Attached files
| file | filename |
|---|---|
| 8-K - 8-K - TESARO, Inc. | a16-5231_18k.htm |
| EX-99.1 - EX-99.1 - TESARO, Inc. | a16-5231_1ex99d1.htm |
Exhibit 99.2
Fourth-Quarter 2015 Results February 25, 2016

Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding TESARO, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "anticipate," "estimate," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. Examples of forward-looking statements contained in this press release include, among others, statements regarding our projected VARUBI P&L break-even revenue run rate, our vision of becoming a leading oncology-focused biopharmaceutical company, our plans to expand niraparib development into additional therapeutic areas, our expectation to have NOVA and QUADRA data in the second quarter of 2016, the expected timing of the oral rolapitant MAA filing, the submission of the NDA for IV rolapitant, the submission of the NDA and MAA for niraparib, and other regulatory filings with respect to our product candidates, the expected timing of data from our various clinical trials, our plans regarding future clinical trials with niraparib, statements regarding our various 2016 corporate goals, the estimated time periods when we expect clinical trials to commence or be completed, statements regarding our expectations about the timing of both the selection of clinical candidates from our immuno-oncology programs and the commencement of clinical testing for those candidates, and statements regarding the potential market opportunity for our products or product candidates. Forward-looking statements in this release involve substantial risks and uncertainties that could cause our research and pre-clinical development programs, clinical development programs, future results, performance, or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the