Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Aralez Pharmaceuticals Inc. | a16-9841_18k.htm |
Exhibit 99.1
“Corporate Overview & Strategic Vision” Bloom Burton Healthcare Conference May 2, 2016

This presentation includes certain statements that constitute “forward-looking statements” within the meaning of applicable securities laws. Forward-looking statements include, but are not limited to, statements regarding our 2016 financial guidance, the anticipated launch of BLEXTEN, promotional and sales initiatives related to Fibricor and growing Fibricor use in the U.S., the anticipated YOSPRALA launch in 4Q 2016, pending FDA approval, YOSPRALA having the ability to achieve mid single digit market share in the secondary prevention patient population, our plan to expand to up to 300 sales professionals, YOSPRALA capturing a quarter to a third of secondary prevention patients, the continued financially disciplined build up of our Company, the successful integration of Tribute, opportunistic and transformative M&A that are near-term revenue generating and accretive, our expectation to be profitable by 2018, our plan to submit YOSPRALA in Europe in 3Q 2016, MT400 (Treximet) and YOSPRALA in Canada in 2017 in the near term, and other statements that are not historical facts, and such statements are typically identified by use of terms such as “may,” “will,” “would,” “should,” “could,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “likely,” “potential,” “continue” or the negative or similar words, variations of these words or other comparable words or phrases, although some forward-looking statements are expressed differently. You should be aware that the forward-looking statements included herein represent management’s current judgment and expectations, and are based on current estimates and assumptions made by management in light of its experience and perception of historical trends, current conditions and expected future developments, as well as other factors that it believes are appropriate and reasonable under the circumstances, but there can be no assurance that such estimates and assumptions will prove to be correct and, as a result, the forward-looking statements based on those assumptions could prove to be incorrect. Accordingly, actual results, level of activity, performance or achievements or future events or developments could differ materially from those expressed or implied in the forward-looking statements. Material factors or assumptions that were applied in providing financial guidance for the year ending December 31, 2016, including with respect to the statements that Aralez’s net revenues are expected to be in the range of $48 million to $58 million, non-GAAP SG&A expenses are expected to be in the range of $85 million to $100 million and non-GAAP R&D expenses are expected to be in the range of $8 million to $12 million, include, but are not limited to, the material factors and assumptions outlined in this presentation and under the caption “Cautionary Note Regarding Forward-Looking Statements” in the Company’s press release dated March 15, 2016 announcing results for the fourth quarter and full-year ended December 31, 2015, and the Company’s 2016 financial guidance. Readers are cautioned that actual future operating results and economic performance of the Company, including with respect to our net revenues, non-GAAP SG&A expenses and non-GAAP R&D expense for the year ending December 31, 2016, are subject to a number of risks and uncertainties, including, among other things described below, our inability to build, acquire or contract with a sales force of sufficient scale for the commercialization of YOSPRALA™ in a timely and cost-effective manner, our failure to successfully