Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ignyta, Inc. | d136279d8k.htm |

RXDX-105 Alexander Drilon MD Thoracic Oncology Service and Developmental Therapeutics Memorial Sloan Kettering Cancer Center Exhibit 99.1
RXDX-105 Orally bioavailable multikinase inhibitor Potent inhibitor of RET Active against BRAF and EGFR Clinical development Currently in phase 1/1b testing: patients with advanced solid tumors Enrichment for tumors with RET or BRAF fusions/mutations
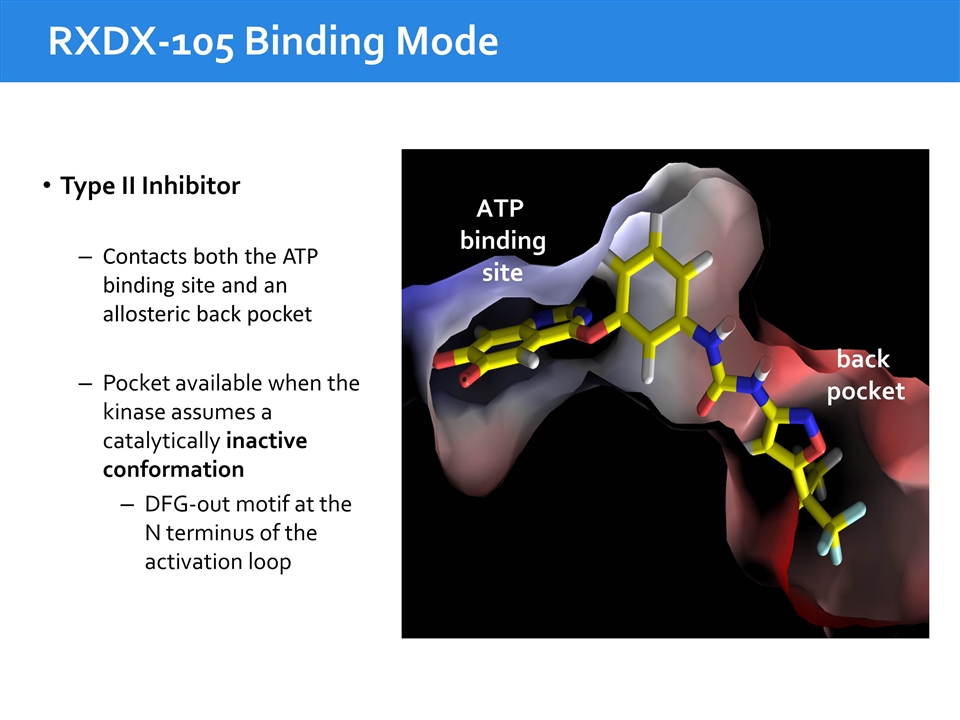
RXDX-105 Binding Mode ATP binding site back pocket Type II Inhibitor Contacts both the ATP binding site and an allosteric back pocket Pocket available