Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ANTARES PHARMA, INC. | atrs-8k_20160309.htm |

NASDAQ: ATRS Cowen and Company 36th Annual Healthcare Conference March 10, 2016 Robert F. Apple President & Chief Executive Officer Exhibit 99.1

Safe Harbor Statement This presentation contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the timing of the launch of VIBEX® Sumatriptan Injection USP and the amount of revenue from the same, the timing and results of the phase 3 studies for QuickShot® Testosterone (QS T) and acceptance of the data by the U.S. Food and Drug Administration (FDA); the Company’s ability to successfully complete a New Drug Application for QS T and submit to the FDA and approval of the same by the FDA; Teva’s ability to adequately and timely respond to the Complete Response Letter received from the FDA for the VIBEX® epinephrine pen ANDA and approval by the FDA of the same, the timing and therapeutic equivalence rating thereof, and any revenue pre or post FDA approval; FDA action with respect to Teva’s ANDA filed for the exenatide pen; continued growth of prescriptions and sales of OTREXUP™; the timing and results of research projects, clinical trials, and product candidates in development including the development project with AMAG Pharmaceuticals for a subcutaneous auto injector for their product Makena and the undisclosed Pen 1 project with Teva; actions by the FDA or other regulatory agencies with the respect to the Company’s products or product candidates of its partners; continued growth in product, development, licensing and royalty revenue; the Company’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities and other statements regarding matters that are not historical facts, and involve predictions. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, achievements or prospects expressed in or implied by such forward-looking statements. In some cases you can identify forward-looking statements by terminology such as ''may'', ''will'', ''should'', ''would'', ''expect'', ''intend'', ''plan'', ''anticipate'', ''believe'', ''estimate'', ''predict'', ''potential'', ''seem'', ''seek'', ''future'', ''continue'', or ''appear'' or the negative of these terms or similar expressions, although not all forward-looking statements contain these identifying words. Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward-looking statements is contained in the "Risk Factors" section of the Company's Annual Report on Form 10-K for the year ended December 31, 2015, and in the Company's other periodic reports and filings with the Securities and Exchange Commission. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this presentation. All forward-looking statements are based on information currently available to the Company on the date hereof, and the Company undertakes no obligation to revise or update these forward-looking statements to reflect events or circumstances after the date of this presentation, except as required by law. ©2015 Copyright Antares Pharma, Inc. All Rights Reserved.

Antares Pharma A Growing, Revenue Generating State-of-the-Art Specialty Pharmaceutical Company A Leader In Self-Administered Injection Pharmaceuticals Proven Track Record – Four Drug/Drug-Device Products FDA Approved Since 2012 Novel, Virtually Painless Drug Delivery Technology Can Provide Life Cycle Management Solutions Poised To Achieve Additional Value Creating Milestones In 2016
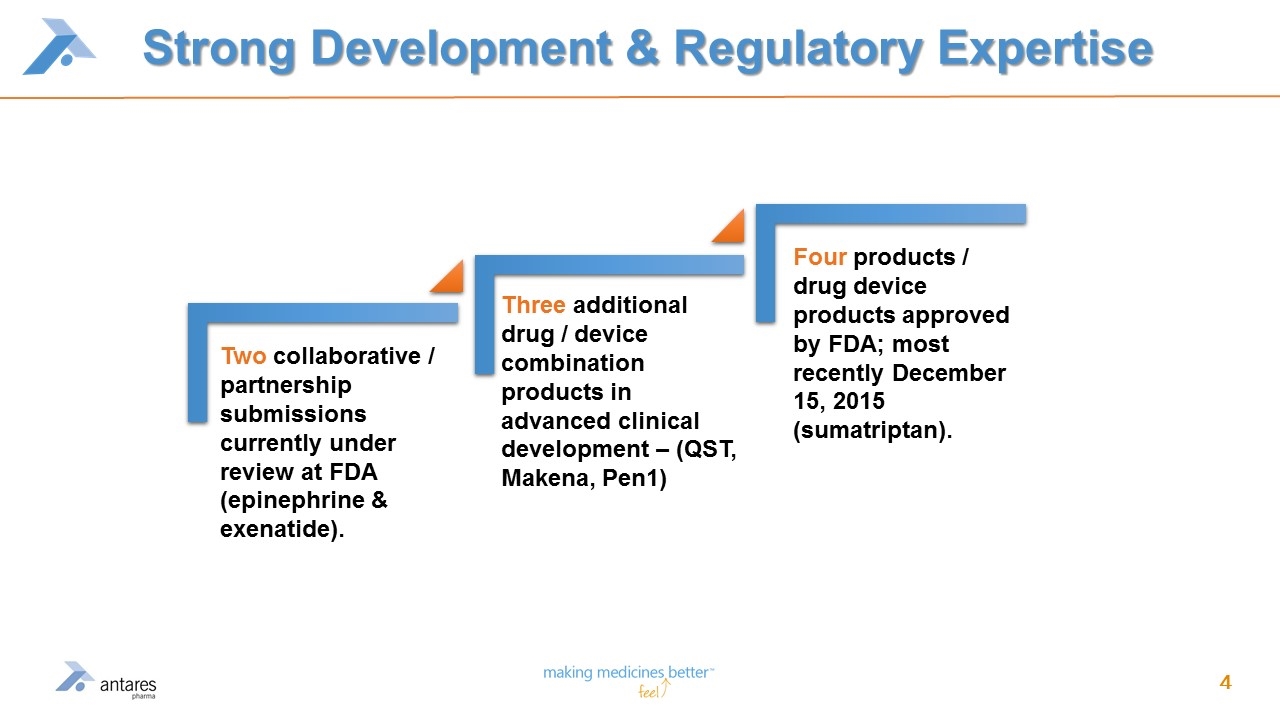
Strong Development & Regulatory Expertise Three additional drug / device combination products in advanced clinical development – (QST, Makena, Pen1) Two collaborative / partnership submissions currently under review at FDA (epinephrine & exenatide). Four products / drug device products approved by FDA; most recently December 15, 2015 (sumatriptan).

