Attached files
| file | filename |
|---|---|
| EX-2.1 - EX-2.1 - ABBOTT LABORATORIES | a16-9714_1ex2d1.htm |
| EX-99.1 - EX-99.1 - ABBOTT LABORATORIES | a16-9714_1ex99d1.htm |
| 8-K - 8-K - ABBOTT LABORATORIES | a16-9714_18k.htm |
Exhibit 99.2
Creating A Premier Medical Device Leader April 28, 2016

Information Related to This Communication Important Additional Information In connection with the proposed transaction, Abbott intends to file a registration statement on Form S-4 with the SEC which will include a document that serves as a prospectus of Abbott and a proxy statement of St. Jude Medical (the "proxy statement/ prospectus"), and each party will file other documents regarding the proposed transaction with the SEC. Investors and security holders of St. Jude Medical are urged to carefully read the entire registration statement and proxy statement/prospectus and other relevant documents filed with the SEC when they become available, because they will contain important information. A definitive proxy statement/prospectus will be sent to St. Jude Medical's shareholders. Investors and security holders will be able to obtain the registration statement and the proxy statement/prospectus free of charge from the SEC's website or from Abbott or St. Jude Medical as described in the paragraphs below. The documents filed by Abbott with the SEC may be obtained free of charge at Abbott's website at www.abbott.com or at the SEC's website at www.sec.gov. These documents may also be obtained free of charge from Abbott by requesting them by mail at Abbott Laboratories, 6100 Abbott Park Road, Abbott Park, IL 60064-6400, Attention Investor Relations, or by telephone at (224) 667-8945. The documents filed by St. Jude Medical with the SEC may be obtained free of charge at St. Jude Medical's website at www.sjm.com or at the SEC's website at www.sec.gov. These documents may also be obtained free of charge from St. Jude Medical by requesting them by mail at St. Jude Medical, One St. Jude Medical Drive, St. Paul, MN 55117. Attention: Investor Relations, or by telephone at (651) 756-4347. Participants in the Solicitation St. Jude Medical, Abbott and certain of their directors, executive officers and employees may be deemed participants in the solicitation of proxies from St. Jude Medical shareholders in connection with the proposed transaction. Information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of the shareholders of St. Jude Medical in connection with the proposed transaction, including a description of their direct or indirect interests, by security holdings or otherwise, will be set forth in the proxy statement/prospectus when it is filed with the SEC. Information about the directors and executive officers of Abbott and their ownership of Abbott common shares is set forth in the definitive proxy statement for Abbott's 2016 annual meeting of shareholders, as previously filed with the SEC on March 18, 2016. Information about the directors and executive officers of St. Jude Medical and their ownership of St. Jude Medical common shares is set forth in the definitive proxy statement for St. Jude Medical's 2016 annual meeting of shareholders, as previously filed with the SEC on March 22, 2016. Free copies of these documents may be obtained as described in the paragraphs above. 2 Creating A Premier Medical Device Leader | April 27, 2016

Forward-Looking Statements Private Securities Litigation Reform Act of 1995 Caution Concerning Forward-Looking Statements Some statements in this news release may be forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995. Abbott and St. Jude Medical caution that these forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those indicated in the forward-looking statements, including but not limited to the ability of the parties to consummate the proposed transaction on a timely basis or at all, the ability of the parties to satisfy the conditions precedent to consummation of the proposed transaction, including the ability to secure the required regulatory approvals on the terms expected, at all or in a timely manner, the ability of Abbott to successfully integrate St. Jude Medical's operations, and the ability of Abbott to implement its plans, forecasts and other expectations with respect to St. Jude Medical's business after the completion of the transaction and realize expected synergies. Economic, competitive, governmental, technological and other factors that may affect Abbott's and St. Jude Medical's operations are discussed in Item 1A, "Risk Factors,'' in each of Abbott's Annual Report on Securities and Exchange Commission Form 10-K for the year ended Dec. 31, 2015, and St. Jude Medical's Annual Report on Securities and Exchange Commission Form 10-K for the year ended Jan. 2, 2016, respectively, and are incorporated by reference. Abbott and St. Jude Medical undertake no obligation to release publicly any revisions to forward-looking statements as a result of subsequent events or developments, except as required by law. 3 Creating A Premier Medical Device Leader | April 27, 2016