uncertainties inherent in the execution and completion of clinical trials, uncertainties surrounding the timing of availability of data from our clinical trials, risks regarding ongoing discussions with and actions by regulatory authorities, patient accrual rates for clinical trials, risks from competitors, and other matters that could affect the timing of availability of data from or initiation of our clinical trials, uncertainties regarding regulatory approvals, uncertainties regarding certain expenditures, and other matters that could affect the availability or commercial potential of our drug candidates. TESARO undertakes no obligation to update or revise any forward-looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the Company in general, see TESARO's Annual Report on Form 10-K for the year ended December 31, 2014 and its Quarterly Report on Form 10-Q for the quarter ended September 30, 2015.

Lonnie Moulder Chief Executive Officer

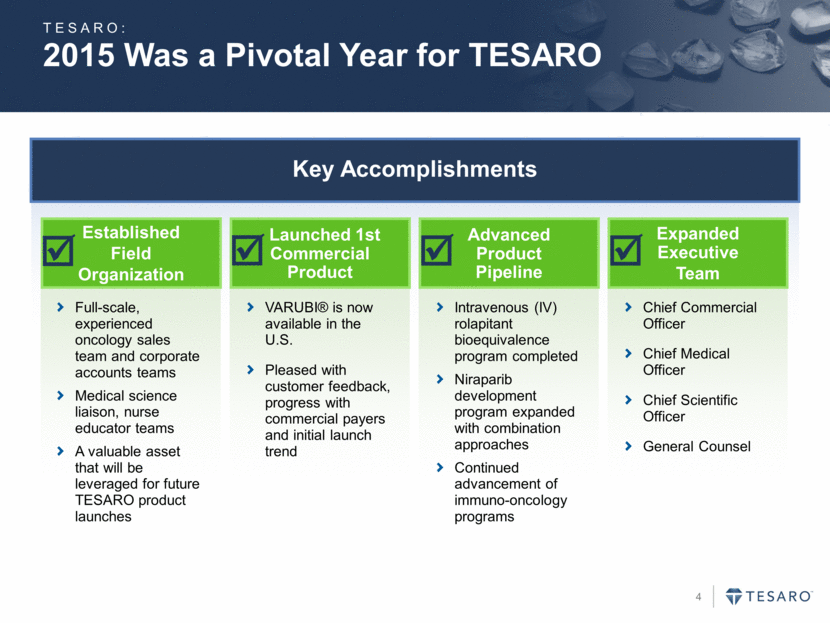
2015 Was a Pivotal Year for TESARO Key Accomplishments Established Field Organization Launched 1st Commercial Product Advanced Product Pipeline Expanded Executive Team Full-scale, experienced oncology sales team and corporate accounts teams Medical science liaison, nurse educator teams A valuable asset that will be leveraged for future TESARO product launches VARUBI® is now available in the U.S. Pleased with customer feedback, progress with commercial payers and initial launch trend Intravenous (IV) rolapitant bioequivalence program completed Niraparib development program expanded with combination approaches Continued advancement of immuno-oncology programs Chief Commercial Officer Chief Medical Officer Chief Scientific Officer General Counsel TESARO:
