Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Aclaris Therapeutics, Inc. | acrs-20160527ex9913b224b.htm |
| 8-K - 8-K - Aclaris Therapeutics, Inc. | acrs-20160527x8k.htm |
|
|
© Copyright 2016 Aclaris Therapeutics. All rights reserved. A-1 1 May 2016 Corporate Presentation |
|
|
This presentation, the information contained herein and the materials accompanying it (collectively, this “presentation”) constitutes confidential information and is provided to you on the condition that you agree that you will hold it in strict confidence and not reproduce, disclose, forward or distribute it in whole or in part without the prior written consent of Aclaris Therapeutics, Inc. (“Aclaris”) and is intended for the recipient hereof only. The presentation is for information purposes only. This presentation contains forward-looking statements, including statements regarding the treatment and market opportunity for SK, common warts and alopecia areata, and the future operations of Aclaris. These statements involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements in this presentation represent our views as of the date of this presentation. For further information regarding these risks, uncertainties and other factors you should read Aclaris’ Annual Report on Form 10-K for the year ended December 31, 2015 and Aclaris’ other filings it makes with the Securities and Exchange Commission from time to time. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Disclaimer May 2016 2 |
|
|
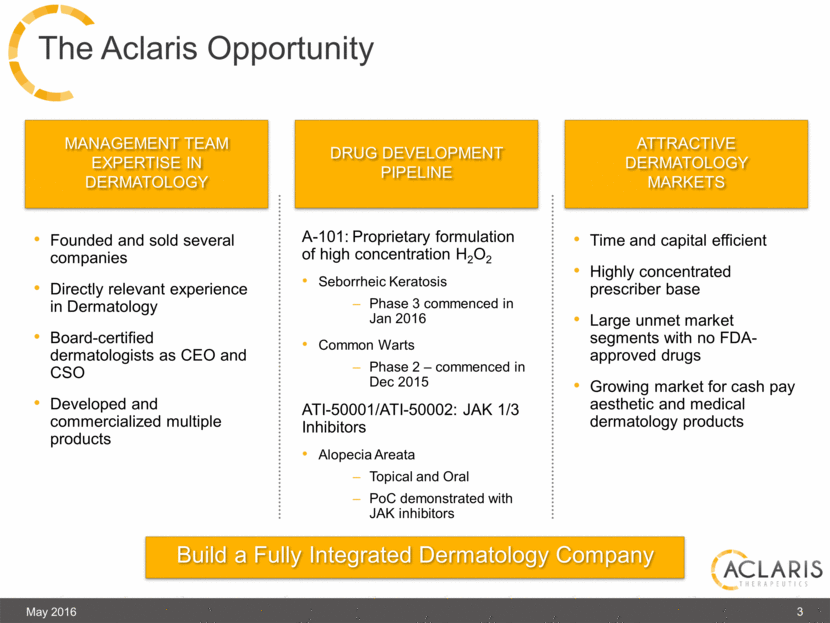
The Aclaris Opportunity May 2016 3 Time and capital efficient Highly concentrated prescriber base Large unmet market segments with no FDA-approved drugs Growing market for cash pay aesthetic and medical dermatology products A-101: Proprietary formulation of high concentration H2O2 Seborrheic Keratosis Phase 3 commenced in Jan 2016 Common Warts Phase 2 – commenced in Dec 2015 ATI-50001/ATI-50002: JAK 1/3 Inhibitors Alopecia Areata Topical and Oral PoC demonstrated with JAK inhibitors Founded and sold several companies Directly relevant experience in Dermatology Board-certified dermatologists as CEO and CSO Developed and commercialized multiple products Build a Fully Integrated Dermatology Company Management team expertise in dermatology DRUG Development PIPELINe Attractive Dermatology markets |