Investment Considerations A growing, revenue generating company – 2015 total revenue of $45.7 million vs. $26.5 million in 2014 – a 72% year-over-year increase Multiple development pipeline products targeting therapeutic markets with ~ $8 Billion in annual revenue over next five years Several potential meaningful value drivers in 2016 Strong balance sheet – ~$48 million in cash and no debt at December 31, 2015 (Q4 2015 cash burn ~$3 million)
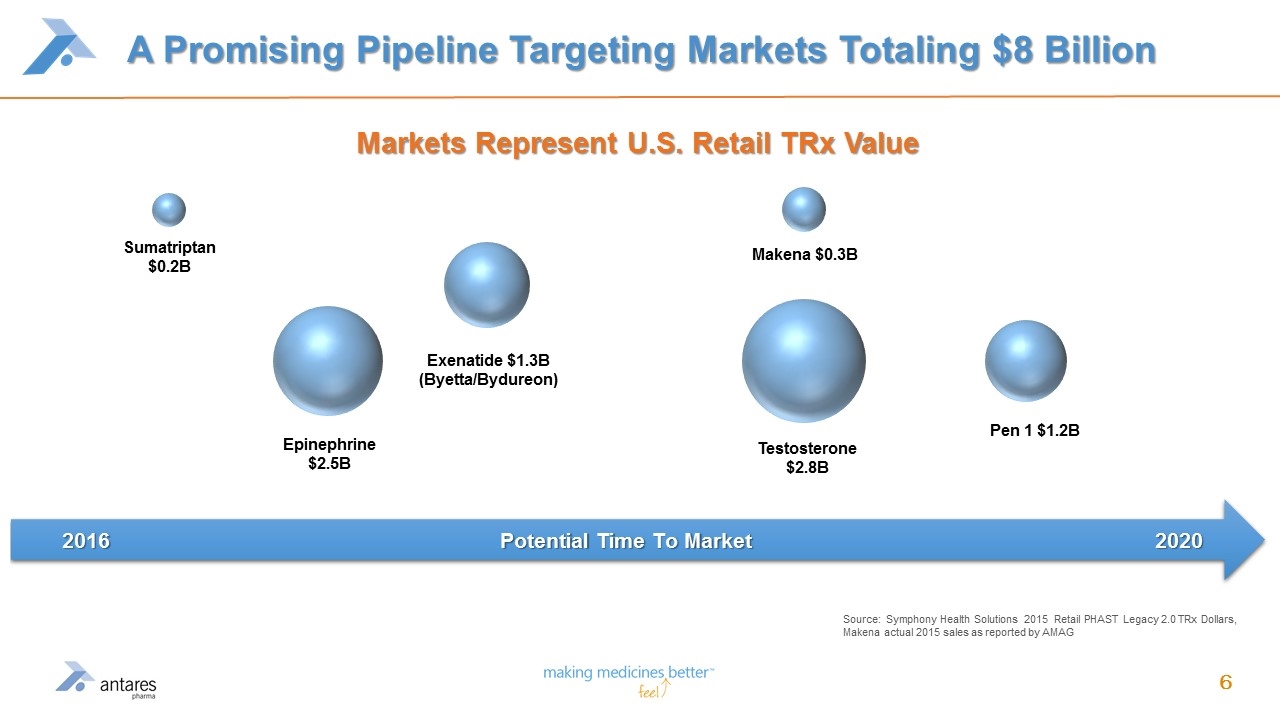
A Promising Pipeline Targeting Markets Totaling $8 Billion Source: Symphony Health Solutions 2015 Retail PHAST Legacy 2.0 TRx Dollars, Makena actual 2015 sales as reported by AMAG Epinephrine $2.5B Exenatide $1.3B (Byetta/Bydureon) Testosterone $2.8B Pen 1 $1.2B Makena $0.3B Sumatriptan $0.2B Markets Represent U.S. Retail TRx Value
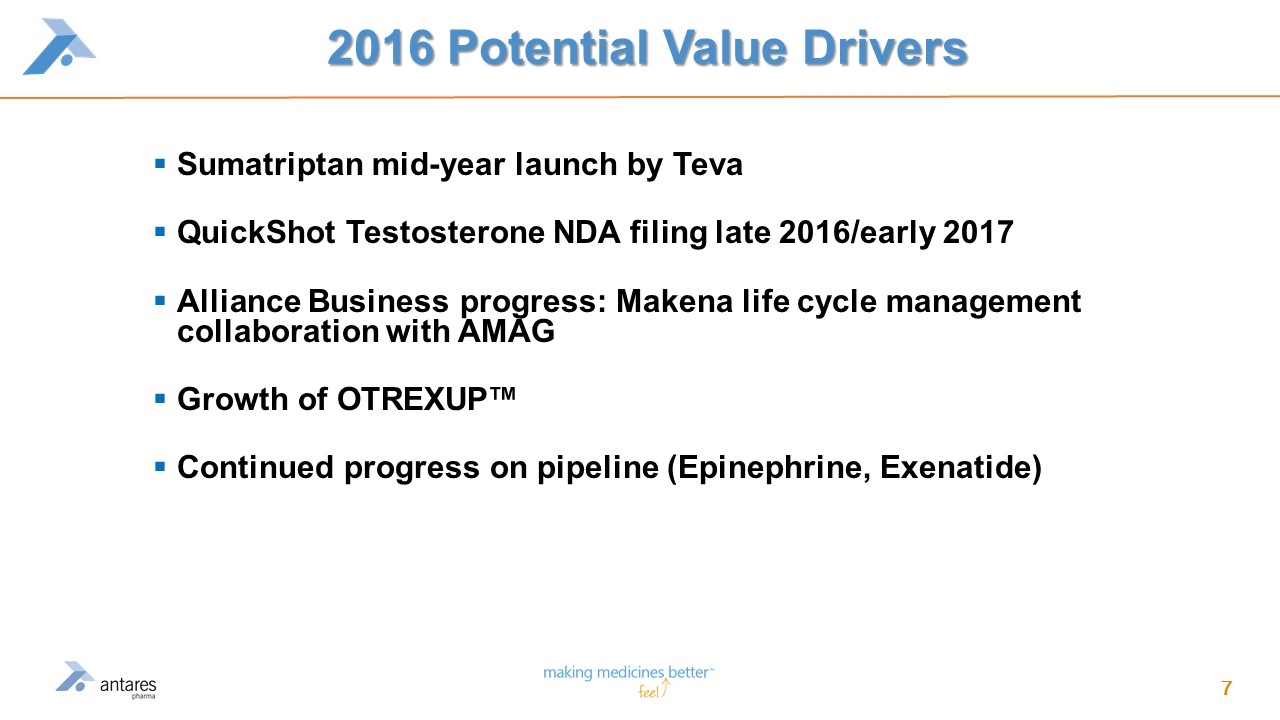
2016 Potential Value Drivers Sumatriptan mid-year launch by Teva QuickShot Testosterone NDA filing late 2016/early 2017 Alliance Business progress: Makena life cycle management collaboration with AMAG Growth of OTREXUP™ Continued progress on pipeline (Epinephrine, Exenatide)
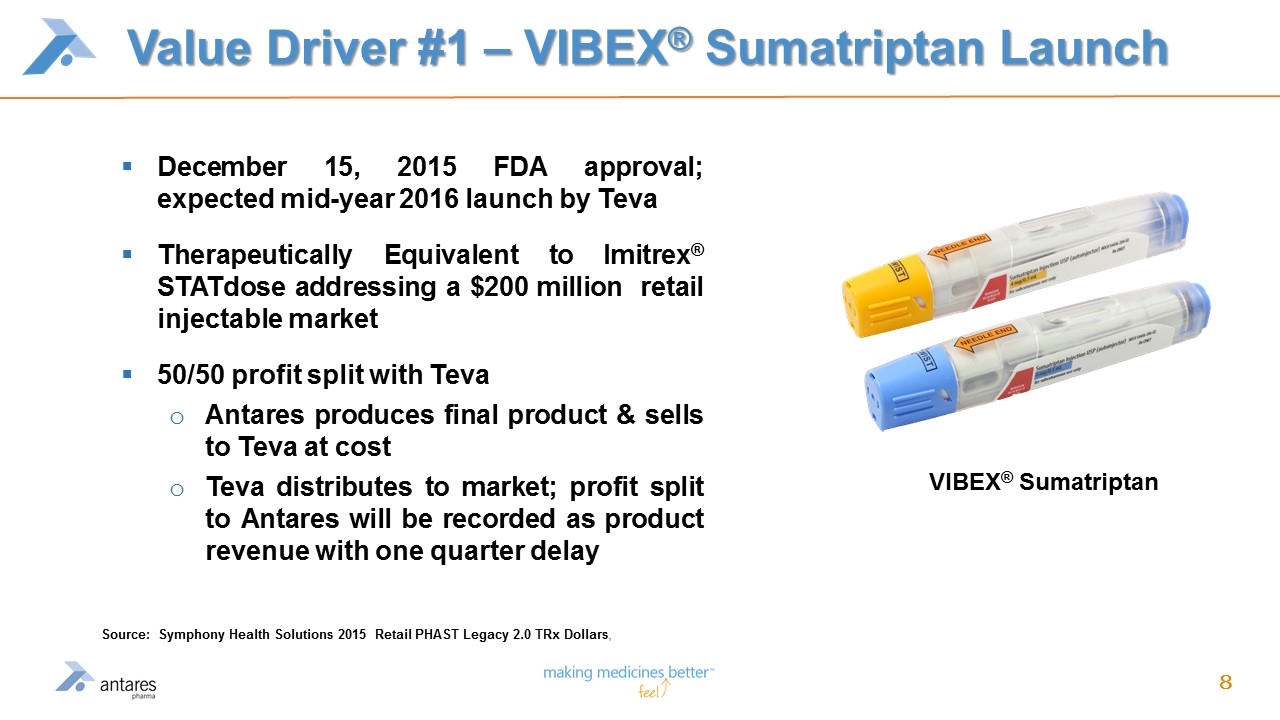
