exchange or otherwise; | |
| · | an exchange distribution in accordance with the rules of the applicable exchange; | |
| · | privately negotiated transactions; | |
| · | short sales; | |
| · | broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share; | |
| · | pursuant to the Registration Statement on Form 8-A that was filed with the SEC on August 29, 2014; | |
| · | a combination of any such methods of sale; and | |
| · | any other method permitted pursuant to applicable law. |
The selling stockholders may also sell shares under Rule 144 under the Securities Act, if available, rather than under this prospectus. Rule 144 under the Securities Act, which generally permits the resale, subject to various terms and conditions, of restricted securities after they have been held for six months will not immediately apply to our common stock because we were at one time designated as a “shell company” under SEC regulations. Pursuant to Rule 144(i), securities issued by a current or former shell company that otherwise meet the holding period and other requirements of Rule 144 nevertheless cannot be sold in reliance on Rule 144 until one year after the date on which the issuer filed current “Form 10 information” (as defined in Rule 144(i)) with the SEC reflecting that it ceased being a shell company, and provided that at the time of a proposed sale pursuant to Rule 144, the issuer has satisfied certain reporting requirements under the Exchange Act. The filing of our Current Report on Form 8-K on August 29, 2013, with the SEC started the running of such one-year period.
The selling stockholders may also engage in short sales against the box, puts and calls and other transactions in our securities or derivatives of our securities and may sell or deliver shares in connection with these trades.
Broker-dealers engaged by the selling stockholders may arrange for other broker-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The selling stockholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved. Any profits on the resale of shares of our common stock by a broker-dealer acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. Discounts, concessions, commissions and similar selling expenses, if any, attributable to the sale of shares will be borne by a selling stockholder. The selling stockholders may agree to indemnify any agent, dealer or broker-dealer that participates in transactions involving sales of the shares if liabilities are imposed on that person under the Securities Act.
| 84 |
In connection with the sale of the shares of our common stock or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the shares of our common stock in the course of hedging in positions they assume. The selling stockholders may also sell shares of our common stock short and deliver shares of our common stock covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. The selling stockholders may also loan or pledge shares of our common stock to broker-dealers that in turn may sell such shares.
The selling stockholders may from time to time pledge or grant a security interest in some or all of the shares of our common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of our common stock from time to time under this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus.
The selling stockholders also may transfer the shares of our common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus and may sell the shares of our common stock from time to time under this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgees, transferees or other successors in interest as selling stockholders under this prospectus. The selling stockholders also may transfer and donate the shares of our common stock in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The selling stockholders and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions paid, or any discounts or concessions allowed to, such broker-dealers or agents and any profit realized on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the shares of our common stock is made, a prospectus supplement, if required, will be distributed which will set forth the aggregate amount of shares of our common stock being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling stockholders and any discounts, commissions or concessions allowed or re-allowed or paid to broker-dealers. Under the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the shares of our common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with. There can be no assurance that any selling stockholder will sell any or all of the shares of our common stock registered pursuant to the shelf registration statement, of which this prospectus forms a part.
Each selling stockholder has informed us that it does not have any agreement or understanding, directly or indirectly, with any person to distribute our common stock. None of the selling stockholders who are affiliates of broker-dealers, other than the initial purchasers in private transactions, purchased the shares of common stock outside of the ordinary course of business or, at the time of the purchase of the common stock, had any agreements, plans or understandings, directly or indirectly, with any person to distribute the securities.
We are required to pay all fees and expenses incident to the registration of the shares of common stock. Except as provided for indemnification of the selling stockholders, we are not obligated to pay any of the expenses of any attorney or other advisor engaged by a selling stockholder. We have agreed to indemnify the selling stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
If we are notified by any selling stockholder that any material arrangement has been entered into with a broker-dealer for the sale of shares of common stock, we will file a post-effective amendment to the registration statement. If the selling stockholders use this prospectus for any sale of the shares of our common stock, they will be subject to the prospectus delivery requirements of the Securities Act.
The anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of our common stock and activities of the selling stockholders, which may limit the timing of purchases and sales of any of the shares of common stock by the selling stockholders and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of the shares of common stock to engage in passive market-making activities with respect to the shares of common stock. Passive market making involves transactions in which a market maker acts as both our underwriter and as a purchaser of our common stock in the secondary market. All of the foregoing may affect the marketability of the shares of common stock and the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.
Once sold under the registration statement, of which this prospectus forms a part, the shares of common stock will be freely tradable in the hands of persons other than our affiliates.
| 85 |
Our common stock is currently quoted on the OTC Market and trades below $5.00 per share; therefore, our common stock is considered a “penny stock” and subject to SEC rules and regulations which impose limitations upon the manner in which such shares may be publicly-traded. These regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure
schedule explaining the penny stock market and the associated risks. Under these regulations, certain brokers who recommend such securities to persons other than established customers or certain accredited investors must make a special written suitability determination regarding such a purchaser and receive such purchaser’s written agreement to a transaction prior to sale. These regulations have the effect of limiting the trading activity of the common stock and reducing the liquidity of an investment in the common stock.
USE OF PROCEEDS
We will not receive proceeds from sales of our common stock made under this prospectus.
DETERMINATION OF OFFERING PRICE
There currently is a limited public market for our common stock. The selling stockholders will determine at what price they may sell the offered shares, and such sales may be made at prevailing market prices or at privately negotiated prices. See “Plan of Distribution” below for more information.
LEGAL MATTERS AND INTERESTS OF NAMED EXPERTS
The validity of the securities being offered by this prospectus will be passed upon for us by CKR Law LLP, New York, New York. CKR Law LLP and its principals have accepted our common stock in exchange for services rendered to us in the past and, although the law firm and its principals are under no obligation to do so, they may continue to accept our common stock for services rendered by them.
| 86 |
* Shares

Common Stock
PROSPECTUS
, 2016
| 87 |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following is an itemized statement of all expenses, all of which we will pay, in connection with the registration of the common stock offered hereby:
| Amount | ||||
| SEC registration fee | $ | 2,375.40 | ||
| Nasdaq Capital Market listing fee | 50,000 | |||
| FINRA filing fee | ||||
| Print fees | 2,500 | * | ||
| Legal fees | 500,000 | * | ||
| Accounting fees and expenses | 100,000 | * | ||
| Transfer Agent Fees and Expenses | * | |||
| Miscellaneous Fees and Expenses | * | |||
| Total | $ | 686,000 | * | |
* Estimated.
Item 14. Indemnification of Directors and Officers.
We are subject to the laws of Delaware on corporate matters, including its indemnification provisions. Section 145 of the General Corporation Law of Delaware (the “DGCL”) provides that Delaware corporations are empowered, subject to certain procedures and limitations, to indemnify any person against expenses (including attorney’s fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by him in connection with any threatened, pending, or completed action, suit, or proceeding (including a derivative action) in which such person is made a party by reason of his being or having been a director, officer, employee, or agent of the company (each, an “Indemnitee”); provided that the right of an Indemnitee to receive indemnification is subject to the following limitations: (i) an Indemnitee is not entitled to indemnification unless he acted in good faith and in a manner that he reasonably believed to be in, or not opposed to, the best interests of the company, and, with respect to any criminal action or proceeding, had no reasonable cause to believe such conduct was unlawful, and (ii) in the case of a derivative action, an Indemnitee is not entitled to indemnification in the event that he is judged to be liable to the company (unless and only to the extent that the court determines that the Indemnitee is fairly and reasonably entitled to indemnification for such expenses as the court deems proper). The statute provides that indemnification pursuant to our provisions is not exclusive of other rights of indemnification to which a person may be entitled under any bylaw, agreement, vote of stockholders, or disinterested directors, or otherwise.
Pursuant to Article Seven of our Certificate of Incorporation (“Article Seven”), we are authorized to provide indemnification of (and advancement of expenses to) directors, officers, employees and agents (and any other persons to which Delaware law permits us to provide indemnification) through bylaw provisions, agreements with such agents or other persons, vote of stockholders or disinterested directors or otherwise, in excess of the indemnification and advancement otherwise permitted by Section 145 of the DGCL, subject only to limits created by applicable Delaware law (statutory or non-statutory), with respect to action for breach of duty to us, our stockholders and others.
Article Seven also states that our directors shall not be personally liable to us or any stockholder for monetary damages for breach of fiduciary duty as a director, except of any matter in respect of which such director shall be liable under Section 174 of the DGCL or shall be liable because the director (1) shall have breached his duty of loyalty to us or our stockholders, (2) shall have acted in a manner involving intentional misconduct or a knowing violation of law or, in failing to act, shall have acted in a manner involving intentional misconduct or a knowing violation of law, or (3) shall have derived an improper personal benefit. Article Seven further states that the liability of our directors shall be eliminated or limited to the fullest extent permitted by the DGCL, as amended.
Under Article Eight of our Certificate of Incorporation, any person who was or is made a party or is threatened to be made a party to or is in any way involved in any threatened, pending or completed action suit or proceeding, whether civil, criminal, administrative or investigative, including any appeal therefrom, by reason of the fact that he is or was a director or officer of ours or was serving at our request as a director or officer of another entity or enterprise (including any subsidiary), shall be indemnified and held harmless by us and we are required to advance all expenses incurred by such person in defense of any such proceeding prior to its final determination, to the fullest extent authorized by the DGCL. These rights are not exclusive of any other rights to which those seeking indemnification may otherwise be entitled.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. No pending material litigation or proceeding involving our directors, executive officers, employees or other agents as to which indemnification is being sought exists, and we are not aware of any pending or threatened material litigation that may result in claims for indemnification by any of our directors or executive officers.