|
|
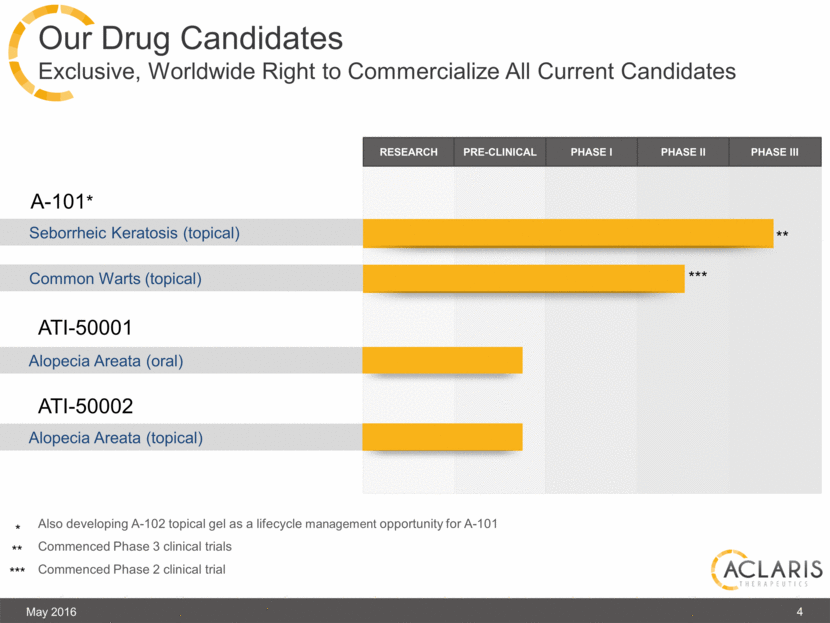
Our Drug Candidates Exclusive, Worldwide Right to Commercialize All Current Candidates May 2016 4 Seborrheic Keratosis (topical) A-101* RESEARCH PRE-CLINICAL PHASE I PHASE II PHASE III Common Warts (topical) ATI-50001 Alopecia Areata (oral) ATI-50002 Alopecia Areata (topical) Also developing A-102 topical gel as a lifecycle management opportunity for A-101 Commenced Phase 3 clinical trials Commenced Phase 2 clinical trial * ** *** ** *** |
|
|
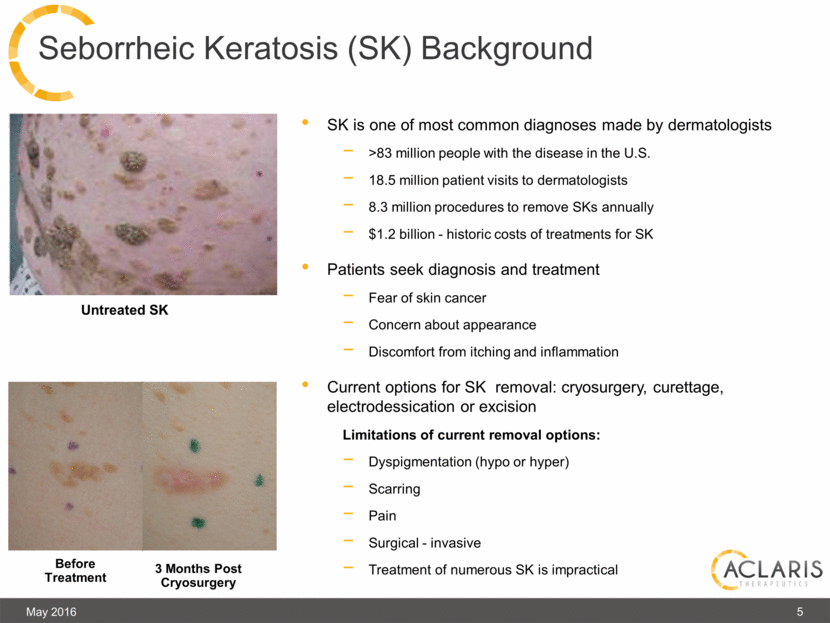
3 Months Post Cryosurgery Before Treatment SK is one of most common diagnoses made by dermatologists >83 million people with the disease in the U.S. 18.5 million patient visits to dermatologists 8.3 million procedures to remove SKs annually $1.2 billion - historic costs of treatments for SK Patients seek diagnosis and treatment Fear of skin cancer Concern about appearance Discomfort from itching and inflammation Current options for SK removal: cryosurgery, curettage, electrodessication or excision Limitations of current removal options: Dyspigmentation (hypo or hyper) Scarring Pain Surgical - invasive Treatment of numerous SK is impractical May 2016 5 Seborrheic Keratosis (SK) Background Untreated SK |
|
|
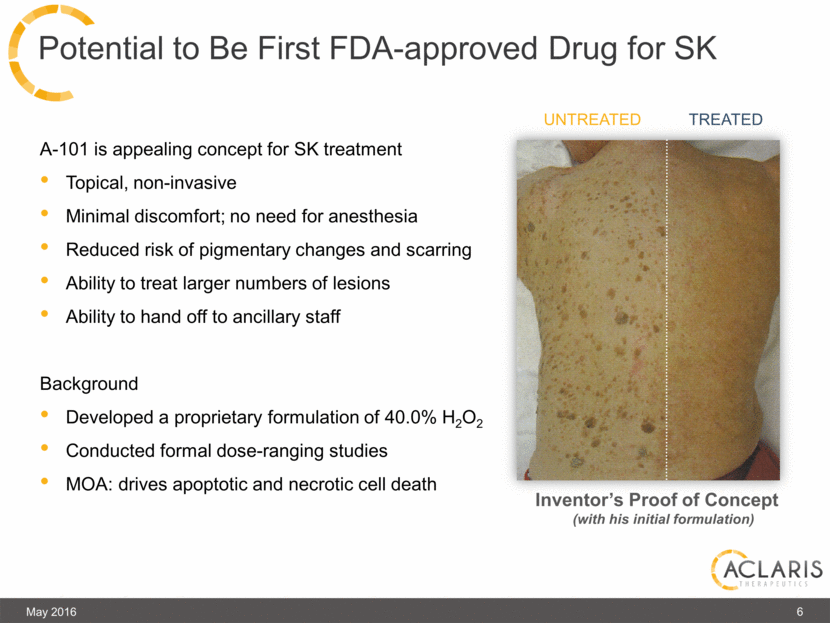
A-101 is appealing concept for SK treatment Topical, non-invasive Minimal discomfort; no need for anesthesia Reduced risk of pigmentary changes and scarring Ability to treat larger numbers of lesions Ability to hand off to ancillary staff Background Developed a proprietary formulation of 40.0% H2O2 Conducted formal dose-ranging studies MOA: drives apoptotic and necrotic cell death Potential to Be First FDA-approved Drug for SK Untreated Treated May 2016 6 Inventor’s Proof of Concept (with his initial formulation) |
|
|
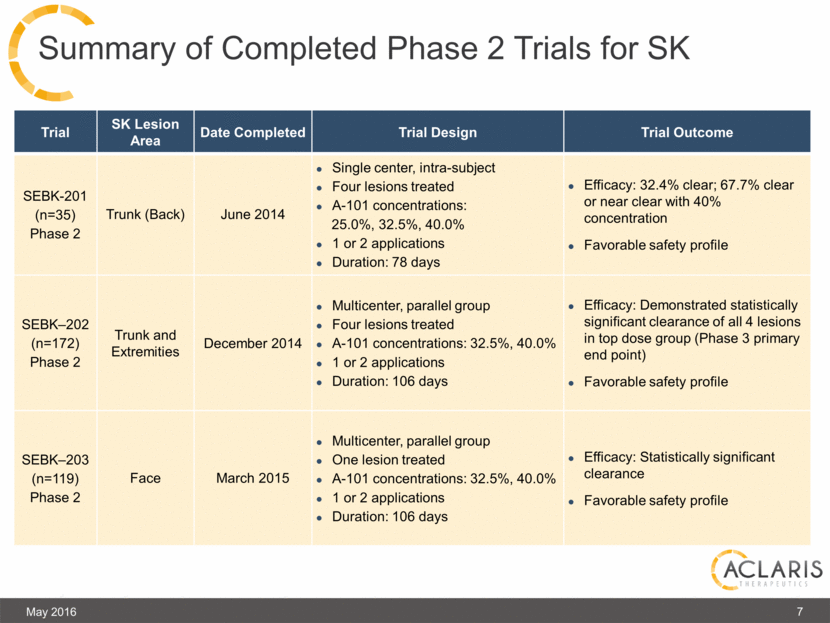
