Attached files
| file | filename |
|---|---|
| EX-31.1 - CERTIFICATION - PREMIER BIOMEDICAL INC | biei_ex311.htm |
| EX-31.2 - CERTIFICATION - PREMIER BIOMEDICAL INC | biei_ex312.htm |
| EX-32.1 - CERTIFICATION - PREMIER BIOMEDICAL INC | biei_ex321.htm |
| EX-32.2 - CERTIFICATION - PREMIER BIOMEDICAL INC | biei_ex322.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2015
OR
¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from_____________ to _____________.
Commission file number 000-54563

Premier Biomedical, Inc. |
(Exact name of registrant as specified in its charter) |
Nevada |
| 27-2635666 |
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. mployer Identification No.) |
|
|
|
P.O. Box 31374 El Paso, TX |
| 79930 |
(Address of principal executive offices) |
| (Zip Code) |
Registrant's telephone number, including area code (814) 786-8849
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of each exchange on which registered | |
None | None |
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, par value $0.00001
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10 K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | ¨ | Accelerated filer | ¨ |
Non-accelerated filer | ¨ | Smaller reporting company | x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter. $9,269,548 based on the closing price of $0.12 on June 30, 2015. The voting stock held by non-affiliates on March 14, 2016 consisted of 77,246,236 shares of common stock.
Applicable Only to Registrants Involved in Bankruptcy Proceedings During the Preceding Five Years:
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ¨ No ¨
(Applicable Only to Corporate Registrants)
Indicate the number of shares outstanding of each of the registrant's classes of common stock, as of the latest practicable date. As of March 14, 2016, there were 89,579,908 shares of common stock, par value $0.001, issued and outstanding.
Documents Incorporated by Reference
List hereunder the following documents if incorporated by reference and the Part of the Form 10-K (e.g., Part I, Part II, etc.) into which the document is incorporated: (1) Any annual report to security holders; (2) Any proxy or information statement; and (3) Any prospectus filed pursuant to rule 424(b) or (c) of the Securities Act of 1933. The listed documents should be clearly described for identification purposes (e.g., annual report to security holders for fiscal year ended December 24, 1980). None.
PREMIER BIOMEDICAL, INC.
FORM 10-K ANNUAL REPORT
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2015
TABLE OF CONTENTS
PART I | |||||
|
|
|
| ||
ITEM 1 – | BUSINESS |
|
| 3 |
|
ITEM 1A – | RISK FACTORS |
|
| 19 |
|
ITEM 1B – | UNRESOLVED STAFF COMMENTS |
|
| 31 |
|
ITEM 2 – | PROPERTIES |
|
| 31 |
|
ITEM 3 – | LEGAL PROCEEDINGS |
|
| 31 |
|
ITEM 4 – | MINE SAFETY DISCLOSURES |
|
| 31 |
|
|
|
|
|
| |
PART II | |||||
|
|
|
|
| |
ITEM 5 – | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
|
| 32 |
|
ITEM 6 – | SELECTED FINANCIAL DATA |
|
| 34 |
|
ITEM 7 – | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
|
| 34 |
|
ITEM 7A – | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
|
| 43 |
|
ITEM 8 – | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
|
| 43 |
|
ITEM 9 – | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
|
| 44 |
|
ITEM 9A – | CONTROLS AND PROCEDURES |
|
| 44 |
|
ITEM 9B – | OTHER INFORMATION |
|
| 46 |
|
|
|
|
|
| |
PART III | |||||
|
|
|
|
| |
ITEM 10 – | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
|
| 47 |
|
ITEM 11 – | EXECUTIVE COMPENSATION |
|
| 50 |
|
ITEM 12 – | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
|
| 53 |
|
ITEM 13 – | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
|
| 55 |
|
ITEM 14 – | PRINCIPAL ACCOUNTING FEES AND SERVICES |
|
| 58 |
|
|
|
|
|
| |
PART IV | |||||
|
|
|
|
| |
ITEM 15 – | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
|
| 59 |
|
| 2 |
PART I
Cautionary Statement Regarding Forward Looking Statements
This Annual Report includes forward looking statements within the meaning of the Securities Exchange Act of 1934 (the "Exchange Act"). These statements are based on management's beliefs and assumptions, and on information currently available to management. Forward looking statements include the information concerning possible or assumed future results of operations of the Company set forth under the heading "Management's Discussion and Analysis of Financial Condition or Plan of Operation." Forward looking statements also include statements in which words such as "expect," "anticipate," "intend," "plan," "believe," "estimate," "consider" or similar expressions are used.
Forward looking statements are not guarantees of future performance. They involve risks, uncertainties and assumptions. The Company's future results and shareholder values may differ materially from those expressed in these forward looking statements. Readers are cautioned not to put undue reliance on any forward looking statements.
ITEM 1 – BUSINESS
Corporate History
We were incorporated on May 10, 2010 in the State of Nevada.
Overview
We are a research-based company that intends to discover and develop medical treatments for humans, specifically targeting the treatment of:
- Cancer | - Fibromyalgia |
- Multiple Sclerosis (MS) | - Traumatic Brain Injury (TBI) |
- Neuropathic Pain | - Alzheimer's Disease (AD) |
- Amyotrophic Lateral Sclerosis (ALS/Lou Gehrig's Disease) | - Blood Sepsis and Viremia |
We have a two-fold corporate focus:
One is to target Cancer, Alzheimer's disease, ALS, Blood Sepsis, Leukemia, and other life-threatening cancers, and to do this we intend to develop our proprietary Sequential-Dialysis Technique. The methodology involved in this technique is largely unexplored and has been described by scientists as the "wild west" of modern medicine. Consequently, our first entry into the therapeutics market for medications that work against cancer, Multiple Sclerosis, infectious diseases, Alzheimer's disease, strokes and traumatic brain injury carries significant obstacles before reaching the opportunities of a $700 billion industry.
| 3 |
The other is the development of our proprietary drug candidate Feldetrex™, a potential treatment for Multiple Sclerosis, Fibromyalgia, Neuropathic Pain and Traumatic Brain Injury. The formulation used in the current Feldetrex™ will be individually tailored to the various illnesses we intend to target, with each formulation being given a unique proprietary brand name. The annual market size of MS treatment is $500 million and the annual market size for all proposed Feldetrex™ market segments is $16 billion.
To overcome the significant obstacles inherent to the development of our Sequential-Dialysis Technique and Feldetrex™ candidate drug, we are seeking to partner with prestigious institutions and pharmaceutical companies with the substantial infrastructure and resource capacity to perform experimentation and to engage in product development in an inexpensive and efficient manner.
SEQUENTIAL-DIALYSIS TECHNIQUE
Our proprietary Sequential-Dialysis Technique is a methodology for the removal of those molecules which are harmful and responsible for causing diseases. A significant disappointment in the practice of modern medicine is that the capabilities do exist to eliminate the presence of most illnesses, including life-threatening diseases such as AIDS and cancer, but with a caveat that the process of treatment comes with catastrophic side effects that can and often do kill the patient.
Our development is that the innovative Sequential-Dialysis Methodology is done extracorporeally (outside the body). This is a truly unique and innovative method for alleviating disease.
We believe that this methodology can be used for the prevention of cancer metastasis, for directly attacking the causation of intractable seizures, for preventing the death of anterior motor neurons in ALS, for preventing the cause of the neuropathological changes in Alzheimer's disease and Traumatic Brain Injury and for eradicating the causations of infectious diseases, and our intention is that the effectiveness of this technique will be demonstrated and supported in future clinical studies.
Through our Sequential-Dialysis Technique, we ultimately hope to provide a cure for cancer if not only to dramatically extend the lives of suffering patients. Our initial focus is on lab and animal tests. Clinical trials, as required, will be undertaken subsequently.
| 4 |
CANCER
Description of Illness
Cancer is a class of diseases in which a group of cells display 1) uncontrolled growth beyond the normal limits of cell reproduction, 2) invasion and destruction of adjacent tissues, and sometimes 3) metastasis or spread to other locations in the body via lymph or blood. These three properties of malignant cancers differentiate them from benign tumors which are self-limited and do not invade or metastasize. Most cancers form a tumor, but some, like leukemia, do not.1
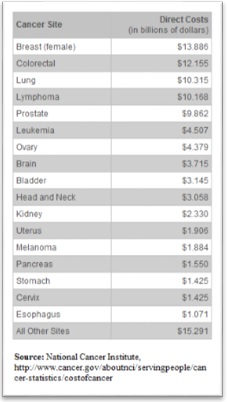
Cancer may affect people at all ages but the risk for most varieties of cancer increases with age. In the United States, cancer accounts for nearly 1 in 4 deaths. According to the American Cancer Society about 577,190 Americans will die of Cancer this year, making the death toll a staggering 1,500 people per day.2 Globally and on average, 7.5 million people die of Cancer every year. There are about 12.7 million people living with cancer in the United States.3
Nearly all cancers are caused by abnormalities in the genetic material of the transformed cells. These abnormalities may be due to the effect of carcinogens, such as tobacco smoke, radiation, chemicals, or infectious agents. Other cancer-promoting genetic abnormalities may be randomly acquired through errors in DNA replication, or are inherited, and thus are present in all cells from birth. The heritability of cancers is usually affected by complex interactions between carcinogens and the host's genome. New aspects of the genetics of cancer pathogenesis, such as DNA methylation and microRNAs are increasingly recognized as important.
____________________
| 1 | http://www.news-medical.net/health/What-is-Cancer.aspx |
| 2 | http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf |
| 3 | http://costprojections.cancer.gov/ |
| 5 |
Conventional Method of Treatment
Cancer can be treated today by surgery, chemotherapy, radiation, immunotherapy, monoclonal antibody therapy, or other methods. The choice of therapy depends upon the location and grade of the tumor and the stage of the disease, as well as the general state of the patient. A number of experimental cancer treatments are also under development. Complete removal of the cancer without damage to the rest of the body is the goal of treatment. Sometimes this can be accomplished by surgery, but the propensity of cancers to invade adjacent tissue or to spread to distant sites by microscopic metastasis often limits its effectiveness. The effectiveness of chemotherapy is often limited by toxicity to other tissues in the body. Radiation damages normal tissue.4
Potential for Sequential Dialysis Technique
We intend to develop a methodology for treating cancer which is completely different from the standard treatments of chemotherapy and radiation therapy that are now being utilized. Due to the fact that all presently known treatments directly inject chemotherapeutic agents into the body of a patient and/or directly irradiate the patient, there is a very high level of adverse side effects, such as kidney failure, encephalopathy, neuropathy, heart toxicity, and other severe morbidities.
We intend to develop our intellectual property applications for utilizing a proprietary methodology in which the cancer patient's blood or other bodily fluid is utilized to remove metastatic cancer cells or other disease causing antigens. This is accomplished by sequentially dialyzing the patient's blood or other bodily fluid extracorporeally. The method will utilize designer antibodies to physically remove the pathophysiologic basis of the disease. For example, in sepsis there will be the physical attachment and removal of bacteria. In cancer treatment, there will be the physical attachment and then physical removal of metastasizing cancer cells. There will also be the physical attachment, and removal of those proteins which allow cancer to metastasize successfully and then thrive-such as angiogenic proteins. To date there has been no specific clinical evidence to support a conclusion that this treatment is effective for premetastatic or metastatic cancers. We hope to demonstrate this in future lab and animal experiments. Through this process, the cancer can be targeted through a number of innovative techniques being developed by the Company. |
|
|
This extracorporeal sequential dialysis methodology for cancer treatment has an enormous potentiality for decreasing the side effects of chemotherapy and radiation treatment in cancer patients. Our methodology may also increase the efficacy of cancer treatment by allowing for much higher dosages of anti-neoplastic agents to be used through this extracorporeal methodology. Due to the fact that this methodology completely avoids exposure of the patient's body to these anti-cancer agents, dosages that cannot be normally tolerated can now be utilized in fighting the cancer.
____________________
| 4 | http://www.kfshrcj.org/NR/rdonlyres/AC6F2108-37CB-4C32-999B-B292ED658481/2846/CancerTreatment.pdf |
| 6 |
ALZHEIMER'S DISEASE
Description of Illness
Alzheimer's disease is a dementing illness, which induces a progressive impairment of intellectual functioning, including a loss of short term memory. There is also a progressive impairment in executive functioning with occasional psychiatric manifestations such as depression and delirium. Delirium is characterized by an acute confusion. Oftentimes patients have language impairment and apraxia—an inability to perform previously learned tasks. Patients also often times show agnosia, an inability to recognize objects, and patients have a loss of visual-spatial abilities, for example oftentimes becoming lost in familiar surroundings. Occasionally hallucinations occur in severe forms of Alzheimer's disease.
Alzheimer's disease comes from neuropathic changes in the brain which includes the accumulation of neurofibrillary tangles and amyloid plaques in the cortex of the brain.5 Neurofibrillary tangles are composed of Tau proteins, which are deposited within the neurons of the brain. Thus, Tau proteins are the causation of Alzheimer's disease and other Tauopathies such as Pick's Disease, Tuberous Sclerosis and certain forms of Parkinson's disease.
A report by the Alzheimer's Association examining the current trajectory of Alzheimer's disease shows that the number of Americans age 65 and older who have Alzheimer's disease will increase from 5.1 million in 2010 to 13.5 million by mid-century. The report entitled "Changing the Trajectory of Alzheimer's Disease: A National Imperative" shows that "in the absence of disease-modifying treatments, the cumulative cost of care for people with Alzheimer's from 2010 to 2050 will exceed $20 trillion, in today's dollars. Total costs of care for individuals with Alzheimer's disease by all payers will soar from $172 billion in 2010 to more than $1 trillion in 2050." During this time, the Medicare costs of Alzheimer's disease will soar 600% to $627 billion and the Medicaid costs of Alzheimer's disease will soar 400% to $178 billion.6
Conventional Method of Treatment
Presently, there is no cure for Alzheimer's disease. Treatments exist but only target the symptoms of Alzheimer's disease without targeting the underlying progression of the disease. Consequently the projected future life span of an individual diagnosed with Alzheimer's disease is 5 to 7 years.7
Potential for Sequential-Dialysis Technique
We believe that our proprietary Sequential-Dialysis Technique can be used to prevent the onset of Alzheimer's disease. This would be done by removing the proteins responsible for the pathologic changes in the brain, namely the protein Tau; thus, preventing the cause of the neuropathic changes that cause Alzheimer's disease. The Tau protein will be removed from the cerebral spinal fluid in which it resides utilizing a designer antibody (an antibody genetically engineered for a specific purpose) which will allow for the efficacious removal of the protein. We hope to demonstrate this in future lab and animal experiments. We believe that our Sequential-Dialysis Technique can also be used in this fashion to treat Traumatic Brain Injury.
____________________
| 5 | McPhee, Stephen J. Current Medical Diagnosis & Treatment. 47th ed. New York: McGraw Hill, 2008. Print. |
| 6 | http://www.alz.org/documents_custom/FINAL_Trajectory_Report_Release-EMB_5-11-10.pdf |
| 7 | http://www.webmd.com/alzheimers/news/20100413/formula-predicts-alzheimers-longevity |
| 7 |
ALS
Description of Illness
Amyotrophic Lateral Sclerosis (ALS) is a progressive neurodegenerative disease that affects nerve cells in the brain and spinal cord. While the cause of ALS is uncertain, the process of ALS is known to occur as motor neurons in affected patients progressively degenerate until death. As motor neurons degenerate, they can no longer send impulses to muscle fibers that normally result in muscle movement. Eventually, the motor neurons die and the ability of the brain to initiate and control muscle movements is lost. With voluntary muscle action progressively affected, patients in the later stages of the disease become totally paralyzed.8
ALS has been frequently referred to as Lou Gehrig's disease, after the famous New York Yankees baseball player diagnosed with the disease in 1939.
Annually, about 5,600 people are diagnosed with ALS in the United States. The projected future life span of a diagnosed patient is two to five years. It is projected that of the current US population, 300,000 people will die of ALS before a cure is found.
The financial cost to families of persons with ALS is exceedingly high. In the advanced stages, care can cost up to $200,000 a year. Entire savings of relatives of patients are quickly depleted because of the extraordinary cost involved in the care of ALS patients.9
|
|
|
Conventional Method of Treatment
There is currently no cure or treatment that halts or reverses ALS. However, there is one FDA approved drug, Riluzole, which modestly slows the progression of ALS.
Potential for Sequential-Dialysis Technique
Numerous medical studies have proven that the causation of ALS is an over excitation of the anterior motor neurons in the spinal cord. Our Sequential-Dialysis Technique method removes those excitatory neural transmitters that cause the death of those cells. The method will utilize designer antibodies to physically remove excitatory neurotransmitters such as glutamate from cerebrospinal fluid. We hope to demonstrate this methodology in future lab and animal experiments. Thus, we hope to prevent the development of ALS; thereby, giving hope to patients of a currently unconquerable disease.
____________________
| 8 | http://www.alsa.org/als/what.cfm |
| 9 | http://www.focusonals.com/alsfacts.htm |
| 8 |
BLOOD SEPSIS AND VIREMIA
Description of Illness
Blood Sepsis, also known as Blood Poisoning, is an infection of the blood stream. Sepsis is caused when toxin releasing bacteria, such as Staphylococcus, enter the blood. Blood Sepsis is a particularly devastating disease due to the domino-effect of organ shutdown which causes multiple organ failure. Blood Sepsis causes a whole body inflammatory state called Systemic Inflammatory Response Syndrome (SIRS).
Blood Sepsis first results in the shutdown of kidneys; thus patients require standard dialysis immediately to prevent death. As the disease progresses, vital signs collapse—the foremost of these being blood pressure. Subsequently, symptoms of Sepsis include elevated temperature, elevated heart rate, respiratory collapse, further organ failures, altered mental status and cardiac failure.
Septicemia is a major cause of death in the United States and puts people in the intensive care unit at a very high rate. Only about 1-2% of all hospitalizations in the United States are attributed to Septicemia, though Septicemia accounts for as much as 25% of bed-utilization in intensive-care units. |
|
|
Conventional Method of Treatment
The traditional therapy of Blood Sepsis (bacteremia) relies on intravenous treatment using multiple antibiotics. However, in intensive care units, even with today's treatment, approximately 35% of patients with severe sepsis and 60% of patients with septic shock die within 30 days.
Septicemia is of particular concern because of the exceedingly high cost of treatment for Septicemia patients. A typical stay in the intensive care unit costs $10,000 per day with testing. Consequently, the treatment of Blood Sepsis is one of the most costly expenditures for hospitals in America.
Potential for Sequential-Dialysis Technique
We hope to conquer Blood Sepsis and Viremia (a disease having symptoms similar to Sepsis but caused by virus) by using our proprietary Sequential-Dialysis Technique. If proven successful, this technique would dialyze the toxin producing bacteria out of the blood by using antibodies; thus saving countless lives while also providing significant cost savings to hospitals around the country. The method will utilize designer antibodies to physically remove the toxin producing bacteria out of the blood. The designer antibodies will attach to the toxin producing bacteria or virus, and then the antibody-antigen compound will be efficaciously dialyzed out of the blood extracorporeally. We hope to demonstrate this methodology in future lab and animal experiments.
| 9 |
FELDETREX™
Although a combination of generic medications, we have a U.S. Patent (No. 8,865,733) on our Feldetrex™ candidate drug. In this way, Feldetrex™ is similar to ViagraÒ, which was a proprietary cardiac drug prior to its current use and ownership by Pfizer. Consequently, we have one pending patent application for our Feldetrex™ candidate drug—intending to increase our Feldetrex™ related patent applications to three in the near future.
Feldetrex™ may serve as an additional medication utilized by physicians for the treatment of Multiple Sclerosis, Fibromyalgia, or Traumatic Brain Injury, and is designed to decrease symptomatology in those conditions. Feldetrex™ will not compete against our proprietary Sequential-Dialysis Technique in the market to treat Traumatic Brain Injury, but rather the two will work conjunctively.
Feldetrex™ utilizes a low dosage of Naltrexone which has been shown in multiple medical articles, in the medical literature, to increase endogenous enkephalins10 (endogenous enkephalins are pain-relieving pentapeptides produced in the body, located in the pituitary gland, brain, and GI tract. Axon terminals that release enkephalins are concentrated in the posterior horn of the gray matter of the spinal cord, in the central part of the thalamus, and in the amygdala of the limbic system of the cerebrum. Endogenous Enkephalins function as neurotransmitters that inhibit neurotransmitters in the pathway for pain perception, thereby reducing the emotional as well as the physical impact of pain). We have not independently conducted medical or laboratory tests to show the mechanism of action of this medication. While Naltrexone in high dosages acts as an opioid antagonist, it inhibits opiate receptors. Naltrexone in low dosages causes a compensatory upregulation (increase in the number of receptors) of native endorphins and enkephalins, which last beyond the effects of the Naltrexone itself. We believe that this means, paradoxically, that a daily dose of low dose Naltrexone can be used to chronically increase endorphin and enkephalin levels. We believe that by utilizing a low dosage, Naltrexone has a unique ability to increase enkephalins and other neurotransmitters in the brainstem of patients.
____________________
| 10 | A. | Bowling, Allen C.. "Low-dose naltrexone (LDN) The "411" on LDN" National Multiple Sclerosis Society. http://www.nationalmssociety.org/multimedia-library/momentum-magazine/back-issues/momentum-spring-09/index.aspx. Retrieved 6 July 2011. |
| B. | Bourdette, Dennis. "Spotlight on Low Dose Naltrexone (LDN)". US Department of Veteran Affairs. http://www.va.gov/MS/articles/Spotlight_on_Low_Dose_Naltrexone_LDN.asp. Retrieved 5 July 2011. | |
| C. | Giesser, Barbara S. (2010). Primer on Multiple Sclerosis. New York: Oxford University Press US. pp. 377. ISBN 978-0-19-536928-1. | |
| D. | Moore, Elaine A. 1948. The promise of low dose naltrexone therapy: potential benefits in cancer, autoimmune, neurological and infectious disorders. Elaine A. Moore and Samantha Wilkinson. ISBN 978-0-7864-3715-3. | |
| A. | Moore, Elaine A. 1948. The promise of low dose naltrexone therapy: potential benefits in cancer, autoimmune, neurological and infectious disorders. Elaine A. Moore and Samantha Wilkinson. ISBN 978-0-7864-3715-3 | |
| B. | Crain SM, Shen K-F (1995). Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci USA 92: 10540–10544. | |
| C. | Powell KJ, Abul-Husn NS, Jhamandas A, Olmstead MC, Beninger RJ, et al. (2002). Paradoxical effects of the opioid antagonist naltrexone on morphine analgesia, tolerance, and reward in rats. J Pharmacol Exp Ther 300: 588–596. | |
| D. | Wang H-Y, Friedman E, Olmstead MC, Burns LH (2005). Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in Mu opioid receptor-G protein coupling and Gβγ signaling. Neuroscience 135: 247–261 |
| 10 |
MULTIPLE SCLEROSIS
Description of Illness
Multiple Sclerosis (MS) is a devastating inflammatory neurologic disease in which white matter, known as myelin, is damaged—causing episodic or neurological symptoms. The destruction of myelin inhibits communications between the nerves in the brain.
Symptoms of Multiple Sclerosis include extreme fatigue, numbness, weakness, difficulty with eyesight, spasticity, speech problems, and problems with coordination. Multiple Sclerosis has its greatest incidence in young adults and patients are usually diagnosed at less than 55 years of age at the onset of the illness.
The cause of Multiple Sclerosis is unknown, although the disease is believed to be an autoimmune problem triggered by a virus—meaning that the patient's immune cells attack and destroy the patient's myelin. In the United States, there are approximately 400,000 patients diagnosed with MS and approximately 200 new patients are diagnosed every week.11 Globally, Multiple Sclerosis is believed to effect 2.1 million people, mostly of European origin.
Conventional Method of Treatment
'ABC' drugs Avonex, Beta-Seron, Copaxone are used to treat Multiple Sclerosis but have been shown to barely beat out placebos in efficacy and are not approved in England for government subsidy. The drug Tysavri was deemed dangerous and was taken off the market for over a year.
Another treatment of MS is high dose steroids; though, this treatment simply decreases symptoms without curing MS.
Potential for Feldetrex™
Our proprietary Feldetrex™ candidate drug will not compete with typical treatment methods for Multiple Sclerosis, but rather, is simply an add-on drug to increase the effectiveness of treatment.
____________________
| 11 | http://www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/who-gets-ms/index.aspx |
| 11 |
FIBROMYALGIA
Description of Illness
Fibromyalgia is a common illness affecting approximately 2% of the general population, most common amongst women 20 to 50 years of age. Approximately five million Americans suffer from the debilitating illness. The cause of Fibromyalgia is officially unknown and diagnosis of Fibromyalgia is a 'diagnosis of exclusion'—meaning that Fibromyalgia is diagnosed as an illness after Rheumatoid Arthritis and Lupus have been ruled out with a blood test.
Patients with Fibromyalgia suffer from debilitating fatigue, numbness, headaches, and chronic widespread musculoskeletal pain with multiple tender points. Fibromyalgia is a chronic condition lasting 6 months to many years. Patients commonly complain of chronic aching, pain, stiffness, sleep difficulty, headaches, and irritable bowel syndrome. Consequently, approximately 25% of patients with Fibromyalgia are work disabled. The direct and indirect costs of Fibromyalgia are, on average, $5,945 per patient.12 |
|
|
Conventional Method of Treatment
Lyrica (Pregabalin) is the only FDA approved medication for the treatment of Fibromyalgia. However, Lyrica oftentimes has side effects of dizziness, drowsiness and dry mouth. Rarely, Lyrica can cause suicidal ideation and severe agitation.
Potential for Feldetrex™
Our Feldetrex™ candidate drug will not compete with currently existing treatments of Fibromyalgia, but, rather, would be an add-on-drug to increase the effectiveness of treatment.
____________________
| 12 | http://www.cdc.gov/arthritis/basics/fibromyalgia.htm |
| 12 |
TRAUMATIC BRAIN INJURY
Description of Illness
Traumatic Brain Injury (TBI) occurs when an external force traumatically injures the brain. TBI is a major cause of death and disability worldwide, especially in children and young adults. Causes of TBI include falls, vehicle accidents, and violence. Three separate processes of Traumatic Brain Injury work to injure the brain: 1) bruising (bleeding), 2) tearing, and 3) swelling. Brain trauma can be caused by a direct impact or by acceleration alone. In addition to the damage caused at the moment of injury, brain trauma causes 'secondary injury', a variety of events that take place in the minutes and days following the injury. These processes, which include alterations in the cerebral blood flow and the pressure within the skull contribute substantially to the damage from the initial injury. |
|
|
Each year, an estimated two million TBI-related deaths, hospitalizations, and emergency department visits occur in the United States. Of these patients, 56,000 die and 300,000 are hospitalized. 1.7 million patients are treated and released from an emergency department.13
Conventional Method of Treatment
The present treatment methodology for Traumatic Brain Injury is centered on the treatment of symptoms. There are currently no treatments that target the underlying pathology of Traumatic Brain Injury.
Currently used medications used to treat Traumatic Brain Injury, such as narcotics and antidepressants, have many side effects including addiction, arrhythmia, liver and kidney damage, abdominal problems, nausea, and vomiting.
Potential for Feldetrex™
Our proprietary Feldetrex™ candidate drug has a mechanism of action via a manipulation of central nervous system neurotransmitters, which involves the cerebral cortex, limbic system, and spinothalamic tracts. Feldetrex™ utilizes a low dosage of Naltrexone which has been shown in multiple medical articles, in the medical literature, to increase endogenous enkephalins. We have not independently conducted medical or laboratory tests to show the mechanism of action of this medication.
____________________
| 13 | http://www.caregiver.org/caregiver/jsp/content_node.jsp?nodeid=441 |
| 13 |
Development Plans
Central Nervous System Disorders
Feldetrex™
Feldetrex™, our candidate drug, is a treatment for decreasing the morbidity of Multiple Sclerosis patients. Multiple Sclerosis is a chronic, progressive illness that affects the nerves in the brain, spinal cord, and other parts of the central nervous system. Multiple Sclerosis affects over 400,000 people in the United States and may affect 2.5 million people worldwide. The licensor under our License Agreements has two pending provisional patent applications with variations featuring oral and/or possibly transdermal medication applications. We intend to compare the efficacy of Feldetrex™ to the standard injectable medications currently utilized to treat Multiple Sclerosis, such as Betaseron (Interferon beta-1b), Avonex (Interferon beta-1a), and Copaxone (Glatiramer acetate). Additionally, we plan on utilizing Feldetrex™ in peer reviewed medical studies at major medical centers to ascertain the efficacy of Feldetrex™ in symbiotically increasing the efficacy of the standard FDA approved Multiple Sclerosis injection drugs.
Feldetrex™ Development Plans
We don't have the financial ability to conduct the necessary clinical trials for FDA approval of our Feldetrex™ product candidate. We intend to enter into agreements with larger pharmaceutical companies as collaboration partners, in part to help cover the cost of such processes.
At the conclusion of the studies involving Feldetrex™, we plan to publish the results in established medical journals and subsequently contact the large pharmaceutical firms for a possible sale or license of the rights to conduct clinical trials, manufacture, and distribute Feldetrex™.
Infectious Diseases
The licensor under our License Agreements has filed a provisional patent application for the dialyzation of blood from a patient who is dying from septicemia. Septicemia is a rapidly fatal condition in which bacteria have infected the bloodstream of a patient. These patients subsequently suffer from rapid renal failure, encephalopathy, and heart failure unless the septicemia is quickly reversed. Each year approximately 200,000 Americans die from septicemia.14 The traditional treatment of septicemia has been intravenous antibiotics, which have limited efficacy and are highly toxic.