Item 15. Recent Sales of Unregistered Securities.
During the past three years, the registrant has issued and sold the following unregistered securities:
Common Stock and Warrants
On March 6, 2013, the Company issued a warrant (the “Antoline Warrant”) to an affiliate of Steve Antoline, a director of the Company, to purchase up to 44,000 shares of the Company’s common stock at an exercise price of $27.50 per share pursuant to that certain Warrant Purchase and Reimbursement Agreement, dated March 6, 2013 by and between the Company and Steve Antoline (the “Reimbursement Agreement”), a director of the Company, as consideration for Mr. Antoline’s agreement to issue a letter of credit for the benefit of MPR Associates, Inc. for the purpose of guaranteeing an amount equal to $600,000 due by the Company to MPR in connection with the production of certain LAESI instruments.
The Antoline Warrant was issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that it was issued to an accredited investor and the Company did not engage in any general solicitation, the purchaser was the sole offeree and had full access to all information concerning the Company that was requested. No commissions were paid in connection with the issuance of the Antoline Warrant.
On March 21, 2013, the Company entered into a Securities Purchase Agreement (the “March Purchase Agreement”) with El Coronado pursuant to which El Coronado was granted an option to purchase, through May 31, 2013, up to 240,000 shares of common stock and warrants to purchase up to 180,000 shares of common stock at a purchase price of $12.50 per share for an aggregate purchase price equal to $3,000,000 pursuant to which the Company issued 54,800 shares of common stock (the “2013 El Coronado Shares”) and warrants to purchase 41,100 shares of common stock (the “2013 El Coronado Warrants”) for aggregate gross proceeds of $685,000. The 2013 El Coronado Warrants are exercisable at an exercise price of $27.50 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof. The 2013 El Coronado Shares and Warrants were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that they were issued to an accredited investor and the Company did not engage in any general solicitation. The Company paid cash commissions equal to $54,800 in connection with the issuance of the 2013 El Coronado Shares and 2013 El Coronado Warrants.
On April 5, 2013, the Company issued 6,000 shares of common stock at $12.50 per share and warrants to purchase 7,500 shares of common stock for $27.50 per share for services rendered pursuant to the terms of a Consulting Agreement by and between the Company and the consultant, dated March 25, 2013. The warrants are exercisable for a term of five years from the issue date of the warrants. The shares and warrants were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that the investor was the sole offeree, had access to all information concerning the Company and the Company did not engage in any general solicitation.
On April 17, 2013, as a result of the sale of shares of common stock of the Company at a per share price less than $50.00 per share, pursuant to the terms and conditions of triggering the anti-dilution provisions set forth in those certain subscription agreements and warrants (the “Offering Documents”) issued in connection with that certain Private Placement prospectus dated August 6, 2012, the Company issued 6,000 shares of common stock for no additional consideration to certain investors that purchased securities pursuant to the Offering Documents.
From April 23, 2013 to June 5, 2013, in connection with Company direct issuances (the “Spring 2013 Company Issuances”), the Company issued an aggregate of 122,400 shares of the Company’s common stock and warrants to purchase an aggregate of 91,800 shares of common stock to certain accredited investors for aggregate gross proceeds equal to $1,530,000 pursuant to the terms and conditions of a Securities Purchase Agreement and a warrant (the “Spring 2013 Warrants”). The Spring 2013 Warrants are exercisable for a term of five years from the issue date, and each purchaser has the right to purchase the number of shares of common stock equal to 75% of the number of shares purchased by such investor, at an exercise price of $27.50 per share. The shares and Spring 2013 Warrants are subject to certain anti-dilution rights in accordance with the terms of the Securities Purchase Agreement, if at any time after the issue date of the securities through December 31, 2013, the Company issues or sells any additional shares of common stock at a purchase price less than $12.50 per share.
II-2
The shares and Spring 2013 Warrants were issued pursuant to the exemption from registration provided by Rule 506 of Regulation D under the Securities Act in that they were issued to accredited investors, the Company did not engage in any general advertisement or general solicitation in connection with the offering of the securities, and the Company was available to answer any questions by and purchaser. Cash commissions were not paid in connection with the sale of the shares and Spring 2013 warrants.
On July 17, 2013, the Company issued an aggregate of 15,100 shares of common stock for no additional consideration to certain stockholders in connection with the anti-dilution rights applicable to such investor’s investments. In addition, the exercise price of the warrants issued to such investors in connection with the anti-dilution rights were reduced from $56.25 to $28.00 per share.
On July 23, 2013, in connection with a private placement offering, the Company issued an aggregate of 12,000 shares of common stock at $12.50 per share and warrants to purchase up to 9,000 shares of common stock at a purchase price of $150,000. The warrants are exercisable for five years from date of issuance at an exercise price of $27.50 per share. The shares and warrants were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that the investors were the sole offerees, had access to all information concerning the Company and the Company did not engage in any general solicitation. Cash commissions were not paid in connection with the sale of the shares and warrants.
On November 1, 2013, the Company issued 4,000 shares of common stock to El Coronado for aggregate proceeds of $50,000. The issuance also included two warrants, (a) a 1-year warrant to purchase 4,000 shares of common stock at an exercise price of $12.50 per share and (b) a 5-year warrant to purchase 2,000 shares of common stock at an exercise price of $18.75 per share.
On November 1, 2013, the Company received $2,104,965 in aggregate gross cash proceeds from 36 accredited investors in connection with the sale of approximately 21 units (each a “ 2013 Unit” and collectively, the “ 2013 shares”) in an offering (the “ 2013 Offering”) of a minimum of $2,000,000 and up to a maximum of $6,000,000, in 2013 shares). Each 2013 Unit consists of (i) 8,000 shares of common stock, (ii) and two warrants, including (a) a 1 year warrant to purchase 8,000 shares of common stock at an exercise price of $12.50 per share (the “A Warrants”) and (b) a 5 year warrant to purchase 4,000 shares of common stock at an exercise price of $18.75 per share (the “B Warrants” and together with the A Warrants the “Investor Warrants”). In addition, the Company issued an aggregate of approximately 18.77 in additional 2013 shares to certain existing note holders, in connection with the conversion of $1,876,828 in outstanding principal and accrued unpaid interest in convertible promissory notes. The Company also agreed to issue warrants to purchase an aggregate of 42,099 shares of common stock to the placement agent (or its designees).
On November 18, 2013, the Company received $440,000 in aggregate gross cash proceeds from 9 accredited investors in connection with the sale of approximately 4.4 in additional 2013 shares. In addition, the Company also issued an aggregate of approximately 1.56 in additional 2013 shares to certain existing note holders, in connection with the conversion of $156,477.78 in outstanding principal and accrued unpaid interest in convertible promissory notes. The Company also agreed to issue warrants to purchase an aggregate of 10,365 shares of common stock to the placement agent (or its designees).
On November 27, 2013, the Company received $847,465 in aggregate gross cash proceeds from 25 accredited investors in connection with the sale of approximately 8.5 in additional 2013 shares. The Company also agreed to issue warrants to purchase an aggregate of 16,949 shares of common stock to the placement agent (or its designees).
On December 10, 2013, the Company received $1,180,263 in aggregate gross cash proceeds from 32 accredited investors in connection with the sale of approximately 11.8 in additional 2013 shares. The Company also agreed to issue warrants to purchase an aggregate of 23,605 shares of common stock to the placement agent (or its designees), representing 10% of the number of shares of Common Stock underlying the shares sold in the Subsequent Closing of the Offering.
On December 30, 2013, the Company received $876,280 in aggregate gross cash proceeds from 29 accredited investors in connection with the sale of approximately 8.76 in additional 2013. In addition, the Company issued an aggregate of approximately 2.8 in additional 2013 shares to certain existing note holders, in connection with the conversion of $280,042 in outstanding principal and accrued unpaid interest in convertible promissory notes. In addition, the Company also agreed to issue warrants to purchase an aggregate of 20,326 shares of common stock to the placement agent (or its designees).
On June 13, 2014 (the “Closing Date”), Protea Biosciences, Inc. (“Protea Sub”) the wholly owned subsidiary of Protea Biosciences Group, Inc. (the “Company”), completed the sale of 100% of the issued and outstanding capital stock of ProteaBio Europe SAS, a wholly-owned subsidiary of Protea Sub (“Protea Europe”) to AzurRx BioPharma, Inc. (the “Buyer”) pursuant to the terms of a Stock Purchase and Sale Agreement (the “SPA”), dated as of May 21, 2014, with AzurRx (such transaction, the “Purchase”).
Under the terms of the SPA, on the Closing Date, the Company received the following consideration (collectively, the “Purchase Price”):
| (i) | an aggregate amount of $300,000 including $200,000 in cash and $100,000 from the forgiveness of outstanding indebtedness of the Company owed to AzurRx; |
| (ii) | 100 shares of Series A Preferred Stock of AzurRx (the “Preferred Stock”) that is convertible into 33% of the issued and outstanding common stock of AzurRx; and |
II-3
| (iii) | the right to receive certain other contingent consideration to be paid upon the satisfaction of certain events, including (a) a one-time milestone payment of $2,000,000 due within (10) days of receipt of the first approval by the FDA of a New Drug Application or Biologics License Application for a Business Product (as such term is defined in the SPA); (b) royalty payments equal to 2.5% of net sales of Business Product up to $100,000,000 and 1.5% of net sales of Business Product in excess of $100,000,000 and (c) ten percent (10%) of the Transaction Value (as defined in the SPA) received in connection with a sale or transfer of the pharmaceutical development business of Protea Europe. |
Following the Closing Date, in the event AzurRx has not raised gross proceeds from an equity or equity-linked financing (the “AzurRx Buyer Financing”) of AzurRx of at least $2 million on or before the date that is 6 months following the Closing Date (“Reversion Date”), all funds AzurRx has raised in such financing through the Reversion Date will revert to the Company and the Preferred Stock issued to the Company will be forfeited (the “Reversion”). In the event of a Reversion, the Company will issue shares of its common stock to AzurRx equal to the total dollar amount raised by AzurRx through the Reversion Date plus the cash portion of the Purchase Price and the $300,000 option fee paid on March 27, 2014, at a per share price equal to the greater of $0.55 or the 20 day volume weighted average price of the Company’s common stock (the “Reversion Shares”).