when the kinase assumes a catalytically inactive conformation DFG-out motif at the N terminus of the activation loop
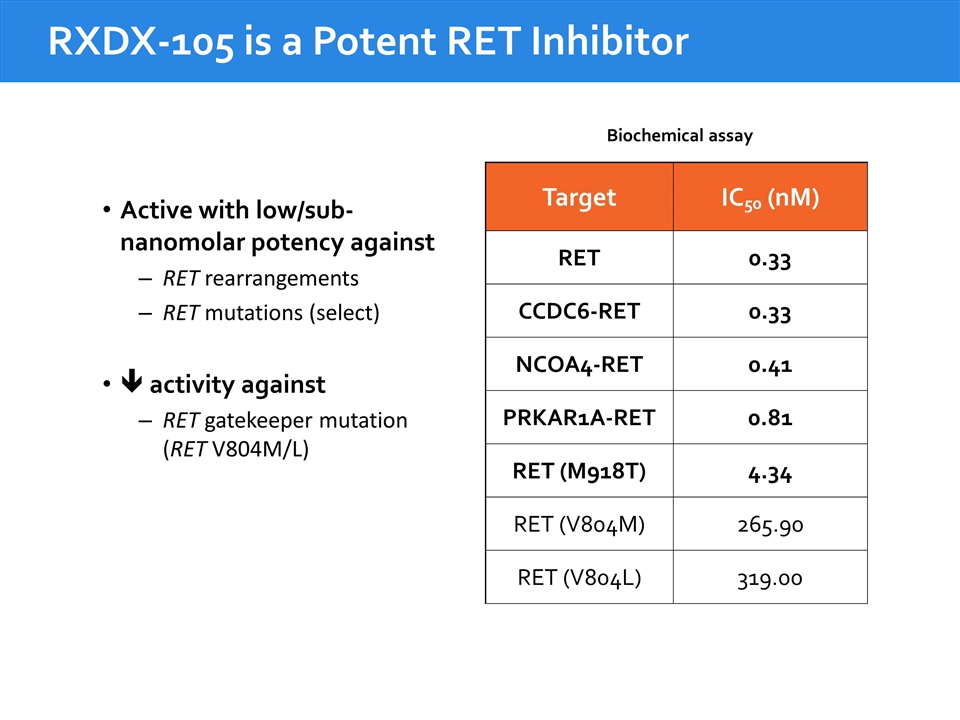
RXDX-105 is a Potent RET Inhibitor Target IC50 (nM) RET 0.33 CCDC6-RET 0.33 NCOA4-RET 0.41 PRKAR1A-RET 0.81 RET (M918T) 4.34 RET (V804M) 265.90 RET (V804L) 319.00 Biochemical assay Active with low/sub- nanomolar potency against RET rearrangements RET mutations (select) ê activity against RET gatekeeper mutation (RET V804M/L)
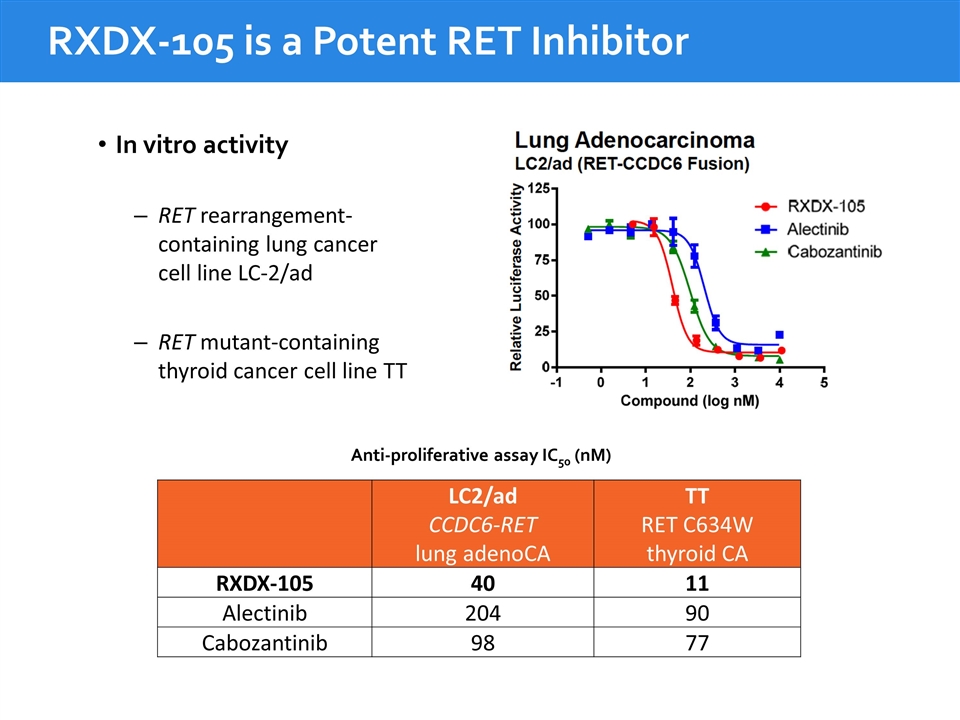
RXDX-105 is a Potent RET Inhibitor LC2/ad CCDC6-RET lung adenoCA TT RET C634W thyroid CA RXDX-105 40 11 Alectinib 204 90 Cabozantinib 