Our method of treatment is to use an extracorporeal methodology of blood dialysis in which the bacteria-infected blood is sterilized through a proprietary methodology of dialyzation in which the blood is placed into contact with antibiotics within the dialysis lumen for a specified period, which subsequently kills the bacterial pathogen with a sequential removal of the antibiotic in order to negate any toxicity to the treated patient. We anticipate that this method could be utilized in place of the standard methodology or could be utilized as an adjunctive treatment for intensive care unit patients.
____________________
14 | Longo, Dan (2011). Harrison's principles of internal medicine. (18th ed.). New York: McGraw-Hill. p. Chapter 271. |
| 14 |
Development Plans
Initial concept experimentations began in 2012. After proof of concept experimentations, we intend to implement a clinical hospital/doctor test plan for patients using the same contacts developed in the studies described above for Feldetrex™. We anticipate that these studies will be initiated after successful laboratory testing no earlier than 2014. Actual clinical trials with patients, conducted in conjunction with our collaboration partners, may continue for many years. We plan to contact the large medical firms, such as Johnson and Johnson, Pfizer, and Eli Lilly, to attempt to sell the rights to use our technology and conduct the anticipated clinical trials, beginning as early as mid-to-late 2014.
Oncology
The licensor under our License Agreements is currently developing applications for utilizing a proprietary methodology in which the cancer patient's blood is utilized to remove metastatic cancer cells. This is accomplished by dialyzing the patient's blood extracorporeally, and, through our proprietary methodology, placing the cancer cells in contact with anti-neoplastic agents within the lumen of the dialysis apparatus. We believe this extracorporeal methodology for cancer treatment has an enormous potentiality for decreasing the side effects of chemotherapy and irradiation treatment in cancer patients. Our methodology may also increase the efficacy of cancer treatment by allowing for much higher dosages of anti-neoplastic agents to be used through this extracorporeal methodology. Because this method completely avoids exposure of the patient's corpus to these anti-cancer agents, we believe dosages that cannot be normally tolerated can now be utilized in fighting the cancer.
Development Plans
We initiated laboratory tests to prove our cancer-fighting technology in late 2012. In 2013, we completed two breast oncology studies to test the effectiveness of a treatment proposed by Premier Biomedical, Inc. on small mice populations. Premier Biomedical, Inc. believes that the results of these studies will be published in a scientific journal in January 2014, although the results will ultimately be published at the discretion of the University of Texas at El Paso. We plan to undertake additional studies at a university/hospital during 2014. We estimate the cost for each of these studies to be between $300,000 and $500,000, including actual testing with cancer patients, which we anticipate funding from additional capital raises. At the anticipated successful conclusion of these studies, we plan to contact the large pharmaceutical/medical devices firms, such as Johnson & Johnson, Boston Scientific, Medtronics, Pfizer, and E. I. Lilly, to attempt to negotiate a partnership and/or sale of the technology.
Research and Development
Innovation by our research and development operations is very important to our success. Our goal is to discover, develop and bring to market innovative products and treatments that address major unmet medical needs, including initially, Multiple Sclerosis, Septicemia, and Cancer. We expect this goal to be supported by substantial research and development investments.
We plan on conducting research internally and may also through contracts with third parties, through collaborations with universities and biotechnology companies and in cooperation with pharmaceutical firms. We may also seek out promising compounds and innovative technologies developed by third parties to incorporate into our discovery or development methods and procedures or projects, as well as our future product lines, through acquisition, licensing or other arrangements.
| 15 |
In addition to discovering and developing new products, methods and procedures of treatment and treatments, we expect our research operations to add value to our existing products and methods and procedures of treatment in development by improving their effectiveness and by discovering new uses for them.
Marketing
Currently, we manage our marketing responsibilities internally. We intend to seek a partnership with and/or sale of our product candidates/technologies to large pharmaceutical and/or medical devices firms. These firms have the ability to effectively promote our product candidates to healthcare providers and patients. Through their marketing organizations, they can explain the approved uses, benefits and risks of our product candidates to healthcare providers, such as doctors, nurse practitioners, physician assistants, pharmacists, hospitals, Pharmacy Benefit Managers (PBMs), Managed Care Organizations (MCOs), employers and government agencies. They also market directly to consumers in the U.S. through direct-to-consumer advertising that communicates the approved uses, benefits, and risks of our product candidates while continuing to motivate people to have meaningful conversations with their doctors. In addition, they sponsor general advertising to educate the public on disease awareness, important public health issues, and patient assistance programs.
The large pharmaceutical/medical devices firms principally sell their products to wholesalers, but they also sell directly to retailers, hospitals, clinics, government agencies and pharmacies and also work with MCOs, PBMs, employers and other appropriate healthcare providers to assist them with disease management, patient education and other tools that help their medical treatment routines.
Patents and Intellectual Property Rights
We have licensed three U.S. patents: Sequential Extracorporeal Treatment of Bodily Fluids, U.S. Patent No. 9,216,386 and Utilization of Stents for the Treatment of Blood Borne Carcinomas, U.S. Patent No. 8,758,287 (both from Marv Enterprises, LLC), and Medication and Treatment for Disease, U.S. Patent No. 8,865,733 (from Altman Enterprises, LLC), in the areas of cancer, sepsis, and multiple sclerosis. We expect these patents to cover the medical treatments discussed above for Multiple Sclerosis, Blood Sepsis, and Cancer and be effective until 2029. Marv and Altman have licensed these technologies to us pursuant to the terms of the License Agreements. Because our license agreements cover the patents and "all applications of the United States and foreign countries that claim priority to the above PCT applications, including any non-provisionals, continuations, continuations-in-part, divisions, reissues, re-examinations or extensions thereof," we anticipate that other technologies that derive from these patents will also belong to us and are covered by the license agreements.
Patents extend for twenty years from the date of patent filing. The actual protection afforded by a patent, which can vary from country to country, depends upon the type of patent, the scope of its coverage and the availability of legal remedies in the country.
| 16 |
Dr. Felder is the owner of the Feldetrex mark, and has also licensed this to us pursuant to the terms of the License Agreements.
We expect our patent and related rights to be of material importance to our business.
Competition
Our business is conducted in an intensely competitive and often highly regulated market. Our treatments face competition in the form of branded drugs, generic drugs and the currently practiced treatments for Multiple Sclerosis, Blood Sepsis, and Cancer. The principal forms of competition include efficacy, safety, ease of use, and cost effectiveness. Where possible, companies compete on the basis of the unique features of their products, such as greater efficacy, better patient ease of use or fewer side effects. A lower overall cost of therapy is also an important factor. Products that demonstrate fewer therapeutic advantages must compete for inclusion based primarily on price. Though the means of competition vary among product categories, demonstrating the value of our medications and procedures will be a critical factor for our success.
Our competitors include large worldwide research-based drug companies, smaller research companies with more limited therapeutic focus, and generic drug manufacturers. We compete with other companies that manufacture and sell products that treat similar diseases as our major medications and procedures.
Environment
Our business may be subject to a variety of federal, state and local environmental protection measures. We intend to comply in all material respects with applicable environmental laws and regulations.
Regulation
We expect our business to be subject to varying degrees of governmental regulation in the United States and any other countries in which our operations are conducted. In the United States, regulation by various federal and state agencies has long been focused primarily on product safety, efficacy, manufacturing, advertising, labeling and safety reporting. The exercise of broad regulatory powers by the FDA continues to result in increases in the amounts of testing and documentation required for FDA clearance of new drugs and devices and a corresponding increase in the expense of product introduction. Likewise, the approval process with the FDA is estimated to take approximately seven (7) years from the time it is started. Similar trends are also evident in major markets outside of the United States.
| 17 |
Clinical trials are a set of procedures in medical research conducted to allow safety (or more specifically, information about adverse drug reactions and adverse effects of other treatments) and efficacy data to be collected for health interventions (e.g., drugs, diagnostics, devices, therapy protocols). These trials can take place only after satisfactory information has been gathered on the quality of the non-clinical safety, and Health Authority/Ethics Committee approval is granted in the country where the trial is taking place.
Depending on the type of product and the stage of its development, investigators enroll healthy volunteers and/or patients into small pilot studies initially, followed by larger scale studies in patients that often compare the new product with the currently prescribed treatment. As positive safety and efficacy data are gathered, the number of patients is typically increased. Clinical trials can vary in size from a single center in one country to multicenter trials in multiple countries.
Due to the sizable cost a full series of clinical trials may incur, the burden of paying for all the necessary people and services is usually borne by the sponsor who may be a governmental organization, a pharmaceutical, or biotechnology company. Since the diversity of roles may exceed resources of the sponsor, often a clinical trial is managed by an outsourced partner such as a contract research organization or a clinical trials unit in the academic sector.
The regulatory agencies under whose purview we intend to operate have administrative powers that may subject us to such actions as product withdrawals, recalls, seizure of products and other civil and criminal sanctions.
Because we intend to seek a partnership with and/or sale of our product candidates/technologies to large pharmaceutical and/or medical devices firms, we anticipate that a larger pharmaceutical company will undertake to navigate the regulatory pathway, including conducting clinical trials, for a product such as Feldetrex™.
Employees
As of the date hereof, we do not have any employees other than our two officers and four additional directors that work on a part-time basis. Our officers and directors will continue to work for us for the foreseeable future. We anticipate hiring appropriate personnel on an as-needed basis, and utilizing the services of independent contractors as needed.
| 18 |
ITEM 1A. – RISK FACTORS.
As a smaller reporting company we are not required to provide a statement of risk factors. Nonetheless, we are voluntarily providing risk factors herein.
Any investment in our common stock involves a high degree of risk. You should consider carefully the following information, together with the other information contained in this Annual Report, before you decide to buy our common stock. If one or more of the following events actually occurs, our business will suffer, and as a result our financial condition or results of operations will be adversely affected. In this case, the market price, if any, of our common stock could decline, and you could lose all or part of your investment in our common stock.
We are developing medical treatments for Alzheimer's disease, multiple sclerosis, amyotrophic lateral sclerosis, fibromyalgia, traumatic brain injury, blood sepsis and virema, and cancer. We face risks in developing our product candidates and services and eventually bringing them to market. We also face risks that our business model may become obsolete. The following risks are material risks that we face. If any of these risks occur, our business, our ability to achieve revenues, our operating results and our financial condition could be seriously harmed.
Risk Factors Related to the Business of the Company
We have a limited operating history and our financial results are uncertain.
We have a limited history and face many of the risks inherent to a new business. As a result of our limited operating history, it is difficult to accurately forecast our potential revenue. We were incorporated in Nevada in 2010. Our revenue and income potential is unproven and our business model is still emerging. Therefore, there can be no assurance that we will provide a return on investment in the future. An investor in our common stock must consider the challenges, risks and uncertainties frequently encountered in the establishment of new technologies, products and processes in emerging markets and evolving industries. These challenges include our ability to:
· execute our business model; · create brand recognition; · manage growth in our operations; · create a customer base in a cost-effective manner; · retain customers; · access additional capital when required; and · attract and retain key personnel.
There can be no assurance that our business model will be successful or that it will successfully address these and other challenges, risks and uncertainties.
We will need additional funding in the future, and if we are unable to raise capital when needed, we may be forced to delay, reduce or eliminate our product candidate development programs, commercial efforts, or sales efforts.
Developing products and methods and procedures of treatment and marketing developed products is costly. We will need to raise substantial additional capital in the future in order to execute our business plan and help us and our collaboration partners fund the development and commercialization of our product candidates.
| 19 |
In 2015, we raised funds primarily through convertible debt financing, and in 2014, we raised funds through a public equity offering. We may need to finance future cash needs through public or private equity offerings, debt financings or strategic collaboration and licensing arrangements. To the extent that we raise additional funds by issuing equity securities, our stockholders may experience additional dilution, and debt financing, if available, may involve restrictive covenants and may result in high interest expense. If we raise additional funds through collaboration and licensing arrangements, it may be necessary to relinquish some rights to our product candidates, processes and technologies or our development projects or to grant licenses on terms that are not favorable to us. We cannot be certain that additional funding will be available on acceptable terms, or at all. If adequate funds are not available from the foregoing sources, we may consider additional strategic financing options, including sales of assets, or we may be required to delay, reduce the scope of, or eliminate one or more of our research or development programs or curtail some of our commercialization efforts of our operations. We may seek to access the public or private equity markets whenever conditions are favorable, even if we do not have an immediate need for additional capital.
We do not own our technologies, they are owned by, and licensed from, two entities that are under the control of the Chairman of our Board of Directors.
We do not currently own the technologies necessary to conduct our operations. The patents necessary to pursue our intended business plan are under the control of our Chairman of the Board of Directors. As consideration for the two licenses, we agreed to (i) pay a royalty of five percent (5%) of any sales of products using the technology, with no minimum royalty and (ii) reimburse the licensor for any costs incurred in pursuing its proprietary rights in the licensed technology and pay any costs incurred for maintaining or obtaining the licensors' proprietary rights in the licensed technology in the U.S. and in extending the intellectual property to other countries around the world. The licensor has the sole discretion to select other countries into which exclusive rights in the licensed technology may be pursued, and if we decline to pay those expenses, then the licensor may pay said expenses and our licensed rights in those countries will revert to the licensor. The license agreements contain provisions that require us to indemnify the licensor for any claims, including costs of litigation, brought against them related to the licenses, and require us to maintain insurance that may be burdensome. In the event of a breach of our obligations under the license agreements, the licensors are entitled to various damages and remedies, up to and including termination of said license agreements. The licensors are entities under the control of Dr. Mitchell S. Felder, the Chairman of our Board of Directors. While Dr. Felder is one of our Company's founders and the Chairman of our Board of Directors, there can be no assurance that he will extend the offer to license these technologies to us in the future as currently contemplated.
We do not intend to take our FeldetrexÒ product candidate past the development stage, but instead intend to enter into collaboration agreements with collaboration partners. If we are unable to enter into an agreement with collaboration partners, our FeldetrexÒ product candidate cannot be marketed, and it will not generate revenue for us.
We do not intend to conduct clinical trials on our FeldetrexÒ product candidate. We instead intend to enter into one or more collaboration agreements with third parties to do so. However, we have not entered into any such agreements, or discussions for any such agreements, and we cannot guarantee that we will be successful in doing so. If we do not find a collaboration partner, the FeldetrexÒ product candidate cannot be marketed, and it will not generate any revenue for us.
| 20 |
The failure to generate revenue from our FeldetrexÒ product candidate will have a materially adverse effect on our overall revenues, profitability, and we may not be able to continue operations.
If an individual used any of our product candidates prior to their approval by the FDA or other regulatory authority, we face risks of unforeseen medical problems, and up to a complete ban on the sale of our product candidates.
The efficacy and safety of pharmaceutical products is established through a process of clinical testing under Federal Drug Administration ("FDA") oversight. No individual is authorized or should use any of our products outside of an established process of clinical testing and FDA approval and authorization. If an individual were to use one of our product candidates in such a manner, we cannot predict the potential medical harm to that individual. If such an event were to occur, the FDA or similar regulatory agency might impose a complete ban on the sale or use of our candidate products.
The FDA might not approve our product candidates for marketing and sale.
We intend to enter into agreements with larger pharmaceutical companies as collaboration partners, in part to help cover the cost of seeking regulatory approvals. We believe that FDA approval of some of our product candidates will need to undergo a full investigational new drug (IND) application with the FDA, including clinical trials. There can be no assurance that the FDA will approve our IND application or any other applications. Failure to obtain the necessary FDA approval will have a material negative affect on our operations. While we intend to license our FeldetrexÒ product to a larger pharmaceutical company, they in turn, may not be able to obtain the necessary approval to market and sale the product.
We may fail to deliver commercially successful new product candidates, methods and procedures of treatment, and treatments.
Our technology is at an early stage of research and development. As of the date hereof, we have partnered with the University of Texas at El Paso (UTEP), and protocol for animal testing procedures is being developed in conjunction with UTEP at this time. Subsequent clinical trials on human patients will be coordinated with our development partners in the near future. We have also entered into a Cooperative Research and Development Agreement (the "CRADA") with the Clinical Investigation Regulatory Office U.S. Army Medical Research and Material Command ("CIRO") for performing medical research, development, testing and evaluation. Pursuant to the CRADA, we will collaborate with the U.S. Army Medical Research Material Command at the William Beaumont Army Medical Center ("WBAMC") on the "Clearance of Specific Immunomodulators from Cerebrospinal Fluid via Selective Dialysis," and more specifically, targeting the prevention of suicidal ideation and clinical depression, and assisting in the creation of antibodies in order to obtain a decrease in the neuropathologic findings in traumatic brain injuries.
| 21 |
The development of commercially viable new products and methods and procedures of treatment, as well as the development of additional uses for existing products and methods and procedures of treatment, is critical to our ability to generate sales and/or sell the rights to manufacture and distribute our product and process candidates to another firm. Developing new products and methods and procedures of treatment is a costly, lengthy and uncertain process. A new product or process candidate can fail at any stage of the development or commercialization, and one or more late-stage product or process candidates could fail to receive regulatory approval.
New product and process candidates may appear promising in development, but after significant investment, fail to reach the market or have only limited commercial success. This, for example, could be as a result of efficacy or safety concerns, inability to obtain necessary regulatory approvals, difficulty or excessive costs to manufacture, erosion of patent term as a result of a lengthy development period, infringement of third-party patents or other intellectual property rights of others or inability to differentiate the product or process adequately from those with which it competes.
The commercialization of product and process candidates under development may not be profitable.
In order for the commercialization of our product candidates to be profitable, our product and process candidates must be cost-effective and economical to manufacture on a commercial scale. Furthermore, if our product candidates and methods and procedures of treatment do not achieve market acceptance, we may not be profitable. Subject to regulatory approval, we expect to incur significant development, sales and marketing expenses in connection with the commercialization of our new product and process candidates. Even if we receive additional financing, we may not be able to complete planned development and marketing of any or all of our product or process candidates. Our future profitability may depend on many factors, including, but not limited to:
· the terms and timing of any collaborative, licensing and other arrangements that we may establish; · the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; · the costs of establishing manufacturing and production, sales, marketing and distribution capabilities; and · the effect of competing technological and market developments.
Even if our collaboration partners receive regulatory approval for our product and process candidates, we may not earn significant revenues from such product or process candidates. With respect to the product and methods and procedures of treatment candidates in our development pipeline that are being developed by or in close conjunction with third parties, our ability to generate revenues from such product and process candidates will depend in large part on the efforts of such third parties. To the extent that our collaboration partners are not successful in commercializing our product or process candidates, our revenues will suffer, we will incur significant additional losses and the price of our common stock will be negatively affected.
| 22 |
We may engage in strategic transactions that fail to enhance stockholder value.
From time to time, we may consider possible strategic transactions, including the potential acquisitions or licensing of products or technologies or acquisition of companies, and other alternatives with the goal of maximizing stockholder value. We may never complete a strategic transaction, and in the event that we do complete a strategic transaction, implementation of such transactions may impair stockholder value or otherwise adversely affect our business. Any such transaction may require us to incur non-recurring or other charges and may pose significant integration challenges and/or management and business disruptions, any of which could harm our results of operation and business prospects.
Our business is heavily regulated by governmental authorities, and failure to comply with such regulation or changes in such regulations could negatively impact our financial results.
We must comply with a broad range of regulatory controls on the testing, approval, manufacturing and marketing of our product candidates, procedures and other treatments, particularly in the United States and countries of the European Union, that affect not only the cost of product development but also the time required to reach the market and the uncertainty of successfully doing so. Health authorities have increased their focus on safety when assessing the benefit risk/balance of drugs in the context of not only initial product approval but also in the context of approval of additional indications and review of information regarding marketed products. Stricter regulatory controls also heighten the risk of changes in product profile or withdrawal by regulators on the basis of post-approval concerns over product safety, which could reduce revenues and can result in product recalls and product liability lawsuits. There is also greater regulatory scrutiny, especially in the United States, on advertising and promotion and in particular on direct-to-consumer advertising.
The regulatory process is uncertain, can take many years, and requires the expenditure of substantial resources. In particular, proposed human pharmaceutical therapeutic product requirements set by the FDA in the United States, and similar health authorities in other countries, require substantial time and resources to satisfy. We may never obtain regulatory approval for our product and process candidates.
We may not be able to gain or sustain market acceptance for our services and product candidates.
Failure to establish a brand and presence in the marketplace on a timely basis could adversely affect our financial condition and results of operations. Moreover, there can be no assurance that we will successfully complete our development and introduction of new products or product enhancements, or methods and procedures of treatment or that any such product candidates or methods and procedures of treatment will achieve acceptance in the marketplace. We may also fail to develop and deploy new products and product enhancements on a timely basis.
| 23 |
The market for products, methods and procedures of treatment and services in the pharmaceuticals industry is highly competitive, and we may not be able to compete successfully.
We intend to operate in highly competitive markets. We will likely face competition both from proprietary products of large international manufacturers and producers of generic pharmaceuticals. Most of the competitors in the industry have longer operating histories and significantly greater financial, technical, marketing and other resources than us, and may be able to respond more quickly than we can to new or changing opportunities and customer requirements. Also, many competitors have greater name recognition and more extensive customer bases that they can leverage to gain market share. Such competitors are able to undertake more extensive promotional activities, adopt more aggressive pricing policies and offer more attractive terms to purchasers than we can.
Significant product innovations, technical advances or the intensification of price competition by competitors could adversely affect our operating results. We cannot predict the timing or impact of competitive products or their potential impact on sales of our product candidates.
If any of our major product candidates or methods and procedures of treatment were to become subject to a problem such as unplanned loss of patent protection, unexpected side effects, regulatory proceedings, publicity affecting doctor or patient confidence or pressure from competitive products and methods and procedures of treatment, or if a new, more effective treatment should be introduced, the adverse impact on our revenues and operating results could be significant.
We are dependent on the services of key personnel and failure to attract qualified management could limit our growth and negatively impact our results of operations.
We are highly dependent on the principal members of our management and scientific staff and certain key consultants, including our Chief Executive Officer and the Chairman of our Board of Directors. We will continue to depend on operations management personnel with pharmaceutical and scientific industry experience. At this time, we do not know of the availability of such experienced management personnel or how much it may cost to attract and retain such personnel. The loss of the services of any member of senior management or the inability to hire experienced operations management personnel could have a material adverse effect on our financial condition and results of operations.
If physicians and patients do not accept our current or future product candidates or methods and procedures of treatment, we may be unable to generate significant additional revenue, if any.
The products and methods and procedures of treatment that we may develop or acquire in the future may fail to gain market acceptance among physicians, health care payors, patients and the medical community. Physicians may elect not to recommend these treatments for a variety of reasons, including:
· timing of market introduction of competitive drugs; · lower demonstrated clinical safety and efficacy compared to other drugs or treatments; · lack of cost-effectiveness; · lack of availability of reimbursement from managed care plans and other third-party payors; · lack of convenience or ease of administration; · prevalence and severity of adverse side effects; · other potential advantages of alternative treatment methods; and · ineffective marketing and distribution support.
| 24 |
If our product candidates and processes fail to achieve market acceptance, we would not be able to generate significant revenue.
We are exposed to the risk of liability claims, for which we may not have adequate insurance.
Since we participate in the pharmaceutical industry, we may be subject to liability claims by employees, customers, end users and third parties. We do not currently have product liability insurance. We intend to have proper insurance in place; however, there can be no assurance that any liability insurance we purchase will be adequate to cover claims asserted against us or that we will be able to maintain such insurance in the future. We intend to adopt prudent risk management programs to reduce these risks and potential liabilities; however, we have not taken any steps to create these programs and have no estimate as to the cost or time required to do so and there can be no assurance that such programs, if and when adopted, will fully protect us. We may not be able to put risk management programs in place, or obtain insurance, if we are unable to retain the necessary expertise and/or are unsuccessful in raising necessary capital in the future. Adverse rulings in any legal matters, proceedings and other matters could have a material adverse effect on our business.
Pre-clinical and clinical trials are conducted during the development of potential products and other treatments to determine their safety and efficacy for use by humans. Notwithstanding these efforts, when our treatments are introduced into the marketplace, unanticipated side effects may become evident. Manufacturing, marketing, selling and testing our product candidates under development or to be acquired or licensed, entails a risk of product liability claims. We could be subject to product liability claims in the event that our product candidates, processes, or products under development fail to perform as intended. Even unsuccessful claims could result in the expenditure of funds in litigation and the diversion of management time and resources, and could damage our reputation and impair the marketability of our product candidates and processes. While we plan to maintain liability insurance for product liability claims, we may not be able to obtain or maintain such insurance at a commercially reasonable cost. If a successful claim were made against us, and we don't have insurance or the amount of insurance was inadequate to cover the costs of defending against or paying such a claim or the damages payable by us, we would experience a material adverse effect on our business, financial condition and results of operations.
Other companies may claim that we have infringed upon their intellectual property or proprietary rights.
We do not believe that our product candidates and methods and procedures violate third-party intellectual property rights; however, we have not had an independent party conduct a study of possible patent infringements. Nevertheless, we cannot guarantee that claims relating to violation of such rights will not be asserted by third parties. If any of our product candidates or methods and procedures of treatment are found to violate third-party intellectual property rights, we may be required to expend significant funds to re-engineer or cause to be re-engineered one or more of those product candidates or methods and procedures of treatment to avoid infringement, or seek to obtain licenses from third parties to continue offering our product candidates or methods and procedures of treatment without substantial re-engineering, and such efforts may not be successful.
| 25 |
In addition, future patents may be issued to third parties upon which our product candidates and methods and procedures of treatment may infringe. We may incur substantial costs in defending against claims under any such patents. Furthermore, parties making such claims may be able to obtain injunctive or other equitable relief, which effectively could block our ability to further develop or commercialize some or all of our products or methods and procedures of treatment in the United States or abroad, and could result in the award of substantial damages against us. In the event of a claim of infringement, we may be required to obtain one or more licenses from third parties. There can be no assurance that we will be able to obtain such licenses at a reasonable cost, if at all. Defense of any lawsuit or failure to obtain any such license could be costly and have a material adverse effect on our business.
Our success depends on our ability to protect our proprietary technology.
Our success depends, to a significant degree, upon the protection of our proprietary technology, and that of any licensors. Legal fees and other expenses necessary to obtain and maintain appropriate patent protection could be material. Insufficient funding may inhibit our ability to obtain and maintain such protection. Additionally, if we must resort to legal proceedings to enforce our intellectual property rights, the proceedings could be burdensome and expensive, and could involve a high degree of risk to our proprietary rights if we are unsuccessful in, or cannot afford to pursue, such proceedings.
Our licensors have been granted three U.S. patents: Sequential Extracorporeal Treatment of Bodily Fluids, U.S. Patent No. 9,216,386; Utilization of Stents for the Treatment of Blood Borne Carcinomas, U.S. Patent No. 8,758,287; and Medication and Treatment for Disease, U.S. Patent No. 8,865,733, in the areas of cancer, sepsis, and multiple sclerosis. We expect these patents to cover the medical treatments for Multiple Sclerosis, Blood Sepsis, and Cancer and be effective until 2029. Our licensors have licensed these technologies to us pursuant to the terms of the license agreements. We anticipate that other technologies that derive from these patents will also belong to us and are covered by the license agreements. However, we have not conducted thorough prior art or novelty studies, but we are not aware of existing prior art that would prevent us from obtaining patents on our product candidates or methods and procedures of treatment. Prior art preventing us from obtaining broad patent protection is a possibility. Inability to obtain valid and enforceable patent protection would have a material negative impact on our business opportunities and success. Because the patent positions of pharmaceutical and biotechnology companies are highly uncertain and involve complex legal and factual questions, the patents may not be granted on our applications, and any future patents owned and licensed by us may not prevent other companies from developing competing products or ensure that others will not be issued patents that may prevent the sale of our products or require licensing and the payment of significant fees or royalties. Furthermore, to the extent that: (i) any of our future products or methods are not patentable; (ii) such products or methods infringe upon the patents of third parties; or (iii) our patents or future patents fail to give us an exclusive position in the subject matter to which such patents relate, our business will be adversely affected. We may be unable to avoid infringement of third-party patents and may have to obtain a license, or defend an infringement action and challenge the validity of such patents in court. A license may be unavailable on terms and conditions acceptable to us, if at all. Patent litigation is costly and time consuming, and we may be unable to prevail in any such patent litigation or devote sufficient resources to even pursue such litigation. If we do not obtain a license under such patents, are found liable for infringement and are not able to have such patents declared invalid, we may be liable for significant monetary damages, encounter significant delays in bringing products to market or may be precluded from participating in the manufacture, use or sale of products or methods of treatment requiring such licenses.
| 26 |
We may also rely on trademarks, trade secrets and contract law to protect certain of our proprietary technology. There can be no assurance that any trademarks will be approved, that such contract will not be breached, or that if breached, we will have adequate remedies. Furthermore, there can be no assurance that any of our trade secrets will not become known or independently discovered by third parties.
Additionally, we may, from time to time, support and collaborate in research conducted by universities and governmental research organizations. There can be no assurance that we will have or be able to acquire title or exclusive rights to the inventions or technical information derived from such collaborations, or that disputes will not arise with respect to rights in derivative or related research programs conducted by us or such collaborators.
Our future growth may be inhibited by the failure to implement new technologies.
