Attached files
| file | filename |
|---|---|
| 8-K - 8-K - EAGLE PHARMACEUTICALS, INC. | a8-k060816.htm |

June 2016 Corporate Overview

2 Forward Looking Statements • This presentation contains forward-looking information within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, and other securities laws. Forward-looking statements are statements that are not historical facts. Words such as “will,” “may,” “intends,” “anticipate(s),” “plan,” “enables,” “facilitates,” “potentially,” “look forward,” “on track,” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements regarding future events including, but not limited to: the marketing of Eagle products by the sales team at Spectrum Pharmaceuticals (“Spectrum”); Eagle’s plan to hire 20 direct sales representatives; the marketing, sale, and distribution of Docetaxel Injection Concentrate, Non-Alcohol Formula, under the licensing agreement with Teikoku Pharma USA (“Teikoku); the results of data analysis of the RYANODEX® study and any additional testing; the achievement of milestones under the license agreement with Cephalon, Inc., a wholly-owned subsidiary of Teva Pharmaceutical Industries Ltd (“Teva”), for the U.S. and Canadian rights to BENDEKA™ and their impact on Eagle’s profitability; the replication of the success of our sales of RYANODEX® for our other product candidates; the approval and subsequent marketing of RTU bivalirudin candidate and tentatively-approved liquid bendamustine product in the 500ml bag; and the impact of such anticipated events and outcomes on Eagle’s profitability. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond Eagle’s control, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. Such risks include, but are not limited to: the success of our commercial relationship with the Spectrum sales team; our ability to hire, and the success of, the direct sales representatives we plan to hire; success in gaining timely FDA approval of the rapid infusion bendamustine product for the treatment of patients with CLL and patients with indolent B-cell NHL that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen, and for the RTU bivalirudin product for the treatment of patients (1) undergoing percutaneous coronary intervention (PCI) with use of glycoprotein IIb/IIIa inhibitor, (2) undergoing PCI with, or at risk of, heparin- induced thrombocytopenia and thrombosis syndrome, and/or (3) with unstable angina undergoing percutaneous transluminal coronary angioplasty (PTCA), if at all; the timing and level of success of