Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Bionik Laboratories Corp. | v438911_8k.htm |
Exhibit 99.1

www.bioniklabs.com OTCQX:BNKL Pioneering global medical device and robotics company focused on providing rehabilitation solutions to individuals with neurological disorders Corporate Presentation May 2016

Legal Disclaimer This presentation contains forward - looking statements relating to future events or the future financial performance and operations of Bionik . Forward - looking statements, which involve assumptions and describe Bionik’s intent, belief or current expectations about its business opportunities, prospects, performance and results, are generally identifiable by use of the words “may,” “could,” “should,” “will,” “would,” “expect,” “anticipate,” “plan,” “potential,” “opportunity,” “estimate,” “believe,” “intend,” “project,” “forecast,” the negative of such words and other variations on such words or similar terminology . These forward - looking statements are not guarantees of future performance and by their nature involve known and unknown risks and uncertainties that may cause actual opportunities, prospects, performance and results to vary from those presented in this document, and those variances may be material . In evaluating such statements, prospective investors should carefully consider the various risks and uncertainties identified in Bionik’s public filings, such as market risk, liquidity risk, competitive risk, regulatory risk and other commonly recognized forms of risk relating to Bionik and its securities . In light of these risks, uncertainties and assumptions, the forward - looking events discussed in this document might not occur . Further information regarding these and other risks, as well as other information about the Company, is described from time to time in the Company’s filings with the SEC, which can be accessed at www . sec . gov . Bionik is not obligated to publicly update or revise any forward - looking statements, whether as a result of new information, future events or otherwise . 2

Bionik Investment Highlights • Pipeline of commercial and development stage robotic products addressing both upper and lower body rehabilitation • Recent acquisition of Interactive Motion Technologies adds 3 commercial stage products characterized as Class II medical devices by the U.S. FDA and sold in over 20 countries • 2015 revenues reported at ~$2 million with significant opportunity for growth in near - term • Immediate priority to focus on executing strategy to accelerate market expansion and revenue generation with product integration • Growth strategy focused on developing earlier stage programs through to commercialization and acquiring/licensing synergistic products and technologies • Pioneering team responsible for foundational patents in robotics 3
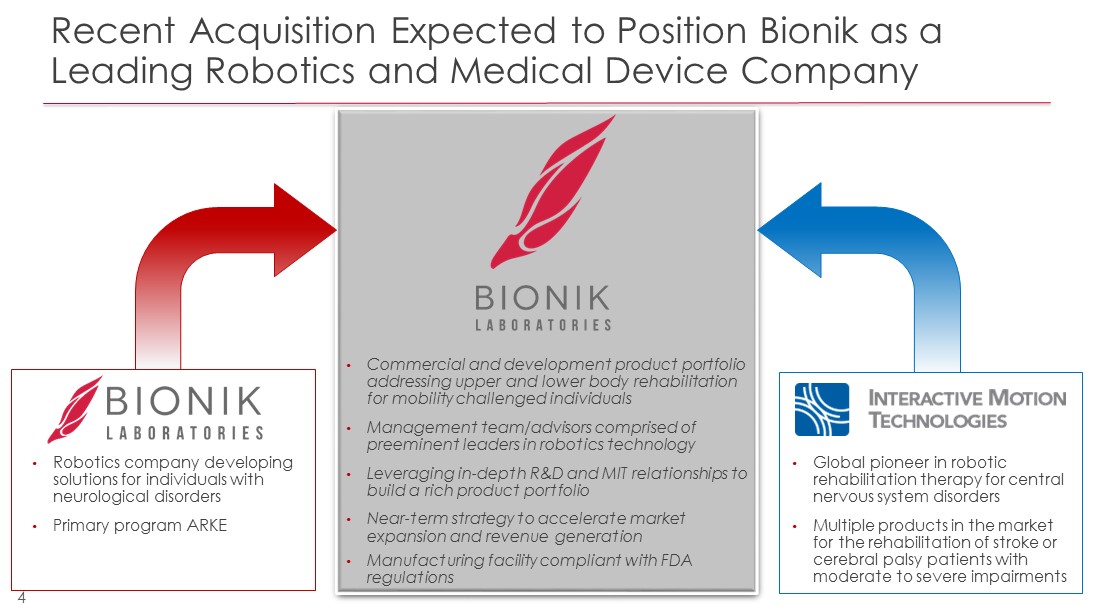
