Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Neurotrope, Inc. | v432481_8k.htm |
Exhibit 99.1

Improving the lives of patients with Alzheimer’s Disease and other Cognitive and Neurodevelopmental disorders February 2016

2 Certain statements in this presentation, particularly those pertaining to our strategy, constitute forward - looking statements . Such statements are based upon the current beliefs and expectations of management and are subject to significant risks and uncertainties . Actual results may differ materially from those set forth in the forward - looking statements . Any statements that are not statements of historical fact (including statements containing the words “ believes, ” “ plans, ” “ anticipates, ” “ expects, ” “ estimates ” and similar expressions) should also be considered to be forward - looking statements . There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements . These factors are contained in Neurotrope Inc . ’s filings with the SEC, including Neurotrope’s Annual Report on Form 10 - K for the year ended December 31 , 2014 and registration statement on Form S - 1 filed on January 14 , 2016 . We encourage all viewers of this presentation to review the aforementioned filings . All statements contained in this presentation are made only as of the date of this presentation, and we do not undertake any obligation to publicly update any forward looking statements . THESE MATERIALS DO NOT CONSTITUTE AN OFFER TO SELL, OR THE SOLICITATION OF ANY OFFER TO BUY, ANY SECURITIES OF THE COMPANY OR ANY ENTITY WHATSOEVER . ANY SUCH OFFER MAY ONLY BE MADE BY A PRIVATE PLACEMENT MEMORANDUM OR PROSPECTUS ISSUED BY THE COMPANY . ANY REPRESENTATION TO THE CONTRARY BY ANY PARTY SHOULD BE IGNORED . The full text of Neurotrope’s SEC filings can be found at the SEC ’ s website (http://www.sec.gov) Safe Harbor Statement
Overview ; Attacking Alzheimer’s disease (AD) with a disruptive therapy – with the objective of reversing disease and slowing progression ; Neurotrope’s Bryostatin approach uses novel mechanisms for treatment of cognitive and neurodevelopmental disorders through Protein Kinase C (PKC) activation, which drives synaptogenesis, thus enhancing memory and learning ; History of clinical progress ; Extensive safety data from over 1,400 patients ; Compassionate