Attached files
| file | filename |
|---|---|
| 8-K - 8-K - TESARO, Inc. | a16-3852_18k.htm |
Exhibit 99.1
Lonnie Moulder, CEO Mary Lynne Hedley, Ph.D., President & COO February 10, 2016
Safe Harbor Statement Statements made in this presentation about TESARO, Inc. that are not descriptions of historical facts are forward-looking statements reflecting the current beliefs and expectations of management. Forward-looking statements are sometimes identified by words such as “plan,” “may,” “will,” “expect,” and similar expressions referencing future events, conditions, or circumstances. Such forward-looking statements include statements regarding the expected timing of the Company’s IND submissions, clinical trial initiations, the data from those trials, the regulatory filings related thereto, and potential product launches; the Company’s expectation to have oral and IV formulations of rolapitant; the potential market opportunity for the Company’s product candidates; and TESARO’s 2016 corporate goals. These forward-looking statements involve substantial risks and uncertainties that could cause actual future results, performance, or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the uncertainties inherent in the execution of our planned commercial launch of VARUBI, actions by regulatory authorities, competitive pressures, and other matters that could affect the commercial launch and/or success of VARUBI, uncertainties inherent in the execution and completion of clinical trials, uncertainties surrounding the timing of availability of data from our clinical trials, ongoing