Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Vitae Pharmaceuticals, Inc | a16-6695_18k.htm |
| EX-99.2 - EX-99.2 - Vitae Pharmaceuticals, Inc | a16-6695_1ex99d2.htm |
Exhibit 99.1
VTP-43742: First-in-Class RORt Inhibitor Ph 2a Proof-of-Concept Results in Psoriasis March 16, 2016

Disclaimer Regarding Forward Looking Statements This presentation includes statements that are, or may be deemed, “forward-looking statements”, within the meaning of Section 27A of the Securities Act of 1933, as amended. All statements, other than statements of historical facts, included in this presentation regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “opportunity,” “proposition,” “strategy,” “potential,” “plan” or the negative of these terms and similar expressions intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the clinical plans for VTP-43742, the potential 12 week efficacy for VTP-43742 and the Company’s projected cash runway. Actual results may be materially different. Certain information in this presentation is from previously published data and literature from prior psoriasis trials of other compounds. Extrapolation of VTP-43742 4 week data to 12 week data is not indicative of any actual clinical results or future trial outcomes. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Forward-looking statements include, but are not limited to, statements about: the timing and success of preclinical studies and clinical trials; the ability to obtain and maintain regulatory approval of our product candidates; the scope, progress, expansion and costs of developing and commercializing our product candidates; our expectations regarding our expenses and revenue; the sufficiency of our cash resources and needs for additional financing; our ability to adequately manufacture our product candidates and the raw materials utilized therein; our ability to obtain and maintain intellectual property protection for our product candidates; our expectations regarding competition; the size and growth of the potential markets for our product candidates and the ability to serve those markets; the rate and degree of market acceptance of any of our product candidates; our anticipated growth strategies; the anticipated trends and challenges in our business and the market in which we operate; our ability to establish and maintain development partnerships; our ability to attract or retain key personnel; our expectations regarding federal, state and foreign regulatory requirements; regulatory developments in the United States and foreign countries and other factors that are described in the "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of our Annual Report on Form 10-K for the year ended December 31, 2015 which has been filed with the Securities and Exchange Commission (SEC) and is available on the SEC's website at www.sec.gov. All written and verbal forward-looking