Value Driver #1 – VIBEX® Sumatriptan Launch December 15, 2015 FDA approval; expected mid-year 2016 launch by Teva Therapeutically Equivalent to Imitrex® STATdose addressing a $200 million retail injectable market 50/50 profit split with Teva Antares produces final product & sells to Teva at cost Teva distributes to market; profit split to Antares will be recorded as product revenue with one quarter delay VIBEX® Sumatriptan Source: Symphony Health Solutions 2015 Retail PHAST Legacy 2.0 TRx Dollars,
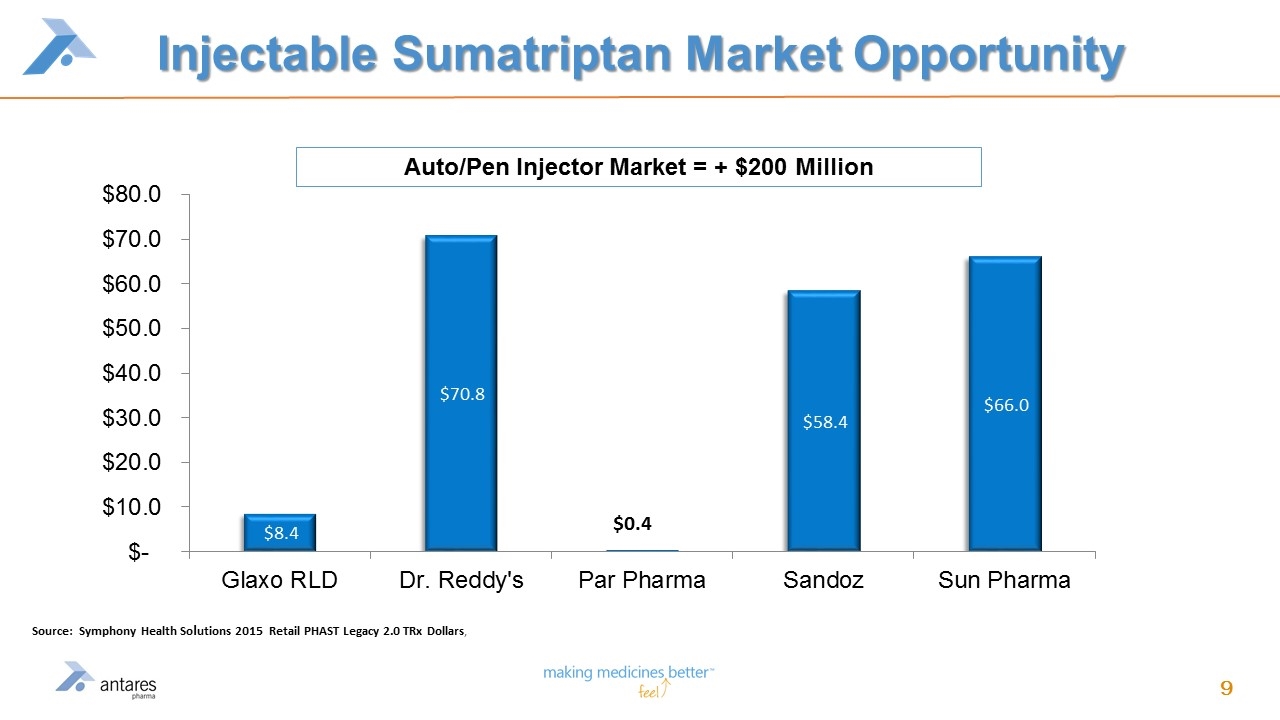
Injectable Sumatriptan Market Opportunity Source: Symphony Health Solutions 2015 Retail PHAST Legacy 2.0 TRx Dollars, Auto/Pen Injector Market = + $200 Million
Value Driver #2 – NDA Filing For QuickShot® Testosterone NDA filing targeted for late Q416 or early Q117 Possible launch in late 2017 / early 2018 Final data from 52 week study , QST-13-003 due Q116 Last patient out of six month supplemental safety study QST-15-005 anticipated late Q216 QuickShot® Testosterone

QuickShot® Testosterone Overview Antares’ QuickShot® is potentially the first at-home auto injector for SC treatment of testosterone deficiency Differentiated Designed to address transference issues experienced with other topical testosterone products Single use, disposable QuickShot® use at-home versus doctor office administration Once Weekly Injection – Peak/Trough ratio reduced versus 1 – 2x per month intramuscular injection Virtually painless subcutaneous injection with 27 gauge needle. Current intramuscular administration requires large-gauge needle Oral option in development requires twice a day dosage with meals QuickShot® Testosterone
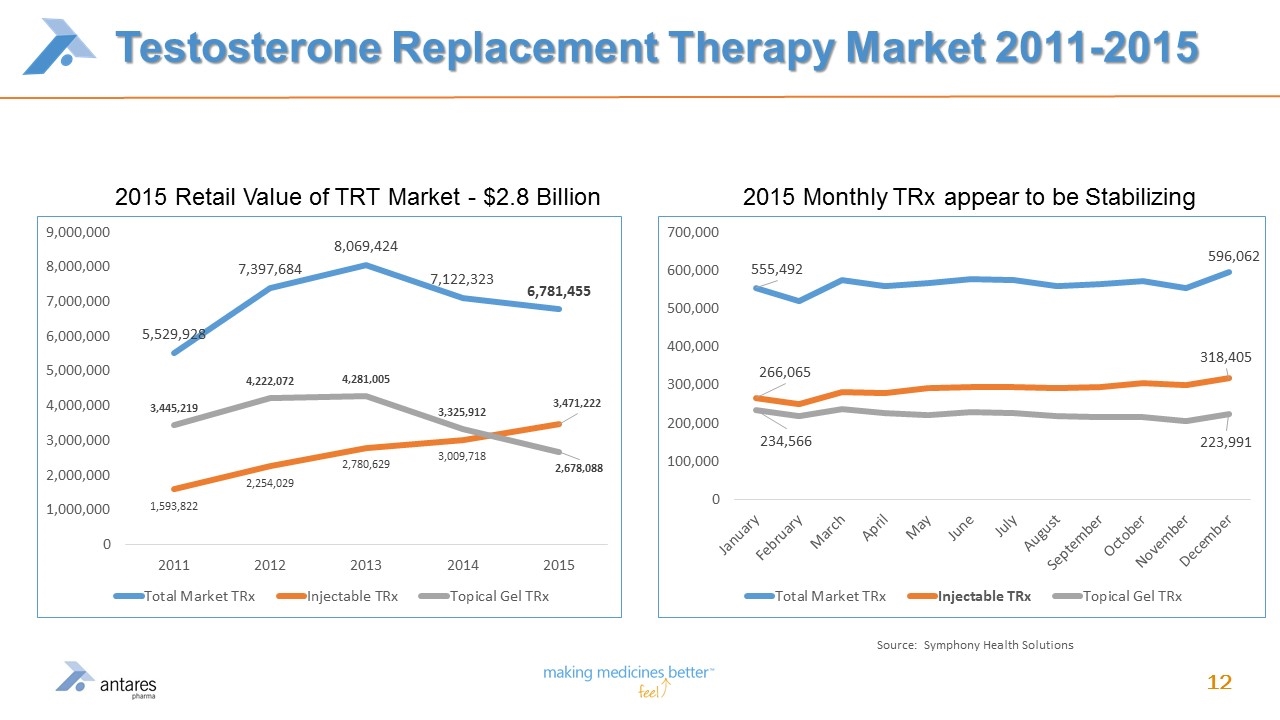
Testosterone Replacement Therapy Market 2011-2015 Source: Symphony Health Solutions 2015 Retail Value of TRT Market - $2.8 Billion 2015 Monthly TRx appear to be Stabilizing
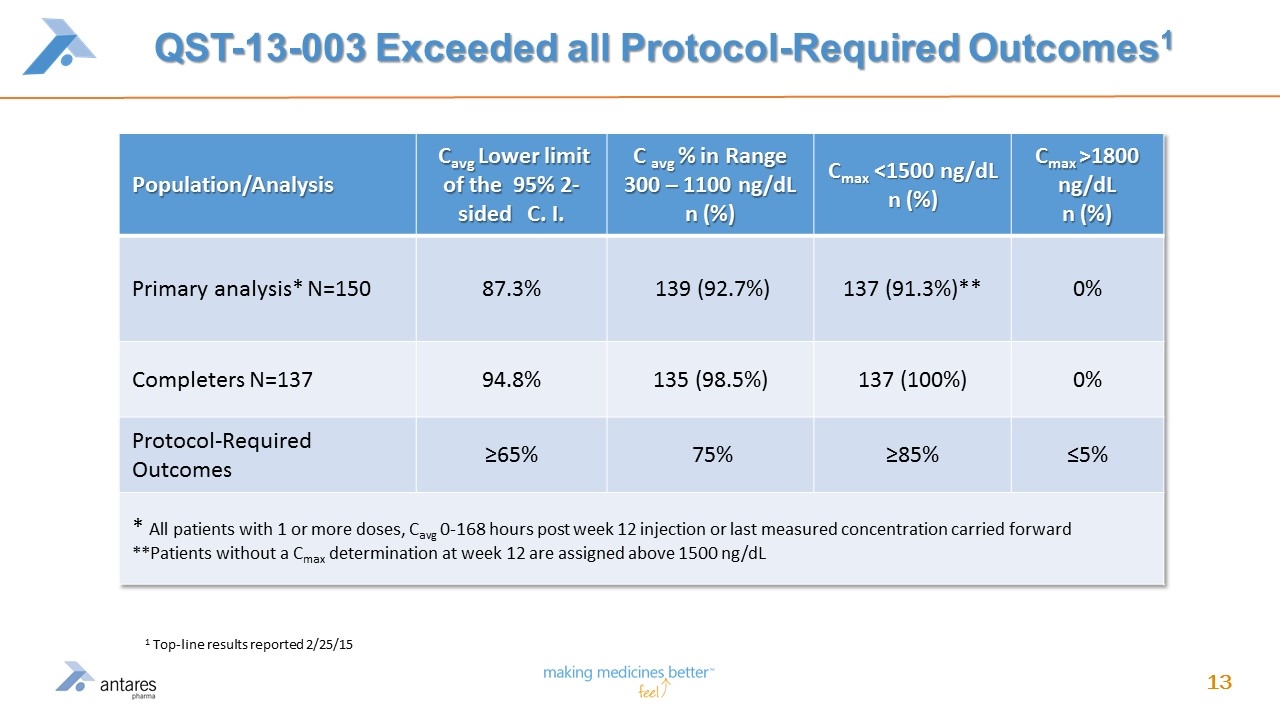
QST-13-003 Exceeded all Protocol-Required Outcomes1 Population/Analysis Cavg Lower limit of the 95% 2-sided C. I. C avg % in Range 300 – 1100 ng/dL n (%) Cmax <1500 ng/dL n (%) Cmax >1800 ng/dL n (%) Primary analysis* N=150 87.3% 139 (92.7%) 137 (91.3%)** 0% Completers N=137 94.8% 135 (98.5%) 137 (100%) 0% Protocol-Required Outcomes ≥65% 75% ≥85% ≤5% * All patients with 1 or more doses, Cavg 0-168 hours post week 12 injection or last measured concentration carried forward **Patients without a Cmax determination at week 12 are assigned above 1500 ng/dL 1 Top-line results reported 2/25/15