Prior to the Reversion Date, AzurRx notified the Company that it raised gross proceeds of at least $2 million in a Buyer Financing. Accordingly, the Reversion is no longer applicable and the Company will not issue the Reversion Shares.
On February 11, 2014, the Company issued warrants to purchase 10,000 shares of common stock to Summit Resources, Inc., an affiliate of Steve Antoline, a director of the Company, in connection with the repayment of the Addendum to the Warrant Purchase and Reimbursement Agreement. The warrant is exercisable for five years from date of issuance at an exercise price of $27.50 per share.
On October 31, 2014, the Company received $2,539,475 in aggregate gross cash proceeds from 47 accredited investors in connection with the sale of approximately 25.4 units (each a “ 2014 Unit” and collectively, the “2014 shares”). Each 2014 Unit consists of (i) 50,000 shares of Series A convertible preferred stock, par value $0.0001 per share (the “Preferred Stock”), (ii) and a 3 year warrant to purchase 8,000 shares of common stock at an exercise price of $9.375 per share for an aggregate of 1,269,738 shares of the Company’s Preferred Stock and warrants to purchase an aggregate of 203,152 shares of the Company’s common stock. In addition, the Company issued an aggregate of approximately 18.9 in additional 2014 shares or 943,525 shares of Preferred Stock and warrants to purchase up to 150,964 shares of common stock, to certain existing convertible promissory note holders, in connection with the conversion of $1,887,053.90 in outstanding principal and accrued unpaid interest in convertible promissory notes.
Each share of Preferred Stock has a stated value equal to $2.00, subject to increase (the “Stated Value”). The Preferred Stock will automatically convert into shares of Common Stock determined by dividing the Stated Value by $6.25 per share on February 17, 2015. Under certain circumstances, the holders of the Preferred Stock have voluntary conversion rights, are entitled to receive dividends at the rate of 6.0% per annum and shall be entitled to certain anti-dilution protections.
The Company agreed to issue a warrant to purchase an aggregate of 59,567 shares of common stock to the placement agent (or its designees).
On November 25, 2014, the Company received $982,500 in aggregate gross cash proceeds from 19 accredited investors in connection with the sale of approximately 9.83 in additional 2014 shares for an aggregate of 491,250 shares of the Company’s Preferred Stock and warrants to purchase an aggregate of 78,600 shares of the Company’s common stock. In addition, the Company issued an aggregate of approximately 2.60 in additional in additional 2014 shares or 129,962 shares of Preferred Stock and warrants to purchase up to 20,794 shares of common stock, to certain existing convertible promissory note holders, in connection with the conversion of $259,922.91 in outstanding principal and accrued unpaid interest in convertible promissory notes. In addition, the Company agreed to issue a warrant to purchase an aggregate of 23,580 shares of common stock to the placement agent (or its designees).
On June 29, 2015, the Company issued 216,103 shares of Common Stock to investors in the Company’s winter 2013 private placement of Common Stock and Common Stock warrants pursuant to anti-dilution rights.
On July 16, 2015, the Company issued a warrant to The George Washington University to purchase 4,000 shares of Common Stock. The warrants are exercisable at an exercise price of $12.50 per share for a five-year term.
On October 12, 2015, the Company issued warrants to purchase 4,000 shares of Common Stock to private lenders. The warrants are exercisable at an exercise price of $12.50 per share for a five year term.
On December 18, 2015, the Company issued 31,200 shares of Common Stock with an aggregate value of $195,000 to non-employee directors of the Company as compensation for their service to the Company.
II-4
On May 22, 2015, the Company entered into a Subscription Agreement with one accredited investor, pursuant to which the Company received an aggregate gross proceeds of $2,000,000 for the sale of an 20% original issue discount unsecured convertible debenture (the “Debenture”) due November 22, 2015. The Company issued (a) the Debenture with a principal amount of $2,500,000 that bears interest at a rate of 10% per annum. On December 31, 2015, the debenture was converted into 424,222 shares of Common Stock at $6.25 per share and included interest of $151,389.
In connection with the Debenture, the Company paid to a FINRA registered broker dealer that acted as the placement agent an aggregate of approximately $440,000 in cash compensation, representing fees and an expense allowance. In addition, the Company agreed to issue a warrant to purchase an aggregate of 62,000 shares of Common Stock to the placement agent (or its designees).
The Company agreed to file a registration statement with the Securities and Exchange Commission to register for resale (i) all common stock issuable upon conversion of the Series A preferred stock and the shares of common stock issuable upon exercise of the Investor Warrants and the Placement Agent Warrants.
In closings between August 26 and December 10, 2015, the Company received $1,422,500 in aggregate gross cash proceeds from 39 accredited investors in connection with the sale of approximately 14.23 units (each a “Unit” and collectively, the “Units”) of its securities in a private placement offering to accredited investors (as defined in Regulation D under the Securities Act). Under the private placement offering (the “2015 Offering”) of a minimum of $4,000,000 and up to a maximum of $4,500,000, each Unit consists of (a) 16,000 shares of the Company’s Common Stock and (b) three year warrant to purchase 8,000 shares of Common Stock (the “Investor Warrant”) at an exercise price of $9.375 per share pursuant to the terms and conditions of a Subscription Agreement and Unit Purchase Agreement. The Company issued an aggregate of 227,680 shares of the Company’s Common Stock and Investor Warrants to purchase an aggregate of 29,640 shares of the Company’s Common Stock issued at each closing in connection with the cash proceeds received.
In connection with each closing, the Company also paid to a FINRA registered broker dealer that acted as the placement agent (the “Placement Agent”) an aggregate of $155,700 in cash compensation, representing (i) 10% of the gross proceeds raised in the Offering from Purchasers introduced to the Company by the Placement Agent; (ii) 2% of the aggregate gross proceeds raised in the 2015 Offering from pre-existing investors of the Company, and (iii) 2% of the aggregate gross proceeds raised in the Offering, in connection with a non-accountable expense allowance. In addition, the Company agreed to issue a warrant (the “Placement Agent Warrant”) to purchase an aggregate of 29,640 shares of Common Stock at exercise price equal to $6.25 per share.
The Company also entered into a Registration Rights Agreement with each of the purchasers of Units in the 2015 Offering, which requires the Company to file a registration statement with the Commission registering for resale (i) all Common Stock issued to the Purchasers, and (ii) all shares of Common stock issuable upon exercise of the Investor Warrants and the Placement Agent Warrants.
In March 2016, May 2016 and June 2016, in connection with the sale of $1,830,000 of bridge notes (described below), the Company issued to the note purchasers and the placement agent, warrants to purchase 305,525 shares of Common Stock.
All of the Units and securities underlying the Units issued in the 2015 Offering were issued to accredited investors in accordance with Rule 506 of Regulation D under the Securities Act. The Company did not engage in any general advertisement or general solicitation in connection with the offering of the Units. The investors received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration or an available exemption from such registration. The sale of the foregoing securities was made without any form of general solicitation or advertising and all of the foregoing securities are deemed restricted securities for purposes of the Securities Act.
Promissory Notes
On January 3, 2013, the Company issued to El Coronado for an aggregate purchase price equal to $125,000 (1) a convertible promissory note in an aggregate principal amount equal to $125,000 (the “El Coronado Note”) and (2) a warrant to purchase 7,500 shares of common stock of the Company (the “El Coronado Note Warrant”). The note accrues simple interest at a rate of 10% per annum and is due and payable on the earlier to occur of (i) April 1, 2013, or (ii) when declared due and payable by the holder upon the occurrence of an event of default. The warrant is exercisable at an exercise price of $27.50 per share any time after the issue date until the earlier of (i) a Qualified Public Offering (as such term is defined in the El Coronado Note Warrant) or (ii) 5:00 p.m. EST on the fifth anniversary of the issue date. As of the date hereof the entire principal amount remains outstanding and no amounts have been paid on the note. On May 1, 2013, the Company and El Coronado extended the maturity date by 60 days to May 31, 2013. The El Coronado Note and El Coronado Note Warrant were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that they were issued to an accredited investor and the Company did not engage in any general solicitation, the purchaser was the sole offeree and had full access to all information concerning the Company that was requested. The Company paid cash commissions equal to $10,000 in connection with the issuance of the El Coronado Note and El Coronado Note Warrant.
During the third quarter of 2013 (the “July and September Note Issue Dates”), the Company issued to eighteen (18) accredited investors (each a “July and September Noteholder” and collectively, the “July and September Noteholders”) (1) 10% convertible 1-year promissory notes (each a “July and September Note” and collectively, the “July and September Notes”) in an aggregate principal amount equal to $1,769,500 and (2) warrants (each a “July and September Noteholder Warrant” and collectively, the “July and September Noteholder Warrants”) for each investor to purchase the number of shares of the Company’s common stock as is equal to 37.5% of the principal amount of the July and September Notes purchased at $27.50 per share, pursuant to the terms and conditions of those certain Note and Warrant Purchase Agreements (the “Purchase Agreements”).
