Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ignyta, Inc. | d183841d8k.htm |
 Entrectinib, an oral pan-Trk, ROS1, and ALK inhibitor
in TKI-naïve patients with advanced solid tumors
harboring gene rearrangements
Exhibit 99.1 Alexander Drilon, Filippo G. De Braud, Salvatore Siena, Sai-Hong I. Ou, Manish Patel, Myung-Ju Ahn, Jeeyun Lee, Todd M. Bauer, Anna F. Farago, Stephen V. Liu, Natasha Reddinger, Rupal Patel, David Luo,
Edna Chow Maneval, Pratik
S. Multani, Robert C. Doebele, Alice T. Shaw
|
 Disclosures • Honoraria: Ignyta,
Exelixis, Genentech/Roche •
Consulting/Advisory Role: Exelixis,
Genentech/Roche, AstraZeneca •
Speaker’s Bureau: Ignyta •
Research Funding: Foundation
Medicine •
Travel/Accomodations: Ignyta, Exelixis, Genentech/Roche, AstraZeneca |
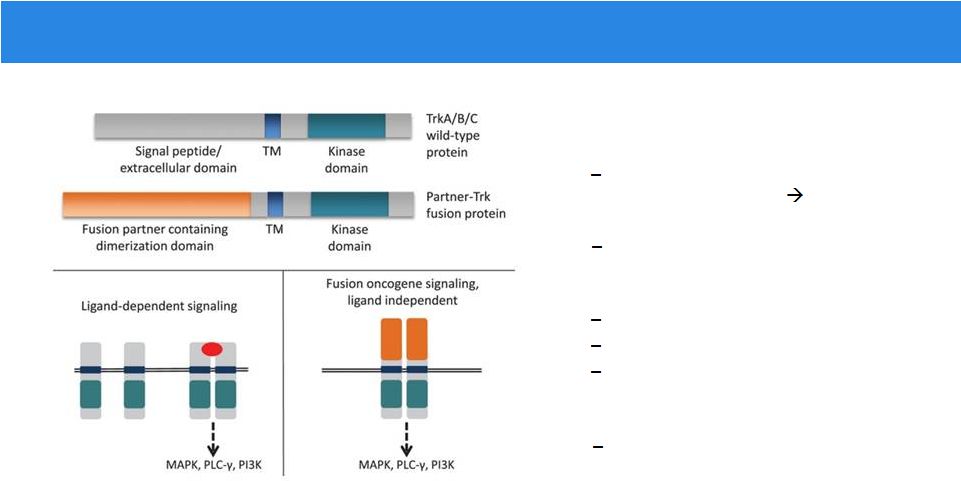 Recurrent Gene Rearrangements
• Oncogenic drivers across a variety of cancers Upstream partner can provide dimerization domains ligand- independent signaling Activation of downstream pathways • Detectable in the clinic FISH RNAseq DNA-based NGS • Select fusions are clinically-actionable responses can be dramatic and durable Farago et al, J Thorac Oncol. 2015 Dec;10(12):1670-4. |
 Recurrent Gene Rearrangements
• NTRK1/2/3, ROS1, and ALK fusions are identified across multiple cancers
lower prevalence in more common cancers
0% 5% 10% 15% 20% NTRK1 NTRK2 NTRK3 ROS1 ALK |
 Recurrent Gene Rearrangements
• NTRK1/2/3, ROS1, and ALK
fusions are identified across multiple cancers
high prevalence events in rare adult and pediatric cancers
Drilon et al, Ann Oncol, 2016 Feb 15.
