Attached files
| file | filename |
|---|---|
| EX-31.2 - EX-31.2 - Mylan II B.V. | d184592dex312.htm |
| EX-31.1 - EX-31.1 - Mylan II B.V. | d184592dex311.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K/A
(Amendment No. 1)
| þ | Annual Report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the Fiscal Year Ended December 31, 2015
OR
| ¨ | Transition Report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from to .
Commission file number 333-199861
MYLAN N.V.
(Exact name of registrant as specified in its charter)
| The Netherlands | 98-1189497 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
Building 4, Trident Place, Mosquito Way, Hatfield, Hertfordshire, AL10 9UL, England
(Address of principal executive offices)
+44 (0) 1707 853 000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class: | Name of Each Exchange on Which Registered: | |
| Ordinary shares, nominal value €0.01 | The NASDAQ Stock Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.:
| Large accelerated filer |
x |
Accelerated filer |
¨ | |||
| Non-accelerated filer |
¨ (Do not check if a smaller reporting company) |
Smaller reporting company |
¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
The aggregate market value of the outstanding ordinary shares, nominal value €0.01, of the registrant other than shares held by persons who may be deemed affiliates of the registrant, as of June 30, 2015, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $33,063,308,366.
The number of ordinary shares outstanding, nominal value €0.01, of the registrant as of April 27, 2016 was 508,342,710.
DOCUMENTS INCORPORATED BY REFERENCE
None.
Table of Contents
EXPLANATORY NOTE
This Amendment No. 1 on Form 10-K/A (this “Amendment”) amends our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, originally filed on February 16, 2016 (the “Original Filing”). We are filing this Amendment to include the information required by Part III and not included in the Original Filing, as we do not intend to file a definitive proxy statement for an annual general meeting of stockholders within 120 days of the end of our fiscal year ended December 31, 2015. In addition, in connection with the filing of this Amendment and pursuant to the rules of the Securities and Exchange Commission (the “SEC”), we are including with this Amendment new certifications of our principal executive officer and principal financial officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. Accordingly, Item 15 of Part IV has also been amended to reflect the filing of these new certifications.
Except as described above, no other changes have been made to the Original Filing. The Original Filing continues to speak as of the date of the Original Filing, and we have not updated the disclosures contained therein to reflect any events which occurred at a date subsequent to the filing of the Original Filing.
As used in this Amendment, unless the context requires otherwise, the “Company,” “Mylan,” “our,” and “we” mean Mylan N.V. and its consolidated subsidiaries, “NASDAQ” means The NASDAQ Stock Market, and “U.S. GAAP” means accounting principles generally accepted in the United States of America.
On February 27, 2015 (the “Closing Date”), Mylan N.V. completed the acquisition (the “EPD Transaction”) of Mylan Inc. and Abbott Laboratories’ (“Abbott”) non-U.S. developed markets specialty and branded generics business (the “EPD Business”). In connection with this transaction, Mylan Inc. and the EPD Business were reorganized under Mylan N.V., a new public company organized in the Netherlands. On February 18, 2015, the Office of Chief Counsel of the Division of Corporation Finance of the Securities and Exchange Commission issued a no-action letter to Mylan Inc. and Mylan N.V. that included its views that the Merger constituted a “succession” for purposes of Rule 12g-3(a) under the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), and that Mylan N.V., as successor to Mylan Inc., is deemed a large accelerated filer for purposes of Exchange Act Rule 12b-2. For purposes of this Amendment, references to the Company for periods prior to the Closing Date refer to Mylan Inc. and its consolidated subsidiaries.
Forward-Looking Statements
This Amendment contains “forward-looking statements.” These statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements may include, without limitation, statements about the proposed acquisition of Meda AB (publ.) (“Meda”) by Mylan (the “Meda Transaction”), Mylan’s related public offer to the shareholders of Meda to acquire all of the outstanding shares of Meda (the “Offer”), the EPD Transaction, the benefits and synergies of the EPD Transaction and the Meda Transaction, future opportunities for Mylan, Meda, or the combined company and products, and any other statements regarding Mylan’s, Meda’s, or the combined company’s future operations, anticipated business levels, future earnings, planned activities, anticipated growth, market opportunities, strategies, competition, and other expectations and targets for future periods. These may often be identified by the use of words such as “will,” “may,” “could,” “should,” “would,” “project,” “believe,” “anticipate,” “expect,” “plan,” “estimate,” “forecast,” “potential,” “intend,” “continue,” “target” and variations of these words or comparable words. Because forward-looking statements inherently involve risks and uncertainties, actual future results may differ materially from those expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to: uncertainties related to the Meda Transaction, including as to the timing of the Meda Transaction, uncertainties as to whether Mylan will be able to complete the Meda Transaction, the possibility that competing offers will be made, the possibility that certain conditions to the completion of the Offer will not be satisfied, and the possibility that Mylan will be unable to obtain regulatory approvals for the Meda Transaction or be required, as a condition to obtaining regulatory approvals, to accept conditions that could reduce the anticipated benefits of the Meda Transaction; the ability to meet expectations regarding the accounting and tax treatments of the EPD Transaction and the Meda Transaction; changes in relevant tax and other laws, including but not limited to changes in the U.S. tax code and
Table of Contents
healthcare and pharmaceutical laws and regulations in the U.S. and abroad; the integration of the EPD Business and Meda being more difficult, time-consuming, or costly than expected; operating costs, customer loss, and business disruption (including, without limitation, difficulties in maintaining relationships with employees, customers, clients, or suppliers) being greater than expected following the EPD Transaction and the Meda Transaction; the retention of certain key employees of the EPD Business and Meda being difficult; the possibility that Mylan may be unable to achieve expected synergies and operating efficiencies in connection with the EPD Transaction and the Meda Transaction within the expected time-frames or at all and to successfully integrate the EPD Business and Meda; expected or targeted future financial and operating performance and results; the capacity to bring new products to market, including but not limited to where Mylan uses its business judgment and decides to manufacture, market, and/or sell products, directly or through third parties, notwithstanding the fact that allegations of patent infringement(s) have not been finally resolved by the courts (i.e., an “at-risk launch”); any regulatory, legal, or other impediments to Mylan’s ability to bring new products to market; success of clinical trials and Mylan’s ability to execute on new product opportunities; any changes in or difficulties with our inventory of, and our ability to manufacture and distribute, the EpiPen® Auto-Injector to meet anticipated demand; the scope, timing, and outcome of any ongoing legal proceedings and the impact of any such proceedings on financial condition, results of operations, and/or cash flows; the ability to protect intellectual property and preserve intellectual property rights; the effect of any changes in customer and supplier relationships and customer purchasing patterns; the ability to attract and retain key personnel; changes in third-party relationships; the impact of competition; changes in the economic and financial conditions of the businesses of Mylan, Meda, or the combined company; the inherent challenges, risks, and costs in identifying, acquiring, and integrating complementary or strategic acquisitions of other companies, products, or assets and in achieving anticipated synergies; uncertainties and matters beyond the control of management; and inherent uncertainties involved in the estimates and judgments used in the preparation of financial statements, and the providing of estimates of financial measures, in accordance with U.S. GAAP and related standards or on an adjusted basis. For more detailed information on the risks and uncertainties associated with Mylan’s business activities, see the risks described in the Original Filing and our other filings with the SEC. These risks and uncertainties also include those risks and uncertainties that are discussed in the offer document that has been filed with the Swedish Financial Supervisory Authority (“SFSA”) and will be published by Mylan upon approval by the SFSA (the “Offer Document”), the Registration Statement on Form S-4 filed with the SEC on April 11, 2016 (as amended from time to time, the “Registration Statement”) and the EU Prospectus that has been filed with the Netherlands Authority for the Financial Markets (“AFM”) and will be published by Mylan upon approval by the AFM (the “EU Prospectus”). You can access Mylan’s filings with the SEC through the SEC website at www.sec.gov, and Mylan strongly encourages you to do so. Mylan undertakes no obligation to update any statements herein for revisions or changes after the filing date of this Amendment.
Additional Information
In connection with the Offer, the Offer Document has been filed with the SFSA and will be published by Mylan upon approval by the SFSA. In addition, Mylan has filed certain materials with the SEC, including, among other materials, the Registration Statement. The EU Prospectus has been filed with the AFM and will be published by Mylan upon approval by the AFM. This Amendment is not intended to be, and is not, a substitute for such documents or for any other document that Mylan may file with the SFSA, the SEC, the AFM or any other competent EU authority in connection with the Offer. INVESTORS AND SECURITYHOLDERS OF MEDA ARE URGED TO READ ANY DOCUMENTS FILED WITH THE SFSA, THE SEC AND THE AFM OR ANY OTHER COMPETENT EU AUTHORITY CAREFULLY AND IN THEIR ENTIRETY BEFORE MAKING AN INVESTMENT DECISION BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT MYLAN, MEDA AND THE OFFER. Such documents are or upon publication will be available free of charge through the website maintained by the SEC at www.sec.gov, on Mylan’s website at medatransaction.mylan.com or, to the extent filed with the AFM, through the website maintained by the AFM at www.afm.nl, or by directing a request to Mylan at +1 724-514-1813 or investor.relations@mylan.com. Any materials filed by Mylan with the SFSA, the SEC, the AFM or any other competent EU authority that are required to be mailed to Meda shareholders will also be mailed to such shareholders.
Table of Contents
Reconciliation of Non-GAAP Financial Measures
This Amendment includes the presentation and discussion of certain financial information that differs from what is reported under U.S. GAAP. These non-GAAP financial measures, including, but not limited to, adjusted diluted earnings per share, adjusted EBITDA, adjusted free cash flow, total adjusted revenues, constant currency third party net sales, constant currency adjusted third party net sales, adjusted third party net sales, cash return on invested capital excluding goodwill, cash return on operating invested capital, and cash return on invested capital are presented in order to supplement investors’ and other readers’ understanding and assessment of Mylan’s financial performance. Management uses these measures internally for forecasting, budgeting, measuring its operating performance, and incentive-based awards. In addition, primarily due to acquisitions, Mylan believes that an evaluation of its ongoing operations (and comparisons of its current operations with historical and future operations) would be difficult if the disclosure of its financial results were limited to financial measures prepared only in accordance with U.S. GAAP. In addition, Mylan believes that including EBITDA and supplemental adjustments applied in presenting adjusted EBITDA pursuant to our debt agreements is appropriate to provide additional information to investors to demonstrate Mylan’s ability to comply with financial debt covenants (which are calculated using a measure similar to adjusted EBITDA) and assess Mylan’s ability to incur additional indebtedness. We also report sales performance using the non-GAAP financial measure of “constant currency” sales and adjusted sales. This measure provides information on the change in net sales assuming that foreign currency exchange rates had not changed between the prior and current period. The comparisons presented as constant currency rates reflect comparative local currency sales at the prior year’s foreign exchange rates. We routinely evaluate our third party net sales performance at constant currency so that sales results can be viewed without the impact of foreign currency exchange rates, thereby facilitating a period-to-period comparison of our operational activities, and we believe that this presentation also provides useful information to investors for the same reason. Appendix A to this Amendment contains reconciliations of such non-GAAP financial measures to the most directly comparable U.S. GAAP financial measures. Investors and other readers are encouraged to review the related U.S. GAAP financial measures and the reconciliations of the non-GAAP measures to their most directly comparable U.S. GAAP measures set forth in Appendix A, and investors and other readers should consider non-GAAP measures only as supplements to, not as substitutes for or as superior measures to, the measures of financial performance prepared in accordance with U.S. GAAP.
Table of Contents
MYLAN N.V.
For the Year Ended December 31, 2015
| Page | ||||||
| PART III | ||||||
| ITEM 10. |
1 | |||||
| ITEM 11. |
Executive Compensation | 12 | ||||
| ITEM 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 51 | ||||
| ITEM 13. |
Certain Relationships and Related Transactions, and Director Independence | 54 | ||||
| ITEM 14. |
Principal Accounting Fees and Services | 56 | ||||
| PART IV | ||||||
| ITEM 15. |
Exhibits | 57 | ||||
| 58 | ||||||
| Appendix A — Reconciliation of Non-GAAP Measures (Unaudited) |
||||||
Table of Contents
PART III
| ITEM 10. | Directors, Executive Officers and Corporate Governance |
Executive Officers
Certain information concerning our Code of Ethics that applies to our Principal Executive Officer, Principal Financial Officer and Corporate Controller is contained in Item 10 of Part III of the Original Filing and is also included below.
The names, ages, and positions of Mylan’s executive officers as of April 29, 2016, are as follows:
| Heather Bresch |
46 |
Chief Executive Officer (principal executive officer) | ||
| Rajiv Malik |
55 |
President | ||
| Anthony Mauro |
43 |
Chief Commercial Officer | ||
| Robert J. Coury |
55 |
Executive Chairman |
Each executive officer listed above was an executive officer of Mylan Inc. on the Closing Date and became an executive officer of Mylan N.V. on such date in connection with the EPD Transaction.
Ms. Bresch and Messrs. Coury and Malik are all also members of Mylan’s Board of Directors (the “Mylan Board”) and a discussion of their respective business experience and other relevant biographical information is provided under “Mylan Board” below.
Mr. Mauro has served as Chief Commercial Officer since January 4, 2016. Prior to that date, Mr. Mauro served as President, North America of Mylan since January 1, 2012. He served as President of Mylan Pharmaceuticals Inc. from 2009 through February 2013. In his 20 years at Mylan, Mr. Mauro has held roles of increasing responsibility, including Chief Operating Officer for Mylan Pharmaceuticals ULC in Canada and Vice President of Strategic Development, North America, and Vice President of Sales, North America for Mylan.
Pursuant to Mylan’s Rules for the Board of Directors of Mylan N.V. (the “Board Rules”), the Mylan Board appoints the Chief Executive Officer and may appoint, or delegate authority to the Chairman or the Chief Executive Officer to appoint, a President, a Chief Financial Officer, a Chief Legal Officer, a Secretary, and any other officers of Mylan as the Mylan Board, the Chairman, or the Chief Executive Officer may desire. Each officer appointed by the Mylan Board, or appointed by the Chairman or the Chief Executive Officer, holds office until his or her successor shall have been appointed, or until his or her death, resignation, or removal. Officers of Mylan who are appointed by the Mylan Board can be removed by the Mylan Board, and the Mylan Board may delegate to the Chairman or the Chief Executive Officer the right to remove any officer the Chairman or the Chief Executive Officer has appointed (but not any officer directly appointed by the Mylan Board). A copy of the Board Rules is available on Mylan’s website at http://www.mylan.com/company/corporate-governance or in print to shareholders upon request, addressed to Mylan N.V.’s Corporate Secretary at Building 4, Trident Place, Mosquito Way, Hatfield, Hertfordshire, AL10 9UL, England.
Mylan Board
The Mylan Board consists of 13 directors, each of whom is either an executive director or a non-executive director pursuant to applicable Dutch law. Executive directors are responsible for the daily management and operation of the Company and non-executive directors are responsible for overseeing and monitoring the performance of the executive directors. Currently, Ms. Bresch and Mr. Malik are executive directors while the other directors listed below are non-executive directors. Consistent with established Dutch law and the Company’s articles of association, executive directors and non-executive directors are appointed by the general meeting from a binding nomination proposed by the Mylan Board. If appointed, each director’s term begins at the general meeting at which he or she is appointed and, unless such director resigns or is suspended or dismissed at an earlier date, his or her term of office lapses immediately after the next annual general meeting held after his or her appointment.
1
Table of Contents
| Name |
Age# | Other Positions with Mylan and Principal Occupation |
Has | |||
| Heather Bresch^ |
46 |
Chief Executive Officer |
2011 | |||
| Wendy Cameron |
56 |
Director and Co-Owner, Cam Land LLC |
2002 | |||
| Hon. Robert J. Cindrich |
72 |
President, Cindrich Consulting; Counsel, Schnader |
||||
| Harrison Segal & Lewis |
2011 | |||||
| Robert J. Coury |
55 |
Executive Chairman |
2002 | |||
| JoEllen Lyons Dillon |
52 |
Executive Vice President, Chief Legal Officer, and |
||||
| Corporate Secretary, The ExOne Company |
2014 | |||||
| Neil Dimick, C.P.A.* |
66 |
Retired Executive Vice President and Chief Financial |
||||
| Officer, AmerisourceBergen Corporation |
2005 | |||||
| Melina Higgins |
48 |
Retired Partner and Managing Director, Goldman |
||||
| Sachs |
2013 | |||||
| Douglas J. Leech, C.P.A.* |
61 |
Founder and Principal, DLJ Advisors |
2000 | |||
| Rajiv Malik^ |
55 |
President |
2013 | |||
| Joseph C. Maroon, M.D. |
75 |
Professor, Heindl Scholar in Neuroscience, and Vice Chairman of the Department of Neurosurgery for the University of Pittsburgh Medical Center; Neurosurgeon for the Pittsburgh Steelers |
2003 | |||
| Mark W. Parrish |
60 |
Chairman and Chief Executive Officer, Trident USA |
||||
| Health Services |
2009 | |||||
| Rodney L. Piatt, C.P.A.* |
63 |
Lead Independent Director and Vice Chairman; President and Owner, Horizon Properties Group, |
||||
| LLC; CEO, Lincoln Manufacturing Inc. |
2004 | |||||
| Randall L. (Pete) Vanderveen, Ph.D., R.Ph. |
65 |
Professor of Pharmaceutical Policy and Economics, Senior Adviser to the Leonard D. Schaeffer Center of Health Policy and Economics, Director of the Margaret and John Biles Center for Leadership, and Senior Adviser to the Dean for Advancement, School of Pharmacy, University of Southern California |
2002 |
| ^ | Refers to an executive director. All other directors listed above are non-executive directors. |
| * | C.P.A. distinctions refer to “inactive” status. |
| # | Ages as of April 29, 2016 |
| ** | Includes service as director of Mylan Inc. and Mylan N.V. Each director listed above was a director of Mylan Inc. on the Closing Date and became a director of Mylan N.V. on such date in connection with the EPD Transaction. |
Heather Bresch. Ms. Bresch has served as Mylan’s Chief Executive Officer (“CEO”) since January 1, 2012. Throughout her 24-year career with Mylan, Ms. Bresch has held roles of increasing responsibility in more than 15 functional areas. Prior to becoming CEO, Ms. Bresch was Mylan’s President commencing in July 2009 and was responsible for the day-to-day operations of the Company. Before that, she served as Mylan’s Chief Operating Officer and Chief Integration Officer from October 2007 to July 2009, leading the successful integration of two transformational international acquisitions – Matrix Laboratories Limited (n/k/a Mylan Laboratories Limited) and Merck KGaA’s generics and specialty pharmaceuticals businesses. Under Ms. Bresch’s leadership, Mylan has continued to expand its portfolio and geographic reach, acquiring Abbott Laboratories’ non-U.S. developed markets specialty and branded generics business, the female healthcare business of Famy Care Ltd., and India-based Agila Specialties, a global leader in injectable products and an innovative respiratory technology platform; partnering on portfolios of biologic and insulin products; entering new commercial markets such as India and Brazil; and expanding its leadership in the treatment of HIV/AIDS
2
Table of Contents
through the distribution of novel testing devices. During her career, Ms. Bresch has championed initiatives aimed at improving product quality and removing barriers to patient access to medicine. Ms. Bresch’s qualifications to serve on the Mylan Board include, among others, her extensive industry, policy, and leadership experience and abilities, as well as her strategic vision, judgment and unique and in-depth knowledge about the Company.
Wendy Cameron. Ms. Cameron has served as Co-Owner and Director of Cam Land LLC, a harness racing business in Washington, Pennsylvania, since January 2003. From 1981 to 1998, she was Vice President, Divisional Sales & Governmental Affairs, Cameron Coca-Cola Bottling Company, Inc. Ms. Cameron served as Chairman of the Washington Hospital Board of Trustees and of the Washington Hospital Executive Committee until she stepped down in 2012. She was a member of the hospital’s Board of Trustees from 1997 through 2012 and a member of the Washington Hospital Foundation Board from 1993 through 2012. In addition to being a business owner and having held an executive position with one of the nation’s largest bottlers for nearly 20 years, Ms. Cameron has invaluable experience and knowledge regarding the business, platforms, strategies, challenges, opportunities, and management of the Company, among other matters. Ms. Cameron’s qualifications to serve on the Mylan Board include, among others, this experience, as well as her independence, business experience, leadership, and judgment.
Hon. Robert J. Cindrich. Since February 2011, Judge Cindrich has been serving as the President of Cindrich Consulting, LLC, a business and healthcare consulting company that advises clients on corporate governance, compliance, and business strategies, and from October 1, 2013 through January 31, 2014 he served as Interim General Counsel for United States Steel Corporation (NYSE: X). Judge Cindrich joined Schnader Harrison Segal & Lewis (“Schnader”), a law firm, as legal counsel in April 2013 and took a temporary leave of absence on October 1, 2013 to join United States Steel as Interim General Counsel, returning to Schnader after his time at United States Steel. In May 2012, he joined the Board of Directors of Allscripts Healthcare Solutions, Inc. (NASDAQ: MDRX), which provides healthcare information technology solutions, where he served until April 2015. From 2011 through 2012, Judge Cindrich served as a senior advisor to the Office of the President of the University of Pittsburgh Medical Center (“UPMC”), an integrated global health enterprise. From 2004 through 2010, Judge Cindrich was a Senior Vice President and the Chief Legal Officer of UPMC. From 1994 through January 2004, Judge Cindrich served as a judge on the United States District Court for the Western District of Pennsylvania. Prior to that appointment, he was active as an attorney in both government and private practice, including positions as the U.S. Attorney for the Western District of Pennsylvania and as the Allegheny County Assistant Public Defender and Assistant District Attorney. Judge Cindrich’s qualifications to serve on the Mylan Board include, among others, his extensive legal and leadership experience and judgment, as well as his independence, and in-depth knowledge of the healthcare industry.
Robert J. Coury. Robert J. Coury has been the Executive Chairman of Mylan and the Mylan Board since January 2012. Under his visionary leadership, Mylan transformed from the third largest generics pharmaceutical company in the U.S. into one of the largest pharmaceutical companies in the world, earning spots in both the S&P 500 and, prior to the Company’s reincorporation outside of the U.S. in 2015, the Fortune 500. Mr. Coury was first elected to the Mylan Board in February 2002, having served since 1995 as a strategic advisor to the Company. He became the Mylan Board’s Vice Chairman shortly after his election and served as CEO of the Company from September 2002 until January 2012.
Since 2007, Mr. Coury has led the Company through a series of transactions totaling approximately $15 billion, which transformed the Company into a global powerhouse within the highly competitive pharmaceutical industry. In 2007, Mylan purchased India-based Matrix Laboratories, a major producer of active pharmaceutical ingredients, and the generics business of Europe-based Merck KGaA. Subsequent acquisitions under Mr. Coury’s leadership further expanded the Company into new therapeutic categories and greatly enhanced its geographic and commercial footprint. For instance, in 2010, Mylan acquired Bioniche Pharma, an injectables business in Ireland, and in 2012, Mylan acquired India-based Agila Specialties, a global injectables company. Most recently, the Company completed its acquisition of Abbott Laboratories’ non-U.S. developed markets specialty and branded generics business.
3
Table of Contents
As a result of this period of expansion, the Company now has in place a high quality foundation supporting Mylan’s mission of providing the world’s 7 billion people with access to high quality medicine.
Before assuming his current role in 2012, Mr. Coury also executed a successful executive leadership transition after cultivating and developing a powerful leadership team. Grooming executive talent from within and recruiting dynamic leaders from outside the Company were both key components of the Company’s past, current and future growth strategies.
Prior to Mylan, Mr. Coury was the principal of Coury Consulting, a boutique business advisory firm he formed in 1989, and The Coury Financial Group, a successful financial and estate planning firm, which he founded in 1984. Mr. Coury earned a Bachelor of Science degree in industrial engineering from the University of Pittsburgh. He has served as a member of the University of Southern California President’s Leadership Council since 2014.
Mr. Coury’s qualifications to serve on the Mylan Board include, among others, his prior business experience, his in-depth knowledge of the industry, the Company, its businesses, and management, and his leadership experience as the Company’s CEO, as well as his judgment, strategic vision, and service and leadership as Vice Chairman and then Chairman of the Mylan Board for more than ten years – the most transformational and successful time in the Company’s history.
JoEllen Lyons Dillon. Ms. Dillon has served as Chief Legal Officer and Corporate Secretary of The ExOne Company (NASDAQ: XONE), a global provider of three-dimensional printing machines, since March 2013, and as Executive Vice President since December 2014. Previously, she was a legal consultant on ExOne’s initial public offering. Prior to that experience, Ms. Dillon was a partner with Reed Smith LLP, a law firm, from 2002 until 2011. She had previously been at the law firm Buchanan Ingersoll & Rooney PC from 1988 until 2002, where she became a partner in 1997. Ms. Dillon is the former Chair, and currently serves as the Audit Committee Chair of, the Allegheny District chapter of the National Multiple Sclerosis Society. Ms. Dillon’s qualifications to serve on the Mylan Board include, among others, this experience, as well as her independence, judgment, and substantial legal and leadership experience.
Neil Dimick, C.P.A.* Currently retired, Mr. Dimick previously served as Executive Vice President and Chief Financial Officer of AmerisourceBergen Corporation (NYSE: ABC), a wholesale distributor of pharmaceuticals, from 2001 to 2002. From 1992 to 2001, he was Senior Executive Vice President and Chief Financial Officer of Bergen Brunswig Corporation, a wholesale drug distributor. Prior to that experience, Mr. Dimick served as a partner with Deloitte & Touche LLP (“Deloitte”) for eight years. Mr. Dimick also serves on the Boards of Directors of WebMD Health Corp. (NASDAQ: WBMD), Alliance HealthCare Services, Inc. (NASDAQ: AIQ), and Resources Connection, Inc. (NASDAQ: RECN). Mr. Dimick also served on the Boards of Directors of Thoratec Corporation from 2003 to October 2015, at which time it was purchased by St. Jude Medical, Inc., and HLTH Corporation from 2002 to 2009, at which time it was merged into WebMD Health Corp. Mr. Dimick has invaluable experience and knowledge regarding the business, platforms, strategies, challenges, opportunities, and management of the Company, among other matters. Mr. Dimick’s qualifications to serve on the Mylan Board include, among others, this experience, as well as his independence, substantial industry experience, judgment, business and accounting background, and judgment.