statements attributable to Vitae or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Vitae cautions investors not to rely too heavily on the forward-looking statements Vitae makes or that are made on its behalf. The information in this presentation is provided only as of the date of this presentation, and Vitae undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
Agenda Welcome and Introduction J. Hatfield In-Depth Description of Data PASI score efficacy Biomarker data Safety / tolerability J. Hatfield R. Gregg External Expert’s Assessment of Data J. Krueger Next Steps / Close J. Hatfield
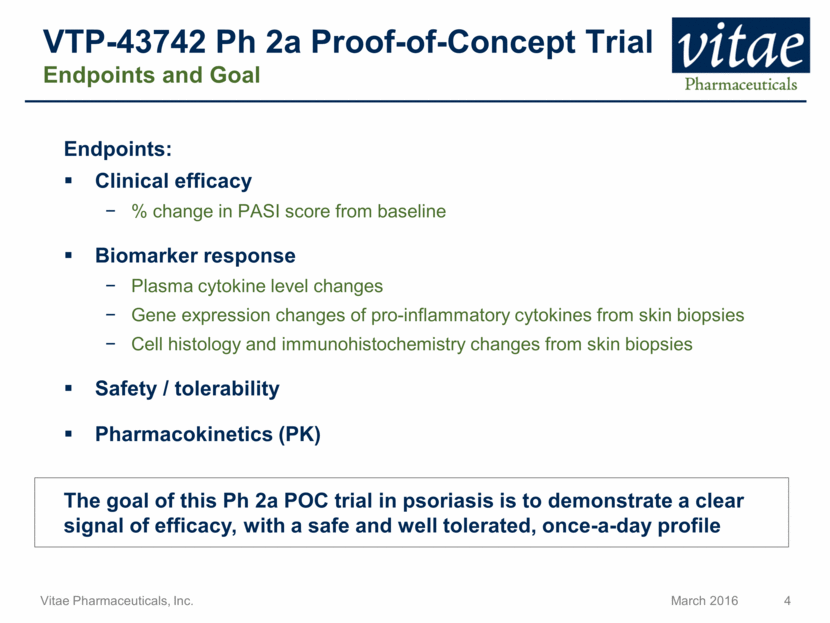
VTP-43742 Ph 2a Proof-of-Concept Trial Endpoints and Goal Endpoints: Clinical efficacy % change in PASI score from baseline Biomarker response Plasma cytokine level changes Gene expression changes of pro-inflammatory cytokines from skin biopsies Cell histology and immunohistochemistry changes from skin biopsies Safety / tolerability Pharmacokinetics (PK) The goal of this Ph 2a POC trial in psoriasis is to demonstrate a clear signal of efficacy, with a safe and well tolerated, once-a-day profile
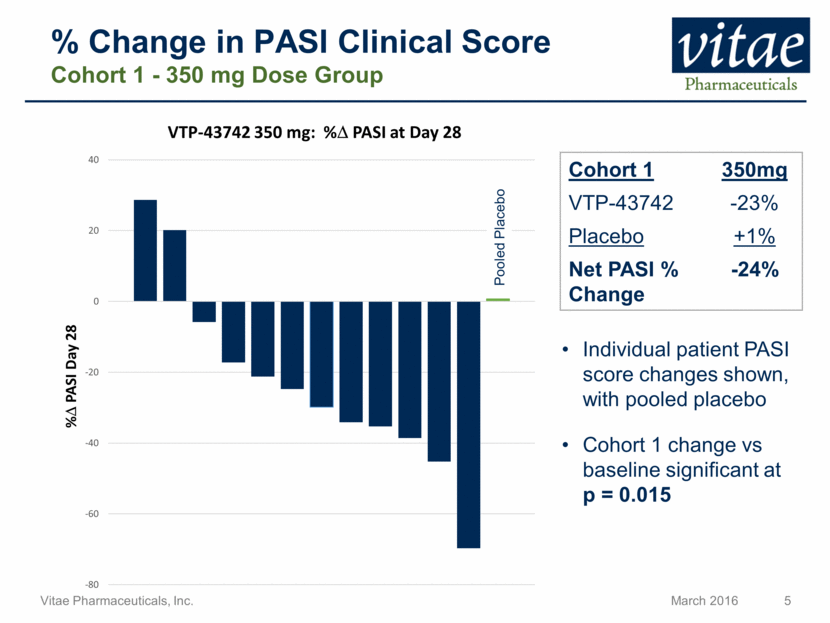
% Change in PASI Clinical Score Cohort 1 - 350 mg Dose Group Cohort 1 350mg VTP-43742 -23% Placebo +1% Net PASI % Change -24% Individual patient PASI score changes shown, with pooled placebo Cohort 1 change vs baseline significant at p = 0.015 Pooled Placebo -80 -60 -40 -20 0 20 40 % D PASI Day 28 VTP - 43742 350 mg: % D PASI at Day 28