Use – Bryostatin indicated disease reversal in severe population ; Phase 2a AD safety trial completed; all primary endpoints met ; Proof - of - Concept phase 2b trial for AD underway; 150 patient study in moderately severe to severe AD ; Active orphan disease programs in Fragile X Syndrome (FXS ) – granted Orphan Drug Designation by FDA - and Niemann - Pick Type C disease (NPC ) 3

The Neurotrope Story ; Founded in 2013 to develop and commercialize Bryostatin as a disruptive approach with the potential to reverse AD ; Patented technology licensed from Blanchette Rockefeller Neurosciences Institute (BRNI) ; Over $200 million invested in R&D by the NINDS of the NIH and BRNI; $40 million invested in NTRP ; Extensive publications in peer reviewed journals 4
Large and Growing Alzheimer’s Market ; 5.1 million Americans age 65+ with Alzheimer’s Disease in 2015; projected to reach 7.1 million by 2025 and 13.8 million by 2050 ; Estimated 700,000 Alzheimer’s deaths among Americans age 65 and older in 2015 ; Alzheimer’s deaths increased 71% from 2000 to 2013 ; Alzheimer’s and other dementias cost U.S. healthcare system $226 billion in 2015; could increase to $1.1 trillion by 2050 5 AD landscape characterized by limited clinical success, creating huge unmet medical need

6 Bryostatin Milestones 2016 First patients dosed in Phase 2b trial 2005 - 2011 Annual NCI safety data available; total >1,400 patients 2012 Neurotrope formed 2013 First financing completed First FDA compassionate use treatment approved 2014 FDA approves Phase 2a trial protocols FDA approves multi - dose six month trial in severe Alzheimer’s Disease patients Compassionate Use treatment continues – beneficial results observed 2015 Phase 2a trial completed Phase 2a safety results available – no adverse events reported; increased PKC ϵ levels Compassionate Use treatment continues – additional beneficial results observed 2017 1Q - Interim Phase 2b data expected 2Q – Full Phase 2B data expected

Bryostatin Background ; Derived from marine organism, Bugula Neritina ; Pioneering science published by CSO, Dr. Daniel Alkon, former Chief, Laboratory of Adaptive Systems, National Institutes of Neurological Diseases and Stroke at NIH; Scientific Director of BRNI; Toyota Chair in Neurodegenerative Disease ; Potent PKC ϵ activator Platform ; Promotes synaptic function, synaptogenesis, amyloid - β degradation, cognitive enhancement ; Potential broad application in other neurodegenerative conditions ; Being synthesized for commercial use under license from Stanford University, led by Dr. Paul Wender, Francis W. Bergstrom Professor of Chemistry 7
