Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Ignyta, Inc. | d149201dex991.htm |
| 8-K - FORM 8-K - Ignyta, Inc. | d149201d8k.htm |

Catalyzing Precision Medicine with Integrated Rx/Dx in Oncology Ignyta’s Strategic Positioning February 23, 2016 Exhibit 99.2

Safe Harbor Statement This document contains forward-looking statements, as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta, Inc. (“us” or the “Company”). Statements that are not purely historical are forward-looking statements. These include statements regarding, among other things: Ignyta’s corporate and scientific vision and goals, including our ability to reduce the size of tumors and to eradicate residual disease; the clinical and/or non-clinical data or plans underlying entrectinib or any of our other development programs; our ability to design and conduct development activities for entrectinib and our other development programs; our ability to develop or access companion diagnostics for our product candidates; our ability to obtain and maintain intellectual property protection for our product candidates; our ability to adequately fund our development programs; our ability to obtain regulatory approvals in order to market any of our product candidates; and our ability to successfully commercialize any approved products. Forward-looking statements involve known and unknown risks that relate to future events or the Company’s future financial performance, some of which may be beyond our control, and the actual results could differ materially from those discussed in this document. Accordingly, the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, this document. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements, include, among others, the potential for results of past or ongoing clinical or non-clinical studies to differ from expectations or previous results; the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical trials and non-clinical studies; regulatory developments; the potential advantages of our product candidates; the markets any approved products are intended to serve; and our capital needs; as well as those set forth under the headings “Special Note Regarding Forward-Looking Statements,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in the Company’s Form 10-K filed with the Securities and Exchange Commission (“SEC”) on March 12, 2015, and similar disclosures made in the Company’s Form 10-Q filings and other SEC filings and press releases. The forward-looking statements contained in this document represent our estimates and assumptions only as of the date of this document, and we undertake no duty or obligation to update or revise publicly any forward-looking statements contained in this document as a result of new information, future events or changes in our expectations. Third-party information included herein has been obtained from sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by, the Company.

Summary of Ignyta’s Strategic Positioning Leading precision oncology company: Aspirational goal is to eradicate residual disease in precisely defined patient populations by 2030 Integrated approach to Rx/Dx development: Focused on molecularly targeted therapies that also intersect with cancer immunotherapies and cancer stem cell targeted therapies Two late stage product candidates: entrectinib and taladegib with compelling Phase 1 clinical proof of concept, in or soon to be in, potentially registration-enabling Phase 2 studies Prioritized pipeline focused on most attractive programs: Molecularly targeted first-in-class and best-in-class product candidates under clinical development in Rx/Dx core puts Ignyta in strong position to generate multiple clinical data readouts in 2016, while building long-term value for patients and shareholders
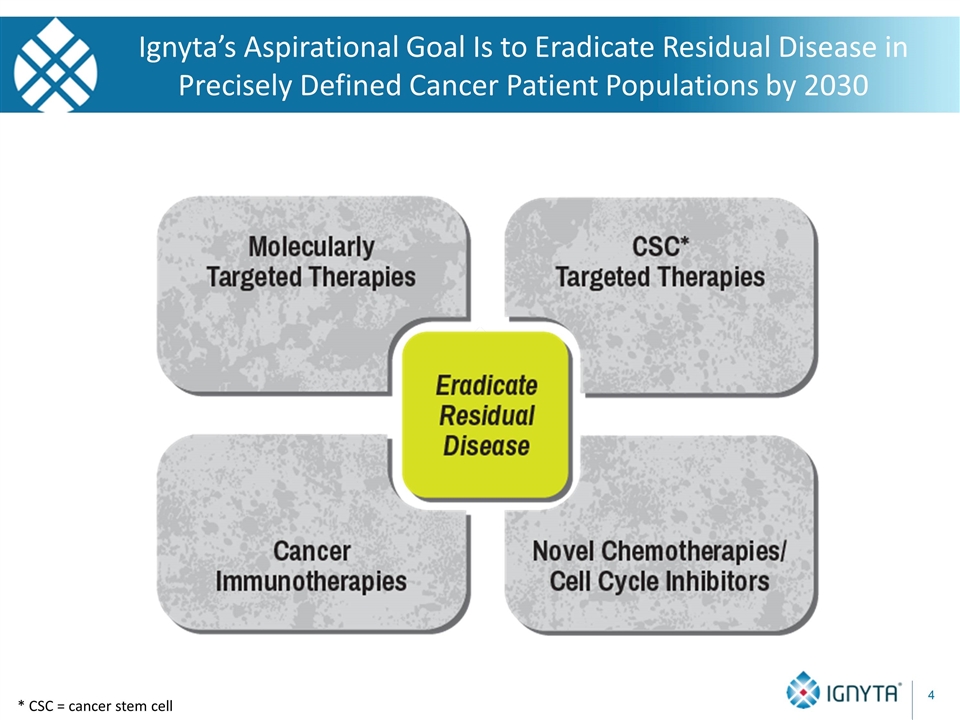
* CSC = cancer stem cell Ignyta’s Aspirational Goal Is to Eradicate Residual Disease in Precisely Defined Cancer Patient Populations by 2030
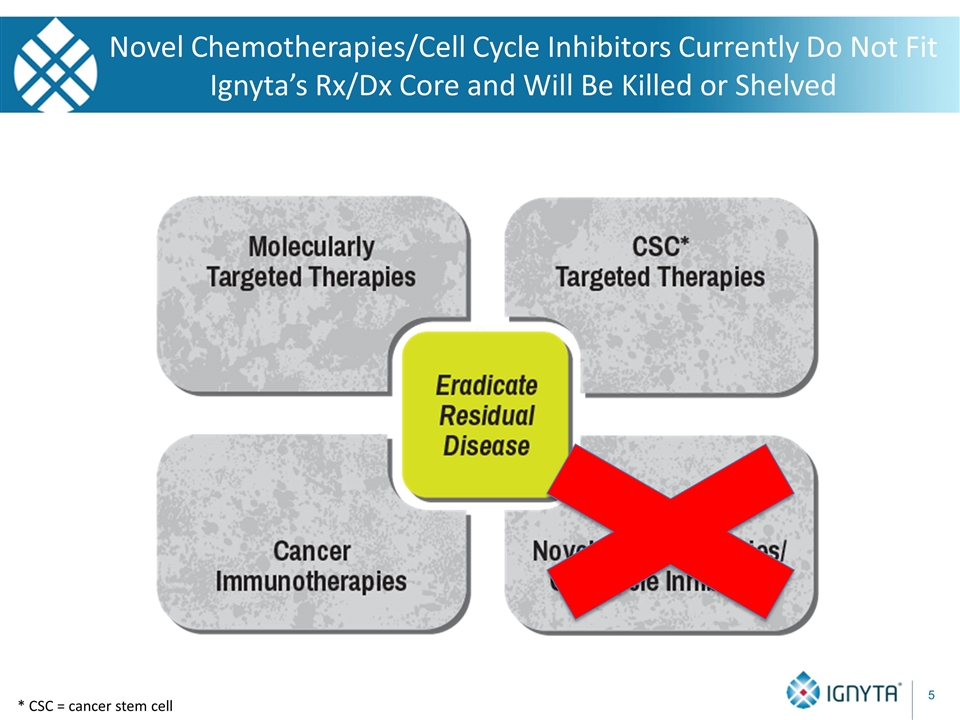
* CSC = cancer stem cell Novel Chemotherapies/Cell Cycle Inhibitors Currently Do Not Fit Ignyta’s Rx/Dx Core and Will Be Killed or Shelved
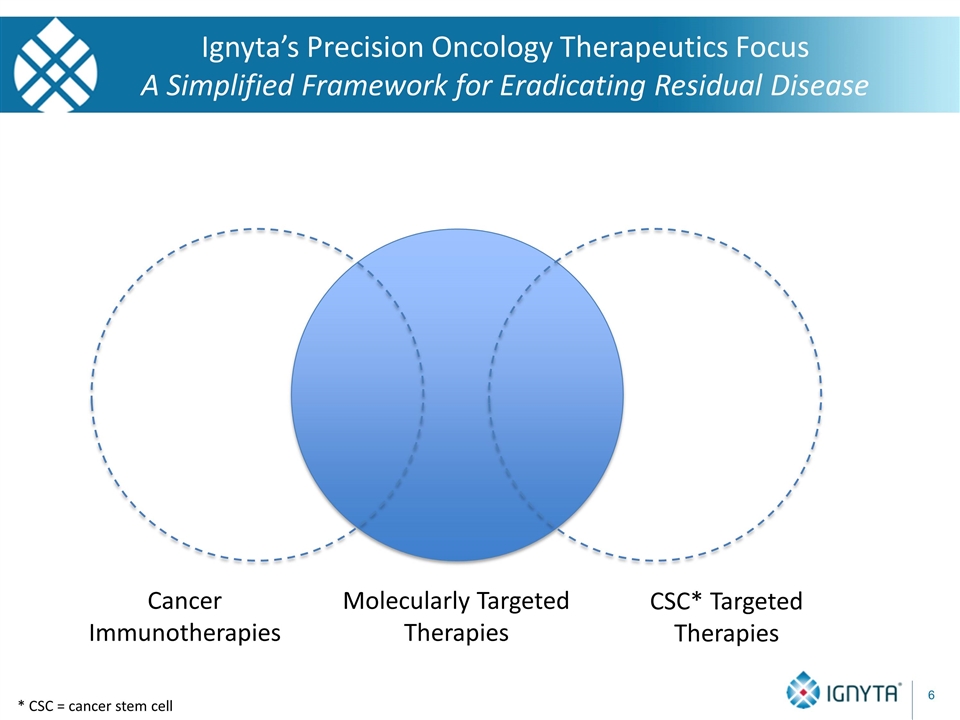
* CSC = cancer stem cell Ignyta’s Precision Oncology Therapeutics Focus A Simplified Framework for Eradicating Residual Disease Molecularly Targeted Therapies Cancer Immunotherapies CSC* Targeted Therapies
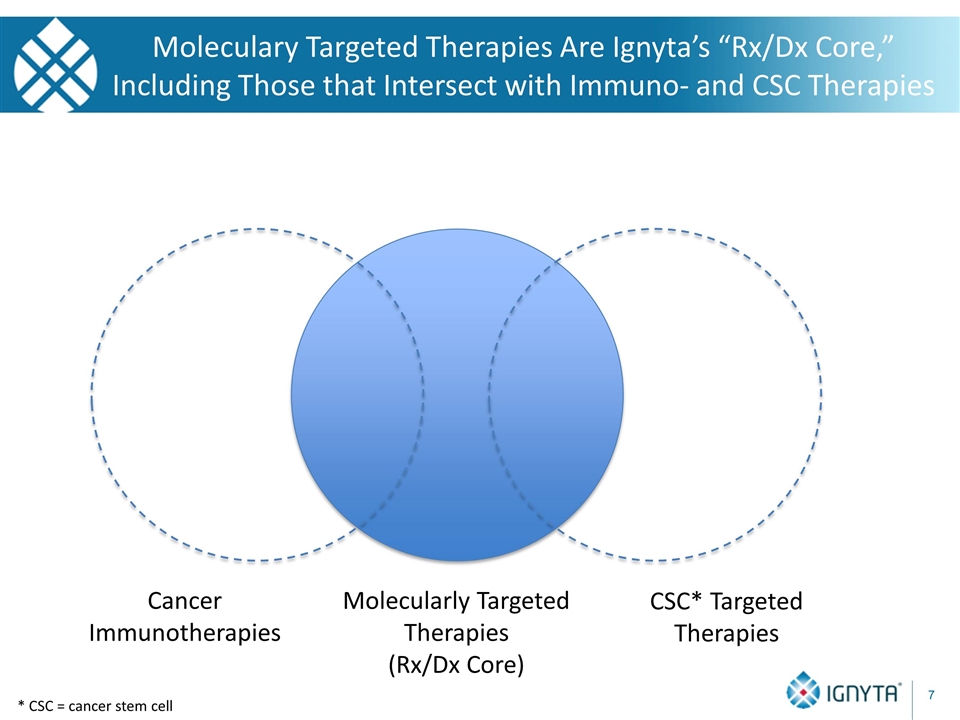
* CSC = cancer stem cell Moleculary Targeted Therapies Are Ignyta’s “Rx/Dx Core,” Including Those that Intersect with Immuno- and CSC Therapies Molecularly Targeted Therapies (Rx/Dx Core) Cancer Immunotherapies CSC* Targeted Therapies