Recent Acquisition Expected to Position Bionik as a Leading Robotics and Medical Device Company 4 • Robotics company developing solutions for individuals with neurological disorders • Primary program ARKE • Global pioneer in robotic rehabilitation therapy for central nervous system disorders • Multiple products in the market for the rehabilitation of stroke or cerebral palsy patients with moderate to severe impairments • Commercial and development product portfolio addressing upper and lower body rehabilitation for mobility challenged individuals • Management team/advisors comprised of preeminent leaders in robotics technology • Leveraging in - depth R&D and MIT relationships to build a rich product portfolio • Near - term strategy to accelerate market expansion and revenue generation • Manufacturing facility compliant with FDA regulations
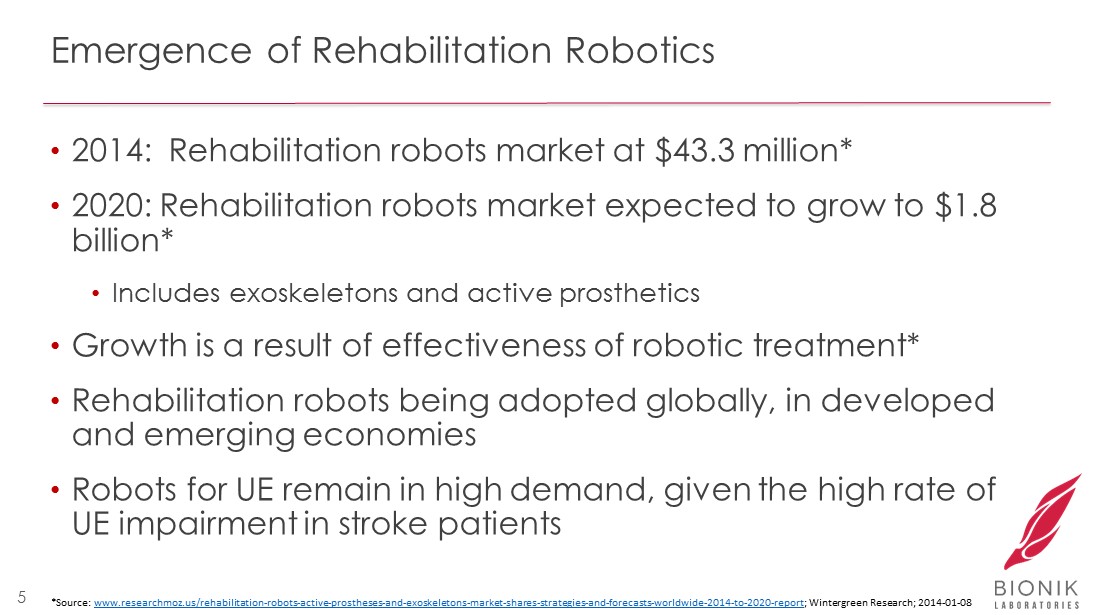
Emergence of Rehabilitation Robotics • 2014: Rehabilitation robots market at $43.3 million* • 2020: Rehabilitation robots market expected to grow to $1.8 billion* • Includes exoskeletons and active prosthetics • Growth is a result of effectiveness of robotic treatment* • Rehabilitation robots being adopted globally, in developed and emerging economies • Robots for UE remain in high demand, given the high rate of UE impairment in stroke patients 5 *Source: www.researchmoz.us/rehabilitation - robots - active - prostheses - and - exoskeletons - market - shares - strategies - and - forecasts - worldwide - 201 4 - to - 2020 - report ; Wintergreen Research; 2014 - 01 - 08
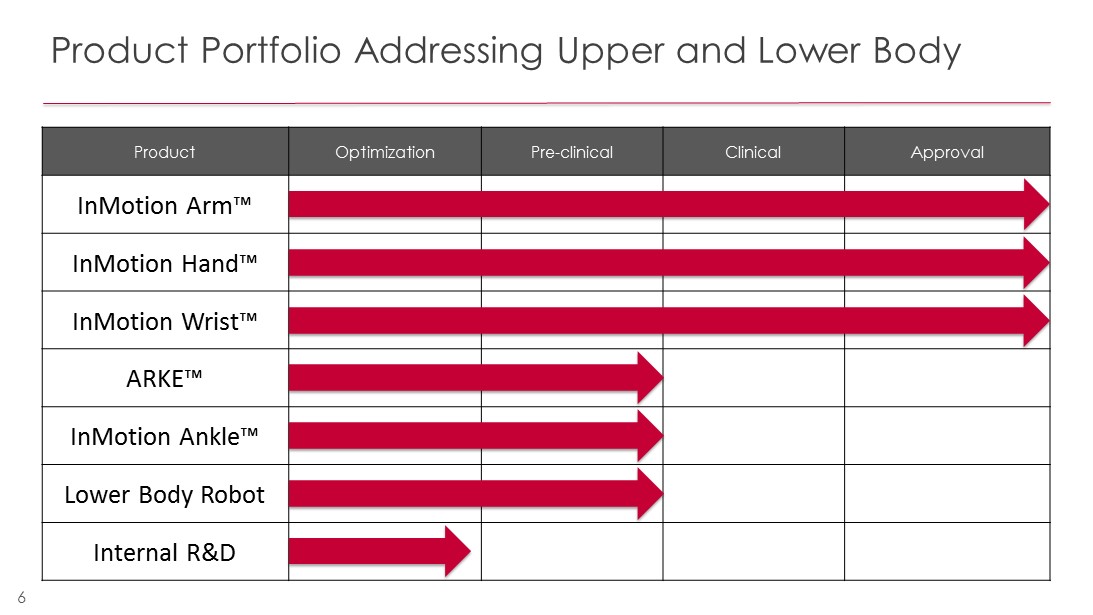
Product Portfolio Addressing Upper and Lower Body 6 Product Optimization Pre - clinical Clinical Approval InMotion Arm™ InMotion Hand™ InMotion Wrist™ ARKE™ InMotion Ankle™ Lower Body Robot Internal R&D

Upper Extremity Commercial - Stage Product Line • InMotion ARM • Designed to rehabilitate stroke patients with upper body neurological limitations • Evidence based, intelligent, interactive technology capable of continuously adapting to and challenging the patient's ability • Allows the clinician to efficiently deliver personalized intensive sensorimotor therapy to neurologic patients • InMotion WRIST • Capable of lifting even a severely impaired neurologic patient's hand against gravity, overcoming most forms of hypertonicity • Accommodates the range of motion of a normal wrist in everyday tasks and can be used by clinicians as a stand - alone treatment option or in addition to the InMotion ARM • InMotion ARM/HAND • Add - on option to the InMotion ARM and capable of continuously adapting to the needs of each patient — delivering customizable therapy • Provides assist - as - needed grasp and release training with flexible positioning 7
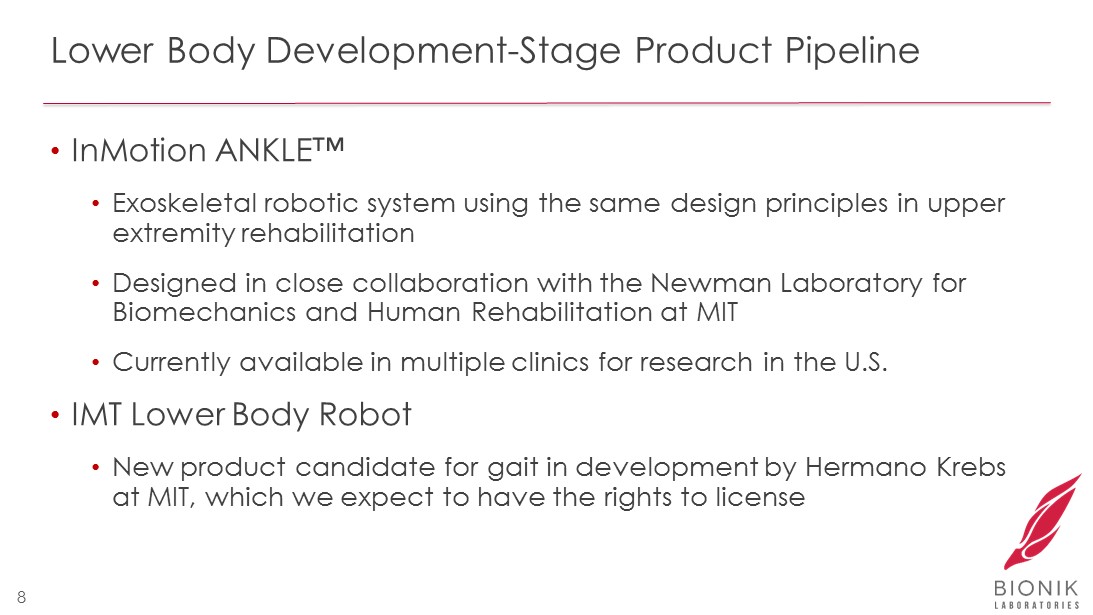