Transaction Overview • • St. Jude Medical shareholders will receive $46.75 in cash and 0.8708 shares of Abbott common stock. At an Abbott stock price of $43.93(1) , this represents a total consideration of $85 per St. Jude Medical share and a total transaction equity value of $25 billion. • The transaction is expected to be accretive to Abbott’s adjusted earnings per share(2) in the first full year, with approximately $0.21 of accretion in 2017 and $0.29 in 2018. The combination is anticipated to result in annual pre-tax synergies of $500 million by 2020, including both sales and operational benefits. • • Abbott will assume or refinance St. Jude Medical’s net debt, currently $5.7 billion, and intends to fund the cash portion of this transaction with debt. Abbott is committed to maintaining an investment grade credit-rating for the combined entity and, following the close of the transaction, will implement a de-leveraging plan to achieve an initial adjusted debt-to-EBITDA ratio of 4.5 and 3.5 in 2018, with further reductions over time.(3) As part of this plan, Abbott will reduce its share repurchase activity, moderate the pace of growth of its dividend, and M&A activity will be minimal until it achieves its leverage targets. • • • The transaction, which has been approved by the boards of directors of St. Jude Medical and Abbott, is subject to the approval of St. Jude Medical shareholders and the satisfaction of customary closing conditions, including specified regulatory approvals. The transaction is expected to close in the fourth quarter of 2016. • (1) Based on Abbott’s 5-day volume weighted average share price of $43.93 as of April 26, 2016. (2) Adjusted earnings per share (EPS) excludes specified items such as amortization of acquired intangibles, inventory step-up, restructuring costs and other costs incurred to execute the transaction. Adjusted EPS is a non-GAAP financial measure and should not be considered a replacement for GAAP results. (3) Adjusted debt-to-EBITDA ratios reflect credit rating agency calculation methodology. 4 Creating A Premier Medical Device Leader | April 27, 2016 Timing Financing Transaction Impact Transaction Terms

Combination of Abbott and St. Jude Medical Creates A Premier Medical Device Leader Combined business will have one of the broadest portfolios of devices and an industry-leading pipeline to help healthcare systems provide better care for patients while increasing efficiencies and reducing costs. Alignment with the most relevant healthcare and demographic trends Together, the company will hold #1 or #2 positions across large and high-growth cardiovascular device areas and will compete in nearly every area of the market – with an aggregate market opportunity of $30 billion. Leadership positions in our core businesses The combination further diversifies and enhances sources of future growth for Abbott. Combined company will have an industry-leading pipeline across cardiovascular, diabetes, neuromodulation, and vision care. Well-managed, diversified business model to deliver reliable growth in a changing world Opportunity to create significant value through enhanced global scale, infrastructure, and capabilities. Strengthens Abbott’s presence in over 100 countries. Strong positions in the largest and fastest-growing geographies 5 Creating A Premier Medical Device Leader | April 27, 2016 4 Global 3 Balanced 2 Leading 1 Aligned Combination of Abbott and St. Jude Medical Abbott’s Strategic Priorities to Deliver Top-Tier Growth and Income

St. Jude Medical Background Founded in 1976, headquartered in St. Paul, MN with ~18,000 employees worldwide. 2015 Revenue(1) $1.5 billion(2) $1.1 billion $0.5 billion $1.2 billion $1.6 billion • Single and dual chamber pacemakers and implantable cardioverter-defibrillators • Cardiac resynchronization therapy • Ventricular assist devices • Pulmonary artery pressure monitors • Spinal cord stimulation • Dorsal root ganglion • Radiofrequency ablation • Deep brain stimulation • Heart valves • PCI optimization • Vascular closure • PFO closure • Percutaneous heart pumps • Atrial fibrillation access, diagnostic, visualization and ablation • Left atrial appendage Product Areas • CardioMEMSTM • MultiPointTM Pacing • HeartMateTM • IllumienTM • TrifectaTM • PorticoTM • AMPLATZERTM • AssurityTM • NanostimTM • EllipseTM • FlexAbilityTM • TactiCathTM • EnSiteTM • ConfirmTM • ProclaimTM • ProtégéTM • ProdigyTM • InfinityTM Key Brands (1) For additional information please see the Form 8-K furnished by St. Jude Medical on January 13, 2016, outlining changes to sales reporting starting in January 2016. (2) 2015 Pro Forma; includes full year revenue for Thoratec of $517 million (acquired in October 2015). 6 Creating A Premier Medical Device Leader | April 27, 2016 Traditional CRM Neuromodulation Atrial Fibrillation Heart Failure Cardiovascular

Together, Abbott and St. Jude Medical Hold Leading Positions Across Large and High-Growth Cardiovascular Device Areas + Cardiovascular Areas of Focus Rank (1) Fractional Flow Reserve and Optical Coherence Tomography 7 Creating A Premier Medical Device Leader | April 27, 2016 Coronary stents #1 PCI Optimization (FFR/OCT) (1) #1 Cardiac Rhythm Management #2 Left Ventricular Assist Devices#1 Surgical Heart Valves #2 Transcatheter Mitral Repair / Replacement #1 Atrial Fibrillation #2 Heart Failure Monitoring 1 Highly Complementary Portfolios with Market Leading Positions in the $30Bn+ Cardiovascular Device Market

Abbott Has A Long And Successful Of Integrations Select examples of Abbott transactions Established Abbott as a top 10 pharmaceutical company in Latin America. 5/16/2014 $2.9 billion • Positioned Abbott as the leading company in the fast-growing Indian pharmaceuticals market. 5/21/2010 $3.7 billion • Significantly strengthened Abbott’s branded generics pharmaceuticals presence in emerging markets. 9/28/2009 $6.2 billion • Broadened Abbott’s position in coronary and endovascular products. 1/08/2006 $4.1 billion 8 Creating A Premier Medical Device Leader | April 27, 2016 Guidant Vascular Business Unit Solvay Pharmaceuticals Piramal CFR Pharmaceuticals Transaction Size Commentary Date Announced

