Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ZOGENIX, INC. | d183795d8k.htm |

Corporate Update April 2016 NASDAQ: ZGNX Exhibit 99.1

Forward Looking Statement Zogenix cautions you that statements included in this presentation that are not a description of historical facts are forward-looking statements. Words such as "believes," "plans," "expects," "will," "potential" and similar expressions are intended to identify forward-looking statements. These statements are based on the company's current beliefs and expectations. These forward-looking statements include statements regarding: the timing of the commencement and completion of clinical trials and regulatory submissions for ZX008 and RELDAY; the Company's cash position related to operating expenses and planned development activities; commercialization plans; orphan drug exclusivity and potential intellectual property protection; and delivery and dosing benefits of ZX008 and RELDAY and the potential to demonstrate that they have differentiated product profiles amongst currently marketed drug products. Actual results may differ from those set forth in this presentation due to the risk and uncertainties inherent in Zogenix's business, including, without limitation: difficulties or delays relating to the development, testing, manufacturing and marketing of any of Zogenix’s product candidates; the potential that earlier clinical trials may not be predictive of future results; Zogenix's reliance on third parties to conduct its clinical trials, enroll patients, manufacture its preclinical and clinical drug supplies and manufacture commercial supplies of its drug products, if approved; Zogenix's ability to fully comply with numerous federal, state and local laws and regulatory requirements that apply to its product development activities; Zogenix’s ability to obtain, and the validity and duration of, patent protection and other intellectual property rights for Relday; Zogenix could spend its available financial resources faster than it currently expects and may be unable to raise additional capital if and when needed, on acceptable terms or at all; and other risks described in the company's filings with the Securities and Exchange Commission (SEC). You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Zogenix undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement.

Late Stage Development Opportunities EPILEPSY (DRAVET SYNDROME) Phase 3 Initiated January 2016 SCHIZOPHRENIA Phase 3 Ready ONCE-MONTHLY ZX008 (low-dose fenfluramine) Products for the Treatment of CENTRAL NERVOUS SYSTEM (CNS) Disorders

The Zogenix Opportunity PROMISING PIPELINE of Differentiated CNS Compounds in Late-Stage Clinical Development ORPHAN DRUG STRATEGY Focused on Solving Significant Treatment Gaps FULLY FUNDED Through 2017 for ZX008 Phase 3 Data, NDA and MAA Submissions PHASE 3 PROGRAM INITIATED for ZX008, Fast Track Designation Granted by FDA EXPERIENCED TEAM with Successful Track Record of Drug Development and Commercialization

Worldwide Rights Orphan Drug Designation in U.S. and E.U. IND Approved Dec. 2015; Phase 3 Initiation Jan. 2016 Fast Track Designation Intend to Commercialize Directly in U.S. and E.U. Asia-Pacific Partnering Opportunity ZX008 Dravet Syndrome