the launch of BENDEKA by Teva; the success of our commercial relationship with Teva and the parties’ ability to work effectively together; whether Eagle and Teva will successfully perform their respective obligations under the license agreement; difficulties or delays in manufacturing; the availability and pricing of third party sourced products and materials; successful compliance with FDA and other governmental regulations applicable to manufacturing facilities, products and/or businesses; general economic conditions; the strength and enforceability of our intellectual property rights; the timing and impact of FDA regulatory decisions on our products; the timing of product launches; the successful marketing of our products; the risks inherent in the early stages of drug development and in conducting clinical trials; the possible outcome risks inherent in the litigation process; and other factors that are discussed in Eagle’s Annual Report on Form 10-K for the year ended December 31, 2015, and its other filings with the U.S. Securities and Exchange Commission. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events.

3 Eagle is a fully commercial specialty company with a broad portfolio and deep near-term pipeline

4 Short-term Activity – Bendeka™ launch with Teva: • Expect near-complete conversion to Bendeka; shared goal with Teva of 90% market share • FDA decision on 3 years of exclusivity pending; seeking to overturn the recent FDA decision to deny 7 years of marketing exclusivity – Federal District Court action filed in April • Existing J-code adequately describes Bendeka – In discussions with FDA regarding RTU Bivalirudin next steps – Right to launch tentatively approved Bendamustine RTD (500mL) May 2016 – Docetaxel launch – first units shipped in January – Ryanodex, Argatroban and Diclofenac currently in-market generating revenue – FDA meeting to discuss potential Ryanodex label expansion for EHS Added pipeline driving growth beyond 2020 – Potential Pemetrexed launch ahead of other generics – Exploring additional Ryanodex label expansion opportunity to treat Ecstasy and Methamphetamine intoxication – Internal and AMRI development programs underway for 4+ product candidates Strong YoY growth anticipated through 2020 WITHOUT deployment of cash or the balance sheet – Buildup of cash plus balance sheet provides opportunity to increase value + significant organic growth already in place Eagle Poised for Strong Growth through 2020
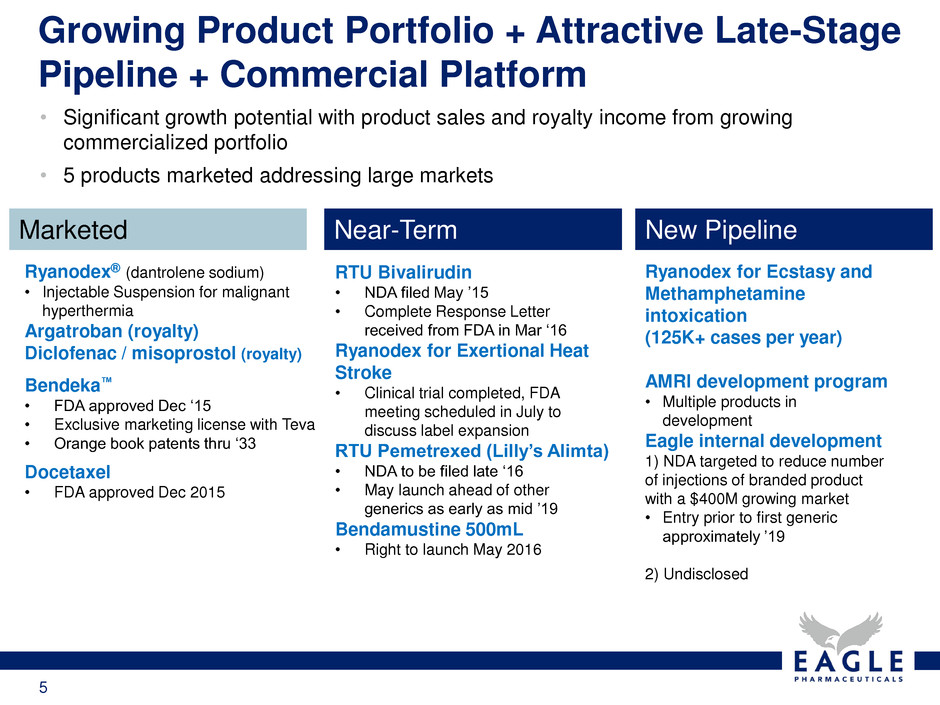
