Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PUMA BIOTECHNOLOGY, INC. | d141281d8k.htm |

Updated Results from ExteNET Trial Using the Event and Censoring Rule Requested by FDA 1 Copyright 2016 Puma Biotechnology Exhibit 99.1

Event and Censoring Rules Original Rule Invasive Disease Free Survival (iDFS) events were defined as all recurrent disease events and deaths occurring within 2 years and 28 days post randomization Revised Rule (as per FDA) iDFS events are defined as all recurrent disease events and deaths occurring within 2 years and 28 days post randomization However, iDFS events observed after missing 2 or more scheduled disease assessments are censored at the last available disease assessment time prior to the event occurrence 2 Copyright 2016 Puma Biotechnology
Patient Populations Intent to Treat Population (ITT) All randomized patients including node negative and node positive patients Amended Intent to Treat Population (aITT) Node positive patients and enrolled less than one year from completion of adjuvant treatment with Herceptin (higher risk population) 3 Copyright 2016 Puma Biotechnology
Analyses Primary Analysis in July 2014 Updated Analysis in December 2015 4 Copyright 2016 Puma Biotechnology
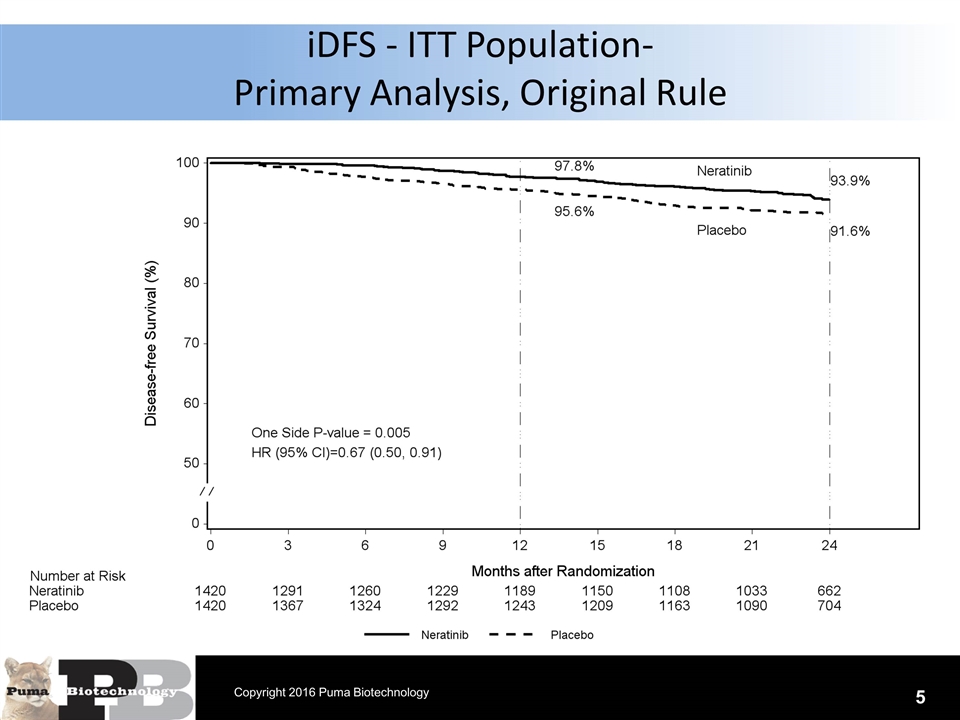
N = 120 patients iDFS - ITT Population- Primary Analysis, Original Rule 5 Copyright 2016 Puma Biotechnology