Dravet Syndrome: A Catastrophic Illness Estimated 16,000 - 29,000 patients in the U.S. and E.U.1 Approximately 75 specialty treatment centers in U.S. and similar in E.U. Does not effectively respond to traditional epilepsy medications Higher incidence of status epilepticus and SUDEP compared to other childhood epilepsies Cognitive, developmental, behavioral, and motor impairment correlated with >5 seizures/month2 Polypharmacy is standard of care but no effective, long-term treatment exists 1. Calculated by patient incidence rates and population in US and in UK, Germany, Italy, Spain, France Bayat A et al; Epilepsia. 2015: 56(4):e36-9 (ISSN:1528-1167) The incidence of SCN1A-related Dravet syndrome in Denmark is 1:22,000: A population-based study from 2004 to 2009. Brunklaus, A. et al, Brain, 2012: 135; 2329-2336. Prognostic, clinical and demographic features in SCN1A mutation-positive Dravet syndrome Hurst, Daniel; Epilepsia. 31(4):397-400, 1990) Epidemiology of Severe Myoclonic Epilepsy of Infancy 2. Wolff M., Dravet C. Epilepsia, 2006; 47 Suppl 2:45-8. Severe myoclonic epilepsy of infants (Dravet syndrome): natural history and neuropsychological findings. Intractable, Severe Epilepsy Which Begins in Infancy

Low Dose Fenfluramine: Long Term Efficacy and Safety in Treating Dravet Syndrome Overview Long-term open-label study of fenfluramine as an adjunctive anticonvulsant therapy Published in Epilepsia in 2012 12 Dravet syndrome patients (11 with confirmatory genetic mutation) Age 9 months to 13 years at treatment initiation Efficacy 70% of patients seizure-free at last assessment Range of 1-19 years of seizure freedom Safety Well tolerated No Signs of Clinically Important Cardiac Valve Disease Clinical Data / IP Licensed by Zogenix