Our future growth is partially tied to our ability to improve our knowledge and implementation of medical and pharmaceutical technologies. The inability to successfully implement commercially viable medical and pharmaceutical technologies in response to market conditions in a manner that is responsive to our customers' requirements could have a material adverse effect on our business.
Risks Related To Our Common Stock
The market price of our common stock may be volatile and may be affected by market conditions beyond our control.
The market price of our common stock is subject to significant fluctuations in response to, among other factors:
· variations in our operating results and market conditions specific to Biomedical Industry companies; · changes in financial estimates or recommendations by securities analysts; · announcements of innovations or new products or services by us or our competitors; · the emergence of new competitors; · operating and market price performance of other companies that investors deem comparable; · changes in our board or management; · sales or purchases of our common stock by insiders; · commencement of, or involvement in, litigation; · changes in governmental regulations; and · general economic conditions and slow or negative growth of related markets.
In addition, if the market for stocks in our industry or the stock market in general, experiences a loss of investor confidence, the market price of our common stock could decline for reasons unrelated to our business, financial condition or results of operations. If any of the foregoing occurs, it could cause the price of our common stock to fall and may expose us to lawsuits that, even if unsuccessful, could be costly to defend and a distraction to the board of directors and management.
| 27 |
If we are unable to pay the costs associated with being a public, reporting company, we may not be able to continue trading on the OTCQB and/or we may be forced to discontinue operations.
Our common stock is listed for trading on the OTCQB. We expect to have significant costs associated with being a public, reporting company, which may raise substantial doubt about our ability to continue trading on the OTCQB and/or continue as a going concern. Our ability to continue trading on the OTCQB and/or continue as a going concern will depend on positive cash flow, if any, from future operations and on our ability to raise additional funds through equity or debt financing. If we are unable to achieve the necessary product sales or raise or obtain needed funding to cover the costs of operating as a public, reporting company, our common stock may be deleted from the OTCQB and/or we may be forced to discontinue operations.
Our common stock is listed for quotation on the OTCQB tier of the marketplace maintained by OTC Markets Group, Inc., which may make it more difficult for investors to resell their shares due to suitability requirements.
Our common stock is currently quoted on the OTCQB tier of the marketplace maintained by OTC Markets Group, Inc. Broker-dealers often decline to trade in over-the-counter stocks given the market for such securities are often limited, the stocks are more volatile, and the risk to investors is greater. These factors may reduce the potential market for our common stock by reducing the number of potential investors. This may make it more difficult for investors in our common stock to sell shares to third parties or to otherwise dispose of their shares. This could cause our stock price to decline.
Our principal stockholders have the ability to exert significant control in matters requiring stockholder approval and could delay, deter, or prevent a change in control of our company.
William A. Hartman and Dr. Mitchell S. Felder collectively own 9,453,944 shares of our outstanding common stock, 2,000,000 shares of our Series A Convertible Preferred Stock (which is convertible into an aggregate of 2,000,000 shares of our common stock), and through the exercise of warrants could acquire another 30,000,000 shares of our common stock. The shares of our Series A Convertible Preferred Stock have 100 votes per share, giving these two shareholders the vast majority of our current voting securities. As a result, they have the ability to influence matters affecting our shareholders, including the election of our directors, the acquisition or disposition of our assets, and the future issuance of our shares. Because they control such shares, investors may find it difficult to replace our management if they disagree with the way our business is being operated. Because the influence by these shareholders could result in management making decisions that are in the best interest of those shareholders and not in the best interest of the investors, you may lose some or all of the value of your investment in our common stock. Investors who purchase our common stock should be willing to entrust all aspects of operational control to our current management team.
We do not intend to pay dividends in the foreseeable future.
We do not intend to pay any dividends in the foreseeable future. We do not plan on making any cash distributions in the manner of a dividend or otherwise. Our Board presently intends to follow a policy of retaining earnings, if any.
| 28 |
We have outstanding convertible debt, which, if repaid will require a significant amount of capital, or if converted into our common stock could have a material adverse effect on our stock price.
As of March 24, 2016, we had six convertible notes outstanding with a cumulative outstanding principal balance of $593,050. Repayment of the notes must be done at a premium to the then-outstanding balance, resulting in the need for approximately $609,000 in liquid capital. If, rather than repay these notes, we allow them to convert into our common stock, which conversions would be done at a discount to the market price of our common stock, resulting in the issuance of approximately 69,684,000 shares of our common stock (based on a conversion price of $0.0087 per share), all of which could be sold into the open market at the time of conversion. The potential dilutive effects of these conversions at various conversion prices below our most recent market price of $0.0174 per share is as follows:
| 100% |
| 75% |
| 50% |
| 25% | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
| $ | 0.0174 |
|
| $ | 0.0131 |
|
| $ | 0.0087 |
|
| $ | 0.0044 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Potential dilutive shares |
|
| 34,841,954 |
|
|
| 46,455,939 |
|
|
| 69,683,908 |
|
|
| 139,367,816 |
|
We have the right to issue additional common stock and preferred stock without consent of stockholders. This would have the effect of diluting investors' ownership and could decrease the value of their investment.
We are authorized to issue up to 1,000,000,000 shares of common stock, of which there are currently 89,579,908 shares issued and outstanding, and up to an additional 47,841,455 of which may be issued and outstanding if all of our currently outstanding preferred stock and warrants were exercised and converted into common stock. We therefore have up to an additional 862,578,637 authorized but unissued shares of our common stock that may be issued by us for any purpose without the consent or vote of our stockholders that would dilute stockholders' percentage ownership of our company.
In addition, our certificate of incorporation authorizes the issuance of shares of preferred stock, the rights, preferences, designations and limitations of which may be set by the Board of Directors. Our certificate of incorporation has authorized the issuance of up to 10,000,000 shares of preferred stock in the discretion of our Board. The shares of authorized but undesignated preferred stock may be issued upon filing of an amended certificate of incorporation and the payment of required fees; no further stockholder action is required. If issued, the rights, preferences, designations and limitations of such preferred stock would be set by our Board and could operate to the disadvantage of the outstanding common stock. Such terms could include, among others, preferences as to dividends and distributions on liquidation.
Our officers and directors can sell some of their stock, which may have a negative effect on our stock price and ability to raise additional capital, and may make it difficult for investors to sell their stock at any price.
Our officers and directors, as a group, are the beneficial owners of 12,529,944 shares of our common stock, representing nearly 14% of our total issued shares, with options and warrants to purchase another 43,245,000 shares of our common stock, representing approximately an additional 28%. Each individual officer and director may be able to sell up to 1% of our outstanding stock (currently approximately 895,799 shares) every 90 days in the open market pursuant to Rule 144, which may have a negative effect on our stock price and may prevent us from obtaining additional capital. In addition, if our officers and directors are selling their stock into the open market, it may make it difficult or impossible for investors to sell their stock at any price.
| 29 |
Our common stock is governed under The Securities Enforcement and Penny Stock Reform Act of 1990.
The Securities Enforcement and Penny Stock Reform Act of 1990 requires additional disclosure relating to the market for penny stocks in connection with trades in any stock defined as a penny stock. The Commission has adopted regulations that generally define a penny stock to be any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. Such exceptions include any equity security listed on NASDAQ and any equity security issued by an issuer that has (i) net tangible assets of at least $2,000,000, if such issuer has been in continuous operation for three years, (ii) net tangible assets of at least $5,000,000, if such issuer has been in continuous operation for less than three years, or (iii) average annual revenue of at least $6,000,000, if such issuer has been in continuous operation for less than three years. Unless an exception is available, the regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the risks associated therewith.
The forward looking statements contained in this annual report may prove incorrect.
This Annual Report contains certain forward-looking statements, including among others: (i) anticipated trends in our financial condition and results of operations; (ii) our business strategy for expanding distribution; and (iii) our ability to distinguish ourselves from our current and future competitors. These forward-looking statements are based largely on our current expectations and are subject to a number of risks and uncertainties. Actual results could differ materially from these forward-looking statements. In addition to the other risks described elsewhere in this "Risk Factors" discussion, important factors to consider in evaluating such forward-looking statements include: (i) changes to external competitive market factors or in our internal budgeting process which might impact trends in our results of operations; (ii) anticipated working capital or other cash requirements; (iii) changes in our business strategy or an inability to execute our strategy due to unanticipated changes in the biotechnology industry; and (iv) various competitive factors that may prevent us from competing successfully in the marketplace. In light of these risks and uncertainties, many of which are described in greater detail elsewhere in this "Risk Factors" discussion, there can be no assurance that the events predicted in forward-looking statements contained in this annual report will, in fact, transpire.
SPECIAL NOTE ABOUT FORWARD-LOOKING STATEMENTS
We have made forward-looking statements in this Annual Report, including the sections entitled "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Business," that are based on our management's beliefs and assumptions and on information currently available to our management. Forward-looking statements include the information concerning our possible or assumed future results of operations, business strategies, financing plans, competitive position, industry environment, potential growth opportunities, the effects of future regulation, and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by the use of forward-looking terminology such as the words "believe," "expect," "anticipate," "intend," "plan," "estimate" or similar expressions. These statements are only predictions and involve known and unknown risks and uncertainties, including the risks outlined under "Risk Factors" and elsewhere in this annual report.
| 30 |
Although we believe that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee future results, events, levels of activity, performance or achievement. We are not under any duty to update any of the forward-looking statements after the date of this annual report to conform these statements to actual results, unless required by law.
ITEM 1B – UNRESOLVED STAFF COMMENTS
This Item is not applicable to us as we are not an accelerated filer, a large accelerated filer, or a well-seasoned issuer; however, we are voluntarily disclosing that we have not received any written comments from the Commission staff within the 180 days before the end of our fiscal year to which this Annual Report relates regarding our periodic or current reports under the Securities Exchange Act of 1934.
ITEM 2 – PROPERTIES
We do not currently lease or use any office space. We have not paid any amounts to Mr. Hartman for the use of his personal office or for reimbursement of personal office expenses incurred by him.
ITEM 3 – LEGAL PROCEEDINGS
We are not a party to or otherwise involved in any legal proceedings.
In the ordinary course of business, we are from time to time involved in various pending or threatened legal actions. The litigation process is inherently uncertain and it is possible that the resolution of such matters might have a material adverse effect upon our financial condition and/or results of operations. However, in the opinion of our management, other than as set forth herein, matters currently pending or threatened against us are not expected to have a material adverse effect on our financial position or results of operations.
ITEM 4 – MINE SAFETY DISCLOSURES
Not applicable.
| 31 |
PART II
ITEM 5 MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock is quoted on the OTCQB tier of the marketplace maintained by OTC Markets Group, Inc. under the symbol "BIEI." Our stock has traded there since February 23, 2012. Our common stock trades on a limited or sporadic basis and should not be deemed to constitute an established public trading market. There is no assurance that there will be liquidity in the common stock.
The following table sets forth the high and low transaction price for each quarter within the fiscal years ended December 31, 2015 and 2014, as provided by OTC Markets Group, Inc. The information reflects prices between dealers, and does not include retail markup, markdown, or commission, and may not represent actual transactions.
| Fiscal Year Ended |
|
|
| Transaction Prices | ||||||
| December 31, |
| Period |
| High |
|
| Low |
| ||
|
|
|
|
|
|
|
|
|
|
|
2016 |
| First Quarter (through March 11, 2016) |
| $ | 0.0410 |
|
| $ | 0.0110 |
|
|
|
|
|
|
|
|
|
|
| |
2015 |
| Fourth Quarter |
| $ | 0.0529 |
|
| $ | 0.0027 |
|
| Third Quarter |
| $ | 0.1200 |
|
| $ | 0.0070 |
| |
| Second Quarter |
| $ | 0.1400 |
|
| $ | 0.1000 |
| |
| First Quarter |
| $ | 0.2250 |
|
| $ | 0.1000 |
| |
|
|
|
|
|
|
|
|
|
| |
2014 |
| Fourth Quarter |
| $ | 0.3300 |
|
| $ | 0.0600 |
|
| Third Quarter |
| $ | 0.4000 |
|
| $ | 0.1000 |
| |
| Second Quarter |
| $ | 0.8500 |
|
| $ | 0.3000 |
| |
| First Quarter |
| $ | 2.6000 |
|
| $ | 0.5000 |
| |
The Securities Enforcement and Penny Stock Reform Act of 1990 requires additional disclosure relating to the market for penny stocks in connection with trades in any stock defined as a penny stock. The Commission has adopted regulations that generally define a penny stock to be any equity security that has a market price of less than $5.00 per share, subject to a few exceptions which we do not meet. Unless an exception is available, the regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the risks associated therewith.
Holders
As of March 14, 2016, there were 89,579,908 shares of our common stock issued and outstanding and held by 130 holders of record, not including shares held in "street name" in brokerage accounts which is unknown.
Dividend Policy
We have not paid any dividends on our common stock and do not expect to do so in the foreseeable future. We intend to apply our earnings, if any, in expanding our operations and related activities. The payment of cash dividends in the future will be at the discretion of the Board of Directors and will depend upon such factors as earnings levels, capital requirements, our financial condition and other factors deemed relevant by the Board of Directors.
| 32 |
Securities Authorized for Issuance under Equity Compensation Plans
We do not currently have a stock option or grant plan.
Recent Issuance of Unregistered Securities
The following sales of equity securities by the Company occurred during the three month period ended December 31, 2015:
Warrant Exercise
On October 1, 2015, we issued 3,000,000 shares of common stock, restricted in accordance with Rule 144, to Mitchell Felder, one of our directors, upon the exercise of warrants at $0.00001 per share. The issuance was exempt from the registration requirements of the Securities Act of 1933 pursuant to Section 4(a)(2) of the Securities Act of 1933. The purchaser was an accredited and sophisticated investor, familiar with our operations, and there was no solicitation.
Director Warrants
Effective as of October 21, 2015, we issued warrants to the following officers and directors, which will allow them to purchase shares of our common stock in the amounts indicated: William Hartman (1,000,000 shares); Mitchell Felder (1,000,000 shares), Heidi Carl (750,000 shares), John Borza (600,000 shares), Richard Najarian (200,000 shares), and Jay Rosen (200,000 shares). We also issued warrants to purchase a total of one million eight hundred thousand (1,800,000) shares of our common stock to six members of our Scientific Advisory Board. The exercise price of the foregoing warrants is Five Cents ($0.05) per share.
One half of the shares underlying each of the respective warrants vest on June 15, 2016, with the balance vesting on December 15, 2016. In order for the warrants to vest on each of the foregoing dates, however, the holders must be serving in the same capacity on behalf of the Company as he or she was serving on October 21, 2015. The issuance of the warrants was fully approved by our Board of Directors on October 21, 2015, the date a fully executed resolution authorizing the issuance was delivered to us. The issuances were exempt from registration pursuant to Section 4(a)(2) of the Securities Act of 1933, the investors are sophisticated and familiar with our operations, and there was no solicitation in connection with the issuance.
| 33 |
Redwood Management, LLC
On December 28, 2015, we entered into a Securities Purchase Agreement with Redwood Management, LLC ("Redwood"), pursuant to which we agreed to sell, and Redwood agreed to purchase, One Million Six Hundred Thousand Dollars ($1,600,000) in 10% Convertible Promissory Notes. On February 26, 2016, and again on March 7, 2016, the Securities Purchase Agreement was amended, and the total amount of funding to which Redwood is obligated was reduced to $525,000. The Notes have an original issue discount of five percent (5%). The first note was issued on December 28, 2015 in the face amount of One Hundred Fifty Seven Thousand Five Hundred Dollars ($157,500), to Redwood Management, LLC. The second note was issued on January 8, 2016, in the face amount of One Hundred Thirty One Thousand Two Hundred Fifty Dollars ($131,250), to Redwood Fund III Ltd. The third note was issued on February 22, 2016, in the face amount of Seventy Eight Thousand Seven Hundred Fifty Dollars ($78,750), to Redwood Management, LLC. The fourth note was issued on March 7, 2016, in the face amount of Seventy Eight Thousand Seven Hundred Fifty Dollars ($78,750), to Redwood Management, LLC. The fifth and final note was issued on March 11, 2016, in the face amount of One Hundred Five Thousand Dollars ($105,000). The maturity date of each note is nine (9) months after its issuance. Each note will be convertible after ninety (90) days into our common stock at a conversion price equal to 60% of the lowest traded price of the Common Stock in the fifteen (15) Trading Days prior to the Conversion Date. The shares of common stock issuable upon conversion of the notes will be restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The notes can be prepaid by us at any time upon ten (10) days written notice to Redwood for a cash amount equal to the sum of the then outstanding principal amount of the note and interest multiplied by 130%. Pursuant to a Registration Rights Agreement, we agreed to register the shares underlying conversion of the notes. The purchase and sale of the initial Note closed on December 28, 2015, the date that the purchase price was delivered to us.The issuance of the Note was exempt from registration pursuant to Rule 506 of Regulation D promulgated under the Securities Act of 1933. The purchaser was an accredited and sophisticated investor, familiar with our operations, and there was no solicitation.
ITEM 6 – SELECTED FINANCIAL DATA
As a smaller reporting company we are not required to provide the information required by this Item.
ITEM 7 – MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
Our Management's Discussion and Analysis contains not only statements that are historical facts, but also statements that are forward-looking (within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934). Forward-looking statements are, by their very nature, uncertain and risky. These risks and uncertainties include international, national and local general economic and market conditions; demographic changes; our ability to sustain, manage, or forecast growth; our ability to successfully make and integrate acquisitions; existing government regulations and changes in, or the failure to comply with, government regulations; adverse publicity; competition; fluctuations and difficulty in forecasting operating results; changes in business strategy or development plans; business disruptions; the ability to attract and retain qualified personnel; the ability to protect technology; and other risks that might be detailed from time to time in our filings with the Securities and Exchange Commission.
Although the forward-looking statements in this Annual Report reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by them. Consequently, and because forward-looking statements are inherently subject to risks and uncertainties, the actual results and outcomes may differ materially from the results and outcomes discussed in the forward-looking statements. You are urged to carefully review and consider the various disclosures made by us in this report and in our other reports as we attempt to advise interested parties of the risks and factors that may affect our business, financial condition, and results of operations and prospects.
| 34 |
Summary Overview
We are a research-based company that intends to discover and develop medical treatments for humans, specifically targeting the treatment of Cancer, Alzheimer's Disease (AD), Amyotrophic Lateral Sclerosis (ALS/Lou Gehrig's Disease), Blood Sepsis, Leukemia, and other life-threatening cancers, Multiple Sclerosis, Fibromyalgia, Neuropathic Pain and Traumatic Brain Injury.
We have not generated any revenue to date, and we do not currently have a product ready for market.
Going Concern
As a result of our financial condition, we have received a report from our independent registered public accounting firm for our financial statements for the years ended December 31, 2015 and 2014 that includes an explanatory paragraph describing the uncertainty as to our ability to continue as a going concern. In order to continue as a going concern we must effectively balance many factors and begin to generate revenue so that we can fund our operations from our sales and revenues. If we are not able to do this we may not be able to continue as an operating company. At our current revenue and burn rate, our cash on hand will last less than one month, and thus we must raise capital by issuing debt or through the sale of our stock. However, there is no assurance that our existing cash flow will be adequate to satisfy our existing operating expenses and capital requirements.
Results of Operations for the Year Ended December 31, 2015 and 2014
Introduction
We had no revenues for the years ended December 31, 2015 and 2014. Our operating expenses were $2,383,282 for the year ended December 31, 2015 compared to $1,368,932 for the year ended December 31, 2014, an increase of $1,014,350, or 74%. Our operating expenses consisted of research and development costs, general and administrative expenses, and professional fees as we incurred additional research and development costs and as we provided stock based compensation to our Officers and Directors.
Revenues and Net Operating Loss
Our revenues, operating expenses, and net operating loss for the years ended December 31, 2015 and 2014 were as follows:
| Year Ended |
|
| Year Ended |
|
|
|
| ||||
| December 31, |
|
| December 31, |
|
| Increase / |
| ||||
| 2015 |
|
| 2014 |
|
| (Decrease) |
| ||||
|
|
|
|
|
|
|
|
| ||||
Revenue |
| $ | - |
|
| $ | - |
|
| $ | - |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 234,095 |
|
|
| 196,179 |
|
|
| 37,916 |
|
General and administrative |
|
| 133,289 |
|
|
| 223,434 |
|
|
| (90,145 | ) |
Professional fees |
|
| 2,015,898 |
|
|
| 949,319 |
|
|
| 1,066,579 |
|
Total operating expenses |
|
| 2,383,282 |
|
|
| 1,368,932 |
|
|
| 1,014,350 |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net operating loss |
|
| (2,383,282 | ) |
|
| (1,368,932 | ) |
|
| 1,014,350 |
|
Other expense |
|
| (198,889 | ) |
|
| (41,582 | ) |
|
| 157,307 |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net loss |
| $ | (2,582,171 | ) |
| $ | (1,410,514 | ) |
| $ | 1,171,657 |
|
| 35 |
Revenues
The Company was established on May 10, 2010, and has had no revenues to date.
Research and Development
Research and development expenses were $234,095 for the year ended December 31, 2015 compared to $196,179 for the year ended December 31, 2014, an increase of $37,916, or 19%. The expenses were the continued research and development costs through our partners, specifically the University of Texas at El Paso, incurred during the year ended December 31, 2015 related to the development of our patented technologies, and increased slightly because we had asked them in 2014 to slow down their efforts when our cash flow would not permit it.
General and Administrative
General and administrative expenses were $133,289 for the year ended December 31, 2015 as compared to $223,434 for the year ended December 31, 2014, a decrease of $90,145, or 40%. The decrease was primarily due to a reduction in the compensation related to stock issuances to Directors incurred during the year ended December 31, 2015 that was not incurred during the comparative year ended December 31, 2014.
Professional Fees
Professional fees expense was $2,015,898 for the year ended December 31, 2015 compared to $949,319 for the year ended December 31, 2014, an increase of $1,066,579, or 112%. The increase was primarily due to increased stock based compensation issued to consultants for services rendered.
Net Operating Loss
Net operating loss for the year ended December 31, 2015 was $2,383,282 compared to a net operating loss of $1,368,932 for the year ended December 31, 2014, an increase of $1,014,350, or 74%. Net operating loss increased, as set forth above, primarily due to increased stock based compensation issued to directors and consultants for services rendered.
Other Expense
Other expense for the year ended December 31, 2015 was $198,889 compared to $41,582 for the year ended December 31, 2014, an increase of $157,307, or 378%. Other expense for both years consisted of interest and finance charges on debt and equity financing.
| 36 |
Net Loss
Net loss for the year ended December 31, 2015 was $2,582,171, or $(0.08) per share, compared to a net loss of $1,410,514, or $(0.07) per share, for the year ended December 31, 2014, an increase of $1,171,657, or 83%. Net loss increased, as set forth above, primarily due to increased stock based compensation issued to directors and consultants for services rendered.
Liquidity and Capital Resources
Introduction
During the year ended December 31, 2015, because we did not generate any revenues, we had negative operating cash flows. Our cash on hand as of December 31, 2015 was $35,414, which was derived from the sale of convertible promissory notes to investors. Our monthly cash flow burn rate has decreased from approximately $53,000 in 2014 to approximately $39,000 in 2015. Although we have moderate short term cash needs, as our operating expenses increase we will face strong medium to long term cash needs. We anticipate that these needs will be satisfied through the issuance of debt or the sale of our securities until such time as our cash flows from operations will satisfy our cash flow needs.
Our cash, current assets, total assets, current liabilities, and total liabilities as of December 31, 2015 and December 31, 2014, respectively, are as follows:
| December 31, |
|
| December 31, |
|
| Change |
| ||||
|
|
|
|
|
|
|
|
| ||||
Cash |
| $ | 35,414 |
|
| $ | 102,599 |
|
| $ | (67,185 | ) |
Total Current Assets |
|
| 66,531 |
|
|
| 115,522 |
|
|
| (48,991 | ) |
Total Assets |
|
| 70,178 |
|
|
| 120,631 |
|
|
| (50,453 | ) |
Total Current Liabilities |
|
| 346,501 |
|
|
| 188,390 |
|
|
| 158,111 |
|
Total Liabilities |
| $ | 346,501 |
|
| $ | 188,390 |
|
| $ | 158,111 |
|
Our cash decreased by $67,185 as of December 31, 2015 as compared to December 31, 2014 because we incurred research and development costs in the fourth quarter of 2015 and weren't able to sell several convertible promissory notes. Our total current assets decreased by $48,991 primarily for the same reason. Our total assets decreased by $50,453 primarily for the same reasons.
Our current liabilities increased by $158,111 as of December 31, 2015 as compared to December 31, 2014 primarily due to an increase in accounts payable of $40,774, accounts payable to related parties of $29,369, accrued interest of $8,241, convertible notes payable of $49,727, and notes payable to related parties of $30,000. Our total liabilities increased by the same $158,111 for the same reasons.
In order to repay our obligations in full or in part when due, we will be required to raise significant capital from other sources. There is no assurance, however, that we will be successful in these efforts.
| 37 |
Cash Requirements
Our cash on hand as of December 31, 2015 was $35,414, which was derived from the sale of convertible promissory notes. Our monthly cash flow burn rate is approximately $39,000. Although we have moderate short term cash needs, as our operating expenses increase we will face strong medium to long term cash needs. We anticipate that these needs will be satisfied through the sale of our securities until such time as our cash flows from operations will satisfy our cash flow needs.
Sources and Uses of Cash
Operations
Our net cash used in operating activities for the years ended December 31, 2015 and 2014 was $462,225 and $639,077, respectively, a decrease of $176,852, or 28%. The decrease primarily consisted of our increased net loss from operations of $1,171,657, offset in part by an increase in amortization of debt discounts of $155,396, an increase of stock based compensation to related parties of $1,134,642, and an increase in accounts payable to related parties of $43,942.
Investments
Our net cash used in investing activities for the years ended December 31, 2015 and 2014 was zero and $2,374, respectively, a decrease of $2,374. The slight decrease reflected a lack of purchases of property and equipment in 2015 compared to 2014.
Financing
Our net cash provided by financing activities for the years ended December 31, 2015 and 2014 was $395,040 and $728,250, respectively, a decrease of $333,210, or 46%. The decrease was a result of an increase in proceeds from the sale of convertible notes payable of $290,000, from $75,000 to $365,000, and an increase in the proceeds from the sale of notes payable to related parties of $30,000, offset by a decrease in repayments in notes payable tor elated parties of $109,000 and a decrease in the proceeds from the sale of common stock of $762,250, to zero.
Critical Accounting Policies and Estimates
Nature of Business
Premier Biomedical, Inc. was incorporated in the state of Nevada on May 10, 2010. The Company was formed to develop and market medications and procedures that address several highly visible health issues. The Company will introduce these medications and procedures to large pharmaceutical firms via publication in medical journals and by direct contact.
These statements reflect all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for fair presentation of the information contained therein.
| 38 |
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
We maintain cash balances in non-interest-bearing accounts, which do not currently exceed federally insured limits. For the purpose of the statements of cash flows, all highly liquid investments with an original maturity of three months or less are considered to be cash equivalents.
Patent rights and applications
Patent rights and applications costs include the acquisition costs and costs incurred for the filing of patents. Patent rights and applications are amortized on a straight-line basis over the legal life of the patent rights beginning at the time the patents are approved. Patent costs for unsuccessful patent applications, or when reviews determine a future economic benefit is uncertain, are expensed when the application is terminated.
Fair Value of Financial Instruments
Under FASB ASC 820-10-05, the Financial Accounting Standards Board establishes a framework for measuring fair value in generally accepted accounting principles and expands disclosures about fair value measurements. This Statement reaffirms that fair value is the relevant measurement attribute. The adoption of this standard did not have a material effect on the Company's financial statements as reflected herein. The carrying amounts of cash, prepaid expenses and accrued expenses reported on the balance sheet are estimated by management to approximate fair value primarily due to the short term nature of the instruments. The Company had no items that required fair value measurement on a recurring basis.
Basic and Diluted Loss Per Share
The basic net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding. Diluted net loss per common share is computed by dividing the net loss adjusted on an "as if converted" basis, by the weighted average number of common shares outstanding plus potential dilutive securities. For the periods presented, potential dilutive securities had an anti-dilutive effect and were not included in the calculation of diluted net loss per common share.
| 39 |
Stock-Based Compensation
Under FASB ASC 718-10-30-2, all share-based payments to employees, including grants of employee stock options, to be recognized in the income statement based on their fair values. Pro forma disclosure is no longer an alternative. The Company's stock based compensation consisted of the following during the years ended December 31, 2015 and 2014, respectively:
| December 31, |
|
| December 31, |
| |||
| 2015 |
|
| 2014 |
| |||
|
|
|
|
|
| |||
Common stock issued for services |
| $ | - |
|
| $ | 263,079 |
|
Warrants issued for services |
|
| 319,986 |
|
|
| 96,767 |
|
Warrants issued for services, related parties |
|
| 1,529,182 |
|
|
| 394,540 |
|
Total stock based compensation |
| $ | 1,849,168 |
|
| $ | 754,386 |
|
Revenue Recognition
Sales on fixed price contracts are recorded when services are earned, the earnings process is complete or substantially complete, and the revenue is measurable and collectability is reasonably assured. Provisions for discounts and rebates to customers, estimated returns and allowances, and other adjustments are provided for in the same period the related sales are recorded. The Company defers any revenue from sales in which payment has been received, but the earnings process has not occurred. No sales have yet commenced.
Advertising and Promotion
All costs associated with advertising and promoting products are expensed as incurred. These expenses were $59,956 and $159,411 for the years ended December 31, 2015 and 2014, respectively.