Lower Body Development - Stage Product Pipeline • InMotion ANKLE™ • Exoskeletal robotic system using the same design principles in upper extremity rehabilitation • Designed in close collaboration with the Newman Laboratory for Biomechanics and Human Rehabilitation at MIT • Currently available in multiple clinics for research in the U.S. • IMT Lower Body Robot • New product candidate for gait in development by Hermano Krebs at MIT, which we expect to have the rights to license 8

ARKE A robotic lower body exoskeleton designed to allow paraplegics as well as other wheelchair users, the ability to rehabilitate through walking TM

ARKE: Product Details • Sensors throughout the device sense body movement and trigger motion • Constructed with carbon fiber, aluminum and steel • Fully customizable to fit patients of all heights and weights • Connects to Bionik’s cloud software where data is analyzed and displayed back to the physiotherapist in real - time • All parameters adjustable through tablet interface very easily – learning curve for therapists is very low • Addresses significant unmet need in rehabilitation and mobility with potential to address many secondary illnesses including a reduction in bone density loss, pressure sores, and urinary tract infections 10
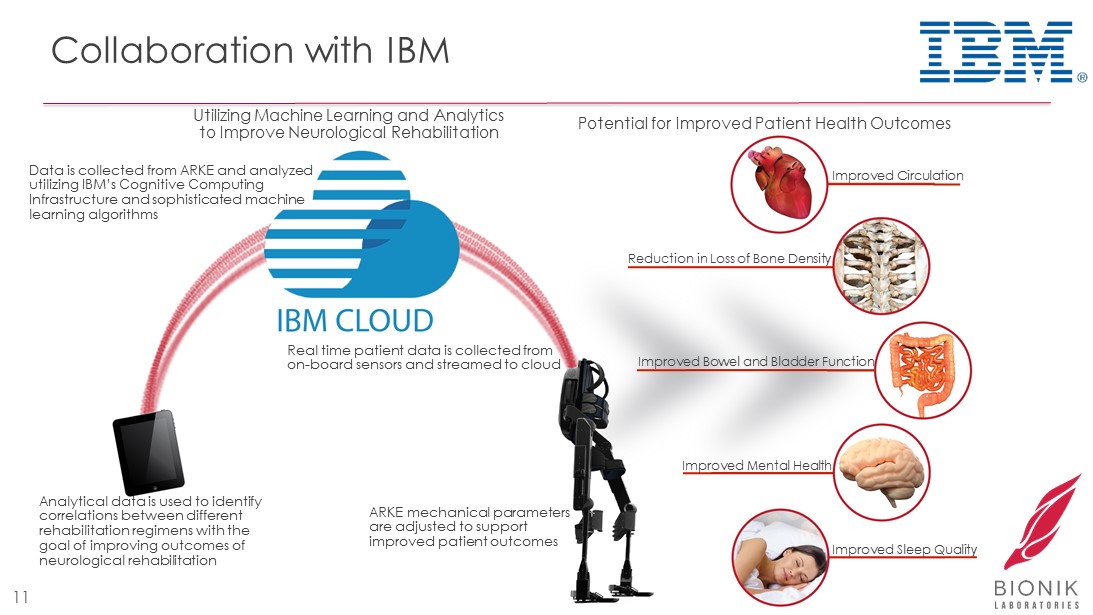
Collaboration with IBM 11 Data is collected from ARKE and analyzed utilizing IBM’s Cognitive Computing Infrastructure and sophisticated machine learning algorithms Analytical data is used to identify correlations between different rehabilitation regimens with the goal of improving outcomes of neurological rehabilitation ARKE mechanical parameters are adjusted to support improved patient outcomes Real time patient data is collected from on - board sensors and streamed to cloud Potential for Improved Patient Health Outcomes Improved Circulation Reduction in Loss of Bone Density Improved Bowel and Bladder Function Improved Mental Health Improved Sleep Quality Utilizing Machine Learning and Analytics to Improve Neurological Rehabilitation

Upper Body Products - Evidence Based and Clinically Proven • Extensive pool of research supports the clinical efficacy of InMotion Robots • Over 1000 patients have been studied in clinical trials at prestigious academic and clinical sites using InMotion systems (formerly known as MIT Manus) • Over 80 published papers reviewing use of InMotion Robots in scientific journals • Studies meet highest standard of scientific rigor – independent, randomized, controlled trial • Outcomes demonstrate clinically significant results in comparison to usual care 12
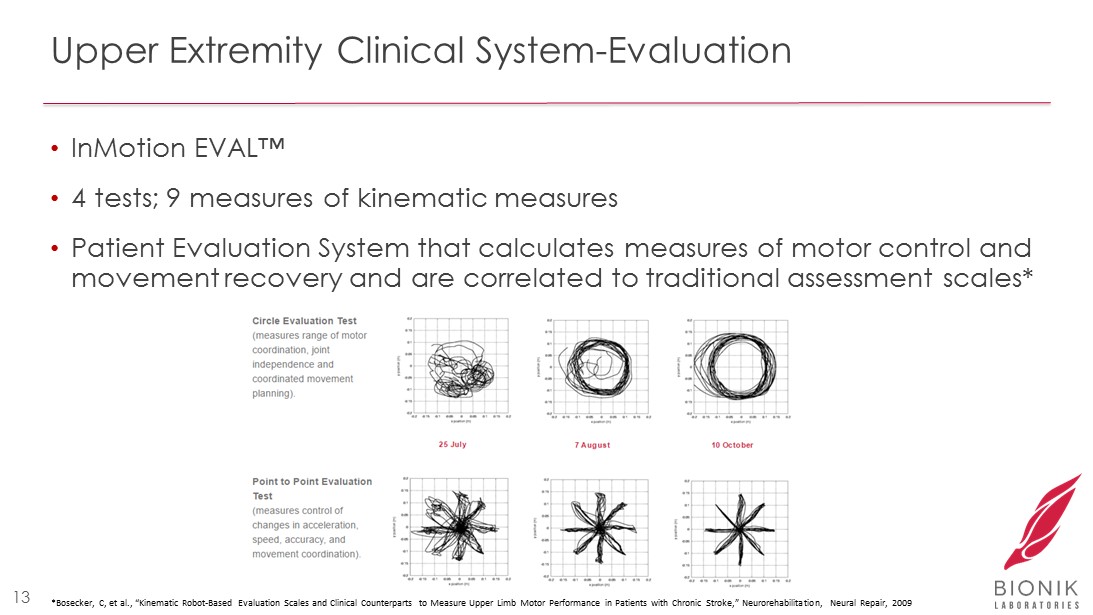
Upper Extremity Clinical System - Evaluation • InMotion EVAL™ • 4 tests; 9 measures of kinematic measures • Patient Evaluation System that calculates measures of motor control and movement recovery and are correlated to traditional assessment scales* 13 * Bosecker , C, et al., “Kinematic Robot - Based Evaluation Scales and Clinical Counterparts to Measure Upper Limb Motor Performance in Patie nts with Chronic Stroke,” Neurorehabilitation , Neural Repair, 2009
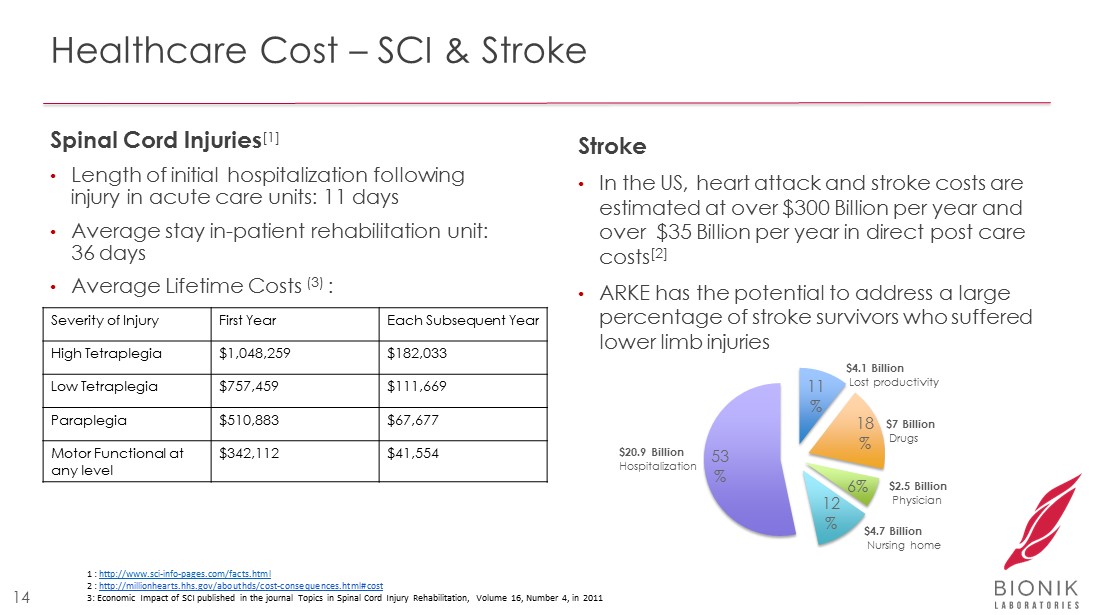
