Attached files
| file | filename |
|---|---|
| 8-K - 8-K STELLAREX DATA - SPECTRANETICS CORP | a20168kstellarexdata.htm |
| EX-99.1 - EXHIBIT 99.1 STELLAREX DATA - SPECTRANETICS CORP | ex9912016stellarexdata.htm |

Charing Cross 2016 Prof. Thomas Zeller Department of Angiology University Heart Center Freiburg‐ Bad Krozingen Bad Krozingen, Germany On behalf of Dr. Andrew Holden, Prof. Yann Gouëffic and the ILLUMENATE Global Investigators ILLUMENATE Global Study Interim Analysis

Charing Cross 2016 Disclosure Speaker name: Prof. Thomas Zeller ................................................................................. I have the following potential conflicts of interest to report: Receipt of grants/research support Receipt of honoraria and travel support Participation in a company sponsored speakers‘ bureau Employment in industry Shareholder in a healthcare company Owner of a healthcare company I do not have any potential conflict of interest

Charing Cross 2016 ILLUMENATE (SFA) Clinical Program 6 Studies: 6000+ Patients

Charing Cross 2016 ILLUMENATE Global Study Overview Prospective, multi‐center, single‐arm, study Patients will be followed for up to 5 years Same rigorous data collection process as randomized trials, independent adjudication by: • Angiographic Core Laboratory1 • Duplex Ultrasound Core Laboratory2 • Clinical Events Committee • Data Safety Monitoring Board Monitoring with 100% source data verification 1. Beth Israel Deaconess Medical Center, Boston, MA 2. VasCore, Boston, MA

Charing Cross 2016 ILLUMENATE Global Study Overview Study Objective: Assess safety and performance of the Stellarex DCB in the SFA and/or popliteal arteries Primary Safety Endpoint: Freedom from device‐ and procedure‐related death through 30 days and freedom from target limb major amputation and CD‐TLR through 12 months Primary Effectiveness Endpoint: Primary patency at 12 months (defined as PSVR ≤ 2.5 and no CD‐ TLR)

Charing Cross 2016 • Low dose paclitaxel, 2 µg/mm2 • Excipient: Polyethylene Glycol (PEG) • Proprietary open‐folded coating technology • High coating stability • Limited drug loss • Effective drug tissue transfer and residency ( 28 days) Stellarex (2 µgr/mm2) DCB Comp. A (3.5 µgr/mm2) DCB Comp. B (2.0 µgr/mm2) Arterial Pharmaco‐Kinetics [2] PTX particulate loss after transit [1] 1. Number of particulates 10µm/mm of DCB length lost during transit. Data on file at Spectranetics 2. Superimposed PK curves from different datasets: R.Melder, EuroPCR 2012; Yazdani et.al. Catheterization and Cardiovascular Interventions 83:132-140 (2014); data on file at Spectranetics Study Device: Stellarex DCB (Spectranetics)

Charing Cross 2016 Key Patient Eligibility Criteria Inclusion Criteria • Rutherford class 2, 3 or 4 • SFA and/or popliteal (down to trifurcation) • Has at least one patent run‐ off below‐the‐knee • 1 or 2 target lesion(s) with cumulative length ≤ 20 cm • Target vessel reference diameter 4‐6 mm Exclusion Criteria • Acute or sub‐acute thrombus in target vessel • Significant inflow disease not successfully treated • In‐stent restenosis • Severe calcification that precludes adequate PTA treatment • Use of adjunctive therapies (i.e. debulking or plaque incision)

Charing Cross 2016 Interim Analysis The following Interim analysis includes data on the first 153 subjects who have been seen for their 12 month follow‐up visit N=153 subjects (of the 371 enrolled) All presented data/images were monitored, adjudicated and assessed by the appropriate core lab as outlined in the protocol ITT analysis Interim Data 42% Data Pending 58%