98 77 Anti-proliferative assay IC50 (nM) In vitro activity RET rearrangement-containing lung cancer cell line LC-2/ad RET mutant-containing thyroid cancer cell line TT
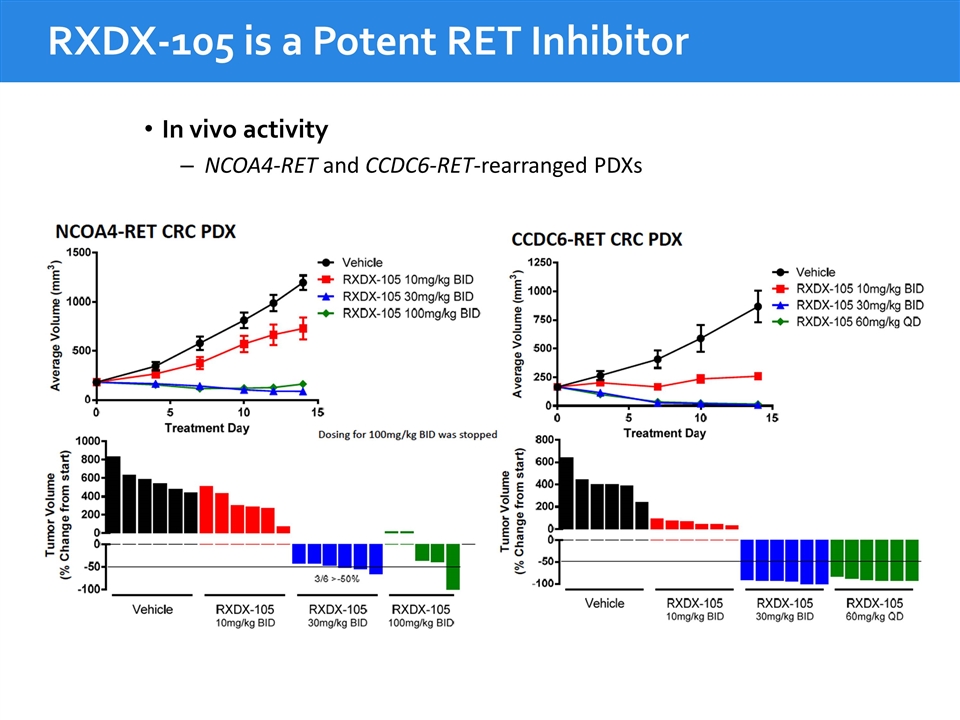
RXDX-105 is a Potent RET Inhibitor In vivo activity NCOA4-RET and CCDC6-RET-rearranged PDXs
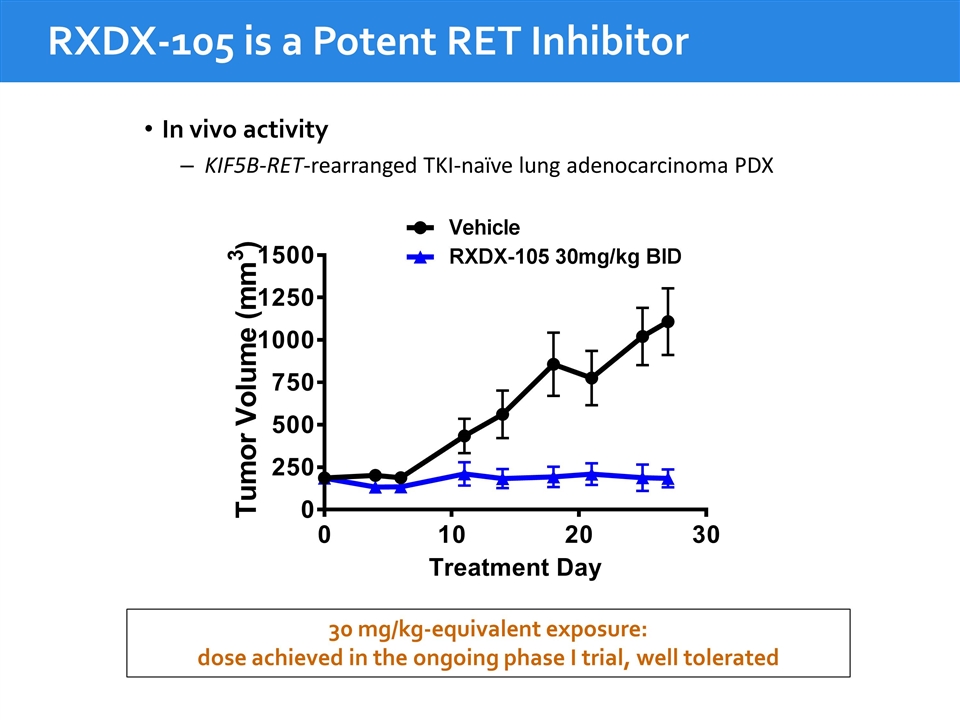
30 mg/kg-equivalent exposure: dose achieved in the ongoing phase I trial, well tolerated RXDX-105 is a Potent RET Inhibitor RXDX-105 induces tumor regression in RET-fusion PDX In vivo activity KIF5B-RET-rearranged TKI-naïve lung adenocarcinoma PDX

RXDX-105: Clinical Development
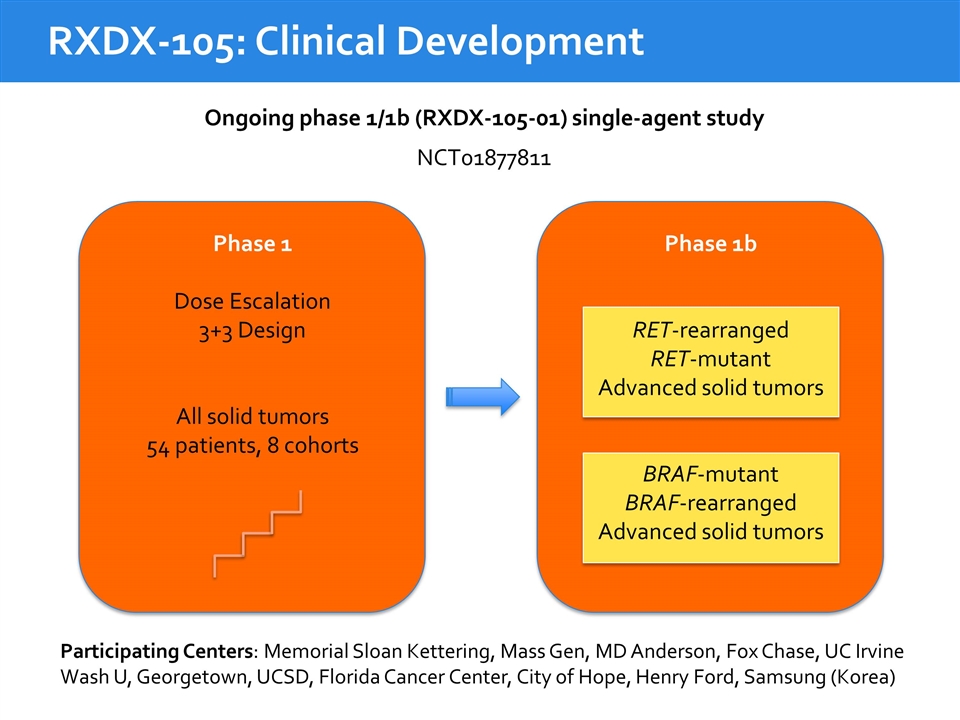
