Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Lipocine Inc. | v436709_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Lipocine Inc. | v436709_ex99-1.htm |

15 th Annual Needham Healthcare Conference Presentation April 2016 Exhibit 99.2

Forward Looking Statements 2 This presentation contains forward - looking statements about Lipocine Inc. (the “Company”). These forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward - looking stat ements relate to the Company’s product candidates, clinical and regulatory processes and objectives, potential benefits of the Compa ny’ s product candidates, intellectual property and related matters, all of which involve known and unknown risks and uncertainties . Actual results may differ materially from the forward - looking statements discussed in this presentation . Accordingly, the Company cautions investors not to place undue reliance on the forward - looking statements contained in, or made in connection with, this presentation . Several factors may affect the initiation and completion of clinical trials, the potential advantages of the Company’s product candidates and the Company’s capital needs. Among other things, the projected commencement and completion of the Company’s clinical trials may be affected by difficulties or delays. In addition, the Company’s results ma y b e affected by its ability to manage its financial resources, difficulties or delays in developing manufacturing processes for its produc t c andidates, preclinical and toxicology testing and regulatory developments. Delays in clinical programs, whether caused by competitive developments, adverse events, patient enrollment rates, regulatory issues or other factors, could adversely affect the Compan y’s financial position and prospects. Prior clinical trial program designs and results are not necessarily predictive of future cli nical trial designs or results. If the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, th ey will not receive regulatory approval and the Company will not be able to market them. The Company may not be able to enter into any strategic partnership agreements. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rates due to changes in corporate priorities, the timing and outcomes of clinical trials, compet iti ve developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly delay, scale ba ck or discontinue one or more of its drug development or discovery research programs. The Company is at an early stage of developm ent and may not ever have any products that generate significant revenue. The forward - looking statements contained in this presentation are further qualified by the detailed discussion of risks and uncertainties set forth in the documents filed by the Company w ith the Securities and Exchange Commission, all of which can be obtained on the Company’s website at www.lipocine.com or on the SEC website at www.sec.gov . The forward - looking statements contained in this document represent the Company’s estimates and assumptions only as of the date of this presentation and the Company undertakes no duty or obligation to update or revise publicly any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations.

Lipocine Investment Highlights First oral TRT option under FDA review with a PDUFA goal date of June 28, 2016 Differentiated product targeting ~$2.0 Billion established US TRT market Targets unmet need with first entrant advantage Robust clinical data with branded market leader as active control Pipeline assets advancing towards “Phase 3 ready” status LPCN 1111 - QD oral TRT option currently in Phase 2b LPCN 1107 Orphan designated oral alternative for the prevention of preterm birth • Potential to optimize clinical outcomes • Avoids painful injections and injection site reaction 3

LPCN: Focused on Innovative Products for Men’s and Women’s Health 4 “Transformative“ Oral Testosterone Franchise Oral Alternative for the Prevention of Pre - Term Birth PRODUCT (Indication) RESEARCH / PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NDA UNDER REVIEW MEN'S HEALTH LPCN 1021 (Oral Testosterone Replacement Therapy) LPCN 1111 (Next Generation Oral T) WOMEN'S HEALTH LPCN 1107 (Prevention of Preterm Birth) PDUFA Goal Date: June 28, 2016

LPCN 1021 - First Oral TRT Option (PDUFA Goal Date of June 28, 2016) ▪ NDA accepted in October 2015 – Targeting Class TRT label – No “black box” warning expected ▪ Potential to be the first approved oral TRT option 5 D IFFERENTIATION vs. MARKET LEADER ▪ Not prone to accidental T transference ▪ Starting dose is the right dose for most patients thereby requiring fewer doctor office visits/testing ▪ Patient preferred oral option ▪ LPCN 1021 met Primary endpoint: 87% response rate vs. FDA requirement of 75% ▪ Secondary endpoints generally consistent with approved products ▪ T levels not affected by food fat content EFFICACY SAFETY ▪ 52 week long term exposure data ▪ Well tolerated ▪ AE profile comparable to active control, including GI ▪ No cardiac, hepatic or drug related SAEs

Undiagnosed Hypogonadism 70% Diagnosed Untreated 19% 68% 23% Treated 11% Treatment-experienced Treatment-naïve ▪ Close to 6M men with diagnosed hypogonadism 3 ▪ 2.2M men being treated 4 ▪ 700,000 patients are new to treatment each year 5 1 US Census data. http://www.infoplease.com/us/census/data/demographic.html. 2. Mulligan T, et al. Int J Clin Pract. 2006 Jul;60(7):762 - 9. 3. Araujo, et al. J Clin Endo Metabol 2007. 92(11):4241 - 7. 4. Symphony Healthcare 2014 for FDA Advisory Meeting. 5. IMS Health Sept 2015. Hypogonadism Affects Up to 20 Million American Men 1,2 Focus on the Addressable Population 6

