Attached files
| file | filename |
|---|---|
| EX-12.1 - EXHIBIT 12.1 - TerraVia Holdings, Inc. | solazyme201510kex121.htm |
| EX-31.1 - EXHIBIT 31.1 - TerraVia Holdings, Inc. | solazyme201510kex311.htm |
| EX-31.2 - EXHIBIT 31.2 - TerraVia Holdings, Inc. | solazyme201510kex312.htm |
| EX-32.1 - EXHIBIT 32.1 - TerraVia Holdings, Inc. | solazyme201510kex321.htm |
| EX-23.1 - EXHIBIT 23.1 - TerraVia Holdings, Inc. | solazyme201510kex231.htm |
| EX-21.1 - EXHIBIT 21.1 - TerraVia Holdings, Inc. | solazyme201510kex211.htm |
| EX-10.23 - EXHIBIT 10.23 - TerraVia Holdings, Inc. | solazyme10k2015ex1023.htm |
| EX-10.24 - EXHIBIT 10.24 - TerraVia Holdings, Inc. | solazyme201510kex1024.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2015
OR
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition Period from to
Commission File Number: 001-35189
Solazyme, Inc.
(Exact name of Registrant as specified in its charter)
Delaware | 33-1077078 | |
(State or Other Jurisdiction of Incorporation or Organization) | (I.R.S. Employer Identification Number) | |
Solazyme, Inc.
225 Gateway Boulevard
South San Francisco, CA 94080
(650) 780-4777
(Address and telephone number principal executive offices)
Securities Registered Pursuant to Section 12(b) of the Exchange Act:
Title of Each Class: | Name of Each Exchange on which Registered: | |
Common Stock, par value $0.001 per share | The NASDAQ Global Select Market | |
Securities Registered Pursuant to Section 12(g) of the Exchange Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one.
Large accelerated filer | ¨ | Accelerated filer | x | |||
Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act.): Yes ¨ No x
The aggregate market value of the registrant’s common stock, $0.001 par value, held by non-affiliates of the registrant as of June 30, 2015, the last business day of our second fiscal quarter, was approximately $231.4 million based on the closing sale price as reported on the Nasdaq Global Select Market.
As of February 29, 2016, there were 81,947,923 shares of the registrant’s common stock, par value $0.001 per share, outstanding.
Documents Incorporated by Reference
Portions of the registrant’s definitive proxy statement for its 2016 Annual Meeting of Stockholders are incorporated by reference into Part III hereof.
Solazyme, Inc.
Annual Report on Form 10-K
For The Year Ended December 31, 2015
INDEX
PART I | ||||
Item 1 | — | |||
Item 1A | — | |||
Item 1B | — | |||
Item 2 | — | |||
Item 3 | — | |||
Item 4 | — | |||
PART II | ||||
Item 5 | — | |||
Item 6 | — | |||
Item 7 | — | |||
Item 7A | — | |||
Item 8 | — | |||
Item 9 | — | |||
Item 9A | — | |||
Item 9B | — | |||
PART III | ||||
Item 10 | — | |||
Item 11 | — | |||
Item 12 | — | |||
Item 13 | — | |||
Item 14 | — | |||
PART IV | ||||
Item 15 | — | |||
Our registered trademarks include Solazyme®, Soladiesel®RD, Soladiesel®BD, Soladiesel®RD, Thrive®, Algenist® and Alguronic Acid®. This Annual Report on Form 10-K also contains trademarks, service marks and trade names owned by us as well as others.
2
The following discussion and analysis should be read together with our audited consolidated financial statements and the related notes and other financial information appearing elsewhere in this Annual Report on Form 10-K. This Annual Report on Form 10-K contains forward-looking statements reflecting our current expectations and involves risks and uncertainties. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential” or “continue” or the negative of these terms or other comparable terminology. For example, statements regarding our expectations as to future financial performance, expense levels, future manufacturing capacity, addressable market size, target average selling prices and liquidity sources are forward-looking statements. Our actual results and the timing of events may differ materially from those discussed in our forward-looking statements as a result of various factors, including those discussed below and those discussed in the section entitled “Risk Factors” included in this Annual Report on Form 10-K and in our other filings with the Securities and Exchange Commission (SEC).
PART I
Item 1. Business
Our Company
Our mission at Solazyme is to make products that are better for people and for the planet.
We are a food, nutrition and specialty ingredients company that harnesses the power of algae, the origin of all plants. Our innovation platform uses microalgae to produce high-value triglyceride oils, proteins, fibers, micronutrients and other ingredients.
We have spent over a decade unlocking the power of algae to find and develop valuable oils and powdered ingredients that offer sustainable solutions for the food, personal care and industrials markets. Moving forward, we are refining our focus to food, nutrition and specialty ingredients, and Solazyme will be known as TerraVia™.
We have developed and are commercializing products for specialty food and nutrition ingredients, animal nutrition ingredients and specialty personal care ingredients. We also recently introduced our first consumer-focused food product formulated with our proprietary ingredients.
The unique compositions of our oils, powdered ingredients and other algae-derived products address specific market and customer needs, offering superior performance characteristics and value. For example, one of our first powdered food ingredients can replace dairy fats and eggs in food products providing food and beverage customers with the opportunity for an improved nutritional profile, a reduction in allergens, with improved or maintained taste and texture. As another example, one of our first oil ingredients can replace or improve upon conventional vegetable oils in major markets by providing customers with better nutrition with increased monounsaturated fat, and reduced saturated and polyunsaturated fat content with improved stability and smoke point. In personal care, our unique oils and ingredients are increasingly important to strategic customers in meeting their product requirements, specifically by improving their product performance and supply chains, and helping to meet their global sustainability goals.
Our high-value oils and powdered ingredients are based on a leading microalgae-based innovation platform that we believe offers the unique opportunity to transform our food system by bringing together better nutrition and great taste, along with economic and environmental sustainability. We believe our solutions can enable our customers to improve product performance and nutrition, reduce processing costs and/or improve their products’ sustainability profile. We are committed to improving the lives of people and the planet through the commercialization of our food and specialty ingredients.
Market Need
Increasing global demand for healthy and sustainable oils and fats as well as plant-based protein sources, amplified by the need for improved nutritional and ecological alternatives in existing supply chains, is driving the market need for new alternatives to incumbent food and specialty ingredients throughout the world.
3
We believe urbanization, improved living standards, and changing diets are consumer forces that will drive demand for our food and specialty ingredient products. As the global population continues to grow and more countries adopt a western-style diet, we expect the demand for healthier oils and fats that are sustainably and economically produced to increase. In addition, the problems of heart health, obesity, diabetes and protein deficiencies are increasingly impacting many global sub-populations. Faced with increasing pressure from consumers to improve the nutritional profile of food and beverage products, governments and manufacturers are increasingly looking for innovation and new food ingredient solutions that will better enable reductions in saturated fat, calories, and cholesterol without compromising performance, taste or cost.
The improvement of living standards globally is also driving a critical trend toward increased consumption of proteins. Many Western consumers consider protein consumption to be an effective strategy for weight and energy management. The environmental impact of addressing the increased demand for protein is important. Animal-derived proteins have a significant impact on land use, deforestation and greenhouse gas emissions. To address this demand for protein, food and beverage manufacturers are looking for new, efficiently-produced plant-based sources of protein.
Initiatives focused on fulfillment of corporate sustainability objectives and government mandates also support an increased use of renewable oils and sustainable ingredients. The ongoing demand to improve the sustainability footprint of supply chains across the globe are being driven by not only social and political drivers, but also the need to improve the overall environmental impacts that originate from increased raw material needs. The ability to convert sustainable carbohydrate-rich plant materials into unique high-value oils and materials offers a solution to global demand for a range of products.
Our Strategy
We intend to be a global market leader delivering much needed innovation and sustainability to food, nutrition and specialty ingredients from microalgae. The principal elements of our strategy are:
• | Prioritize market entry in Food, Nutrition and Specialty Ingredients. We intend to focus on high-value food, nutrition and specialty products that generate attractive margins in our target markets while we continue to scale operations at our manufacturing facilities. We have spent over a decade building a leading innovation platform for food and specialty ingredients, obtaining critical regulatory clearance for our core products, producing at commercial scale and validating our products with customers and consumers. All of these efforts align with our goal to capitalize on market demand for healthier oils and ingredients during a time when key trends of plant-based eating and enhanced nutrition are accelerating. |
• | Execute on our customer-driven approach to technology and product development. We will continue to work closely with our partners and customers to understand their requirements and design products to specifically address their needs. It is our intent to provide both branded and unbranded products within food applications that include AlgaVia™ protein and lipid-rich powders, AlgaWise™ food oils and AlgaPur™ specialty oils for personal care. |
• | Enter into sales agreements and additional partnership agreements to advance commercialization efforts. We are currently engaged in development activities with multiple partners in food, nutrition and specialty ingredients. In addition to funding development work and performing application testing, we expect that our partners will enter into long-term commercial supply agreements with us. |
• | Maximize return on manufacturing assets. We have brought large-scale manufacturing of our products online and have invested to establish our manufacturing footprint. This includes a wholly owned facility for food ingredients and new product development in Peoria Illinois. It also includes the large commercial manufacturing facility in Brazil that was built with our joint venture partner, Bunge. We expect to continue to optimize and scale production in these facilities and to continue to execute a capital-efficient strategy by entering agreements whereby our partners will invest capital and operational resources in building and scaling manufacturing capacity, while also providing us access to feedstock. |
Our Technology
Our process begins by selecting one of our microalgae strains to produce a specific oil or powder (whole algae products and encapsulated oil products). Then, through fermentation, the microalgae convert sugars into the desired end product. Fermentation helps accelerate microalgae’s natural biological process, allowing us to produce large amounts of a desired product in a matter of days. After fermentation, the microalgae go through a few final steps depending on the product, such as
4
drying or standard mechanical oil extraction (pressing), before being shipped to customers in a variety of end markets. Our technology platform enables us to produce a broad array of products and oil profiles with the same infrastructure.
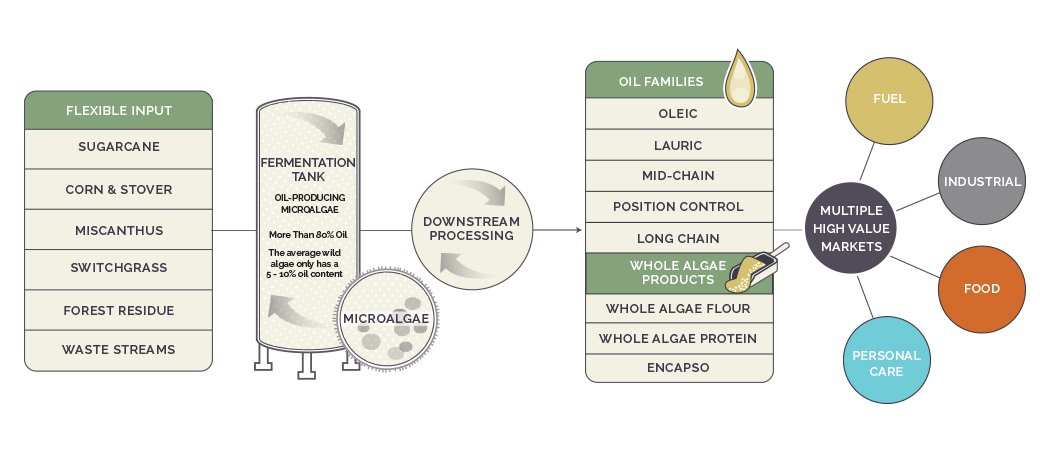
Our platform is feedstock flexible and can utilize a variety of renewable plant-based sugars. We currently use sugarcane-based sucrose and corn-based dextrose as our two primary feedstock sources. Our technology can also support sugar from other sustainable biomass sources including cellulosics, which we believe will represent an important alternative feedstock in the future.
The controlled environment of the standard fermentation tanks enables product purity and allows us to tightly regulate acidity, temperature and other key parameters. Our core competencies include (1) identifying, isolating and further optimizing strains of microalgae to achieve high cell densities, high yields converting sugar to product and high time-based productivity rates compared to other alternatives, and (2) tailoring oil outputs to meet specific market needs.
Some of our oils and all of our AlgaVia™ whole algae products (AlgaVia™ Lipid Rich Powder and AlgaViaTM Protein) are made from native microalgae strains. These strains are selected through a rigorous screening process in which we look at thousands of microalgae, seeking properties that translate into product benefits for our customers.
When we seek to develop unique oils with significant health and performance benefits - for example, oil with a higher flash point to improve the performance of cooking oil or healthier oil with low levels of saturated fat, we apply the tools of biotechnology, including standard genetic engineering techniques, to the algae to develop these oils. These are tools that have been used for decades to generate important drugs, vitamins, foods, hygiene and household products that are broadly used and beneficial for people. We may introduce one or sometimes a few genes from a plant with desired properties, or we might make only modest changes to the microalgae’s existing genes, for example, by shutting off production of an undesired oil component.
We believe that the following advantages of our platform allow us to offer a new source of high-value oil and powdered products to address the major markets served by conventional oils:
• | Large and diverse market opportunities. Because we make oils, we can access the vast markets currently served by petroleum, plant oils and animal fats. In addition, we make proteins, fibers, polysaccharides and micronutrients which are also broadly used in food, nutrition and specialty ingredients. |
• | Cost-competitive at commercial scale. We harness the innate characteristics of microalgae through a proven fermentation process in a controlled environment that we believe is able to produce large volumes of oil, proteins and other macro and micronutrients in a cost-effective, scalable and predictable manner. |
• | Rapid time to market. Our custom-designed oils can improve upon triglyceride oils currently used and can be integrated more quickly into our customers’ platforms because they are chemically similar to existing oils. Additionally, if a customer desires a novel oil profile, our development timelines for new profiles can be on a |
5
scale of several months to several years as opposed to timelines demonstrated with plant-based oils, which are typically on the scale of 10-15 years.
Our Products
Our primary products are oils and fats, powdered ingredients, including proteins, lipids, fibers and micronutrients and other bioproducts. Our products are sold into two main categories as: Food and Nutrition Products and Specialty Ingredients. The inherent flexibility of our technology platform and its broad applicability across multiple industries allow us to approach a wide range of customers across myriad end markets.
Food and Nutrition Products
We believe that some of the largest opportunities and most valuable uses for our oils and powdered ingredients are in food and nutrition. We have developed and commercialized microalgae-based food ingredients that can enhance the nutritional profile and functionality of a wide range of foods and beverages. We currently have two commercial food ingredient platforms with four commercially available products. The AlgaVia™ Microalgae Food Ingredients include a lipid-rich powder and a protein powder, and the AlgaWise™ algae oils family, which currently includes the Ultra Omega-9 Oil and the High Stability Oil. The AlgaVia™ ingredients are new, sustainable sources of food with benefits that can enhance nutrition, performance and taste. AlgaVia™ ingredients are sold to food and beverage manufacturers and foodservice providers through a combination of direct sales, distributors and strategic partnerships. The AlgaWise™ oils are based on unique nutrition and performance characteristics and the oils are among the most sustainably produced cooking oils, with more oil produced per acre of land, and a lower carbon and water footprint than nearly all cooking oils.
Powdered Ingredients
We have developed novel methods of preparing powdered forms of triglyceride oils and vegan proteins, and our powdered ingredients are composed of unmodified whole algae cells. Our whole algae products include:
• | AlgaVia™ Lipid Rich Powder (commonly known as whole algae flour) is a lipid-rich ingredient that can replace or reduce dairy fat, egg yolks and oil in recipes to reduce fat, cholesterol and calories with the added benefits of texture enhancement, water binding and flavor delivery. This is a new fat source that can enable the creation of healthier products with taste and texture similar to or better than foods made with conventional animal or vegetable oils. We believe the total addressable market for our lipid powders is greater than $2.5 billion annually. |
• | AlgaVia™ Protein (commonly known as whole algae protein) is a vegan protein source that is non-allergenic, gluten-free and a sustainable source of high quality protein. This ingredient delivers protein along with a rich collection of fiber, lipids and micronutrients. The protein present in AlgaVia™ protein is protected by the natural cell wall and enables protein fortification in challenging applications such as low pH beverages, dressings and crackers. We believe the total addressable market for our protein powders is greater than $8.0 billion annually. |
Both AlgaVia™ Lipid Rich Powder and Protein can be used across a range of applications such as beverages (ready-to-drink and powdered), bakery, snacks, bars, dressings, sauces and frozen desserts. We have received FDA GRAS “No Questions” letters for each of these powdered ingredients. They may also be sold in other regions/countries including Europe, Mexico, Australia, and New Zealand. Additionally, we successfully produced at scale a new food powder intended for use in animal nutrition applications and this ingredient is intended for commercial launch in 2016.
Oil Ingredients
We have developed a range of food oils that have the potential to improve upon conventionally utilized specialty fats and oils and our high oleic algae oil has received an FDA GRAS "No Questions" letter. Our first high performance food oil captured the 2014 IFT Food Expo Innovation Award. We believe the total addressable market for our food oils is greater than $5.5 billion annually.
Currently, these oils are commercially available in our AlgaWise™ branded food oil platform as:
6
• | AlgaWise™ High Stability algae oil is extremely stable, with zero trans fats and very low levels of polyunsaturates. This oil provides exceptional stability in a wide range of applications such as for use in frying, baking, spreads, coatings, sauces, and dressings. Higher stability means lower systems costs overall and greater simplicity. It can enable longer shelf life with fresher tasting products, longer fry life, and less polymerization in frying and baking. |
• | AlgaWise™ Ultra Omega-9 algae oil exhibits a high level of omega-9 fatty acid, and lowest saturated fat of any commercially available oil. Further, this oil has the highest monounsaturated fat of any commercially available oil and has a neutral flavor with a high smoke point. |
• | Thrive® algae oil, launched as a consumer focused culinary oil, is one of the most sustainably produced cooking oils, with more oil produced per acre of land, and a lower carbon and water footprint than nearly all cooking oils. In 2015, we introduced Thrive® culinary algae oil through a regional test market and via online distribution at www.thrivealgae.com. |
In addition to our commercially available products today, we have a pipeline of additional high value products we expect to launch in the food, nutrition and specialty ingredients markets in 2016 and 2017.
Specialty Ingredients
We believe our renewable oils could be part of the next generation of high performance oils, and our platform enables replacement and enhancement of petroleum, plant, or animal derived oils that are used as raw materials or ingredients. In many cases, we expect to create novel oils and high-performing end products that do not exist in nature or are prohibitively expensive to synthesize today. We are focusing our efforts within the specialty ingredients market predominantly on personal care. These oils typically replace traditional sources that are from plant or animal fats and appear in the home and personal care industries in applications such as surfactants, detergents, soaps, cosmetics and many others. Our specialty ingredients include:
• | AlgaPur™ algae oils are a range of specialty personal care oils including Capric, Lauric and Oleic-based oils that have improved characteristics such as for stability, sensory and hydration for skin and hair benefits. For example, AlgaPur™ high lauric oil is currently used in soaps while AlgaPur™ high stability oleic oil is used in a natural line of body care. |
We have developed a portfolio of innovative and branded microalgae-based products. During our algae strain screening process, we discovered and isolated key compounds that microalgae synthesize to protect themselves against environmental hazards, such as UV exposure, extremes temperatures, and dehydration. Our first major ingredient is Alguronic Acid®, which we have formulated into a full range of skin care products with significant anti-aging benefits. For example, since 2011, we have commercialized our Algenist® anti-aging skin care line currently selling in 21 countries including member countries of the EU, Mexico, Canada and China.
Our Competitive Strengths
We harness the power of microalgae to develop and commercialize breakthrough products. Our key competitive advantages are:
• | Proprietary and innovative technology. We have made significant investments to protect the intellectual property and know-how related to our technology platform, including screening, classical strain development, targeted recombinant strain optimization, product and applications development and manufacturing capabilities. |
• | Premium pricing for custom-designed and unique products. We believe the enhanced value of our oils and powders as compared to conventional ingredients should garner premium pricing. Examples in food and nutrition applications include oils with low levels of polyunsaturates for improved shelf and frying life, structuring fats, like algae-based butter, providing specific melting profiles, and naturally encapsulated protein powders that can enable increased protein incorporation into foods. |
• | Technology proven at scale. We have operated our large-scale fermentations at multiple partner facilities since 2008 and now run our processes routinely in our commercial 128,000 liter fermenters in Peoria and 625,000 liter fermenters at the Solazyme Bunge JV Plant. |
7
• | Established manufacturing capacity. In structuring our capacity and feedstock partnerships, we have deployed a capital efficient strategy to source low-cost financing and predictable feedstock with our partners. For example, for our joint venture with Bunge Global Innovation, LLC in Brazil, significant capital expenditure related to investments for the Solazyme Bunge JV Plant was approved for financing through an 8-year loan from the Brazilian Development Bank (BNDES) at an average interest rate of approximately 4% per annum. |
• | Commercial products. We launched our Algenist® brand within the Personal Care Products market in 2011. The Algenist® product line has grown to 38 SKUs with a number of additional product launches planned for 2016. We commenced commercial scale production of our renewable, custom-designed products manufactured in January 2014 and at the Solazyme Bunge JV Plant in May 2014. As of the end of 2015, we have consolidated commercial production at our Solazyme Bunge JV plant with supplemental product capabilities for AlgaVia™ food powders and developmental oils at our Peoria facility. In addition to our commercially available products today, we have a pipeline of additional high value products we expect to launch in the food, nutrition and specialty ingredients markets in 2016 and 2017. |
• | Feedstock and target market flexibility. Our technology platform provides us with the flexibility to choose from among multiple feedstocks on the input side and multiple specific products (and markets) on the output side, while using the same standard fermentation equipment. A manufacturing facility utilizing a given plant-based sugar feedstock can produce oils with many different products. Conversely, we can produce the same products by processing a variety of plant-based sugar feedstock. This flexibility enables us to choose the optimal feedstock for any particular geography, while also enabling us to produce a variety of products from the same manufacturing facility. |
Significant Partners
Bunge. Since 2011, we have entered multiple Joint Development Agreements (JDAs) with Bunge to jointly develop microbe-derived oils, biomass, and explore the production of such oils and biomass from Brazilian sugarcane feedstock.
In April 2012, we and Bunge formed the Solazyme Bunge JV to build, own and operate the Solazyme Bunge JV Plant, a commercial-scale renewable algae oils production facility adjacent to Bunge's Moema sugar mill in Brazil. Construction of the Solazyme Bunge JV Plant commenced in the second quarter of 2012 and the Solazyme Bunge JV Plant produced its first products on full-scale production lines in May 2014.
In October 2015, we and Bunge entered into an amended and restated joint venture agreement to expand the Solazyme Bunge JV to add worldwide focus on human food and animal nutrition. Also in October 2015, we and Bunge entered into an amended and restated development agreement under which we granted to the Solazyme Bunge JV a worldwide royalty-bearing, field-limited license to all of our technology that is necessary or useful for the manufacture of certain algae oil products. Concurrently with the entry into such agreement, we and Solazyme Bunge JV entered into two funded research programs targeted at completing the development of additional products for the Solazyme Bunge JV. Refer to Note 8 in the accompanying notes to the consolidated financial statements for further discussion on the Solazyme Bunge JV.
ADM. In November 2012, we entered into a strategic collaboration agreement with Archer-Daniels-Midlands Company (“ADM”), establishing a collaboration for the production of algae triglyceride oil products at ADM’s facility in Clinton, Iowa (the “Clinton Facility”). The Clinton Facility produced algae triglyceride oil products using our proprietary technology. In January 2014, we began commercial scale production of our oils at the Clinton Facility. In October 2015, we provided to ADM a notice of termination of the Operating Agreement entered into with ADM in November 2012 related to the production of products at the Clinton Facility. As a result of the termination of the Operating Agreement, the Strategic Collaboration Agreement with ADM terminated on February 26, 2016. Refer to Note 3 and Note 11 in the accompanying notes to our consolidated financial statements for further discussion regarding ADM.
Unilever. In October 2011, we entered into a joint development agreement with Unilever (our fourth agreement altogether), which expanded our current research and development efforts. In September 2015, we and Unilever extended this joint development agreement through September 30, 2017. In September 2013, we and Unilever entered into a commercial supply agreement for up to 10,000 MT of our algae oil. In May 2014, Unilever announced the initial introduction of our sustainable algal oil into one of its biggest soap brands, Lux. In March 2016, we entered into a multi-year global supply agreement with Unilever, which includes a broad portfolio of our algae oils for Unilever to purchase for use in personal care
8
products. Production of these oils will take place at the Solazyme Bunge Renewable Oils facility in Brazil and pricing terms are based upon actual production cost plus a defined contribution margin. The agreement contains certain minimum and maximum sales volumes and is subject to other terms and conditions.
Mitsui. We have a multi-year agreement with Mitsui & Co., Ltd. (Mitsui) to jointly develop triglyceride oils for use primarily in the oleochemical industry. The agreement includes further development of our myristic oil, a valuable raw material in the oleochemical industry, and additional oils that we are developing for the oleochemical and industrial sectors. End use applications may include renewable, high-performance lubricants and other industrial products.
Algenist® Distribution Partners. We have distribution contracts with Sephora S.A. and its affiliates to distribute the Algenist® product line in Sephora stores in the U.S. and other countries. We also sell our Algenist® product line through QVC's multimedia platform. We also have an agreement with ULTA Beauty to sell our Algenist® line in its retail stores throughout the United States.
AkzoNobel. In May 2013, we entered into a joint development agreement with AkzoNobel, a leading global paints and coatings company and a major producer of specialty chemicals, targeting the development and commercial sales of triglyceride oils for use by AkzoNobel in its surface chemistry and decorative paints businesses. Product development efforts began in the second half of 2013, and in July 2014 we entered into a research and development plan with AkzoNobel which extends through June 2017.
Manufacturing Operations
Our process is compatible with commercial-scale, widely-available fermentation and oil recovery equipment. We operate our lab and pilot fermentation and recovery equipment as scaled-down versions of our large commercial engineering designs, such as those used to perform development work under certain agreements with strategic partners and to fulfill commercial supply agreements with certain partners. This allows us to more easily scale up to larger fermentation vessels. We have scaled up our technology platform and have successfully operated at lab (5-15 liter), pilot (600-1,000 liter) and commercial (up to 625,000 liter) fermenter scale. Our existing manufacturing operations are as follows:
• | Our pilot plant in South San Francisco, California, with recovery operations capable of handling material from both 600 and 1,000 liter fermenters, enables us to produce samples of our algae oils for testing and optimization by our partners, as well as to test new process conditions at an intermediate scale. |
• | In 2012, we commissioned our Peoria, Illinois facility (Peoria Facility), to produce algae oil. The Peoria Facility was partially funded with a federal grant that we received from the U.S. Department of Energy (DOE) in December 2009 to demonstrate integrated commercial-scale production of renewable algae-derived fuels. The Peoria Facility has a nameplate capacity of two million liters of oil annually and provides an important platform for continued work on feedstock flexibility and scaling of new algae oils into the marketplace. We have also modified our Peoria Facility to produce food ingredients in conjunction with market development activity. |
• | In May 2014, the Solazyme Bunge JV Plant initiated its first commercial production utilizing full-scale production lines, including 625,000 liter fermenter tanks. The Solazyme Bunge JV Plant leverages our technology and Bunge’s sugarcane milling and natural oil processing capabilities to produce microalgae-based products. In addition, the Solazyme Bunge JV Plant has been designed to be expanded for further production in line with market demand. The facility was constructed as part of our Joint Venture with Bunge, and was financed with equal equity contributions from both Bunge and us and over $100 million (based on the exchange rate at the time of funding approval) in project financing from BNDES. |
• | We utilize contract manufacturing to assist in the production and sale of our Algenist® products, and we closely monitor and advise these contract manufacturers to maintain stringent quality standards for our products. We also produce some active ingredients for Solazyme Personal Care Products at our Peoria Facility. |
Intellectual Property
Our success depends in part upon our ability to obtain and maintain intellectual property protection for our products and technologies, and to operate without infringing the proprietary rights of others. With respect to the former, our policy is to protect our proprietary position through filing for patent applications on inventions, filing for trademark protection on our
9
product names and related materials and methods, and through trade secret protection when and where appropriate. We seek to avoid infringing the proprietary rights of others by: (1) monitoring patents and publications in our product areas; (2) monitoring the technological developments of others; and (3) evaluating and taking appropriate courses of action whenever we identify such developments.
As of December 31, 2015, we own over 50 issued U.S. patents, over 50 issued foreign patents and over 250 pending patent applications filed in the United States and in various foreign jurisdictions. The expiration dates of the issued patents are between 2024 and 2034. Patents that issue, if any, from our currently pending patent applications would be expected to expire twenty years from the date of filing. Our patents and patent applications are directed to compositions such as food products, custom oils, fuel products, chemicals, cosmetics, strains of microbes, and recombinant technologies; methods of manufacturing finished goods and raw materials; and methods of using our raw materials and products. We also protect our proprietary information by requiring our employees, consultants, contractors and other advisors to execute nondisclosure and assignment of invention agreements upon commencement of their respective employments or engagements. Agreements with our employees also prohibit them from bringing the proprietary rights of third parties to us. In addition, we protect our proprietary information through creating written obligations of confidentiality with outside parties who are exposed to confidential information. Where appropriate we also employ material transfer agreements governing the use, intellectual property rights, and transfer of materials such as custom oils when sending them to third parties for purposes such as integration into food applications or conversion into fuels, chemicals and personal care products.
We believe that the creation, when possible and appropriate, of multiple, overlapping mechanisms and forms of protection will offer the possibility of broadest and longest proprietary positions for our products and technologies. It is possible that our current and future patents may be successfully challenged or invalidated in whole or in part. It is also possible that we may not obtain issued patents from our filed applications, and may not be able to obtain patents covering other inventions we seek to protect. Due to uncertainties inherent in prosecuting patent applications, some patent applications may be rejected and we may subsequently abandon them. We may also abandon applications when we determine that a product or method is no longer of interest. It is also possible that we may develop products or technologies that will not be patentable or that the patents of others will limit or preclude our ability to do business. In addition, any patent issued to us may provide us with little or no competitive advantage, in which case we may abandon such patent or license it to another entity.
Government Regulation
Our development and production processes involve the use, generation, handling, storage, transportation and disposal of hazardous chemicals and radioactive and biological materials. We are subject to a variety of environmental, health and safety, federal, state, local and international laws, regulations and permit requirements governing, among other matters, the use, generation, manufacture, transportation, storage, handling and disposal of these materials, in the US, Brazil and other countries where we intend to operate or may operate or sell our products in the future. These laws, regulations and permits can cause delays, require expensive fees, pollution control equipment, capital expenditures or operational changes to limit actual or potential impact of our operations on the environment.
We are also subject to regulation by the Occupational Safety and Health Administration (OSHA), the California and federal Environmental Protection Agency (EPA), and to regulation under the Toxic Substance Control Act (TSCA). OSHA, the California or federal EPA or other government agencies may adopt regulations that affect our research and development programs. In particular, our renewable chemical products may be subject to regulation by government agencies in our target markets. The EPA administers the requirements of the TSCA, which regulates the commercial use of chemicals. Before an entity can manufacture a chemical, it needs to determine whether that chemical is listed in the TSCA inventory. If the substance is listed, then manufacture can commence immediately. If not, then a pre-manufacture notice (PMN) must be filed with the EPA, which has 90 days to review it. Some of the products we produce or plan to produce are on the TSCA inventory, after successful PMN submissions and filed Notice of Commencements (NOC). Others are not yet listed. A similar program exists under the European Chemicals Agency (ECHA) called REACH. Under REACH, we are required to register some of our products with the ECHA, and this process could cause delays or involve significant costs.
The use of recombinant microbes like many of our microbial strains is subject to laws and regulations in many countries. In the US, the EPA regulates the commercial use of recombinant microbes as well as potential products from recombinant microbes. When used in an industrial process, our microalgae strains designed using recombinant technology may be considered new chemicals under TSCA, administered by the EPA. Our microalgae strains designed using recombinant technology will be required to comply with the EPA’s Microbial Commercial Activity Notice (MCAN) process and we have filed MCANs for strains of recombinant microalgae that we use for our chemicals and fuels businesses, which have been dropped from review. We have subsequently filed NOCs for dropped MCANs allowing commercial use of the microalgae in the U.S. In Brazil, engineered microbes are regulated by CTNBio. We have filed applications, and in the future may file additional
10
applications, for approval from CTNBio to import and use engineered microbes in our Brazilian facilities for research and development purposes. In addition, we received several commercial approvals from CTNBio for one of our current microbial strains since October 2013. We expect to encounter regulations concerning engineered microbes in most if not all of the countries in which we may seek to make our fuel and chemical products, however, the scope and nature of these regulations will likely be different from country to country. In February 2014, CTNBio granted a CQB (Certificate of Quality in Biosafety) to the Solazyme Bunge JV Plant for activities including industrial production, import and export, disposal and storage of our key production organisms, allowing the Solazyme Bunge JV Plant to run strains without prior commercial approval, under controlled conditions.
Our fuel products are subject to regulation by various government agencies, including the EPA and the California Air Resources Board in the US and Agencia Nacional do Petroleo in Brazil. We have registered fuels with the EPA and are preparing to secure approval for use of our diesel in Brazil. In addition, we may decide to register our fuel with the California Air Resources Board and the European Commission. Registration with each of these bodies is required for the sale and use of our fuels within their respective jurisdictions. Our jet fuels meet the standards set by ASTM D7566 and may therefore be used in commercial aviation.
The manufacture, sale and use of our foods products are regulated as food ingredients in the United States by the U.S Food and Drug Administration (FDA) under the federal Food, Drug, and Cosmetic Act. Food ingredients are broadly defined as any substance that may become a component, or otherwise affect the characteristics, of food. Food ingredients are regulated as food additives and must be approved through a formal Food Additive Petition (FAP) process or affirmed as substances generally recognized as safe, or GRAS. A substance can be listed or affirmed as GRAS by the FDA or self-affirmed by its manufacturer upon determination by independent qualified experts who generally agree that the substance is GRAS for a particular use. Although the FDA does not officially affirm the GRAS status of ingredients, it does review, at the notifier’s request, the notifier’s determination of ingredients’ GRAS status. FDA endeavors to respond to GRAS notices by issuing a letter that either does not question the basis of the notifier’s determination of GRAS status or concludes that the notice does not provide a sufficient basis for a GRAS determination. Self-affirmation of GRAS status without FDA notification allows the marketing and sale of the ingredient, but reliance on self-affirmation alone may limit its marketability, as many food manufacturers require that the FDA issue a letter confirming that it does not question the notifier’s determination of GRAS status before such manufacturers will purchase food ingredients from third parties. We submitted a GRAS Notice to the FDA for our first algae oil in June 2011, and received a “No Questions” letter from the FDA in June 2012. A panel of qualified experts in the field of food toxicology has determined that an additional oleic algae oil, our second algae oil, is GRAS. We submitted a GRAS Notice for the oleic algae oil in July 2014, and received a “No Questions” letter from the FDA in February 2015. We submitted a GRAS Notice for whole algae flour in the third quarter of 2012 and received a “No Questions” letter from the FDA in June 2013. We also submitted a GRAS Notice for whole algae protein in June 2014 and received a “No Questions” letter from the FDA in December 2014.
Food ingredients that are not suitable for the GRAS affirmation process are regulated as food additives and require the submission of a FAP to the FDA and the FDA’s approval prior to commercialization. The food additive petition process is generally expensive and time consuming, with approval, if secured, taking years. The petition must establish with reasonable certainty that the food additive is safe for its intended use at the level specified in the petition. If a food additive petition is submitted, the FDA may choose to reject the petition or deny any desired labeling claims. Furthermore, the FDA may require the establishment of regulations that necessitate costly and time-consuming compliance procedures. All products may also fall under the jurisdiction of the U.S. Department of Agriculture if the intended applications are for meat, dairy, organic or other specialty food areas.
The sale of ingredients for use in animal feed is regulated by agencies including the FDA Center for Veterinary Medicine, or CVM. CVM requirements for suitability must be met by providing data from studies.
Countries other than the U.S. also regulate the manufacture and sale of food, feed and chemical ingredients. Regulations vary substantially from country to country, and we will be required to comply with applicable regulations in each country in which we choose to market our ingredients. In February 2014, the Sao Paulo State Environmental Department granted a license to operate the Solazyme Bunge JV Plant, which was necessary to begin commercial production. We submitted a dossier for oleic algae oil to the Brazilian food safety agency ANVISA in the first quarter of 2014, and anticipate approval for use in foods in 2016. We received food approval from ANVISA with no restrictions for our whole algae flour in July 2015. We also received a Letter of No Objection from Health Canada for whole algae flour in February 2016.
Our skin and personal care products are also subject to regulation by various government agencies in the countries in which our products are sold. We completed several rounds of testing in connection with launching the Algenist® product line,
11
including Human Repeat Insult Patch Testing. We will continue to evaluate regulatory requirements as we launch new skin and personal care products.
Employees
As of December 31, 2015, we had 229 full-time employees, excluding the employees of our joint venture. Our employees’ roles include research, process development, manufacturing, regulatory affairs, program management, finance, human resources, administration, sales and marketing and business development. None of our employees are covered by collective bargaining agreements and we consider relations with our employees to be good.
Investor Information
Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K and any amendments to those reports are available free of charge on the Investor Relations section of our website at http://investors.solazyme.com/sec.cfm as soon as reasonably practicable after they are electronically filed with or furnished to the Securities and Exchange Commission (SEC). The public may read and copy any materials filed us with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Room 1580, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC at http://www.sec.gov. Except as expressly set forth in this Annual Report on Form 10-K, the contents of these websites are not incorporated into, or otherwise to be regarded as part of this report.
Item 1A. | Risk Factors. |
You should carefully consider the risks and uncertainties described below before investing in our publicly-traded securities. Additional risks and uncertainties not presently known to us or that our management currently deems immaterial also may impair our business operations. If any of the risks described below were to occur, our business, financial condition, operating results, and cash flows could be materially adversely affected. In such an event, the trading price of our common stock could decline and you could lose all or part of your investment. In assessing these risks and uncertainties, you should also refer to the other information contained in this Report, including our consolidated financial statements and related notes. The risks and uncertainties discussed below also include forward-looking statements, and our actual results may differ substantially from those discussed in these forward-looking statements. See Management’s Discussion and Analysis of Financial Condition and Results of Operations-Forward-Looking Statements.
Risks Related to Our Business and Industry
We have a limited operating history and have incurred significant losses to date, anticipate continuing to incur losses and may never achieve or sustain profitability.
We are an emerging growth company with a limited operating history. We only recently began commercializing our products. To date, a substantial portion of our revenues has consisted of funding from third party collaborative research agreements and government grants. We have generated only limited revenues from commercial sales, which have been principally derived from sales of our personal care products. We expect a significant portion of our future revenues to come from commercial sales in food, nutrition and specialty personal care ingredients.
We have incurred substantial net losses since our inception, including a net loss of $141.4 million during the year ended December 31, 2015. We expect these losses may continue as we ramp up our manufacturing capacity and build out our product pipeline. As of December 31, 2015, we had an accumulated deficit of $609.9 million. We expect to incur additional costs and expenses related to the continued development and expansion of our business, including research and development, the operation of our Peoria Facility, the ramp up and operation of the Solazyme Bunge JV production facility (described below) and other commercial facilities. As a result, our annual and quarterly operating losses may continue.
We, along with our development and commercialization partners, will need to develop products successfully, cost effectively produce them in large quantities, and market and sell them profitably. If we fail to become profitable, or if we are unable to fund our continuing losses, we may be unable to continue our business operations. There can be no assurance that we will ever achieve or sustain profitability.
12
We have generated limited revenues from the sale of our products, and our business may fail if we are not able to successfully commercialize these products.
We have had only limited product sales to date. If we are not successful in further advancing our existing commercial arrangements with strategic partners, developing new arrangements, ramping up or otherwise increasing our manufacturing capacity and securing reliable access to sufficient volumes of low-cost feedstock, we will be unable to generate meaningful revenues from our products. We are subject to the substantial risk of failure facing businesses seeking to develop products based on a new technology.
Certain factors that could, alone or in combination, prevent us from successfully commercializing our products include:
• | our ability to secure reliable access to sufficient volumes of low-cost feedstock; |
• | our ability to achieve commercial-scale production of our products on a cost-effective basis and in a timely manner; |
• | technical or operational challenges with our manufacturing processes or with development of new products that we are not able to overcome; |
• | our ability to consistently manufacture our products within specifications; |
• | our ability to establish and maintain successful relationships with development, feedstock, manufacturing and commercialization partners; |
• | our ability to gain market acceptance of our products with customers and maintain customer relationships; |
• | our ability to sell our products at an acceptable price; |
• | our ability to manage our growth; |
• | our ability to meet applicable regulatory requirements for the production, distribution and sale of our products and to comply with applicable laws and regulations; |
• | actions of direct and indirect competitors that may seek to enter the markets in which we expect to compete or that may seek to impose barriers to one or more markets that we intend to target; and |
• | public concerns about the ethical, legal, environmental and social ramifications of the use of targeted recombinant technology, land use and the potential diversion of resources from food production. |
The production of our microalgae-based products requires fermentable feedstock. The inability to obtain feedstock in sufficient quantities or in a timely and cost-effective manner may limit our ability to produce our products.