Melina Higgins. Currently retired, Ms. Higgins held senior roles of increasing responsibility at The Goldman Sachs Group, Inc. (NYSE: GS), including Partner and Managing Director, during her nearly 20-year career at the firm from 1989 to 1992 and 1994 to 2010. During her tenure at Goldman Sachs, Ms. Higgins served as a member of the Investment Committee of the Principal Investment Area, which oversaw and approved global private equity and private debt investments and was one of the largest alternative asset managers in the world. She also served as head of the Americas and as co-chairperson of the Investment Advisory Committee for the GS Mezzanine Partners funds, which managed over $30 billion of assets and were global leaders in their industry. Ms. Higgins also serves on the Women’s Leadership Board of Harvard University’s John F. Kennedy School of
4
Table of Contents
Government. In September 2013, Ms. Higgins joined the Board of Directors of Genworth Financial Inc. (NYSE: GNW), an insurance company. In January 2016, Ms. Higgins became non-executive Chairman of Antares Midco Inc., a private company that provides financing solutions for middle-market, private equity-backed transactions. Ms. Higgins’ qualifications to serve on the Mylan Board include, among others, her independence, broad experience in finance, and judgment.
Douglas J. Leech, C.P.A.* Mr. Leech is the founder and principal of DLJ Advisors. From 1999 to 2011, he was Founder, Chairman, President and Chief Executive Officer of Centra Bank, Inc. and Centra Financial Holdings, Inc., prior to which he was Chief Executive Officer, President of the southeast region, and Chief Operating Officer of Huntington National Bank. Mr. Leech also served on the Board of Directors of United Bankshares, Inc. (NASDAQ: UBSI) from 2011 to 2015. Mr. Leech’s public accounting, audit, and professional experience has provided him financial and business expertise and leadership experience. In addition, Mr. Leech has invaluable experience and knowledge regarding the business, platforms, strategies, challenges, opportunities, and management of the Company, among other matters. Mr. Leech’s qualifications to serve on the Mylan Board include, among others, this experience, as well as his independence, years of business experience, and judgment.
Rajiv Malik. Mr. Malik has served as Mylan’s President since January 1, 2012. Previously, Mr. Malik held various senior roles at Mylan, including Executive Vice President and Chief Operating Officer from July 2009 to December 2012, and Head of Global Technical Operations from January 2007 to July 2009. In addition to his oversight of day-to-day operations of the Company as President, Mr. Malik has been instrumental in identifying, evaluating, and executing on significant business development opportunities, expanding and optimizing Mylan’s product portfolio, and leveraging Mylan’s global research and development capabilities, among other important contributions. Previously, he served as Chief Executive Officer of Matrix Laboratories Limited (n/k/a Mylan Laboratories Limited) from July 2005 to June 2008. Prior to joining Matrix, he served as Head of Global Development and Registrations for Sandoz GmbH from September 2003 to July 2005. Prior to joining Sandoz, Mr. Malik was Head of Global Regulatory Affairs and Head of Pharma Research for Ranbaxy from October 1999 to September 2003. Mr. Malik’s qualifications to serve on the Mylan Board include, among others, his extensive industry and leadership experience, his understanding of the Asia-Pacific region and other growth markets, and his knowledge about the Company and judgment.
Joseph C. Maroon, M.D. Dr. Maroon is Professor, Heindl Scholar in Neuroscience and Vice Chairman of the Department of Neurosurgery, UPMC, and has held other positions at UPMC since 1998. He also has served as the team neurosurgeon for the Pittsburgh Steelers since 1981. From 1995 to 1998, Dr. Maroon was Professor and Chairman of the Department of Surgery at Allegheny General Hospital, and from 1984 to 1999 he was Professor and Chairman of the Department of Neurosurgery at Allegheny General Hospital. Dr. Maroon has earned numerous awards for his contributions to neurosurgery from various national and international neurological societies throughout his career, and patients travel from all over the world to seek his care. In addition, Dr. Maroon has invaluable experience and knowledge regarding the business, platforms, strategies, challenges, opportunities, and management of the Company, among other matters. Dr. Maroon’s qualifications to serve on the Mylan Board include, among others, this experience, as well as his independence, exceptional medical and leadership experience, and judgment.
Mark W. Parrish. Mr. Parrish has served as Chairman and Chief Executive Officer of TridentUSA Health Services, a provider of mobile X-ray and laboratory services to the long-term care industry, since 2008. Since January 2013, Mr. Parrish has also served on the Board of Directors of Omnicell, Inc. (NASDAQ: OMCL), a company that specializes in healthcare technology. Mr. Parrish also serves on the Boards of Directors of Silvergate Pharmaceuticals, a private company that develops and commercializes pediatric medications, and GSMS, a private company that specializes in meeting unique labeling and sizing needs for its customers and pharmaceutical packaging, serialization, and distribution. From 2001 to 2007, Mr. Parrish held management roles of increasing responsibility with Cardinal Health Inc. (NYSE: CAH) and its affiliates, including Chief Executive Officer of Healthcare Supply Chain Services for Cardinal Health from 2006 to 2007. Mr. Parrish also serves as President of the International Federation of Pharmaceutical Wholesalers, an association of pharmaceutical
5
Table of Contents
wholesalers and pharmaceutical supply chain service companies, and senior adviser to Frazier Healthcare Ventures, a healthcare oriented growth equity firm. Mr. Parrish’s qualifications to serve on the Mylan Board include, among others, his independence, extensive industry, business, and leadership experience, knowledge of the healthcare industry, and judgment.
Rodney L. Piatt, C.P.A.* Mr. Piatt is the Lead Independent Director and has served as Vice Chairman of the Mylan Board since May 2009. Since 1996, he has also been President and owner of Horizon Properties Group, LLC, a real estate and development company. Since 2003, Mr. Piatt has also served as Chief Executive Officer and Director of Lincoln Manufacturing Inc., a steel and coal manufacturing company. Mr. Piatt is also on the Board of Directors of AccuTrex Products, Inc., a private company that manufactures a wide range of custom products for diverse and demanding industries throughout the world. Mr. Piatt brings extensive experience to the Mylan Board as an auditor and a successful business owner. In addition, Mr. Piatt has invaluable experience and knowledge regarding the business, platforms, strategies, challenges, opportunities, and management of the Company, among other matters. Mr. Piatt’s qualifications to serve on the Mylan Board include, among others, this experience, as well as his independence, financial and business expertise, leadership experience, and judgment.
Randall L. (Pete) Vanderveen, Ph.D., R.Ph. Dr. Vanderveen is currently Professor of Pharmaceutical Policy and Economics, Senior Adviser to the Leonard D. Schaeffer Center of Health Policy and Economics, Director of the Margaret and John Biles Center for Leadership, and Senior Adviser to the Dean for Advancement at the School of Pharmacy, University of Southern California in Los Angeles, California. Dr. Vanderveen previously served as Dean, Professor and John Stauffer Decanal Chair of the USC School of Pharmacy from 2005 to 2015 where he was named “Outstanding Pharmacy Dean in the Nation” in 2013 by the American Pharmacist Association. From 1998 to 2005, he served as Dean and Professor of Pharmacy of the School of Pharmacy and the Graduate School of Pharmaceutical Sciences at Duquesne University, before which he was Assistant Dean at Oregon State University from 1988 to 1998. Dr. Vanderveen has an extensive pharmaceutical and academic background. In addition, Dr. Vanderveen has invaluable experience and knowledge regarding the business, platforms, strategies, challenges, opportunities, and management of the Company, among other matters. Dr. Vanderveen’s qualifications to serve on the Mylan Board include, among others, this experience, as well as his independence, pharmaceutical and leadership experience, and judgment.
| * | C.P.A. distinctions refer to “inactive” status. |
Meetings of the Mylan Board
The Mylan Board met seven times in 2015, including three meetings of Mylan N.V. (after the Closing Date) and four meetings of Mylan Inc. (prior to the Closing Date). In addition to meetings of the Mylan Board, directors attended meetings of individual Mylan Board committees of which they were members. Each of the directors attended at least 75% of the Mylan Board meetings and meetings of Mylan Board committees of which they were a member during the periods for which they served. Twelve members of the Mylan Board attended Mylan’s extraordinary general meeting on August 28, 2015, which constituted the Company’s 2015 annual meeting of shareholders for the purposes of compliance with NASDAQ listing standards.
Non-management members of the Mylan Board met in executive session from time to time during 2015. As noted, Rodney L. Piatt, the Vice Chairman of the Mylan Board, is the Lead Independent Director and presides at such executive sessions.
Mylan Board Committees
The standing committees of the Mylan Board include the Audit Committee, the Compensation Committee, the Compliance Committee, the Executive Committee, the Finance Committee, the Governance and Nominating Committee, and the Science and Technology Committee. Each committee operates pursuant to a written charter.
6
Table of Contents
The table below provides the current membership and 2015 meeting information for the noted Mylan Board committees of Mylan. The 2015 meeting information includes meetings of Mylan N.V. (after the Closing Date) and meetings of Mylan Inc. (prior to the Closing Date).
| Director |
Audit | Compensation | Compliance | Executive | Finance | Governance and Nominating |
Science and Technology | |||||||
| Heather Bresch |
X | |||||||||||||
| Wendy Cameron (1) |
C | X | ||||||||||||
| Hon. Robert J. Cindrich |
X | X | X | |||||||||||
| Robert J. Coury |
C | |||||||||||||
| JoEllen Lyons Dillon |
X | |||||||||||||
| Neil Dimick |
C | X | X | X | ||||||||||
| Melina Higgins |
X | C | ||||||||||||
| Douglas J. Leech |
X | X | C | |||||||||||
| Rajiv Malik |
X | |||||||||||||
| Joseph C. Maroon, M.D. |
X | X | C | |||||||||||
| Mark W. Parrish |
X | C | X | |||||||||||
| Rodney L. Piatt (1) |
X | X | X | X | ||||||||||
| Randall L. (Pete) Vanderveen, Ph.D., R.Ph. | X | X | ||||||||||||
| Meetings during 2015 |
5 | 7 | 4 | 7 | 2 | 3 | 1 |
| (1) | Mr. Piatt served as the Chair of the Compensation Committee until October 27, 2015, at which time Ms. Cameron was appointed Chair of the Compensation Committee. |
C = Chair
X = Member
Copies of the committee charters of Mylan are available on Mylan’s website at http://www.mylan.com/company/corporate-governance or in print to shareholders upon request, addressed to Mylan N.V.’s Corporate Secretary at Building 4, Trident Place, Mosquito Way, Hatfield, Hertfordshire, AL10 9UL, England.
Audit Committee and Audit Committee Financial Expert
The Audit Committee’s responsibilities include, among others: the appointment (other than the independent auditor of annual accounts prepared in accordance with Dutch law), compensation, retention, oversight, and replacement of the Company’s independent registered public accounting firm; approving the scope, procedures and fees for the proposed audit for the current year and reviewing the scope, conduct and findings of any financial or internal control-related audit performed by the independent registered public accounting firm; reviewing the organization, responsibilities, plans and resources of the internal audit function; reviewing with management both the Company’s financial statements and management’s assessment of the Company’s internal control over financial reporting; reviewing, including reviewing and discussing with management (including the Company’s internal audit function) and the independent registered public accounting firm, as appropriate, the Company’s processes and procedures with respect to risk assessment and risk management; and reviewing, approving, ratifying or rejecting “transactions” between the Company and “related persons” (each as defined in Item 404 of Regulation S-K). All of the members of the Audit Committee are independent directors, as required by and as defined in the audit committee independence standards of the SEC and the applicable NASDAQ listing standards. The Mylan Board has determined that each of the Audit Committee members — Mr. Dimick, Ms. Higgins, Mr. Leech, and Mr. Piatt — is an “audit committee financial expert,” as that term is defined in the rules of the SEC. The Mylan Board has also approved Mr. Dimick’s concurrent service on the audit committees of more than two other public companies.
7
Table of Contents
Compensation Committee
The Compensation Committee’s responsibilities include, among others: reviewing and recommending to the non-executive, independent (in accordance with the NASDAQ listing standards) members of the Mylan Board corporate goals and objectives relevant to the Executive Chairman’s, CEO’s, and other executive directors’ compensation, evaluating such individual’s performance, and determining (with respect to the CEO’s and other executive directors’ compensation) and providing recommendations to the non-executive, independent members of the Mylan Board with respect to such individual’s compensation based on these evaluations. In making such recommendations, the Compensation Committee may consider pay for performance, alignment with long-term shareholder interests, promotion of Company strategic goals, maintenance of the appropriate level of fixed and at-risk compensation, remaining competitive with companies within the Company’s peer group, internal pay equity, an executive’s leadership and mentoring skills and contributions, talent management, the executive’s contributions to establishment or execution of corporate strategy, retention, and recognition of individual performance and contributions, and/or any other factors determined by the Mylan Board or the Compensation Committee to be in the interests of the Company. The Compensation Committee also exercises oversight of, and provides recommendations to the Mylan Board as appropriate regarding, the compensation of the other executive officers of the Company and applicable compensation programs and incentive compensation plans, as well as the compensation of independent directors. All of the members of the Compensation Committee are independent directors as defined in the applicable NASDAQ listing standards.
Compliance Committee
The Compliance Committee oversees the Chief Compliance Officer’s implementation of the Company’s Corporate Compliance Program and, as appropriate, makes recommendations to the Mylan Board with respect to the formulation or re-formulation of, and the implementation, maintenance, and monitoring of, the Company’s Corporate Compliance Program and Code of Business Conduct and Ethics as may be modified, supplemented or replaced from time to time, designed to support and promote compliance with corporate policies and legal rules and regulations. All of the members of the Compliance Committee are independent directors as defined in the NASDAQ listing standards.
Executive Committee
The Executive Committee exercises those powers of the Mylan Board not otherwise limited by a resolution of the Mylan Board or by law.
Finance Committee
The Finance Committee advises the Mylan Board with respect to, and by discharging the duties and responsibilities delegated to it by the Mylan Board in respect of, material financial matters and transactions of the Company including, but not limited to: reviewing and overseeing material mergers, acquisitions, and combinations with other companies; swaps and other derivatives transactions; the establishment of credit facilities; potential financings with commercial lenders; and the issuance and repurchase of the Company’s debt, equity, hybrid or other securities. All of the members of the Finance Committee are independent directors as defined in the applicable NASDAQ listing standards.
Governance and Nominating Committee
The Governance and Nominating Committee advises the Mylan Board with respect to corporate governance matters as well as the nomination or re-nomination of director candidates and its responsibilities also include overseeing both the Mylan Board’s review and consideration of shareholder recommendations for director candidates and the Mylan Board’s annual self-evaluation. Additionally, the Governance and Nominating Committee oversees director orientation and Mylan Board continuing education programs and makes
8
Table of Contents
recommendations to the Mylan Board with respect to the annual evaluation of independence of each director and, as needed, the appointment of directors to committees of the Mylan Board and the appointment of a chair of each committee. All of the members of the Governance and Nominating Committee are independent directors as defined in the applicable NASDAQ listing standards.
Science and Technology Committee
The Science and Technology Committee serves as a sounding board as requested by management and, at the Mylan Board’s request, reviews the Company’s research and development strategy and portfolio from time to time from a scientific and technological perspective.
Consideration of Director Nominees
Consistent with established Dutch law and the Company’s articles of association, executive directors and non-executive directors are appointed by the general meeting from a binding nomination proposed by the Mylan Board. The proposed candidate specified in a binding nomination shall be appointed provided that the requisite quorum is present or represented at the annual general meeting, unless the nomination is overruled by the general meeting voting against the appointment of the candidate by a resolution adopted with a majority of at least two-thirds of the votes cast, representing more than half of the issued share capital. In such event, the Mylan Board may propose a new binding nomination to be submitted at a subsequent general meeting.
The Governance and Nominating Committee will consider for nomination by the Mylan Board potential director candidates properly recommended by shareholders, subject to the discretion of the Mylan Board and to Mylan’s articles of association. In considering candidates recommended by shareholders, the Governance and Nominating Committee will take into consideration, among other matters, the needs of the Mylan Board and Mylan and the qualifications of the candidate, including, among other things, those traits, abilities, and experiences set forth in Mylan’s Corporate Governance Principles. Any submission to the Governance and Nominating Committee of a recommended candidate for consideration must include, among other information, the name of the recommending shareholder and evidence of such person’s ownership of Mylan shares, and the name of the recommended candidate, his or her resume or a statement of his or her principal occupation or employment, and the recommended candidate’s signed consent to be named as a director if recommended by the Governance and Nominating Committee and nominated by the Mylan Board. Any shareholder recommendations for director must be sent to Mylan’s Corporate Secretary at Building 4, Trident Place, Mosquito Way, Hatfield, Hertfordshire, AL10 9UL, England, not later than 120 days prior to the anniversary date of Mylan’s most recent annual general meeting of shareholders.
Board Education
From time-to-time, the Mylan Board or individual Mylan Board members participate in director educational programs.
Mylan Board Leadership Structure
The Mylan Board elects one of its own members as the Chairman of the Mylan Board. Mr. Coury has served as the Chairman of the Board of Mylan Inc. and now Mylan N.V. since being elected in May 2009. Based on significant interaction and experience with Mr. Coury, the independent directors on the Mylan Board continue to believe that Mr. Coury’s highly collaborative relationship with the independent directors, including the Lead Independent Director, his extensive knowledge of the industry, Mylan’s management, businesses and global platform, and the opportunities and challenges anticipated in the future, as well as his proven leadership abilities, vision, and insight, and the continued outstanding performance of the Company, make him the ideal person to lead the Mylan Board. Mr. Coury previously served as CEO of the Company from September 2002 to January 2012.
9
Table of Contents
In his capacity as Executive Chairman, Mr. Coury’s primary responsibilities include providing overall leadership and strategic direction of the Company; providing guidance to the CEO and senior management; coordinating the activities of the Mylan Board; overseeing talent management; communicating with shareholders and other stakeholders; strategic business development; and mergers and acquisitions.
Effective January 1, 2012, the Mylan Board implemented an enhanced management structure, again electing Mr. Coury as Executive Chairman of the Mylan Board (as described above) and appointing Ms. Bresch as CEO and Mr. Malik as President, among other changes described in previous public filings.
In connection with this enhanced management structure implemented in 2012, the Mylan Board also appointed Mr. Piatt as Lead Independent Director based on, among other factors, Mr. Piatt’s independence, outstanding contributions as a director of the Company, excellent business judgment, and recognized leadership abilities. The Mylan Board believes that this appointment only further enhanced the Mylan Board’s already strong independent oversight of the Company. As Lead Independent Director, Mr. Piatt presides at executive sessions of the independent directors, and he has the authority to call meetings of the independent directors. He also serves on the Executive Committee of the Mylan Board. In addition, the Chairman, in consultation with the Lead Independent Director, as applicable, determines the information sent to the Mylan Board, the meeting agendas, and meeting schedules to assure that there is sufficient time for discussion of agenda items. The Lead Independent Director in turn is charged with separately approving information sent to the Mylan Board, its meeting agendas, and its meeting schedules. He also serves as the contact person for stakeholders wishing to communicate with the Mylan Board and as a liaison between the Chairman and independent directors.
As of 2012, in her role as CEO, Ms. Bresch’s primary responsibilities include the day-to-day running and oversight of the Company’s global operations, business, and functions; executing on and overseeing implementation of strategies developed or approved by the Mylan Board; continued oversight of process and operational enhancements; and continued implementation of a blueprint for an organizational design to help ensure the sustainability of our success into the future.
The Mylan Board strongly believes, and the Company’s short- and long-term performance demonstrates, that the current Mylan Board and management structures continue to prove to be ideal for Mylan, and that it has produced outstanding results for shareholders and has benefited the interests of other stakeholders, as illustrated on pages 12 to 23 of this Amendment. We believe that the Company and its stakeholders have benefited, and continue to benefit, from the respective leadership, judgment, vision, experience – and performance – of the existing Mylan Board and management structure, and that the Executive Chairman, Mr. Coury, the CEO, Ms. Bresch, and the President, Mr. Malik, all share a vision for the Company that is consistent with the Mylan Board’s philosophy.
This determination is based on, among other factors, senior management’s demonstrated leadership abilities; the performance of the Company; the Mylan Board’s deep and unique knowledge of the complexity, size, and dramatic growth of the Company, the Company’s businesses, operations, vision, and strategies; the respective talents and capabilities of our fellow directors and management; and the opportunities and challenges anticipated in the future.
Our governance structure also provides effective independent oversight by the Mylan Board in the following additional ways:
| • | ten of the thirteen members of the Mylan Board are independent; |
| • | the Mylan Board has established robust Corporate Governance Principles; |
| • | the Audit, Compensation, Compliance, Finance and Governance and Nominating Committees are all composed entirely of independent directors (as defined in the applicable NASDAQ listing standards); |
10
Table of Contents
| • | the independent directors on the Mylan Board and its committees receive extensive information and input from management and external advisors, engage in detailed discussion and analysis regarding matters brought before them (including in executive session), and consistently and actively engage in the development and approval of significant corporate strategies; |
| • | the Mylan Board and its committees have unrestricted access to management; |
| • | the Mylan Board and its committees (other than the Science and Technology Committee) can retain, at their discretion and at Company expense, any advisors they deem necessary with respect to any matter brought before the Mylan Board or any of its committees (the Science and Technology Committee retains advisors in consultation with the Executive Chairman and the Lead Independent Director); |
| • | the Mylan Board and its committees are intimately familiar with the business and management of the Company and collectively met 36 times in 2015, including 24 times after the Closing Date; and |
| • | in 2015, the Mylan Board held five executive sessions of non-management members while its committees collectively held 16 executive sessions. |
Board of Directors Risk Oversight
The Mylan Board’s Audit Committee is primarily responsible for overseeing the Company’s risk management processes on behalf of the full Mylan Board. The Audit Committee focuses on financial reporting risk and oversight of the internal audit function. It receives reports from management at least quarterly regarding, among other matters, the Company’s assessment of risks and the adequacy and effectiveness of internal controls. The Audit Committee also receives reports from management addressing risks impacting the day-to-day operations of the Company. Mylan’s internal audit function meets with the Audit Committee on at least a quarterly basis to discuss potential risk or control issues. The Audit Committee reports regularly to the full Mylan Board, which also considers the Company’s risk profile. The full Mylan Board focuses on the most significant risks facing the Company and the Company’s general risk management strategy, and also seeks to ensure that risks undertaken by the Company are consistent with the Mylan Board’s risk management expectations. While the Mylan Board oversees the Company’s overall risk management strategy, management is responsible for the day-to-day risk management processes. We believe this division of responsibility continues to remain a highly effective approach for addressing the risks facing the Company and that the Mylan Board’s leadership structure supports this approach.
In addition, the Compensation Committee is responsible for overseeing the Company’s compensation risks as discussed further beginning on page 30 of this Amendment under “Consideration of Risk in Company Compensation Policies.”
Also, the Compliance Committee is responsible for overseeing the Company’s corporate compliance program and related policies and controls.
Code of Ethics; Corporate Governance Principles; Code of Business Conduct and Ethics
The Mylan Board has adopted a Code of Ethics that applies to our Principal Executive Officer, Principal Financial Officer, and Corporate Controller (“Code of Ethics”). The Mylan Board also has adopted Corporate Governance Principles as well as a Code of Business Conduct and Ethics applicable to all directors, officers, and employees.
Copies of the Code of Ethics, the Corporate Governance Principles, and the Code of Business Conduct and Ethics for Mylan N.V. are posted on Mylan N.V.’s website at http://www.mylan.com/company/corporate-governance. Copies of the Code of Ethics, the Corporate Governance Principles, and the Code of Business Conduct and Ethics for Mylan N.V. are also available in print to shareholders upon request, addressed to Mylan N.V.’s Corporate Secretary at Building 4, Trident Place, Mosquito Way, Hatfield, Hertfordshire, AL10 9UL, England. Mylan N.V. intends to post any amendments to and waivers from the Code of Ethics on its website as identified above.
11
Table of Contents
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires all directors and certain executive officers and persons who own more than 10% of a registered class of Mylan’s equity securities to file with the SEC within specified due dates reports of ownership and reports of changes of ownership of Mylan ordinary shares and our other equity securities. These persons are required by SEC regulations to furnish us with copies of all Section 16(a) reports they file. Based on reports and written representations furnished to us by these persons, we believe that all Mylan directors and relevant executive officers complied with these filing requirements during 2015.
| ITEM 11. | Executive Compensation |
Executive Compensation for 2015
Compensation Discussion and Analysis
Executive Summary
The Mylan Board has structured Mylan’s executive compensation programs to create a maximum “return on executive leadership.” Our compensation program is designed to incentivize continued excellence in execution against our long-stated strategy to create a leading, robust, sustainable company, as well as deliver outstanding performance and shareholder value creation over the short- and long-term, and align compensation with performance and shareholder and other stakeholder interests.
As outlined below, Mylan has successfully executed on its clearly articulated strategy, delivering superior long-term shareholder value and continuing to invest in initiatives designed to continue this track record of long-term growth and value creation in the future, all while also meeting and exceeding annual financial and performance targets.
Mylan has long believed that development of, and consistent execution against, a clear and coherent long-term strategy approved by the Mylan Board and executed by senior management is critical to the Company’s success and its ability to consistently create value for shareholders and other stakeholders. Mylan has developed an exceptional global operating and commercial platform and industry-leading product portfolio; attracted and retained highly-talented and motivated leaders to the organization; identified strategic drivers of organic growth and effectively executed against these key growth drivers; and identified exciting external opportunities to further enhance the business and accelerate the Company’s long-term growth trajectory. In pursuing external opportunities, we seek to differentiate the Company by acquiring assets that deliver not only short-term financial benefit, but which also will deliver sustainable long-term value for our business, shareholders and other stakeholders.
Since the beginning of 2015, we have had extensive discussions with holders of over 80% of Mylan’s ordinary shares on a variety of topics. As part of Mylan’s shareholder outreach and engagement, shareholders have consistently communicated to the Mylan Board and senior management that they too believe in the critical importance of focusing on sustainable long-term value creation.
The Mylan Board believes that the success of Mylan’s long-term strategy and the Company’s exceptional financial and operational performance over the past decade clearly reflects the dedication and talents of our employees around the world — and demonstrates the effectiveness of our compensation programs in incentivizing performance and aligning compensation with shareholder and other stakeholder interests.
The Mylan Board also believes that the outstanding long-term growth of Mylan — including the exceptional 25.4% and 20.7% total shareholder return (“TSR”) over the past three and five years, respectively, each of which significantly exceeded the S&P 500 Index and S&P 500 Pharmaceutical Index results over those
12
Table of Contents
periods — is directly related to the effectiveness and the robustness of our compensation program, as well as the talents of Mylan’s global workforce and the extraordinary vision, commitment, and leadership of Mylan’s senior management team.
And the Company’s performance in 2015 again set records to the benefit of shareholders and other stakeholders, with a record high in adjusted diluted earnings per share (“adjusted diluted EPS”) of $4.30, total adjusted revenues of $9.45 billion, and adjusted EBITDA of $3.01 billion (U.S. GAAP diluted earnings per share were $1.70, U.S. GAAP total revenues were $9.43 billion, and U.S. GAAP net earnings attributable to Mylan N.V. ordinary shareholders were $847.6 million in 2015).