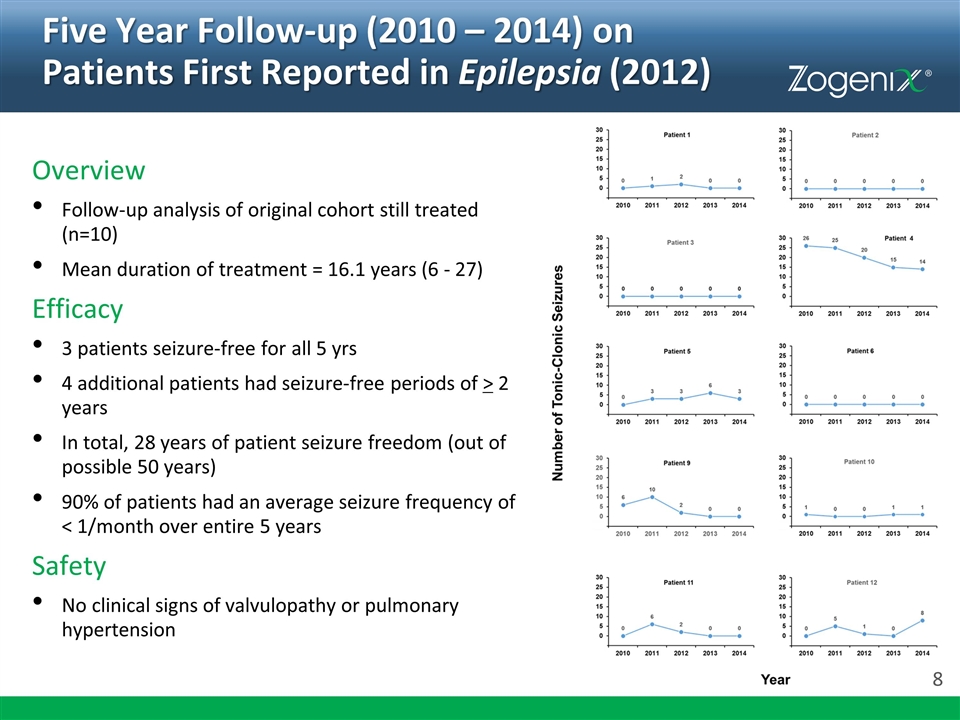
Five Year Follow-up (2010 – 2014) on Patients First Reported in Epilepsia (2012) Overview Follow-up analysis of original cohort still treated (n=10) Mean duration of treatment = 16.1 years (6 - 27) Efficacy 3 patients seizure-free for all 5 yrs 4 additional patients had seizure-free periods of > 2 years In total, 28 years of patient seizure freedom (out of possible 50 years) 90% of patients had an average seizure frequency of < 1/month over entire 5 years Safety No clinical signs of valvulopathy or pulmonary hypertension
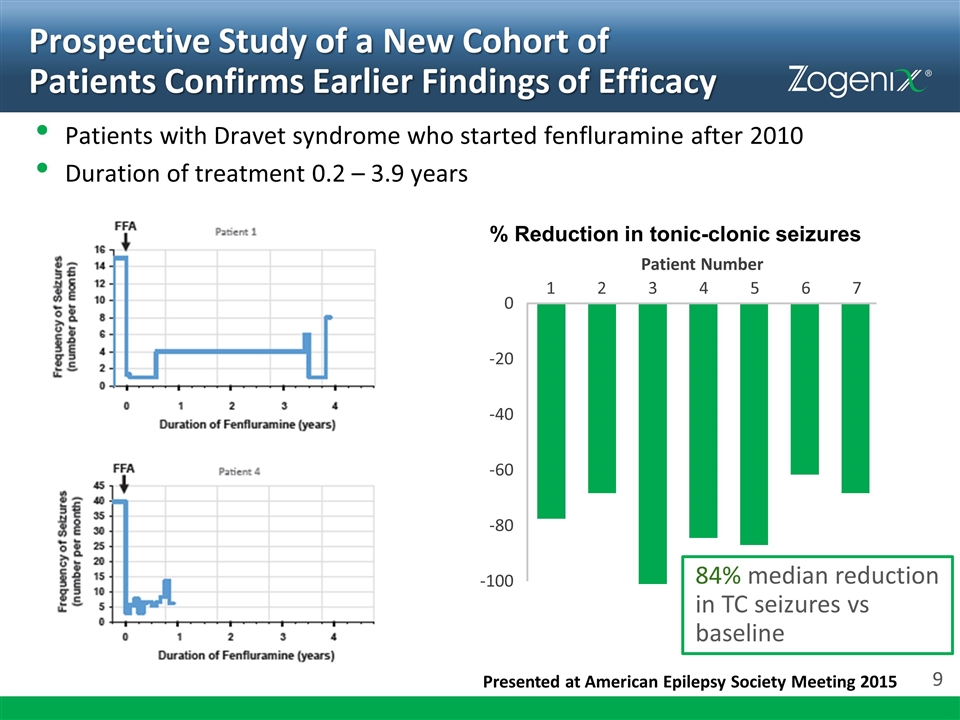
Prospective Study of a New Cohort of Patients Confirms Earlier Findings of Efficacy Presented at American Epilepsy Society Meeting 2015 % Reduction in tonic-clonic seizures Patient Number 0 -20 -40 -60 -80 -100 1 2 3 4 5 6 7 Patients with Dravet syndrome who started fenfluramine after 2010 Duration of treatment 0.2 – 3.9 years 84% median reduction in TC seizures vs baseline
Preclinical & Clinical Evidence for Role of Serotonin in Seizure Control Preclinical data ↑ extracellular 5-HT levels inhibits many types of seizures in rodent models Identification of 5HT receptors involved in Dravet syndrome seizure control Several AED’s (i.e. valproic acid, topirimate) ↑ 5HT Bonnycastle et al., 1957, Bagdy et al., 2007, Grosso et al., 2008 Genetic deletion of 5-HT2C receptors in mice causes audiogenic seizures Genetic knockout of 5-HT1A receptors ↓ threshold for kainate-induced seizures Richerson et al., 2011 Clinical data PET studies in clinical epilepsy demonstrates: ↓ binding of serotonin and 5-HT1A ligands in regions of the brain where seizure