Charing Cross 2016 Baseline Patient Characteristics N (patients) 153 Age 69.3 ± 9.6 (153) Male 72.5% (111/153) Diabetes 35.3% (54/153) Body Mass Index 27.1 ± 4.1 (152) Hypertension 75.8% (116/153) Hyperlipidemia 75.8% (116/153) Current smoker 37.3% (57/153) Previous coronary revasc. 37.3% (57/153) Renal insufficiency 6.5% (10/153) Previous intervention of lower limb 43.1% (66/153) ABI 0.72 ± 0.19 (139) RCC 1 0.7% RCC 2 28.1% RCC 3 60.1% RCC 4 7.2% RCC 5 3.9% Baseline Rutherford Class

Charing Cross 2016 Baseline Angiographic Characteristics N (lesions) 174 De novo1 94.3% (164/174) Lesion Length (cm) 7.3 ± 5.0 (172) Total occlusions 25.6% (44/172) Calcification None/Mild 37.8% (65/172) Moderate 19.8% (34/172) Severe calcification2 42.4% (73/172) Minimum lumen diameter (mm) 1.1 ± 0.9 (172) Reference vessel diameter (mm) 4.9 ± 0.8 (172) Baseline diameter stenosis (%) 78.5 ± 17.9 (172) Eccentric Lesion 60.5% (104/172) 1. Site reported 2. Defined as: Radiopacities noted on both sides of the arterial wall and extending more than one cm of length prior to contrast injection or digital subtraction. Prox SFA 10% Mid SFA 44% Distal SFA 30% Prox Pop 11% Mid Pop 4% Distal Pop 1% Lesion Location

Charing Cross 2016 Procedural Characteristics Pre‐dilatation1 98.9% (172/174) Post‐dilatation1 25.3% (44/174) Provisional stent1 12.6% (22/174) Stent Due to Dissection1 7.5% (13/174) Post‐procedural Dissections Grade D Flow‐limiting (Grade E or F) 16.3% (28/172) 0% (0/172) Post‐procedure MLD (mm) 3.6 ± 0.8 (171) Post‐procedure Diameter Stenosis (%) 26.3 ± 13.1 (171) Lesion Success2 94.7% (162/171) Technical Success3 93.0% (159/171) 1. Site‐reported data 2. Lesion success‐ Final residual %DS ≤ 50% (per angiographic core lab), after using the DCB 3. Technical success‐ Lesion success without a device malfunction. Summary statistics are based on non‐missing data

Charing Cross 2016 Interim 12 Month Safety Outcomes Freedom from Primary Safety Event: 91.0% 12‐month all‐cause mortality rate: 0.7% (1/143) Cause of death: pancreatic cancer Primary Safety Outcome Component Device‐ or procedure‐related death 0 Major target limb amputation 1 (Patient RCC 5 at baseline) Clinically‐driven TLR* 13 * No “non‐clinically‐driven” TLRs reported to date

Charing Cross 2016 Freedom From CD‐TLR* by Clinical Events Committee Adjudication * Defined as revascularization associated with PSVR ≥ 2.5 or >50% stenosis via angiogram and worsening of RCC by more than 1 or ABI decrease of >0.15 from the maximum early post-procedure level, that is clearly referable to the target lesion 93.2% at day 365 Day 0 30 180 360 365 395 At Risk 153 151 143 114 99 38 Event 0 1 4 12 13 13 Survival (%) 100 99.3 97.4 91.8 91.0 91.0 91.0%@ day 365 360

Charing Cross 2016 Freedom From Loss of Primary Patency by Duplex Core Lab Evaluation Primary Patency defined as freedom from Duplex derived restenosis (PSVR ≤ 2.5) and clinically-driven TLR Number at risk at day 395 is 39 since follow-up is still in progress Day 0 30 360 365 395 At Risk 174 171 161 125 109 38 Event 0 2 8 19 24 31 Survival (%) 100 98.9 95.3 88.5 84.7 75.8 84.7%@ day 365 360