A critical component of the production of our microalgae-based products is access to feedstock in sufficient quantities and at an acceptable price to enable commercial production and sale. Other than as described below, we currently purchase feedstock, such as sugarcane-based sucrose and corn-based dextrose, for the production of our products at prevailing market prices.
We do not have any long-term supply agreements or other guaranteed access to feedstock other than for the supply of feedstock to Solazyme Bunge Produtos Renováveis Ltda. (“Solazyme Bunge Renewable Oils” or the “Solazyme Bunge JV”) by our partner, Bunge Global Innovation, LLC and certain of its affiliates (“Bunge”), pursuant to our joint venture arrangement that includes a feedstock supply agreement. As we scale our production, we anticipate that the production of our microalgae-based products will require large volumes of feedstock, and we may not be able to contract with feedstock producers to secure sufficient quantities of feedstock at reasonable costs or at all. For example, sugarcane-based sucrose for the Solazyme Bunge JV facility in Moema, Brazil is being provided by Bunge. Sugar and corn are traded as commodities and are subject to price volatility. While we may seek to manage our exposure to fluctuations in the price of sugar and corn-based dextrose by entering into hedging transactions directly or through our joint venture arrangement, we may not be successful in doing so. If we cannot access feedstock in the quantities we need at acceptable prices, we may not be able to successfully commercialize our food ingredients, fuels, chemicals, encapsulated lubricant and other products, and our business will suffer. If we do not succeed in entering into long-term supply contracts when necessary or successfully hedge against our exposure to fluctuations in the price of feedstock, our costs and profit margins may fluctuate from period to period as we will remain subject to prevailing market prices.
Although our plan is to enter into partnerships, such as the Solazyme Bunge JV, with feedstock providers to supply the feedstock necessary to produce our products, we cannot predict the future availability or price of such feedstock or be sure that
13
our feedstock partners will be able to supply such feedstock in sufficient quantities or in a timely manner. The prices of feedstock depend on numerous factors outside of our or our partners’ control, including weather conditions, government programs and regulations, changes in global demand, rising or falling commodities and equities markets, and availability of credit to producers. Crop yields and sugar content depend on weather conditions such as rainfall and temperature. Variable weather conditions have historically caused volatility in feedstock crop prices due to crop failures or reduced harvests. For example, excessive rainfall can adversely affect the supply of feedstock available for the production of our products by reducing the sucrose content of feedstock and limiting growers’ ability to harvest. Crop disease and pestilence can also occur from time to time and can adversely affect feedstock crop growth, potentially rendering useless or unusable all or a substantial portion of affected harvests. The limited amount of time during which feedstock crops keep their sugar content after harvest poses a risk of spoilage. Also, the fact that many feedstock crops are not themselves traded commodities limits our ability to substitute supply in the event of such an occurrence. If our ability to obtain feedstock crops is adversely affected by these or other conditions, our ability to produce our products will be impaired, and our business will be adversely affected. In the near term we believe Brazilian sugarcane-based sucrose will be an important feedstock for us. Along with the risks described above, Brazilian sugarcane prices may also increase due to, among other things, changes in the criteria set by the Conselho dos Produtores de Cana, Açúcar e Álcool (Council of Sugarcane, Sugar and Ethanol Producers), known as Consecana. Consecana is an industry association of producers of sugarcane, sugar and ethanol that sets market terms and prices for general supply, lease and partnership agreements and may change such prices and terms from time to time. Moreover, Brazil has a developed industry for producing ethanol from sugarcane, and if we have manufacturing operations in Brazil that do not have a partner providing the sugarcane feedstock, such as Bunge as part of the Solazyme Bunge JV, we will need to compete for sugarcane feedstock with ethanol producers. Such changes and competition could result in higher sugarcane prices and/or a significant decrease in the volume of sugarcane available for the production of our products, which could adversely affect our business and results of operations.
We have entered into, and plan to enter into other, arrangements with feedstock producers to co-locate production at their existing mills, and if we are not able to complete and execute on these arrangements in a timely manner and on terms favorable to us, our business will be adversely affected.
In April 2012, we entered into a Joint Venture Agreement with Bunge, forming the Solazyme Bunge JV, which is doing business as Solazyme Bunge Renewable Oils. The Joint Venture Agreement was amended in October 2013 and again in October 2015 to expand the field and product portfolio. The Solazyme Bunge JV produces microalgae-based products in Brazil using our proprietary technology and sugarcane feedstock provided by Bunge. The Solazyme Bunge JV’s production facility is located adjacent to a sugarcane processing mill in Brazil that is owned by Bunge. The acquisition of the facility site by the Solazyme Bunge JV from Bunge has been completed. The purchase of any additional land by the Solazyme Bunge JV would be complex, subject to multiple approvals from governmental authorities and take time to complete. The construction of the Solazyme Bunge JV’s production facility began in June 2012, and the first commercial product from the Solazyme Bunge JV production facility was produced in the second quarter of 2014. Manufacturing operations and processes continue to be optimized as the facility is ramped up. In addition, we have entered into a series of research and development agreements with Bunge and with the Solazyme Bunge JV to, among other things, develop additional products for the Solazyme Bunge JV. The current funded projects extend through December 2018. We intend to continue to expand our manufacturing capacity by entering into additional agreements with feedstock producers that require them to invest some or all of the capital needed to build new production facilities to produce our products. In return, we expect to share in profits anticipated to be realized from the sale of these products.
In November 2012, we and Archer-Daniels-Midland Company ("ADM") entered into a Strategic Collaboration Agreement (“Collaboration Agreement”), establishing a strategic collaboration ("Solazyme/ADM Collaboration") at the ADM facility in Clinton, Iowa (Clinton Facility") for the production of microalgae-based products. Concurrently with the execution of the Collaboration Agreement, we and ADM entered into an operating agreement (the “Operating Agreement”) related to the production of products at the Clinton Facility. On October 29, 2015, we provided notice to ADM of the termination of the Operating Agreement as of February 26, 2016 (the “Termination Date”). On December 28, 2015, we and ADM entered into a Termination Agreement relating to the termination of the Strategic Collaboration. On February 26, 2016, the Operating Agreement and the Collaboration Agreement terminated.
Since the third quarter of 2013, downstream processing of products manufactured at the Clinton Facility had been performed at a finishing facility in Galva, Iowa (“Galva Facility”), which is operated by our long-term partner, a wholly owned subsidiary of American Natural Processors, Inc. (“ANP”) (“Clinton/Galva Facilities”). We and the wholly owned subsidiary of ANP entered a Termination Agreement on December 11, 2015 terminating the contract for services at the Galva Facility as of December 31, 2015. Despite the termination we expect to continue to use the Galva Facility for some downstream processing.
14
Due to the termination of the contracts relating to the Clinton/Galva Facilities, customers that previously had received our products from the Clinton/Galva Facilities may need to qualify our products that are produced at the Solazyme Bunge JV production facility. A failure by the products manufactured at the Solazyme Bunge JV to qualify or otherwise meet the requirements of our customers would adversely affect our business.
There can be no assurance that a sufficient number of other sugar or other feedstock mill owners will accept the opportunity to partner with us for the production of our microalgae-based products. Reluctance on the part of mill owners may be caused, for example, by their failure to understand our technology or product opportunities or their belief that greater economic benefits can be achieved from partnering with others. Mill owners may also be reluctant or unable to obtain needed capital; alternatively, if mill owners are able to obtain debt financing, we may be required to provide a guarantee. Limitations in the credit markets, such as those experienced in the most recent economic downturn or historically in developing nations as a result of government monetary policies designed in response to very high rates of inflation, would impede or prevent this kind of financing and could adversely affect our ability to develop the production capacity needed to allow us to grow our business. Mill owners may also be limited by existing contractual obligations with other third parties, liability, health and safety concerns and additional maintenance, training, operating and other ongoing expenses.
Even if additional feedstock partners are willing to co-locate our production at their mills, they may do so only on economic terms that place more of the cost, or confer less of the economic return, on us than we currently anticipate. If we are not successful in negotiations with mill owners, our cost of securing additional manufacturing capacity may be higher than anticipated in terms of up-front costs, capital expenditure or lost future returns, and we may not gain the manufacturing capacity that we need to grow our business.
Our pursuit of new product opportunities may not be technologically feasible or cost effective, which would limit our ability to expand our product line and sources of revenues.
We have committed, and intend to continue to commit, substantial resources, alone or with collaboration partners, to the development and analysis of new oils and other microalgae-based products by applying classical and recombinant technology to our microalgae strains. There is no guarantee that we will be successful in creating new oil profiles, or other microalgae-based products, that we, our partners or their customers desire. There are significant technological hurdles in successfully applying recombinant and other technology to microalgae, and if we are unsuccessful at developing microalgae strains that produce desirable oils and other microalgae-based products, the number and size of the markets we will be able to address will be limited, our expected profit margins could be reduced and the potential profitability of our business could be compromised.
The successful development of our business depends on our ability to efficiently and cost-effectively produce microalgae-based products at large commercial scale.
Two of the significant drivers of our production costs are the level of productivity and conversion yield of our microalgae strains. For example, with respect to oil, productivity is principally a function of the amount of oil that can be obtained from a given volume over a particular time period. Conversion yield refers to the amount of the desired oil that can be produced from a fixed amount of feedstock. We may not be able to meet our currently expected production cost profile as we ramp up large commercial manufacturing facilities. If we cannot do so, our business could be materially and adversely affected.
Production of both current and future oils and other microalgae-based products will require that our technology and processes be scalable from laboratory, pilot and demonstration projects to large commercial-scale production. We have limited experience constructing, ramping up or managing large, commercial-scale manufacturing facilities. We may not have identified all of the factors that could affect our manufacturing processes. Our technology may not perform as expected when applied at large commercial scale, or we may encounter operational challenges for which we are unable to identify a workable solution. For example, contamination in the production process, equipment failure or accidents, problems with consistent and reliable plant utilities, human error, issues arising from process modifications to reduce costs and adjust product specifications, and other similar challenges could decrease process efficiency, create delays and increase our costs. To date we have employed our technology using fermenters with a capacity of up to approximately 625,000 liters. However, we still need to demonstrate that we can reach our target cost structure, including the achievement of target yields and productivities at approximately 625,000 liter scale in Brazil. We may not be able to scale up our production in a timely manner, on commercially reasonable terms, or at all. If we are unable to manufacture products at a large commercial scale, our ability to commercialize our technology will be adversely affected, and, with respect to any products that we do bring to market, we may not be able to achieve and maintain an acceptable production cost profile, which would adversely affect our ability to reach, maintain and increase the profitability of our business.
We rely in part on third parties for the production and processing of our products. If these parties do not produce and process our products at a satisfactory quality, in a timely manner, in sufficient quantities and at an acceptable cost, our development and commercialization efforts could be delayed or otherwise negatively impacted.
15
Other than our Peoria Facility, we do not wholly own facilities that can produce and process our products other than at small scale. As such, we rely, and we expect to continue to rely, at least partially, on third parties (including partners and contract manufacturers) for the production and processing of our products. We currently have only one manufacturing arrangement for large-scale commercial fermentation: an agreement for the manufacture of certain products by the Solazyme Bunge JV pursuant to a joint venture arrangement. We also have the ability to do smaller-scale commercial fermentation at our Peoria facility.
In addition, we have manufacturing agreements relating to other aspects of our production process. Our current and anticipated future dependence upon our partners and contract manufacturers for the production and processing of our products may adversely affect our ability to develop products on a timely and competitive basis. The failure of any of our counterparties to provide acceptable products could delay the development and commercialization of our products. We or our partners will need to enter into additional agreements for the commercial development, manufacturing and sale of our products. There can be no assurance that we or our partners can do so on favorable terms, if at all. Even if we reach agreements with manufacturing partners to produce and process our products, initially the partners will be unfamiliar with our technology and production processes. We cannot be sure that the partners will have or develop the operational expertise needed to run the equipment and processes required to manufacture our products. Further, we may have limited control over the amount or timing of resources that any partner is able or willing to devote to production and processing of our products.
To date, our products have been produced and processed in quantities sufficient for our development work and initial commercial sales. Even if there is demand for our products at commercial scale, we or our partners may not be able to successfully increase the production capacity for any of our products in a timely or economic manner or at all. In addition, to the extent we are relying on contract manufacturers to produce and process our products, we cannot be sure that such contract manufacturers will have capacity available when we need their services, that they will be willing to dedicate a portion of their production and/or processing capacity to our products or that we will be able to reach acceptable price and other terms with them for the provision of their production and/or processing services. If we, our partners or our contract manufacturers are unable to increase the production capacity for a product when and as needed, the commercial launch of that product may be delayed, or there may be a shortage of supply, which could limit sales, cause us to lose customers and sales opportunities and impair the growth of our business.
In addition, if a facility or the equipment in a facility that produces and/or processes our products is significantly damaged, destroyed or otherwise becomes unavailable, we or our partners may be unable to replace the manufacturing capacity quickly or cost effectively. The inability to enter into manufacturing agreements, the damage or destruction of a facility upon which we or our partners rely for manufacturing or any other delays in obtaining supply would delay or prevent us and/or our partners from further developing and commercializing our products.
We may experience significant delays and/or cost overruns in financing, designing, constructing and ramping up large commercial manufacturing facilities, which could result in harm to our business and prospects.
Our business plan contemplates bringing additional commercial manufacturing capacity online over the next several years. In order to meet our financial requirements for those facilities, we may have to raise additional funds and may be unable to do so in a timely manner, in sufficient amounts and on terms that are favorable to us, if at all. If we fail to raise sufficient funds, our ability to ramp up the Solazyme Bunge JV production facility or construct additional manufacturing facilities could be significantly limited. If this happens, we may be forced to delay the commercialization of our products and we will not be able to successfully execute our business plan, which would harm our business.
Manufacturing operations have begun at the Solazyme Bunge JV production facility adjacent to Bunge’s Moema sugarcane mill in Brazil. The first products from the Solazyme Bunge JV production facility were produced in the second quarter of 2014, and manufacturing operations at the facility are in the process of being optimized and ramped up. We do not expect the facility to reach target nameplate capacity in the near term as the Solazyme Bunge JV continues to optimize manufacturing operations and focuses production on high margin products, and additional capital expenditures may be required to reach nameplate capacity depending on the product mix produced at the Solazyme Bunge JV production facility. Under the joint venture agreements, Bunge has agreed to provide feedstock as well as utility services to the Solazyme Bunge JV production facility. The production facility has experienced, and may continue to experience, intermittent supply of power and steam from Bunge. Bunge and the Solazyme Bunge JV have completed a number of power and steam improvement projects, including the construction of an electrical grid tie-in and the tie-in and activation of a second steam boiler. The Solazyme Bunge JV continues to evaluate the performance of these projects and may take additional actions in the future to further improve power and steam reliability, if necessary. Without consistent and reliable supplies of power and steam to the production facility, production yields will be lower, the ramp up and optimization of the Solazyme Bunge JV production facility will be delayed, our costs will increase and our business and results of operations will be adversely affected.
16
In February 2013, the Solazyme Bunge JV entered into a loan agreement with the Brazilian Development Bank (“BNDES”) for project financing. Funds borrowed under the loan agreement have supported the production facility in Brazil, including a portion of the construction costs of the facility. We have used a portion of our $35.0 million revolving and term loan credit facility (the “HSBC facility”) with HSBC Bank, USA, National Association (“HSBC”) to support a bank guarantee of the BNDES loan. The HSBC facility expires on May 31, 2016, and we may not be successful in replacing the bank guarantee or the facility to support the bank guarantee on substantially similar terms; negotiating the terms of the bank guarantee or the new facility may take longer than anticipated and may contain terms that are not favorable to us. In addition, we may be required to provide a corporate guarantee of a portion of the BNDES loan (in an amount that, when added to the amount supported by our bank guarantee, does not exceed our ownership percentage in the Solazyme Bunge JV). Negotiating the terms of the corporate guarantee documentation may take longer than anticipated and may contain terms that are not favorable to us. The Solazyme Bunge JV may in the future seek additional financing and may not be able to raise sufficient additional funds on favorable terms, if at all. If the Solazyme Bunge JV is unable to secure additional financing, we will be required to fund our portion of the Solazyme Bunge JV’s capital requirements either from existing sources or seek additional financing. The acquisition of the facility site by the Solazyme Bunge JV from Bunge has been completed. The purchase of any additional land by the Solazyme Bunge JV would be complex, subject to multiple approvals of governmental authorities and take time to complete.
We will need to construct, or otherwise secure access to, and fund, additional capacity significantly greater than what we currently have as we continue to commercialize our products. Some of our customers may ultimately require that we acquire access to additional production facilities in order to diversify our manufacturing base. We expect to bring online additional facilities in the future. Although we intend to enter into arrangements with third parties to meet our capacity targets, it is possible that we will need to construct our own facility or facilities to meet a portion or all of these targets. We have limited experience in the construction of commercial production facilities and, if we decide to construct our own facility, we will need to secure necessary funding, complete design and other plans needed for the construction of such facility and secure the requisite permits, licenses and other governmental approvals, and we may not be successful in doing so. The construction of any such facility would have to be completed on a timely basis and within an acceptable budget. In addition, there may be delays related to the acquisition of facility sites, which could delay the development and commercialization of our products, as well as delays in deliveries of materials for the construction of such manufacturing facilities in more remote locations. Any facility, whether owned by a third party or by us, must perform as designed once it is operational. If we encounter significant delays, cost overruns, engineering or utility problems, equipment damage, accidents, equipment supply constraints or other serious challenges in bringing any of these facilities online, we may be unable to meet our production goals in the time frame we have planned. In addition, we have limited experience in the management of manufacturing operations at large scale. We may not be successful in producing the amount and quality of oil or other microalgae-based products we anticipate in the facilities and our results of operations may suffer as a result. We have limited experience producing our products at commercial scale, and we will not succeed if we cannot maintain or decrease our production costs and effectively scale our technology and manufacturing processes.
We face financial risk associated with ramping up production to reduce our per-unit production costs. To reduce per-unit production costs, we must increase production to achieve economies of scale. However, if we do not sell production output in a timely manner or in sufficient volumes at sufficient prices, our investment in production will harm our cash position and generate losses. Due to decreases in the prices of petroleum and certain plant oils, on which products competitive with our own depend, we have determined not to manufacture certain of our products because the production and sale of such products at a loss would adversely affect our business. Therefore, we expect the time required to ramp up the Solazyme Bunge JV production facility and to achieve positive cash flows at such facility will be more than we previously anticipated. Further delays would materially adversely affect our business.
If we fail to maintain and successfully manage our existing, or enter into new, strategic collaborations, we may not be able to develop and commercialize many of our products and achieve or sustain profitability.
Our ability to enter into, maintain and manage collaborations in our target markets is fundamental to the success of our business. We currently have joint venture, research and development, supply and/or distribution agreements with various strategic partners. We currently rely on our partners, in part, for manufacturing and sales or marketing services and intend to continue to do so for the foreseeable future, and we intend to enter into other strategic collaborations to produce, market and sell other products we develop. However, we may not be successful in entering into collaborative arrangements with third parties for the production and sale and marketing of other products. Any failure to enter into collaborative arrangements on favorable terms could delay or hinder our ability to develop and commercialize our products and could increase our costs of development and commercialization.
In the food, animal nutrition, fuels and chemicals markets, we have entered into a joint venture arrangement with Bunge that is focused on the manufacture of products in Brazil and development agreements with various other partners. In addition, we have entered into a commercial supply agreement with Unilever. In the skin and personal care market, we have entered into
17
arrangements with Sephora S.A. and its affiliates (“Sephora”), QVC, Inc. and others. There can be no guarantee that we can successfully manage these strategic collaborations. Under our agreement with Sephora, we bear a significant portion of the costs and risk of marketing the products, but do not exercise sole control of marketing strategy. In some cases, we will need to meet certain milestones to continue our activities with these partners. Moreover, the exclusivity provisions of certain strategic arrangements limit our ability to otherwise commercialize our products.
Pursuant to the agreements listed above and similar arrangements that we may enter into in the future, we may have limited or no control over the amount or timing of resources that any partner is able or willing to devote to our products or collaborative efforts. Any of our partners may fail to perform their obligations as expected. These partners may breach or terminate their agreements with us or otherwise fail to conduct their collaborative activities successfully and in a timely manner. Further, our partners may not develop products arising out of our arrangements or devote sufficient resources to the development, manufacture, marketing, or sale of our products. Dependence on collaborative arrangements will also subject us to other risks, including:
• | we may be required to relinquish important rights, including intellectual property, marketing and distribution rights; |
• | we may disagree with our partners as to rights to intellectual property we develop, or their research programs or commercialization activities; |
• | we may have lower revenues than if we were to market and distribute such products ourselves; |
• | a partner could separately develop and market a competing product either independently or in collaboration with others, including our competitors; |
• | a partner could divest assets that are critical to our or our joint venture’s operations to a third party that is less willing to cooperate with us or is less incentivized or able to manage such assets in a way that helps us achieve our operational and financial goals; |
• | our partners could become unable or less willing to expend their resources on research and development, commercialization efforts or the maintenance or supply of production services due to general market conditions, their financial condition or other circumstances beyond our control; |
• | we may be unable to manage multiple simultaneous partnerships or collaborations; and |
• | our partners may operate in countries where their operations could be adversely affected by changes in the local regulatory environment or by political unrest. |
Moreover, disagreements with a partner or former partner could develop, and any conflict with a partner or former partner could reduce our ability to enter into future collaboration agreements and negatively impact our relationships with one or more existing partners. If any of these events occurs, or if we fail to maintain our agreements with our partners, we may not be able to commercialize our existing and potential products, grow our business or generate sufficient revenues to support our operations. In addition, disagreements with a partner or former partner could result in disputes or litigation. Formal dispute resolution and litigation can require substantial time and resources, and the resolution of disputes and litigation may result in settlements or judgments that have a materially adverse impact on our results of operations or our financial condition. We are currently engaged in legal proceedings with our former partner Roquette Frères, S.A. For additional information regarding the Roquette proceedings, see Item 3. Legal Proceedings and Note 12 - Commitments and Contingencies of the notes to the consolidated financial statements.
Additionally, our business could be negatively impacted if any of our partners undergoes a change of control or were to otherwise assign the rights or obligations under any of our agreements to a competitor of ours or to a third party who is not willing to work with us on the same terms or commit the same resources as the current partner.
Our relationship with our strategic partner Bunge may not prove successful.
We have entered into a joint venture with Bunge that is focused on the production of certain microalgae-based products in Brazil. In connection with the establishment of the Solazyme Bunge JV, we entered into a development agreement and other agreements with Bunge and the Solazyme Bunge JV.
Our ability to generate value from the Solazyme Bunge JV depends on, among other things, our ability to work cooperatively with Bunge and the Solazyme Bunge JV for the commercialization of the Solazyme Bunge JV’s products. We may not be able to do so. For example, under the joint venture agreements, Bunge has agreed to provide feedstock as well as
18
utility services to the Solazyme Bunge JV production facility. Since originally establishing the Solazyme Bunge JV we have expanded the products and fields in which the joint venture is operating.
In addition, Bunge has announced that it is actively pursuing strategic alternatives for its Brazilian sugarcane business, which could involve the divestment, in whole or in part, of the assets of such business. While a new controlling entity would remain subject to the terms of the feedstock and utility supply agreements, that entity may be less willing to cooperate with us or the Solazyme Bunge JV, which may adversely affect the development and commercialization of the Solazyme Bunge JV’s products.
We and Bunge each provide various administrative services to the Solazyme Bunge JV, and Bunge also provides working capital to the Solazyme Bunge JV through a revolving loan facility. Bunge does not have previous experience working with our technology, and we cannot be sure that the Solazyme Bunge JV will be successful in commercializing its products. In addition, there may be delays or cost overruns in connection with the ramp up and optimization of the Solazyme Bunge JV production facility. There may also be delays in our negotiation of the bank and corporate loan guarantees in connection with the loan agreement with BNDES. In addition, we will be required to maintain the required license, granted by the Sao Paulo State Environmental Department, to operate the production facility. Any negative event with respect to these issues would delay the development and commercialization of the Solazyme Bunge JV products. Furthermore, the agreements governing our partnership are complex and cover a range of future activities, and disputes may arise between us and Bunge that could delay completion of the Solazyme Bunge JV facility and/or the expansion of the Solazyme Bunge JV’s capacity and the development and commercialization of the Solazyme Bunge JV’s products or cause the dissolution of the Solazyme Bunge JV.
Our joint venture with Roquette has been dissolved. We are currently in litigation with Roquette and we may have other disputes with Roquette related to the joint venture's business.
In 2010, we entered into a 50/50 joint venture with Roquette Frères, S.A. (“Roquette”). As part of this relationship, we and Roquette formed Solazyme Roquette Nutritionals, LLC (“SRN”) through which both we and Roquette agreed to pursue certain opportunities in microalgae-based products for the food, nutraceuticals and animal feed markets. In June 2013, we and Roquette agreed to dissolve SRN and on July 18, 2013, SRN was dissolved. As a result of the dissolution, the joint venture and operating agreement between us and Roquette, and the license agreement, whereby we licensed to SRN certain of our intellectual property, automatically terminated.
We and Roquette engaged in an arbitration proceeding concerning the proper assignment of the intellectual property of SRN. In February 2015 the arbitration panel awarded all such intellectual property to us, and this award was confirmed by the U.S. District Court for the District of Delaware in December 2015. In addition, Roquette commenced two separate actions in the U.S. District Court for the District of Delaware for declarations that, among other things, the arbitrators exceeded their authority by failing to render a timely arbitration award and as a result any orders or awards issued by the arbitrators are void. Summary judgment in these matters requested by Roquette was denied by the U.S. District Court for the District of Delaware in December 2015. We have counterclaimed for damages for misappropriation of trade secrets, misuse of confidential information and breach of contract. In turn Roquette has counterclaimed that we have misused certain Roquette trade secrets. The proceedings are ongoing. See Item 3. Legal Proceedings and Note 12 - Commitments and Contingencies of the notes to the consolidated financial statements for more information regarding our proceedings with Roquette. We cannot be sure that other disputes will not arise between us and Roquette related to the joint venture's business. Such disagreements and disputes are costly, time-consuming to resolve and distracting to our management.
Disputes regarding our intellectual property rights, and the rights of others (including Roquette) to manufacture and sell the products included in the SRN joint venture could delay or negatively impact our commercialization of products in the markets SRN was targeting. Any such disputes could be costly, time-consuming to resolve and distracting to our management. In addition, if our commercialization in these markets is delayed or unsuccessful, our financial results could be negatively impacted.
We cannot be sure that our products will meet necessary standards or be approved or accepted by customers in our target markets.
If we are unable to convince our potential customers or end users of our products that we are a reliable supplier, that our products are comparable or superior to the products that they currently use, or that the use of our products is otherwise beneficial to them, we will not be successful in entering our target markets and our business will be adversely affected.
In the food and nutrition market, our food ingredients and products will compete with oils and other food ingredients currently in use. Potential customers may not perceive a benefit to microalgae-based ingredients as compared to existing ingredients or may be otherwise unwilling to adopt their use. If consumer packaged goods (“CPG”) companies do not accept our food ingredients as ingredients for their widely distributed finished products, or if end customers are unwilling to purchase
19
finished products made using our products, we will not be successful in competing in the nutrition market and our business will be adversely affected. Customers in the nutrition market also may require lengthy and complex qualification procedures with respect to our products, our manufacturing capabilities, regulatory issues and other matters.
In the skin and personal care market, our branded products are marketed directly to potential consumers, but we cannot be sure that consumers will continue to be attracted to our brands, be attracted to our new brands or products, or purchase our products on an ongoing basis. As a result, our branded products may not be successful, distribution partners may decide to discontinue marketing our products and our business will be adversely affected.
In the chemicals market, the potential customers for our or the Solazyme Bunge JV’s products are generally companies that have well-developed manufacturing processes and arrangements with suppliers for the chemical components of their products and may resist changing these processes and components. These potential customers frequently impose lengthy and complex product qualification procedures on their suppliers, influenced by consumer preference, manufacturing considerations, supplier operating history, regulatory issues, product liability and other factors, many of which are unknown to, or not well understood by, us. Satisfying these processes may take many months or years.
Although we produce products for the fuels market that comply with industry specifications, potential fuels customers may be reluctant to adopt new products. In addition, our fuels may need to satisfy product certification requirements of equipment manufacturers. For example, diesel engine manufacturers may need to certify that the use of diesel fuels produced from our oils in their equipment will not invalidate product warranties.
In the oil field services market, Encapso™ competes with incumbent drilling lubricants and other specialty lubricants. Potential customers may be reluctant to adopt an algae-based product because of their unfamiliarity with our technology. Encapso™ has been subjected only to a limited number of on-site drilling trials, and certain customers may require further data and operating history prior to committing to purchase.
We have entered into a limited number of binding, definitive commercial supply agreements that contain minimum volume commitments. We also periodically enter into non-binding letters of intent with third parties regarding purchase of our products, but these agreements do not unconditionally obligate the other party to purchase any quantities of any products at this time. There can be no assurance that non-binding letters of intent will lead to unconditional definitive agreements to purchase our products.
We have limited experience in structuring arrangements with customers for the purchase of our microalgae-based products, and we may not be successful in this essential aspect of our business.
We expect that our customers will include large companies that sell food products, personal care products and chemical products, as well as large users of oils for fuels and lubricants for oil field operations and other applications. Because we began commercializing our personal care products in the last few years, have only recently begun to commercialize lubricants for oil field operations and our own food ingredient products, and are still in the process of developing our products for the food, nutrition and skin and personal care, fuels and chemicals, oil field services and other markets, we have limited experience operating in our customers’ industries and interacting with the customers that we intend to target. Developing the necessary expertise may take longer than we expect and will require that we expand and improve our sales and marketing capability, which could be costly. These activities could delay our ability to capitalize on the opportunities that we believe our technology and products present, and may prevent us from successfully commercializing our products. Further, we ultimately aim to sell large amounts of our products, and this will require that we effectively negotiate and manage contracts for these purchase and sale relationships. The companies with which we aim to have arrangements are generally much larger than we are and have substantially longer operating histories and more experience in their industries than we have. As a result, we may not succeed in establishing relationships with these companies and, if we do, we may not be effective in negotiating or managing the terms of such relationships, which could adversely affect our future results of operations.
We may be subject to product liability claims and other claims of our customers and partners.
The design, development, production and sale of our products involve an inherent risk of product liability claims and the associated adverse publicity. Because some of our ultimate products in each of our target markets are used by consumers, and because use of those ultimate products may cause injury to those consumers and damage to property, we are subject to a risk of claims for such injuries and damages. In addition, we may be named directly in product liability suits relating to our products or third-party products integrating our products, even for defects resulting from errors of our partners, contract manufacturers or other third parties working with our products. These claims could be brought by various parties, including customers who are purchasing products directly from us or other users who purchase products from our customers or partners. We could also be named as co-parties in product liability suits that are brought against manufacturing partners that produce our products.
20
In addition, our customers and partners may bring suits against us alleging damages for the failure of our products to meet stated claims, specifications or other requirements. Any such suits, even if not successful, could be costly, disrupt the attention of our management and damage our negotiations with other partners and/or customers. Although we often seek to limit our product liability in our contracts, such limits may not be enforceable or may be subject to exceptions. Our current product liability and umbrella insurance for our business may be inadequate to cover all potential liability claims. Insurance coverage is expensive and may be difficult to obtain. Also, insurance coverage may not be available in the future on acceptable terms and may not be sufficient to cover potential claims. We cannot be sure that our contract manufacturers or manufacturing partners who produce our ultimate products will have adequate insurance coverage to cover against potential claims. If we experience a large insured loss, it may exceed our coverage limits, or our insurance carrier may decline to further cover us or may raise our insurance rates to unacceptable levels, any of which could impair our financial position and potentially cause us to go out of business.
We will face risks associated with our international business in developing countries and elsewhere.
For the foreseeable future, our business plan will likely subject us to risks associated with essential manufacturing, sales and operations in developing countries. We have limited experience to date manufacturing and selling internationally and such expansion will require us to make significant expenditures, including the hiring of local employees and establishing facilities, in advance of generating any revenue. The economies of many of the countries in which we or our joint ventures operate or will operate have been characterized by frequent and occasionally extensive government intervention and unstable economic cycles.
In addition, in Brazil, where the Solazyme Bunge JV is located, there are restrictions on the foreign ownership of land, which may affect the Solazyme Bunge JV's ownership rights in the facility site or in any additional land purchased.
International business operations are subject to local legal, political, regulatory and social requirements and economic conditions and our business, financial performance and prospects may be adversely affected by, among others, the following factors:
• | political, economic, diplomatic or social instability; |
• | land reform movements; |
• | tariffs, export or import restrictions, restrictions on remittances abroad or repatriation of profits, duties or taxes that limit our ability to move our products out of these countries or interfere with the import of essential materials into these countries; |
• | inflation, changing interest rates and exchange controls; |
• | tax burden and policies; |
• | delays or failures in securing licenses, permits or other governmental approvals necessary to build and operate facilities and use our microalgae strains to produce products; |
• | the imposition of limitations on products or processes and the production or sale of those products or processes; |
• | uncertainties relating to foreign laws, including labor laws, regulations and restrictions, and legal proceedings; |
• | foreign ownership rules and changes in regard thereto; |
• | an inability, or reduced ability, to protect our intellectual property, including any effect of compulsory licensing imposed by government action; |
• | successful compliance with U.S. and foreign laws that regulate the conduct of business abroad, including the Foreign Corrupt Practices Act; |
• | insufficient investment in developing countries in public infrastructure, including transportation infrastructure, and disruption of transportation and logistics services; and |
• | difficulties and costs of staffing and managing foreign operations. |
These and other factors could have a material adverse impact on our results of operations and financial condition.
21
Our international operations may expose us to the risk of fluctuation in currency exchange rates and rates of foreign inflation, which could adversely affect our results of operations.
We currently incur some costs and expenses in Euros and Brazilian Reals and expect in the future to incur additional expenses in these and other foreign currencies, and also derive a portion of our revenues in the local currencies of customers throughout the world. As a result, our revenues and results of operations are subject to foreign exchange fluctuations, which we may not be able to manage successfully. During the past few decades, the Brazilian currency in particular has faced frequent and substantial exchange rate fluctuations in relation to the U.S. dollar and other foreign currencies. For example, the value of the Brazilian Real has fallen significantly against the U.S. dollar over the past few years. There can be no assurance that the Real or the Euro will not significantly appreciate or depreciate against the U.S. dollar in the future. We bear the risk that the rate of inflation in the foreign countries where we incur costs and expenses or the decline in value of the U.S. dollar compared to those foreign currencies will increase our costs as expressed in U.S. dollars. Future measures by foreign governments to control inflation, including interest rate adjustments, intervention in the foreign exchange market and changes to the fixed value of their currencies, may trigger increases in inflation. We may not be able to adjust the prices of our products to offset the effects of inflation on our cost structure, which could increase our costs and reduce our net operating margins. If we do not successfully manage these risks through hedging or other mechanisms, our revenues and results of operations could be adversely affected.
We may encounter difficulties managing our growth, and we will need to properly prioritize our efforts in our target markets as our business grows. If we are unable to do so, our business, financial condition and results of operations may be adversely affected.
Our business has grown rapidly. Continued growth may place a strain on our human and capital resources. If we grow too rapidly or if our headcount or other aspects of our operating structure become misaligned with our strategy, we may need to reduce headcount or other operating costs. For example, in December 2014, as part of an adjustment to our operating and expense strategy related to the ramping up of the Solazyme Bunge JV production facility, we announced the intention to decrease operating expenses through a reduction in workforce and other cost-cutting measures. See the risk factor titled “We may experience significant delays and/or cost overruns in financing, designing, constructing and ramping up large commercial manufacturing facilities, which could result in harm to our business and prospects” above for more information. Such reductions in workforce can have an adverse effect on our business.
Furthermore, we intend to conduct our business internationally and anticipate business operations in the United States, Europe, Latin America and elsewhere. These diversified, global operations place increased demands on our limited resources and may require us to substantially expand the capabilities of our administrative and operational resources and will require us to attract, train, manage and retain qualified management, technicians, scientists and other personnel. As our operations expand domestically and internationally, we will need to continue to manage multiple locations and additional relationships with various customers, partners, suppliers and other third parties across several product categories and markets.
Our business has taken place across several target markets: food and nutrition, skin and personal care, fuels and chemicals, and oil field services. We will be required to prioritize our limited financial and managerial resources as we pursue particular development and commercialization efforts in each target market. Any resources we expend on one or more of these efforts could be at the expense of other potentially profitable opportunities. If we focus our efforts and resources on one or more of these markets and they do not lead to commercially viable products, our revenues, financial condition and results of operations could be adversely affected. Furthermore, as our operations continue to grow, the simultaneous management of development, production and commercialization across our target markets will become increasingly complex and may result in less than optimal allocation of management and other administrative resources, increase our operating expenses and harm our operating results.
Our ability to effectively manage our operations, growth and various projects across our target markets will require us to make additional investments in our infrastructure to continue to improve our operational, financial and management controls and our reporting systems and procedures and to attract and retain sufficient numbers of talented employees, which we may be unable to do effectively. We may be unable to successfully manage our expenses in the future, which may negatively impact our gross margins or operating margins in any particular quarter.
In addition, we may not be able to improve our management information and control systems, including our internal control over financial reporting, to a level necessary to manage our growth and we may discover deficiencies in existing systems and controls that we may not be able to remediate in an efficient or timely manner.
We are involved, and may become involved in the future, in legal proceedings that, if adversely adjudicated or settled, could adversely affect our financial results.
22
We, and certain of our officers and directors, are involved, and may be involved in the future, in legal proceedings and claims arising in the course of our business. Such matters are subject to many uncertainties and there can be no assurance that such legal proceedings will not have a material adverse effect on our business, results of operations, financial position or cash flows. See Item 3 - Legal Proceedings and Note 12 - Commitments and Contingencies of the notes to the consolidated financial statements of this report for a description of the material legal proceedings in which we are currently engaged.
We cannot predict the outcome of any legal proceeding in which we are engaged. Moreover, any conclusion of legal proceedings in a manner adverse to us could have an adverse effect on our financial condition and business. Even if we are successful in litigation, we could incur substantial costs, suffer a significant adverse impact on our reputation and divert management’s attention and resources from other priorities, including the execution of business plans and strategies that are important to our ability to grow our business, any of which could have an adverse effect on our business. Legal proceedings may result in significant legal expenses, settlement costs or damage awards that are not covered by, or exceed the limits of, our liability insurance, which could adversely impact our financial condition, results of operations or cash flow.
Our success depends in part on recruiting and retaining key personnel and, if we fail to do so, it may be more difficult for us to execute our business strategy. We are currently a small organization and will need to hire additional personnel to execute our business strategy successfully.
Our success depends on our continued ability to attract, retain and motivate highly qualified management, business development, manufacturing and scientific personnel and directors, and on our ability to develop and maintain important relationships with leading academic institutions and scientists. We are highly dependent upon a number of key members of our senior management, including manufacturing, business development and scientific personnel, and on our directors. If any of such persons left, our business could be harmed. All of our employees and directors are at-will and may resign at any time. The loss of the services of one or more of our key employees, or directors could delay or have an impact on the successful commercialization of our products. We do not maintain any key man insurance. Competition for qualified personnel in the biotechnology field is intense, particularly in the San Francisco Bay Area. We may not be able to attract and retain qualified personnel on acceptable terms given the competition for such personnel. In addition, the restructuring that we implemented in January 2016 could have an adverse impact on our ability to retain and recruit qualified personnel. If we are unsuccessful in our recruitment efforts, we may be unable to execute our strategy.
We may not be able to meet applicable regulatory requirements for the sale of our microalgae-based products and the operation of production facilities, and, even if requirements are met, complying on an ongoing basis with the numerous regulatory requirements applicable to our various product categories and facilities will be time-consuming and costly.
Our chemical products may be subject to government regulation in our target markets. In the United States, the EPA administers the Toxic Substances Control Act (“TSCA”), which regulates the commercial registration, distribution, and use of chemicals. TSCA will require us to comply with the Microbial Commercial Activity Notice (“MCAN”) process to manufacture and distribute products made from our recombinant microalgae strains. An MCAN is not required for non-recombinant strains. To date, we have filed MCANs for certain of our recombinant microalgae strains, all of which have been dropped from review. Our subsequent filing of Notices of Commencement (NOC) relating to previously filed MCANs allows us to commercially use these strains. We expect to file additional MCANs in the future.