Bryostatin and PKC ϵ Activation ; Studies demonstrate that PKC ϵ plays a pivotal role in learning and memory ; Bryostatin , a small molecule, penetrates the blood - brain barrier and activates PKC ϵ , resulting in ; Improved synaptic function ; Promotion of new synapse formation ; Maturation of immature synapses ; Repair of damaged synapses ; PKC ϵ protects against synaptic and neuronal loss, degrades toxic beta amyloid, enhances new synapses and memory, prevents neurofibrillary tangle formation 8 Other Alzheimer’s drugs focus on single pathway; Bryostatin targets multiple pathways
Developmental History ; Preclinical work at BRNI demonstrated improved memory and learning in two different AD mouse models and several other animal models ; Clinical Work ; Safety data from >1,400 patients in NCI - sponsored clinical trials ; FDA approved several Compassionate Use protocols in severe AD patients; three patients treated, longest dosed patient approximately one year ; S ignificant improvement in cognition and activities of daily living over treatment period ; Phase 2a clinical trial completed ; B ryostatin achieved primary safety endpoint ; Demonstrated activation of PKC ϵ target engagement ; Phase 2b proof of concept trial initiated ; Estimated cost - $12 million 9
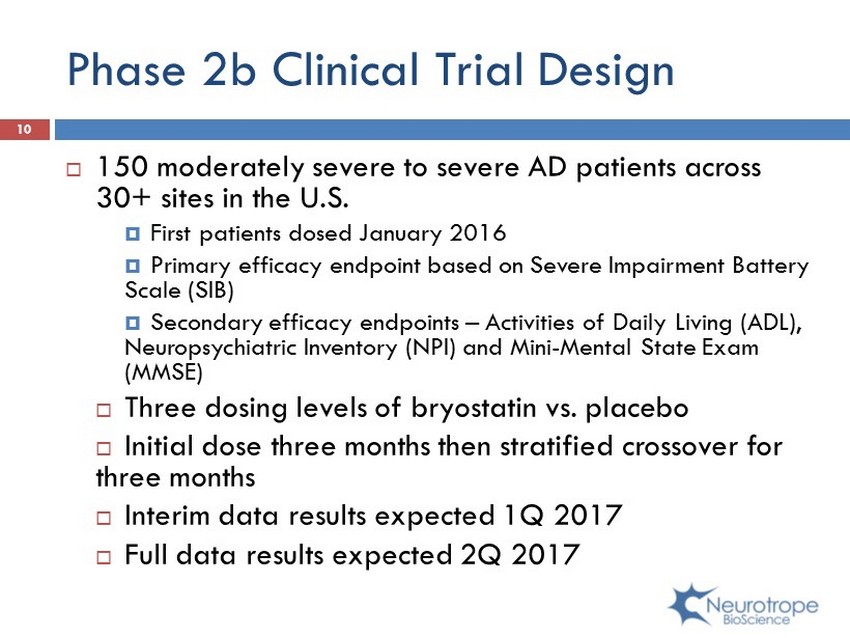
Phase 2b Clinical Trial Design ; 150 moderately severe to severe AD patients across 30+ sites in the U.S. ; First patients dosed January 2016 ; Primary efficacy endpoint based on Severe Impairment Battery Scale (SIB) ; Secondary efficacy endpoints – Activities of Daily Living (ADL), Neuropsychiatric Inventory (NPI) and Mini - Mental State Exam (MMSE) ; Three dosing levels of bryostatin vs. placebo ; Initial dose three months then stratified crossover for three months ; Interim data results expected 1Q 2017 ; Full data results expected 2Q 2017 10
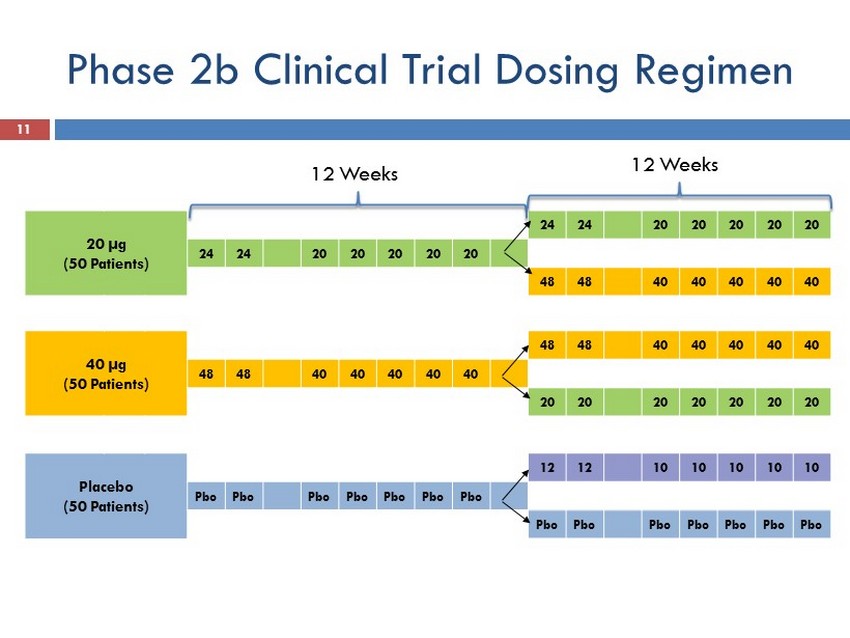