Approved by FDA on September 1, 2015 1 Provides protection from CINV for the full delayed risk period (25–120 hours) 2 Single oral dose administration just prior to chemotherapy 6 Included in NCCN® Antiemesis Guidelines with a Category 1 Level of Evidence and Consensus for MEC and HEC 3 No dose adjustment for dexamethasone, a CYP3A4 substrate 4 Flexibility to use with any 5-HT3 receptor antagonist 5 IV formulation in development to address entire market VARUBI®: TESARO’S FIRST MARKETED PRODUCT

Strategy to Drive Growth VARUBI®: Establish Oral, Take Share Establish IV, Take Share Grow NK-1 Market Opportunity Each Initial VARUBI Dose May Lead to An Additional Three to Four Doses During an Average Course of Chemotherapy Year 1 Year 2 Year 3 Year 4 Volume Oral IV
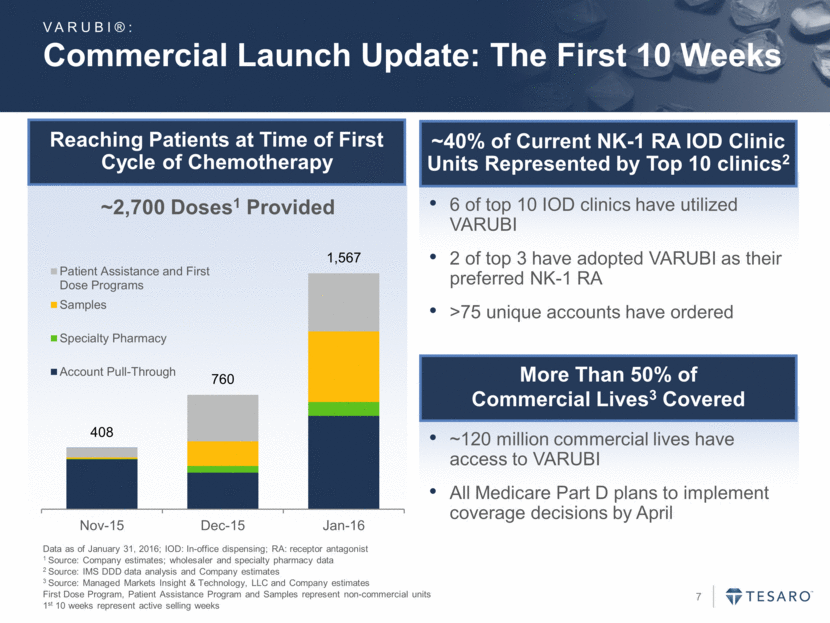
Commercial Launch Update: The First 10 Weeks Data as of January 31, 2016; IOD: In-office dispensing; RA: receptor antagonist 1 Source: Company estimates; wholesaler and specialty pharmacy data 2 Source: IMS DDD data analysis and Company estimates 3 Source: Managed Markets Insight & Technology, LLC and Company estimates First Dose Program, Patient Assistance Program and Samples represent non-commercial units 1st 10 weeks represent active selling weeks VARUBI®: Reaching Patients at Time of First Cycle of Chemotherapy ~40% of Current NK-1 RA IOD Clinic Units Represented by Top 10 clinics2 6 of top 10 IOD clinics have utilized VARUBI 2 of top 3 have adopted VARUBI as their preferred NK-1 RA >75 unique accounts have ordered More Than 50% of Commercial Lives3 Covered ~120 million commercial lives have access to VARUBI All Medicare Part D plans to implement coverage decisions by April ~2,700 Doses1 Provided 408 760 1,567 Nov-15 Dec-15 Jan-16 Patient Assistance and First Dose Programs Samples Specialty Pharmacy Account Pull-Through
Tim Pearson Chief Financial Officer

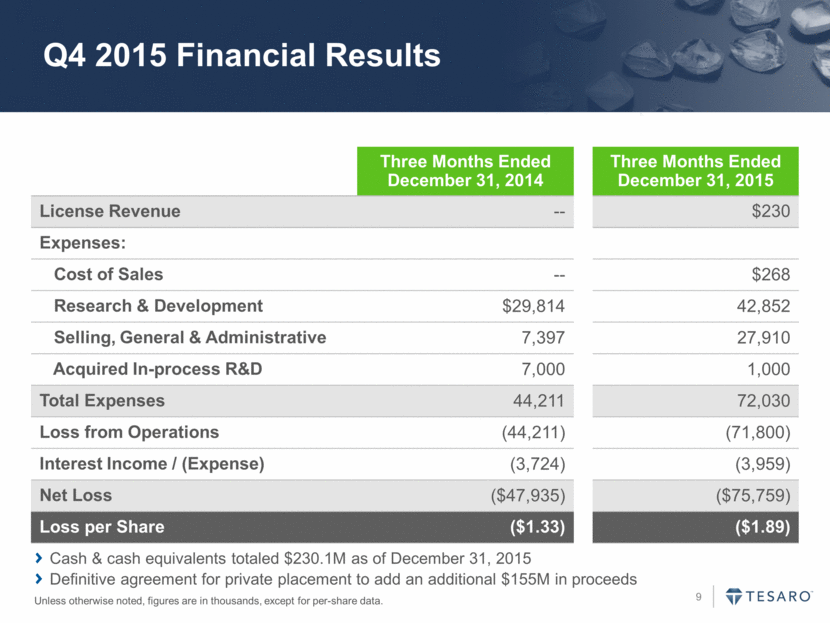