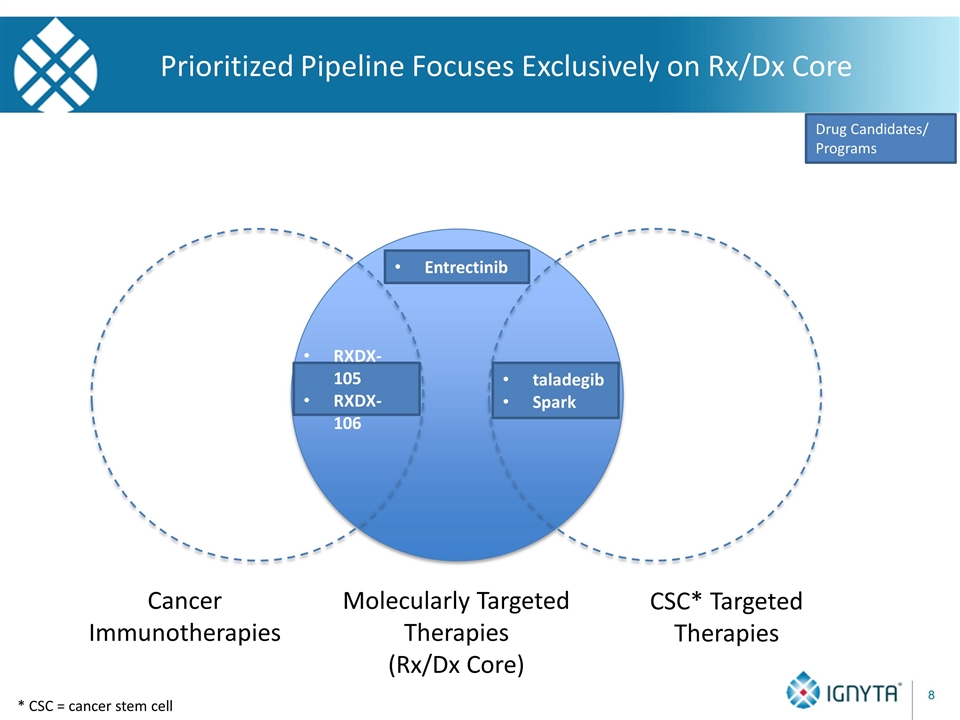
* CSC = cancer stem cell Prioritized Pipeline Focuses Exclusively on Rx/Dx Core Molecularly Targeted Therapies (Rx/Dx Core) Cancer Immunotherapies CSC* Targeted Therapies taladegib Spark Drug Candidates/ Programs Entrectinib RXDX-105 RXDX-106
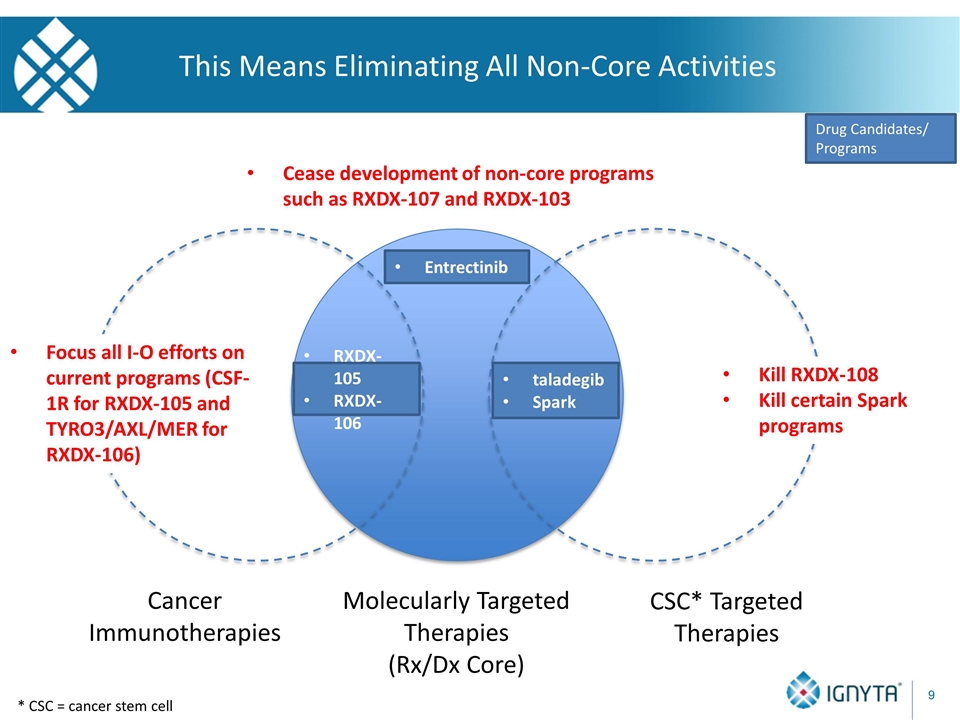
* CSC = cancer stem cell This Means Eliminating All Non-Core Activities Molecularly Targeted Therapies (Rx/Dx Core) Cancer Immunotherapies CSC* Targeted Therapies Drug Candidates/ Programs Entrectinib Cease development of non-core programs such as RXDX-107 and RXDX-103 Focus all I-O efforts on current programs (CSF-1R for RXDX-105 and TYRO3/AXL/MER for RXDX-106) Kill RXDX-108 Kill certain Spark programs taladegib Spark RXDX-105 RXDX-106
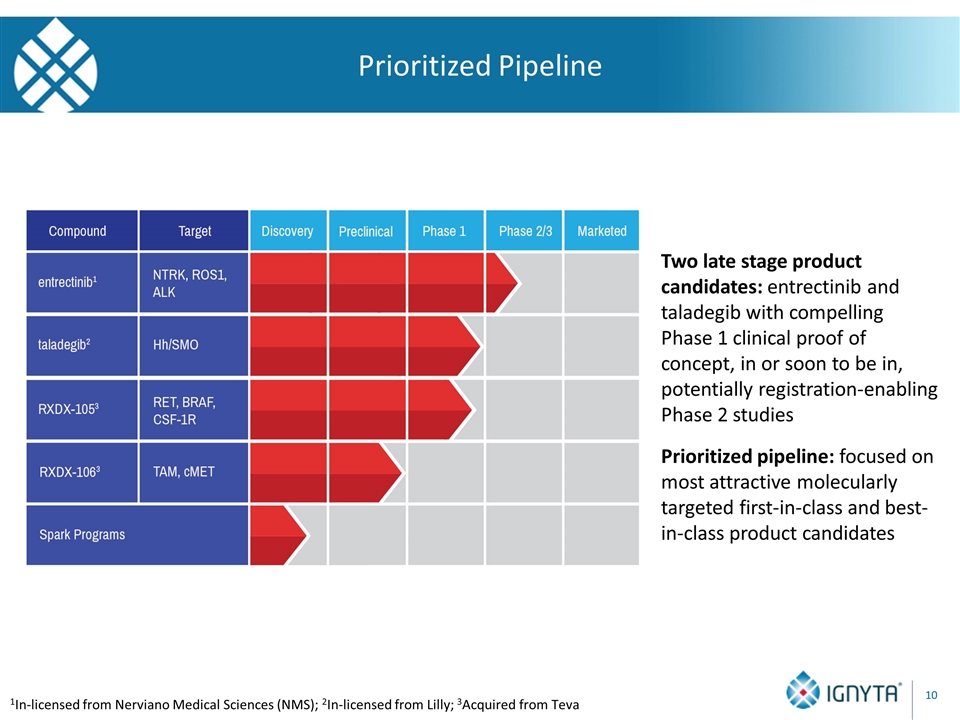
Prioritized Pipeline 1In-licensed from Nerviano Medical Sciences (NMS); 2In-licensed from Lilly; 3Acquired from Teva Two late stage product candidates: entrectinib and taladegib with compelling Phase 1 clinical proof of concept, in or soon to be in, potentially registration-enabling Phase 2 studies Prioritized pipeline: focused on most attractive molecularly targeted first-in-class and best-in-class product candidates