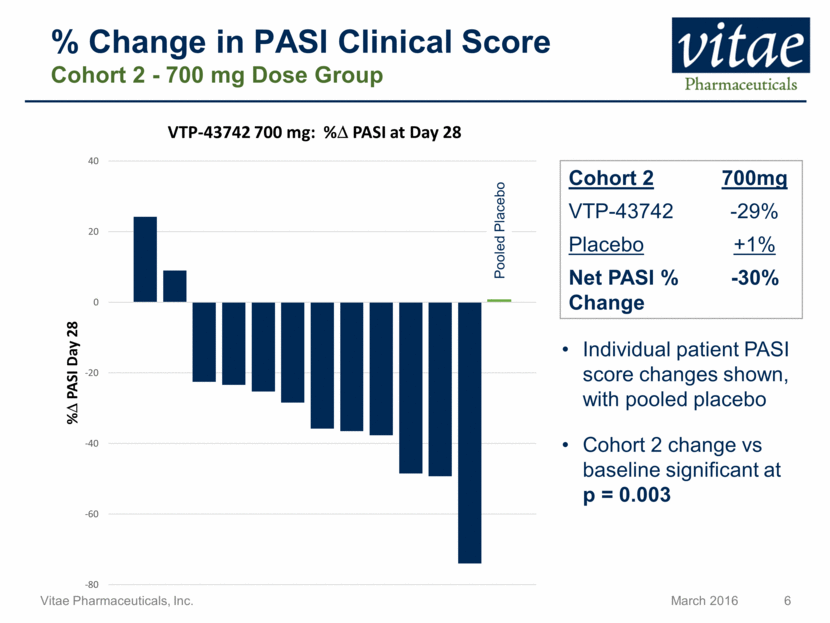
% Change in PASI Clinical Score Cohort 2 - 700 mg Dose Group Cohort 2 700mg VTP-43742 -29% Placebo +1% Net PASI % Change -30% Individual patient PASI score changes shown, with pooled placebo Cohort 2 change vs baseline significant at p = 0.003 Pooled Placebo -80 -60 -40 -20 0 20 40 % D PASI Day 28 VTP - 43742 700 mg: % D PASI at Day 28
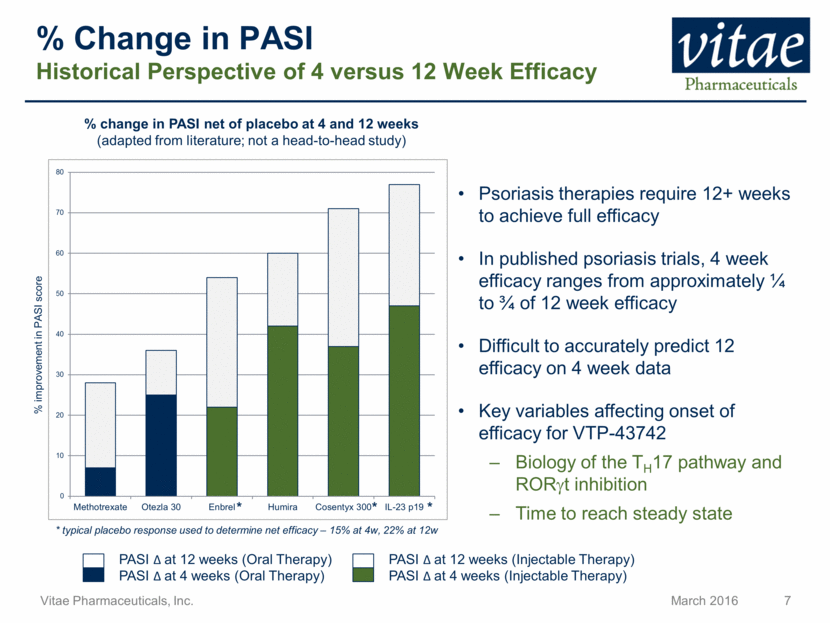
PASI at 12 weeks (Injectable Therapy) % Change in PASI Historical Perspective of 4 versus 12 Week Efficacy PASI at 4 weeks (Oral Therapy) Psoriasis therapies require 12+ weeks to achieve full efficacy In published psoriasis trials, 4 week efficacy ranges from approximately ¼ to ¾ of 12 week efficacy Difficult to accurately predict 12 efficacy on 4 week data Key variables affecting onset of efficacy for VTP-43742 Biology of the TH17 pathway and RORt inhibition Time to reach steady state % change in PASI net of placebo at 4 and 12 weeks (adapted from literature; not a head-to-head study) * * * * typical placebo response used to determine net efficacy – 15% at 4w, 22% at 12w % improvement in PASI score PASI at 12 weeks (Oral Therapy) PASI at 4 weeks (Injectable Therapy) 0 10 20 30 40 50 60 70 80 Methotrexate Otezla 30 Enbrel Humira Cosentyx 300 IL-23 p19
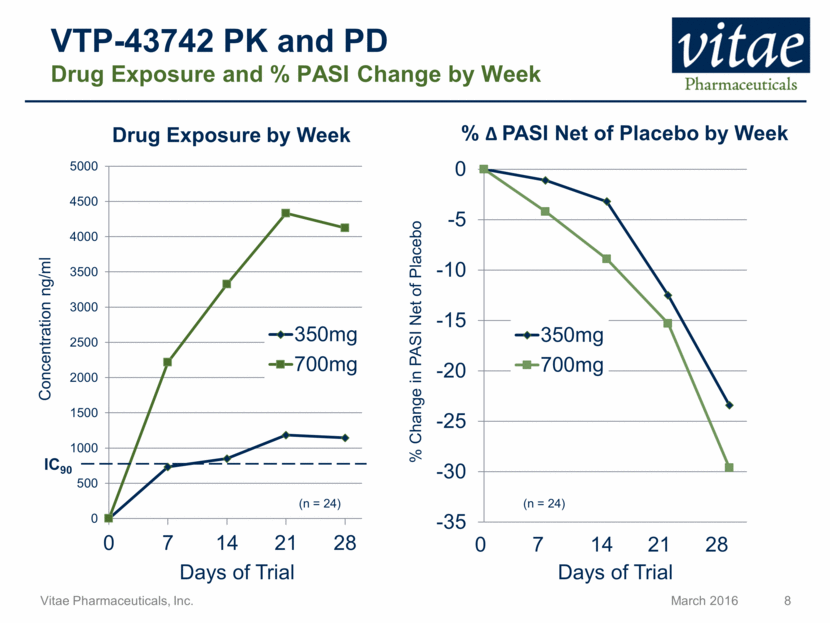