activity is emanating 5HT1A receptor abnormalities in epileptogenic areas and areas of seizure propagation Chugani et.al., 2005, Toczek et.al., 2003 Genetic variation of the 5-HT2A receptor affects age at onset in patients with Temporal Lobe Epilepsy Manna et al, 2012 Net: Preclinical and clinical exploratory studies show 5-HT manipulations reduce seizures, but approved serotonergic drugs (e.g. fluoxetine) do not function as general anti-epileptic agents
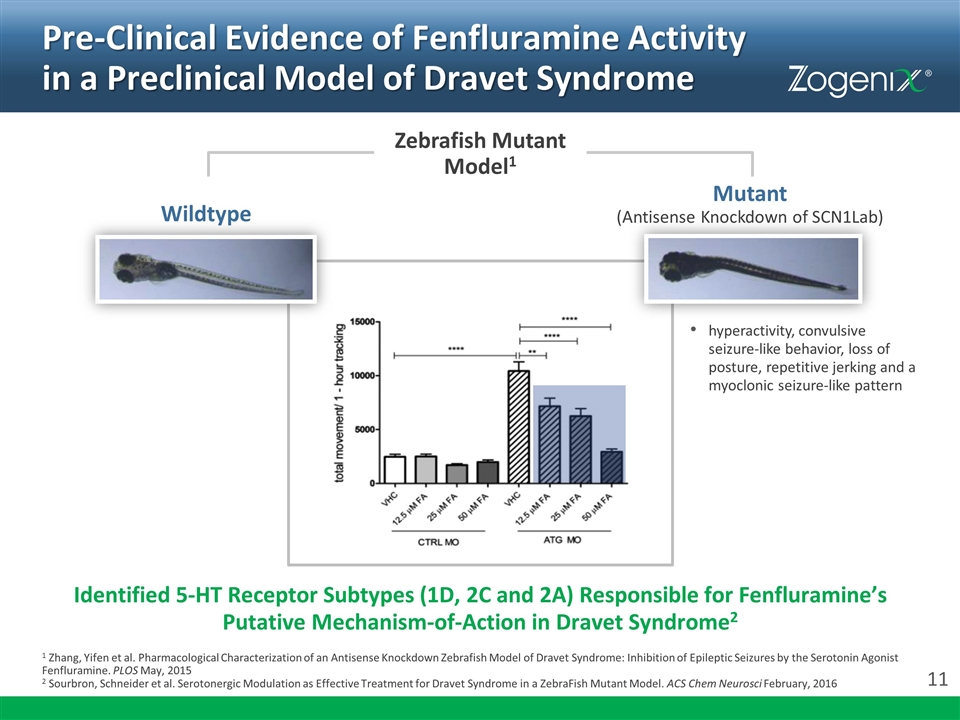
Pre-Clinical Evidence of Fenfluramine Activity in a Preclinical Model of Dravet Syndrome Identified 5-HT Receptor Subtypes (1D, 2C and 2A) Responsible for Fenfluramine’s Putative Mechanism-of-Action in Dravet Syndrome2 1 Zhang, Yifen et al. Pharmacological Characterization of an Antisense Knockdown Zebrafish Model of Dravet Syndrome: Inhibition of Epileptic Seizures by the Serotonin Agonist Fenfluramine. PLOS May, 2015 2 Sourbron, Schneider et al. Serotonergic Modulation as Effective Treatment for Dravet Syndrome in a ZebraFish Mutant Model. ACS Chem Neurosci February, 2016 hyperactivity, convulsive seizure-like behavior, loss of posture, repetitive jerking and a myoclonic seizure-like pattern Wildtype Mutant (Antisense Knockdown of SCN1Lab) Zebrafish Mutant Model1
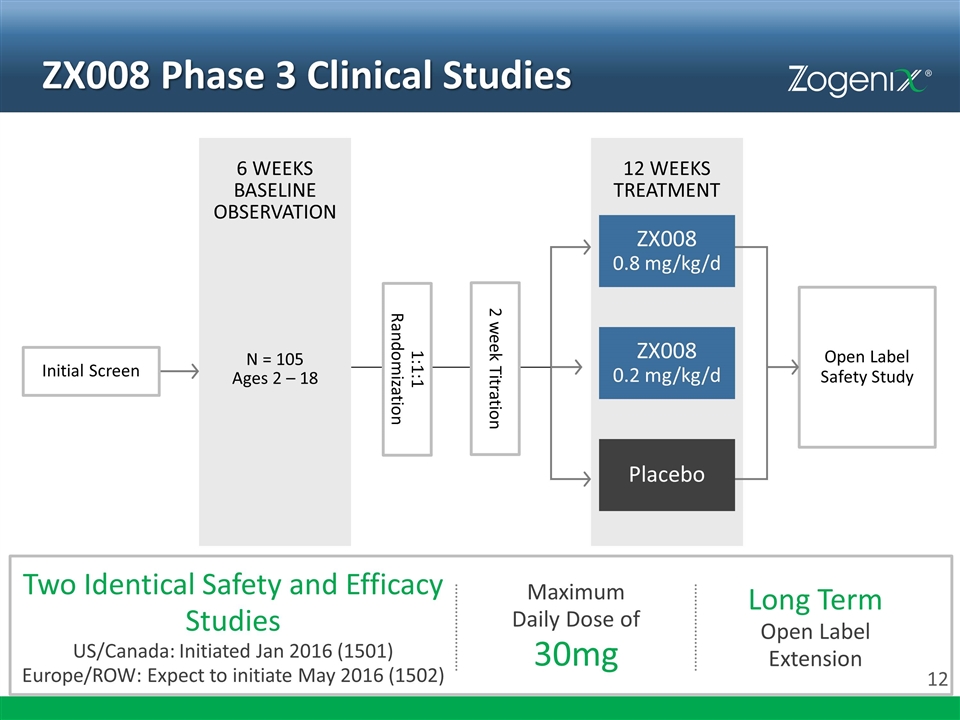
ZX008 Phase 3 Clinical Studies 6 WEEKS BASELINE OBSERVATION 12 WEEKS TREATMENT Initial Screen Placebo Open Label Safety Study N = 105 Ages 2 – 18 ZX008 0.8 mg/kg/d ZX008 0.2 mg/kg/d 1:1:1 Randomization 2 week Titration Two Identical Safety and Efficacy Studies US/Canada: Initiated Jan 2016 (1501) Europe/ROW: Expect to initiate May 2016 (1502) Maximum Daily Dose of 30mg Long Term Open Label Extension