Healthcare Cost – SCI & Stroke Spinal Cord Injuries [1] • Length of initial hospitalization following injury in acute care units: 11 days • Average stay in - patient rehabilitation unit: 36 days • Average Lifetime Costs (3) : Stroke • In the US, heart attack and stroke costs are estimated at over $300 Billion per year and over $35 Billion per year in direct post care costs [2] • ARKE has the potential to address a large percentage of stroke survivors who suffered lower limb injuries 11 % 18 % 6% 12 % 53 % $20.9 Billion Hospitalization $4.7 Billion Nursing home $7 Billion Drugs $2.5 Billion Physician $4.1 Billion Lost productivity 1 : http://www.sci - info - pages.com/facts.html 2 : http://millionhearts.hhs.gov/abouthds/cost - consequences.html#cost 3: E conomic Impact of SCI published in the journal Topics in Spinal Cord Injury Rehabilitation, Volume 16, Number 4, in 2011 14 Severity of Injury First Year Each Subsequent Year High Tetraplegia $1,048,259 $182,033 Low Tetraplegia $757,459 $111,669 Paraplegia $510,883 $67,677 Motor Functional at any level $342,112 $41,554
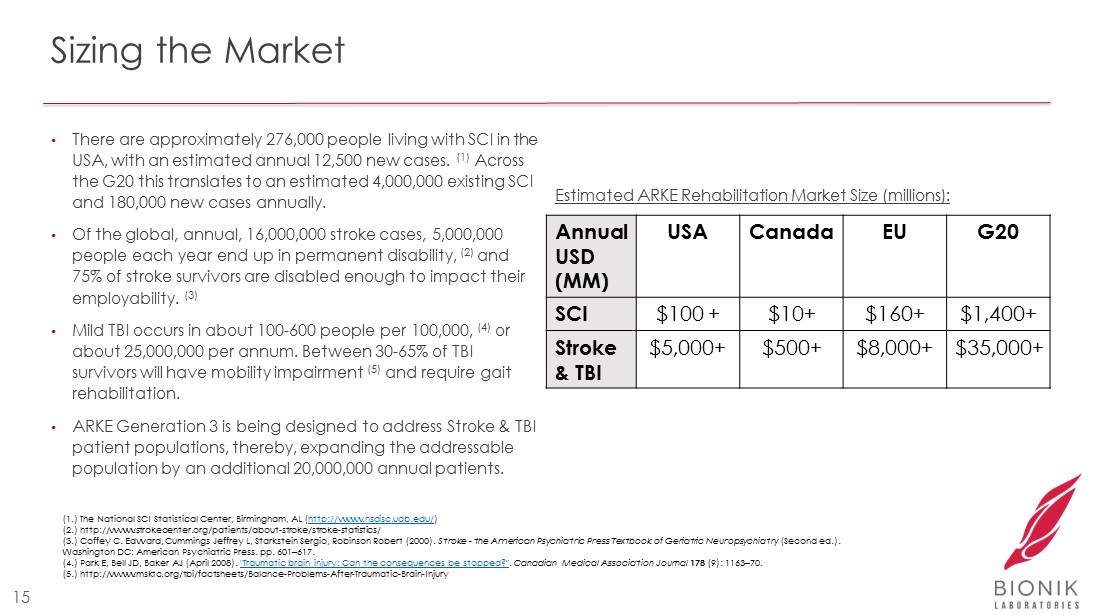
Sizing the Market • There are approximately 276,000 people living with SCI in the USA, with an estimated annual 12,500 new cases. (1) Across the G20 this translates to an estimated 4,000,000 existing SCI and 180,000 new cases annually. • Of the global, annual, 16,000,000 stroke cases, 5,000,000 people each year end up in permanent disability, (2) and 75% of stroke survivors are disabled enough to impact their employability. (3) • Mild TBI occurs in about 100 - 600 people per 100,000, (4) or about 25,000,000 per annum. Between 30 - 65% of TBI survivors will have mobility impairment (5) and require gait rehabilitation. • ARKE Generation 3 is being designed to address Stroke & TBI patient populations, thereby, expanding the addressable population by an additional 20,000,000 annual patients. 15 (1.) The National SCI Statistical Center, Birmingham, AL ( http://www.nscisc.uab.edu/ ) (2.) http:// www.strokecenter.org /patients/about - stroke/stroke - statistics/ (3.) Coffey C. Edward, Cummings Jeffrey L, Starkstein Sergio, Robinson Robert (2000). Stroke - the American Psychiatric Press Textbook of Geriatric Neuropsychiatry (Second ed.). Washington DC: American Psychiatric Press. pp. 601 – 617. (4.) Park E, Bell JD, Baker AJ (April 2008). "Traumatic brain injury: Can the consequences be stopped?" . Canadian Medical Association Journal 178 (9): 1163 – 70. (5.) http:// www.msktc.org / tbi /factsheets/Balance - Problems - After - Traumatic - Brain - Injury Annual USD (MM) USA Canada EU G20 SCI $100 + $10+ $160+ $1,400+ Stroke & TBI $5,000+ $500+ $8,000+ $35,000+ Estimated ARKE Rehabilitation Market Size (millions):
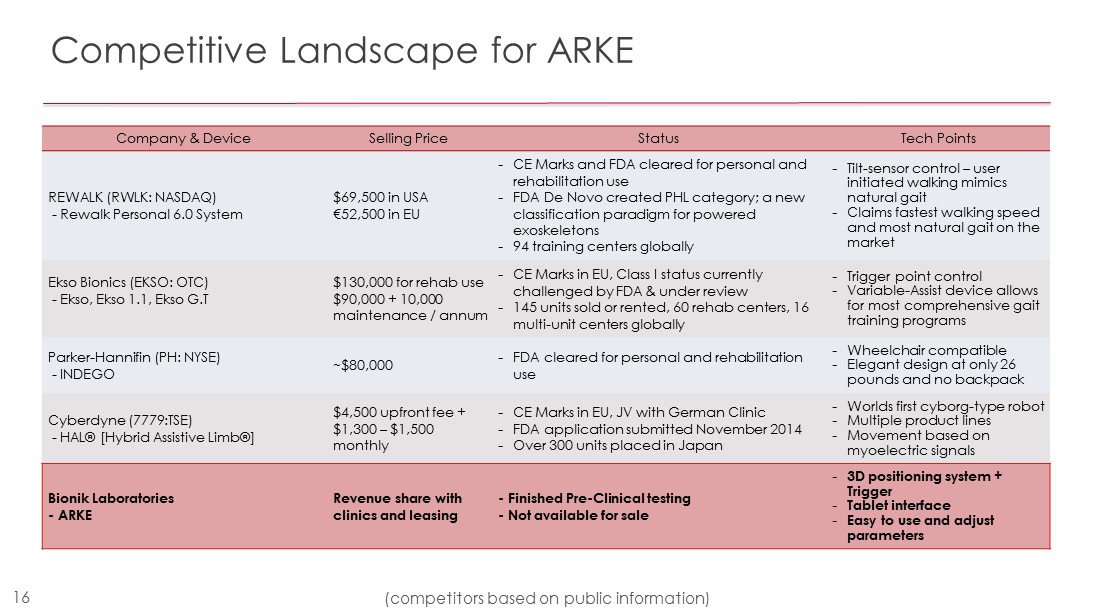
