Attached files
| file | filename |
|---|---|
| EX-5.1 - EXHIBIT 5.1 - Neurotrope, Inc. | v427866_ex5-1.htm |
| EX-23.1 - EXHIBIT 23.1 - Neurotrope, Inc. | v427866_ex23-1.htm |
As filed with the Securities and Exchange Commission on January 14, 2016
Registration No. 333-208502
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1 to
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933

Neurotrope, Inc.
(Exact name of registrant as specified in its charter)
| Nevada | 2834 | 46-3522381 |
|
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
50 Park Place, Suite 1401
Newark, New Jersey 07102
973-242-0005
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
Charles S. Ramat
Neurotrope, Inc.
50 Park Place, Suite 1401
Newark, New Jersey 07102
973-242-0005
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
Copy to:
Kenneth R. Koch, Esq.
Jeffrey P. Schultz, Esq.
Mintz, Levin, Cohn, Ferris, Glovsky and
Popeo, P.C.
666 Third Avenue
New York, New York 10017
212-935-3000
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. þ
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
(Check one):
| Large accelerated filer ¨ | Accelerated filer ¨ |
| Non-accelerated filer ¨ (Do not check if a smaller reporting company) | Smaller reporting company þ |
CALCULATION OF REGISTRATION FEE
Title of Each Class of Securities to be Registered | Amount to be Registered (1) | Proposed Maximum Offering Price Per Share (2) | Proposed Maximum Aggregate Offering Price | Amount of Registration Fee (3) | ||||||||||
| Common stock, par value $0.0001 per share | 240,024,699 shares | $ | 0.505 | $ | 121,212,473 | $ | 12,206.10 | |||||||
| (1) | Consists of 150% of each of the following: (a) 26,234,940 shares of our common stock issuable upon conversion of 262,349.4 outstanding shares of our Series B Stock, (b) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series A warrants to purchase our common stock having an exercise price of $0.80 per share, (c) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series B warrants to purchase our common stock having an exercise price of $0.80 per share, (d) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series C warrants to purchase our common stock having an exercise price of $1.25 per share, (e) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series D warrants to purchase our common stock having an exercise price of $1.00 per share, (f) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series E warrants to purchase our common stock having an exercise price of $1.50 per share, (g) 363,456 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.01 per share, (h) 1,090,370 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.60 per share, and (i) 1,153,000 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $1.50 per share, subject to adjustment to $0.80 per share upon the exercise of all Series A Warrants or all Series B Warrants. Pursuant to Rule 416 under the Securities Act of 1933, as amended, the securities being registered hereunder include such indeterminate number of additional shares of common stock as may be issued after the date hereof as a result of stock splits, stock dividends, or similar transactions. |
| (2) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act of 1933, as amended, based on the average of the high and low prices of the registrant’s common stock as reported by the OTCQB marketplace (the “OTC Market”) on December 9, 2015, which date is within five business days prior to the initial filing of this registration statement. The shares offered hereunder may be sold by the selling stockholders from time to time in the open market, through privately negotiated transactions or a combination of these methods, at market prices prevailing at the time of sale or at negotiated prices. |
| (3) | Previously paid. |
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. The selling stockholders may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and the selling stockholders are not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to completion, dated January 14, 2016
Neurotrope, Inc.
Prospectus
240,024,699 Shares
Common Stock
This prospectus relates to the sale of up to 240,024,699 shares of our common stock, par value $0.0001 per share, by the selling stockholders of Neurotrope, Inc., a Nevada corporation, named in this prospectus. The shares being offered consist of 150% of each of the following: (a) 26,234,940 shares of our common stock issuable upon conversion of 262,349.4 outstanding shares of our Series B Stock, (b) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series A warrants to purchase our common stock having an exercise price of $0.80 per share, (c) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series B warrants to purchase our common stock having an exercise price of $0.80 per share, (d) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series C warrants to purchase our common stock having an exercise price of $1.25 per share, (e) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series D warrants to purchase our common stock having an exercise price of $1.00 per share, (f) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series E warrants to purchase our common stock having an exercise price of $1.50 per share, (g) 363,456 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.01 per share, (h) 1,090,370 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.60 per share, and (i) 1,153,000 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $1.50 per share, subject to adjustment to $0.80 per share upon the exercise of all Series A Warrants or all Series B Warrants. The shares offered by this prospectus were issued in connection with our private placement of securities, which was completed in November 2015 (the “November 2015 Private Placement”), and may be sold by the selling stockholders from time to time in the open market, through privately negotiated transactions or a combination of these methods, at market prices prevailing at the time of sale or at negotiated prices.
Our common stock is traded on the OTCQB marketplace, or the OTC Market, under the symbol “NTRP.” On January 6, 2016, the last reported sale price for our common stock was $0.50 per share.
The distribution of the shares by the selling stockholders is not subject to any underwriting agreement. We will not receive any proceeds from the sale of the shares by the selling stockholders. We will bear all expenses of registration incurred in connection with this offering, but all selling and other expenses incurred by the selling stockholders will be borne by them.
Our business and an investment in our securities involve a high degree of risk. Before making any investment in our securities, you should read and carefully consider the risks described in the “Risk Factors” section beginning on page 12 of this prospectus.
You should rely only on the information contained in this prospectus and any prospectus supplement or amendment. We have not authorized anyone to provide you with different information. This prospectus may only be used where it is legal to sell these securities. The information in this prospectus is only accurate on and as of the date of this prospectus, regardless of the time of any sale of securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
This prospectus is dated January , 2016
| 1 |
You should rely only on the information contained in this prospectus or any prospectus supplement or amendment. We have not authorized any other person to provide you with information that is different from, or adds to, that contained in this prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. The selling stockholders are offering to sell and seeking offers to buy our common stock only in jurisdictions where offers and sales are permitted. You should assume that the information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date. We are not making an offer of any securities in any jurisdiction.
TABLE OF CONTENTS
| 2 |
The following summary highlights information contained elsewhere in this prospectus. This summary is not complete and does not contain all of the information that should be considered before investing in our common stock. Potential investors should read the entire prospectus carefully, including the more detailed information regarding our business provided below in the “Description of Business” section, the risks of purchasing our common stock discussed under the “Risk Factors” section, and our consolidated financial statements and the accompanying notes to the consolidated financial statements.
Unless the context indicates otherwise, all references in this registration statement to “Neurotrope,” the “Company,” “we,” “us” and “our” refer to Neurotrope, Inc. and its wholly-owned consolidated operating subsidiary, Neurotrope BioScience, Inc. All references in this prospectus to “Neurotrope BioScience” refer solely to Neurotrope BioScience, Inc.
Overview
We are a biopharmaceutical company with product candidates in pre-clinical and clinical development. Neurotrope BioScience began operations in October 2012. We are principally focused on developing a product platform based upon a drug candidate called bryostatin for the treatment of Alzheimer’s disease, or AD, which is in the clinical testing stage. We are also developing bryostatin for other neurodegenerative or cognitive diseases and dysfunctions, which are in pre-clinical testing. We have a technology license and services agreement, or the BRNI License, with the Blanchette Rockefeller Neurosciences Institute, and its affiliate NRV II, LLC, which we collectively refer to herein as BRNI, pursuant to which we have an exclusive non-transferable license to certain patents and technologies required to develop our proposed products. Neurotrope BioScience was formed for the primary purpose of commercializing the technologies initially developed by BRNI for therapeutic applications for AD or other cognitive dysfunctions. These technologies have been under development by BRNI since 1999 and, up until March 2013, have been financed by BRNI through funding from a variety of non-investor sources (which include not-for-profit foundations, the National Institutes of Health (which is part of the U.S. Department of Health and Human Services) and individual philanthropists). From March 2013 forward, development of the licensed technology has been funded principally through collaboration by BRNI with Neurotrope BioScience.
According to the Alzheimer’s Association, an estimated 36 million people worldwide had AD in 2010. The prevalence of AD is independent of race, ethnicity, geography, lifestyle and, to a large extent, genetics. The most common cause of developing AD is old age. In developing countries, where the median age of death is less than 65 years old, AD is rarely recognized or diagnosed. In the U.S., 5.2 million people were estimated to have AD in 2013, and 96% of these people were older than 65 years of age.
Researchers have explored and continue to explore a wide range of drug mechanisms in hopes of developing drugs to combat AD. We believe that our approach, which involves the activation of an enzyme called protein kinase C epsilon, or PKCε, represents a novel mechanism in potential AD drug therapies.
BRNI is conducting an enhanced access program, formerly known as compassionate use, of Bryostatin-1 in patients with advanced AD. Thus far, four patients with advanced AD have been treated, three of which were treated under an Investigational New Drug Application, IND, cleared by the FDA which is held by BRNI. One of these patients, who had familiar AD, has died, but the death was not drug-related. The study for another one of these patients has concluded after almost one year on the protocol. A fourth patient has recently commenced treatment. On the basis of communication from caregivers and treating physicians, BRNI, with our support, has decided to enroll one additional patient in the extended access program. We are providing limited funding and personnel support under the terms of our agreement with BRNI for this modest expansion of this clinical effort in AD in the 2015 to 2016 timeframe.
