Attached files
| file | filename |
|---|---|
| 8-K - 8-K - TREVENA INC | a16-10317_18k.htm |
| EX-99.1 - EX-99.1 - TREVENA INC | a16-10317_1ex99d1.htm |
Exhibit 99.2
Biased Ligands. Better Drugs. May 2, 2016
Forward looking statements and other important cautions To the extent that statements contained in this presentation are not descriptions of historical facts regarding Trevena, Inc., they are forward-looking statements reflecting management’s current beliefs and expectations. Forward-looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. You can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “intends,” or “continue,” or the negative of these terms or other comparable terminology. Forward-looking statements contained in this presentation include, but are not limited to, (i) statements regarding the timing of anticipated clinical trials for our product candidates; (ii) the timing of receipt of clinical data for our product candidates; (iii) our expectations regarding the potential safety, efficacy, or clinical utility of our product candidates; (iv) the size of patient populations targeted by our product candidates and market adoption of our potential drugs by physicians and patients; (v) the timing or likelihood of regulatory filings and approvals; and (vi) our cash needs and potential payments under our agreements with Allergan plc. Various factors may cause differences between our expectations and actual results, including unexpected safety or efficacy data, unexpected side effects observed during preclinical studies or in clinical trials, lower than expected enrollment rates in clinical trials, changes in expected or existing competition, changes in the regulatory environment for our drug candidates and our need for future capital, the inability to protect our intellectual property, and the risk that we become a