discussions with and actions by regulatory authorities, patient accrual rates for clinical trials, and other matters that could affect the timing of data, the potential regulatory approval, or the commercial availability of the Company’s product candidates. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the Company in general, see TESARO's Annual Report on Form 10-K for the year ended December 31, 2014, and its Quarterly Report on Form 10-Q for the quarter ended September 30, 2015.

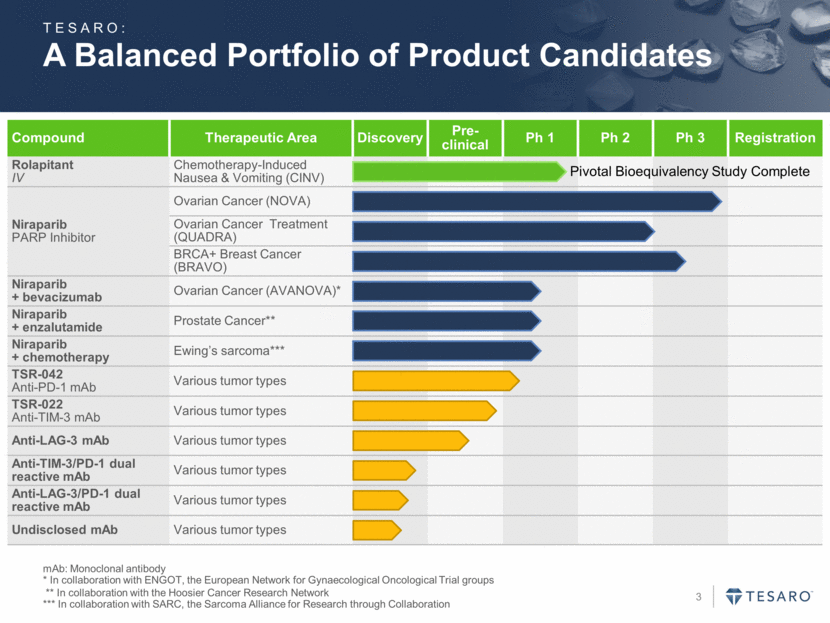
A Balanced Portfolio of Product Candidates mAb: Monoclonal antibody * In collaboration with ENGOT, the European Network for Gynaecological Oncological Trial groups ** In collaboration with the Hoosier Cancer Research Network *** In collaboration with SARC, the Sarcoma Alliance for Research through Collaboration Compound Therapeutic Area Discovery Pre-clinical Ph 1 Ph 2 Ph 3 Registration Rolapitant IV Chemotherapy-Induced Nausea & Vomiting (CINV) Niraparib PARP Inhibitor Ovarian Cancer (NOVA) Ovarian Cancer Treatment (QUADRA) BRCA+ Breast Cancer (BRAVO) Niraparib + bevacizumab Ovarian Cancer (AVANOVA)* Niraparib + enzalutamide Prostate Cancer** Niraparib + chemotherapy Ewing’s sarcoma*** TSR-042 Anti-PD-1 mAb Various tumor types TSR-022 Anti-TIM-3 mAb Various tumor types Anti-LAG-3 mAb Various tumor types Anti-TIM-3/PD-1 dual reactive mAb Various tumor types Anti-LAG-3/PD-1 dual reactive mAb Various tumor types Undisclosed mAb Various tumor types TESARO: Pivotal Bioequivalency Study Complete
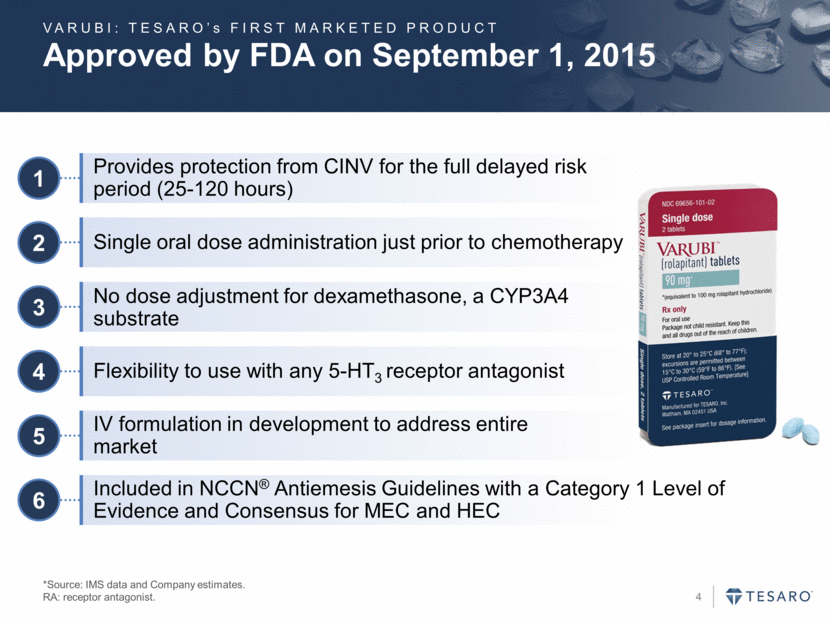