PMID: 26884591
MASC (Mammary Analogue
Secretory Carcinoma)
0% 20% 40% 60% 80% 100% MASC Breast Secretory CA Congenital Mesoblastic Nephroma Congenital Fibrosarcoma NTRK3 5’ 3’ Chromosome 12 Chromosome 15 NTRK3 ETV6 RTK |
 Entrectinib (RXDX-101) • Highly-potent, orally-available, ATP-competitive tyrosine kinase inhibitor Low to sub-nanomolar efficacy against 5 kinases Results in decreased downstream effector activity PLC , MAPK and PI3K/AKT pathways • Active in vitro and in vivo
NTRK1/2/3-rearranged cancers
ROS1-rearranged cancers
ALK-rearranged cancers
Kinase
IC50 (nM) TrkA 1.7 TrkB 0.1 TrkC 0.1 ROS1 0.2 ALK 1.6 |
 Entrectinib (RXDX-101) • Active against potential Trk inhibitor resistance mutations at clinically achievable exposures NTRK1 F589L (gatekeeper) NTRK1 V573M NTRK1 G667S TrkA Kinase domain Phe589 Val573 Gly667 Mutation in TrkA LOXO-101 IC 50 (nM) Entrectinib IC 50 (nM) Entrectinib Human Exposure Equivalent (nM) F589L 959.4 9.7 58.2 V573M 534.5 24.2 145.2 G667S 185.3 14.6 87.6 Wildtype 15.0 2.3 13.8 AACR Abstract 2136, Data generated by Ignyta |
 Phase I Development
STARTRK-1 and ALKA-372-001 |
 Entrectinib: Phase I Studies
STARTRK-1 (n=65)
• Dosing: continuous • NTRK/ROS1/ALK alterations • US, EU, Asia • Ignyta initiated in July 2014 ALKA-372-001 (n=54) • Dosing: intermittent & continuous • NTRK/ROS1/ALK alterations • Italy • FIH study: Nerviano Medical Sciences in October 2012 Ignyta assumed responsibility in November 2013 • RP2D: 600 mg PO once daily, continuous • Total clinical experience: n=119 patients
• Updated safety and efficacy data • Data cut-off: March 7, 2016 |
 ALKA-372-001
(n=54) STARTRK-1 (n=65) TOTAL (n=119) Age, years, median (range) 53 (46-63) 57 (46-66) 55 (46-66) Sex, male/female (%) 44/56 48/52 46/54 ECOG performance status, n (%) 0 30 (56) 22 (34) 52 (44) 1 21 (39) 41 (63) 62 (52) 2 2 (4) 2 93) 4 (3) Unknown 1 (2) 0 1 (1) Prior Cancer Therapies, n (%) 0 0 6 (9) 6 (5) 1 -2 0 15 (23) 15 (13) 3 - 4 3 (6) 25 (39) 28 (24) > 4 51 (94) 19 (29) 70 (59) Tumor type, n (%) NSCLC 35 (65) 36 (56) 71 (60) Gastrointestinal Tract 9 (17) 9 (14) 18 (15) CNS 4 (7) 1 (2) 5 (4) Head & Neck 1 (2) 4 (6) 5 (4) Other (e.g., sarcomas, breast, melanoma, RCC, ovarian) 5 (9) 15 (23) 20 (17) Baseline Characteristics |
 Safety All patients in dose escalation and expansion phases -advanced solid tumor -NTRK1/2/3-, ROS1-, or ALK alteration |
 Treatment-Related Adverse Events
• AEs were classified via CTCAE v4.0; all reversible with dose modifications
• No evidence of cumulative hepatic or renal toxicity, or QTc prolongation • Only 2 DLTs occurred (STARTRK-1): grade 3 cognitive disturbance, grade 3 idiopathic eosinophilic myocarditis Adverse Events (AEs) in >10% of Patients at Any Dose Level (n=119) Adverse Event Term, n (%) Grades 1-2 Grade 3 Total Fatigue/Asthenia 47 (40) 5 (4) 52 (44) Dysgeusia 49 (41) 49 (41) Paresthesia 33 (28) 33 (28) Nausea 29 (24) 29 (24) Myalgia 26 (22) 26 (22) Diarrhea 22 (19) 1 (1) 23 (19) Dizziness 19 (16) 19 (16) Arthralgia 17 (14) 1 (1) 18 (15) Vomiting 18 (15) 18 (15) Constipation 14 (12) 14 (12) |
