Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Nuwellis, Inc. | a16-5939_18k.htm |
Exhibit 99.1
This image cannot currently be display ed. Corporate Update March 2016 www.sunshineheart.com

TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. Forward Looking Statement • This presentation contains forward-looking statements. All forward-looking statements are management’s present expectations of future events and are subject to a number of risks and uncertainties. Various factors could cause actual results to differ materially from these statements including timing, clinical enrollment, clinical results, financing availability, product sales and marketing or efficacy of products, and the other risks set forth under the caption “Risk Factors” and elsewhere in our periodic and other reports filed with the U.S. Securities and Exchange Commission, including our Annual Report or Form 10-K for the fiscal year ended December 31, 2014. Although the Company believes that the forward-looking statements are reasonable and based on information currently available, it can give no assurances that the Company’s expectations are correct. All forward looking statements are expressly qualified in their entirety by this cautionary statement. Caution: C-Pulse ® is an investigational device. The device is limited by federal (United States) law to investigational use only. C-Pulse is a registered trademark of Sunshine Heart Inc. • • • © 2016 Sunshine Heart, Inc. 2
TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. Our Vision Offering minimally invasive therapies for moderate to severe heart failure that provide: symptomatic relief improved quality of life slowed disease progression, and reduced re-hospitalization rates © 2016 Sunshine Heart, Inc. 3

TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. Business Update • John Erb named full-time CEO on March 1, 2016 • On March 3, 2016 announced strategic changes focused on pursuing a more optimal clinical strategy that expedites approval of C-Pulse therapy • Announced stopping enrollment in the US COUNTER HF clinical trial and the EU OPTIONS HF post-market study in order to re-focus resources • Recently published data from OPTIONS HF trial shows meaningful improvements in LVEF and strong trends of improved functional capacity • Research supports belief that C-Pulse mechanism of action may be both hemodynamic and a neuromodulation effect • Ended 2015 with $23.1M in cash and $8M in debt. Reducing operating cash-burn to extend need to raise additional equity into back half of 2016 © 2016 Sunshine Heart, Inc. 4

TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. COUNTER HF US IDE Trial • The Challenges: – Slow enrollment would extend the trial >7 years • Trial requires 388 randomized patients; since September of 2012 only 38 patients have been randomized Implanting current C-Pulse device usually requires an invasive sternotomy (“cracking the chest”) Length of trial increased risk of infection from external drive line Invasiveness and risks resulted in physicians/patients choosing to wait, resulting in sicker patients or less optimal candidates Invasive procedure, external drive line, and length of trial deter Class III patients Principal investigators have recommended protocol changes – – – – – © 2016 Sunshine Heart, Inc. 5
TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. OPTIONS HF Post-market EU Study • Data from 15 patient implants: – – – – – – Short hospital stays and minimal perioperative One patient weaned - asymptomatic complications Clinically significant improvements in ejection fraction Most patients have experienced a reduction in HF class No strokes, clots, bleeding or heart attacks No re-hospitalization for worsening heart failure months 13.3% exit site infection rate in first 6 – © 2016 Sunshine Heart, Inc. 6
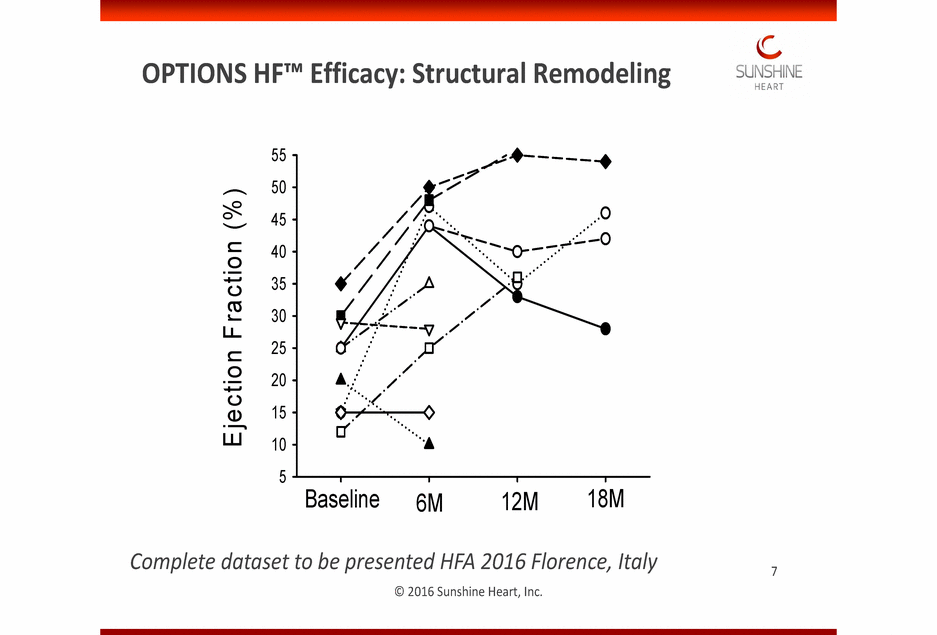
TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. OPTIONS HF™ Efficacy: Structural Remodeling 55 50 45 40 35 30 25 20 15 10 5 Baseline 18M 12M 6M Complete dataset to be presented HFA 2016 Florence, Italy © 2016 Sunshine Heart, Inc. 7 E jection F rac tion (% )
TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. Revised Clinical Strategy • The Company’s Feasibility Study (20 patients) and OPTIONS HF post-marketing study (15 patients) provided very positive results for counterpulsation, but still represents limited clinical evidence A shorter clinical trial can generate needed incremental data demonstrating clinical benefits, which will expedite the US regulatory approval of the fully implantable device Physician investigators