Before we can manufacture or distribute significant volumes of a chemical, we need to determine whether that chemical is listed in the TSCA inventory. If the substance is listed, then manufacture or distribution can commence immediately. If not, then a pre-manufacture notice (“PMN”) must be filed with the EPA for a review period of up to 90 days excluding extensions. We have filed PMNs for certain of our products and expect to file additional PMNs in the future. Some of the products we produce or plan to produce are already on the TSCA inventory due to our successful PMN submissions and filed NOCs. Others are not yet listed. We may not be able to expediently receive approval from the EPA to list the chemicals we would like to make on the TSCA inventory, resulting in delays or significant increases in testing requirements. A similar program exists in the European Union, called REACH (Registration, Evaluation, Authorization, and Restriction of Chemical Substances). We are required to register some of our products with the European Commission, and this process could cause delays or significant costs. We have determined that some of our algae oils are exempt from REACH registration requirements. To the extent that other geographies, such as Brazil, may rely on the TSCA or REACH for chemical registration in their geographies, delays with the U.S. or European authorities may subsequently delay entry into these markets as well. Furthermore, other geographies may have their own chemical inventory requirements, which may delay entry into these markets, irrespective of U.S. or European approval.
Our food and nutrition products are subject to regulation by various government agencies, including the U.S. Food and Drug Administration (“FDA”), state and local agencies and similar agencies outside the United States. In the U.S., food ingredients are regulated either as food additives or as substances generally recognized as safe, or GRAS. A GRAS self-
23
determination can be made with respect to a substance by its manufacturer upon the receipt of an opinion from a panel of independent qualified experts who determine that the substance is GRAS for its intended conditions of use. A GRAS Notice for one algae oil was submitted to the FDA in June 2011, and a “no questions” letter was received from the FDA in June 2012. A GRAS Notice for each of whole algae flour and whole algae protein was submitted to the FDA, and a “no questions” letter was received from the FDA in 2013 for whole algae flour and in 2014 for whole algae protein. A GRAS Notice for our second algae oil, an oleic algae oil, was submitted in July 2014 and received a “no questions” letter in February 2015. If the FDA were to disagree with the conclusions in future GRAS Notices, they could ask that the products be voluntarily withdrawn from the market or could initiate legal action to halt their sale. Such actions by the FDA could have an adverse effect on our business, financial condition, and results of our operations. Food ingredients that are not GRAS are regulated as food additives and require FDA approval prior to commercialization. The food additive petition process is generally expensive and time consuming, with approval, if secured, taking years. In Brazil, we submitted applications to the Brazilian Health Surveillance Agency (ANVISA) for approval of various food products. We received food approval with no restrictions for our whole algae flour in July 2015. In Canada, we received a Letter of No Objection for whole algae flour in February 2016. Other products may or may not be approved in the future. Any significant delay or disapproval by ANVISA, Health Canada or other government agencies of our food products would adversely affect our food and nutrition business in these countries.
The sale and/or use of diesel and jet fuels produced from our oils are subject to regulation by various government agencies, including the Environmental Protection Agency (“EPA”) and the California Air Resources Board in the United States. To date, we have registered our Soladiesel®RD fuel in the United States and submitted an application for registering Soladiesel®BD in December 2015. We or our refining or commercialization partners or customers may be required to register our fuel in the United States, with the European Commission and elsewhere before selling our products.
The sale of ingredients for use in animal feed is regulated by agencies including the FDA's Center for Veterinary Medicine (“CVM”). Regulatory requirements for suitability must be met by providing data from studies, which may cause delays and the incursion of additional costs.
Our personal care products are subject to regulation by various government agencies both within and outside the United States. Such regulations principally relate to the ingredients, labeling, packaging and marketing of our personal care products.
Changes in regulatory requirements, laws and policies, or evolving interpretations of existing regulatory requirements, laws and policies, may result in increased compliance costs, delays, capital expenditures and other financial obligations that could adversely affect our business or financial results.
We expect to encounter regulations in most if not all of the countries in which we may seek to sell our products, and we cannot be sure that we will be able to obtain necessary approvals in a timely manner or at all. If our products do not meet applicable regulatory requirements in a particular country or at all, then we may not be able to commercialize them and our business will be adversely affected. The various regulatory schemes applicable to our products will continue to apply following initial approval for sale. Monitoring regulatory changes and ensuring our ongoing compliance with applicable requirements will be time-consuming and may affect our results of operations. If we fail to comply with such requirements on an ongoing basis, we may be subject to fines or other penalties, or may be prevented from selling our products, and our business may be harmed.
The construction and operation of our, our partners’ or our joint ventures’ production facilities are likely to require government approvals. If we are not able to obtain or maintain the necessary approvals in a timely manner or at all, our business will be adversely affected. In February 2014, the Sao Paulo State Environmental Department granted a license to operate the Solazyme Bunge JV facility, which was necessary to begin commercial production.
We may incur significant costs complying with environmental, health and safety laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities.
We use hazardous chemicals and radioactive and biological materials in our business and are subject to a variety of federal, state, local and international laws and regulations governing, among other matters, the use, generation, manufacture, transportation, storage, handling, disposal of, and human exposure to, these materials both in the U.S. and outside the U.S., including regulation by governmental regulatory agencies, such as the Occupational Safety and Health Administration and the EPA. We have incurred, and will continue to incur, capital and operating expenditures and other costs in the ordinary course of our business in complying with these laws and regulations.
Although we have implemented safety procedures for handling and disposing of these materials and waste products in an effort to comply with these laws and regulations, we cannot be sure that our safety measures will be compliant or capable of eliminating the risk of injury or contamination from the generation, manufacturing, use, storage, transportation, handling, disposal of, and human exposure to, hazardous materials. Failure to comply with environmental, health and safety laws could subject us to liability and resulting damages. There can be no assurance that violations of environmental, health and safety laws
24
will not occur as a result of human error, accident, equipment failure or other causes. Compliance with applicable environmental laws and regulations may be expensive, and the failure to comply with past, present, or future laws could result in the imposition of fines, regulatory oversight costs, third party property damage, product liability and personal injury claims, investigation and remediation costs, the suspension of production, or a cessation of operations, and our liability may exceed our total assets. Liability under environmental laws, such as the Comprehensive Environmental Response Compensation and Liability Act in the United States, can impose liability for the full amount of damages, without regard to comparative fault for the investigation and cleanup of contamination and impacts to human health and for damages to natural resources. Contamination at properties we own and operate, and at properties to which we send hazardous materials, may result in liability for us under environmental laws and regulations.
Our business and operations will be affected by other new environmental, health and safety laws and regulations, which may affect our research and development and manufacturing programs, and environmental laws could become more stringent over time, requiring us to change our operations, or resulting in greater compliance costs and increasing risks and penalties associated with violations, which could impair our research, development or production efforts and harm our business. The costs of complying with environmental, health and safety laws and regulations, and any claims concerning noncompliance, or liability with respect to contamination in the future could have a material adverse effect on our financial condition or operating results.
Changes in government regulations, including subsidies and economic incentives, could have a material adverse effect on demand for our products, business or results of operations.
The market for renewable fuels is heavily influenced by foreign, federal, state and local government regulations and policies. Changes to existing, or adoption of new, domestic or foreign federal, state or local legislative initiatives that impact the production, distribution, sale or import and export of renewable fuels may harm our business. For example, in 2007, the Energy Independence and Security Act (“EISA”) of 2007 set targets for alternative sourced liquid transportation fuels (approximately 14 billion gallons in 2011, increasing to 36 billion gallons by 2022). Of the 2022 target amount, a minimum of 21 billion gallons must be advanced biofuels. In the U.S. and in a number of other countries, these regulations and policies have been modified in the past and may be modified again in the future. For example, in 2015, the U.S. Environmental Protection Agency (EPA) announced a reduction in the volume of total renewable fuel from the 2015 statutory target of 20.5 billion gallons to 16.3 billion gallons. The elimination of, or any additional reduction in, mandated requirements for fuel alternatives and additives to gasoline may cause demand for biofuels to decline and deter investment in the research, development or commercial adoption of renewable fuels.
In addition, the U.S. Congress has passed legislation that extends tax credits to blenders of certain renewable fuel products. However, there is no assurance that this or any other favorable legislation will remain in place. For example, the biofuel tax credit expires annually, and is therefore at risk every year for delay of approval. Any reduction in, phasing out or elimination of existing tax credits, subsidies and other incentives in the U.S. and foreign markets for renewable fuels, or any inability of our customers to access such credits, subsidies and incentives, may adversely affect demand for our fuel products and increase the overall cost of commercialization of our renewable fuels, which would adversely affect our business.
Furthermore, market uncertainty regarding future policies may also affect our ability to develop new renewable products, license our technologies to third parties and sell products to end customers. Any inability to address these requirements and any regulatory or policy changes could have a material adverse effect on our business, financial condition and results of operations.
Conversely, government programs could increase investment and competition in the markets for our products. For example, various governments have announced a number of spending programs focused on the development of clean technology, including alternatives to petroleum-based fuels and materials and the reduction of greenhouse gas (“GHG”) emissions, which could lead to increased funding for us or our competitors, or the rapid increase in the number of competitors within our markets.
Concerns associated with renewable fuels, including land usage, national security interests and food crop usage, are receiving legislative, industry and public attention. This could result in future legislation, regulation and/or administrative action that could adversely affect our business. When and how these requirements and any regulatory or policy changes are addressed could have a material adverse effect on our business, financial condition and results of operations.
Future government policies may adversely affect the supply of sugarcane, corn or cellulosic sugars, restricting our ability to use these feedstocks to produce our products, and negatively impact our revenues and results of operations.
25
We may face risks relating to the use of our targeted recombinant microalgae strains, and if we are not able to meet applicable regulatory requirements for the use of these strains or if we face material ethical, legal and social concerns about our use of targeted recombinant technology, our business could be adversely affected.
The use of microorganisms designed using targeted recombinant technology, such as some of our microalgae strains, is subject to laws and regulations in many states and countries, some of which are new and still evolving and interpreted by fact specific application. In the United States, the EPA regulates the commercial use of microorganisms designed using targeted recombinant technology as well as potential products derived from them.
We expect to encounter regulations of microorganisms designed using targeted recombinant technology in most if not all of the countries in which we may seek to establish manufacturing operations, and the scope and nature of these regulations will likely be different from country to country. For example, in the U.S., when used in an industrial process, our microalgae strains designed using targeted recombinant technology may be considered new chemicals under the TSCA, administered by the EPA. We will be required to comply with the EPA’s process. In Brazil, microorganisms designed using targeted recombinant technology are regulated by the National Biosafety Technical Commission, or CTNBio. In March 2013, we submitted an application for approval from CTNBio to use a specific microalgae strain designed using targeted recombinant technology in a contained environment in order to use these microalgae for research and development and commercial production purposes in any facilities we establish in Brazil. We obtained approval for this application from CTNBio in October 2013, and we have since received several approvals related to additional applications we had submitted. In February 2014, CTNBio granted a CQB (Certificate of Quality in Biosafety) to the Solazyme Bunge JV facility for activities including industrial production, import and export, disposal and storage of our key production organisms. If we cannot meet the applicable requirements in countries in which we intend to produce microalgae-based products, or if it takes longer than anticipated to obtain such approvals, our business could be adversely affected.
The subject of organisms designed using targeted recombinant technology has received negative publicity, which has aroused public debate. Public attitudes about the safety and environmental hazards of, and ethical concerns over, genetic research and microorganisms designed using targeted recombinant technology could influence public acceptance of our technology and products. In addition, shifting public attitudes regarding, and potential changes to laws governing, ownership of genetic material could harm our intellectual property rights with respect to our genetic material and discourage collaborators from supporting, developing, or commercializing our products, processes and technologies. Governmental reaction to negative publicity concerning organisms designed using targeted recombinant technology could result in greater government regulation of or trade restrictions on imports of genetic research and derivative products. If we and/or our collaborators are not able to overcome the ethical, legal, and social concerns relating to the use of targeted recombinant technology, our products and processes may not be accepted or we could face increased expenses, delays or other impediments to their commercialization.
We expect to face competition for our food, nutrition and skin and personal care products from other companies in these fields, many of whom have greater resources and experience than we do. If we cannot compete effectively against these companies or their products, we may not be successful in selling our products or further growing our business.
We expect that our food, nutrition and skin and personal care products will compete with providers in both the specialty and mass food ingredient markets. Many of these companies, such as Cargill, Incorporated, Monsanto Company, Syngenta AG and Roquette Frères, S.A., are larger than we are, have well-developed distribution systems and networks for their products and have valuable historical relationships with the potential customers and distributors we hope to serve. We may also compete with companies seeking to produce food and nutrition products based on renewable oils, including DSM Food Specialties and DuPont Nutrition & Health. Our success in the development of food and nutrition products will depend on our ability to effectively compete with established companies and successfully commercialize our products.
In the skin and personal care market, we expect to compete with established companies and brands with loyal customer followings. The market for skin and personal care products is characterized by strong established brands, loyal brand following and heavy brand marketing. We will compete with companies with well-known brands such as Kinerase®, Perricone MD®, and StriVectin®. These companies have greater sales and marketing resources than us. We will also compete in the mass consumer market. Some of our competitors in this market have well-known brands such as Meaningful Beauty® and Principal Secret® and have substantially greater sales and marketing resources than us. We have limited experience in the skin and personal care market. We will need to continue to devote substantial resources to the marketing of our products and there can be no assurance that we will be successful.
26
We expect to face competition for our products in the fuels and chemicals markets from providers of products based on petroleum, plant oils and animal fats and from other companies seeking to provide alternatives to these products, many of whom have greater resources and experience than we do. If we cannot compete effectively against these companies or products, our business could be harmed and certain of our strategic alternatives could be adversely impacted.
In the chemical market, we will compete with the established providers of oils currently used in chemical products. Producers of these incumbent products include global oil companies, including those selling agricultural products such as palm oil, palm kernel oil, castor bean oil and sunflower oil, large international chemical companies and other companies specializing in specific products or essential oils. We may also compete in one or more of these markets with manufacturers of other products such as highly refined petrochemicals, synthetic polymers and other petroleum-based fluids and lubricants as well as new market entrants offering renewable products.
In the transportation fuels market, we expect to compete with independent and integrated oil refiners, large oil and gas companies and, in certain fuels markets, with other companies producing advanced biofuels. The refiners compete with us by selling conventional fuel products, and some are also pursuing hydrocarbon fuel production using non-renewable feedstocks, such as natural gas and coal, as well as production using renewable feedstocks, such as vegetable oil and biomass. We also expect to compete with companies that are developing the capacity to produce diesel and other transportation fuels from renewable resources in other ways. These include advanced biofuels companies using specific engineered enzymes that they have developed to convert cellulosic biomass, which is non-food plant material such as wood chips, corn stalks and sugarcane bagasse, into fermentable sugars and ultimately, renewable diesel and other fuels. Biodiesel companies convert vegetable oils and animal oils into diesel fuel and some are seeking to produce diesel and other transportation fuels using thermochemical methods to convert biomass into renewable fuels.
We believe the primary competitive factors in both the fuels and chemicals markets are product price, product performance, sustainability, availability of supply and compatibility of products with existing infrastructure.
The oil companies, large chemical companies and well-established agricultural products companies with whom we expect to compete are much larger than we are, have, in most cases, well-developed distribution systems and networks for their products, have valuable historical relationships with the potential customers we are seeking to serve and have much more extensive sales and marketing programs in place to promote their products. Some of our competitors may use their influence to impede the development and acceptance of our products. Our limited resources relative to many of our competitors may cause us to fail to anticipate or respond adequately to new developments and other competitive pressures. In the nascent markets for renewable fuels and chemicals, it is difficult to predict which, if any, market entrants will be successful, and we may lose market share to competitors producing new or existing renewable products. If we are unsuccessful in competing in the fuels and chemicals markets, our business could be harmed and certain of our strategic alternatives could be adversely impacted.
We expect to face competition in the oil field services market.
We expect that Encapso™ will compete with incumbent drilling lubricant products that are marketed by larger companies with significantly greater resources and experience. Such competitors compete vigorously on fluids performance and price. These companies have broad product and service offerings in addition to their drilling fluids. These larger companies may attempt to compete by offering discounts to customers to use multiple products and services, some of which we do not offer. We may also compete with regional companies that compete on price, performance and local relationships. Our success in this target market will depend on our ability to effectively compete with these established companies. If we are unable to do so effectively, our business could be harmed and certain of our strategic alternatives could be adversely impacted.
A decline in the price of petroleum and petroleum-based products, plant oils or other commodities may reduce demand for our products and may otherwise adversely affect our business.
We anticipate that most of our oils, and in particular those used to produce fuels, will be marketed as alternatives to corresponding products based on petroleum and plant oils. When the price of any of these oils falls, as they have recently, we may be unable to produce algae oils or other products that are cost-effective alternatives to their petroleum or plant oil-based counterparts. Declining oil prices, or the perception of a future decline in oil prices, may adversely affect the prices we can obtain from our potential customers or prevent potential customers from entering into agreements with us to buy our products. During sustained periods of lower oil prices we may be unable to sell our products, which could materially and adversely affect our operating results. For example, in part as a result of the recent drop in the prices of petroleum and certain plant oils, the ramp up of the Solazyme Bunge JV’s production facility in Brazil will be slower than, and the mix of products manufactured in that facility will be different from, what we previously anticipated as production will be focused primarily on high margin products.
27
Petroleum prices have been extremely volatile, and this volatility is expected to persist. Lower petroleum prices over extended periods of time may change the perceptions in government and the private sector that cheaper, more readily available energy alternatives should be developed and produced. If petroleum prices remain at present levels or decline to lower levels for extended periods of time, the demand for renewable fuels could be reduced, and our business and revenue may be harmed.
Prices of plant oils have also experienced significant volatility. If prices for oils such as palm kernel were to materially decrease in the future, there may be less demand for oil alternatives, which could reduce demand for our products and harm our business. The prices of commodities that serve as food ingredients have also been volatile. To the extent that the prices of these commodities decline and remain at lower levels for extended periods of time, the demand for our food, nutrition and skin and personal care products may be reduced, and our ability to successfully compete in this market may be harmed.
Our information technology systems, processes and sites may suffer a significant breach or disruption that may adversely affect our ability to conduct our business.
Our information technology systems, some of which are dependent on services provided by third parties, provide critical data and services for internal and external users, including procurement and inventory management, transaction processing, financial, commercial and operational data, human resources management, legal and tax compliance information and other information and processes necessary to operate and manage our business. Our information technology and infrastructure may experience attacks by hackers, breaches or other failures or disruptions that could compromise our systems and the information stored there. While we have implemented security measures and disaster recovery plans designed to protect the security and continuity of our networks and critical systems, these measures may not adequately prevent adverse events such as breaches or failures from occurring or mitigate their severity if they do occur. If our information technology systems are breached, damaged or fail to function properly due to any number of causes, such as security breaches or cyber-based attacks, systems implementation difficulties, catastrophic events or power outages, and our security, contingency or disaster recovery plans do not effectively mitigate these occurrences on a timely basis, we may experience a material disruption in our ability to manage our business operations. We may also be subject to legal claims or proceedings, liability under laws that protect the privacy of personal information, potential regulatory penalties and damage to our reputation. The occurrence of any of these events may adversely impact our business, results of operations and financial condition, as well as our competitive position.
Our facilities in California are located near an earthquake fault, and an earthquake or other natural disaster or resource shortage could disrupt our operations.
Important documents and records, such as hard copies of our laboratory books and records for our products and some of our manufacturing operations, are located in our corporate headquarters in South San Francisco, California, near active earthquake zones. In the event of a natural disaster, such as an earthquake, drought or flood, or localized extended outages of critical utilities or transportation systems, we do not have a formal business continuity or disaster recovery plan, and could therefore experience a significant business interruption. In addition, California from time to time has experienced shortages of water, electric power and natural gas. Future shortages and conservation measures could disrupt our operations and could result in additional expense. Although we maintain business interruption insurance coverage, we do not maintain earthquake or flood coverage.
Risks Related to Our Intellectual Property
Our competitive position depends on our ability to effectively obtain and enforce patents related to our products, manufacturing components and manufacturing processes. If we or our licensors fail to adequately protect this intellectual property, our ability and/or our partners’ ability to commercialize products could suffer.
Our success depends in part on our ability to obtain and maintain patent protection sufficient to prevent others from utilizing our manufacturing components, manufacturing processes or marketing our products, as well as to successfully defend and enforce our patents against infringement by others. In order to protect our products, manufacturing components and manufacturing processes from unauthorized use by third parties, we must hold patent rights that cover our products, manufacturing components and manufacturing processes.
The patent position of biotechnology and bio-industrial companies can be highly uncertain because obtaining and determining the scope of patent rights involves complex legal and factual questions. The standards applied by the U.S. Patent and Trademark Office and foreign patent offices in granting patents are not always applied uniformly or predictably. There is no uniform worldwide policy regarding patentable subject matter, the scope of claims allowable in biotechnology and bio-industrial patents, or the formal requirements to obtain such patents. Consequently, patents may not issue from our pending patent applications. Furthermore, in the process of seeking patent protection or even after a patent is granted, we could become subject to expensive and protracted proceedings, including patent interference, opposition, post-grant review and re-
28
examination proceedings, which could invalidate or narrow the scope of our patent rights. As such, we do not know nor can we predict the scope and/or breadth of patent protection that we might obtain on our products and technology.
Changes either in patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property rights. In the U.S., depending on the decisions and actions taken by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. In foreign jurisdictions, depending on the decisions and actions taken by the foreign government, the judicial system of the jurisdiction, and its patent office, the laws and regulations governing patents could change in unpredictable ways that could weaken our ability to obtain new patents or to enforce our existing patents or patents that we might obtain in the future.
The America Invents Act (“AIA”), which was signed into law on September 16, 2011, brought a number of changes to the U.S. patent system and affects the way patents are prosecuted, challenged and litigated. Among the changes that went into effect on September 16, 2012, one of the most significant involves the implementation of a reformed post-grant review system. Other changes, which went into effect on March 16, 2013, include the transition from a “first-to-invent” to “first-to-file” system that attempts to harmonize the U.S. with most of the world. Lack of precedential interpretation of the new provisions of the AIA through specific cases or through guidelines promulgated by the U.S. Patent and Trademark Office and the lack of binding precedent from the courts increase the uncertainty of the impact of the AIA. Together, these changes may increase the costs of prosecution and enforcement of U.S. patents. While it is currently unclear what impact these changes will have on the operation of our business, they may favor companies able to dedicate more resources to patent filings and challenges.
Risks associated with enforcing our intellectual property rights in the United States.
If we were to initiate legal proceedings against a third party to enforce a patent claiming one of our technologies, the defendant could counterclaim that our patent is invalid and/or unenforceable or assert that the patent does not cover its manufacturing processes, manufacturing components or products. Proving patent infringement may be difficult, especially where it is possible to manufacture a product by multiple processes or when a patented process is performed by multiple parties. Patent litigation is also costly, time-consuming and distracting to our management. Furthermore, in patent litigation in the United States or elsewhere, defendant counterclaims alleging both invalidity and unenforceability are commonplace. Although we believe that we have conducted our patent prosecution in accordance with the duty of candor and in good faith, the outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity of our patent rights, we cannot be certain, for example, that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would not be able to exclude others from practicing the inventions claimed therein. Such a loss of patent protection could have a material adverse effect on our business. Defendant counterclaims of antitrust or other anti-competitive conduct are also commonplace.
Even if our patent rights are found to be valid and enforceable, patent claims that survive litigation may not cover commercially viable products or prevent competitors from importing or marketing products similar to our own, or using manufacturing processes or manufacturing components similar to our own.
Although we believe we have obtained valid assignments of patent rights from all inventors, if an inventor did not adequately assign their patent rights to us, a third party could obtain a license to the patent from such inventor. This could preclude us from enforcing the patent against such third party.
We may not be able to enforce our intellectual property rights throughout the world.
The laws of some foreign countries where we intend to produce and use our proprietary strains in collaboration with sugar mills or other feedstock suppliers do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of certain countries, including Brazil, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology and/or bio-industrial technologies. This could make it difficult for us to stop the infringement of our patents or misappropriation of our intellectual property rights in these countries. Proceedings to enforce our patent rights in certain foreign jurisdictions are unpredictable and could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate.
29
Third parties may misappropriate our proprietary strains, information, or trade secrets despite a contractual obligation not to do so.
Third parties (including joint venture, collaboration, development and feedstock partners and former partners, contract manufacturers, and other contractors and shipping agents) often have custody or control of our proprietary microbe strains. If our proprietary microbe strains were stolen, misappropriated or reverse engineered, they could be used by other parties who may be able to use our strains or reverse-engineered strains for their own commercial gain. It is difficult to prevent misappropriation or subsequent reverse engineering. In the event that our proprietary microbe strains are misappropriated, it could be difficult for us to challenge the misappropriation or prevent reverse engineering, especially in countries with limited legal and intellectual property protection.
Confidentiality agreements with employees and third parties may not prevent unauthorized disclosure of proprietary information and trade secrets.
In addition to patents, we rely on confidentiality agreements to protect our technical know-how and other proprietary information. Confidentiality agreements are used, for example, when we talk to potential strategic partners. In addition, each of our employees signed a confidentiality agreement upon joining our company. Nevertheless, there can be no guarantee that an employee or an outside party will not make an unauthorized disclosure or use of our proprietary confidential information. This might happen intentionally or inadvertently. It is possible that a competitor will make use of such information, and that our competitive position will be compromised, in spite of any legal action we might take against persons making such unauthorized disclosures.
We also keep as trade secrets certain technical and proprietary information where we do not believe patent protection is appropriate, desirable or obtainable. However, trade secrets are difficult to protect. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, outside scientific collaborators, partners, former partners and other advisors may unintentionally or willfully disclose our trade secrets to competitors or otherwise use misappropriated trade secrets to compete with us. It can be expensive and time consuming to enforce a claim that a third party illegally obtained and is using our trade secrets. Furthermore, the outcome of such claims is unpredictable. In addition, courts outside the United States may be less willing to or may not protect trade secrets. Moreover, our competitors may independently design around our intellectual property or develop equivalent knowledge, methods and know-how without misappropriating or otherwise violating our trade secret rights. Where a third party independently designs around our intellectual property or develops equivalent knowledge, methods and know-how without misappropriating or otherwise violating our trade secret rights, they may be able to seek patent protection for such equivalent knowledge, methods and know-how. This could prohibit us from practicing our trade secrets.
Claims by patent holders that our products or manufacturing processes infringe their patent rights could result in costly litigation or could require substantial time and money to resolve, whether or not we are successful, and an unfavorable outcome in these proceedings could have a material adverse effect on our business.
Our ability to commercialize our technology depends on our ability to develop, manufacture, market and sell our products without infringing the proprietary rights of patent holders or their authorized agents. An issued patent does not guarantee us the right to practice or utilize the patented inventions or commercialize the patented product. Third parties may have blocking patents that may prevent us from commercializing our patented products and utilizing our patented manufacturing components and manufacturing processes. In the event that we are made aware of blocking third party patents, we cannot be sure that licenses to the blocking third-party patents would be available or obtainable on terms favorable to us or at all.
Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, relate to (1) the production of bio-industrial products, including edible ingredients, oils, chemicals, drilling fluids and biofuels, and (2) the use of microalgae strains, such as microalgae strains containing genes to alter oil composition. As such, there could be existing valid patents that our manufacturing processes, manufacturing components, or products may inadvertently infringe. There could also be existing invalid or unenforceable patents that could nevertheless be asserted against us and would require expenditure of resources to defend. In addition, there are pending patent applications that are currently unpublished and therefore unknown to us that may later result in issued patents that are infringed by our products, manufacturing processes or other aspects of our business.
We may be exposed to future litigation based on claims that our products, manufacturing processes or manufacturing components infringe the intellectual property rights of others. There is inevitable uncertainty in any litigation, including patent litigation. Defending against claims of patent infringement is costly and time consuming, regardless of the outcome. Thus, even if we were to ultimately prevail, or to settle at an early stage of litigation, such litigation could burden us with substantial unanticipated costs. Some of our competitors are larger than we are and have substantially greater resources. These competitors
30
are, therefore, likely to be able to sustain the costs of complex patent litigation longer than we could. In addition, the costs and uncertainty associated with patent litigation could have a material adverse effect on our ability to continue our internal research and development programs, in-license needed technology, or enter into strategic partnerships that would help us commercialize our technologies. In addition, litigation or threatened litigation could result in significant demands on the time and attention of our management team, distracting them from the pursuit of other company business.
If a party successfully asserts a patent or other intellectual property rights against us, we might be barred from using certain of our manufacturing processes or manufacturing components, or from developing and commercializing related products. Injunctions against using specified processes or components, or prohibitions against commercializing specified products, could be imposed by a court or by a settlement agreement between us and a third party. In addition, we may be required to pay substantial damage awards to the third party, including treble or enhanced damages if we are found to have willfully infringed the third party’s intellectual property rights. We may also be required to obtain a license from the third party in order to continue manufacturing and/or marketing the products that were found to infringe. It is possible that the necessary license will not be available to us on commercially acceptable terms, or at all. This could limit our ability to competitively commercialize some or all of our products.
During the course of any patent litigation, there could be public announcements of the results of hearings, rulings on motions, and other interim proceedings in the litigation. If securities analysts or investors regard these announcements as negative, the perceived value of our products, technology or intellectual property could be diminished. Accordingly, the market price of our common stock may decline.
We have received government funding in connection with the development of certain of our proprietary technologies, which could negatively affect our intellectual property rights in such technologies.
Some of our proprietary technology was developed with U.S. federal government funding. When new technologies are developed with U.S. government funding, the government obtains certain rights in any resulting patents, including a nonexclusive license authorizing the government to use the invention for non-commercial purposes. These rights may permit the government to disclose our confidential information to third parties and to exercise “march-in” rights to use or allow third parties to use our patented technology. The government can exercise its march-in rights if it determines that action is necessary because we fail to achieve practical application of the U.S. government-funded technology, because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations, or to give preference to U.S. industry. In addition, U.S. government-funded inventions must be reported to the government and U.S. government funding must be disclosed in any resulting patent applications. In addition, our rights in such inventions are subject to government license rights and foreign manufacturing restrictions. Any exercise by the government of such rights could harm our competitive position or impact our operating results.
In addition, some of our technology was funded by a grant from the State of California. Inventions funded by this grant may be subject to forfeiture if we do not seek to patent or practically apply them. Any such forfeiture could have a materially adverse effect on our business. For proprietary technology developed with funding from the State of California, certain confidential information may be disclosed to third parties by the State of California. Our rights in such inventions are subject to State of California license and march-in rights. Any exercise by the State of California of such rights could harm our competitive position or impact our operating results.
Risks Related to Our Finances and Capital Requirements
Our financial results could vary significantly from quarter to quarter and are difficult to predict.
Our revenues and results of operations could vary significantly from quarter to quarter because of a variety of factors, many of which are outside of our control. As a result, comparing our results of operations on a period-to-period basis may not be meaningful. Factors that could cause our quarterly results of operations to fluctuate include:
• | achievement, or failure to achieve, technology or product development milestones needed to allow us to enter target markets on a cost effective basis; |
• | delays or greater than anticipated expenses or time associated with the completion of new manufacturing facilities and the ramp up to nameplate capacity and optimization of production following completion of a new manufacturing facility; |
• | delays or greater than anticipated expenses associated with the provision of key support and/or operational services to manufacturing facilities; |
• | our capital requirements or capital requirements of our joint ventures; |
31
• | our ability to effectively manage larger working capital positions as we increase commercial production and distribution of our products; |
• | disruptions in the production process at any facility where we produce our products, including due to equipment failure or accidents; |
• | the timing, size and mix of sales to customers for our products; |
• | increases in price or decreases in availability of feedstocks; |
• | fluctuations in the price of, and demand for, products based on petroleum or other oils for which our products are alternatives; |
• | the unavailability of contract manufacturing capacity altogether or at anticipated cost; |
• | fluctuations in foreign currency exchange rates; |
• | seasonal production and sale of our products; |
• | the effects of competitive pricing pressures, including decreases in average selling prices of our products; |
• | unanticipated expenses associated with changes in governmental regulations and environmental, health and safety requirements; |
• | reductions or changes to existing regulations and policies that impact the fuel, chemical, food, nutrition and skin and personal care, and oil field services markets; |
• | departure of key employees; |
• | business interruptions, such as earthquakes and other natural disasters; |
• | our ability to integrate businesses that we may acquire; |
• | risks associated with the international aspects of our business; and |
• | changes in general economic, industry and market conditions, both domestically and in foreign markets in which we operate. |
Due to these factors and others the results of any quarterly or annual period may not meet our expectations or the expectations of our investors and may not be meaningful indications of our future performance.
We may require additional financing in the future and may not be able to obtain such financing on favorable terms, if at all, which could force us to delay, reduce or eliminate our research and development or commercialization activities.
To date, we have financed our operations primarily through our initial public offering, completed in June 2011, public and private placements of our equity and convertible debt securities, credit facilities, government grants and funding from strategic partners. In January 2013 we issued $125.0 million aggregate principal amount of convertible senior subordinated notes due 2018 (the “2018 Notes”). The 2018 Notes bear interest at a rate of 6.00% per year, payable in cash semi-annually. In April 2014 we issued 5,750,000 shares of our common stock and $149.5 million aggregate principal amount of convertible senior subordinated notes due 2019 (the “2019 Notes”). The 2019 Notes bear interest at a rate of 5.00% per year, payable in cash semi-annually. As of December 31, 2015, approximately $61.6 million aggregate principal amount of the 2018 Notes was outstanding and approximately $149.5 million aggregate principal amount of the 2019 Notes was outstanding. We have also entered into the HSBC facility that provides for a $35.0 million revolving facility for working capital and letters of credit. While we plan to enter into relationships with partners or collaborators for them to provide some portion or all of the capital needed to build production facilities, we may determine that it is more advantageous for us to provide some portion or all of the financing for new production facilities. Some of our previous funding has come from government grants; however, our future ability to obtain government grants is uncertain due to the competitive bid process and other factors.
In addition, we may have to raise additional funds through public or private debt or equity financings to meet our capital requirements, including our portion of joint venture funding requirements. For example, if the Solazyme Bunge JV needs and is unable to secure additional financing, we will be required to fund our portion of the Solazyme Bunge JV’s capital requirements from existing sources or seek additional financing. In addition, our working capital requirements and the working capital
32
requirements of the Solazyme Bunge JV may increase as we and the Solazyme Bunge JV each increase production due to an increase in inventory and the manufacture of out-of-specification product during the ramp-up of commercial production.
Although we believe that our current cash, cash equivalents, marketable securities and revenue from product sales will be sufficient to fund our current operations for at least the next 12 months, our liquidity assumptions may prove to be wrong, and we could utilize our available financial resources sooner than we currently expect. We may not be able to raise sufficient additional funds on terms that are favorable to us, if at all. If we fail to raise sufficient funds and continue to incur losses, our ability to fund our operations, take advantage of strategic opportunities, develop and commercialize products or technologies, or otherwise respond to competitive pressures could be significantly limited. If this happens, we may be forced to delay or terminate research and development programs or the commercialization of products resulting from our technologies, curtail or cease operations or obtain funds through collaborative and licensing arrangements that may require us to relinquish commercial rights, or grant licenses on terms that are not favorable to us. If adequate funds are not available, we will not be able to successfully execute our business plan or continue our business, and doubts may be raised regarding our ability to continue as a going concern.
Servicing our debt will require a significant amount of cash, and we may not have sufficient cash flow from our business to pay amounts due under our indebtedness.
As of December 31, 2015, our total consolidated indebtedness was $211.1 million. Of our $211.1 million of indebtedness, none is currently secured. We also may be required to provide a corporate guarantee with respect to the portion of the BNDES loan to the Solazyme Bunge JV that, when added to our bank guarantee, does not exceed our percentage ownership in the Solazyme Bunge JV.
Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our indebtedness, including the 2018 Notes and the 2019 Notes, depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not continue to generate cash flow from operations in the future sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional equity capital on terms that may be onerous or highly dilutive. Our ability to refinance our indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
Despite our current debt levels, we may still incur substantially more debt or take other actions which would intensify the risks discussed above.
Despite our current consolidated debt levels, we and our subsidiaries may be able to incur substantial additional debt in the future, subject to the restrictions contained in our debt instruments, some of which may be secured debt. We are not restricted under the terms of the indentures governing the 2018 Notes and 2019 Notes from incurring additional debt, securing existing or future debt, recapitalizing our debt or taking a number of other actions that are not limited by the terms of the indentures governing the 2018 Notes and the 2019 Notes that could have the effect of diminishing our ability to make payments on the notes when due. Our existing HSBC facility restricts our ability to incur additional indebtedness, including secured indebtedness, but if the facility matures or is repaid, we may not be subject to such restrictions under the terms of any subsequent indebtedness.
We have received government grant funding and entered contracts with government agencies, and may pursue government grant funding or contracts in the future. Our receipt of government funds through grants and contracts subjects us to additional regulatory oversight.
We have received government grants and have entered contracts with government agencies in the past. Activities funded by a government grant or pursuant to government contracts are subject to audits by government agencies. As part of an audit, these agencies may review our performance, cost structures and compliance with applicable laws, regulations and standards. Grant funds must be applied by us toward the research and development programs specified by the granting agency, rather than for all of our programs generally. If any of our grant-funded costs are found to be allocated improperly, the costs may not be reimbursed and any costs already reimbursed may have to be refunded. Accordingly, an audit could result in an adjustment to our revenues and results of operations. We are also subject to additional regulations based on our receipt of government grant funding and entry into government contracts. If we fail to comply with the requirements under our grants or contracts, we may face penalties or other negative consequences, and in such event we may not be awarded government funding or contracts in the future.
33
If we engage in any acquisitions, we will incur a variety of costs and may potentially face numerous risks that could adversely affect our business and operations.
If appropriate opportunities become available, we may seek to acquire additional businesses, assets, technologies or products to enhance our business. In connection with any acquisitions, we could issue additional equity or equity-linked securities such as the 2018 Notes or 2019 Notes, which would dilute our stockholders, incur substantial debt to fund the acquisitions, or assume significant liabilities.
Acquisitions involve numerous risks, including problems integrating the purchased operations, technologies or products, unanticipated costs and other liabilities, diversion of management’s attention from our core businesses, adverse effects on existing business relationships with current and/or prospective collaborators, customers and/or suppliers, risks associated with entering markets in which we have no or limited prior experience and potential loss of key employees. Acquisitions may also require us to record goodwill and non-amortizable intangible assets that will be subject to impairment testing on a regular basis and potential periodic impairment charges, incur amortization expenses related to certain intangible assets, and incur write offs and restructuring and other related expenses, any of which could harm our operating results and financial condition. If we fail in our integration efforts with respect to any of our acquisitions and are unable to efficiently operate as a combined organization, our business and financial condition may be adversely affected.
Raising additional funds may cause dilution to our stockholders or require us to relinquish valuable rights.
If we elect to raise additional funds through equity offerings or offerings of equity-linked securities, our stockholders would likely experience dilution. Debt financing, if available, may subject us to restrictive covenants that could limit our flexibility in conducting future business activities. For example, the HSBC facility contains financial covenants that, if breached, would require us to secure our obligations thereunder. To the extent that we raise additional funds through collaboration and licensing arrangements, it may be necessary for us to share a portion of the margin from the sale of our products. We may also be required to relinquish or license on unfavorable terms our rights to technologies or products that we otherwise would seek to develop or commercialize ourselves.
If we fail to maintain an effective system of internal controls, we might not be able to report our financial results accurately or prevent fraud; in that case, our stockholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our stock.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. In addition, Section 404 of the Sarbanes-Oxley Act of 2002 requires us and our independent registered public accounting firm to evaluate and report on our internal control over financial reporting, and have our chief executive officer and chief financial officer certify as to the accuracy and completeness of our financial reports. The process of implementing our internal controls and complying with Section 404 is expensive and time consuming, and requires significant attention from management. We cannot be certain that these measures will ensure that we implement and maintain adequate controls over our financial processes and reporting in the future.
Our management has concluded that there were no material weaknesses in our internal controls over financial reporting as of December 31, 2015. However, there can be no assurance that our controls over financial processes and reporting will be effective in the future or that material weaknesses or significant deficiencies in our internal controls will not be discovered in the future. Because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause us to fail to meet our reporting obligations. If we or our independent registered public accounting firm discover a material weakness, the disclosure of that fact, even if quickly remedied, could reduce the market’s confidence in our financial statements and harm our stock price.
Risks Relating to Securities Markets and Investment in Our Stock
The price of our common stock may be volatile.
Stock markets have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock. In addition, the average daily trading volume of the securities of small companies, particularly small technology companies, can be very low. Limited trading volume of our stock may contribute to its volatility. Price declines in our common stock could result from general market and economic conditions and a variety of other factors, including any of the risk factors described in this report.
These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. The market price of our common stock could also be affected by possible sales of our common stock by
34
investors who view our convertible notes as a more attractive means of equity participation in us and by hedging or arbitrage trading activity that we expect involving our common stock.
The sale or issuance by us of substantial amounts of our common stock could adversely impact the trading price of our common stock.
A substantial number of shares of our common stock may be issued in connection with the exercise of options outstanding under our equity incentive plans, the vesting of restricted stock awards and restricted stock units, the exercise of outstanding warrants, the conversion of or exchange for outstanding 2018 Notes and 2019 Notes. See Note 2 in the accompanying notes to our consolidated financial statements for additional information regarding the number of outstanding shares of potentially dilutive securities. Also see Note 11 in the accompanying notes to our consolidated financial statements for information regarding the possible conversion of the 2018 Notes and 2019 Notes into shares of our common stock. In addition, we expect to issue additional shares under our equity incentive plans and employee stock purchase plan in the future. In the future, we may issue additional shares of common stock or other equity-linked securities to raise additional capital.