5 Growing Product Portfolio + Attractive Late-Stage Pipeline + Commercial Platform • Significant growth potential with product sales and royalty income from growing commercialized portfolio • 5 products marketed addressing large markets Marketed Near-Term New Pipeline Ryanodex® (dantrolene sodium) • Injectable Suspension for malignant hyperthermia Argatroban (royalty) Diclofenac / misoprostol (royalty) Docetaxel • FDA approved Dec 2015 Bendeka™ • FDA approved Dec ‘15 • Exclusive marketing license with Teva • Orange book patents thru ‘33 RTU Bivalirudin • NDA filed May ’15 • Complete Response Letter received from FDA in Mar ‘16 Ryanodex for Exertional Heat Stroke • Clinical trial completed, FDA meeting scheduled in July to discuss label expansion RTU Pemetrexed (Lilly’s Alimta) • NDA to be filed late ‘16 • May launch ahead of other generics as early as mid ’19 Bendamustine 500mL • Right to launch May 2016 Ryanodex for Ecstasy and Methamphetamine intoxication (125K+ cases per year) AMRI development program • Multiple products in development Eagle internal development 1) NDA targeted to reduce number of injections of branded product with a $400M growing market • Entry prior to first generic approximately ’19 2) Undisclosed

6 • 32 proven and seasoned Spectrum professionals focused in the hematology and oncology space – Devote 80% of time to selling up to six Eagle products – Target infusion centers, hospitals and oncology purchasing groups • Attractive financial terms for both parties – Eagle to pay Spectrum potential total payment of up to $22 million in base fee and specified milestones over 18 mos. – Option for 6 month renewal periods upon mutual agreement – Agreement will maximize product revenue stream at low risk / cost Eagle Will Have Over 50 Sales Reps Calling on Hospitals and Cancer Centers • Eagle hired 12 of 20 new direct sales reps and 2 district managers who will work with Spectrum Sales Team. VP sales and VP marketing also hired. – Will form core of Eagle’s internal sales team – Positions Eagle for successful transition from development-stage company to commercial provider External Internal

7 Significant market opportunity: • Current shared market share goal with Teva is 90% – Achieved 71% total market share • $25M in additional milestone payments subject to: – Achievement of certain sales levels • Royalty payments: – 20% royalty on net sales of Bendeka (50mL) – 20% royalty owed by Eagle to Teva if we take Bendeka back • Option to terminate agreement when competitive product launches • Option to launch at any time tentatively approved Bendamustine RTD (500mL) product in the U.S. • Six patents issued and listed in Orange Book covering periods ending within 2026-2033 Exclusive Marketing Agreement with Teva for Bendeka™ (50 mL Rapid Infusion)