QST-15-005 Supplemental Safety Study Study helps ensure we satisfy the FDA’s recommendation that we have a safety database of approximately 350 subjects exposed to QS T in total with approximately 200 subjects exposed for six months and approximately 100 subjects exposed at twelve months Enrollment completed ahead of schedule in November 2015; 133 patients are enrolled in the study and will receive treatment for six months We anticipate the last patient in the study will complete their final visit by late Q216

Value Driver #3 – Grow Alliance Business AMAG Makena alliance (began in 2014) Developing a subcutaneous auto injector device Better patient compliance Potentially less painful injection (small gauge needle) and easier administration Currently Makena is ~ $250 million product opportunity, expected to grow to approximately $310 - $340* in 2016 AMAG estimate sNDA filing in 1Q17 with a 6 to10 month regulatory review Antares will sell devices to AMAG and will receive royalties and certain milestone payments based upon net sales benchmarks Secure additional alliance business in 2016 * AMAG 2016 Makena Revenue Guidance Issued 1/11/16
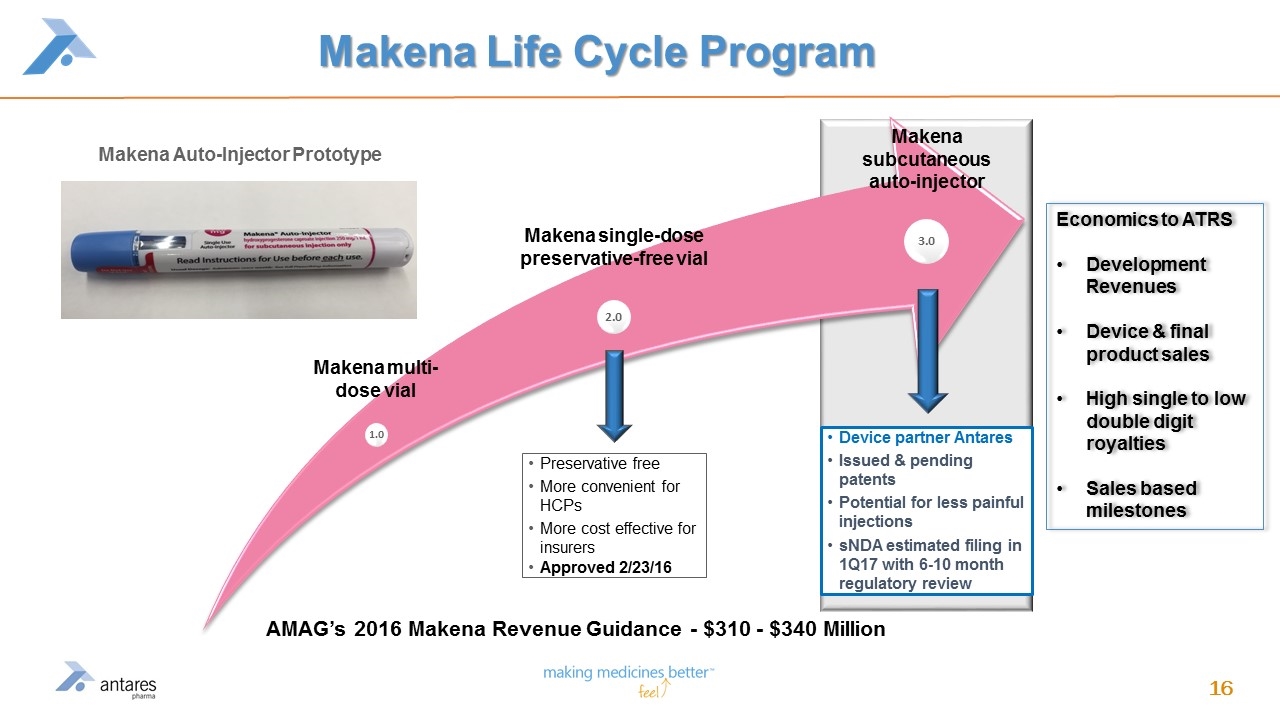
Makena Life Cycle Program Preservative free More convenient for HCPs More cost effective for insurers Approved 2/23/16 Device partner Antares Issued & pending patents Potential for less painful injections sNDA