Any future sale or issuance of common stock could adversely impact the trading price of our common stock.
If the market price of our common stock were to cease to be quoted on a national exchange, the market price of our common stock and our reputation would be negatively impacted.
If we are unable to meet the stock price listing requirements of NASDAQ, including the requirement that our consolidated closing bid price not be below $1.00 per share for 30 consecutive business days, NASDAQ may issue a deficiency notice providing a 180 day compliance period prior to our common stock being subject to delisting from the NASDAQ Global Select Market. If our common stock were delisted from the NASDAQ Global Select Market, among other things, this could result in a number of negative implications, including reduced price and liquidity in our common stock as a result of the loss of market efficiencies associated with NASDAQ and the loss of federal preemption of state securities laws, as well as the potential loss of confidence by suppliers, customers and employees, the loss of institutional investor interest, the loss of the ability to raise future capital, less coverage by analysts, fewer business development opportunities and greater difficulty in obtaining financing. The threat of delisting may also lead to a “reverse split” to increase the per share price of our common stock.
Our certificate of incorporation, our bylaws and Delaware law contain provisions that could discourage another company from acquiring us and may prevent attempts by our stockholders to replace or remove our current management.
Provisions of Delaware law (where we are incorporated), our certificate of incorporation and bylaws may discourage, delay or prevent a merger or acquisition that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace or remove our board of directors. These provisions include:
• | authorizing the issuance of “blank check” preferred stock without any need for action by stockholders; |
• | requiring supermajority stockholder voting to effect certain amendments to our certificate of incorporation and bylaws; |
• | eliminating the ability of stockholders to call special meetings of stockholders; |
• | prohibiting stockholder action by written consent; |
• | establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted on by stockholders at stockholder meetings; and |
• | dividing our board of directors into three classes so that only one third of our directors will be up for election in any given year. |
In addition, we are subject to Section 203 of the Delaware General Corporation Law, which, under certain circumstances, may make it more difficult for a person who would be an “interested stockholder,” as defined in Section 203, to effect various business combinations with us for a three-year period. Our certificate of incorporation and bylaws do not exclude us from the restrictions imposed under Section 203. These provisions could impede a merger, takeover or other business combination involving us or discourage a potential acquirer from making a tender offer for our common stock, which, under certain circumstances, could reduce the market price of our common stock.
35
We incur significant expenses as a result of being a public company.
As a public company, we incur significant additional legal, accounting and other expenses. For example, as a public company, we have adopted internal and disclosure controls and procedures and bear all of the internal and external costs of preparing and distributing periodic public reports in compliance with our obligations under applicable securities laws.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 and related regulations implemented by the SEC and the NASDAQ-GS, create uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. We evaluate and monitor developments with respect to new and proposed rules and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors, particularly to serve on our audit committee and compensation committee, and attract and retain qualified executive officers. If these requirements divert our management’s attention from other business concerns, they could have a material adverse effect on our business, financial condition and results of operations.
If securities or industry analysts do not continue to publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us or our business. If securities or industry analysts do not continue coverage of our company, the trading price for our common stock would be negatively impacted. If one or more of the analysts who cover us downgrade our common stock or publish inaccurate or unfavorable research about our business, the price of our common stock would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our common stock could decrease, which might cause the price of our common stock and its trading volume to decline.
We do not anticipate paying cash dividends, and accordingly, stockholders must rely on stock appreciation for any return on their investment.
We do not anticipate paying cash dividends in the foreseeable future. As a result, only appreciation of the price of our common stock, which may never occur, would provide a return to stockholders. Our HSBC facility restricts our ability to pay cash dividends, and we may be subject to additional dividend restrictions under the terms of future indebtedness. Investors seeking cash dividends should not invest in our common stock.
36
Item 1B.Unresolved Staff Comments.
None.
Item 2. Properties.
We currently lease an aggregate of approximately 106,000 square feet of office and laboratory facilities, including a pilot plant, in South San Francisco, California. The South San Francisco location is comprised of two buildings and is covered by a lease agreement that expires in January 2018.
In September 2014, we entered into a lease agreement for approximately 5,000 square feet of office space located in Glendale, California. The term of the Glendale lease commenced in November 2014 and ends in October 2017.
In May 2011, we purchased the Peoria Facility in Peoria, Illinois. Our wholly-owned subsidiary, Solazyme Brazil Renewable Oils and Bioproducts Limitada (Solazyme Brazil), leased approximately 14,100 square feet of space in Campinas, Brazil that was used as general office and lab space pursuant to a lease that was terminated in June 2015 as part of the Company's 2014 Restructuring Plan (See Note 3 - Restructuring of the notes to the consolidated financial statements of this Annual Report on Form 10-K).
We believe that our current facilities are suitable and adequate to meet our current needs. As our needs change and as our business grows, we intend to build, lease or acquire additional manufacturing capacity on our own and with partners. We believe that additional space and facilities will be available.
Item 3. Legal Proceedings.
Roquette Frères, S.A.
In September 2013, an arbitration (the “Roquette Arbitration”) was initiated with Roquette Frères, S.A. (“Roquette”) in connection with the dissolution of a joint venture between the Company and Roquette known as Solazyme Roquette Nutritionals L.L.C. (“SRN”). The Company sought a declaration that, in accordance with the terms of the joint venture agreement between the parties, the Company should be assigned all improvements made by or on behalf of SRN to the Company’s intellectual property. On February 19, 2015 the arbitration panel released its decision, ordering, inter alia, the assignment to the Company of (i) all SRN patent applications, (ii) all SRN know-how related to high lipid algae flour and high protein algae powder and (iii) all Roquette patent applications filed since November 2010 relating to algae food and food ingredients, as well as methods for making and using them. In addition, the arbitration panel ordered Roquette to pay to the Company, $2.3 million in legal costs and fees. The arbitration award was confirmed by order of the U.S. District Court for the District of Delaware on December 21, 2015. Roquette has appealed the confirmation of the arbitration award to the U.S. Court of Appeals for the Third Circuit. Pending this appeal, the confirmation of the arbitration award has been stayed by order of the U.S. District Court for the District of Delaware by order dated January 12, 2016, in which the Court granted not only a stay but also enjoined Roquette from pursuing any further commercialization of any technology arguably within the ambit of the arbitral decision, including the sale of products. The Company expects the appeal of the confirmation of the arbitration award to be heard later in 2016.
In November 2014 Roquette filed an action against the Company in U.S. District Court for the District of Delaware for declaratory judgment related to the Roquette Arbitration. Roquette sought a declaration that (i) the arbitrators in the Roquette Arbitration exceeded their authority by failing to render a timely arbitration award, (ii) any award issued by the arbitrators is void and (iii) all intangible assets of SRN should be assigned jointly to Roquette and the Company. Other than seeking its attorney fees and costs in the action, Roquette did not make any monetary claims against the Company. The Company filed an Answer to the Complaint in January 2015, denying substantially all of Roquette’s claims and all of its prayers for relief. In February 2015 Roquette filed a second action against the Company in U.S. District Court for the District of Delaware for declaratory judgment related to the Roquette Arbitration. Roquette sought a declaration that (A) the order of the arbitrators in the Roquette Arbitration for more discovery and new hearings was unenforceable and (B) in the alternative, the proposed new discovery and hearings concerned an issue that was outside the scope of the arbitration. Other than seeking its attorney fees and costs in the action, Roquette did not make any monetary claims against the Company. The two Delaware actions were consolidated in February 2015. The Company filed its Answer to the second Complaint on February 26, 2015, denying all claims made in the Complaint and all related prayers for relief. In addition, the Company cross-claimed for (x) confirmation of the arbitration award, (y) an order compelling Roquette to comply with the arbitration award and (z) damages for misappropriation of the Company’s trade secrets, misuse of the Company’s confidential information and breach of contract. In April 2015 Roquette filed motions for summary judgment in each of the two declaratory judgment actions commenced by Roquette and a motion to vacate the award rendered in the Roquette Arbitration, which included counterclaims alleging the
37
Company misused certain Roquette trade secrets. The summary judgment motions made by Roquette were denied in an opinion of the court dated December 21, 2015. All further proceedings under the declaratory actions have been stayed pending the Third Circuit appeal described above.
Securities Class Action Litigation
In June 2015, a securities class action complaint entitled Norfolk County Retirement System v. Solazyme, Inc. et al. was filed against the Company, its CEO, Jonathan Wolfson, its CFO/COO, Tyler Painter, certain of its current and former directors, and the underwriters of its March 2014 equity and debt offerings, Goldman, Sachs & Co., Inc. and Morgan Stanley & Co. LLC, in the U.S. District Court for the Northern District of California. The complaint asserts claims for alleged violations of Sections 11, 12(a)(2), and 15 of the Securities Act of 1934, as well as Sections 10(b) and 20(a) of the Securities Exchange Act of 1934. The complaint seeks unspecified damages on behalf of a purported class that would comprise all individuals who acquired the Company's securities (i) between February 27, 2014 and November 5, 2014 and (ii) pursuant and/or traceable to the Company's public equity and debt offerings in March 2014. The complaint alleges that investors were misled by statements made during that period about the construction progress, development, and production capacity associated with the production facility located in Brazil owned by the Company’s joint venture, Solazyme Bunge Produtos Renovaveis Ltda. In October 2015, the lead plaintiff was selected in action and an amended complaint was filed on December 15, 2015. The Company filed a Motion to Dismiss the action on February 12, 2016 and it expects to be heard in May 2016. The Company believes the complaint lacks merit, and intends to defend itself vigorously.
Derivative Litigation
In July 2015, a complaint entitled Jim Bertonis, derivatively on behalf of Solazyme, Inc. v. Jonathan Wolfson et al. was filed in the Superior Court of California, County of San Mateo. The complaint seeks unspecified damages, purportedly on behalf of the Company from certain of its current and former directors and officers and alleges these defendants breached their fiduciary duties to the Company and unjustly enriched themselves by making allegedly false and misleading statements and omitting certain material facts in the Company's securities filings and other public disclosures. This purported stockholder derivative action is based on substantially the same facts as the securities class action described above. Based on a review of the plaintiffs’ allegations, the Company believes that the plaintiff has not demonstrated standing to sue on its behalf.
In August 2015, a complaint entitled Gregory M. Miller, derivatively on behalf of Solazyme, Inc. v. Jonathan S. Wolfson et al. was filed in the U.S. District Court for the Northern District of California. The complaint seeks unspecified damages, purportedly on behalf of the Company from certain of its current and former directors and officers and alleges these defendants breached their fiduciary duties to the Company and aided and abetted the Company in making allegedly false and misleading statements and omitting certain material facts in the Company's securities filings and other public disclosures. This purported stockholder derivative action is based on substantially the same facts as the previously filed securities class action and derivative action. Based on a review of the plaintiffs’ allegations, the Company believes that the plaintiff has not demonstrated standing to sue on its behalf.
The Company may be involved, from time to time, in additional legal proceedings and claims arising in the ordinary course of its business. Such matters are subject to many uncertainties and outcomes are not predictable with assurance. While there can be no assurances as to the ultimate outcome of any legal proceeding or other loss contingencies involving the Company, management does not believe any pending matters individually and in the aggregate will be resolved in a manner that would have a material effect on the Company’s consolidated financial position, results of operations or cash flows.
Item 4. Mine Safety Disclosures.
Not applicable.
38
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock is traded on The NASDAQ Global Select Market, or NASDAQ, under the symbol “SZYM.” The following table sets forth the high and low sales prices per share of the common stock as reported on NASDAQ. Such prices represent inter dealer prices without retail markup, markdown or commission and may not necessarily represent actual transactions.
Fiscal 2014 | High | Low | |||||
First Quarter | $ | 15.00 | $ | 9.32 | |||
Second Quarter | 12.44 | 8.90 | |||||
Third Quarter | 12.08 | 7.31 | |||||
Fourth Quarter | 8.00 | 2.15 | |||||
Fiscal 2015 | High | Low | |||||
First Quarter | $ | 3.49 | $ | 2.00 | |||
Second Quarter | 4.74 | 2.82 | |||||
Third Quarter | 3.34 | 1.89 | |||||
Fourth Quarter | 3.52 | 2.32 | |||||
Holders
As of February 29, 2016 there were 80 stockholders of record of our common stock. A substantially greater number of stockholders may be beneficial holders, whose shares are held of record by banks, brokers and other financial institutions in “street name.”
Dividend Policy
We have never declared or paid any dividends on our common stock or any other securities. We currently intend to retain our future earnings, if any, for use in the expansion and operation of our business and therefore do not anticipate paying cash dividends on our common stock in the foreseeable future. Any future determination relating to our dividend policy will be made at the discretion of our board of directors, based upon our financial condition, results of operations, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant. In addition, the terms of our loan facility require that we obtain consent from the lender prior to any payment of dividends.
Stock Performance Graph
The following graph compares our total common stock return with the total return for (i) the S&P SmallCap 600 Index and (ii) the NASDAQ Clean Edge Green Energy Index for the period from May 27, 2011 (the date our common stock commenced trading on the NASDAQ) through December 31, 2015. The figures represented below assume an investment of $100 in our common stock at the closing price of $20.71 on May 27, 2011 and in the S&P SmallCap Index and the NASDAQ Clean Edge Green Energy Index on May 27, 2011 and the reinvestment of dividends into shares of common stock. The comparisons in the table are required by the SEC and are not intended to forecast or be indicative of possible future performance of our common stock. This graph shall not be deemed “soliciting material” or to be “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any of our filings under the Securities Act or the Exchange Act.
39
Recent Sales of Unregistered Securities
1.In January 2013, we issued $125.0 million aggregate principal amount of convertible notes (2018 Notes) that will mature on February 1, 2018. See Note 11 of our consolidated financial statements for additional detail. We had $61.6 million aggregate principal amount of 2018 Notes outstanding as of December 31, 2015.
2.On October 24, 2013, pursuant to a Research and Development Agreement with Warner Babcock Institute for Green Chemistry LLC (WBI), we issued 39,578 shares of our common stock to WBI. All of these shares have fully vested. Pursuant to the Research and Development Agreement, WBI provided research and development services to us.
3.On November 4, 2013, we issued 423,278 shares of our common stock to Archer-Daniels-Midland Company (ADM) upon the exercise by ADM of a warrant to receive $4,500,000, payable at our election in cash, stock or a combination thereof, for use and operation of a portion of ADM’s facility in Clinton, Iowa under the Strategic Collaboration Agreement between us and ADM. The Strategic Collaboration agreement terminated on February 26, 2016.
4.On April 1, 2014, we issued $149.5 million aggregate principal amount of convertible notes (2019 Notes) that will mature on October 1, 2019. See Note 11 of our consolidated financial statements for additional detail. We had $149.5 million aggregate principal amount of 2019 Notes outstanding as of December 31, 2015.
5.On October 29, 2015, we provided to ADM a notice of termination of the Operating Agreement entered into with ADM in November 2012 related to the production of products at the Clinton Facility. On December 28, 2015, we entered into a warrant exchange agreement (Exchange Agreement) with ADM pursuant to which ADM agreed to exchange (x) a warrant covering 500,000 shares of Company common stock, (y) a warrant in the face amount of $5.1 million and (z) two warrants in the face amount of $6.5 million each (the Warrants) for (i) 1,121,914 shares of our common stock, which is equal to $3.0 million divided by the average daily closing share price of our common stock over the five consecutive trading days ending on the trading day prior to December 28, 2015 and (ii) a number of shares of common stock equal to $2.5 million divided by the average daily closing share price of our common stock over the five consecutive trading days ending on the trading day prior to February 17, 2016. The settlement of the first tranche of shares covered by the Exchange occurred on December 29, 2015.
On February 17, 2016 we and ADM amended the Exchange Agreement (Amended Exchange Agreement) to provide that the Company’s exchange consideration for the February issuance portion would instead be (x) $1.25 million paid to ADM in cash no later than February 19, 2016, and (y) the issuance of a number of shares of Common Stock (expected to occur on April 1, 2016) equal to $1.25 million divided by the average daily closing share price of our common Stock over the five consecutive
40
trading days ending on the trading day prior to April 1, 2016. We may choose to pay cash in lieu of all or a portion of the shares issuable to ADM pursuant to the Amended Exchange Agreement.
6.On January 29, 2016, pursuant to a Consulting Agreement with Mark Schuett, we issued 111,386 shares of our common stock to Mr. Schuett. Pursuant to the Consulting Agreement, Mr. Schuett is providing consulting services related to manufacturing processes.
The issuances of securities described in paragraphs 1 and 4 above were made pursuant to Rule 144A under the Securities Act in private offerings to qualified institutional buyers. The issuances of securities described in paragraphs 2, 3 and 6 above were deemed to be exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act and Regulation D promulgated thereunder as transactions by an issuer not involving a public offering. The recipients of securities in each such transaction represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the electronic records representing such securities in such transactions. All recipients received adequate information about us. The issuance of securities described in paragraph 5 above was made in reliance on the exemption from registration contained in Section 3(a)(9) of the Securities Act of 1933, as amended.
41
Item 6. Selected Financial Data
Year Ended December 31, | |||||||||||||||||||
2015 | 2014 | 2013 | 2012 | 2011 | |||||||||||||||
(In thousands, except share and per share data) | |||||||||||||||||||
Statement of Operations Data: | |||||||||||||||||||
Total revenues | $ | 46,131 | $ | 60,391 | $ | 39,750 | $ | 44,108 | $ | 38,966 | |||||||||
Cost of product revenue | 18,179 | 20,612 | 6,385 | 5,311 | 2,420 | ||||||||||||||
Research and development | 48,094 | 81,680 | 66,572 | 66,384 | 45,613 | ||||||||||||||
Sales, general and administrative | 80,733 | 90,266 | 62,933 | 57,516 | 41,426 | ||||||||||||||
Restructuring charges | 4,953 | 3,514 | — | — | — | ||||||||||||||
Total costs and operating expenses(1) | 151,959 | 196,072 | 135,890 | 129,211 | 89,459 | ||||||||||||||
Loss from operations | (105,828 | ) | (135,681 | ) | (96,140 | ) | (85,103 | ) | (50,493 | ) | |||||||||
Total other income (expense), net | (35,619 | ) | (26,460 | ) | (20,249 | ) | 1,971 | (3,408 | ) | ||||||||||
Net loss from continuing operations | (141,447 | ) | (162,141 | ) | (116,389 | ) | (83,132 | ) | (53,901 | ) | |||||||||
Accretion of redeemable convertible preferred stock | — | — | — | — | (60 | ) | |||||||||||||
Net loss attributable to Solazyme, Inc. common stockholders | $ | (141,447 | ) | $ | (162,141 | ) | $ | (116,389 | ) | $ | (83,132 | ) | $ | (53,961 | ) | ||||
Basic and diluted net loss per share attributable to Solazyme, Inc. common stockholders(2) | $ | (1.76 | ) | $ | (2.14 | ) | $ | (1.81 | ) | $ | (1.37 | ) | $ | (1.35 | ) | ||||
Shares used in computation of basic and diluted net loss per share(2) | 80,165,402 | 75,879,208 | 64,211,958 | 60,509,048 | 39,934,013 | ||||||||||||||
(1) | Includes stock-based compensation expense of $15.7 million, $25.5 million, $18.7 million, $15.4 million and $10.9 million for the years ended December 31, 2015, 2014, 2013, 2012 and 2011, respectively. |
(2) | See notes to our consolidated financial statements for an explanation of the method used to calculate basic and diluted net loss per share of common stock and the weighted-average number of shares used in computation of the per share amounts. |
As of December 31, | |||||||||||||||||||
2015 | 2014 | 2013 | 2012 | 2011 | |||||||||||||||
(In thousands) | |||||||||||||||||||
Balance Sheet Data: | |||||||||||||||||||
Cash and cash equivalents | $ | 46,966 | $ | 42,689 | $ | 54,977 | $ | 30,818 | $ | 28,780 | |||||||||
Marketable securities | 51,009 | 164,619 | 112,544 | 118,187 | 214,944 | ||||||||||||||
Working capital | 93,614 | 210,473 | 166,523 | 140,341 | 229,681 | ||||||||||||||
Total assets | 182,423 | 312,589 | 258,705 | 217,024 | 285,224 | ||||||||||||||
Total debt, net of unamortized discounts and loan fees | 202,466 | 200,097 | 93,522 | 14,968 | 20,252 | ||||||||||||||
Accumulated deficit | (609,905 | ) | (468,458 | ) | (306,317 | ) | (189,928 | ) | (106,796 | ) | |||||||||
Total stockholders’ (deficit) equity | (46,475 | ) | 86,376 | 138,948 | 183,311 | 240,558 | |||||||||||||
42
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Forward-Looking Statements
The following discussion and analysis should be read together with our audited consolidated financial statements and the other financial information appearing elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements reflecting our current expectations and involves risks and uncertainties. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential” or “continue” or the negative of these terms or other comparable terminology. For example, statements regarding our expectations as to future financial and operating performance, future selling prices and margins for our products, attributes and performance of our products, manufacturing capacity, expense levels and liquidity sources are forward-looking statements. Our actual results and the timing of events may differ materially from those discussed in our forward-looking statements as a result of various factors, including those discussed below and those discussed in the section entitled “Risk Factors” included in this Annual Report on Form 10-K and in our other filings with the Securities and Exchange Commission (SEC).
Overview
We are a food, nutrition and specialty ingredients company that harnesses the power of algae, the origin of all plants. Our innovative platform uses microalgae to produce high-value triglyceride oils, proteins, fibers, micronutrients and other ingredients. The inherent flexibility of our technology platform and the broad usage of these materials across multiple industries, allow us to approach a wide range of customers across and end markets. Moving forward, Solazyme will be known as TerraVia™.
The unique composition of our oils, powders and other algae-derived products address specific customer requirements. We are commercializing high-value oils and powder products to companies that primarily use them as ingredients. We have developed and are commercializing products for specialty food ingredients, animal nutrition ingredients, consumer food products and specialty personal care ingredients. Over our history, we have invested in and developed products, technology and market opportunities in the industrials area, which includes fuels, industrial oils, and the oilfield/Encapso™ business. In line with our strategy to focus our commercial efforts on food and specialty personal care ingredients, we expect to pursue strategic alternatives for the industrial business and our objective will be to identify partners who have the operational capabilities needed to realize the potential of those businesses.
Our food oils are formulated to offer a variety of functional benefits such as enhanced structuring capabilities and stability while providing robust formulation and process flexibility. These food oils have the potential to improve upon conventionally utilized specialty fats and oils and our high oleic algae oil has received an FDA GRAS "No Questions" letter. Currently, these oils are commercially available in our AlgaWise™ branded food oil platform and in our consumer culinary oil Thrive®. In addition, we have developed novel methods of preparing powdered forms of triglyceride oils and vegan proteins, and our powdered ingredients are composed of unmodified whole algae cells. AlgaVia® Lipid Powder (commonly known as whole algae flour) and AlgaVia® Protein (commonly known as whole algae protein) are whole algae ingredients that can improve the nutritional profile of foods and beverages. AlgaVia®Lipid Powder is a new fat source that allows for the reduction or replacement of dairy fats, oils, and eggs. AlgaVia® Protein is a new vegan source of protein that is free of known allergens and gluten. Both AlgaVia® Lipid Powder and Protein can be used across a range of applications such as beverages (ready-to-drink and powdered), bakery, snacks, bars, dressings, sauces and frozen desserts.
Our process is compatible with commercial-scale, widely-available fermentation and oil recovery equipment. We operate our lab and pilot fermentation and recovery equipment as scaled-down versions of our large commercial engineering designs, such as those used to perform development work under certain agreements with strategic partners and to fulfill commercial supply agreements. We have scaled up our technology platform and have successfully operated at lab (5-15 liter), pilot (600-1,000 liter), demonstration/small commercial (120,000 liter) and large commercial (approximately 500,000 liter and above) fermenter scale. The fermentation equipment used to achieve commercial scale at the Clinton Facility is comparable to the fermentation equipment at the Solazyme Bunge JV Plant in Brazil. Our existing manufacturing operations are as follows:
• | Our pilot plant in South San Francisco, California, with recovery operations capable of handling material from both 600 and 1,000 liter fermenters, enables us to produce samples of our algae oils for testing and optimization by our partners, as well as to test new process conditions at an intermediate scale. |
• | In 2012, we successfully commissioned our Peoria, Illinois facility (the Peoria Facility), to produce algae oil. The Peoria Facility was partially funded with a federal grant that we received from the U.S. Department of Energy |
43
(DOE) in December 2009 to demonstrate integrated commercial-scale production of renewable algae-derived fuels. The Peoria Facility provides an important platform for continued work on feedstock flexibility and scaling of new algae oils and whole algae ingredients into the marketplace. We have also modified our Peoria Facility to produce food and specialty ingredients in conjunction with market development activity.
• | In April 2012, we executed a joint venture agreement with Bunge Global Innovation, LLC and certain of its affiliates (collectively, Bunge) (Joint Venture Agreement), a leading agribusiness and food company with integrated operations in approximately 40 countries, establishing a joint venture (Solazyme Bunge JV) to construct and operate a purpose-built production facility (the Solazyme Bunge JV Plant) adjacent to Bunge’s sugarcane mill in Moema, Brazil. The Solazyme Bunge JV Plant leverages our technology and Bunge’s sugarcane milling and natural oil processing capabilities to produce microalgae-based products. In addition, the Solazyme Bunge JV Plant has been designed to be expanded for further production in line with market demand. Additional capital expenditures may be required to reach nameplate capacity depending on the product mix produced at the plant. See “Significant Partner Agreements.” |
• | In November 2012, we executed a strategic collaboration agreement with Archer-Daniels-Midland Company (ADM) to produce algae triglyceride oil products at ADM’s facility in Clinton, Iowa (Clinton Facility). In January 2014, we commenced commercial operations at both the Clinton Facility and the downstream companion facility operated by American Natural Processors, Inc. (ANP). We, along with ADM and ANP, have manufactured five distinct products at the facilities, and products were sold and distributed from January 2014 to December 2015. On October 29, 2015, we provided to ADM a notice of termination of the Operating Agreement entered into with ADM in November 2012 related to the production of products at the Clinton Facility. As a result of the termination of the Operating Agreement on February 26, 2016, the Strategic Collaboration Agreement with ADM automatically terminated on the same date. We have product inventories manufactured prior to the termination that we expect to sell in 2016. See Note 10 in the accompanying notes to our consolidated financial statements. |
Our research and development programs have been conducted primarily under agreements with strategic partners to fund development work and to perform application testing. We focus our innovation efforts on creating a broad suite of algae products that meet market needs. We are currently engaged in development activities with multiple partners. We intend to continue to work closely with our partners and customers to understand their requirements and design products to specifically address their needs.
Our main commercial focus is to sell high-value oils and whole algae powdered products to companies that use them as ingredients, and our first major ingredient in the personal care market was Alguronic Acid®, which was formulated into a full range of Algenist® skin care products.
We have historically focused our efforts on R&D in the development of our oils, powders and bioproduct platform and in the first quarter of 2011 introduced our first commercially available consumer product with Algenist. After securing commercial production capacity, we were able to more broadly commercialize our technology with ingredients primarily targeted into lower margin applications within the industrial, fuel and volume personal care markets. In late 2014, we undertook a strategy to focus our efforts on commercializing high value products with the intent to upgrade the margin profile of our ingredients. In line with this strategy, we began to more broadly commercialize our food powders and food oil ingredients in 2015. Consistent with the ongoing strategy to maximize the return on our production assets, we continue to streamline the business around our core application within food and specialty ingredients and on a go forward basis, will continue to develop and commercialize products for food and nutrition ingredients, animal nutrition ingredients, specialty personal care ingredients and consumer food product applications.
We have entered into sales and partnership agreements to advance commercialization efforts of our ingredient products. In addition to development agreements to fund development work and new product application testing, we expect that our partners will enter into long-term purchase agreements with us, or the Solazyme Bunge JV.
We expect the average margins on ingredient products will be lower than those of our consumer-focused products.
44
Financial Operations Overview
Revenues
Our total revenues are generated from (1) sales of our products and (2) funding from our R&D agreements with strategic partners, including the Solazyme Bunge JV. Our total revenues were $46.1 million, $60.4 million, and $39.8 million in 2015, 2014, and 2013, respectively.
Product revenues consist of sales of consumer-focused branded Algenist® skin and personal care products and ingredient products targeted at customers in the Industrial, Specialty Personal Care and Food markets. We began to sell ingredient products more broadly into the Industrial Products markets in the first quarter of 2014, as we began to commercially produce and distribute products from our Clinton/Galva Facilities. Product revenues represented 72%, 62%, and 50% of our total revenues for 2015, 2014 and 2013, respectively.
Our R&D program revenues relate to collaboration agreements with strategic partners that generally provide payment in return for research and development activities over a contractually defined period. We are required to perform research and development activities as specified in each respective agreement based on the terms and performance periods set forth in the agreements. Our development agreements fluctuate depending on timing of the development work performed and achievement of contract milestones defined in these agreements. R&D program revenues represented 28%, 38%, and 50% of our total revenues for 2015, 2014 and 2013, respectively.
Cost of Product Revenue
Cost of product revenue consist primarily of third-party contractor costs associated with the production, distribution and packaging of our products, as well as shipping, supplies, internal labor and other overhead costs. Prior to our products' meeting any applicable regulatory requirements, all manufacturing and related production costs are recorded as research and development expenses.
Operating Expenses
•Research and Development
Our research and development efforts are directed at (1) identifying, isolating and further optimizing strains of microalgae to achieve high cell densities, high yield converting sugar to product and high productivity rates compared to other alternatives; (2) tailoring the oil outputs to meet specific market needs; (3) product and process development projects aimed at reducing the cost of oil production; and (4) scale-up of commercial scale production as well as product and process development activities at our production facilities.
We expense our research and development costs as they are incurred. Our research and development programs are undertaken to advance our overall technology platform that enables us to produce high-value algae oils. Although our partners fund certain development activities, they benefit from advances in our technology platform as a whole, including costs funded by other development programs. Therefore, costs for such activities have not been separated as these costs have all been determined to be part of our total research and development related activity.
Research and development expenses consist primarily of personnel and related costs including non-cash stock-based compensation, third party contract manufacturers, reimbursable equipment and other costs associated with our work on R&D programs associated with our collaboration agreements with strategic partners. In addition, research and development expenses include certain costs associated with contract manufacturers' facilities, feedstock and supplies, depreciation and amortization of property and equipment used in the development of our algae oil products as well as manufacturing process as we scale up our manufacturing facilities to commercial scale production.
•Sales, General and Administrative
Sales, general and administrative expenses consist primarily of personnel and related costs including non-cash stock-based compensation related to our executive management, corporate administration, sales, marketing and business development functions, professional services, marketing programs and samples, facility and administrative overhead expenses and unabsorbed production costs associated with excess capacity. Professional services consist primarily of consulting, external accounting, legal and investor relations fees associated with operating as a publicly-traded company.
Critical Accounting Policies and Estimates
45
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of our consolidated financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures. The results of our analysis form the basis for making assumptions about the carrying values of assets and liabilities that are not readily apparent from other sources. Our actual results may differ from these estimates under different assumptions or conditions. We evaluate our estimates, assumptions and judgments on an ongoing basis.
We believe the following critical accounting policies involve significant areas of management’s judgments and estimates in the preparation of our consolidated financial statements.
Revenue Recognition
We currently recognize revenues from commercial sales of our products, and from R&D programs that consist of collaborative research and development agreements with commercial and strategic partners and related parties. Product revenues are recognized when all of the following criteria are met: persuasive evidence of an arrangement exists, risk of loss and title has transferred to the customer or services have been rendered, the fee is fixed or determinable and collectability is reasonably assured.
Revenues from collaborative research and development are recognized as the services are performed consistent with the performance requirements of the agreement. In cases where the planned levels of research and development fluctuate over the research term, we recognize revenues using the proportionate performance method based upon actual efforts to date relative to the amount of expected effort to be incurred by us. When up-front payments are received and the planned levels of research and development do not fluctuate over the research term, revenues are recorded on a ratable basis over the arrangement term, up to the amount of cash received. When up-front payments are received and the planned levels of research and development fluctuate over the research term, revenues are recorded using the proportionate performance method, up to the amount of cash received. Where arrangements include milestones that are determined to be substantive and at risk at the inception of the arrangement, revenues are recognized upon achievement of the milestone and are limited to those amounts for which collectability is reasonably assured. If these conditions are not met, we defer the milestone payment and recognize it as revenue over the estimated period of performance under the contract as we complete our performance obligations.
If collaborative research and development or sales agreements contain multiple elements, we evaluate whether the components of each arrangement represent separate units of accounting. We have determined that all of our revenue arrangements should be accounted for as a single unit of accounting. Application of revenue recognition standards requires subjective determination and requires management to make judgments about the fair values of each individual element and whether it is separable from other aspects of the contractual relationship.
Impairment Assessment of Equity Method Investment
We periodically review if a series of operating losses or other factors might indicate that a decrease in value of our equity method investment has occurred that is other than temporary and that shall be recognized even though the decrease in value is in excess of what would otherwise be recognized by application of the equity method. Fair value is estimated based on discounted future cash flows. See Note 8 of the accompanying notes to our consolidated financial statements for impairment assessment of the Solazyme Bunge JV as of December 31, 2015. There were no equity method investment impairment charges incurred for the years ended December 31, 2015, 2014 and 2013.
We assessed the recoverability of our $35.9 million equity investment in Solazyme Bunge JV as of December 31, 2015. Based upon such assessment, we expect to recover the carrying amount of our equity investment and concluded that our equity investment was not impaired.
In order for the Solazyme Bunge JV to achieve sufficient cash flows to enable us to fully recover our equity investment, the Solazyme Bunge JV must:
• | Increase production volumes by: |
• | Optimizing plant throughput |
• | Improving lipid and oil content output |
• | Increasing final recovery yields |
• | Maintain access to low-cost cane sugar feedstock and power |
• | Commercialize and sell its high value products |
46
Convertible Debt and Embedded Derivative
In January 2013, we issued $125.0 million aggregate principal amount of 2018 Notes, and in April 2014, we issued $149.5 million aggregate principal amount of 2019 Notes (collectively the Notes). Each of the 2018 Notes and the 2019 Notes contain an early conversion payment feature pursuant to which a holder may convert its Notes into shares of our common stock. With respect to any conversion of 2018 Notes prior to November 1, 2016 or any conversion of 2019 Notes prior to January 1, 2018 (other than conversions in connection with certain fundamental changes), in addition to the shares deliverable upon conversion, holders are entitled to receive an early conversion payment equal to $83.33 per $1,000 principal amount of Notes surrendered for conversion that may be settled, at our election, in cash or, subject to satisfaction of certain conditions, in shares of our common stock. These early conversion payment features have been identified as embedded derivatives and are separated from the host contracts, the Notes, and carried at fair value at each reporting period. We use a Monte Carlo simulation model to estimate the fair values of the embedded derivatives related to the early conversion payment features of the Notes using the “with-and-without method”.
Stock-Based Compensation
We recognize compensation expense related to stock-based compensation, including the awarding of employee stock options and restricted stock units, based on the grant date estimated fair value. We amortize the fair value of the employee stock options on a straight-line basis over the requisite service period of the award, which is generally the vesting period.
We estimate the fair value of stock-based compensation awards using the Black-Scholes option pricing model, which requires the following inputs: expected life, expected volatility, risk-free interest rate, expected dividend yield rate, exercise price and closing price of our common stock on the date of grant. Due to our limited history of grant activity, we calculate our expected term utilizing the “simplified method” permitted by the SEC, which is the average of the total contractual term of the option and its vesting period.
We account for stock options issued to non-employees based on their estimated fair value determined using the Black-Scholes option-pricing model. We account for restricted stock units and restricted stock awards issued to nonemployees based on the estimated fair value of our common stock. The measurement of stock-based compensation for nonemployees is subject to periodic adjustments as the underlying equity instruments vest, and the resulting change in value, if any, is recognized in our consolidated statements of operations during the period the related services are rendered.
Results of Operations
Comparison of Years Ended December 31, 2015 and 2014
Revenues
Year ended December 31, | |||||||||||
2015 | 2014 | $ Change | |||||||||
(In thousands) | |||||||||||
Revenues: | |||||||||||
Product revenues | $ | 33,300 | $ | 37,346 | $ | (4,046 | ) | ||||
Research and development programs | 12,831 | 23,045 | (10,214 | ) | |||||||
Total revenues | $ | 46,131 | $ | 60,391 | $ | (14,260 | ) | ||||
We have two reportable segments for financial statement reporting purposes: Algenist® and Ingredients and Other (previously named Intermediates/Ingredients and Other). The Ingredients and Other segment includes sales of our Encapso™ product, oils and fuel blend sales related to building our fuels marketing and commercial development programs. Our discussions below surrounding changes in product revenue and gross margin are based on these two reportable segments.
47
Product Revenues and Cost of Product Revenues
Product revenues and cost of product revenues by segment for the years ended December 31, 2015 and 2014 were as follows:
Year Ended December 31, | |||||||||||
Algenist® | 2015 | 2014 | $ Change | ||||||||
Product revenue | $ | 23,278 | $ | 24,429 | $ | (1,151 | ) | ||||
Cost of product revenue | 7,616 | 7,746 | (130 | ) | |||||||
Gross profit | $ | 15,662 | $ | 16,683 | $ | (1,021 | ) | ||||
Gross margin | 67 | % | 68 | % | (1 | )% | |||||
Ingredients and Other | |||||||||||
Product revenue | $ | 10,022 | $ | 12,917 | $ | (2,895 | ) | ||||
Cost of product revenue | 10,563 | 12,866 | (2,303 | ) | |||||||
Gross profit | $ | (541 | ) | $ | 51 | $ | (592 | ) | |||
Gross margin | (5 | )% | — | % | (5 | )% | |||||
Algenist®
Algenist® product revenues and cost of product revenues decreased $1.2 million and $0.1 million, respectively, in 2015 compared to 2014 primarily as a result of lower units sold to QVC and customers located in EMEA in 2015. Algenist® gross margin decreased to 67% in 2015 from 68% in 2014 due primarily to product and customer mix.
Ingredients and Other
Ingredients and Other product revenues decreased $2.9 million in 2015 compared to 2014 due to decreased product sales related to our Personal Care Products and fuels marketing and commercial development program, consistent with our strategy to focus on high value product sales.
During scale-up of the manufacturing process at the ADM Clinton and ANP Galva Facilities in 2014 and 2015, certain production costs were charged to research and development and selling, general and administrative expenses. Gross margins for our Ingredients and Other products would have been lower in 2015 if such production costs had not been charged to operating expenses.
The gross margin for Ingredients and Other product sales was a negative gross margin of 5% in 2015 compared to a 0% gross margin in 2014, primarily due to product mix, and consistent with our strategy to focus on high value product sales. Gross margin was lower in 2015 due primarily to lower industrial sales which have higher average selling prices, compared to the same period in 2014.
We plan to focus our production at the Solazyme Bunge JV facility and at our Peoria facility in the immediate future and believe this strategic decision will better align our immediate production assets with our operating strategy while minimizing production costs. We expect costs of production will be higher for ingredient products as compared to Algenist® cost of production.
Research and Development Programs Revenue
We are currently engaged in development activities with multiple strategic partners and the Solazyme Bunge JV, and although we expect funded program revenue to remain an important indication of strategic commitment from partners and a source of future customers, we expect funded program revenue to become a less meaningful part of our overall revenue as our focus shifts to commercialization and product revenues. In line with this strategy, research and development programs revenue decreased by $10.2 million in 2015 compared to 2014, due primarily to decreased revenues from development agreements with the Solazyme Bunge JV and development agreements with strategic partners. Our revenues from development agreements with the Solazyme Bunge JV and strategic partners fluctuate due to timing and terms of the development work performed and achievement of contract milestones defined in these agreements. Revenues from the Solazyme Bunge JV decreased in 2015 compared to 2014, due primarily to a reduction of revenue associated with technical and commercial services related to the
48
operations of the Solazyme Bunge JV Plant and support for its commercial activities as the plant ramps up, partially offset by revenue associated with new development agreements entered into with Solazyme Bunge JV in the fourth quarter of 2015.
As we enter into new agreements with strategic partners or government programs, we expect that results may fluctuate based on the timing of program activities with our strategic partners.
Operating Expenses
Year ended December 31, | |||||||||||
2015 | 2014 | $ Change | |||||||||
(In thousands) | |||||||||||
Operating expenses: | |||||||||||
Research and development | $ | 48,094 | $ | 81,680 | $ | (33,586 | ) | ||||
Sales, general and administrative | 80,733 | 90,266 | (9,533 | ) | |||||||
Restructuring charges | 4,953 | 3,514 | 1,439 | ||||||||
Total operating expenses | $ | 133,780 | $ | 175,460 | $ | (41,680 | ) | ||||
Research and Development Expenses
Research and development expenses in 2015 compared to 2014 decreased by $33.6 million, due primarily to a decrease in scale-up production costs related to operations at the Clinton/Galva facilities of $17.2 million as well as decreased personnel-related and costs of $9.7 million, product development costs of $3.3 million, consumables and supplies cost of $1.2 million, travel of $1.0 million and third party contract manufacturing and facilities costs of $1.1 million. The reduction in personnel related costs are related primarily to our restructuring activities implemented starting in late December 2014. Personnel-related costs include non-cash stock-based compensation expense of $4.6 million in 2015 compared to $7.4 million in 2014. Scale up costs decreased as we had limited operations at the Clinton/Galva Facilities in 2015 compared to 2014.
