Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Lipocine Inc. | v439253_8k.htm |

Long - term Safety and Tolerability of Oral Testosterone (LPCN 1021) in Hypogonadal Men: Results from the 52 - Week Phase 3 Study Mohit Khera, MD 1 , Christina Wang, MD 2 , Jed C. Kaminetsky, MD 3 , Martin M. Miner, MD 4 , Adrian S Dobs, MD, MHS 5 , Nachiappan Chidambaram 6 , Anthony DelConte, MD 6,7 , Satish Nachaegari 6 , Mahesh Patel 6 1 Baylor College of Medicine, Houston, TX, 2 Harbor - UCLA Medical Center and Los Angeles Biomedical Research Institute, Torrance, CA, 3 University Urology Associates, New York, NY, 4 Brown University and the Miriam Hospital, Providence, RI, 5 Johns Hopkins University School of Medicine, Baltimore, MD, 6 Lipocine, Inc. Salt Lake City, UT, 7 Saint Joseph’s University, Philadelphia, PA 1 Exhibit 99.1
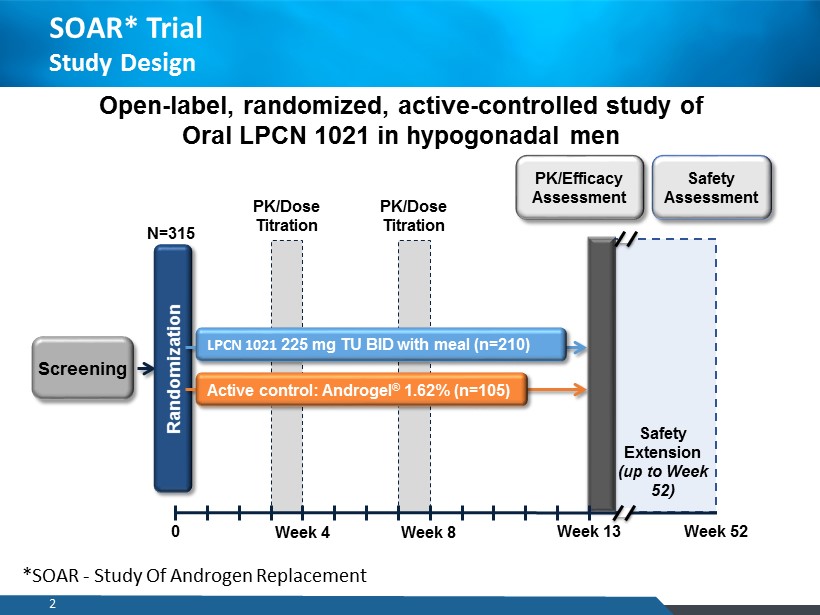
PK/Dose Titration PK/Dose Titration 2 Screening N=315 Randomization LPCN 1021 225 mg TU BID with meal (n=210) Active control: Androgel ® 1.62% (n=105) PK/Efficacy Assessment Safety Assessment Safety Extension (up to Week 52) 0 Week 4 Week 8 Week 13 Week 52 Open - label, randomized, active - controlled study of Oral LPCN 1021 in hypogonadal men SOAR* Trial Study Design *SOAR - Study Of Androgen Replacement 2