Summary of Completed Phase 2 Trials for SK Trial SK Lesion Area Date Completed Trial Design Trial Outcome SEBK-201 (n=35) Phase 2 Trunk (Back) June 2014 Single center, intra-subject Four lesions treated A-101 concentrations: 25.0%, 32.5%, 40.0% 1 or 2 applications Duration: 78 days Efficacy: 32.4% clear; 67.7% clear or near clear with 40% concentration Favorable safety profile SEBK–202 (n=172) Phase 2 Trunk and Extremities December 2014 Multicenter, parallel group Four lesions treated A-101 concentrations: 32.5%, 40.0% 1 or 2 applications Duration: 106 days Efficacy: Demonstrated statistically significant clearance of all 4 lesions in top dose group (Phase 3 primary end point) Favorable safety profile SEBK–203 (n=119) Phase 2 Face March 2015 Multicenter, parallel group One lesion treated A-101 concentrations: 32.5%, 40.0% 1 or 2 applications Duration: 106 days Efficacy: Statistically significant clearance Favorable safety profile May 2016 7 |
|
|
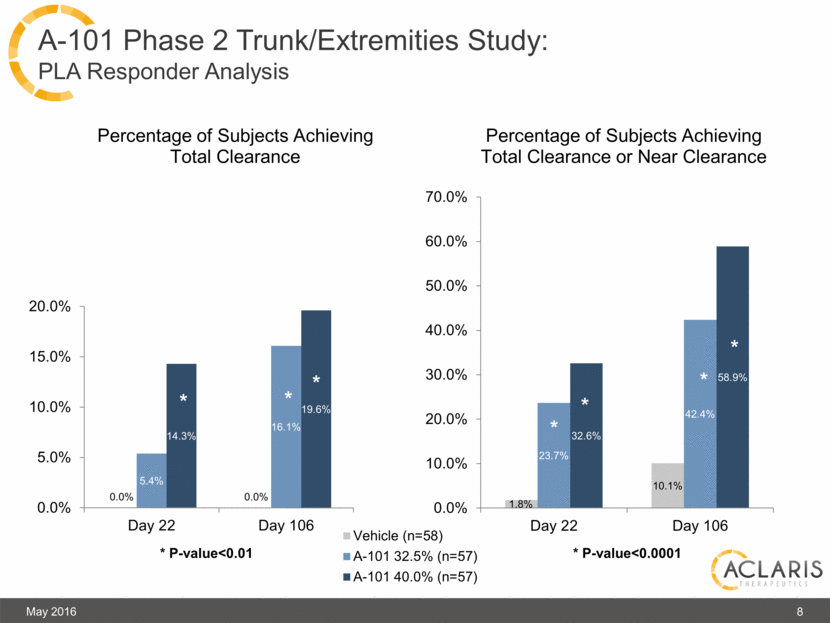
A-101 Phase 2 Trunk/Extremities Study: PLA Responder Analysis * 8 May 2016 |
|
|
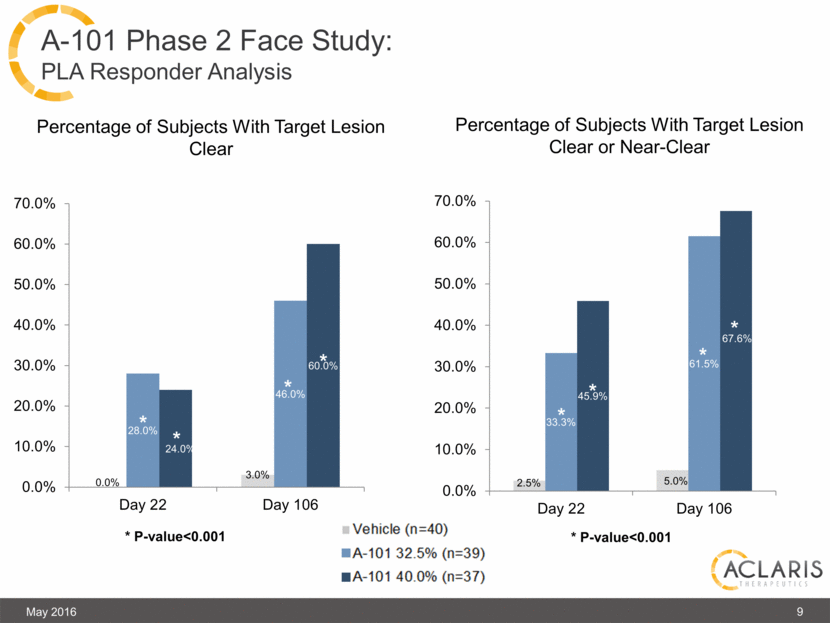
A-101 Phase 2 Face Study: PLA Responder Analysis Percentage of Subjects With Target Lesion Clear Percentage of Subjects With Target Lesion Clear or Near-Clear * * * P-value<0.001 May 2016 9 * P-value<0.001 0.0% 3.0% 28.0% 46.0% 24.0% 60.0% 0.0% 10.0% 20.0% 30.0% 40.0% 50.0% 60.0% 70.0% Day 22 Day 106 * * * * 2.5% 5.0% 33.3% 61.5% 45.9% 67.6% 0.0% 10.0% 20.0% 30.0% 40.0% 50.0% 60.0% 70.0% Day 22 Day 106 * * * * |
|
|
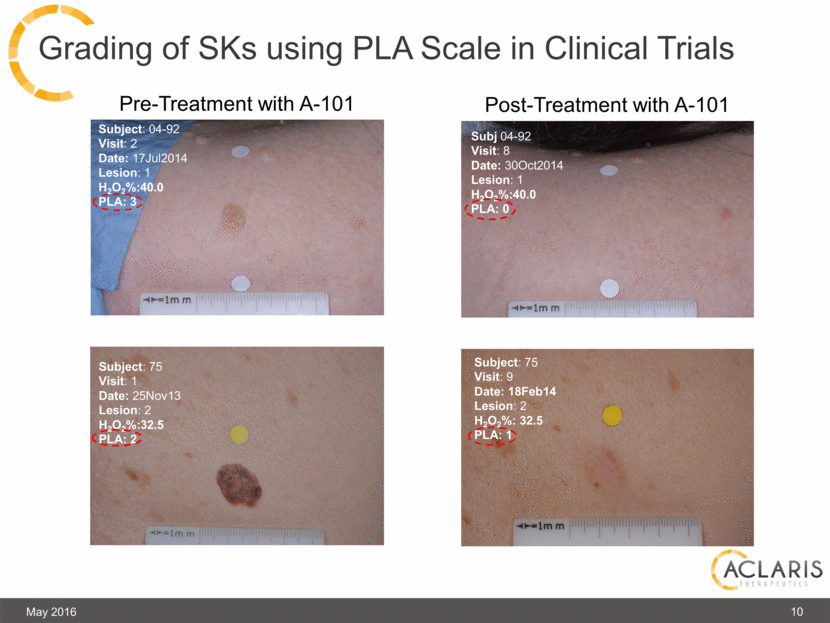
Grading of SKs using PLA Scale in Clinical Trials Subject: 04-52 Visit: 8 Date: 23Oct2014 Lesion: 3 H2O2%: 40.0 PLA: 1 Subject: 04-52 Visit: 2 Date: 07Jul2014 Lesion: 3 H2O2%: 40.0 PLA: 3 Subject: 04-52 Visit: 2 Date: 07Jul2014 Lesion: 4 H2O2%: 40.0 PLA: 3 Subj 04-92 Visit: 8 Date: 30Oct2014 Lesion: 1 H2O2%:40.0 PLA: 0 Subject: 04-92 Visit: 2 Date: 17Jul2014 Lesion: 1 H2O2%:40.0 PLA: 3 Subject: 75 Visit: 1 Date: 25Nov13 Lesion: 2 H2O2%:32.5 PLA: 2 Subject: 75 Visit: 9 Date: 18Feb14 Lesion: 2 H2O2%: 32.5 PLA: 1 May 2016 10 Pre-Treatment with A-101 Post-Treatment with A-101 |
|
|
A-101 40.0% is being used for Phase 3 clinical testing Initiated Phase 3 program – January 2016 Pivotal trials (SEBK-301/302): Two identical Phase 3 trials 4 lesions treated in total with at least one on face and one on trunk or extremities 400 subjects each Primary endpoint: Proportion of subjects with clear on PLA scale 3 month drug-free follow-up Open-label (SEBK-303): 4 SK lesions Up to four applications 200 subjects Plan to submit NDA – 4Q 2016 A-101 Next Steps: Phase 3 Overview May 2016 11 |