Phase 2b Clinical Trial Dosing Regimen 11 20 µg (50 Patients) 24 24 20 20 20 20 20 24 24 20 20 20 20 20 48 48 40 40 40 40 40 40 µg (50 Patients) 48 48 40 40 40 40 40 48 48 40 40 40 40 40 20 20 20 20 20 20 20 Placebo (50 Patients) 12 12 10 10 10 10 10 Pbo Pbo Pbo Pbo Pbo Pbo Pbo Pbo Pbo Pbo Pbo Pbo Pbo Pbo 12 Weeks 12 Weeks
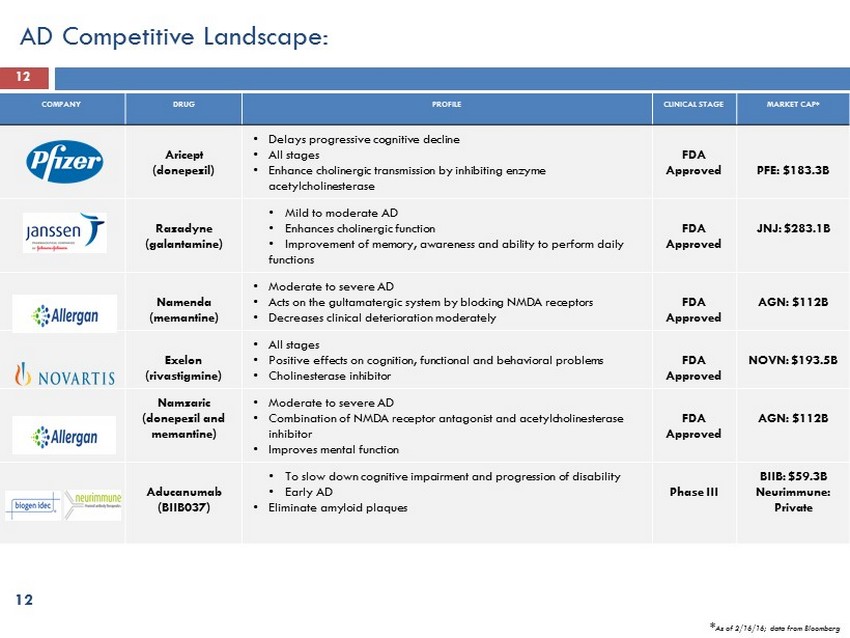
12 COMPANY DRUG PROFILE CLINICAL STAGE MARKET CAP* Aricept (donepezil) • Delays progressive cognitive decline • All stages • Enhance cholinergic transmission by inhibiting enzyme acetylcholinesterase FDA Approved PFE: $183.3B Razadyne (galantamine) • Mild to moderate AD • Enhances cholinergic function • Improvement of memory, awareness and ability to perform daily functions FDA Approved JNJ: $283.1B Namenda (memantine) • Moderate to severe AD • Acts on the gultamatergic system by blocking NMDA receptors • Decreases clinical deterioration moderately FDA Approved AGN: $112B Exelon (rivastigmine) • All stages • Positive effects on cognition, functional and behavioral problems • Cholinesterase inhibitor FDA Approved NOVN: $193.5B Namzaric (donepezil and memantine ) • Moderate to severe AD • Combination of NMDA receptor antagonist and acetylcholinesterase inhibitor • Improves mental function FDA Approved AGN: $112B Aducanumab (BIIB037) • To slow down cognitive impairment and progression of disability • Early AD • Eliminate amyloid plaques Phase III BIIB: $59.3B Neurimmune: Private AD Competitive Landscape: 12 * As of 2/16/16 ; data from Bloomberg
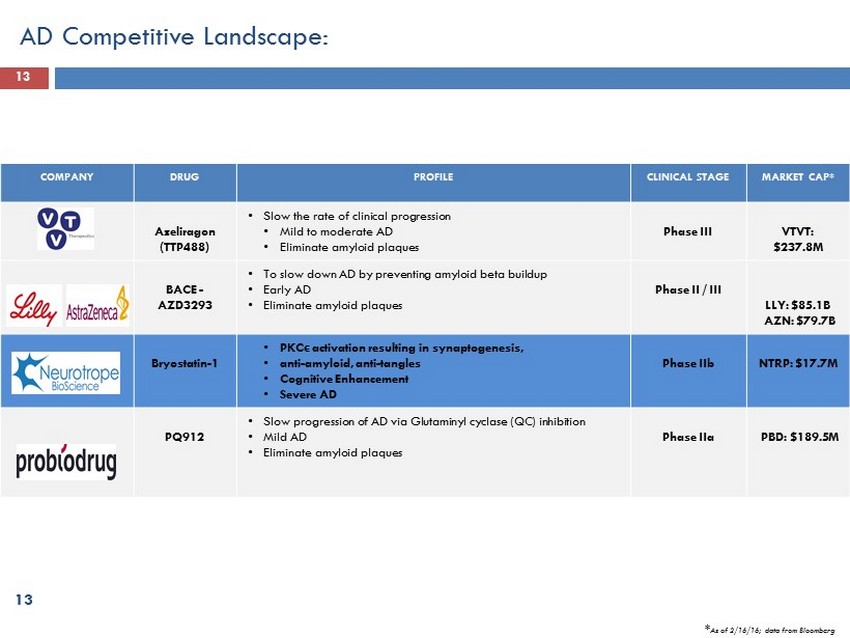
13 COMPANY DRUG PROFILE CLINICAL STAGE MARKET CAP* Azeliragon (TTP488) • Slow the rate of clinical progression • Mild to moderate AD • Eliminate amyloid plaques Phase III VTVT: $237.8M BACE - AZD3293 • To slow down AD by preventing amyloid beta buildup • Early AD • Eliminate amyloid plaques Phase II / III LLY: $85.1B AZN: $79.7B Bryostatin - 1 • PKC ϵ activation resulting in synaptogenesis, • anti - amyloid, anti - tangles • Cognitive Enhancement • Severe AD Phase IIb NTRP: $17.7M PQ912 • Slow progression of AD via Glutaminyl cyclase (QC) inhibition • Mild AD • Eliminate amyloid plaques Phase IIa PBD: $189.5M AD Competitive Landscape: 13 * As of 2/16/16 ; data from Bloomberg