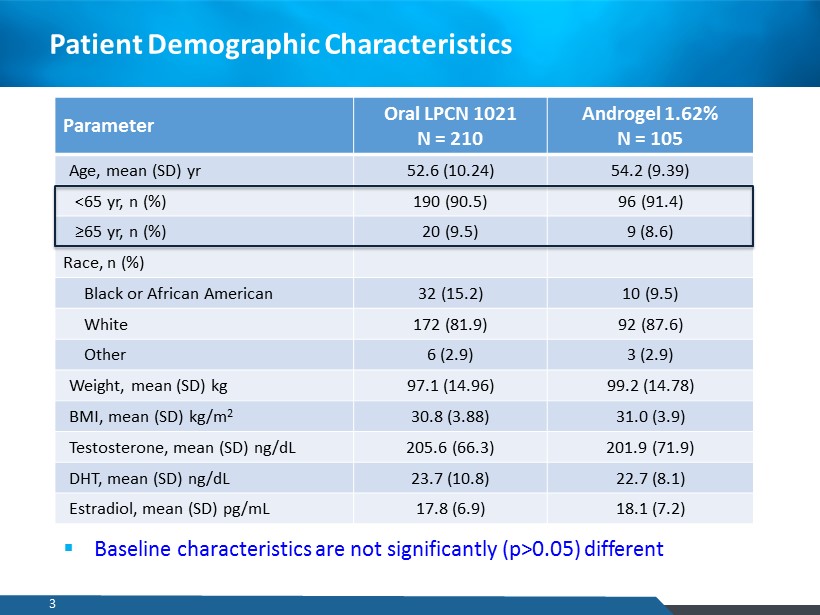
Patient Demographic Characteristics Parameter Oral LPCN 1021 N = 210 Androgel 1.62% N = 105 Age, mean (SD) yr 52.6 (10.24) 54.2 (9.39) <65 yr , n (%) 190 (90.5) 96 (91.4) ≥65 yr , n (%) 20 (9.5) 9 (8.6) Race, n (%) Black or African American 32 (15.2) 10 (9.5) White 172 (81.9) 92 (87.6) Other 6 (2.9) 3 (2.9) Weight, mean (SD) kg 97.1 (14.96) 99.2 (14.78) BMI, mean (SD) kg/m 2 30.8 (3.88) 31.0 (3.9) Testosterone, mean (SD) ng/dL 205.6 (66.3) 201.9 (71.9) DHT, mean (SD) ng/dL 23.7 (10.8) 22.7 (8.1) Estradiol, mean (SD) pg /mL 17.8 (6.9) 18.1 (7.2) ▪ Baseline characteristics are not significantly (p>0.05) different 3
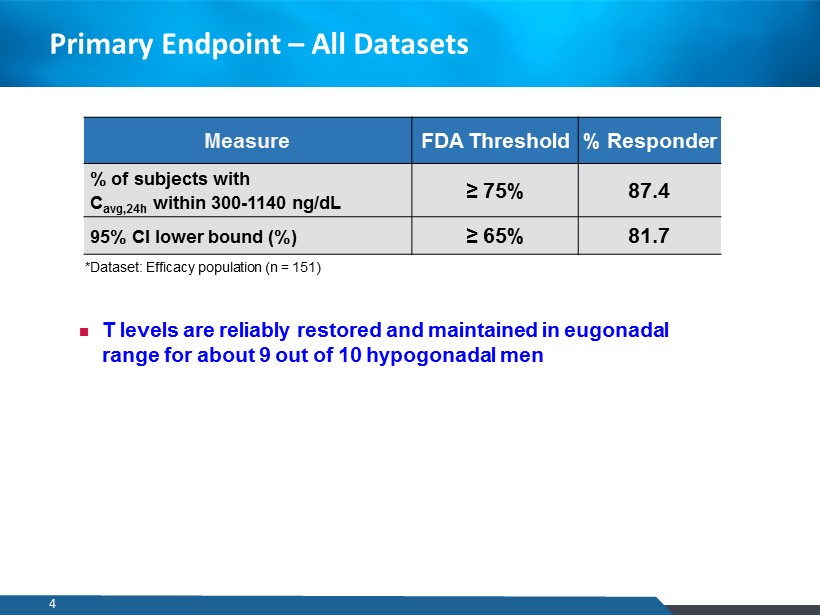
Primary Endpoint – All Datasets Measure FDA Threshold % Responder % of subjects with C avg,24h within 300 - 1140 ng/dL ≥ 75% 87.4 95% CI lower bound (%) ≥ 65% 81.7 *Dataset: Efficacy population (n = 151) T levels are reliably restored and maintained in eugonadal range for about 9 out of 10 hypogonadal men 4