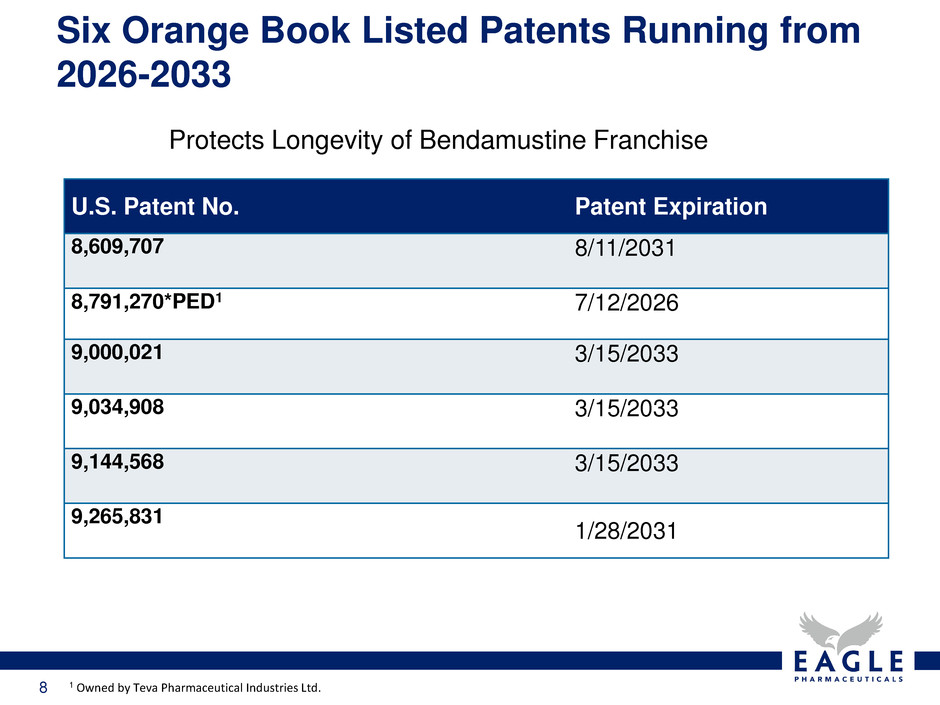
8 Six Orange Book Listed Patents Running from 2026-2033 Protects Longevity of Bendamustine Franchise U.S. Patent No. Patent Expiration 8,609,707 8/11/2031 8,791,270*PED1 7/12/2026 9,000,021 3/15/2033 9,034,908 3/15/2033 9,144,568 3/15/2033 9,265,831 1/28/2031 1 Owned by Teva Pharmaceutical Industries Ltd.
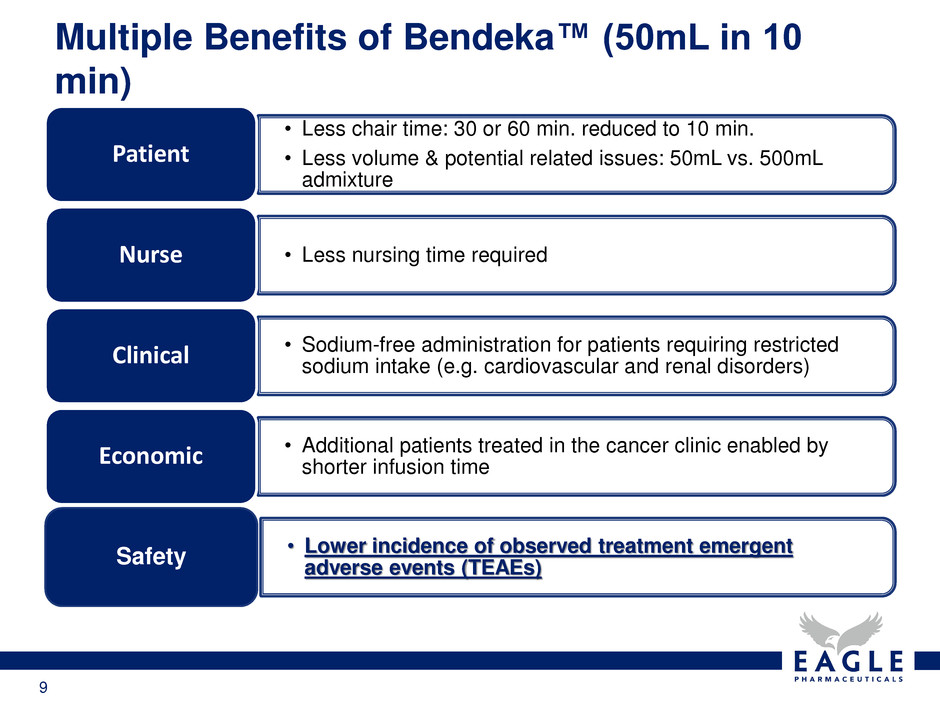
9 Multiple Benefits of Bendeka™ (50mL in 10 min) • Less chair time: 30 or 60 min. reduced to 10 min. • Less volume & potential related issues: 50mL vs. 500mL admixture Patient • Less nursing time required Nurse • Sodium-free administration for patients requiring restricted sodium intake (e.g. cardiovascular and renal disorders) Clinical • Additional patients treated in the cancer clinic enabled by shorter infusion time Economic • Lower incidence of observed treatment emergent adverse events (TEAEs) Safety
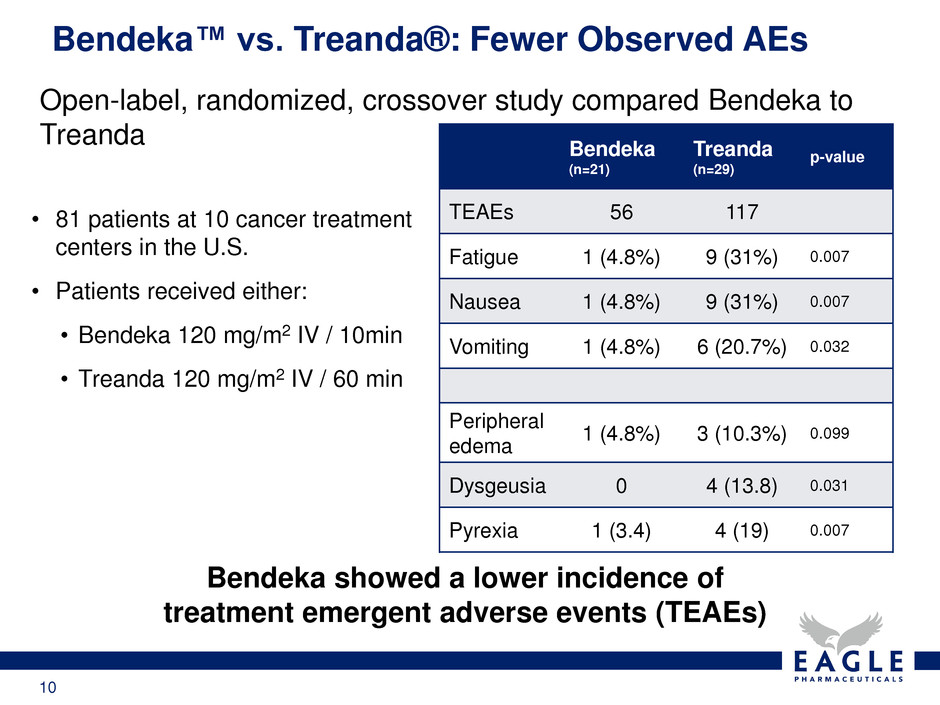
10 Bendeka™ vs. Treanda®: Fewer Observed AEs Bendeka showed a lower incidence of treatment emergent adverse events (TEAEs) Bendeka (n=21) Treanda (n=29) p-value TEAEs 56 117 Fatigue 1 (4.8%) 9 (31%) 0.007 Nausea 1 (4.8%) 9 (31%) 0.007 Vomiting 1 (4.8%) 6 (20.7%) 0.032 Peripheral edema 1 (4.8%) 3 (10.3%) 0.099 Dysgeusia 0 4 (13.8) 0.031 Pyrexia 1 (3.4) 4 (19) 0.007 Open-label, randomized, crossover study compared Bendeka to Treanda • 81 patients at 10 cancer treatment centers in the U.S. • Patients received either: • Bendeka 120 mg/m2 IV / 10min • Treanda 120 mg/m2 IV / 60 min
11 RTU Bivalirudin • Stable, liquid intravenous formulation of Angiomax1 – Brand sales over $635M2 • Limited market offers significant opportunity – Hospira’s marketed generic at risk on 1st day of launch due to Appellate Court decision being vacated – Sandoz’s authorized generic can come off the market contractually at Medicines Co. discretion if Hospira exits the market – Additional generics are not likely to enter the market until the appellate situation is resolved • Two patents issued; third patent filed Q1 2014 • Complete Response Letter received March 2016 1 Angiomax® (bivalirudin) / Angiox® (bivalirudin) (The Medicines Company) 2 Worldwide Angiomax/Angiox sales in 2014 of $635.7 million, source: The Medicines Company press release dated 2/18/2015.