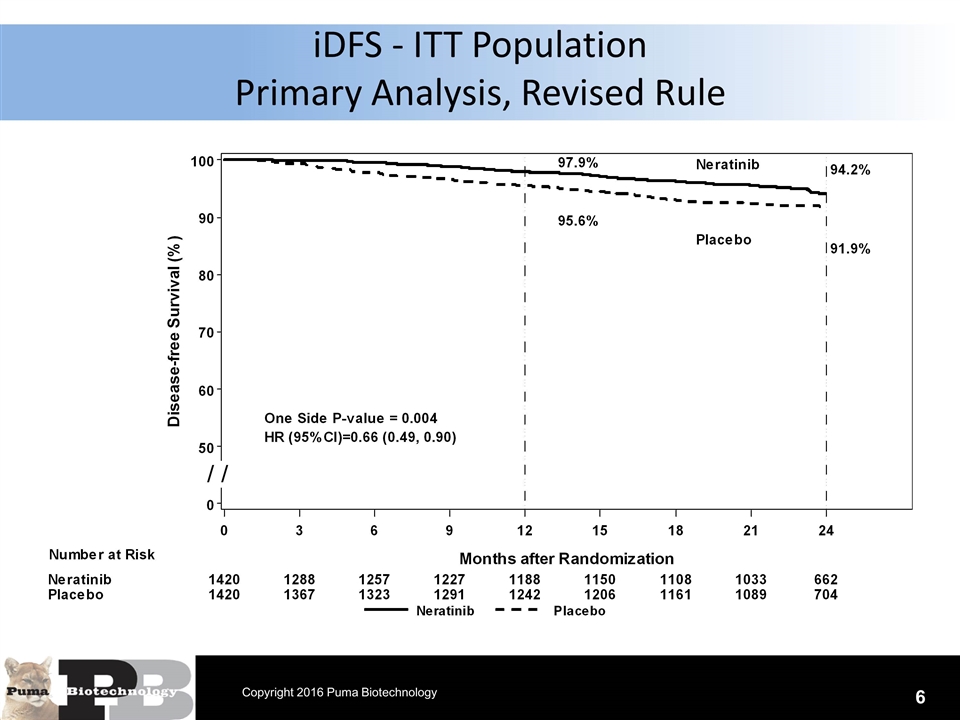
N = 120 patients iDFS - ITT Population Primary Analysis, Revised Rule 6 Copyright 2016 Puma Biotechnology
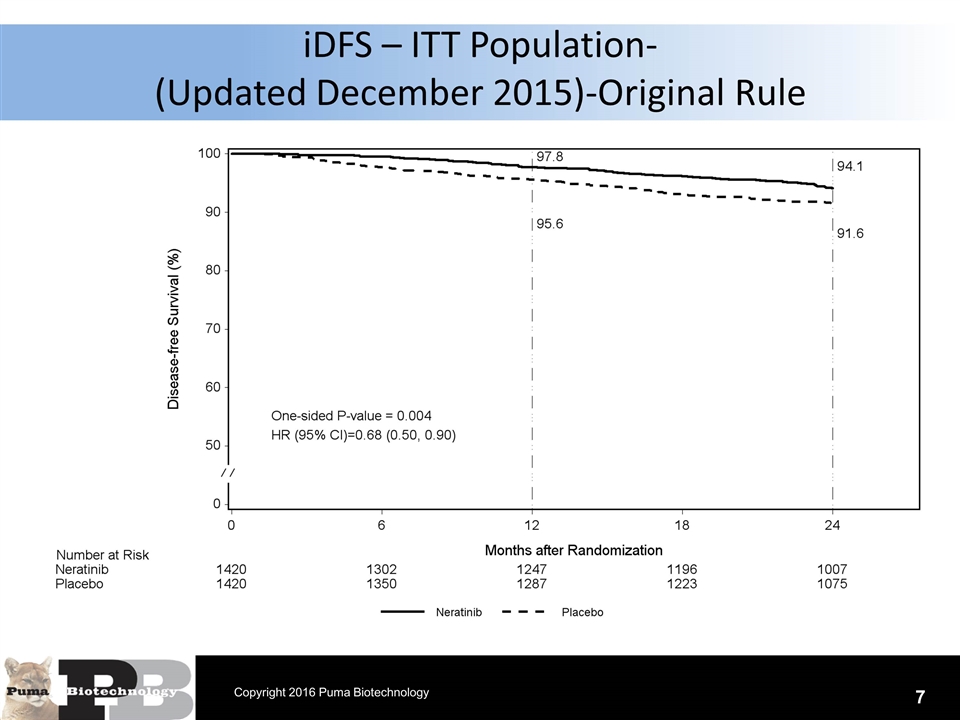
N = 120 patients iDFS – ITT Population- (Updated December 2015)-Original Rule 7 Copyright 2016 Puma Biotechnology
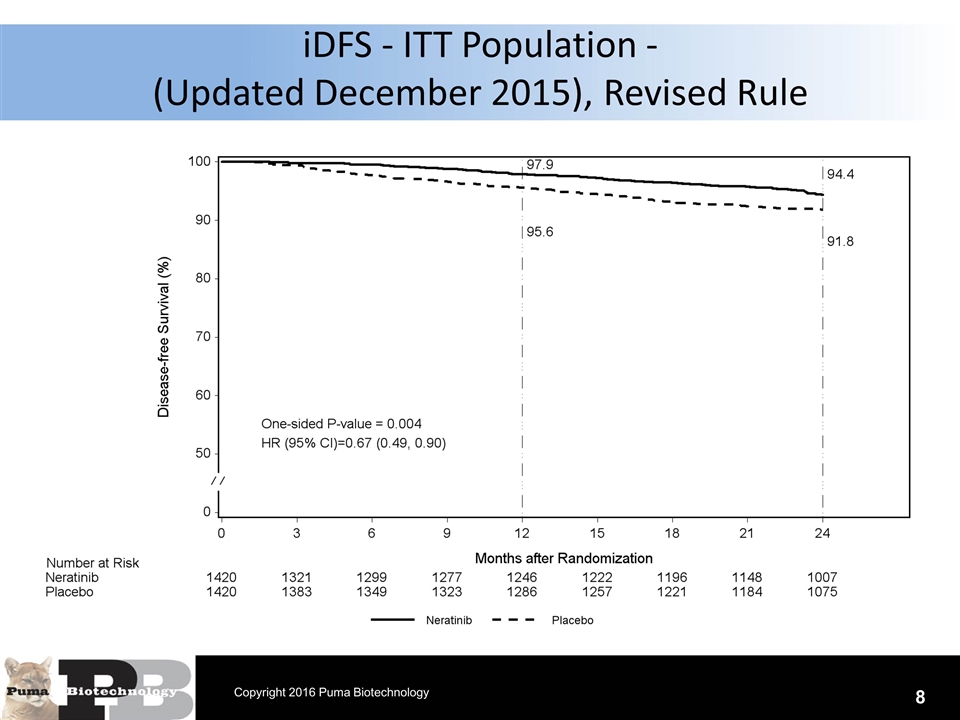
N = 120 patients iDFS - ITT Population - (Updated December 2015), Revised Rule 8 Copyright 2016 Puma Biotechnology
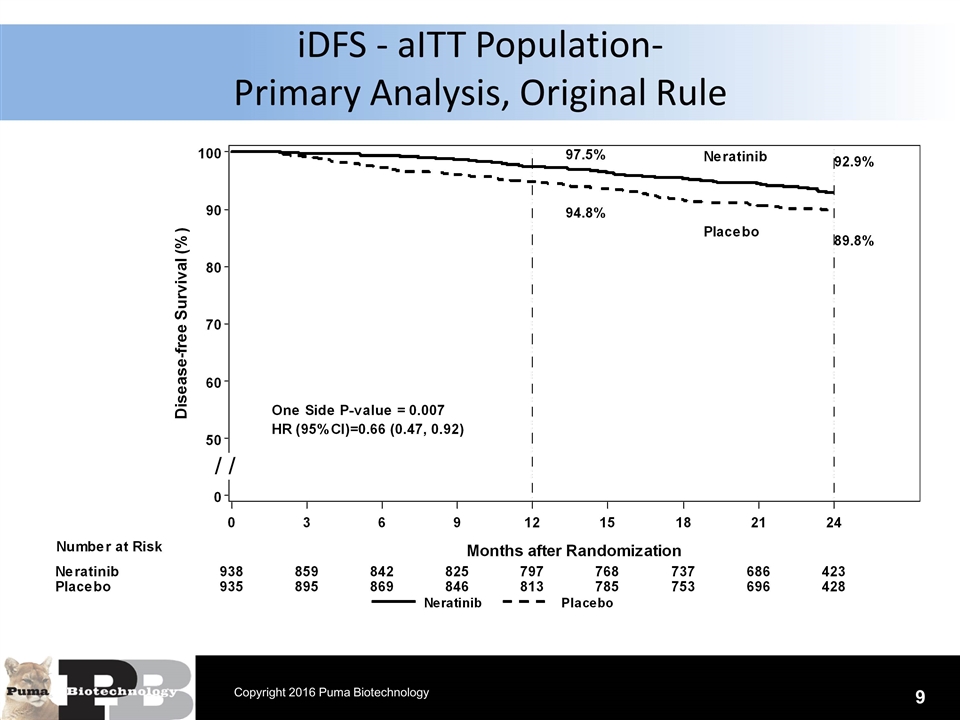