commercialize our product candidates; costs and delays in the development and/or FDA approval of our product candidates (including YOSPRALA), including as a result of the need to conduct additional studies or due to issues with third-party API or finished product manufacturers, or the failure to obtain such approval of our product candidates for all expected indications, including as a result of changes in regulatory standards or the regulatory environment during the development period of any of its product candidates; the inability to maintain or enter into, and the risks resulting from our dependence upon, collaboration or contractual arrangements necessary for the development, manufacture, commercialization, marketing, sales and distribution of any products, including our dependence on AstraZeneca and Horizon for the sales and marketing of VIMOVO, our dependence on Patheon for the manufacture of YOSPRALA™ 81/40 and YOSPRALA™ 325/40; our ability to protect our intellectual property and defend our patents; regulatory obligations and oversight; failure to make, integrate and maintain new acquisitions, such as the integration of Tribute; fluctuations in the value of certain foreign currencies, including the Canadian dollar, in relation to the U.S. dollar, and other world currencies; changes in government regulations, including tax laws and unanticipated tax liabilities; and general adverse economic, market and business conditions, and could differ materially from what is currently expected as set out in this presentation. Our operations involve risks and uncertainties, many of which are outside of our control, and any one or any combination of these risks and uncertainties could also affect whether the forward-looking statements ultimately prove to be correct and could cause our actual results, level of activity, performance or achievements or future events or developments to differ materially from those expressed or implied by the forward-looking statements. These risks and uncertainties include those risks detailed from time-to-time under the caption “Risk Factors” and elsewhere in the Company’s SEC filings and reports and Canadian securities law filings, including in our Annual Report on Form 10-K for the year ended December 31, 2015 which are available on EDGAR at www.sec.gov, on SEDAR at www.sedar.com, and on the Company’s website at www.aralez.com, and those described from time to time in our future reports filed with the Securities and Exchange Commission and applicable securities regulatory authorities in Canada. You should not place undue importance on forward-looking statements and should not rely upon this information as of any other date. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law. Disclaimer

The Aralez Investment Thesis Aralez is a new global specialty pharmaceutical company designed to: Maximize the value of an expanded portfolio, growing revenue base and geographic footprint across North America and Europe Build on our anchor positions in cardiovascular disease and pain management and opportunistically expand into other specialty therapeutic areas Access high potential growth opportunities through aggressive BD&L and strategic M&A Build value organically and leverage competitive platform to accelerate transformation and execute growth strategy Maintain a lean, nimble and performance-oriented operating model Align with shareholder interests with a strong focus on creating shareholder value