Competitive Landscape for ARKE Company & Device Selling Price Status Tech Points REWALK (RWLK: NASDAQ) - Rewalk Personal 6.0 System $ 69,500 in USA € 52,500 in EU - CE Marks and FDA cleared for personal and rehabilitation use - FDA De Novo created PHL category; a new classification paradigm for powered exoskeletons - 94 training centers globally - Tilt - sensor control – user initiated walking mimics natural gait - Claims fastest walking speed and most natural gait on the market Ekso Bionics (EKSO: OTC) - Ekso, Ekso 1.1, Ekso G.T $130,000 for rehab use $90,000 + 10,000 maintenance / annum - CE Marks in EU, Class I status currently challenged by FDA & under review - 145 units sold or rented, 60 rehab centers, 16 multi - unit centers globally - Trigger point control - Variable - Assist device allows for most comprehensive gait training programs Parker - Hannifin (PH: NYSE) - INDEGO ~$80,000 - FDA cleared for personal and rehabilitation use - Wheelchair compatible - Elegant design at only 26 pounds and no backpack Cyberdyne (7779:TSE) - HAL® [Hybrid Assistive Limb®] $4,500 upfront fee + $1,300 – $1,500 monthly - CE Marks in EU, JV with German Clinic - FDA application submitted November 2014 - Over 300 units placed in Japan - Worlds first cyborg - type robot - Multiple product lines - Movement based on myoelectric signals Bionik Laboratories - ARKE Revenue share with clinics and leasing - Finished Pre - Clinical testing - Not available for sale - 3D positioning system + Trigger - Tablet interface - Easy to use and adjust parameters 16 (competitors based on public information)
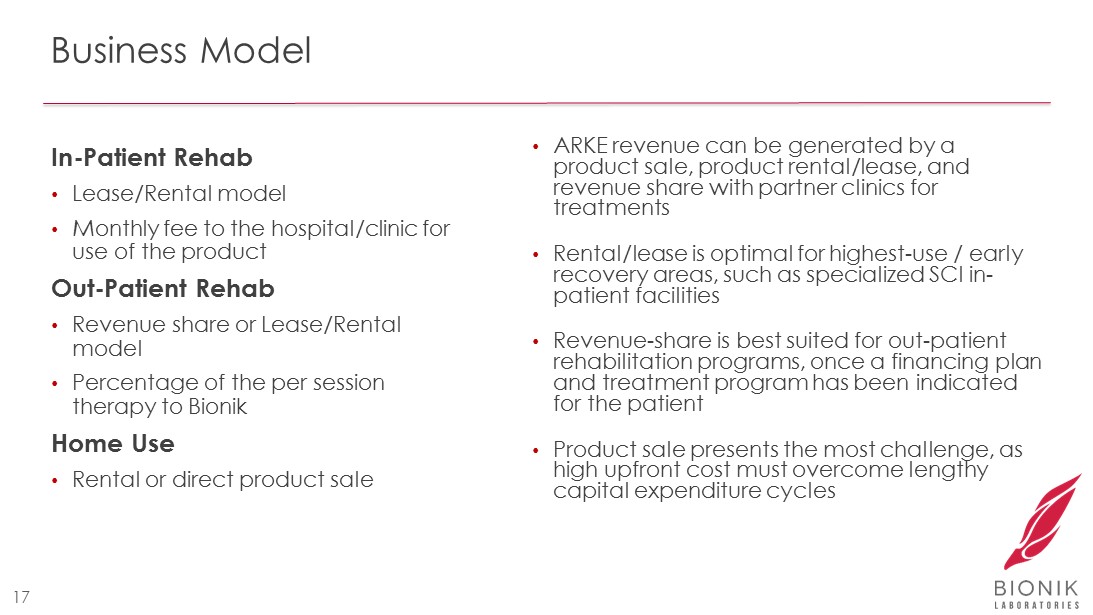
Business Model 17 In - Patient Rehab • Lease/Rental model • Monthly fee to the hospital/clinic for use of the product Out - Patient Rehab • Revenue share or Lease/Rental model • Percentage of the per session therapy to Bionik Home Use • Rental or direct product sale • ARKE revenue can be generated by a product sale, product rental/lease, and revenue share with partner clinics for treatments • Rental/lease is optimal for highest - use / early recovery areas, such as specialized SCI in - patient facilities • Revenue - share is best suited for out - patient rehabilitation programs, once a financing plan and treatment program has been indicated for the patient • Product sale presents the most challenge, as high upfront cost must overcome lengthy capital expenditure cycles
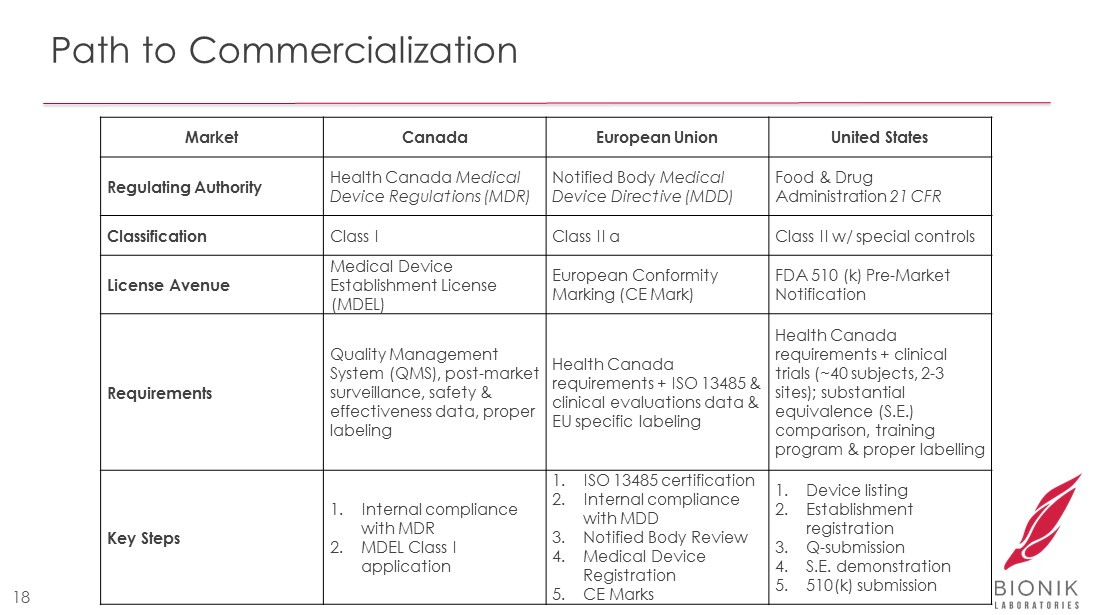
Path to Commercialization 18 Market Canada European Union United States Regulating Authority Health Canada Medical Device Regulations (MDR) Notified Body Medical Device Directive (MDD) Food & Drug Administration 21 CFR Classification Class I Class II a Class II w/ special controls License Avenue Medical Device Establishment License (MDEL) European Conformity Marking (CE Mark) FDA 510 (k) Pre - Market Notification Requirements Quality Management System (QMS), post - market surveillance, safety & effectiveness data, proper labeling Health Canada requirements + ISO 13485 & clinical evaluations data & EU specific labeling Health Canada requirements + clinical trials (~40 subjects, 2 - 3 sites); substantial equivalence (S.E.) comparison, training program & proper labelling Key Steps 1. Internal compliance with MDR 2. MDEL Class I application 1. ISO 13485 certification 2. Internal compliance with MDD 3. Notified Body Review 4. Medical Device Registration 5. CE Marks 1. Device listing 2. Establishment registration 3. Q - submission 4. S.E. demonstration 5. 510(k) submission