N = 120 patients iDFS - aITT Population- Primary Analysis, Original Rule 9 Copyright 2016 Puma Biotechnology
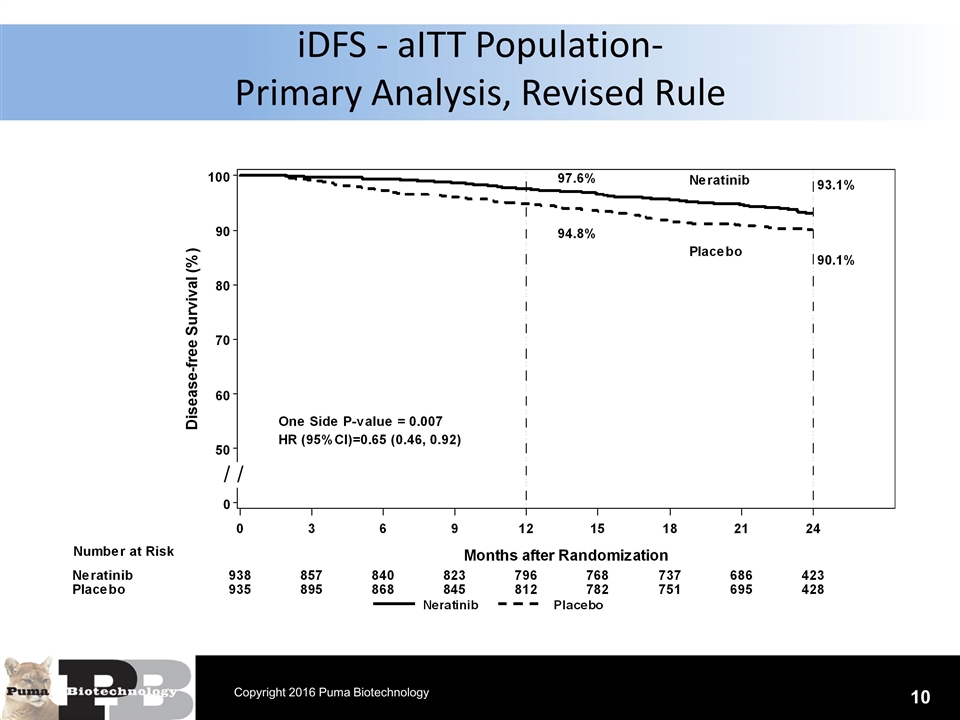
N = 120 patients iDFS - aITT Population- Primary Analysis, Revised Rule 10 Copyright 2016 Puma Biotechnology
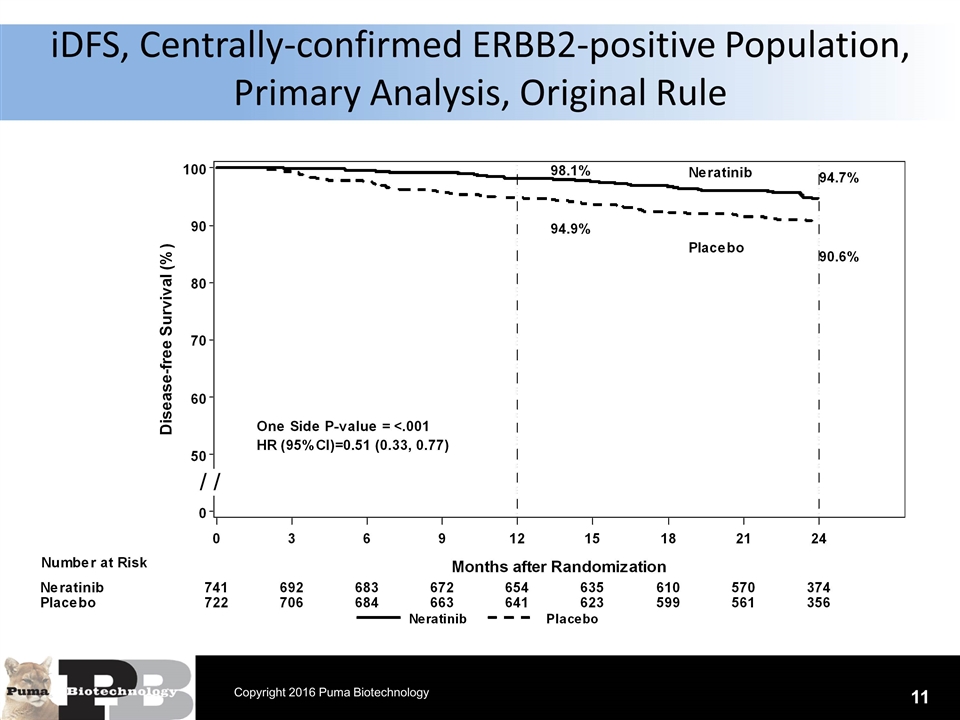
iDFS, Centrally-confirmed ERBB2-positive Population, Primary Analysis, Original Rule 11 Copyright 2016 Puma Biotechnology
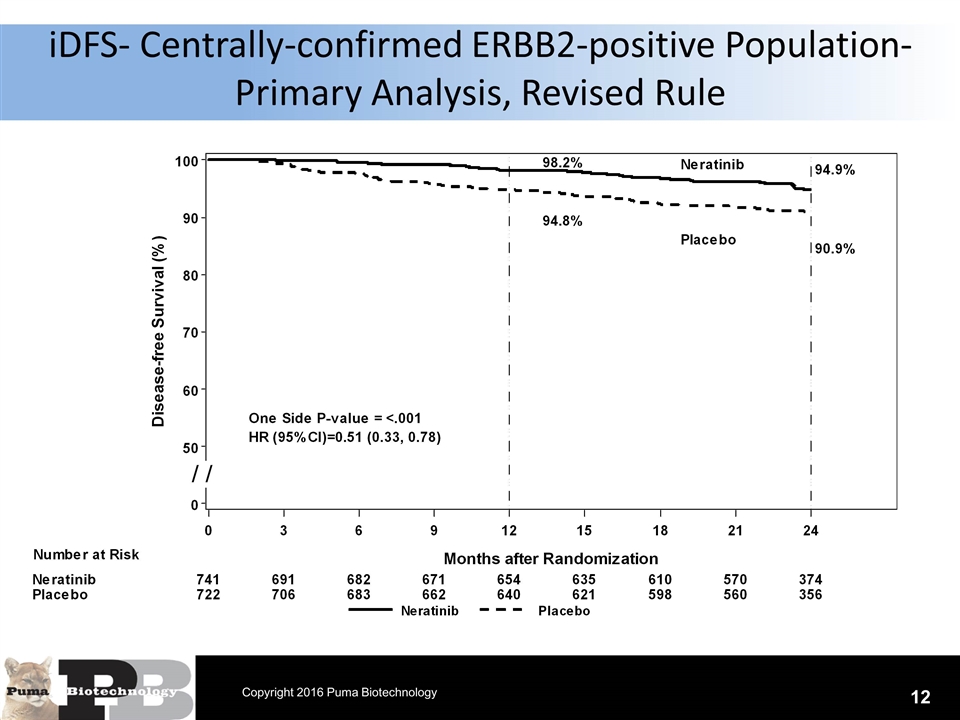