Our senior leadership team is led by Mr. Coury, Ms. Bresch, and Mr. Malik. Together, Mr. Coury, Ms. Bresch, Mr. Malik, and the Mylan Board have successfully developed and executed on a vision and strategy to position Mylan as a global leader in its industry and significantly enhance Mylan’s long-term growth prospects.
In addition to creating extraordinary long-term shareholder value over the last three and five years (and longer), Mylan has also delivered exceptional results for other stakeholders, including customers, patients, employees, and the broader community.
Named Executive Officers for 2015
Mylan’s named executive officers (“NEOs”) for 2015 were:
| Heather Bresch |
Chief Executive Officer | |
| John D. Sheehan, C.P.A (1) |
Former EVP and Chief Financial Officer | |
| Rajiv Malik |
President | |
| Anthony Mauro |
Chief Commercial Officer | |
| Robert J. Coury |
Executive Chairman |
| (1) |
Mr. Sheehan retired effective April 1, 2016. |
Outstanding 2015 and Long-Term Financial and Operational Performance
2015 represented yet another remarkable year for Mylan on many fronts, not the least of which was our continued outstanding execution on the Mylan Board’s long-standing strategy and vision. We made progress against the key pillars of this strategy by further strengthening our exceptional and differentiated global operating platform; continuing to diversify our product portfolio, which is already one of the industry’s broadest; and further building out our powerful commercial infrastructure. We also continued to position the company for long-term growth through significant investment in our organic growth drivers, which we complemented by executing on value-creating, strategic acquisitions and other business development opportunities.
Outstanding 2015 Financial Results
In addition to our continued focus on building a long-term sustainable business, we did not take our eye off the ball with regard to the Company’s day-to-day core business and short-term execution, as our financial results for 2015 clearly demonstrate.
In 2015, we delivered total adjusted revenues of nearly $9.45 billion, adjusted EBITDA of $3.01 billion, and adjusted diluted EPS of $4.30, an increase of 22%, 27%, and 21%, respectively, compared to our very strong performance in 2014 (U.S. GAAP total revenues were $9.43 billion, U.S. GAAP net earnings attributable to Mylan N.V. ordinary shareholders were $847.6 million, and U.S. GAAP diluted earnings per share were $1.70 in 2015, an increase of 22%, decrease of 9%, and decrease of 27%, respectively). All of our regions and businesses contributed to this strong growth, and we achieved it despite strong foreign-currency headwinds.
13
Table of Contents
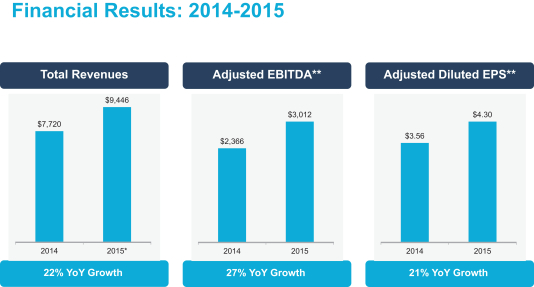
$ million except per share amount
* 2015 represents Total Adjusted Revenues. See Appendix A for a reconciliation to the most comparable GAAP measure
** See Appendix A for a reconciliation to the most comparable GAAP measures
For 2015, U.S. GAAP total revenues were $9,429 million. For 2014 and 2015, U.S. GAAP net earnings attributable to Mylan N.V. ordinary shareholders were $929.4 million and $847.6 million, respectively (down 9% year-over-year). For 2014 and 2015, U.S. GAAP diluted earnings per share were $2.34 and $1.70, respectively (down 27% year-over-year).
Continued Outstanding Performance in Regions and Segments
In our North America generics segment, third party net sales totaled $3.9 billion, a 16% increase compared to 2014. Growth came mainly from sales of new products, and to a lesser extent, from the EPD Business. Also contributing were higher volumes on existing products, partially offset by lower pricing.
In Europe, adjusted third party net sales totaled $2.2 billion in 2015, a year-over-year constant currency increase of 67% on an adjusted basis (U.S. GAAP third party net sales for Europe totaled $2.2 billion, a year-over-year increase of 49%). Growth came primarily from sales generated by the EPD Business and, to a lesser extent, from new products. Higher volumes on existing products, mainly in France and Italy, were offset by lower pricing throughout the region.
In the Rest of World, third party net sales totaled $2.1 billion, a year-over-year increase of 38% on a constant currency basis (a year-over-year increase of 27% for U.S. GAAP third party net sales). The growth came from the EPD Business; new product launches primarily in Australia and Japan; and higher volumes in India – predominately of antiretroviral medications. Increases were offset somewhat by lower pricing in the region.
Our Specialty business delivered revenues of $1.2 billion in 2015, an increase of 1% compared to 2014, driven by the continued strong performance of EpiPen® Auto-Injector, as well as strong sales of Perforomist and ULTIVA, which increased by double-digit percentage points from the prior year.
It is noteworthy that our EPD Business grew 2% year over year on a constant currency basis, demonstrating again our ability to take a declining business and drive growth ahead of our expectations.
This strong performance and continued dedication to operational excellence and execution allowed us to deliver a record year with respect to adjusted free cash flow, which more than doubled over the prior year (U.S. GAAP net cash provided by operating activities increased 98%). Specifically, we realized approximately $1.85 billion of adjusted free cash flow in 2015.
14
Table of Contents
In addition, continuing our commitment to strong balance sheet management, the Company maintained its investment grade rating from Standard & Poor’s and Moody’s, the two principal ratings agencies. As a result of maintaining a strong balance sheet and having an investment grade rating, we were able to reduce our cost of borrowing in 2015, providing additional financial flexibility.
Outstanding Long-Term Financial Results
As shown in the tables below, our consistent execution and strong performance in 2015 continued a long-term track record of exceptional business execution, delivering superior financial results for shareholders, and contributing to a long-term compound annual growth rate (“CAGR”) in adjusted diluted EPS of 27% since 2008.
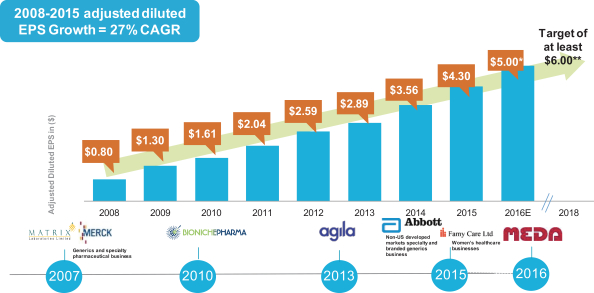
Adjusted diluted EPS is a non-GAAP financial measure. See Appendix for reconciliation of adjusted diluted EPS to the most directly comparable GAAP measure
* Midpoint of 2018 guidance range
** Stated 2018 target, this is a long-term only and does not represent company guidance
U.S. GAAP diluted earnings per share in 2008 were $(1.10), 2009 were $0.30, 2010 were $0.68, 2011 were $1.22, 2012 were $1.52, 2013 were $1.58, 2014 were $2.34, and 2015 were $1.70. The midpoint of forecasted U.S. GAAP diluted earnings per share for 2016 is $2.41.
15
Table of Contents
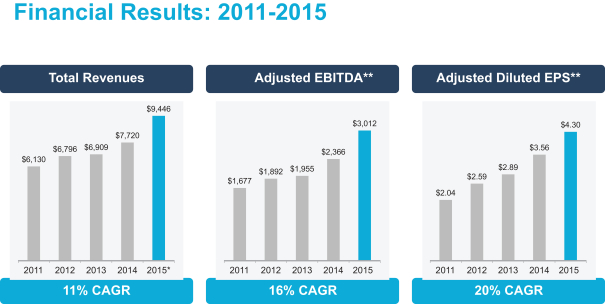
$ million except per share amounts
* 2015 represents Total Adjusted Revenues. See Appendix A for a reconciliation to the most comparable GAAP measure.
** See Appendix A for a reconciliation to the most comparable GAAP measures.
For 2015, U.S. GAAP total revenues were $9,429 million. U.S. GAAP net earnings attributable to Mylan N.V. ordinary shareholders were $536.8 million in 2011, $640.9 million in 2012, $623.7 million in 2013, $929.4 million in 2014, and $847.6 million in 2015 (12% CAGR). U.S. GAAP diluted earnings per share were $1.22 in 2011, $1.52 in 2012, $1.58 in 2013, $2.34 in 2014, and $1.70 in 2015 (9% CAGR).
Our track record of excellence in business execution and outstanding performance is further demonstrated by the long-term shareholder value creation that our exceptional team has achieved. As shown below, Mylan’s TSR over the last three and five years has significantly outperformed both the S&P 500 Index and the S&P 500 Pharmaceuticals Index.
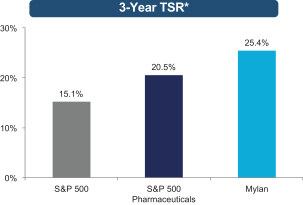
| * | TSR data is from Bloomberg and reflects total return (including price appreciation and reinvested dividends) as of December 31, 2015. |
16
Table of Contents
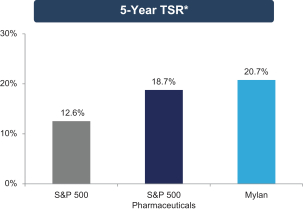
| * | TSR data is from Bloomberg and reflects total return (including price appreciation and reinvested dividends) as of December 31, 2015. |
In addition to outstanding long-term shareholder value creation, we have also generated exceptional returns on our invested capital. In 2015, cash return on operating invested capital, cash return on invested capital excluding goodwill, and cash return on total invested capital were 52%, 22%, and 15%, respectively, continuing our outstanding long-term performance in those areas as well (see Appendix A for a reconciliation to the most directly comparable U.S. GAAP measure).
Strengthening and Expanding our Exceptional and Differentiated Global Platform and Portfolio
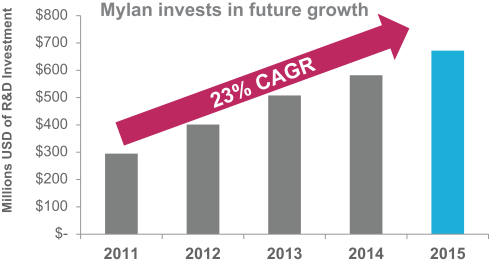
Mylan has earned a well-deserved reputation for innovation within the generics and specialty pharmaceuticals spaces. Our executive leadership team, together with Mylan’s outstanding workforce, has a track record of successfully developing and bringing to market products that are difficult to formulate or manufacture – to which the many innovative companies that have made Mylan a partner of choice can attest. To remain at the industry’s forefront, we continue to invest heavily in research and development and in strengthening our already-powerful manufacturing and commercial infrastructure.
17
Table of Contents
As a result of this R&D investment, in 2015, we submitted 167 regulatory applications to bring new high-quality medicines to market around the world. Currently, we have 270 new drug applications pending U.S. Food and Drug Administration approval, representing $101.6 billion in annual brand sales, according to IMS Health. Fifty of these pending Abbreviated New Drug Applications are potential first-to-file opportunities, representing $35.8 billion in annual brand sales for the twelve months ending June 30, 2015, according to IMS Health. Globally, we have more than 4,100 new product submissions pending regulatory approval around the world, up from 2,100 just two and a half years ago.
Commercially, our portfolio of marketed products rose from more than 1,300 to more than 1,400 and our commercial footprint expanded from approximately 140 countries and territories to approximately 165.
Advancement of Strategic Organic Growth Drivers
In addition to the continued expansion of our global portfolio, operating platform, and commercial footprint, the Mylan Board and management have carefully identified several key strategic opportunities for growth within our sector and focused resources on these exciting and powerful growth drivers, which we expect will result in continued and sustained business performance and value creation over both short- and long-term periods.
During 2015, Mylan continued to execute on several of these growth drivers:
Continued Growth of EpiPen® Auto-Injector Franchise
EpiPen® Auto-Injector continues to be an important and growing product and remains the number one dispensed epinephrine auto-injector. As a global franchise, EpiPen® Auto-Injector reached $1 billion in annual net sales for the second year in a row in 2015.
During the year, we continued to advocate for legislation to increase access to “stock,” or undesignated, epinephrine auto-injectors in schools and other public places to ensure availability of this life-saving treatment for severe allergic reactions, or anaphylaxis.
We also continued to invest in our EpiPen4Schools® program, which provides free EpiPen® and EpiPen Jr® Auto-Injectors to qualified schools. To date, more than the 63,000 U.S. schools are participating in the program, and Mylan has distributed more than 500,000 free EpiPen® and EpiPen Jr® Auto-Injectors.
The Allergy & Asthma Network recognized Mylan in May 2015 with its “Most Outstanding Partner in Advocacy” award for our leadership in advocating for stock epinephrine. Food Allergy Research & Education (“FARE”) recognized the Company in June 2014 with its “FARE Vision Award” for working to make the world safer for people with food allergies and supporting FARE in its mission.
In addition, in 2015, Mylan continued a multi-year strategic alliance agreement with Walt Disney Parks and Resorts to help increase awareness of anaphylaxis. Maps in Disney’s domestic theme parks and on its cruise ships, as well as signage in the parks, highlight locations with EpiPen® and EpiPen Jr® Auto-Injectors help to ensure visitor access to these products.
Through Mylan’s new “On Location” program, which we will begin piloting next year with EpiPen®, we hope to provide enhanced access to this product in more public places around the U.S.
We also remain committed to continuing to invest in education and building awareness about the need for access to these life-saving treatments for anaphylaxis.
18
Table of Contents
Advances in the Treatment of Infectious Diseases
Throughout 2015, we also worked with a variety of stakeholders in our continued fight against HIV/AIDS and other infectious diseases. We announced, for example, that we expect to be the first to launch TLE400, an antiretroviral medication we developed in partnership with the Clinton Health Access Initiative, for $99 per patient, per year.
Today more than 50% of patients in the developing world receiving treatment for HIV/AIDS rely on a Mylan product.
We expanded our Hepatitis C licensing agreement with Gilead Sciences, a valued partner since 2006. As Gilead’s exclusive branded-medicine distribution partner in India, we launched its Solvaldi® product there in 2015, as well as introducing MyHepTM, the generic form of Solvaldi®, in India and other emerging markets. Early this year, we announced the launch of MyHep LVIRTM, the generic form of Harvoni®, in India.
Creating a Leading Respiratory Franchise
Toward the end of 2015, we filed an Abbreviated New Drug Application with the U.S. Food and Drug Administration for generic Advair Diskus® and on February 19, 2016, Mylan announced that its application had been accepted for filing, putting Mylan in a position to potentially become the first company to bring generic Advair® to the U.S. market in 2017.
We announced a partnership with Theravance Biopharma whose goal is to develop and commercialize Revefenacin, a once-daily nebulized product used to treat chronic obstructive pulmonary disease, or COPD, and other respiratory diseases.
Through our partnership with Prosonix, we continued work on our generic Flovent® program for the United States. The product’s counterpart in Europe, generic Flixotide®, is on track to be approved this year. In addition, we launched SirduplaTM, the generic version of Seretide® Evohaler®, in the United Kingdom.
Creating a World Leader in Biosimilars and Insulin Analogs
In partnership with Biocon, we progressed our biologics and insulin-analogs programs and expect this year to submit in the United States and Europe three biosimilar applications – Pegfilgrastim, Trastuzumab, and Adalimumab – and an interchangeable glargine application.
Some of the key milestones in these programs in 2015 include:
| • | Completed enrollment for Phase III study of trastuzumab; launched Hertraz™ in 10 countries with multiple new launches planned in 2016, building on our successful commercialization over the past two years; |
| • | Completed Phase I clinical trial & enrollment in Phase III trials for pegfilgrastim; |
| • | Completed Phase I clinical trial & initiated Phase III for Adalimumab; and |
| • | Completed enrollment for two Phase III clinical trials for an insulin analog to Lantus®; continued to pursue interchangeability discussions with the U.S. Food and Drug Administration. We also completed and qualified a state-of-the-art manufacturing facility in Malaysia. |
In addition, in early 2016 we announced an exclusive global agreement with Momenta that expands our portfolio of biologics with up to six additional products and broadens the scope and scale of our capability. We believe this collaboration with Momenta, which is highly complementary to our partnership with Biocon, will position us to become a world leader in biosimilars, with a broad portfolio of 15 biosimilar/insulin analog generic products in development.
19
Table of Contents
Capitalizing on Strategic Inorganic Growth Opportunities
Mylan for many years has been a strategic participant in the consolidation occurring in the industry. We seek to differentiate the Company by acquiring assets that deliver not only short-term financial benefit, but also long-term value for the business, shareholders, and other stakeholders. We look beyond what targeted assets have achieved on their own to how we can leverage them using our exceptional platform and excellence in execution. Our approach to acquisitions has delivered time and again, and we are confident that the transactions we completed or initiated in 2015 represent excellent new examples of Mylan’s commitment to long-term value creation.
In that regard, 2015 was a year of unprecedented activity for Mylan in identifying and executing on significant M&A opportunities. In 2015, we completed or initiated three significant M&A transactions with an aggregate enterprise value of approximately $17 billion. Each of these strategic transactions represents yet another step in the long-term strategy of the Mylan Board to create an unparalleled worldwide leader in our industry and continues our track record of pursuing high-quality businesses and assets in a disciplined and focused manner to accomplish that goal. We believe that each of the three transactions described below will create long-term shareholder value and further extend Mylan’s product portfolio and geographic footprint.
EPD Transaction
In February 2015, we consummated the acquisition of the EPD Business via the EPD Transaction. The EPD Transaction accomplished a number of important strategic and financial objectives for Mylan.
| • | Increased Mylan’s geographic footprint and infrastructure in key markets |
The EPD Transaction significantly increased the Company’s presence and revenues in some of our largest markets outside of the United States, including Italy, the United Kingdom, Germany, France, Spain, Portugal, Canada, Japan, Australia, and New Zealand, and provided Mylan with entry into new markets in Central and Eastern Europe.
| • | Extended and strengthened Mylan’s product portfolio |
The EPD Business provided the Company with a portfolio of more than 100 specialty, branded generic and over-the-counter (“OTC”) pharmaceutical products in five major therapeutic areas (cardio/metabolic, gastrointestinal, anti-infective/respiratory, CNS/pain, and women’s and men’s health). The portfolio includes several patent-protected, novel, and/or hard-to-manufacture products with durable growth potential. Key products include Creon®, Influvac®, Brufen®, Amitiza®, and Androgel®, among others.
| • | Significantly expanded commercial and manufacturing platform and capabilities |
The EPD Business provided Mylan with an active sales organization of approximately 2,000 representatives serving more than 40 non-U.S. markets. The EPD Business’s strong sales force in key developed markets enhanced the Company’s reach with physicians and patients and complemented our existing strength in pharmacies. This platform provided Mylan with the enhanced infrastructure and expertise to even more effectively execute on existing growth opportunities that require access to the physician channel, such as the anticipated launch of biologics and respiratory products, including generic Seretide® and generic Advair®. The EPD Business also included two high-quality manufacturing facilities in France and Japan.
| • | Provided additional financial flexibility and related strategic benefits |
The EPD Transaction provided Mylan with a number of important financial and other strategic benefits, including increased revenues, liquidity, and an optimized tax structure. Notably, the EPD Transaction provided us with over $1.4 billion of revenue in a 100% stock transaction and without the incurrence of additional debt, which further strengthened Mylan’s balance sheet. These benefits will enable us to invest further in our business in the future.
20
Table of Contents
The integration of the EPD Business to date has gone well, and the EPD Business’s performance has exceeded our own expectations. As noted earlier, we reversed a decline in the business and grew the business by 2% year over year in 2015 on a constant currency basis, demonstrating again our ability to take an underappreciated asset and drive expansion within our platform.
Famy Care Transaction
In November 2015, we acquired certain female healthcare businesses from Famy Care, a valued partner since 2008. The transaction brought Mylan a broad portfolio of products (primarily oral contraceptives), strong technical capabilities, and dedicated hormone manufacturing. By combining these assets with the Company’s global commercial footprint and supply chain infrastructure, we look forward to creating a leading women’s healthcare franchise and providing enhanced access to these products around the world.
Recently Announced Meda Transaction
In early 2016, we took the next step in executing on our vision by announcing that Mylan would acquire Meda, a leading international specialty pharmaceutical company, which has served as our marketing and distribution partner for EpiPen® in Europe since 2011.
The combination will create a global pharmaceutical leader with 2015 combined revenues of nearly $12 billion,1 a diversified portfolio of more than 2,000 products, and critical mass across all commercial channels, including a $1 billion OTC business. Geographically, we gain a more balanced and expanded global footprint with an even stronger presence across Europe; a leading U.S. specialty business; and an expanded presence in emerging markets, including several new and attractive ones such as China, Southeast Asia, Russia, the Middle East, and Mexico. We also will become a leader in the global respiratory/allergy market and achieve scale in many other therapeutic areas, including dermatology and pain, providing even greater opportunities for growth in these therapeutic franchises.
Completion of the Meda Transaction is subject to a public offer to the shareholders of Meda to acquire all of Meda’s outstanding shares and other customary closing conditions. We expect to complete the transaction by the end of the third quarter in 2016.
Investing in Our People
Achievement of our goals relies on Mylan’s dedicated global workforce, which now numbers nearly 35,000 talented individuals. We consider them to be Mylan’s most important asset.
As Mylan continues to grow in scope and scale, the Mylan Board has tasked management with investing further in retaining, recruiting, and motivating our workforce and in developing individuals for key management roles in Mylan’s increasingly global platform.
During 2015 and early 2016, Mylan had several important developments in this regard. As just one example, in early 2016, Mylan promoted Anthony Mauro to the role of Chief Commercial Officer. Over his 20 years at Mylan, Mr. Mauro has led several important business units, functions, and geographies. In his new role, Mr. Mauro will be responsible for overseeing global sales excellence and operations of all commercial markets around the world.
Mylan’s commitment to employees can also be seen in its focus on employee safety and employee wellness initiatives. For example, five Mylan sites in India have been certified as meeting the internationally recognized benchmark, BS OHSAS 18001, for superior performance in occupational health and safety management systems.
| 1 | Combined company figures represent an aggregation of Mylan figures derived from financial information prepared in accordance with U.S. GAAP and Meda figures derived from financial information prepared in accordance with International Financial Reporting Standards as adopted by the European Union and do not reflect pro forma adjustments (including no elimination of transactions between Mylan and Meda). |
21
Table of Contents
In addition, in the last year, Mylan has offered its U.S.-based employees more innovative options to cover them and their families’ healthcare needs, including incentives for weight loss, regular exercise, and preventative medicine.
In order to continue to execute against our mission, strategy, and financial objectives, our employees must be incentivized to deliver against both our short-term goals and priorities, as well as our long-term vision and mission. To this end, we focused throughout 2015 on driving even greater focus, accountability, and alignment across our leadership and entire workforce.
Continued Strong Alignment of CEO Pay with Company Performance
As discussed above, Mylan’s compensation philosophy is designed to reward employees for delivering against our short- and long-term performance goals. As demonstrated below, this philosophy was clearly successful in 2015 and over the past several years, as compensation has remained closely aligned with the outstanding performance noted above.
| • | Outstanding performance against goals: The Company exceeded each of the rigorous performance-based goals set by the Compensation Committee relating to adjusted diluted EPS, global regulatory submissions, and adjusted free cash flow in 2015. As a result, the NEOs received maximum payouts of annual incentive awards for 2015. These achievements are discussed in more detail on pages 24 to 25 of this Amendment. |
| • | Continued strong alignment between realizable pay and performance relative to peers. The total compensation realizable by Mylan’s CEO over a three-year period is fully aligned with Mylan’s TSR relative to the Company’s peer group, as shown below. |
Alignment of CEO Realizable Pay* with TSR Performance
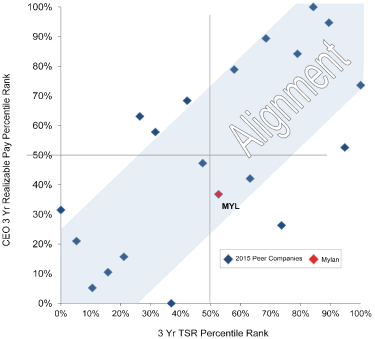
| * | Realizable pay includes cumulative salary and annual incentives paid for the most recent three years, plus current value (as of December 31, 2015) of options (intrinsic value) as well as time-based restricted stock/units granted during the most recent three years, plus the value of performance-based long-term incentive |
22
Table of Contents
| awards earned (which excludes awards granted in connection with the One-Time Special Performance-Based Incentive Program (as defined below), which are still subject to performance-based criteria), plus change in pension value and all other compensation for the most recent three years. TSR data is from the S&P Research Insight Database. Peer companies in this chart reflect the 2015 peer companies listed on page 29 of this Amendment (excluding Hospira Inc., which was acquired in September 2015, and Teva Pharmaceutical Industries Ltd., for whom sufficient information was not publicly available, and including Allergan plc in place of Actavis plc and Allergan, Inc.). |
2015 Elements of Compensation and Certain Compensation Policies
Our 2015 compensation performance-related metrics helped drive yet another outstanding year of operational and financial performance. The Compensation Committee continues to aim to align executive compensation with Company performance and, in 2015, we delivered on that goal — as demonstrated by the close correlation between Mylan’s TSR performance and CEO realizable pay relative to our peer group, as well as the Company’s record adjusted diluted EPS, total adjusted revenues and adjusted EBITDA. For the remainder of this Compensation Discussion and Analysis and for the compensation tables and related narratives that follow, references to Mylan will include Mylan and its subsidiaries and affiliates, including Mylan Inc.
In 2015, the NEOs were compensated through base salary, an annual incentive, a long-term incentive, employee benefits, and perquisites. Approximately 84% of annual NEO target compensation was tied to Mylan’s ordinary share price or the achievement of key financial and operational performance goals, thereby closely aligning compensation with both the success of Mylan’s business strategy and objectives, as well as the value realized by shareholders. The following graphs show the relative weight of the base salary, target annual incentive, and target long-term incentive (based on grant date fair value) components:
|
|
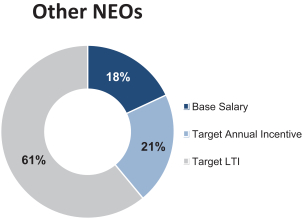
|
Base Salary Compensation
The Compensation Committee considers a variety of factors in deciding base salary, including, among others: individual performance, responsibilities, and expected future performance; Company performance; management structure; marketplace practices; internal equity considerations; and the executive’s experience, tenure, and leadership. The Compensation Committee also considers what the marketplace would require in terms of the replacement costs to hire a qualified individual to replace an executive, as well as the fact that a new executive would lack the critical knowledge base regarding Mylan as compared to the executive he or she would be replacing.