In January 2016, we implemented additional cost-cutting measures to streamline operations, and as a result reduced headcount by more than 20%. We expect overall research and development costs to decrease in 2016, compared to 2015, in particular personnel-related costs, as a result of the reduction in workforce and other cost-cutting measures we implemented in December 2014 and January 2016. We plan to continue to make investments in research and development for the foreseeable future, but at a lower rate, as we continue to (1) identify, isolate and further optimize strains of microalgae to achieve high cell densities, high yield converting sugar to product and high productivity rates compared to other alternatives; (2) customize oil outputs to meet specific market needs; and (3) engage in product and process development projects aimed at reducing the cost of oil production.
Sales, General and Administrative Expenses
Sales, general and administrative expenses in 2015 compared to 2014 decreased $9.5 million, reflecting a $12.0 million decrease in personnel-related costs, $6.1 million of decreased costs from the 2014 litigation settlement and other external legal costs, lower travel costs of $0.8 million, lower professional and outside services of $0.8 million and lower marketing and promotional costs of $0.5 million,. These decreases were partially offset by a $10.2 million increase in unallocated fixed costs for the Clinton/Galva facilities associated with the facilities not operating at full capacity and $0.5 million of increased facility costs. Personnel-related costs decreased due to decreased non-cash stock-based compensation expense and decreased personnel costs as a result of restructuring activities implemented in December 2014. Personnel-related costs include non-cash stock-based compensation of $11.1 million in 2015 compared to $18.1 million in 2014. Stock-based compensation decreased in 2015 compared to 2014 primarily due to the restructuring activities implemented in December 2014 and stock option modification expense recorded in 2014.
During 2015, our facilities were not operating at full capacity as we managed limited production campaigns at the Clinton/Galva Facilities to focus on establishing operations at the Solazyme Bunge JV Plant pursuant to our 2014 Restructuring Plan. We plan to continue to invest in commercialization of our high value products within food and specialty ingredients markets, which may increase our overall selling, general and administrative expense, but expect personnel-related expenses to decrease as a result of a reduction in workforce and other cost-cutting measures we implemented starting in December 2014 to January 2016.
49
Restructuring Charges
In December 2014 we took steps to decrease operating expenses through a reduction in workforce and other cost-cutting measures (2014 Restructuring Plan). These targeted reductions were designed to enable us to achieve sustainable cash flow in the future.
In October 2015, we made a strategic decision to terminate our current manufacturing agreements at the ADM Clinton and ANP Galva facilities to better align our immediate production assets with our operating strategy while minimizing production costs (2015 Exit Plan). On October 29, 2015, we provided to ADM a notice of termination of the Operating Agreement entered into with ADM in 2012 related to the production of products at the Clinton Facility, and as a result, the Strategic Collaboration Agreement with ADM automatically terminated on February 26, 2016. In December 2015, we entered into a termination agreements with ADM Clinton and ANP Galva (collectively, the ADM and ANP Termination Agreements).
Restructuring charges in 2015 consist primarily of $0.4 million of one-time employee severance costs and asset accelerated depreciation charges related to the 2014 Restructuring Plan and $4.6 million of facility closure costs, asset impairment charges and other costs related to the 2015 Exit Plan. Under the ADM and ANP Termination Agreements, we made cash payments of approximately $2.4 million and issued $3.1 million in our common stock in December 2015. We will also make a combination of payments in cash and in our common stock in the amount of $3.4 million in 2016. We also recognized facility closure costs comprised of $0.6 million of costs offset by income related to the reversal of deferred rent liabilities of $7.3 million.
In January 2016, we took additional steps to decrease operating expenses (2016 Restructuring Plan). As a result, we anticipate reductions in annualized cash operating expenses as well as investments in the Solazyme Bunge JV of approximately $40.0 million in 2016.
Other Income (Expense), Net
Year ended December 31, | |||||||||||
2015 | 2014 | $ Change | |||||||||
(In thousands) | |||||||||||
Other income (expense): | |||||||||||
Interest and other income, net | $ | 929 | $ | 1,310 | $ | (381 | ) | ||||
Interest expense | (14,160 | ) | (13,477 | ) | 683 | ||||||
Loss from equity method investments | (22,389 | ) | (23,037 | ) | (648 | ) | |||||
Gain from change in fair value of warrant liability | — | 688 | (688 | ) | |||||||
Gain from change in fair value of derivative liability | 1 | 8,056 | (8,055 | ) | |||||||
Total other income (expense), net | $ | (35,619 | ) | $ | (26,460 | ) | $ | 9,159 | |||
Interest and other income, net
Interest and other income, net decreased by $0.4 million in 2015 compared to 2014, primarily due to interest income earned on lower investment balances. Our interest income will vary for each reporting period depending on our average investment balances during the period and market interest rates.
Interest expense
Interest expense increased by $0.7 million in 2015 compared to 2014, due primarily to increased interest expense as a result of the 2019 Notes issued in April 2014, partially offset by $1.8 million of debt conversion expense incurred in 2014. We expect interest expense to remain relatively flat during 2016, but could decrease if holders of our Notes elect to early convert such Notes prior to their final maturity dates.
Loss from Equity Method Investment
Loss from equity method investments decreased by $0.6 million in 2015 compared to 2014. We expect the loss from our equity method investment to decrease as the Solazyme Bunge JV continues optimization of the Solazyme Bunge JV Plant and once commercial-scale production is achieved.
50
We assessed the recoverability of our equity investment in the Solazyme Bunge JV as of December 31, 2015 and concluded that we expect to recover the carrying amount of our equity investment and our equity investment was not impaired. See discussion of how we evaluate the Solazyme Bunge JV for impairment in Note 8 of our notes to the consolidated financial statements.
Gain from Change in Fair Value of Warrant Liability
Gain from the change in fair value of warrant liability was $0 in 2015 compared to a $0.7 million gain from the change in fair value of warrant liability in 2014. The change in fair value of warrant liability is related to the fair value of the unvested warrant issued to Bunge Limited.
Gain from Change in Fair Value of Derivative Liability
There was very little change in the gain from change in fair value of derivative liabilities $1,000 during 2015 due primarily to the change in the fair value of the embedded derivatives related to the 2018 Notes and 2019 Notes (Notes) issued in January 2013 and April 2014, compared to a $8.1 million gain in 2014 due primarily to the decline in our stock price. We expect that gain/loss from change in fair value of derivative liabilities will fluctuate each reporting period as we remeasure these embedded derivatives at fair value, which is included as a component of convertible debt on our consolidated balance sheets.
Results of Operations
Comparison of Years Ended December 31, 2014 and 2013
Revenues
Year ended December 31, | |||||||||||
2014 | 2013 | $ Change | |||||||||
(In thousands) | |||||||||||
Revenues: | |||||||||||
Product revenues | $ | 37,346 | $ | 19,962 | $ | 17,384 | |||||
Research and development programs | 23,045 | 19,788 | 3,257 | ||||||||
Total revenues | $ | 60,391 | $ | 39,750 | $ | 20,641 | |||||
We have two reportable segments for financial statement reporting purposes: Algenist® and Ingredients and Other. The Ingredients and Other segment includes sale of our Encapso™ product and oils. Our discussions below surrounding changes in product revenue and gross margin are based on these two reportable segments.
Product Revenues and Cost of Product Revenues
Product revenues and cost of product revenues by segment for the years ended December 31, 2015 and 2014 were as follows:
51
Year Ended December 31, | |||||||||||
Algenist® | 2014 | 2013 | $ Change | ||||||||
Product revenue | $ | 24,429 | $ | 19,856 | $ | 4,573 | |||||
Cost of product revenue | 7,746 | 6,338 | 1,408 | ||||||||
Gross profit | $ | 16,683 | $ | 13,518 | $ | 3,165 | |||||
Gross margin | 68 | % | 68 | % | — | % | |||||
Ingredients and Other | |||||||||||
Product revenue | $ | 12,917 | $ | 106 | $ | 12,811 | |||||
Cost of product revenue | 12,866 | 47 | 12,819 | ||||||||
Gross profit | $ | 51 | $ | 59 | $ | (8 | ) | ||||
Gross margin | — | % | 56 | % | (56 | )% | |||||
Our total revenues increased by $20.6 million in 2014 compared to 2013, due to $17.4 million of increased product sales and $3.3 million of increased R&D program revenues in 2014 compared to 2013. The increase in product revenues was due to Ingredient product sales of $12.7 million, including $2.9 million of product revenue from the Solazyme Bunge JV in 2014 and a $4.7 million increase in skin care product sales primarily due to new retail customers, new product offerings and increased consumer demand. We began to sell Ingredient products more broadly starting in the first quarter of 2014 into Industrial Products markets. These sales included our commercial launch of our Encapso™ product and Tailored™ oils as well as fuel blend sales as part of our effort to build fuels marketing and commercial development programs, which preliminarily includes the sale and transfer of blended fuels to private (non-government) customers.
R&D program revenues increased by $3.3 million, due primarily to an increase in revenues from development agreements with the Solazyme Bunge JV, offset by a decrease in revenues from development agreements with strategic partners, due to the timing of milestone achievement, which included milestone achievement recognized from a strategic partner of $1.5 million during 2014 as compared to a $4.0 million milestone achievement recognized during 2013.
Cost of Product Revenues
Year ended December 31, | |||||||||||
2014 | 2013 | Change | |||||||||
(In thousands) | |||||||||||
Cost of revenue: | |||||||||||
Product | $ | 20,612 | $ | 6,385 | $ | 14,227 | |||||
Gross profit: | |||||||||||
Product | $ | 16,734 | $ | 13,577 | $ | 3,157 | |||||
Gross margin: | |||||||||||
Product | 45 | % | 68 | % | (23 | )% | |||||
Cost of product revenue increased $14.2 million in 2014 compared to 2013 due to sales of Tailored™ oil and blended fuel sales that began in early 2014 and increased Algenist® product sales. Gross margins decreased from 68% during 2013 to 45% in 2014 due primarily due to the higher mix of lower gross margin ingredient products. Gross margin percentage on Algenist® product sales remained constant at 68% in 2014 and 2013. The gross margin for Ingredient product sales was 11% in 2014 impacted favorably by the sale of inventories that were expensed previously to research and development expense in 2013. In addition, the gross margin on Ingredient product sales excludes certain production costs related to the scale-up of plant operations which are recorded to research and development expense.
Operating Expenses
52
Year ended December 31, | |||||||||||
2014 | 2013 | $ Change | |||||||||
(In thousands) | |||||||||||
Operating expenses: | |||||||||||
Research and development | $ | 81,680 | $ | 66,572 | $ | 15,108 | |||||
Sales, general and administrative | 90,266 | 62,933 | 27,333 | ||||||||
Restructuring charges | 3,514 | — | 3,514 | ||||||||
Total operating expenses | $ | 175,460 | $ | 129,505 | $ | 45,955 | |||||
Research and Development Expenses
Our research and development expenses increased by $15.1 million in 2014 compared to 2013, due primarily to $7.3 million of costs related to development of new algae oils and the scale up of commercial production at the Clinton/ Galva Facilities, increased personnel-related and facilities-related costs of $5.0 million and $1.6 million, respectively and increased research program expenses of $1.8 million due primarily to timing of such programs.
Personnel-related and facilities-related costs increased as a result of headcount growth to support the Clinton, Galva and Solazyme Bunge JV activities as well as Peoria manufacturing and collaborative research activities. Personnel-related costs include non-cash stock-based compensation expense of $7.4 million in 2014 compared to $5.9 million in 2013.
Sales, General and Administrative Expenses
Our sales, general and administrative expenses increased by $27.3 million in 2014 compared to 2013, primarily due to increased personnel-related and facilities-related costs of $12.5 million and $0.7 million, respectively, net litigation settlement costs of $4.5 million, increased marketing and promotional costs of $4.6 million and increased external legal costs and outside services of $3.3 million. During 2014, we and Therabotanics, LLC agreed to settle our litigation for $4.8 million, net of insurance reimbursements of $0.3 million.
Personnel-related and facilities-related costs increased due to headcount growth primarily related to commercialization of our products. Personnel-related costs include non-cash stock-based compensation of $18.1 million in 2014 compared to $12.7 million in 2013. Stock-based compensation in 2014 includes $2.3 million of costs related to the modification of a former executive's equity awards. Marketing and promotional costs increased mainly due to Algenist® product and ingredients product launches during 2014 compared to 2013.
Restructuring Charges
In December 2014 we took steps to decrease operating expenses through a reduction in workforce and other cost-cutting measures (2014 Restructuring Plan). Restructuring charges consist primarily of one-time employee severance costs of $2.0 million and asset impairment charges of $1.5 million.
Other Income (Expense), Net
Year ended December 31, | |||||||||||
2014 | 2013 | $ Change | |||||||||
(In thousands) | |||||||||||
Other income (expense): | |||||||||||
Interest and other income, net | $ | 1,310 | $ | 1,369 | $ | (59 | ) | ||||
Interest expense | (13,477 | ) | (7,136 | ) | 6,341 | ||||||
Loss from equity method investments | (23,037 | ) | (8,237 | ) | 14,800 | ||||||
Gain from change in fair value of warrant liability | 688 | 147 | 541 | ||||||||
Gain (loss) from change in fair value of derivative liability | 8,056 | (6,392 | ) | (14,448 | ) | ||||||
Total other income (expense), net | $ | (26,460 | ) | $ | (20,249 | ) | $ | 6,211 | |||
Interest expense
Interest expense increased by $6.3 million in 2014 compared to 2013, primarily due to $6.8 million of increased interest expense as a result of the 2019 Notes and $1.8 million of debt conversion expense incurred in 2014, partially offset by
53
$2.6 million of reduced interest expense resulting from early conversions of the 2018 Notes and $10.4 million paydown of our credit facility with HSBC. In June 2014, we entered into note exchange agreements with certain holders of our 2018 Notes pursuant to which such holders agreed to exchange approximately $17.5 million in aggregate principal amount of 2018 Notes, together with accrued interest thereon through the settlement date of the Exchange, with us for a total 2.4 million shares of our common stock. Debt conversion expense represents the fair value of all common stock transferred in the Exchange in excess of the fair value of common stock issuable pursuant to the original conversion terms. The fair value of the common stock was measured as of the date the Exchange offer was accepted by the holders.
Loss from Equity Method Investment
Loss from equity method investments increased by $14.8 million in 2014 compared to 2013, primarily due to a $16.2 million increase in our proportionate share of the net loss from the Solazyme Bunge JV, partially offset by a $1.4 million loss recognized in 2013 related to the dissolution of SRN.
Gain from Change in Fair Value of Warrant Liability
There was a $0.7 million gain from the change in fair value of warrant liability in 2014 compared to a $0.1 million gain in 2013. The change in fair value of warrant liability is related to the fair value of the unvested warrant issued to Bunge Limited.
Gain (Loss) from Change in Fair Value of Derivative Liability
Gain from change in fair value of derivative liabilities of $8.1 million in 2014 was due primarily to the change in the fair value of the embedded derivatives related to the early conversion payment features of the 2018 Notes and 2019 Notes (Notes) issued in January 2013 and April 2014, compared to a $6.4 million loss in 2013.
Liquidity and Capital Resources
Total cash and cash equivalents and marketable securities available-for-sale were:
December 31, 2015 | December 31, 2014 | ||||||
(In thousands) | |||||||
Cash and cash equivalents | $ | 46,966 | $ | 42,689 | |||
Marketable securities available-for-sale | 51,009 | 164,619 | |||||
Total cash and cash equivalents and marketable securities | $ | 97,975 | $ | 207,308 | |||
Cash, cash equivalents and marketable securities decreased by $109.3 million in 2015, primarily due to cash used in operating activities of $85.1 million, $22.3 million of capital contributed to the Solazyme Bunge JV and $1.6 million of property and equipment purchases.
The following table shows a summary of our cash flows for the periods indicated:
Year ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
(In thousands) | |||||||||||
Net cash used in operating activities | $ | (85,115 | ) | $ | (118,113 | ) | $ | (74,790 | ) | ||
Net cash provided by (used in) investing activities | $ | 89,117 | $ | (95,091 | ) | $ | (20,341 | ) | |||
Net cash provided by financing activities | $ | 708 | $ | 201,082 | $ | 119,379 | |||||
Liquidity
We are an emerging growth company with a limited operating history. We only recently began commercializing our products. To date, a substantial portion of revenues has consisted of funding from third party collaborative research agreements and government grants. We have generated limited revenues from commercial sales, principally derived from sales of personal care products. A significant portion of future revenues are expected to come from commercial sales in the food and nutrition ingredients and specialty personal care products.
54
Net losses may continue as we ramp up manufacturing capacity and build out our product pipeline. We expect to incur additional costs and expenses related to the continued development and expansion of our business, including research and development, the operation of our Peoria Facility and the ramp up and operation of the Solazyme Bunge JV Plant in Brazil.
We, along with our development and commercialization partners, need to develop products successfully, cost effectively produce them in large quantities and market and sell such products profitably. Our failure to generate sufficient revenues, achieve planned gross margins, control operating costs or raise sufficient additional funds may require us to modify, delay or abandon our planned operations, which could have a material adverse effect on the business, operating results, financial condition and ability to achieve intended business objectives. We may be required to seek additional funds through collaborations, public or private debt or equity financings or government programs, and may also seek to reduce expenses related to the our operations. There can be no assurance that any financing will be available or on acceptable terms.
Based on current cash utilization, management believes that our cash and cash flows from operations will be sufficient to fund operations through March 31, 2017.
Our future capital requirements and the adequacy of available funds will depend on many factors, including those set forth under “Risk Factors” elsewhere in this Annual Report on Form 10-K. If we raise additional funds by issuing equity securities, dilution to our existing stockholders may result.
Cash Flows from Operating Activities
Cash used in operating activities of $85.1 million in 2015 primarily reflects a loss of $141.4 million, aggregate non-cash charges of $49.8 million and a net change of $6.5 million in our net operating assets and liabilities. Non-cash charges included loss from equity method investments, stock-based compensation, depreciation and amortization, restructuring charges, net amortization of premiums on marketable securities and debt discount and loan fee amortization.
The net change in our operating assets and liabilities in 2015 was primarily a result of decreased inventories of $3.3 million, increased other current and long-term liabilities of $5.4 million and increased deferred revenue of $3.5 million, partially offset by increased accounts receivables and unbilled revenues of $2.5 million, increased prepaid expenses and other current assets of $0.8 million and decreased accounts payable and accrued liabilities of $2.3 million. Inventories decreased primarily due to reduced production at the Clinton/Galva Facilities. Deferred revenue increased due to the timing of payments received on new development agreements entered into during the fourth quarter of 2015. Accounts receivable and unbilled revenues increased due to timing of payments received. Prepaid expenses increased due primarily to an increase of promotional supplies. Accounts payable and accrued liabilities decreased due to cash payments of severance related to the 2014 Restructuring Plan and decreased production costs incurred.
Cash used in operating activities of $118.1 million in 2014 primarily reflects a loss of $162.1 million, aggregate non-cash charges of $55.5 million and a net change of $11.5 million in our net operating assets and liabilities. Non-cash charges included stock-based compensation, loss from equity method investments, revaluations of our warrant liability and derivative liabilities, depreciation and amortization, net amortization of premiums on marketable securities, restructuring charges, debt conversion expense and debt discount and loan fee amortization. The net change in our operating assets and liabilities in 2014 was primarily a result of decreased accounts payable and accrued liabilities of $1.5 million, decreased other assets of $3.6 million, decreased accounts receivable and unbilled revenue of $11.3 million, partially offset by increased inventories of $5.5 million. Accounts payable and accrued liabilities decreased due to decreased scale-up activities at the Clinton/Galva Facilities and decreased bonus accrual, partially offset by additional interest expense accrued as a result of the issuance of 2019 Notes in April 2014 and restructuring charges accrued as a result of the 2014 Restructuring Plan. Accounts receivables and unbilled revenues decreased due primarily to the timing of payments received under our R&D programs. Inventories increased due primarily to commercial launch of ingredient products, including EncapsoTM, TailoredTM oils and fuels.
Cash used in operating activities of $74.8 million in 2013 reflects a loss of $116.4 million, and a net change of $0.4 million in our net operating assets and liabilities, partially offset by aggregate non-cash charges of $42.0 million. Non-cash charges included stock-based compensation, loss from equity method investments, revaluation of our derivative liability, depreciation and amortization, net amortization of premiums on marketable securities and debt discount and loan fee amortization. The net change in our operating assets and liabilities was primarily a result of increased accounts receivable and unbilled revenue of $5.2 million, increased deferred revenues of $2.0 million, increased inventories of $2.9 million, increased accounts payable and accrued liabilities of $3.4 million and decreased other assets of $3.3 million. Accounts receivable and unbilled revenue increased primarily due to billing related to research and development agreements entered into in 2013 and timing of payments received on accounts receivables from strategic partners. Deferred revenues increased due primarily to the
55
timing of payments received under our R&D programs. Inventories increased due to the expansion of and increased demand in our skin care line. The net increase in accounts payable and accrued liabilities was due primarily to increased scale-up activities at the Clinton Facility and interest accrued on the Notes.
Cash Flows from Investing Activities
In 2015, cash provided by investing activities was $89.1 million, primarily as a result of $112.6 million of net proceeds from marketable securities maturities and sales, partially offset by $22.3 million of capital contributed to the Solazyme Bunge JV and $1.6 million of equipment purchases.
In 2014, cash used in investing activities was $95.1 million, primarily as a result of $54.0 million of net marketable securities purchases, $32.6 million of capital contributed to the Solazyme Bunge JV, $7.2 million of capital expenditures related primarily to equipment installed at the Peoria and Clinton/Galva Facilities, a $0.7 million letter of credit obtained for a lease agreement and $0.6 million of interest capitalized related to the Solazyme Bunge JV.
In 2013, cash used in investing activities was $20.3 million, primarily as a result of $3.4 million of net proceeds from marketable securities maturities and sales, offset by $12.4 million of capital contributed to the Solazyme Bunge JV and the Solazyme Roquette JV, $10.2 million of capital expenditures related primarily to equipment installed at the Clinton Facility, and $1.1 million of capitalized interest related to the Solazyme Bunge JV.
Cash Flows from Financing Activities
In 2015, cash provided by financing activities was $0.7 million, primarily due to proceeds received from common stock issuances pursuant to our equity plans.
In 2014, cash provided by financing activities was $201.1 million, primarily due to $202.6 million of proceeds received from the issuance of the 2019 Notes and Common Stock Offering, net of underwriting discounts and offering issue costs and $8.9 million of proceeds received from common stock issuances pursuant to our equity plans, partially offset by $10.4 million of principal debt payments.
In 2013, cash provided by financing activities was $119.4 million, primarily due to $119.2 million of proceeds received from the issuance of the 2018 Notes, net of debt discounts and debt issue costs, $10.4 million of loan proceeds received from HSBC and $4.8 million received from common stock issuances pursuant to our equity plans, partially offset by $14.9 million of principal debt payments.
Commercial Bank
The Company has a credit facility with HSBC (the HSBC facility), which provides for a $35.0 million revolving facility for working capital, letters of credit denominated in U.S. dollars or a foreign currency and other general corporate purposes. Approximately $26.1 million of the HSBC facility remained available as of December 31, 2015, and we were in compliance with the financial covenants of the HSBC facility. The HSBC facility is unsecured unless (i) we take action that could cause or permit obligations under the HSBC facility not to constitute senior debt (as defined in the indenture), (ii) we breach financial covenants that require us and our subsidiaries to maintain cash and unrestricted cash equivalents at all times of not less than $35.0 million plus 110% of the aggregate dollar equivalent amount of outstanding advances and letters of credit under the HSBC facility, or (iii) there is a payment default under the HSBC facility or bankruptcy or insolvency events relating to us. A portion of the HSBC facility supports the bank guarantee issued to BNDES in May 2013. The HSBC Facility expires on May 31, 2016, and the Company expects to replace the HSBC Facility to support the BNDES bank guarantee on substantially similar terms.
BNDES Loan
In April 2012, we entered into the Solazyme Bunge JV, which is jointly capitalized by us and Bunge, which operates an oil production facility in Brazil. Through December 31, 2015 we contributed $97.9 million in capital to the Solazyme Bunge JV, and we may need to contribute additional capital to this project. In February 2013, the Solazyme Bunge JV entered a loan agreement with the Brazilian Development Bank (BNDES) under which it may borrow up to R$245.7 million (approximately USD $62.0 million based on the exchange rate as of December 31, 2015). As of December 31, 2015, approximately $53.4 million was outstanding under the BNDES loan based on the exchange rate as of December 31, 2015. In addition to the bank guarantee described above, we may be required to provide a corporate guarantee for a portion of the loan (in an amount that when added to the amount supported by our bank guarantee does not to exceed our ownership percentage in the Solazyme Bunge JV). We expect to evaluate the optimal amount of Solazyme Bunge JV-related capital expenditures that we agree to fund
56
on a case-by-case basis. These events may require us to access additional capital through equity or debt offerings. If we are unable to access additional capital, our growth may be limited due to the inability to build out additional manufacturing capacity.
Convertible Debt
2018 Notes—In January 2013, we issued $125.0 million aggregate principal amount of 2018 Notes that will mature on February 1, 2018 as described in more detail in Note 11. We had $61.6 million aggregate principal amount of 2018 Notes outstanding as of December 31, 2015.
2019 Notes—On April 1, 2014, we issued $149.5 million aggregate principal amount of 2019 Notes that will mature on October 1, 2019 as described in more detail in Note 11. We had $149.5 million aggregate principal amount of 2019 Notes outstanding as of December 31, 2015.
Subsequent Event
On March 10, 2016, the Company entered into a Stock Purchase Agreement with certain strategic investors and entrepreneurs in food, nutrition and innovation in connection with the private placement of 27,850 shares of its Series A Convertible Preferred Stock, par value $0.001 per share (the “Series A Convertible Preferred Stock”), for a per share purchase price of $1,000 per share, with a conversion price of $2.00 per common share. The 27,850 shares of Series A Preferred Stock that are expected to be issued will be initially convertible into an aggregate of 13.9 million shares of common stock. The Company expects to receive gross proceeds of $27.9 million in cash from the private placement and intends to use such proceeds for capital expenditures, working capital and general corporate purposes.
The private placement is expected to close in March 2016, subject to the satisfaction of customary closing conditions.
Contractual Obligations and Commitments
The following is a summary of our contractual obligations and commitments as of December 31, 2015 (in thousands)
Total | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 and beyond | |||||||||||||||||||||
Principal payments on long-term debt | $ | 211,132 | $ | — | $ | — | $ | 61,632 | $ | 149,500 | $ | — | $ | — | |||||||||||||
Interest payments on long-term debt, fixed rate | 39,145 | 11,173 | 11,173 | 9,324 | 7,475 | — | — | ||||||||||||||||||||
Non-cancelable operating leases | 9,293 | 4,400 | 4,527 | 366 | — | — | — | ||||||||||||||||||||
Purchase obligations | 83 | 83 | — | — | — | — | — | ||||||||||||||||||||
Total | $ | 259,653 | $ | 15,656 | $ | 15,700 | $ | 71,322 | $ | 156,975 | $ | — | $ | — | |||||||||||||
Off-Balance Sheet Arrangements
For information on variable interest entities and guarantees, refer to Note 8 and Note 12, respectively, in the accompanying notes to our consolidated financial statements.
Recent Accounting Pronouncements
Refer to Note 2 in the accompanying notes to our consolidated financial statements for a discussion of recent accounting pronouncements.
57
Item 7A. Quantitative and Qualitative Disclosures about Market Risk.
We are exposed to financial market risks, primarily changes in interest rates, currency exchange rates and commodity prices. All of the potential changes noted below are based on sensitivity analyses performed on our financial positions as of December 31, 2015. Actual results may differ materially.
Interest Rate Risk
Our exposure to market risk for changes in interest rates relates primarily to our investment portfolio and our outstanding debt obligations. We generally invest our cash in investments with short maturities or with frequent interest reset terms. Accordingly, our interest income fluctuates with short-term market conditions. As of December 31, 2015, our investment portfolio consisted primarily of corporate debt obligations, U.S. government agency securities, asset-backed and mortgaged-backed securities, municipal bonds and money market funds, which are held for working capital purposes. We believe we do not have material exposure to changes in fair value as a result of changes in interest rates. Our marketable securities were comprised primarily of fixed-term securities as of December 31, 2015. Due to the short-term nature of these instruments, we do not believe that there would be a significant negative impact to our condensed consolidated financial position or results of operations as a result of interest rate fluctuations in the financial markets. Our outstanding debt as of December 31, 2015 consists of fixed-rate debt, and therefore, is not subject to fluctuations in market interest rates.
Foreign Currency Risk
Our operations include manufacturing and sales activities primarily in the United States, as well as research activities primarily in the United States. We are actively expanding outside the United States, in particular in Brazil through our Solazyme Bunge JV. We sell our Algenist® products in Europe and conduct operations in Brazil. As we expand internationally, our results of operations and cash flows will become increasingly subject to fluctuations due to changes in foreign currency exchange rates. For example, our operations in Brazil and/or potential expansion elsewhere in Latin America or increasing Euro denominated product sales to European distributors, will result in our use of currencies other than the U.S. dollar. In addition, the local currency is the functional currency of our Brazil subsidiary and the Solazyme Bunge JV (an unconsolidated joint venture). The assets and liabilities of the Brazil subsidiary are translated from its functional currency to U.S. dollars at the exchange rate in effect at the balance sheet date, with resulting foreign currency translation adjustments recorded in accumulated other comprehensive income (loss) in the condensed consolidated statements of comprehensive loss. The assets and liabilities of the Solazyme Bunge JV are also translated to U.S. dollars similar to our Brazil subsidiary, and we adjust our investment in the Solazyme Bunge JV and cumulative translation adjustment in equity for our ownership portion of the cumulative translation gain or loss recognized on the Solazyme Bunge JV's financial statements. As a result, our comprehensive income (loss), cash flows and expenses are subject to fluctuations due to changes in foreign currency exchange rates. In periods when the U.S. dollar declines in value as compared to the foreign currencies in which we incur expenses, our foreign-currency based expenses increase when translated into U.S. dollars. A hypothetical 10% adverse change in foreign currency exchange rate would have had a $1.9 million impact on our net loss for the year ended December 31, 2015. We have not hedged our foreign currency since the exposure has not been material to our historical operating results. Although substantially all of our sales are currently denominated in U.S. dollars, future fluctuations in the value of the U.S. dollar may affect the price competitiveness of our products outside the United States. We may consider hedging our foreign currency risk as we continue to expand internationally.
Commodity Price Risk
Our exposure to market risk for changes in commodity prices currently relates primarily to our purchases of plant sugar feedstock, and fuel in connection with our blended fuels marketing and commercial development programs. A hypothetical 10% change in the cost of plant sugar feedstock would have had approximately a $0.4 million impact on our share of loss from equity method investment in the Solazyme Bunge JV for the year ended December 31, 2015. We have not historically hedged the price volatility of plant sugar feedstock. Also, fluctuations in the prices of petroleum or certain plant oils may also impact our business to the extent our products compete with petroleum or plant-oil-derived products. In the future, we may manage our exposure to these risks by hedging the price volatility of such products, principally through futures contracts, and entering into joint venture agreements that would enable us to obtain secure access to feedstock. See also “Risk Factors-Risks Related to Our Business and Industry," - A decline in the price of petroleum and petroleum-based products, plant oils or other commodities may reduce demand for our products and may otherwise adversely affect our business.”
58
59
Item 8. Financial Statements and Supplementary Data.
CONSOLIDATED FINANCIAL STATEMENTS
Solazyme, Inc.
Index
CONSOLIDATED FINANCIAL STATEMENTS AS OF DECEMBER 31, 2015 AND 2014 AND FOR THE YEARS ENDED DECEMBER 31, 2015, 2014 AND 2013: | |
60
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Solazyme, Inc.
We have audited the accompanying consolidated balance sheets of Solazyme, Inc. and subsidiaries (the “Company”) as of December 31, 2015 and 2014, and the related consolidated statements of operations, comprehensive loss, stockholders’ (deficit) equity, and cash flows for each of the three years in the period ended December 31, 2015. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the consolidated financial position of Solazyme, Inc. and subsidiaries as of December 31, 2015 and 2014, and the results of its operations and their cash flows for each of the three years in the period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2015, based on the criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 14, 2016, expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/ Deloitte & Touche LLP
San Francisco, California
March 14, 2016
61
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Solazyme, Inc.
We have audited the internal control over financial reporting of Solazyme, Inc. and subsidiaries (the “Company”) as of December 31, 2015, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained, in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements as of and for the year ended December 31, 2015, of the Company and our report dated March 14, 2016, expressed an unqualified opinion on those consolidated financial statements.
/s/ Deloitte & Touche LLP
San Francisco, California
March 14, 2016
62
SOLAZYME, INC.
CONSOLIDATED BALANCE SHEETS
In thousands, except share and per share amounts
December 31, 2015 | December 31, 2014 | ||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 46,966 | $ | 42,689 | |||
Marketable securities available-for-sale | 51,009 | 164,619 | |||||
Accounts receivable, net | 3,552 | 4,598 | |||||
Unbilled revenues | 1,036 | 3,002 | |||||
Inventories | 12,018 | 15,334 | |||||
Prepaid expenses and other current assets | 4,363 | 3,685 | |||||
Total current assets | 118,944 | 233,927 | |||||
Property, plant and equipment, net | 26,344 | 36,080 | |||||
Investment in Solazyme Bunge JV | 35,910 | 40,934 | |||||
Other assets | 1,225 | 1,648 | |||||
Total assets | $ | 182,423 | $ | 312,589 | |||
LIABILITIES AND STOCKHOLDERS’ (DEFICIT) EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 7,016 | $ | 8,319 | |||
Accrued liabilities | 14,155 | 14,079 | |||||
Current portion of long-term debt | — | 6 | |||||
Deferred revenue | 4,159 | 1,050 | |||||
Total current liabilities | 25,330 | 23,454 | |||||
Deferred revenue | 500 | 150 | |||||
Convertible debt | 202,466 | 200,091 | |||||
Other liabilities | 602 | 2,518 | |||||
Total liabilities | 228,898 | 226,213 | |||||
Commitments and contingencies (Note 12) | |||||||
Stockholders’ (deficit) equity: | |||||||
Preferred stock, par value $0.001—5,000,000 shares authorized; 0 shares issued and outstanding | — | — | |||||
Common stock, par value $0.001—150,000,000 shares authorized; 81,734,078 and 79,388,069 shares issued and outstanding at December 31, 2015 and 2014, respectively | 82 | 79 | |||||
Additional paid-in capital | 585,679 | 565,769 | |||||
Accumulated other comprehensive loss | (22,331 | ) | (11,014 | ) | |||
Accumulated deficit | (609,905 | ) | (468,458 | ) | |||
Total stockholders’ (deficit) equity | (46,475 | ) | 86,376 | ||||
Total liabilities and stockholders’ (deficit) equity | $ | 182,423 | $ | 312,589 | |||
See accompanying notes to the consolidated financial statements.
63
SOLAZYME, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
In thousands, except share and per share amounts
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Revenues: | |||||||||||
Product revenues | $ | 33,300 | $ | 37,346 | $ | 19,962 | |||||
Research and development programs | 12,831 | 23,045 | 19,788 | ||||||||
Total revenues | 46,131 | 60,391 | 39,750 | ||||||||
Costs and operating expenses: | |||||||||||
Cost of product revenue | 18,179 | 20,612 | 6,385 | ||||||||
Research and development | 48,094 | 81,680 | 66,572 | ||||||||
Sales, general and administrative | 80,733 | 90,266 | 62,933 | ||||||||
Restructuring charges | 4,953 | 3,514 | — | ||||||||
Total costs and operating expenses | 151,959 | 196,072 | 135,890 | ||||||||
Loss from operations | (105,828 | ) | (135,681 | ) | (96,140 | ) | |||||
Other income (expense): | |||||||||||
Interest and other income, net | 929 | 1,310 | 1,369 | ||||||||
Interest expense | (14,160 | ) | (13,477 | ) | (7,136 | ) | |||||
Loss from equity method investments | (22,389 | ) | (23,037 | ) | (8,237 | ) | |||||
Gain from change in fair value of warrant liability | — | 688 | 147 | ||||||||
Gain (loss) from change in fair value of derivative liability | 1 | 8,056 | (6,392 | ) | |||||||
Total other expense, net | (35,619 | ) | (26,460 | ) | (20,249 | ) | |||||
Net loss | $ | (141,447 | ) | $ | (162,141 | ) | $ | (116,389 | ) | ||
Net loss per share, basic and diluted | $ | (1.76 | ) | $ | (2.14 | ) | $ | (1.81 | ) | ||
Weighted average number of common shares used in loss per share computation, basic and diluted | 80,165,402 | 75,879,208 | 64,211,958 | ||||||||
See accompanying notes to the consolidated financial statements.
64
SOLAZYME, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
In thousands
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Net loss | $ | (141,447 | ) | $ | (162,141 | ) | $ | (116,389 | ) | ||
Other comprehensive income (loss), net: | |||||||||||
Change in unrealized gain/loss on available-for-sale securities | 228 | (312 | ) | (240 | ) | ||||||
Foreign currency translation adjustment | (11,545 | ) | (6,908 | ) | (3,155 | ) | |||||
Other comprehensive loss | (11,317 | ) | (7,220 | ) | (3,395 | ) | |||||
Total comprehensive loss | $ | (152,764 | ) | $ | (169,361 | ) | $ | (119,784 | ) | ||
See accompanying notes to the consolidated financial statements.
65
SOLAZYME, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITY
In thousands, except share and per share amounts
Common Stock | Additional Paid-In Capital | Accumulated Other Comprehensive Loss | Accumulated Deficit | Total Stockholders’ (Deficit) Equity | ||||||||||||||||||
Shares | Amount | |||||||||||||||||||||
December 31, 2012 | 61,000,724 | $ | 61 | $ | 373,577 | $ | (399 | ) | $ | (189,928 | ) | $ | 183,311 | |||||||||
Issuance of common stock under stock plans, net of shares used for tax withholdings | 1,347,617 | 1 | 5,325 | 5,326 | ||||||||||||||||||
Common stock issued pursuant to vesting of restricted stock, restricted stock units and performance stock units | 83,835 | 5,209 | 5,209 | |||||||||||||||||||
Stock-based compensation expense | 13,444 | 13,444 | ||||||||||||||||||||
Common stock issued upon early conversion of Senior Convertible Notes | 5,541,597 | 6 | 44,212 | 44,218 | ||||||||||||||||||
Common stock issued in connection with use and operation of the Clinton Facility | 770,761 | 1 | 7,125 | 7,126 | ||||||||||||||||||
Vesting of warrant shares issued in connection with use and operation of the Clinton Facility | 98 | 98 | ||||||||||||||||||||
Change in unrealized loss on available-for-sale securities | (240 | ) | (240 | ) | ||||||||||||||||||
Foreign currency translation adjustment | (3,155 | ) | (3,155 | ) | ||||||||||||||||||
Net loss | (116,389 | ) | (116,389 | ) | ||||||||||||||||||
December 31, 2013 | 68,744,534 | 69 | 448,990 | (3,794 | ) | (306,317 | ) | 138,948 | ||||||||||||||
Issuance of common stock under stock plans, net of shares used for tax withholdings | 1,367,075 | 1 | 8,879 | 8,880 | ||||||||||||||||||
Issuance of common stock pursuant to secondary offering | 5,750,000 | 6 | 59,203 | 59,209 | ||||||||||||||||||
Common stock issued pursuant to vesting of restricted stock and restricted stock units | 782,985 | 1 | 8,517 | 8,518 | ||||||||||||||||||
Stock-based compensation expense | 17,032 | 17,032 | ||||||||||||||||||||
Common stock issued upon early conversion of Senior Convertible Notes (see Note 11) | 2,743,475 | 2 | 22,592 | 22,594 | ||||||||||||||||||
Vesting of warrant shares issued in connection with use and operation of the Clinton Facility (see Note 10) | 556 | 556 | ||||||||||||||||||||
Change in unrealized loss on available-for-sale securities | (312 | ) | (312 | ) | ||||||||||||||||||
Foreign currency translation adjustment | (6,908 | ) | (6,908 | ) | ||||||||||||||||||
Net loss | (162,141 | ) | (162,141 | ) | ||||||||||||||||||
December 31, 2014 | 79,388,069 | 79 | 565,769 | (11,014 | ) | (468,458 | ) | 86,376 | ||||||||||||||
Issuance of common stock under stock plans, net of shares used for tax withholdings | 416,008 | 1 | 836 | 837 | ||||||||||||||||||
Common stock issued pursuant to vesting of restricted stock and restricted stock units | 808,087 | 1 | 5,632 | 5,633 | ||||||||||||||||||
Stock-based compensation expense | 10,052 | 10,052 | ||||||||||||||||||||
Vesting of warrant shares issued in connection with use and operation of the Clinton Facility (see Note 10) | 97 | 97 | ||||||||||||||||||||
Cancellation of ADM warrants (See Note 3 and Note 10) | 242 | 242 | ||||||||||||||||||||
Common stock issued pursuant to ADM termination agreement | 1,121,914 | 1 | 3,051 | 3,052 | ||||||||||||||||||
Change in unrealized loss on available-for-sale securities | 228 | 228 | ||||||||||||||||||||
Foreign currency translation adjustment | (11,545 | ) | (11,545 | ) | ||||||||||||||||||
Net loss | (141,447 | ) | (141,447 | ) | ||||||||||||||||||
December 31, 2015 | 81,734,078 | $ | 82 | $ | 585,679 | $ | (22,331 | ) | $ | (609,905 | ) | $ | (46,475 | ) | ||||||||
See accompanying notes to the consolidated financial statements.