N = 120 patients iDFS- Centrally-confirmed ERBB2-positive Population- Primary Analysis, Revised Rule 12 Copyright 2016 Puma Biotechnology
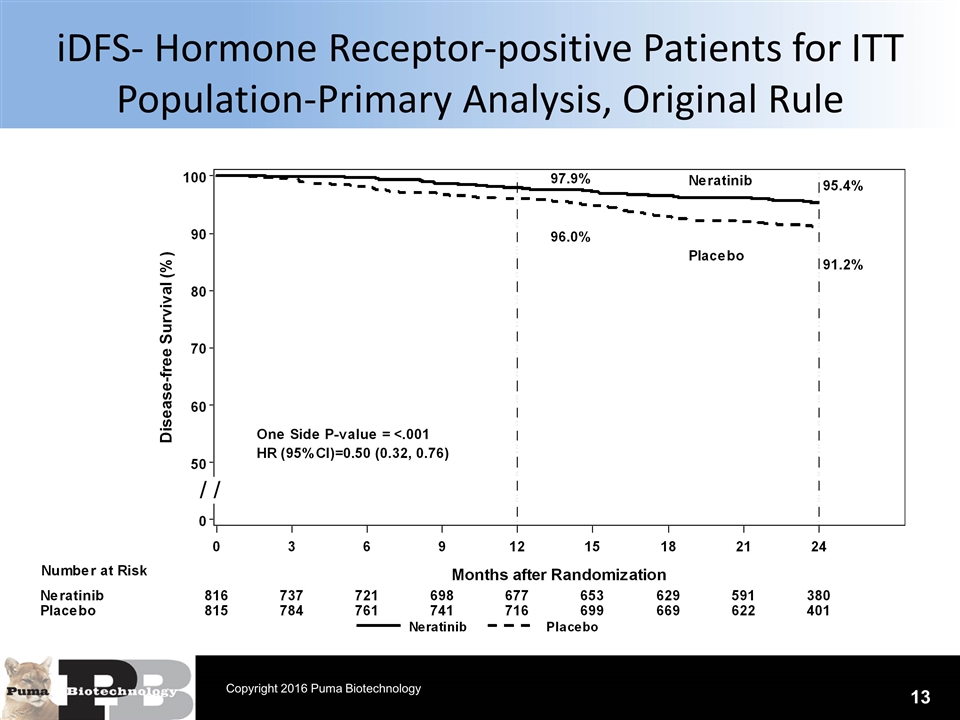
iDFS- Hormone Receptor-positive Patients for ITT Population-Primary Analysis, Original Rule 13 Copyright 2016 Puma Biotechnology
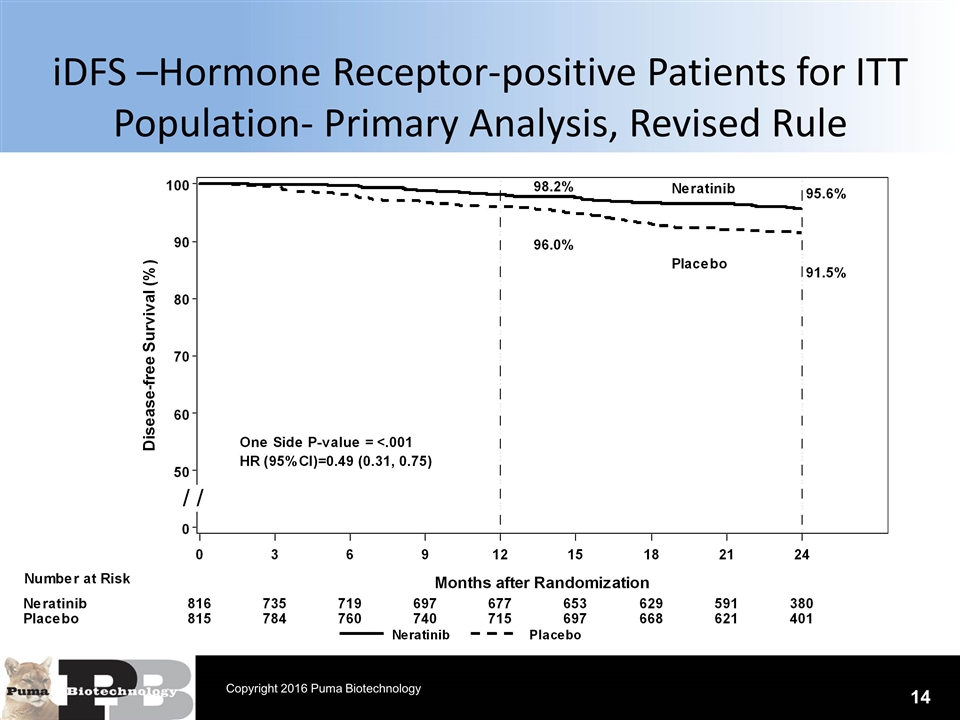
iDFS –Hormone Receptor-positive Patients for ITT Population- Primary Analysis, Revised Rule 14 Copyright 2016 Puma Biotechnology