Ongoing phase 1/1b (RXDX-105-01) single-agent study RXDX-105: Clinical Development Phase 1b RET-rearranged RET-mutant Advanced solid tumors BRAF-mutant BRAF-rearranged Advanced solid tumors Phase 1 Dose Escalation 3+3 Design All solid tumors 54 patients, 8 cohorts NCT01877811 Participating Centers: Memorial Sloan Kettering, Mass Gen, MD Anderson, Fox Chase, UC Irvine Wash U, Georgetown, UCSD, Florida Cancer Center, City of Hope, Henry Ford, Samsung (Korea)
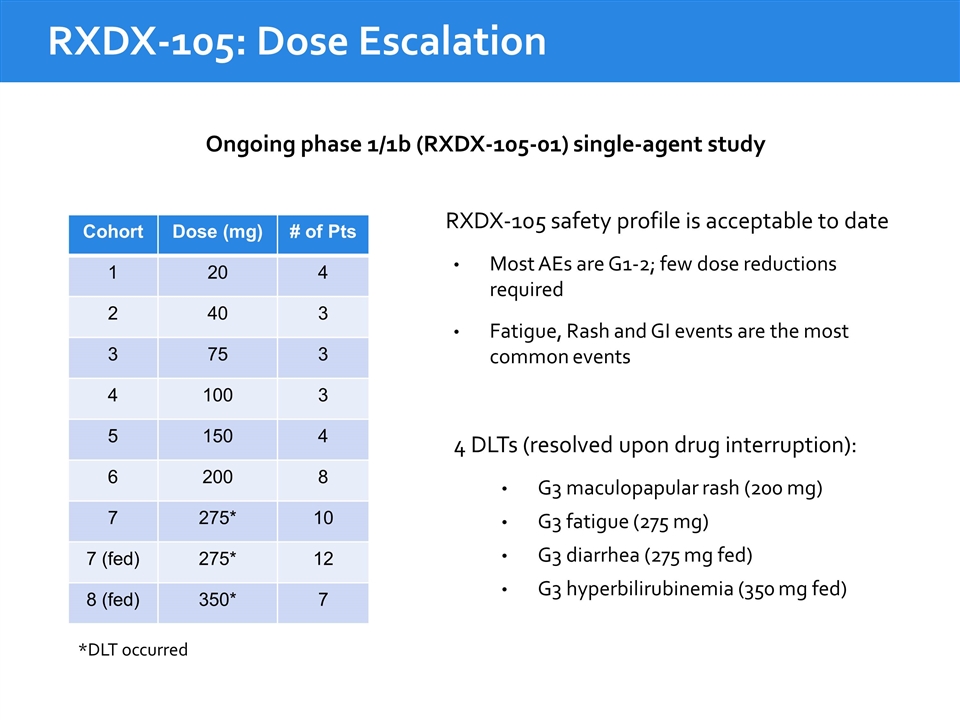
Ongoing phase 1/1b (RXDX-105-01) single-agent study RXDX-105 safety profile is acceptable to date Most AEs are G1-2; few dose reductions required Fatigue, Rash and