VTP-43742 PK and PD Drug Exposure and % PASI Change by Week Concentration ng/ml Days of Trial 0 7 14 21 28 Days of Trial % Change in PASI Net of Placebo Drug Exposure by Week % PASI Net of Placebo by Week IC90 (n = 24) (n = 24) -35 -30 -25 -20 -15 -10 -5 0 350mg 700mg
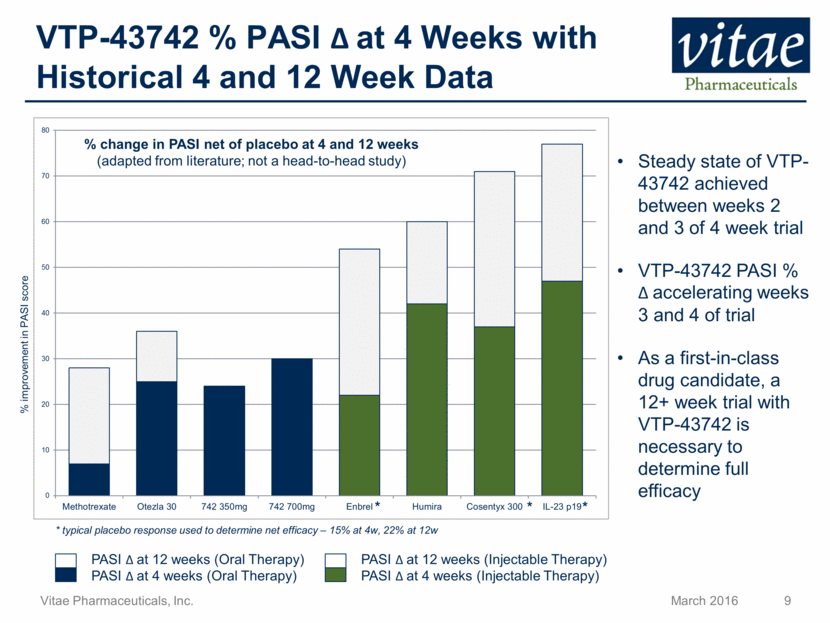
VTP-43742 % PASI at 4 Weeks with Historical 4 and 12 Week Data * * * % improvement in PASI score PASI at 12 weeks (Injectable Therapy) PASI at 4 weeks (Oral Therapy) * typical placebo response used to determine net efficacy – 15% at 4w, 22% at 12w PASI at 12 weeks (Oral Therapy) PASI at 4 weeks (Injectable Therapy) % change in PASI net of placebo at 4 and 12 weeks (adapted from literature; not a head-to-head study) Steady state of VTP-43742 achieved between weeks 2 and 3 of 4 week trial VTP-43742 PASI % accelerating weeks 3 and 4 of trial As a first-in-class drug candidate, a 12+ week trial with VTP-43742 is necessary to determine full efficacy 0 10 20 30 40 50 60 70 80 Methotrexate Otezla 30 742 350mg 742 700mg Enbrel Humira Cosentyx 300 IL-23 p19
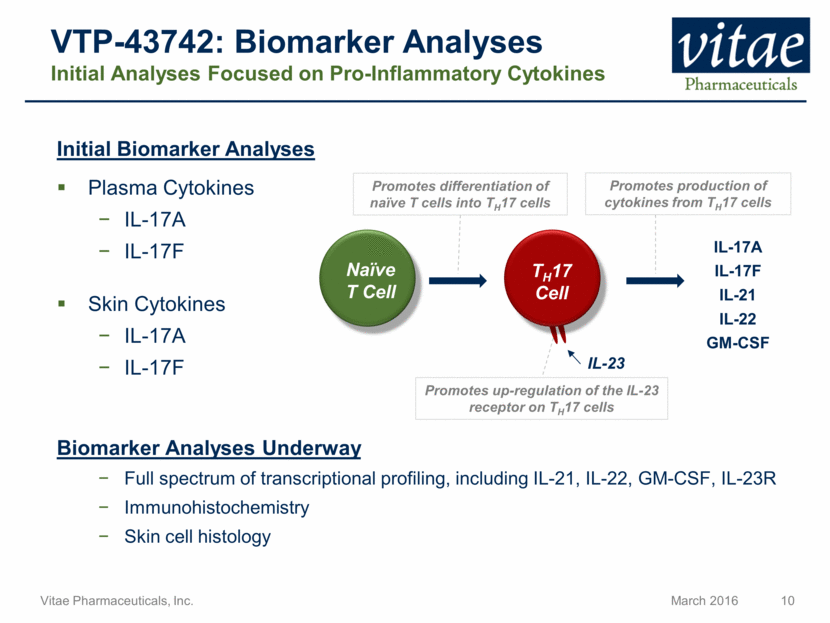
VTP-43742: Biomarker Analyses Initial Analyses Focused on Pro-Inflammatory Cytokines Initial Biomarker Analyses Plasma Cytokines IL-17A IL-17F Skin Cytokines IL-17A IL-17F Biomarker Analyses Underway Full spectrum of transcriptional profiling, including IL-21, IL-22, GM-CSF, IL-23R Immunohistochemistry Skin cell histology IL-17A IL-17F IL-21 IL-22 GM-CSF Promotes production of cytokines from TH17 cells Promotes differentiation of naïve T cells into TH17 cells Promotes up-regulation of the IL-23 receptor on TH17 cells Naïve T Cell TH17 Cell IL-23