In October 2015, we announced the initiation of a Phase 2b clinical trial to evaluate bryostatin for the treatment of patients with moderately severe to severe AD. We have commenced enrollment and plan to enroll a total of up to 150 patients in the randomized, double-blind, placebo-controlled, study at approximately 30 sites. The primary objective of the clinical trial will be to assess the appropriate dose of bryostatin and preliminary evaluation of the safety and efficacy of several doses of bryostatin in the patient population. We believe bryostatin may restore synaptic structures and functions damaged by AD, leading to improvements in cognition and memory. Beyond AD, we believe that several other neurodegenerative diseases, such as Fragile X Syndrome and Niemann Pick Type C Disease (which we are pursuing) ischemic stroke, traumatic brain injury, depression and aging in the brain, may be amenable to treatment with bryostatin.
| 3 |
To the extent resources permit, we intend to pursue development of selected technology platforms with applications related to the treatment of AD and other neurodegenerative disorders based on our current licensed technology or technology available from third party licensors or collaborators.
Financings to Date
In February 2013, through a private placement, Neurotrope BioScience issued 9,073,300 shares of its Series A convertible preferred stock, or Neurotrope BioScience Series A Stock, at $1.00 per share, resulting in gross proceeds of $9,073,300. In May 2013, Neurotrope BioScience issued an additional 1,313,325 shares of Neurotrope BioScience Series A Stock at $1.00 per share, resulting in gross proceeds of $1,313,325. In August 2013, Neurotrope BioScience issued 11,533,375 of Neurotrope BioScience Series A Stock at $1.00 per share, resulting in gross proceeds of $11,533,375. All of the outstanding shares of Neurotrope BioScience Series A Stock were converted on a one-for-one basis into shares of Neurotrope, Inc.’s Series A convertible preferred stock, or Series A Stock, in connection with the Reverse Merger (as defined below) in August 2013. In October 2013, we issued 1,080,000 additional shares of our Series A Stock at $1.00 per share, resulting in gross proceeds of $1,080,000, for a total of $23,000,000 of gross proceeds raised between February and October 2013.
In the November 2015 Private Placement, we sold 26,234,940 units at $0.60 per unit with each unit consisting of Series B convertible preferred stock, or the Series B Stock, together with Series A through Series E warrants to purchase common stock, and certain placement agent warrants, resulting in gross proceeds of $15,640,963. The private placement was completed in two closings, which took place on November 13, 2015 and November 30, 2015. In connection with this private placement, effective as of November 13, 2015, the holders of all 16,656,894 shares of our Series A Stock converted their shares into 19,864,971 shares of our common stock, which included 3,208,077 shares of our common stock issued in accordance with anti-dilution provisions relating to the Series A Stock.
Organizational History
We were incorporated as BlueFlash Communications, Inc. in Florida on January 11, 2011. Prior to the Reverse Merger (as defined below) and Split-Off (as defined below), our business was to provide software solutions to deliver geo-location targeted coupon advertising to mobile internet devices.
On August 9, 2013, we reincorporated in the State of Nevada by merging into a newly-formed special-purpose subsidiary, Neurotrope, Inc., which was incorporated on June 13, 2013 and was the surviving corporation in such reincorporation merger, or the Reincorporation Merger. As a result of the Reincorporation Merger, (i) we changed our name to Neurotrope, Inc., (ii) we changed our jurisdiction of incorporation from Florida to Nevada, (iii) we increased our authorized capital stock from 300,000,000 shares of common stock, par value $0.0001 per share, to 300,000,000 shares of common stock, par value $0.0001 per share, and 50,000,000 shares of “blank check” preferred stock, par value $0.0001 per share, (iv) each share of BlueFlash Communications, Inc. common stock outstanding at the time of the Reincorporation Merger was automatically converted into 2.242 shares of Neurotrope, Inc. common stock, our common stock, with the result being that the 10,200,000 shares of common stock of BlueFlash Communications, Inc. outstanding immediately prior to the Reincorporation Merger were converted into 22,868,400 shares of common stock of Neurotrope, Inc. outstanding immediately thereafter. All share and per share numbers in this prospectus relating to the common stock of Neurotrope, Inc., prior to the Reincorporation Merger have been adjusted to give effect to this conversion, unless otherwise stated.
In connection with the Reincorporation Merger, we changed our fiscal year from a fiscal year ending on January 31 of each year to one ending on December 31 of each year.
On August 23, 2013, our wholly-owned subsidiary, Neurotrope Acquisition, Inc., or Acquisition Sub, a corporation formed in the State of Nevada on August 15, 2013 merged with and into Neurotrope BioScience, a corporation incorporated in the State of Delaware on October 31, 2012. Neurotrope BioScience was the surviving corporation in the merger, or the Reverse Merger, and became our wholly-owned subsidiary. All of the outstanding shares of Neurotrope BioScience common stock, or Neurotrope BioScience Common Stock, were converted into shares of our common stock, par value $0.0001 per share, and all of the outstanding shares of Neurotrope BioScience Series A Stock were converted into shares of our Series A Stock, in each case on a one-for-one basis.
In connection with the Reverse Merger and pursuant to a split-off agreement, or Split-Off, we transferred our pre-Reverse Merger business to Marissa Watson, our pre-Reverse Merger majority stockholder, in exchange for the surrender and cancellation of 20,178,000 shares of our common stock owned by her.
| 4 |
As a result of the Reverse Merger and Split-Off, we discontinued our pre-Reverse Merger business and acquired the business of Neurotrope BioScience. Following the Reverse Merger and Split-off, we have undertaken the business operations of Neurotrope BioScience as a publicly-traded company under the name Neurotrope, Inc., through Neurotrope BioScience, which is now our wholly-owned subsidiary.
In accordance with “reverse merger” accounting treatment, our historical financial statements as of period ends, and for periods ended, prior to the Reverse Merger have been and will be replaced with the historical financial statements of Neurotrope BioScience prior to the Reverse Merger in all applicable filings with the Securities and Exchange Commission, or the SEC.
Capital Needs
With the capital raised to date as described above and a cash and cash equivalent position of approximately $11.2 million as of January 6, 2016, we believe we have funding for approximately the next 12 months based upon our current product development plans. However, as described in this prospectus, we are currently reviewing our strategic plans. We intend to adjust our operating plans, to the extent possible, by rationalizing non-contractual variable overhead expenses so that we have at least 12 months of cash for operations at the end of fiscal year 2015. In addition, we may choose to further reevaluate our strategic plan in the future. There can be no assurance that our current funding will even last for 12 months. We will need to raise additional funds based upon advancing certain product development programs in our current and future pipeline. We will determine when and if to proceed with future fundraising efforts from time to time. There can be no assurance financing will be available to us when required on acceptable terms or at all.
About This Offering
This prospectus relates to the offering, which is not being underwritten, by the selling stockholders listed in this prospectus, of up to 240,024,699 shares of our common stock. The shares being offered consist of 150% of each of the following: (a) 26,234,940 shares of our common stock issuable upon conversion of 262,349.4 outstanding shares of our Series B Stock, (b) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series A warrants to purchase our common stock having an exercise price of $0.80 per share, (c) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series B warrants to purchase our common stock having an exercise price of $0.80 per share, (d) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series C warrants to purchase our common stock having an exercise price of $1.25 per share, (e) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series D warrants to purchase our common stock having an exercise price of $1.00 per share, (f) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series E warrants to purchase our common stock having an exercise price of $1.50 per share, (g) 363,456 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.01 per share, (h) 1,090,370 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.60 per share, and (i) 1,153,000 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $1.50 per share, subject to adjustment to $0.80 per share upon the exercise of all Series A Warrants or all Series B Warrants. The shares offered by this prospectus were issued in connection with the November 2015 Private Placement, and may be sold by the selling stockholders from time to time in the open market, through negotiated transactions or otherwise at market prices prevailing at the time of sale or at negotiated prices. We will receive none of the proceeds from the sale of the shares by the selling stockholders. We are registering 150% of the shares of common stock underlying the Series B Stock and the warrants described above pursuant to the Registration Rights Agreement, dated as of November 13, 2015, between us and the investors in the November 2015 Private Placement. We will bear all expenses of registration incurred in connection with this offering, but all selling and other expenses incurred by the selling stockholders will be borne by them.