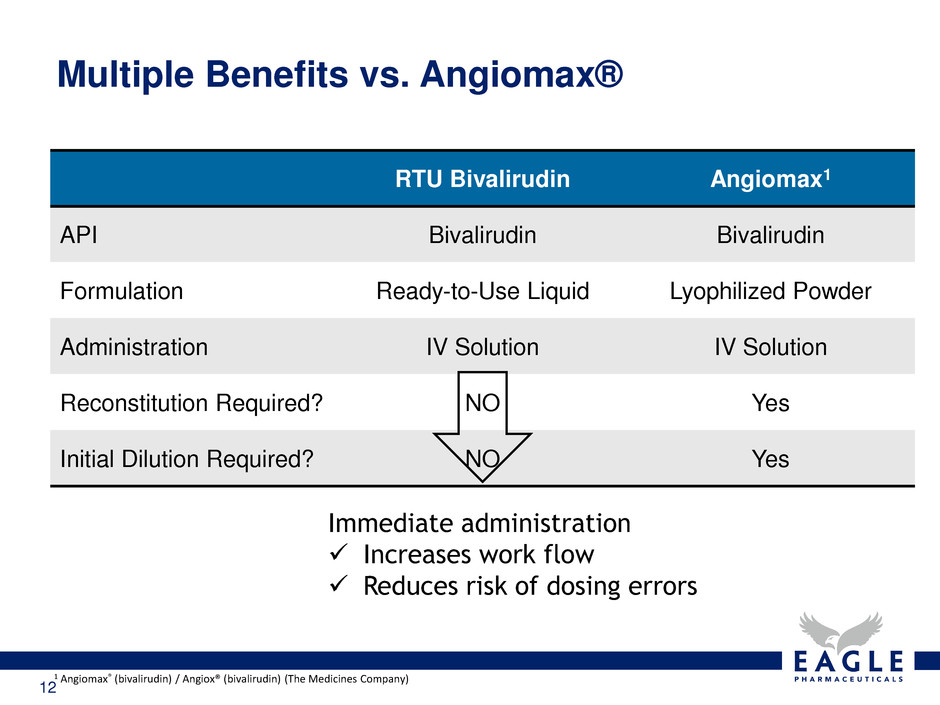
12 Multiple Benefits vs. Angiomax® RTU Bivalirudin Angiomax1 API Bivalirudin Bivalirudin Formulation Ready-to-Use Liquid Lyophilized Powder Administration IV Solution IV Solution Reconstitution Required? NO Yes Initial Dilution Required? NO Yes 1 Angiomax® (bivalirudin) / Angiox® (bivalirudin) (The Medicines Company) Immediate administration Increases work flow Reduces risk of dosing errors
13 • No change to Standard of Care (SOC) 1 for MH treatment in over 30 years – Often fatal if untreated • Ryanodex approved in July 2014 – Optimized formulation of dantrolene sodium – Reduces to 5mL (1 vial) vs. 720 mL (12 vials) for old product • Breakthrough change • Protected market position – Five patents issued + two filed – Orphan drug designation for MH (U.S. & Europe) • Granted seven years market exclusivity in US Ryanodex® (dantrolene sodium) for Malignant Hyperthermia (MH) 1SOC: body cooling by physical methods (e.g. cold water immersion, cold water mist, ice packs application) and supportive measures
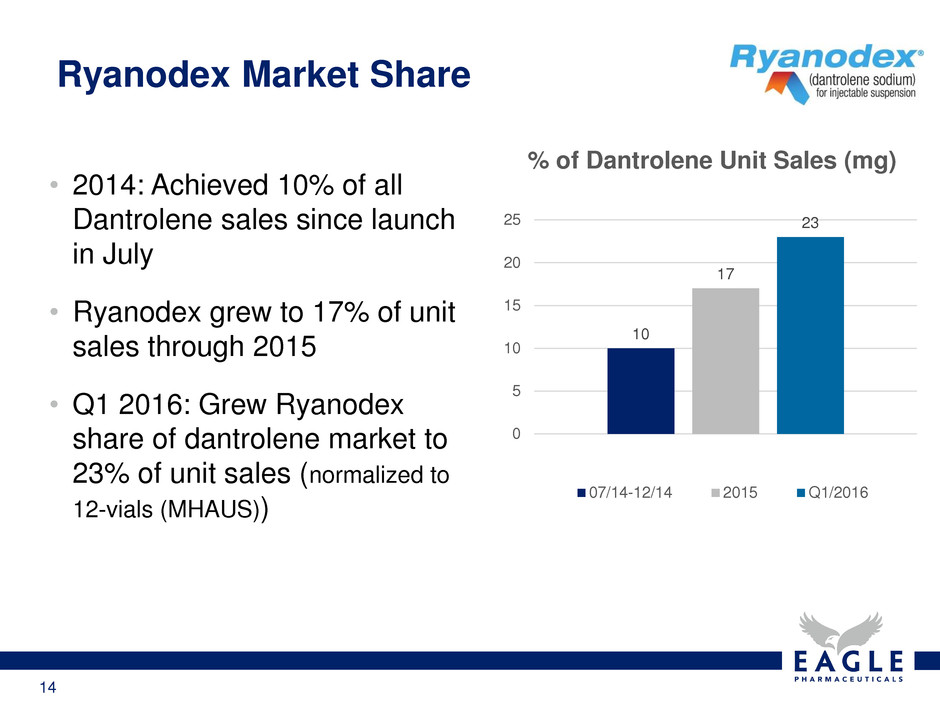
14 • 2014: Achieved 10% of all Dantrolene sales since launch in July • Ryanodex grew to 17% of unit sales through 2015 • Q1 2016: Grew Ryanodex share of dantrolene market to 23% of unit sales (normalized to 12-vials (MHAUS)) Ryanodex Market Share 10 17 23 0 5 10 15 20 25 % of Dantrolene Unit Sales (mg) 07/14-12/14 2015 Q1/2016