Total Testosterone Levels 0 100 200 300 400 500 600 700 800 900 1000 0 26 39 52 Mean (+ SD) Total T (ng/dL) Time (weeks) LPCN Androgel Serum concentrations obtained 3 to 6 hours post morning dose 5

Free Testosterone Levels 0 2 4 6 8 10 12 14 16 0 26 39 52 Calculated Mean (+ SD) Free T (ng/dL) Time (weeks) LPCN Androgel * P = 0.002 * P = 0.003 NS NS 6

Sex Hormone Binding Globulin (SHBG) 0 10 20 30 40 50 60 70 80 0 26 39 52 Mean (SD) SHBG (nmol/L) Time (weeks) LPCN Androgel * P <0.0001 * P <0.0001 * P <0.0001 NS ▪ Changes from baseline to end of study (single blood draw) for DHT, and E2 were comparable between treatment groups. 7
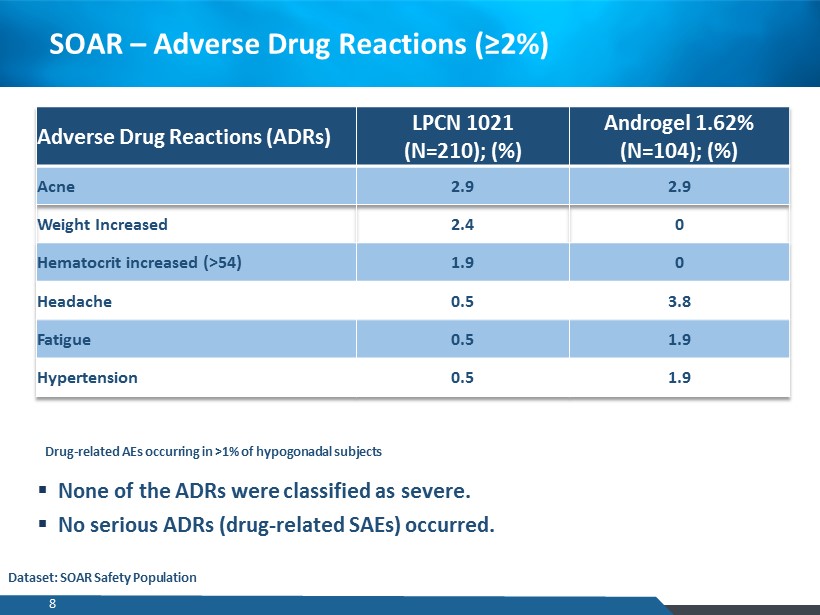
SOAR – Adverse Drug Reactions (≥2%) Adverse Drug Reactions (ADRs) LPCN 1021 (N=210); (%) Androgel 1.62% (N=104); (%) Acne 2.9 2.9 Weight Increased 2.4 0 Hematocrit increased (>54) 1.9 0 Headache 0.5 3.8 Fatigue 0.5 1.9 Hypertension 0.5 1.9 ▪ None of the ADRs were classified as severe . ▪ No serious ADRs (drug - related SAEs) occurred. Drug - related AEs occurring in >1% of hypogonadal subjects Dataset: SOAR Safety Population 8

Androgenic Parameters (Baseline to EOS) 0 1 2 3 4 5 6 7 8 9 HCT (%) HGB (g/dL) PLATELET (K/Cu mm) PROTHROMBIN (sec) PSA (ng/ml) Mean Change from Baseline at Week 52 LPCN 1021 Androgel® 1.62% NS NS NS NS NS Dataset: SOAR Safety Population 9

Lipid Analysis -30 -20 -10 0 10 20 30 HDL LDL Triglyceride Cholesterol Mean Change from Baseline at Week 52 (mg/ dL ) Oral LPCN Androgel® 1.62% P < 0.01 NS NS NS Dataset: SOAR Safety Population; EOS – End of Study 10

HDL/LDL (Baseline to Week 52) 0 10 20 30 40 50 60 70 80 90 100 110 120 0 Week 7 Week 13 Week 26 Week 39 Week 52 HDL/LDL (mg/ dL ) LPCN 1021 Androgel 1.62% Column1 Column2 . Total cholesterol/HDL ratio from baseline to EOS • LPCN 1021: 4.3 to 4.5 • Androgel 1.62%: 4.3 to 4.4 HDL LDL 11

-20.0 -15.0 -10.0 -5.0 0.0 5.0 10.0 15.0 20.0 ALT (U/L) GGT (U/L) Alkaline Phosphate (U/L) Bilirubin (mg/dL) Mean Change from Baseline at Week 52 Liver Enzyme Parameters LPCN 1021 Androgel® 1.62% Liver Enzymes (Baseline to EOS) NS NS NS P < 0.01 Dataset: SOAR Safety Population 12

Oral LPCN 1021 AEs by System Organ Class in Hypogonadal Subjects 0 5 10 15 20 25 % Subjects TEAEs by System Organ Class LPCN 1021 Androgel 1.62% Dataset: SOAR Safety Population 13

Oral LPCN 1021 AEs by System Organ Class in Hypogonadal Subjects (cont’d) 0 5 10 15 20 25 % Subjects TEAEs by System Organ Class LPCN 1021 Androgel 1.62% Dataset: SOAR Safety Population 14

Conclusion ▪ LPCN 1021 was well tolerated and had a favorable safety profile in the long - term management of hypogonadal subjects. ▪ Notably, no hepatic safety concerns were identified and gastrointestinal AEs with oral LPCN 1021 were generally comparable to active control 15