|
|
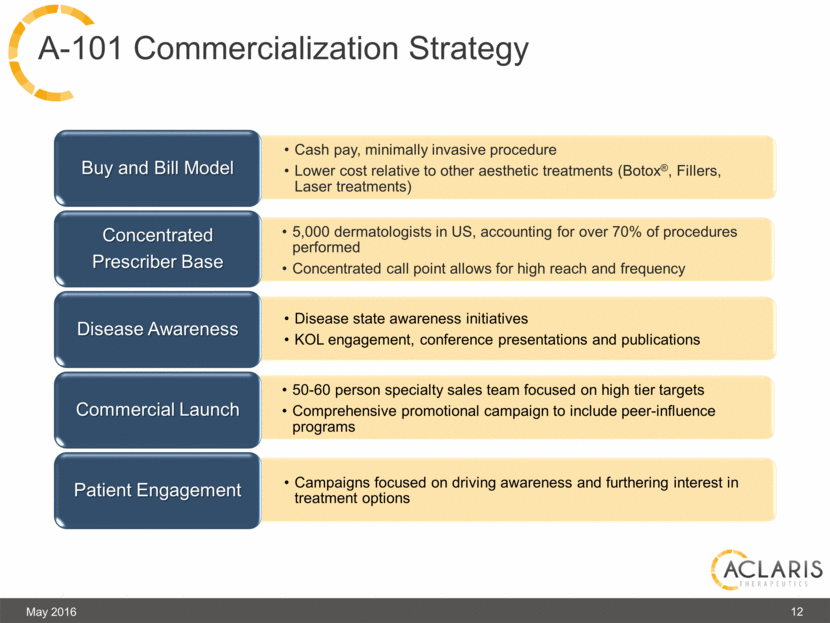
May 2016 12 A-101 Commercialization Strategy Cash pay, minimally invasive procedure Lower cost relative to other aesthetic treatments (Botox®, Fillers, Laser treatments) Buy and Bill Model 5,000 dermatologists in US, accounting for over 70% of procedures performed Concentrated call point allows for high reach and frequency Concentrated Prescriber Base Disease state awareness initiatives KOL engagement, conference presentations and publications Disease Awareness 50-60 person specialty sales team focused on high tier targets Comprehensive promotional campaign to include peer-influence programs Commercial Launch Campaigns focused on driving awareness and furthering interest in treatment options Patient Engagement |
|
|
ATI-50001/ATI-50002 Candidates for Alopecia Areata September 2015 CONFIDENTIAL 13 |
|
|
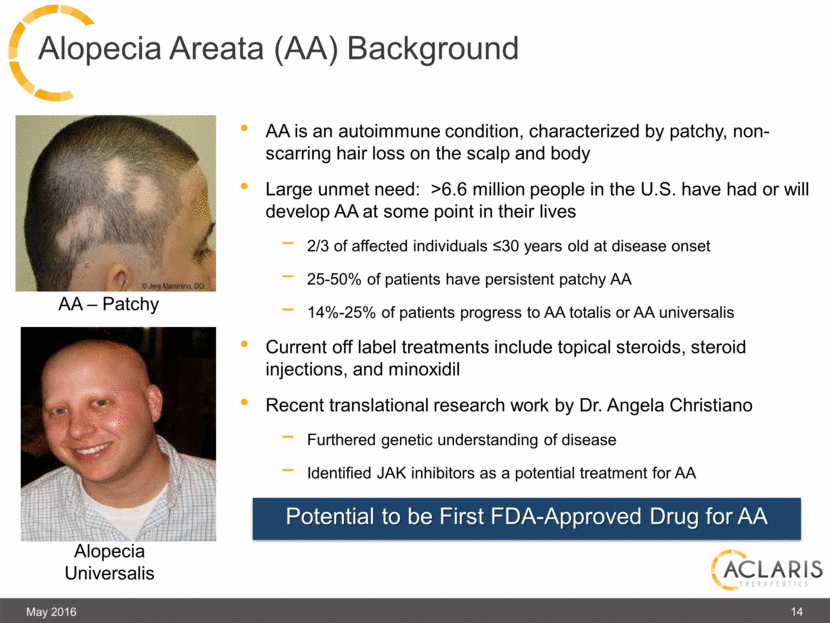
AA is an autoimmune condition, characterized by patchy, non-scarring hair loss on the scalp and body Large unmet need: >6.6 million people in the U.S. have had or will develop AA at some point in their lives 2/3 of affected individuals ≤30 years old at disease onset 25-50% of patients have persistent patchy AA 14%-25% of patients progress to AA totalis or AA universalis Current off label treatments include topical steroids, steroid injections, and minoxidil Recent translational research work by Dr. Angela Christiano Furthered genetic understanding of disease Identified JAK inhibitors as a potential treatment for AA May 2016 14 Alopecia Areata (AA) Background Potential to be First FDA-Approved Drug for AA AA – Patchy Alopecia Universalis |
|
|
Lead asset: Selective JAK 1/3 inhibitor from Rigel Exclusive, worldwide license and development collaboration Oral and topical rights Known mechanism of action and biological response in humans Promoted hair regrowth in mouse model of AA Drug Candidates: ATI-50001 for oral administration in Alopecia Totalis and Alopecia Universalis ATI-50002 for topical administration in Patchy Alopecia Areata Development Strategy Planned submission of IND: 2H 2016 Initiation of Clinical Trial: 1H 2017 May 2016 15 ATI-50001/ATI-50002: JAK Inhibitors in Alopecia Areata |