Selected Risks Associated with Our Business and Our Common Stock
Our business is subject to numerous risks described in the section entitled “Risk Factors” and elsewhere in this prospectus, which you should review carefully. You should carefully consider these risks before making an investment. Some of these risks include the following:
| 5 |
| · | We will need additional financing to continue our operations. If we are unable to obtain additional financing on acceptable terms, we may have to curtail or cease our development plans and operations under such circumstances. |
| · | We cannot guarantee that we will continue as a going concern because we have not yet been successful in establishing profitable operations. |
| · | Our ongoing viability and ability to continue as a going concern depends on our ability to raise more capital, and to successfully develop and commercialize our licensed technologies. |
| · | If the BRNI License were terminated, we would lose rights to a substantial portion of the intellectual property currently being developed by us. As a result, we may be required to cease operations. |
| · | We are dependent on BRNI to conduct clinical and non-clinical studies of our drug candidates and to provide services for certain core aspects of our business. Under certain conditions, we may engage other third parties to provide such services in the future. Any interruption or failure by BRNI or any third parties to meet their obligations pursuant to various agreements with us could have a material adverse effect on our business, results of operations and financial condition. The BRNI License limits our ability to make certain decisions without BRNI’s consent. |
| · | We have relied (without independent third-party verification) on the representations and materials provided by BRNI, including scientific, peer-reviewed and non-peer reviewed publications, abstracts, slides, internal documents, verbal communications, patents and related patent filings, with respect to the results of its research related to our proposed products. Some of BRNI’s results have been included in this prospectus. |
| · | We have a limited operating history upon which investors can evaluate our future prospects. It is difficult to judge our prospects. |
| · | If we do not obtain the necessary regulatory approvals in the United States and/or other countries, we will not be able to sell our drug candidates. |
| · | We have not generated any revenues since our inception and we do not expect to generate revenue for the foreseeable future. If we do not generate revenues and achieve profitability, we will likely have to curtail or cease our development plans and operations. |
| · | Our commercial success will depend, in part, on our ability, and the ability of our licensors, to obtain and maintain patent protection. Our licensors’ failure to obtain and maintain patent protection for our products may have a material adverse effect on our business. |
| · | Changes in our ownership could limit our ability to utilize net operating loss carry forwards. |
| · | Our licensed patented technologies may infringe on other patents, which may expose us to costly litigation. |
| · | We may not be able to protect our trade secrets and other unpatented proprietary technologies, which could give our competitors an advantage over us. |
| · | We are dependent on Charles S. Ramat, our President and Chief Executive Officer, for the successful execution of our business plan. The loss of Mr. Ramat or other key members of our management team could have a material adverse effect on our business prospects. |
| · | If we are unable to hire additional qualified personnel our business prospects may suffer. |
| · | We may not be able to in-license or acquire new development-stage products or technologies. |
| · | We are dependent upon the National Cancer Institute, or the NCI, to supply bryostatin for our clinical trials. |
| 6 |
| · | We expect to rely on third parties to manufacture our proposed products and, as a result, we may not be able to control our product development. |
| · | We may rely on third parties for marketing and sales and our revenue prospects may depend on their efforts. |
| · | If our products are not accepted by patients, the medical community or health insurance companies, our business prospects will suffer. |
| · | The branded prescription segment of the pharmaceutical industry in which we operate is competitive, and we are particularly subject to the risks of such competition. |
| · | Our business exposes us to potential product liability risks, which could result in significant product liability exposure. |
| · | Although we have clinical trial product liability insurance and comprehensive insurance, a successful liability claim against us could exceed the limits of such coverages and have a material adverse effect on our financial condition. |
| · | Reforms in the health care industry and the uncertainty associated with pharmaceutical pricing, reimbursement and related matters could adversely affect the marketing, pricing and demand for our product candidates, if approved. |
| · | Consolidation in the pharmaceutical industry could materially affect our ability to operate as an independent entity. |
| · | There currently is a limited public market for our common stock. Failure to develop or maintain an active trading market could negatively affect the value of our common stock and make it difficult or impossible for you to sell your shares. |
| · | We cannot assure you that our common stock will become liquid or that it will be listed on a securities exchange. |
| · | Our common stock is subject to the “penny stock” rules of the SEC and the trading market in the securities is limited, which makes transactions in the stock cumbersome and may reduce the value of an investment in our common stock. |
| · | Volatility in the price of our common stock could lead to losses by investors and costly securities litigation. |
| · | We do not anticipate dividends to be paid on our common stock, and investors may lose the entire amount of their investment. |
| · | If securities analysts do not initiate coverage or continue to cover our common stock or publish unfavorable research or reports about our business, this may have a negative impact on the market price of our common stock. |
| · | Because state securities “Blue Sky” laws prohibit trading absent compliance with individual state laws, State Blue Sky registration requirements could limit resale of the shares. |
| · | You may experience dilution of your ownership interests because of the future issuance of additional shares of our common stock. |
| · | The conversion of our issued and outstanding shares of Series B Stock could have the effect of diluting the voting power of common stockholders. |
| 7 |
| · | We may obtain additional capital through the issuance of preferred stock, which may limit your rights as a holder of our common stock. |
| · | Being a public company is expensive and administratively burdensome. |
| · | Any failure to maintain effective internal control over our financial reporting could materially adversely affect us. |
| · | BRNI and its affiliate Neurosciences Research Ventures, Inc. are able to exercise substantial control over our business. |
Corporate Information
Our principal executive offices are located at 50 Park Place, Suite 1401, Newark, New Jersey 07102. Our telephone number is 1-973-242-0005. Our website address is http://www.neurotropebioscience.com. The information on, or that can be accessed through, our website is not part of this prospectus.
| 8 |
| Common stock currently outstanding | 49,172,026 shares (1) | |
| Series B Stock currently outstanding | 262,349.4 shares (2) | |
| Series A through Series E Warrants currently outstanding | Warrants to purchase an aggregate of 131,174,700 shares of our common stock | |
| Placement Agent Warrants currently outstanding | Warrants to purchase an aggregate of 2,276,311 shares of our common stock, which reflects the exercise of placement agent warrants to purchase 330,515 shares of our common stock | |
| Common stock offered by the Company | None | |
| Common stock offered by the selling stockholders | Up to 240,024,699 shares (3) | |
| Use of proceeds | We will not receive any of the proceeds from the sales of our common stock by the selling stockholders. | |
| OTC Market symbol | NTRP | |
| Risk Factors | You should carefully consider the information set forth in this prospectus and, in particular, the specific factors set forth in the “Risk Factors” section beginning on page 12 of this prospectus before deciding whether or not to invest in shares of our common stock. |
| (1) | As of January 6, 2016, and excludes warrants to purchase 1,375,432 shares of our common stock (1,325,000 at $1.00 and 50,432 at $0.01 per share) and options to purchase 8,582,952 shares of our common stock (with exercise prices ranging from $0.60 to $2.22 per share). |
| (2) | Currently, our Series B Stock is convertible into our common stock at the option of the holder currently on a 100-for-one basis. However, each one one-hundredth share of our Series B Stock will convert into more than one share of our common stock in the event that we sell certain securities of the Company at a price per share that is less than the conversion price, which is currently set at $0.60 per share. |
| (3) | Consists of 150% of each of the following: (a) 26,234,940 shares of our common stock issuable upon conversion of 262,349.4 outstanding shares of our Series B Stock, (b) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series A warrants to purchase our common stock having an exercise price of $0.80 per share, (c) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series B warrants to purchase our common stock having an exercise price of $0.80 per share, (d) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series C warrants to purchase our common stock having an exercise price of $1.25 per share, (e) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series D warrants to purchase our common stock having an exercise price of $1.00 per share, (f) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series E warrants to purchase our common stock having an exercise price of $1.50 per share, (g) 363,456 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.01 per share, (h) 1,090,370 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.60 per share, and (i) 1,153,000 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $1.50 per share, subject to adjustment to $0.80 per share upon the exercise of all Series A Warrants or all Series B Warrants. |
| 9 |
Summary Financial Information
| Fiscal Year Ended December 31, 2014 | Fiscal Year Ended December 31, 2013 | Nine Months Ended September 30, 2015 | Nine Months Ended September 30, 2014 | |||||||||||||
| Statement of Operations Data | ||||||||||||||||
| Revenues | $ | — | $ | — | $ | — | $ | — | ||||||||
| Total operating expenses | $ | 9,267,120 | $ | 7,590,782 | $ | 6,911,018 | $ | 6,870,607 | ||||||||
| Net loss | $ | (9,253,323 | ) | $ | (7,589,404 | ) | $ | (6,908,174 | ) | $ | (6,859,017 | ) | ||||
| Statement of Cash Flows Data | ||||||||||||||||
| Cash used in operating activities | $ | (7,152,576 | ) | $ | (5,533,441 | ) | $ | (6,475,749 | ) | $ | (5,097,100 | ) | ||||
| Cash used in investing activities | $ | (54,943 | ) | $ | — | $ | (11,827 | ) | $ | (48,720 | ) | |||||
| Cash provided by financing activities | $ | 6,132 | $ | 20,745,185 | $ | 20,756 | $ | — | ||||||||
| At December 31, 2014 | At December 31, 2013 | At September 30, 2015 | At September 30, 2014 | |||||||||||||
| Balance Sheet Data | ||||||||||||||||
| Total current assets | $ | 8,107,430 | $ | 15,298,803 | $ | 1,891,468 | $ | 10,220,536 | ||||||||
| Total assets | $ | 8,161,048 | $ | 15,298,803 | $ | 1,952,254 | $ | 10,268,643 | ||||||||
| Total current liabilities | $ | 1,289,188 | $ | 408,416 | $ | 1,598,978 | $ | 1,281,400 | ||||||||
| Total liabilities | $ | 1,289,188 | $ | 408,416 | $ | 1,598,978 | $ | 1,281,400 | ||||||||
| Convertible redeemable preferred stock | $ | 18,524,163 | $ | 19,943,572 | $ | 14,522,010 | $ | 19,639,022 | ||||||||
| Total stockholders’ deficit | $ | (11,652,303 | ) | $ | (5,053,185 | ) | $ | (14,168,734 | ) | $ | (10,651,779 | ) | ||||
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements, including, without limitation, in the section captioned “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and elsewhere. Any and all statements contained in this prospectus that are not statements of historical fact may be deemed forward-looking statements. Terms such as “may,” “might,” “would,” “should,” “could,” “project,” “estimate,” “pro-forma,” “predict,” “potential,” “strategy,” “anticipate,” “attempt,” “develop,” “plan,” “help,” “believe,” “continue,” “intend,” “expect,” “future,” and terms of similar import (including the negative of any of the foregoing) may be intended to identify forward-looking statements. However, not all forward-looking statements may contain one or more of these identifying terms. Forward-looking statements in this prospectus may include, without limitation, statements regarding (i) the plans and objectives of management for future operations, including plans or objectives relating to the development of commercially viable pharmaceuticals, (ii) a projection of income (including income/loss), earnings (including earnings/loss) per share, capital expenditures, dividends, capital structure or other financial items, (iii) our future financial performance, including any such statement contained in a discussion and analysis of financial condition by management or in the results of operations included pursuant to the rules and regulations of the SEC, and (iv) the assumptions underlying or relating to any statement described in points (i), (ii) or (iii) above.