Charing Cross 2016 ILLUMENATE Global Interim Data In Context with Core Lab* Adjudicated Patency Rates Schroeder H, et al. Catheter Cadiovasc Interv 86;278‐286 (2015). Tepe G. IN.PACT SFA 1‐year primary outcomes. Oral presentation . Charing Cross. April 5–8, 2014. London, UK, 2014. Rosenfield K, Jaff MR, White CJ et al. NEJM 2015;373:145‐53. *VasCore (Boston, MA); PSVR: 2.5 M e a n l e s i o n l e n g t h ( c m ) P a t e n t ( % ) Single‐ arm N=153 Single‐ arm N=50 RCT N=220 RCT N=316 ILLUMENATE Global Interim Stellarex 2µg/mm2 ILLUMENATE FIH Stellarex 2µg/mm2 IN.PACT SFA IN.PACT Admiral 3.5µg/mm2 LEVANT 2 Lutonix 2µg/mm2 Data overview for informational purposes only and not for head-to head comparison 88.5% @ day 360 84.7% @ day 365 89.5% @ day 365 89.8% @ day 360 73.5% @ day 365 7.3 7.2 8.9 6.3 0 2 4 6 8 10 12 14 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Single‐ arm N=153 Single‐ arm N=50 RCT N=220 RCT N=316