15 • Exertional Heat Stroke (EHS) ‒ Sudden, unpredictable and life-threatening condition ‒ Patient population impacted: military, student athletes, athletes, construction workers, migrant workers, and firemen ‒ A leading cause of student athlete death (US) & non-combat military deaths ‒ Similarities to MH • Potential for Ryanodex to be first to market for EHS • Orphan drug designation for EHS – Potential for 7 years of exclusivity • Market estimated at $400M worldwide • FDA meeting to discuss potential Ryanodex label expansion for EHS Initial Label Expansion Opportunity

16 • Completed Safety & Efficacy study to evaluate Ryanodex for treatment of EHS during during Hajj pilgrimage in Saudi Arabia (Sept. 22-27) • 34 patients randomized 1:1 to receive SOC or SOC + Ryanodex • Inclusion protocol criteria required that patients showed hallmark clinical features of EHS including: – 18-45 years of age who experienced exertional physical activity within previous 24 hours; – Presence of neurological impairment, evaluated using the Glasgow Coma Scale; – Core body temperature of at least 104 degrees Fahrenheit; and – Tachycardia (at least 100 heart beats per minute) EHS Clinical Study: September 2015
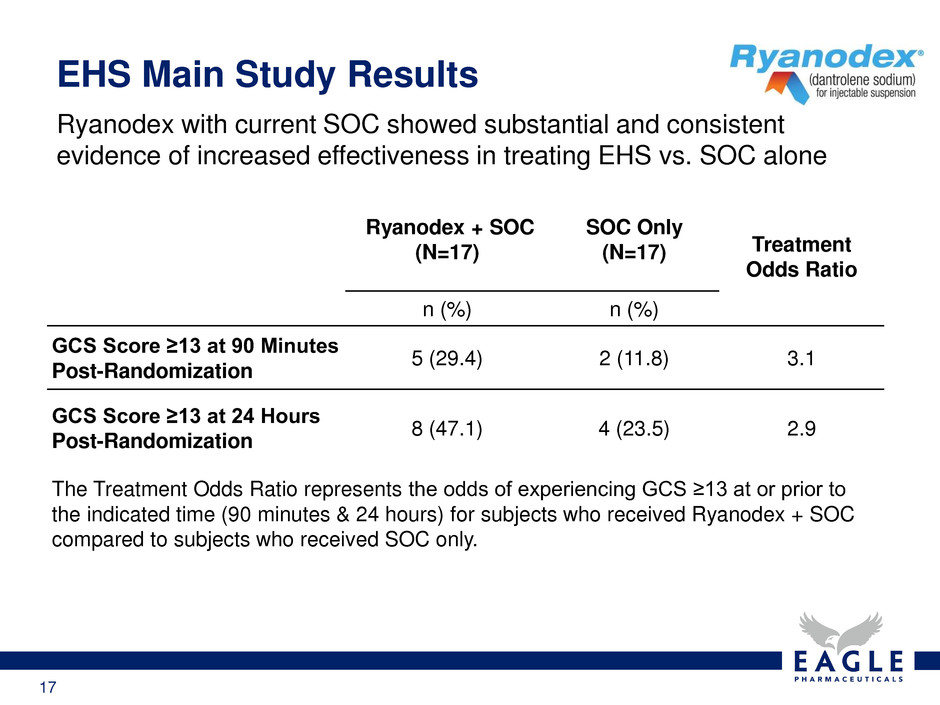
17 Ryanodex with current SOC showed substantial and consistent evidence of increased effectiveness in treating EHS vs. SOC alone EHS Main Study Results Ryanodex + SOC (N=17) SOC Only (N=17) Treatment Odds Ratio n (%) n (%) GCS Score ≥13 at 90 Minutes Post-Randomization 5 (29.4) 2 (11.8) 3.1 GCS Score ≥13 at 24 Hours Post-Randomization 8 (47.1) 4 (23.5) 2.9 The Treatment Odds Ratio represents the odds of experiencing GCS ≥13 at or prior to the indicated time (90 minutes & 24 hours) for subjects who received Ryanodex + SOC compared to subjects who received SOC only.