Approved by FDA on September 1, 2015 *Source: IMS data and Company estimates. RA: receptor antagonist. 1 Provides protection from CINV for the full delayed risk period (25-120 hours) 2 Single oral dose administration just prior to chemotherapy 6 Included in NCCN® Antiemesis Guidelines with a Category 1 Level of Evidence and Consensus for MEC and HEC 3 No dose adjustment for dexamethasone, a CYP3A4 substrate 4 Flexibility to use with any 5-HT3 receptor antagonist 5 IV formulation in development to address entire market VARUBI: TESARO’s FIRST MARKETED PRODUCT
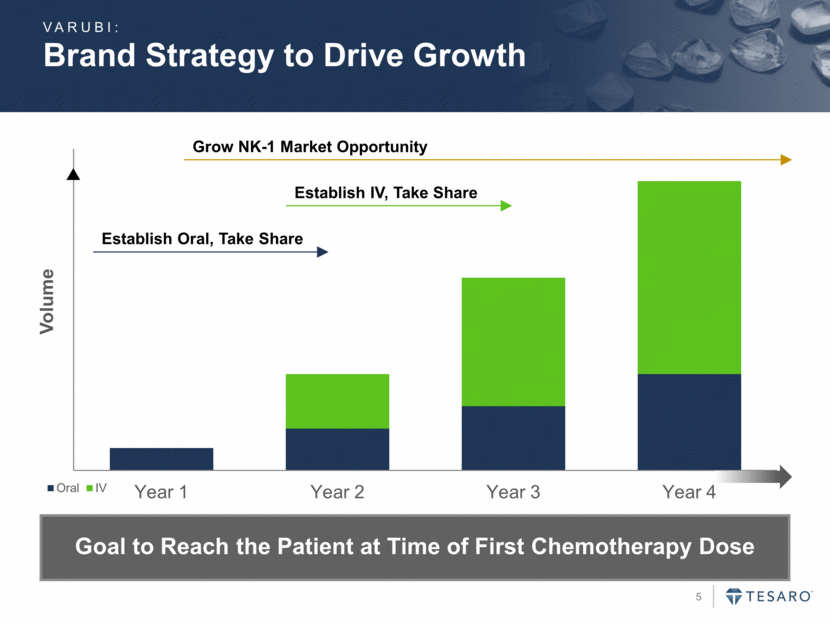
Brand Strategy to Drive Growth VARUBI: Establish Oral, Take Share Establish IV, Take Share Grow NK-1 Market Opportunity Goal to Reach the Patient at Time of First Chemotherapy Dose Year 1 Year 2 Year 3 Year 4 Volume Oral IV
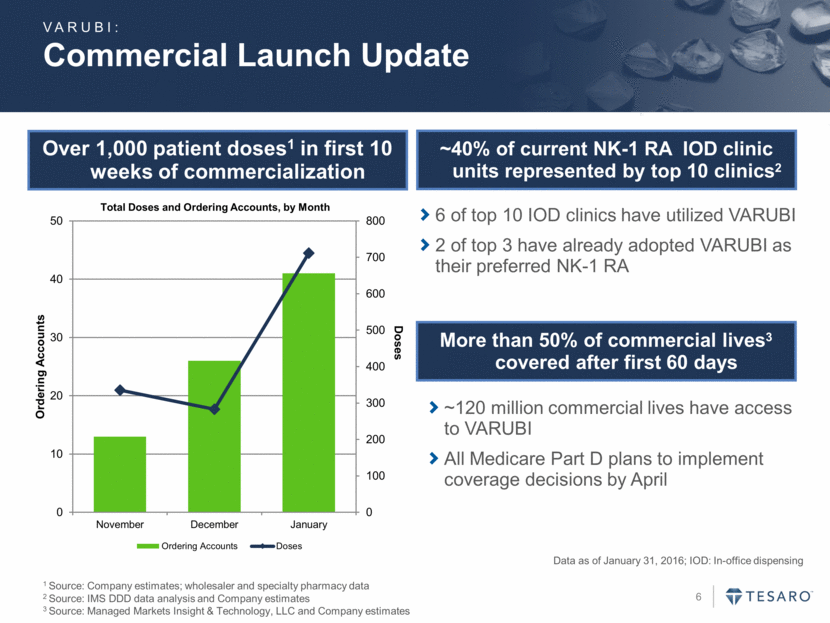
Commercial Launch Update Over 1,000 patient doses1 in first 10 weeks of commercialization More than 50% of commercial lives3 covered after first 60 days Data as of January 31, 2016; IOD: In-office dispensing 1 Source: Company estimates; wholesaler and specialty pharmacy data 2 Source: IMS DDD data analysis and Company estimates 3 Source: Managed Markets Insight & Technology, LLC and Company estimates ~120 million commercial lives have access to VARUBI All Medicare Part D plans to implement coverage decisions by April VARUBI: ~40% of current NK-1 RA IOD clinic units represented by top 10 clinics2 6 of top 10 IOD clinics have utilized VARUBI 2 of top 3 have already adopted VARUBI as their preferred NK-1 RA 0 100 200 300 400 500 600 700 800 0 10 20 30 40 50 November December January Doses Ordering Accounts Total Doses and Ordering Accounts, by Month Ordering Accounts Doses
