Attached files
| file | filename |
|---|---|
| EX-31.1 - CEO CERTIFICATION - LUMOS PHARMA, INC. | nlnk-20160331xex311.htm |
| EX-32.1 - SECTION 1350 CERTIFICATION - LUMOS PHARMA, INC. | nlnk-20160331xex321.htm |
| EX-31.2 - CFO CERTIFICATION - LUMOS PHARMA, INC. | nlnk-20160331xex312.htm |
| EX-10.9 - EXHIBIT 10.9 - LUMOS PHARMA, INC. | nlnk-20160331xex109.htm |
| EX-10.10 - EXHIBIT 10.10 - LUMOS PHARMA, INC. | nlnk-20160331xex1010.htm |
| 10-Q - MARCH 31, 2016 10-Q - LUMOS PHARMA, INC. | nlnk-20160331x10q.htm |
[*] Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 10.8
SIXTH AMENDMENT TO LICENSE AGREEMENT
This Sixth Amendment to License Agreement (“Sixth Amendment”) is effective as of March 15, 2016 (the “Sixth Amendment Effective Date”), by and between Augusta University Research Institute, Inc. (formerly known as Georgia Regents Research Institute, Inc. which was formerly known as Georgia Health Sciences University Research Institute, Inc. which was formerly known as Medical College of Georgia Research Institute, Inc.) (“AURI”) and NewLink Genetics Corporation (“NewLink”). AURI and NewLink are sometimes referred to herein individually as a “Party” and collectively as the “Parties.”
WHEREAS, AURI and NewLink are parties to that certain License Agreement dated as of September 13, 2005, and amended on March 28, 2006, April 27, 2006, February 13, 2007, July 12, 2013, and July 10, 2014 (the “Agreement”); and
WHEREAS, the Parties desire to amend the Agreement in accordance with Section 14.8 thereof;
NOW THEREFORE, in consideration of the premises and mutual covenants contained in this Sixth Amendment, the Parties agree as follows:
1. | All references in the Agreement to MCGRI, GHSURI or GRRI are hereby deemed to be references to AURI. |
2. | The following definitions shall be added to Article 1 in the applicable alphabetical order therein: |
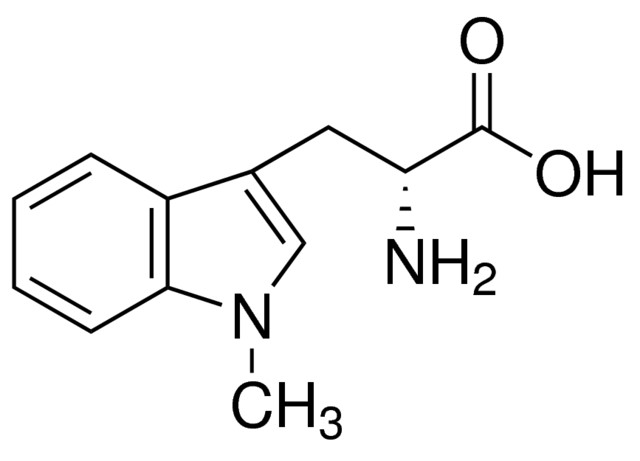
“Indoximod” shall mean the small molecule indoleamine 2,3-dioxygenase (IDO) pathway inhibitor known as indoximod, or 1-methyl-D-tryptophan, having CAS Number 110117-83-4 and the chemical structure as set forth below, or any enantiomer, polymorph, salt form, base, acid, racemate, isomer, tautomer, solvate, or hydrate thereof:
“Indoximod Prodrug” is a medication or compound that is administered in an inactive or less than fully active form, and is intended to be converted to Indoximod [*]. For clarity, Indoximod Prodrug [*].
[*] Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 10.8
3. | The definitions of “Improvement” and “Licensed Product(s)” in Article 1 are hereby deleted and replaced with the following: |
“Improvement” shall mean any invention, that is conceived or reduced to practice in the laboratory of any Inventor (or of his/their collaborators) while employed at Augusta University, that relates to an invention claimed in or covered by the Licensed Patents or which is a modification of the inventions claimed in or covered by the Licensed Patents.
“Licensed Product(s)” shall mean any process, service, or product, the manufacture, use or sale of which is covered by a Valid Claim, or incorporates or uses any Licensed Technology. The Parties acknowledge and agree that, for the purposes of Sections 4.2, 4.3, 4.4 and 4.6, an Indoximod Prodrug shall be deemed to be a Licensed Product with respect to activities in a particular country at a time when there is a Valid Claim in such country that covers Indoximod or the applicable use of Indoximod. For clarity, no payments will be due pursuant to Section 4.2, 4.3 or 4.6 for any activity (including sales made or milestone events achieved) involving an Indoximod Prodrug at a time when there is no Valid Claim in the relevant country that covers Indoximod or the applicable use of Indoximod or such Indoximod Prodrug.