66
SOLAZYME, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
In thousands
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Operating activities: | |||||||||||
Net loss | $ | (141,447 | ) | $ | (162,141 | ) | $ | (116,389 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||
Depreciation and amortization | 5,845 | 6,283 | 5,108 | ||||||||
Gain on sale of available for sale securities | (49 | ) | (7 | ) | — | ||||||
Net amortization of premiums on marketable securities | 1,053 | 1,588 | 1,685 | ||||||||
Amortization of debt discount and loan fees | 2,554 | 2,206 | 1,529 | ||||||||
Issuance of common stock in connection with professional services rendered | — | — | 452 | ||||||||
Warrant expense related to vesting of ADM Warrant (see Note 10) | 97 | 556 | 98 | ||||||||
Debt conversion expense | — | 1,766 | — | ||||||||
Restructuring charges | 2,622 | 2,900 | — | ||||||||
Stock-based compensation expense | 15,684 | 25,549 | 18,653 | ||||||||
Loss from equity method investments | 21,994 | 23,432 | 8,237 | ||||||||
Revaluation of warrant liability | — | (688 | ) | (147 | ) | ||||||
Revaluation of derivative liabilities | (1 | ) | (8,056 | ) | 6,392 | ||||||
Changes in operating assets and liabilities: | |||||||||||
Accounts receivable | (4,436 | ) | (9,441 | ) | (7,268 | ) | |||||
Unbilled revenues | 1,966 | (1,901 | ) | 2,045 | |||||||
Inventories | 3,315 | (5,497 | ) | (2,946 | ) | ||||||
Prepaid expenses and other current assets | (815 | ) | (842 | ) | (764 | ) | |||||
Other assets | (42 | ) | 3,582 | 3,262 | |||||||
Accounts payable | 739 | 3,575 | (2,563 | ) | |||||||
Accrued liabilities | (3,010 | ) | (2,107 | ) | 5,955 | ||||||
Deferred revenue | 3,460 | (1,075 | ) | 1,983 | |||||||
Other current and long-term liabilities | 5,356 | 2,205 | (112 | ) | |||||||
Net cash used in operating activities | (85,115 | ) | (118,113 | ) | (74,790 | ) | |||||
Investing activities: | |||||||||||
Purchases of property, plant and equipment | (1,579 | ) | (7,208 | ) | (10,217 | ) | |||||
Proceeds received from the sale of equipment | 114 | — | — | ||||||||
Purchases of marketable securities | (24,146 | ) | (195,987 | ) | (133,601 | ) | |||||
Proceeds from maturities of marketable securities | 105,589 | 135,464 | 126,073 | ||||||||
Proceeds from sales of marketable securities | 31,196 | 6,541 | 10,890 | ||||||||
Capital contributions in unconsolidated joint ventures | (22,344 | ) | (32,550 | ) | (12,431 | ) | |||||
Capitalized interest related to unconsolidated joint venture | — | (620 | ) | (1,055 | ) | ||||||
Restricted certificates of deposit | 287 | (731 | ) | — | |||||||
Net cash provided by (used in) investing activities | 89,117 | (95,091 | ) | (20,341 | ) | ||||||
Financing activities: | |||||||||||
Repayments under loan agreements | (6 | ) | (10,433 | ) | (14,917 | ) | |||||
Proceeds from the issuance of senior subordinated convertible notes, net of discount | — | 143,894 | 119,750 | ||||||||
Proceeds from the issuance of common stock, net of repurchases | 746 | 8,945 | 4,718 | ||||||||
Proceeds from borrowings under loan agreements | — | — | 10,369 | ||||||||
Payment for loan costs and fees | — | (465 | ) | (541 | ) | ||||||
Proceeds from issuance of common stock in a public offering, net of underwriting discounts and commission | — | 59,209 | — | ||||||||
Cash settlement of vested restricted stock units | (32 | ) | (68 | ) | — | ||||||
Net cash provided by financing activities | 708 | 201,082 | 119,379 | ||||||||
Effect of exchange rate changes on cash and cash equivalents | (433 | ) | (166 | ) | (89 | ) | |||||
Net increase (decrease) of cash and cash equivalents | 4,277 | (12,288 | ) | 24,159 | |||||||
Cash and cash equivalents—beginning of period | 42,689 | 54,977 | 30,818 | ||||||||
Cash and cash equivalents—end of period | $ | 46,966 | $ | 42,689 | $ | 54,977 | |||||
Supplemental disclosures of cash flow information: | |||||||||||
Interest paid in cash, net of capitalized interest | $ | 11,175 | $ | 7,558 | $ | 3,499 | |||||
Supplemental disclosure of noncash investing and financing activities: | |||||||||||
Purchase of property, plant and equipment in accounts payable and accrued liabilities | $ | 232 | $ | 154 | $ | 3,413 | |||||
Capital contribution to unconsolidated joint venture settled with reduction of receivable | $ | 5,480 | $ | 15,300 | $ | — | |||||
Common stock issued to Archer-Daniels-Midlands Company pursuant to Termination Agreement (see Note 3) | $ | 3,052 | $ | — | $ | — | |||||
Common stock issued in connection with use and operation of the Clinton Facility (see Note 10) | $ | — | $ | — | $ | 7,125 | |||||
Conversion of Senior Convertible Notes to common stock | $ | — | $ | 2,461 | $ | 40,616 | |||||
Early conversion on Senior Convertible Notes settled in common stock | $ | — | $ | 217 | $ | 3,602 | |||||
Conversion of Senior Convertible Notes pursuant to inducement settled in common stock | $ | — | $ | 18,125 | $ | — | |||||
See accompanying notes to the consolidated financial statements.
67
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1. THE COMPANY
Nature of Business—Solazyme, Inc. (the “Company”) was incorporated in the State of Delaware on March 31, 2003. The Company creates food, nutrition and specialty ingredients from algae. Moving forward, the Company will be known as TerraVia™.
The Company’s proprietary technology uses microalgae to produce high-value triglyceride oils, proteins, fibers, micronutrients and other ingredients. The Company has developed and is commercializing products for food and nutrition ingredients, animal nutrition ingredients and specialty personal care applications and its sustainable products can replace or enhance products derived from the world’s three existing oil sources: petroleum, plants and animal fats. The Company's technology platform harnesses the oil, protein and polysaccharide-producing characteristics of microalgae and the Company is able to tailor the composition of its oils, powders and other bioproducts to address specific customer requirements. The Company uses standard fermentation equipment to convert sugars into the desired end product. By feeding plant-based sugars to the Company’s proprietary microalgae in enclosed fermentation tanks, the Company is in effect utilizing “indirect photosynthesis.”
The Company is involved in a highly competitive industry that is characterized by the risks of changing technologies, market conditions and regulatory requirements. Penetration into markets requires investment of considerable resources and continuous development efforts. The Company’s future success depends upon several factors, including the technological quality, price, and performance of its products and services relative to those of its competitors, scaling up of production for commercial sale, ability to secure adequate project financing at appropriate terms, and the nature of regulation in its target markets.
Liquidity—The Company has incurred substantial net losses since its inception; the Company incurred net losses of $141.4 million, $162.1 million and $116.4 million during the years ended December 31, 2015, 2014 and 2013, respectively. Accumulated deficit was $609.9 million as of December 31, 2015. Net cash used in operating activities was $85.1 million, $118.1 million and $74.8 million during the years ended December 31, 2015, 2014 and 2013, respectively. Cash and cash equivalents and marketable securities available for sale were $98.0 million as of December 31, 2015.
The Company is an emerging growth company with a limited operating history. The Company only recently began commercializing its products. To date, a substantial portion of revenues has consisted of funding from third party collaborative research agreements and government grants. The Company has generated limited revenues from commercial sales, principally derived from sales of personal care products. A significant portion of future revenues are expected to come from commercial sales in the food and nutrition ingredients and specialty personal care products.
Net losses may continue as the Company ramps up manufacturing capacity and builds out its product pipeline. The Company expects to incur additional costs and expenses related to the continued development and expansion of its business, including research and development, the operation of its commercial production facility in Peoria, Illinois ("Peoria Facility"), the ramp up and operation of the commercial production facility in Brazil ("Solazyme Bunge JV") through its joint venture with Bunge Global Innovation, LLC (together with its affiliates, "Bunge") and other commercial facilities.
The Company, along with its development and commercialization partners, needs to develop products successfully, cost effectively produce them in large quantities and market and sell such products profitably. The Company’s failure to generate sufficient revenues, achieve planned gross margins, control operating costs or raise sufficient additional funds may require it to modify, delay or abandon its planned operations, which could have a material adverse effect on the business, operating results, financial condition and ability to achieve intended business objectives. The Company may be required to seek additional funds through collaborations, public or private debt or equity financings or government programs, and may also seek to reduce expenses related to the Company’s operations. There can be no assurance that any financing will be available or on acceptable terms.
Based on current cash utilization, management believes that its cash and cash flows from operations will be sufficient to fund operations through March 31, 2017.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND RECENT ACCOUNTING PRONOUNCEMENTS
68
Basis of Presentation—The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and include all adjustments necessary for the fair presentation of the Company’s consolidated financial position, results of operations and cash flows for the periods presented. The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, Solazyme Brazil Renewable Oils and Bioproducts Limitada (“Solazyme Brazil”) and Solazyme Manufacturing 1, L.L.C., the latter of which owns the Company's facility located in Peoria, Illinois ("Peoria Facility"). All intercompany accounts and transactions have been eliminated in consolidation.
The Company entered into a joint venture agreement ("Joint Venture Agreement") with Bunge, which is a variable interest entity ("VIE") that is 50.1% owned by the Company and 49.9% owned by Bunge. The Company determined that it was not required to consolidate the 50.1% ownership in this joint venture and, therefore, accounts for this joint venture under the equity method of accounting (see Note 8).
Use of Estimates—Financial statements prepared in conformity with GAAP require management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ materially from those estimates.
Foreign Currency Translation—The assets and liabilities of the Company’s foreign subsidiary, Solazyme Brazil, and the Solazyme Bunge JV, where the local currency is the functional currency, are translated from their respective functional currency into U.S. dollars at the exchange rate in effect at the balance sheet date, with resulting foreign currency translation adjustments recorded in accumulated other comprehensive income (loss) in the consolidated statements of comprehensive loss. The Company also adjusts its investment in the Solazyme Bunge JV and cumulative translation adjustment in equity for its ownership portion of the cumulative translation gain or loss recognized on the Solazyme Bunge JV's financial statements. Revenues and expense amounts are translated at average rates during the period.
Cash Equivalents—All highly liquid investments with original or remaining maturities of three months or less at the time of purchase are classified as cash equivalents. Cash equivalents primarily consist of money market funds, commercial paper and U.S. treasury notes.
Marketable Securities—Investments with original maturities greater than three months at the time of purchase and maturing less than one year from the consolidated balance sheet date are classified as marketable securities. The Company classifies marketable securities as short-term based upon whether such assets are reasonably expected to be used in current operations. The Company invests its excess cash balances primarily in corporate bonds, United States Government and Agency securities, asset-backed securities, mortgage-backed securities, commercial paper, municipal bonds, certificates of deposit and floating rate notes. The Company classifies its marketable securities as available-for-sale, and records them at estimated fair value in the consolidated balance sheets, with unrealized gains and losses, if any, reported as a component of accumulated other comprehensive income (loss) in the consolidated statements of comprehensive loss. Marketable securities classified as available-for-sale are adjusted for amortization of premiums and accretion of discounts and such amortization and accretion are reported as components of interest and other income. Realized gains and losses and declines in value that are considered to be other-than-temporary are recognized in interest and other income. The cost of all securities sold is based on the specific identification method.
Restricted Certificates of Deposit—The Company maintained certificates of deposits classified in other long-term assets of $0.7 million and $1.0 million as of December 31, 2015 and 2014, respectively. These certificates of deposits were required to be pledged as collateral related to the Company’s facility lease in South San Francisco.
Deferred Financing Costs—Issuance fees or direct costs relating to its credit facilities are deferred and amortized to interest expense over the contractual or expected term of the related debt using the effective interest method. The Company classifies deferred financing costs in other long-term assets.
Debt Discounts—Debt discounts incurred with the issuance of the Company’s debt are recorded in the consolidated balance sheets as a reduction to associated debt balances. The Company amortizes debt discount to interest expense over the contractual or expected term of the debt using the effective interest method.
Accounts Receivable—Accounts receivable represents amounts owed to the Company for product revenues, collaborative research and development agreements and agreements with related parties. The Company had no amounts reserved for doubtful accounts as of December 31, 2015 and 2014, as the Company expected full collection of its accounts receivable balances. The Company reserves for estimated product returns as reductions of accounts receivable and product
69
revenues. As of December 31, 2015 and 2014, the reserve for product returns was $1.8 million and $1.6 million, respectively. The Company monitors actual return history and reassesses its return reserve as return experience develops. Related party receivables were $12,000 and $0.4 million as of December 31, 2015 and 2014, respectively.
Unbilled Revenues—Unbilled revenues represent fees earned but not yet billed under certain research and development programs including agreements with related parties.
Fair Value of Financial Instruments—The Company measures certain financial assets and liabilities at fair value based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. Where available, fair value is based on, or derived from, observable market prices or other observable inputs. Where observable prices or inputs are not available, valuation models are applied. These valuation techniques involve some level of management estimation and judgment, the degree of which is dependent on the price transparency for the instruments or market and the instruments’ complexity. While the Company believes that its valuation methods are appropriate and consistent with other market participants, it recognizes that the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different estimate of fair value at the reporting date.
The carrying amount of certain of the Company’s financial instruments, including cash and cash equivalents, restricted certificates of deposit, accounts receivable, unbilled revenues, prepaid expenses, accounts payable and accrued liabilities, approximates fair value due to their relatively short maturities. The fair value of the Company’s debt obligations, warrant liability and derivative financial instruments were determined using unobservable inputs (Level 3 inputs), as defined in FASB ASC 820, Fair Value Measurement (see Note 5).
Derivative Financial Instruments—FASB ASC 815, Derivatives and Hedging ("ASC 815"), establishes accounting and reporting standards for derivative instruments. The accounting standards require companies to bifurcate conversion options from their host instruments and account for them as free standing derivative financial instruments according to certain criteria. The fair value of the derivative is remeasured to fair value at each balance sheet date, with a resulting non-cash gain or loss related to the change in the fair value of the derivative being charged to earnings (loss). The Company has determined that it must bifurcate and account for the early conversion payment features in its 6.00% convertible senior subordinated notes due 2018 (“2018 Notes”) and its 5.00% convertible senior subordinated notes due 2019 ("2019 Notes" and, collectively with the 2018 Notes, the "Notes") as embedded derivatives in accordance with ASC 815 (see Note 5 and Note 11). The Company recorded these embedded derivative liabilities as non-current liabilities on its consolidated balance sheets with a corresponding debt discount at the date of issuance that is netted against the principal amount of the Notes. The Company estimates the fair value of these liabilities using a Monte Carlo simulation model.
Concentration of Credit Risk—Financial instruments that potentially subject the Company to a concentration of credit risk consist of cash and cash equivalents, marketable securities, accounts receivables and restricted certificates of deposit. The Company places its cash equivalents and investments with high credit quality financial institutions and, by policy, limits the amounts invested with any one financial institution or issuer. Deposits held with banks may exceed the amount of insurance provided on such deposits. The Company has not experienced any losses on its deposits of cash and cash equivalents.
The Company had 17 customers accounting for 92% of the receivable balance as of December 31, 2015. The Company had 12 customers accounting for 95% of the receivable balance as of December 31, 2014. The Company does not believe the accounts receivable from these customers represent a significant credit risk based on past collection experiences and the general creditworthiness of these customers. In 2015, three customers accounted for 20%, 14% and 13% of total net revenues. In 2014, three customers accounted for 27%, 16%, and 13% of total net revenues. In 2013, four customers accounted for 22%, 21%, 20% and 15% of total net revenues.
Inventories—Inventories are stated at the lower of cost or market. Cost is determined using the first-in, first-out basis. Inventory cost consists of raw materials, third-party contractor costs associated with packaging, distribution and production of products, supplies, shipping costs and other overhead costs associated with manufacturing. If inventory costs exceed expected market value due to obsolescence or lack of demand, inventory write-downs may be recorded as deemed necessary by management for the difference between the cost and the market value in the period that impairment is first recognized. Beginning in 2014, inventories also include manufacturing and third-party contract costs associated with the production of the Company's ingredient products that met applicable regulatory requirements. Prior to products' meeting any applicable regulatory requirements, and during scale-up of the manufacturing process to nameplate capacity, a portion of the manufacturing and associated production costs are charged to research and development expenses.
70
Property, Plant and Equipment—Property, plant and equipment are recorded at cost, less accumulated depreciation. Depreciation is calculated on a straight-line basis over the following estimated ranges of useful lives:
Asset classification | Estimated useful life | |
Plant equipment | 5 – 20 years | |
Lab equipment | 3 – 7 years | |
Leasehold improvements | Shorter of useful life or life of lease | |
Building and improvements | 7 – 20 years | |
Computer equipment and software | 3 – 7 years | |
Furniture and fixtures and automobiles | 5 – 7 years | |
Long-Lived Assets—The Company periodically reviews long-lived assets, including property, plant and equipment, for impairment whenever events or changes in business circumstances indicate that the carrying amount of an asset is impaired or the estimated useful lives are no longer appropriate. If indicators of impairment exist and the undiscounted projected cash flows associated with such assets are less than the carrying amount of the asset, an impairment loss is recorded to write the asset down to its estimated fair value. Fair value is estimated based on discounted future cash flows. See Note 3 for information on impairment charges recognized in years ended December 31, 2015 and 2014. There were no asset impairment charges incurred for the year ended December 31, 2013.
Segment Reporting—The chief operating decision maker is the Chief Executive Officer of the Company.
The Company derives revenue from two principal activities: commercial product sales and collaborative research and development programs with strategic and government entities. The Company’s commercial product sales are focused on Algenist® products and high-value oils, encapsulated oils and whole algae powdered products to companies that use them as ingredients.
The Company has two operating segments for financial statement reporting purposes: Algenist® and Ingredients & Other (previously called Intermediates/Ingredients & Other). The Company’s chief operating decision maker reviews and monitors gross margin by segment, however, the Company does not allocate its operating expenses between its different segments and its collaborative research and development programs, and therefore the chief operating decision maker does not evaluate financial performance beyond product gross margin.
The following table reflects revenues and gross margins for the Company's operating segments for the years ended December 31, 2015, 2014 and 2013, reconciled to the Company’s total product revenue and cost of product revenue as shown in its consolidated statements of operations (in thousands):
Year ended December 31, 2015 | Algenist® | Ingredients & Other | Total | ||||||||
Product revenue | $ | 23,278 | $ | 10,022 | $ | 33,300 | |||||
Cost of product revenue | 7,616 | 10,563 | 18,179 | ||||||||
Segment gross margin | $ | 15,662 | $ | (541 | ) | $ | 15,121 | ||||
Year ended December 31, 2014 | |||||||||||
Product revenue | $ | 24,429 | $ | 12,917 | $ | 37,346 | |||||
Cost of product revenue | 7,746 | 12,866 | 20,612 | ||||||||
Segment gross margin | $ | 16,683 | $ | 51 | $ | 16,734 | |||||
Year ended December 31, 2013 | |||||||||||
Product revenue | $ | 19,856 | $ | 106 | $ | 19,962 | |||||
Cost of product revenue | 6,338 | 47 | 6,385 | ||||||||
Segment gross margin | $ | 13,518 | $ | 59 | $ | 13,577 | |||||
A reconciliation of total gross margin to operating loss is as follows (in thousands):
71
Year ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Gross margin | $ | 15,121 | $ | 16,734 | $ | 13,577 | |||||
Research and development programs revenue | 12,831 | 23,045 | 19,788 | ||||||||
Research and development expense | 48,094 | 81,680 | 66,572 | ||||||||
Sales, general and administrative expense | 80,733 | 90,266 | 62,933 | ||||||||
Restructuring charges | 4,953 | 3,514 | — | ||||||||
Loss from operations | $ | (105,828 | ) | $ | (135,681 | ) | $ | (96,140 | ) | ||
The Company does not allocate any assets to its operating segments.
Geographic Data
Geographic revenues are identified by the location in which the research and development program revenue and product sales were originated. Total revenues of $45.7 million, $56.3 million, and $38.1 million for the years ended December 31, 2015, 2014 and 2013, respectively, originated in the United States. Total revenues of $0.4 million, $4.1 million and $1.7 million for the years ended December 31, 2015, 2014 and 2013, respectively, originated in Brazil. Long-lived assets, net of accumulated depreciation, located in the United States were $26.3 million and $35.3 million as of December 31, 2015 and 2014, respectively. Long-lived assets, net of accumulated depreciation, located in Brazil were $23,000 and $0.8 million as of December 31, 2015 and 2014, respectively.
Revenue Recognition—Revenues are recognized when the following criteria are met: (1) persuasive evidence of an arrangement exists; (2)transfer of title has been completed or services have been rendered; (3)the fee is fixed or determinable; and (4) collectability is reasonably assured. The Company’s primary sources of revenues are revenues from research and development programs and product sales. If sales arrangements contain multiple elements, the Company evaluates whether the components of each arrangement represent separate units of accounting. To date, the Company has determined that all revenue arrangements should be accounted for as a single unit of accounting.
Product Revenue—Product revenue is recognized from the sale of the Company's personal care products, which includes its Algenist® skin care line and ingredient products including its Tailored™ oil products and blended fuel sales, the latter of which is part of the Company's fuels marketing and development programs.
Research and development program revenues
• | Collaborative Research and Development—Collaborative research and development programs with commercial and strategic partners typically provide the Company with multiple revenue streams, which may include up-front non-refundable fees for licensing and reimbursement for research and development activities; cost reimbursement fees may include reimbursement for full-time employee equivalents (“FTE”), contingent milestone payments upon achievement of contractual criteria, licensing fees and commercialization royalty fees. Such revenues are recognized as the services are performed over a performance period, as specified in the respective agreements. When up-front payments are combined with funded research services in a single unit of accounting, the Company recognizes the payments using the proportional performance method of revenue recognition based upon the actual amount of research and development labor hours and research expenses incurred relative to the amount of the total expected labor hours and research expenses estimated to be incurred, but not greater than the amount of the research and development program fee as specified under such agreements. The Company is required to make estimates of total labor hours and research and development expenses required to perform the Company’s obligations under each research and development program; the Company evaluates the appropriate period based on research progress attained and reevaluates the period when significant changes occur. Where arrangements include milestones that are determined to be substantive and at risk at the inception of the arrangement, revenues are recognized upon achievement of the milestone and are limited to those amounts for which collectability is reasonably assured. If these conditions are not met, the milestone payments are deferred and recognized as revenue over the estimated period of performance under the contract as completion of performance obligations occur. |
• | Government Programs—Revenues from research and development programs with governmental entities generally provide cost reimbursement for certain types of expenditures in return for research and |
72
development activities over a contractually defined period. Revenues from government programs are recognized in the period during which the related costs are incurred, provided that the conditions under which the government program activities were provided have been met and only perfunctory obligations are outstanding.
Research and Development—Research and development costs associated with research performed pursuant to research and development programs with government entities and commercial and strategic partners (“partners”) and the Company’s internal projects are expensed as incurred, and include, but are not limited to, personnel and related expenses, facility costs and overhead, depreciation and amortization of plant, property and equipment used in development, laboratory supplies, and scale-up research manufacturing and consulting costs.
Restructuring Charges—The Company accounts for restructuring activities in accordance with FASB ASC 420, Exit or Disposal Cost Obligations. Under the guidance, for the cost of restructuring activities that do not constitute a discontinued operation, the liability for the current fair value of expected future costs associated with such restructuring activity are recognized in the period in which the liability is incurred.
Advertising Costs—Advertising costs are expensed as incurred. Advertising expense was $1.7 million, $2.4 million, and $3.1 million for 2015, 2014 and 2013, respectively.
Patent Costs—All costs related to filing and pursuing patent applications are expensed as incurred as recoverability of such expenditures is uncertain and the underlying technologies are under development. Patent-related legal costs incurred are recorded in selling, general and administrative expenses.
Income Taxes—The Company accounts for income taxes under the asset and liability method, which requires, among other things, that deferred income taxes be provided for temporary differences between the tax basis of the Company’s assets and liabilities and the financial statement reported amounts. A valuation allowance is provided against deferred tax assets when it is more likely than not that they will not be realized.
The Company provides for reserves necessary for uncertain tax positions taken or expected to be taken on tax filings. First, the Company determines if the weight of available evidence indicates that a tax position is more likely than not to be sustained upon audit. Second, based on the largest amount of benefit that is more likely than not to be realized on ultimate settlement, the Company recognizes any such differences as a liability. Because the Company’s unrecognized tax benefits offset deferred tax assets for which the Company has not realized benefit in the financial statements, none of the unrecognized tax benefits through December 31, 2015, if recognized, would affect the Company’s effective tax rate.
Stock-Based Compensation—The Company recognizes stock-based compensation for awards to employees based on the estimated fair value of the awards granted. The Company uses the Black-Scholes option-pricing model to estimate the fair value of awards granted to employees, and the requisite fair value is recognized as expense on a straight-line basis over the service period of the award.
The Black-Scholes option pricing model requires the following inputs: expected life, expected volatility, risk-free interest rate, expected dividend yield rate, exercise price and closing price of the Company’s common stock on the date of grant. Due to the Company’s limited history of grant activity, the Company calculates its expected term utilizing the “simplified method” permitted by the Securities and Exchange Commission (“SEC”), which is the average of the total contractual term of the option and its vesting period. The Company believes historical volatility data of the Company's share price provides the best estimate for the expected volatility of the underlying share price that marketplace participants would most likely use in determining an exchange price for an option. As such, beginning in the second quarter of 2014 the Company began weighting the historical volatility data of its share price in proportion to the number of years of information it has available to the total expected term of stock options. The remaining volatility weight is allocated evenly among selected comparable public companies within its industry. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for zero coupon U.S. Treasury notes with maturities similar to the option’s expected term. The expected dividend yield was assumed to be zero, as the Company has not paid, nor does it anticipate paying, cash dividends on shares of its common stock. The Company estimates its forfeiture rate based on an analysis of its actual forfeitures and will continue to evaluate the appropriateness of the forfeiture rate based on actual forfeiture experience, analysis of employee turnover and other factors.
The Company accounts for restricted stock units and restricted stock awards issued to employees based on the quoted market price of the Company’s common stock on the date of grant that are expensed on a straight-line basis over the service period.
73
The Company uses the Black-Scholes option-pricing model to estimate the fair value of awards granted to nonemployees. The Company accounts for restricted stock units and restricted stock awards issued to nonemployees based on the estimated fair value of the Company’s common stock on the date of grant. The measurement of stock-based compensation for nonemployees is subject to periodic adjustments as the underlying equity instruments vest, and the resulting change in value, if any, is recognized in the Company’s consolidated statements of operations during the period the related services are rendered.
Accumulated Other Comprehensive Loss—The components of accumulated other comprehensive income loss consisted of the following (in thousands):
Foreign Currency Translation Adjustments | Change in unrealized gain/loss on available-for-sale securities | Total Accumulated Other Comprehensive Loss | |||||||||
Balance at December 31, 2012 | $ | (725 | ) | $ | 326 | $ | (399 | ) | |||
Current period other comprehensive loss | (3,155 | ) | (240 | ) | (3,395 | ) | |||||
Balance at December 31, 2013 | (3,880 | ) | 86 | (3,794 | ) | ||||||
Current period other comprehensive loss | (6,908 | ) | (312 | ) | (7,220 | ) | |||||
Balance at December 31, 2014 | (10,788 | ) | (226 | ) | (11,014 | ) | |||||
Current period other comprehensive loss | (11,545 | ) | 228 | (11,317 | ) | ||||||
Balance at December 31, 2015 | $ | (22,333 | ) | $ | 2 | $ | (22,331 | ) | |||
Net Loss per Share—Basic net loss per share is computed by dividing the Company’s net loss by the weighted-average number of common shares outstanding during the period. Diluted net loss per share is computed by giving effect to all potentially dilutive securities, including stock options, common stock issuable pursuant to the 2011 Employee Stock Purchase Plan, restricted stock, restricted stock units and common stock warrants. Basic and diluted net loss per share was the same for all periods presented as the inclusion of all potentially dilutive securities outstanding was anti-dilutive.
The following table summarizes the Company’s calculation of basic and diluted net loss per share (in thousands, except share and per share amounts):
Year ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Numerator | |||||||||||
Net loss | $ | (141,447 | ) | $ | (162,141 | ) | $ | (116,389 | ) | ||
Denominator | |||||||||||
Weighted-average number of common shares used in net loss per share calculation | 80,165,402 | 75,879,721 | 64,228,387 | ||||||||
Less: Weighted-average shares subject to repurchase | — | (513 | ) | (16,429 | ) | ||||||
Denominator: basic and diluted | 80,165,402 | 75,879,208 | 64,211,958 | ||||||||
Net loss per share, basic and diluted | $ | (1.76 | ) | $ | (2.14 | ) | $ | (1.81 | ) | ||
The following outstanding shares of potentially dilutive securities were excluded from the calculation of diluted net loss per share for the periods presented as the effect was anti-dilutive:
Year ended December 31, | ||||||||
2015 | 2014 | 2013 | ||||||
Options to purchase common stock | 11,900,671 | 13,740,204 | 9,957,367 | |||||
Common stock subject to repurchase | — | — | 2,942 | |||||
Restricted stock units | 1,883,552 | 1,812,332 | 1,871,907 | |||||
Warrants to purchase common stock | 750,000 | 1,250,000 | 1,385,000 | |||||
Shares of common stock to be issued upon conversion of the Notes | 18,790,996 | 18,790,996 | 9,905,521 | |||||
Total | 33,325,219 | 35,593,532 | 23,122,737 | |||||
74
The table above does not reflect early conversion payment features of the Notes (see Notes 5 and 11) that may be settled, at the Company’s election, in cash or, subject to satisfaction of certain conditions, in shares of the Company’s common stock.
Recent Accounting Pronouncements—In May 2014, the FASB issued Accounting Standards Update ("ASU") 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASU 2014-09”), which supersedes the revenue recognition requirements in FASB ASC 605, Revenue Recognition. ASU 2014-09 is based on the principle that revenue is recognized to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. ASU 2014-09 also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to obtain or fulfill a contract. This new guidance is effective for interim and annual reporting periods beginning after December 15, 2017, and early adoption is permitted, but not before December 15, 2016. The Company is currently assessing the potential impact of this new guidance on its consolidated financial statements.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements - Going Concern (Subtopic 205-40); Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern, which requires management of a company to evaluate whether there is substantial doubt about the Company’s ability to continue as a going concern. This standard will be effective for the Company beginning in the first quarter of 2017. The Company is currently assessing the potential impact of this new guidance on its consolidated financial statements.
In April 2015, the FASB issued ASU 2015-03, Interest - Imputation of Interest (Subtopic 835-30), which requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. This standard requires retrospective adoption and will be effective for the Company beginning in its first quarter of 2016. In August 2015, the FASB issued ASU 2015-15, Interest - Imputation of Interest (Subtopic 835-30): Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements - Amendments to SEC Paragraphs Pursuant to Staff Announcement at 18 June 2015 EITF Meeting. ASU 2015-15 incorporates the SEC staff's announcement that clarifies the exclusion of line-of-credit arrangements from the scope of ASU 2015-03. In the first quarter of 2016, the Company will reclassify debt issuance costs from other long-term assets to convertible debt as required under this standard.
In July 2015, the FASB issued ASU 2015-11, Inventory (Topic 330): Simplifying the Measurement of Inventory. Topic 330, Inventory, currently requires an entity to measure inventory at the lower of cost or market, with market value represented by replacement cost, net realizable value or net realizable value less a normal profit margin. The amendments in ASU 2015-11 require an entity to measure inventory at the lower of cost or net realizable value. This standard requires retrospective adoption and will be effective for the Company beginning in its first quarter of 2017. The Company is currently assessing the potential impact of this new guidance on its consolidated financial statements.
In November 2015, the FASB issued ASU 2015-17, Balance Sheet Classification of Deferred Taxes, which requires companies to classify all deferred tax assets and liabilities as noncurrent. In addition, companies will no longer allocate valuation allowances between current and noncurrent deferred tax assets because those allowances also will be classified as noncurrent. This standard applies to the Company beginning in its first quarter of 2017 and the Company has early adopted this standard on a prospective basis effective December 31, 2015. Prior periods were not retrospectively adjusted. The adoption of this standard did not have a significant impact on the Company's consolidated financial statements.
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments-Overall: Recognition and Measurement of Financial Assets and Financial Liabilities. The new standard principally affects accounting standards for equity investments, financial liabilities where the fair value option has been elected, and the presentation and disclosure requirements for financial instruments. Upon the effective date of the new standards, all equity investments in unconsolidated entities, other than those accounted for using the equity method of accounting, will generally be measured at fair value through earnings. There will no longer be an available-for-sale classification and therefore, no changes in fair value will be reported in other comprehensive income for equity securities with readily determinable fair values. The new guidance on the classification and measurement will be effective for public business entities in fiscal years beginning after December 15, 2017, including interim periods within those fiscal years and early adoption is permitted. The Company is in the process of evaluating the impact of the adoption of this new guidance on its consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842) which, for operating leases, requires a lessee to recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments, in its balance sheet. The standard also requires a lessee to recognize a single lease cost, calculated so that the cost of the lease is allocated over the lease term, on a generally straight-line basis. The ASU is effective for public companies for fiscal years beginning after December
75
15, 2018, including interim periods within those fiscal years. Early adoption is permitted. The Company is in the process of evaluating the impact of the adoption of this new guidance on its consolidated financial statements.
3. RESTRUCTURING CHARGES
2014 Restructuring Plan
On December 18, 2014 the Company took steps to decrease operating expenses through a reduction in workforce and other cost-cutting measures (“2014 Restructuring Plan”). These targeted reductions were designed to enable the Company to achieve sustainable cash flow in the future.
A summary of the costs, which were recorded to Restructuring Charges in the consolidated statements of operations during the years ended December 31, 2015 and 2014 associated with the 2014 Restructuring Plan are as follows (in thousands):
Expensed in Year Ended December 31, 2014 | Expensed in Year Ended December 31, 2015 | Total | |||||||||
Employee termination costs | $ | 1,962 | $ | (22 | ) | $ | 1,940 | ||||
Asset impairment | 1,552 | — | 1,552 | ||||||||
Other exit costs | — | 394 | 394 | ||||||||
Total | $ | 3,514 | $ | 372 | $ | 3,886 | |||||
Costs associated with exit or disposal activities are recorded when the liability is incurred. Below is a roll forward of the liabilities recognized on the consolidated balance sheet as of December 31, 2015, related to the 2014 Restructuring Plan (in thousands):
Liability at December 31, 2014 | Additions/Adjustments | Payments | Liability at December 31, 2015 | ||||||||||||
Employee termination costs | $ | 1,348 | $ | (22 | ) | $ | (1,326 | ) | $ | — | |||||
Other exit costs | — | 12 | (12 | ) | — | ||||||||||
Total(1) | $ | 1,348 | $ | (10 | ) | $ | (1,338 | ) | $ | — | |||||
(1) The accrued costs as of December 31, 2014 are recorded in the current portion of the consolidated balance sheets under “Accrued liabilities,” as they were paid in 2015.
2015 Exit Activities
In October 2015, the Company made a strategic decision to terminate its current manufacturing agreements at the ADM Clinton and American Natural Processors ("ANP") Galva facilities to better align the Company's immediate production assets with its operating strategy while minimizing production costs. On October 29, 2015, the Company provided to ADM a notice of termination of the Operating Agreement entered into with ADM in November 2012 related to the production of products at the Clinton Facility (see Note 11). On February 26, 2016, the Operated Agreement and the Strategic Collaboration Agreement with ADM terminated. In December 2015, the Company entered into a termination agreement with ADM ("ADM Termination Agreement"). In connection with this exit activity, the Company made cash payments of approximately $2.4 million and issued $3.1 million in the Company's common stock in December 2015. The Company will also make a combination of payments in cash and the Company's common stock in the amount of $3.4 million during the year ended December 31, 2016 associated with the ADM and ANP termination arrangements.
76
A summary of the costs, which were recorded to Restructuring Charges in the consolidated statements of operations during the year ended December 31, 2015 are as follows (in thousands):
Expensed in Year Ended December 31, 2015 | ||||
Facility closure costs | $ | 495 | ||
Asset impairment | 4,931 | |||
Other exit costs(1) | 6,429 | |||
Reversal of deferred rent liabilities | (7,273 | ) | ||
Total(2) | $ | 4,582 | ||
(1)Other exit costs consists primarily of $3.1 million of the Company's common stock issued to ADM, a $2.5 million payment to be made to ADM in 2016 with a combination of cash and common stock, $0.6 million cash payment to be made to ANP during 2016 and a $0.3 million payment to be made in common stock to the principal of ANP in 2016.
(2)Reflected in the Company's Ingredients & Other reporting segment.
Costs associated with exit or disposal activities are recorded when the liability is incurred. Below is a roll forward of the 2015 Restructuring Plan expensed during the year ended December 31, 2015 to liabilities on the consolidated balance sheet as of December 31, 2015 (in thousands):
2015 Expense | Deductions/Payments | Liability as of December 31, 2015 | ||||||||||
Facility closure costs | $ | 495 | $ | (495 | ) | $ | — | |||||
Asset impairment | 4,931 | (4,931 | ) | — | ||||||||
Other exit costs | 6,429 | (3,029 | ) | 3,400 | ||||||||
Reversal of deferred rent liabilities | (7,273 | ) | 7,273 | — | ||||||||
Total(1) | $ | 4,582 | $ | (1,182 | ) | $ | 3,400 | |||||
(1) The accrued costs as of December 31, 2015 are recorded in the current portion of the consolidated balance sheets under “Accrued liabilities,” as the remaining balance is expected to be paid in 2016.
2016 Restructuring Plan
As part of the Company's continuing strategy to focus its operations on targeted, higher-value product categories, the Company streamlined operations by reducing workforce by approximately 20% in January 2016. As a result, the Company expects to record a restructuring charge of approximately $1.0 million to $1.5 million in the three months ended March 31, 2016.
4. MARKETABLE SECURITIES
Marketable securities classified as available-for-sale consisted of the following (in thousands):
December 31, 2015 | |||||||||||||||
Amortized Cost | Gross Unrealized Gain | Gross Unrealized Loss | Fair Value | ||||||||||||
Corporate bonds | $ | 25,608 | $ | 122 | $ | (73 | ) | $ | 25,657 | ||||||
Asset-backed securities | 12,424 | — | (31 | ) | 12,393 | ||||||||||
Mortgage-backed securities | 4,800 | 2 | (23 | ) | 4,779 | ||||||||||
Government and agency securities | 5,705 | 16 | (9 | ) | 5,712 | ||||||||||
Municipal bonds | 2,470 | — | (2 | ) | 2,468 | ||||||||||
Total | $ | 51,007 | $ | 140 | $ | (138 | ) | $ | 51,009 | ||||||
77
December 31, 2014 | |||||||||||||||
Amortized Cost | Gross Unrealized Gain | Gross Unrealized Loss | Fair Value | ||||||||||||
Corporate bonds | $ | 62,208 | $ | 16 | $ | (134 | ) | $ | 62,090 | ||||||
Asset-backed securities | 49,343 | 5 | (38 | ) | 49,310 | ||||||||||
Mortgage-backed securities | 19,280 | 25 | (114 | ) | 19,191 | ||||||||||
Commercial paper | 18,698 | 2 | — | 18,700 | |||||||||||
Government and agency securities | 11,868 | 14 | (4 | ) | 11,878 | ||||||||||
Municipal bonds | 3,448 | 3 | (1 | ) | 3,450 | ||||||||||
Total | $ | 164,845 | $ | 65 | $ | (291 | ) | $ | 164,619 | ||||||
The following table summarizes the amortized cost and fair value of the Company’s marketable securities, classified by maturity as of December 31, 2015 and 2014 (in thousands):
December 31, 2015 | December 31, 2014 | ||||||||||||||
Amortized Cost | Fair Value | Amortized Cost | Fair Value | ||||||||||||
Marketable securities | |||||||||||||||
Due in 1 year or less | $ | 17,783 | $ | 17,870 | $ | 46,759 | $ | 46,754 | |||||||
Due in 1-2 years | 15,900 | 15,858 | 53,698 | 53,639 | |||||||||||
Due in 2-3 years | 7,959 | 7,934 | 30,558 | 30,505 | |||||||||||
Due in 3-4 years | 2,399 | 2,408 | 11,277 | 11,275 | |||||||||||
Due in 4-9 years | 2,844 | 2,843 | 7,280 | 7,275 | |||||||||||
Due in 9-20 years | 1,397 | 1,394 | 1,257 | 1,266 | |||||||||||
Due in 20-35 years | 2,725 | 2,702 | 14,016 | 13,905 | |||||||||||
$ | 51,007 | $ | 51,009 | $ | 164,845 | $ | 164,619 | ||||||||
Marketable securities classified as available-for-sale are carried at fair value as of December 31, 2015 and 2014. Realized gains and losses from sales and maturities of marketable securities were not significant in the periods presented.