Key Assessments Adverse events Laboratory safety parameters (hematology, chemistry, urinalysis) Vital signs Cardiac examination (baseline, midway, and endpoint) 3 Doppler echocardiograms 12-lead electrocardiogram SAFETY Analyses for ZX008 0.8 mg/kg/day vs. Placebo, and ZX008 0.2 mg/kg/day vs. Placebo: Change in frequency of convulsive seizures* Patients who achieve ≥40% and ≥50% reduction in convulsive seizure frequency Longest convulsive seizure-free internal Clinical global impression (as assessed by parent / caregiver and investigator) * Change in frequency of convulsive seizures in ZX008 0.8 mg/kg/day vs. Placebo is the primary outcome measure EFFICACY

Other ZX008 Clinical Studies Focused on Market Access in Europe Maintaining orphan exclusivity in EU requires a demonstration of incremental benefit over stiripentol Stiripentol is an approved orphan drug for the adjunctive treatment of Dravet syndrome in a specific combination with valproate and clobazam Conducting a Phase 3 safety and efficacy study of ZX008 in Dravet syndrome patients who are poor responders to the stiripentol standard regimen (Study 1504) Cohort 1 establishes ZX008 pharmacokinetics in children with Dravet syndrome on stiripentol Cohort 2 provides safety and efficacy data for ZX008 as adjunctive treatment with stiripentol regimen Data is also expected to support future pricing and reimbursement