party to unexpected litigation or other disputes. You should read our filings with the Securities and Exchange Commission, including the Risk Factors set forth in our Annual Report on Form 10-K and our Quarterly Reports on Form 10-Q filed with the Securities and Exchange Commission and other filings the Company makes with the Securities and Exchange Commission from time to time, completely and with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. 2

INTRODUCTORY COMMENTS 3

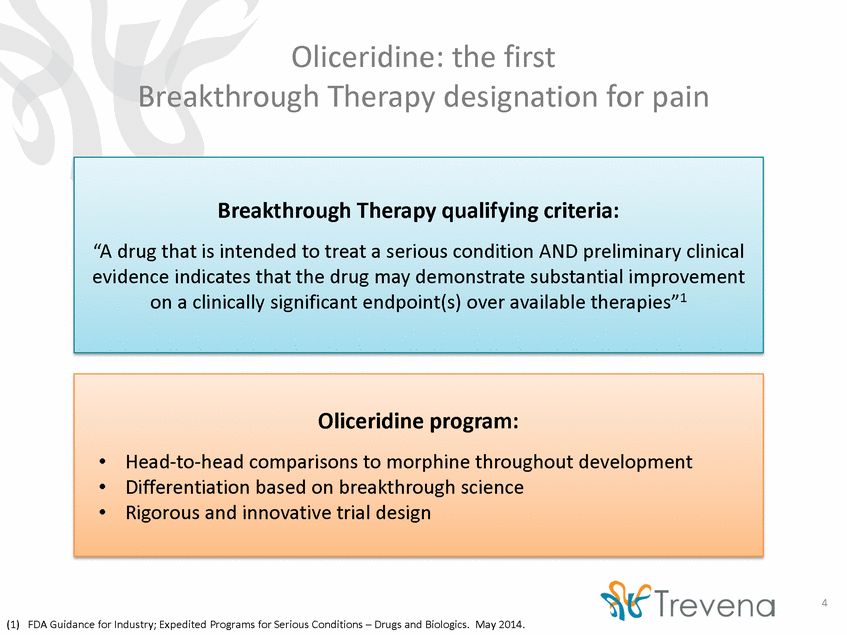
Oliceridine: the first Breakthrough Therapy designation for pain 4 (1) FDA Guidance for Industry; Expedited Programs for Serious Conditions – Drugs and Biologics. May 2014. Oliceridine program: •Head-to-head comparisons to morphine throughout development •Differentiation based on breakthrough science •Rigorous and innovative trial design Breakthrough Therapy qualifying criteria: “A drug that is intended to treat a serious condition AND preliminary clinical evidence indicates that the drug may demonstrate substantial improvement on a clinically significant endpoint(s) over available therapies”1
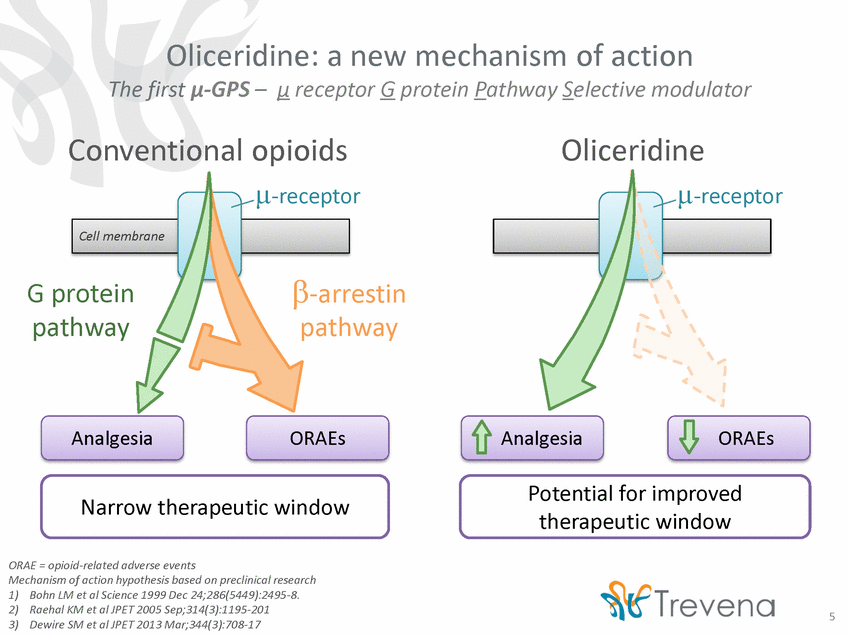
Oliceridine: a new mechanism of action The first µ-GPS – µ receptor G protein Pathway Selective modulator Conventional opioids Oliceridine µ-receptor µ-receptor β-arrestin pathway G protein pathway Analgesia ORAEs Analgesia ORAEs Potential for improved therapeutic window Narrow therapeutic window ORAE = opioid-related adverse events Mechanism of action hypothesis based on preclinical research 1) 2) 3) Bohn LM et al Science 1999 Dec 24;286(5449):2495-8. Raehal KM et al JPET 2005 Sep;314(3):1195-201 Dewire SM et al JPET 2013 Mar;344(3):708-17 5 Cell membrane
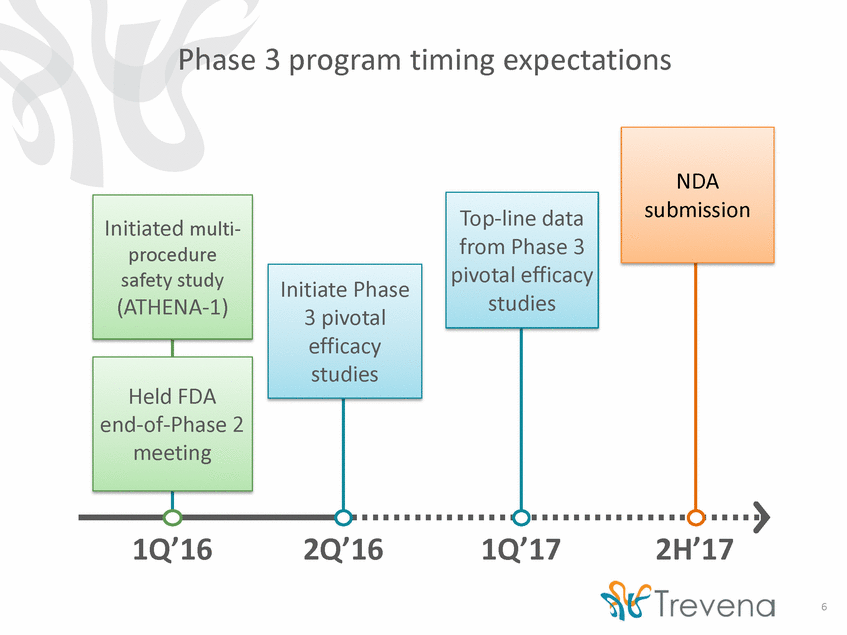