For 2015, the base salaries of Ms. Bresch and Mr. Malik increased 8.3% and 11.1%, respectively, reflecting their continued growth and experience in the roles that they assumed in 2012, as well as their performance and
23
Table of Contents
leadership, among other factors. For 2015, the base salary of Mr. Mauro increased 13.6%, reflecting his performance and increased role, among other factors. The base salaries of the other NEOs were not changed in 2015.
| 2014 | 2015 | Change in Base Salary |
||||||||||||
| Heather Bresch |
Chief Executive Officer | $ | 1,200,000 | $ | 1,300,000 | 8.3 | % | |||||||
| John D. Sheehan |
Former EVP and Chief Financial Officer | 650,000 | 650,000 | 0 | % | |||||||||
| Rajiv Malik |
President | 900,000 | 1,000,000 | 11.1 | % | |||||||||
| Anthony Mauro |
Chief Commercial Officer | 550,000 | 625,000 | 13.6 | % | |||||||||
| Robert J. Coury |
Executive Chairman | 1,350,000 | 1,350,000 | 0 | % | |||||||||
| Total |
$ | 4,650,000 | $ | 4,925,000 | 5.9 | % | ||||||||
The base salary earned by each of the NEOs for 2015 is set forth in the column entitled “Salary” in the Summary Compensation Table on page 36 of this Amendment.
Annual Incentive Compensation
Mylan’s annual incentive compensation for executive officers consists of performance-based annual cash awards that are intended to align the interests of executives and investors by providing incentives based on a set of operational and financial measures identified by the Mylan Board, as discussed above, as critical to the successful execution of Mylan’s business strategy — and which are expected to impact shareholder value and the interest of other stakeholders.
Performance Measures. For 2015, annual incentives were based on adjusted diluted EPS, global regulatory submissions, and adjusted free cash flow. These measures represent key performance indicators of the current and future strength of our business.
| • | Adjusted diluted EPS is an important metric for Mylan and its shareholders because earnings are expected to have a direct relationship to the price of Mylan’s ordinary shares. |
| • | The global regulatory submissions metric measures the number of filings submitted to global regulatory agencies for new products. This is also an important metric because approval and commercialization of new products yield new revenue sources, are essential for Mylan to remain competitive in a constantly evolving industry, and are therefore fundamental to our short- and long-term strategy for growth. |
| • | Adjusted free cash flow also is an important metric because it captures the potential impact of all types of business transactions on the generation of adjusted operating cash flow, not merely changes in working capital. Adjusted free cash flow is defined as adjusted operating cash flow less net capital expenditures. |
The Compensation Committee set adjusted diluted EPS and adjusted free cash flow targets at double digit percentage increases over prior year performance, and also took into account Mylan’s increased size following the EPD Transaction. The Compensation Committee set the global regulatory submissions target based on total submissions in our portfolio as of year-end 2014. The following tables show the 2015 threshold, target, and maximum goals and the relative weightings of each metric:
| Goal |
Weighting | Threshold | Target | Maximum | ||||||||||||
| Adjusted diluted EPS |
50 | % | $ | 4.00 | $ | 4.15 | $ | 4.30 | ||||||||
| Global regulatory submissions |
25 | % | 135 | 150 | 165 | |||||||||||
| Adjusted free cash flow (millions) |
25 | % | $ | 1,050 | $ | 1,150 | $ | 1,250 | ||||||||
24
Table of Contents
No annual incentives are paid if threshold performance is not achieved.
Potential Opportunities Subject to Performance. Set forth below are the 2015 threshold, target, and maximum award opportunities for the NEOs:
| Threshold (% of Salary) | Target (% of Salary) | Maximum (% of Salary) | ||||
| Heather Bresch |
75.0% | 150% | 300% | |||
| John D. Sheehan |
50.0% | 100% | 200% | |||
| Rajiv Malik |
62.5% | 125% | 250% | |||
| Anthony Mauro |
57.5% | 115% | 230% | |||
| Robert J. Coury |
62.5% | 125% | 250% |
Incentive payouts. The annual incentives earned for 2015 were determined based on the annual performance criteria, relative weightings, and Company results set forth in the table below. The Company exceeded each of the rigorous performance-based goals set by the Compensation Committee relating to adjusted diluted EPS, global regulatory submissions, and adjusted free cash flow in 2015. As a result, the NEOs received maximum payouts of annual incentive awards for 2015.
| Goal |
Weighting | Outcome | Weighted Score | |||||
| Adjusted diluted EPS* |
50% | $ | 4.41 | 100% | ||||
| Global regulatory submissions |
25% | 167 | 50% | |||||
| Adjusted free cash flow (millions) |
25% | $ | 1,854 | 50% | ||||
| 2015 Company Performance Score |
200% | |||||||
| * | The adjusted diluted EPS amount is calculated from Mylan’s audited financial statements in the same manner as Mylan publicly reports adjusted diluted EPS (which for 2015 is reconciled to the most directly comparable U.S. GAAP measure in Appendix A), but is measured on a constant currency basis. Adjusted free cash flow is calculated from Mylan’s audited financial statements in the same manner as Mylan publicly reports adjusted free cash flow (which for 2015 is reconciled to the most directly comparable U.S. GAAP measure in Appendix A). |
The annual incentive compensation earned by each of the NEOs for 2015 is set forth in the column entitled “Non-Equity Incentive Plan Compensation” in the Summary Compensation Table on page 36 of this Amendment.
The Compensation Committee has committed to not using its discretion to upwardly adjust annual incentive award amounts generated by the performance metrics.
Long-Term Incentive Compensation
The Compensation Committee believes that the value of long-term incentives should be directly related to the performance of Mylan’s ordinary shares, as well as other operational and financial measures associated with the growth and success of Mylan. The long-term equity grants awarded to the NEOs in 2015 under the Company’s Amended and Restated 2003 Long-Term Incentive Plan (the “Amended 2003 Plan”) included:
| • | Performance-based restricted stock units (“PRSUs”) that cliff-vest after the end of the applicable performance period, assuming specified performance criteria are met and provided that the NEO remains continually employed by Mylan; |
| • | Stock options with an exercise price equal to the closing price of Mylan’s ordinary shares on the date of grant, which vest in three equal installments, provided that the NEO remains continually employed by Mylan; and |
| • | Restricted stock units (“RSUs”) that vest in three equal installments, provided that the NEO remains continually employed by Mylan. |
25
Table of Contents
Timing of Equity Award Grants. The Compensation Committee has historically approved annual equity grants in the first quarter of the fiscal year, which grants were made following the release of year-end audited financial results, with exceptions for new hires, promotions, and other special awards, grants, or circumstances, although there is no exact date for the making of these equity grants each year. In 2015, equity grants for Mylan employees other than the NEOs were made in the first quarter of the fiscal year, consistent with past practice. The Compensation Committee seeks the advice of external experts in executive compensation and reviews its equity grant practices from time to time with respect to corporate best practices.
Performance Metrics. Again consistent with comments received during investor outreach over the past several years, the Compensation Committee utilizes performance metrics for the long-term incentive program that compare the Company’s performance against that of its peers. Since 2013, the Compensation Committee has used the TSR of Mylan’s ordinary shares relative to that of peer companies as one of the metrics to which the PRSUs are subject. The other metric designated by the Compensation Committee for periods through December 31, 2015 was Cash Return on Operating Invested Capital, excluding goodwill and intangibles (“ROIC”). TSR and ROIC are each weighted 50% in the determination of the percentage of PRSUs that can be earned. The Compensation Committee believes that the use of these two metrics provides balance by rewarding the NEOs both for our performance relative to peer companies and for the returns generated by investments in our business. The following table shows the 2015 threshold, target, and maximum goals and relative weightings.
| Metric |
Weighting | Threshold | Target | Maximum | ||||
|
ROIC* |
50% | 34% | 38% | 42% | ||||
| Relative TSR* * |
50% | 25th Percentile of Peer Group |
50th Percentile of Peer Group |
75th Percentile of Peer Group | ||||
| Opportunity |
N/A | 50% | 100% | 150% |
| * | ROIC is calculated from Mylan’s audited financial statements in the same manner as set forth in the reconciliations provided in Appendix A. |
| ** | Relative TSR is calculated by comparing the difference between Mylan’s 30-day trailing average closing ordinary share price at the beginning of the performance period and the end of the performance period plus any dividends paid during the performance period against the same metric for each company in our life sciences peer group. |
2015 Long-Term Incentive Awards. Consistent with comments from shareholders during outreach to investors over the past several years, the Compensation Committee again maintained an increased percentage of performance-based equity awards compared to the percentage of time-based equity awards. In 2015, each NEO received a grant of long-term equity awards with a targeted value at grant equal to a percentage of their base salary. The allocation of the award among PRSUs, stock options, and RSUs for each NEO was 60%, 20%, and 20%, respectively, of the NEO’s total long-term incentive award. The Compensation Committee believes that maintaining a higher percentage of the total NEO’s award that is specifically performance based further supports alignment between the Company’s performance, shareholder interests, and executive compensation.
Each PRSU entitles the recipient to a number of ordinary shares equal to between 50% and 150% of the target award, depending on actual achievement of the performance metrics outlined above over a two-year period. Achievement of threshold goals results in delivery of ordinary shares with respect to 50% of PRSUs granted, achievement of target goals results in delivery of ordinary shares with respect to 100% of PRSUs granted, and achievement of maximum goals results in delivery of ordinary shares with respect to 150% of PRSUs granted.
Other Benefits and Agreements
Mylan provides additional benefits to the NEOs in the form of:
| • | Perquisites |
| • | Retirement Benefits |
26
Table of Contents
Perquisites. Perquisites include the following:
| • | Each NEO receives the use of a Company car or a car allowance. The NEOs are responsible for paying any taxes incurred relating to this perquisite. |
| • | Our senior executives take an extraordinarily active approach to overseeing and managing our global operations, which necessitates a significant amount of domestic and international travel time due to our diverse set of business centers, manufacturing and other facilities, and many client and vendor locations around the world. Mylan provides management access to corporate aircraft to assist in the management of Mylan’s global platform by providing a more efficient and secure traveling environment, including where sensitive business issues may be discussed or reviewed, as well as maximum flexibility to our executives in the conduct of Company business. For reasons of business efficiency and continued security-related concerns (including personal security, especially given the global nature of Mylan’s business, as well as privacy of business information and communications), we require Mr. Coury and Ms. Bresch to use Mylan aircraft for business and personal purposes. During 2015, other executives from time to time also were authorized to have personal use of the corporate aircraft for similar reasons. The Compensation Committee monitors business and personal aircraft usage on a periodic basis. To the extent any travel on the corporate aircraft results in imputed taxable income to a NEO, Mylan does not provide gross-up payments to cover the NEO’s personal income tax obligation due to such imputed income. For a summary of how this perquisite is calculated, see footnote (b) to the Summary Compensation Table on page 37 of this Amendment. |
| • | Executives will also receive tax equalization payments for incremental tax liabilities, if any, incurred as a result of attendance at board meetings in the United Kingdom. |
Retirement Benefits. Mylan has entered into RBAs (as defined below) with four of the NEOs — Ms. Bresch and Messrs. Coury, Sheehan, and Malik — in recognition of their service to Mylan, to encourage their retention and to provide a supplemental form of retirement and death benefit. For a detailed description of the RBAs, see the section below entitled “Retirement Benefit Agreements” beginning on page 43 of this Amendment. Mylan also maintains a 401(k) Restoration Plan (the “Restoration Plan”) and an Income Deferral Plan permitting senior level employees to elect to defer the receipt of a portion of their compensation and, in the case of the Restoration Plan, providing matching contributions to employees that make such an election; however, effective April 1, 2013, Mylan modified the Restoration Plan so that U.S. employees with an RBA would no longer receive matching contributions under the Restoration Plan.
The Compensation Committee approved an amendment to Mr. Coury’s RBA in October 2011, in connection with his retention and the enhanced management transition, to provide a retention incentive in his newly created role as Executive Chairman of the Mylan Board. Mr. Coury’s RBA vested in full on January 1, 2014. On February 24, 2014, the Compensation Committee approved an additional amendment to Mr. Coury’s RBA to provide a fixed discount rate to be used for purposes of determining the present value of the retirement benefits provided to Mr. Coury pursuant to the RBA, so that Mylan’s obligation would no longer be subject to variations in the appropriate discount rate.
When Mr. Malik joined Mylan in January 2007, Mylan established a nonqualified deferred compensation plan on his behalf. Although Mylan no longer contributes to the account, the plan account will be distributed to him upon Mylan’s termination of Mr. Malik’s employment, or upon other qualifying distribution events, such as his retirement, disability, or death, or Mylan’s termination of the plan.
The Summary Compensation Table includes changes in pension values calculated based on certain actuarial assumptions regarding discount rates. As discussed above, Mr. Coury’s RBA was amended to fix the discount rate used in determining his pension value. In computing these amounts, we used the same assumptions that were used to determine the expense amounts recognized in our 2015 financial statements. In 2015, the impact of a decrease in
27
Table of Contents
the applicable discount rates led to an increase in the present value of accumulated benefits of approximately $129,954 for Ms. Bresch, approximately $13,091 for Mr. Sheehan, and approximately $37,751 for Mr. Malik.
Each of the relevant NEOs executed a one-time waiver providing that the EPD Transaction did not constitute a change in control for purposes of the RBAs.
Employment Agreements. We believe it is important to have employment agreements with our executive officers and other key employees. These agreements memorialize certain key terms of employment, including termination rights and obligations, non-competition and other restrictive covenants, and compensation and perquisites, and we believe thereby enhance the stability and continuity of our employment relationships. Each of the NEOs is party to an Executive Employment Agreement with Mylan. For a detailed description, see the section below entitled “Employment Agreements” beginning on page 45 of this Amendment. See also page 34 of this Amendment for a discussion of the 2015 and 2016 extensions of the Executive Employment Agreement for Mr. Mauro.
Transition and Succession Agreements. Mylan is party to separate Transition and Succession Agreements with each NEO with an aim to assuring that Mylan will have the NEO’s full attention and dedication to Mylan during the pendency of a possible change in control transaction that might optimize shareholder value, and to provide the officer with compensation and benefits in connection with a change in control. These agreements are independent of each such NEO’s employment agreement. Subsequent to the execution of these agreements, Mylan adopted a policy that no new Transition and Succession Agreements will provide for an excise tax gross–up for golden parachute payments. For legal and other considerations, the Transition and Succession Agreements currently in effect and executed prior to the new policy are not subject to that policy. Mylan does not have the right to unilaterally abrogate pre-existing binding contracts with its executives, and does not believe it would be in shareholders’ best interests to expend funds to “buy out” the executives from these rights. Since implementation of the new policy, no new or amended Transition and Succession Agreements with excise tax gross-up provisions have been executed and several have expired as executives have retired from Mylan (as was the case with the retirements of Hal Korman and John Sheehan over the last several years).
For a detailed description of these Transition and Succession Agreements, see below, under “Termination Under Transition and Succession Agreements (Change in Control)” beginning on page 48 of this Amendment.
Each of the relevant executive officers executed a one-time waiver providing that the EPD Transaction did not constitute a change in control for purposes of the Transition and Succession Agreements.
Compensation Committee Considerations in Evaluating Compensation
Our culture and our success continue to depend on our ability to attract and retain talented people in critical roles. The independent Directors believe that the remarkable growth and performance of Mylan during the past decade is directly related to the unique leadership of Mr. Coury, Ms. Bresch, and Mr. Malik, and the talents of Mylan’s other senior executives, as well as Mylan’s outstanding workforce around the world.
The decisions of the Compensation Committee and the independent Directors relating to executive compensation each year reflect a variety of subjective considerations, in addition to raw metrics. Our determinations reflect our individual and collective experience and business judgment, and are based on our extensive interactions with, and observations of, management, and our assessment of some or all of the following factors, among others:
| • | Company performance (relative to peers and budget); |
| • | Value realized by shareholders; |
| • | Individual performance and contributions to the success of Mylan; |
28
Table of Contents
| • | Responsibilities of, and future expectations for, the individual; |
| • | Short-, medium-, and long-term personnel needs of Mylan; |
| • | The need to reward and retain our uniquely talented NEOs and other key employees; |
| • | Other qualitative contributions of each executive, including, among others, the actual and potential value and impact of his or her leadership style, strategic vision and execution, talent development, and ability to adapt to and drive the change necessary to our success; and |
| • | Peer group pay levels and published survey data. |
We consider these and other qualitative and quantitative factors from time-to-time in assessing our compensation philosophy and approach, in addition to using these factors to make individual compensation decisions. The Compensation Committee and the independent Directors believe that, while peer groups may be helpful reference points, they do not substitute for the individual and collective judgment and experience of independent Directors who are intimately familiar with, among other matters that the Mylan Board oversees and opines on, Mylan, its business, its strategies, its challenges, its opportunities, and the unique respective talents, contributions, leadership, responsibilities, and future expectations of the executives who drive performance and long-term sustainability.
Peer Group
While the competitive market for our executives is one factor the Compensation Committee considers when making compensation decisions, the Compensation Committee does not target compensation of NEOs within a specific percentile of any set of peer companies. As noted, we use peer groups as one of many factors considered when determining compensation.
After review and consideration of these factors and consultation with experts in executive compensation, we developed the peer group listed below for 2015. The Compensation Committee refers to the peer group as a reference point when evaluating executive pay and performance. As was the case previously, pay is not formulaically tied to a particular percentile of the peer group. Instead, this group is considered as part of the overall mix of subjective, qualitative, and quantitative information considered by the Compensation Committee.
This group consists of companies with revenues ranging from approximately 0.5x-2.5x Mylan’s revenue. Because the generic pharmaceutical market is limited, we include companies in the following GICS industries — Pharmaceuticals, Health Care Equipment & Supplies, Biotechnology, and Life Sciences Tools & Services:
| AbbVie Inc. |
Boston Scientific Corp. |
Perrigo Company plc | ||
| Actavis plc* |
Bristol-Myers Squibb Company |
St. Jude Medical Inc. | ||
| Agilent Technologies Inc. |
Celgene Corp. |
Stryker Corp. | ||
| Allergan, Inc.* |
Eli Lilly and Company |
Teva Pharmaceutical Industries Ltd. | ||
| Amgen Inc. |
Endo International plc |
Thermo Fisher Scientific Inc. | ||
| Baxter International Inc. |
Gilead Sciences, Inc. |
Zimmer Biomet Holdings, Inc. | ||
| Becton Dickinson & Co. |
Hospira Inc.* |
|||
| Biogen Inc. |
Medtronic plc |
|||
| * | Hospira Inc. has since been acquired and Actavis plc acquired Allergan, Inc. and became Allergan plc. |
Role of Compensation Committee, Consultants, and Management
In 2015, the Compensation Committee retained Meridian Compensation Partners, LLC (“Meridian”) to provide advice and information regarding the design and implementation of Mylan’s executive compensation
29
Table of Contents
programs. Meridian also provided information to the Compensation Committee regarding regulatory and other technical developments that may be relevant to Mylan’s executive compensation programs. In addition, Meridian provided the Compensation Committee with competitive market information, analyses, and trends on executive base salary, annual incentives, long-term incentives, benefits, and perquisites.
The Compensation Committee and management also receive advice from outside counsel including, but not limited to, Cravath, Swaine & Moore LLP.
The Compensation Committee also receives input from management; however, decisions on these matters are made solely by the Compensation Committee and/or the independent Directors.
The Compensation Committee performs an annual review of the independence of its outside advisors, consistent with NASDAQ requirements and the Compensation Committee charter.
Consideration of Risk in Company Compensation Policies
Management and the Compensation Committee have considered and discussed the risks inherent in our business and the design of our compensation plans, policies, and programs that are intended to drive the achievement of our business objectives. We believe that the nature of our business, and the material risks we face, are such that the compensation plans, policies, and programs we have put in place are not reasonably likely to give rise to risks that would have a material adverse effect on our business. We believe that the mix and design of the elements of executive compensation do not encourage management to assume excessive risks. In addition, the Chairmen of the Audit and Compliance Committees serve on the Compensation Committee, giving Mylan the benefit of the breadth of their perspective regarding the impact of compensation-related decisions on the Company. Finally, as described in this Compensation Discussion and Analysis, our compensation programs and decisions include qualitative factors which we believe restrain the influence that an overly formulaic approach may have on excessive risk-taking by management.
Deductibility Cap on Executive Compensation
Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”), restricts the deductibility for federal income tax purposes of the compensation paid to the CEO and each of the other NEOs (other than our Chief Financial Officer) for any fiscal year to the extent that such compensation for such executive exceeds one million dollars and does not qualify as “performance-based” compensation as defined under Section 162(m) of the Code. The Compensation Committee generally takes available opportunities to be able to deduct compensation paid to NEOs for federal income tax purposes. The Compensation Committee, however, reserves the right to grant compensation to our executives that is not deductible, including but not limited to when necessary to comply with contractual commitments, or to maintain the flexibility needed to attract talent, promote retention, or recognize and reward desired performance.
Clawback Policy
The Mylan Board has approved a clawback policy relating to incentive compensation programs. The provisions of the policy allow Mylan to recoup certain bonus and equity-based incentive compensation gains resulting from specified misconduct that causes Mylan to materially restate its financial statements. The Mylan Board intends to review and consider updates to this policy from time to time. In addition, to the extent that the SEC adopts rules for clawback policies that require changes to our policy, we will revise our policy accordingly.
Anti-Hedging and Pledging Policy
The Mylan Board has approved a securities trading policy that prohibits Directors and certain executive officers from engaging in any transaction designed to limit or eliminate economic risks associated with the ownership of our equity or debt securities by trading in certain types of hedging instruments relating to any of our securities. Hedging instruments include prepaid variable forward contracts, equity swaps, collars, exchange
30
Table of Contents
funds, insurance contracts, short sales, options, puts, calls, or other instruments designed to hedge or offset movements in the price of our stock or debt. The policy also prohibits Directors and certain executive officers from entering into transactions that involve the holding of Mylan securities in margin accounts (other than the “cashless exercise” of stock options) or the pledging of Mylan equity or debt securities as collateral for loans, with certain exceptions approved by the Compensation Committee if the executive demonstrates that he or she has the continuing financial capacity to repay any underlying loan or potential margin call without resorting to Mylan equity or debt securities. To the extent that the SEC adopts rules for anti-hedging and pledging policies that require changes to our policy, we will revise our policy accordingly.
Ordinary Share Ownership Requirements for NEOs
The ownership requirements are expressed as a multiple of base salary as follows:
| Position |
Ownership Requirement (multiple of base salary) | |
| Executive Chairman |
6x | |
| CEO |
6x | |
| President |
4x | |
| Other NEOs |
3x |
In addition to the NEOs, Mylan’s ordinary share ownership policy covers approximately 150 of the most senior executives at Mylan to promote an ownership culture and stronger alignment with the interests of shareholders among the broader leadership team. Each executive generally has five years from the adoption of the policy to achieve the minimum ownership requirement. Ordinary shares actually owned by the executive (including ordinary shares held by the executive in Mylan’s 401(k) and Profit Sharing Plan), as well as restricted ordinary shares and unvested RSUs and PRSUs count toward compliance with these requirements.
As of December 31, 2015, all of the NEOs were in compliance with these requirements.
Significant Recent Compensation Actions and Strong Shareholder Support
and Approval for These Actions
Mylan implemented several important compensation actions in 2014 and 2015. Among these were the implementation of the One-Time Special Performance-Based Five-Year Realizable Value Incentive Program (the “One-Time Special Performance-Based Incentive Program”), described in further detail in the Form 10-K/A for Mylan Inc.’s fiscal year ended December 31, 2014 and certain actions taken in connection with the EPD Transaction, as described below. Consistent with Mylan’s robust shareholder outreach program over the past few years, Mylan approached each of these actions with a view toward the best interests of shareholders, and Mylan Inc.’s shareholders were strongly supportive of these actions. The One-Time Special Performance-Based Incentive Program was described in extensive detail in the Proxy Statement for Mylan Inc.’s 2014 Annual Meeting of Shareholders. At that meeting the advisory proposal on compensation matters received the strong support and approval of Mylan’s shareholders. In addition, the compensation actions taken in connection with the EPD Transaction were described in extensive detail in the Proxy Statement for Mylan Inc.’s Special Meeting of Shareholders regarding the EPD Transaction. At that meeting the advisory proposal on compensation matters relating to the EPD Transaction received the overwhelming support and approval by over 80% of Mylan’s shareholders.
Treatment of Equity-Based Awards in Connection with the EPD Transaction
The compensation actions described below, which were taken in connection with the EPD Transaction, were extensively described in the Proxy Statement for Mylan Inc.’s Special Meeting of Shareholders regarding the EPD Transaction. At that meeting the advisory proposal on compensation matters received the overwhelming support and approval of Mylan’s shareholders.
31
Table of Contents
As explained in the Form 10-K/A for Mylan Inc.’s fiscal year ending December 31, 2014, the EPD Transaction had certain implications under Mylan’s compensation plans and programs and individual arrangements with certain employees of Mylan (including the NEOs) and also implicated the excise tax under Section 4985 of the Code on the value of certain equity-based awards held by the Directors and NEOs (the “Transaction-Related Excise Tax”). Section 4985 of the Code imposes the Transaction-Related Excise Tax (15% in 2014 and 2015) on the value of certain equity-based compensation held at any time during the six months before and six months after the closing of certain inversion transactions by individuals who were and/or are directors or executive officers of the parties to the transactions and subject to the reporting requirements of Section 16 of the Exchange Act during the same period. The Transaction-Related Excise Tax applies to all payments (or rights to payment) granted to such persons by the party to the transaction to which the individual provides services and its affiliates in connection with the performance of such services if the value of such payment or right is based on (or determined by reference to) the value (or change in value) of stock in the applicable entity or its affiliates (excluding incentive stock options (“ISOs”) and holdings in tax-qualified plans), which would include any outstanding (i) unexercised vested or unvested nonqualified stock options or stock appreciation rights (“SARs”), (ii) unvested restricted stock awards, (iii) RSUs and PRSUs, and (iv) other equity compensation, in each case, held by such directors and executive officers during this twelve-month period. However, the Transaction-Related Excise Tax does not apply to (i) any stock option or SAR that is exercised prior to the closing of the inversion transaction if income is recognized under Section 83 of the Code on or before such date with respect to the shares acquired as a result of such exercise and (ii) any other specified equity-based compensation that is exercised, sold, exchanged, distributed, cashed out, or otherwise paid prior to the closing in a transaction in which income, gain, or loss is recognized in full.