Reimbursement: Landmark Policy Granted Dec. 2015 • Currently no universal coverage; however successful reimbursement on a case - by - case has been achieved • Etiology of injury impacts financing plan – e.g. vehicle accidents (automobile liability and Personal Injury Protection); workplace (workman’s compensation); fall at home (home insurance); etc. • Case Managers & Social Workers work with patients to prepare a comprehensive financing plan from multiple sources • Industry - wide efforts, all competitors, academics, etc., drive the case for universal reimbursement (indicated by granting of HCPCS Code), whenever they demonstrate beneficial outcomes in published studies • Successful cases include: broad coverage at Bronx - VA, top 3 private insurers in Germany, & German Statutory Accident Insurance (DGUV) • E.g. DGUV offers reimbursement of EUR 500 / session for up to 61 sessions (1) • Applicable Center for Medicare & Medicaid Services HCPCS codes currently include: Therapeutic Exercises, Neuromuscular re - education, Therapeutic activities, Gait training, Physical performance tests, assistive technology assessments, orthotic management/training and prosthetic management training. Average Reimbursement of $34.03 / 15 minute interval (2) 19 (1.) http:// www.cyberdyne.jp / english /services/ HALTherapy.html (2.) Centers for Medicare and Medicaid Services U.S. Department of Veterans Affairs issued the first exclusive national policy for the evaluation, training and procurement of ReWalk’s personal exoskeleton systems for all qualifying veterans who have suffered spinal cord injury across the US
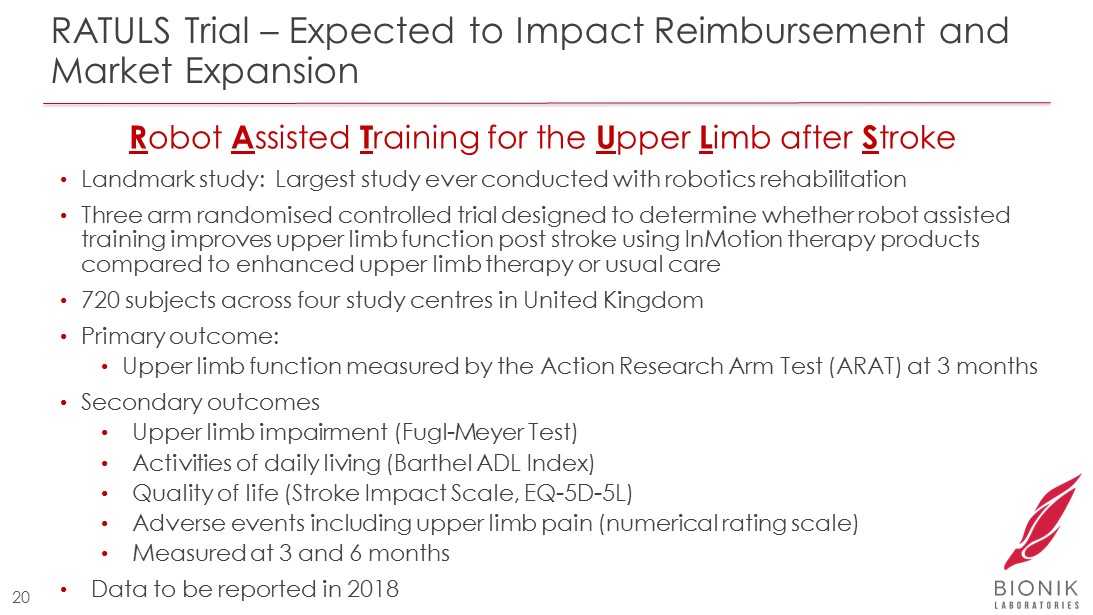
RATULS Trial – Expected to Impact Reimbursement and Market Expansion • Landmark study: Largest study ever conducted with robotics rehabilitation • Three arm randomised controlled trial designed to determine whether robot assisted training improves upper limb function post stroke using InMotion therapy products compared to enhanced upper limb therapy or usual care • 720 subjects across four study centres in United Kingdom • Primary outcome: • Upper limb function measured by the Action Research Arm Test (ARAT) at 3 months • Secondary outcomes • Upper limb impairment ( Fugl - Meyer Test) • Activities of daily living ( Barthel ADL Index) • Quality of life (Stroke Impact Scale, EQ - 5D - 5L) • Adverse events including upper limb pain (numerical rating scale) • Measured at 3 and 6 months • Data to be reported in 2018 20 R obot A ssisted T raining for the U pper L imb after S troke