| 10 |
The forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon our current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences, many of which we have no control over. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation, our inability to obtain adequate financing, the significant length of time associated with drug development and related insufficient cash flows and resulting illiquidity, our inability to expand our business, significant government regulation of pharmaceuticals and the healthcare industry, lack of product diversification, volatility in the price of our raw materials, existing or increased competition, results of arbitration and litigation, stock volatility and illiquidity, and our failure to implement our business plans or strategies. A description of some of the risks and uncertainties that could cause our actual results to differ materially from those described by the forward-looking statements in this prospectus appears in the section captioned “Risk Factors” and elsewhere in this prospectus. Readers should carefully review this prospectus in its entirety, including, but not limited to, our financial statements and the notes thereto and the risks described herein. We advise you to carefully review the reports and documents we file from time to time with the SEC, particularly our annual reports on Form 10-K, our quarterly reports on Form 10-Q and our current reports on Form 8-K.
Readers are cautioned not to place undue reliance on forward-looking statements because of the risks and uncertainties related to them and to the risk factors. We disclaim any obligation to update the forward-looking statements contained in this prospectus to reflect any new information or future events or circumstances or otherwise.
| 11 |
An investment in shares of our common stock is highly speculative and involves a high degree of risk. We face a variety of risks that may affect our operations and financial results and many of those risks are driven by factors that we cannot control or predict. Before investing in our common stock you should carefully consider the following risks, together with the financial and other information contained in this prospectus. If any of the following risks actually occurs, our business, prospects, financial condition and results of operations could be materially adversely affected. In that case, the trading price of our common stock would likely decline and you may lose all or a part of your investment. Only those investors who can bear the risk of loss of their entire investment should invest in our common stock.
Risks Related to Our Business and Financial Condition
We will need additional financing to continue our operations. If we are unable to obtain additional financing on acceptable terms, we will need to curtail or cease our development plans and operations.
As of January 6, 2016, we had approximately $11.2 million of available cash and cash equivalents. We are currently reviewing our current operating plans, and we will require additional capital. Additional funds may be raised through the issuance of equity securities and/or debt financing, there being no assurance that any type of financing on terms acceptable to us will be available or otherwise occur. Debt financing must be repaid regardless of whether we generate revenues or cash flows from operations and may be secured by substantially all of our assets. Any equity financing or debt financing that requires the issuance of warrants or other equity securities to the lender would cause the percentage ownership by our current stockholders to be diluted, which dilution may be substantial. Also, any additional equity securities issued may have rights, preferences or privileges senior to those of existing stockholders. Any equity financing at a price below the then current conversion price of our Series B Stock will result in an adjustment to the conversion ratio, applicable to such securities, resulting in the issuance of additional shares of our common stock upon the conversion of our Series B Stock, which would further dilute our other stockholders. If such financing is not available when required or is not available on acceptable terms, we may be required to reduce or eliminate certain product candidates and development activities, including those related to bryostatin, the “bryologs” or polyunsaturated fatty acid analogs, and it may ultimately require us to suspend or cease operations, which could cause investors to lose the entire amount of their investment.
We cannot guarantee that we will continue as a going concern because we have not yet been successful in establishing profitable operations.
We received a report from our independent registered public accounting firm on our financial statements for fiscal years ended December 31, 2014 and 2013, which contained emphasis of matter language indicating substantial doubt about the Company’s ability to continue as a going concern. In addition, the footnotes to our financial statements contained in this prospectus list factors, including substantial losses, substantial contractual commitments, and failure to generate revenues, which raise substantial doubt about our ability to continue as a going concern.
Our ongoing viability as a company depends on our ability to successfully develop and commercialize our licensed technology.
We are principally focused on developing a drug, bryostatin, for the treatment of AD and other diseases, which is still in the clinical testing stage and has not yet been fully developed. Our potential success is highly uncertain since our principal product candidate (bryostatin to treat AD) is in Phase 2 of development. Our other product candidates (use of bryostatin to treat Niemann Pick Type-C and Fragile X Syndrome) are even earlier in their development cycles. Bryostatin is also subject to regulatory approval. Our potential success depends upon our ability to raise more capital, complete development of and successfully commercialize bryostatin in a timely manner for the treatment of AD or other diseases. We must develop bryostatin, successfully test it for safety and efficacy in the targeted patient population, and manufacture the finished dosage form on a commercial scale to meet regulatory standards and receive regulatory approvals. The development and commercialization process is both time-consuming and costly, and involves a high degree of business risk. Bryostatin is still at an early stage in its product development cycle, and any follow-on product candidates are still at the concept stage. The results of pre-clinical and clinical testing of our product candidates are uncertain and we cannot assure anybody that we will be able to obtain regulatory approvals of our product candidates. If obtained, regulatory approval may take longer or be more expensive than anticipated. Furthermore, even if regulatory approvals are obtained, our products may not perform as we expect and we may not be able to successfully and profitably produce and market any products. Delays in any part of the process or our inability to obtain regulatory approval of our products could adversely affect our future operating results by restricting (or even prohibiting) the introduction and sale of our products.
| 12 |
If the BRNI License were terminated, we may be required to cease operations.
Our rights to develop, commercialize and sell certain of our proposed products, including bryostatin, is, in part, dependent upon the BRNI License. BRNI has the right to terminate this agreement after 30 days prior notice in certain circumstances, including if we were to materially breach any provisions of the agreement after a 60-day cure period for breaches that are capable of being cured, in the event of certain bankruptcy or insolvency proceedings. Additionally, the BRNI License provides that the license may not be assigned, including by means of a change of control of the Company, or sublicensed without the consent of BRNI. For additional information regarding the BRNI License, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Overview.” If the BRNI License were terminated, we would lose rights to a substantial portion of the intellectual property currently being developed by us and no longer have the rights to develop, commercialize and sell some of our proposed products. As a result, we may be required to cease operations under such circumstance.
We currently rely on BRNI, and may also rely on other independent third-party contract research organizations, to perform clinical and non-clinical studies of our drug candidate and to perform other research and development services.
The BRNI License requires us to use BRNI to provide research and development services and other scientific assistance and support services, including clinical trials, under certain conditions. The BRNI License limits our ability to make certain decisions, including those relating to our drug candidate, without BRNI’s consent. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Overview.” Under certain conditions, we may, however, also rely on independent third-party contract research organizations, or a CRO, to perform clinical and non-clinical studies of our drug candidate. Many important aspects of the services that may be performed for us by CROs would be out of our direct control. If there were to be any dispute or disruption in our relationship with such CROs, the development of our drug candidate may be delayed. Moreover, in our regulatory submissions, we would expect to rely on the quality and validity of the clinical work performed by our CROs. If any of our CROs’ processes, methodologies or results were determined to be invalid or inadequate, our own clinical data and results and related regulatory approvals could be materially adversely impacted.
We have relied on the representations and materials provided by BRNI, including scientific, peer-reviewed and non-peer reviewed publications, abstracts, slides, internal documents, verbal communications, patents and related patent filings, with respect to the results of its research related to our proposed products.
BRNI began the development of the intellectual property that forms the basis for our proposed products in 1999. We have relied on the quality and validity of the research results obtained by BRNI with respect to this intellectual property, and we have conducted limited verification of the raw preclinical and clinical data produced by BRNI. No independent third-party has verified any such data. If any of BRNI’s basic processes, methodologies or results were determined to be invalid or inadequate, our own clinical data and results and related regulatory approvals, could be materially adversely impacted.
We have a limited operating history upon which investors can evaluate our future prospects.