II-5
During the fourth quarter of 2013 (the “October Note Issue Dates”), the Company issued to three (3) accredited investors (each an “October Noteholder” and collectively, the “October Noteholders”) (1) 10% convertible I-year promissory notes (each an “October Note” and collectively, the “October Notes”) in an aggregate principal amount equal to $225,000 and (2) warrants (each an “October Noteholder Warrant” and collectively, the “October Noteholder Warrants”) for each investor to purchase the number of shares of the Company’s common stock as is equal to 37.5% of the principal amount of the October Notes purchased by it, divided by $12.50, pursuant to the terms and conditions of those certain Note and Warrant Purchase Agreements (the “October Purchase Agreements”).
During the fourth quarter of 2013 (the “Related Party Note Issue Dates”) the Company issued Promissory Notes (the “Related Party Notes” and together with the July and September Notes and the October Notes, the “2013 Notes”) to two (2) board members’ affiliated companies in the aggregate amount of $275,000. The notes mature on October 25, 2014 and accrue simple interest of 10% per annum.
The 2013 Notes accrue simple interest at a rate of 10% per annum and are due and payable on the one-year anniversary of their respective Issue Dates. As of December 31, 2013, the 2013 Notes and all accrued unpaid interest thereon were converted into the 2013 Offering at a rate of eight percent of each share of the Company’s common stock and a) a 1-year warrant to purchase two shares of common stock at an exercise price of $12.50 per share for each $1 invested and b) a 5-year warrant to purchase one share of common stock exercisable at $18.75 per share for each $1.00 invested.
The Notes and the July and September Noteholder Warrants and the October Noteholder Warrants were issued to accredited investors in accordance with Rule 506 of Regulation D under the Securities Act in that the Company did not engage in any general advertisement or general solicitation in connection with the offering of the Notes, the July and September Noteholder Warrants and the October Noteholder Warrants, and the Company was available to answer any questions from any purchaser. Cash commissions were not paid in connection with the sale of the Notes, the July and September Noteholder Warrants and the October Noteholder Warrants.
On August 6, 2013, the Company entered into the Note and Warrant Purchase Agreement (the “Summit Agreement”) with Summit Resources, Inc. (“Summit”), an affiliate of Steve Antoline, a director of the Company pursuant to which Summit acquired (a) a promissory note (the “Summit Note”) in an aggregate principal amount of $600,000 and (b) a five year warrant to purchase up to an aggregate of 10,000 shares of common stock for each $150,000 borrowed of common stock at an exercise price of $27.50 per share for up to a maximum of 40,000 shares of common stock as consideration for up to $600,000 in advances made or to be made to the Company as consideration for advances of up to $600,000, of which $550,000 was advanced to the Company as of June 30, 2013. In accordance with the terms of the Summit Agreement, up to $150,000 of the gross proceeds received by the Company from the sale of each LAESI instrument beginning with the sale of the fourth LAESI instrument sold following the effective date shall be used to repay the principal amounts due under the Summit Note. The Summit Note and warrants were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that the investor was the sole offeree, had access to all information concerning the Company that was requested and the Company did not engage in any general solicitation.
On September 11, 2013, the Company issued a Promissory Note for $315,000 and a warrant to purchase up to 9,450 shares of the Company’s common stock to El Coronado Holdings, LLC and, entered into a Note and Warrant Purchase Agreement by and between the Company and El Coronado Holdings, LLC.
On September 20, 2013, the Company issued a Promissory Note for $300,000 and a warrant to purchase up to 9,000 shares of the Company’s common stock to El Coronado Holdings, LLC and, entered into a Note and Warrant Purchase Agreement by and between the Company and El Coronado Holdings, LLC.
On October 7, 2013, the Company issued a Promissory Note for $125,000 and a warrant to purchase up to 3,750 shares of the Company’s common stock to Summit Resources, Inc. and, entered into a Note and Warrant Purchase Agreement by and between the Company and Summit Resources, Inc. On November 1, 2013, the note converted into 1.26 shares issued in the Offering consisting of common stock and warrants issued in connection with the conversion of the principal and interest underlying the note.
On October 25, 2013, the Company issued a Promissory Note for $125,000 to El Coronado Holdings, LLC and, entered into a Note and Warrant Purchase Agreement by and between the Company and El Coronado Holdings, LLC. On December 30, 2013, the note converted into 1.53 shares issued in the Offering consisting of common stock and warrants issued in connection with the conversion of the principal and interest underlying the note.
On October 25, 2013, the Company issued a Promissory Note for $150,000 to Summit Resources, Inc. and, entered into a Note and Warrant Purchase Agreement by and between the Company and Summit Resources, Inc.
From April 2, 2014 until June 30, 2014, the Company entered into a Note Purchase Agreement, and issued convertible one-year promissory notes (the “2014 Q2 Notes”) to eight related parties in the aggregate principal amount of $1,378,500. From July 1, 2014 until June 30, 2015, the Company entered into a Note Purchase Agreement, and issued convertible one-year promissory notes (the “2014 Q3 Notes”) to ten related parties in the aggregate principal amount of $817,500. The 2014 Q2 Notes and 2014 Q3 Notes converted into the 2014 Offering.
II-6
On July 1, 2015, the Company received an aggregate gross proceeds of $200,000 from one director and issued a 10% Convertible Promissory Note due on December 31, 2015. The note is convertible into Common Stock at a conversion price of $8.25 per share. The Company also issued 3,636 shares of Common Stock as commitment fee to the director with a price per share of $8.25.
In March 2016, the Company sold $655,000 principal amount of original issue discount unsecured notes due six months from funding and providing an estimated 23% yield to maturity (the “Bridge Note”) to one accredited investor, pursuant to which the Company received an aggregate gross proceeds of $500,000. The Bridge Note does not accrue additional interest and included legal fees of $5,000, which was added to the principal amount. The Company also issued to the investor an aggregate of 4,347 shares of our common stock and five year warrants to purchase up to 65,500 additional shares of common stock at an exercise price of $18.75, subject to adjustment in certain events as provided therein.
If we fail to pay the Bridge Notes on or before its maturity date or otherwise commit an event of default, as defined in the Bridge Notes, the holder may convert the outstanding principal amount of the Bridge Notes into our common stock at a conversion price initially equal to 70% of the lowest closing bid price for the common stock in the twenty trading days immediately preceding the conversion, subject to adjustment in certain events as provided therein.
In connection with our sale of the 2016 Bridge Notes, the Company paid to Laidlaw & Company (UK) Ltd., who acted as the placement agent, an aggregate of approximately $60,000 in cash compensation, representing fees and an expense allowance. In addition, we issued to the placement agent (or its designees) a five-year warrant to purchase an aggregate of approximately 19,650 shares of our common stock at an exercise price of $6.25 per share.
In May and June 2016, the Company received an aggregate of $1,175,000 in gross cash proceeds from 22 accredited investors in connection with the sale of original issue discount unsecured short-term convertible notes due November 20, 2016, November 30, 2016 and December 10, 2016, being six months following the dates of issuance of each of the notes. The Company notes (a) were issued with a total principal amount of $1,468,750 (b) bears interest at a rate of 10% per annum, and (c) are convertible at any time by the holders into our common stock at a conversion price of $6.25. In addition, we issued to the note holders three-year warrants to purchase up to 176,260 shares of common stock of the Company at an exercise price of $8.13 per share.
In connection with our sale of the May and June 2016 Bridge Notes, we paid to Laidlaw & Company (UK) Ltd., who acted as the placement agent, an aggregate of approximately $141,000 in cash compensation, representing fees and an expense allowance. In addition, we issued to the placement agent (or its designees) a five-year warrant to purchase an aggregate of approximately 41,125 shares of our common stock at an exercise price of $0.325 per share.
Conversion of Promissory Notes
As of June 30, 2013, the Company entered into conversion agreements (the “Conversion Agreements”) with certain related party holders (the “Existing Noteholders”) of its existing convertible promissory notes with an aggregate principal amount of $3,003,216 (the “Existing Notes”) pursuant to which the Company agreed to issue 5 year warrants (the “Conversion Warrants”) to purchase up to 75% of the number of shares of common stock into which the Existing Notes were convertible, at an exercise price of $27.50 per share, provided that the conversion of the Existing Notes was exercised on or prior to June 30, 2013. In accordance with the terms and conditions of the Conversion Agreements, on June 30, 2013 the Existing Noteholders notified the Company of their desire to convert the Existing Notes into an aggregate of 266,528 shares (the “Conversion Shares”). On July 23 and 29, 2013, the Company issued the Conversion Shares and Conversion Warrants to purchase up to an aggregate of 199,896 shares of common stock. The Conversion Shares and Conversion Warrants were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that the investors were the sole offerees, had access to all information concerning the Company and the Company did not engage in any general solicitation. Cash commissions were not paid in connection with the sale of the Conversion Shares and Conversion Warrants.