Q4 2015 Financial Results Unless otherwise noted, figures are in thousands, except for per-share data. Three Months Ended December 31, 2014 Three Months Ended December 31, 2015 License Revenue -- $230 Expenses: Cost of Sales -- $268 Research & Development $29,814 42,852 Selling, General & Administrative 7,397 27,910 Acquired In-process R&D 7,000 1,000 Total Expenses 44,211 72,030 Loss from Operations (44,211) (71,800) Interest Income / (Expense) (3,724) (3,959) Net Loss ($47,935) ($75,759) Loss per Share ($1.33) ($1.89) Cash & cash equivalents totaled $230.1M as of December 31, 2015 Definitive agreement for private placement to add an additional $155M in proceeds
Mary Lynne Hedley, Ph.D. President & Chief Operating Officer

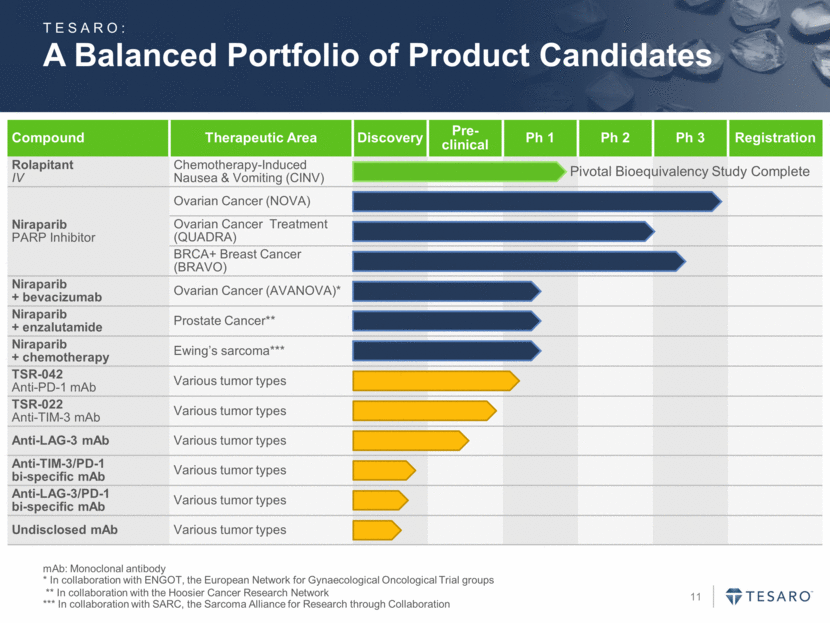
A Balanced Portfolio of Product Candidates mAb: Monoclonal antibody * In collaboration with ENGOT, the European Network for Gynaecological Oncological Trial groups ** In collaboration with the Hoosier Cancer Research Network *** In collaboration with SARC, the Sarcoma Alliance for Research through Collaboration Compound Therapeutic Area Discovery Pre-clinical Ph 1 Ph 2 Ph 3 Registration Rolapitant IV Chemotherapy-Induced Nausea & Vomiting (CINV) Niraparib PARP Inhibitor Ovarian Cancer (NOVA) Ovarian Cancer Treatment (QUADRA) BRCA+ Breast Cancer (BRAVO) Niraparib + bevacizumab Ovarian Cancer (AVANOVA)* Niraparib + enzalutamide Prostate Cancer** Niraparib + chemotherapy Ewing’s sarcoma*** TSR-042 Anti-PD-1 mAb Various tumor types TSR-022 Anti-TIM-3 mAb Various tumor types Anti-LAG-3 mAb Various tumor types Anti-TIM-3/PD-1 bi-specific mAb Various tumor types Anti-LAG-3/PD-1 bi-specific mAb Various tumor types Undisclosed mAb Various tumor types TESARO: Pivotal Bioequivalency Study Complete
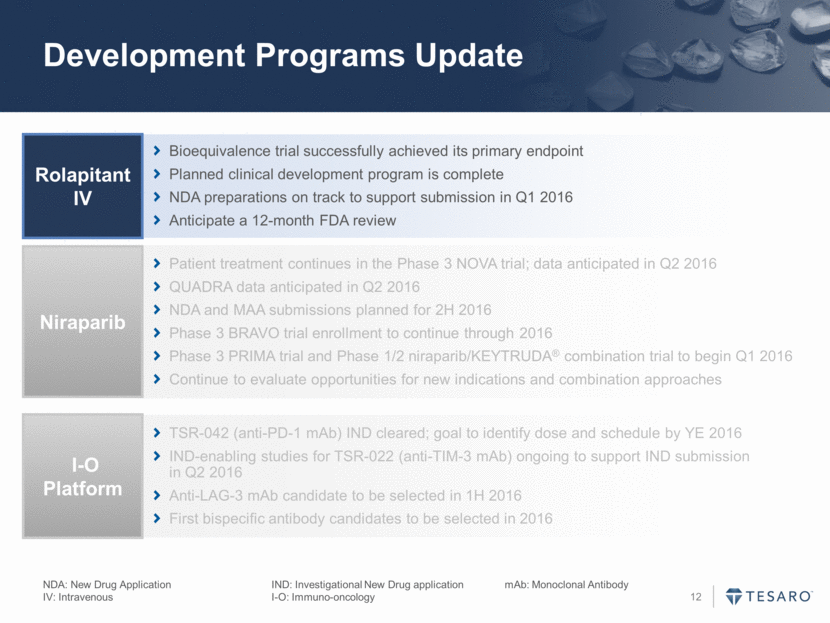
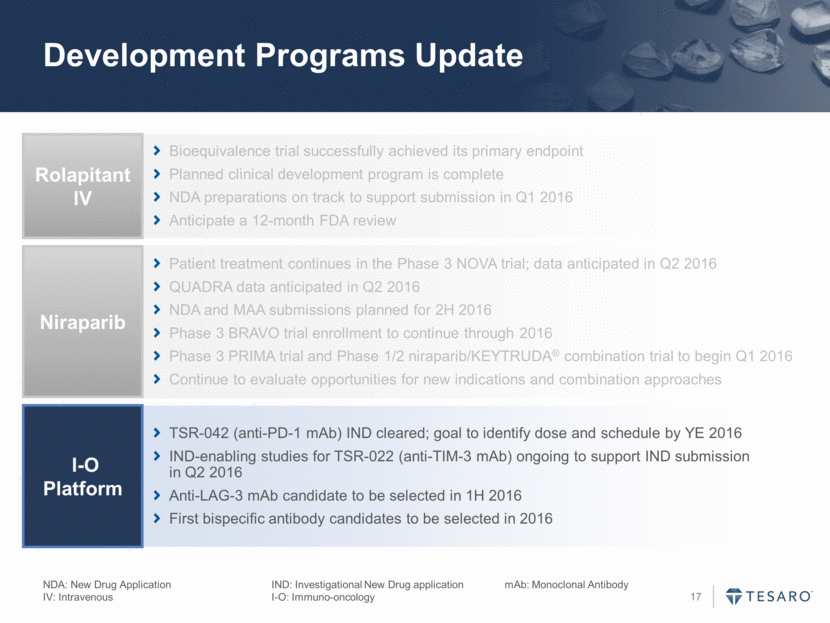