estimated filing in 1Q17 with 6-10 month regulatory review Makena subcutaneous auto-injector 1.0 2.0 3.0 Makena single-dose preservative-free vial Makena multi-dose vial AMAG’s 2016 Makena Revenue Guidance - $310 - $340 Million Makena Auto-Injector Prototype Economics to ATRS Development Revenues Device & final product sales High single to low double digit royalties Sales based milestones

Value Driver #4 - OTREXUP™ Growth First approved methotrexate for subcutaneous injection in the U.S. Single-use, disposable & easy to use Collar activated, no push button, easy to grip and virtually painless Needle guard prevents accidental sticks Audible click followed by red indicator to confirm injection is complete Approved in 7.5, 10, 15, 20 & 25 mg color-coded doses
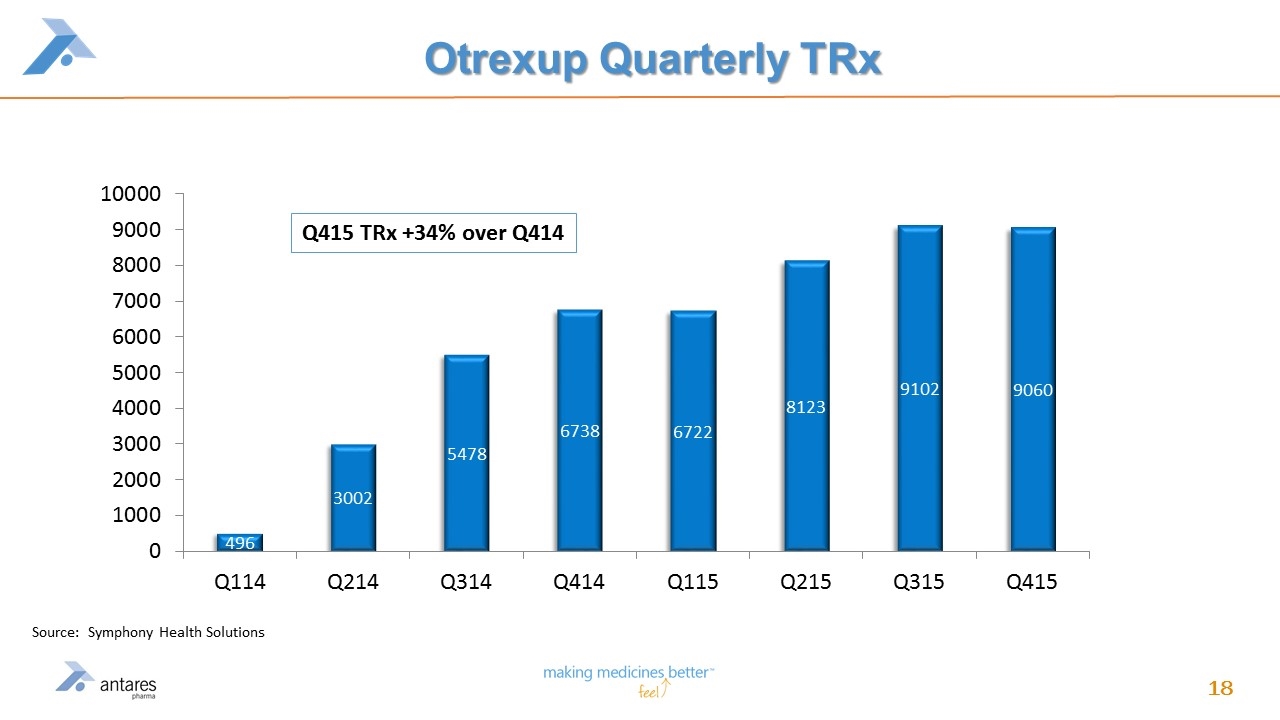
Otrexup Quarterly TRx Source: Symphony Health Solutions Q415 TRx +34% over Q414
Near Term Pipeline Opportunities Epinephrine Auto Injector Exenatide

VIBEX® Epinephrine Auto Injector 2015 Epinephrine retail sales were $2.5 billion per Symphony Health Solutions retail PHAST legacy 2.0 TRx dollars – EpiPen retail sales were $2.2 billion Teva filed major amendment with FDA in December 2014, Complete Response Letter issued February 2016 citing major deficiencies – Teva