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. A valuation allowance is provided for significant deferred tax assets when it is more likely than not, that such asset will not be recovered through future operations.
| 40 |
Uncertain Tax Positions
In accordance with ASC 740, "Income Taxes" ("ASC 740"), the Company recognizes the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be capable of withstanding examination by the taxing authorities based on the technical merits of the position. These standards prescribe a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. These standards also provide guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition.
Various taxing authorities periodically audit the Company's income tax returns. These audits include questions regarding the Company's tax filing positions, including the timing and amount of deductions and the allocation of income to various tax jurisdictions. In evaluating the exposures connected with these various tax filing positions, including state and local taxes, the Company records allowances for probable exposures. A number of years may elapse before a particular matter, for which an allowance has been established, is audited and fully resolved. The Company has not yet undergone an examination by any taxing authorities.
The assessment of the Company's tax position relies on the judgment of management to estimate the exposures associated with the Company's various filing positions.
Recently Issued Accounting Pronouncements
In January 2016, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") No. 2016-16, Financial Instruments - Overall (Subtopic 825-10), Recognition and Measurement of Financial Assets and Financial Liabilities. The provisions of the update require equity investments to be measured at fair value with changes in fair value recognized in net income. However, an entity may choose to measure equity investments that do not have readily determinable fair values at cost minus impairment. The update also simplifies the impairment assessment of equity investments without readily determinable fair values by requiring a qualitative assessment to identify impairment. It also eliminates the requirement to disclose the fair value of financial instruments measured at amortized cost for entities that are not public business entities, and eliminates the requirement for public business entities to disclose the methods and significant assumptions used to estimate the fair value for financial instruments measured at amortized cost on the balance sheet. ASU No. 2016-16 requires public business entities to use the exit price notion when measuring the fair value of financial instruments for disclosure purposes. It also requires an entity to present separately in other comprehensive income the portion of the total change in the fair value of a liability resulting from a change in the instrument-specific credit risk when the entity has elected to measure the liability at fair value in accordance with the fair value option for financial instruments. The update requires separate presentation of financial assets and financial liabilities by category and form on the balance sheet or the accompanying notes to the financial statements. In addition, the update clarifies that an entity should evaluate the need for a valuation allowance on a deferred tax asset related to available-for-sale securities in combination with the entity's other deferred tax assets. For public business entities, the amendments in the update are effective for fiscal years beginning after December 15, 2017, including interim periods. The adoption of this ASU is not expected to have a material impact on the Company's financial statements.
In November 2015, the FASB issued an ASU No. 2015-17, Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes ("ASU 2015-17"). Under current GAAP, deferred income tax assets and liabilities are separated into current and noncurrent amounts in the balance sheet. ASU 2015-17 requires all deferred assets and liabilities be classified as noncurrent in the balance sheet. The standard will be effective for periods beginning after December 15, 2016, including interim periods within that reporting period. The adoption of this ASU is not expected to have a material impact on the Company's financial statements.
| 41 |
In September 2015, the FASB issued ASU No. 2015-16, Business Combinations (Topic 805), Simplifying the Accounting for Measurement-Period Adjustments ("ASU 2015-16"), which require an acquiring Company to recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. GAAP requires that during the measurement period, the acquirer retrospectively adjust the provisional amounts recognized at the acquisition date with a corresponding adjustment to goodwill. Those adjustments are required when new information is obtained about facts and circumstances that existed as of the acquisition date that if known, would have affected the measurement of the amounts initially recorded. To simplify the accounting for adjustments made to provisional amounts recognized in a business combination, the amendments in the update eliminate the requirement to retrospectively account for those adjustments. This ASU is effective for public entities for fiscal years beginning after December 15, 2015, including interim periods within those years. Disclosure of the nature and reason for the change should be made in the first period, including interim periods, there is a measurement period adjustment.
In April 2015, the FASB issued ASU No. 2015-03, Interest–Imputation of Interest (Subtopic 835-30) ("ASU 2015-03"), which changes the presentation of debt issuance costs in financial statements. ASU 2015-03 requires an entity to present such costs in the balance sheet as a direct deduction from the related debt liability rather than as an asset. Amortization of the costs will continue to be reported as interest expense. For public business entities, ASU 2015-03 is effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. For all other entities, ASU 2015-03 is effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within fiscal years beginning after December 15, 2016. Early adoption is permitted for financial statements that have not been previously issued. The new guidance should be applied on a retrospective basis. The Company will adopt ASU 2015-03 on its balance sheets retrospectively during the interim period ending March 31, 2016.
In August 2014, the FASB issued ASU No. 2014-15, Presentation of Financial Statements - Going Concern: Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern ("ASU 2014-15"). This guidance clarifies that an entity's management should evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity's ability to continue as a going concern within one year after the date that the financial statements are issued. The amendments in this update are effective for annual reporting periods ending after December 15, 2016, and annual and interim periods thereafter, and early application is permitted. The Company is currently in the process of evaluating the impact of adoption of ASU 2014-15 on its financial statements.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers ("ASU 2014-09"), which supersedes the revenue recognition requirements in Topic 605, Revenue Recognition, including most industry-specific revenue recognition guidance throughout the Industry Topics of the Codification. The core principle of ASU 2014-09 is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration that is expected to be received for those goods or services. ASU 2014-09 is effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period, and early application is not permitted. ASU 2014-09 allows for either full retrospective or modified retrospective adoption. We do not expect the adoption of the new provisions to have a material impact on our financial condition or results of operations.
No other new accounting pronouncements, issued or effective during the year ended December 31, 2015, have had or are expected to have a significant impact on the Company's financial statements.
| 42 |
ITEM 7A – QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a smaller reporting company we are not required to provide the information required by this Item.
ITEM 8 – FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Report of Independent Registered Public Accounting Firm |
|
| F-1 |
|
|
|
|
| |
Balance Sheets as of December 31, 2015 and 2014 (Audited) |
|
| F-2 |
|
|
|
|
| |
Statements of Operations for the years ended December 31, 2015 and 2014 (Audited) |
|
| F-3 |
|
|
|
|
| |
Statement of Stockholders' Equity (Deficit) for the years ended December 31, 2015 and 2014 (Audited) |
|
| F-4 |
|
|
|
|
| |
Statements of Cash Flows for the years ended December 31, 2015 and 2014 (Audited) |
|
| F-5 |
|
|
|
|
| |
Notes to Financial Statements |
|
| F-6 to F-34 |
|
| 43 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
Premier Biomedical, Inc.
We have audited the accompanying balance sheets of Premier Biomedical, Inc. (the Company) as of December 31, 2015 and 2014, and the related statements of operations, changes in stockholders' deficit, and cash flows for the years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Premier Biomedical, Inc. as of December 31, 2015 and 2014, and the results of its operations, changes in stockholders' deficit and cash flows for the periods described above in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has insufficient working capital, which raises substantial doubt about its ability to continue as a going concern. Management's plans regarding those matters also are described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ M&K CPAS, PLLC
www.mkacpas.com
Houston, Texas
March 30, 2016
| 6F-1 |
PREMIER BIOMEDICAL, INC. | ||||||||
BALANCE SHEETS | ||||||||
|
|
|
|
|
| |||
| December 31, |
|
| December 31, |
| |||
| 2015 |
|
| 2014 |
| |||
| ||||||||
ASSETS | ||||||||
|
|
|
|
|
| |||
Current assets: |
|
|
|
|
|
| ||
Cash |
| $ | 35,414 |
|
| $ | 102,599 |
|
Prepaid expenses |
|
| 9,166 |
|
|
| 9,450 |
|
Loan origination costs |
|
| 21,951 |
|
|
| 3,473 |
|
Total current assets |
|
| 66,531 |
|
|
| 115,522 |
|
|
|
|
|
|
|
|
| |
Property and equipment, net |
|
| 3,647 |
|
|
| 5,109 |
|
|
|
|
|
|
|
|
| |
Total assets |
| $ | 70,178 |
|
| $ | 120,631 |
|
|
|
|
|
|
|
|
| |
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) | ||||||||
|
|
|
|
|
|
|
| |
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
| $ | 188,265 |
|
| $ | 147,491 |
|
Accounts payable, related parties |
|
| 54,668 |
|
|
| 25,299 |
|
Accrued interest |
|
| 7,792 |
|
|
| 721 |
|
Accrued interest, related parties |
|
| 1,170 |
|
|
| - |
|
Convertible notes payable, net of discounts of $223,394 and $71,621 at December 31, 2015 and 2014, respectively |
| 64,606 |
|
|
| 14,879 |
| |
Notes payable, related parties |
|
| 30,000 |
|
|
| - |
|
Total current liabilities |
|
| 346,501 |
|
|
| 188,390 |
|
|
|
|
|
|
|
|
| |
Total liabilities |
|
| 346,501 |
|
|
| 188,390 |
|
|
|
|
|
|
|
|
| |
Commitments and contingencies |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
| |
Stockholders' equity (deficit): |
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value, 10,000,000 shares authorized, no shares issued and outstanding |
| - |
|
|
| - |
| |
Common stock, $0.00001 par value, 1,000,000,000 shares authorized, 82,331,062 and 21,757,175 shares issued and outstanding at December 31, 2015 and 2014, respectively |
| 823 |
|
|
| 218 |
| |
Additional paid in capital |
|
| 10,500,651 |
|
|
| 8,127,649 |
|
Accumulated deficit |
|
| (10,777,797 | ) |
|
| (8,195,626 | ) |
Total stockholders' equity (deficit) |
|
| (276,323 | ) |
|
| (67,759 | ) |
|
|
|
|
|
|
|
| |
Total liabilities and stockholders' equity (deficit) |
| $ | 70,178 |
|
| $ | 120,631 |
|
The accompanying notes are an integral part of these financial statements.
F-2
PREMIER BIOMEDICAL, INC. | ||||||||
STATEMENTS OF OPERATIONS | ||||||||
|
|
|
|
|
| |||
For the Years | ||||||||
| 2015 |
|
| 2014 |
| |||
|
|
|
|
|
| |||
Revenue |
| $ | - |
|
| $ | - |
|
|
|
|
|
|
|
|
| |
Operating expenses: |
|
|
|
|
|
|
|
|
Research and development |
|
| 234,095 |
|
|
| 196,179 |
|
General and administrative |
|
| 133,289 |
|
|
| 223,434 |
|
Professional fees |
|
| 2,015,898 |
|
|
| 949,319 |
|
Total operating expenses |
|
| 2,383,282 |
|
|
| 1,368,932 |
|
|
|
|
|
|
|
|
| |
Net operating loss |
|
| (2,383,282 | ) |
|
| (1,368,932 | ) |
|
|
|
|
|
|
|
| |
Other expense: |
|
|
|
|
|
|
|
|
Interest expense |
|
| (198,889 | ) |
|
| (41,582 | ) |
Total other expenses |
|
| (198,889 | ) |
|
| (41,582 | ) |
|
|
|
|
|
|
|
| |
Net loss |
| $ | (2,582,171 | ) |
| $ | (1,410,514 | ) |
|
|
|
|
|
|
|
| |
Weighted average number of common shares outstanding - basic and fully diluted |
| 31,264,255 |
|
|
| 20,408,658 |
| |
|
|
|
|
|
|
|
| |
Net loss per share - basic and fully diluted |
| $ | (0.08 | ) |
| $ | (0.07 | ) |
The accompanying notes are an integral part of these financial statements.
| F-3 |
PREMIER BIOMEDICAL, INC. | ||||||||||||||||||||||||||||
STATEMENT OF STOCKHOLDERS' EQUITY (DEFICIT) | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| Additional Paid-In Capital |
|
|
|
|
| Total |
| ||||||||
| Preferred Stock |
|
| Common Stock |
|
|
|
| Accumulated Deficit |
|
| Stockholders' |
| |||||||||||||||
| Shares |
|
| Amount |
|
| Shares |
|
| Amount |
|
|
|
|
|
| Equity (Deficit) |
| ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
Balance, December 31, 2013 |
|
| - |
|
| $ | - |
|
|
| 18,355,819 |
|
| $ | 184 |
|
| $ | 6,535,913 |
|
| $ | (6,785,112 | ) |
| $ | (249,015 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Imputed interest on non-interest bearing related party debts |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 134 |
|
|
| - |
|
|
| 134 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Warrants granted as debt discount on convertible note |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 37,981 |
|
|
| - |
|
|
| 37,981 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Exercise of warrants at $0.75 per share |
|
| - |
|
|
| - |
|
|
| 15,000 |
|
|
| - |
|
|
| 11,250 |
|
|
| - |
|
|
| 11,250 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Common stock sold for cash to Kodiak Capital Group, LLC |
|
| - |
|
|
| - |
|
|
| 2,022,894 |
|
|
| 20 |
|
|
| 699,980 |
|
|
| - |
|
|
| 700,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Common stock sold for cash at $0.10 per share |
|
| - |
|
|
| - |
|
|
| 510,000 |
|
|
| 5 |
|
|
| 50,995 |
|
|
| - |
|
|
| 51,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Common stock issued for services |
|
| - |
|
|
| - |
|
|
| 853,462 |
|
|
| 9 |
|
|
| 263,070 |
|
|
| - |
|
|
| 263,079 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Amortization of warrants granted for services, related parties |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 394,540 |
|
|
| - |
|
|
| 394,540 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Amortization of warrants granted for services |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 96,767 |
|
|
| - |
|
|
| 96,767 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Beneficial conversion feature of convertible note |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 37,019 |
|
|
| - |
|
|
| 37,019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net loss for the year ended December 31, 2014 |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (1,410,514 | ) |
|
| (1,410,514 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2014 |
|
| - |
|
| $ | - |
|
|
| 21,757,175 |
|
| $ | 218 |
|
| $ | 8,127,649 |
|
| $ | (8,195,626 | ) |
| $ | (67,759 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Common stock issued on debt conversions |
|
| - |
|
|
| - |
|
|
| 56,723,887 |
|
|
| 567 |
|
|
| 226,821 |
|
|
| - |
|
|
| 227,388 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Exercise of warrants at $0.00001 per share, related party |
|
| - |
|
|
| - |
|
|
| 4,000,000 |
|
|
| 40 |
|
|
| - |
|
|
| - |
|
|
| 40 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Common stock cancelled |
|
| - |
|
|
| - |
|
|
| (150,000 | ) |
|
| (2 | ) |
|
| 2 |
|
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Amortization of warrants granted for services, related parties |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,529,182 |
|
|
| - |
|
|
| 1,529,182 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Amortization of warrants granted for services |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 319,986 |
|
|
| - |
|
|
| 319,986 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Beneficial conversion feature of convertible note |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 297,011 |
|
|
| - |
|
|
| 297,011 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Net loss for the year ended December 31, 2015 |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (2,582,171 | ) |
|
| (2,582,171 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Balance, December 31, 2015 |
|
| - |
|
| $ | - |
|
|
| 82,331,062 |
|
| $ | 823 |
|
| $ | 10,500,651 |
|
| $ | (10,777,797 | ) |
| $ | (276,323 | ) |
The accompanying notes are an integral part of these financial statements.
F-4
PREMIER BIOMEDICAL, INC. | ||||||||
STATEMENTS OF CASH FLOWS | ||||||||
|
|
|
|
|
| |||
For the Years | ||||||||
| 2015 |
|
| 2014 |
| |||
CASH FLOWS FROM OPERATING ACTIVITIES |
|
|
|
|
|
| ||
Net (loss) |
| $ | (2,582,171 | ) |
| $ | (1,410,514 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
| |
Depreciation |
|
| 1,462 |
|
|
| 954 |
|
Amortization of loan origination costs |
|
| 10,272 |
|
|
| 527 |
|
Imputed interest on non-interest bearing related party debts |
|
| - |
|
|
| 134 |
|
Amortization of debt discounts |
|
| 166,275 |
|
|
| 10,879 |
|
Stock based compensation, related parties |
|
| 1,529,182 |
|
|
| 394,540 |
|
Stock based compensation |
|
| 319,986 |
|
|
| 359,846 |
|
Decrease (increase) in assets: |
|
|
|
|
|
|
|
|
Prepaid expenses |
|
| 284 |
|
|
| (3,450 | ) |
Increase (decrease) in liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
| 40,774 |
|
|
| 25,157 |
|
Accounts payable, related parties |
|
| 29,369 |
|
|
| (14,573 | ) |
Accrued interest |
|
| 21,172 |
|
|
| (2,577 | ) |
Accrued interest, related parties |
|
| 1,170 |
|
|
| - |
|
Net cash used in operating activities |
|
| (462,225 | ) |
|
| (639,077 | ) |
|
|
|
|
|
|
|
| |
CASH FLOWS FROM INVESTING ACTIVITIES |
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
| - |
|
|
| (2,374 | ) |
Net cash used in investing activities |
|
| - |
|
|
| (2,374 | ) |
|
|
|
|
|
|
|
| |
CASH FLOWS FROM FINANCING ACTIVITIES |
|
|
|
|
|
|
|
|
Proceeds from exercise of warrants, related party |
|
| 40 |
|
|
| - |
|
Proceeds from convertible notes payable |
|
| 365,000 |
|
|
| 75,000 |
|
Proceeds from notes payable, related parties |
|
| 30,000 |
|
|
| - |
|
Repayments on notes payable, related parties |
|
| - |
|
|
| (109,000 | ) |
Proceeds from the sale of common stock |
|
| - |
|
|
| 762,250 |
|
Net cash provided by financing activities |
|
| 395,040 |
|
|
| 728,250 |
|
|
|
|
|
|
|
|
| |
NET CHANGE IN CASH |
|
| (67,185 | ) |
|
| 86,799 |
|
CASH AT BEGINNING OF PERIOD |
|
| 102,599 |
|
|
| 15,800 |
|
|
|
|
|
|
|
|
| |
CASH AT END OF PERIOD |
| $ | 35,414 |
|
| $ | 102,599 |
|
|
|
|
|
|
|
|
| |
SUPPLEMENTAL INFORMATION: |
|
|
|
|
|
|
|
|
Interest paid |
| $ | - |
|
| $ | 32,619 |
|
Income taxes paid |
| $ | - |
|
| $ | - |
|
|
|
|
|
|
|
|
| |
NON-CASH INVESTING AND FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
Discount on beneficial conversion feature on convertible note |
| $ | 297,011 |
|
| $ | 37,019 |
|
Value of shares issued for conversion of debt |
| $ | 227,388 |
|
| $ | - |
|
Cashless exercise of common stock warrants, 250,000 warrants exercised |
| $ | - |
|
| $ | 37,981 |
|
The accompanying notes are an integral part of these financial statements.
| F-5 |
Premier Biomedical, Inc.
Notes to Financial Statements
Note 1 – Nature of Business and Significant Accounting Policies
Nature of Business
Premier Biomedical, Inc. ("the Company") was incorporated in the State of Nevada on May 10, 2010 ("Inception"). The Company was formed to develop and market medications and procedures that address a significant number of the most highly visible health issues currently affecting mankind. The Company will market these medications and procedures to leading worldwide pharmaceutical firms via publication in medical journals and by direct contact.
These statements reflect all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for fair presentation of the information contained therein.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
We maintain cash balances in non-interest-bearing accounts, which do not currently exceed federally insured limits. For the purpose of the statements of cash flows, all highly liquid investments with an original maturity of three months or less are considered to be cash equivalents.
Patent Rights and Applications
Patent rights and applications costs include the acquisition costs and costs incurred for the filing of patents. Patent rights and applications are amortized on a straight-line basis over the legal life of the patent rights beginning at the time the patents are approved. Patent costs for unsuccessful patent applications are expensed when the application is terminated.
Fair Value of Financial Instruments
Under FASB ASC 820-10-05, the Financial Accounting Standards Board establishes a framework for measuring fair value in generally accepted accounting principles and expands disclosures about fair value measurements. This Statement reaffirms that fair value is the relevant measurement attribute. The adoption of this standard did not have a material effect on the Company's financial statements as reflected herein. The carrying amounts of cash, prepaid expenses and accrued expenses reported on the balance sheet are estimated by management to approximate fair value primarily due to the short term nature of the instruments.
Basic and Diluted Loss Per Share
The basic net loss per common share is computed by dividing the net loss by the weighted average number of common shares outstanding. Diluted net loss per common share is computed by dividing the net loss adjusted on an "as if converted" basis, by the weighted average number of common shares outstanding plus potential dilutive securities. For the periods presented, potential dilutive securities had an anti-dilutive effect and were not included in the calculation of diluted net loss per common share.
| F-6 |
Premier Biomedical, Inc.
Notes to Financial Statements
Stock-Based Compensation
Under FASB ASC 718-10-30-2, all share-based payments to employees, including grants of employee stock options, to be recognized in the income statement based on their fair values. Pro forma disclosure is no longer an alternative. The Company's stock based compensation consisted of the following during the years ended December 31, 2015 and 2014, respectively:
| December 31, |
|
| December 31, |
| |||
| 2015 |
|
| 2014 |
| |||
|
|
|
|
|
| |||
Common stock issued for services |
| $ | - |
|
| $ | 263,079 |
|
Warrants issued for services |
|
| 319,986 |
|
|
| 96,767 |
|
Warrants issued for services, related parties |
|
| 1,529,182 |
|
|
| 394,540 |
|
Total stock based compensation |
| $ | 1,849,168 |
|
| $ | 754,386 |
|
Revenue Recognition
Sales on fixed price contracts are recorded when services are earned, the earnings process is complete or substantially complete, and the revenue is measurable and collectability is reasonably assured. Provisions for discounts and rebates to customers, estimated returns and allowances, and other adjustments are provided for in the same period the related sales are recorded. The Company defers any revenue from sales in which payment has been received, but the earnings process has not occurred. Sales have not yet commenced.
Advertising and Promotion
All costs associated with advertising and promoting products are expensed as incurred. These expenses were $59,956 and $159,411 for the years ended December 31, 2015 and 2014, respectively.
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. A valuation allowance is provided for significant deferred tax assets when it is more likely than not, that such asset will not be recovered through future operations.
Uncertain Tax Positions
In accordance with ASC 740, "Income Taxes" ("ASC 740"), the Company recognizes the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be capable of withstanding examination by the taxing authorities based on the technical merits of the position. These standards prescribe a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. These standards also provide guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition.
Various taxing authorities periodically audit the Company's income tax returns. These audits include questions regarding the Company's tax filing positions, including the timing and amount of deductions and the allocation of income to various tax jurisdictions. In evaluating the exposures connected with these various tax filing positions, including state and local taxes, the Company records allowances for probable exposures. A number of years may elapse before a particular matter, for which an allowance has been established, is audited and fully resolved. The Company has not yet undergone an examination by any taxing authorities.
The assessment of the Company's tax position relies on the judgment of management to estimate the exposures associated with the Company's various filing positions.
| F-7 |
Premier Biomedical, Inc.
Notes to Financial Statements
Recently Issued Accounting Pronouncements
In January 2016, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") No. 2016-16, Financial Instruments - Overall (Subtopic 825-10), Recognition and Measurement of Financial Assets and Financial Liabilities. The provisions of the update require equity investments to be measured at fair value with changes in fair value recognized in net income. However, an entity may choose to measure equity investments that do not have readily determinable fair values at cost minus impairment. The update also simplifies the impairment assessment of equity investments without readily determinable fair values by requiring a qualitative assessment to identify impairment. It also eliminates the requirement to disclose the fair value of financial instruments measured at amortized cost for entities that are not public business entities, and eliminates the requirement for public business entities to disclose the methods and significant assumptions used to estimate the fair value for financial instruments measured at amortized cost on the balance sheet. ASU No. 2016-16 requires public business entities to use the exit price notion when measuring the fair value of financial instruments for disclosure purposes. It also requires an entity to present separately in other comprehensive income the portion of the total change in the fair value of a liability resulting from a change in the instrument-specific credit risk when the entity has elected to measure the liability at fair value in accordance with the fair value option for financial instruments. The update requires separate presentation of financial assets and financial liabilities by category and form on the balance sheet or the accompanying notes to the financial statements. In addition, the update clarifies that an entity should evaluate the need for a valuation allowance on a deferred tax asset related to available-for-sale securities in combination with the entity's other deferred tax assets. For public business entities, the amendments in the update are effective for fiscal years beginning after December 15, 2017, including interim periods. The adoption of this ASU is not expected to have a material impact on the Company's financial statements.
In November 2015, the FASB issued an ASU No. 2015-17, Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes ("ASU 2015-17"). Under current GAAP, deferred income tax assets and liabilities are separated into current and noncurrent amounts in the balance sheet. ASU 2015-17 requires all deferred assets and liabilities be classified as noncurrent in the balance sheet. The standard will be effective for periods beginning after December 15, 2016, including interim periods within that reporting period. The adoption of this ASU is not expected to have a material impact on the Company's financial statements.
In September 2015, the FASB issued ASU No. 2015-16, Business Combinations (Topic 805), Simplifying the Accounting for Measurement-Period Adjustments ("ASU 2015-16"), which require an acquiring Company to recognize adjustments to provisional amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. GAAP requires that during the measurement period, the acquirer retrospectively adjust the provisional amounts recognized at the acquisition date with a corresponding adjustment to goodwill. Those adjustments are required when new information is obtained about facts and circumstances that existed as of the acquisition date that if known, would have affected the measurement of the amounts initially recorded. To simplify the accounting for adjustments made to provisional amounts recognized in a business combination, the amendments in the update eliminate the requirement to retrospectively account for those adjustments. This ASU is effective for public entities for fiscal years beginning after December 15, 2015, including interim periods within those years. Disclosure of the nature and reason for the change should be made in the first period, including interim periods, there is a measurement period adjustment.
In April 2015, the FASB issued ASU No. 2015-03, Interest–Imputation of Interest (Subtopic 835-30) ("ASU 2015-03"), which changes the presentation of debt issuance costs in financial statements. ASU 2015-03 requires an entity to present such costs in the balance sheet as a direct deduction from the related debt liability rather than as an asset. Amortization of the costs will continue to be reported as interest expense. For public business entities, ASU 2015-03 is effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. For all other entities, ASU 2015-03 is effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within fiscal years beginning after December 15, 2016. Early adoption is permitted for financial statements that have not been previously issued. The new guidance should be applied on a retrospective basis. The Company will adopt ASU 2015-03 on its balance sheets retrospectively during the interim period ending March 31, 2016.
| F-8 |
Premier Biomedical, Inc.
Notes to Financial Statements
In August 2014, the FASB issued ASU No. 2014-15, Presentation of Financial Statements - Going Concern: Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern ("ASU 2014-15"). This guidance clarifies that an entity's management should evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity's ability to continue as a going concern within one year after the date that the financial statements are issued. The amendments in this update are effective for annual reporting periods ending after December 15, 2016, and annual and interim periods thereafter, and early application is permitted. The Company is currently in the process of evaluating the impact of adoption of ASU 2014-15 on its financial statements.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers ("ASU 2014-09"), which supersedes the revenue recognition requirements in Topic 605, Revenue Recognition, including most industry-specific revenue recognition guidance throughout the Industry Topics of the Codification. The core principle of ASU 2014-09 is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration that is expected to be received for those goods or services. ASU 2014-09 is effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period, and early application is not permitted. ASU 2014-09 allows for either full retrospective or modified retrospective adoption. We do not expect the adoption of the new provisions to have a material impact on our financial condition or results of operations.
No other new accounting pronouncements, issued or effective during the year ended December 31, 2015, have had or are expected to have a significant impact on the Company's financial statements.
Note 2 – Going Concern
As shown in the accompanying financial statements, the Company has no revenues, incurred net losses from operations resulting in an accumulated deficit of $10,777,797, and had negative working capital of ($279,970) at December 31, 2015. These factors raise substantial doubt about the Company's ability to continue as a going concern. Management is actively pursuing new products and services to begin generating revenues. In addition, the Company is currently seeking additional sources of capital to fund short term operations. The Company, however, is dependent upon its ability to secure equity and/or debt financing and there are no assurances that the Company will be successful; therefore, without sufficient financing it would be unlikely for the Company to continue as a going concern.
The financial statements do not include any adjustments that might result from the outcome of any uncertainty as to the Company's ability to continue as a going concern. The financial statements also do not include any adjustments relating to the recoverability and classification of recorded asset amounts, or amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern.
Note 3 – Related Party
Accounts Payable
The Company owed $37,486 and $11,069 as of December 31, 2015 and 2014, respectively, to entities owned by the Chairman of the Board of Directors. The amounts are related to patent costs paid by the Chairman on behalf of the Company.
The Company owed $3,265 and $465 as of December 31, 2015 and 2014, respectively, to the Company's CEO for reimbursable expenses.
The Company owed $13,917 and $13,765 as of December 31, 2015 and 2014, respectively, amongst members of the Company's Board of Directors for reimbursable expenses.
| F-9 |
Premier Biomedical, Inc.
Notes to Financial Statements
Notes Payable
From time to time, the Company has received short term loans from officers and directors as disclosed in Note 7 below.
Common Stock Warrants Exercised
On October 1, 2015, the Company issued 3,000,000 shares of common stock pursuant to the exercise of warrants by the Company's Chairman of the Board at $0.00001 per share for total proceeds of $30.
On September 10, 2015, the Company issued 1,000,000 shares of common stock pursuant to the exercise of warrants by the Company's Chairman of the Board at $0.00001 per share for total proceeds of $10.