Development Programs Update Bioequivalence trial successfully achieved its primary endpoint Planned clinical development program is complete NDA preparations on track to support submission in Q1 2016 Anticipate a 12-month FDA review Rolapitant IV Patient treatment continues in the Phase 3 NOVA trial; data anticipated in Q2 2016 QUADRA data anticipated in Q2 2016 NDA and MAA submissions planned for 2H 2016 Phase 3 BRAVO trial enrollment to continue through 2016 Phase 3 PRIMA trial and Phase 1/2 niraparib/KEYTRUDA® combination trial to begin Q1 2016 Continue to evaluate opportunities for new indications and combination approaches Niraparib TSR-042 (anti-PD-1 mAb) IND cleared; goal to identify dose and schedule by YE 2016 IND-enabling studies for TSR-022 (anti-TIM-3 mAb) ongoing to support IND submission in Q2 2016 Anti-LAG-3 mAb candidate to be selected in 1H 2016 First bispecific antibody candidates to be selected in 2016 I-O Platform NDA: New Drug Application IND: Investigational New Drug application mAb: Monoclonal Antibody IV: Intravenous I-O: Immuno-oncology
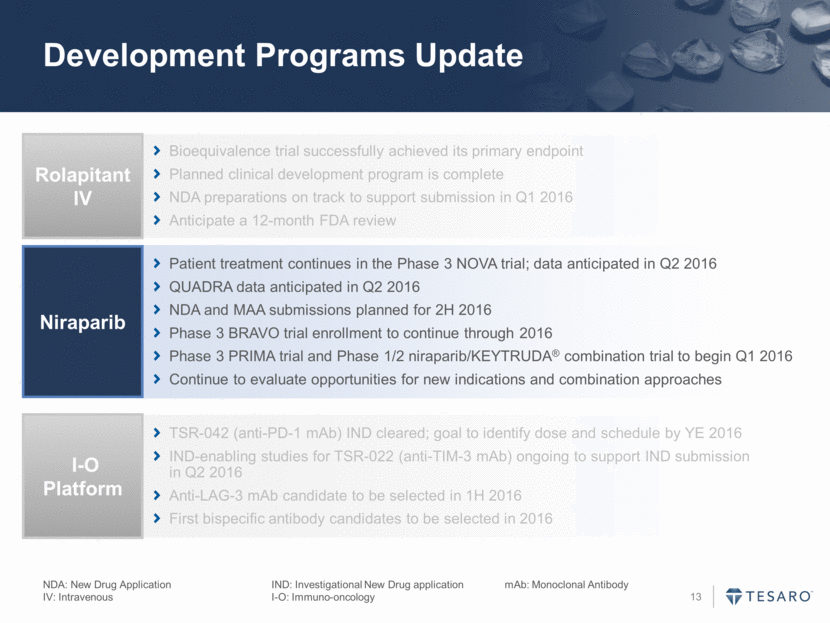
Development Programs Update Bioequivalence trial successfully achieved its primary endpoint Planned clinical development program is complete NDA preparations on track to support submission in Q1 2016 Anticipate a 12-month FDA review Rolapitant IV Patient treatment continues in the Phase 3 NOVA trial; data anticipated in Q2 2016 QUADRA data anticipated in Q2 2016 NDA and MAA submissions planned for 2H 2016 Phase 3 BRAVO trial enrollment to continue through 2016 Phase 3 PRIMA trial and Phase 1/2 niraparib/KEYTRUDA® combination trial to begin Q1 2016 Continue to evaluate opportunities for new indications and combination approaches Niraparib TSR-042 (anti-PD-1 mAb) IND cleared; goal to identify dose and schedule by YE 2016 IND-enabling studies for TSR-022 (anti-TIM-3 mAb) ongoing to support IND submission in Q2 2016 Anti-LAG-3 mAb candidate to be selected in 1H 2016 First bispecific antibody candidates to be selected in 2016 I-O Platform NDA: New Drug Application IND: Investigational New Drug application mAb: Monoclonal Antibody IV: Intravenous I-O: Immuno-oncology
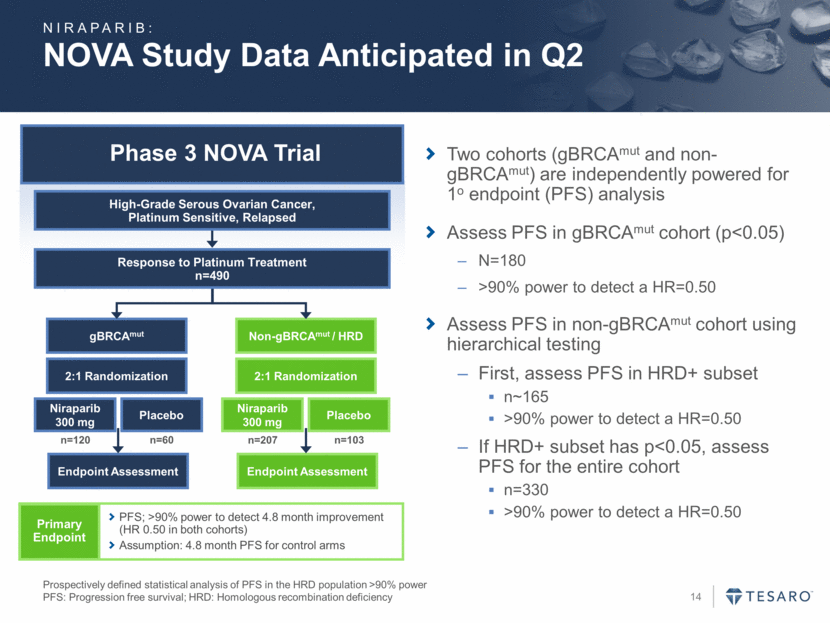
Phase 3 NOVA Trial NOVA Study Data Anticipated in Q2 Prospectively defined statistical analysis of PFS in the HRD population >90% power PFS: Progression free survival; HRD: Homologous recombination deficiency gBRCAmut Endpoint Assessment Niraparib 300 mg Placebo Non-gBRCAmut / HRD Endpoint Assessment Niraparib 300 mg Placebo 2:1 Randomization 2:1 Randomization n=120 n=60 n=207 n=103 Response to Platinum Treatment n=490 High-Grade Serous Ovarian Cancer, Platinum Sensitive, Relapsed Primary Endpoint PFS; >90% power to detect 4.8 month improvement (HR 0.50 in both cohorts) Assumption: 4.8 month PFS for control arms Two cohorts (gBRCAmut and non-gBRCAmut) are independently powered for 1o endpoint (PFS) analysis Assess PFS in gBRCAmut cohort (p<0.05) N=180 >90% power to detect a HR=0.50 Assess PFS in non-gBRCAmut cohort using hierarchical testing First, assess PFS in HRD+ subset n~165 >90% power to detect a HR=0.50 If HRD+ subset has p<0.05, assess PFS for the entire cohort n=330 >90% power to detect a HR=0.50 NIRAPARIB:
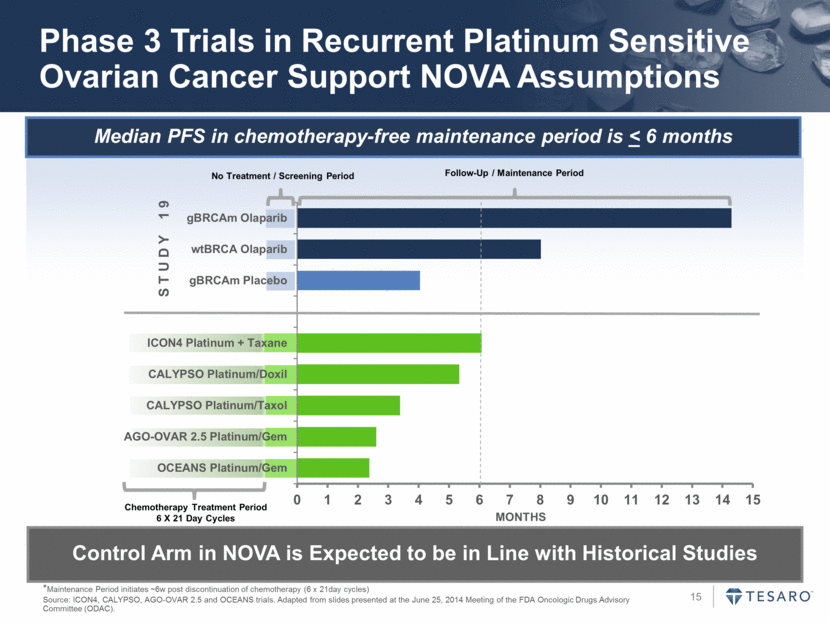
Median PFS in chemotherapy-free maintenance period is < 6 months Phase 3 Trials in Recurrent Platinum Sensitive Ovarian Cancer Support NOVA Assumptions *Maintenance Period initiates ~6w post discontinuation of chemotherapy (6 x 21day cycles) Source: ICON4, CALYPSO, AGO-OVAR 2.5 and OCEANS trials. Adapted from slides presented at the June 25, 2014 Meeting of the FDA Oncologic Drugs Advisory Committee (ODAC). Control Arm in NOVA is