The aggregate fair value of available-for-sale securities with unrealized losses was $44.5 million as of December 31, 2015. Gross unrealized losses on available-for-sale securities were $0.1 million as of December 31, 2015, and the Company believes the gross unrealized losses are temporary. In determining that the decline in fair value of these securities was temporary, the Company considered the length of time each security was in an unrealized loss position and the extent to which the fair value was less than cost. The aggregate fair value and unrealized loss of available-for-sale securities which had been in a continuous loss position for more than 12 months was $1.9 million and $12,000 as of December 31, 2015, respectively. In addition, the Company does not intend to sell these securities and it is more likely than not that the Company will not be required to sell these securities before the recovery of their amortized cost basis.
5. FAIR VALUE OF FINANCIAL INSTRUMENTS
Assets and liabilities recorded at fair value in the consolidated financial statements are categorized based upon the level of judgment associated with the inputs used to measure their fair value. Hierarchical levels that are directly related to the amount of subjectivity associated with the inputs to the valuation of these assets or liabilities are as follows:
• | Level 1—Observable inputs, such as quoted prices in active markets for identical assets or liabilities. |
• | Level 2—Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
• | Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Level 3 assets and liabilities include those whose fair value measurements are determined using pricing models, discounted cash flow methodologies or similar valuation techniques and significant management judgment or estimation. |
78
The following tables present the Company’s financial instruments that were measured at fair value on a recurring basis as of December 31, 2015 and 2014 by level within the fair value hierarchy (in thousands):
December 31, 2015 | |||||||||||||||
Level 1 | Level 2 | Level 3 | Total | ||||||||||||
Financial Assets | |||||||||||||||
Cash equivalents | $ | 3 | $ | 18,900 | $ | — | $ | 18,903 | |||||||
Marketable securities | 3,722 | 47,287 | — | 51,009 | |||||||||||
Total | $ | 3,725 | $ | 66,187 | $ | — | $ | 69,912 | |||||||
Financial Liabilities | |||||||||||||||
Derivative liabilities | $ | — | $ | — | $ | 82 | $ | 82 | |||||||
December 31, 2014 | |||||||||||||||
Level 1 | Level 2 | Level 3 | Total | ||||||||||||
Financial Assets | |||||||||||||||
Cash equivalents | $ | 1,880 | $ | 7,828 | $ | — | $ | 9,708 | |||||||
Marketable securities | 4,897 | 159,722 | — | 164,619 | |||||||||||
Total | $ | 6,777 | $ | 167,550 | $ | — | $ | 174,327 | |||||||
Financial Liabilities | |||||||||||||||
Derivative liabilities | $ | — | $ | — | $ | 83 | $ | 83 | |||||||
Other than assets impaired as a result of the 2014 and 2015 Restructuring Plans (see Note 3), the Company had no transactions measured at fair value on a nonrecurring basis as of December 31, 2015 and 2014.
Cash Equivalents and Marketable Securities - Cash equivalents and marketable securities classified within Level 2 of the fair value hierarchy are valued based on other observable inputs, including broker or dealer quotations or alternative pricing sources. When quoted prices in active markets for identical assets or liabilities are not available, the Company relies on non-binding quotes, which are based on proprietary valuation models of independent pricing services. These models generally use inputs such as observable market data, quoted market prices for similar instruments, historical pricing trends of a security as relative to its peers and internal assumptions of the independent pricing services. The Company corroborates the reasonableness of non-binding quotes received from the independent pricing services by comparing them to quotes of identical or similar instruments from other pricing sources.
Derivative Liabilities - The 2018 Notes and the 2019 Notes contain early conversion payment features pursuant to which a holder may convert its Notes into shares of the Company's common stock. With respect to any conversion of 2018 Notes prior to November 1, 2016 or any conversion of 2019 Notes prior to January 1, 2018, in addition to the shares deliverable upon conversion, holders are entitled to receive an early conversion payment equal to $83.33 per $1,000 principal amount of Notes surrendered for conversion that may be settled, at the Company’s election, in cash or in shares of the Company’s common stock. These early conversion payment features have been identified as embedded derivatives and are separated from the host contracts, the Notes, and recorded at fair value each reporting period.
The Company used a Monte Carlo simulation model to estimate the fair values of the embedded derivatives related to the early conversion payment features of the Notes using a "with-and-without method".
The following tables set forth the Level 3 inputs to the Monte Carlo simulation models that were used to determine the fair values of the embedded derivatives for the Notes:
Constant Inputs | 2018 Notes | 2019 Notes | |||||
Conversion rate | 121.1240 | 75.7576 | |||||
Conversion price | $ | 8.26 | $ | 13.20 | |||
Maturity date of the Notes | February 1, 2018 | October 1, 2019 | |||||
Maturity date of early payment feature | November 1, 2016 | January 1, 2018 | |||||
79
Variable Inputs | December 31, 2015 | December 31, 2015 | December 31, 2014 | December 31, 2014 | |||||||||||
2018 Notes | 2019 Notes | 2018 Notes | 2019 Notes | ||||||||||||
Stock price | $ | 2.48 | $ | 2.48 | $ | 2.58 | $ | 2.58 | |||||||
Estimated credit spread | 4,685 basis points | 3,978 basis points | 2,450 basis points | 2,900 basis points | |||||||||||
Estimated stock volatility | 55 | % | 55 | % | 55 | % | 55 | % | |||||||
The following table sets forth the estimated fair values of the embedded derivatives (in thousands):
December 31, 2015 | December 31, 2014 | |||||||
2018 Notes | $ | 26 | $ | 35 | ||||
2019 Notes | $ | 56 | $ | 48 | ||||
The total net decrease in the estimated fair value of the Level 3 embedded derivative for the Notes between December 31, 2014 and December 31, 2015 represents an unrealized gain that has been recorded as a gain from change in fair value of derivative liabilities in the consolidated statements of operations for the year ended December 31, 2015.
As of December 31, 2015 and 2014, the carrying values of the Company’s accounts receivables and secured and unsecured debt obligations, excluding the Notes, approximated their fair values. The Company has estimated the fair value of the Notes to be $105.9 million at December 31, 2015 based upon Level 2 inputs using the market price of the Notes derived from actual trades quoted from Bloomberg, and the fair value of the Notes to be $127.1 million at December 31, 2014 using a midmarket pricing convention (the midpoint price between bid and ask prices) quoted from Bloomberg.
6. INVENTORIES
Inventories consisted of the following (in thousands):
December 31, 2015 | December 31, 2014 | ||||||
Raw materials | $ | 1,837 | $ | 1,555 | |||
Work in process | 6,621 | 8,544 | |||||
Finished goods | 3,560 | 5,235 | |||||
Total inventories | $ | 12,018 | $ | 15,334 | |||
7. PROPERTY, PLANT AND EQUIPMENT—NET
Property, plant and equipment—net consisted of the following (in thousands):
December 31, 2015 | December 31, 2014 | ||||||
Plant equipment | $ | 24,824 | $ | 30,213 | |||
Building and improvements | 5,810 | 5,807 | |||||
Lab equipment | 7,495 | 7,904 | |||||
Leasehold improvements | 1,876 | 1,935 | |||||
Computer equipment and software | 4,159 | 3,936 | |||||
Furniture and fixtures | 669 | 638 | |||||
Land | 430 | 430 | |||||
Automobiles | 194 | 194 | |||||
Construction in progress | 342 | 1,926 | |||||
Total | 45,799 | 52,983 | |||||
Less: accumulated depreciation and amortization | (19,455 | ) | (16,903 | ) | |||
Property, plant and equipment—net | $ | 26,344 | $ | 36,080 | |||
80
Depreciation and amortization expense was $5.8 million, $6.3 million and $5.1 million for the years ended December 31, 2015, 2014 and 2013, respectively.
8. INVESTMENT IN SOLAZYME BUNGE JOINT VENTURE
Background and Operations
In April 2012, the Company and Bunge formed the Solazyme Bunge JV to build, own and operate the Solazyme Bunge JV Plant, a commercial-scale renewable algae oils production facility adjacent to Bunge’s Moema sugarcane mill in Brazil, leveraging the Company's technology. The Solazyme Bunge JV is 50.1% owned by the Company and 49.9% owned by Bunge and is governed by a six member board of directors, three from each investor.
Solazyme Bunge JV's operational focus from inception through 2015 was primarily on supporting the construction, ramp up and optimization of the commercial-scale renewable algae oils production facility. Construction of the Solazyme Bunge JV Plant commenced in the second quarter of 2012. By the end of the second quarter of 2014, the Solazyme Bunge JV Plant was operational including large fermenters and formal operations began with production of saleable product. By the second quarter of 2015, the Solazyme Bunge JV Plant completed the construction and activation of the electrical grid tie-in and activation of a second steam boiler which allowed for improvements on up-time and downstream recovery efficiency. While the Solazyme Bunge JV has incurred significant losses to date, the Company believes that the overall long-term expectation of profitability will drive positive cash flows sufficient for the Company to recover its investment in the Solazyme Bunge JV.
In October 2015, the Company and Bunge entered into an amended and restated joint venture agreement to expand the Solazyme Bunge JV to add a worldwide focus on human food and animal nutrition. Also in October 2015, the Company and Bunge entered into an amended and restated Development Agreement under which the Company granted to the Solazyme Bunge JV a worldwide royalty-bearing, field-limited license to all of its technology that is necessary or useful for the manufacture of certain algae oil products. Concurrently with the entry into such agreements, the Company and Solazyme Bunge JV entered into two funded research programs targeted at completing the development of additional products for the Solazyme Bunge JV; pursuant to these agreements:
Solazyme Bunge JV will:
• | continue to use the Company's proprietary technology to produce a range of algae-based oils and products from cane sugar through microbe-based catalysis. |
• | pay the Company a royalty for certain products sold by the joint venture. |
• | pay the Company a technology maintenance fee in recognition of the Company's ongoing research investment in technology. |
The Company will:
• | provide sales, marketing and application development for certain oils and technical expertise in regard to the implementation of its technology. |
• | provide access to the Company's proprietary technology for the production of certain oils and structuring fats for the food and animal nutrition markets. |
• | retain co-primary sales rights for certain products. |
Bunge will:
• | continue to provide cane sugar feedstock and utilities to the Solazyme Bunge JV Plant from Bunge's adjacent sugar cane processing mill. |
• | provide sales, marketing and application development for certain food oils and will also provide oil processing, global distribution and logistics. |
• | serve as the primary sales channel for some of the joint venture's products, with the Company as an additional sales channel, in each case in exchange for a distribution fee. |
• | continue to provide working capital to the Solazyme Bunge JV through a revolving loan facility. |
81
The Company contributed $22.3 million, $32.6 million and $12.4 million during the years ended December 31, 2015, 2014 and 2013, respectively. The Company also contributed $5.5 million and $15.3 million in the years ended December 31, 2015 and 2014, respectively, to the Solazyme Bunge JV through a reduction in the Company’s receivables due from the Solazyme Bunge JV.
Equity Accounting
The Company accounts for its interest in the Solazyme Bunge JV under the equity method of accounting. The Company's equity investment in the Solazyme Bunge JV was $35.9 million and $40.9 million as of December 31, 2015 and 2014, respectively. During the years ended December 31, 2015, 2014 and 2013, the Company recognized $22.4 million, $23.0 million and $6.8 million of losses, respectively, related to its equity method investment in the Solazyme Bunge JV.
The Company has determined that the Solazyme Bunge JV is a VIE based on the insufficiency of each party’s equity investment at risk to absorb losses and the Company’s share of the respective expected losses of the Solazyme Bunge JV. The optimization and ramping up of the Solazyme Bunge JV Plant is the activity of the Solazyme Bunge JV that most significantly impacts its current economic performance. Although the Company has the obligation to absorb losses and the right to receive benefits of the Solazyme Bunge JV that could potentially be significant to the Solazyme Bunge JV, each of the Company and Bunge has equally shared decision–making powers over certain significant activities of the Solazyme Bunge JV, including those related to the construction, optimization and ramping up of the Solazyme Bunge JV. Therefore, as of December 31, 2015, the Company does not consider itself to be the Solazyme Bunge JV’s primary beneficiary, and as such has not consolidated the financial results of the Solazyme Bunge JV. Consolidation may be required in the future due to changes in events and circumstances impacting the power to direct the activities that most significantly affect the Solazyme Bunge JV’s economic performance. The Company will continue to reassess its potential designation as the primary beneficiary of the Solazyme Bunge JV.
The following table summarizes the carrying amounts of the assets and the fair value of the liabilities included in the Company’s consolidated balance sheets and the maximum loss exposure related to the Company’s interest in the Solazyme Bunge JV as of December 31, 2015 and 2014 (in thousands):
As of December 31, 2015 | |||||||||||||||||||
Assets | Liabilities | ||||||||||||||||||
VIE | Accounts Receivable | Unbilled Revenues | Investments in Unconsolidated Joint Ventures | Loan Guarantee | Maximum Exposure to Loss(1) | ||||||||||||||
Solazyme Bunge JV | $ | 12 | $ | 839 | $ | 35,910 | $ | — | $ | 45,692 | |||||||||
As of December 31, 2014 | |||||||||||||||||||
Assets | Liabilities | ||||||||||||||||||
VIE | Accounts Receivable | Unbilled Revenues | Investments in Unconsolidated Joint Ventures | Loan Guarantee | Maximum Exposure to Loss(2) | ||||||||||||||
Solazyme Bunge JV | $ | 446 | $ | 2,435 | $ | 40,934 | $ | — | $ | 57,618 | |||||||||
(1) | Includes maximum exposure to loss attributable to the Company’s bank guarantee required to be provided for the Solazyme Bunge JV of $8.9 million (based on the exchange rate at December 31, 2015). |
(2) Includes maximum exposure to loss attributable to the Company’s bank guarantee required to be provided for the Solazyme Bunge JV of $13.2 million and non-cancelable purchase obligations of $0.6 million (based on the exchange rate at December 31, 2014).
The Company may be required to contribute additional capital to the VIE which would increase the Company’s maximum exposure to loss. These future contribution amounts cannot be quantified at this time.
Summarized Financial Information
Summarized information on the Solazyme Bunge JV’s balance sheets and income statements as of December 31, 2015, 2014 and 2013 and for the years ended December 31, 2015, 2014 and 2013, respectively, was as follows (in thousands):
82
As of December 31, 2015 | As of December 31, 2014 | ||||||
Current assets | $ | 5,654 | $ | 4,339 | |||
Property, plant and equipment, net | 100,755 | 141,147 | |||||
Recoverable taxes(1) | 16,144 | 20,604 | |||||
Total assets | $ | 122,553 | $ | 166,090 | |||
Current liabilities | $ | 23,009 | $ | 24,881 | |||
Noncurrent liabilities | 43,054 | 78,666 | |||||
JV’s partners’ capital, net | 56,490 | 62,543 | |||||
Total liabilities and partners’ capital, net | $ | 122,553 | $ | 166,090 | |||
(1)Recoverable taxes are comprised of value-added taxes paid upon the acquisition of property, plant and equipment items and other goods and services, and other transactional taxes which can be recovered in cash or as compensation against income taxes or other taxes owed by Solazyme Bunge JV in Brazil. The realization of these recoverable tax payments could take in excess of five years.
Year ended December 31, 2015 | Year ended December 31, 2014 | Year ended December 31, 2013 | |||||||||
Net sales | $ | 3,713 | $ | 805 | $ | — | |||||
Net losses | $ | (42,398 | ) | $ | (46,165 | ) | $ | (16,280 | ) | ||
During the year ended December 31, 2013, the Solazyme Bunge JV entered into a loan agreement with the Brazilian Development Bank ("BNDES" or "BNDES Loan") under which it may borrow up to $62.0 million (based on the exchange rate as of December 31, 2015). Outstanding borrowings were $53.4 million and $91.6 million as of December 31, 2015 and 2014, respectively. The Company has provided a bank guarantee equal to 14.39% of the total amount available under the BNDES Loan and may be required to provide a corporate guarantee equal to 35.71% of the total amount available under the BNDES Loan (with the total amount covered by the guarantees not to exceed the Company’s ownership percentage in the Solazyme Bunge JV). The BNDES funding has supported the construction of the Solazyme Bunge JV’s production facility. The term of the BNDES Loan is eight years and the loan has an average interest rate of approximately 4.0% per annum. As of December 31, 2015, the Company’s bank guarantee was in place and the corporate guarantee was not in place. The fees incurred on the cancelable bank guarantee were not material during the year ended December 31, 2015, 2014 and 2013.
Impairment Assessment
The Company assessed the recoverability of its $35.9 million equity investment in Solazyme Bunge JV as of December 31, 2015 using a discounted cash flow analysis. Based upon such analysis, the Company expects to recover the carrying amount of its equity investment and concluded that its equity investment was not impaired.
The process of evaluating the potential impairment is subjective and requires significant estimates and assumptions. The Company’s estimated future cash flows are based on assumptions that are consistent with its annual planning process and include estimates for revenue and operating margins and future economic and market conditions. Actual future results may differ significantly from those estimates. Changes in assumptions or circumstances could result in an impairment in the period the change occurs and in future years. Management’s conclusion that its equity investment was not impaired as of December 31, 2015 was based upon the following critical estimates and assumptions:
• | No significant adverse change in the regulatory or economic environment in Brazil or other countries, as applicable |
• | No significant difficulties as production increases from minimal capacity to full capacity over the next several years |
• | Sales mix of products currently commercially produced and sold to existing customers as well as certain oil products for food and animal nutrition markets under development and expected to be commercialized in 2016 and 2017 |
• | Average selling prices based on current contracted prices and at or above market prices for comparable products |
• | Additional capital investment to increase plant capacity for new products and process improvements of approximately $50.0 million in total |
• | Increased fermentation and recovery efficiencies over the next 5 years based on strain and process improvements |
• | Reduction to production costs based on ramp up of production volume to an aggregate maximum plant capacity in line with sales volume |
• | Discount rate of approximately 14% |
83
In order for the Solazyme Bunge JV to achieve sufficient cash flows to enable the Company to fully recover its equity investment, the Solazyme Bunge JV must:
• | Increase production volumes by: |
◦ | Optimizing plant throughput |
◦ | Improving lipid and oil content output |
◦ | Increasing final recovery yields |
• | Maintain access to low-cost cane sugar feedstock and power |
• | Commercialize and sell its high value products |
The estimates used for cash flow forecasts required significant exercise of judgment and are subject to change in future reporting periods as facts and circumstances change. Additionally, the Company may make changes to its business plan that could result in changes to the expected cash flows. As a result, it is possible that an equity method investment may be impaired in future reporting periods.
Joint Development and Other Agreements
The Company has three agreements with the Solazyme Bunge JV:
• | Development Agreement with the Solazyme Bunge JV to continue to conduct research and development activities that are intended to benefit the Solazyme Bunge JV, including activities in the areas of strain development, molecular biology and process development |
• | Technology Service Agreement with the Solazyme Bunge JV for technical services related to the operations of the production facility |
• | Commercial support services agreement |
These agreements with Solazyme Bunge JV require the Solazyme Bunge JV to pay the Company for such research activities and support services. The Company accounts for these Agreements as an obligation to perform research and development services for others in accordance with FASB ASC 730-20, Research and Development Arrangements, and recorded the payments for the performance of these services as revenue in its consolidated statement of operations. The Company recognized revenue on these Agreements based on proportionate performance of actual efforts to date relative to the amount of expected effort incurred. The cumulative amount of revenue recognized under the Agreements were limited by the amounts the Company was contractually obligated to receive as cash reimbursements.
In October 2015, the Company and Solazyme Bunge JV entered into an amended and restated joint development agreement (“JDA”), and project plans thereunder, incorporating development work from a previous JDA with Bunge associated with the development of a unique food ingredient, as well as new development work on nutrition products intended to benefit the Solazyme Bunge JV. This JDA provides that the Solazyme Bunge JV will provide research funding to the Company covering periods through December 2018, payable quarterly in advance throughout the research term.
Research and development program revenues include $6.3 million, $13.6 million and $8.1 million from the Solazyme Bunge JV for the years ended December 31, 2015, 2014 and 2013, respectively. Product sales include $1.6 million and $2.9 million from the Solazyme Bunge JV during the years ended December 31, 2015 and 2014, respectively, and none during the year ended December 31, 2013. The Company had receivables of $12,000 and $0.4 million due from the Solazyme Bunge JV as of December 31, 2015 and 2014, respectively. Unbilled revenues from the Solazyme Bunge JV was $0.8 million and $2.4 million as of December 31, 2015 and 2014, respectively. Deferred revenues from Solazyme Bunge JV were $4.2 million as of December 31, 2015.
Accounting for Bunge Warrant
In 2011, the Company granted Bunge a warrant (the “Bunge Warrant”) to purchase 1,000,000 shares of its common stock at an exercise price of $13.50 per share. The Bunge Warrant was to vest (i) 25% on the date that Solazyme and Bunge entered into a joint venture agreement to construct and operate a commercial-scale renewable oil production facility; (ii) 50% upon the commencement of construction of the production facility; and (iii) 25% on the date upon which the aggregate output of triglyceride oil reached certain levels.
84
The Company accounted for the Bunge Warrant pursuant to FASB ASC 505-50, Equity-Based Payments to Non-Employees, which establishes that share-based payment transactions with nonemployees shall be measured at the fair value of the consideration received or the fair value of the equity instruments issued (whichever is more reliably measurable).
In 2012, the Company recorded an investment in the Solazyme Bunge JV of $10.4 million, equal to the fair value of the Bunge Warrant, and recorded a corresponding $7.3 million of additional paid-in capital for the first two vested tranches of the Bunge Warrant. The fair value of the Bunge Warrant was determined using the Black-Scholes option pricing model. As of December 31, 2015 and 2014, 750,000 of the Bunge Warrant shares had vested, and the remaining 250,000 shares under the third tranche could no longer vest; accordingly, no warrant liability associated with the Bunge Warrant shares was recorded as of December 31, 2015 and 2014. The Company recorded a net unrealized gain related to the change in the fair value of the warrant liability of $0.7 million and $0.1 million during the years ended December 31, 2014 and 2013, respectively.
9. ACCRUED LIABILITIES
Accrued liabilities consisted of the following (in thousands):
December 31, 2015 | December 31, 2014 | ||||||
Accrued compensation and related liabilities | $ | 6,270 | $ | 6,956 | |||
Accrued interest | 3,495 | 3,495 | |||||
Accrued restructuring costs | 3,400 | 1,348 | |||||
Other accrued liabilities | 990 | 2,280 | |||||
Total accrued liabilities | $ | 14,155 | $ | 14,079 | |||
10. COLLABORATIVE RESEARCH AND DEVELOPMENT AGREEMENTS, DISTRIBUTION AGREEMENTS AND LICENSES
Unilever—In October 2011, the Company entered into a joint research and development agreement with Unilever (the Company’s fourth agreement with Unilever), which expanded its current research and development efforts. In September 2015, the Company and Unilever extended this joint development agreement through September 30, 2017. In September 2013, the Company and Unilever entered into a commercial supply agreement for at least 10,000 MT of the Company’s algae oil. In May 2014, Unilever announced the initial introduction of the Company's sustainable algae oil into one of its biggest soap brands, Lux. In March 2016, the Company entered into a multi-year global supply agreement with Unilever, which includes a broad portfolio of our algae oils for Unilever to purchase for use in personal care products. Production of these oils will take place at the Solazyme Bunge Renewable Oils facility in Brazil and pricing terms are based upon actual production cost plus a defined contribution margin. The agreement contains certain minimum and maximum sales volumes and is subject to other terms and conditions.
Algenist® Distribution Partners—The Company has distribution contracts with Sephora S.A. to distribute the Algenist® product line in Sephora stores in the U.S. and other countries. The Company is obligated to fund minimum marketing expenditures under the agreement with Sephora EMEA. The Company has also granted a license to Sephora Americas and Sephora EMEA to use the Algenist® trademarks and logos to advertise and promote the product line. In March 2011, the Company entered into an agreement with QVC, Inc. (“QVC”) and launched the sale of its Algenist® product line through QVC’s multimedia platform. In July 2014, the Company entered into an agreement with ULTA Beauty to sell the Algenist® line in its retail stores throughout the United States.
Bunge—In May 2011, the Company entered into a joint development agreement (“JDA”) with Bunge, a global agribusiness and food company, that extended through May 2013. In September 2013, the Company and Bunge agreed to extend the JDA, effective from May 2013 through September 2014. Pursuant to the JDA, the Company and Bunge jointly developed microbe-derived oils and explored the production of such oils from Brazilian sugarcane feedstock. The JDA also provided for Bunge to provide research funding to the Company through September 2014, payable quarterly in advance throughout the research term.
In March 2015, the Company entered into an additional JDA with Bunge to jointly develop a unique food ingredient. Research funding of $2.0 million was paid to the Company through September 30, 2015 related to this JDA. The additional JDA was rolled into a project plan between the Solazyme Bunge JV and the Company in October 2015 and that the Solazyme Bunge JV will provide research funding to the Company covering periods through December 2018, payable quarterly in
85
advance throughout the research term. Unamortized deferred revenue balance related to this JDA agreement with Bunge of $0.7 million as of October 2015 is being recognized over the remaining term of the project plan with Solazyme Bunge JV through December 31, 2018.
ADM—In November 2012, the Company and ADM entered into the Collaboration Agreement, establishing a collaboration for the production of algae triglyceride oil products at the Clinton Facility. In January 2014, the Company began commercial scale production of its products at the Clinton Facility using the Company’s proprietary technology. Under the terms of the Collaboration Agreement, the Company paid ADM annual fees for use and operation of a portion of the Clinton Facility, a portion of which could be paid in Company common stock. In March 2013, the Company issued a series of warrants to ADM for payment in stock, in lieu of cash, at its election, of future annual fees for use and operation of a portion of the Clinton Facility. Downstream processing of products produced at the Clinton Facility is being done at a facility in Galva, Iowa ("Galva Facility") operated by a wholly owned subsidiary of ANP. On October 29, 2015, the Company provided to ADM a notice of termination of the Operating Agreement entered into with ADM in November 2012 related to the production of products at the Clinton Facility. As a result of the termination of the Operating Agreement, the Strategic Collaboration Agreement with ADM automatically terminated on February 26, 2016. In December 2015, the Company entered a termination agreement with ADM. The Company has made net cash payments of approximately $2.4 million in the fourth quarter of 2015 and another $3.4 million in common stock and cash to be paid during the year ended December 31, 2016 associated with the ADM and ANP termination arrangements. See 2015 Exit Plan in Note 3.
In January 2013, the Company granted to ADM a warrant (“ADM Warrant”) to purchase 500,000 shares of the Company’s common stock, which vests in equal monthly installments over five years, commencing in November 2013. In addition, the Company shall grant to ADM a warrant (“ADM Extension Warrant”) covering an additional 500,000 shares of the Company’s common stock upon the extension of the Collaboration Agreement for each further five year term. The measurement date of the ADM Warrant was established in July 2013 when the Company agreed that vesting of the ADM Warrant would commence in November 2013. The Company recognized on a straight-line basis, the fair value of the ADM Warrant to rent expense beginning on the measurement date and over the lease term. As part of the termination of the Operating Agreement and Strategic Collaboration Agreement with ADM in December 2015, the Company also entered into a warrant exchange agreement ("Warrant Exchange") with ADM, as amended on February 17, 2016, whereby ADM agreed to exchange all warrants outstanding from the Company for (1) 1,121,914 shares of the Company's common stock, which is equal to $3.1 million divided by the average daily closing share price of the Company's common stock over the five consecutive trading days ending on the trading day prior to December 28, 2015, (2) $1.25 million paid to ADM in cash no later than February 19, 2016 and (3) a number of shares of the Company's common stock equal to $1.25 million divided by the average daily closing share price of the Company's common stock over five consecutive trading days ending on the trading day prior to April 1, 2016. The Company may choose to pay cash in lieu of all or a portion of the second tranche of shares. All outstanding warrants issued to ADM, including the ADM Warrants, were canceled and no longer exercisable effective on December 28, 2015.
Mitsui—In February 2013, the Company entered into a multi-year agreement with Mitsui & Co., Ltd. (“Mitsui”) to jointly develop a suite of triglyceride oils for use primarily in the oleochemical industry. The Company recognized $2.1 million and $1.5 million of revenue related to substantive milestones achieved under the Mitsui joint development agreement during the years ended December 31, 2015 and 2014, respectively.
86
11. DEBT
A summary of the Company’s debt as of December 31, 2015 and 2014 is as follows (in thousands):
December 31, 2015 | December 31, 2014 | ||||||
Convertible debt: | |||||||
2018 Notes | $ | 61,632 | $ | 61,632 | |||
2019 Notes | 149,500 | 149,500 | |||||
Equipment note | — | 6 | |||||
Total debt | 211,132 | 211,138 | |||||
Add: | |||||||
Fair value of embedded derivative | 82 | 83 | |||||
Less: | |||||||
Unamortized debt discount | (8,749 | ) | (11,124 | ) | |||
Current portion of debt | — | (6 | ) | ||||
Long-term portion of debt | $ | 202,465 | $ | 200,091 | |||
Total interest costs incurred related to the Company’s total debt was $11.2 million, $10.0 million and $6.3 million for the years ended December 31, 2015, 2014 and 2013, respectively. Total interest costs capitalized during the year ended December 31, 2015 and 2014 was zero and $0.6 million, respectively, related to the Company’s investment in the Solazyme Bunge JV, accounted for under the equity method, which had activities in progress necessary to commence its planned principal operations through May 2014. The Company was in compliance with all debt covenants as of December 31, 2015 and 2014.
Convertible Senior Subordinated Notes -2018 Notes—On January 24, 2013 the Company issued $125.0 million aggregate principal amount of 2018 Notes in a private offering to qualified institutional buyers pursuant to Rule 144A under the Securities Act of 1933, as amended. The 2018 Notes bear interest at a fixed rate of 6.00% per year, payable semiannually in arrears on August 1 and February 1 of each year. The 2018 Notes are convertible into the Company’s common stock and may be settled as described below. The 2018 Notes will mature on February 1, 2018, unless earlier repurchased or converted. The Company may not redeem the 2018 Notes prior to maturity.
The 2018 Notes are convertible at the option of the holders at any time prior to the close of business on the scheduled trading day immediately preceding February 1, 2018 into shares of the Company’s common stock at the then-applicable conversion rate. The conversion rate is initially 121.1240 shares of common stock per $1,000 principal amount of 2018 Notes (equivalent to an initial conversion price of approximately $8.26 per share of common stock). With respect to any conversion prior to November 1, 2016 (other than conversions in connection with certain fundamental changes where the Company may be required to increase the conversion rate as described below), in addition to the shares deliverable upon conversion, holders are entitled to receive an early conversion payment equal to $83.33 per $1,000 principal amount of 2018 Notes surrendered for conversion that may be settled, at the Company’s election, in cash or, subject to satisfaction of certain conditions, in shares of the Company’s common stock.
On June 19, 2014, the Company entered into note exchange agreements (the “Exchange Agreements”) with certain holders of the 2018 Notes pursuant to which such holders agreed to exchange approximately $17.5 million in aggregate principal amount of their 2018 Notes, together with accrued interest thereon through the settlement date of the Exchange Agreements, with the Company for 2,409,964 shares of the Company's common stock. The Exchange Agreements settled on June 30, 2014. As the Exchange Agreements were considered induced conversions under the applicable accounting guidance, the Company recognized $1.8 million of debt conversion expense reflected in interest expense in 2014, representing the fair value of the securities transferred in excess of the fair value of the securities issuable upon the original conversion terms of the 2018 Notes. During the years ended December 31, 2014 and 2013, $20.2 million and $43.2 million, respectively, of the 2018 Notes were converted into the Company’s common stock and were reclassified from long-term debt to stockholders’ equity in the consolidated balance sheets. During the years ended December 31, 2014 and 2013, the Company issued 2,743,475 and 5,541,597 shares of its common stock, respectively, to settle both the 2018 Note conversions and early conversion payments, including the settlements under the Exchange Agreements. The Company had $61.6 million aggregate principal amount of 2018 Notes outstanding as of December 31, 2015.
Convertible Senior Subordinated Notes - 2019 Notes—On April 1, 2014, the Company issued $149.5 million aggregate principal amount of 5.00% Convertible Senior Subordinated 2019 Notes in a public offering. The 2019 Notes bear interest at a
87
fixed rate of 5.00% per year, payable semiannually in arrears on April 1 and October 1 of each year. The 2019 Notes are convertible into the Company's common stock and may be settled early as described below. The 2019 Notes will mature on October 1, 2019, unless earlier repurchased or converted. The Company may not redeem the 2019 Notes prior to maturity.
The 2019 Notes are convertible at the option of the holders on any day prior to and including the scheduled trading day prior to October 1, 2019. The 2019 Notes will initially be convertible at a conversion rate of 75.7576 shares of Common Stock per $1,000 principal amount of 2019 Notes (equivalent to an initial conversion price of $13.20 per share of Common Stock), subject to adjustment upon the occurrence of certain events. With respect to any conversion prior to January 1, 2018 (other than conversions in connection with certain fundamental changes where the Company may be required to increase the conversion rate as described below), in addition to the shares deliverable upon conversion, holders are entitled to receive an early conversion payment equal to $83.33 per $1,000 principal amount of 2019 Notes surrendered for conversion that may be settled, at the Company’s election, in cash or shares of the Company's common stock. The Company had $149.5 million aggregate principal amount of 2019 Notes outstanding as of December 31, 2015.
The Company issued the Notes pursuant to indenture agreements by and between the Company and Wells Fargo Bank, National Association, as trustee. The indentures provide for customary events of default, including cross acceleration to certain other indebtedness of the Company and its significant subsidiaries.
If the Company undergoes a fundamental change (as defined), holders may require the Company to repurchase for cash all or part of their Notes at a purchase price equal to100% of the principal amount of the Notes to be repurchased, plus accrued and unpaid interest to, but excluding, the fundamental change repurchase date. In addition, if certain fundamental changes occur, the Company may be required in certain circumstances to increase the conversion rate for any Notes converted in connection with such fundamental changes by a specified number of shares of its common stock.
The Notes are the general unsecured obligations of the Company and will be subordinated in right of payment to any senior debt outstanding. The Notes will be equal or senior in right of payment to any of the Company’s indebtedness other than senior debt. The Notes will effectively rank junior in right of payment to any of the Company’s secured indebtedness to the extent of the value of the assets securing such indebtedness and be structurally junior to all indebtedness and other liabilities of the Company’s subsidiaries, including trade payables.
HSBC Facility—The Company has a loan and security agreement with HSBC Bank, USA, National Association (“HSBC”) that provides for a $35.0 million revolving facility (the “HSBC facility”) for working capital, letters of credit denominated in U.S. dollars or a foreign currency and other general corporate purposes. A portion of the HSBC facility also supports the bank guarantee issued to BNDES in May 2013 (see Note 8). Therefore, approximately $26.1 million of the HSBC facility remained available as of December 31, 2015. The HSBC Facility expires on May 31, 2016, and the Company expects to replace the HSBC Facility to support the BNDES bank guarantee on substantially similar terms.
The HSBC facility is unsecured unless (i) the Company takes action that could cause or permit obligations under the HSBC facility not to constitute Senior Debt (as defined in the indenture), (ii) the Company breaches financial covenants that require the Company and its subsidiaries to maintain cash and unrestricted cash equivalents at all times of not less than $35.0 million plus 110% of the aggregate dollar equivalent amount of outstanding advances and letters of credit under the HSBC facility, or (iii) there is a payment default under the facility or bankruptcy or insolvency events relating to the Company.
Advances under the HSBC facility will bear interest at a variable interest rate based on, at the Company’s option at the time an advance is requested, either (i) the Base Rate (as defined in the HSBC facility) plus the applicable Base Rate Margin (as defined in the HSBC facility), or (ii) the Eurodollar Rate (as defined in the HSBC facility) plus the applicable Eurodollar Rate Margin (as defined in the HSBC facility). The Company pays HSBC a fee of two and one-half percent (2.50%) per annum with respect to letters of credit issued. Upon an event of default, outstanding obligations under the HSBC facility will bear interest at a rate of two percent (2.00%) per annum above the rates described in (i) and (ii) above. If on the maturity date (or earlier termination date of the HSBC facility), there are any outstanding letters of credit, the Company will be required to provide HSBC with cash collateral in the amount of (i) for letters of credit denominated in U.S. dollars, up to one hundred five percent (105%), and (ii) for letters of credit denominated in a foreign currency, up to one hundred ten percent (110%), of the dollar equivalent of the face amount of all such letters of credit plus all interest, fees and costs.
In addition to the financial covenants and covenants related to the indenture referenced above, the Company is subject to customary affirmative and negative covenants and events of default under the HSBC facility including certain restrictions on borrowing. If an event of default occurs and continues, HSBC may declare all outstanding obligations under the HSBC facility immediately due and payable, with all obligations being immediately due and payable without any action by HSBC upon the occurrence of certain events of default or if the Company becomes insolvent.
88
A summary of debt maturity follows (in thousands):
December 31, 2015 | |||
2016 | $ | — | |
2017 | — | ||
2018 | 61,632 | ||
2019 | 149,500 | ||
Total | $ | 211,132 | |
12. COMMITMENTS AND CONTINGENCIES
Operating Lease Agreements
The Company records rent expense under its lease agreements on a straight-line basis. Differences between actual lease payments and rent expense recognized under these leases results in a deferred rent asset or a deferred rent liability at each reporting period.
The Company currently leases 106,000 square feet of office and laboratory space located in two buildings on adjacent properties in South San Francisco (“SSF”), California. The term of the current lease will end in January 2018. This lease agreement includes scheduled rent increases over the lease term, and the Company was required to provide the landlord with letters of credit amounting to $0.7 million, which was recorded to restricted certificates of deposit in the consolidated balance sheets as of December 31, 2015. In addition, the landlord will reimburse the Company up to $1.6 million of improvements to the leased property, subject to certain requirements. This reimbursement is considered a lease incentive and shall be recognized as a deferred lease incentive liability and recognized as a reduction of rent expense by the Company on a straight-line basis over the term of the lease.
Future minimum lease payments under non-cancellable operating leases are as follows as of December 31, 2015 (in thousands):
2016 | $ | 4,400 | |
2017 | 4,527 | ||
2018 | 366 | ||
Total minimum lease payments | $ | 9,293 | |
Rent expense was $10.7 million, $10.5 million and 7.1 million for the years ended December 31, 2015, 2014 and 2013, respectively.
Contractual Obligations—The Company has various manufacturing, research, and other contracts with vendors in the conduct of the normal course of its business. All contracts are terminable with varying provisions regarding termination. If a contract with a specific vendor were to be terminated, the Company would only be obligated for the products or services that the Company had received at the time the termination became effective.
Guarantees and Indemnifications—The Company makes certain indemnities, commitments, and guarantees under which it may be required to make payments in relation to certain transactions. The Company, as permitted under Delaware law and in accordance with its amended and restated certificate of incorporation and amended and restated bylaws, indemnifies its officers and directors for certain events or occurrences, subject to certain limits, while the officer or director is or was serving at the Company’s request in such capacity. The duration of these indemnifications, commitments, and guarantees varies and, in certain cases, is indefinite. The maximum amount of potential future indemnification is unlimited; however, the Company has a director and officer insurance policy that may enable it to recover all or a portion of any future amounts paid. The Company believes the fair value of these indemnification agreements is minimal. The Company has not recorded any liability for these indemnities in the accompanying consolidated balance sheets. However, the Company accrues for losses for any known contingent liability, including those that may arise from indemnification provisions, when future payment is probable. No such losses have been recorded to date.
Legal Matters—The Company may be involved, from time to time, in legal proceedings and claims arising in the ordinary course of its business. Such matters are subject to many uncertainties and outcomes are not predictable with assurance.
89
The Company accrues amounts, to the extent they can be reasonably estimated, that it believes are adequate to address any liabilities related to legal proceedings and other loss contingencies that the Company believes will result in a probable loss that is reasonably estimable.