|
|
Vixen (Columbia University IP) and Key Organics/JAKPharm Broadens our IP estate Methods of use covering JAK inhibitors for the treatment of: Alopecia Areata Androgenetic alopecia (female and male pattern hair loss) Additional hair loss disorders Next generation JAK inhibitors Covalently bound highly selective JAK3 inhibitors Opportunity to broaden our target indications May 2016 16 Recent Business Development Transactions |
|
|
December 2015 CONFIDENTIAL 17 Indication Expansion |
|
|
Androgenic Alopecia December 2015 CONFIDENTIAL 18 |
|
|
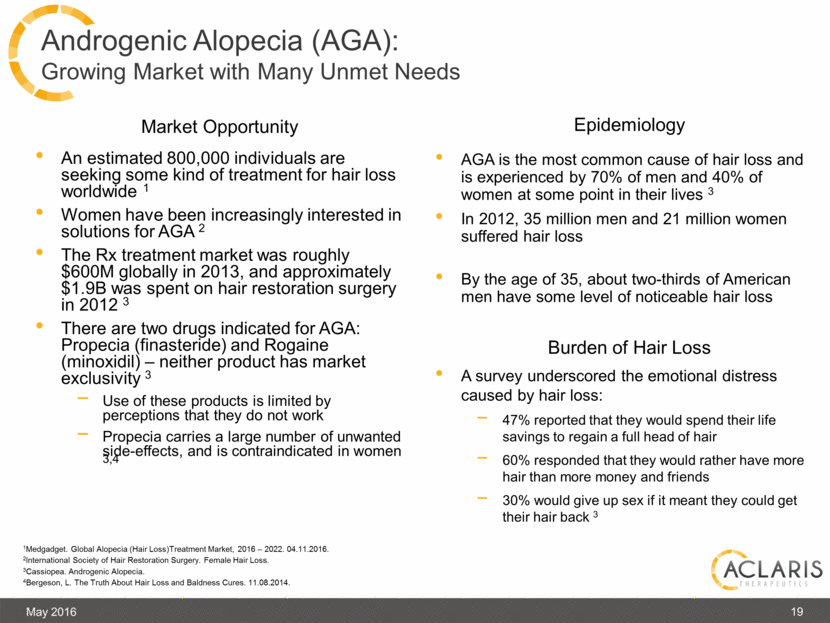
An estimated 800,000 individuals are seeking some kind of treatment for hair loss worldwide 1 Women have been increasingly interested in solutions for AGA 2 The Rx treatment market was roughly $600M globally in 2013, and approximately $1.9B was spent on hair restoration surgery in 2012 3 There are two drugs indicated for AGA: Propecia (finasteride) and Rogaine (minoxidil) – neither product has market exclusivity 3 Use of these products is limited by perceptions that they do not work Propecia carries a large number of unwanted side-effects, and is contraindicated in women 3,4 May 2016 19 Androgenic Alopecia (AGA): Growing Market with Many Unmet Needs Market Opportunity Epidemiology Burden of Hair Loss 1Medgadget. Global Alopecia (Hair Loss)Treatment Market, 2016 – 2022. 04.11.2016. 2International Society of Hair Restoration Surgery. Female Hair Loss. 3Cassiopea. Androgenic Alopecia. 4Bergeson, L. The Truth About Hair Loss and Baldness Cures. 11.08.2014. AGA is the most common cause of hair loss and is experienced by 70% of men and 40% of women at some point in their lives 3 In 2012, 35 million men and 21 million women suffered hair loss By the age of 35, about two-thirds of American men have some level of noticeable hair loss A survey underscored the emotional distress caused by hair loss: 47% reported that they would spend their life savings to regain a full head of hair 60% responded that they would rather have more hair than more money and friends 30% would give up sex if it meant they could get their hair back 3 |
|
|
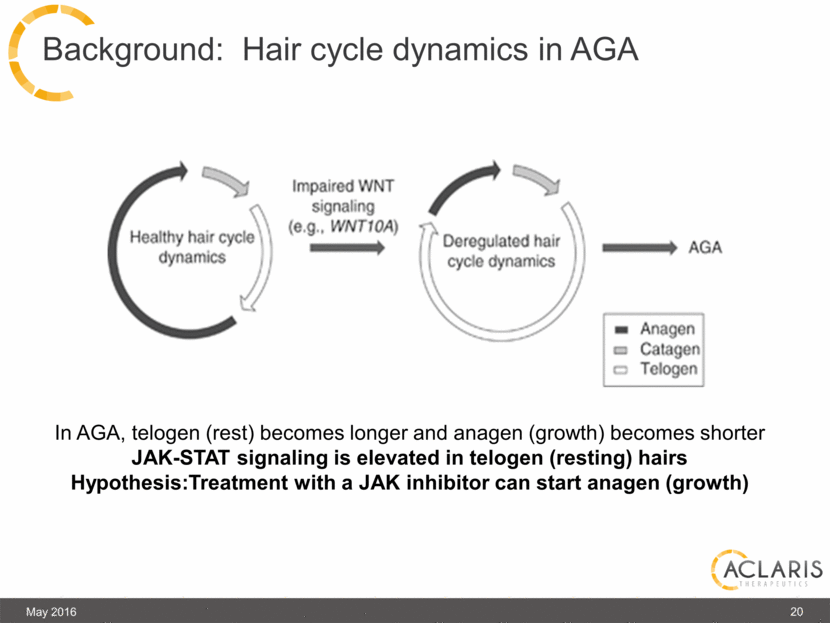