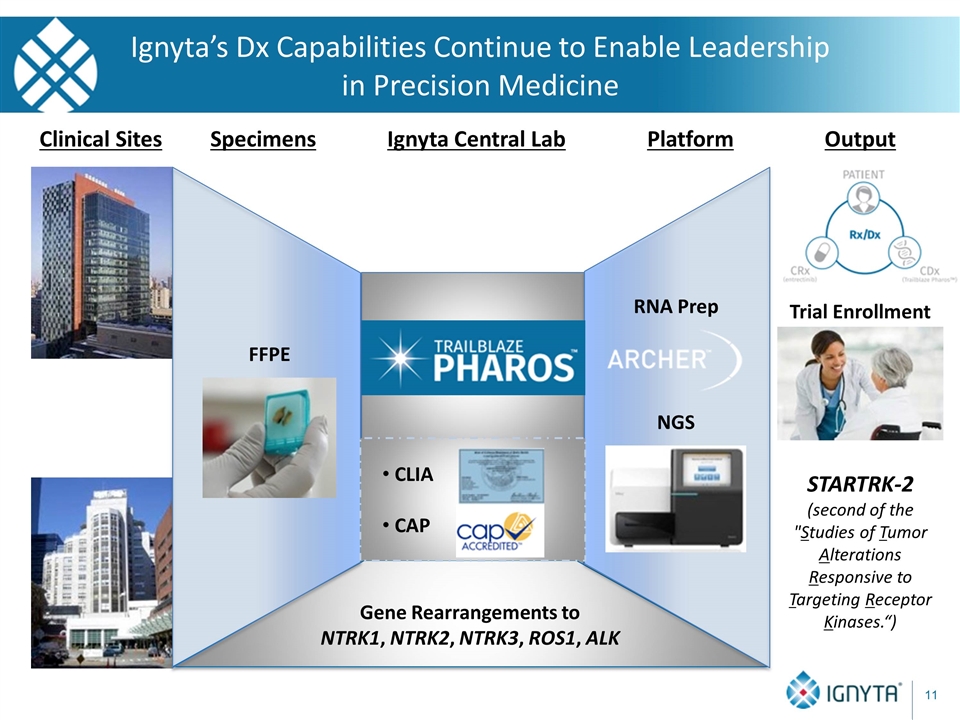
Gene Rearrangements to NTRK1, NTRK2, NTRK3, ROS1, ALK Clinical Sites Specimens Platform Output FFPE NGS Trial Enrollment Ignyta Central Lab CLIA CAP RNA Prep STARTRK-2 (second of the "Studies of Tumor Alterations Responsive to Targeting Receptor Kinases.“) Ignyta’s Dx Capabilities Continue to Enable Leadership in Precision Medicine
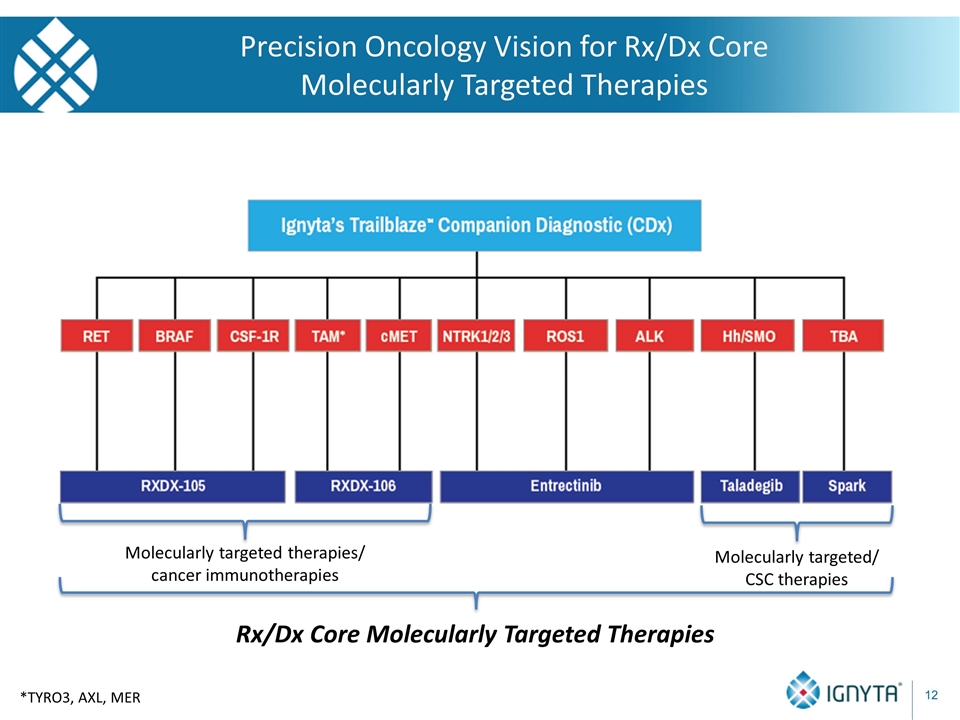
Precision Oncology Vision for Rx/Dx Core Molecularly Targeted Therapies *TYRO3, AXL, MER Molecularly targeted therapies/ cancer immunotherapies Molecularly targeted/ CSC therapies Rx/Dx Core Molecularly Targeted Therapies

2016 Corporate Milestones & Clinical Updates Revised 2030 Vision 2016 Milestones Continue to enroll STARTRK-2 global potentially registration-enabling study for entrectinib, ongoing Identify RP2D for RXDX-105 and initiate Study 105-01 Ph 1b RET+ and BRAF+ solid tumors, 1Q16 Complete tech transfer for taladegib, 1H16 File IND for RXDX-106, 2H16 Initiate Ph 2 study(ies) for taladegib, 2H16 Complete Ph 1a and identify RP2D for RXDX-107, 4Q16 Clinical study updates or data for entrectinib, RXDX-105, -107, +/- taladegib, at AACR/ASCO, 1H16 and/or ESMO/ENA, 2H16 2016 Clinical Updates 2011 – 2015 Advance clinical pipeline 2016 – 2020 Commercialize Rx/ Dx products 2021 – 2025 Scale pipeline revenue 2026 – 2030 Drive sustainable profitability Leading Precision Oncology Company that Eradicates Residual Disease

Summary of Ignyta’s Strategic Positioning Leading precision oncology company: Aspirational goal is to eradicate residual disease in precisely defined patient populations by 2030 Integrated approach to Rx/Dx development: Focused on molecularly targeted therapies that also intersect with cancer immunotherapies and cancer stem cell targeted therapies Two late stage product candidates: entrectinib and taladegib with compelling Phase 1 clinical proof of concept, in or soon to be in, potentially registration-enabling Phase 2 studies Prioritized pipeline focused on most attractive programs: Molecularly targeted first-in-class and best-in-class product candidates under clinical development in Rx/Dx core puts Ignyta in strong position to generate multiple clinical data readouts in 2016, while building long-term value for patients and shareholders