2014 2015 Source: IMS Dec. 2015 TRT Market Monthly TRx Trend 350 375 400 425 450 475 500 525 550 575 Sep 14 Oct 14 Nov 14 Dec 14 Jan 15 Feb 15 Mar 15 Apr 15 May 15 Jun 15 Jul 15 Aug 15 Sep 15 Oct 15 Nov 15 Dec 15 Jan 16 Feb 16 Monthly TRx (000s) FDA Label Guidance 7 x No TRx impact of FDA TRT label change x Monthly TRx stable around 500,000/month 2016

Testosterone Replacement Therapy Market 8 Source: IMS Health Dec 2015 $0.0 $0.5 $1.0 $1.5 $2.0 $2.5 2013 2014 2015 Billions ($) Gross Sales Testosterone - Injectable Depo-Testosterone Testopel Testosterone - Transdermal Fortesta Androderm Testim Axiron AndroGel 1.62% AndroGel 1% 0 1 2 3 4 5 6 7 8 2013 2014 2015 Millions TRx $ 2.3 $ 2.1 $2.0 *Sales reported as invoice data from wholesalers to the various channels. Note: Testopel is excluded because it is a buy - and - bill product. 7.3 6.4 6.2 x Androgel 1.62% has significant portion of $ sales x TRx’s in 2015 evenly split between topicals and injectables

9 Issues with Current TRT Therapies Transfer potential to children and partner No freedom to use around pregnant loved ones Skin irritation potential Messy to apply and wait to dress Pulmonary embolism potential Pain from injection Needle phobia, needle fatigue Scarring/injection site reactions Risk of infection Not flexible for dose reversals Topicals Injectables / Implants

TRT Market: “On Therapy” Persistency Based on New - to - Market Cohorts (July 2013) Median Persistency on a TRT Brand is 3 - 4 Months (~100 Days) MABI’s TRT Patient Metrics (Powered by Source Healthcare Analytics Patient Data). All trademarks acknowledged. Adherence to TRT Treatment Is Poor 10.90% 23.40% 16.10% 19.20% 36.70% 16.30% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% M+1 M+2 M+3 M+4 M+5 M+6 M+7 M+8 M+9 M+10 M+11 M+12 Percentage of Patients Remaining “On Therapy” Androderm AndroGel 1.62% Axiron Fortesta Injectables Testim 10

LPCN 1021: First Oral TRT Option Challenges of Oral T Product ▪ Native testosterone has poor oral bioavailability with a very short half life (~30 min) – Impractical daily doses would be required to obtain effective levels – Inconsistent and unpredictable performance – Methyl testosterone ▪ Liver toxicity ▪ Unsafe for chronic use LPCN 1021 Advantage ▪ Novel product primarily directing Testosterone Undecanoate (TU) into the lymph – Maintains effective T blood levels in eugonadal range when dosed twice daily – Consistent and predictable performance – By - passes liver in first pass metabolism 11

12 LPCN 1021 (First Oral TRT Option): Primary Efficacy Results in Phase 3 Trial “Reliably Restores and Maintain Testosterone in Vast Majority of Patients” Measure FDA Targets Efficacy Population* 1,2 Full Analysis Set #1 Number of subjects 151 193 % subjects with C avg w ithin normal range (300 - 1140 ng/ dL ) ≥75% 87.4% 87.0% 95 % CI lower bound ≥ 65% 81.7% 82.0% x LPCN 1021 met both the primary endpoint targets x C avg and overall variability comparable or better than marketed products Parameter Mean (CV) Mean (CV) C avg ( ng / dL ) 446 (38%) 471 (41%) * Subjects randomized into the study with at least one PK profile and no significant protocol deviations # Subjects randomized into the study with at least one post - baseline efficacy variable response 1 Missing data imputed by LOCF 2 3.3% of subjects were non - responders ( C avg <300 ng/ dL at highest (300 mg) dose)

LPCN 1021 (First Oral TRT Option): Attributes of Special Interest 13 Efficacy Robust to Sensitivity Analysis No cardiac, hepatic or drug related SAE Comparable to Active Control No Change from Baseline Mean Maintenance Dose Comparable to Starting Dose T Levels not Sensitive to Fat Content Yes LPCN 1021 ATTRIBUTES Consistent Inter - day/ Intra - day Performance Meal Fat Effect Safety DHT Levels Blood Pressure Dose

LPCN 1111 (Next Generation Oral TRT Option): “Once Daily” ▪ Novel prodrug of testosterone for oral delivery through proprietary drug delivery technology ▪ Once daily is expected to sustain and improve market share or oral T franchise ▪ Once daily feasibility established – Positive Phase 2a study results in hypogonadal men ▪ Single daily oral dose provides T levels in the eugonadal range ▪ No subject exceeded peak T levels of 1500 ng/ dL ▪ Development status – Phase 2b PK dose finding study – ongoing – Next step: Top - line results expected – 3Q 2016 14