GI events are the most common events 4 DLTs (resolved upon drug interruption): G3 maculopapular rash (200 mg) G3 fatigue (275 mg) G3 diarrhea (275 mg fed) G3 hyperbilirubinemia (350 mg fed) Cohort Dose (mg) # of Pts 1 20 4 2 40 3 3 75 3 4 100 3 5 150 4 6 200 8 7 275* 10 7 (fed) 275* 12 8 (fed) 350* 7 RXDX-105: Dose Escalation *DLT occurred
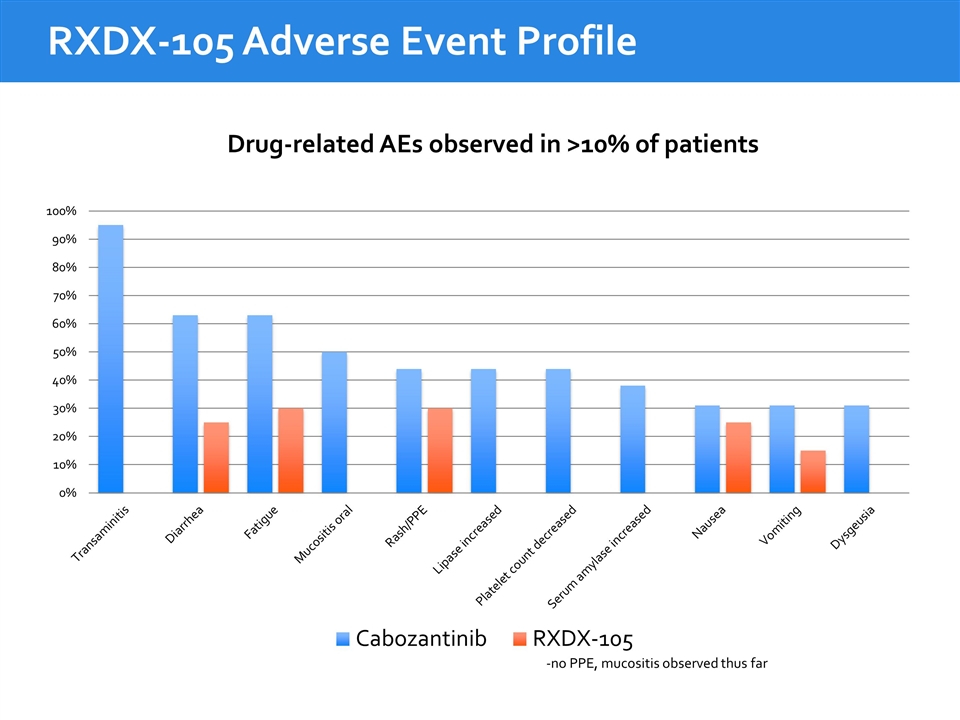
RXDX-105 Adverse Event Profile Drug-related AEs observed in >10% of patients -no PPE, mucositis observed thus far
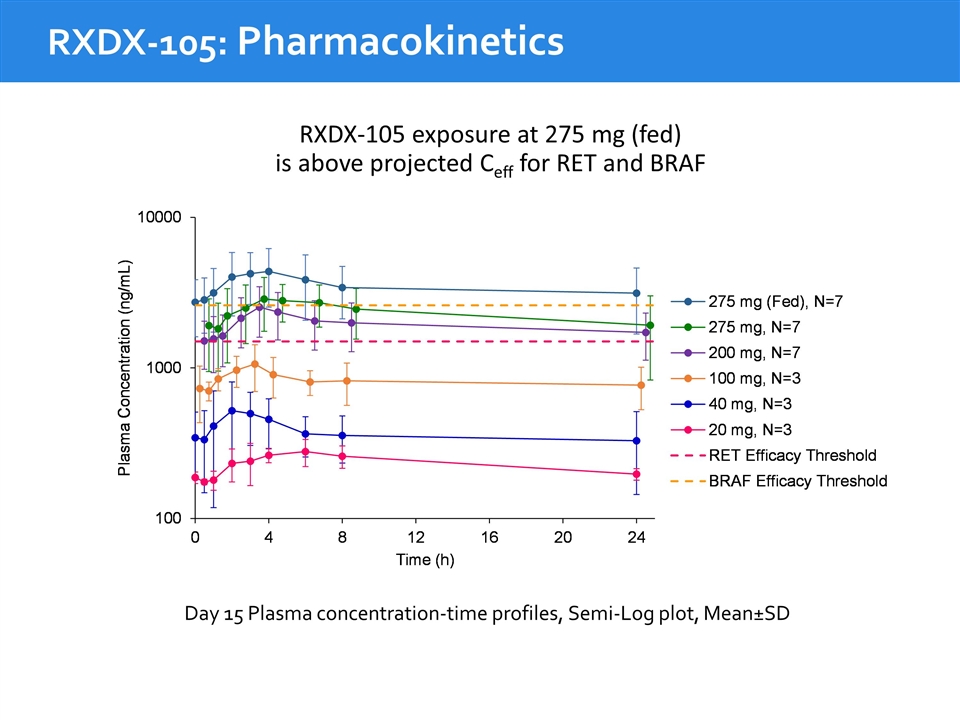
RXDX-105: Pharmacokinetics RXDX-105 exposure at 275 mg (fed) is above projected Ceff for RET and BRAF Day 15 Plasma concentration-time profiles, Semi-Log plot, Mean±SD