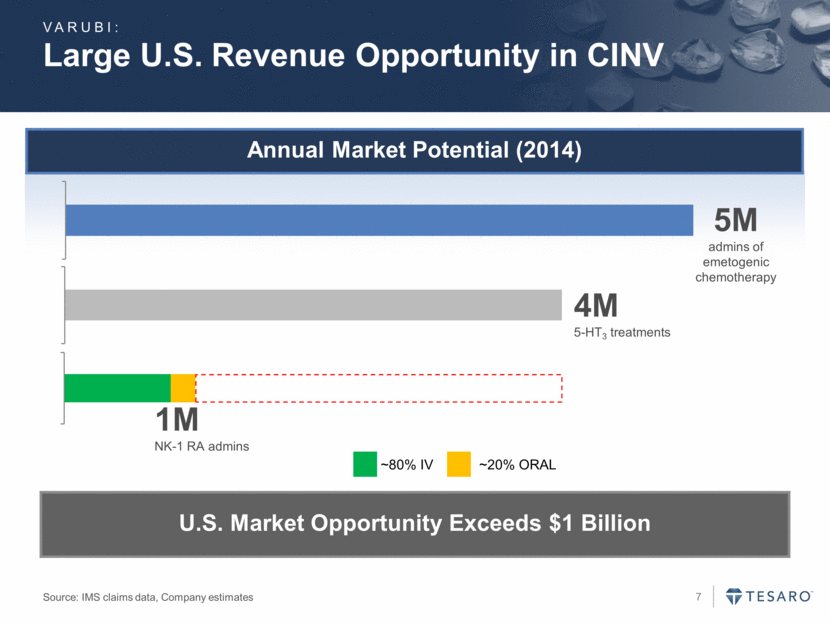
Annual Market Potential (2014) Large U.S. Revenue Opportunity in CINV Source: IMS claims data, Company estimates 5M admins of emetogenic chemotherapy 4M 5-HT3 treatments 1M NK-1 RA admins ~20% ORAL ~80% IV U.S. Market Opportunity Exceeds $1 Billion VARUBI: - 1,000,000.00 2,000,000.00 3,000,000.00 4,000,000.00 5,000,000.00 6,000,000.00
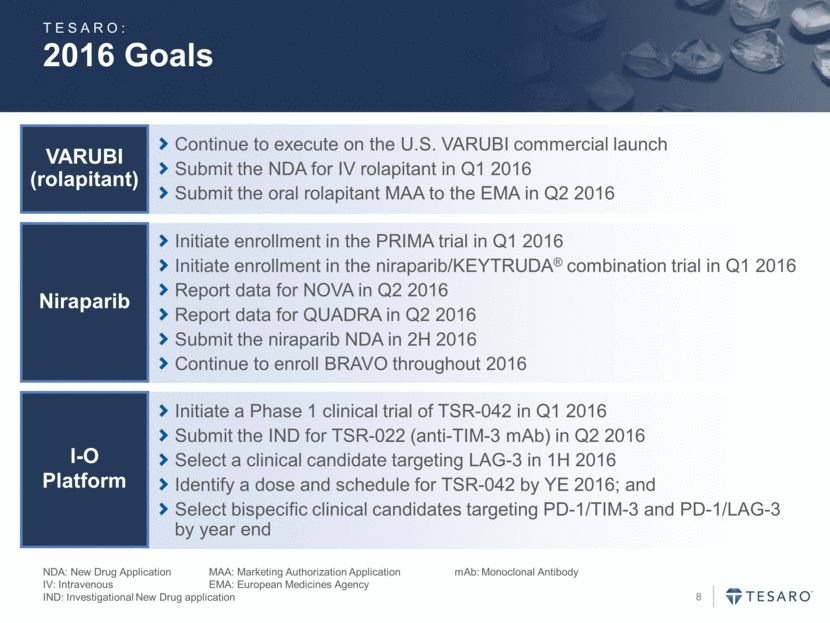
2016 Goals NDA: New Drug Application MAA: Marketing Authorization Application mAb: Monoclonal Antibody IV: Intravenous EMA: European Medicines Agency IND: Investigational New Drug application Continue to execute on the U.S. VARUBI commercial launch Submit the NDA for IV rolapitant in Q1 2016 Submit the oral rolapitant MAA to the EMA in Q2 2016 VARUBI (rolapitant) Initiate a Phase 1 clinical trial of TSR-042 in Q1 2016 Submit the IND for TSR-022 (anti-TIM-3 mAb) in Q2 2016 Select a clinical candidate targeting LAG-3 in 1H 2016 Identify a dose and schedule for TSR-042 by YE 2016; and Select bispecific clinical candidates targeting PD-1/TIM-3 and PD-1/LAG-3 by year end I-O Platform Initiate enrollment in the PRIMA trial in Q1 2016 Initiate enrollment in the niraparib/KEYTRUDA® combination trial in Q1 2016 Report data for NOVA in Q2 2016 Report data for QUADRA in Q2 2016 Submit the niraparib NDA in 2H 2016 Continue to enroll BRAVO throughout 2016 Niraparib TESARO:
Lonnie Moulder, CEO Mary Lynne Hedley, Ph.D., President & COO February 10, 2016