Securities Class Action Litigation
In June 2015, a securities class action complaint entitled Norfolk County Retirement System v. Solazyme, Inc. et al. was filed against the Company, its CEO, Jonathan Wolfson, its CFO/COO, Tyler Painter, certain of its current and former directors, and the underwriters of its March 2014 equity and debt offerings, Goldman, Sachs & Co., Inc. and Morgan Stanley & Co. LLC, in the U.S. District Court for the Northern District of California. The complaint asserts claims for alleged violations of Sections 11, 12(a)(2), and 15 of the Securities Act of 1934, as well as Sections 10(b) and 20(a) of the Securities Exchange Act of 1934. The complaint seeks unspecified damages on behalf of a purported class that would comprise all individuals who acquired the Company's securities (i) between February 27, 2014 and November 5, 2014 and (ii) pursuant and/or traceable to the Company's public equity and debt offerings in March 2014. The complaint alleges that investors were misled by statements made during that period about the construction progress, development, and production capacity associated with the production facility located in Brazil owned by the Company’s joint venture, Solazyme Bunge Produtos Renovaveis Ltda. In October 2015, the lead plaintiff was selected in the action and an amended complaint was filed on December 15, 2015. The Company filed a Motion to Dismiss the action on February 12, 2016 that it expects to be heard in May 2016. The Company believes the complaint lacks merit, and intends to defend itself vigorously.
Derivative Litigation
In July 2015, a complaint entitled Jim Bertonis, derivatively on behalf of Solazyme, Inc. v. Jonathan Wolfson et al. was filed in the Superior Court of California, County of San Mateo. The complaint seeks unspecified damages, purportedly on behalf of the Company from certain of its current and former directors and officers and alleges these defendants breached their fiduciary duties to the Company and unjustly enriched themselves by making allegedly false and misleading statements and omitting certain material facts in the Company's securities filings and other public disclosures. This purported stockholder derivative action is based on substantially the same facts as the securities class action described above. Based on a review of the plaintiffs’ allegations, the Company believes that the plaintiff has not demonstrated standing to sue on its behalf.
In August 2015, a complaint entitled Gregory M. Miller, derivatively on behalf of Solazyme, Inc. v. Jonathan S. Wolfson et al. was filed in the U.S. District Court for the Northern District of California. The complaint seeks unspecified damages, purportedly on behalf of the Company from certain of its current and former directors and officers and alleges these defendants breached their fiduciary duties to the Company and aided and abetted the Company in making allegedly false and misleading statements and omitting certain material facts in the Company's securities filings and other public disclosures. This purported stockholder derivative action is based on substantially the same facts as the securities class action and derivative action described above. Based on a review of the plaintiffs’ allegations, the Company believes that the plaintiff has not demonstrated standing to sue on its behalf.
Roquette Frères, S.A.
In September 2013, an arbitration (the “Roquette Arbitration”) was initiated with Roquette Frères, S.A. (“Roquette”) in connection with the dissolution of a joint venture between the Company and Roquette known as Solazyme Roquette Nutritionals L.L.C. (“SRN”). The Company sought a declaration that, in accordance with the terms of the joint venture agreement between the parties, the Company should be assigned all improvements made by or on behalf of SRN to the Company’s intellectual property. On February 19, 2015 the arbitration panel released its decision, ordering, inter alia, the assignment to the Company of (i) all SRN patent applications, (ii) all SRN know-how related to high lipid algae flour and high protein algae powder and (iii) all Roquette patent applications filed since November 2010 relating to algae food and food ingredients, as well as methods for making and using them. In addition, the arbitration panel ordered Roquette to pay to the Company, $2.3 million in legal costs and fees. The arbitration award was confirmed by order of the U.S. District Court for the District of Delaware on December 21, 2015. Roquette has appealed the confirmation of the arbitration award to the U.S. Court of Appeals for the Third Circuit. Pending this appeal, the confirmation of the arbitration award has been stayed by order of the U.S. District Court for the District of Delaware by order dated January 12, 2016, in which the Court granted not only a stay but also enjoined Roquette from pursuing any further commercialization of any technology arguably within the ambit of the arbitral decision, including the sale of products. The Company expects the appeal of the confirmation of the arbitration award to be heard later in 2016.
90
In November 2014, Roquette filed an action against the Company in U.S. District Court for the District of Delaware for declaratory judgment related to the Roquette Arbitration. Roquette seeks a declaration that (i) the arbitrators in the Roquette Arbitration exceeded their authority by failing to render a timely arbitration award, (ii) any award issued by the arbitrators is void and (iii) all intangible assets of SRN should be assigned jointly to Roquette and the Company. Other than seeking its attorney fees and costs in the action, Roquette did not make any monetary claims against the Company. The Company filed an Answer to the Complaint in January 2015, denying substantially all of Roquette’s claims and all of its prayers for relief.
In February 2015 Roquette filed a second action against the Company in U.S. District Court for the District of Delaware for declaratory judgment related to the Roquette Arbitration. Roquette sought a declaration that (A) the order of the arbitrators in the Roquette Arbitration for more discovery and new hearings was unenforceable and (B) in the alternative, the proposed new discovery and hearings concerned an issue that was outside the scope of the arbitration. Other than seeking its attorney fees and costs in the action, Roquette did not make any monetary claims against the Company. The two Delaware actions were consolidated in February 2015. The Company filed its Answer to the second Complaint on February 26, 2015, denying all claims made in the Complaint and all related prayers for relief. In addition, the Company cross-claimed for (x) confirmation of the arbitration award, (y) an order compelling Roquette to comply with the arbitration award and (z) damages for misappropriation of the Company’s trade secrets, misuse of the Company’s confidential information and breach of contract. In April 2015 Roquette filed motions for summary judgment in each of the two declaratory judgment actions commenced by Roquette and a motion to vacate the award rendered in the Roquette Arbitration, which included counterclaims alleging the Company misused certain Roquette trade secrets. The summary judgment motions made by Roquette were denied in an opinion of the court dated December 21, 2015. All further proceedings under the declaratory actions have been stayed pending the Third Circuit appeal described above.
Therabotanics, LLC and Solabotanics, LLC
In July 2012, a complaint was filed in the Los Angeles Superior Court by Therabotanics, LLC and Solabotanics, LLC against the Company, Sephora USA, Inc. and the Company's Senior Vice President Frederic Stoeckel alleging various causes of action in connection with the Company’s joint venture with Therabotanics. The Plaintiffs alleged that the Company misappropriated assets of the joint venture and failed to produce an infomercial with the joint venture. The Plaintiffs claimed unspecified damages and injunctive relief. In June 2013, the Company filed an Answer in which it denied each and every allegation made by the Plaintiffs in their Complaint and filed a Cross-Complaint against Therabotanics and certain named individuals. In January 2014, the Plaintiffs filed a Third Amended Complaint in which the Company's Chief Executive Officer, Jonathan Wolfson, and Sephora, S.A. were added as additional defendants. In September 2014 the parties agreed to settle the litigation in exchange for a settlement payment from the Company to Therabotanics of $4.8 million, which was paid in October 2014. During the year ended December 31, 2014, the Company recorded $4.8 million of litigation expense to sales, general and administrative expenses in its consolidated statements of operations. As part of the settlement the Company denied all claims made by the Plaintiffs and all parties granted releases to all other parties. The Company maintains corporate insurance and the insurer reimbursed a portion of both the litigation settlement and external legal fees in the amount of $0.4 million during the year ended December 31, 2014.
The Company may be involved, from time to time, in additional legal proceedings and claims arising in the ordinary course of its business. Such matters are subject to many uncertainties and outcomes are not predictable with assurance. While there can be no assurances as to the ultimate outcome of any legal proceeding or other loss contingencies involving the Company, management does not believe any pending matters individually and in the aggregate will be resolved in a manner that would have a material effect on the Company’s consolidated financial position, results of operations or cash flows.
13. COMMON STOCK
Common Stock—The authorized number of shares of common stock is 150 million. The holder of each share of common stock is entitled to one vote.
On April 1, 2014, the Company issued 5,750,000 shares of its common stock, par value $0.001 per share, at $11.00 per share (the "Common Stock Offering"). The net proceeds from the Common Stock Offering were approximately $59.2 million, after deducting underwriter discounts and commissions and estimated offering expenses payable by the Company.
During the years ended December 31, 2014 and 2013, $22.6 million and $44.2 million, respectively, of the 2018 Notes, accrued interest and early conversion payments, net of related deferred offering costs and debt discounts, were converted into the Company’s common stock and were reclassified from long-term debt, accrued interest and other assets to stockholders’ equity in the consolidated balance sheets. The Company settled the early conversion payments in shares of the Company’s common stock. The Company issued 2,743,475 and 5,541,597 shares of its common stock to settle both the 2018 Note
91
conversions and early conversion payments, including the settlements under the Exchange Agreements, during the years ended December 31, 2014 and 2013, respectively (see Note 11).
14. STOCK-BASED COMPENSATION
Second Amended and Restated 2004 Equity Incentive Plan—The Company’s Second Amended and Restated Equity Incentive Plan (the “2004 EIP”) was adopted by the Board of Directors in February 2008 (termination date of January 4, 2014). Pursuant to the 2004 EIP, the Company may grant options, restricted stock and stock purchase rights to employees, directors, or consultants of the Company. Options granted may be either incentive stock options or nonstatutory stock options. Incentive stock options may be granted to employees (including offices and directors, who are also employees). Nonstatutory stock options may be granted to employees, directors or consultants. In March 2011, the Company’s Board of Directors approved a 2,000,000 increase in the options reserved for issuance under the Company’s 2004 EIP. On May 25, 2011, in conjunction with the Company’s initial public offering, the 2004 EIP terminated so that no further awards may be granted under the 2004 EIP. Although the 2004 EIP terminated, all outstanding awards will continue to be governed by their existing terms.
2011 Equity Incentive Plan—On May 26, 2011, the Company’s 2011 Equity Incentive Plan (the “2011 EIP”, and together with the 2004 EIP (the “Plans”)) became effective. The Company initially reserved 7,000,000 shares of common stock for issuance under the 2011 EIP. Starting on May 26, 2011, any shares subject to outstanding awards granted under the 2004 EIP that expire or terminate for any reason prior to the issuance of shares shall become available for issuance under the 2011 EIP. The 2011 EIP also provides for automatic annual increases in the number of shares reserved for future issuance, and during the year ended December 31, 2015 an additional 3,969,403 shares were reserved under the 2011 EIP as a result of this provision. As of December 31, 2015 there were 8,130,211 shares available for issuance under the 2011 EIP.
Options under the Plans may be granted for periods up to ten years. All options issued to date have had up to a ten year life. The exercise price of incentive and nonstatutory stock options shall not be less than 100% of the fair market value of the shares on the date of grant. The Board of Directors determines the vesting period of stock-based awards. The Company’s stock options generally vest over four years. Restricted stock awards are subject to forfeiture if certain vesting requirements are not met. The Company issues new common stock from authorized shares upon the exercise of stock options.
2011 Employee Stock Purchase Plan—On May 26, 2011, the Company’s 2011 Employee Stock Purchase Plan (the “2011 ESPP”) became effective. The Company initially reserved 750,000 shares of common stock for issuance under the 2011 ESPP. The purchase price of the common stock under the Employee Stock Purchase Plan is 85% of the lower of the fair market value of a share of common stock on the first day of the offering period or the last day of the purchase period. The 2011 ESPP also provides for automatic annual increases in the number of shares reserved for future issuance, and during the year ended December 31, 2015 an additional 793,880 shares were reserved under the 2011 ESPP as a result of this provision. As of December 31, 2015 there were 2,749,083 shares available for issuance under the 2011 ESPP.
The Company recognized stock-based compensation expense related to its 2011 ESPP of $0.1 million, $0.7 million and $0.6 million for the years ended December 31, 2015, 2014 and 2013, respectively.
Stock Options
A summary of the Company’s stock option activity under the Plans and related information is as follows:
Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value(1) | |||||||||
(in thousands) | ||||||||||||
Balance at December 31, 2014 | 13,740,204 | $ | 7.82 | 7.7 | $ | 1,680 | ||||||
Granted | 5,915,112 | $ | 2.67 | |||||||||
Exercised | (157,975 | ) | $ | 1.32 | ||||||||
Forfeited, canceled or expired | (7,596,670 | ) | $ | 9.62 | ||||||||
Balance at December 31, 2015 | 11,900,671 | $ | 4.20 | 7.7 | $ | 999 | ||||||
Options vested and exercisable at December 31, 2015 | 4,260,008 | $ | 6.43 | 6.5 | $ | 945 | ||||||
Options vested and expected to vest as of December 31, 2015 | 10,443,746 | $ | 4.37 | 7.7 | $ | 989 | ||||||
92
(1) | The aggregate intrinsic value represents the value by which the Company’s closing stock price on the last trading day of the year ended December 31, 2015 exceeds the exercise price of the stock multiplied by the number of options outstanding or exercisable, excluding any that have a negative intrinsic value. |
The weighted-average grant date fair value of options granted was $1.68, $3.98 and $5.07 for the years ended December 31, 2015, 2014 and 2013, respectively. The total intrinsic value of options exercised was $251,000, $5.8 million and $7.5 million for the years ended December 31, 2015, 2014 and 2013, respectively.
The total fair value of options vested was $3.8 million, $14.7 million and $12.9 million for the years ended December 31, 2015, 2014 and 2013, respectively.
The following table presents the composition of options outstanding and vested and exercisable as of December 31, 2015:
Options Outstanding | Options Vested and Exercisable | ||||||||||||||||
Range of Exercise Prices | Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term (in years) | Number of Vested and Exercisable Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term (in years) | |||||||||||
$0.044-2.35 | 1,268,956 | $ | 1.73 | 4.2 | 1,180,523 | $ | 1.69 | 3.9 | |||||||||
$2.46-2.54 | 3,108,991 | $ | 2.47 | 8.9 | 691,191 | $ | 2.47 | 8.6 | |||||||||
$2.58-3.60 | 4,965,565 | $ | 2.70 | 8.3 | 186,560 | $ | 3.06 | 9.3 | |||||||||
$6.79-9.72 | 1,173,475 | $ | 8.12 | 6.5 | 990,176 | $ | 8.12 | 6.2 | |||||||||
$10.56-27.03 | 1,383,684 | $ | 12.41 | 7.1 | 1,211,558 | $ | 12.46 | 7.0 | |||||||||
Totals | 11,900,671 | $ | 4.20 | 7.7 | 4,260,008 | $ | 6.43 | 6.5 | |||||||||
Stock-based compensation expense related to stock-based awards granted to employees and nonemployees were allocated to research and development and sales, general and administrative expense as follows (in thousands):
Year ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Research and development | $ | 4,562 | $ | 7,407 | $ | 5,917 | |||||
Sales, general and administrative | 11,122 | 18,142 | 12,736 | ||||||||
Total | $ | 15,684 | $ | 25,549 | $ | 18,653 | |||||
No income tax benefits have been recognized related to stock-based compensation expense for the years ended December 31, 2015, 2014 and 2013.
Employee Stock-Based Compensation—Stock-based compensation expense was $14.7 million, $23.9 million and $17.3 million for the years ended December 31, 2015, 2014 and 2013, respectively, for stock-based awards granted to employees. There was unrecognized stock-based compensation cost of $12.0 million related to nonvested stock options granted to employees as of December 31, 2015, and the Company expects to recognize this cost over a weighted-average period of 1.5 years as of December 31, 2015. The grant date fair value of employee stock-based awards was estimated using the following weighted-average assumptions:
Year ended December 31, | |||||
2015 | 2014 | 2013 | |||
Expected term (in years) | 5.3-6.0 | 5.3-6.0 | 5.1-6.0 | ||
Volatility | 67%-73% | 51%-68% | 60%-62% | ||
Risk-free interest rate | 0.86%-1.85% | 1.58%-2.0% | 0.8%-2.1% | ||
Dividend yield | —% | —% | —% | ||
Nonemployee Stock-Based Compensation—Stock-based compensation expense was $1.0 million, $1.7 million and $1.4 million for the years ended December 31, 2015, 2014 and 2013, respectively, for stock-based awards granted to nonemployees. There was unrecognized stock-based compensation cost of approximately $0.6 million related to nonvested stock options
93
granted to nonemployees as of December 31, 2015, and the Company expects to recognize this cost over a weighted-average period of 0.9 year as of December 31, 2015. The fair value of non-employee stock-based awards was estimated using the following weighted-average assumptions:
Year ended December 31, | |||||
2015 | 2014 | 2013 | |||
Expected term (in years) | 8.2-8.6 | 8.3-8.6 | 7.8-8.9 | ||
Volatility | 65%-67% | 53%-64% | 60%-61% | ||
Risk-free interest rate | 1.83%-2.21% | 2.0%-2.5% | 1.6%-2.8% | ||
Dividend yield | —% | —% | —% | ||
Restricted Stock Units—A summary of the Company's restricted stock unit activity is as follows:
Number of Shares | Weighted Average Grant Date Fair Value | |||||
Unvested at December 31, 2014 | 1,812,332 | $ | 10.91 | |||
Granted | 1,139,531 | $ | 2.82 | |||
Vested | (820,401 | ) | $ | 2.78 | ||
Canceled | (247,910 | ) | $ | 8.66 | ||
Unvested at December 31, 2015 | 1,883,552 | $ | 9.85 | |||
Stock-based compensation expense related to RSUs was $5.6 million, $8.6 million and $5.5 million for the years ended December 31, 2015, 2014 and 2013, respectively.
Stock Option Modification—In October 2014, the Company modified an employee’s stock options which resulted in the Company recording $2.3 million of additional stock-based compensation expense in the year ended December 31, 2014.
Employee Stock Option Exchange Program-In January 2015, the Company commenced an exchange offer to allow employees the opportunity to exchange, on a grant-by-grant basis, their outstanding eligible options that had an exercise price per share equal to or greater than $6.79 for new stock options on a two-for-one basis that the Company granted under its 2011 EIP. Generally, all employees with options were eligible to participate in the program, which expired on February 18, 2015. Non-employee members of Solazyme’s Board of Directors were not eligible to participate. Each new stock option has an exercise price of $2.58, the last reported sale price per share of Solazyme common stock on the NASDAQ Global Select Market on the new stock option grant date, which was February 19, 2015.
Each new stock option has a maximum term that is equal to the remaining term of the corresponding eligible option. Each new stock option has the same final vesting date as the corresponding eligible option. Each new stock option has the same rate of vesting, from the same vesting commencement date, as the corresponding eligible option, provided that any vesting that would have occurred prior to January 1, 2016 cumulates and cliff vests on January 1, 2016. This is the case even if the eligible options were fully vested on the date of the exchange. The optionee must be employed by the Company on January 1, 2016 to benefit from this new option cliff vest.
On February 19, 2015, the Company granted new options to eligible options holders to purchase 2,745,279 shares of common stock in exchange for the cancellation of the tendered options. The Company will record a charge of approximately $0.5 million associated with the stock option modification over the vesting periods of the new options which range from ten months to four years. This modification charge was recorded as additional stock-based compensation expense beginning in the first quarter of 2015.
15. INCOME TAXES
The Company has incurred net operating losses for the years ended December 31, 2015, 2014 and 2013, and therefore has no provision for income taxes recorded for such years. The Company had federal, state and foreign net operating loss
94
carryforwards of $497.2 million, $245.4 million and $10.7 million, respectively, as of December 31, 2015. Federal net operating loss carryforwards expire beginning in 2024 and state net operating loss carryforwards expire beginning in 2016, if not utilized. The Company had federal and state research and development tax credit carryforwards of $4.5 million and $2.7 million, respectively, as of December 31, 2015. The federal research and development tax credit carryforwards will expire starting in 2028 if not utilized. The state research and development tax credit carryforwards can be carried forward indefinitely.
Utilization of the net operating loss and research and development credit carryforwards may be subject to an annual limitation due to the ownership percentage change limitations provided by Section 382 of the Internal Revenue Code and similar state provisions. The annual limitation may result in the expiration of the net operating losses and research and development credit carryforwards before utilization.
The components of loss before income taxes are as follows for the years ended December 31, 2015, 2014 and 2013 (in thousands):
Years Ending December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
United States | $ | (138,424 | ) | $ | (159,370 | ) | $ | (111,791 | ) | ||
Foreign | (3,023 | ) | (2,771 | ) | (4,598 | ) | |||||
Loss before income taxes | $ | (141,447 | ) | $ | (162,141 | ) | $ | (116,389 | ) | ||
Reconciling items from income tax computed at the statutory federal rate for the years ended December 31, 2015, 2014 and 2013, were as follows:
Years Ending December 31, | ||||||||
2015 | 2014 | 2013 | ||||||
Federal income tax statutory rate | 34.0 | % | 34.0 | % | 34.0 | % | ||
State income taxes, net of federal benefits | 2.2 | 1.6 | 4.1 | |||||
Revalued derivative liability | — | 0.6 | (1.8 | ) | ||||
Incentive stock option compensation | (0.4 | ) | (1.3 | ) | (1.6 | ) | ||
Research tax credits | 0.4 | 0.8 | 1.6 | |||||
Other | (0.1 | ) | 0.3 | 1.9 | ||||
Valuation allowance | (36.1 | )% | (36.0 | )% | (38.2 | )% | ||
Effective income tax rate | — | % | — | % | — | % | ||
95
The tax effects of temporary differences and carry forwards that give rise to significant portions of the deferred tax assets are as follows (in thousands):
December 31, | |||||||
2015 | 2014 | ||||||
Net operating loss carry forwards | $ | 187,169 | $ | 140,185 | |||
Capitalized start-up costs | 7,475 | 8,051 | |||||
Research and development credits | 6,320 | 5,603 | |||||
Stock compensation | 12,683 | 10,630 | |||||
Other | 6,875 | 6,634 | |||||
Total deferred tax assets | 220,522 | 171,103 | |||||
Valuation allowance | (216,220 | ) | (166,180 | ) | |||
Deferred tax liability—fixed assets | (3,056 | ) | (3,346 | ) | |||
Deferred tax liability—derivative liability | (1,246 | ) | (1,577 | ) | |||
Net deferred tax assets, after valuation allowance | $ | — | $ | — | |||
The Company is tracking the portion of its deferred tax assets attributable to excess stock option benefits in accordance with FASB ASC 718-740-45, Other Presentation Matters, and therefore, these amounts are not included in the Company’s deferred tax assets. The deferred tax assets attributable to excess stock option benefits total $6.7 million at December 31, 2015, and the benefit related thereto will only be recognized when it reduces cash taxes payable.
The Company’s deferred tax assets represent an unrecognized future tax benefit. The Company has provided a full valuation allowance on its deferred tax assets as of December 31, 2015 and 2014, as management believes it is more likely than not that the related deferred tax asset will not be realized. The net valuation allowance increased by $50.0 million during the year ended December 31, 2015.
Uncertain Tax Positions
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
Balance as of December 31, 2012 | $ | 619 | |
Addition based on tax positions related to current year | 441 | ||
Additions based on tax positions related to prior year | 428 | ||
Balance as of December 31, 2013 | 1,488 | ||
Addition based on tax positions related to current year | 6,411 | ||
Additions based on tax positions related to prior year | 4,042 | ||
Balance as of December 31, 2014 | 11,941 | ||
Addition based on tax positions related to current year | 184 | ||
Reduction based on tax positions related to prior year | (9,799 | ) | |
Additions based on tax positions related to prior year | 86 | ||
Balance as of December 31, 2015 | $ | 2,412 | |
During the year ended December 31, 2015, unrecognized tax benefits of $9.8 million were resolved in connection with the outcome of a California Supreme Court case of Gillette and Company et al. v. Franchise Tax Board that concluded on a methodology which follows that certain of the Company’s net operating losses cannot be sustained. The decision had no impact on the Company’s gross deferred tax assets as presented, as the Company’s deferred tax asset for net operating losses was previously reported, net of a reserve for this same item.
96
The Company’s policy is to include interest and penalties related to unrecognized tax benefits within the provision for income taxes. Management has determined that no accrual for interest and penalties was required as of December 31, 2015. The Company does not anticipate the total amounts of unrecognized tax benefits will significantly increase or decrease in the next twelve months.
The Company is subject to taxation in the U.S., various states and a foreign jurisdiction. As of December 31, 2015, the Company’s tax years 2004 and thereafter remain subject to examination by the tax authorities.
16. EMPLOYEE BENEFIT PLAN
In January 2007, the Company adopted a 401(k) plan for its employees whereby eligible employees may contribute up to 90% of their compensation, on a pretax basis, subject to the maximum amount permitted by the Internal Revenue Code. The Company has not contributed to, nor is it required to contribute to, the 401(k) plan since its inception.
97
17. SUBSEQUENT EVENT
On March 10, 2016, the Company entered into a Stock Purchase Agreement with certain strategic investors and entrepreneurs in food, nutrition and innovation in connection with the private placement of 27,850 shares of its Series A Convertible Preferred Stock, par value $0.001 per share (the “Series A Convertible Preferred Stock”), for a per share purchase price of $1,000 per share, with a conversion price of $2.00 per common share. The 27,850 shares of Series A Preferred Stock that are expected to be issued will be initially convertible into an aggregate of 13.9 million shares of common stock. The Company expects to receive gross proceeds of $27.9 million in cash from the private placement and intends to use such proceeds for capital expenditures, working capital and general corporate purposes.
The private placement is expected to close in March 2016, subject to the satisfaction of customary closing conditions.
98
18. SELECTED QUARTERLY FINANCIAL DATA (Unaudited)
The following table provides the selected quarterly financial data for 2015 and 2014 (in thousands, except per share amounts):
SOLAZYME, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
Quarter Ended | |||||||||||||||||||||||||||||||
December 31, 2015 | September 30, 2015 | June 30, 2015 | March 31, 2015 | December 31, 2014 | September 30, 2014 | June 30, 2014 | March 31, 2014 | ||||||||||||||||||||||||
Revenues: | |||||||||||||||||||||||||||||||
Product revenue | $ | 7,039 | $ | 9,133 | $ | 8,307 | $ | 8,821 | $ | 9,353 | $ | 11,623 | $ | 9,022 | $ | 7,348 | |||||||||||||||
Research and development programs | 3,348 | 2,266 | 3,433 | 3,784 | 5,149 | 5,936 | 6,917 | 5,043 | |||||||||||||||||||||||
Total revenues | 10,387 | 11,399 | 11,740 | 12,605 | 14,502 | 17,559 | 15,939 | 12,391 | |||||||||||||||||||||||
Costs and operating expenses: | |||||||||||||||||||||||||||||||
Cost of product revenue | 4,578 | 4,570 | 4,361 | 4,670 | 6,154 | 6,598 | 4,470 | 3,390 | |||||||||||||||||||||||
Research and development | 9,586 | 13,207 | 12,747 | 12,554 | 18,210 | 20,571 | 22,064 | 20,835 | |||||||||||||||||||||||
Sales, general and administrative | 18,888 | 19,596 | 20,981 | 21,268 | 22,139 | 25,883 | 21,637 | 20,607 | |||||||||||||||||||||||
Restructuring charges | 4,581 | (21 | ) | (31 | ) | 424 | 3,514 | — | — | — | |||||||||||||||||||||
Total costs and operating expenses | 37,633 | 37,352 | 38,058 | 38,916 | 50,017 | 53,052 | 48,171 | 44,832 | |||||||||||||||||||||||
Loss from operations | (27,246 | ) | (25,953 | ) | (26,318 | ) | (26,311 | ) | (35,515 | ) | (35,493 | ) | (32,232 | ) | (32,441 | ) | |||||||||||||||
Other income (expense): | |||||||||||||||||||||||||||||||
Interest and other (expense) income, net | (3,323 | ) | (3,225 | ) | (3,410 | ) | (3,273 | ) | (3,205 | ) | (3,188 | ) | (4,662 | ) | (1,112 | ) | |||||||||||||||
Loss from equity method investment | (4,098 | ) | (5,916 | ) | (7,309 | ) | (5,066 | ) | (7,724 | ) | (7,201 | ) | (4,278 | ) | (3,834 | ) | |||||||||||||||
Gain from change in fair value of warrant liability | — | — | — | — | — | — | — | 688 | |||||||||||||||||||||||
Gain (loss) from change in fair value of derivative liability | (26 | ) | 176 | (134 | ) | (15 | ) | 1,578 | 6,205 | (1,745 | ) | 2,018 | |||||||||||||||||||
Total other income (expense) | (7,447 | ) | (8,965 | ) | (10,853 | ) | (8,354 | ) | (9,351 | ) | (4,184 | ) | (10,685 | ) | (2,240 | ) | |||||||||||||||
Net loss | (34,693 | ) | (34,918 | ) | (37,171 | ) | (34,665 | ) | (44,866 | ) | (39,677 | ) | (42,917 | ) | (34,681 | ) | |||||||||||||||
Net loss per share, basic and diluted | $ | (0.43 | ) | $ | (0.43 | ) | $ | (0.46 | ) | $ | (0.44 | ) | $ | (0.57 | ) | $ | (0.50 | ) | $ | (0.56 | ) | $ | (0.50 | ) | |||||||
Weighted-average number of shares used in loss per share computation, basic and diluted | 80,600 | 80,298 | 80,098 | 79,650 | 79,330 | 78,867 | 75,963 | 69,213 | |||||||||||||||||||||||
99
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosures.
None.
Item 9A. Controls and Procedures.
Our management, including our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2015. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable, and not absolute, assurance of achieving the desired objectives. In reaching a reasonable level of assurance, management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 31, 2015 at the reasonable assurance level.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2015 based on the guidelines established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Our internal control over financial reporting includes policies and procedures that provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with U.S. generally accepted accounting principles.
Based on the results of our evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2015. We reviewed the results of management’s assessment with our Audit Committee. The effectiveness of our internal control over financial reporting as of December 31, 2015 has been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report which is included in this Annual Report on Form 10-K.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the quarterly period ended December 31, 2015 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including our Chief Executive Officer and Chief Financial Officer, believes that our disclosure controls and procedures and internal control over financial reporting are designed to provide reasonable assurance of achieving their objectives and are effective at the reasonable assurance level. However, our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by management override of the controls. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes
100
in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost effective control system, misstatements due to error or fraud may occur and not be detected.
Item 9B. Other Information.
None.
101
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this item concerning our directors, executive officers, compliance with Section 16 of the Exchange Act, our Code of Business Conduct and Ethics and other governance matters is incorporated by reference from the information set forth in the sections under the headings “Directors, Executive Officers and Corporate Governance” and “Section 16(a) Beneficial Ownership Reporting Compliance” in our Definitive Proxy Statement to be filed with the Securities and Exchange Commission pursuant to Regulation 14A within 120 days after our fiscal year end of December 31, 2015 in connection with the Annual Meeting of Stockholders to be held in 2016 (the “2016 Proxy Statement”).
Item 11. Executive Compensation.
The information required by this item is incorporated by reference from the information in the 2016 Proxy Statement under the headings “Executive Compensation,” “Directors, Executive Officers and Corporate Governance—Non-Employee Director Compensation” and “Directors, Executive Officers and Corporate Governance—Compensation Committee Interlocks and Insider Participation.”
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item concerning securities authorized for issuance under equity compensation plans and security ownership of certain beneficial owners and management is incorporated by reference from the information in the 2016 Proxy Statement under the headings “Equity Compensation Plan Information” and “Security Ownership of Certain Beneficial Owners and Officers and Directors.”
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item concerning transactions with related persons and director independence is incorporated by reference from the information in the 2016 Proxy Statement under the headings “Certain Relationships and Related Party Transactions” and “Directors, Executive Officers and Corporate Governance.”
Item 14. Principal Accounting Fees and Services.
The information required by this item is incorporated by reference from the information in the 2016 Proxy Statement under the heading “Ratification of Appointment of Independent Registered Public Accounting Firm—Fees Paid to Auditors.”
102
PART IV
Item 15. Exhibits and Financial Statement Schedules.
1. Our consolidated financial statements are filed as part of this Annual Report on Form 10-K in Item 8. Financial Statements and Supplementary Data, and a list of the consolidated financial statements are found on page 60 of this report.
2. Exhibits: The exhibits listed in the accompanying index to exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K.
103
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
SOLAZYME, INC. | |
By:/S/ JONATHAN S. WOLFSON | |
Name: Jonathan S. Wolfson | |
Title: Chief Executive Officer and Chairman of the Board | |
Date: March 14, 2016 | |
POWER OF ATTORNEY
Each person whose individual signature appears below hereby authorizes and appoints Jonathan S. Wolfson and Tyler W. Painter, and each of them, with full power of substitution and resubstitution and full power to act without the other, as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file any and all amendments to this annual report on Form 10-K and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or his substitute or substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ Jonathan S. Wolfson | Chief Executive Officer and Chairman of the Board (Principal Executive Officer) | March 14, 2016 | ||
Jonathan S. Wolfson | ||||
/s/ Tyler W. Painter | Chief Financial Officer and Chief Operating Officer (Principal Financial and Accounting Officer) | March 14, 2016 | ||
Tyler W. Painter | ||||
/s/ Michael V. Arbige | Director | March 14, 2016 | ||
Michael V. Arbige | ||||
/s/ Ian T. Clark | Director | March 14, 2016 | ||
Ian T. Clark | ||||
/s/ James R. Craigie | Director | March 14, 2016 | ||
James R. Craigie | ||||
/s/ Jerry Fiddler | Director | March 14, 2016 | ||
Jerry Fiddler | ||||
/s/ Gary Pfeiffer | Director | March 14, 2016 | ||
Gary Pfeiffer | ||||
Director | ||||
Irene Chang Britt | ||||
104
EXHIBIT INDEX
Exhibit Number | Description | Incorporated by Reference | Filed Herewith with this 10-K | ||||||||||
Form | File No. | Filing Date | Exhibit | ||||||||||
3.1 | Amended and Restated Certificate of Incorporation | 10-K/A | 001-35189 | April 27, 2012 | 3.1 | ||||||||
3.2 | Amended and Restated Bylaws | 10-K/A | 001-35189 | April 27, 2012 | 3.2 | ||||||||
4.1 | Indenture dated as of January 24, 2013 between Wells Fargo Bank, National Association, as Trustee, and Solazyme, Inc. | 8-K | 001-35189 | January 24, 2013 | 4.1 | ||||||||
4.2 | Indenture dated as of April 1, 2014 between Wells Fargo Bank, National Association, as Trustee, and Solazyme, Inc. | 8-K | 001-35189 | April 1, 2014 | 4.1 | ||||||||
10.1§ | Form of Director/Officer Indemnification Agreement entered into by and between Solazyme, Inc. and each of its directors and executive officers | S-1 | 333-172790 | May 4, 2011 | 10.1 | ||||||||
10.2§ | Second Amended and Restated 2004 Equity Incentive Plan and forms of notice of stock option grant, stock option agreement and restricted stock agreement | S-1 | 333-172790 | March 11, 2011 | 10.2 | ||||||||
10.3§ | 2011 Equity Incentive Plan | S-1 | 333-172790 | May 4, 2011 | 10.3 | ||||||||
10.4§ | 2011 Employee Stock Purchase Plan | S-1 | 333-172790 | May 4, 2011 | 10.4 | ||||||||
10.5§ | Executive Severance and Change of Control Plan | S-1 | 333-172790 | May 23, 2011 | 10.17 | ||||||||
10.6§ | Amended and Restated Employment Agreement dated May 19, 2011, by and between Solazyme, Inc. and Jonathan S. Wolfson | S-1 | 333-172790 | May 23, 2011 | 10.18 | ||||||||
10.7§ | Amended and Restated Employment Agreement dated May 19, 2011, by and between Solazyme, Inc. and Tyler W. Painter | S-1 | 333-172790 | May 23, 2011 | 10.20 | ||||||||
10.8§ | Amended and Restated Employment Agreement dated May 19, 2011, by and between Solazyme, Inc. and Peter J. Licari | S-1 | 333-172790 | May 23, 2011 | 10.21 | ||||||||
10.9§ | Amended and Restated Employment Agreement dated May 19, 2011, by and between Solazyme, Inc. and Paul T. Quinlan | S-1 | 333-172790 | May 23, 2011 | 10.22 | ||||||||
10.10 | Warrant for the Purchase of Shares of Common Stock of Solazyme, Inc. by Bunge Limited | S-1 | 333-172790 | May 4, 2011 | 10.24 | ||||||||
10.11 | Amendment No. 1 to Warrant for the Purchase of Shares of Common Stock of Solazyme, Inc. dated August 5, 2011 by and between Solazyme, Inc. and Bunge Limited | 10-Q | 001-35189 | November 8, 2011 | 10.20 | ||||||||
10.12§ | Form of 2011 Equity Incentive Plan Stock Option Agreement | 10-K | 001-35189 | March 15, 2012 | 10.30 | ||||||||
10.13§ | Form of 2011 Equity Incentive Plan Restricted Stock Agreement | 10-K | 001-35189 | March 15, 2012 | 10.31 | ||||||||
10.14§ | Form of 2011 Equity Incentive Plan Restricted Stock Unit Agreement | 10-K | 001-35189 | March 13, 2013 | 10.23 | ||||||||
10.15† | Strategic Collaboration Agreement dated as of November 13, 2012 by and between Solazyme, Inc. and Archer-Daniels-Midland Company | 10-K | 001-35189 | March 13, 2013 | 10.26 | ||||||||
10.16 | Loan and Security Agreement dated as of March 26, 2013 between HSBC National Bank, USA, National Association and Solazyme, Inc. | 10-Q | 001-35189 | May 8, 2013 | 10.30 | ||||||||
105
Exhibit Number | Description | Incorporated by Reference | Filed Herewith with this 10-K | ||||||||||
Form | File No. | Filing Date | Exhibit | ||||||||||
10.17 | Credit Facility Agreement No. 12.2.1149.1, Executed Between Banco Nacional de Desenvolvimento Economico e Social—BNDES and Solazyme Bunge Produtos Renovaveis Ltda., dated February 14, 2013 | 10-Q | 001-35189 | August 7, 2013 | 10.10 | ||||||||
10.18 | First Amendment to Loan and Security Agreement by and between HSBC Bank USA, National Association and Solazyme, Inc., dated May 9, 2013 | 10-Q | 001-35189 | August 7, 2013 | 10.20 | ||||||||
10.19 | 1st Amendment to Credit Facility Agreement No., 12.2.1149.1, Executed Between Banco Nacional de Desenvolvimento Economico e Social-BNDES and Solazyme Bunge Produtos Renovaveis Ltda., dated January 16, 2014 | 10-Q | 001-35189 | May 8, 2014 | 10.2 | ||||||||
10.20 | Second Amendment to Loan and Security Agreement by and between HSBC Bank USA, National Association and Solazyme, Inc., dated March 31, 2014 | 10-Q | 001-35189 | May 8, 2014 | 10.3 | ||||||||
10.21 | Lease, dated as of July 22, 2014, by and between Britannia Gateway II Limited Partnership and Solazyme, Inc. | 10-Q | 001-35189 | November 6, 2014 | 10.1 | ||||||||
10.22 | Letter of Termination of Operating Agreement by and between Solazyme, Inc. and Archer-Daniels-Midland Company entered into as of November 13, 2012 | 8-K | 001-35189 | October 30, 2015 | 10.1 | ||||||||
10.23† | Amended and Restated Joint Venture Agreement entered into as of October 27, 2015 by and among Bunge Global Innovation, LLC, Solazyme, Inc. and certain other parties | X | |||||||||||
10.24† | Amended and Restated Solazyme Development Agreement entered into as of October 27, 2015 by and among Solazyme Bunge Renováveis Ltda., Solazyme, Inc. and certain other parties. | X | |||||||||||
12.1 | Computation of Ratio of Earnings to Fixed Charges | X | |||||||||||
21.1 | List of subsidiaries of the Registrant | X | |||||||||||
23.1 | Consent of Deloitte & Touche LLP, Independent Registered Public Accounting Firm | X | |||||||||||
24.1 | Power of Attorney (found on Signature Page) | X | |||||||||||
31.1 | Certification of Chief Executive Officer, as required by Section 302 of the Sarbanes-Oxley Act of 2002 | X | |||||||||||
31.2 | Certification of Chief Financial Officer, as required by Section 302 of the Sarbanes-Oxley Act of 2002 | X | |||||||||||
32.1 | Certification of the Chief Executive Officer and Chief Financial Officer, as required by Section 906 of the Sarbanes-Oxley Act of 2002 | X | |||||||||||
106
Exhibit Number | Description | Incorporated by Reference | Filed Herewith with this 10-K | ||||||||||
Form | File No. | Filing Date | Exhibit | ||||||||||
101 | The following financial statements, formatted in XBRL: (i) Consolidated Balance Sheets at December 31, 2015 and December 31, 2014, (ii) Consolidated Statements of Operations for each of the three years in the period ended December 31, 2015, (iii) Consolidated Statements of Comprehensive Loss for each of the three years in the period ended December 31, 2015, (iv) Consolidated Statements of Stockholders’ Equity for each of the three years in the period ended December 31, 2015, (v) Consolidated Statements of Cash Flows for each of the three years in the period ended December 31, 2015; and (vi) Notes to the Consolidated Financial Statements | X | |||||||||||
† | Confidential treatment has been granted, or requested, with respect to portions of the exhibit. A complete copy of the agreement, including the redacted terms, has been separately filed with the SEC. |
§ | Management contract or compensatory plan or arrangement. |
107