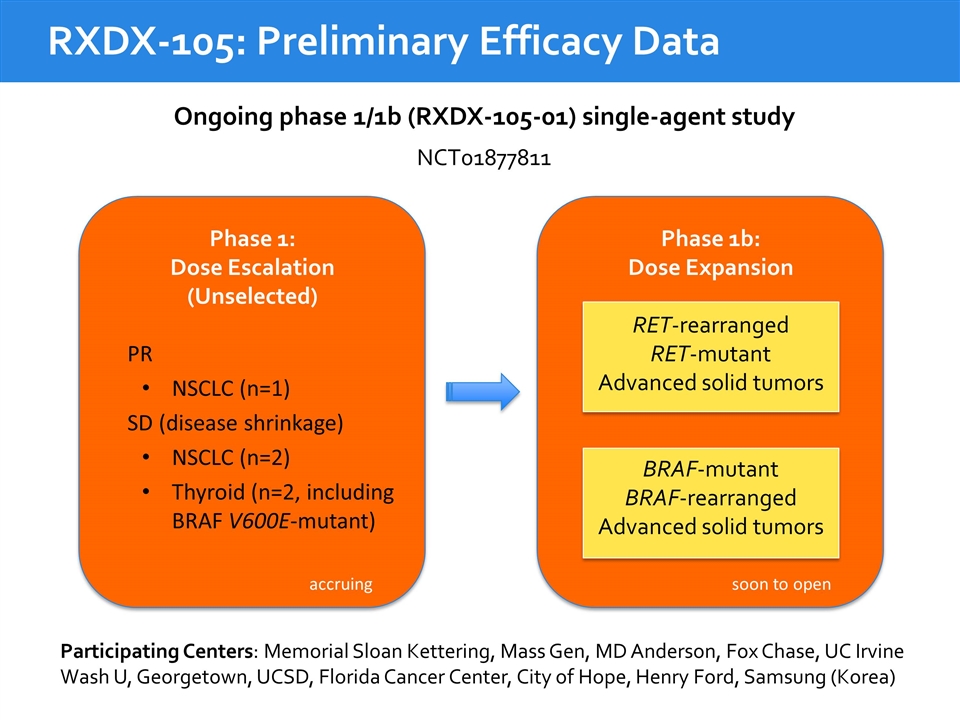
RXDX-105: Preliminary Efficacy Data Ongoing phase 1/1b (RXDX-105-01) single-agent study Phase 1b: Dose Expansion RET-rearranged RET-mutant Advanced solid tumors BRAF-mutant BRAF-rearranged Advanced solid tumors Phase 1: Dose Escalation (Unselected) NCT01877811 PR NSCLC (n=1) SD (disease shrinkage) NSCLC (n=2) Thyroid (n=2, including BRAF V600E-mutant) accruing soon to open Participating Centers: Memorial Sloan Kettering, Mass Gen, MD Anderson, Fox Chase, UC Irvine Wash U, Georgetown, UCSD, Florida Cancer Center, City of Hope, Henry Ford, Samsung (Korea)
RXDX-105 is a multi-kinase inhibitor that has demonstrated potent inhibition of RET. RXDX-105 is also active against BRAF RXDX-105 has demonstrated potent tumor growth inhibition in multiple preclinical RET fusion-containing models In an ongoing Phase 1 study, several patients have achieved clinical benefit, including 1 PR, and the predicted efficacious concentration from preclinical models has been observed at the 275 mg fed dose The clinical safety profile to date indicates that RXDX-105 is tolerable at doses above the projected Ceff for RET and BRAF A Phase 1b basket study in patients with RET or BRAF alterations will open, following RP2D selection Conclusions

Thank You