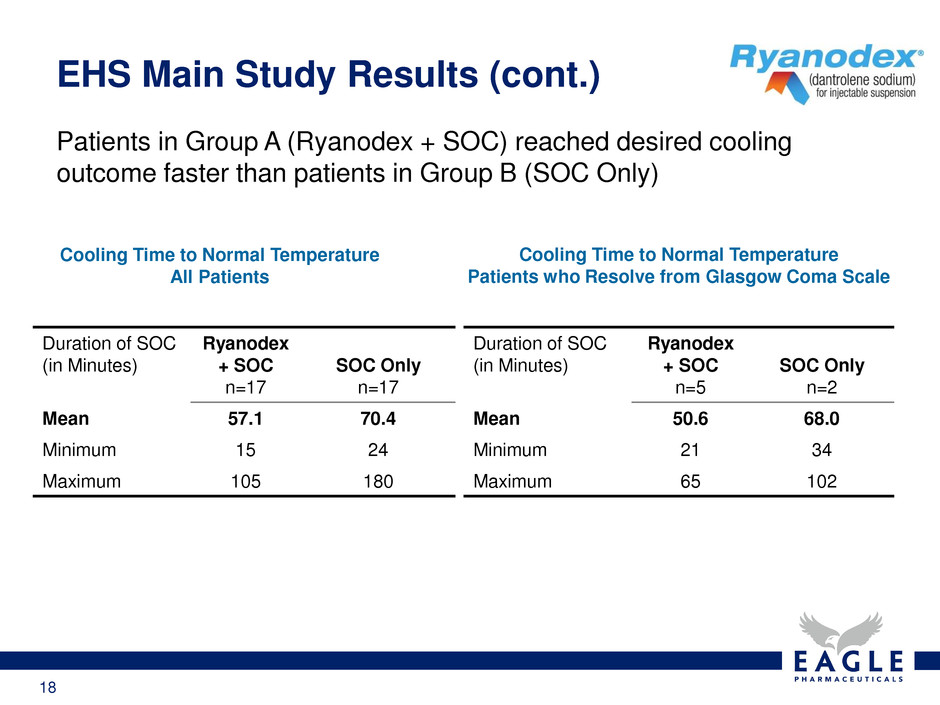
18 Patients in Group A (Ryanodex + SOC) reached desired cooling outcome faster than patients in Group B (SOC Only) EHS Main Study Results (cont.) Cooling Time to Normal Temperature All Patients Duration of SOC (in Minutes) Ryanodex + SOC n=17 SOC Only n=17 Mean 57.1 70.4 Minimum 15 24 Maximum 105 180 Cooling Time to Normal Temperature Patients who Resolve from Glasgow Coma Scale Duration of SOC (in Minutes) Ryanodex + SOC n=5 SOC Only n=2 Mean 50.6 68.0 Minimum 21 34 Maximum 65 102
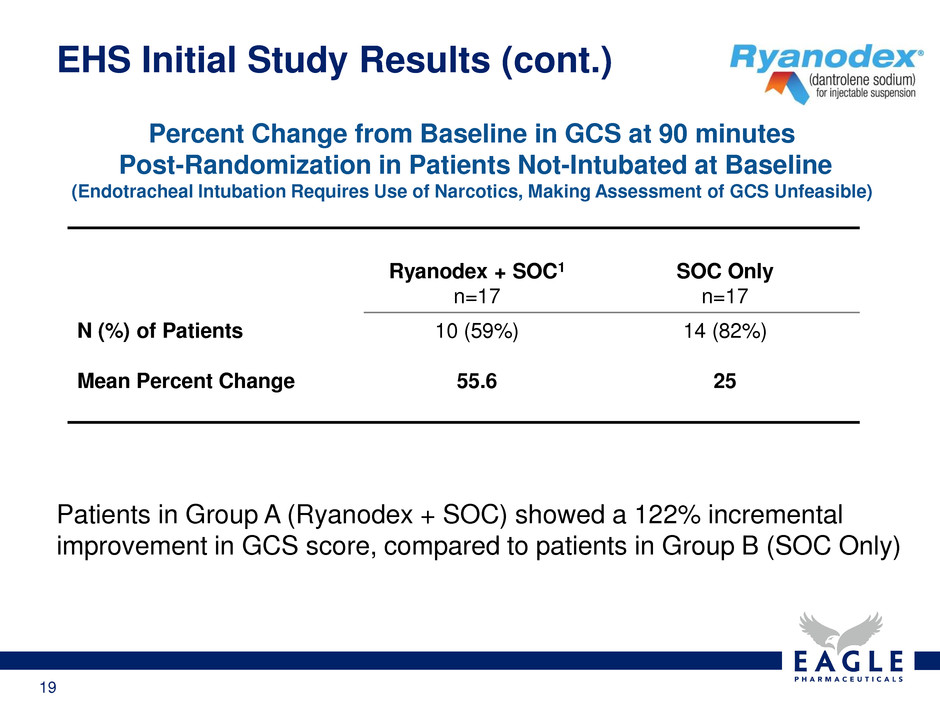
19 EHS Initial Study Results (cont.) Ryanodex + SOC1 n=17 SOC Only n=17 N (%) of Patients Mean Percent Change 10 (59%) 55.6 14 (82%) 25 Percent Change from Baseline in GCS at 90 minutes Post-Randomization in Patients Not-Intubated at Baseline (Endotracheal Intubation Requires Use of Narcotics, Making Assessment of GCS Unfeasible) Patients in Group A (Ryanodex + SOC) showed a 122% incremental improvement in GCS score, compared to patients in Group B (SOC Only)

20 EHS Initial Study Results (cont.) Safety Summary • Overall incidence of adverse events (AEs) was comparable in both treatment groups. • Most AEs in Group A (Ryanodex + SOC) were mild to moderate and assessed as not related to study drug. • Safety profile of Ryanodex in EHS patients was consistent with its known and well characterized safety profile. Type of Event Ryanodex and SOC1 (N=17) n (%) SOC Only (N=17) n (%) Any Adverse Event 11 (64.7) 13 (76.5) Any Serious Adverse Event 4 (23.5) 3 (17.6) Any Adverse Event (AE) by Maximum Intensity Severe AE 2 (11.8) 2 (11.8) Moderate AE 2 (11.8) 5 (29.4) Mild AE 7 (41.2) 6 (35.3) Any Treatment-Related Adverse Event 0 0 Any Treatment-Related Serious Adverse Event 0 0

21 • Joint effort with the NIH to explore the potential of Ryanodex to treat hyperthermia related to Ecstasy and Methamphetamine intoxication – 125,000 emergency room visits in 2011 due to Ecstasy and Methamphetamine use1 – Brain hyperthermia is one of the leading causes of death in Ecstasy and Methamphetamine intoxication • Preclinical studies to be conducted by NIDA beginning summer 2016 – Well-characterized animal model to be used – Initial results anticipated late 2016 / early 2017 • Positive preclinical results would facilitate FDA meeting and potentially lead to a rapid transition to short-duration pivotal clinical trials Eagle & NIH/NIDA Agreement 1Source Drug Abuse Warning Network
22 • Lilly’s Alimta patent infringement lawsuit win should prevent current ANDA filers from launching until May 24, 2022 • Eagle plans to file Pemetrexed RTU NDA in late 2016 • Registration batches have been produced • We believe our patent position may enable us to bring the product to market as early as Q4 2017 • 30 month stay to expire 1st half of 2019 • $1.2B market opportunity1 Pemetrexed 1 Alimta® (pemetrexed) (Eli Lilly & Co.). Source: Eli Lilly & Co. press release dated 1/30/2015: (U.S. sales)