Common Stock Warrants
On October 7, 2015, the Company granted warrants to the following officers and directors, which will allow them to purchase shares of our common stock in the amounts indicated: William Hartman (1,000,000 shares); Mitchell Felder (1,000,000 shares), Heidi Carl (750,000 shares), John Borza (600,000 shares), Richard Najarian (200,000 shares), and Jay Rosen (200,000 shares). The exercise price of the foregoing warrants is five cents ($0.05) per share. The warrants are exercisable over ten (10) years. The total fair value of the 3,750,000 common stock warrants using the Black-Scholes option-pricing model is $31,109, or $0.0083 per share, based on a volatility rate of 232%, a risk-free interest rate of 1.75% and an expected term of 10.0 years, and is being amortized over the implied service term, or vesting period, of the warrants.
One half of the shares underlying each of the respective warrants vest on June 15, 2016, with the balance vesting on December 15, 2016. In order for the warrants to vest on each of the foregoing dates, however, the holders must be serving in the same capacity on behalf of the Company as he or she was serving on October 21, 2015. The issuance of the warrants was fully approved by our Board of Directors on October 21, 2015, the date a fully executed resolution authorizing the issuance was delivered. The issuances were exempt from registration pursuant to Section 4(a)(2) of the Securities Act of 1933, the investors are sophisticated and familiar with our operations, and there was no solicitation in connection with the issuance.
On November 18, 2014, the Company granted common stock warrants to the Company's CEO to purchase a total of 1,600,000 shares of common stock at $0.25 per share for his services as an Officer. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The warrants are exercisable over seven (7) years. The fair value of the 1,600,000 common stock warrants using the Black-Scholes option-pricing model is $356,771, or $0.22298 per share, based on a volatility rate of 192%, a risk-free interest rate of 0.96% and an expected term of 3.54 years, and is being amortized over the implied service term, or vesting period, of the warrants.
On November 18, 2014, the Company granted common stock warrants to the Company's Chairman of the Board of Directors to purchase a total of 1,600,000 shares of common stock at $0.25 per share for his services as the Chairman of the Board. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The warrants are exercisable over seven (7) years. The fair value of the 1,600,000 common stock warrants using the Black-Scholes option-pricing model is $356,771, or $0.22298 per share, based on a volatility rate of 192%, a risk-free interest rate of 0.96% and an expected term of 3.54 years, and is being amortized over the implied service term, or vesting period, of the warrants.
On November 18, 2014, the Company granted common stock warrants to one of the Directors to purchase a total of 1,400,000 shares of common stock at $0.25 per share over a seven year period from the grant date for their services as Directors. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The total fair value of the 1,400,000 common stock warrants using the Black-Scholes option-pricing model is $312,174, or $0.22298 per share, based on a volatility rate of 192%, a risk-free interest rate of 0.96% and an expected term of 3.54 years, and is being amortized over the implied service term, or vesting period, of the warrants.
| F-10 |
Premier Biomedical, Inc.
Notes to Financial Statements
On November 18, 2014, the Company granted 1,200,000 common stock warrants to each of three Directors to purchase a total of 3,600,000 shares of common stock at $0.25 per share over a seven year period from the grant date for their service as Directors. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The total fair value of the 3,600,000 common stock warrants using the Black-Scholes option-pricing model is $802,734, or $0.22298 per share, based on a volatility rate of 192%, a risk-free interest rate of 0.96% and an expected term of 3.54 years, and is being amortized over the implied service term, or vesting period, of the warrants.
On November 18, 2014, the Company granted common stock warrants to one of the Directors to purchase a total of 400,000 shares of common stock at $0.25 per share over a seven year period from the grant date for their services as Directors. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The total fair value of the 400,000 common stock warrants using the Black-Scholes option-pricing model is $89,193, or $0.22298 per share, based on a volatility rate of 192%, a risk-free interest rate of 0.96% and an expected term of 3.54 years, and is being amortized over the implied service term, or vesting period, of the warrants.
A total of $1,529,182 and $394,540 of previously issued warrants were amortized and expensed to professional fees as stock-based compensation during the years ended December 31, 2015 and 2014, respectively.
Note 4 – Patent Rights and Applications
The Company amortizes its patent rights and applications on a straight line basis over the expected useful technological or economic life of the patents, which is typically 17 years from the legal approval of the patent applications when there is probable future economic benefits associated with the patent. The Company has elected to expense all of their patent rights and application costs due to difficulties associated with having to prove the value of their future economic benefits. All patent applications are currently pending and the Company has no patents that have yet been approved. It is the Company's policy that it performs reviews of the carrying value of its patent rights and applications on an annual basis.
On March 4, 2015, we entered into a Patent License Agreement ("PLA") with the University of Texas at El Paso ("UTEP") regarding our joint research and development of CTLA-4 Blockade with Metronomic Chemotherapy for the Treatment of Breast Cancer. This is the first PLA with UTEP following our Collaborative Agreement with them dated May 9, 2012, and memorializes the joint ownership of the applicable patent and the financial and other terms related thereto.
On June 19, 2015, we entered into Amendment No. 1 to this Agreement, pursuant to which we explicitly included Provisional Patent Application No. 62/161,116 entitled, "Anti-CTLA-4 Blockade" (the "Application") under the definition of "Patent Rights" as set forth in the PLA. The Application was filed with the United States Patent and Trademarks Office on May 13, 2015; the underlying technology was invented by Robert Kirken and Georgialina Rodriguez, and is solely-owned by The Board of Regents of The University of Texas System.
| F-11 |
Premier Biomedical, Inc.
Notes to Financial Statements
Note 5 – Fair Value of Financial Instruments
Under FASB ASC 820-10-5, fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date (an exit price). The standard outlines a valuation framework and creates a fair value hierarchy in order to increase the consistency and comparability of fair value measurements and the related disclosures. Under GAAP, certain assets and liabilities must be measured at fair value, and FASB ASC 820-10-50 details the disclosures that are required for items measured at fair value.
The Company has certain financial instruments that must be measured under the new fair value standard. The Company's financial assets and liabilities are measured using inputs from the three levels of the fair value hierarchy. The three levels are as follows:
Level 1 - Inputs are unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date.
Level 2 - Inputs include quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (e.g., interest rates, yield curves, etc.), and inputs that are derived principally from or corroborated by observable market data by correlation or other means (market corroborated inputs).
Level 3 - Unobservable inputs that reflect our assumptions about the assumptions that market participants would use in pricing the asset or liability.
The following schedule summarizes the valuation of financial instruments at fair value on a recurring basis in the balance sheets as of December 31, 2015 and 2014, respectively:
Fair Value Measurements at December 31, 2015 | ||||||||||||
| Level 1 |
|
| Level 2 |
|
| Level 3 |
| ||||
Assets |
|
|
|
|
|
|
|
|
| |||
Cash |
| $ | 35,414 |
|
| $ | - |
|
| $ | - |
|
Total assets |
|
| 35,414 |
|
|
| - |
|
|
| - |
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
Convertible note payable, net of discounts |
|
| - |
|
|
| 64,606 |
|
|
| - |
|
Notes payable, related parties |
|
| - |
|
|
| 30,000 |
|
|
| - |
|
Total liabilities |
|
| - |
|
|
| 94,606 |
|
|
| - |
|
| $ | 35,414 |
|
| $ | (94,606 | ) |
| $ | - |
| |
Fair Value Measurements at December 31, 2014 | ||||||||||||
| Level 1 |
|
| Level 2 |
|
| Level 3 |
| ||||
Assets |
|
|
|
|
|
|
|
|
| |||
Cash |
| $ | 102,599 |
|
| $ | - |
|
| $ | - |
|
Total assets |
|
| 102,599 |
|
|
| - |
|
|
| - |
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
Convertible note payable, net of discounts |
|
| - |
|
|
| 14,879 |
|
|
| - |
|
Total liabilities |
|
| - |
|
|
| 14,879 |
|
|
| - |
|
| $ | 102,599 |
|
| $ | (14,879 | ) |
| $ | - |
| |
The fair values of our related party debts are deemed to approximate book value, and are considered Level 2 inputs as defined by ASC Topic 820-10-35.
There were no transfers of financial assets or liabilities between Level 1, Level 2 and Level 3 inputs for the years ended December 31, 2015 or the year ended December 31, 2014.
| F-12 |
Premier Biomedical, Inc.
Notes to Financial Statements
Note 6 – Convertible Notes Payable
Convertible notes payable consist of the following at December 31, 2015 and 2014, respectively:
| December 31, |
|
| December 31, |
| |||
| 2015 |
|
| 2014 |
| |||
|
|
|
|
|
| |||
On December 28, 2015, the Company received net proceeds of $130,000 in exchange for a 10% interest bearing; unsecured convertible promissory note dated December 28, 2015 with a face value of $157,500 ("First Redwood Note"), which matures on September 28, 2016. The principal and interest is convertible into shares of common stock at the discretion of the note holder at a price equal to the greater of (i) 60% of the lowest traded price over the 15 days prior to conversion or (ii) a fixed $0.00005 per share. The note carries liquidated damages of $1,000 per day for failure to provide certificates, and compensation for Buy-In on failure to timely deliver certificates. Principal and interest is due upon default at 50% of the lowest traded price over the previous fifteen (15) days, and an additional interest rate equal to the lesser of 2% per month (24% per annum) or the maximum rate per applicable law. |
| $ | 157,500 |
|
| $ | - |
|
|
|
|
|
|
|
|
| |
On December 15, 2015, the Company received net proceeds of $25,000 in exchange for a non-interest bearing, unsecured convertible promissory note with a face value of $27,500 ("Second JMJ Note"), which matures on December 15, 2016, as part of a larger financing agreement that enables the Company to draw total proceeds of $250,000 at the discretion of the lender. The principal and interest is convertible into shares of common stock at the discretion of the note holder at a price equal to the greater of sixty percent (60%) of the lowest trading price of the Company's common stock over the twenty five (25) trading days prior to the conversion request date, or a fixed rate of $0.00005 per share, as amended within the original promissory note on September 8, 2015 (In the case that conversion shares are not deliverable by DWAC an additional 10% discount will apply; and if the shares are ineligible for deposit into the DTC system and only eligible for Xclearing deposit an additional 5% discount shall apply; in the case of both an additional cumulative 15% discount shall apply). The note carries a one-time twelve percent (12%) of principal interest charge in the event of default, and the debt holder is limited to owning 4.99% of the Company's issued and outstanding shares. The promissory note carries a $2,500 Original Issue Discount that is being amortized over the life of the loan on the straight line method, which approximates the effective interest method. The Company reserved at least 24 million shares of common stock for potential conversions. |
|
| 27,500 |
|
|
| - |
|
|
|
|
|
|
|
|
| |
On September 21, 2015, the Company received net proceeds of $45,000 in exchange for an 8% interest bearing; unsecured convertible promissory note dated September 3, 2015 with a face value of $48,000 ("First Vis Vires Note"), which matures on June 8, 2016. The principal and interest is convertible into shares of common stock at the discretion of the note holder at a price equal to the greater of fifty eight percent (58%) of the average of the three (3) lowest closing bid prices over the 10 days prior to conversion, or a fixed rate of $0.00001 per share. The note includes various prepayment penalties ranging from 112% through 130%, and default provisions of 150% of the then outstanding principal and interest, and an interest rate of 22% thereafter. The debt holder is limited to owning 4.99% of the Company's issued and outstanding shares. The Company must at all times reserve at least 20,250,000 shares of common stock for potential conversions. |
|
| 48,000 |
|
|
| - |
|
| F-13 |
Premier Biomedical, Inc.
Notes to Financial Statements
|
| December 31, |
| December 31, |
| |||
|
| 2015 |
|
| 2014 |
| ||
|
|
|
|
|
|
| ||
On September 2, 2015, the Company received net proceeds of $50,000 in exchange for a non-interest bearing, unsecured convertible promissory note with a face value of $55,000 ("First JMJ Note"), which matures on September 1, 2016, as part of a larger financing agreement that enables the Company to draw total proceeds of $250,000 at the discretion of the lender. The principal and interest is convertible into shares of common stock at the discretion of the note holder at a price equal to the greater of sixty percent (60%) of the lowest trading price of the Company's common stock over the twenty five (25) trading days prior to the conversion request date, or a fixed rate of $0.00005 per share, as amended within the original promissory note on September 8, 2015 (In the case that conversion shares are not deliverable by DWAC an additional 10% discount will apply; and if the shares are ineligible for deposit into the DTC system and only eligible for Xclearing deposit an additional 5% discount shall apply; in the case of both an additional cumulative 15% discount shall apply). The note carries a one-time twelve percent (12%) of principal interest charge in the event of default, and the debt holder is limited to owning 4.99% of the Company's issued and outstanding shares. The promissory note carries a $5,000 Original Issue Discount that is being amortized over the life of the loan on the straight line method, which approximates the effective interest method. The Company reserved at least 24 million shares of common stock for potential conversions. |
|
| 55,000 |
|
|
| - |
|
|
|
|
|
|
|
|
| |
On February 24, 2015, we entered into a Securities Purchase Agreement with Adar Bays, LLC ("Adar Bays"), pursuant to which we sold to Adar Bays an 8% Convertible Promissory Note in the original principal amount of $44,100.00 (the "First Adar Note"). The Note has a maturity date of February 24, 2016, and is convertible after 180 days into our common stock at the higher of (i) $0.001 cents per share or (ii) 70% of the average of the two (2) lowest closing prices of our common stock for the fifteen (15) trading days prior to receipt of a conversion notice from Adar Bays. The shares of common stock issuable upon conversion of the Note will be restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The Note can be prepaid by us at a premium as follows: (a) between 1 and 30 days after issuance – 115% of the principal amount; (b) between 31 and 60 days after issuance – 121% of the principal amount; (c) between 61 and 90 days after issuance – 127% of the principal amount; (d) between 91 and 120 days after issuance – 133% of the principal amount; (e) between 121 and 150 days after issuance – 139% of the principal amount; and (f) between 151 and 180 days after issuance – 140% of the principal amount. There is no right to pre-payment after 180 days. The purchase and sale of the Note closed on March 2, 2015, the date that the purchase price was delivered to us. The note holder elected to convert a total of $46,355, consisting of $44,100 of principal and $2,255 of interest, in exchange for 15,772,387 shares of common stock on various dates between September, 2015 and November 12, 2015. |
|
| - |
|
|
| - |
|
| F-14 |
Premier Biomedical, Inc.
Notes to Financial Statements
|
| December 31, |
|
| December 31, |
| ||
| 2015 |
|
| 2014 |
| |||
|
|
|
|
|
|
| ||
On January 30, 2015, we entered into a Securities Purchase Agreement with LG Capital Funding, LLC ("LG Capital"), pursuant to which we sold to LG Capital a 8% Convertible Promissory Note in the original principal amount of $82,687.00 (the " Second LG Note"). The Note has a maturity date of January 29, 2016, and is convertible after 180 days into our common stock at the higher of (i) $0.001 cents per share or (ii) 70% of the average of the two (2) lowest closing bid prices of our common stock for the fifteen (15) trading days prior to receipt of a conversion notice from LG Capital. The shares of common stock issuable upon conversion of the Note will be restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The Note can be prepaid by us at a premium as follows: (a) between 1 and 30 days after issuance – 115% of the principal amount; (b) between 31 and 60 days after issuance – 121% of the principal amount; (c) between 61 and 90 days after issuance – 126% of the principal amount; (d) between 91 and 120 days after issuance – 132% of the principal amount; (e) between 121 and 150 days after issuance – 138% of the principal amount; and (f) between 151 and 180 days after issuance – 140% of the principal amount. There is no right to pre-payment after 180 days. The purchase and sale of the Note closed on January 30, 2015, the date that the purchase price was delivered to us. The note holder elected to convert a total of $87,679, consisting of $82,687 of principal and $4,992 of interest, in exchange for a total of 23,099,120 shares of common stock on various dates between August 4, 2015 and December 15, 2015. |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
| |
On November 25, 2014, the Company received an unsecured loan from Typenex Co-Investment, LLC ("First Typenex Note") in the amount of $86,500, bearing interest at 10%, maturing on August 25, 2015, in exchange for net proceeds of $75,000 after the deduction of $4,000 of loan origination costs and an original issue discount ("OID") of $7,500. The Company also issued Typenex warrants to purchase 351,455 shares of common stock at a strike price of $0.18 per share over a five year term from the date of investment. The principal and interest is convertible into shares of common stock at the discretion of the note holder at the lesser of (i) $0.18 per share, or (ii) 70% (the "Conversion Factor") multiplied by the Market Price (as defined in the Note). If the Market Price of our common stock falls below $0.10 per share after the issuance of the Note, the Conversion Factor will automatically be reduced by 5% for all conversions completed while the Market Price is below $0.10 per share. Notwithstanding the foregoing, so long as no Event of Default has occurred, the Conversion Price shall be not less than $0.0001 (the "Conversion Floor"). For the avoidance of doubt, upon the occurrence of an Event of Default, the Conversion Floor shall not apply to any future Conversions and shall be of no further force or effect. The note can be prepaid upon notice to Typenex any time prior to the first conversion at a premium of 120% of the then outstanding balance of the Note. The note carries a default interest rate of 22% per annum. The note holder elected to convert a total of $93,354, consisting of $86,500 of principal and $6,854 of interest, in exchange for 17,852,380 shares of common stock on various dates between June 3, 2015 and December 1, 2015. |
|
| - |
|
|
| 86,500 |
|
|
|
|
|
|
|
|
| |
Total convertible notes payable |
|
| 288,000 |
|
|
| 86,500 |
|
Less unamortized debt discounts: |
|
|
|
|
|
|
|
|
Discount on beneficial conversion feature |
|
| 210,230 |
|
|
| 32,137 |
|
Original issue discount |
|
| 13,164 |
|
|
| 6,511 |
|
Discount on warrants |
|
| - |
|
|
| 32,973 |
|
Convertible notes payable |
|
| 64,606 |
|
|
| 14,879 |
|
Less: current portion |
|
| 64,606 |
|
|
| 14,879 |
|
Convertible notes payable, less current portion |
| $ | - |
|
| $ | - |
|
| F-15 |
Premier Biomedical, Inc.
Notes to Financial Statements
The Company recognized interest expense for the years ended December 31, 2015 and 2014, respectively, as follows:
| December 31, |
|
| December 31, |
| |||
| 2015 |
|
| 2014 |
| |||
|
|
|
|
|
| |||
Interest on convertible notes |
| $ | 21,172 |
|
| $ | 721 |
|
Interest and penalties on related party loans |
|
| 1,170 |
|
|
| 29,455 |
|
Amortization of loan origination costs |
|
| 10,272 |
|
|
| 527 |
|
Amortization of beneficial conversion feature |
|
| 118,918 |
|
|
| 4,882 |
|
Amortization of OID |
|
| 14,384 |
|
|
| 989 |
|
Amortization of warrants |
|
| 32,973 |
|
|
| 5,008 |
|
Total interest expense |
| $ | 198,889 |
|
| $ | 41,582 |
|
In addition, the Company recognized and measured the embedded beneficial conversion feature present in the convertible debts by allocating a portion of the proceeds equal to the intrinsic value of the feature to additional paid-in-capital. The intrinsic value of the feature was calculated on the commitment date using the effective conversion price of the convertible debt. This intrinsic value is limited to the portion of the proceeds allocated to the convertible debt.
The aforementioned accounting treatment resulted in debt discounts equal to $318,048 and $82,500 during the years ended December 31, 2015 and 2014, respectively. The discount, including Original Issue Discounts of $21,037 and $7,500 and Warrant Discounts of $-0- and $37,981 during the years ended December 31, 2015 and 2014, respectively, is amortized on a straight line basis from the dates of issuance until the earlier of the stated redemption date of the debts, as noted above or the actual settlement date. During the years ended December 31, 2015 and 2014, the Company recorded debt amortization expense in the amount of $166,275 and $10,879, respectively, attributed to the aforementioned debt discounts.
The convertible notes, consisting of total original face values of $157,500 from Redwood Fund III Ltd., $48,000 from Vis Vires Group, Inc., $27,500 and $55,000 from JMJ Financial, $44,100 from Adar Bays, LLC, $82,687 from LG Capital Funding, LLC, and $86,500 from Typenex Co-Investment, LLC that created the beneficial conversion features carried a default provision that placed a "maximum share amount" on the note holder that can be owned as a result of the conversions to common stock by the note holder of 4.99% of the issued and outstanding shares of the Company.
First Redwood Convertible Note
On December 28, 2015, we entered into and received proceeds from a Securities Purchase Agreement with Redwood Management, LLC, pursuant to which we agreed to sell, and Redwood Management, LLC, pursuant to which we sold to Redwood Fund III Ltd. a 10% Convertible Promissory Note ("First Redwood Note") in the original principal amount of $157,500. The First Redwood Note has a maturity date of September 28, 2016, and is convertible after 90 days into our common stock at a conversion price equal to 60% of the lowest traded price of the common stock in the fifteen (15) trading days prior to the conversion date.
The shares of common stock issuable upon conversion of the First Redwood Note are restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The issuance of the First Redwood Note was exempt from the registration requirements of the Securities Act of 1933 pursuant to Rule 506 of Regulation D promulgated thereunder. The purchaser was an accredited and sophisticated investor, familiar with our operations, and there was no solicitation.
The Company evaluated the First Redwood Note and determined that the shares issuable pursuant to the conversion option were determinate due to the Fixed Conversion Price and, as such, did not constitute a derivative liability. The beneficial conversion feature discount resulting from the conversion price of $0.03810 below the market price on December 28, 2015 of $0.0435 provided a value of $130,000, of which $1,418 and $-0- was amortized during the years ended December 31, 2015 and 2014, respectively.
| F-16 |
Premier Biomedical, Inc.
Notes to Financial Statements
Second JMJ Financial Convertible Note
On December 15, 2015, we entered into a Securities Purchase Agreement with JMJ Financial, pursuant to which we sold to JMJ Financial a 12% Convertible Promissory Note ("Second JMJ Note") in the original principal amount of $27,500. The Second JMJ Note has a maturity date of December 15, 2016, and is convertible after 180 days into our common stock at the higher of (i) $0.00005 cents per share or (ii) sixty percent (60%) of the lowest trading price of the Company's common stock over the twenty five (25) trading days prior to receipt of a conversion notice.
The shares of common stock issuable upon conversion of the Second JMJ Note are restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The issuance of the Second JMJ Note was exempt from the registration requirements of the Securities Act of 1933 pursuant to Rule 506 of Regulation D promulgated thereunder. The purchaser was an accredited and sophisticated investor, familiar with our operations, and there was no solicitation.
The Company evaluated the Second JMJ Note and determined that the shares issuable pursuant to the conversion option were determinate due to the Fixed Conversion Price and, as such, did not constitute a derivative liability. The beneficial conversion feature discount resulting from the conversion price of $0.03262 below the market price on December 15, 2015 of $0.034 provided a value of $25,000, of which $1,096 and $-0- was amortized during the years ended December 31, 2015 and 2014, respectively.
Vis Vires Group, Inc. Convertible Note
On September 21, 2015, we received proceeds from a Securities Purchase Agreement we entered into on September 3, 2015 with VIS Vires Group, Inc., pursuant to which we sold to VIS Vires an 8% Convertible Promissory Note ("First VIS Vires Note") in the original principal amount of $48,000. The First VIS Vires Note has a maturity date of June 8, 2016, and is convertible after 180 days into our common stock at the higher of (i) $0.00001 cents per share or (ii) fifty eight percent (58%) of the average of the three (3) lowest closing bid prices over the 10 days prior to receipt of a conversion notice.
The shares of common stock issuable upon conversion of the First Vis Vires Note are restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The issuance of the First Vis Vires Note was exempt from the registration requirements of the Securities Act of 1933 pursuant to Rule 506 of Regulation D promulgated thereunder. The purchaser was an accredited and sophisticated investor, familiar with our operations, and there was no solicitation.
The Company evaluated the First Vis Vires Note and determined that the shares issuable pursuant to the conversion option were determinate due to the Fixed Conversion Price and, as such, did not constitute a derivative liability. The beneficial conversion feature discount resulting from the conversion price of $0.00747 below the market price on September 21, 2015 of $0.016 provided a value of $39,448, of which $15,265 and $-0- was amortized during the years ended December 31, 2015 and 2014, respectively.
| F-17 |
Premier Biomedical, Inc.
Notes to Financial Statements
First JMJ Financial Convertible Note
On September 2, 2015, we entered into a Securities Purchase Agreement with JMJ Financial, pursuant to which we sold to JMJ Financial a 12% Convertible Promissory Note ("First JMJ Note") in the original principal amount of $55,000. The First JMJ Note has a maturity date of September 1, 2016, and is convertible after 180 days into our common stock at the higher of (i) $0.00005 cents per share or (ii) sixty percent (60%) of the lowest trading price of the Company's common stock over the twenty five (25) trading days prior to receipt of a conversion notice.
The shares of common stock issuable upon conversion of the First JMJ Note are restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The issuance of the First JMJ Note was exempt from the registration requirements of the Securities Act of 1933 pursuant to Rule 506 of Regulation D promulgated thereunder. The purchaser was an accredited and sophisticated investor, familiar with our operations, and there was no solicitation.
The Company evaluated the First JMJ Note and determined that the shares issuable pursuant to the conversion option were determinate due to the Fixed Conversion Price and, as such, did not constitute a derivative liability. The beneficial conversion feature discount resulting from the conversion price of $0.0194 below the market price on September 2, 2015 of $0.032 provided a value of $50,000, of which $16,439 and $-0- was amortized during the years ended December 31, 2015 and 2014, respectively.
Adar Bays, LLC Convertible Note
On February 24, 2015, we entered into a Securities Purchase Agreement with Adar Bays, LLC, pursuant to which we sold to Adar Bays an 8% Convertible Promissory Note ("First Adar Bays Note") in the original principal amount of $44,100. The First Adar Bays Note has a maturity date of February 24, 2016, and is convertible after 180 days into our common stock at the higher of (i) $0.001 cents per share or (ii) 70% of the average of the two (2) lowest closing prices of our common stock for the fifteen (15) trading days prior to receipt of a conversion notice.
The shares of common stock issuable upon conversion of the First Adar Bays Note are restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The issuance of the First Adar Bays Note was exempt from the registration requirements of the Securities Act of 1933 pursuant to Rule 506 of Regulation D promulgated thereunder. The purchaser was an accredited and sophisticated investor, familiar with our operations, and there was no solicitation.
The Company evaluated the First Adar Bays Note and determined that the shares issuable pursuant to the conversion option were determinate due to the Fixed Conversion Price and, as such, did not constitute a derivative liability. The beneficial conversion feature discount resulting from the conversion price of $0.0473 below the market price on February 24, 2015 of $0.14 provided a value of $20,420, of which $20,420 and $-0- was amortized during the years ended December 31, 2015 and 2014, respectively.
LG Capital Funding, LLC Convertible Note
On January 30, 2015, we entered into a Securities Purchase Agreement with LG Capital Funding, LLC, pursuant to which we sold to LG Capital an 8% Convertible Promissory Note ("Second LG Capital Note") in the original principal amount of $82,687. The Second LG Capital Note has a maturity date of January 29, 2016, and is convertible after 180 days into our common stock at the higher of (i) $0.001 cents per share or (ii) 70% of the average of the two (2) lowest closing prices of our common stock for the fifteen (15) trading days prior to receipt of a conversion notice.
The shares of common stock issuable upon conversion of the Second LG Capital Note are restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The issuance of the Second LG Capital Note was exempt from the registration requirements of the Securities Act of 1933 pursuant to Rule 506 of Regulation D promulgated thereunder. The purchaser was an accredited and sophisticated investor, familiar with our operations, and there was no solicitation.
The Company evaluated the Second LG Capital Note and determined that the shares issuable pursuant to the conversion option were determinate due to the Fixed Conversion Price and, as such, did not constitute a derivative liability. The beneficial conversion feature discount resulting from the conversion price of $0.042 below the market price on January 30, 2015 of $0.14 provided a value of $32,143, of which $32,143 and $-0- was amortized during the years ended December 31, 2015 and 2014, respectively.
| F-18 |
Premier Biomedical, Inc.
Notes to Financial Statements
Typenex Co-Investment, LLC Convertible Note
On November 25, 2014, we entered into a Securities Purchase Agreement with Typenex Co-Investment, LLC, pursuant to which we sold to Typenex a 10% Convertible Promissory Note ("First Typenex Note") in the original principal amount of $86,500. The First Typenex Note has a maturity date of August 25, 2015, and is convertible into our common stock at the lesser of (i) $0.18 per share, or (ii) 70% (the "Conversion Factor") multiplied by the Market Price (as defined in the Note). If the Market Price of our common stock falls below $0.10 per share after the issuance of the Note, the Conversion Factor will automatically be reduced by 5% for all conversions completed while the Market Price is below $0.10 per share. Notwithstanding the foregoing, so long as no Event of Default has occurred, the Conversion Price shall be not less than $0.0001 (the "Conversion Floor"). For the avoidance of doubt, upon the occurrence of an Event of Default, the Conversion Floor shall not apply to any future Conversions and shall be of no further force or effect.
The shares of common stock issuable upon conversion of the First Typenex Note are restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The issuance of the First Typenex Note is exempt from the registration requirements of the Securities Act of 1933 pursuant to Rule 506 of Regulation D promulgated thereunder. The purchaser was an accredited and sophisticated investor, familiar with our operations, and there was no solicitation.
The Company evaluated the First Typenex Note and determined that the shares issuable pursuant to the conversion option were determinate due to the Fixed Conversion Price and, as such, does not constitute a derivative liability. The beneficial conversion feature discount resulting from the conversion price of $0.1019 below the market price on November 25, 2014 of $0.225 provided a value of $37,019, of which $32,137 and $4,882 was amortized during the years ended December 31, 2015 and 2014, respectively.