4. | Section 2.2 is hereby deleted and replaced with the following: |
2.2 Sublicensing. Licensee and its Affiliates may sublicense to one or more third parties the rights granted under this Agreement, subject to the prior approval of AURI, not to be unreasonably withheld or delayed, provided, however, that no such prior approval is required for the grant of a sublicense after the Sixth Amendment Effective Date [*]. If this Agreement is terminated for any reason, any such sublicenses granted shall remain in full force and effect and be directly enforceable by AURI. Licensee or an Affiliate shall provide to AURI a copy of any such sublicense and any amendment thereto, including all attachments, exhibits, and/or addendums, within [*] of execution; provided, however, such copies to AURI may be redacted to exclude confidential information of the applicable Sublicensee or of LICENSEE to the extent not relevant to AURI, but such copies shall not be redacted to the extent that it impairs AURI’s ability to ensure compliance with this Agreement.
5. | Section 3.2 is hereby deleted and replaced with the following: |
3.2 For as long as Indoximod is a Licensed Product, Licensee agrees to provide to AURI an annual report regarding Licensee’s (or its Affiliates’ or Sublicensees’) progress in Indoximod development outside of cancer. AURI has the sole right to determine if non-cancer areas are receiving due diligence in Indoximod development in accordance with standards common to the industry, taking into account efficacy, the competitiveness of alternative products in the marketplace, the patent and other proprietary position of Indoximod, the likelihood of regulatory approval given the regulatory structure involved, the profitability of Indoximod and alternative products and all other relevant factors. If Licensee has not met basic product development milestones with respect to Indoximod, and does not remedy that failure within [*] days after written notice from AURI, Licensee’s right and license in Section 2.1 with respect to Indoximod in that area of the Field of Use (specifically, infectious disease
[*] Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 10.8
or diagnostics) will revert from exclusive to non-exclusive for that specific application.
6. | Section 4.2 is hereby deleted and replaced with the following: |
4.2 LICENSEE shall pay AURI [*] of any fees or payments or remuneration paid to LICENSEE or an Affiliate of LICENSEE by a Sublicensee in relation to a Licensed Product for rights to all or part of the Licensed Patents with respect to a Licensed Product, which payments or remuneration are received at a time when there is at least one Valid Claim in such Licensed Patents that covers such Licensed Product in the relevant country, and which payments are other than: research funding (including purchase price of Licensed Products to be used by Sublicensee in connection with research and development activities), equity, loans, or patent costs or fee reimbursements. Such percentage shall decrease [*] for each year of the term of this Agreement in which Licensee expends at least [*] towards the development of Licensed Products, but not to go below a floor of [*]. The Parties acknowledge and agree that, as of the Sixth Amendment Effective Date, [*] is the applicable percentage for payments under this Section 4.2. The Parties also acknowledge and agree that: (a) upon expiration of the last Valid Claim in the Licensed Patents that covers a particular Licensed Product (or Indoximod Prodrug if it is deemed to be a Licensed Product) in a particular country, LICENSEE’s payment obligations pursuant to this Section 4.2 shall expire with respect to such Licensed Product or Indoximod Prodrug in such country; and the license granted pursuant to Section 2.1 with respect to such Licensed Product or Indoximod Prodrug in such country shall become fully-paid, perpetual and irrevocable, subject to AURI’s retained license in Section 2.3 of this Agreement which shall remain unaffected; and (b) no payments are due pursuant to this Section 4.2 with respect to amounts received by Licensee or its Affiliate prior to the Sixth Amendment Effective Date pursuant to the [*] because [*]. For clarity, Licensee shall only become obligated to make payments pursuant to this Section 4.2 with respect to amounts received by Licensee or its Affiliate pursuant to the [*] if [*], such payments are received by Licensee or its Affiliate [*], and such payments meet the criteria set forth in the first sentence of this Section 4.2.
7. | The last sentence of Section 4.3 is hereby deleted and replaced with the following: |
Royalties shall be payable on a Licensed Product-by-Licensed Product and country-by-country basis from first commercial sale of a Licensed Product in a country until the expiration of the last to expire Valid Claim of the Licensed Patents claiming the manufacture, use or sale of such Licensed Product in such country. Upon expiration of such royalty term with respect to a Licensed Product (or Indoximod Prodrug if it is deemed to be a Licensed Product) in a particular country, the license granted pursuant to Section 2.1 with respect to such Licensed Product or Indoximod Prodrug in such country shall become fully-paid, perpetual and irrevocable subject to AURI’s retained license in Section 2.3 of this Agreement which shall remain unaffected.