Robust Intellectual Property Area Of IP Status Filing Year Algorithms & Control Systems Filed US & International 2012 Sensory Technology Filed US & International 2012 Robotics Filed US & International 2012 Robotics Filed US & International 2012 Robotics Filed US & International 2012 Robotics US Provisional 2014 Cloud Computing US Provisional 2014 Cloud Computing US Provisional 2014 Robotics US Provisional 2014 Robotics US Provisional 2014 Robotics US Provisional 2014 Algorithms & Control Systems US Provisional 2014 Algorithms & Control Systems US Provisional 2014 Sensory Technology US Provisional 2014 Algorithms & Control Systems US Provisional 2014 Robotics US Provisional 2014 Robotics US Provisional 2014 Algorithms & Control Systems US Provisional 2014 Energy US Provisional 2014 ROBOTICS SENSORY TECHNOLOGY ALGORITHMS & CONTROL SYSTEMS ENERGY CLOUD COMPUTING 21 Bionik is working towards becoming a leader in the robotics rehabilitation market. We have a strong focus on intellectual property generation and consolidation. IMT brings strong data and licensed intellectual property for certain of its products including three patents with exclusivity through 2029 and 2033
University Networks Expanded and Focused Development Programs In - house R&D Licensing / Acquisitions Industry Partners MIT Technology Growth Strategy Focused on Further Pipeline Expansion • Leverage our in - depth research and development relationships within the space along with our relationship with professors at MIT to identify additional synergistic robotic technologies to continue building a rich product portfolio • Team dedicated to expanding product pipeline through strategic partnering and acquisitions with products and enabling software 22

World Class Management Team 23 • Peter Bloch, CPA, CA – Chief Executive Officer • Over 25 years of executive management experience with proven track record of building public and private technology companies. Former CFO and joint Interim CEO of Sanofi Canada, CFO of Intellivax , Gennum TSX:GNM (n/k/a Semtech NASDAQ:SMTC), Just Energy (NYSE/TSX:JE); and Founder of Tribute Pharmaceuticals • Michal Prywata – Co - Founder and Chief Operating Officer • Biomedical engineering experience with a track record of winning technology showcases and developing technologies that address significant and untapped markets • Hermano Krebs, Ph.D., M.S. – Chief Science Officer • Pioneer in the design, development, clinical use, and research of robots used to administer rehabilitation therapy to patients with neurological injury. Principal Research Scientist and Lecturer at the Massachusetts Institute of Technology • Jules Fried – VP, U.S. Operations • Serial entrepreneur, having built and led a two - time Inc. 500 capital equipment manufacturer as a founding management team member. Founder and member of the Board of Directors of First Commons Bank. Former partner in the law firm of McDermott, Will & Emery • Leslie N. Markow, CPA, CA, CPA(Illinois) C.Dir – Chief Financial Officer • Over 25 years of finance/accounting leadership experience. Former CFO of SunOpta (NASDAQ: STKL)