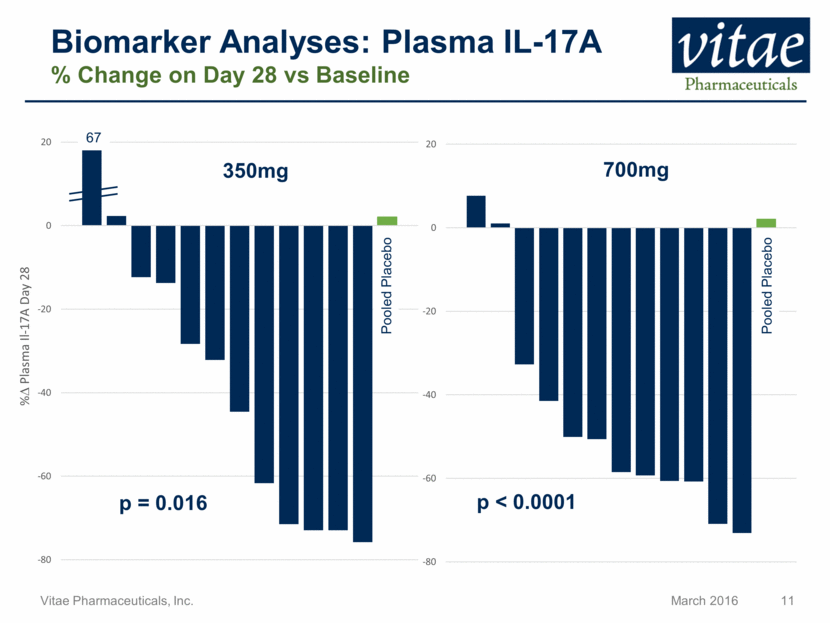
Biomarker Analyses: Plasma IL-17A % Change on Day 28 vs Baseline Pooled Placebo 700mg p < 0.0001 67 Pooled Placebo 350mg p = 0.016 -80 -60 -40 -20 0 20 -80 -60 -40 -20 0 20 % D Plasma Il - 17A Day 28
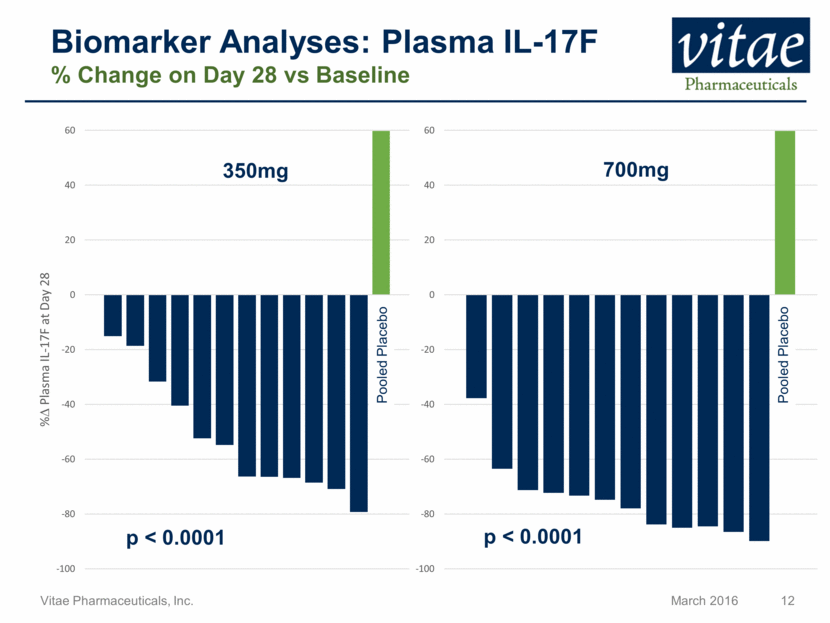
Biomarker Analyses: Plasma IL-17F % Change on Day 28 vs Baseline Pooled Placebo Pooled Placebo 350mg 700mg p < 0.0001 p < 0.0001 -100 -80 -60 -40 -20 0 20 40 60 % D Plasma IL - 17F at Day 28 -100 -80 -60 -40 -20 0 20 40 60
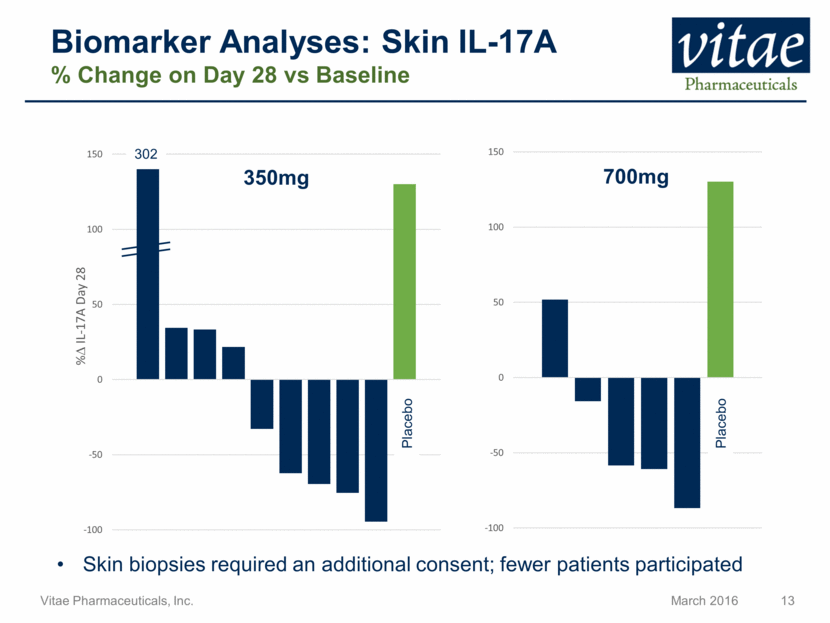
Biomarker Analyses: Skin IL-17A % Change on Day 28 vs Baseline 302 Placebo 350mg 700mg Placebo Skin biopsies required an additional consent; fewer patients participated -100 -50 0 50 100 150 -100 -50 0 50 100 150 % D IL - 17A Day 28
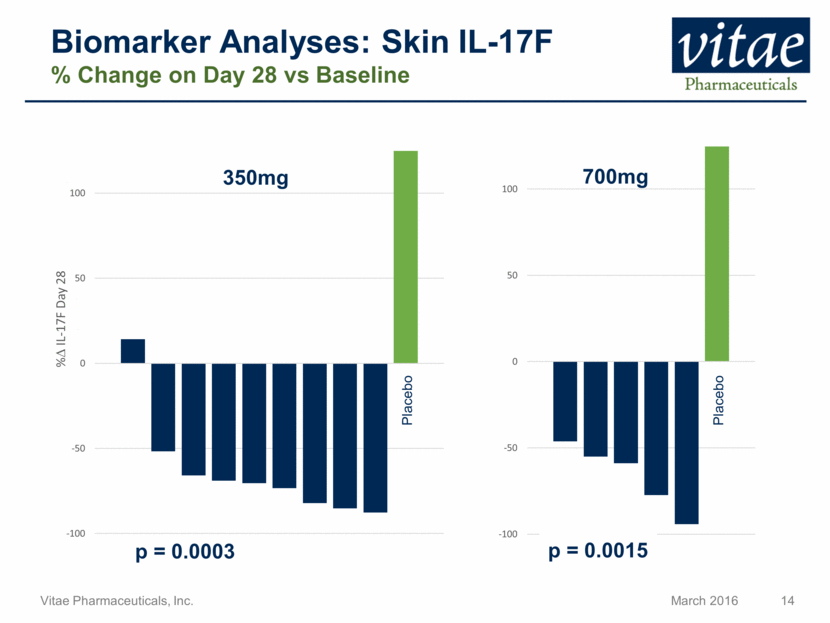
Biomarker Analyses: Skin IL-17F % Change on Day 28 vs Baseline Placebo 350mg 700mg Placebo p = 0.0003 p = 0.0015 -100 -50 0 50 100 -100 -50 0 50 100 % D IL - 17F Day 28
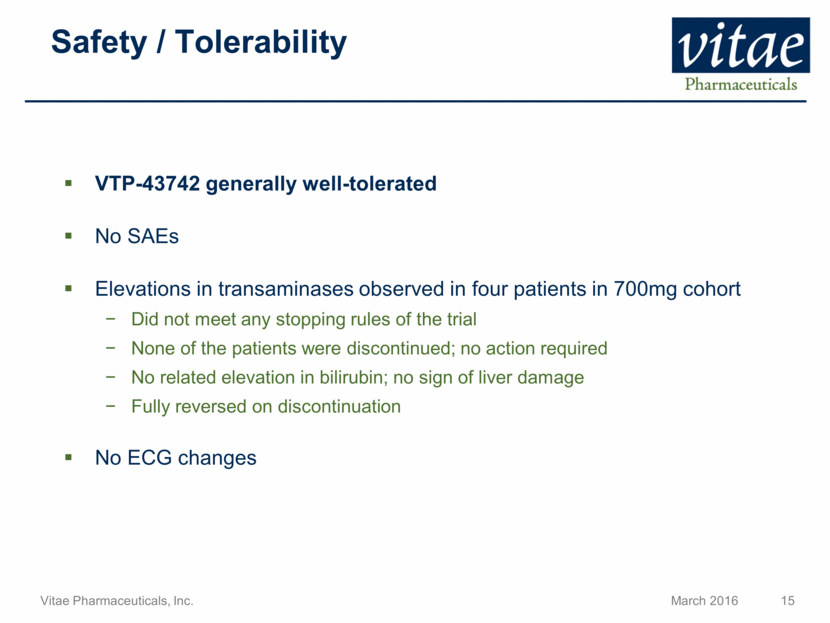
Safety / Tolerability VTP-43742 generally well-tolerated No SAEs Elevations in transaminases observed in four patients in 700mg cohort Did not meet any stopping rules of the trial None of the patients were discontinued; no action required No related elevation in bilirubin; no sign of liver damage Fully reversed on discontinuation No ECG changes