Phase 3 program timing expectations Initiate Phase udies votal cacy udies Phase 2 eting 1Q’16 2Q’16 1Q’17 2H’17 6 NDA submission Initiated multi-procedure safety study (ATHENA-1) Top-line data from P pivotal st hase 3 efficacy 3 pi effi st Held end-of-me FDA
END-OF-PHASE 2 MEETING OUTCOME 7

Successful outcome for the end-of-phase 2 meeting for the oliceridine program • • General agreement was reached to move oliceridine in to Phase 3 General agreement was reached that the oliceridine Phase 3 program could support an approval for the target indication – Management of moderate-to-severe acute pain where parenteral therapy is warranted • Key elements of Phase 3 program: – – – Two pivotal efficacy studies with PCA dosing to support potential finding of efficacy Bunionectomy and abdominoplasty populations appropriate for target indication Safety database with PCA and bolus dosing >1,100 patients •Including a sufficient number of patients with higher exposures and longer durations of oliceridine therapy Key clinical pharmacology, nonclinical toxicology, CMC activities to support NDA – 8

OLICERIDINE PHASE 3 PROGRAM OVERVIEW 9

Pivotal efficacy studies to support potential approval • Two pivotal studies: same surgical models as in Phase 2 – – – APOLLO-1: 48 hour treatment following bunionectomy APOLLO-2: 24 hour treatment following abdominoplasty Each study will include 375 patients, 75/group • Primary endpoint: efficacy of oliceridine vs. placebo • Secondary endpoints: oliceridine vs. morphine – – – Efficacy, including pain intensity difference and time to onset Safety, including respiratory safety burden based on hypoventilation events Tolerability, including nausea and vomiting 10
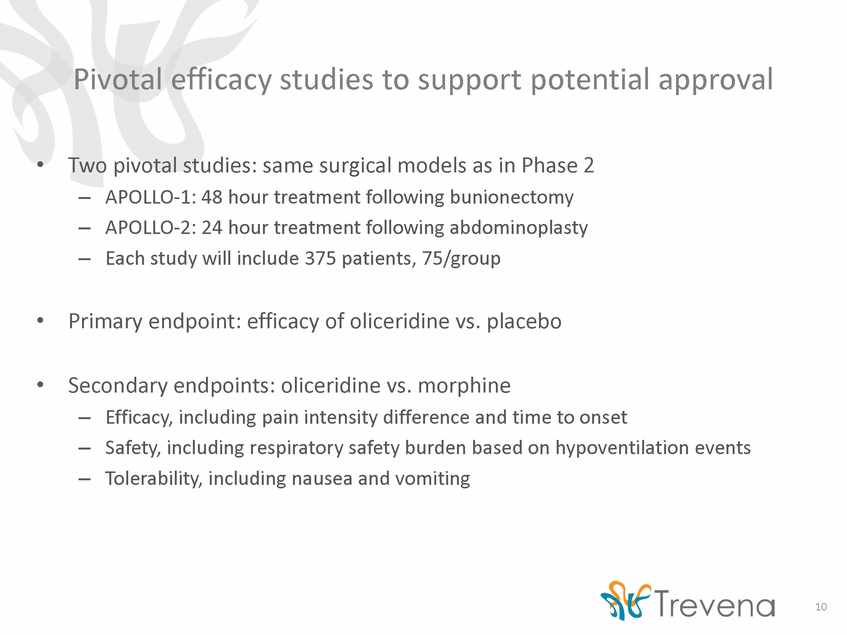