Fragile X Syndrome - Orphan Drug ; Most common single gene cause of intellectual disability ; Part of autism spectrum ; ~135,000 patients in U.S. ; Preclinical cognitive, behavioral, cellular studies performed at BRNI ; Preclinical additional behavioral studies performed at University of Santiago de Chile ; Funded by FRAXA (Premier advocacy group) ; Orphan Drug Designation granted Q1 2015 ; Clinical trial pending appropriate funding 14
Niemann - Pick Type C Disease (NPC) – Orphan Drug ; Lysosomal storage disorder primarily affecting children ; Often causes death within the first two decades of life ; Originates from a gene defect that results in the inability to transport lipids within and between cells ; NTRP signed exclusive licensing agreement with Mt. Sinai School of Medicine (NYC) for the work of Dr. Yiannis Ioannou, an expert in NPC, for use of bryostatin in NPC ; Dr. Ioannou has shown bryostatin can correct the lipid transport defect in NPC cell lines ; NTRP and Mt. Sinai currently investigating bryostatin in NPC mouse model 15

World - Class Scientific Collaborations 16

Leadership Team □ Charles S. Ramat – President, Chief Executive Officer and Board member □ Extensive operational and general business experience in both public and private companies □ Founding investor in Neurotrope □ Track record of increasing shareholder value at micro - and small - cap companies □ J.D. from Columbia Law School □ Paul Freiman – Chairman of the Board □ Seasoned pharmaceutical executive □ Former Chairman and CEO Syntex – Sold to Roche for $5 billion+ □ Board member NovaBay Pharmaceutical ( NYSE MKT: NBY) □ Chairman Chronix BioMedical □ B.S. in pharmacy from Fordham University 17

Leadership Team (cont.) □ Dr. Daniel Alkon – Chief Scientific Officer □ Graduate of Cornell Medical School □ Mt. Sinai Hospital (NY) Internship in Medicine □ 30 year career as Medical Director and Lab Chief in US NIH specializing in memory disorders □ 14 years as founding Scientific Director of BRNI □ Toyota Chair in Neurodegenerative Disease □ Author of over 300 peer - reviewed publications □ Dr. Warren W. Wasiewski – Acting Chief Medical Officer □ Board certified in Neurology and Pediatrics □ Over 30 years of medical and pharmaceutical experience □ Extensive clinical & regulatory experience in Neurology, Pediatrics and Orphan disease space at AstraZeneca, InfaCare and Alexion □ Robert Weinstein, CPA, MBA – Chief Financial Officer □ Experienced healthcare industry CFO and consultant □ Successful healthcare private equity fund manager & investment banker □ MBA from University of Chicago Graduate School of Business 18