On March 20, 2015, the Company’s Board of Directors authorized the conversion of an aggregate of $341,084.81 principal amount of, and $192,784.63 of accrued interest on promissory notes issued by the Company to Summit Resources, Inc. (“Summit”), the president of which is Steven Antoline, who is a director of the Company and an affiliate of Summit, and $16,392.14 of accounts payable by the Company to Summit, into 2,201,046 units of securities, each unit consisting of one share of the Company’s Common Stock and a five-year warrant to purchase one-half share of Common Stock, at an exercise price of $12.50 per whole share, at a conversion price of $6.25 per unit. The issuance of these shares and warrants was exempt from registrations pursuant to Section 4(a)(2) of the Securities Act as not involving any public offering.
Consultant Shares
On June 5, 2013, the Company issued 1,920 shares of common stock at $12.50 per share for services rendered pursuant to the terms of a Consulting Termination Agreement by and between the Company and the consultant, dated April 15, 2013.
On December 10, 2013, the Company issued 15,000 shares of common stock at $12.50 per share for services rendered pursuant to the terms of a Consulting Agreement by and between the Company and the consultant dated March 25, 2013.
During the first quarter of 2014, the Company issued 1,000 shares of common stock at $12.50 per share for services rendered pursuant to the terms of a Consulting Agreement by and between the Company and a consultant, dated February 13, 2014.
On March 3, 2014, the Company issued warrants to purchase 6,700 shares of common stock in connection with services rendered pursuant to the terms of a Consulting Agreement by and between the Company and a consultant, dated March 3, 2014. The warrants contain an exercise price of $27.50 per share and are exercisable for five years from date of issuance.
II-7
During the quarter ended June 30, 2014, the Company issued 21,494 shares of common stock for services rendered by attorneys and two consultants.
On April 30, 2014, the Company issued warrants to purchase 7,500 shares of common stock in connection with services rendered pursuant to the terms of a Consulting Agreement by and between the Company and a consultant, dated October 17, 2013. The warrants contain an exercise price of $27.50 per share and are exercisable for five years from date of issuance.
During the quarter ended June 30, 2015, the Company issued 10,000 shares of common stock having a value of $220,000 to three consultants for services rendered.
On March 20, 2015, the Company issued warrants to purchase 6,700 shares of Common Stock in connection with services rendered pursuant to the terms of a Consulting Agreement by and between the Company and a consultant, dated March 3, 2014. The warrants contain an exercise price of $27.50 per share and are exercisable for five years from date of issuance.
On March 20, 2015, the Company granted options to purchase 6,700 shares of Common Stock in connection with services rendered pursuant to the terms of a Consulting Agreement by and between the Company and a consultant, dated March 3, 2014. The options contain an exercise price of $13.75 per share and are exercisable for ten years from date of issuance.
On June 11, 2015, the Company issued 12,000 shares of Common Stock with a value of $93,000 to a consultant for services rendered.
On October 28, 2015, the Company issued 15,600 shares of Common Stock and warrants to purchase 38,000 shares of Common Stock to a consultant for services retained. The warrants are exercisable at an exercise price of $12.50 per share for a five year term.
On December 14, 2015, the Company issued 12,000 shares of Common Stock to a consultant for services rendered during the year with a value of $93,000.
The shares issued to consultants as described above were issued in connection with the exemption from the registration requirements of the Securities Act pursuant to Section 4(2) thereof and the rules promulgated thereunder. The investor received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration or an available exemption from such registration. The sale of the foregoing securities was made without any form of general solicitation or advertising and all of the foregoing securities are deemed restricted securities for purposes of the Securities Act.
Options
During the quarter ended March 31, 2013, the Company granted options to purchase an aggregate of 3,480 shares of common stock in connection with the Company’s 2013 Equity Incentive Plan at an exercise price of $12.50 per share and 42,000 shares of common stock in accordance with the 2013 Equity Incentive Plan at an exercise price of $0.55 per share.
During the quarter ended June 30, 2013, the Company granted options to purchase an aggregate of 367,000 shares of common stock in connection with the Company’s 2013 Equity Incentive Plan at an exercise price of $13.75 per share.
During the quarter ended September 30, 2013, the Company granted options to purchase an aggregate of 10,080 shares of common stock in connection with the Company’s 2013 Equity Incentive Plan at an exercise price of $13.75 per share.
During the quarter ended March 31, 2014, the Company granted options to purchase an aggregate of 52,180 shares of common stock in connection with the Company’s 2013 Equity Incentive Plan at an exercise price of $13.75 per share.
During the quarter ended June 30, 2014, the Company granted options to purchase an aggregate of 41,700 shares of common stock in connection with the Company’s 2013 Equity Incentive Plan at an exercise price of $13.75 per share to the Chief Scientific Officer, Vice President, Corporate Finance & Development and various employees. The options expire five years from date of issuance.
During the quarter ended September 30, 2014, the Company granted options to purchase an aggregate of 2,300 shares of common stock in connection with the Company’s 2013 Equity Incentive Plan at an exercise price of $13.75 per share to various employees. The options expire ten years from date of issuance. In addition, 1,000 options were exercised at $13.75 per share during the quarter.
On July 16, 2015 (the “Issuance Date”), the Company issued an incentive stock option to Dave Halverson, Vice President of Sales, under the Company’s 2013 Equity Incentive Plan (the “Plan”), to purchase 6,000 shares of Common Stock. The option shall be exercisable at fair market value, as defined in the Plan, and shall vest as to 25% of the shares at the end of each of the first four anniversaries from the Issuance Date, becoming fully vested and exercisable four years from the Issuance Date.
On October 12, 2015, the Company granted non-qualified options to purchase an aggregate of 4,880 shares of Common Stock to certain individuals in recognition of their services to the Company at an exercise price of $13.75 per share.
II-8
On December 18, 2015, the Board of Directors approved an increase of 300,000 shares of Common Stock issuable under the 2013 Equity Incentive Plan.
On December 31, 2015, the Company granted non-qualified options to purchase an aggregate of 21,333 shares of Common Stock to non-employee directors of the Company in recognition of their service to the Company at an exercise price of $6.25 per share.
During the quarter ended December 31, 2015, the Company granted options to purchase an aggregate of 68,800 shares of Common Stock to several key employees in recognition of their services to the Company at an exercise price of $6.25 per share.
Consulting Agreement
On March 25, 2013, the Company entered into a consulting agreement (the “Consulting Agreement”) with an existing shareholder to provide business and management consulting services pursuant to which the Company agreed to issue to the consultant and its designees an aggregate of 12,000 shares of common stock and five year warrants to purchase up to 15,000 shares of common stock at an exercise price of $27.50 per share, of which 50% of the shares of common stock and warrants were payable upon execution of the Consulting Agreement and the balance is payable on the one year anniversary of the Consulting Agreement. On April 5, 2013, the Company issued an aggregate of 6,000 shares of common stock and a warrant to purchase up to 7,500 shares of common stock to the consultant. The shares of common stock and warrants issued pursuant to the Consulting Agreement were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that they were issued to an accredited investor, the Company did not engage in any general solicitation, the purchaser was the sole offeree and had full access to all information concerning the Company that was requested. No cash commissions were paid in connection with the issuance of the securities pursuant to the Consulting Agreement.
Advertising Services Agreement
On March 16, 2016, the Company entered into an agreement with an advertising firm for certain services to be rendered to the Company over an estimated ninety (90) day period. Besides cash compensation, the agreement required the Company to issue 150,000 shares of Common Stock to the advertising firm as of the execution date of the agreement and an additional 150,000 shares to be issued to the advertising firm thirty (30) days from the execution date of the agreement, assuming the advertising firm is providing the services contemplated and the agreement has not otherwise been terminated.
Related Party Debt
During the three months ended March 31, 2016, the Company received advances equal to an aggregate of $109,500 from various directors and current stockholders of the Company. The Company repaid $57,500 to three directors during this period, which included payment of $15,000 of the $2,547,500 in aggregate advances and other loans payable to stockholders outstanding as of December 31, 2015. As of March 31, 2016, the outstanding balance of advances and other loans payable was $2,626,500, which included two interest-bearing promissory notes with a combined outstanding balance of $1,117,000 with two stockholders and $1,509,000 in outstanding advances from stockholders that have no terms of repayment and do not bear interest.
As of the date of this prospectus, we are indebted to certain members of our board of directors or their affiliates for loans and advances made to our Company over the past five years in the aggregate amount of $2,676,500. We are currently in discussions with members of our board of directors, including Stephen Turner, our President and CEO, to repay (on a pro-rata basis) out of the net proceeds of this offering, $669,125 of the total of $2,676,500 of related party advances from such persons. See "Use of Proceeds". The balance of such indebtedness to our directors and their affiliates would be evidenced by interest-bearing notes due as to principal and accrued interest over a two - three year period on a date which is one year following the date of this registration statement. There can be no assurance that we will be successful in completing the contemplated agreements or the terms and conditions of any such arrangements. Any such agreements may be subject to completion of the offering of 3,100,000 shares of our common stock included in the prospectus forming a part of this registration statement.
Item 16. Exhibits and Financial Statement Schedules.
The following exhibits are filed as part of this registration statement.