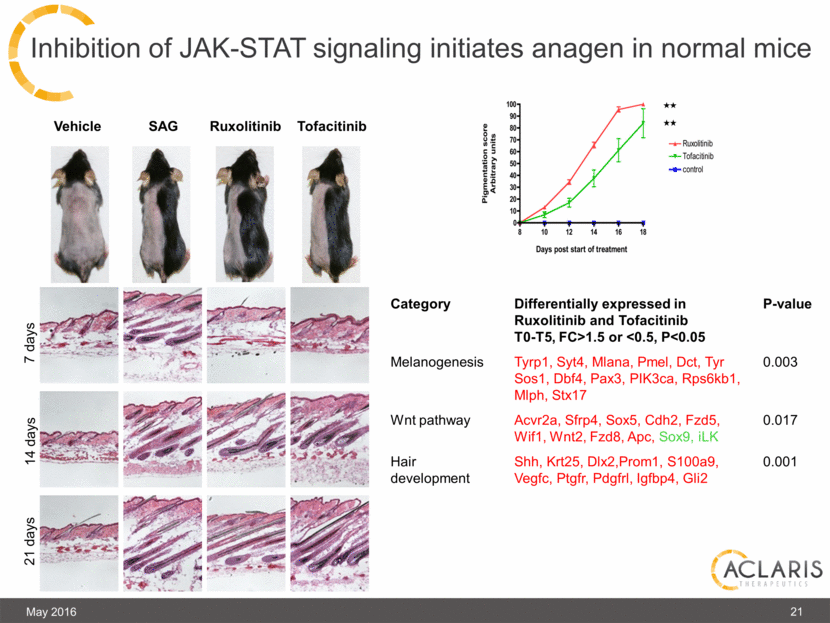
In AGA, telogen (rest) becomes longer and anagen (growth) becomes shorter JAK-STAT signaling is elevated in telogen (resting) hairs Hypothesis:Treatment with a JAK inhibitor can start anagen (growth) Background: Hair cycle dynamics in AGA 20 May 2016 |
|
|
Inhibition of JAK-STAT signaling initiates anagen in normal mice Tofacitinib Vehicle SAG Ruxolitinib 7 days 14 days 21 days Category Differentially expressed in Ruxolitinib and Tofacitinib T0-T5, FC>1.5 or <0.5, P<0.05 P-value Melanogenesis Tyrp1, Syt4, Mlana, Pmel, Dct, Tyr Sos1, Dbf4, Pax3, PIK3ca, Rps6kb1, Mlph, Stx17 0.003 Wnt pathway Acvr2a, Sfrp4, Sox5, Cdh2, Fzd5, Wif1, Wnt2, Fzd8, Apc, Sox9, iLK 0.017 Hair development Shh, Krt25, Dlx2,Prom1, S100a9, Vegfc, Ptgfr, Pdgfrl, Igfbp4, Gli2 0.001 21 May 2016 |
|
|
Dynamics of hair cycle In Androgenetic Alopecia (AGA), the duration of anagen decreases with each successive hair cycle, whereas the duration of telogen becomes prolonged. This leads to a reduction in the anagen-to-telogen ration and corresponds to periods of excessive hair shedding. Prolongation of telogen increases the proportion of vellus hairs on the scalp, further contributing to the AGA process. Most research and pharmaceutical development has focused on the role of androgens in AGA and hair follicle miniaturization, however, other factors such as stem cell quiescence may play a role in telogen arrest. New discoveries in AGA Inhibition of JAK-STAT signaling: Initiates anagen hair growth in mice Promotes hair elongation in humans and improves dermal papilla inductivity Acts directly on activating hair follicle bulge stem cells, independent of androgens Can induce anagen hair growth from telogen stage hairs, suggesting it may have efficacy in disorders of arrested telogen, such as AGA 22 Pathophysiology and Mechanism May 2016 |
|
|
Vitiligo December 2015 CONFIDENTIAL 23 |
|
|
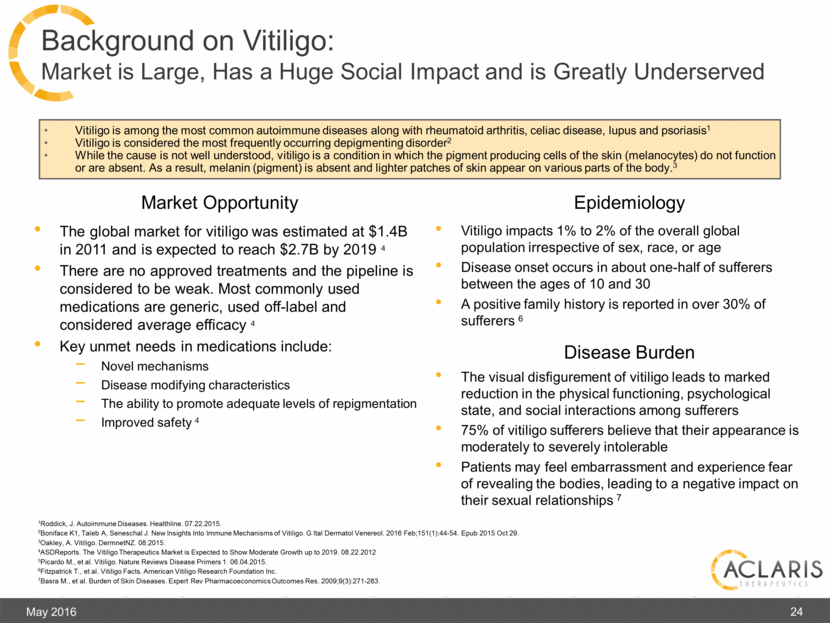