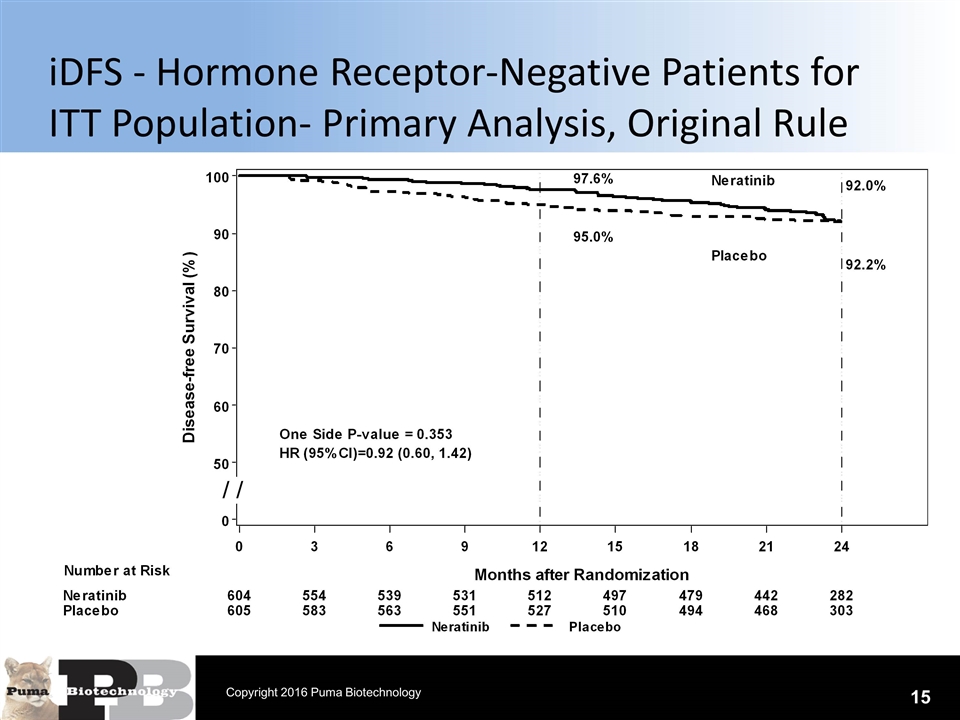
iDFS - Hormone Receptor-Negative Patients for ITT Population- Primary Analysis, Original Rule 15 Copyright 2016 Puma Biotechnology
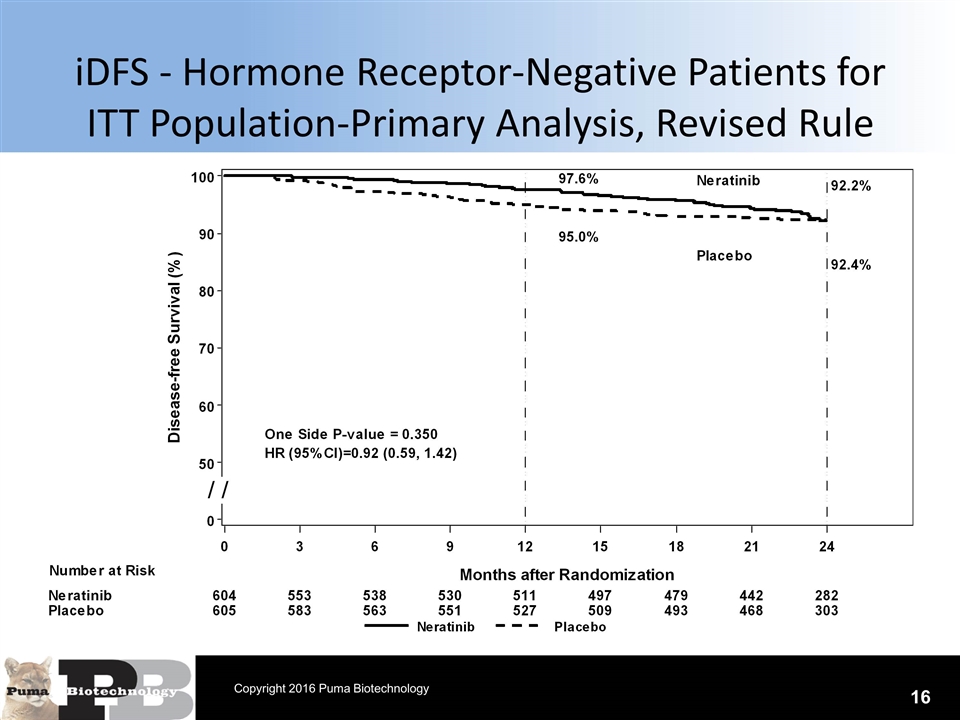
iDFS - Hormone Receptor-Negative Patients for ITT Population-Primary Analysis, Revised Rule 16 Copyright 2016 Puma Biotechnology
Conclusions The primary analysis results of the trial do not appear to be materially altered due to this change in event and censoring rule per FDA. ITT population applying the original rule Hazard ratio=0.67 (0.50, 0.91), 1-sided P=0.005 2-year iDFS rates 93.9% vs 91.6% ITT population applying revised rule Hazard ratio=0.66 (0.49, 0.90), 1-sided P=0.004 2-year iDFS rates 94.2% vs 91.9% Analysis results of prospectively defined subgroups do not appear to be materially altered due to change in the event and censoring rule 17 Copyright 2016 Puma Biotechnology