Focus on Cardiovascular & Pain Therapies VIMOVO®: (partnered): Combination of esomeprazole and naproxen for osteoarthritis in patients at risk of developing NSAID associated gastric ulcers Horizon Pharma (US) – 10% on US sales; annual minimum of $7.5M; Patented until 2031 AstraZeneca (ex-US) – 50+ countries; 10% on all territory sales Total combined royalties of $21.4M in 2015 TREXIMET®/MT400: (US partnered; ex-US Aralez): Combination of sumatriptan and naproxen sodium for acute migraine in patients 12 years of age and older Pernix (US) – Royalty sold to CPPIB; receive 20% of CPPIB receipts starting April 2018 Treximet® – Adolescent Dose recently FDA approved Plans to submit MT400 to Canada in the near-term
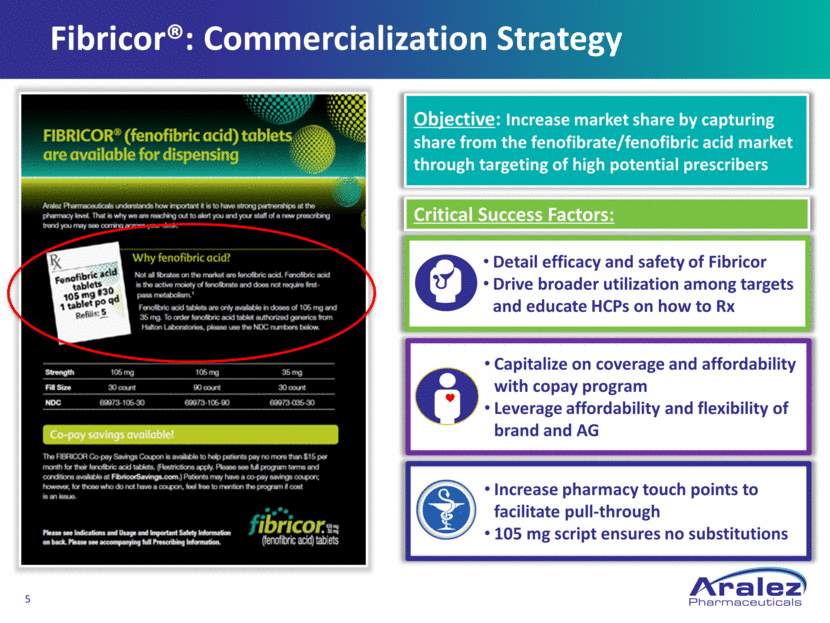
Fibricor®: Commercialization Strategy Detail efficacy and safety of Fibricor Drive broader utilization among targets and educate HCPs on how to Rx Capitalize on coverage and affordability with copay program Leverage affordability and flexibility of brand and AG Increase pharmacy touch points to facilitate pull-through 105 mg script ensures no substitutions Objective: Increase market share by capturing share from the fenofibrate/fenofibric acid market through targeting of high potential prescribers Critical Success Factors:
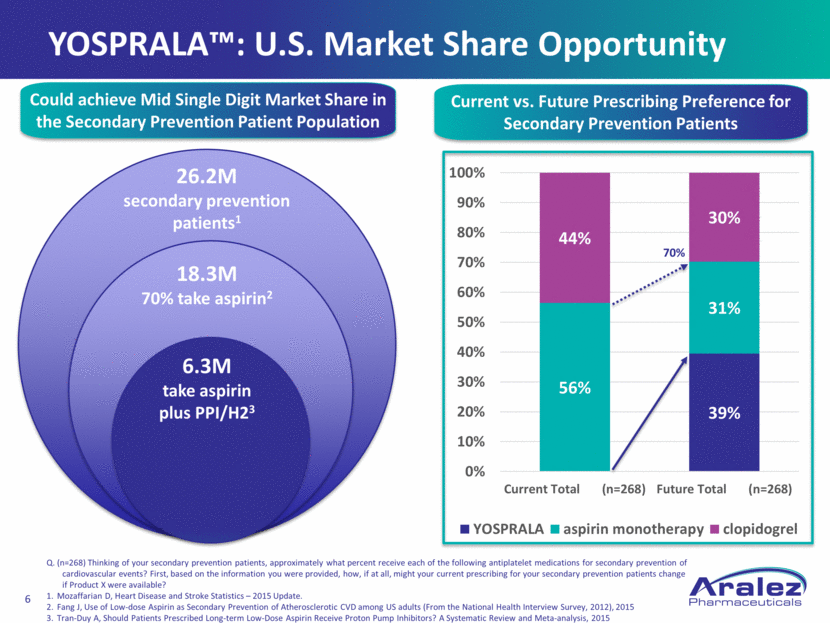
18.3M 70% take aspirin2 6.3M take aspirin plus PPI/H23 26.2M secondary prevention patients1 Could achieve Mid Single Digit Market Share in the Secondary Prevention Patient Population Current vs. Future Prescribing Preference for Secondary Prevention Patients Q. (n=268) Thinking of your secondary prevention patients, approximately what percent receive each of the following antiplatelet medications for secondary prevention of cardiovascular events? First, based on the information you were provided, how, if at all, might your current prescribing for your secondary prevention patients change if Product X were available? Mozaffarian D, Heart Disease and Stroke Statistics – 2015 Update. Fang J, Use of Low-dose Aspirin as Secondary Prevention of Atherosclerotic CVD among US adults (From the National Health Interview Survey, 2012), 2015 Tran-Duy A, Should Patients Prescribed Long-term Low-Dose Aspirin Receive Proton Pump Inhibitors? A Systematic Review and Meta-analysis, 2015 YOSPRALA™: U.S. Market Share Opportunity 39% 56% 31% 44% 30% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Current Total (n=268) Future Total (n=268) YOSPRALA aspirin monotherapy clopidogrel 70%
YOSPRALA: Commercialization Strategy Upon FDA approval, plan to launch YOSPRALA with ~110 sales professionals (including 25 previously hired for Fibricor), targeting top 29% of secondary prevention specialist (CV, IM and PCP) physicians; Target ~15,000 physicians initially, including 8,000 cardiologists “Invest into the opportunity” and plan to expand to up to 300 sales professionals over time targeting over 40% of the defined market; Target ~35,000 physicians, including 14,000 cardiologists New guidelines published in the Journal of The American College of Cardiology provide opportunity for other advocacy groups to refresh their recommendations Aspirin is assumed in EVERY instance of Dual Antiplatelet therapy (DAPT) Aspirin remains the cornerstone of antiplatelet therapy Patients almost always need to stay on aspirin indefinitely Commercialization Strategy Market Environment

YOSPRALA: Pre-Launch Preparations Confirmed launch pricing for YOSPRALA Received feedback from KOL, Professional, and Advocacy groups Developed comprehensive and logical commercial messaging platform Developed and tested compelling branded and unbranded platforms Received positive feedback regarding optimal promotional messaging bundle Completed quantitative forecast research Scientific educational platform established Scientific advisory panel development Medical science liaison team recruitment Data publication / presentation planning complete Health economic activities underway Marketing Preparations Medical Affairs Preparations