Note 7 – Notes Payable, Related Parties
Notes payable, related parties consist of the following at December 31, 2015 and 2014, respectively:
| December 31, |
|
| December 31, |
| |||
| 2015 |
|
| 2014 |
| |||
|
|
|
|
|
| |||
On July 6, 2015, the Company received an unsecured loan in the amount of $10,000, due on demand, bearing interest at a simple interest rate of 8%, from the Company's CEO. |
| $ | 10,000 |
|
| $ | - |
|
|
|
|
|
|
|
|
| |
On July 6, 2015, the Company received an unsecured loan in the amount of $10,000, due on demand, bearing interest at a simple interest rate of 8%, from the Company's Chairman of the Board. |
|
| 10,000 |
|
|
| - |
|
|
|
|
|
|
|
|
| |
On July 6, 2015, the Company received an unsecured loan in the amount of $10,000, due on demand, bearing interest at a simple interest rate of 8%, from one of the Company's Directors. |
|
| 10,000 |
|
|
| - |
|
|
|
|
|
|
|
|
| |
On November 18, 2013, the Company received an unsecured, 8% interest bearing loan in the amount of $50,000, due on August 18, 2014, or three business days following the receipt of one million dollars in funding, net of expenses, from the Company's CEO. The note carries an additional prepayment premium of 35% of the principal if the note is not paid prior to maturity, and whereby the note holder is entitled to additional interest on the principal pursuant to the schedule listed below if the note is paid prior to maturity: |
|
|
|
|
|
| ||
No. of days after issuance date: |
| Prepayment Premium: |
|
|
|
|
|
|
|
|
0-30 days 31-60 days 61-90 days 91-120 days 121 days or more |
| 15% 20% 25% 30% 35% |
|
|
|
|
|
|
|
|
The note was repaid in full on March 12, 2014, in the total amount of $66,381, consisting of $50,000 of principal, $1,381 of interest and $15,000 of prepayment premium. |
|
| - |
|
|
| - |
|
| F-19 |
Premier Biomedical, Inc.
Notes to Financial Statements
|
| December 31, |
|
| December 31, |
| ||
|
| 2015 |
|
| 2014 |
| ||
|
|
|
|
|
|
|
|
|
On November 18, 2013, the Company received an unsecured, 8% interest bearing loan in the amount of $50,000, due on August 18, 2014, or three business days following the receipt of one million dollars in funding, net of expenses, from one of the Company's Directors. The note carries an additional prepayment premium of 35% of the principal if the note is not paid prior to maturity, and whereby the note holder is entitled to additional interest on the principal pursuant to the same schedule listed above in the $50,000 note from the Company's CEO. The note was repaid in full on March 12, 2014, in the total amount of $66,238, consisting of $50,000 of principal, $1,238 of interest and $15,000 of prepayment premium. |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
| |
On May 4, 2012, the Company received an unsecured, non-interest bearing loan in the amount of $3,000, due on demand from the Company's CEO. The note was repaid in full on March 7, 2014. |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
| |
On May 4, 2012, the Company received an unsecured, non-interest bearing loan in the amount of $3,000, due on demand and a from the Company's Chairman of the Board of Directors. The note was repaid in full on March 12, 2014. |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
| |
On May 7, 2012, the Company received an unsecured, non-interest bearing loan in the amount of $3,000, due on demand from one of the Company's directors. The note was repaid in full on March 12, 2014. |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
Total notes payable, related parties |
|
| 30,000 |
|
|
| - |
|
Less: current portion |
|
| 30,000 |
|
|
| - |
|
Notes payable, related parties, less current portion |
| $ | - |
|
| $ | - |
|
The Company recorded interest expense in the amount of $1,170 and $29,455 for the years ended December 31, 2015 and 2014, respectively related to notes payable, related parties, including imputed interest expense in the amount of $134 for the year ended December 31, 2014.
| F-20 |
Premier Biomedical, Inc.
Notes to Financial Statements
Note 8 – Commitments and Contingencies
Collaborative Patent License Agreements
On May 9, 2012, the Company entered into a Collaborative Agreement with the University of Texas at El Paso. Pursuant to the terms of the Agreement, the Company will work jointly with the University to develop a series of research and development programs around its sequential-dialysis technology in the areas of Alzheimer's Disease, Traumatic Brain Injury (TBI), Chronic Pain Syndrome, Fibromyalgia, Multiple Sclerosis, Amyotrophic Lateral Sclerosis (ALS or Lou Gehrig's disease), Blood Sepsis, Cancer, Heart Attacks and Strokes. The programs will utilize the facilities at one or more of the University of Texas' campuses. The Company will pay the University's actual overhead for the projects, plus a negotiated facility and administration overhead expense, and 10% of all gross revenues associated with the sale, license and/or royalties of all products and treatment procedures directly affiliated with programs. Intellectual property jointly invented and developed as a result of the projects will be owned jointly by the University and the Company. The Agreement has an initial term of five (5) years, and is renewable upon mutual agreement of the parties.
On March 4, 2015, we entered into a Patent License Agreement (PLA) with the University of Texas at El Paso (UTEP) regarding our joint research and development of CTLA-4 Blockade with Metronomic Chemotherapy for the Treatment of Breast Cancer. This is the first PLA with UTEP following our Collaborative Agreement with them dated May 9, 2012, and memorializes the joint ownership of the applicable patent and the financial and other terms related thereto.
On June 19, 2015, we entered into Amendment No. 1 to this Agreement, pursuant to which we explicitly included Provisional Patent Application No. 62/161,116 entitled, "Anti-CTLA-4 Blockade" (the "Application") under the definition of "Patent Rights" as set forth in the PLA. The Application was filed with the United States Patent and Trademarks Office on May 13, 2015; the underlying technology was invented by Robert Kirken and Georgialina Rodriguez, and is solely-owned by The Board of Regents of The University of Texas System.
Common Stock Commitments
On July 3, 2015, we entered into a consulting agreement with FBROCCO ASSESSORIA EMPRES ARIAL LTDA ASSESSORIA EMPRESARIAL LTDA, a Brazilian company ("FBROCCO"), pursuant to which FBROCCO will provide certain consulting services to us, which shall include (i) developing a relationship between us and a Brazilian-based entity that is interested in entering into a joint venture where the purpose is to import, market and sell our products in Brazil and other South American countries; and (ii) facilitating a trip for one of our officers to travel to Brazil and meet with the proposed joint venture partner and various governmental officials who have relationships that would be advantageous to the formation and success of the anticipated joint venture.
In addition to a $10,000 payment made in July, we agreed to pay FBROCCO a total of 1,500,000 shares of our common stock if a successful joint venture is formed and generates One Million U.S. Dollars ($1,000,000) in gross revenues by June 23, 2016.
Note 9 – Equity Line of Credit
On January 17, 2014, the Company's registration statement became effective whereby it registered 15 million shares of common stock that it would sell to Kodiak Capital Group, LLC ("Kodiak") over time pursuant to an Investment Agreement entered into on December 5, 2013 wherein Kodiak agreed to invest up to five million dollars ($5,000,000). The offering was to terminate on the earlier of (i) when all 15 million shares are sold, (ii) when the maximum offering amount of $5,000,000 has been achieved, or (iii) on January 17, 2016, unless the Company terminated it earlier. The investment Agreement included a termination date of July 17, 2014, but on July 9, 2014 it was extended to run through July 17, 2015. On October 16, 2014, we exercised our right to terminate the contract upon written notice to Kodiak.
The Company could not submit a new put notice until after the closing of the previous notice. The purchase price for the shares pursuant to the put notice was to be equal to seventy-five percent (75%) of the lowest closing best bid price of the common stock during the five consecutive trading days immediately following the date of our notice to Kodiak ("Put Notice") of our election to put shares pursuant to the Investment Agreement, subject to a limitation, whereby Kodiak's holdings could not exceed 9.9% of the outstanding shares of common stock. The shares had to be paid for and share certificates delivered within the "pricing period," which was five (5) trading days from the date the put notice is delivered ("Put Date").
On October 15, 2013, the Company paid to Kodiak an initial fee of $10,000 and issued 391,398 shares of common stock following execution of the Agreement, along with the issuance of another 167,742 shares to Manners, Inc. as commitment fees. In addition, the Company agreed to pay a consulting firm, Cambridge Partners Atlanta Group, LLC ("Cambridge"), an introduction fee payable as follows: (i) ten percent (10%) of the first $1,000,000 in total draws, (ii) seven percent (7%) on the draws from $1,000,000 to $1,500,000; and (iii) five percent (5%) on all draws in excess of $1,500,000. Cambridge was entitled to these introduction fees on all proceeds received from Kodiak until the termination date of the consulting agreement, which was September 26, 2015 until we exercised our right to terminate the contract on October 16, 2014.
The Company used the proceeds from the sale of common stock pursuant to the agreement to pay down debt, research and development activities, general corporate and working capital purposes, and for other purposes that the board of directors deems to be in the best interest of the Company.
As of the date of this report, the Company had sold a total of 2,022,894 shares of common stock in exchange for total proceeds of $700,000 pursuant to the investment agreement, in addition to the initial issuance of 559,140 common shares as a commitment fee.
| F-21 |
Premier Biomedical, Inc.
Notes to Financial Statements
Note 10 – Stockholders' Equity
Convertible Preferred Stock, Series A
The Company has 10,000,000 authorized shares of Preferred Stock, of which 2,000,000 shares of $0.001 par value Series A Convertible Preferred Stock ("Series A Preferred Stock") have been designated. Each share of Series A Preferred Stock is convertible, at the option of the holder thereof, at any time after the issuance of such share into one (1) fully paid and non-assessable share of Common Stock. Each outstanding share of Series A Preferred Stock is entitled to one hundred (100) votes per share on all matters to which the shareholders of the Corporation are entitled or required to vote. The Company shall reserve and keep available out of its authorized but unissued shares of Common Stock such number of shares sufficient to effect the conversions. No shares of Series A Preferred Stock have been granted as of December 31, 2015.
Common Stock
The Company has one billion authorized shares of $0.00001 par value Common Stock, as amended from 300 million shares effective February 9, 2016.
Common Stock Issuances for Debt Conversions (2015)
On December 15, 2015, the Company issued 1,762,516 shares of common stock pursuant to the conversion of $16,039, consisting of $15,000 of principal and $1,039 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On December 8, 2015, the Company issued 2,794,392 shares of common stock pursuant to the conversion of $18,094, consisting of $16,946 of principal and $1,148 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On December 4, 2015, the Company issued 1,526,070 shares of common stock pursuant to the conversion of $3,258, consisting of $3,054 of principal and $204 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On December 2, 2015, the Company issued 3,022,017 shares of common stock pursuant to the conversion of $5,183, consisting of $4,860 of principal and $323 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On December 1, 2015, the Company issued 4,441,702 shares of common stock pursuant to the conversion of $7,413, consisting of $559 of principal and $6,854 of interest, on the First Typenex Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On November 25, 2015, the Company issued 3,003,665 shares of common stock pursuant to the conversion of $4,941, consisting of $4,640 of principal and $301 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On November 17, 2015, the Company issued 2,261,963 shares of common stock pursuant to the conversion of $3,721, consisting of $3,500 of principal and $221 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On November 16, 2015, the Company issued 3,000,000 shares of common stock pursuant to the conversion of $5,007 of principal on the First Typenex Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
| F-22 |
Premier Biomedical, Inc.
Notes to Financial Statements
On November 12, 2015, the Company issued 2,400,940 shares of common stock pursuant to the conversion of $4,370, consisting of $2,115 of principal and $2,255 of interest, on the First Adar Bay Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On November 11, 2015, the Company issued 2,259,167 shares of common stock pursuant to the conversion of $3,716, consisting of $3,500 of principal and $216 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On November 11, 2015, the Company issued 2,375,275 shares of common stock pursuant to the conversion of $4,323 of principal on the First Adar Bay Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On November 9, 2015, the Company issued 2,375,000 shares of common stock pursuant to the conversion of $4,322 of principal on the First Adar Bay Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On November 9, 2015, the Company issued 3,510,000 shares of common stock pursuant to the conversion of $6,234 of principal on the First Typenex Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On November 6, 2015, the Company issued 1,979,568 shares of common stock pursuant to the conversion of $3,256, consisting of $3,070 of principal and $186 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On November 2, 2015, the Company issued 2,168,067 shares of common stock pursuant to the conversion of $5,160 of principal on the First Adar Bay Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On October 27, 2015, the Company issued 1,839,530 shares of common stock pursuant to the conversion of $4,700 of principal on the First Adar Bay Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On October 26, 2015, the Company issued 1,620,522 shares of common stock pursuant to the conversion of $3,630, consisting of $3,430 of principal and $200 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On October 26, 2015, the Company issued 1,753,425 shares of common stock pursuant to the conversion of $4,480 of principal on the First Adar Bay Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On October 26, 2015, the Company issued 3,091,128 shares of common stock pursuant to the conversion of $7,700 of principal on the First Typenex Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On October 19, 2015, the Company issued 1,284,109 shares of common stock pursuant to the conversion of $6,000 of principal on the First Adar Bay Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On October 13, 2015, the Company issued 1,076,992 shares of common stock pursuant to the conversion of $4,222, consisting of $4,000 of principal and $222 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On October 8, 2015, the Company issued 1,116,799 shares of common stock pursuant to the conversion of $6,000 of principal on the First Adar Bay Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
| F-23 |
Premier Biomedical, Inc.
Notes to Financial Statements
On October 1, 2015, the Company issued 2,344,032 shares of common stock pursuant to the conversion of $13,000 of principal on the First Typenex Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On September 18, 2015, the Company issued 681,800 shares of common stock pursuant to the conversion of $6,300, consisting of $6,000 of principal and $300 of interest on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On September 3, 2015, the Company issued 459,242 shares of common stock pursuant to the conversion of $7,000 of principal on the First Adar Bay Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On August 26, 2015, the Company issued 446,711 shares of common stock pursuant to the conversion of $6,270, consisting of $6,000 of principal and $270 of interest on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On August 25, 2015, the Company issued 823,121 shares of common stock pursuant to the conversion of $13,500 of principal on the First Typenex Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On August 17, 2015, the Company issued 371,556 shares of common stock pursuant to the conversion of $5,215, consisting of $5,000 of principal and $215 of interest on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On August 4, 2015, the Company issued 292,181 shares of common stock pursuant to the conversion of $3,835, consisting of $3,687 of principal and $148 of interest, on the First LG Capital Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On July 23, 2015, the Company issued 260,866 shares of common stock pursuant to the conversion of $13,000 of principal on the First Typenex Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On June 25, 2015, the Company issued 208,719 shares of common stock pursuant to the conversion of $15,000 of principal on the First Typenex Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
On June 3, 2015, the Company issued 172,812 shares of common stock pursuant to the conversion of $12,500 of principal on the First Typenex Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
Common Stock Issuances for Exercise of Warrants, Related Party (2015)
On October 1, 2015, the Company issued 3,000,000 shares of common stock pursuant to the exercise of warrants by the Company's Chairman of the Board at $0.00001 per share for total proceeds of $30.
On September 10, 2015, the Company issued 1,000,000 shares of common stock pursuant to the exercise of warrants by the Company's Chairman of the Board at $0.00001 per share for total proceeds of $10.
Common Stock Cancellations (2015)
On August 12, 2015, the Company cancelled and returned to treasury a total of 150,000 shares of common stock previously issued to a consultant for services provided.
| F-24 |
Premier Biomedical, Inc.
Notes to Financial Statements
Common Stock (2014)
On October 2, 2014, the Company sold 500,000 shares of common stock in exchange for total proceeds of $50,000.
On October 2, 2014, the Company sold 10,000 shares of common stock in exchange for total proceeds of $1,000.
On September 19, 2014, the Company granted 70,000 shares of common stock for services performed. The total fair value of the common stock was $7,959 based on the closing price of the Company's common stock on the date of grant. The shares were subsequently issued on October 6, 2014.
On September 19, 2014, the Company granted 100,000 shares of common stock for services performed. The total fair value of the common stock was $11,370 based on the closing price of the Company's common stock on the date of grant. The shares were subsequently issued on October 15, 2014.
On September 13, 2014, the Company granted 15,000 shares of common stock for services performed. The total fair value of the common stock was $2,250 based on the closing price of the Company's common stock on the date of grant.
On September 6, 2014, the Company granted 120,000 shares of common stock for services performed. The total fair value of the common stock was $19,200 based on the closing price of the Company's common stock on the date of grant. The shares were subsequently issued on November 3, 2014.
On September 6, 2014, the Company granted another 120,000 shares of common stock for services performed. The total fair value of the common stock was $19,200 based on the closing price of the Company's common stock on the date of grant. The shares were subsequently issued on November 3, 2014.
On September 6, 2014, the Company granted 70,000 shares of common stock for services performed. The total fair value of the common stock was $11,200 based on the closing price of the Company's common stock on the date of grant. The shares were subsequently issued on November 3, 2014.
On August 12, 2014, the Company granted 75,000 shares of common stock for services performed. The total fair value of the common stock was $12,000 based on the closing price of the Company's common stock on the date of grant.
On June 19, 2014, the Company granted 100,000 shares of common stock for services performed. The total fair value of the common stock was $42,400 based on the closing price of the Company's common stock on the date of grant.
On May 8, 2014, we sold 588,235 shares of our common stock to Kodiak in exchange for proceeds of $100,000 pursuant to our fifth Put Notice under our equity line of credit as delivered on April 30, 2014.
On April 10, 2014, we sold 211,641 shares of our common stock to Kodiak in exchange for proceeds of $100,000 pursuant to our fourth Put Notice under our equity line of credit as delivered on April 2, 2014.
On April 3, 2014, the Company granted 50,000 shares of common stock for services performed. The total fair value of the common stock was $40,500 based on the closing price of the Company's common stock on the date of grant.
On March 19, 2014, the Company granted 100,000 shares of common stock for services performed. The total fair value of the common stock was $70,000 based on the closing price of the Company's common stock on the date of grant. The shares were subsequently issued on April 17, 2014.
On March 14, 2014, we sold 181,819 shares of our common stock to Kodiak in exchange for proceeds of $100,000 pursuant to our third Put Notice under our equity line of credit as delivered on March 6, 2014.
On March 7, 2014, the Company granted 15,000 shares of common stock for services performed. The total fair value of the common stock was $15,000 based on the closing price of the Company's common stock on the date of grant.
| F-25 |
Premier Biomedical, Inc.
Notes to Financial Statements
On February 24, 2014, we sold 374,532 shares of our common stock to Kodiak in exchange for proceeds of $250,000 pursuant to our second Put Notice under our equity line of credit as delivered on February 12, 2014.
On February 20, 2014, a warrant holder elected to exercise warrants consisting of 10,000 shares of its common stock pursuant to a unit offering previously sold on March 11, 2013 in exchange for proceeds of $7,500.
On February 12, 2014, a warrant holder elected to exercise warrants consisting of 5,000 shares of its common stock pursuant to a unit offering previously sold on February 20, 2013 in exchange for proceeds of $3,750.
On February 10, 2014, the Company granted 18,462 shares of common stock for services performed. The total fair value of the common stock was $12,000 based on the closing price of the Company's common stock on the date of grant.
On February 3, 2014, we sold 666,667 shares of our common stock to Kodiak in exchange for proceeds of $150,000 pursuant to our first Put Notice under our equity line of credit as delivered on January 25, 2014.
Beneficial Conversion Feature
On December 28, 2015, the Company entered into a convertible promissory note with Redwood Fund III, Ltd. The beneficial conversion feature discount resulting from the conversion price that was $0.03810 below the market price of $0.0435 on the origination date of December 28, 2015, resulted in a debt discount value of $130,000 that was recognized as additional paid in capital and was amortized on a straight line basis over the life of the loan.
On December 24, 2015, the Company entered into a convertible promissory note with JMJ Financial. The beneficial conversion feature discount resulting from the conversion price that was $0.03262 below the market price of $0.034 on the origination date of December 24, 2015, resulted in a debt discount value of $25,000 that was recognized as additional paid in capital and was amortized on a straight line basis over the life of the loan.
On September 21, 2015, the Company entered into a convertible promissory note with Vis Vires Group, Inc. The beneficial conversion feature discount resulting from the conversion price that was $0.00747 below the market price of $0.016 on the origination date of September 21, 2015, resulted in a debt discount value of $39,448 that was recognized as additional paid in capital and was amortized on a straight line basis over the life of the loan.
On September 2, 2015, the Company entered into a convertible promissory note with JMJ Financial. The beneficial conversion feature discount resulting from the conversion price that was $0.0194 below the market price of $0.032 on the origination date of September 2, 2015, resulted in a debt discount value of $50,000 that was recognized as additional paid in capital and was amortized on a straight line basis over the life of the loan.
On February 24, 2015, the Company entered into a convertible promissory note with Adar Bays, LLC. The beneficial conversion feature discount resulting from the conversion price that was $0.0473 below the market price of $0.14 on the origination date of February 24, 2015, resulted in a debt discount value of $20,420 that was recognized as additional paid in capital and was amortized on a straight line basis over the life of the loan.
On January 30, 2015, the Company entered into a convertible promissory note with LG Capital Funding, LLC. The beneficial conversion feature discount resulting from the conversion price that was $0.042 below the market price of $0.14 on the origination date of January 30, 2015, resulted in a debt discount value of $32,143 that was recognized as additional paid in capital and was amortized on a straight line basis over the life of the loan.
On November 25, 2014, the Company entered into a convertible promissory note with Typenex Co-Investments, LLC. The beneficial conversion feature discount resulting from the conversion price that was $0.1019 below the market price of $0.225 on the November 25, 2014 origination date resulted in a debt discount value of $37,019 that was recognized as additional paid in capital and was amortized on a straight line basis over the life of the loan.
| F-26 |
Premier Biomedical, Inc.
Notes to Financial Statements
Note 11 – Series A Convertible Preferred Stock Warrants
Series A Convertible Preferred Stock Warrants Granted
No series A preferred stock warrants were granted during the years ended December 31, 2015 and 2014.
Series A Preferred Stock Warrants Cancelled
No series A preferred stock warrants were cancelled during the years ended December 31, 2015 and 2014.
Series A Preferred Stock Warrants Expired
No series A preferred stock warrants were expired during the years ended December 31, 2015 and 2014.
Series A Preferred Stock Warrants Exercised
No series A preferred stock warrants were exercised during the years ended December 31, 2015 and 2014.
The following is a summary of information about the Series A Preferred Stock Warrants outstanding at December 31, 2015.
Shares Underlying Warrants Outstanding | Shares Underlying Warrants Exercisable | |||||||||||||||||||
|
|
|
|
|
| |||||||||||||||
|
| Underlying |
|
| Weighted |
| Weighted |
|
| Shares |
|
| Weighted |
| ||||||
Range of |
|
| Shares |
|
| Remaining |
| Average |
|
| Underlying |
|
| Average |
| |||||
Exercise |
|
| Warrants |
|
| Contractual |
| Exercise |
|
| Warrants |
|
| Exercise |
| |||||
Prices |
|
| Outstanding |
|
| Life |
| Price |
|
| Exercisable |
|
| Price |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
| $ | 0.001 |
|
|
| 2,000,000 |
|
| 4.5 years |
| $ | 0.001 |
|
|
| 2,000,000 |
|
| $ | 0.001 |
|
The fair value of each warrant grant is estimated on the date of grant using the Black-Scholes option pricing model with the following weighted-average assumptions used for grants under the fixed option plan:
| December 31, |
| December 31, |
| |
| 2015 |
| 2014 |
| |
|
|
|
|
| |
Average risk-free interest rates |
| N/A |
| N/A |
|
Average expected life (in years) |
| N/A |
| N/A |
|
The Black-Scholes option pricing model was developed for use in estimating the fair value of short-term traded options that have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including expected stock price volatility. Because the Company's series A preferred stock warrants have characteristics significantly different from those of traded options and because changes in the subjective input assumptions can materially affect the fair value estimate, in management's opinion the existing models do not necessarily provide a reliable single measure of the fair value of its series A preferred stock warrants. During the years ended December 31, 2015 and 2014 there were no warrants granted with an exercise price below the fair value of the underlying stock at the grant date.
There were no series A preferred stock warrants granted during the years ended December 31, 2015 and 2014.
| F-27 |
Premier Biomedical, Inc.
Notes to Financial Statements
The following is a summary of activity of outstanding series A preferred stock warrants:
|
|
|
| Weighted |
| |||
|
|
|
| Average |
| |||
| Number of |
|
| Exercise |
| |||
| Shares |
|
| Price |
| |||
|
|
|
|
|
| |||
Balance, December 31, 2013 |
|
| 2,000,000 |
|
| $ | 0.001 |
|
Warrants expired |
|
| - |
|
|
| - |
|
Warrants granted |
|
| - |
|
|
| - |
|
Warrants exercised |
|
| - |
|
|
| - |
|
Balance, December 31, 2014 |
|
| 2,000,000 |
|
| $ | 0.001 |
|
Warrants expired |
|
| - |
|
|
| - |
|
Warrants granted |
|
| - |
|
|
| - |
|
Warrants exercised |
|
| - |
|
|
| - |
|
Balance, December 31, 2015 |
|
| 2,000,000 |
|
| $ | 0.001 |
|
|
|
|
|
|
|
|
| |
Exercisable, December 31, 2015 |
|
| 2,000,000 |
|
| $ | 0.001 |
|
Note 12 – Common Stock Warrants
Common Stock Warrants Granted (2015)
On October 7, 2015, the Company granted warrants to the following officers and directors, which will allow them to purchase shares of our common stock in the amounts indicated: William Hartman (1,000,000 shares); Mitchell Felder (1,000,000 shares), Heidi Carl (750,000 shares), John Borza (600,000 shares), Richard Najarian (200,000 shares), and Jay Rosen (200,000 shares). The exercise price of the foregoing warrants is five cents ($0.05) per share. The warrants are exercisable over ten (10) years. The total fair value of the 3,750,000 common stock warrants using the Black-Scholes option-pricing model is $31,109, or $0.0083 per share, based on a volatility rate of 232%, a risk-free interest rate of 1.75% and an expected term of 10 years, and is being amortized over the implied service term, or vesting period, of the warrants. One half of the shares underlying each of the respective warrants vest on June 15, 2016, with the balance vesting on December 15, 2016. In order for the warrants to vest on each of the foregoing dates, however, the holders must be serving in the same capacity on behalf of the Company as he or she was serving on October 21, 2015. The issuance of the warrants was fully approved by our Board of Directors on October 21, 2015, the date a fully executed resolution authorizing the issuance was delivered. The Company recognized a total of $6,079 and $-0- of professional fee expense during the years ended December 31, 2015 and 2014, respectively.
On October 7, 2015, we also issued warrants to purchase a total of one million eight hundred thousand (1,800,000) shares of our common stock amongst six members of our Scientific Advisory Board. The exercise price of the foregoing warrants is five cents ($0.05) per share. The warrants are exercisable over ten (10) years. The total fair value of the 1,800,000 common stock warrants using the Black-Scholes option-pricing model is $14,931, or $0.0083 per share, based on a volatility rate of 232%, a risk-free interest rate of 1.75% and an expected term of 10 years. In accordance with Accounting Standards Codification ("ASC") 505-50, non-employee stock based compensation awards are re-measured at each period. One half of the shares underlying each of the respective warrants vest on June 15, 2016, with the balance vesting on December 15, 2016. In order for the warrants to vest on each of the foregoing dates, however, the holders must be serving in the same capacity on behalf of the Company as he or she was serving on October 21, 2015. The issuance of the warrants was fully approved by our Board of Directors on October 21, 2015, the date a fully executed resolution authorizing the issuance was delivered. The Company recognized a total of $14,421 and $-0- of professional fee expense during the years ended December 31, 2015 and 2014, respectively.
| F-28 |
Premier Biomedical, Inc.
Notes to Financial Statements
On July 25, 2015, the Company granted cashless common stock warrants to an independent contractor to purchase a total of 500,000 shares of common stock at $0.10 per share for advisory services. The warrants are exercisable over seven (7) years from July 25, 2015. The warrants vest in full on December 1, 2015. The initial estimated value using the Black-Scholes Pricing Model, based on a volatility rate of 204% and a call option value of $0.0497, was $24,872. In accordance with Accounting Standards Codification ("ASC") 505-50, non-employee stock based compensation awards are re-measured at each period. The total fair value of the 500,000 common stock warrants using the Black-Scholes option-pricing model was re-measured at $4,720, or $0.0094 per share as of September 30, 2015, based on a volatility rate of 232%, a risk-free interest rate of 1.75% and an expected term of 7 years. The Company recognized a total of $11,943 and $-0- of professional fee expense during the years ended December 31, 2015 and 2014, respectively.
On May 30, 2015, the Company granted cashless common stock warrants to an independent contractor to purchase a total of 500,000 shares of common stock at $0.25 per share for advisory services. The warrants are exercisable over seven (7) years from May 30, 2015. The warrants vest in full on December 1, 2015. The initial estimated value using the Black-Scholes Pricing Model, based on a volatility rate of 199% and a call option value of $0.13751, was $68,756. In accordance with Accounting Standards Codification ("ASC") 505-50, non-employee stock based compensation awards are re-measured at each period. The total fair value of the 500,000 common stock warrants using the Black-Scholes option-pricing model was re-measured at $4,706, or $0.0094 per share as of September 30, 2015, based on a volatility rate of 232%, a risk-free interest rate of 1.75% and an expected term of 7 years. The Company recognized a total of $11,939 and $-0- of professional fee expense during the years ended December 31, 2015 and 2014, respectively.