AD Clinical Advisory Board □ Dr . Jeffrey L. Cummings , MD, ScD, CCF - Chairman □ Director of Cleveland Clinic Lou Ruvo Center for Brain Health □ Professor of Neurology and Psychiatry, Director of MSE Center for AD Research and Director of the DFJ Center for Neurotherapeutics at UCLA □ Expert in clinical trial design & analysis, global trial implementation, outcome measures □ Authored or edited 30 books and published 600 peer - reviewed papers □ Past President of Behavioral Neurology Society & American Neuropsych. Association. □ Dr . Martin R. Farlow , MD □ Professor and Vice Chairman of Research, Dept. of Neurology Indiana University □ Associate Co - Director of the Indiana AD Center and member of gov’t. AD task force □ Principal Investigator of the Indiana site of the AD Cooperative Study Unit □ Published 200 peer - reviewed papers. □ Dr . Samuel E. Gandy , MD, PhD □ Mount Sinai Chair in AD Research, Professor of Neurology and Psychiatry at Mount Sinai □ Director of the Mount Sinai Center for Cognitive Health and NFL Neurological Care □ Former Chairman of the National Medical & Scientific Advisory Council of the AD Association □ Founding Director of the Farber Institute of Neurosciences, Jefferson Medical College □ Discovered PKC regulation of amyloid precursor phosphorylation and processing. 19

AD Clinical Advisory Board (cont.) □ Dr. Cristina Sampaio , MD, PhD □ Chief Clinical Officer at CHDI Foundation □ Professor of Clinical Pharmacology and Therapeutics University of Lisbon, Portugal □ Former member of the Committee of Proprietary Medicinal Products and the Scientific Advice Working Party at the European Medicines Agency. □ Dr. Michael Weiner, MD □ Professor UCSF School of Medicine in Radiology and Biomedical Imaging □ Principle Investigator of the AD Neuroimaging Initiative □ Educated at Mount Sinai and Yale, previously Assistant Professor of Medicine at Stanford □ Established the Magnetic Resonance Unit at the San Francisco VA Medical Center . 20
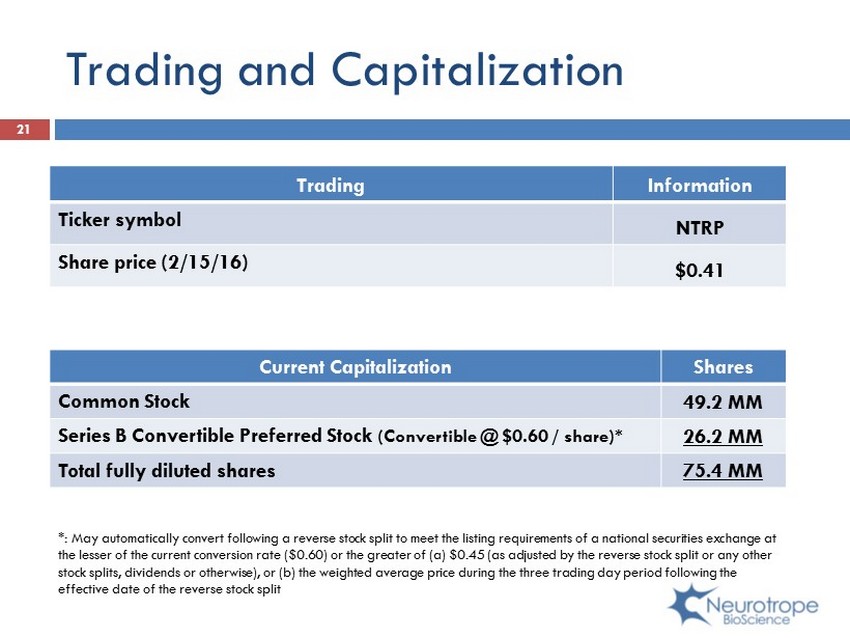