Charing Cross 2016 0% 1% 30% 57% 8% 4% 63% 23% 7% 6% 0% 1% RCC 0 RCC 1 RCC 2 RCC 3 RCC 4 RCC 5 Baseline 12 Months Functional Outcomes 0.72 0.93 0.92 0 0.2 0.4 0.6 0.8 1 1.2 Baseline n=139 6 months n=125 12 months n=121 Mean ABI Rutherford Classification* 74.8% of subjects had an improvement in ABI at 12 months compared to baseline *analysis includes patients with RCC data at baseline and 12 months (n=135) P values based on paired data at baseline and follow‐up P<0.001 P<0.001 P<0.001 94.1% of subjects had an improvement in Rutherford class at 12 months compared to baseline
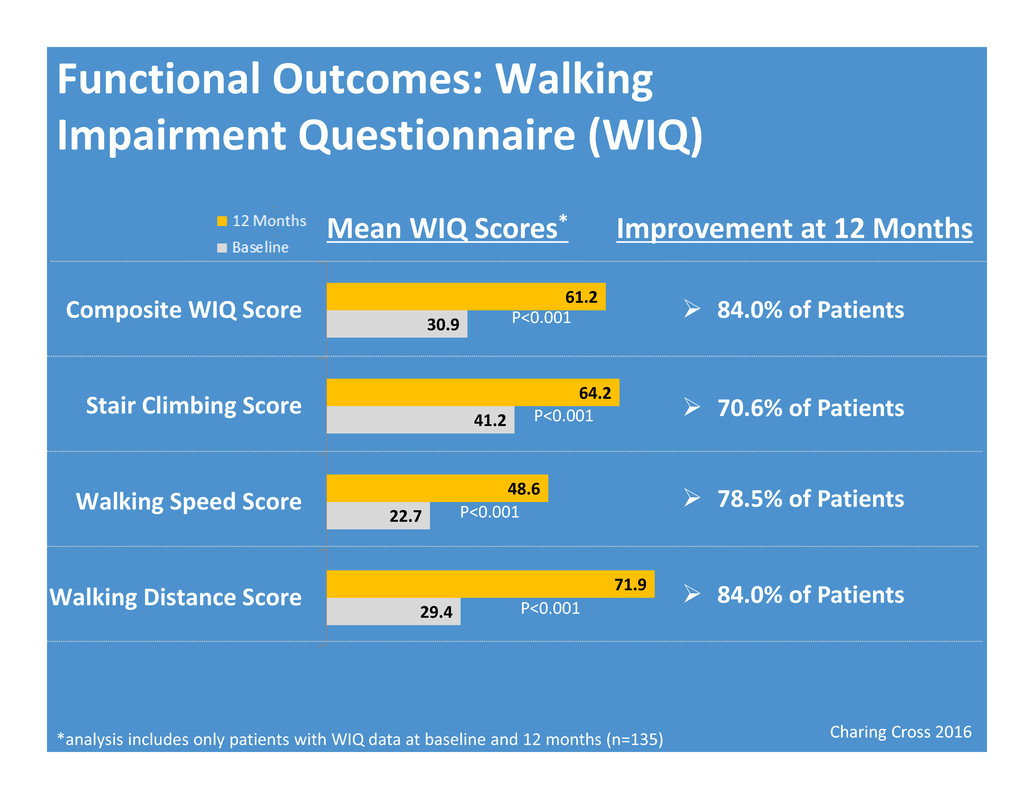
Charing Cross 2016 Functional Outcomes: Walking Impairment Questionnaire (WIQ) Mean WIQ Scores* *analysis includes only patients with WIQ data at baseline and 12 months (n=135) 29.4 22.7 41.2 30.9 71.9 48.6 64.2 61.2 Walking Distance Score Walking Speed Score Stair Climbing Score Composite WIQ Score Improvement at 12 Months P<0.001 P<0.001 P<0.001 P<0.001 84.0% of Patients 70.6% of Patients 78.5% of Patients 84.0% of Patients
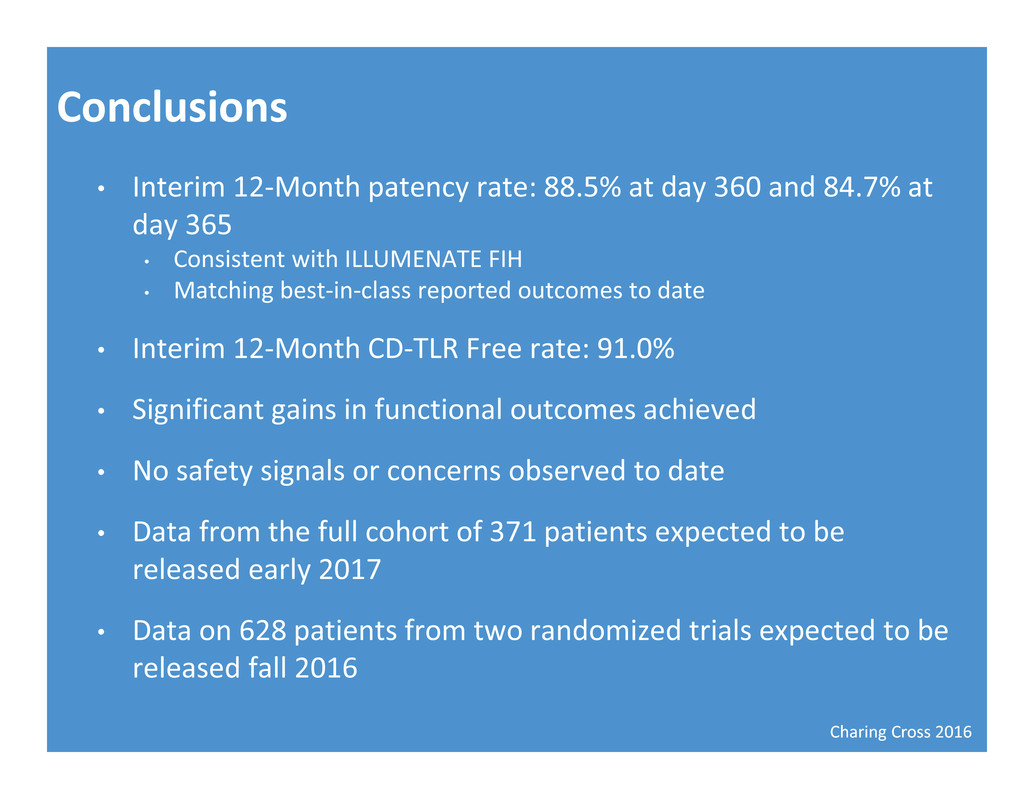
Charing Cross 2016 Conclusions • Interim 12‐Month patency rate: 88.5% at day 360 and 84.7% at day 365 • Consistent with ILLUMENATE FIH • Matching best‐in‐class reported outcomes to date • Interim 12‐Month CD‐TLR Free rate: 91.0% • Significant gains in functional outcomes achieved • No safety signals or concerns observed to date • Data from the full cohort of 371 patients expected to be released early 2017 • Data on 628 patients from two randomized trials expected to be released fall 2016

Charing Cross 2016 Prof. Thomas Zeller Department of Angiology University Heart Center Freiburg‐ Bad Krozingen Bad Krozingen, Germany On behalf of Dr. Andrew Holden, Prof. Yann Gouëffic and the ILLUMENATE Global Investigators ILLUMENATE Global Study Interim Analysis