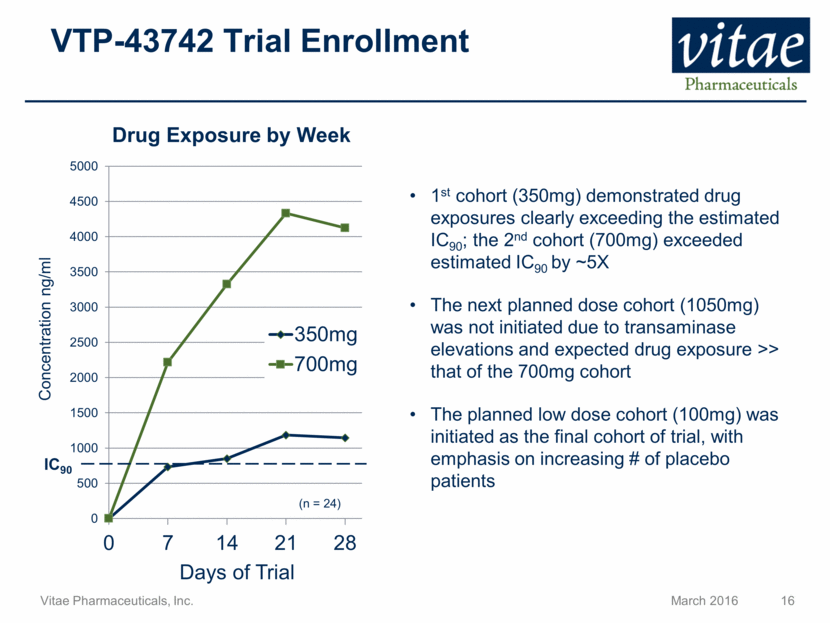
VTP-43742 Trial Enrollment Concentration ng/ml Days of Trial Drug Exposure by Week IC90 1st cohort (350mg) demonstrated drug exposures clearly exceeding the estimated IC90; the 2nd cohort (700mg) exceeded estimated IC90 by ~5X The next planned dose cohort (1050mg) was not initiated due to transaminase elevations and expected drug exposure >> that of the 700mg cohort The planned low dose cohort (100mg) was initiated as the final cohort of trial, with emphasis on increasing # of placebo patients (n = 24)
Dr. Krueger Call-In Discussion Leading Advisor to Industry for Psoriasis, TH17 Pathway What is your assessment of the results from this initial proof-of-concept trial with first-in-class RORt inhibitor VTP-43742? PASI % change efficacy Biomarker data Overall assessment Calling in: James G. Krueger, M.D., Ph.D. Director, Milstein Medical Research Program Senior Attending Physician D. Martin Carter Professor in Clinical Investigation Laboratory of Investigative Dermatology Rockefeller University

Next Steps The Company believes these data validate RORt as a therapeutic target for autoimmune disorders Next Steps Initiate 16 week trial to assess full efficacy of first-in-class RORt inhibitor VTP-43742, planned by end of year 2016
Summary