stated due to major nature of CRL, launch will not take place before 2017 We will assist Teva to address device related comments from the FDA’s CRL Shipped $9.9 million in devices to Teva in 2015; expect to complete delivery of pre- launch requirements 1H16 EpiPen® is a registered trademark of the Mylan Companies

Exenatide – “Pen 2” Teva submitted ANDA to FDA 10/14 – filed 12/14 AstraZeneca and Amylin filed Paragraph IV certification 12/14 – 30 month clock to mid-2017 begins According to Symphony Health Solutions retail PHAST legacy 2.0 TRx dollars, 2015 Byetta/Bydureon (exenatide) retail sales were $1.3 billion ($0.3 Byetta/$1.0 Bydureon) ATRS agreement with Teva – ATRS will receive margin on our supply agreement and single digit to mid-teens royalty on overall product sales EpiPen® is a registered trademark of the Mylan Companies
Financial Overview Sources of Revenue Financials Investment Considerations
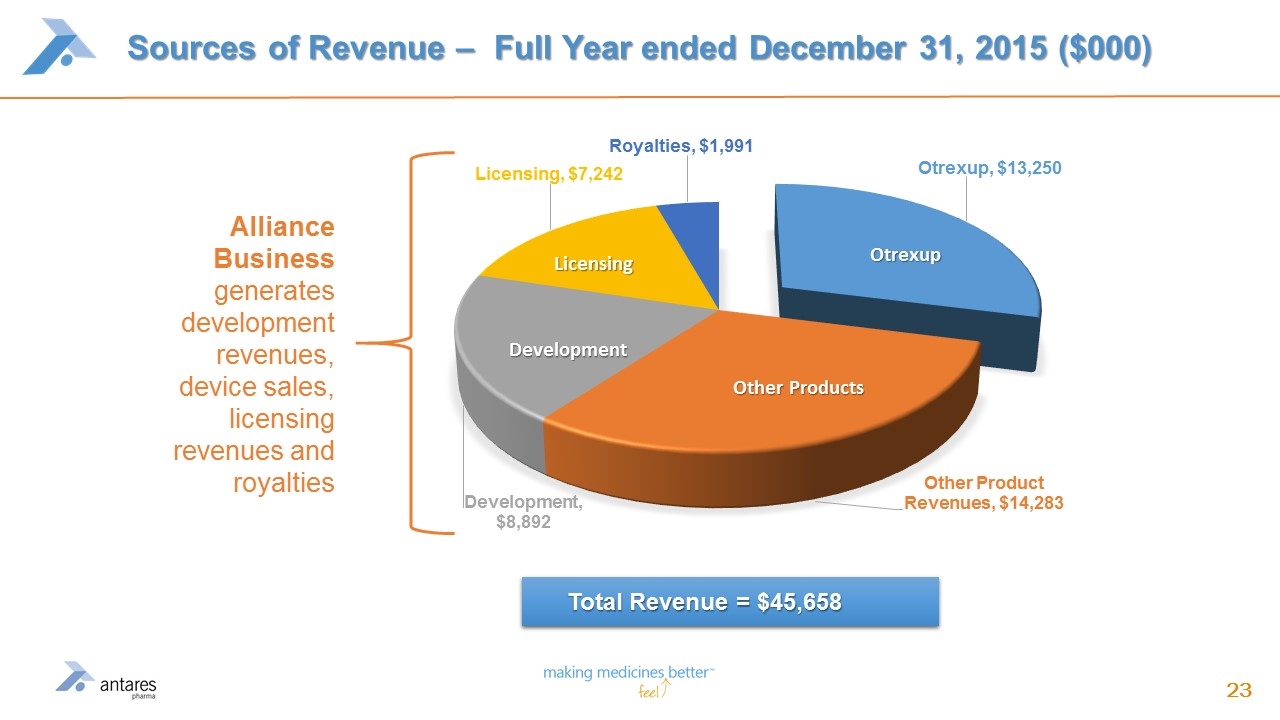
Sources of Revenue – Full Year ended December 31, 2015 ($000) Alliance Business generates development revenues, device sales, licensing revenues and royalties Total Revenue = $45,658
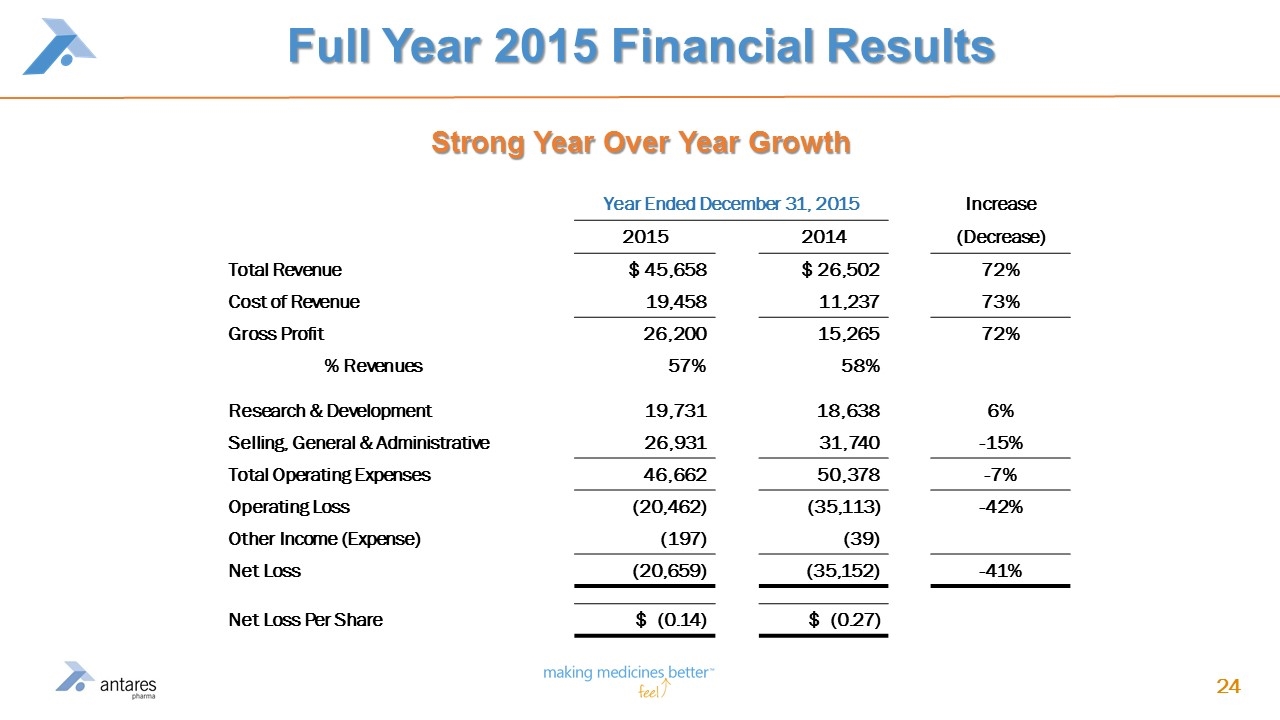