 Adverse Events (AEs) in >10% of Patients at the RP2D (n=45)
Adverse Event Term, n (%) Grades 1-2 Grade 3 Total Dysgeusia 21 (47) 21 (47) Fatigue/Asthenia 17 (38) 1 (2) 18 (40) Constipation 10 (22) 10 (22) Weight Increased 8 (18) 1 (2) 9 (20) Diarrhea 7 (16) 1 (2) 9 (18) Nausea 8 (18) 8 (18) Myalgia 7 (16) 7 (16) Paresthesia 7 (16) 7 (16) Dizziness 6 (13) 6 (13) Peripheral Sensory Neuropathy 4 (9) 2 (4) 6 (13) Anemia 2 (4) 3 (7) 5 (11) Dysphagia 4 (9) 1 (2) 5 (11) Vomiting 5 (11) 5 (11) Treatment-Related Adverse Events |
 Efficacy Phase 2-Eligible Population |
 Population “Phase 2-Eligible Population” (n=25) - NTRK1/2/3-,
ROS1-, or
ALK-rearranged solid tumor - TKI treatment-naïve - treated at or above RP2D Molecular Testing:
local testing performed -
FISH - next-generation sequencing Response Evaluation - RECIST v1.1, locally assessed and confirmed (n=24) - volumetric assessment (n=1; primary brain tumor*) * RECIST criteria not validated in primary brain tumors (FDA-AACR Brain Tumor Endpoints Workshop 2006)
|
 Fusion Confirmed Responses (n) ORR (%) NTRK1/3 3/3 100% ROS1 12/14 86% ALK 4/7 57% PD PR CR Antitumor Activity Best Response in TKI Treatment-Naïve NTRK-,
ROS1-, and ALK-rearranged Tumors (n=24) -100 -90 -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30 |
 Fusion Confirmed Responses (n) ORR (%) NTRK1/3 3/3 100% 1 additional patient with NTRK+ astrocytoma • SD by RECIST (not validated for primary brain tumors) • 45% by exploratory 3-D volumetric assessment PD PR CR Antitumor Activity Best Response in TKI Treatment-Naïve NTRK-,
ROS1-, and ALK-rearranged Tumors (n=24) -100 -90 -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30 |
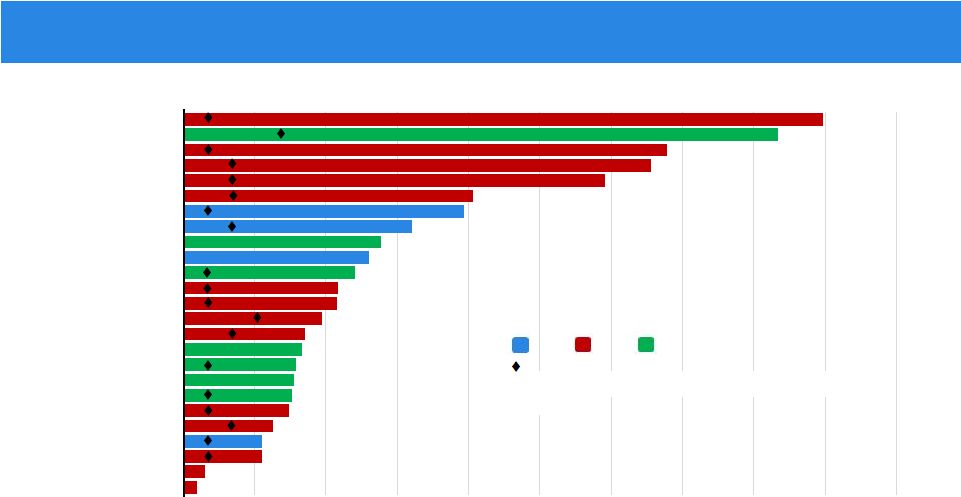 NSCLC NSCLC NSCLC NSCLC NSCLC NSCLC NSCLC MASC NSCLC Astrocytoma RCC NSCLC NSCLC Melanoma NSCLC Unknown Primary CRC NSCLC NSCLC NSCLC NSCLC CRC NSCLC NSCLC NSCLC 0 3 6 9 12 15 18 21 24 27 30 Time on Study (months) X X X X X X X X . . X = off study . = progression by RECIST, continued due to clinical benefit Duration of Clinical Benefit NTRK ALK ROS1 . = time to response TKI Treatment-Naïve NTRK-, ROS1-, and ALK-rearranged Tumors (n=25)
. |
 • Response achieved in 100% of tumors – Rapid (within 1 month of treatment) and prolonged ( ~1 year, ongoing) responses were observed • Response achieved in a variety of histologies and fusion types – CRC: LMNA-NTRK1 – Astrocytoma: BCAN-NTRK1 – NSCLC: SQSTM1-NTRK1
– MASC: ETV6-NTRK3
NTRK-Rearranged Cancers 3-D
volumetric assessment (courtesy
of P. Brastianos MD, MGH) SD by RECIST v1.1 Best Response in TKI Treatment-Naïve NTRK-rearranged Tumors (n=4)