VTP-43742 demonstrated a clear signal of efficacy Biomarker data support, align with clinical results VTP-43742 generally well-tolerated Company believes these data validate RORt as a therapeutic target Company intends to move first-in-class VTP-43742 forward into a 16 week trial to assess full efficacy response VTP-43742 POC Trial Summary Topline Summary
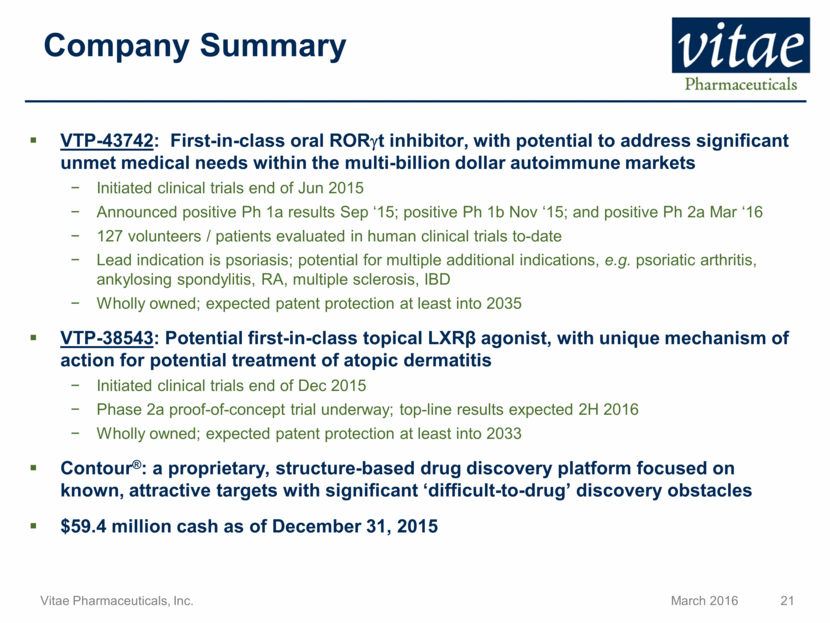
VTP-43742: First-in-class oral RORgt inhibitor, with potential to address significant unmet medical needs within the multi-billion dollar autoimmune markets Initiated clinical trials end of Jun 2015 Announced positive Ph 1a results Sep ‘15; positive Ph 1b Nov ‘15; and positive Ph 2a Mar ‘16 127 volunteers / patients evaluated in human clinical trials to-date Lead indication is psoriasis; potential for multiple additional indications, e.g. psoriatic arthritis, ankylosing spondylitis, RA, multiple sclerosis, IBD Wholly owned; expected patent protection at least into 2035 VTP-38543: Potential first-in-class topical LXR agonist, with unique mechanism of action for potential treatment of atopic dermatitis Initiated clinical trials end of Dec 2015 Phase 2a proof-of-concept trial underway; top-line results expected 2H 2016 Wholly owned; expected patent protection at least into 2033 Contour®: a proprietary, structure-based drug discovery platform focused on known, attractive targets with significant ‘difficult-to-drug’ discovery obstacles $59.4 million cash as of December 31, 2015 Company Summary