Our drug product, bryostatin, is in an early development stage and we are subject to all of the risks inherent in the establishment of a new business enterprise. While development of our product candidates was started in 1999 by BRNI, Neurotrope BioScience was incorporated on October 31, 2012 and on that same date entered into the Technology License and Services Agreement with BRNI and NRV II, LLC for the continuing development and commercialization of our product candidates, and, therefore, we have a limited operating history. Our proposed products are currently in the research and development stage and we have not generated any revenues, nor do we expect our products to generate revenues for the near term, if ever. As a result, any investment in our securities must be evaluated in light of the potential problems, delays, uncertainties and complications encountered in connection with a newly established pharmaceutical development business. The risks include, but are not limited to, the possibilities that any or all of our potential products will be found to be unsafe, ineffective or, that the products once developed, although effective, are not economical to market; that our competitors hold proprietary rights that preclude us from marketing such products; that our competitors market a superior or equivalent product; or the failure to receive necessary regulatory clearances for our proposed products. To achieve profitable operations, we must successfully develop, obtain regulatory approval for, introduce and successfully market, sell or license at a profit product candidates that are currently in the research and development phase. We only have one product candidate in clinical development, i.e., bryostatin to treat AD. Much of the clinical development work and testing for our product candidates remains to be completed. No assurance can be given that our research and development efforts will be successful, that required regulatory approvals will be obtained, that any of our candidates will be safe and effective, that any products, if developed and introduced, will be successfully marketed, sold or licensed or achieve market acceptance or that products will be marketed at prices necessary to generate profits. Failure to successfully develop, obtain regulatory approvals for, or introduce and market, sell or license our products would have material adverse effects on our business prospects, financial condition and results of operations.
| 13 |
If we do not obtain the necessary regulatory approvals in the United States and/or other countries, we will not be able to sell our drug candidates.
We cannot assure you that we will receive the approvals necessary to commercialize bryostatin, or any other potential drug candidates we acquire or attempt to develop in the future. We will need approval from the FDA to commercialize our drug candidates in the U.S. and approvals from similar regulatory authorities in foreign jurisdictions to commercialize our drug candidates in those jurisdictions. In order to obtain FDA approval of bryostatin or any other drug candidate for the treatment of AD, we must submit first an Investigational New Drug, or IND, application and then a New Drug Application, or NDA, to the FDA, demonstrating that the drug candidate is safe, pure and potent, and effective for its intended use. This demonstration requires significant research including completion of clinical trials. Satisfaction of the FDA’s regulatory requirements typically takes many years, depending upon the type, complexity and novelty of the drug candidate and requires substantial resources for research, development and testing. We cannot predict whether our clinical trials will demonstrate the safety and efficacy of our drug candidates or if the results of any clinical trials will be sufficient to advance to the next phase of development or for approval from the FDA. We also cannot predict whether our research and clinical approaches will result in drugs or therapeutics that the FDA considers safe and effective for the proposed indications. The FDA has substantial discretion in the drug approval process. The approval process may be delayed by changes in government regulation, future legislation or administrative action or changes in FDA policy that occur prior to or during our regulatory review. Delays in obtaining regulatory approvals may prevent or delay commercialization of, and our ability to derive revenues from, our drug candidates and diminish any competitive advantages that we may otherwise believe that we hold. Even if we comply with all FDA requests, the FDA may ultimately reject one or more of our applications. We may never obtain regulatory clearance for any of our drug candidates. Failure to obtain FDA approval of our drug candidates will leave us without a saleable product and therefore without any source of revenues. In addition, the FDA may require us to conduct additional clinical testing or to perform post-marketing studies, as a condition to granting marketing approval of a drug product or permit continued marketing, if previously approved. If conditional marketing approval is obtained, the results generated after approval could result in loss of marketing approval, changes in product labeling, and/or new or increased concerns about the side effects or efficacy of a product. The FDA has significant post-market authority, including the explicit authority to require post-market studies and clinical trials, labeling changes based on new safety information and compliance with FDA-approved risk evaluation and mitigation strategies. The FDA’s exercise of its authority has in some cases resulted, and in the future could result, in delays or increased costs during product development, clinical trials and regulatory review, increased costs to comply with additional post-approval regulatory requirements and potential restrictions on sales of approved drugs. In foreign jurisdictions, the regulatory approval processes generally include the same or similar risks as those associated with the FDA approval procedures described above. We cannot assure you that we will receive the approvals necessary to commercialize our drug candidates for sale either within or outside the United States.
We have not generated any revenues since our inception and we do not expect to generate revenue for the foreseeable future. If we do not generate revenues and achieve profitability, we will likely need to curtail or cease our development plans and operations.
Our ability to generate revenues depends upon many factors, including our ability to complete our currently planned clinical study and development of our proposed products, our ability to obtain necessary regulatory approvals for our proposed products and our ability to successfully commercialize market and sell our products. We have not generated any revenues since we began operations on October 31, 2012. We expect to incur significant operating losses over the next several years. If we do not generate revenues, do not achieve profitability and do not have other sources of financing for our business, we will likely need to curtail or cease our development plans and operations, which could cause investors to lose the entire amount of their investment.
| 14 |
Our commercial success will depend, in part, on our ability, and the ability of our licensors, to obtain and maintain patent protection. Our licensors’ failure to obtain and maintain patent protection for our products may have a material adverse effect on our business.
Pursuant to the BRNI License, we have obtained rights to certain patents owned by BRNI or licensed to NRV II, LLC by BRNI as of or subsequent to October 31, 2012. For additional information regarding the BRNI License, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Overview.” In the future, we may seek rights from third parties to other patents or patent applications. Our success will depend, in part, on our ability and the ability of our licensors to maintain and/or obtain and enforce patent protection for our proposed products and to preserve our trade secrets, and to operate without infringing upon the proprietary rights of third parties. Patent positions in the field of biotechnology and pharmaceuticals are generally highly uncertain and involve complex legal and scientific questions. We cannot be certain that we or our licensors were the first inventors of inventions covered by our licensed patents or that we or they were the first to file. Accordingly, the patents licensed to us may not be valid or afford us protection against competitors with similar technology. The failure to maintain and/or obtain patent protection on the technologies underlying our proposed products may have material adverse effects on our competitive position and business prospects.
Changes in our ownership could limit our ability to utilize net operating loss carryforwards.
As of September 30, 2015 we had aggregate federal and state net operating loss carryforwards of $20.2 million, which begin to expire in fiscal 2032. Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, changes in our ownership may limit the amount of our net operating loss carryforwards that could be utilized annually to offset our future taxable income, if any. This limitation would generally apply in the event of a cumulative change in ownership of our company of more than 50% within a three-year period. Any such limitation may significantly reduce our ability to utilize our net operating loss carryforwards and tax credit carryforwards before they expire. Any such limitation, whether as the result of future offerings, prior private placements, sales of our common stock by our existing stockholders or additional sales of our common stock by us in the future (through the conversion of preferred stock, the exercise of outstanding warrants, or otherwise), could have a material adverse effect on our results of operations in future years. We have not completed a study to assess whether an ownership change for purposes of Section 382 has occurred, or whether there have been multiple ownership changes since our inception, due to the significant costs and complexities associated with such study.
Our licensed patented technologies may infringe on other patents, which may expose us to costly litigation.
It is possible that our licensed patented technologies may infringe on patents or other rights owned by others. We may have to alter our products or processes, pay additional licensing fees, pay to defend an infringement action or challenge the validity of the patents in court or cease activities altogether because of patent rights of third parties, thereby causing additional unexpected costs and delays to us. Patent litigation is costly and time consuming, and we may not have sufficient resources to pay for such litigation. Pursuant to the BRNI License, BRNI has the exclusive right (but not the obligation) to apply for, file, prosecute or maintain patents and patent applications for our licensed technologies. However, in order to maintain our rights to use our licensed technologies, we must reimburse BRNI for all of the attorney’s fees and other costs and expenses related to any of the foregoing. For additional information regarding the BRNI License, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Overview.” If the patents licensed to us are determined to infringe a patent owned by a third party and we do not obtain a license under such third-party patents, or if we are found liable for infringement or are not able to have such third-party patents declared invalid, we may be liable for significant money damages, we may encounter significant delays in bringing products to market or we may be precluded from participating in the manufacture, use or sale of products or methods of treatment requiring such licenses.
We may not be able to protect our trade secrets and other unpatented proprietary technologies, which could give our competitors an advantage over us.
In addition to our reliance on patents and pending patents owned by BRNI, we rely upon trade secrets and other unpatented proprietary technologies. We may not be able to adequately protect our rights with regard to such unpatented proprietary technologies or competitors may independently develop substantially equivalent technologies. We seek to protect trade secrets and proprietary knowledge, in part through confidentiality agreements with our employees, consultants, advisors and collaborators. Nevertheless, these agreements may not effectively prevent disclosure of our confidential information and may not provide us with an adequate remedy in the event of unauthorized disclosure of such information and, as a result, our competitors could gain a competitive advantage over us.
| 15 |
We are dependent on Charles S. Ramat, our President and Chief Executive Officer for the successful execution of our business plan. The loss of Mr. Ramat or other key members of our management team could have a material adverse effect on our business prospects.
We are highly dependent on Charles S. Ramat, our President and Chief Executive Officer. We are dependent on Charles S. Ramat’s and our directors’ networks of contacts and experience to recruit key talent to the Company. We do not have key-man insurance on any of our officers. Loss of the services of Charles S. Ramat or other key members of our management team or our Board of Directors’ ability to identify and hire key talent could have a material adverse effect on our business prospects, financial condition and results of operations.
If we are unable to hire additional qualified personnel, our business prospects may suffer.