RECIST v1.1 -100% -90% -80% -70% -60% -50% -40% -30% -20% -10% 0% CRC Astrocytoma NSCLC MASC |
 Response to Entrectinib
34/F with metastatic ETV6- NTRK3-rearranged
MASC
Resected stage III disease and
post-operative RT in 2006
Recurred in 2011 and treated
with 3 lines of cytotoxic
chemotherapy and RT
NGS revealed an ETV6-NTRK3 rearrangement Enrolled onto STARTRK-1 in 2015 durable PR, 10 months
of
entrectinib treatment Images: Drilon, MSKCC baseline 9 weeks on therapy |
 CNS Activity |
 CNS Disease in Cancer
Brain metastases
-20-40% of all patients
with cancer -lung (up to 50%) -breast -melanoma Primary brain tumors -astrocytoma (NTRK2 fusions: 3%) -glioblastoma (NTRK1 fusions: 1-2%) -pediatric gliomas (NTRK3 fusions: 7%)
Optimal therapy would address both systemic and CNS disease
|
 CNS Activity of Entrectinib
• Entrectinib was designed to cross the blood brain barrier Blood/brain ratios Mouse 0.4 Rat 0.6 – 1.0 Dog 1.4 – 1.2 • Preclinical CNS activity – cells injected intracranially – treated with entrectinib orally vs vehicle for 10 days EML4-ALK rearranged NCI-H228 - |
 Response to Entrectinib
46/M with metastatic SQSTM1-NTRK1-rearranged NSCLC – Diagnosed in November 2013 with widely metastatic disease – 4 prior therapies including anti-PD-1: carboplatin/pemetrexed, pembrolizumab,
docetaxel, vinorelbine
– Poor baseline performance status (ECOG 2), on supplemental O 2 and in hospice – Enrolled in STARTRK-1 March 2015 Farago et al, J Thorac Oncol. 2015 Dec;10(12):1670-4. |
 Extracranial Response to Entrectinib
Baseline Day 26: - 47% response Day 317: - 79% response Images: Farago and Shaw, MGH |
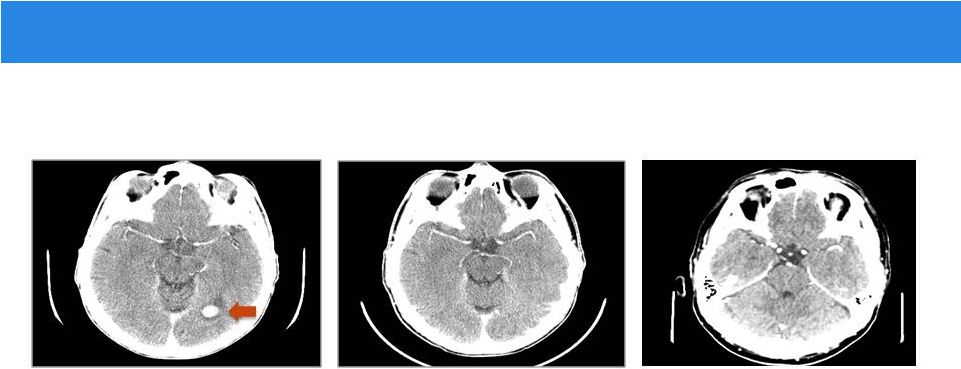 Intracranial Response to Entrectinib
Baseline Day 26: complete response Day 317: complete response Images: Farago and Shaw, MGH Remains on entrectinib and clinically progression-free > 12 months |
 0 2 4 6 8 10 12 14 • 57/M with low-grade astrocytoma harboring a BCAN-
NTRK1 gene rearrangement – Unresectable pontine tumor – SD by RECIST (not validated for primary brain tumors) – Exploratory 3-Dimensional volumetric tumor assessment performed showed a 45% decrease in tumor burden – Improvements in clinical symptoms of ataxia and diplopia entrectinib initiated Non-Enhancing Volume (cm³) Enhancing Volume (cm³) 11.66 cm³ 6.45 cm³ Tumor 3-D volumetric assessments courtesy of P. Brastianos MD (MGH) Jul 2015 Feb 2016 Primary Brain Tumor Response to Entrectinib |
 • 20 month-old boy with recurrent, metastatic infantile fibrosarcoma harboring
an
ETV6-NTRK3 gene rearrangement – Presented at birth with left leg mass, requiring through-the-knee amputation
• Age 4 months, large metastases to left lung identified 24-weeks of chemotherapy • Age 12 months, large right frontal intracranial tumor identified resected, followed by 5 cycles of salvage chemotherapy • Recurrent CNS disease with lesions in the right frontal and temporal lobes, as well