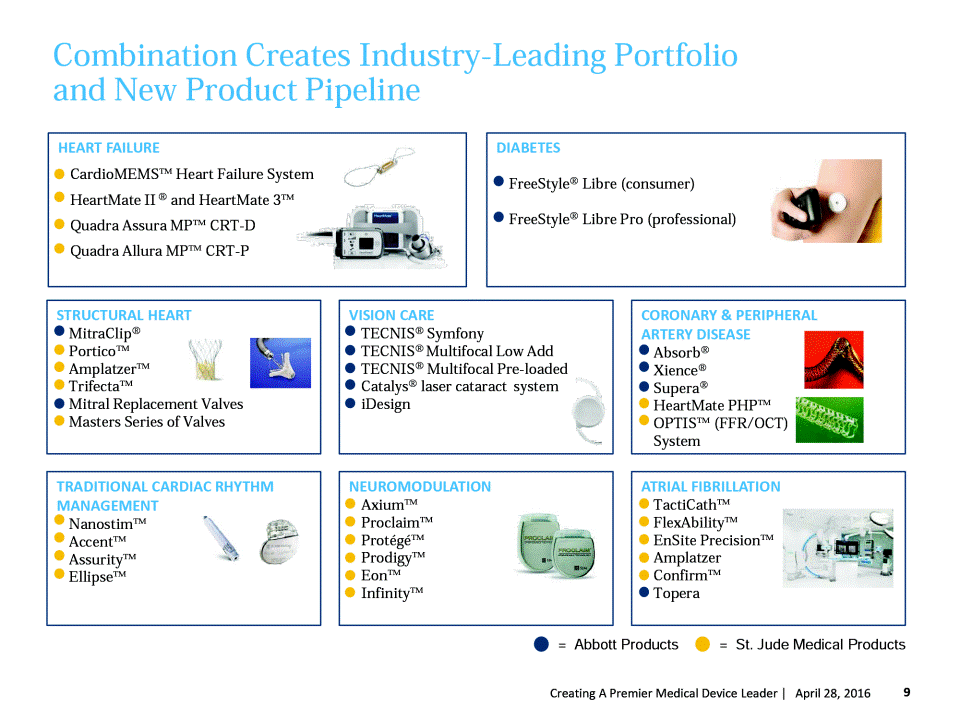
Combination Creates Industry-Leading Portfolio and New Product Pipeline = Abbott Products = St. Jude Medical Products 9 Creating A Premier Medical Device Leader | April 27, 2016 ATRIAL FIBRILLATION TactiCath™ FlexAbility™ EnSite Precision™ AmplatzerAmulet™ Confirm™ Topera TRADITIONAL CARDIAC RHYTHM MANAGEMENT Nanostim™ Accent™ Assurity™ Ellipse™ NEUROMODULATION Axium™ Proclaim™ Protégé™ Prodigy™ Eon™ Infinity™ CORONARY & PERIPHERAL ARTERY DISEASE Absorb® Xience® Supera® HeartMate PHP™ OPTIS™ (FFR/OCT) System VISION CARE TECNIS® Symfony TECNIS® Multifocal Low Add TECNIS® Multifocal Pre-loaded Catalys® laser cataract system iDesign STRUCTURAL HEART MitraClip® Portico™ Amplatzer™ Trifecta™ Mitral Replacement Valves Masters Series of Valves DIABETES • FreeStyle® Libre (consumer) • FreeStyle® Libre Pro (professional) HEART FAILURE CardioMEMS™ Heart Failure System HeartMate II ® and HeartMate 3™ Quadra Assura MP™ CRT-D Quadra Allura MP™ CRT-P
Highly Strategic and Financially Compelling Combination • Advances Abbott’s strategic and competitive position Creates a premier medical device leader well-positioned for long-term growth with access to new, high growth therapy areas Diversifies Abbott’s revenue mix and future sources of growth Provides operational and revenue synergies to create shareholder value Delivers adjusted earnings per share accretion in the first full year • Delivers immediate value through upfront cash consideration Opportunity to participate in the significant upside potential of the combined business • • • • • Adjusted earnings per share (EPS) excludes specified items such as amortization of acquired intangibles, inventory step-up, restructuring costs and other costs incurred to execute the transaction. Adjusted EPS is a non-GAAP financial measure and should not be considered a replacement for GAAP results. 10 Creating A Premier Medical Device Leader | April 27, 2016 St. Jude Medical Shareholders Abbott Shareholders

Summary Immediately creates a premier medical device leader with top positions in attractive markets and an industry-leading pipeline Highly complementary combination that is well-aligned with Abbott’s strategic priorities Significant value creation through enhanced global scale and capabilities, and broader portfolio of best-in-class products Strengthens Abbott’s long-term growth potential Offers compelling financial profile and value creation 11 Creating A Premier Medical Device Leader | April 27, 2016