Our success and achievement of our business plans depend upon our ability to recruit, hire, train and retain other highly qualified technical and managerial personnel. Competition for qualified employees among pharmaceutical and biotechnology companies is intense, and the loss of any of such persons, such as the recent departure of NBI’s Executive Vice President, Development or an inability to attract, retain and motivate any additional highly skilled employees required for the implementation of our business plans and activities could have a material adverse effect on us. Our inability to attract and retain the necessary technical and managerial personnel and consultants and scientific and/or regulatory consultants and advisors could have a material adverse effect on our business prospects, financial condition and results of operations.
We may not be able to in-license or acquire new development-stage products or technologies.
Our product commercialization strategy relies, to some extent, on our ability to in-license or acquire product formulation techniques, new chemical entities, or related know-how that has proprietary protection. If resources permit, we may also seek to acquire, by license or otherwise, other development stage products that are consistent with our product portfolio objectives and commercialization strategy. The acquisition of products requires the identification of appropriate candidates, negotiation of terms of acquisition, and financing for the acquisition and integration of the candidates into our portfolio. Failure to accomplish any of these tasks may diminish our growth rate and adversely alter our competitive position.
We are dependent upon the NCI to supply bryostatin for our clinical trials.
BRNI has entered into a material transfer agreement with the NCI, pursuant to which the NCI has agreed to supply bryostatin required for our pre-clinical research and clinical trials. This agreement does not provide for a sufficient amount of bryostatin to support the completion of our clinical trials that we are required to conduct in order to seek FDA approval of bryostatin for the treatment of AD. Therefore, BRNI or we will have to enter into one or more subsequent agreements with the NCI for the supply of additional amounts of bryostatin. If BRNI or we are unable to secure such additional agreements or if the NCI otherwise discontinues for any reason supplying us with bryostatin, then we would have to either secure another source of bryostatin or discontinue our efforts to develop and commercialize bryostatin for the treatment of AD. There can be no assurance that we will be able to secure future bryostatin supplies from any source on commercially reasonable terms, if at all.
We expect to rely on third parties to manufacture our proposed products and, as a result, we may not be able to control our product development or commercialization.
We currently do not have an FDA approved manufacturing facility. We expect to rely on contract manufacturers to produce quantities of products and substances necessary for product commercialization. See also the risk factor above captioned “We are dependent upon the NCI to supply bryostatin for our clinical trials.” Contract manufacturers that we use must adhere to current good manufacturing practice regulations enforced by the FDA through its facilities inspection program. If the facilities of such manufacturers cannot pass a pre-approval plant inspection, the FDA pre-market approval of our products will not be granted. As a result:
| · | there are a limited number of manufacturers that could produce the products for us and we may not be able to identify and enter into acceptable agreements with any manufacturers; |
| · | the products may not be produced at costs or in quantities necessary to make them commercially viable; |
| · | the quality of the products may not be acceptable to us and/or regulatory authorities; |
| 16 |
| · | our manufacturing partners may go out of business or file for bankruptcy; |
| · | our manufacturing partners may decide not to manufacture our products for us; |
| · | our manufacturing partners could fail to manufacture to our specifications; |
| · | there could be delays in the delivery of quantities needed; |
| · | we could be unable to fulfill our commercial needs in the event we obtain regulatory approvals and there is strong market demand; or |
| · | ongoing inspections by the FDA or other regulatory authorities may result in suspensions, seizures, recalls, fines, injunctions, revocations and/or criminal prosecutions. |
If we are unable to engage contract manufacturers or suppliers to manufacture or package our products, or if we are unable to contract for a sufficient supply of required products and substances on acceptable terms, or if we encounter delays or difficulties in our relationships with these manufacturers, or with a regulatory agency, then the submission of products for regulatory approval and subsequent sales of such products would be delayed. Any such delay may have a material adverse effect on our business prospects, financial condition and results of operations.
We may rely on third parties for marketing and sales and our revenue prospects may depend on their efforts.
We currently have no experience in sales, marketing or distribution. We do not anticipate having the resources in the foreseeable future to allocate to the sales and marketing of our proposed products. As a result, if our product development is successful, our future success will likely depend, in part, on our ability to enter into and maintain collaborative relationships with one or more third parties for sales, marketing or distribution, on the collaborator’s strategic interest in the products we have under development and on such collaborator’s ability to successfully market and sell any such products. We intend to pursue collaborative arrangements regarding the sales and marketing of our products as appropriate. However, we may not be able to establish or maintain such collaborative arrangements or, if we are able to do so, they may not have effective sales forces. To the extent that we decide not to, or are unable to, enter into collaborative arrangements with respect to the sales and marketing of our proposed products, significant capital expenditures, management resources and time will be required to establish and develop an in-house marketing and sales force with technical expertise. To the extent that we depend on third parties for marketing and distribution, any revenues received by us will depend upon the efforts of such third parties, which may not be successful.
If our products are not accepted by patients, the medical community or health insurance companies, our business prospects will suffer.
Commercial sales of any products we successfully develop will substantially depend upon the products’ efficacy and on their acceptance by patients, the medical community, providers of comprehensive healthcare insurance, healthcare benefit plan managers, the Centers for Medicare and Medicaid Services, or CMS (which is the U.S. federal agency which administers Medicare, Medicaid and the State Children’s Health Insurance Program), and other organizations. Widespread acceptance of our products will require educating patients, the medical community and third-party payors of medical treatments as to the benefits and reliability of the products. Our proposed products may not be accepted, and, even if they are accepted, we are unable to estimate the length of time it would take to gain such acceptance.
The branded prescription segment of the pharmaceutical industry in which we operate is competitive, and we are particularly subject to the risks of such competition.
The branded prescription segment of the pharmaceutical industry in which we operate is competitive, in part, because the products that are sold require extensive sales and marketing resources invested in their commercialization. The increasing cost of prescription pharmaceuticals has caused providers of comprehensive healthcare insurance, healthcare benefit plan managers, CMS, as well as other organizations, collectively known as third-party payors, to tightly control and dictate their drug formulary plans to control the costs associated with the use of prescription pharmaceutical products by enrollees in these plans. Our ability to gain formulary access to drug plans supported by these third-party payors is substantially dependent on the differentiated patient benefit that our proposed products can provide, compared closely to similar products claiming the same benefits or advantages. We may not be able to differentiate our proposed products from those of our competitors, successfully develop or introduce new products that are less costly or offer better performance than those of our competitors, or offer purchasers of our proposed products payment and other commercial terms as favorable as those offered by our competitors. We expect that some of our proposed products, even if successfully developed and commercialized, will eventually face competition from a significant number of biotechnology or large pharmaceutical companies. Because most of our competitors have substantially greater financial and other resources than we have, we are particularly subject to the risks inherent in competing with them. The effects of this competition could materially adversely affect our business prospects, financial condition and results of operations.
| 17 |
We compete with many companies, research institutes, hospitals, governments and universities that are working to develop products and processes to treat or diagnose AD. We believe that others are doing research on Fragile X Syndrome and Niemann Pick disease. Many of these entities have substantially greater financial, technical, manufacturing, marketing, distribution and other resources than we do. However, there has been a dearth of new product introductions in the last 20 years for the treatment of AD symptoms in patients who begin exhibiting the memory and cognitive disorders associated with the disease. All of the products introduced to date for the treatment of AD have yielded negative or marginal results with little effect on the progression of AD and no improvement in the memory or cognitive performance of the patients receiving these therapies. The absolute determination of AD in patients is currently achieved only upon autopsy. We believe we are the only company currently pursuing PKCε activation as a mechanism to treat AD and neurodegenerative diseases. Although we believe that we have no direct competitors working in this same field on product candidates using the same mechanism of action, we cannot provide assurance that our competitors will not discover compounds or processes that may be competitive with our products and introduce such products or processes before us.
We are developing our product candidates to address unmet medical needs in the treatment of AD and other neurodegenerative diseases. Our competition will be determined in part by the potential indications for which drugs are developed and ultimately approved by regulatory authorities. Additionally, the timing of market introduction of some of our potential products or of competitors’ products may be an important competitive factor. Accordingly, the relative speed with which we can develop our product candidates, complete preclinical testing, clinical trials and approval processes and supply commercial quantities to market are expected to be important competitive factors. We expect that competition among products approved for sale will be based on various factors, including product efficacy, safety, reliability, availability, price and patent position.
Our business will expose us to potential product liability risks, which could result in significant product liability exposure.
Our business will expose us to potential product liability risks that are inherent in the testing, designing, manufacturing and marketing of human therapeutic products. Product liability insurance in the pharmaceutical industry is generally expensive, and we may not be able to obtain or maintain product liability insurance in the future on acceptable terms or with adequate coverage against potential liabilities, if at all. A successful products liability claim brought against us could have a material adverse effect on our business prospects, financial condition and results of operations.
A successful clinical trial liability claim against us could have a material adverse effect on our financial condition even with such insurance coverage.
Our business will expose us to potential liability that results from risks associated with conducting clinical trials of our product candidates. Although we have procured clinical trial product liability insurance coverage for our bryostatin product candidate with coverages and deductibles we believe are adequate, there is no guarantee that our coverage will be adequate to satisfy any liability we may incur. We do not currently have insurance with respect to any other drug product. A successful clinical trial liability claim brought against us could have a material adverse effect on our business prospects, financial condition and results of operations even if we successfully obtain clinical trial insurance.