21 Current Capitalization Shares Common Stock 49.2 MM Series B Convertible Preferred Stock (Convertible @ $0.60 / share)* 26.2 MM Total fully diluted shares 75.4 MM Trading Information Ticker symbol NTRP Share price (2/15/16) $0.41 Trading and Capitalization *: May automatically convert following a reverse stock split to meet the listing requirements of a national securities exchan ge at the lesser of the current conversion rate ($0.60) or the greater of (a) $0.45 (as adjusted by the reverse stock split or any oth er stock splits, dividends or otherwise), or (b) the weighted average price during the three trading day period following the effective date of the reverse stock split

Key Takeaways 22 ; Late - stage biotech with Bryostatin - 1, a novel compound for Alzheimer’s Disease – Lead compound in PKC Activator Platform ; New paradigm - p rimary focus is synaptogenesis through PKC ϵ activation while also inhibiting Amyloid Plaque and Tau ; Leveraging >$200 million R&D investment from NINDS (NIH Institute) and Blanchette Rockefeller Neurosciences Institute (BRNI) ; Pioneering science developed by Neurotrope CSO and leading neuroscientist Dr. Daniel Alkon, former Chief of Laboratory of Adaptive Systems, National Institutes of Neurological Diseases and Stroke at NIH, current Director, BRNI ; Demonstrated improvement in compassionate use cases ; Currently enrolling severe patients in Phase 2b proof - of - concept trial; interim data expected 1Q 2017

Appendix
Bryostatin Mechanism of Action ; Activates Synaptic Growth Factors – BDNF, NGF, IGF, others ; Activates All amyloid - β Degrading Enzymes (ECE, Neprilysin, IDE) ; Activates α - secretase, which reduces formation of neurotoxic amyloid - β formed by γ - secretase and BACE ; A poE3 induces PKC ϵ ; by activating PKC ϵ , there is an increase in BDNF expression ; Bryostatin blocks ApoE4 reduction of BDNF via HDAC inhibition ; Bryostatin normalizes GSK3 - β , thereby inhibiting pathological Tau protein transformation into neurofibrillary tangles (NFTs) 24 Activation of Protein Kinase C epsilon (PKC ϵ )
Causes and Consequences of Neurodegeneration 25 ; Numerous elements influence the causes of Alzheimer’s Disease (AD): ; Causal Genes (PS1, PS2): 5% cases ; Risk Genes (ApoE4): 35% cases ; Age: >50% cases ; Two Chief Pathologic Consequences resulting of AD: ; Synaptic Loss ; Neuronal Loss Bryostatin is the only drug in clinical trials that blocks ApoE4