Phase 3 program dosing and administration • • Goal: dosing and administration labeling for as-needed dosing (bolus or PCA) APOLLO dosing: patient controlled analgesia (PCA), as in Phase 2b study – If PCA dosing does not adequately control pain, supplemental study drug can be given as often as every hour •If pain still not adequately controlled, rescue NSAID analgesic available 3 oliceridine dose arms planned in each APOLLO study: – • ATHENA safety study: PCA and as-needed bolus dosing intended to complement PCA dosing data from APOLLO studies 11 RegimenNLoading dose (mg)Demand dose (mg)Supplemental dose (mg) Placebo75---Oliceridine 0.1 mg751.50.10.75 Oliceridine 0.35 mg751.50.350.75 Oliceridine 0.5 mg751.50.50.75 Morphine754.01.02.0
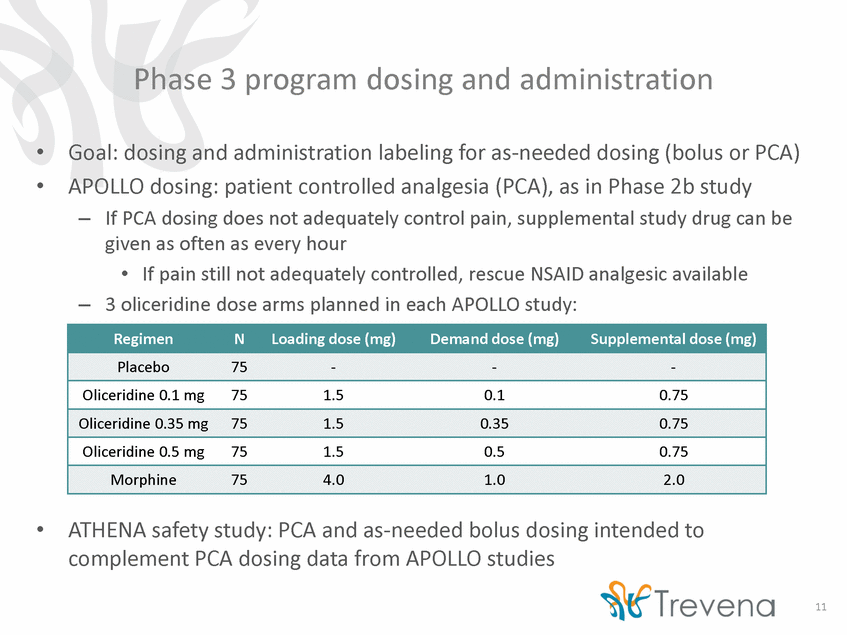
Pivotal efficacy studies: primary endpoint Same measure as Phase 2: pain intensity assessed with a visual analog scale • • Phase 3 will use a responder analysis as proposed by Trevena – Defined as >30% improvement in sum of pain intensity difference from baseline (SPID) without early discontinuation and without rescue pain medication – Rationale: • • • More straightforward clinical interpretation than pain intensity difference Incorporates an element of safety/tolerability High power based on post-hoc analysis of Phase 2b abdominoplasty data Post-hoc analysis of phase 2b abdominoplasty data using Phase 3 primary endpoint 4.0 mg load, 12 Post-hoc analysis of Phas Placebo (n = 39) e 2b dvaotlaume matched Oliceridine (n = 39) 1.5 mg load, 0.1 mg demand Oliceridine (n = 39) 1.5 mg load, 0.35 mg demand Morphine (n = 83) 1.0 mg demand Responders, n (%) 12 (30.8%) 25 (64.1%) 28 (71.8%) 55 (66.3%) p-value vs. placebo 0.0005 0.0004 0.0003
Pivotal efficacy studies: secondary endpoints • Efficacy measures vs. placebo, including sum of pain intensity difference analyses and time to meaningful pain relief, to support primary analysis • Efficacy, safety, and tolerability comparisons to morphine allow demonstration of potential clinical advantages of oliceridine – Efficacy measures vs. morphine include: • Responder rate: same evaluation as primary endpoint – Non-inferiority and superiority vs. morphine Time to onset of meaningful pain relief Time to use, total use, and prevalence of use for rescue analgesics • • – Safety/tolerability measures vs. morphine include: • • Spontaneously reported nausea/vomiting and rescue anti-emetic use Respiratory safety burden 13
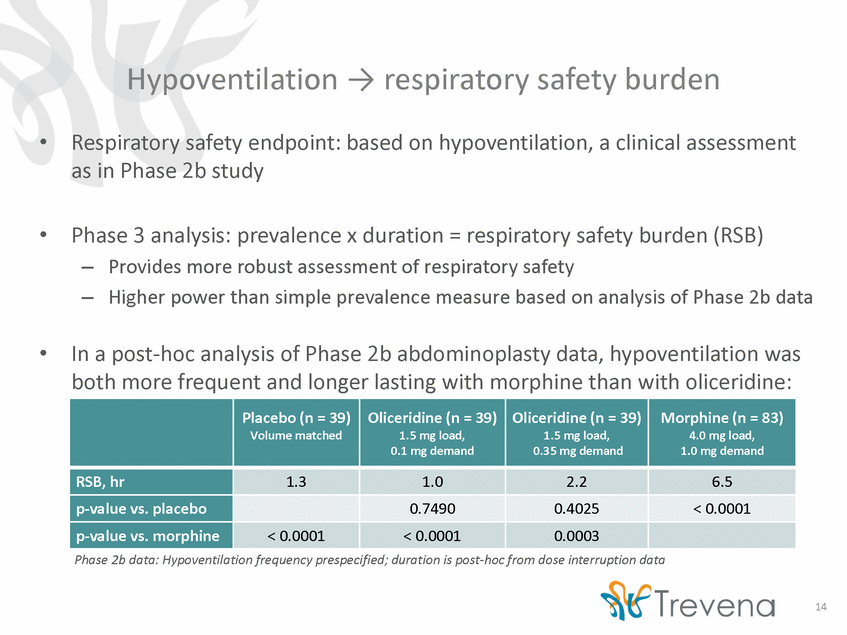
Hypoventilation respiratory safety burden • Respiratory safety endpoint: based on hypoventilation, a clinical assessment as in Phase 2b study • Phase 3 analysis: prevalence x duration = respiratory safety burden (RSB) – – Provides more robust assessment of respiratory safety Higher power than simple prevalence measure based on analysis of Phase 2b data • In a post-hoc analysis of Phase 2b abdominoplasty data, hypoventilation was both more frequent and longer lasting with morphine than with oliceridine: Phase 2b data: Hypoventilation frequency prespecified; duration is post-hoc from dose interruption data 14 Placebo (n = 39) Volume matched Oliceridine (n = 39) 1.5 mg load, 0.1 mg demand Oliceridine (n = 39) 1.5 mg load, 0.35 mg demand Morphine (n = 83) 4.0 mg load, 1.0 mg demand RSB, hr 1.3 1.0 2.2 6.5 p-value vs. placebo 0.7490 0.4025 < 0.0001 p-value vs. morphine < 0.0001 < 0.0001 0.0003
Summary Successful end-of-phase 2 meeting • – – – Appropriate to move oliceridine to Phase 3 Agreed upon key elements of Phase 3 program Collaborative discussion with FDA • Phase 3 program overview – APOLLO studies designed to support approval and differentiation •Endpoints and analysis are well informed by the Phase 2 program ATHENA-1 study is underway APOLLO studies to commence this quarter – – • Breakthrough Therapy designation offers opportunity for ongoing dialogue 15
Biased Ligands. Better Drugs. 16