As explained in the Form 10-K/A for Mylan Inc.’s fiscal year ending December 31, 2014, the Board of Mylan Inc. carefully reviewed the two primary approaches taken by other issuers in transactions similar to the EPD Transaction with respect to the Transaction-Related Excise Tax: (i) accelerating the vesting of equity-based awards such that stock options may be exercised, and other equity-based awards are settled, prior to the transaction so that the Transaction-Related Excise Tax does not apply to them or (ii) providing Directors and NEOs with a tax reimbursement payment for the cost of the Transaction-Related Excise Tax. After such review, including consultation with external experts, the Board of Mylan Inc. determined that neither approach alone would be in the interests of Mylan nor accomplish and appropriately balance the objectives of minimizing costs, maintaining proper incentives, and not diminishing the retentive and motivating effect of the Directors’ and NEOs’ equity awards by depriving them of a substantial portion of the value. In particular, the Board of Mylan Inc. determined that, given the unique terms and structure of the EPD Transaction, it would be an inefficient use of shareholder resources to provide the Directors and NEOs with a tax reimbursement payment covering all outstanding equity-based awards, especially when some of the covered awards were already vested or would vest in the ordinary course in a relatively short period following the EPD Transaction. As a result, the Board of Mylan Inc. determined to utilize a hybrid of these two approaches, which took into account a variety of factors, including the purpose of the types of equity-based awards held by the Directors and NEOs and the remaining vesting period of the applicable awards.
In reaching this determination, the Board of Mylan Inc. carefully considered the appropriate manner in which to treat the equity-based awards of the Directors and NEOs in connection with the EPD Transaction and determined that the overall treatment described below served to: (i) minimize cost to Mylan; (ii) maintain proper incentives for the affected individuals to remain with Mylan and to continue achieving exceptional operating performance, long-term financial objectives, and the creation of shareholder value as they have consistently done in the past; and (iii) ensure that the Directors and NEOs did not bear the burden of the Transaction-Related Excise Tax, which did not apply to other Mylan shareholders and would have deprived them of a substantial portion of the value of their equity-based awards, when they were critically important to Mylan’s past success and in negotiating the transformative opportunity for Mylan represented by the EPD Transaction and continue to be critically important to its successful implementation and execution, and our future strategy and performance.
32
Table of Contents
Ordinary Course Annual Equity-Based Awards Other than Stock Options Granted in 2014. The Board of Mylan Inc. determined that the vesting of all unvested stock options, RSUs, and PRSUs granted to Directors and NEOs as part of Mylan’s ordinary course annual equity compensation program, other than ISOs (which are not subject to the Transaction-Related Excise Tax) and the stock options granted in 2014 (because of their recent grant and, therefore, strong incentive for retention and shareholder value creation), would be accelerated prior to the closing of the EPD Transaction. The Board of Mylan Inc. believed that this approach was advisable and in the best interests of Mylan because it avoided the expense to Mylan of providing a tax reimbursement payment for the Transaction-Related Excise Tax with respect to these awards, which the Board of Mylan Inc. believed the Directors and NEOs would likely have eventually received even absent the EPD Transaction given Mylan’s expected future performance.
One-Time Special Performance-Based Incentive Program and 2014 Stock Option Grants. As discussed in further detail in the Form 10-K/A for Mylan Inc.’s fiscal year ended December 31, 2014, in March 2014, the Board of Mylan Inc. granted awards under the One-Time Special Performance-Based Incentive Program to retain and further align more than 100 key employees with long-term shareholder interests and further motivate them to achieve Mylan’s ambitious goals of achieving at least $6.00 of adjusted diluted EPS by the end of 2018 and deliver significant additional shareholder value over that period. In addition, because of their recent grant and, therefore, strong incentive for retention and shareholder value creation, the vesting of awards granted under the One-Time Special Performance-Based Incentive Program and the stock options granted in 2014 were not accelerated in connection with the EPD Transaction. Instead, the Board of Mylan Inc. determined that the Directors and NEOs would receive a tax reimbursement payment from Mylan with respect to the Transaction-Related Excise Tax imposed on awards granted under the One-Time Special Performance-Based Incentive Program and the stock options granted in 2014, so that, on a net after-tax basis, they would be in the same position as if the Transaction-Related Excise Tax had not been imposed. The Board of Mylan Inc. believed that the exceptional and unique nature of this program and the strong incentives inherent in the stock options granted in 2014 warranted the limited cost of the tax reimbursement payment, particularly when viewed in relation to both the anticipated benefits of the EPD Transaction and, with respect to the awards under the One-Time Special Performance-Based Incentive Program, the shareholder value that is expected to be created if the goal of achieving adjusted diluted EPS of at least $6.00 by the end of 2018 is achieved. Payment of the tax reimbursement resulted in no unique benefit to the Directors and NEOs but only placed them in the same position as other equity-based award holders after the EPD Transaction. The Board of Mylan Inc. determined that no director or NEO would receive a tax reimbursement payment for any Transaction-Related Excise Tax imposed on stock options granted prior to 2014 that such director or NEO was able to but chose not to exercise prior to the closing of the EPD Transaction.
Update on One-Time Special Performance-Based Five-Year Realizable Value Incentive Program
As described in the Proxy Statement for Mylan Inc.’s 2014 Annual Meeting of Shareholders, in February 2014 Mylan adopted the One-Time Special Performance-Based Incentive Program to retain more than 100 key employees and to incentivize them toward the achievement of Mylan’s ambitious long-term objective of achieving adjusted diluted EPS of at least $6.00 by the end of 2018, which the Mylan Board believed and continues to believe will lead to the corresponding creation of significant shareholder value. This innovative, new, wholly performance-based program was a continuation of Mylan’s robust pay-for-performance philosophy. At Mylan Inc.’s 2014 Annual Meeting of Shareholders, shareholders strongly supported the advisory vote on compensation, which included the One-Time Special Performance-Based Incentive Program.
As demonstrated above, Mylan has made great progress toward the achievement of its long-term objective of achieving adjusted diluted EPS of at least $6.00 by the end of 2018, which the Mylan Board believes is linked, in no small part, to the powerful design and incentives provided by this program. During 2015, because Mylan’s share price exceeded the target share price under the program for 10 consecutive trading days, the SARs held by the NEOs converted to a fixed number of restricted Mylan ordinary shares. These ordinary shares remain unvested, however, and will be earned by the NEOs in full only if Mylan reaches its adjusted diluted EPS target
33
Table of Contents
and the NEOs remain with Mylan through the end of 2018 (subject to certain limited exceptions). Participants will be eligible for 50% vesting if we achieve 90% of our adjusted diluted EPS target ($5.40 per share), with linear interpolation between $5.40 and $6.00 per share.
Evaluation with Respect to Performance Award Granted to Mr. Coury in 2014
As described in the Proxy Statement for Mylan Inc.’s 2014 Annual Meeting of Shareholders, in February 2014, Mr. Coury was granted a $20 million performance incentive opportunity in connection with the extension of his employment agreement. The award will be earned only if Mr. Coury satisfactorily performs his key leadership responsibilities and the requirements of his employment agreement through December 31, 2016 and remains employed by Mylan through such date. At Mylan Inc.’s 2014 Annual Meeting of Shareholders, shareholders strongly supported the advisory vote on compensation, which included Mr. Coury’s performance incentive award.
The Mylan Board continues to evaluate Mr. Coury’s performance to assess, among other things, whether Mr. Coury is satisfying the performance requirements of this award. In evaluating Mr. Coury’s performance through 2015, the Mylan Board noted Mr. Coury’s significant leadership achievements since the award was granted, including, among others, his continued strong overall leadership of Mylan, mentorship of executives, shareholder engagement, and unique and successful strategic vision that has led to the continued growth of Mylan’s global operating platform and strong financial results over the past several years, including significant short- and long-term value creation for shareholders and other stakeholders, and his execution, completion, and/or possible completion of the EPD Transaction, Famy Care Transaction, and Meda Transaction, each of which is expected to have a significant role in the creation of shareholder value over the long term.
Developments in Early 2016
Promotion of Anthony Mauro and Extension of Executive Employment Agreement
In early 2015, Mylan took action to extend the term of Mr. Mauro’s Executive Employment Agreement with Mylan through the end of 2015. As noted above, in recognition of Mr. Mauro’s performance in his role as President, North America and increasing role with Mylan, in early 2016, Mr. Mauro was promoted to Chief Commercial Officer, effective January 4, 2016, and his Executive Employment Agreement with Mylan was amended and restated effective January 1, 2016. The Amended and Restated Executive Employment Agreement automatically renews on each anniversary of the effective date unless earlier terminated by Mr. Mauro or Mylan. The Amended and Restated Executive Employment Agreement contains substantially the same terms as Mr. Mauro’s previous contract, except that Mr. Mauro’s base salary was increased to $700,000 in connection with his promotion to Chief Commercial Officer.
Retirement of Chief Financial Officer
John D. Sheehan, former Executive Vice President and Chief Financial Officer of Mylan, retired effective April 1, 2016. In connection with Mr. Sheehan’s retirement from the Company, to secure certain consulting services, and in order to facilitate the transition of Mr. Sheehan’s responsibilities, Mr. Sheehan and Mylan Inc. entered into a Retirement and Consulting Agreement. The agreement provides that Mr. Sheehan will provide consulting services to Mylan for one year following his retirement date. Pursuant to the agreement, Mr. Sheehan will receive an amount equal to his annual base salary, payable in four equal installments on or around the end of the first four fiscal quarters following his retirement date, and COBRA health and welfare benefits during the consulting period, and he will be treated as retirement eligible for purposes of his outstanding stock options. Mr. Sheehan will also remain subject to all restrictive covenants with Mylan pursuant to their terms.
34
Table of Contents
Compensation Best Practices
Based on shareholder outreach over the past several years, as well as the Mylan Board’s own independent analysis and initiatives, we have implemented numerous robust compensation-related policies, including, among others, those noted below.
| What We Do |
What We Don’t Do | |
| Maintain a significant portion of compensation aligned with shareholder interests and tied to stock price or financial and operational business performance |
No exercise of positive discretion in determining annual incentive payouts | |
| Balance annual and long-term incentives |
No re-pricing of stock options | |
| Employ balanced and different metrics for annual and long-term incentives |
No pledging of Company ordinary shares | |
| Long-term incentives heavily weighted to performance-based metrics: 60% PRSUs, 20% RSUs and 20% stock options |
No new 280G tax gross ups | |
| Double-trigger vesting for annual equity-based awards upon a change in control |
No Company matching contributions to the Restoration Plan for NEOs with RBAs | |
| Consider peer groups and market data in determining compensation |
||
| Retain an independent compensation consultant that reports directly to the Compensation Committee |
||
| Maintain robust stock ownership guidelines, which our senior management significantly exceeds |
||
| Maintain a robust clawback policy |
||
| Conduct an annual compensation-related risk review to ensure that compensation is aligned with shareholder interests |
||
Compensation Committee Report
We have reviewed and discussed the Compensation Discussion and Analysis with management. Based on such review and discussions, we recommended to the Mylan Board that the Compensation Discussion and Analysis be included in this Amendment.
Respectfully submitted,
Wendy Cameron
Neil Dimick
Mark W. Parrish
35
Table of Contents
Summary Compensation Table
The following summary compensation table sets forth the cash and non-cash compensation paid to or earned by the NEOs for 2015, 2014, and 2013.
| Name and |
Fiscal Year |
Salary ($)(2) |
Bonus ($) |
Stock Awards ($)(3) |
Option Awards ($)(4) |
Non-Equity Incentive Plan Compensation ($)(5) |
Changes in Pension Value and Non- qualified Deferred Compensation Earnings ($)(6) |
All Other Compensation ($)(7) |
Total ($) |
Total without Transaction- Related Excise Tax Reimbursement ($)(8) |
||||||||||||||||||||||||||||||
| Heather Bresch Chief Executive Officer |
2015 | 1,330,769 | — | 5,200,046 | 1,300,007 | 3,900,000 | 768,216 | 6,432,030 | 18,931,068 | 13,102,073 | ||||||||||||||||||||||||||||||
| 2014 | 1,180,769 | — | 4,800,007 | 14,401,997 | 3,259,800 | 1,546,776 | 633,477 | 25,822,826 | 25,822,826 | |||||||||||||||||||||||||||||||
| 2013 | 1,080,769 | — | 3,960,020 | 995,198 | 2,200,000 | 339,202 | 471,971 | 9,047,160 | 9,047,160 | |||||||||||||||||||||||||||||||
| John D. Sheehan Former Executive Vice President and Chief Financial Officer (1) |
2015 | 675,000 | — | 1,560,074 | 390,008 | 1,300,000 | 355,679 | 1,235,718 | 5,516,479 | 4,447,422 | ||||||||||||||||||||||||||||||
| 2014 | 650,000 | — | 1,300,011 | 2,682,497 | 1,177,150 | 341,795 | 177,821 | 6,329,274 | 6,329,274 | |||||||||||||||||||||||||||||||
| 2013 | 650,000 | — | 1,299,994 | 326,706 | 1,040,000 | 237,114 | 216,469 | 3,770,283 | 3,770,283 | |||||||||||||||||||||||||||||||
| Rajiv Malik President |
2015 | 1,019,231 | — | 3,200,041 | 800,017 | 2,500,000 | 970,676 | 11,411,770 | 19,901,735 | 15,042,664 | ||||||||||||||||||||||||||||||
| 2014 | 890,385 | — | 2,520,003 | 11,946,006 | 1,874,385 | 649,051 | 7,284,822 | 25,164,652 | 25,164,652 | |||||||||||||||||||||||||||||||
| 2013 | 840,385 | — | 2,380,011 | 598,127 | 1,564,000 | 429,750 | 2,384,328 | 8,196,601 | 8,196,601 | |||||||||||||||||||||||||||||||
| Anthony Mauro Chief Commercial Officer |
2015 | 634,615 | 1,250,036 | 312,517 | 1,437,500 | — | 1,216,500 | 4,851,168 | 3,830,446 | |||||||||||||||||||||||||||||||
| 2014 | 545,192 | — | 879,983 | 2,577,505 | 996,050 | — | 240,881 | 5,239,611 | 5,239,611 | |||||||||||||||||||||||||||||||
| Robert J. Coury Executive Chairman |
2015 | 1,401,923 | — | 4,860,067 | 1,215,004 | 3,375,000 | 1,606,533 | 5,242,131 | 17,700,658 | 13,428,369 | ||||||||||||||||||||||||||||||
| 2014 | 1,350,000 | — | 4,320,005 | 10,510,001 | 3,056,063 | 2,404,435 | 883,086 | 22,523,590 | 22,523,590 | |||||||||||||||||||||||||||||||
| 2013 | 1,350,000 | — | 4,319,975 | 1,085,667 | 2,700,000 | 4,796,967 | 1,157,391 | 15,410,000 | 15,410,000 | |||||||||||||||||||||||||||||||
| (1) |
Mr. Sheehan retired from the Company effective April 1, 2016. |
| (2) | Represents the value of the base salary actually paid to the NEOs in 2015. The annual base salary approved by the Compensation Committee for each of the NEOs is payable in accordance with the Company’s normal payroll practices for its senior executives, so that an NEO’s total base salary amount is paid to him or her in 26 equal bi-weekly installments. 2015 included an additional payment date (a total of 27 payments were made in 2015), therefore the amounts shown for 2015 are greater than the applicable NEO’s annual base salary. |
| (3) | Represents the grant date fair value of the stock awards granted to the NEO in 2015, 2014, and 2013, as applicable. For information regarding assumptions used in determining such expense, please refer to Note 11 to the Company’s Consolidated Financial Statements contained in the Original Filing. |
| (4) | Represents the grant date fair value of the option awards granted to the NEO in 2015, 2014, and 2013, as applicable. For information regarding assumptions used in determining such expense, please refer to Note 11 to the Company’s Consolidated Financial Statements contained in the Original Filing. For 2014, also includes the grant date fair value of SARs granted under the One-Time Special Performance-Based Incentive Program, which were as follows: $13,202,000 for Ms. Bresch; $11,316,000 for Mr. Malik; $9,430,000 for Mr. Coury; and $2,357,500 for Messrs. Sheehan and Mauro. |
| (5) | Represents amounts paid under the Company’s non-equity incentive compensation plan. For a discussion of this plan, see the Compensation Discussion and Analysis set forth above. |
36
Table of Contents
| (6) | Represents the aggregate change in present value of the applicable NEO’s accumulated benefit under his or her respective RBA or the Amended Retirement Benefit Agreement (“Amended RBA”) for Mr. Coury. In computing these amounts, we used the same assumptions that were used to determine the expense amounts recognized in our 2015 financial statements. In 2015, the impact of a decrease in the applicable discount rates led to an increase in the present value of accumulated benefits of approximately $129,954 for Ms. Bresch, approximately $13,091 for Mr. Sheehan, and approximately $37,751 for Mr. Malik. For further information concerning the RBAs, see the Pension Benefits for 2015 table set forth below and the discussion under “Retirement Benefit Agreements” beginning on page 43 of this Amendment. |
| (7) | Amounts shown in this column are detailed in the chart on the next page. |
| (8) | In order to show the effect that the one-time tax reimbursement with respect to the Transaction-Related Excise Tax had on total compensation, as determined under applicable SEC rules, we have included an additional column to show total compensation less this item. The amounts reported in the Total without Transaction-Related Excise Tax Reimbursement column differ substantially from the amounts reported in the Total column required under SEC rules and are not a substitute for total compensation. Total without Transaction-Related Excise Tax Reimbursement represents total compensation, as determined under applicable SEC rules, minus, for 2015 only, the value of the one-time tax reimbursement with respect to the Transaction-Related Excise Tax reported in the All Other Compensation column. The tax reimbursement with respect to the Transaction-Related Excise Tax was a one-time payment so that, on a net after-tax basis, the NEO would be in the same position as if the Transaction-Related Excise Tax had not been imposed. |
| Fiscal Year |
Use
of Company- Provided Automobile ($)(a) |
Personal Use of Company Aircraft ($)(b) |
Lodging Reimbursement ($)(c) |
Expatriate Benefits ($)(d) |
401(k) and Profit Sharing Plan Matching and Profit Sharing Contribution ($)(e) |
Restoration Plan Contribution ($)(f) |
Transaction- Related Excise Tax Reimbursement ($)(g) |
Other ($)(h) |
||||||||||||||||||||||||||||
| Heather Bresch |
2015 | 19,200 | 310,312 | — | — | 28,792 | 218,454 | 5,828,995 | 26,277 | |||||||||||||||||||||||||||
| 2014 | 19,200 | 319,050 | — | — | 27,280 | 224,054 | — | 43,893 | ||||||||||||||||||||||||||||
| 2013 | 19,200 | 137,137 | — | — | 27,308 | 270,051 | — | 18,275 | ||||||||||||||||||||||||||||
| John D. Sheehan |
2015 | 19,200 | 4,506 | — | — | 28,800 | 100,100 | 1,069,057 | 14,055 | |||||||||||||||||||||||||||
| 2014 | 19,200 | — | — | — | 28,250 | 114,100 | — | 16,271 | ||||||||||||||||||||||||||||
| 2013 | 19,200 | 2,268 | — | — | 27,700 | 161,363 | — | 5,938 | ||||||||||||||||||||||||||||
| Rajiv Malik |
2015 | 23,392 | 29,557 | 50,000 | 6,333,891 | — | — | 4,859,071 | 115,859 | |||||||||||||||||||||||||||
| 2014 | 29,992 | 32,234 | 50,000 | 7,076,038 | — | — | — | 96,558 | ||||||||||||||||||||||||||||
| 2013 | 19,200 | 25,671 | 50,000 | 2,183,224 | — | — | — | 106,233 | ||||||||||||||||||||||||||||
| Anthony Mauro |
2015 | 19,200 | — | — | — | 28,800 | 131,918 | 1,020,722 | 15,860 | |||||||||||||||||||||||||||
| 2014 | 19,200 | — | — | 77,267 | 28,250 | 106,222 | — | 9,942 | ||||||||||||||||||||||||||||
| Robert J. Coury |
2015 | 38,931 | 605,255 | — | — | 28,800 | 265,300 | 4,272,289 | 31,556 | |||||||||||||||||||||||||||
| 2014 | 40,114 | 498,636 | — | — | 28,250 | 301,088 | — | 14,998 | ||||||||||||||||||||||||||||
| 2013 | 39,047 | 542,296 | — | — | 27,700 | 515,968 | — | 32,380 | ||||||||||||||||||||||||||||
| (a) | In the case of Ms. Bresch and Messrs. Sheehan and Mauro, these numbers represent a vehicle allowance. In the case of Messrs. Malik and Coury, this number represents the cost of a vehicle (based on lease value), insurance, and, in the case of Mr. Coury only, ancillary expenses associated with such vehicle. |
| (b) | Amounts disclosed represent the actual aggregate incremental costs incurred by Mylan associated with the personal use of the Company’s aircraft. Incremental costs include annual average hourly fuel and maintenance costs, landing and parking fees, customs and handling charges, passenger catering and ground transportation, crew travel expenses, away from home hanger fees, and other trip-related variable costs. Because the aircrafts are used primarily for business travel, incremental costs exclude fixed costs that do not change based on usage, such as pilots’ salaries, aircraft purchase or lease costs, home-base hangar costs, and certain maintenance fees. Aggregate incremental cost as so determined with respect to personal deadhead |
37
Table of Contents
| flights is allocable to the NEO. In certain instances where there are both business and personal passengers, the incremental costs per hour are pro-rated. |
| (c) | Represents a housing allowance afforded to Mr. Malik. |
| (d) | Expatriate benefits for Mr. Malik represent income taxes paid by Mylan in connection with Mr. Malik’s expatriate assignment to the United States from India effective January 1, 2012. Specifically, Mr. Malik is responsible for, and has continued to pay taxes equal to those he would have been obligated for had he maintained his principal work location and residence in India rather than having transferred, at Mylan’s request, to the United States, while Mylan has responsibility for all additional taxes, including Mr. Malik’s tax obligations on the imputed income associated with Mylan’s payment of taxes on his behalf. Amounts shown for 2015, 2014, and 2013 for Mr. Malik are net of Mylan’s estimated tax refunds for each year. Estimated refunds were approximately $1.1 million for 2015, $1.5 million for 2014, and $0.3 million for 2013. Expatriate benefits for Mr. Mauro represent income taxes paid by the Company in connection with certain equity awards held by Mr. Mauro relating to a period when he provided services in Canada. |
| (e) | In 2015, amounts disclosed for Ms. Bresch included a matched contribution of $10,592, and a profit sharing contribution from the Company of $18,200. In 2015, such amounts for each of Messrs. Sheehan, Mauro, and Coury were $10,600 and $18,200, respectively. In 2014, amounts disclosed for Ms. Bresch included the total of a $17,850 matched contribution and $9,430 in Company profit sharing, and for each of Messrs. Sheehan, Mauro, and Coury such amounts were $17,850 and $10,400, respectively. In 2013, amounts disclosed for Ms. Bresch included the total of a $9,808 matched contribution and $17,500 in Company profit sharing, and for each of Messrs. Sheehan and Coury such amounts were $10,200 and $17,500, respectively. Effective April 1, 2013, Ms. Bresch and Messrs. Sheehan and Coury are no longer eligible to receive matching contributions under the Restoration Plan. |
| (f) | Represents profit sharing contribution under the Restoration Plan. Effective April 1, 2013, Ms. Bresch and Messrs. Sheehan and Coury are no longer eligible to receive matching contributions under the Restoration Plan. See page 43 of this Amendment for further information regarding Restoration Plan contributions. |
| (g) | Represents the one-time tax reimbursement payment with respect to the Transaction-Related Excise Tax imposed on awards granted under the One-Time Special Performance-Based Incentive Program and the stock options granted in 2014, so that, on a net after-tax basis, the NEO would be in the same position as if the Transaction-Related Excise Tax had not been imposed. See pages 31 to 33 of this Amendment for further discussion of this payment. |
| (h) | Represents out-of-pocket medical, vision, health insurance, long-term disability, and life insurance retention plan premiums. For Mr. Malik, it also represents employee contributions to the Provident Fund, a statutory plan in India, and a health insurance premium. Also includes: events and memberships; certain security services; life insurance retention plan premium for Ms. Bresch and Mr. Mauro; long-term disability premium for Ms. Bresch and Messrs. Sheehan, Mauro, and Coury; and executive physicals for Ms. Bresch and Mr. Sheehan. |
38
Table of Contents
Grants of Plan-Based Awards for 2015
The following table summarizes grants of plan-based awards made to each NEO during 2015.