23 • Need for alcohol-free docetaxel after FDA issued Drug Safety warning that docetaxel may cause symptoms of alcohol intoxication after treatment • Some U.S. hospitals and clinics require patients to wait two or more hours after treatment • For treatment of breast cancer, non-small cell lung cancer, prostate cancer, gastric adenocarcinoma, and head and neck cancer • Approved by the FDA in Dec ‘15 and shipped in January • Eagle holds exclusive right to market, sell and distribute in the U.S. • Market for generic docetaxel is approximately $75M First Docetaxel Injection Concentrate, Non-Alcohol Formulation Approved in the U.S.

24 Building Pipeline for Long Term Growth • Ryanodex for the treatment of Ecstasy and Methamphetamine intoxication (over 125,000 new cases per year) • Label expansion opportunity with potential fourth indication • AMRI Development Program • Joint development program for several new product candidates • AMRI to provide drug development and manufacturing once approved by the FDA • Eagle responsible clinical trials, regulatory submissions, and commercial distribution in the U.S. • New NDA under development targeted to reduce number of injections of $400M branded product in a growing market • Entry prior to first generic approximately 2019 • Undisclosed next project

25 Short-term Activity – Bendeka™ launch with Teva: • Expect near-complete conversion to Bendeka; shared goal with Teva of 90% market share • FDA decision on 3 years of exclusivity pending; seeking to overturn the recent FDA decision to deny 7 years of marketing exclusivity – Federal District Court action filed in April • Existing J-code adequately describes Bendeka – In discussions with FDA regarding RTU Bivalirudin next steps – Right to launch tentatively approved Bendamustine RTD (500mL) May 2016 – Docetaxel launch – first units shipped in January – Ryanodex, Argatroban and Diclofenac currently in-market generating revenue – FDA meeting to discuss potential Ryanodex label expansion for EHS Added pipeline driving growth beyond 2020 – Potential Pemetrexed launch ahead of other generics – Exploring additional Ryanodex label expansion opportunity to treat Ecstasy and Methamphetamine intoxication – Internal and AMRI development programs underway for 4+ product candidates Strong YoY growth anticipated through 2020 WITHOUT deployment of cash or the balance sheet – Buildup of cash plus balance sheet provides opportunity to increase value + significant organic growth already in place Eagle Poised for Strong Growth through 2020