Expected to be in Line with Historical Studies No Treatment / Screening Period Chemotherapy Treatment Period 6 X 21 Day Cycles Follow-Up / Maintenance Period S T U D Y 1 9 MONTHS 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 OCEANS Platinum/Gem AGO-OVAR 2.5 Platinum/Gem CALYPSO Platinum/Taxol CALYPSO Platinum/Doxil ICON4 Platinum + Taxane gBRCAm Placebo wtBRCA Olaparib gBRCAm Olaparib
Additional Monotherapy and Combination Trials NIRAPARIB: QUADRA trial of niraparib for ovarian cancer treatment PRIMA trial of niraparib for first line ovarian cancer BRAVO trial of niraparib for patients with gBRCAmut advanced/metastatic breast cancer treatment Niraparib + bevacizumab (AVANOVA) combination for ovarian cancer Niraparib + pembrolizumab combination for ovarian or triple negative breast cancer Niraparib + enzalutamide combination for metastatic castration-resistant prostate cancer Currently Ongoing Trials Other tumor types to potentially include: Non-small cell lung cancer Small cell lung cancer Prostate cancer Combination and monotherapy approaches Planned Trials
Development Programs Update NDA: New Drug Application IND: Investigational New Drug application mAb: Monoclonal Antibody IV: Intravenous I-O: Immuno-oncology Bioequivalence trial successfully achieved its primary endpoint Planned clinical development program is complete NDA preparations on track to support submission in Q1 2016 Anticipate a 12-month FDA review Rolapitant IV Patient treatment continues in the Phase 3 NOVA trial; data anticipated in Q2 2016 QUADRA data anticipated in Q2 2016 NDA and MAA submissions planned for 2H 2016 Phase 3 BRAVO trial enrollment to continue through 2016 Phase 3 PRIMA trial and Phase 1/2 niraparib/KEYTRUDA® combination trial to begin Q1 2016 Continue to evaluate opportunities for new indications and combination approaches Niraparib