| Estimated Future Payments Under Non-Equity Incentive Plan Awards(1) |
Estimated Future Payments Under Equity Incentive Plan Awards(2) |
All
Other Stock Awards: Number of Shares of Stock or Units (#)(3) |
All
Other Option Awards: Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($/Sh) |
Grant Date Fair Value of Stock and Option Awards ($)(5) |
|||||||||||||||||||||||||||||||||||||||||||
| Grant | Approval | Threshold | Target | Maximum | Threshold | Target | Maximum | |||||||||||||||||||||||||||||||||||||||||
| Name |
Date | Date | ($) | ($) | ($) | (#) | (#) | (#) | ||||||||||||||||||||||||||||||||||||||||
| Heather Bresch |
975,000 | 1,950,000 | 3,900,000 | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | 38,492 | 76,984 | 115,476 | — | — | — | 3,900,009 | |||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | — | — | — | 25,662 | — | — | 1,300,037 | |||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | — | — | — | — | 67,659 | 50.66 | 1,300,007 | |||||||||||||||||||||||||||||||||||||
| John D. Sheehan |
325,000 | 650,000 | 1,300,000 | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | 11,548 | 23,096 | 34,644 | — | — | — | 1,170,043 | |||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | — | — | — | 7,699 | — | — | 390,031 | |||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | — | — | — | — | 20,298 | 50.66 | 390,008 | |||||||||||||||||||||||||||||||||||||
| Rajiv Malik |
625,000 | 1,250,000 | 2,500,000 | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | 23,688 | 47,375 | 71,063 | — | — | — | 2,400,018 | |||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | — | — | — | 15,792 | — | — | 800,023 | |||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | — | — | — | — | 41,637 | 50.66 | 800,017 | |||||||||||||||||||||||||||||||||||||
| Anthony Mauro |
359,375 | 718,750 | 1,437,500 | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | 9,253 | 18,506 | 27,759 | — | — | — | 937,514 | |||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | — | — | — | 6,169 | — | — | 312,522 | |||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | — | — | — | — | 16,265 | 50.66 | 312,517 | |||||||||||||||||||||||||||||||||||||
| Robert J. Coury |
843,750 | 1,687,500 | 3,375,000 | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | 35,976 | 71,951 | 107,927 | — | — | — | 3,645,038 | |||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | — | — | — | 23,984 | — | — | 1,215,029 | |||||||||||||||||||||||||||||||||||||
| 11/17/2015 | 11/17/2015 | — | — | — | — | — | — | — | 63,235 | 50.66 | 1,215,004 | |||||||||||||||||||||||||||||||||||||
| (1) | The performance goals under the annual incentive compensation program applicable to the NEOs during 2015 are described above in the Compensation Discussion and Analysis. |
| (2) | Consist of PRSUs awarded under the Amended 2003 Plan. The vesting terms applicable to these awards are described above in the Compensation Discussion and Analysis and below following the Outstanding Equity Awards at the End of 2015 table. |
| (3) | Consist of RSUs awarded under the Amended 2003 Plan. The vesting terms applicable to these awards are described below following the Outstanding Equity Awards at the End of 2015 table. |
| (4) | Represents the grant of ten-year stock options awarded under the Amended 2003 Plan. Stock options were granted with an exercise price equal to the closing price of the Company’s ordinary shares on the date of grant. The vesting terms applicable to these awards are described below following the Outstanding Equity Awards at the End of 2015 table. |
| (5) | Represents the grant date fair value of the specific award granted to the NEO. For information regarding assumptions used in determining such value, please refer to Note 11 to the Company’s Consolidated Financial Statements contained in the Original Filing. |
39
Table of Contents
Outstanding Equity Awards at the End of 2015
The following table sets forth information concerning all of the outstanding equity-based awards held by each NEO as of December 31, 2015.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable (1) |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested (#)(2) |
Market Value of Shares or Units of Stock That Have Not Vested ($)(3) |
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(3) |
||||||||||||||||||||||||
| Heather Bresch |
14,196 | — | 21.13 | 3/3/2020 | — | — | — | — | ||||||||||||||||||||||||
| 4,413 | — | 22.66 | 3/2/2021 | — | — | — | — | |||||||||||||||||||||||||
| 4,266 | — | 23.44 | 2/22/2022 | — | — | — | — | |||||||||||||||||||||||||
| — | 3,236 | 30.90 | 3/6/2023 | — | — | — | — | |||||||||||||||||||||||||
| 21,834 | 43,668 | 55.84 | 3/5/2024 | — | — | — | — | |||||||||||||||||||||||||
| — | 67,659 | 50.66 | 11/17/2025 | — | — | — | — | |||||||||||||||||||||||||
| — | — | — | — | — | — | 378,071 | (4) | 20,442,299 | ||||||||||||||||||||||||
| — | — | — | — | 25,662 | 1,387,544 | 76,984 | (5) | 4,162,525 | ||||||||||||||||||||||||
| John D. Sheehan |
13,239 | — | 22.66 | 3/2/2021 | — | — | — | — | ||||||||||||||||||||||||
| 4,266 | — | 23.44 | 2/22/2022 | — | — | — | — | |||||||||||||||||||||||||
| — | 3,236 | 30.90 | 3/6/2023 | — | — | — | — | |||||||||||||||||||||||||
| 5,914 | 11,826 | 55.84 | 3/5/2024 | — | — | — | — | |||||||||||||||||||||||||
| — | 20,298 | 50.66 | 11/17/2025 | — | — | — | — | |||||||||||||||||||||||||
| — | — | — | — | — | — | 67,512 | (4) | 3,650,374 | ||||||||||||||||||||||||
| — | — | — | — | 7,699 | 416,285 | 23,096 | (5) | 1,248,801 | ||||||||||||||||||||||||
| Rajiv Malik |
11,463 | 22,926 | 55.84 | 3/5/2024 | — | — | — | — | ||||||||||||||||||||||||
| — | 41,637 | 50.66 | 11/17/2025 | — | — | — | — | |||||||||||||||||||||||||
| — | — | — | — | — | — | 324,061 | (4) | 17,521,978 | ||||||||||||||||||||||||
| — | — | — | — | 15,792 | 853,873 | 47,375 | (5) | 2,561,566 | ||||||||||||||||||||||||
| Anthony Mauro |
4,757 | — | 22.66 | 3/2/2021 | — | — | — | — | ||||||||||||||||||||||||
| 4,266 | — | 23.44 | 2/22/2022 | — | — | — | — | |||||||||||||||||||||||||
| — | 3,236 | 30.90 | 3/6/2023 | — | — | — | — | |||||||||||||||||||||||||
| 4,003 | 8,006 | 55.84 | 3/5/2024 | — | — | — | — | |||||||||||||||||||||||||
| — | 16,265 | 50.66 | 11/17/2025 | — | — | — | — | |||||||||||||||||||||||||
| — | — | — | — | — | — | 67,512 | (4) | 3,650,374 | ||||||||||||||||||||||||
| — | — | — | — | 6,169 | 333,558 | 18,506 | (5) | 1,000,619 | ||||||||||||||||||||||||
| Robert J. Coury |
14,196 | — | 21.13 | 3/3/2020 | — | — | — | — | ||||||||||||||||||||||||
| 4,413 | — | 22.66 | 3/2/2021 | — | — | — | — | |||||||||||||||||||||||||
| 4,266 | — | 23.44 | 2/22/2022 | — | — | — | — | |||||||||||||||||||||||||
| — | 3,236 | 30.90 | 3/6/2023 | — | — | — | — | |||||||||||||||||||||||||
| 19,651 | 39,301 | 55.84 | 3/5/2024 | — | — | — | — | |||||||||||||||||||||||||
| — | 63,235 | 50.66 | 11/17/2025 | — | — | — | — | |||||||||||||||||||||||||
| — | — | — | — | — | — | 270,051 | (4) | 14,601,658 | ||||||||||||||||||||||||
| — | — | — | — | 23,984 | 1,296,815 | 71,951 | (5) | 3,890,391 | ||||||||||||||||||||||||
| (1) | Vesting dates applicable to unvested stock options are as follows, in each case subject to continued employment with Mylan: the unvested options at the $30.90 exercise price for Ms. Bresch and Messrs. Sheehan, Mauro, and Coury vested on March 6, 2016; one-half of the unvested options at the $55.84 exercise price for Ms. Bresch and Messrs. Sheehan, Malik, Mauro, and Coury vested on March 5, 2016, and the remaining options will vest on March 5, 2017; one-third of the unvested options at the $50.66 exercise price vested on March 4, 2016, and the remaining options will vest 50% on each of March 4, 2017 and 2018 except that, in each case, all of Mr. Sheehan’s unvested stock options were vested as of April 21, 2016 in accordance with the terms of his Retirement and Consulting Agreement. Subject to applicable employment agreement provisions, following termination of employment, vested stock options will generally remain exercisable for 30 days following termination, except that (i) in the case of termination because of disability, 100% of options become vested and vested options will remain exercisable for two years following termination; (ii) in the case of a termination due to a reduction in force, vested options will remain exercisable for one year following termination; and (iii) in the case of death or retirement, or a participant’s death within two years following termination because of disability, 100% of options become vested and vested options will remain exercisable for the remainder of the original term. In the case of options granted |
40
Table of Contents
| in 2011, 2012, 2013, 2014, and 2015 to Mr. Coury, in 2013, 2014, and 2015 to Ms. Bresch, and in 2014 and 2015 to Mr. Malik, following termination of employment without “cause” or resignation for “good reason” (as determined pursuant to the applicable employment agreement), 100% of options become vested and vested options will remain exercisable for one year following termination. |
| (2) | All of the RSUs shown in this column vested one-third on March 4, 2016, and the remaining RSUs will vest 50% on each of March 4, 2017 and 2018 except that Mr. Sheehan’s unvested RSUs were forfeited in connection with his retirement. In accordance with their terms, all of these awards would vest upon an involuntary termination without cause or a voluntary resignation for good reason occurs within two years following the change in control (double trigger awards). In the case of awards granted to Ms. Bresch and Messrs. Malik and Coury, the awards would also vest upon the executive’s retirement, termination without “cause” or resignation for “good reason” as defined in the applicable employment agreement. |
| (3) | The market value of restricted ordinary shares, RSUs and PRSUs was calculated using the closing price of the Company’s ordinary shares as of December 31, 2015. |
| (4) | On June 10, 2015, the SARs granted under the One-Time Special Performance-Based Incentive Program converted into restricted ordinary shares pursuant to the terms of the program, with any fractional shares converted into restricted cash awards. The restricted ordinary shares (and the restricted cash awards) remain subject to forfeiture and additional vesting conditions, including achievement of adjusted diluted EPS of $6.00 and continued service through December 31, 2018, and the other terms and conditions of the program. The One-Time Special Performance-Based Incentive Program is described in detail in the Form 10-K/A for Mylan’s fiscal year ending December 31, 2014. In accordance with their terms, the restricted ordinary shares (and restricted cash awards) would vest upon a change in control. In the case of awards granted to Ms. Bresch and Messrs. Malik and Coury, the restricted ordinary shares (and restricted cash awards) would also vest upon the executive’s termination without “cause” or resignation for “good reason” as defined in the applicable employment agreement, subject to the achievement of the applicable performance goals, except that, in the case of Ms. Bresch and Mr. Malik, if such termination or resignation occurs prior to January 1, 2017, only a pro-rated portion of the restricted ordinary shares will vest. Mr. Sheehan’s restricted ordinary shares (and his restricted cash awards) were forfeited in connection with his retirement. |
| (5) | The vesting of all of the PRSUs shown in this column is subject to the attainment of performance goals. On March 4, 2018, Ms. Bresch is expected to vest in 76,984 shares, Mr. Malik is expected to vest in 47,375 shares, Mr. Mauro is expected to vest in 18,506 shares, and Mr. Coury is expected to vest in 71,951 shares. Mr. Sheehan’s PRSUs were forfeited in connection with his retirement. The PRSUs are expected to vest upon the earliest to occur of (i) March 4, 2018, provided that the performance goals have been satisfied, (ii) an involuntary termination without cause or a voluntary resignation for good reason within two years following the change in control, (iii) the executive’s death or disability, and (iv) in the case of awards granted to Ms. Bresch and Messrs. Malik and Coury, the executive’s retirement, termination without “cause”, or resignation for “good reason” as defined in the applicable employment agreement. Any outstanding shares subject to the award that remain unvested as of March 4, 2018 will be forfeited. |
41
Table of Contents
Option Exercises and Stock Vested for 2015
The following option awards and stock awards were exercised or became vested for the NEOs during 2015 in connection with the EPD Transaction, as described above in the Compensation Discussion and Analysis:
| Option Awards | Stock Awards | |||||||||||||||
| Name |
Number of Shares Acquired on Exercise (#) |
Value Realized on Exercise ($) |
Number of Shares Acquired on Vesting (#)(1) |
Value Realized on Vesting ($) |
||||||||||||
| Heather Bresch |
557,583 | 16,174,466 | 287,427 | 15,775,431 | ||||||||||||
| John D. Sheehan |
120,710 | 3,473,014 | 100,842 | 5,534,713 | ||||||||||||
| Rajiv Malik |
382,372 | 11,178,305 | 173,327 | 9,513,052 | ||||||||||||
| Anthony Mauro |
37,347 | 951,471 | 57,236 | 3,141,398 | ||||||||||||
| Robert J. Coury |
1,133,138 | 34,011,112 | 335,104 | 18,392,183 | ||||||||||||
| (1) | This number of vested ordinary shares includes PRSU awards described above in the Compensation Discussion and Analysis. The number of PRSUs that vested at target amounts were 225,913 for Ms. Bresch, 136,409 for Mr. Malik, 79,344 for Mr. Sheehan, 45,086 for Mr. Mauro, and 263,666 for Mr. Coury. |
Pension Benefits for 2015
The following table summarizes the benefits accrued by the NEOs as of December 31, 2015 under the RBA (or deferred compensation plan, in the case of Mr. Malik) in effect with the NEO. The Company does not sponsor any other defined benefit pension programs covering the NEOs.
| Name |
Plan Name(1) |
Number of Years Credited Service (#) |
Present Value of Accumulated Benefit ($)(2) |
Payments During Last Fiscal Year ($) |
||||||||||
| Heather Bresch |
Retirement Benefit Agreement | 11 | 6,426,561 | — | ||||||||||
| John D. Sheehan |
Retirement Benefit Agreement | 5 | 1,213,431 | — | ||||||||||
| Rajiv Malik |
The Executive Plan for Rajiv Malik(3) | N/A | 281,445 | — | ||||||||||
| Rajiv Malik |
Retirement Benefit Agreement | 9 | 3,684,867 | — | ||||||||||
| Anthony Mauro |
N/A | N/A | — | — | ||||||||||
| Robert J. Coury |
Retirement Benefit Agreement | 14 | 50,437,336 | — | ||||||||||
| (1) | Mr. Mauro is not party to an RBA. |
| (2) | See page 37 of this Amendment for further information on the value of the accumulated pension benefit. |
| (3) | This is a deferred compensation plan established for the benefit of Mr. Malik. The Company is no longer contributing to this plan. |
42
Table of Contents
Nonqualified Deferred Compensation
The following table sets forth information relating to the Restoration Plan for 2015. There was no NEO participation in the Mylan Executive Income Deferral Plan in 2015.
| Name |
Aggregate Balance at Last FYE ($) |
Executive Contributions in Last FY ($) |
Company Profit Sharing Contributions in Last FY ($) |
Aggregate Earnings (Loss) in Last FY ($)(1) |
Aggregate Withdrawals/ Distributions ($) |
Aggregate Balance at FYE ($) |
||||||||||||||||||
| Heather Bresch |
2,116,823 | 258,045 | 218,454 | (40,599 | ) | — | 2,552,723 | |||||||||||||||||
| John D. Sheehan |
1,192,277 | 72,600 | 100,100 | (19,814 | ) | — | 1,345,163 | |||||||||||||||||
| Anthony Mauro |
772,552 | 105,157 | 131,918 | (12,907 | ) | — | 996,720 | |||||||||||||||||
| Robert J. Coury |
4,035,641 | 122,027 | 265,300 | 70,170 | — | 4,493,138 | ||||||||||||||||||
| (1) | These amounts include earnings (losses), dividends, and interest provided on account balances, including the change in value of the underlying investments in which our NEOs are deemed to be invested. These amounts are not reported in the Summary Compensation Table. |
Restoration Plan
The Restoration Plan permits employees (including certain NEOs) who earn compensation in excess of the limits imposed by Section 401(a)(17) of the Code to (i) defer a portion of base salary and bonus compensation, (ii) be credited with a Company matching contribution in respect of deferrals under the Restoration Plan, and (iii) be credited with Company non-elective contributions (to the extent so made by Mylan), in each case, to the extent that participants otherwise would be able to defer or be credited with such amounts, as applicable, under Mylan’s Profit Sharing 401(k) Plan if not for the limits on contributions and deferrals imposed by the Code. Company matching contributions immediately vest and Company profit sharing contributions are subject to an initial three-year vesting period. Upon a change in control (as defined in the Restoration Plan), a participant will become 100% vested in any unvested portion of his or her matching contributions or non-elective contributions. In connection with the EPD Transaction, the Board of Mylan Inc. amended the Restoration Plan to provide that the transaction would not be considered a change in control. Distributions of a participant’s vested account balance will be made in a lump sum within sixty days following a participant’s separation from service (or such later date as may be required by Section 409A of the Code).
Ms. Bresch and Messrs. Sheehan, Malik, and Coury are no longer eligible to receive matching contributions under the Restoration Plan because they are party to RBAs with Mylan.
Retirement Benefit Agreements
In December 2004, Mylan entered into an RBA with Mr. Coury. This RBA has been modified from time to time (as defined above, the “Amended RBA”). Additionally, Mylan entered into RBAs with Ms. Bresch and Mr. Malik in August 2009, and Mr. Sheehan in February 2011 (together with Mr. Coury’s Amended RBA, the “RBAs”). The information below is based upon the RBAs in effect as of December 31, 2015.
Pursuant to the Amended RBA, upon any termination of employment, Mr. Coury is entitled to receive a lump sum retirement benefit (the “Retirement Benefit”) equal to the present value of a monthly retirement benefit equal to 50% of the sum of his base salary as of December 31, 2011, and the average of the three highest annual cash bonuses paid to Mr. Coury during the five years preceding January 1, 2012, for a period of 15 years, discounted to Mr. Coury’s current age from 55. As a result of his years of service, Mr. Coury has fully vested in his Retirement Benefit. Pursuant to the terms of the Amended RBA, Mr. Coury is eligible to receive a supplemental retirement benefit equal to 20% of the sum of his base salary as of December 31, 2011 and the average of the three highest annual cash bonuses paid to Mr. Coury in the five years preceding January 1, 2012
43
Table of Contents
(the “Supplemental Retirement Benefit”). The Supplemental Retirement Benefit vested 50% on January 1, 2013 and 50% on January 1, 2014. In connection with the extension of his employment agreement in 2014, Mr. Coury’s RBA was amended to fix the discount rate used for purposes of the RBA, so that Mylan’s obligation would no longer be subject to variations in the appropriate discount rate.
Pursuant to the RBAs of Ms. Bresch and Messrs. Sheehan and Malik, upon retirement following completion of ten or more years of service, each executive would be entitled to receive a lump sum retirement benefit equal to the present value of an annual payment of 20%, 15%, and 15%, respectively, of the sum of their base salary and target annual bonus on the date of retirement, for a period of 15 years, discounted to the executive’s current age from age 55. Having completed ten years of continuous service as an executive, Ms. Bresch is now vested 100% in her retirement benefit. Mr. Sheehan completed his fifth year of service with the Company on April 1, 2015, and vested 50% in his retirement benefit on such date, with an additional 10% vesting on April 1, 2016, at which time Mr. Sheehan retired from the Company and ceased additional vesting in his retirement benefit. Mr. Malik has completed nine years of continuous service with the Company, and has vested 90% in his retirement benefit, with an additional 10% of the retirement benefit vesting after each year of service for up to one additional year.
Upon the occurrence of a change in control of the Company, each executive would become fully vested in his or her retirement benefit and would be entitled to receive a lump sum payment equal to the net present value of the retirement benefit, further discounted to the executive’s current age from age 55, as soon as practicable following any subsequent termination of employment. If an executive dies while employed by Mylan, the executive’s beneficiary would be entitled to receive a lump sum payment equal to the greater of (i) two times the executive’s current base salary or (ii) the net present value of the retirement benefit. As described above, each of the relevant executive officers executed a one-time waiver providing that the EPD Transaction did not constitute a change in control for purposes of the RBAs.
Ms. Bresch and Messrs. Sheehan and Malik’s RBAs provide that if the executive’s employment is terminated without cause or for good reason, the executive will receive additional years of service credit corresponding to the applicable severance multiplier under his or her Transition and Succession Agreement.
Each of the RBAs provides that the executive is prohibited for one year following termination from engaging in activities that are competitive with the Company’s activities, provided that this provision will have no effect if, after the occurrence of a change in control, Mylan refuses, fails to make, or disputes any payments to be made to the executive under the RBA, whether or not the executive actually receives payments under the RBA.
Ms. Bresch and Messrs. Sheehan and Malik’s RBAs provide that during the five-year period following termination, except for any termination occurring following a change in control, Mylan may request that the executive provide consulting services for the Company, which services will be reasonable in scope, duration, and frequency, and not to exceed 20 hours per month. The hourly rate for such consulting services will be determined by the parties at the time, but may not be less than $500 per hour, payable monthly. The executive would also be entitled to reimbursement of all out-of-pocket expenses incurred in the course of providing these services.
Information concerning the estimated value of benefits under the RBAs assuming retirement as of December 31, 2015 is at “Potential Payments Upon Termination or Change in Control” beginning on page 45 of this Amendment.
In 2007, Mylan established a nonqualified deferred compensation plan for Mr. Malik, who was then living outside the United States and therefore unable to participate in Mylan’s 401(k) plan. Although Mylan no longer contributes to the account, the plan account will be distributed to Mr. Malik upon Mylan’s termination of the plan, the termination of Mr. Malik’s employment, or other qualifying distribution events, such as his retirement, disability, or death.
44
Table of Contents
Employment Agreements
Mylan was party to employment agreements with each of the NEOs in 2015. The information below is based on the employment agreements in effect as of December 31, 2015. For a further description of the employment agreement with Mr. Mauro, see “Promotion of Anthony Mauro and Extension of Executive Employment Agreement” on page 34 of this Amendment.
Mr. Coury. Mylan and Mr. Coury entered into an employment agreement in February 2014, effective January 1, 2014. Mr. Coury’s employment agreement has a term of five years (through January 1, 2019, unless earlier terminated or extended in accordance with its terms). As Mylan’s most senior leader, Mr. Coury is responsible for the overall strategic direction of Mylan, leadership of the Mylan Board, oversight of talent management and retention, shareholder outreach, business development, and mergers and acquisitions. Pursuant to his employment agreement in effect as of December 31, 2015, Mr. Coury is entitled to an annual base salary of $1,350,000 and is eligible for an annual performance-based target bonus of at least 125% of base salary which will be payable upon the achievement of the performance targets. Mr. Coury is also entitled to participate in long-term incentive and equity plans of the Company at the discretion of the Compensation Committee and to receive employee benefits and other fringe benefits no less favorable than the benefits to which he was entitled under his original employment agreement. Throughout the term of the agreement and for a period of two years following Mr. Coury’s termination of employment for any reason, he may not engage in activities that are competitive with the Company’s activities and may not solicit the Company’s customers or employees.
For a description of the termination provisions of Mr. Coury’s employment agreement in effect as of December 31, 2015, please see below, at “Potential Payments Upon Termination or Change in Control” on page 45 of this Amendment.
Ms. Bresch and Messrs. Sheehan, Malik, and Mauro. Mylan entered into amended and restated employment agreements with Ms. Bresch and Mr. Malik in February 2014, effective January 1, 2014 (through December 31, 2018, unless earlier terminated or extended in accordance with its terms), entered into an amended and restated employment agreement with Mr. Sheehan in July 2013, and entered into an amended and restated employment agreement with Mr. Mauro in October 2011, effective January 1, 2012, which was further amended on April 10, 2015 and January 8, 2016, as described above. Each of these agreements provides for the payment of a minimum base salary as of December 31, 2015 of $1,300,000, $650,000, $1,000,000, and $625,000, with respect to Ms. Bresch and Messrs. Sheehan, Malik, and Mauro, respectively, subject to reduction only in the event of similar decreases among Mylan’s executives in the case of Ms. Bresch and Messrs. Malik, and Mauro. Each employment agreement also provides for the executive’s eligibility to receive a discretionary bonus and fringe benefits of employment as are customarily provided to senior executives of Mylan.
The agreements provide for a target bonus equal to 150%, 100%, 125%, and 115% of base salary with respect to Ms. Bresch and Messrs. Sheehan, Malik, and Mauro, respectively. Each of Ms. Bresch, Messrs. Sheehan, Malik, and Mauro’s agreements also provide that throughout the term of the agreement and for a period of one year following the executive’s termination of employment for any reason, the executive may not engage in activities that are competitive with the Company’s activities and may not solicit the Company’s customers or employees.
For a description of the termination provisions under these agreements, please see immediately below, at “Potential Payments Upon Termination or Change in Control.”
Potential Payments Upon Termination or Change in Control
The following discussion summarizes the termination and change in control-related provisions of the employment agreements, RBAs, and transition and succession agreements entered into between Mylan and the applicable NEO and in effect as of December 31, 2015, and termination of employment and change in control
45
Table of Contents
provisions under the Amended 2003 Plan. In the discussions that follow, all amounts payable upon termination or change in control that include the value of equity, include the value, if any, attributable to the One-Time Performance-Based Awards.
Termination Under Employment Agreements
Mr. Coury. Under Mr. Coury’s employment agreement in effect as of December 31, 2015, if Mr. Coury’s employment were terminated for any reason, he will be entitled to a payment equal to three times his “annual cash compensation” (defined as the sum of Mr. Coury’s base salary as in effect on December 31, 2011, plus the higher of (i) the average annual bonus awarded to Mr. Coury with respect to 2009, 2010, and 2011 or (ii) Mr. Coury’s 2011 target bonus), and a pro-rated annual bonus for the year of termination based on actual performance, which would be reduced by Company-provided death benefits in the event of the termination of Mr. Coury’s employment due to death. Mr. Coury will also be provided with continued health and other benefits and aircraft usage for three years following any such termination of employment, and will be eligible to participate in Mylan’s Supplemental Health Insurance Plan. In addition, if Mr. Coury’s employment is terminated without “cause” or for “good reason” (each as defined under his employment agreement in effect as of December 31, 2015), all equity-based awards and Mr. Coury’s $20 million performance incentive award will fully vest.
If Mr. Coury’s employment with Mylan had terminated on December 31, 2015, by Mylan without cause or by Mr. Coury for good reason prior to a change in control, under his current employment agreement he would have been entitled to cash severance payments and other benefits having an aggregate value of $45,578,100, and equity awards having an intrinsic value as of December 31, 2015 of approximately $20,079,472 would have become vested. If Mr. Coury’s employment with Mylan had terminated on December 31, 2015, because of his death, he would have been entitled to cash severance payments and other benefits under his employment agreement in effect as of December 31, 2015 (including equity awards) having an aggregate value of $61,413,783. If Mr. Coury’s employment with Mylan had terminated on December 31, 2015, because of his disability, he would have been entitled to cash severance payments and other benefits under his employment agreement in effect as of December 31, 2015 (including equity awards) having an estimated aggregate value as of December 31, 2015 of $65,657,572.
Ms. Bresch. Under Ms. Bresch’s employment agreement in effect as of December 31 2015, if Ms. Bresch were to resign for “good reason” or be terminated by Mylan without “cause” (each as defined in her employment agreement in effect as of December 31, 2015), or if her employment were terminated due to death or disability, in each case, prior to a change in control, she would be entitled to a lump sum payment equal to two times her annual base salary, two years of health benefits at Mylan’s cost, and a pro rata bonus based upon the actual bonus she would have been entitled to receive for the fiscal year in which the termination occurs. Such payments and benefits would be reduced by Company-provided death or disability benefits in the event of termination of Ms. Bresch’s employment due to death or disability. Pursuant to the applicable individual award agreements, if Ms. Bresch’s employment is terminated without cause or for good reason, all outstanding equity-based awards granted to Ms. Bresch would have fully vested. Pursuant to the terms of Ms. Bresch’s employment agreement in effect as of December 31, 2015, if the term of employment were not extended or renewed, she would have been entitled to the same payments and benefits as if she had been terminated without cause. If Mylan had offered to renew Ms. Bresch’s term of employment on substantially similar terms and conditions, and Ms. Bresch rejected such offer, she would have been entitled to a lump sum payment equal to 12 months’ continuation of base salary and health benefits at Mylan’s cost.
If Ms. Bresch’s employment had been terminated on December 31, 2015, by Mylan without cause or by Ms. Bresch for good reason prior to a change in control, she would have been entitled to cash severance and other benefits under her employment agreement in effect as of December 31, 2015 and equity awards having an estimated aggregate value of $20,593,879. If Ms. Bresch’s employment with Mylan had terminated on December 31, 2015 because of her death or disability, she would have been entitled to cash severance payments and other benefits under her employment agreement in effect as of December 31, 2015 and equity awards having an aggregate value of $20,593,879.