Full Year 2015 Financial Results Year Ended December 31, 2015 Increase 2015 2014 (Decrease) Total Revenue $ 45,658 $ 26,502 72% Cost of Revenue 19,458 11,237 73% Gross Profit 26,200 15,265 72% % Revenues 57% 58% Research & Development 19,731 18,638 6% Selling, General & Administrative 26,931 31,740 -15% Total Operating Expenses 46,662 50,378 -7% Operating Loss (20,462) (35,113) -42% Other Income (Expense) (197) (39) Net Loss (20,659) (35,152) -41% Net Loss Per Share $ (0.14) $ (0.27) Strong Year Over Year Growth
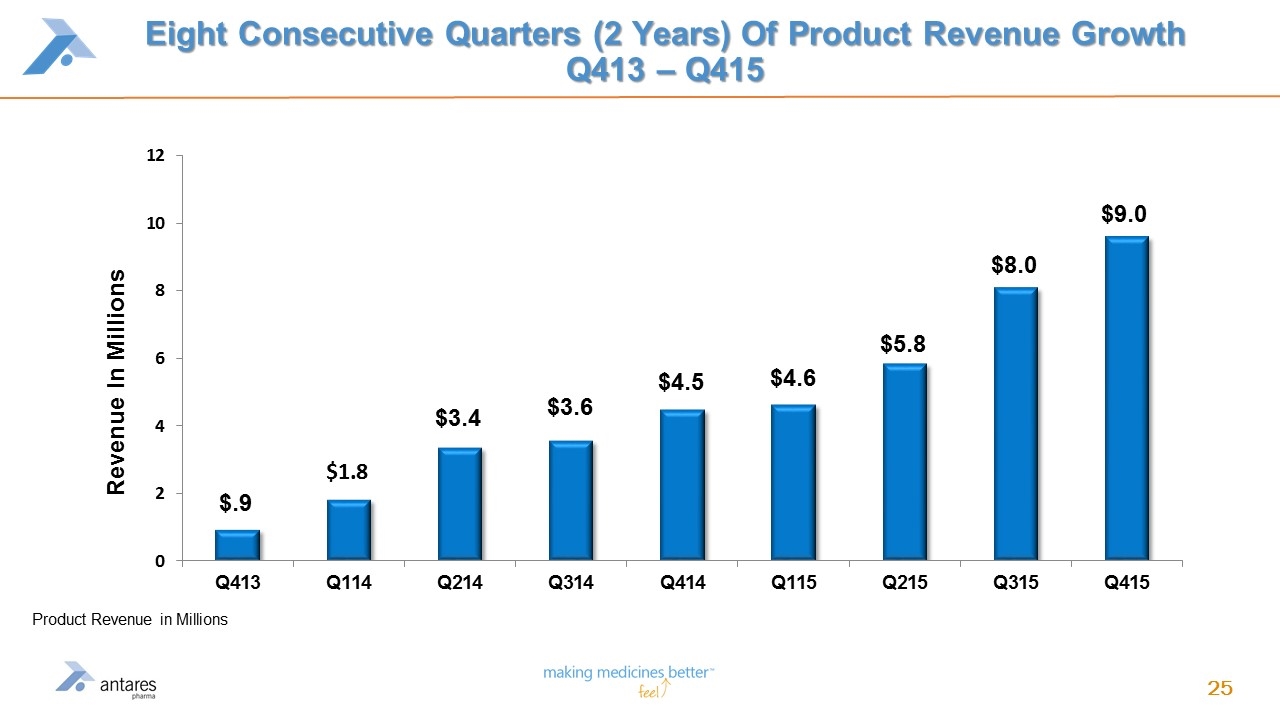
Eight Consecutive Quarters (2 Years) Of Product Revenue Growth Q413 – Q415 Product Revenue in Millions

Investment Considerations A growing, revenue generating company - $45.7 million in 2015 – a 72% increase over last year with 8 consecutive quarters of product revenue growth Multiple development pipeline products targeting therapeutic markets with ~ $8 Billion in annual revenue over next five years Several potential value drivers in 2016: Sumatriptan launch Clinical data on QS T program and possible NDA filing Growing Alliance Business Growth of OTREXUP™ Strong balance sheet – ~$48 million in cash and no debt at December 31, 2015 (Q4 2015 cash burn ~$3 million)

NASDAQ: ATRS Cowen and Company 36th Annual Healthcare Conference March 10, 2016 Robert F. Apple President & Chief Executive Officer