Preterm Birth (PTB) Represents a Significant Unmet Medical Need ▪ 11.7% of all US pregnancies 2 result in PTB (< 37 weeks) - a leading cause of neonatal mortality and morbidity 3 ▪ ~10x more first year medical costs are for PTB infants than for full term infants 4 ▪ ≥ $26 billion economic impact: 4 $1 billion market opportunity 5 15 1 Pediatric Research (2006) 60, 775 – 776 2 CDC (2010) 3 J . Maternal - Fetal and Neonatal Medicine, Dec. 2006, 19(12), 773 – 782 4 Institute of Medicine of the National Academies. Jul.2006 5 AMAG Pharmaceuticals presentation 09/29/2014 One preterm infant per minute in the U.S. 1

LPCN 1107 (First Oral PTB Candidate): “Addresses Unmet Need” 16 ▪ Potential to be the first oral standard - of care therapy – Elimination of 18 - 22 injections – Potential for optimizing clinical outcomes ▪ Orphan drug designation – A major contribution to patient care ▪ Development status – Multi - dose PK dose finding study – Complete – Next step: Request End of Phase 2 Meeting - 2Q 2016

LPCN 1107 (First Oral PTB Candidate): Single and Multi - dose Study Results 17 ▪ Relevant hydroxyprogesterone caproate levels achieved following oral administration ▪ Amenable to therapy improvement ▪ Well - tolerated with no serious adverse events or adverse drug reactions 1 Caritis SN, Venkataramanan R, Thom E, et al. Relationship between 17 - alpha hydroxyprogesterone caproate concentration and spontaneous preterm birth. Am J Obstet Gynecol 2014;210(2):128.

LPCN 1107 (First Oral PTB Candidate): Potential to Optimize Clinical Outcomes Limitations of Current Injectable Therapy Potential Advantages of LPCN 1107 18 ▪ Several weeks to get to steady state HPC levels ▪ “One size fits all” fixed single dosing option ▪ Weekly visits to care giver ▪ Steady state levels in seven days ▪ Amenable to customize dose – Enables sooner desired HPC levels in patients – Maintain patient at desired levels through dose titration independent of pre - pregnancy BMI, age, race, etc. – No lingering HPC levels post delivery

Several Near Term Value Drivers 19 Event Expected Timing LPCN 1107: Request End of Phase 2 Meeting 2Q16 LPCN 1021: FDA PDUFA Date June 28, 2016 LPCN 1111: Top - line Results from Phase 2b Study 3Q16 LPCN 1111: End of Phase 2 Meeting 2H16 LPCN 1021: File NDS in Canada 2H16
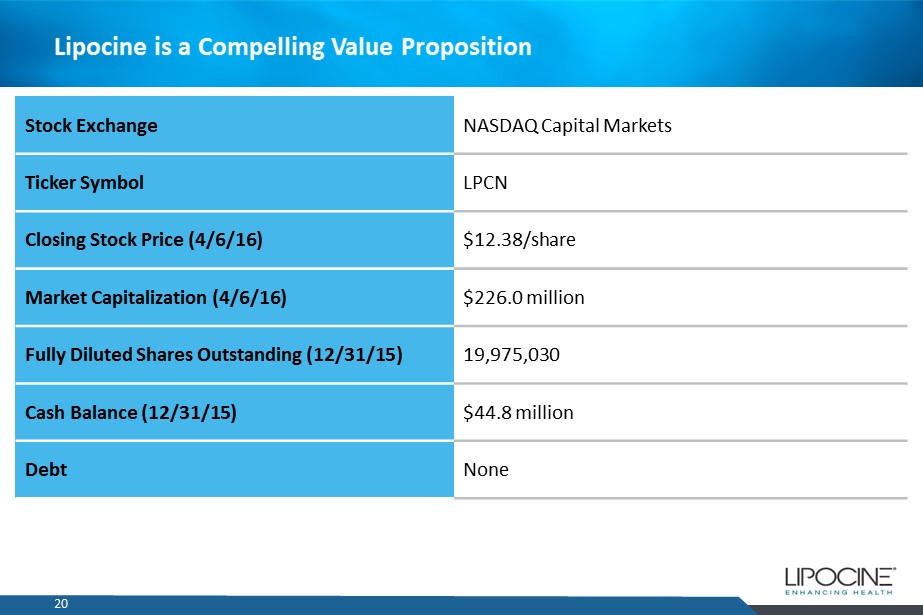
20 Lipocine is a Compelling Value Proposition Stock Exchange NASDAQ Capital Markets Ticker Symbol LPCN Closing Stock Price (4/6/16) $12.38/share Market Capitalization (4/6/16) $226.0 million Fully Diluted Shares Outstanding (12/31/15) 19,975,030 Cash Balance (12/31/15) $44.8 million Debt None

Lipocine Investment Highlights First oral TRT option under FDA review with a PDUFA goal date of June 28, 2016 Differentiated product targeting ~$2.0 Billion established US TRT market Targets unmet need with first entrant advantage Robust clinical data with branded market leader as active control Pipeline assets advancing towards “Phase 3 ready” status LPCN 1111 - QD oral TRT option currently in Phase 2b LPCN 1107 Orphan designated oral alternative for the prevention of preterm birth • Potential to optimize clinical outcomes • Avoids painful injections and injection site reaction 21