Disease Awareness Initiative Key Communication Goals: Raise awareness among prescribers of the gastrointestinal (GI) side effects of aspirin and their impact on outcomes There may be a hole in his aspirin therapy Journals Ads Website Direct Mail Banner Ads E-blast

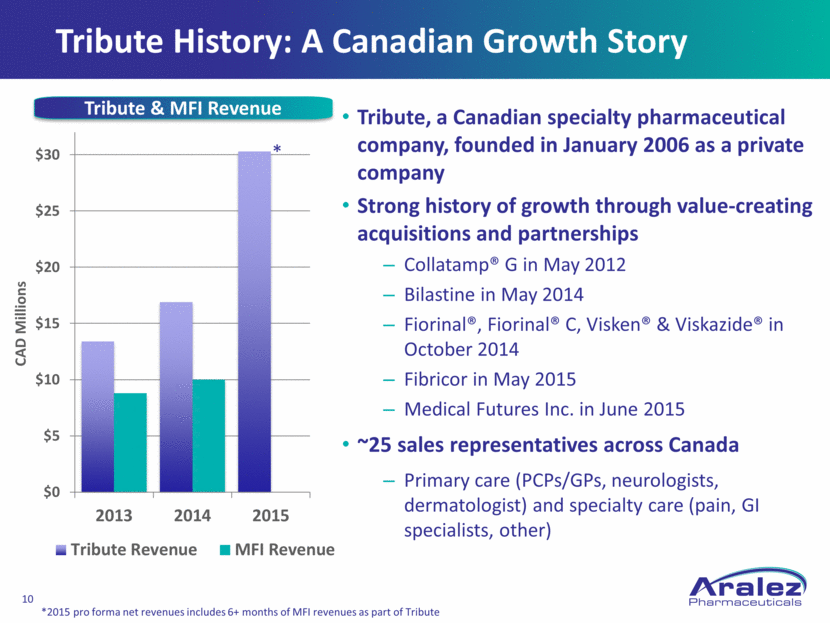
Tribute History: A Canadian Growth Story Tribute, a Canadian specialty pharmaceutical company, founded in January 2006 as a private company Strong history of growth through value-creating acquisitions and partnerships Collatamp® G in May 2012 Bilastine in May 2014 Fiorinal®, Fiorinal® C, Visken® & Viskazide® in October 2014 Fibricor in May 2015 Medical Futures Inc. in June 2015 ~25 sales representatives across Canada Primary care (PCPs/GPs, neurologists, dermatologist) and specialty care (pain, GI specialists, other) CAD Millions Tribute & MFI Revenue * *2015 pro forma net revenues includes 6+ months of MFI revenues as part of Tribute $0 $5 $10 $15 $20 $25 $30 2013 2014 2015 Tribute Revenue MFI Revenue
BLEXTEN™ - New Product Opportunity in Canada BLEXTEN (bilastine 20 mg oral tablet) is a second generation antihistamine drug for the treatment of the symptoms of Seasonal Allergic Rhinitis (SAR) and Chronic Spontaneous Urticaria (CSU) (such as itchiness and hives) developed in Spain by FAES Farma, S.A. Demonstrated somnolence rates similar to placebo representing a potentially non-sedating effect at therapeutic doses BLEXTEN, is an innovative drug, and as such is entitled to an eight-year term of data protection (market exclusivity) under section C.08.004.1 of the Food and Drug Regulations The Canadian antihistamine market is currently valued at approximately $115M per year Represents an opportunity to introduce the first new antihistamine in Canada in over 15 years Planning for the launch of BLEXTEN to support the organic growth of the Canadian business At launch, Aralez is expected to be the only company promoting an antihistamine and providing promotional support and samples to doctors in Canada As a prescription medication, BLEXTEN has the potential to be a covered medication to those Canadians enrolled in a drug plan Commercialization Strategy Market Opportunity Product Profile
Aralez: Canadian Portfolio Growth Drivers Cambia® Exclusive license in Canada from Depomed, Inc. Cambia (diclofenac) was approved by Health Canada in 2009 for the acute treatment of migraine attached with or without aura in adults 18 years of age or older Durela® Exclusive license in Canada from Cipher Pharmaceuticals Inc. Durela (tramadol) was approved by Health Canada in 2011 for the management of moderate to moderately severe pain in adults who require continuous treatment for several days or more Soriatane® Soriatane (acitretin) was approved by Health Canada in 1994 for the treatment for severe psoriasis (includes erythrodermic and pustular types) and other disorders of keratinization The first and only oral retinoid indicated for severe psoriasis
2016 Financial Guidance Measure 2016 Guidance Net Revenues* $48 million to $58 million Non-GAAP SG&A Expenses** $85 million to $100 million Non-GAAP R&D Expenses ** $8 million to $12 million * Guidance on net revenues includes revenues from Tribute from February 6, 2016 through December 31, 2016, assuming foreign currency exchange rates remain at or near current levels. ** Excludes share-based compensation expense and discrete items, such as merger and acquisition- related expenses, including transaction fees and severance. GAAP to Non-GAAP reconciliations are included within the tables accompanying our press release issued today on Current Report on Form 8-K, a copy of which is available on EDGAR at www.sec.gov, on SEDAR at www.sedar.com and on our website at www.Aralez.com.