In reviewing the agreements included (or incorporated by reference) as exhibits to this registration statement, please remember that they are included to provide you with information regarding their terms and are not intended to provide any other factual or disclosure information about us or the other parties to the agreements. The agreements may contain representations and warranties by each of the parties to the applicable agreement. These representations and warranties have been made solely for the benefit of the parties to the applicable agreement and:
| · | should not in all instances be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate; | |
| · | have been qualified by disclosures that were made to the other party in connection with the negotiation of the applicable agreement, which disclosures are not necessarily reflected in the agreement; |
II-9
| · | may apply standards of materiality in a way that is different from what may be viewed as material to you or other investors; and | |
| · | were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement and are subject to more recent developments. |
Accordingly, these representations and warranties may not describe the actual state of affairs as of the date they were made or at any other time. Additional information about us may be found elsewhere in this registration statement and our other public filings, which are available without charge through the SEC’s website at http://www.sec.gov.
| Exhibit No. | Description | Exhibit Location | ||
| 2.1 | Merger Agreement by and among Protea Biosciences, Inc., SRKP 5, Inc., and SRKP 5 Acquisition Corp. Inc. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 2.2 | Share Purchase Agreement among Protea Biosciences Group, Inc., vivoPharm PTY Ltd., Dr. Ralf Brandt and the Brandt Family Trust, South Australian Life Science Advancement Partnership, LP, Terra Rossa Capital PTY Ltd, Royal Melbourne Institute of Technology. | Form 8-K filed with the Securities and Exchange Commission on April 6, 2015 and incorporated herein by this reference. | ||
| 3.1 | Certificate of Incorporation. | Form 10-SB filed with the Securities and Exchange Commission on August 3, 2005 and incorporated herein by this reference. | ||
| 3.2 | Amended and Restated Bylaws. | Filed herewith. | ||
| 3.3 | Certificate of Ownership and Merger filed with the Office of Secretary of State of Delaware on September 2, 2011. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 3.4 | Certificate of Amendment to Certificate of Incorporation. | Form 8-K filed with the Securities and Exchange Commission on June 24, 2013 and incorporated herein by this reference. | ||
| 3.5 | Certificate of Amendment to Certificate of Incorporation | Form 8-K filed with the Securities and Exchange Commission on July 8, 2015 and incorporated herein by this reference. | ||
| 3.6 | Certificate of Designation, Preferences and Rights of Series A Convertible Preferred Stock. | Form 8-K filed with the Securities and Exchange Commission on November 5, 2014 and incorporated herein by this reference. | ||
| 3.7 | Certificate of Amendment to Certificate of Designation, Preferences and Rights of Series A Convertible Preferred Stock | Form 8-K filed with the Securities and Exchange Commission on March 18, 2015 and incorporated herein by this reference. | ||
| 4.1 | Form of Warrant issued by Protea Biosciences, Inc. in connection with sale of convertible debentures. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 4.2 | Form of Warrant issued by Protea Biosciences, Inc. in connection with sale of Class A common stock. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 4.3 | Promissory Note issued by Protea Biosciences, Inc. to West Virginia Economic Development Authority, dated August 3, 2009. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 4.4 | Promissory Note issued by Protea Biosciences, Inc. to West Virginia Water Development Authority, acting by and on behalf of the West Virginia Infrastructure and Jobs Development Council, dated August 3, 2009. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 4.5 | Promissory Note issued by Protea Biosciences, Inc. to West Virginia Economic Development Authority, dated October 21, 2010. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. |
II-10
| 4.6 | Promissory Note issued by Protea Biosciences, Inc. to West Virginia Water Development Authority, acting by and on behalf of the West Virginia Infrastructure and Jobs Development Council, dated December 10, 2010. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 4.7 | Commercial Promissory Note between Protea Biosciences, Inc. and United Bank (formerly Centra Bank, Inc.), dated August 27, 2009. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 4.8 | Form of Promissory Note issued by Protea Biosciences, Inc. in connection with the sale of promissory notes. | Form 10-Q filed with the Securities and Exchange Commission on August 5, 2014 and incorporated herein by this reference. | ||
| 4.9 | Form of Warrant issued by Protea Biosciences, Inc. in connection with the sale of promissory notes | Form 10-Q filed with the Securities and Exchange Commission on August 5, 2014 and incorporated herein by this reference. | ||
| 4.10 | Form of Convertible Promissory Note issued by Protea Biosciences, Inc. in connection with the sale of convertible promissory notes | Form 10-Q filed with the Securities and Exchange Commission on August 5, 2014 and incorporated herein by this reference. | ||
| 4.11 | Form of Warrant issued to El Coronado Holdings, LLC. | Form 8-K filed with the Securities and Exchange Commission on March 27, 2013 and incorporated herein by this reference. | ||
| 4.12 | Warrant to purchase common stock of the Company issued to Summit Resources, Inc. in connection with Warrant and Reimbursement Agreement. | Form 10-Q filed with the Securities and Exchange Commission on May 10, 2013 and incorporated herein by this reference. | ||
| 4.13 | Form of Placement Agent Warrant issued in connection with the Fall 2012 Offering. | Form 10-Q filed with the Securities and Exchange Commission on May 10, 2013 and incorporated herein by this reference. | ||
| 4.14 | Form of Warrants to purchase common stock issued in connection with the Spring 2013 Direct Issuances. | Form 10-Q filed with the Securities and Exchange Commission on May 10, 2013 and incorporated herein by this reference. | ||
| 4.15 | Form of Promissory Note for $1,500,000 Offering. | Form 10-Q filed with the Securities and Exchange Commission on August 5, 2014 and incorporated herein by this reference. | ||
| 4.16 | Form of Warrant for $1,500,000 Offering. | Form 10-Q filed with the Securities and Exchange Commission on August 5, 2014 and incorporated herein by this reference. | ||
| 4.17 | Form of Convertible Promissory Note for $2,000,000 Offering. | Form 10-Q filed with the Securities and Exchange Commission on August 5, 2014 and incorporated herein by this reference. |
| 4.18 | Form of Underwriting Agreement between the registrant and Laidlaw & Co (UK) Ltd. | Filed herewith | ||
| 4.19 | Form of Warrant issued to the Representative of the several underwriters | Filed herewith |
| 5.1 | Legal Opinion of CKR Law LLP. | To be filed by amendment. | ||
| 10.1 | Share Cancellation Agreement dated as of September 2, 2011 by and between the registrant and the Persons signatory thereto. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.2 | Amended and Restated Exclusive License Agreement between George Washington University and Protea Biosciences, Inc., dated February 22, 2010. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. |
II-11
| 10.3 | Second Amendment to Exclusive License Agreement between George Washington University and Protea Biosciences, Inc., dated January 14, 2011. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.4 | Third Amendment to Exclusive License Agreement between George Washington University and Protea Biosciences, Inc., dated April 27, 2011. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.5 | Fourth Amendment to Exclusive License Agreement between George Washington University and Protea Biosciences, Inc., dated June 21, 2011. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.6 | Fifth Amendment to Exclusive License Agreement between George Washington University and Protea Biosciences, Inc., dated September 10, 2012. | Form S-1 filed with the Securities and Exchange Commission on February 13, 2015 and incorporated herein by this reference. | ||
| 10.7 | Sixth Amendment to Exclusive License Agreement between George Washington University and Protea Biosciences, Inc., dated July 30, 2013 | Form S-1 filed with the Securities and Exchange Commission on February 13, 2015 and incorporated herein by this reference. | ||
| 10.8 | Exclusive License Agreement between Johns Hopkins University and Protea Biosciences, Inc., dated June 9, 2009. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.9 | Exclusive Option Agreement between West Virginia University and Protea Biosciences, Inc., dated September 19, 2001. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.10 | Exclusive License Agreement between West Virginia University and Protea Biosciences, Inc., dated December 21, 2005. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. |
| 10.11 | 1st Amendment of Exclusive License Agreement between West Virginia University and Protea Biosciences, Inc., dated March 8, 2006. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.12 | 2nd Amendment of Exclusive License Agreement between West Virginia University and Protea Biosciences, Inc., dated November 20, 2006. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.13 | 3rd Amendment of Exclusive License Agreement between West Virginia University and Protea Biosciences, Inc., dated April 11, 2007. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.14 | 4th Amendment of Exclusive License Agreement between West Virginia University and Protea Biosciences, Inc., dated January 24, 2008. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.15 | Joint Research and Development Agreement between Laboratories Mayoly Spindler SAS, Protea Biosciences, Inc. and Proteabio Europe SAS, dated March 22, 2010. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. |
II-12