as leptomeningeal involvement
– Received entrectinib starting February 2016 Response to Entrectinib |
 Response to Entrectinib
massive peritumoral edema, midline shift,
transtentorial herniation, progressive lethargy
decreased tumor and edema, patient with
increased alertness, resumed eating and crawling
ETV6-NTRK3 gene rearranged metastatic
fibrosarcoma in 20-month old |
 Summary |
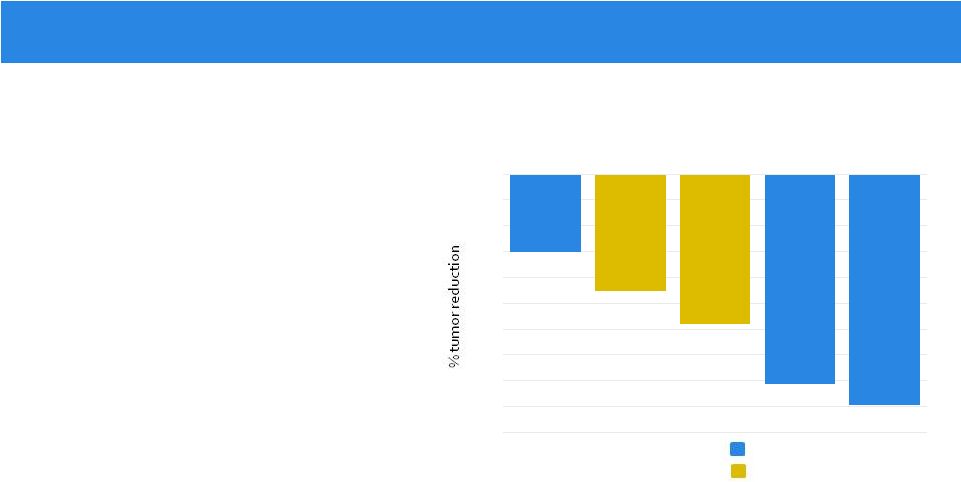 • Entrectinib is a potent TrkA/B/C Inhibitor – Large safety experience (119 patients) – Rapid and durable responses • Response in 100% (5/5) of patients achieved in a variety of histologies and fusion types – CRC: LMNA-NTRK1 – Astrocytoma: BCAN-NTRK1 – Infantile fibrosarcoma: ETV6-NTRK3 – NSCLC: SQSTM1-NTRK1
– MASC: ETV6-NTRK3
• Dramatic intracranial activity in 100% of patients with CNS disease (3/3) – 3/5 of patients treated in Phase 1 setting had primary or metastatic CNS disease – only Trk inhibitor with demonstrated CNS activity thus far NTRK-Rearranged Cancers
* 3-D volumetric assessment (courtesy of P. Brastianos MD, MGH) ** estimated from radiology assessment RECIST v1.1 -100 -90 -80 -70 -60 -50 -40 -30 -20 -10 0 mCRC Astrocytoma Fibrosarcoma NSCLC MASC * ** Response in TKI Treatment-Naïve NTRK-rearranged Tumors (n=5)
|
 -100 -90 -80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30 ROS1-Rearranged Cancers
• Response achieved in 86% (12/14) of TKI-naïve tumors – Two complete responders – Rapid (within 1 month of treatment) and prolonged responses were observed – In NSCLC, ORR of 85% (11/13 patients) – One additional response in melanoma – Longest ongoing response approaching 2 years and 3+ months Best Response in TKI Treatment-Naïve ROS1-rearranged Tumors (n=14)
PR CR |
 Conclusions • Entrectinib is safe and well-tolerated. – 119 patients have been treated: 45 patients at the RP2D of 600 mg daily – therapy duration: 19 patients > 6 months (11 patients > 1 year, including 3 patients > 2 years)
• Entrectinib is an active targeted therapy for NTRK-, ROS1-, and ALK-rearranged cancers.
– confirmed responses observed in 19/24 (79%) patients with extracranial solid tumors; in addition,
evidence of tumor shrinkage observed in a patient with NTRK+ astrocytoma –
brisk (within 4 weeks) and durable (up to 2 years and 3+ months) responses were
achieved –
NTRK-rearranged tumors
•
response achieved in 5 different histologies in both adult and pediatric
patients •
Entrectinib is highly CNS-penetrant. – durable responses in both primary brain tumors and metastatic disease – complete response observed in the CNS |
 Current Directions
STARTRK-2 An Open-Label, Multicenter, Global Phase 2 Basket Study of Entrectinib for the Treatment of Patients with Locally Advanced or Metastatic Solid Tumors that Harbor NTRK1/2/3,
ROS1, or ALK Gene |
 Thank You |