The global market for vitiligo was estimated at $1.4B in 2011 and is expected to reach $2.7B by 2019 4 There are no approved treatments and the pipeline is considered to be weak. Most commonly used medications are generic, used off-label and considered average efficacy 4 Key unmet needs in medications include: Novel mechanisms Disease modifying characteristics The ability to promote adequate levels of repigmentation Improved safety 4 May 2016 24 Background on Vitiligo: Market is Large, Has a Huge Social Impact and is Greatly Underserved Market Opportunity Epidemiology Disease Burden 1Roddick, J. Autoimmune Diseases. Healthline. 07.22.2015. 2Boniface K1, Taïeb A, Seneschal J. New Insights Into Immune Mechanisms of Vitiligo. G Ital Dermatol Venereol. 2016 Feb;151(1):44-54. Epub 2015 Oct 29. 3Oakley, A. Vitiligo. DermnetNZ. 08.2015. 4ASDReports. The Vitiligo Therapeutics Market is Expected to Show Moderate Growth up to 2019. 08.22.2012 5Picardo M., et al. Vitiligo. Nature Reviews Disease Primers 1. 06.04.2015. 6Fitzpatrick T., et al. Vitiligo Facts. American Vitiligo Research Foundation Inc. 7Basra M., et al. Burden of Skin Diseases. Expert Rev Pharmacoeconomics Outcomes Res. 2009;9(3):271-283. Vitiligo impacts 1% to 2% of the overall global population irrespective of sex, race, or age Disease onset occurs in about one-half of sufferers between the ages of 10 and 30 A positive family history is reported in over 30% of sufferers 6 The visual disfigurement of vitiligo leads to marked reduction in the physical functioning, psychological state, and social interactions among sufferers 75% of vitiligo sufferers believe that their appearance is moderately to severely intolerable Patients may feel embarrassment and experience fear of revealing the bodies, leading to a negative impact on their sexual relationships 7 Vitiligo is among the most common autoimmune diseases along with rheumatoid arthritis, celiac disease, lupus and psoriasis1 Vitiligo is considered the most frequently occurring depigmenting disorder2 While the cause is not well understood, vitiligo is a condition in which the pigment producing cells of the skin (melanocytes) do not function or are absent. As a result, melanin (pigment) is absent and lighter patches of skin appear on various parts of the body.3 |
|
|
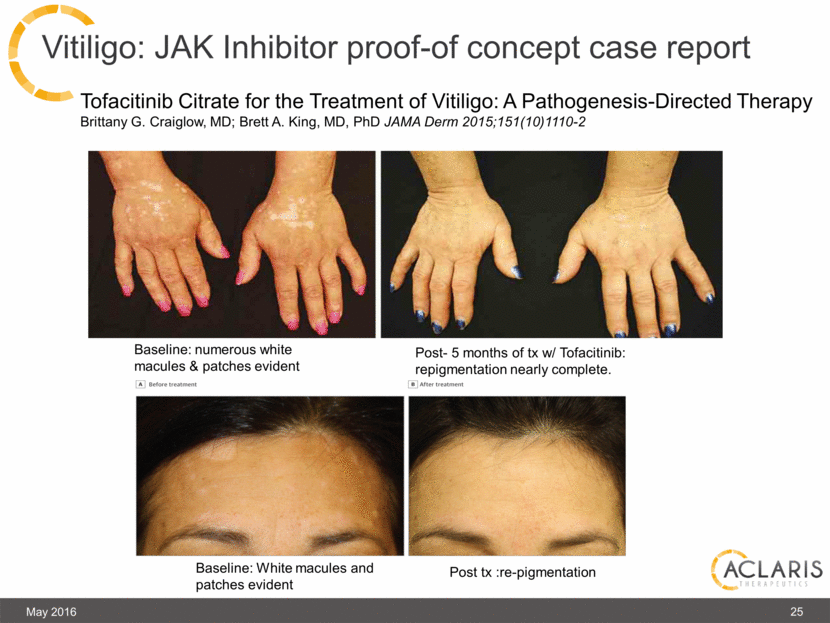
Vitiligo: JAK Inhibitor proof-of concept case report Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy Brittany G. Craiglow, MD; Brett A. King, MD, PhD JAMA Derm 2015;151(10)1110-2 Baseline: White macules and patches evident Baseline: numerous white macules & patches evident Post- 5 months of tx w/ Tofacitinib: repigmentation nearly complete. Post tx :re-pigmentation May 2016 25 |