Establishing Product Protection ORPHAN DRUG STATUS Provides 10 Years of Market Exclusivity in E.U. and 7 Years in U.S. If Obtained in Japan, ODS Will Provide 10 Years Exclusivity PATENTS PENDING(1) Use of Fenfluramine in Dravet Syndrome and Elements of a Future REMS Program Ongoing Preclinical and Development Work Providing Additional IP Opportunities PRODUCT SPECIFIC REMS Will Include Patient Registry and Cardiac Monitoring Difficult to Circumvent and Expensive to Replicate (1) Method For the Treatment of Dravet Syndrome – (13/887,014), (EP 2014/058954), (14/447/253), (14/447/303), (14/447,369)
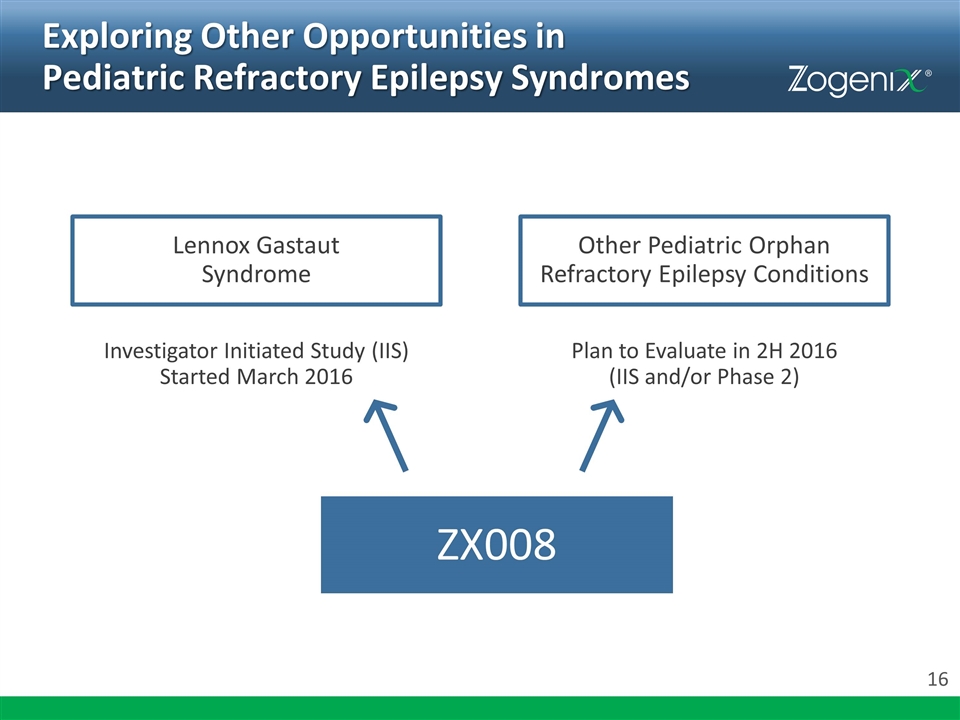
Exploring Other Opportunities in Pediatric Refractory Epilepsy Syndromes ZX008 Lennox Gastaut Syndrome Other Pediatric Orphan Refractory Epilepsy Conditions Investigator Initiated Study (IIS) Started March 2016 Plan to Evaluate in 2H 2016 (IIS and/or Phase 2)

Proprietary Subcutaneous Once-Monthly Antipsychotic Highly Differentiated Profile Worldwide Rights Search for Development and Commercialization Partner Initiated Schizophrenia PHASE 3 READY
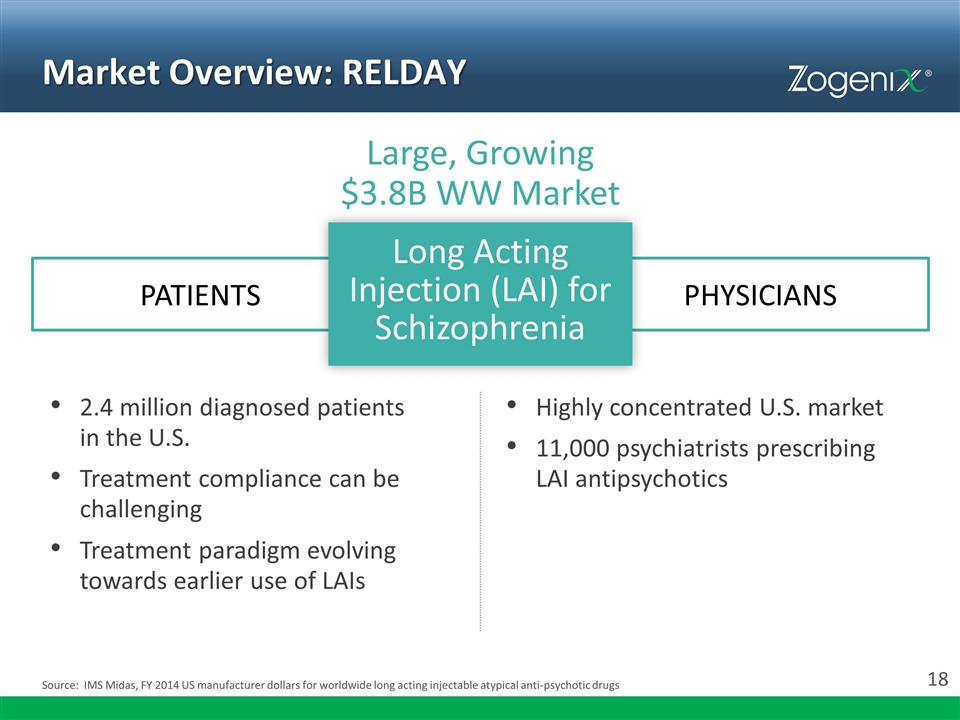
Market Overview: RELDAY 2.4 million diagnosed patients in the U.S. Treatment compliance can be challenging Treatment paradigm evolving towards earlier use of LAIs Long Acting Injection (LAI) for Schizophrenia PATIENTS PHYSICIANS Large, Growing $3.8B WW Market Highly concentrated U.S. market 11,000 psychiatrists prescribing LAI antipsychotics Source: IMS Midas, FY 2014 US manufacturer dollars for worldwide long acting injectable atypical anti-psychotic drugs
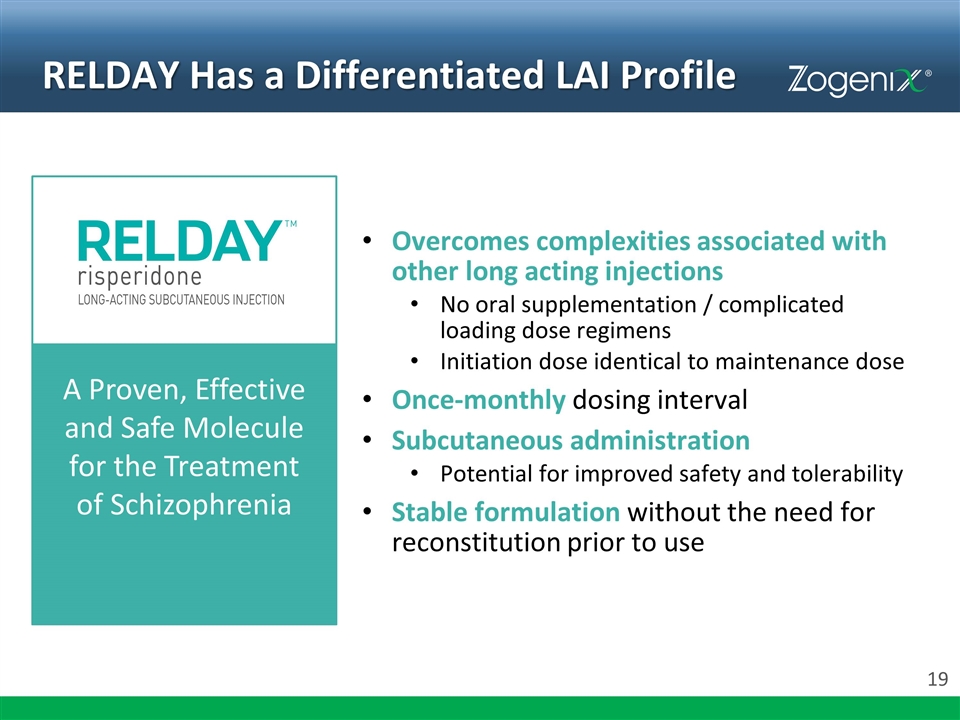
RELDAY Has a Differentiated LAI Profile Overcomes complexities associated with other long acting injections No oral supplementation / complicated loading dose regimens Initiation dose identical to maintenance dose Once-monthly dosing interval Subcutaneous administration Potential for improved safety and tolerability Stable formulation without the need for reconstitution prior to use A Proven, Effective and Safe Molecule for the Treatment of Schizophrenia