A successful liability claim against us could have a material adverse effect on our financial condition.
Our business and actions can expose us to potential liability risks that are inherent in business, generally, and in the pharmaceutical industry, specifically. While we maintain commercial general liability insurance with coverages and deductibles we believe are adequate, there is no guarantee that our coverage will be adequate to satisfy any liability we may incur. A successful liability claim brought against us could have a material adverse effect on our business prospects, financial condition and results of operations.
| 18 |
Reforms in the health care industry and the uncertainty associated with pharmaceutical and laboratory test pricing, reimbursement and related matters could adversely affect the marketing, pricing and demand for our products.
Public and private entities are seeking ways to reduce or contain increasing health care costs. All generic pharmaceutical manufacturers whose products are covered by the Medicaid program are required to rebate to each state a percentage of their “average manufacturer price” for the products in question. The extension of prescription drug coverage to all Medicare recipients was approved by Congress several years ago. Numerous other proposals to curb rising pharmaceutical prices have also been introduced or proposed in Congress and in some state legislatures. We cannot predict the nature of the measures that may be adopted or their effect on our competitive position. Our ability to market our products depends, in part, on reimbursement levels for them and related treatment established by health care providers, private health insurers and other organizations, including health maintenance organizations and managed care organizations. In the event that governmental authorities enact additional legislation or adopt regulations that affect third party coverage and reimbursement, demand for our products may be reduced, which may materially adversely affect our business prospects, financial condition and results of operations.
Consolidation in the pharmaceutical industry could materially affect our ability to operate as an independent entity.
The pressure to grow revenues while containing the escalating costs of basic research and development has resulted in an increase in mergers and acquisitions in our industry. More consolidation in the pharmaceutical industry is expected over the next five years. We could become an acquisition target by a larger competitor and, as a consequence, suffer serious disruptions to our business model or even lose control of our ability to operate as an independent entity. Such events could have a material adverse effect on our product development efforts or the commercialization of our proposed products.
Risks Related to Our Common Stock
There currently is a limited public market for our common stock. Failure to develop or maintain an active trading market could negatively affect the value of our common stock and make it difficult or impossible for you to sell your shares.
There is currently a limited public market for shares of our common stock, and an active trading market may never develop or, if developed, may not be maintained. Our common stock is not listed on a stock exchange. Our common stock is quoted on the OTC Market. The OTC Market is a thinly traded market and lacks the liquidity of certain other public markets with which some investors may have more experience. The average daily trading volume in our common stock was approximately 12,500 shares during the 90-day period ended January 6, 2016. We do not currently and may not ever be able to satisfy the listing requirements for our common stock to be listed on a national securities exchange, which are often a more widely-traded and liquid market. Some, but not all, of the factors which may delay or prevent the listing of our common stock on a more widely-traded and liquid market include the following: our stockholders’ equity may be insufficient; the market value of our outstanding securities may be too low; our net income from operations may be too low; our common stock may not be sufficiently widely held; we may not be able to secure market makers for our common stock; and we may fail to meet the rules and requirements mandated by the several exchanges and markets to have our common stock listed. Should we fail to satisfy the initial listing standards of the national exchanges, or our common stock is otherwise rejected for listing and remains listed on the OTC Market or suspended from the OTC Market, the trading price of our common stock could suffer and the trading market for our common stock may be less liquid and our common stock price may be subject to increased volatility.
We cannot assure you that our common stock will become liquid or that it will be listed on a securities exchange.
Until our common stock is listed on a national securities exchange, such as The New York Stock Exchange or The Nasdaq Stock Market, we expect our common stock to remain eligible for quotation on the OTC Market, or on another over-the-counter quotation system, or in the “pink sheets.” In those venues, however, an investor may find it difficult to obtain accurate quotations as to the market value of our common stock. In addition, if we fail to meet the criteria set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling our common stock, which may further affect the liquidity of our common stock. This would also make it more difficult for us to raise capital.
| 19 |
Our common stock is subject to the “penny stock” rules of the SEC and the trading market in the securities is limited, which makes transactions in the stock cumbersome and may reduce the value of an investment in our common stock.
The SEC has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require:
| · | that a broker or dealer approve a person’s account for transactions in penny stocks; and |
| · | that the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased. |
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must:
| · | obtain financial information and investment experience objectives of the person; and |
| · | make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks |
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth:
| · | the basis on which the broker or dealer made the suitability determination; and |
| · | that the broker or dealer received a signed, written agreement from the investor prior to the transaction. |
Generally, brokers and potential investors may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for existing stockholders to dispose of such securities and cause a decline in the market value of such securities.
Rule 15g-2 requires that disclosure has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. If our securities are subject to the penny stock rules, investors will find it more difficult to dispose of our securities.
Volatility in the price of our common stock could lead to losses by investors and costly securities litigation.
The trading price of our common stock is likely to be highly volatile and could fluctuate in response to factors such as:
| · | additions or departures of key personnel; |
| · | actual or anticipated variations in our operating results; |
| · | announcements of developments by us or our competitors; |
| 20 |
| · | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| · | adoption of new accounting standards affecting our industry; |
| · | sales of our common stock or other securities in the open market or in any publicized transaction; |
| · | changes in our industry; |
| · | regulatory and economic developments, including our ability to obtain working capital financing; |
| · | shares of our common stock are saleable under Rule 144 of the Securities Act of 1933, as amended, or the Securities Act, and as a result, potential and actual sales of our common stock by our present stockholders may have a depressive effect on the price of our common stock in the marketplace; |
| · | on August 29, 2014, we filed a Registration Statement on Form 8-A with the SEC, and as a result, potential and actual sales of our common stock by our present stockholders may have a depressive effect on the price of our common stock in the marketplace; |
| · | on April 8, 2015, we filed a Post-Effective Amendment No. 1 to Registration Statement on Form S-1 (Registration No. 333- 200664), which became effective on May 11, 2015. Potential and actual sales of our common stock by our present stockholders pursuant to such Registration Statement may have a depressive effect on the price of our common stock in the marketplace; |
| · | our ability to execute our business plan; |
| · | other events or factors, many of which are beyond our control; and |
| · | announcement of clinical trial results. |
The stock market is subject to significant price and volume fluctuations. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been initiated against the public company. Litigation initiated against us, whether or not successful, could result in substantial costs and diversion of our management’s attention and resources, which could harm our business and financial condition.
We do not anticipate dividends to be paid on our common stock, and investors may lose the entire amount of their investment.
Cash dividends have never been declared or paid on our common stock, and we do not anticipate such a declaration or payment for the foreseeable future. We expect to use future earnings, if any, to fund business growth. Therefore, stockholders will not likely receive any funds absent a sale of their shares. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates. We cannot assure stockholders of a positive return on their investment when they sell their shares, nor can we assure that stockholders will not lose the entire amount of their investment.
If securities analysts do not initiate coverage or continue to cover our common stock or if they publish unfavorable research or reports about our business, there could be a negative impact on the market price of our common stock.
The trading market for our common stock may depend, in part, on the research and reports that securities analysts publish about our business and the Company. It is often more difficult to obtain analyst coverage for companies whose securities are traded on the OTC Market. We do not have any control over securities analysts. There is no guarantee that securities analysts will cover our common stock. If securities analysts do not cover our common stock, the lack of research coverage may adversely affect its market price. If we are covered by securities analysts, and our stock is the subject of an unfavorable report, our stock price and trading volume would likely decline. If one or more of these analysts ceases to cover the Company or fails to publish regular reports on the Company, we could lose visibility in the financial markets, which could cause our stock price or trading volume to decline.
| 21 |
Because state securities “Blue Sky” laws prohibit trading absent compliance with individual state laws, state Blue Sky registration requirements could limit resale of the shares.
Transfer of our common stock may be restricted under the securities laws and regulations promulgated by various states and foreign jurisdictions, commonly referred to as “Blue Sky” laws. Absent compliance with such individual “Blue Sky” laws, our common stock may not be traded in such jurisdictions. We currently maintain information which permits sales of securities pursuant to the “manuals exemption”. This manuals exemption permits a security to be sold by stockholders in a particular state without being registered if the company issuing the security has a listing for that security in a securities manual recognized by that state. The listing entry must contain (i) the names of issuers, officers, and directors, (ii) an issuer’s balance sheet, and (iii) a profit and loss statement for either the fiscal year preceding the balance sheet or for the most recent fiscal year of operations. The principal accepted manuals are those published by Standard and Poor’s, and Mergent, Inc. Many states expressly recognize these manuals. Certain states either do not recognize principal accepted manuals or do not expressly recognize the manuals exemption. These states include: Alabama, California, Illinois, Kentucky, Louisiana, Missouri, New Hampshire, New York, Tennessee and Virginia. Registration of the securities is required in these states in order for such securities to be sold by stockholders in such states. As a result, it will not be possible for persons to resell shares of our common stock pursuant to this registration statement in these states without such registration. There is no assurance that the state securities divisions will approve these registrations. Accordingly, investors should consider the secondary market for our securities to be a limited one.
You may experience dilution of your ownership interests because of the future issuance of additional shares of our common stock.