On March 20, 2015, the Company granted cashless common stock warrants to an independent contractor to purchase a total of 500,000 shares of common stock at $0.20 per share for investor relation services. The warrants are exercisable over five (5) years from March 20, 2015. The warrants vest in accordance with the schedule presented below, whereby the price per share is defined by the closing bid price over three consecutive trading days:
| · | 125,000 warrants will vest at $0.20 per share |
|
|
|
| · | 125,000 warrants will vest at $0.30 per share |
|
|
|
| · | 125,000 warrants will vest at $0.40 per share |
|
|
|
| · | 125,000 warrants will vest at $0.50 per share |
The estimated value using the Black-Scholes Pricing Model, based on a volatility rate of 204% and a call option value of $0.1166, was $58,301. The vesting period is indeterminate, therefore, the Company recognized the entire $58,301 of stock based compensation expense during the years ended December 31, 2015 and 2014, respectively.
A total of $1,849,169 and $506,979 of warrants were amortized and expensed to professional fees as stock-based compensation during the years ended December 31, 2015 and 2014, respectively, including $1,529,182 and $394,540 during the years ended December 31, 2015 and 2014, respectively, related to warrants issued to related parties.
| F-29 |
Premier Biomedical, Inc.
Notes to Financial Statements
Common Stock Warrants Granted (2014)
On November 25, 2014, the Company issued warrants to purchase 351,455 shares of common stock, exercisable at $0.18 per share over a five (5) year period pursuant to a convertible debenture offering in exchange for net proceeds of $75,000 with an $86,500 face value. The fair value of the 351,455 common stock warrants using the Black-Scholes option-pricing model is $76,950, or $0.21895 per share based on a volatility rate of 193%, a risk-free interest rate of 1.58% and an expected term of 5 years. The proceeds received were allocated between the debt and warrants on a relative fair value basis, resulting in a debt discount of $37,981, which is being amortized over the life of the loan. The Company recognized $32,973 and $5,008 of amortization from the debt discount recorded to interest expense during the years ended December 31, 2015 and 2014, respectively.
On November 18, 2014, the Company granted common stock warrants to the Company's CEO to purchase a total of 1,600,000 shares of common stock at $0.25 per share for his services as an Officer. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The warrants are exercisable over seven (7) years. The fair value of the 1,600,000 common stock warrants using the Black-Scholes option-pricing model is $356,771, or $0.22298 per share, based on a volatility rate of 192%, a risk-free interest rate of 0.96% and an expected term of 3.54 years, and is being amortized over the implied service term, or vesting period, of the warrants. The Company recognized $283,368 and $73,403 of amortization recorded to professional fee expense during the years ended December 31, 2015 and 2014, respectively.
On November 18, 2014, the Company granted common stock warrants to the Company's Chairman of the Board of Directors to purchase a total of 1,600,000 shares of common stock at $0.25 per share for his services as the Chairman of the Board. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The warrants are exercisable over seven (7) years. The fair value of the 1,600,000 common stock warrants using the Black-Scholes option-pricing model is $356,771, or $0.22298 per share, based on a volatility rate of 192%, a risk-free interest rate of 0.96% and an expected term of 3.54 years, and is being amortized over the implied service term, or vesting period, of the warrants. The Company recognized $283,368 and $73,403 of amortization recorded to professional fee expense during the years ended December 31, 2015 and 2014, respectively.
On November 18, 2014, the Company granted common stock warrants to one of the Directors to purchase a total of 1,400,000 shares of common stock at $0.25 per share over a seven year period from the grant date for their services as Directors. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The total fair value of the 1,400,000 common stock warrants using the Black-Scholes option-pricing model is $312,174, or $0.22298 per share, based on a volatility rate of 192%, a risk-free interest rate of 0.96% and an expected term of 3.54 years, and is being amortized over the implied service term, or vesting period, of the warrants. The Company recognized a total of $247,947 and $64,227 of amortization recorded to professional fee expense during the years ended December 31, 2015 and 2014, respectively.
On November 18, 2014, the Company granted 1,200,000 common stock warrants to each of three Directors to purchase a total of 3,600,000 shares of common stock at $0.25 per share over a seven year period from the grant date for their service as Directors. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The total fair value of the 3,600,000 common stock warrants using the Black-Scholes option-pricing model is $802,734, or $0.22298 per share, based on a volatility rate of 192%, a risk-free interest rate of 0.96% and an expected term of 3.54 years, and is being amortized over the implied service term, or vesting period, of the warrants. The Company recognized a total of $637,578 and $165,156 of amortization recorded to professional fee expense during the years ended December 31, 2015 and 2014, respectively.
On November 18, 2014, the Company granted common stock warrants to one of the Directors to purchase a total of 400,000 shares of common stock at $0.25 per share over a seven year period from the grant date for their services as Directors. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The total fair value of the 400,000 common stock warrants using the Black-Scholes option-pricing model is $89,193, or $0.22298 per share, based on a volatility rate of 192%, a risk-free interest rate of 0.96% and an expected term of 3.54 years, and is being amortized over the implied service term, or vesting period, of the warrants. The Company recognized a total of $70,842 and $18,351 of amortization recorded to professional fee expense during the years ended December 31, 2015 and 2014, respectively.
On November 18, 2014, the Company granted 700,000 common stock warrants to each of three new advisors to purchase a total of 2,100,000 shares of common stock at $0.25 per share for services provided. The warrants carry a vesting period of 50% on January 15, 2015, and the remaining 50% vest on June 15, 2015. The warrants are exercisable over seven (7) years. In accordance with Accounting Standards Codification ("ASC") 505-50, non-employee stock based compensation awards are re-measured at each period. The total fair value of the 2,100,000 common stock warrants using the Black-Scholes option-pricing model is $228,516, or $0.1088 per share as of December 31, 2014, based on a volatility rate of 201%, a risk-free interest rate of 1.97% and an expected term of 7 years. The Company recognized a total of $223,383 and $47,016 of professional fee expense during the years ended December 31, 2015 and 2014, respectively.
| F-30 |
Premier Biomedical, Inc.
Notes to Financial Statements
Common Stock Warrants Cancelled
No warrants were cancelled during the years ended December 31, 2015 and 2014.
Common Stock Warrants Expired
A total of 50,000 and 95,000 warrants exercisable at $0.25 and $0.75 per share expired during years ended December 31, 2015 and 2014, respectively.
Common Stock Issuances for Exercise of Warrants, Related Party (2015)
On October 1, 2015, the Company issued 3,000,000 shares of common stock pursuant to the exercise of warrants by the Company's Chairman of the Board at $0.00001 per share for total proceeds of $30.
On September 10, 2015, the Company issued 1,000,000 shares of common stock pursuant to the exercise of warrants by the Company's Chairman of the Board at $0.00001 per share for total proceeds of $10.
Common Stock Warrants Exercised (2014)
On February 20, 2014, a warrant holder elected to exercise warrants consisting of a total of 10,000 shares of its common stock pursuant to a unit offering previously sold on March 11, 2013, in exchange for total proceeds of $7,500.
On February 12, 2014, a warrant holder elected to exercise warrants consisting of a total of 5,000 shares of its common stock pursuant to a unit offering previously sold on February 20, 2013 in exchange for total proceeds of $3,750.
The following is a summary of information about the Common Stock Warrants outstanding at December 31, 2015.
Shares Underlying Warrants Outstanding | Shares Underlying Warrants Exercisable | |||||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
|
|
|
|
|
| Weighted |
|
|
|
|
|
|
|
|
| |||||
|
|
| Shares |
|
| Average |
| Weighted |
|
| Shares |
|
| Weighted |
| |||||
Range of |
|
| Underlying |
|
| Remaining |
| Average |
|
| Underlying |
|
| Average |
| |||||
Exercise |
|
| Warrants |
|
| Contractual |
| Exercise |
|
| Warrants |
|
| Exercise |
| |||||
Prices |
|
| Outstanding |
|
| Life |
| Price |
|
| Exercisable |
|
| Price |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
$ | 0.00001 – $1.45 |
|
|
| 53,591,455 |
|
| 5.27 years |
| $ | 0.14633 |
|
|
| 47,541,455 |
|
| $ | 0.15701 |
|
The fair value of each warrant grant is estimated on the date of grant using the Black-Scholes option pricing model with the following weighted-average assumptions used for grants under the fixed option plan:
| December 31, |
|
| December 31, |
| |||
| 2015 |
|
| 2014 |
| |||
|
|
|
|
|
| |||
Average risk-free interest rates |
|
| 1.75 | % |
|
| 1.18 | % |
Average expected life (in years) |
|
| 9.22 |
|
|
| 4.24 |
|
The Black-Scholes option pricing model was developed for use in estimating the fair value of short-term traded options that have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including expected stock price volatility. Because the Company's common stock warrants have characteristics significantly different from those of traded options and because changes in the subjective input assumptions can materially affect the fair value estimate, in management's opinion the existing models do not necessarily provide a reliable single measure of the fair value of its common stock warrants. During the years ended December 31, 2015 and 2014 there were no warrants granted with an exercise price below the fair value of the underlying stock at the grant date.
| F-31 |
Premier Biomedical, Inc.
Notes to Financial Statements
The weighted average fair value of warrants granted with exercise prices at the current fair value of the underlying stock was approximately $0.0784 and $0.2256 per warrant granted during the years ended December 31, 2015 and 2014, respectively.
The following is a summary of activity of outstanding common stock warrants:
|
|
|
| Weighted |
| |||
|
|
|
| Average |
| |||
| Number of |
|
| Exercise |
| |||
| Shares |
|
| Price |
| |||
|
|
|
|
|
| |||
Balance, December 31, 2013 |
|
| 39,650,000 |
|
| $ | 0.1172 |
|
Warrants cancelled |
|
| (95,000 | ) |
|
| (0.75 | ) |
Warrants granted |
|
| 11,051,455 |
|
|
| 0.2477 |
|
Warrants exercised |
|
| (15,000 | ) |
|
| (0.75 | ) |
Balance, December 31, 2014 |
|
| 50,591,455 |
|
| $ | 0.1443 |
|
Warrants cancelled |
|
| (50,000 | ) |
|
| (0.25 | ) |
Warrants granted |
|
| 7,050,000 |
|
|
| 0.0784 |
|
Warrants exercised |
|
| (4,000,000 | ) |
|
| (0.00001 | ) |
Balance, December 31, 2015 |
|
| 53,591,455 |
|
| $ | 0.1463 |
|
|
|
|
|
|
|
|
| |
Exercisable, December 31, 2015 |
|
| 47,541,455 |
|
| $ | 0.15701 |
|
Note 13 – Income Taxes
The Company accounts for income taxes under FASB ASC 740-10, which requires use of the liability method. FASB ASC 740-10-25 provides that deferred tax assets and liabilities are recorded based on the differences between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes, referred to as temporary differences.
For the years ended December 31, 2015 and 2014, the Company incurred a net operating loss and, accordingly, no provision for income taxes has been recorded. In addition, no benefit for income taxes has been recorded due to the uncertainty of the realization of any tax assets. At December 31, 2015 and December 31, 2014, the Company had approximately $2,662,993 and $1,930,314 of federal net operating losses, respectively. The net operating loss carry forwards, if not utilized, will begin to expire in 2031.
The components of the Company's deferred tax asset are as follows:
| December 31, |
|
| December 31, |
| |||
| 2015 |
|
| 2014 |
| |||
Deferred tax assets: |
|
|
|
|
|
| ||
Net operating loss carry forwards |
| $ | 932,050 |
|
| $ | 675,610 |
|
|
|
|
|
|
|
|
| |
Net deferred tax assets before valuation allowance |
| $ | 932,050 |
|
| $ | 675,610 |
|
Less: Valuation allowance |
|
| (932,050 | ) |
|
| (675,610 | ) |
Net deferred tax assets |
| $ | - |
|
| $ | - |
|
Based on the available objective evidence, including the Company's history of losses, management believes it is more likely than not that the net deferred tax assets will not be fully realizable. Accordingly, the Company provided for a full valuation allowance against its net deferred tax assets at December 31, 2015 and 2014, respectively.
| F-32 |
Premier Biomedical, Inc.
Notes to Financial Statements
A reconciliation between the amounts of income tax benefit determined by applying the applicable U.S. and State statutory income tax rate to pre-tax loss is as follows:
| December 31, |
|
| December 31, |
| |||
| 2015 |
|
| 2014 |
| |||
|
|
|
|
|
| |||
Federal and state statutory rate |
|
| 35 | % |
|
| 35 | % |
Change in valuation allowance on deferred tax assets |
| (35 | %) |
| (35 | %) | ||
In accordance with FASB ASC 740, the Company has evaluated its tax positions and determined there are no uncertain tax positions.
Note 14 – Subsequent Events
Convertible Debts
On December 28, 2015, the Company entered into a Securities Purchase Agreement with Redwood Management, LLC, pursuant to which we agreed to sell, and Redwood Management, LLC, or its assigns, agreed to purchase, One Million Six Hundred Thousand Dollars ($1,600,000) in 10% Convertible Promissory Notes. On February 26, 2016, and again on March 7, 2016, the Securities Purchase Agreement was amended, and the total amount of funding to which Redwood is obligated was reduced to $525,000. The Notes have an original issue discount of five percent (5%). The following notes were issued subsequent to the original convertible note with a face value of $157,500 issued on December 28, 2015:
· $131,250, Redwood Fund III Ltd. originated on January 8, 2016, maturing on October 8, 2016 · $ 78,750, Redwood Fund III Ltd. originated on February 22, 2016, maturing on November 22, 2016 · $ 78,750, Redwood Fund III Ltd. originated on March 7, 2016, maturing on December 7, 2016 · $105,000, Redwood Fund III Ltd. originated on March 11, 2016, maturing on December 11, 2016
These notes are convertible after ninety (90) days into our common stock at a conversion price equal to 60% of the lowest traded price of the Common Stock in the fifteen (15) Trading Days prior to the Conversion Date. The shares of common stock issuable upon conversion of the notes will be restricted securities as defined in Rule 144 promulgated under the Securities Act of 1933. The notes can be prepaid by us at any time upon ten (10) days written notice to Redwood for a cash amount equal to the sum of the then outstanding principal amount of the note and interest multiplied by 130%. Pursuant to a Registration Rights Agreement, we agreed to register the shares underlying conversion of the notes.
Convertible Debt Repayments
On January 19, 2016, the Company repaid the First Vis Vires Note that originated on September 3, 2015 out of proceeds received from the Redwood Fund notes. The repayment consisted of $61,262, consisted of $48,000 of principal and $13,262 of interest and prepayment penalties.
On March 1, 2016, the Company repaid the Second JMJ Note that originated on December 15, 2016 out of proceeds received from the Redwood Fund notes. The repayment consisted of $27,500 of principal.
| F-33 |
Premier Biomedical, Inc.
Notes to Financial Statements
Change in Common Stock Authorization
On February 9, 2016, the Company's amendment to its articles of incorporation to increase the authorized shares from three hundred million to one billion authorized shares of $0.00001 par value Common Stock became effective.
Common Stock Issuances for Exercise of Preferred Stock Warrants, Related Parties
On January 29, 2016, the Company issued 1,000,000 shares of series A preferred stock pursuant to the exercise of warrants by the Company's CEO at $0.001 per share for total proceeds of $1,000. Each share of Series A Convertible Preferred Stock is convertible, at the election of the holder thereof, into one (1) share of our common stock, and has one hundred (100) votes per share.
On January 29, 2016, the Company issued 1,000,000 shares of series A preferred stock pursuant to the exercise of warrants by the Company's Chairman of the Board at $0.001 per share for total proceeds of $1,000. Each share of Series A Convertible Preferred Stock is convertible, at the election of the holder thereof, into one (1) share of our common stock, and has one hundred (100) votes per share.
Common Stock Issuances for Exercise of Common Stock Warrants
On February 10, 2016, the Company issued 2,248,846 shares of common stock pursuant to the cashless exercise of 2,250,000 warrants by the Company's securities attorney at $0.00001 per share.
Common Stock Issuances for Debt Conversions
On March 2, 2016, the Company issued 2,000,000 shares of common stock pursuant to the conversion of $13,200 of principal on the First JMJ Financial Note. The note was converted in accordance with the conversion terms; therefore no gain or loss has been recognized.
Common Stock Issuances for Debt Financing
On February 10, 2016, the Company issued 600,000 shares of our common stock to a third-party for services rendered in connection with our recent financing transactions with Redwood Fund III, Ltd. The total fair value of the common stock was $9,600 based on the closing price of the Company's common stock on the date of grant.
On February 10, 2016, the Company issued 2,400,000 shares of our common stock to a third-party for services rendered in connection with our recent financing transactions with Redwood Fund III, Ltd. The total fair value of the common stock was $38,400 based on the closing price of the Company's common stock on the date of grant.
F-34
ITEM 9 CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
There are no events required to be disclosed under this Item.
ITEM 9A - CONTROLS AND PROCEDURES
(a) Disclosure Controls and Procedures
We conducted an evaluation, with the participation of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as of December 31, 2015, to ensure that information required to be disclosed by us in the reports filed or submitted by us under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Securities Exchange Commission's rules and forms, including to ensure that information required to be disclosed by us in the reports filed or submitted by us under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that as of December 31, 2015, our disclosure controls and procedures were not effective at the reasonable assurance level due to the material weaknesses identified and described in Item 9A(b).
Our principal executive officers do not expect that our disclosure controls or internal controls will prevent all error and all fraud. Although our disclosure controls and procedures were designed to provide reasonable assurance of achieving their objectives and our principal executive officers have determined that our disclosure controls and procedures are effective at doing so, a control system, no matter how well conceived and operated, can provide only reasonable, not absolute assurance that the objectives of the system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented if there exists in an individual a desire to do so. There can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
| 44 |
(b) Management Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act, as amended, as a process designed by, or under the supervision of, our principal executive and principal financial officer and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States and includes those policies and procedures that:
· Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and any disposition of our assets; · Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and · Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2015. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on this assessment, Management identified the following two material weaknesses that have caused management to conclude that, as of December 31, 2015, our disclosure controls and procedures, and our internal control over financial reporting, were not effective at the reasonable assurance level:
We do not have written documentation of our internal control policies and procedures. Written documentation of key internal controls over financial reporting is a requirement of Section 404 of the Sarbanes-Oxley Act. Management evaluated the impact of our failure to have written documentation of our internal controls and procedures on our assessment of our disclosure controls and procedures and has concluded that the control deficiency that resulted represented a material weakness. We do not have sufficient segregation of duties within accounting functions, which is a basic internal control. Due to our size and nature, segregation of all conflicting duties may not always be possible and may not be economically feasible. However, to the extent possible, the initiation of transactions, the custody of assets and the recording of transactions should be performed by separate individuals. Management evaluated the impact of our failure to have segregation of duties on our assessment of our disclosure controls and procedures and has concluded that the control deficiency that resulted represented a material weakness.
1. 2.
| 45 |
To address these material weaknesses, management performed additional analyses and other procedures to ensure that the financial statements included herein fairly present, in all material respects, our financial position, results of operations and cash flows for the periods presented. Accordingly, we believe that the financial statements included in this report fairly present, in all material respects, our financial condition, results of operations and cash flows for the periods presented.
This Annual Report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by our registered public accounting firm pursuant to the rules of the Securities and Exchange Commission that permit us to provide only our management's report in this Annual Report.
(c) Remediation of Material Weaknesses
To remediate the material weakness in our documentation, evaluation and testing of internal controls we plan to engage a third-party firm to assist us in remedying this material weakness once resources become available.
We also intend to remedy our material weakness with regard to insufficient segregation of duties by hiring additional employees in order to segregate duties in a manner that establishes effective internal controls once resources become available.
(d) Changes in Internal Control over Financial Reporting
No change in our system of internal control over financial reporting occurred during the period covered by this report, fourth quarter of the fiscal year ended December 31, 2015, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B – OTHER INFORMATION
There are no events required to be disclosed by the Item.
| 46 |
PART III
ITEM 10 – DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors and Executive Officers
The following table sets forth the names, ages, and biographical information of each of our current directors and executive officers, and the positions with the Company held by each person, and the date such person became a director or executive officer of the Company. Our executive officers are elected annually by the Board of Directors. The directors serve one-year terms until their successors are elected. The executive officers serve terms of one year or until their death, resignation or removal by the Board of Directors. Family relationships among any of the directors and officers are described below.
Name | Age | Position(s) | ||
William A. Hartman | 74 | President, Chief Executive Officer, and Director (June 2010) | ||
Dr. Mitchell S. Felder | 61 | Chairman of the Board of Directors and the Scientific Advisory Board (June 2010) | ||
Heidi H. Carl | 45 | Secretary and Treasurer, Director (June 2010) | ||
Jay Rosen | 60 | Director (June 2010) | ||
John S. Borza | 62 | Director (August 2012) |
William A. Hartman, age 74, is our President and Chief Executive Officer and a member of our Board of Directors. From March 2008 until June 2010, Mr. Hartman was not directly employed but was planning the formation of Premier Biomedical, Inc. From October 2006 to March 2008, Mr. Hartman was the Chief Operating Officer of Nanologix, Inc. From July 1991 to July 2000, Mr. Hartman was a Director at TRW Automotive. From 1984 to 1991, Mr. Hartman was Chief Engineer at TRW Automotive and from 1979 to 1984 he was Division Quality Compliance Manager at Ford Motor Company. At TRW Automotive, Mr. Hartman was one of the auto industry pioneers of the concept of grouping related components into systems and modules and shipping just-in-time to the vehicle assembly plants. He founded and headed a separate business group within TRW Automotive with plants in the U.S., Mexico and Europe with combined annual sales of $1.3 Billion. Academic credentials include a BSME degree from Youngstown State University and a MSIA degree (Industrial Administration/Management) from the University of Michigan.
Dr. Mitchell S. Felder, age 61, is our Chairman of the Board of Directors and our Scientific Advisory Board. Dr. Felder is a practicing Board Certified Neurologist. Dr. Felder acquired a B.A. Degree from the University of Pennsylvania in 1975 and an M.D. Degree from the University of Rome, Faculty of Medicine in 1983. He has been Board Certified by both the American Academy of Clinical Neurology and the American Board of Psychiatry and Neurology. Dr. Felder has authored or co-authored six publications, three studies, and has 18 issued patents. Dr. Felder is the former President, Chairman, and founder of Infectech/Nanologix (from its founding in 1989 through March 2007)—growing the company from startup to a $100 million market cap. During the past five years, Dr. Felder has had as his principal occupation and employment work as an attending neurologist. Dr. Felder is presently an attending neurologist at the William Beaumont Army Medical Center in El Paso, Texas. Dr. Felder has more than 20 years of management experience.
| 47 |
Heidi H. Carl, age 45, is our Chief Financial Officer, Secretary, Treasurer, and a member of our Board of Directors. From May 2009 until June 2010, Ms. Carl was not directly employed but was working with Mr. Hartman in planning the formation of Premier Biomedical, Inc. From June 2007 to May 2009, Ms. Carl was the Product Development Specialist at General Motors Corporation. From May 2006 to May 2007, Ms. Carl was the Associate Marketing Manager at General Motors Corporation. From May 2003 to May 2006, Ms. Carl was the Marketing Specialist at General Motors Corporation and from May 1999 to May 2003, Ms. Carl was the District Area Parts Manager at General Motors Corporation. Academic credentials include a BSBA degree from Madonna University and an ASBA degree from Oakland Community College.
Jay Rosen, age 60, has been a member of our Board of Directors since our inception in June 2010. Mr. Rosen has been a partner at Rosen Associates, a real estate holding and management company, since 1971. He is also a partner at Midway Industrial Terminal, a real estate holding and management company, and has been since 2005. Mr. Rosen privately owns and manages the Rosen Farm, cellular towers and various other real estate properties, is the President of XintCorp, a small start-up company for developing intellectual property, and is a former member of the NY Mercantile Exchange and the New York Futures Exchange. Mr. Rosen studied economics and finance at New York University and Columbia University.
John S. Borza, PE, AVS, age 62, was appointed to our Board of Directors on August 17, 2012. Mr. Borza is currently the President and Chief Executive Officer of Value Innovation, LLC, a consulting firm focused on value engineering and creative problem solving, where he has served since August 2009. Prior to Value Innovation, Mr. Borza was a Specialist with TRW Automotive from September 2007 to September 2009, and a Director at TRW Automotive from May 1999 to September 2007. Earlier in his career, Mr. Borza worked in R&D for 12 years on a variety of products and technologies in various capacities ranging from Engineer to Chief Engineer, before moving into launch and production support roles. Mr. Borza is an Altshuller Institute certified TRIZ Practitioner, and a SAVE International certified Associate Value Specialist. He is active in the local chapter of SAVE International. Mr. Borza holds a BS degree in Electrical Engineering and an MBA from the University of Michigan.
Family Relationships
Heidi H. Carl is the daughter of William A. Hartman.
Other Directorships; Director Independence
Other than as set forth above, none of our officers and directors is a director of any company with a class of securities registered pursuant to section 12 of the Exchange Act or subject to the requirements of section 15(d) of such Act or any company registered as an investment company under the Investment Company Act of 1940.
| 48 |
For purposes of determining director independence, we have applied the definitions set out in NASDAQ Rule 5605(a)(2). The OTCQB on which shares of common stock are quoted does not have any director independence requirements. The NASDAQ definition of "Independent Officer" means a person other than an Executive Officer or employee of the Company or any other individual having a relationship which, in the opinion of the Company's Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. According to the NASDAQ definition, Mssrs. Rosen and Borza are independent directors.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires the Company's directors and executive officers and persons who own more than ten percent of a registered class of the Company's equity securities to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities of the Company. Officers, directors and greater than ten percent shareholders are required by SEC regulations to furnish the Company with copies of all Section 16(a) forms they file.
To the Company's knowledge, the following is a list of individuals that have not filed, or filed late, a report reflecting a change in ownership as required pursuant to Section 16(a) of the Securities Act of 1934:
Name of Individual |
| Number of |
|
| Number of |
| ||
|
|
|
|
|
| |||
Justin Felder |
|
| 5 |
|
|
| 7 |
|
Board Committees
Our Board of Directors does not maintain a separate audit, nominating or compensation committee. Functions customarily performed by such committees are performed by its Board of Directors as a whole. We are not required to maintain such committees under the applicable rules of the Over-the-Counter Bulletin Board. We do not currently have an "audit committee financial expert" since we currently do not have an audit committee in place. We intend to create board committees, including an independent audit committee, in the near future.
We do not currently have a process for security holders to send communications to the Board.
During the fiscal years ended December 31, 2015 and 2014, the Board of Directors met approximately on a bi-weekly basis.
| 49 |
Involvement in Certain Legal Proceedings
None of our officers or directors has, in the past ten years, filed bankruptcy, been convicted in a criminal proceeding or named in a pending criminal proceeding, been the subject of any order, judgment, or decree of any court permanently or temporarily enjoining him or her from any securities activities, or any other disclosable event required by Item 401(f) of Regulation S-K.
Code of Ethics
We have not adopted a written code of ethics, primarily because we believe and understand that our officers and directors adhere to and follow ethical standards without the necessity of a written policy.
ITEM 11 EXECUTIVE COMPENSATION
Narrative Disclosure of Executive Compensation
Effective on September 28, 2012, we entered into employment agreements with our President and Chief Executive Officer, William A. Hartman, and our Chairman of the Board of Directors and Chairman of the Scientific Advisory Board, Dr. Mitchell S. Felder. In December 2012, the Company and Dr. Felder agreed to terminate his employment agreement, effective as of its date of inception.
Pursuant to the employment agreement with Hartman, he will be compensated in the amount of $150,000 per year for the duration of the agreement. Pursuant to the agreement, Hartman has waived the salary and the accrual thereof in exchange for being issued a Common Stock Purchase Warrant whereby Hartman may purchase a maximum of 105,000 shares of our common stock at a purchase price of $1.45 per share. The agreement has a one-year term and provides for two (2) years of severance in the event Hartman is terminated due to death, disability or without cause.
We do not currently have written employment agreements with our other executives. All are at-will employees or consultants whose compensation is set forth in the Summary Compensation Table below.
| 50 |
Summary Compensation Table
The following table sets forth information with respect to compensation earned by our Chief Executive Officer, President, and Chief Financial Officer for the years ended December 31, 2015, 2014, and 2013.
Name and |
| Year |
| Salary ($) |
|
| Bonus ($) |
|
| Stock Awards ($) |
|
| Option Awards ($) |
|
| Non-Equity Incentive Plan Compensation ($) |
|
| Nonqualified Deferred Compensation ($) |
|
| All Other Compensation ($) |
|
| Total ($) |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||
William A. |
| 2015 (2) |
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| 8,296 |
|
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| 8,296 |
|
Hartman, CEO (1) |
| 2014 (2) |
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| 356,771 |
|
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| 356,771 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Heidi H. Carl |
| 2015 (4) |
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| 6,222 |
|
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| 6,222 |
|
Secretary/Treasurer (3) |
| 2014 (4) |
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| 312,174 |
|
|
| -0- |
|
|
| -0- |
|
|
| -0- |
|
|
| 312,174 |
|
____________________
Mr. Hartman does not receive a salary for his services as Chief Executive Officer. Option awards consist of warrants to purchase 1,600,000 shares of our common stock at an exercise price of $0.25 over seven (7) years from the grant date of November 18, 2014, vesting in two (2) equal tranches, on January 15, 2015 and June 15, 2015, each with the condition that the individual is an employee of or rendering services to the Company on such date. Ms. Carl does not receive a salary for her services as Secretary and Treasurer. Ms. Carl received warrants to purchase 70,000 shares of our common stock at an exercise price of $1.45, vesting in two (2) tranches, on January 15, 2013 and June 15, 2013, each with the condition that the individual is an employee of or rendering services to the Company on such date. In order to exercise the warrants, our common stock must reach a closing bid price of Three Dollars ($3.00) per share and remain at or above Three Dollars ($3.00) per share for thirty (30) consecutive trading days prior to exercise. Option awards consist of warrants to purchase 1,400,000 shares of our common stock at an exercise price of $0.25 over seven (7) years from the grant date of November 18, 2014, vesting in two (2) equal tranches, on January 15, 2015 and June 15, 2015, each with the condition that the individual is an employee of or rendering services to the Company on such date.