| 10.16 | Loan Agreement between West Virginia Economic Development Authority and Protea Biosciences, Inc., dated August 3, 2009. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.17 | Security Agreement between Protea Biosciences, Inc. and West Virginia Economic Development Authority, dated August 3, 2009. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.18 | Loan Agreement between West Virginia Water Development Authority, acting by and on behalf of the West Virginia Infrastructure and Jobs Development Council and Protea Biosciences, Inc., dated August 3, 2009. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.19 | Intercreditor Agreement between West Virginia Water Development Authority, acting by and on behalf of the West Virginia Infrastructure and Jobs Development Council, West Virginia Economic Development Authority and Protea Biosciences, Inc., dated August 3, 2009. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.20 | Security Agreement between Protea Biosciences, Inc. and West Virginia Water Development Authority, acting by and on behalf of the West Virginia Infrastructure and Jobs Development Council, dated August 3, 2009. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.21 | Loan Agreement between West Virginia Economic Development Authority and Protea Biosciences, Inc., dated October 21, 2010. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.22 | Security Agreement between Protea Biosciences, Inc. and West Virginia Economic Development Authority, dated October 21, 2010. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.23 | Loan Agreement between West Virginia Water Development Authority, acting by and on behalf of the West Virginia Infrastructure and Jobs Development Council and Protea Biosciences, Inc., dated December 10, 2010. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.24 | Intercreditor Agreement between West Virginia Water Development Authority, acting by and on behalf of the West Virginia Infrastructure and Jobs Development Council, West Virginia Economic Development Authority and Protea Biosciences, Inc., dated December 10, 2010. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.25 | Amended and Restated Security Agreement between Protea Biosciences, Inc. and West Virginia Water Development Authority, acting by and on behalf of the West Virginia Infrastructure and Jobs Development Council, dated December 10, 2010. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.26 | Commercial Loan Agreement between United Bank (formerly Centra Bank, Inc.) and Protea Biosciences, Inc., dated August 27, 2009. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. |
II-13
| 10.27 | First Amendment to Exclusive Option Agreement between West Virginia University and Protea Biosciences, Inc., dated December 11, 2002. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.28 | Equipment Lease Agreement between Monogalia County Development Authority and Protea Biosciences, Inc., dated March 12, 2007. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. |
| 10.29 | Form of Convertible Promissory Notes, dated December 20, 2011*. | Form 8-K filed with the Securities and Exchange Commission on December 28, 2011 and amended on April 16, 2012 and incorporated herein by this reference. | ||
| 10.30 | Share Cancellation Agreement dated as of September 2, 2011. | Form 8-K filed with the Securities and Exchange Commission on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference. | ||
| 10.31 | Lease Agreement, dated January 25, 2012, by and between Protea Biosciences, Inc. and White Birch Properties LLC. | Form 8-K filed with the Securities and Exchange Commission on January 25, 2012 and incorporated herein by this reference. | ||
| 10.32 | Form of Convertible Promissory Notes, dated as of April 16, 2012, issued to directors and certain related parties*. | Form 8-K filed with the Securities and Exchange Commission on April 21, 2012 as Exhibit 10.1 and amended on June 15, 2012 and incorporated herein by this reference. | ||
| 10.33 | 10% Convertible Debenture, dated as of April 18, 2012, issued to the West Virginia Jobs Investment Trust Board. | Form 8-K filed with the Securities and Exchange Commission on April 21, 2012 as Exhibit 10.2 and amended on October 31, 2012 and incorporated herein by this reference. | ||
| 10.34 | Secured Convertible Note and Investment Agreement, dated as of May 24, 2012, issued to the West Virginia High Technology Consortium Foundation. | Form 8-K filed with the Securities and Exchange Commission on June 15, 2012 as Exhibit 10.1 and incorporated herein by this reference. | ||
| 10.35 | Security Agreement, dated as of May 24, 2012, issued to the West Virginia High Technology Consortium Foundation. | Form 8-K filed with the Securities and Exchange Commission on June 15, 2012 as Exhibit 10.2 and incorporated herein by this reference. | ||
| 10.36 | Loan Agreement, dated as of June 11, 2012, issued to the West Virginia Economic Development Authority Foundation. | Form 8-K filed with the Securities and Exchange Commission on June 15, 2012 as Exhibit 10.3 and incorporated herein by this reference. | ||
| 10.37 | Promissory Note, dated as of June 11, 2012, issued to the West Virginia Economic Development Authority Foundation. | Form 8-K filed with the Securities and Exchange Commission on June 15, 2012 as Exhibit 10.4 and incorporated herein by this reference. | ||
| 10.38 | Security Agreement, dated as of June 11, 2012, issued to the West Virginia Economic Development Authority Foundation. | Form 8-K filed with the Securities and Exchange Commission on June 15, 2012 as Exhibit 10.5 and incorporated herein by this reference. | ||
| 10.39 | Guaranty, dated as of May 22, 2012, issued to the West Virginia High Technology Consortium Foundation. | Form 8-K filed with the Securities and Exchange Commission on June 15, 2012 as Exhibit 10.6 and incorporated herein by this reference. |
| 10.40 | Guaranty, dated as of June 6, 2012, issued to the West Virginia Economic Development Authority Foundation. | Form 8-K filed with the Securities and Exchange Commission on June 15, 2012 as Exhibit 10.7 and incorporated herein by this reference. | ||
| 10.41 | Convertible Promissory Note Addendum, dated as of June 15, 2012, executed by Summit Resources, Inc. with respect to the December Note by and between the Company and Summit Resources, Inc.* | Form 8-K filed with the Securities and Exchange Commission on July 16, 2012 as Exhibit 10.1 and amended on September 25, 2012 and incorporated herein by this reference. |
II-14
| 10.42 | Convertible Promissory Note Addendum, dated as of June 15, 2012, executed by Stanley Hostler with respect to the December Note by and between the Company and Stanley Hostler. * | Form 8-K filed with the Securities and Exchange Commission on July 16, 2012 as Exhibit 10.2 and amended on September 25, 2012 and incorporated herein by this reference. | ||
| 10.43 | Convertible Promissory Note Addendum, dated as of June 15, 2012, executed by Summit Resources, Inc. with respect to the April Note by and between the Company and Summit Resources, Inc.* | Form 8-K filed with the Securities and Exchange Commission on July 16, 2012 as Exhibit 10.3 and amended on September 25, 2012 and incorporated herein by this reference. | ||
| 10.44 | Convertible Promissory Note Addendum, dated as of June 15, 2012, executed by Scott Segal with respect to the April Note by and between the Company and Scott Segal.* | Form 8-K filed with the Securities and Exchange Commission on July 16, 2012 as Exhibit 10.4 and amended on September 25, 2012 and incorporated herein by this reference. | ||
| 10.45 | Convertible Promissory Note Addendum, dated as of June 15, 2012, executed by Stanley Hostler with respect to the April Note by and between the Company and Stanley Hostler.* | Form 8-K filed with the Securities and Exchange Commission on July 16, 2012 as Exhibit 10.5 and amended on September 25, 2012 and incorporated herein by this reference. | ||
| 10.46 | Convertible Promissory Note Addendum, dated as of June 18, 2012, executed by Virginia Child with respect to the April Note by and between the Company and Virginia Child. | Form 8-K filed with the Securities and Exchange Commission on July 16, 2012 as Exhibit 10.6 and amended on September 25, 2012 and incorporated herein by this reference. | ||
| 10.47 | Convertible Promissory Note Addendum, dated as of June 22, 2012, executed by Nancy Turner with respect to the April Note by and between the Company and Nancy Turner. | Form 8-K filed with the Securities and Exchange Commission on July 16, 2012 as Exhibit 10.7 and amended on September 25, 2012 and incorporated herein by this reference. | ||
| 10.48 | Convertible Promissory Note Addendum, dated as of July 2, 2012, executed by Leonard Harris with respect to the April Note by and between the Company and Leonard Harris.* | Form 8-K filed with the Securities and Exchange Commission on July 16, 2012 as Exhibit 10.8 and amended on September 25, 2012 and incorporated herein by this reference. | ||
| 10.49 | Letter Agreement, dated June 18, 2012, by and between the Company and the WVJITB. | Form 8-K filed with the Securities and Exchange Commission on July 16, 2012 as Exhibit 10.7 and amended on September 25, 2012 and incorporated herein by this reference. | ||
| 10.50 | Convertible Promissory Note Addendum, dated as of September 25, 2012, executed by Stanley Hostler with respect to the December Note by and between the Company and Stanley Hostler.* | Form 8-K filed with the Securities and Exchange Commission on October 4, 2012 as Exhibit 10.2 and incorporated herein by this reference. | ||
| 10.51 | Convertible Promissory Note Addendum, dated as of September 25, 2012, executed by Summit Resources, Inc. with respect to the December Note by and between the Company and Summit Resources, Inc.* | Form 8-K filed with the Securities and Exchange Commission on October 4, 2012 as Exhibit 10.3 and incorporated herein by this reference. | ||
| 10.52 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Summit Resources, Inc. with respect to the April Note by and between the Company and Summit Resources, Inc.* | Form 8-K filed with the Securities and Exchange Commission on November 7, 2012 as Exhibit 10.1 and incorporated herein by this reference. |
II-15
| 10.53 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Scott S. Segal with respect to the April Note by and between the Company and Scott S. Segal.* | Form 8-K filed with the Securities and Exchange Commission on November 7, 2012 as Exhibit 10.2 and incorporated herein by this reference. | ||
| 10.54 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Stanley M. Hostler with respect to the April Note by and between the Company and Stanley M. Hostler.* | Form 8-K filed with the Securities and Exchange Commission on November 7, 2012 as Exhibit 10.3 and incorporated herein by this reference. | ||
| 10.55 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Virginia E. Child with respect to the April Note by and between the Company and Virginia E. Child. | Form 8-K filed with the Securities and Exchange Commission on November 7, 2012 as Exhibit 10.4 and incorporated herein by this reference. | ||
| 10.56 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Nancy Turner with respect to the April Note by and between the Company and Nancy Turner. | Form 8-K filed with the Securities and Exchange Commission on November 7, 2012 as Exhibit 10.5 and incorporated herein by this reference. | ||
| 10.57 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Leonard P. Harris with respect to the April Note by and between the Company and Leonard P. Harris.* | Form 8-K filed with the Securities and Exchange Commission on November 7, 2012 as Exhibit 10.6 and incorporated herein by this reference. | ||
|
10.58
|