RELDAY Development Progress Strong physician and payer interest Single-dose study successfully completed Multi-dose study successfully completed => Phase 3 ready Issued and pending patents through at least 2034 Formulation technology also amenable to three-month dosing interval for future development and life-cycle management
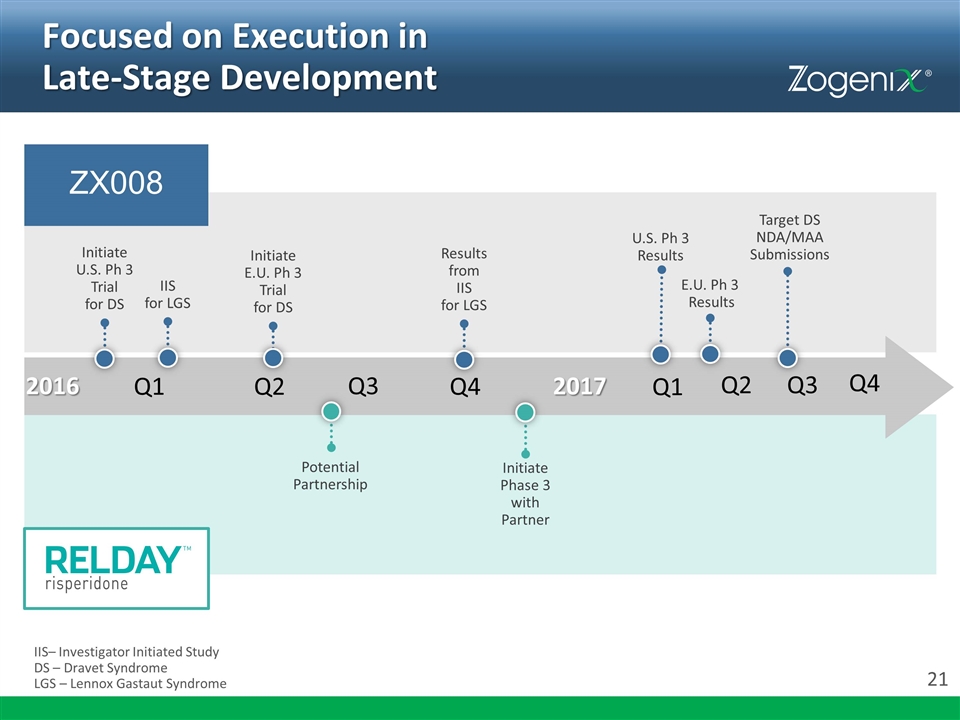
Focused on Execution in Late-Stage Development ZX008 IIS– Investigator Initiated Study DS – Dravet Syndrome LGS – Lennox Gastaut Syndrome Q1 Q2 Q3 Q4 2017 U.S. Ph 3 Results Results from IIS for LGS Initiate E.U. Ph 3 Trial for DS IIS for LGS Initiate U.S. Ph 3 Trial for DS E.U. Ph 3 Results Potential Partnership Initiate Phase 3 with Partner 2016 Q1 Q2 Q3 Q4 Target DS NDA/MAA Submissions
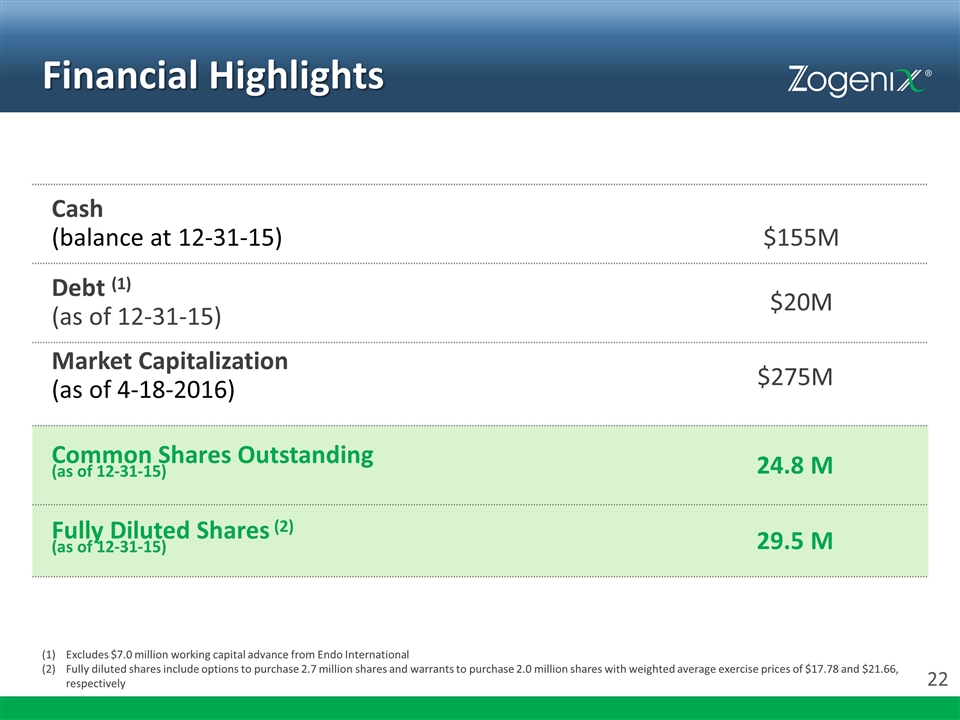
Financial Highlights Cash (balance at 12-31-15) $155M Debt (1) (as of 12-31-15) $20M Market Capitalization (as of 4-18-2016) $275M Common Shares Outstanding (as of 12-31-15) 24.8 M Fully Diluted Shares (2) (as of 12-31-15) 29.5 M Excludes $7.0 million working capital advance from Endo International Fully diluted shares include options to purchase 2.7 million shares and warrants to purchase 2.0 million shares with weighted average exercise prices of $17.78 and $21.66, respectively

PROMISING PIPELINE PHASE 3 PROGRAM INITIATED FOR ZX008 EXPERIENCED TEAM IN PLACE FULLY FUNDED CLINICAL PROGRAMS SIGNIFICANT NEAR-TERM MILESTONES NASDAQ: ZGNX

NASDAQ: ZGNX NASDAQ: ZGNX