believe current CP-1 implant procedure modified to be minimally invasive with a 12-month drive line limit would have commercial value as an acute therapy Our short-term clinical plan is to submit an application for a US IDE clinical study in 2016 utilizing the CP-1 device to gain the needed counterpulsation clinical evidence and approval to commercialize in the US Our longer-term clinical plan is to submit an application for a US IDE clinical study utilizing the fully implantable device for approval to commercialize in the US © 2016 Sunshine Heart, Inc. • • • • 8
TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. New 2016 US Clinical Trial Objectives • • • • Implant will be a minimally invasive procedure External drive line will be removed after 12-months Quantify staying power of > 6-12 month patient benefits Results to be demonstrated in a shorter time frame than COUNTER by winning primary endpoint well-known to predict patient outcome Further establish the benefits of both acute and chronic C-Pulse therapy using objective variables and control (therapy off) study periods • © 2016 Sunshine Heart, Inc. 9
TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. C-Pulse: Unique Therapy for HF • Placement on ascending aorta optimal location for hemodynamic and neural effects; strong IP protection • Compatible with any device and pharmacologic therapy • Mechanical compression and rapid pressure changes optimal for stimulation of baroreceptors • Automatic ‘dose’ mode: increased demand met by increased frequency of counterpulsation • Automatic adjustment to CRT timing changes • Ideal system to implement weaning protocol due to modular nature, non-obligatory therapy, extra-vascular implant © 2016 Sunshine Heart, Inc. 10
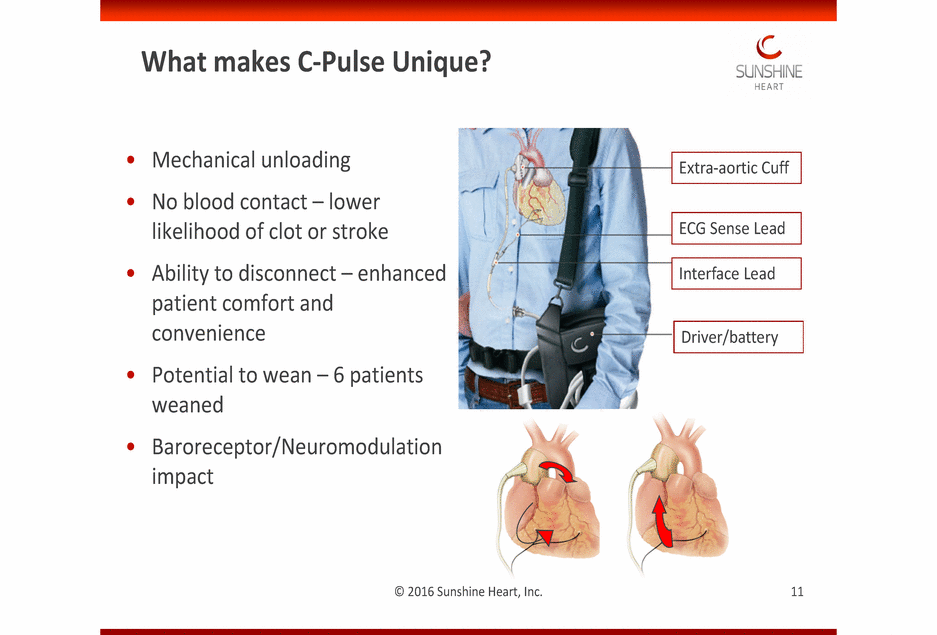
TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. What makes C-Pulse Unique? • • Mechanical unloading No blood contact – lower likelihood of clot or stroke Ability to disconnect – enhanced patient comfort and convenience Potential to wean – 6 patients weaned Baroreceptor/Neuromodulation impact • • • © 2011 Sunshine Heart, Inc. © 2016 Sunshine Heart, Inc. 11 Driver/battery Interface Lead ECG Sense Lead Extra-aortic Cuff
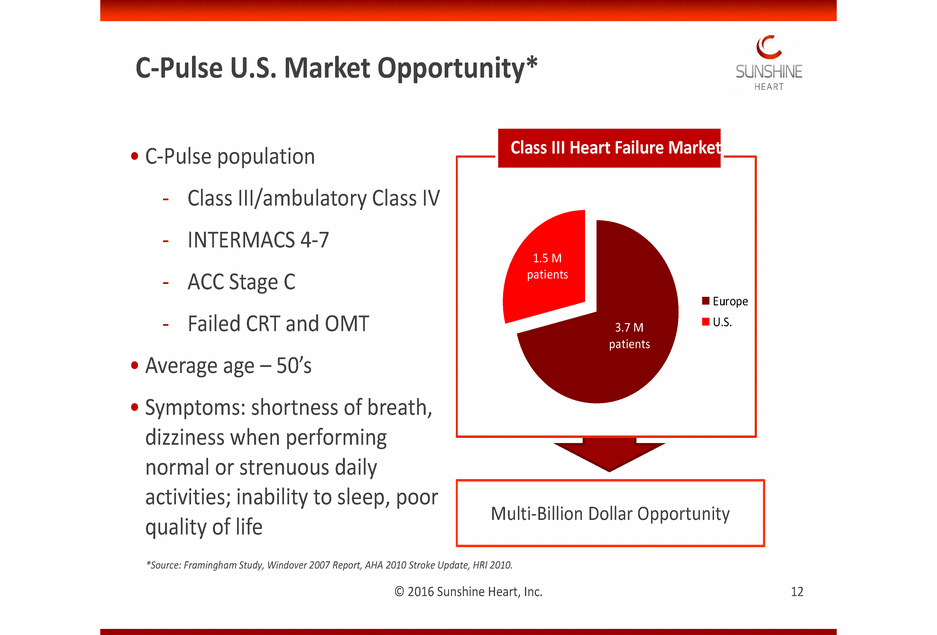
TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. C-Pulse U.S. Market Opportunity* • C-Pulse population Class III Heart Failure Market - - - - Class III/ambulatory INTERMACS 4-7 ACC Stage C Failed CRT and OMT Class IV 1.5 M patients Europe U.S. 3.7 M patients • • Average age – 50’s Symptoms: shortness of breath, dizziness when performing normal or strenuous daily activities; inability to sleep, poor quality of life Multi-Billion Dollar Opportunity *Source: Framingham Study, Windover 2007 Report, AHA 2010 Stroke Update, HRI 2010. © 2016 Sunshine Heart, Inc. 12
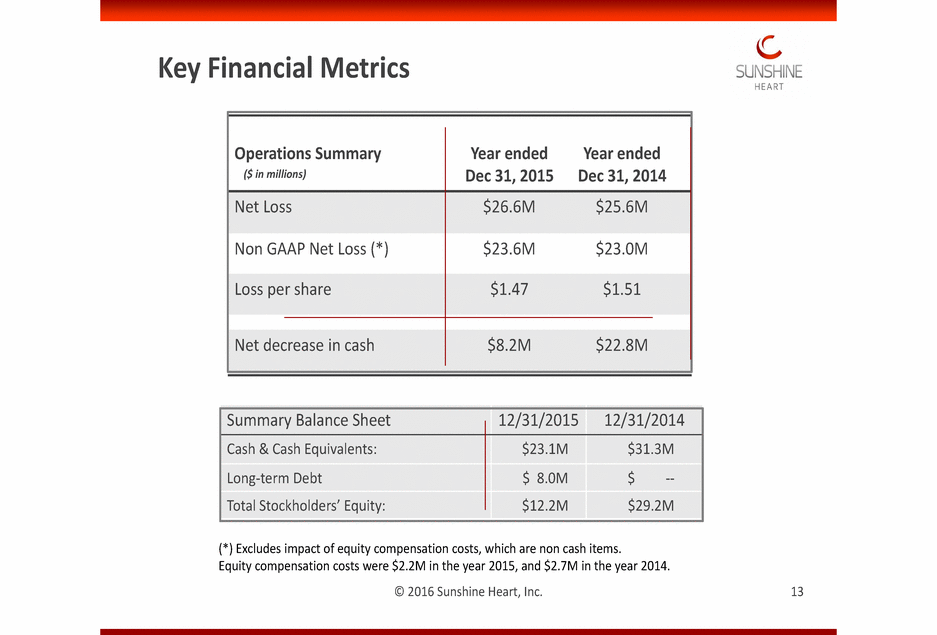
TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. Key Financial Metrics Summary Balance Sheet (*) Excludes impact of equity compensation costs, which are non cash items. Equity compensation costs were $2.2M in the year 2015, and $2.7M in the year 2014. © 2016 Sunshine Heart, Inc. 13 12/31/2015 12/31/2014 Cash & Cash Equivalents: $23.1M $31.3M Long-term Debt $ 8.0M $--Total Stockholders’ Equity: $12.2M $29.2M Operations Summary ($ in millions) Year endedYear ended Dec 31, 2015Dec 31, 2014 Net Loss $26.6M$25.6M Non GAAP Net Loss (*) $23.6M$23.0M Loss per share $1.47$1.51 ($ in thousands) Net decrease in cash $8.2M$22.8M
TThhisis imimagagee ccaannnnoott ccuurrrrenenttllyy bbee didissppllaayy eed.d. Conclusion • Clinical Benefits – C-Pulse provides benefit based on traditional concepts of counterpulsation; increase coronary perfusion and afterload reduction Advanced hemodynamic analysis from patients indicates afterload reduction due to peripheral effects, similar to IABP Late systolic reduction associated with marked vasodilation hypothesized mediated by aortic and carotid baroreceptors Chronic therapy with enhanced coronary perfusion, peripheral vascular unloading, and neurohormonal modulation may provide substrate for chronic remodeling and myocardial recovery – – – • Strategic Direction – – – Implement CP-1 changes to insure minimally invasive and reduced drive line use Increase investment in the development of the fully implantable device Implement a 2-stage clinical strategy • 2016 initiation of US IDE trial for an acute therapy with CP-1 • When ready, initiate US IDE trial for a chronic therapy with fully implantable Research and develop counterpulsation and its neuromodulation effect – © 2016 Sunshine Heart, Inc. 14