Board and Advisors Strengthens Capabilities 24 Advisors • Neville Hogan, Ph.D., M.S, M.E. • Sun Jae Professor of Mechanical Engineering, Professor of Brain and Cognitive Sciences, and Director of the Newman Laboratory for Biomechanics and Human Rehabilitation at MIT • Gary Henley • Significant medtech experience • Former CEO of United Orthopedic Group, Wright Medical Technology, CeCorp and Electronic Systems • Board Director of numerous companies • Dr. Isador Lieberman, MD MBA FRCSC • Medical Director, Clinical Advisor; Texas Back Institute Orthopedic and Spinal Surgeon • Dr. Edward Lemaire • Clinical Trial Advisor & Investigator; Clinical Investigator, Centre for Rehabilitation Research, Clinical Epidemiology, Ottawa Hospital Research Institute • Dr. Kaamran Raahemifar • Control Systems & Electronics Advisor; PhD in Biomedical Engineering; Electrical & Computer Eng. professor at Ryerson University • Dr. Dany Gagnon • Clinical Trial Advisor & Investigator; Clinical Investigator: Centre de Recherche Interdisciplinaire en readaptation du Grand Montreal Board of Directors • Dr. Robert Hariri • Independent Director • Chairman, Founder, Chief Scientific Officer, and former Chief Executive Officer of Celgene Cellular Therapeutics; Board member of Myos Corporation and Provista Diagnostics • Marc Mathieu • Independent Director • Chief Marketing Officer of Samsung North America; Former Senior Vice President of Global Marketing at Unilever; Chairman and Co - founder of We&Co • Peter Bloch, CPA, CA • Chairman and Chief Executive Officer • Michal Prywata • Co - Founder and Chief Operating Officer • Thiago Caires • Co - Founder
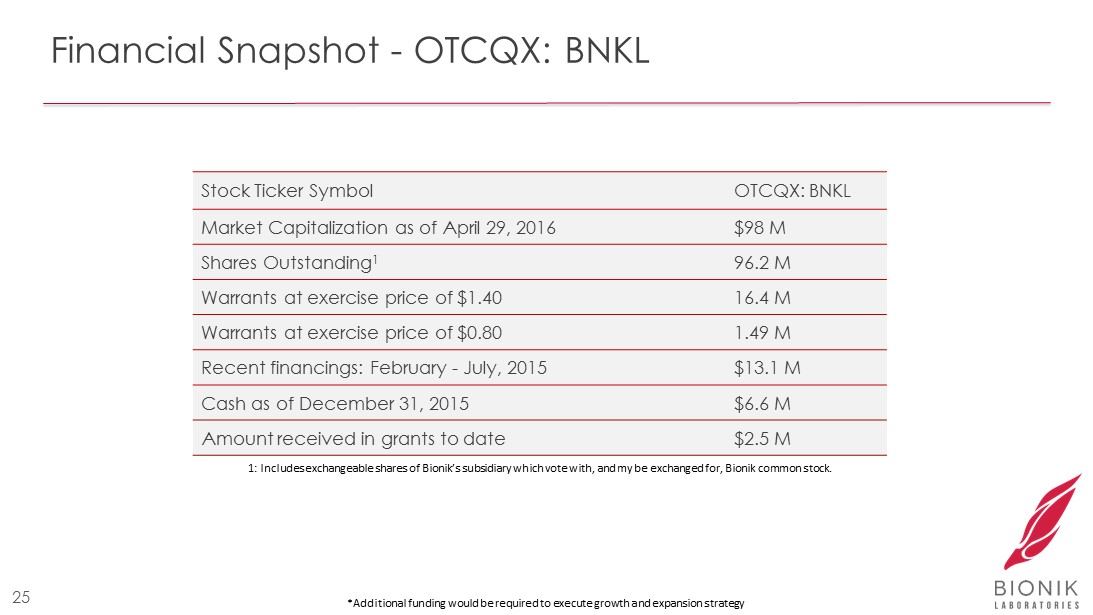
Financial Snapshot - OTCQX: BNKL Stock Ticker Symbol OTCQX: BNKL Market Capitalization as of April 29, 2016 $98 M Shares Outstanding 1 96.2 M Warrants at exercise price of $1.40 16.4 M Warrants at exercise price of $0.80 1.49 M R ecent financings: February - July , 2015 $13.1 M Cash as of December 31 , 2015 $6.6 M Amount received in grants to date $2.5 M *Additional funding would be required to execute growth and expansion strategy 25 1: Includes exchangeable shares of Bionik’s subsidiary which vote with, and my be exchanged for, Bionik common stock.

2016 Expected Milestones Expected to Drive Value 26 • Complete integration of IMT • Appoint Chief Commercial Officer • Focus on revenue generation and market expansion • Advance development products through to commercialization • Further expand product portfolio through acquisitions/licensing • Strengthen team with additional independent Board Members • Uplist to National Exchange 2016

www.bioniklabs.com OTCQX:BNKL Pioneering global medical device and robotics company focused on providing rehabilitation solutions to individuals with neurological disorders Corporate Presentation