|
|
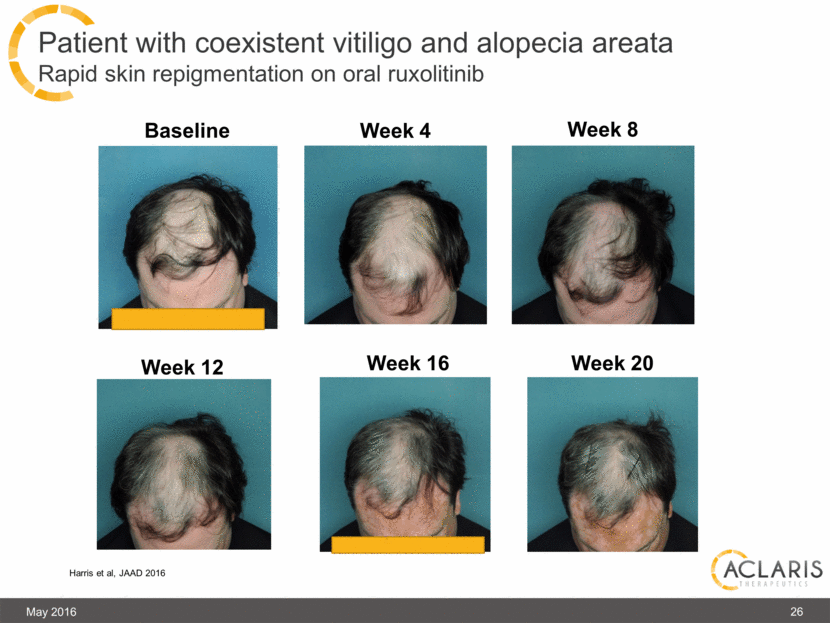
Patient with coexistent vitiligo and alopecia areata Rapid skin repigmentation on oral ruxolitinib May 2016 Baseline Week 4 Week 8 Week 12 Week 16 Week 20 Harris et al, JAAD 2016 26 |
|
|
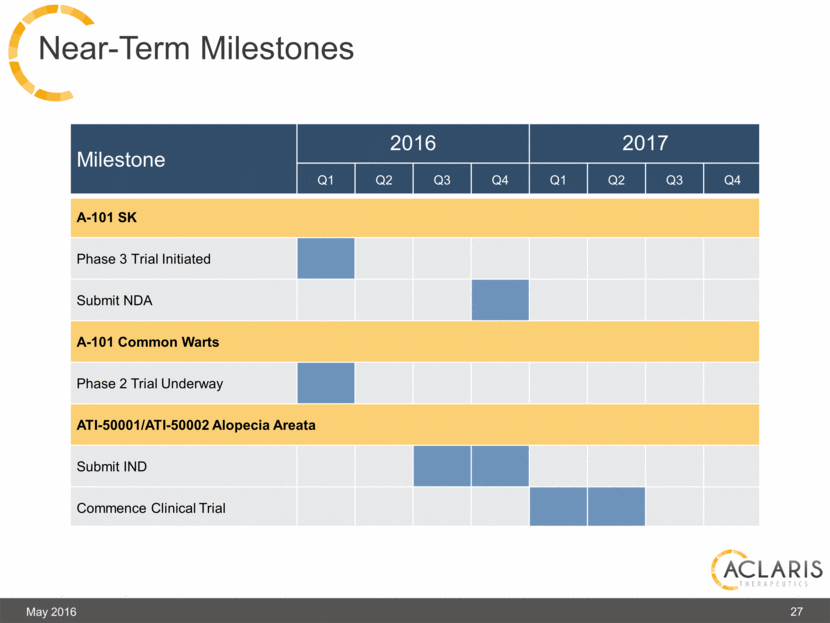
Milestone 2016 2017 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 A-101 SK Phase 3 Trial Initiated Submit NDA A-101 Common Warts Phase 2 Trial Underway ATI-50001/ATI-50002 Alopecia Areata Submit IND Commence Clinical Trial May 2016 27 Near-Term Milestones |
|
|
© Copyright 2016 Aclaris Therapeutics. All rights reserved. THANK YOU www.aclaristx.com 28 |
|
|
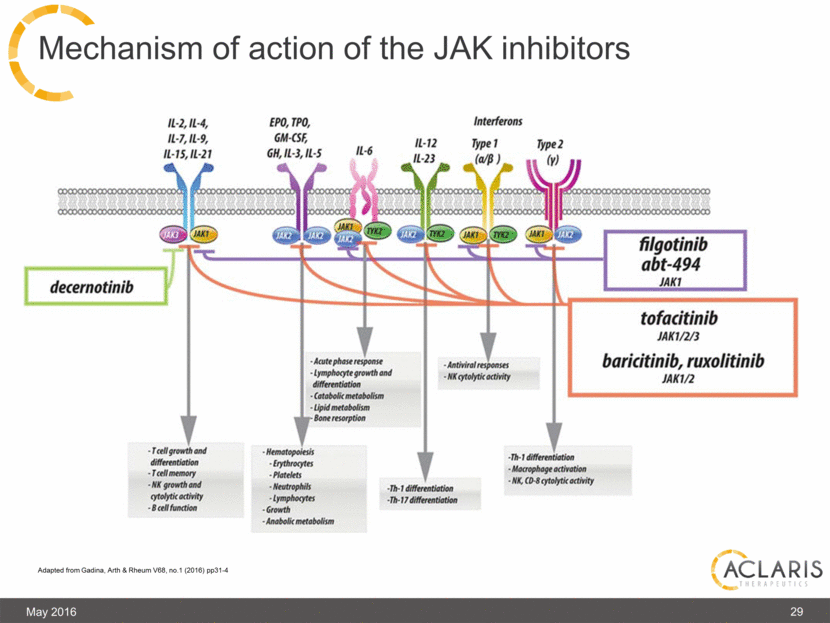
29 Mechanism of action of the JAK inhibitors May 2016 Adapted from Gadina, Arth & Rheum V68, no.1 (2016) pp31-4 |
|
|
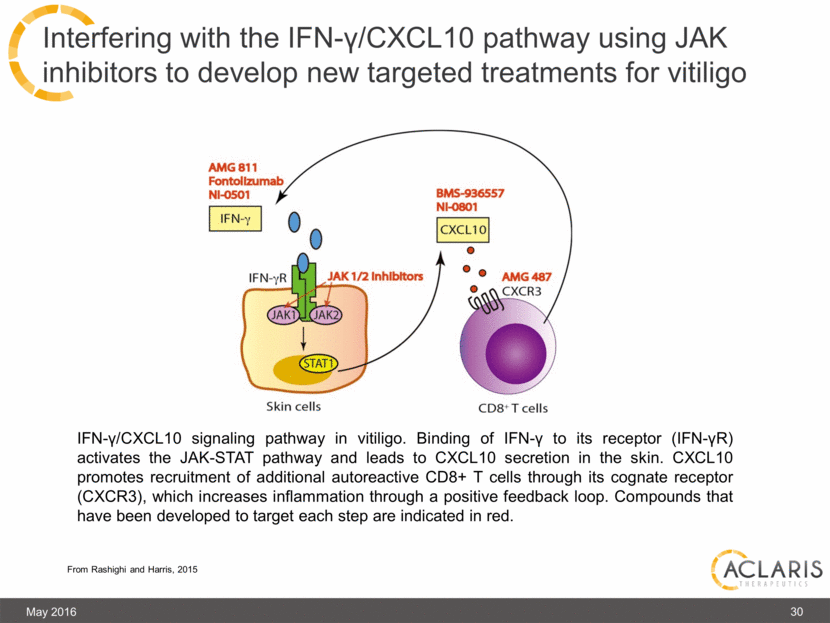
Interfering with the IFN-γ/CXCL10 pathway using JAK inhibitors to develop new targeted treatments for vitiligo May 2016 IFN-γ/CXCL10 signaling pathway in vitiligo. Binding of IFN-γ to its receptor (IFN-γR) activates the JAK-STAT pathway and leads to CXCL10 secretion in the skin. CXCL10 promotes recruitment of additional autoreactive CD8+ T cells through its cognate receptor (CXCR3), which increases inflammation through a positive feedback loop. Compounds that have been developed to target each step are indicated in red. From Rashighi and Harris, 2015 30 |
|
|
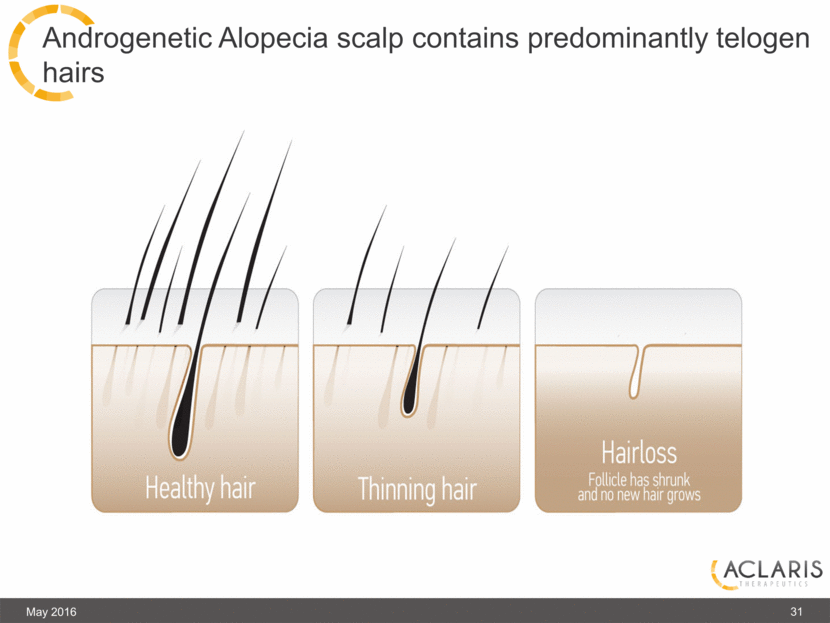
Androgenetic Alopecia scalp contains predominantly telogen hairs May 2016 31 |
|
|
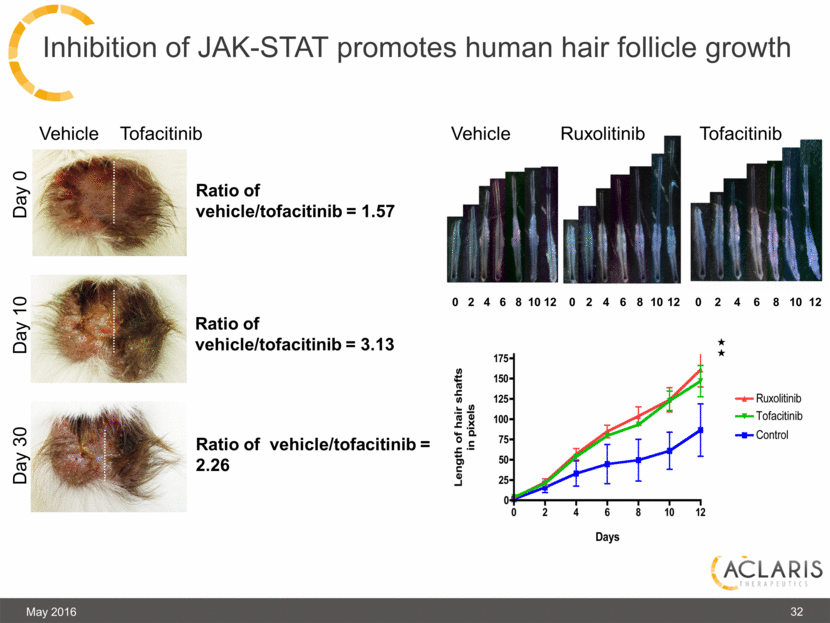
Inhibition of JAK-STAT promotes human hair follicle growth May 2016 Day 0 Day 10 Day 30 Vehicle Tofacitinib Ratio of vehicle/tofacitinib = 1.57 Ratio of vehicle/tofacitinib = 3.13 Ratio of vehicle/tofacitinib = 2.26 Tofacitinib Ruxolitinib Vehicle 32 0 2 4 6 8 10 12 0 2 4 6 8 10 12 0 2 4 6 8 10 12 |
|
|
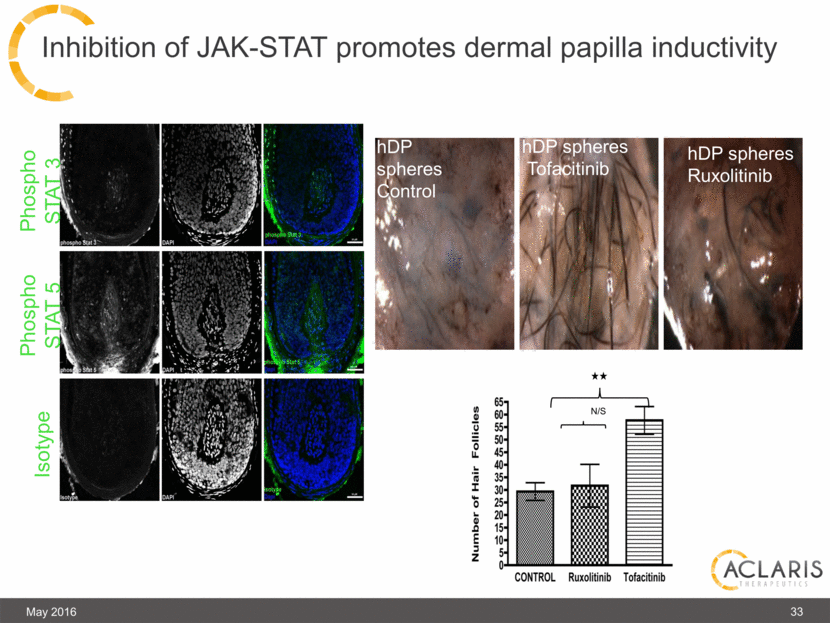
Inhibition of JAK-STAT promotes dermal papilla inductivity May 2016 Phospho STAT 3 Phospho STAT 5 Isotype hDP spheres Control hDP spheres Tofacitinib N/S hDP spheres Ruxolitinib 33 |