(1) (2) (3) (4)
| 51 |
Director Compensation
On November 18, 2014, we issued warrants to the following officers and directors, which will allow them to purchase shares of our common stock in the amounts indicated: William Hartman (1,600,000 shares); Richard Najarian (1,200,000 shares); John Borza (1,200,000 shares); Justin Felder (1,200,000 shares); Jay Rosen (400,000 shares); Heidi Carl (1,400,000 shares); and Mitchell Felder (1,600,000 shares). One half of the shares underlying each of the respective warrants vest on January 15, 2015, with the balance vesting on June 15, 2015. In order for the warrants to vest on each of the foregoing dates, however, the holders must be serving in the same capacity on behalf of the Company as he or she was serving on November 18, 2014, i.e., the effective date of the grant. The issuance of the warrants was fully approved by our Board of Directors on December 9, 2014, the date a fully executed resolution authorizing the issuance was delivered to us, and issued on December 10, 2014.
On October 21, 2015, we issued warrants to the following officers and directors, which will allow them to purchase shares of our common stock in the amounts indicated: William Hartman (1,000,000 shares); Mitchell Felder (1,000,000 shares), Heidi Carl (750,000 shares), John Borza (600,000 shares), Richard Najarian (200,000 shares), and Jay Rosen (200,000 shares). One half of the shares underlying each of the respective warrants vest on June 15, 2016, with the balance vesting on December 15, 2016. In order for the warrants to vest on each of the foregoing dates, however, the holders must be serving in the same capacity on behalf of the Company as he or she was serving on October 21, 2015. The issuance of the warrants was fully approved by our Board of Directors on October 21, 2015, the date a fully executed resolution authorizing the issuance was delivered to us.
We do not currently have an established policy to provide compensation to members of our Board of Directors for their services in that capacity. We intend to develop such a policy in the near future.
Outstanding Equity Awards at Fiscal Year-End
We do not currently have a stock option or grant plan.
52
ITEM 12 SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth, as of March 10, 2016, certain information with respect to the Company's equity securities owned of record or beneficially by (i) each Officer and Director of the Company; (ii) each person who owns beneficially more than 5% of each class of the Company's outstanding equity securities; and (iii) all Directors and Executive Officers as a group.
Name and Address (1) |
| Common stock Ownership |
|
| Percentage of Common stock Ownership (2) |
|
| Series A Preferred Stock Ownership |
|
| Percentage of Series A Preferred Stock Ownership (3) |
| ||||
|
|
|
|
|
|
|
|
|
|
|
| |||||
William A. Hartman (4)(7) |
|
| 23,755,000 | (5) |
|
| 21.53 | % |
|
| 1,000,000 |
|
|
| 50.0 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Dr. Mitchell S. Felder (4) P.O. Box 1332 Hermitage, PA 16148 |
|
| 23,103,944 | (6) |
|
| 21.75 | % |
|
| 1,000,000 |
|
|
| 50.0 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Heidi H. Carl (4)(7) |
|
| 3,270,000 | (10) |
|
| 3.56 | % |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Jay Rosen (4) |
|
| 1,670,000 | (11) |
|
| 1.85 | % |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
John S. Borza (4) |
|
| 3,976,000 | (8) |
|
| 4.30 | % |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Richard T. Najarian |
|
| 5,061,200 | (9) |
|
| 5.50 | % |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
All Officers and Directors as a Group (5 Persons) |
|
| 55,774,944 | (5)(6)(8)(9)(10)(11) |
|
| 42.00 | % |
|
| 2,000,000 |
|
|
| 100.0 | % |
____________________
| (1) | Unless otherwise indicated, the address of the shareholder is c/o Premier Biomedical, Inc. |
| (2) | Unless otherwise indicated, based on 89,579,908 shares of common stock issued and outstanding. Shares of common stock subject to options or warrants currently exercisable, or exercisable within 60 days, are deemed outstanding for purposes of computing the percentage of the person holding such options or warrants, but are not deemed outstanding for purposes of computing the percentage of any other person. |
| (3) | Unless otherwise indicated, based on 2,000,000 shares of Series A Convertible Preferred Stock issued and outstanding. |
| 53 |
| (4) | Indicates one of our officers or directors. |
| (5) | Includes 17,000,000 shares of common stock that may be acquired upon the exercise of warrants at $0.00001 per share, 1,000,000 shares of common stock that may be acquired upon the conversion of 1,000,000 shares of Series A Convertible Preferred Stock, 50,000 shares of common stock that may be acquired at $1.45 per share, 105,000 shares of common stock that may be acquired at $1.45 per share if the Company's common stock reaches a closing bid price of $3.00 per share and remains at or above $3.00 per share for thirty (30) consecutive trading days on any and all markets or exchanges on which the Company's common stock is traded, 1,600,000 shares that may be acquired upon the exercise of warrants at $0.25 per share, and 1,000,000 shares that may be acquired upon the exercise of warrants at $0.05 per share. |
| (6) | Includes 13,000,000 shares of common stock that may be acquired upon the exercise of warrants at $0.00001 per share, 1,000,000 shares of common stock that may be acquired upon the conversion of 1,000,000 shares of Series A Convertible Preferred Stock, 50,000 shares of common stock that may be acquired at $1.45 per share, 105,000 shares of common stock that may be acquired at $1.45 per share if the Company's common stock reaches a closing bid price of $3.00 per share and remains at or above $3.00 per share for thirty (30) consecutive trading days on any and all markets or exchanges on which the Company's common stock is traded, 1,600,000 shares that may be acquired upon the exercise of warrants at $0.25 per share, and 1,000,000 shares that may be acquired upon the exercise of warrants at $0.05 per share. |
| (7) | William A. Hartman is the father of Heidi H. Carl. Mr. Hartman disclaims ownership of shares held by his daughter. |
| (8) | Includes 20,000 shares owned by Mr. Borza's spouse, 1,050,000 shares of common stock that may be acquired by Mr. Borza at $1.45 per share, 70,000 shares of common stock that can be acquired at $1.45 per share if the Company's common stock reaches a closing bid price of $3.00 per share and remains at or above $3.00 per share for thirty (30) consecutive trading days on any and all markets or exchanges on which the Company's common stock is traded, 1,200,000 shares that may be acquired upon the exercise of warrants at $0.25 per share, and 600,000 shares that may be acquired upon the exercise of warrants at $0.05 per share. |
| (9) | Includes 1,050,000 shares of common stock that may be acquired upon the exercise of warrants at $1.45 per share, and 70,000 shares that can be acquired at $1.45 per share if the Company's common stock reaches a closing bid price of $3.00 per share and remains at or above $3.00 per share for thirty (30) consecutive trading days on any and all markets or exchanges on which the Company's common stock is traded. Also includes 30,000 shares owned by each of Mr. Najarian's spouse, and two minor children (a total of 90,000 shares), 1,200,000 shares that may be acquired upon the exercise of warrants at $0.25 per share, and 200,000 shares that may be acquired upon the exercise of warrants at $0.05 per share. |
| (10) | Includes 50,000 shares of common stock that may be acquired upon the exercise of warrants at $1.45 per share, 70,000 shares of common stock that can be acquired at $1.45 per share if the Company's common stock reaches a closing bid price of $3.00 per share and remains at or above $3.00 per share for thirty (30) consecutive trading days on any and all markets or exchanges on which the Company's common stock is traded, 1,400,000 shares that may be acquired upon the exercise of warrants at $0.25 per share, and 750,000 shares that may be acquired upon the exercise of warrants at $0.05 per share. |
| (11) | Includes 50,000 shares of common stock that may be acquired upon the exercise of warrants at $1.45 per share, 400,000 shares that may be acquired upon the exercise of warrants at $0.25 per share, and 200,000 shares that may be acquired upon the exercise of warrants at $0.05 per share. |
The issuer is not aware of any person who owns of record, or is known to own beneficially, five percent or more of the outstanding securities of any class of the issuer, other than as set forth above.
There are no current arrangements which will result in a change in control.
| 54 |
ITEM 13 CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Employment Agreements
Effective on September 28, 2012, we entered into employment agreements with our President and Chief Executive Officer, William A. Hartman, and our Chairman of the Board of Directors and Chairman of the Scientific Advisory Board, Dr. Mitchell S. Felder. In December 2012, the Company and Dr. Felder agreed to terminate his employment agreement, effective as of its date of inception.
Pursuant to the employment agreement with Hartman, he will be compensated in the amount of $150,000 per year for the duration of the agreement. Pursuant to the agreement, Hartman has waived the salary and the accrual thereof in exchange for being issued a Common Stock Purchase Warrant whereby Hartman may purchase a maximum of 105,000 shares of our common stock at a purchase price of $1.45. The agreement has a one-year term and provides for two (2) years of severance in the event Hartman is terminated due to death, disability or without cause.
License Agreements
On May 12, 2010, we entered into two separate License Agreements. One License Agreement was entered into with Altman Enterprises, LLC, wherein we obtained certain exclusive rights in (i) proprietary technology that is the subject of one pending PCT patent application relating to the treatment of auto-immune diseases, and (ii) the "Feldetrex" trademark. The other License Agreement was entered into with Marv Enterprises, LLC, wherein we obtained certain exclusive rights in proprietary technology that is the subject of two PCT patent applications relating to the treatment of blood borne carcinomas and sequential extracorporeal treatment of blood. Authority to execute the License Agreements on behalf of Altman and Marv is vested in Dr. Mitchell S. Felder, the Chairman of our Board of Directors. Because the licensors are controlled by one of our directors, there may exist a conflict of interest in decisions made by the Company with respect to the licenses.
As consideration for the two licenses, we agreed to (i) pay a royalty of five percent (5%) of any sales of products using the technology, with no minimum royalty, and (ii) reimburse the licensor for any costs already incurred in pursuing its proprietary rights in the licensed technology and pay any costs incurred for maintaining or obtaining the licensors' proprietary rights in the licensed technology in the U.S. and in extending the intellectual property to other countries around the world. Licensor shall have sole discretion to select other countries into which exclusive rights in the licensed technology may be pursued, and if we decline to pay those expenses, then licensor may pay said expenses and our licensed rights in those countries will revert to the licensor.
As of December 31, 2015, we have not generated any revenues and thus have not paid any license fees. We have, however, paid $142,873 in reimbursement of expenses associated with the technology we have licensed, and owe them an additional $37,486.
| 55 |
Stock Issuances
Preferred Stock
On January 2, 2016, two of our officers and directors, William A. Hartman and Mitchell Felder, exercised warrants to acquire one million (1,000,000) shares of Series A Convertible Preferred Stock each. Each share of Series A Convertible Preferred Stock is convertible, at the election of the holder thereof, into one (1) share of our common stock, and has one hundred (100) votes per share. We issued the warrants on June 21, 2010 and they have an exercise price of $0.001 per share.
The Preferred Stock is convertible, at the option of the holder, into one share of common stock for each share of Preferred Stock converted. The holders of our Preferred Stock also have 100 votes per share of Preferred Stock that they hold. It also contains protective provisions as follows:
The Company may not take any of the following actions without the approval of a majority of the holders of the outstanding Series A Convertible Preferred Stock: (i) effect a sale of all or substantially all of the Company's assets or which results in the holders of the Company's capital stock prior to the transaction owning less than fifty percent (50%) of the voting power of the Company's capital stock after the transaction, (ii) alter or change the rights, preferences, or privileges of the Series A Convertible Preferred Stock, (iii) increase or decrease the number of authorized shares of Series A Convertible Preferred Stock, (iv) authorize the issuance of securities having a preference over or on par with the Series A Convertible Preferred Stock, or (v) effectuate a forward or reverse stock split or dividend of the Company's common stock.
Common stock
On October 21, 2015, we issued warrants to the following officers and directors, which will allow them to purchase shares of our common stock in the amounts indicated: William Hartman (1,000,000 shares); Mitchell Felder (1,000,000 shares), Heidi Carl (750,000 shares), John Borza (600,000 shares), Richard Najarian (200,000 shares), and Jay Rosen (200,000 shares). We also issued warrants to purchase a total of one million eight hundred thousand (1,800,000) shares of our common stock to six members of our Scientific Advisory Board.
On October 1, 2015, we issued 3,000,000 shares of common stock, restricted in accordance with Rule 144, to Mitchell Felder, one of our directors, upon the exercise of warrants at $0.00001 per share.
On September 10, 2015, we issued warrants to purchase five hundred thousand (500,000) shares of our common stock to a new member of our Scientific Advisory Board. The exercise price of the warrants is Ten Cents ($0.10) per share. The warrants are vested immediately.
On September 8, 2015, we issued 1,000,000 shares of common stock, restricted in accordance with Rule 144, to Mitchell Felder, one of our directors, upon the exercise of warrants at $0.00001 per share.
On November 18, 2014, we issued warrants to the following officers and directors, which will allow them to purchase shares of our common stock in the amounts indicated: William Hartman (1,600,000 shares); Richard Najarian (1,200,000 shares); John Borza (1,200,000 shares); Justin Felder (1,200,000 shares); Jay Rosen (400,000 shares); Heidi Carl (1,400,000 shares); and Mitchell Felder (1,600,000 shares).
| 56 |
On September 25, 2013, we issued 1,000,000 shares of our common stock, restricted in accordance with Rule 144, to Richard T. Najarian, one of the members of our Board of Directors, for total consideration of $50,000. The issuance was exempt from registration pursuant to Section 4(a)(2) of the Securities Act of 1933, the investor was accredited, and there was no solicitation in connection with the offering.
On February 20, 2013 and February 25, 2013, we sold an aggregate of 3,000,000 shares of our common stock, at a price of $0.05 per share, to three members of our Board of Directors as follows: Richard T. Najarian purchased 1,500,000 shares, John S. Borza purchased 1,000,000 shares, and Mitchell Felder purchased 500,000 shares, for total cash consideration of $150,000. The issuances were exempt from registration pursuant to Section 4(2) of the Securities Act of 1933, and all parties receiving shares of common stock were accredited investors because they are members of our Board of Directors.
On October 1, 2012, we issued Common stock Purchase Warrants to Richard T. Najarian and John S. Borza for their services as members of our Board of Directors. Pursuant to the warrants, both will be able to purchase a maximum of 1,000,000 shares of our common stock at an exercise price of $1.45. The warrants may be exercised immediately. The issuance of these warrants is exempt from registration pursuant to Section 4(2) of the Securities Act of 1933, and all parties receiving warrants were accredited investors because they are members of our Board of Directors.
On September 28, 2012, we issued warrants to each of the nine (9) members of our Board of Directors as compensation. Each Director received warrants to purchase 50,000 shares of our common stock at an exercise price of $1.45, vesting in two (2) tranches, on January 15, 2013 and June 15, 2013, each with the condition that the individual is a member of our Board of Directors on such date.
On September 28, 2012, we issued warrants to each of the following six (6) executives as compensation: Hartman, Dr. Felder, Heidi H. Carl, Justin Felder, Richard T. Najarian and John S. Borza. Hartman and Dr. Felder each received warrants to purchase 105,000 shares of our common stock at an exercise price of $1.45, vesting in two (2) tranches, on January 15, 2013 and June 15, 2013, each with the condition that our common stock reach a closing bid price of Three Dollars ($3.00) per share and remain at or above Three Dollars ($3.00) per share for thirty (30) consecutive trading days and that the individual is an employee of or rendering services to the Company on such date. In December 2012, the Company and Dr. Felder agreed to terminate his employment agreement, effective as of its date of inception. The other four (4) executives received warrants to purchase 70,000 shares of our common stock at an exercise price of $1.45, also vesting in two (2) tranches, on January 15, 2013 and June 15, 2013, each with the condition that our common stock reach a closing bid price of Three Dollars ($3.00) per share and remain at or above Three Dollars ($3.00) per share for thirty (30) consecutive trading days and that the individual is an employee of or rendering services to the Company on such date.
On January 20, 2011, we issued 500,000 founders shares of our common stock to Scott Barnes, for services rendered as a director. The shares are restricted in accordance with Rule 144. The issuances were exempt from registration pursuant to Section 4(2) of the Securities Act of 1933 since the shareholder was a sophisticated investor, had access to the type of information that is normally available in a prospectus, and agreed not to resell or distribute the securities to the public.
| 57 |
Directors Notes
On December 2, 2013, we entered into a Directors Bridge Loan Agreement Promissory Note dated November 18, 2013 with each of William A. Hartman, one of our officers and directors, and John S. Borza, one of our directors. Pursuant to each Promissory Note, we borrowed Fifty Thousand Dollars ($50,000). The principal amount of each Promissory Note, plus the Prepayment Premium (defined below), shall be due and payable on or before the earlier of (a) the date which is nine (9) months from the date of the note, or (b) three (3) business days following the receipt by us of funding (net of offering expenses, including finders fees, commissions, legal and other fees, and discounts) from any source, of at least One Million Dollars ($1,000,000) (the "Maturity Date"). The Prepayment Premium shall be determined by multiplying the then-outstanding principal amount of the Promissory Note by the Prepayment Premium based on the following schedule:
No. of Days After Issue Date: |
| Prepayment Premium: |
| |
0-30 days |
|
| 115 | % |
31-60 days |
|
| 120 | % |
61-90 days |
|
| 125 | % |
91-120 days |
|
| 130 | % |
121 days or more |
|
| 135 | % |
In the event the Promissory Note is not prepaid prior to the Maturity Date, the Prepayment Premium of 135% shall apply. Interest shall accrue on the outstanding principal amount on an annual basis at a rate of eight percent (8.0%).
ITEM 14 – PRINCIPAL ACCOUNTING FEES AND SERVICES
M&K CPAS, PLLC ("M&K") was the Company's independent registered public accounting firm for the years ended December 31, 2015 and 2014 and has served the Company as its independent registered public accounting firm since our inception.
Audit and Non-Audit Fees
The following table presents fees for professional services rendered by M&K for the audit of the Company's annual financial statements for the years ended December 31, 2015 and 2014.
| Years Ended December 31, |
| ||||||
|
| 2015 |
|
| 2014 |
| ||
Audit Fees (1) |
| $ | 12,350 |
|
| $ | 9,850 |
|
Audit Related Fee (2) |
|
| 9,650 |
|
|
| 8,150 |
|
Tax Fees |
|
| - |
|
|
| - |
|
All Other Fees |
|
| - |
|
|
| - |
|
Total |
| $ | 22,000 |
|
| $ | 18,000 |
|
____________________
Audit fees were principally for audit services. Audit related fees were principally for work performed in the preparation and review of the Company's quarterly reports on Form 10-Q.
(1) (2)
Of the fees described above for the years ended December 31, 2015 and 2014, all were approved by the entire Board of Directors.
58
PART IV
ITEM 15 – EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements
The following financial statements are filed as part of this report:
Report of Independent Registered Public Accounting Firm |
|
| F-1 |
|
|
|
|
| |
Balance Sheets as of December 31, 2015 and 2014 (Audited) |
|
| F-2 |
|
|
|
|
| |
Statements of Operations for the years ended December 31, 2015 and 2014 (Audited) |
|
| F-3 |
|
|
|
|
| |
Statement of Stockholders' Equity (Deficit) for the years ended December 31, 2015 and 2014 (Audited) |
|
| F-4 |
|
|
|
|
| |
Statements of Cash Flows for the years ended December 31, 2015 and 2014 (Audited) |
|
| F-5 |
|
|
|
|
| |
Notes to Financial Statements |
|
| F-6 to F-34 |
|
(a)(2) Financial Statement Schedules
We do not have any financial statement schedules required to be supplied under this Item.
(a)(3) Exhibits
Refer to (b) below.
(b) Exhibits
3.1 (1) | Articles of Incorporation of Premier Biomedical, Inc. | |
3.2 (2) | Amendment to Articles of Incorporation of Premier Biomedical, Inc. | |
3.2 (1) | Bylaws of Premier Biomedical, Inc., as amended | |
3.3 (1) | Certificate of Designation of Series A Convertible Preferred Stock | |
5.1 (2) | Legal Opinion of Clyde Snow & Sessions, PC | |
10.1 (1) | License Agreement dated May 12, 2010 with Altman Enterprises, Inc. | |
10.2 (1) | License Agreement dated May 12, 2010 with Marv Enterprises, LLC. | |
10.3 (1) | Preferred Stock Purchase Warrant issued to Mitchell Felder | |
10.4 (1) | Preferred Stock Purchase Warrant issued to William A. Hartman |
| 59 |
10.5 (1) | Common Stock Purchase Warrant issued to Mitchell Felder | |
10.6 (1) | Common Stock Purchase Warrant issued to William A. Hartman | |
10.7 (1) | Common Stock Purchase Warrant issued to The Lebrecht Group, APLC | |
10.8 (1) | Promissory Note issued to William A. Hartman dated December 31, 2010 | |
10.9 (1) | Promissory Note issued to Mitchell Felder dated December 31, 2010 | |
10.10 (1) | Promissory Note issued to William A. Hartman dated March 31, 2011 | |
10.11 (1) | Promissory Note issued to Mitchell Felder dated March 31, 2011 | |
10.12 (1) | Form of Warrant Sold in Private Placement | |
10.13 (3) | First Addendum to License Agreement dated August 17, 2011 with Marv Enterprises, LLC. | |
10.14 (3) | Frist Addendum to License Agreement dated August 17, 2011 with Altman Enterprises, LLC. | |
10.15 (4) | Collaboration Agreement with the University of Texas System dated May 9, 2012 | |
10.16 (5) | Employment Agreement with William A. Hartman, dated September 28, 2012 | |
10.17 (6) | Form of Directors and Officers Warrant | |
10.18 (7) | Form of Directors Stock Purchase Agreement | |
10.19 (8) | Cooperative Research and Development Agreement with U.S. Army Medical Research and Material Command | |
10.20 (9) | Securities Purchase Agreement dated August 13, 2013 | |
10.21 (9) | Convertible Promissory Note dated August 13, 2013 | |
10.22 (10) | Form of Directors Bridge Loan Agreement Promissory Note dated November 18, 2013 | |
10.23 (11) | Securities Purchase Agreement dated November 25, 2014 | |
10.24 (11) | Convertible Promissory Note dated November 25, 2014 |
| 60 |
10.25 (12) | Securities Purchase Agreement dated January 30, 2015 | |
10.26 (12) | Convertible Promissory Note dated January 30, 2015 | |
10.27 (13) | Securities Purchase Agreement dated February 24, 2015 | |
10.28 (13) | Convertible Promissory Note dated February 24, 2015 | |
10.29 (14) | Amendment to Note dated March 4, 2015 | |
10.30 (15) | Patent License Agreement dated March 4, 2015 | |
10.31 (16) | Common Stock Purchase Warrant dated March 20, 2015 | |
10.32 (17) | Amendment No. 1 to Patent License Agreement dated June 19, 2015. | |
10.33 (18) | Common Stock Purchase Warrant issued to Ryan Fields dated March 30, 2015 | |
10.34 (19) | Consulting Agreement with FBROCCO dated June 23, 2015 | |
10.35 (20) | Convertible Promissory Note dated September 2, 2015 | |
10.36 (21) | Securities Purchase Agreement dated September 3, 2015 with Vis Vires Group, Inc. | |
10.37 (21) | Convertible Promissory Note dated September 3, 2015 with Vis Vires Group, Inc. | |
10.38 (22) | Securities Purchase Agreement dated December 28, 2015 with Redwood Management, LLC | |
10.39 (22) | Convertible Promissory Note dated December 28, 2015 with Redwood Management, LLC | |
10.40 (22) | Registration Rights Agreement dated December 28, 2015 with Redwood Management, LLC | |
10.41 (23) | Convertible Promissory Note dated January 8, 2016, with Redwood Fund III Ltd. | |
10.42 (24) | Convertible Promissory Note dated February 22, 2016 with Redwood Management, LLC | |
10.43 (25) | First Amendment to Securities Purchase Agreement dated February 22, 2016 | |
10.44 (25) | Second Amendment to Securities Purchase Agreement dated March 7, 2016 |
| 61 |
10.45 (25) | Convertible Promissory Note dated March 7, 2016 with Redwood Management, LLC | |
10.46 (26) | Convertible Promissory Note dated March 11, 2016 with Redwood Management, LLC | |
31.1 | Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer | |
31.2 | Rule 13a-14(a)/15d-14(a) Certification of Chief Financial Officer | |
32.1 | Chief Executive Officer Certification Pursuant to 18 USC, Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
32.2 | Chief Financial Officer Certification Pursuant to 18 USC, Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| (1) | Incorporated by reference from our Registration Statement on Form S-1 dated June 13, 2011, filed with the Commission on June 14, 2011. |
| (2) | To be filed by amendment. |
| (3) | Incorporated by reference from our Registration Statement on Form S-1/A dated and filed with the Commission on October 4, 2011. |
| (4) | Incorporated by reference from our Current Report on Form 8-K filed with the Commission on May 14, 2012. |
| (5) | Incorporated by reference from our Current Report on Form 8-K filed with the Commission on October 10, 2012. |
| (6) | Incorporated by reference from our Annual Report on Form 10-K filed with the Commission on April 1, 2013. |
| (7) | Incorporated by reference from our Current Report on Form 8-K dated February 20, 2013, filed with the Commission on February 27, 2013. |
| (8) | Incorporated by reference from our Current Report on Form 8-K dated and filed with the Commission on June 12, 2013. |
| (9) | Incorporated by reference from our Current Report on Form 8-K dated August 22, 2013, filed with the Commission on August 28, 2013. |
| (10) | Incorporated by reference from our Current Report on Form 8-K dated December 6, 2013, filed with the Commission on December 9, 2013. |
| 62 |
| (11) | Incorporated by reference from our Current Report on Form 8-K dated December 1, 2014, filed with the Commission on December 2, 2014. |
| (12) | Incorporated by reference from our Current Report on Form 8-K dated February 2, 2015, filed with the Commission on February 4, 2015. |
| (13) | Incorporated by reference from our Current Report on Form 8-K dated March 2, 2015, filed with the Commission on March 4, 2015. |
| (14) | Incorporated by reference from our Current Report on Form 8-K dated and filed with the Commission on March 6, 2015. |
| (15) | Incorporated by reference from our Current Report on Form 8-K dated March 16, 2015, filed with the Commission on March 18, 2015. |
| (16) | Incorporated by reference from our Quarterly Report on Form 10-Q dated May 14, 2015, filed with the Commission on May 15, 2015. |
| (17) | Incorporated by reference from our Current Report on Form 8-K dated June 19, 2015, filed with the Commission on June 23, 2015. |
| (18) | Incorporated by reference from our Quarterly Report on Form 10-Q dated and filed with the Commission on August 14, 2015. |
| (19) | Incorporated by reference from our Current Report on Form 8-K dated July 6, 2015, filed with the Commission on July 9, 2015. |
| (20) | Incorporated by reference from our Current Report on Form 8-K dated September 8, 2015, filed with the Commission on September 9, 2015. |
| (21) | Incorporated by reference from our Current Report on Form 8-K dated September 21, 2015, filed with the Commission on September 23, 2015. |
| (22) | Incorporated by reference from our Current Report on Form 8-K dated December 30, 2015, filed with the Commission on December 31, 2015. |
| (23) | Incorporated by reference from our Current Report on Form 8-K dated January 11, 2016, filed with the Commission on January 12, 2016. |
| (24) | Incorporated by reference from our Current Report on Form 8-K dated March 1, 2016, filed with the Commission on March 3, 2016. |
| (25) | Incorporated by reference from our Current Report on Form 8-K dated and filed with the Commission on March 11, 2016. |
| (26) | Incorporated by reference from our First Amended Registration Statement on Form S-1/A dated and filed with the Commission on March 15, 2016. |
| 63 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Premier Biomedical, Inc. | |||
| |||
Dated: March 30, 2016 | By: | /s/ William A. Hartman |
|
| Name: | William A. Hartman |
| |
Its: | Chief Executive Officer |
| |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Dated: March 30, 2016 | By: | /s/ William A. Hartman |
|
| Name: | William A. Hartman |
| |
Its: | Chief Executive Officer and Director |
| |
| |||
Dated: March 30, 2016 | By: | /s/ Heidi H. Carl |
|
| Name: | Heidi H. Carl |
| |
Its: | Chief Financial Officer, Treasurer and Principal Accounting Officer |
| |
| |||
Dated: March 30, 2016 | By: | /s/ Dr. Mitchell S. Felder |
|
| Name: | Dr. Mitchell S. Felder |
| |
Its: | Director |
| |
| |||
Dated: March 30, 2016 | By: | /s/ Jay Rosen |
|
| Name: | Jay Rosen |
| |
Its: | Director |
| |
| |||
Dated: March 30, 2016 | By: | /s/ John S. Borza |
|
| Name: | John S. Borza |
| |
| Its: | Director |
|
64