TSR-042 (anti-PD-1 mAb) IND cleared; goal to identify dose and schedule by YE 2016 IND-enabling studies for TSR-022 (anti-TIM-3 mAb) ongoing to support IND submission in Q2 2016 Anti-LAG-3 mAb candidate to be selected in 1H 2016 First bispecific antibody candidates to be selected in 2016 I-O Platform
Lonnie Moulder Chief Executive Officer

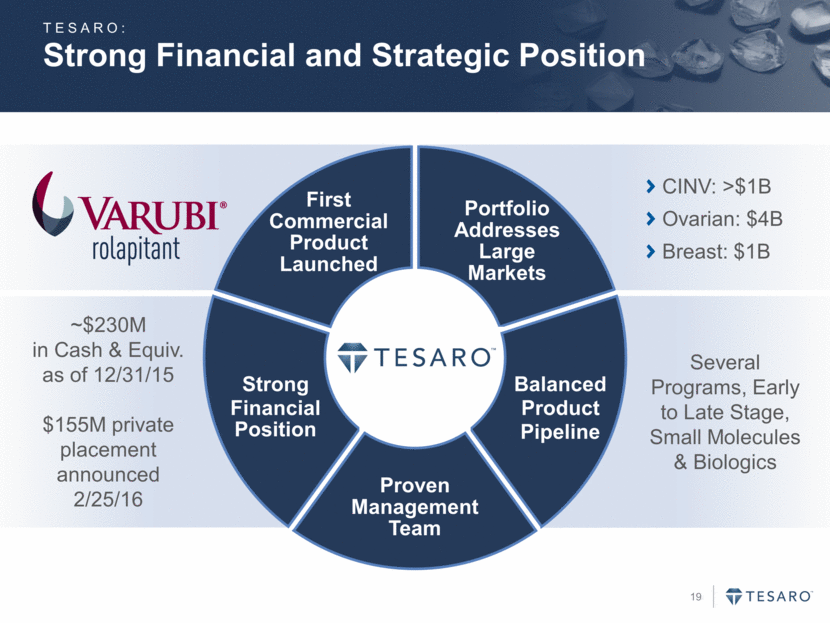
Strong Financial and Strategic Position TESARO: First Commercial Product Launched Portfolio Addresses Large Markets Balanced Product Pipeline Strong Financial Position Proven Management Team CINV: >$1B Ovarian: $4B Breast: $1B Several Programs, Early to Late Stage, Small Molecules & Biologics ~$230M in Cash & Equiv. as of 12/31/15 $155M private placement announced 2/25/16
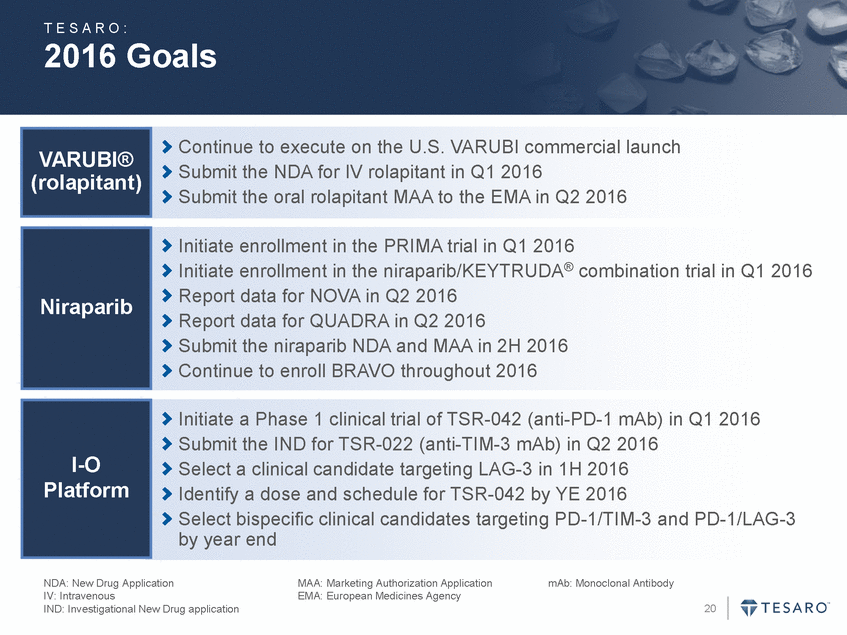
T E S A R O : 2016 Goals Continue to execute on the U.S. VARUBI commercial launch Submit the NDA for IV rolapitant in Q1 2016 Submit the oral rolapitant MAA to the EMA in Q2 2016 VARUBI® (rolapitant) Initiate enrollment in the PRIMA trial in Q1 2016 Initiate enrollment in the niraparib/KEYTRUDA® combination trial in Q1 2016 Report data for NOVA in Q2 2016 Report data for QUADRA in Q2 2016 Submit the niraparib NDA and MAA in 2H 2016 Continue to enroll BRAVO throughout 2016 Niraparib Initiate a Phase 1 clinical trial of TSR-042 (anti-PD-1 mAb) in Q1 2016 Submit the IND for TSR-022 (anti-TIM-3 mAb) in Q2 2016 Select a clinical candidate targeting LAG-3 in 1H 2016 Identify a dose and schedule for TSR-042 by YE 2016 Select bispecific clinical candidates targeting PD-1/TIM-3 and PD-1/LAG-3 by year end I-O Platform NDA: New Drug Application IV: Intravenous IND: Investigational New Drug application MAA: Marketing Authorization Application EMA: European Medicines Agency mAb: Monoclonal Antibody 20
[LOGO]

Fourth-Quarter 2015 Results February 25, 2016