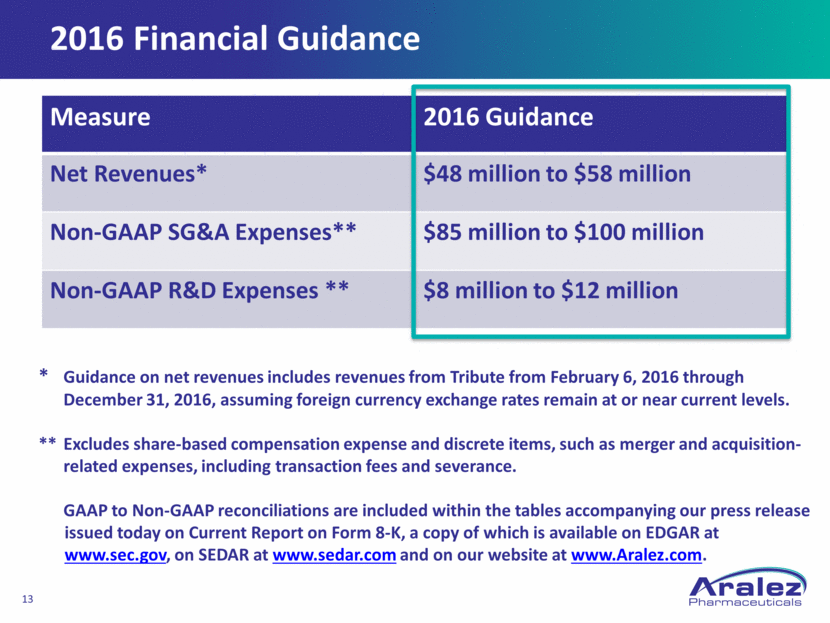
Aralez: A Compelling Platform for Growth Canadian domiciled with an Irish presence Aralez Pharmaceuticals Trading DAC holds IP & plans to execute acquisition and operating strategy Corporate structure offers competitive advantage Strong balance sheet & financial position At Feb. 5, $150M of new capital $75M equity/ $75M convert) $200M committed credit facility for acquisitions Expect to be profitable on an adjusted EBITDA basis by 2018 assuming only the projected base business and not taking into account any Business Development upside Goal is to accelerate profitability through aggressive Business Development and M&A Strong focus on building out a diversified growing revenue base with a North American focus All numbers $ U.S. millions Aralez 2015 Pro Forma *2015 pro forma net revenues includes 6+ months of MFI revenues as part of Tribute 2015 $21.4 $23.7 POZEN Tribute
Business Development: Framework and Focus Near-term EBITDA accretive* and revenue generating products Approved products in Cardiovascular and Pain anchor areas Opportunistic approach to other specialty therapeutic areas meeting specified criteria US, Canada and ex-North American geographies Aligned, opportunistic and transformative M&A Value Creation Strategy: Focus Areas and Priorities Corporate Structure with Strong Financial Position Enhances Competitiveness * Excluding discrete items, including merger and acquisition-related expenses.

Aralez - Our Near Term Priorities Continued financially disciplined build up of organization Commercial execution of the recent promotional launch of Fibricor YOSPRALA PDUFA goal date of September 14, 2016; target launch in 4Q 2016, pending FDA approval Plan to submit YOSPRALA in Europe in 3Q 2016 and MT400 (Treximet) and YOSPRALA in Canada in the near-term Plan for launch of BLEXTEN™, recently approved by Health Canada, for the treatment of the symptoms of Seasonal Allergic Rhinitis and Chronic Spontaneous Urticaria in Canada Actively assess and execute Business Development and M&A opportunities that are near-term revenue generating and accretive Shareholder Value Creation

“Corporate Overview & Strategic Vision” Bloom Burton Healthcare Conference May 2, 2016