Therapeutic effects of PKC ϵ activation on AD 26 ; ApoE 4: Predominant genetic risk factor for AD ; Bryostatin blocks ApoE 4 via regulation of epigenetic factors, HDACs, and resulting stimulation of PKC ϵ expression, BDNF synthesis, and synaptogenesis ; PKC ϵ deficits observed in AD brains at Autopsy (Harvard Brain/ BRNI) and, in vivo , in cells of AD patients (Diagnostic Biomarkers) ; PKC ϵ protects against synaptic and neuronal loss, degrades toxic beta amyloid, enhances new synapses and memory, prevents neurofibrillary tangle formation ; Bryostatin augments ApoE3 producing increased BDNF levels ; Bryostatin blocks ApoE4 inhibitory effects on BDNF levels
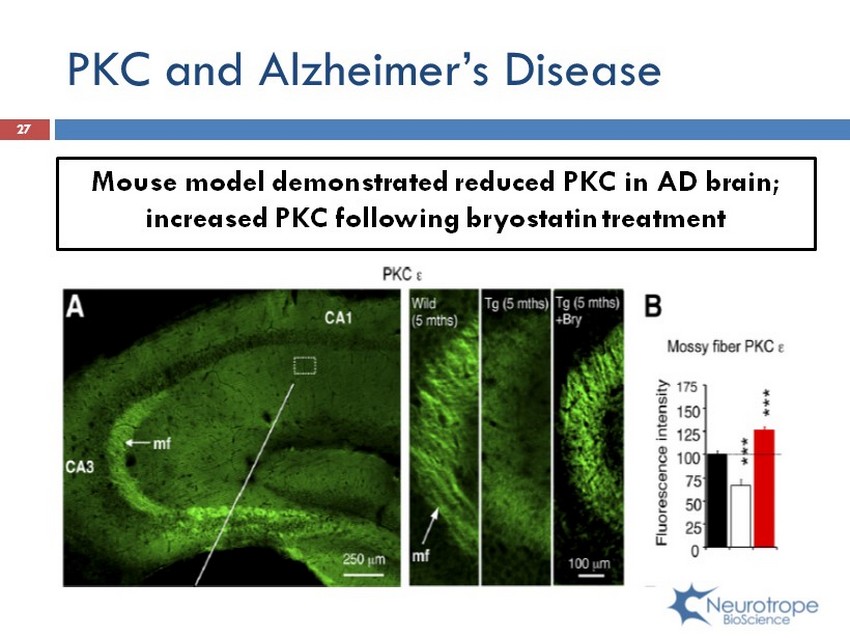
PKC and Alzheimer’s Disease Mouse model demonstrated reduced PKC in AD brain; increased PKC following b ryostatin treatment 27
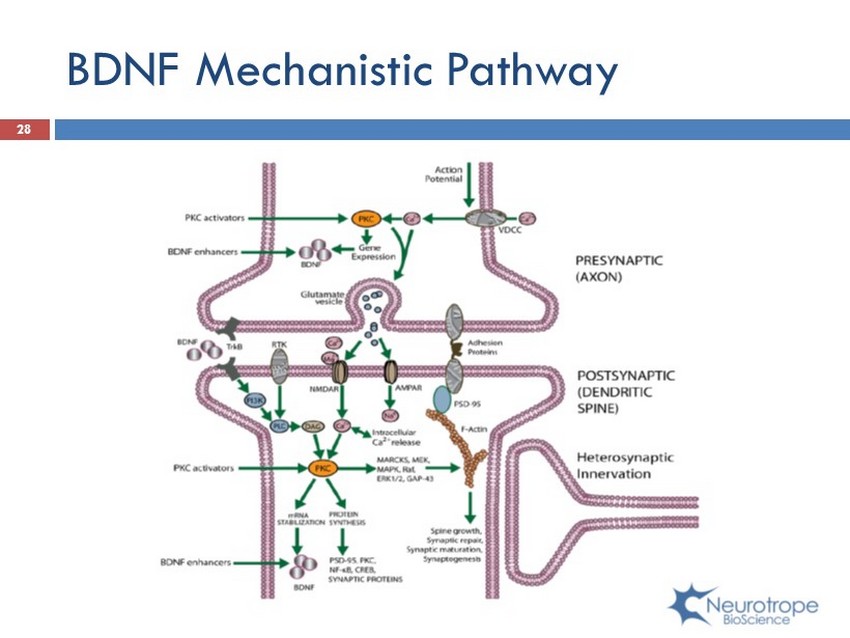
BDNF Mechanistic Pathway 28
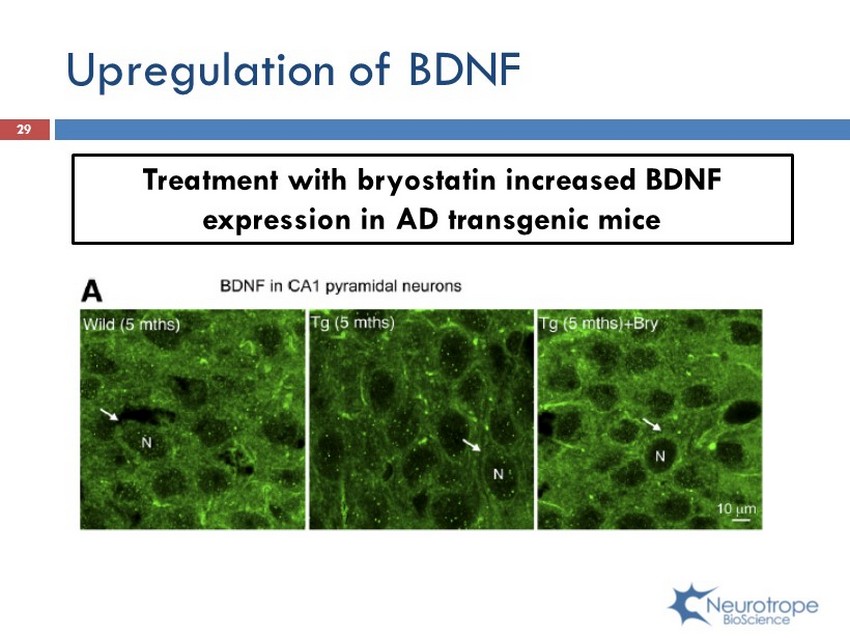
Upregulation of BDNF Treatment with bryostatin increased BDNF expression in AD transgenic mice 29
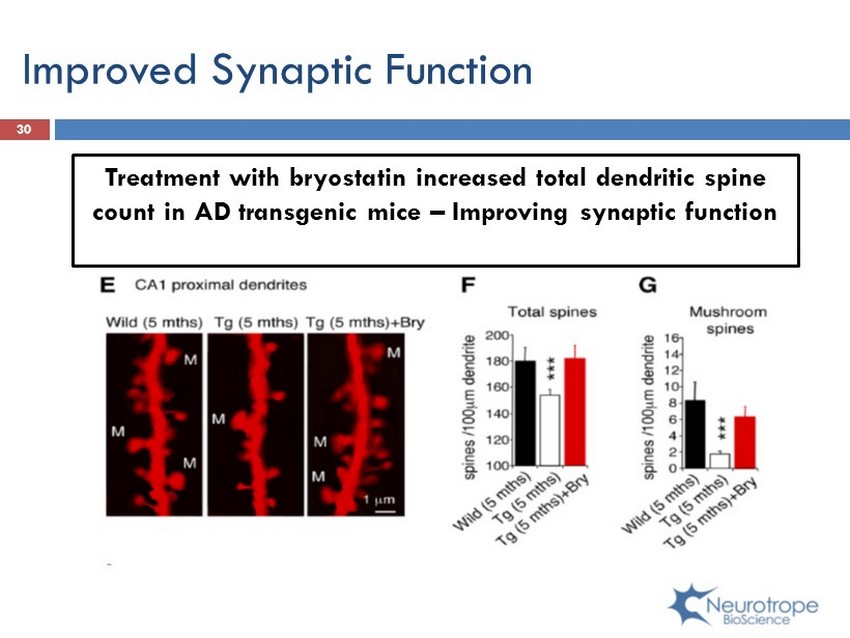
Improved Synaptic F unction Treatment with bryostatin increased total dendritic spine count in AD transgenic mice – Improving synaptic function 30
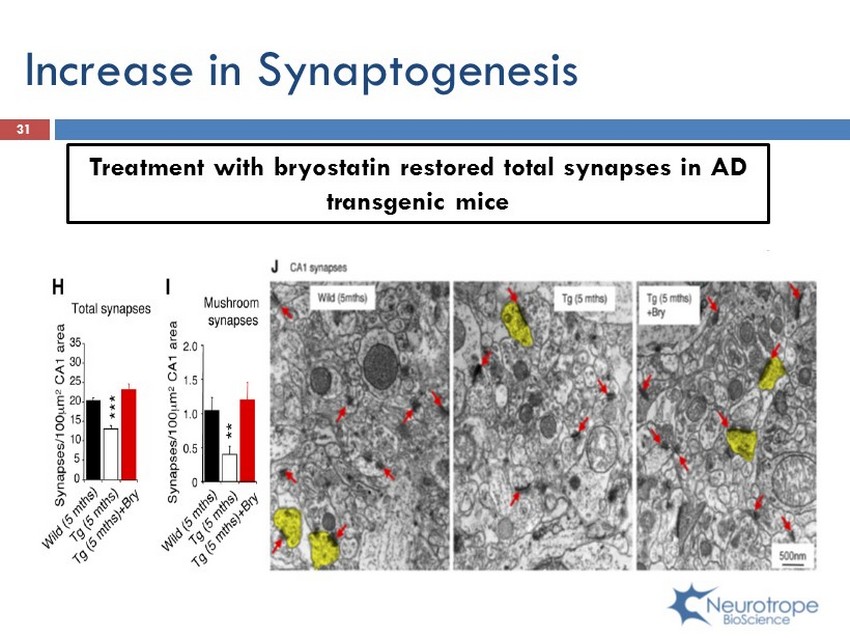
Increase in Synaptogenesis Treatment with bryostatin restored total synapses in AD transgenic mice 31
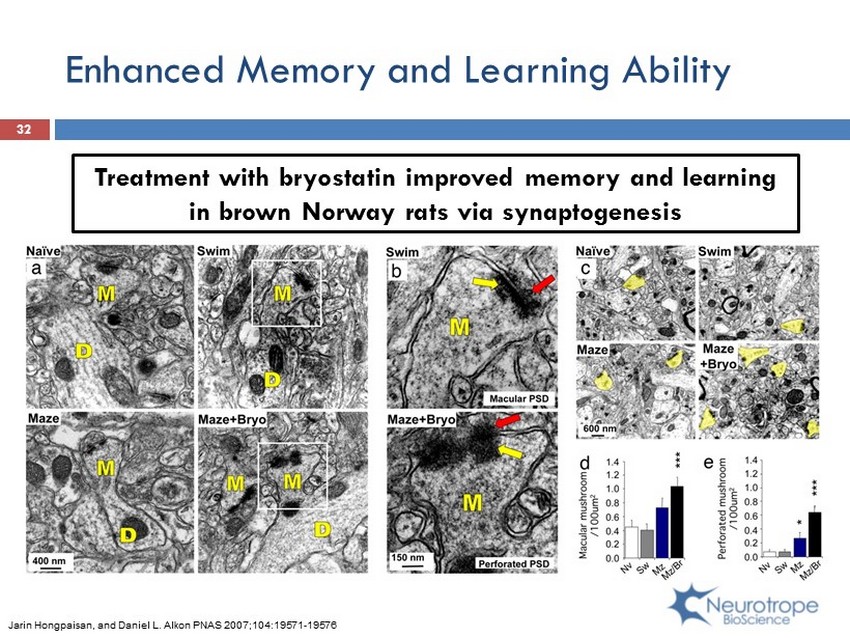
Enhanced Memory and Learning Ability 32 Treatment with bryostatin improved memory and learning in brown Norway rats via synaptogenesis Jarin Hongpaisan, and Daniel L. Alkon PNAS 2007;104:19571 - 19576
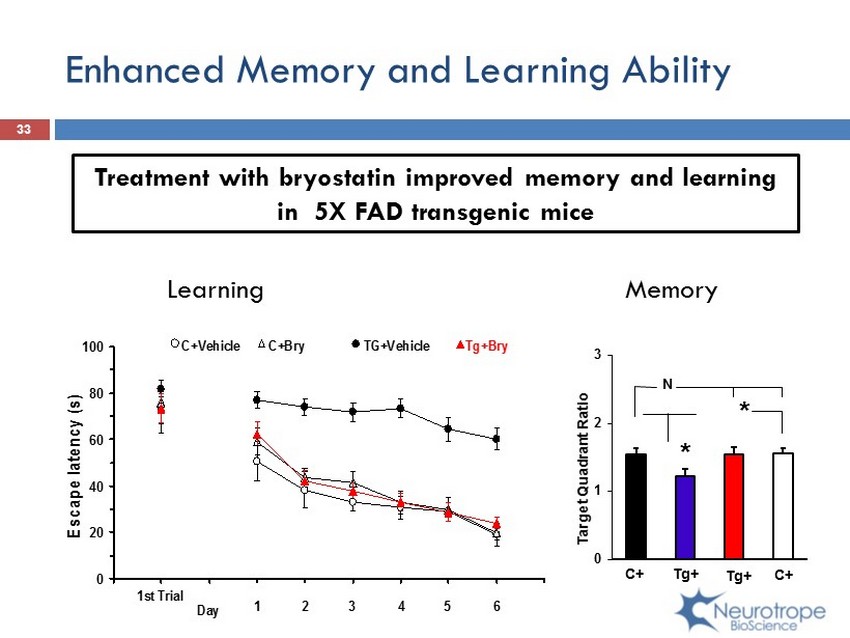
Enhanced Memory and Learning Ability 33 0 20 40 60 80 100 Escape latency (s) C+Vehicle Tg+BryTG+Vehicle 1st Trial Day 1 2 3 4 5 6 C+Bry Treatment with bryostatin improved memory and learning in 5X FAD transgenic mice 0 1 2 3 Target Quadrant Ratio N C+ Tg + Tg + C+ * * Learning Memory
Fragile X Mouse Model Treatment with bryostatin for 13 weeks beginning at two months ; Biochemical and morphologic effects at the synaptic level ; Improved synaptic function ; Synaptic maturation ; Increased synapse formation Synaptic - level effects resulted in improved cognition 34

Fragile X Mouse Model Treatment with bryostatin in FXS mouse restored synapses, improved memory & learning 35