8. | The following is hereby added to the end of Section 4.6: |
Notwithstanding anything to the contrary in this Section 4.6, to the extent any sublicensing
[*] Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 10.8
fee payable to AURI pursuant to Section 4.2 is based upon a milestone payment with respect to a Licensed Product that is made in connection with an event that is substantially similar to an event requiring the payment of a milestone under this Section 4.6, LICENSEE will pay AURI the greater of: (a) the applicable percentage of such sublicensing fee pursuant to Section 4.2, and (b) the applicable milestone payment under this Section 4.6. For clarity, AURI will be entitled to payment under either Section 4.2 or this Section 4.6, but not both, with respect to any milestone payment received from a Sublicensee.
9. | The following is hereby added to the end of Section 12.1: |
The license granted pursuant to Section 2.1 shall, with respect to the Licensed Technology, survive such expiration and become fully-paid, perpetual and irrevocable subject to AURI’s retained license in Section 2.3 of this Agreement which shall remain unaffected.
10. | Section 12.8 is hereby deleted and replaced with the following: |
12.8 Effect. In the event this Agreement is terminated for any reason whatsoever, LICENSEE shall return, or at AURI’s direction destroy, all plans, drawings, papers, notes, writings and other documents, samples, organisms, biological materials and models pertaining to the Licensed Patents and Licensed Technology, retaining no copies, and shall refrain from using or publishing any portion of the Licensed Patents or Licensed Technology as provided in Article 8 of this Agreement. Upon termination of this Agreement, LICENSEE shall cease manufacturing, processing, producing, using, Selling, or distributing Licensed Products (other than those Licensed Products for which the license granted in Section 2.1 has become fully-paid, perpetual and irrevocable subject to AURI’s retained license in Section 2.3 of this Agreement which shall remain unaffected); provided, however, that LICENSEE may continue to Sell in the ordinary course of business for a period of one (1) year reasonable quantities of Licensed Products which are fully manufactured and in LICENSEE's normal inventory at the date of termination if (a) all monetary obligations of LICENSEE to AURI have been satisfied and (b) royalties on such sales are paid to AURI in the amounts and in the manner provided in this Agreement. The provisions of Articles 9, 10, and 11 of this Agreement shall remain in full force and effect notwithstanding the termination of this Agreement.
11. | Exhibit A to the Agreement is hereby deleted in its entirety and replaced with Exhibit A attached hereto. |
12. | To the best of each Party’s knowledge, as of the Sixth Amendment Effective Date, (a) such Party is not aware of any material noncompliance by Licensee with respect to Licensee’s obligations under this Agreement and (b) such Party is not aware of any fact or circumstance that would permit AURI to terminate the Agreement or to provide a notice to Licensee of AURI’s election to terminate the Agreement. Without limiting the foregoing, the Parties acknowledge and agree that, prior to the Sixth Amendment Effective Date, Licensee fulfilled the obligations set forth in Section 3.1, and Licensee paid in full the initial license fee set forth in Section 4.1, and the milestone payments set forth in Sections 4.6.1.1 and 4.6.1.2 with respect to cancer. AURI acknowledges that as of the Sixth Amendment Effective Date Licensee has not previously provided AURI with annual reports |
[*] Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 10.8
regarding Licensee’s (or its Affiliates’ or Sublicensees’) progress in non-cancer areas of Licensed Product development as set forth in Section 3.2 of the Agreement and AURI hereby waives such obligation of Licensee prior to the Sixth Amendment Effective Date.
13. | In consideration of the modifications to the Agreement as set forth in this Sixth Amendment, NewLink shall pay AURI a milestone payment of [*] within [*] days of the Sixth Amendment Effective Date. |
14. | Except as expressly amended hereby, the terms and conditions of the Agreement shall remain unchanged and in full force and effect. In the event of any conflict between the terms of this Sixth Amendment and the terms of the Agreement, the terms of this Sixth Amendment shall govern. The amendments made herein shall be effective as of the Sixth Amendment Effective Date. Capitalized terms used in this Sixth Amendment that are not otherwise defined herein shall have the same meanings as such terms are given in the Agreement. For clarity, any cross-references to Agreement Sections refer to those Agreement Sections as amended by this Sixth Amendment. This Sixth Amendment may be executed in counterparts, each of which shall be deemed an original but all of which shall be considered one and the same instrument. |
15. |
[Signatures are on next page]
IN WITNESS WHEREOF, the Parties have executed this Sixth Amendment by their duly authorized officers as of the date set forth above.
Augusta University Research Institute, Inc. By:_/s/ Sarah White_____________________ Name:_Sarah White_____________________ Title:_Executive Director_________________ READ AND UNDERSTOOD: By:_/s/ David Munn__________________ Name:David H. Munn, M.D. | NewLink Genetics Corporation By:_/s/ Charles Link____________________ Name:_Charles Link_____________________ Title:_Chief Executive Officer_____________ |
EXHIBIT A
[*] Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 10.8
LICENSED PATENTS
[*]