46
Table of Contents
Mr. Sheehan. Under Mr. Sheehan’s employment agreement as in effect on December 31, 2015, if Mr. Sheehan were to resign for “good reason” or be terminated by Mylan without “cause” (each as defined in his employment agreement in effect as of December 31, 2015), or if his employment were terminated due to death or disability, in each case, prior to a change in control, he would be entitled to a lump sum payment equal to his annual base salary, 12 months of health benefits at Mylan’s cost, plus a pro rata bonus equal to the bonus he would have been entitled to receive for the fiscal year in which the termination occurs. Such payments and benefits would be reduced by Company-provided death or disability benefits in the event of termination of Mr. Sheehan’s employment due to death or disability. If Mylan had failed to extend or renew the term of employment in Mr. Sheehan’s employment agreement as in effect on December 31, 2015 on terms mutually acceptable to him and Mylan, by the terms of his employment agreement in effect on December 31, 2015, he would be entitled to a lump sum payment equal to 12 months’ continuation of base salary and health benefits at Mylan’s cost.
If Mr. Sheehan’s employment had been terminated on December 31, 2015, by Mylan without cause or by Mr. Sheehan for good reason prior to a change in control, he would have been entitled to cash severance and other benefits under his employment agreement in effect on such date having an estimated aggregate value of $1,982,307. If Mr. Sheehan’s employment with Mylan had terminated on December 31, 2015, because of his death or disability, he would have been entitled to cash severance payments and other benefits under his employment agreement in effect on such date and equity awards having an aggregate value of $3,791,587. Mr. Sheehan did not receive any payments or benefits from Mylan under his employment agreement in connection with his retirement.
Mr. Malik. Under Mr. Malik’s employment agreement in effect as of December 31, 2015, if Mr. Malik were to resign for “good reason” or be terminated by Mylan without “cause” (each as defined in his employment agreement in effect as of December 31, 2015), or if his employment were terminated due to death or disability, in each case, prior to a change in control, he would be entitled to a lump sum payment equal to one-and-one-half times his annual base salary, 18 months of health benefits at Mylan’s cost, and a pro rata bonus based upon the actual bonus he would have been entitled to receive for the fiscal year in which the termination occurs. Such payments and benefits would be reduced by Company-provided death or disability benefits in the event of termination of Mr. Malik’s employment due to death or disability. Pursuant to the applicable individual award agreements, if Mr. Malik were to resign for good reason or be terminated by Mylan without cause, all outstanding equity-based awards granted to Mr. Malik beginning in 2013 would have fully vested. Pursuant to the terms of Mr. Malik’s employment agreement in effect as of December 31, 2015, if the terms of employment were not extended or renewed, he would have been entitled to the same payments and benefits as if he had been terminated without cause. If Mylan had offered to renew Mr. Malik’s term of employment on substantially similar terms and conditions, and Mr. Malik rejected such offer, he would have been entitled to a lump sum payment equal to 12 months’ continuation of base salary and health benefits at Mylan’s cost.
If Mr. Malik’s employment had been terminated on December 31, 2015, by Mylan without cause or by Mr. Malik for good reason prior to a change in control, he would have been entitled to cash severance and other benefits under his employment agreement in effect as of December 31, 2015 and equity awards having an estimated aggregate value of $14,612,047. If Mr. Malik’s employment with Mylan had terminated on December 31, 2015, because of his death or disability, he would have been entitled to cash severance payments and other benefits under his current employment agreement and equity awards having an aggregate value of $14,612,047.
Mr. Mauro. Under Mr. Mauro’s employment agreement in effect on December 31, 2015, if Mr. Mauro were to be discharged by Mylan without “cause” (as defined in his employment agreement in effect on December 31, 2015) or if his employment were terminated due to death or disability, in each case, prior to a change in control, he would be entitled to a lump sum payment equal to his annual base salary, 12 months of health benefits at Mylan’s cost and a pro rata bonus equal to the bonus he would have been entitled to receive for the fiscal year in
47
Table of Contents
which the termination occurs. Such payments and benefits would be reduced by Company-provided death or disability benefits in the event of termination of Mr. Mauro’s employment due to death or disability. If the term of employment in Mr. Mauro’s employment agreement in effect on December 31, 2015 was not extended or renewed, he would have been entitled to the same payments and benefits as if he had been terminated without cause.
If Mr. Mauro’s employment had been terminated on December 31, 2015, by Mylan without cause, he would have been entitled to cash severance and other benefits under his current employment agreement having an estimated aggregate value of $2,094,807. If Mr. Mauro’s employment with Mylan had been terminated on December 31, 2015, because of his death or disability, he would have been entitled to benefits under his current employment agreement and equity awards having an aggregate value of $3,559,426.
Retirement Benefit Agreements
Mr. Coury and Ms. Bresch. If the employment of each of Mr. Coury and Ms. Bresch had terminated for any reason on December 31, 2015, each of the executives would have been entitled to an estimated lump sum payment under their Amended RBA or RBA, as applicable, equal to $50,437,336 and $6,426,561, respectively.
Messrs. Sheehan and Malik. If the employment of each of Messrs. Sheehan and Malik had terminated for any reason on December 31, 2015, each of the executives would have been entitled to lump sum payments having the following estimated values under their respective RBAs: (i) in the case of termination by Mylan for “cause” or by the executive without “good reason,” as defined in the executive’s employment agreement, $1,213,431 and $3,684,866, respectively; (ii) in the case of a termination by Mylan without cause or by the executive for good reason, $1,941,490 and $4,094,295, respectively; and (iii) in the case of termination because of death or disability, $2,426,862 and $4,094,295, respectively. If a change in control had occurred on December 31, 2015, each of Messrs. Sheehan and Malik would be entitled upon a simultaneous termination of employment to the benefit the executive would have been entitled to under his RBA in the case of termination because of death or disability. As discussed above, in connection with the EPD Transaction, the executive officers executed a one-time waiver of accelerated vesting of the benefits under their RBAs. In connection with his retirement on April 1, 2016, Mr. Sheehan will receive a lump sum payment equal to the vested benefit under his RBA as of such date.
Termination Under Transition and Succession Agreements (Change in Control)
Mr. Coury. Pursuant to Mr. Coury’s prior employment agreement, Mr. Coury waived his right to the cash severance payments and continuation benefits under his Transition and Succession Agreement dated December 2, 2004, as amended. This waiver does not apply to Mr. Coury’s right under the Transition and Succession Agreement to receive from Mylan a gross-up payment for any excise tax on “excess parachute payments” and reimbursement of legal fees associated with good faith disputes regarding termination of employment, in seeking benefits under the Transition and Succession Agreement.
Pursuant to Mr. Coury’s employment agreement in effect as of December 31, 2015, if a change in control had occurred on December 31, 2015, and Mr. Coury’s employment had been terminated without cause or for good reason on the same date, then Mr. Coury would not have been entitled to payment of cash severance or continuation benefits under his Transition and Succession Agreement. However, under his employment agreement in effect as of December 31, 2015, he would be entitled to the cash severance and other benefits described above under “Employment Agreements.” If a change in control had occurred on December 31, 2015, and Mr. Coury’s employment had been terminated without cause or for good reason on the same date, Mr. Coury would not have been subject to the excise tax under Section 280G of the Code and therefore would not have been entitled to a gross-up payment.
Ms. Bresch and Messrs. Sheehan, Malik, and Mauro. The Transition and Succession Agreements with the other NEOs provide that if the executive’s employment is terminated other than for cause (including death or disability) or if the executive terminates his or her employment for good reason, in each case prior to a change in
48
Table of Contents
control under certain circumstances (such as in the event the termination arose in connection with the change in control) or within two years following the occurrence of a change in control, or, under certain circumstances, for any reason within 90 days following the first anniversary of a change in control, the executive would become entitled to receive a lump sum severance payment, equal to, in the case of Ms. Bresch and Messrs. Sheehan and Malik, the higher of (i) the compensation and benefits payable under his or her employment agreement as if the change in control were deemed to be a termination without cause under the employment agreement and (ii) a lump sum severance payment in an amount equal to three times the sum of base salary and highest bonus paid to the executive under the employment agreement or the Transition and Succession Agreement, or, in the case of Mr. Mauro, a lump sum severance payment in an amount equal to the greater of three times the sum of base salary and the higher cash bonus paid to Mr. Mauro by Mylan as reflected on Mr. Mauro’s W-2 in (a) the tax year immediately preceding the year in which the date of termination occurs or (b) the year in which the change in control occurs. Such payments and benefits would be reduced by Company-provided death or disability benefits in the event of the executive’s termination due to death or disability. Each executive would additionally be entitled to continuation of health and insurance benefits for a period of three years. The Transition and Succession Agreements for each of these NEOs also provide for a gross-up payment for any excise tax on “excess parachute payments.”
If a change in control had occurred on December 31, 2015, and the employment of each of Ms. Bresch and Messrs. Sheehan, Malik, and Mauro had been terminated on such date under circumstances entitling them to payments under their Transition and Succession Agreements, the executives would have been entitled to cash severance and other benefits (which includes the vesting of equity awards and the valuation of other perquisites and are in addition to the Retirement Benefit which they would receive as described above) having an estimated aggregate value as follows: for Ms. Bresch, $44,383,299; for Mr. Sheehan, $12,840,389; for Mr. Malik, $32,579,780; and for Mr. Mauro, $13,142,454. Ms. Bresch and Messrs. Sheehan, Malik, and Mauro would also have been entitled to a gross-up payment for excise taxes estimated at $17,166,142, $6,030,373, $11,266,557, and $5,463,896, respectively. As a result of his retirement from the Company on April 1, 2016, Mr. Sheehan is no longer eligible to receive any cash severance or other benefits under his Transition and Succession Agreement.
As described above, subsequent to the execution of these agreements, Mylan adopted a policy that no new Transition and Succession Agreements will provide for an excise tax gross–up for golden parachute payments. For legal and other considerations, the Transition and Succession Agreements currently in effect and executed prior to the new policy are not subject to that policy. Mylan does not have the right to unilaterally abrogate pre-existing binding contracts with its executives, and does not believe it would be in shareholders’ best interests to expend funds to “buy out” the executives from these rights. Since implementation of the new policy, no new or amended Transition and Succession Agreements with excise tax gross-up provisions have been executed and several have expired as executives have retired from Mylan (as was the case with the retirements of Hal Korman and John Sheehan over the last several years).
2003 Long-Term Incentive Plan, as amended
The Amended 2003 Plan provides that, unless otherwise provided in an award agreement, at the time of a change in control (as defined in the Amended 2003 Plan), (i) each stock option and SAR outstanding will become immediately and fully exercisable, (ii) all restrictions applicable to awards of restricted stock and RSUs will terminate in full, (iii) all performance awards (with certain limited exceptions) will become fully payable at the maximum level, and (iv) all other stock-based awards will become fully vested and payable.
Annual equity awards contain “double trigger” vesting provisions that provide for accelerated vesting only if (i) there has been a change in control and (ii) an involuntary termination without cause or a voluntary resignation for good reason occurs within two years following the change in control, unless otherwise specifically determined by the Compensation Committee.
49
Table of Contents
A description of the material terms that apply to the equity awards held by the NEOs, including the awards granted under the One-Time Special Performance-Based Incentive Program, may be found in the footnotes to the Outstanding Equity Awards at the End of 2015 table.
If a change in control and qualifying termination had occurred on December 31, 2015, the intrinsic value of vesting equity-based awards held by the NEOs would have equaled approximately: for Mr. Coury, $20,079,472; for Ms. Bresch, $26,298,063; for Mr. Sheehan, $5,459,654; for Mr. Malik, $21,079,400; and for Mr. Mauro, $5,114,993.
Compensation Committee Interlocks and Insider Participation
None of the members of the Compensation Committee during 2015 (Ms. Cameron, Mr. Dimick, Mr. Parrish, and Mr. Piatt) was an officer or employee of the Company, and no executive officer of the Company served on the compensation committee or board of any company that employed any member of the Compensation Committee or the Mylan Board.
Non-Employee Director Compensation for 2015
The following table sets forth information concerning the compensation earned by the Directors who are not employees of the Company or Mylan Inc. (the “Non-Employee Directors”) for 2015. Directors who are employees of Mylan Inc. do not receive any consideration for their service on the Mylan Board. A discussion of the elements of Non-Employee Director compensation follows the table.
| Name |
Fees Earned or Paid in Cash ($) |
RSUs ($)(1) | Option Awards ($)(1) |
All
Other Compensation ($)(2) |
Total ($) | |||||||||||||||
| Wendy Cameron |
121,167 | 165,050 | 50,014 | 21,453 | 357,684 | |||||||||||||||
| Hon. Robert J. Cindrich |
120,000 | 165,050 | 50,014 | 21,453 | 356,517 | |||||||||||||||
| JoEllen Lyons Dillon |
110,000 | 165,050 | 50,014 | 21,946 | 347,010 | |||||||||||||||
| Neil Dimick |
175,000 | 165,050 | 50,014 | 21,679 | 411,743 | |||||||||||||||
| Melina Higgins |
132,000 | 165,050 | 50,014 | 23,067 | 370,131 | |||||||||||||||
| Douglas J. Leech |
125,000 | 165,050 | 50,014 | 22,055 | 362,119 | |||||||||||||||
| Joseph C. Maroon, M.D. |
127,000 | 165,050 | 50,014 | 21,453 | 363,517 | |||||||||||||||
| Mark W. Parrish |
145,000 | 165,050 | 50,014 | 20,515 | 380,579 | |||||||||||||||
| Rodney L. Piatt |
232,833 | 165,050 | 50,014 | 21,453 | 469,350 | |||||||||||||||
| Randall L. (Pete) Vanderveen, Ph.D., R.Ph. |
113,000 | 165,050 | 50,014 | 23,934 | 351,998 | |||||||||||||||
| (1) | Represents the grant date fair value of the specific award granted to the Non-Employee Director. Option awards and RSU awards granted in 2015 vest on May 1, 2016. For information regarding assumptions used in determining the amounts reflected in the table above, please refer to Note 11 to the Company’s Consolidated Financial Statements contained in the Original Filing. The aggregate number of ordinary shares subject to stock options held by the Non-Employee Directors as of December 31, 2015 were as follows: Ms. Cameron, 5,577; Judge Cindrich, 5,577; Ms. Dillon, 5,577; Mr. Dimick, 5,577; Ms. Higgins, 12,200; Mr. Leech, 5,577; Dr. Maroon, 5,577; Mr. Parrish, 5,577; Mr. Piatt, 82,128; and Dr. Vanderveen, 5,577. The number of unvested RSUs held by each of the non-employee Directors, as of December 31, 2015, was 3,258. |
| (2) | Represents the tax reimbursement payment from Mylan with respect to the Transaction-Related Excise Tax (as defined above) imposed on stock options granted in 2014, so that, on a net after-tax basis, the Non-Employee Director would be in the same position as if the Transaction-Related Excise Tax had not been imposed. |
Non-Employee Directors receive $100,000 per year in cash compensation for their service on the Mylan Board. Non-Employee Directors are also reimbursed for actual expenses relating to meeting attendance.
50
Table of Contents
In addition:
| • | The Chair of the Audit Committee receives an additional fee of $30,000 per year; |
| • | The Chair of the Compensation Committee receives an additional fee of $25,000 per year; |
| • | The Chair of the Compliance Committee receives an additional fee of $30,000 per year; |
| • | The Chair of the Finance Committee receives an additional fee of $20,000 per year; |
| • | The Chair of the Governance and Nominating Committee receives an additional fee of $10,000 per year; |
| • | The Chair of the Science and Technology Committee receives an additional fee of $10,000 per year; |
| • | Each member of the Executive Committee who is a Non-Employee Director receives an additional fee of $30,000 per year; |
| • | Each member of the Audit Committee and Compensation Committee receives an additional fee of $12,000 per year; |
| • | Each member of the Compliance Committee receives an additional fee of $10,000 per year; |
| • | Each member of the Governance and Nominating Committee receives an additional fee of $7,000 per year; |
| • | Each member of the Finance Committee and the Science and Technology Committee receives an additional fee of $3,000 per year; and |
| • | Mr. Piatt, as the Lead Independent Director, receives an additional fee of $60,000 per year. |
Non-Employee Directors are eligible to receive stock options or other grants under the Amended 2003 Plan. In November 2015, each Non-Employee Director was granted an option to purchase 2,603 ordinary shares, at an exercise price of $50.66 per share, the closing price per share of the Company’s ordinary shares on the date of grant, which option vests on May 1, 2016, and 3,258 RSUs, also vesting on May 1, 2016. Non-Employee Directors will also receive tax equalization payments for incremental tax liabilities, if any, incurred as a result of attendance at board meetings in the United Kingdom.
Stock Ownership Requirements. In February 2013, the Board of Mylan Inc. adopted stock ownership requirements for Non-Employee Directors, requiring Non-Employee Directors to hold shares valued at three times their annual retainer as long as they remain on the Mylan Board, and the Board of Mylan Inc. increased this ownership requirement to four times the annual retainer in April 2014. Mylan N.V. adopted the Mylan Inc. stock ownership requirement. Non-Employee Directors who were members of the Board of Mylan Inc. when this policy was initially adopted have until January 1, 2018 to comply, while each other Non-Employee Director has five years from his or her initial election to the Mylan Board or the Board of Mylan Inc., as applicable, to achieve this requirement. The policy was adopted to further demonstrate the alignment of Directors’ interests with shareholders for the duration of their service. As of April 29, 2016, all Non-Employee Directors, except for Ms. Higgins, satisfied the ownership requirement. Ms. Higgins joined the Mylan Board in September 2013, and she has until September 2018 to satisfy this ownership requirement.
| ITEM 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Certain information concerning our executive officers is contained in the discussion entitled “Equity Compensation Plan Information” in Item 12 of Part III of the Original Filing.
51
Table of Contents
The following table sets forth information regarding the beneficial ownership of ordinary shares of Mylan N.V. as of April 27, 2016 by (i) Mylan N.V.’s directors and NEOs, and (ii) all directors and executive officers of Mylan N.V. as a group (based on 508,342,710 ordinary shares of Mylan N.V. outstanding as of such date). For purposes of this table, and in accordance with the rules of the SEC, shares are considered “beneficially owned” if the person, directly or indirectly, has sole or shared voting or investment power over such shares. A person is also considered to beneficially own shares that he or she has the right to acquire within 60 days of April 27, 2016. To Mylan N.V.’s knowledge, the persons in the following table have sole voting and investment power, either directly or through one or more entities controlled by such person, with respect to all of the shares shown as beneficially owned by them, unless otherwise indicated in the footnotes below.
| Name of Beneficial Owner |
Amount and Nature of Beneficial Ownership |
Options Exercisable and Restricted Shares Vesting within 60 days |
Percent of Class | |||||||||
| Heather Bresch |
929,675 | (1)(6) | 92,332 | * | ||||||||
| Wendy Cameron |
66,677 | 8,835 | * | |||||||||
| Hon. Robert J. Cindrich |
10,322 | 8,835 | * | |||||||||
| Robert J. Coury |
1,339,263 | (2)(6) | 86,491 | * | ||||||||
| JoEllen Lyons Dillon |
3,609 | 8,835 | * | |||||||||
| Neil Dimick |
38,587 | 8,835 | * | |||||||||
| Melina Higgins |
19,000 | (3) | 15,458 | * | ||||||||
| Douglas J. Leech |
42,380 | 8,835 | * | |||||||||
| Rajiv Malik |
816,846 | (6) | 36,805 | * | ||||||||
| Joseph C. Maroon, M.D. |
16,124 | 8,835 | * | |||||||||
| Anthony Mauro |
152,440 | (4)(6) | 25,687 | * | ||||||||
| Mark W. Parrish |
31,365 | 8,835 | * | |||||||||
| Rodney L. Piatt |
33,135 | 85,386 | * | |||||||||
| John D. Sheehan, C.P.A. (5) |
126,220 | 38,038 | * | |||||||||
| Randall L. (Pete) Vanderveen, Ph.D., R.Ph. |
35,787 | 8,835 | * | |||||||||
| All directors and executive officers as a group (14 persons, but not including Mr. Sheehan (5)) |
3,535,210 | (7) | 412,839 | * | ||||||||
| * | Less than 1%. |
| (1) | Includes 1,157 shares held in Ms. Bresch’s 401(k) account. |
| (2) | Includes 4,957 shares held in Mr. Coury’s 401(k) account and 1,000,000 shares held in a grantor retained annuity trust of which Mr. Coury is the sole trustee. |
| (3) | Includes 19,000 shares held by Ms. Higgins’ spouse. |
| (4) | Includes 5,574 shares held in Mr. Mauro’s 401(k) account. |
| (5) |
Mr. Sheehan retired from Mylan effective April 1, 2016. |
| (6) | Includes restricted ordinary shares issued on June 10, 2015 upon conversion of SARs pursuant to the terms of Mylan’s One-Time Special Performance-Based Incentive Program (as defined above) implemented in 2014 (as described in detail in “Executive Compensation for 2015”). The restricted ordinary shares remain subject to forfeiture and additional vesting conditions, including achievement of adjusted diluted earnings per share of $6.00 and continued service, and the other terms and conditions of the program. |
| (7) | Includes 11,688 shares held in the executive officers’ 401(k) accounts. |
52
Table of Contents
Security Ownership of Certain Beneficial Owners
The following table lists the names and addresses of the shareholders known to management to own beneficially more than five percent of the ordinary shares of Mylan N.V. as of April 27, 2016 (based on 508,342,710 ordinary shares of Mylan N.V. outstanding as of such date):
| Name and Address of Beneficial Owner |
Amount and Nature of Beneficial Ownership |
Percent of Class |
||||||
| Subsidiaries of Abbott Laboratories (1) c/o Abbott Laboratories, 100 Abbott Park Road, Abbott Park, IL 60064-6092 |
69,750,000 | (2) | 13.7 | % | ||||
| Wellington Management Company LLP and affiliates, 280 Congress Street, Boston, MA 02210 |
44,793,344 | (3) | 8.8 | % | ||||
| BlackRock, Inc. 55 East 52nd Street, New York, NY 10055 |
33,735,289 | (4) | 6.6 | % | ||||
| (1) | Abbott and its subsidiaries that own ordinary shares of Mylan N.V. are subject to the terms of the Shareholder Agreement (the “Shareholder Agreement”), dated February 27, 2015, by and among Mylan N.V., Abbott, Laboratoires Fournier S.A.S. (“Abbott France”), Abbott Established Products Holdings (Gibraltar) Limited (“Abbott Gibraltar”), and Abbott Investments Luxembourg S.à.r.l. (“Abbott Luxembourg” and, together with Abbott France and Abbott Gibraltar, the “Abbott Subsidiaries”). According to Item 4 of the Schedule 13D/A filed by Abbott on August 10, 2015, Abbott Gibraltar distributed 62,782,018 Mylan ordinary shares to Abbott Products on July 28, 2015 (the “Distribution”). Contemporaneously with the Distribution, Abbott Products became a party to the Shareholder Agreement by executing a joinder agreement thereto. As a result of the Distribution, Abbott Gibraltar no longer beneficially owns any Mylan ordinary shares. The Shareholder Agreement will terminate when Abbott no longer beneficially owns any of the ordinary shares of Mylan N.V. issued to it in connection with the EPD Transaction. So long as Abbott beneficially owns at least five percent of the ordinary shares of Mylan N.V., Abbott is required to vote each Mylan N.V. voting security (a) in favor of all those persons nominated and recommended to serve as directors of the Mylan Board or any applicable committee thereof and (b) with respect to any other action, proposal, or matter to be voted on by the shareholders of Mylan N.V. (including through action by written consent), in accordance with the recommendation of the Mylan Board or any applicable committee thereof. However, Abbott is free to vote at its discretion in connection with any proposal submitted for a vote of the Mylan N.V. shareholders in respect of (a) the issuance of equity securities in connection with any merger, consolidation, or business combination of Mylan N.V., (b) any merger, consolidation, or business combination of Mylan N.V., or (c) the sale of all or substantially all the assets of Mylan N.V., except where such proposal has not been approved or recommended by the Mylan Board, in which event Abbott must vote against the proposal. |
| (2) | Based on Schedule 13D/A filed by Abbott, Abbott Luxembourg and Abbott Products with the SEC on August 10, 2015 (the “Schedule 13D/A”), Abbott has sole voting power over 0 shares, shared voting power over 69,750,000 shares, sole dispositive power over 0 shares, and shared dispositive power over 69,750,000 shares; Abbott France has sole voting power, shared voting power, sole dispositive power and shared dispositive power over 0 shares; Abbott Luxembourg has sole voting power over 0 shares, shared voting power over 6,967,982 shares, sole dispositive power over 0 shares, and shared dispositive power over 6,967,982 shares; and Abbott Products has sole voting power over 0 shares, shared voting power over 62,782,018 shares, sole dispositive power over 0 shares and shared dispositive power over 62,782,018 shares. |
| (3) | Based on Schedule 13G/A filed by Wellington Management Group LLP, Wellington Group Holdings LLP, Wellington Investment Advisors Holdings LLP and Wellington Management Company LLP with the SEC on February 11, 2016, Wellington Management Group LLP has sole voting power over 0 shares, shared voting power over 13,546,750 shares, sole dispositive power over 0 shares, and shared dispositive power over 44,793,344 shares; Wellington Group Holdings LLP has sole voting power over 0 shares, shared voting |
53
Table of Contents
| power over 13,546,750 shares, sole dispositive power over 0 shares, and shared dispositive power over 44,793,344 shares; Wellington Investment Advisors Holdings LLP has sole voting power over 0 shares, shared voting power over 13,546,750 shares, sole dispositive power over 0 shares, and shared dispositive power over 44,793,344 shares; and Wellington Management Company LLP has sole voting power over 0 shares, shared voting power over 12,489,471 shares, sole dispositive power over 0 shares, and shared dispositive power over 42,867,413 shares. Based on the Schedule 13G/A, the securities as to which the Schedule 13G/A was filed are owned of record by clients of one or more investment advisers identified therein directly or indirectly owned by Wellington Management Group LLP. Those clients have the right to receive, or the power to direct the receipt of, dividends from, or the proceeds from the sale of, such securities. No such client is known to have such right or power with respect to more than five percent of this class of securities. |
| (4) | Based on Schedule 13G filed by BlackRock, Inc. with the SEC on February 9, 2016, BlackRock, Inc. has sole voting power over 30,656,253 shares, shared voting power over 0 shares, sole dispositive power over 33,735,289 shares, and shared dispositive power over 0 shares. |
| ITEM 13. | Certain Relationships and Related Transactions, and Director Independence |
The Mylan Board annually reviews certain relationships and related party transactions, with respect to directors, as part of its assessment of each director’s independence. Based on a review of the transactions between Mylan and its directors and executive officers, their immediate family members, and their affiliated entities, Mylan has determined that since the beginning of 2015, it was a party to the following transactions in which the amount involved exceeded $120,000 and in which any of Mylan’s directors, executive officers, or greater than five percent shareholders, or any of their immediate family members or affiliates, have or had a direct or indirect material interest:
As previously disclosed, Mylan has engaged Coury Financial Group, LP (“CFG”), Coury Investment Advisors, Inc. (“CIA”), and Coury Consulting, L.P. (“Coury Consulting”), the principals of which are brothers of Robert J. Coury, Executive Chairman, to provide certain services to Mylan. CFG, CIA, and Coury Consulting are in the business of providing strategic business consulting and corporate benefits advice and services, among others. Since approximately 1995, CFG and CIA have served as the broker in connection with several of the Company’s employee benefit programs. Effective January 1, 2015, Mylan’s arrangements with CFG and CIA provided for a fixed base fee of $37,500 per month to be paid by Mylan for a period of three years, corresponding to the term of agreements negotiated with certain benefit plan carriers and capping payments over that time period. However, where required by law, CFG and CIA will continue to receive commissions directly from certain other benefit plan carriers, and in 2015 and early 2016, received payments totaling approximately $311,000 in commissions for these services directly from the insurance carriers (including payments for 2014 business paid in 2015).