Letter Agreement, dated as of October 31, 2012, executed by the Company and WVJITB. | Form 8-K filed with the Securities and Exchange Commission on November 7, 2012 as Exhibit 10.7 and incorporated herein by this reference. | ||
| 10.59 | Form of Convertible Promissory Notes, dated as of November 30, 2012, issued to directors and certain related parties.* | Form 8-K filed with the Securities and Exchange Commission on December 6, 2012 as Exhibit 10.1 and incorporated herein by this reference. | ||
| 10.60 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Stanley M. Hostler with respect to the September Note by and between the Company and Stanley M. Hostler.* | Form 8-K filed with the Securities and Exchange Commission on December 6, 2012 as Exhibit 10.2 and incorporated herein by this reference. | ||
| 10.61 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Scott Segal with respect to the September Note by and between the Company and Scott Segal.* | Form 8-K filed with the Securities and Exchange Commission on December 6, 2012 as Exhibit 10.3 and incorporated herein by this reference. | ||
| 10.62 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Leo Harris with respect to the September Note by and between the Company and Leo Harris.* | Form 8-K filed with the Securities and Exchange Commission on December 6, 2012 as Exhibit 10.4 and incorporated herein by this reference. | ||
| 10.63 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Virginia Child with respect to the September Note by and between the Company and Virginia Child. | Form 8-K filed with the Securities and Exchange Commission on December 6, 2012 as Exhibit 10.5 and incorporated herein by this reference. |
II-16
| 10.64 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Summit Resources, Inc. with respect to the September Note by and between the Company and Summit Resources, Inc.* | Form 8-K filed with the Securities and Exchange Commission on December 6, 2012 as Exhibit 10.6 and incorporated herein by this reference. | ||
| 10.65 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Ed Roberson with respect to the September Note by and between the Company and Ed Roberson.* | Form 8-K filed with the Securities and Exchange Commission on December 6, 2012 as Exhibit 10.7 and incorporated herein by this reference. | ||
| 10.66 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Stanley Hostler and Virginia Child with respect to the September Note by and between the Company, Stanley Hostler and Virginia Child.* | Form 8-K filed with the Securities and Exchange Commission on December 6, 2012 as Exhibit 10.8 and incorporated herein by this reference. | ||
| 10.67 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Stephen Turner with respect to the September Note by and between the Company and Stephen Turner.* | Form 8-K filed with the Securities and Exchange Commission on December 6, 2012 as Exhibit 10.9 and incorporated herein by this reference. | ||
| 10.68 | Convertible Promissory Note Due April 1, 2013, executed by Josiah T. Austin, Managing Member of El Coronado Holdings, LLC with respect to the note by and between the Company and El Coronado Holdings, LLC.* | Form 8-K filed with the Securities and Exchange Commission on January 9, 2013 as Exhibit 10.1 and incorporated herein by this reference. | ||
| 10.69 | Warrant to Purchase common stock, executed by Josiah T. Austin, Managing Member of El Coronado Holdings, LLC with respect to the warrant by and between the Company and El Coronado Holdings, LLC.* | Form 8-K filed with the Securities and Exchange Commission on January 9, 2013 as Exhibit 10.1 and incorporated herein by this reference. | ||
| 10.70 | Securities Purchase Agreement, dated as of March 21, 2013 by and between the Company and El Coronado Holdings, LLC.* | Form 8-K filed with the Securities and Exchange Commission on January 24, 2013 as Exhibit 10.1 and incorporated herein by this reference. | ||
| 10.71 | Warrant and Reimbursement Agreement, dated March 6, 2013 by and between the Company and Steve Antoline.* | Form 10-Q filed with the Securities and Exchange Commission on May 10, 2013 and incorporated herein by this reference. | ||
| 10.72 | 2013 Equity Incentive Plan.* | Form 10-Q filed with the Securities and Exchange Commission on May 10, 2013 and incorporated herein by this reference. | ||
| 10.73 | Form of Securities Purchase Agreement by and between the Company and investors in the Spring 2013 Direct Issuances. | Form 10-Q filed with the Securities and Exchange Commission on May 10, 2013 and incorporated herein by this reference. | ||
| 10.74 | Form of Conversion Agreement. | Form 8-K filed with the Securities and Exchange Commission on July 23, 2013 and incorporated herein by this reference. | ||
| 10.75 | Form of Conversion Warrant. | Form 8-K filed with the Securities and Exchange Commission on July 23, 2013 and incorporated herein by this reference. | ||
| 10.76 | Form of Bridge Notes Purchase Agreement. | Form 8-K filed with the Securities and Exchange Commission on July 23, 2013 and incorporated herein by this reference. |
II-17
| 10.77 | Form of Bridge Notes. | Form 8-K filed with the Securities and Exchange Commission on July 23, 2013 and incorporated herein by this reference. | ||
| 10.78 | Form of Bridge Notesholder Warrant. | Form 8-K filed with the Securities and Exchange Commission on July 23, 2013 and incorporated herein by this reference. | ||
| 10.79 | Convertible Promissory Note Addendum, dated as of August 6, 2013, executed by Nancy Turner with respect to the September 2012 Note by and between the Company and Nancy Turner. | Form 8-K filed with the Securities and Exchange Commission on August 12, 2013 and incorporated herein by this reference. | ||
| 10.80 | Form of Related Party Advance Note Purchase Agreement.* | Form 8-K filed with the Securities and Exchange Commission on October 30, 2013 and incorporated herein by this reference. | ||
| 10.81 | Form of Related Party Advance Note.* | Form 8-K filed with the Securities and Exchange Commission on October 30, 2013 and incorporated herein by this reference. | ||
| 10.82 | Patent License Agreement between the Company and George Washington University. | Form 10-K filed with the Securities and Exchange Commission on March 27, 2013 and incorporated herein by this reference. | ||
| 10.83 | Memorandum of Understanding, dated March 19, 2014, by and between the Company and BioPharma d’Azur, Inc. | Form 10-Q filed with the Securities and Exchange Commission on May 9, 2014 and incorporated herein by this reference. | ||
| 10.84 | Option Agreement, dated March 27, 2014, by and between the Company, ProteaBio Europe SAS and BioPharma d’Azur, Inc. | Form 10-Q filed with the Securities and Exchange Commission on May 9, 2014 and incorporated herein by this reference. | ||
| 10.85 | Stock Purchase and Sale Agreement, dated May 21, 2014, by and among the Company, Protea Biosciences, Inc., ProteaBio Europe SAS, and AzurRx BioPharma, Inc. | Form 10-Q filed with the Securities and Exchange Commission on August 5, 2014 and incorporated herein by this reference. | ||
| 10.86 | Form of Unit Purchase Agreement among Protea Biosciences Group, Inc., and investors in the Company’s winter 2014-2015 private placement offering | Form 8-K filed with the Securities and Exchange Commission on April 6, 2015 and incorporated herein by this reference. | ||
| 10.86 | Form of Subscription Agreement among Protea Biosciences Group, Inc., and investors in the Company’s winter 2014-2015 private placement offering | Form 8-K filed with the Securities and Exchange Commission on April 6, 2015 and incorporated herein by this reference. | ||
| 10.87 | Form of Registration Rights Agreement among Protea Biosciences Group, Inc., and investors in the Company’s winter 2014-2015 private placement offering | Form 8-K filed with the Securities and Exchange Commission on April 6, 2015 and incorporated herein by this reference. |
| 10.88 | Form of Warrant issued to investors in the Company’s winter 2014-2015 private placement offering | Form S-1 filed with the Securities and Exchange Commission on February 13, 2015 and incorporated herein by this reference. | ||
| 10.89 | Employment Agreement by and between Stephen Turner and Protea Biosciences Group, Inc. | Form 8-K filed with the Securities and Exchange Commission on April 6, 2015 and incorporated herein by this reference. | ||
| 10.90 | Media Advertising Agreement by and between Al & J Media Inc., and Protea Biosciences Group, Inc., dated March 16, 2016. | Form 10-Q filed with the Securities and Exchange Commission on May 13, 2016 and incorporated herein by this reference. |
II-18
| 10.91 | Exclusive License Agreement by and between Yale University and Protea Biosciences Group, Inc., dated April 12, 2016. | Filed herewith. | ||
10.92 |
Letter, dated June 20, 2016 between United Bank and Protea Biosciences Group, Inc. extending maturity date under line of credit to July 12, 2018 |
Filed herewith . | ||
| 14.1 | Code of Ethics. | Filed herewith. | ||
| 21.1 | List of Subsidiaries. | Filed with Form S-1 registration statement dated May 27, 2016. | ||
| 23.1 | Consent of Schneider Downs & Co., Inc. | Filed herewith. | ||
| 23.2 | Consent of CKR Law LLP (included in Exhibit 5.1). | To be filed by amendment. | ||
| 24.1 | Power of Attorney. | Included in the signature page hereto. | ||
| EX-101.INS | XBRL Instance Document | Filed herewith. | ||
| EX-101.SCH | XBRL Taxonomy Extension Schema | Filed herewith. | ||
| EX-101.CAL | XBRL Taxonomy Extension Calculation Linkbase | Filed herewith. | ||
| EX-101.DEF | XBRL Taxonomy Extension Definition Linkbase | Filed herewith. | ||
| EX-101.LAB | XBRL Taxonomy Extension Label Linkbase | Filed herewith. | ||
| EX-101.PRE | XBRL Taxonomy Extension Presentation Linkbase | Filed herewith. |
* Management contract or compensatory plan or arrangement.
Item 17. Undertakings.
| (a) | The undersigned registrant hereby undertakes: |
| 1. | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| i. | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| ii. | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
| iii. | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
II-19
| 2. | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| 3. | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (b) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities, other than the payment by the registrant of expenses incurred and paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding, is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue. |
| (c) | Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
II-20
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Morgantown, State of West Virginia, on June 21, 2016.
| PROTEA BIOSCIENCES GROUP, INC. | ||
| By: | /s/ Stephen Turner | |
| Stephen Turner | ||
| Chief Executive Officer, President and Director | ||
| (Principal Executive Officer, Interim Principal Financial and Accounting Officer) | ||
| Stephen Turner, as attorney-in-fact and agent | ||
II-21