Since approximately 2000, Coury Consulting from time to time has been engaged to provide specialized consulting and advisory services to Mylan. Most recently, Mylan engaged Coury Consulting to provide consulting and advisory services with regard to Mylan’s Human Resources function as well as certain of Mylan’s compensation, benefits, and health care related programs. Beginning on January 1, 2015, Mylan paid Coury Consulting $40,000 per month for 12 months, with the possibility of a performance payment at the end of the term in Mylan’s discretion. In 2016, Mylan made a performance payment of $500,000 for that contract term based on, among other factors, significant work completed, increase in scope, exceptional performance, and results. Mylan renewed the agreement with Coury Consulting effective January 1, 2016 at a rate of $80,000 per month for 18 months. The renewed agreement does not provide for a performance payment.
As discussed on page 20 of this Amendment, on February 27, 2015 the EPD Transaction was completed pursuant to which Mylan N.V. issued 110,000,000 ordinary shares (worth approximately $6.31 billion) to various Abbott affiliates and pursuant to which Abbott became a holder of over 5% of Mylan N.V.’s outstanding ordinary shares. As previously disclosed, at the closing of the EPD Transaction, Mylan, Abbott and certain of their affiliates also entered into ancillary agreements providing for transition services, manufacturing
54
Table of Contents
relationships, and license arrangements. In addition to these ancillary agreements, since the beginning of 2015, Abbott and Mylan have entered into or engaged in ordinary course, arms length transactions with each other. From January 1, 2015 to early 2016, Mylan has received inventory and services from Abbott pursuant to those ancillary agreements, and also received inventory and services pursuant to separate ordinary course, arms length transactions, totaling approximately $183 million (substantially all of which related to the ancillary agreements). During this time period, Mylan has also provided inventory and services pursuant to those ancillary agreements to Abbott totaling approximately $55 million.
On April 3, 2015, Mylan entered into a call option agreement with the Foundation pursuant to which Mylan granted the Foundation a call option to acquire from time to time, at an exercise price of €0.01 per share, Mylan preferred shares up to a maximum number at any time equal to the total number of Mylan ordinary shares issued at such time. On July 23, 2015, in response to Teva Pharmaceutical Industries Ltd.’s unsolicited expression of interest in acquiring Mylan, the Foundation exercised its call option and acquired 488,388,431 Mylan preferred shares (which represented 100% of the class of Mylan preferred shares) pursuant to the terms of the call option agreement. Each Mylan ordinary share and preferred share is entitled to one vote on each matter properly brought before a general meeting of shareholders so that beginning on July 23, 2015, the Foundation was the beneficial owner of more than five percent of a class of Mylan’s voting securities and therefore a “related person” of Mylan. The Foundation is an independent entity with its own independent directors and advisers and is entitled to determine, in its sole discretion, subject to the limits set by its governing documents and applicable Dutch law, whether or not to exercise the call option and any resulting voting power. On September 19, 2015, the Foundation requested the redemption of the Mylan preferred shares issued on July 23, 2015 and holders of Mylan shares approved the redemption of the Mylan preferred shares on January 7, 2016 at an extraordinary general meeting. The redemption of the Mylan preferred shares became effective on March 17, 2016. The Foundation will continue to have the right to exercise its call option in the future consistent with its governing documents and applicable Dutch law. Since the beginning of 2015, Mylan has made payments to the Foundation totaling approximately $12.7 million. Such payments were made in satisfaction of Mylan’s contractual obligation to pay the Foundation’s expenses. Although the Foundation is under no obligation to do so, it has informed Mylan that these expenses were primarily fees for the Foundation’s independent legal and financial advisors. During that period, the Foundation also paid Mylan approximately $1.3 million to purchase the preferred shares.
Mr. Piatt, like each member of the Mylan Board, is party to an indemnification agreement with the Company. In accordance with such agreement, the Company made payments of approximately $63,000 in 2015 and early 2016 for written claims for repayment or advancement of expenses presented by Mr. Piatt related to the previously disclosed SEC investigation and anticipates making additional such payments of approximately $275,000 for legal fees and expenses incurred during 2015 through early 2016. Mylan expects that Mr. Piatt will make additional written claims for repayment or advancement of expenses during the pendency of the SEC investigation and anticipates that it will make payments for any such claims.
In 2013, the Mylan Board approved a written related party transactions policy that establishes guidelines for reviewing and approving transactions involving any director or certain executives in which (1) the aggregate amount involved will or may be expected to exceed $25,000; (2) Mylan or an affiliate of Mylan is a participant; and (3) any related party has or will have a direct or indirect interest.
Director Independence
The Mylan Board has determined that Ms. Cameron, Judge Cindrich, Ms. Dillon, Mr. Dimick, Ms. Higgins, Mr. Leech, Dr. Maroon, Mr. Parrish, Mr. Piatt, and Dr. Vanderveen are independent directors under the applicable NASDAQ listing standards. In making these determinations, the Mylan Board considered, with respect to Dr. Maroon’s independence, that his daughter has worked for Mylan during one or more of the past several years. With respect to Mr. Piatt’s independence, the Mylan Board considered that in 2015 and earlier years, Mylan paid minimal membership costs for several employees and sponsored events at a facility indirectly owned, in part, by Mr. Piatt. The Mylan Board also considered that Mr. Piatt is a prominent member of the Southpointe community, in which Mylan’s headquarters is located, and that he has, and has had in the past,
55
Table of Contents
ownership interests in certain properties in the Southpointe community; Mr. Piatt has also been involved in the development of Southpointe and in various routine matters related to the upkeep and maintenance of the neighborhood and associated utilities, as has Mylan. With regard to both Dr. Maroon and Mr. Piatt, the Mylan Board determined that any such arrangements, transactions, or relationships do not interfere with the exercise of independent judgment by these directors in carrying out their responsibilities as a director of Mylan. Mr. Coury, Ms. Bresch, and Mr. Malik are not independent directors due to their current service as Mylan’s Executive Chairman, CEO, and President, respectively. A majority of the Mylan Board is also independent within the meaning of best practice provision III.2.2 of the Dutch Corporate Governance Code.
| ITEM 14. | Principal Accounting Fees and Services |
Deloitte served as Mylan’s independent registered public accounting firm during 2015 and 2014, and no relationship exists other than the usual relationship between independent registered public accounting firm and client. Details about the nature of the services provided by, and the fees the Company paid to, Deloitte and affiliated firms for such services during 2015 and 2014 are set forth below.
| Dollars in Millions | ||||||||
| 2015 | 2014 | |||||||
| Audit Fees (1) |
$ | 8.5 | $ | 5.8 | ||||
| Audit-Related Fees (2) |
0.5 | 0.4 | ||||||
| Tax Fees (3) |
0.1 | 0.3 | ||||||
| All Other Fees (4) |
0.1 | — | ||||||
| Total Fees |
$ | 9.2 | $ | 6.5 | ||||
| (1) | Represents fees for professional services provided for the audit of the Company’s annual consolidated financial statements and Dutch Annual Accounts, the audit of the Company’s internal control over financial reporting as required by Section 404 of the Sarbanes-Oxley Act of 2002, reviews of the Company’s quarterly condensed consolidated financial statements, audit services provided in connection with other statutory or regulatory filings, and accounting, reporting and disclosure matters. |
| (2) | Represents fees for assurance services related to the audit of the Company’s annual consolidated financial statements, including the audit of the Company’s employee benefit plans, comfort letters, certain SEC filings and other agreed upon procedures. |
| (3) | Represents fees related primarily to tax return preparation, tax planning and tax compliance support services. |
| (4) | Represents fees related primarily to advisory services. |
Audit Committee Pre-Approval Policy
The Audit Committee has a policy regarding pre-approval of audit, audit-related, tax, and other services that the independent registered public accounting firm may perform for the Company. Under the policy, the Audit Committee must pre-approve on an individual basis any requests for audit, audit-related, tax, and other services not covered by certain services that are pre-approved annually by the Audit Committee. The policy also prohibits the engagement of the independent registered public accounting firm for non-audit related financial information systems design and implementation, for certain other services considered to have an impact on independence, and for all services prohibited by the Sarbanes-Oxley Act of 2002. All services performed by Deloitte during 2015 and 2014 were pre-approved by the Audit Committee in accordance with its policy.
56
Table of Contents
PART IV
| ITEM 15. | Exhibits |
| 31.1 | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| 31.2 | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
57
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: April 29, 2016 |
MYLAN N.V. | |||||||
| By: | /s/ Paul B. Campbell | |||||||
| Paul B. Campbell Senior Vice President, Controller and Chief Accounting Officer (Principal Accounting Officer) | ||||||||
58
Table of Contents
EXHIBIT INDEX
| 31.1 | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| 31.2 | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
59
Table of Contents
Appendix A — Reconciliation of Non-GAAP Measures (Unaudited)
Adjusted Third Party Net Sales — Europe
| Year Ended December 31, | ||||||||
| (Unaudited; in millions) | 2015 | 2014 | ||||||
| U.S. GAAP third party net sales from Europe |
$ | 2,205.6 | $ | 1,476.8 | ||||
| Add: |
||||||||
| Acquisition related customer incentive |
17.1 | — | ||||||
|
|
|
|
|
|||||
| Adjusted third party net sales from Europe |
$ | 2,222.7 | $ | 1,476.8 | ||||
|
|
|
|
|
|||||
Adjusted Third Party Net Sales — Generic
| Year Ended December 31, | ||||||||
| (Unaudited; in millions) | 2015 | 2014 | ||||||
| U.S. GAAP Generics segment third party net sales |
$ | 8,157.8 | $ | 6,459.3 | ||||
| Add: |
||||||||
| Acquisition related customer incentive |
17.1 | — | ||||||
|
|
|
|
|
|||||
| Adjusted Generics segment third party net sales |
$ | 8,174.9 | $ | 6,459.3 | ||||
|
|
|
|
|
|||||
Adjusted Third Party Net Sales
| Year Ended December 31, | ||||||||
| (Unaudited; in millions) | 2015 | 2014 | ||||||
| GAAP third party net sales |
$ | 9,362.6 | $ | 7,646.5 | ||||
| Add: |
||||||||
| Acquisition related customer incentive |
17.1 | — | ||||||
|
|
|
|
|
|||||
| Adjusted third party net sales |
$ | 9,379.7 | $ | 7,646.5 | ||||
|
|
|
|
|
|||||
Adjusted Revenue
| Year Ended December 31, | ||||||||
| (Unaudited; in millions) | 2015 | 2014 | ||||||
| U.S. GAAP Total Revenues |
$ | 9,429.3 | $ | 7,719.6 | ||||
| Add: |
||||||||
| Acquisition related customer incentive |
17.1 | — | ||||||
|
|
|
|
|
|||||
| Adjusted total revenues |
$ | 9,446.4 | $ | 7,719.6 | ||||
|
|
|
|
|
|||||
Table of Contents
Adjusted Diluted EPS
| Year Ended December 31, | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (Unaudited; in millions, except per share amounts) |
2015 | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| U.S. GAAP net earnings attributable to Mylan N.V. and U.S. GAAP diluted EPS |
$ | 848 | $ | 1.70 | $ | 929 | $ | 2.34 | $ | 624 | $ | 1.58 | $ | 641 | $ | 1.52 | $ | 537 | $ | 1.22 | $ | 224 | $ | 0.68 | $ | 94 | $ | 0.30 | $ | (335 | ) | $ | (1.10 | ) | ||||||||||||||||||||||||||||||
| Purchase accounting related amortization (primarily included in cost of sales) (a) |
901 | 419 | 371 | 391 | 365 | 309 | 283 | 489 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Goodwill impairment charges |
— | — | — | — | — | — | — | 385 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bystolic revenue |
— | — | — | — | — | — | — | (468 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Litigation settlements, net |
(97 | ) | 48 | (10 | ) | (3 | ) | 49 | 127 | 226 | 17 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interest expense, primarily amortization of convertible debt discount |
46 | 46 | 38 | 36 | 49 | 60 | 43 | 30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-cash accretion and fair value adjustments of contingent consideration liability |
38 | 35 | 35 | 39 | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clean energy investments pre-tax loss (b) |
93 | 79 | 22 | 17 | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Financing related costs (included in other expense (income), net) (c) |
112 | 33 | 73 | — | 34 | 37 | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Acquisition related costs (primarily included in cost of sales and selling, general and administrative expense) |
438 | 140 | 50 | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Acquisition related customer incentive (included in third party net sales) |
17 | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Acceleration of deferred revenue |
— | — | — | — | — | — | (29 | ) | — | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-controlling interest |
— | — | — | — | — | — | 9 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Restructuring and other special items included in: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cost of sales |
36 | 45 | 49 | 66 | 8 | 7 | 33 | 53 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Research and development expense |
20 | 18 | 52 | 12 | 4 | 10 | 22 | 14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selling, general and administrative expense |
48 | 67 | 71 | 105 | 45 | 63 | 49 | 89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other income (expense), net |
7 | (11 | ) | 25 | (1 | ) | — | 1 | (13 | ) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tax effect of the above items and other income tax related items (d) |
(370 | ) | (432 | ) | (260 | ) | (216 | ) | (198 | ) | (253 | ) | (273 | ) | (31 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Preferred dividend (e) |
— | — | — | — | — | 122 | 139 | — | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Adjusted net earnings attributable to Mylan N.V. and adjusted diluted EPS |
$ | 2,137 | $ | 4.30 | $ | 1,416 | $ | 3.56 | $ | 1,140 | $ | 2.89 | $ | 1,087 | $ | 2.59 | $ | 893 | $ | 2.04 | $ | 707 | $ | 1.61 | $ | 583 | $ | 1.30 | $ | 244 | $ | 0.80 | ||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||
| Weighted average diluted common shares outstanding (e) |
$ | 497 | $ | 398 | $ | 395 | $ | 420 | $ | 439 | $ | 438 | $ | 450 | $ | 304 | ||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| (a) | Adjustment for purchase accounting related amortization expense for the year ended December 31, 2015, 2014, 2013, 2012, and 2011, respectively includes $31 million, $28 million, $18 million, $42 million and $16 million of intangible asset impairment charges. |
| (b) | Adjustment represents exclusion of the pre-tax loss related to Mylan’s clean energy investments, the activities of which qualify for income tax credits under section 45 of the U.S. Internal Revenue Code of 1986, as amended (the “Code”). The amount is included in other expense (income), net in the Consolidated Statements of Operations. |
| (c) | Adjustment represents approximately $71.2 million related to the termination of certain interest rate swaps and changes of approximately $40.8 million related to the redemption of the Company’s 7.875% Senior Notes due to 2020 for the year ended December 31, 2015. |
| (d) | Adjustment for other income tax related items includes the exclusion from adjusted net earnings of the tax benefit of approximately $156 million related to the merger of the Company’s wholly owned subsidiaries, Agila Specialties Private Limited and Onco Therapies Limited, into Mylan Laboratories Limited for the year ended December 31, 2014. |
Table of Contents
| (e) | Adjusted diluted EPS for the year ended December 31, 2010, includes the full effect of the conversion of the company’s preferred stock into 125.2 million shares of common stock on November 15, 2010. Adjusted diluted EPS for the period ended December 31, 2009 was calculated under the “if-converted method” which assumes conversion of the Company’s preferred stock into shares of common stock, based on an average share price, and excludes the preferred dividend from the calculation, as the “if-converted method” is more dilutive. |
Summary of Adjusted Total Revenues by Segment
| Year Ended December 31, |
Year
Ended Percentage Change |
|||||||||||||||
| 2015 | 2014 | Actual | Constant Currency (1) |
|||||||||||||
| Generics (adjusted): |
||||||||||||||||
| Third party net sales |
||||||||||||||||
| North America |
$ | 3,895.6 | $ | 3,361.2 | 16 | % | 16 | % | ||||||||
| Europe (adjusted) (2) |
2,222.7 | 1,476.8 | 51 | % | 67 | % | ||||||||||
| Rest of World |
2,056.6 | 1,621.3 | 27 | % | 38 | % | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Adjusted total third party net sales (2) |
8,174.9 | 6,459.3 | 27 | % | 33 | % | ||||||||||
|
|
|
|
|
|||||||||||||
| Other third party revenues |
40.8 | 51.1 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Adjusted total third party revenues |
8,215.7 | 6,510.4 | ||||||||||||||
| Intersegment sales |
6.3 | 4.7 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Adjusted Generics total revenues |
8,222.0 | 6,515.1 | ||||||||||||||
| Specialty: |
||||||||||||||||
| Third party net sales |
1,204.8 | 1,187.2 | 1 | % | 1 | % | ||||||||||
|
|
|
|
|
|||||||||||||
| Other third party revenues |
25.9 | 22.0 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Total third party revenues |
1,230.7 | 1,209.2 | ||||||||||||||
| Intersegment sales |
10.9 | 9.0 | ||||||||||||||
|
|
|
|
|
|||||||||||||
| Specialty total revenues |
1,241.6 | 1,218.2 | ||||||||||||||
| Elimination of intersegment sales |
(17.2 | ) | (13.7 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Adjusted consolidated total revenues (2) |
$ | 9,446.4 | $ | 7,719.6 | 22 | % | 28 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | The constant currency percent change is derived by translating third party net sales for the current period at prior year comparative period exchange rates. |
| (2) | Refer to the non-GAAP reconciliations for reconciliations of adjusted third party net sales from Europe, Generics segment adjusted third party net sales, adjusted third party net sales and adjusted total revenues to the most directly comparable GAAP financial measures for the year ended December 31, 2015. For the year ended December 31, 2014, GAAP third party net sales from Europe, GAAP Generics segment third party net sales, GAAP third party new sales and GAAP total revenues were the same as the corresponding adjusted measures. |
Table of Contents
Adjusted EBITDA
| Year Ended December 31, | ||||||||||||||||||||
| (Unaudited; in millions, except %) | 2015 | 2014 | 2013 | 2012 | 2011 | |||||||||||||||
| U.S. GAAP net earnings attributable to Mylan N.V. ordinary shareholders |
$ | 847.6 | $ | 929.4 | $ | 623.7 | $ | 640.9 | $ | 536.8 | ||||||||||
| Add adjustments: |
||||||||||||||||||||
| Net contribution attributable to the noncontrolling interest and equity method investments |
105.2 | 95.1 | 37.1 | 17.9 | 2.0 | |||||||||||||||
| Income taxes |
67.7 | 41.4 | 120.8 | 161.2 | 115.8 | |||||||||||||||
| Interest expense |
339.4 | 333.2 | 313.3 | 308.7 | 335.9 | |||||||||||||||
| Depreciation and purchase accounting amortization, including product and IPR&D asset impairments |
1,032.1 | 566.6 | 516.0 | 546.6 | 510.7 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| EBITDA |
$ | 2,392.0 | $ | 1,965.7 | $ | 1,610.9 | $ | 1,675.3 | $ | 1,501.2 | ||||||||||
| Add / (Deduct) adjustments: |
||||||||||||||||||||
| Share-based compensation expense |
92.8 | 65.9 | 47.0 | 42.6 | 42.0 | |||||||||||||||
| Litigation settlements, net |
(97.4 | ) | 47.9 | (9.9 | ) | (3.1 | ) | 48.6 | ||||||||||||
| Restructuring & other special items |
624.7 | 286.4 | 306.7 | 176.0 | 84.0 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Adjusted EBITDA |
$ | 3,012.1 | $ | 2,365.9 | $ | 1,954.7 | $ | 1,890.8 | $ | 1,675.8 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Adjusted Pre-tax Income
| Year
Ended December 31, 2015 |
||||
| (Unaudited; in millions) | ||||
| U.S. GAAP pre-tax income (loss) |
$ | 915.4 | ||
| Pre-tax loss related to the clean energy investments |
93.2 | |||
| Purchase accounting related amortization, including product and IPR&D asset impairments |
900.9 | |||
| Acquisition related costs (primarily included in selling, general and administrative expense) |
438.0 | |||
| Acquisition related customer incentive (included in third party net sales) |
17.1 | |||
| Litigation settlements, net |
(97.4 | ) | ||
| Financing-related costs |
112.0 | |||
| Interest expense, primarily accretion of contingent consideration and amortization of convertible debt discount |
84.0 | |||
| Restructuring and other special items |
112.1 | |||
|
|
|
|||
| Adjusted pre-tax income |
$ | 2,575.3 | ||
|
|
|
|||
Table of Contents
Adjusted Interest Expense
| (Unaudited; in millions) | Year Ended December 31, 2015 |
|||
| U.S. GAAP interest expense |
$ | 339.4 | ||
| Deduct: |
||||
| Interest expense related to clean energy investments (a) |
(16.4 | ) | ||
| Non-cash accretion of contingent consideration liability |
(38.4 | ) | ||
| Non-cash interest |
(29.2 | ) | ||
| Acquisition financing costs |
(56.9 | ) | ||
|
|
|
|||
| Adjusted interest expense |
$ | 198.5 | ||
|
|
|
|||
| (a) | Adjustment represents exclusion of activity related to Mylan’s clean energy investments, the activities of which qualify for income tax credits under Section 45 of the Internal Revenue Code of 1986, as amended. |
Adjusted Income Tax Expense
| Year
Ended December 31, 2015 |
||||
| (Unaudited; in millions) | ||||
| U.S. GAAP income tax provision |
$ | 67.7 | ||
| Deduct: |
||||
| Tax effect of adjustments to pre-tax income and other income tax related items |
(370.1 | ) | ||
|
|
|
|||
| Adjusted income tax provision |
$ | 437.8 | ||
|
|
|
|||
| Adjusted effective tax rate |
17 | % | ||
Adjusted Operating Cash Flow and Free Cash Flow
| Year Ended December 31, | ||||||||
| (Unaudited; in millions) | 2015 | 2014 | ||||||
| U.S. GAAP net cash provided by operating activities |
$ | 2,008 | $ | 1,015 | ||||
| (Deduct) / Add: |
||||||||
| (Receipt) / payment of litigation settlements |
(113 | ) | 96 | |||||
| Financing Fees |
137 | 24 | ||||||
| Acquisition related costs |
191 | 64 | ||||||
| R&D expense |
12 | 21 | ||||||
| Income tax items |
(22 | ) | (13 | ) | ||||
| Other |
4 | 3 | ||||||
|
|
|
|
|
|||||
| Adjusted cash provided by operating activities |
$ | 2,217 | $ | 1,210 | ||||
|
|
|
|
|
|||||
| (Deduct) / Add: |
||||||||
| Capital expenditures |
(363 | ) | (325 | ) | ||||
| Proceeds from sale of property plant and equipment |
— | 9 | ||||||
|
|
|
|
|
|||||
| Adjusted free cash flow |
$ | 1,854 | $ | 894 | ||||
|
|
|
|
|
|||||
Table of Contents
Return on Invested Capital
| Year
Ended December 31, 2015 |
||||||||||
| (Unaudited; in millions, except %) | ||||||||||
| Adjusted pre-tax income |
$ | 2,575 | ||||||||
| Adjusted interest expense |
199 | |||||||||
|
|
|
|||||||||
| Adjusted income before interest and tax |
2,774 | |||||||||
| Estimated adjusted income tax expense (a) |
(472 | ) | ||||||||
|
|
|
|||||||||
| Adjusted net operating profit after tax |
$ | 2,302 | ||||||||
| As of December 31, 2014 |
||||||||||
| Total assets |
$ | 20,878 | ||||||||
| Cash and near cash items |
(553 | ) | ||||||||
| Short-term investments |
(71 | ) | ||||||||
| Deferred income taxes |
(470 | ) | ||||||||
| Cash Convertible Note hedge |
(1,105 | ) | ||||||||
| Forward starting swaps |
45 | |||||||||
| Clean energy investments |
(422 | ) | ||||||||
| Restricted cash |
(124 | ) | ||||||||
|
|
|
|||||||||
| Total invested assets |
$ | 18,178 | ||||||||
|
|
|
|||||||||
| Accounts payable |
(1,070 | ) | ||||||||
| Other current liabilities |
(1,615 | ) | ||||||||
| Income taxes payable |
(98 | ) | ||||||||
|
|
|
|||||||||
| Total invested capital |
$ | 15,395 | ||||||||
|
|
|
|||||||||
| Intangible assets |
(6,011 | ) | ||||||||
| Goodwill |
(4,977 | ) | ||||||||
|
|
|
|
||||||||
| Operational invested capital |
$ | 4,407 | ||||||||
|
|
|
|||||||||
| Total invested capital excluding goodwill |
$ | 10,418 | ||||||||
|
|
|
|
||||||||
| Cash Return on Total Invested Capital (b) |
15 | % | ||||||||
| Cash Return on Operating Invested Capital (b) |
52 | % | ||||||||
| Cash Return on Invested Capital Excluding Goodwill (b) |
22 | % | ||||||||
| Weighted Average Cost of Capital |
8 | % | ||||||||
| (a) | Estimated adjusted income tax expense is the adjusted income tax rate multiplied by adjusted income before interest and tax. |
| (b) | Calculated using current year Net Operating Profit After Tax / 5 point average (Prior Year Ending Invested Capital + quarterly Invested Capital for Current Year) |
Table of Contents
Reconciliation of Forecasted GAAP Net Earnings and GAAP Diluted EPS to Adjusted Net Earnings and Adjusted Diluted EPS
| Twelve Months Ended December 31, 2016 | ||||||||||||||||
| (Unaudited; in millions) | Lower | Upper | ||||||||||||||
| GAAP net earnings attributable to Mylan N.V. and GAAP diluted EPS |
$ | 1,235 | $ | 2.38 | $ | 1,290 | $ | 2.43 | ||||||||
| Purchase accounting related amortization |
1,000 | 1,050 | ||||||||||||||
| Interest expense, primarily amortization of convertible debt discount |
60 | 70 | ||||||||||||||
| Pre-tax loss of clean energy investments |
90 | 100 | ||||||||||||||
| R&D milestone payments |
100 | 125 | ||||||||||||||
| Restructuring, acquisition and other special items |
270 | 375 | ||||||||||||||
| Tax effect of the above items and other income tax related items |
(230 | ) | (285 | ) | ||||||||||||
|
|
|
|
|
|||||||||||||
| Adjusted net earnings attributable to Mylan N.V. and adjusted diluted EPS |
$ | 2,525 | $ | 4.85 | $ | 2,725 | $ | 5.15 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||