Any future issuance of our equity or equity-backed securities will dilute then-current stockholders’ ownership percentages and could also result in a decrease in the fair market value of our equity securities, because our assets would be owned by a larger pool of outstanding equity. As described above, we will need additional financing to continue our operations and may raise additional capital through public or private offerings of our common or preferred stock or other securities that are convertible into or exercisable for our common or preferred stock. We may also issue such securities in connection with hiring or retaining employees and consultants (including stock options and other equity compensation issued under our equity incentive plans), as payment to providers of goods and services, in connection with future acquisitions or for other business purposes. Our Board of Directors may at any time authorize the issuance of additional common or preferred stock without common stockholder approval, subject only to the total number of authorized common and preferred shares set forth in our Articles of Incorporation. The terms of equity securities issued by us in future transactions may be more favorable to new investors, and may include dividend and/or liquidation preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have a further dilutive effect. Also, the future issuance of any such additional shares of our common or preferred stock or other securities may create downward pressure on the trading price of our common stock. There can be no assurance that any such future issuances will not be at a price (or exercise prices) below the price at which shares of our common stock are then traded.
The conversion of our issued and outstanding shares of Series B Stock will have the effect of diluting the voting power of existing common stockholders.
The conversion of shares of our Series B Stock could have the effect of changing or preventing a change of control of us and will dilute your interests. Our authorized capital stock includes 50,000,000 shares of preferred stock, of which 333,333 shares are designated as Series B Stock. Currently, our Series B Stock converts into common stock on a one-hundred-for-one basis. However, each one-hundredth share of our Series B Stock will convert into more than one share of our common stock in the event that we sell certain securities of the Company at a price per share that is less than the conversion price, which is currently set at $0.60 per share. The 45-day average price per share of our common stock for the 45-day time period ended January 6, 2016 and the 90-day average price per share of our common stock for the 90-day time period ended January 6, 2016 were $0.50 and $0.54, respectively. On January 6, 2016, the last reported sales price for our common stock was $0.50 per share.
The effects of the conversion of shares of our Series B Stock upon the rights of our common stockholders might include, among other things, restricting dividends on our common stock, diluting the voting power of our common stockholders, reducing the market price of our common stock, or impairing the liquidation rights of our common stock.
| 22 |
We may obtain additional capital through the issuance of preferred stock, which may limit your rights as a holder of our common stock.
Without any stockholder vote or action, our Board of Directors may designate and approve for issuance shares of our preferred stock. The terms of any preferred stock may include priority claims to assets and dividends and special voting rights which could limit the rights of the holders of our common stock. The designation and issuance of preferred stock favorable to current management or stockholders could make any possible takeover of us or the removal of our management more difficult.
Being a public company is expensive and administratively burdensome.
Public reporting companies are subject to the information and reporting requirements of the Securities Act, the Securities Exchange Act of 1934, as amended, or the Exchange Act, and other federal securities laws, rules and regulations related thereto, including compliance with the Sarbanes-Oxley Act. Prior to August 29, 2014, we voluntarily filed certain reports with the SEC pursuant to the Exchange Act. On August 29, 2014, we filed a Registration Statement on Form 8-A with the SEC, which subjects us to the rules and regulations of the Exchange Act, including those requiring annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K. Complying with these laws and regulations requires the time and attention of our Board of Directors and management, and increases our expenses. Among other things, public reporting companies must:
| · | maintain and evaluate a system of internal controls over financial reporting in compliance with the requirements of Section 404 of the Sarbanes-Oxley Act and the related rules and regulations of the SEC and the Public Company Accounting Oversight Board; |
| · | maintain policies relating to disclosure controls and procedures; |
| · | prepare and distribute periodic reports in compliance with our obligations under federal securities laws; |
| · | institute a more comprehensive compliance function, including with respect to corporate governance; and |
| · | involve, to a greater degree, our outside legal counsel and accountants in the above activities. |
The costs of preparing and filing annual and quarterly reports, proxy statements and other information with the SEC and furnishing audited reports to stockholders is expensive and much greater than that of a privately-held company, and compliance with these rules and regulations may require us to hire additional financial reporting, internal controls and other finance personnel, and will involve a material increase in regulatory, legal and accounting expenses and the attention of management. We currently do not comply with all of these regulations. See below Risk Factor entitled “Any failure to maintain effective internal control over our financial reporting could materially adversely affect us.” There can be no assurance that we will be able to comply with the applicable regulations in a timely manner, if at all. In addition, being a public company has made it more expensive for us to obtain director and officer liability insurance. In the future, we may be required to accept reduced coverage or incur substantially higher costs to obtain this coverage. These factors could also make it more difficult for us to attract and retain qualified executives and members of our Board of Directors, particularly directors willing to serve on the audit and compensation committees.
Any failure to maintain effective internal control over our financial reporting could materially adversely affect us.
Section 404 of the Sarbanes-Oxley Act, or Section 404, requires us to include in our annual reports on Form 10-K an assessment by management of the effectiveness of our internal control over financial reporting. In addition, at such time, if any, as we are no longer a “smaller reporting company,” our independent auditors will have to attest to and report on management’s assessment of the effectiveness of such internal control over financial reporting. We have limited experience operating as a public reporting company under the level of internal control over financial reporting required by the Sarbanes-Oxley Act. We performed an evaluation under the supervision and with the participation of our Chief Executive Officer and our Chief Financial Officer, our principal executive officer and principal financial officer, respectively, of the effectiveness of our disclosure controls and procedures and internal controls over financial reporting. Based on their evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures and internal controls over financial reporting are not effective due to the material weakness resulting from a limited segregation of duties among our employees with respect to our control activities. This deficiency is the result of our limited number of employees. This deficiency may affect management’s ability to determine if errors or inappropriate actions have taken place. Management is required to apply its judgment in evaluating the cost-benefit relationship of possible changes in our disclosure controls and procedures.
| 23 |
Even in the event that our management concludes that our internal control over financial reporting becomes effective, if our independent auditors are not satisfied with the adequacy of our internal control over financial reporting, or if the independent auditors interpret the requirements, rules or regulations differently than we do, then (to the extent we are no longer a “smaller reporting company”) they may decline to attest to management’s assessment or may issue a report that is qualified. Any of these events could result in a loss of investor confidence in the reliability of our financial statements, which in turn could negatively impact the price of our common stock.
We must perform system and process evaluation and testing of our internal control over financial reporting to allow management and (if required in the future) our independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting, as required by Section 404. Our compliance with Section 404 may require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and we will need to retain the services of additional accounting and financial staff or consultants with appropriate public company experience and technical accounting knowledge to satisfy the ongoing requirements of Section 404.
Neurosciences Research Ventures, Inc. will be able to exercise substantial control over our business.
BRNI, through its affiliate Neurosciences Research Ventures, Inc., or NRV, owns 9,025,000 shares of our common stock and options to purchase 1,662,500 shares of our common stock. In the aggregate, such securities represented beneficial ownership of approximately 21.7% of our common stock as of January 6, 2016. Additionally, pursuant to a certain letter agreement dated November 12, 2015 between the Company and NRV, NRV has the right to nominate two representatives for election to our Board of Directors. Pursuant to this right, William S. Singer and James Gottlieb are members of our Board of Directors. As a result of the foregoing, BRNI and NRV are able to exercise substantial influence over our business, policies and practices.
In addition, Dr. Daniel Alkon, our Chief Scientific Officer, founding Scientific Director of BRNI and the Toyota Chair in Neuroscience at BRNI, owns 950,000 shares of our common stock and options to purchase 175,000 shares of our common stock. In the aggregate, such securities represented beneficial ownership of approximately 2.3% of our common stock as of January 6, 2016.
| 24 |
This prospectus relates to the sale of up to 240,024,699 shares of our common stock, par value $0.0001 per share, by the selling stockholders named in this prospectus.
In November 2015, through a private placement, we sold an aggregate of 26,234,940 units at $0.60 per unit, with each unit consisting of shares of our Series B Stock together with Series A through Series E warrants to purchase common stock, and certain placement agent warrants, resulting in gross proceeds to the Company of $15,640,963. The private placement was completed in two closings, which took place on November 13, 2015 and November 30, 2015 pursuant to a securities purchase agreement, or the Securities Purchase Agreement, among the Company and the selling stockholders, dated as of November 13, 2015.
In accordance with the terms of a registration rights agreement among the Company and the selling stockholders entered into in connection with the Securities Purchase Agreement, we are hereby registering, and the shares being offered by this prospectus consist of, 150% of each of the following securities issued in connection with the Securities Purchase Agreement: (a) 26,234,940 shares of our common stock issuable upon conversion of 262,349.4 outstanding shares of our Series B Stock, (b) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series A warrants to purchase our common stock having an exercise price of $0.80 per share, (c) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series B warrants to purchase our common stock having an exercise price of $0.80 per share, (d) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series C warrants to purchase our common stock having an exercise price of $1.25 per share, (e) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series D warrants to purchase our common stock having an exercise price of $1.00 per share, (f) 26,234,940 shares of our common stock issuable upon exercise of the outstanding Series E warrants to purchase our common stock having an exercise price of $1.50 per share, (g) 363,456 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.01 per share, (h) 1,090,370 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.60 per share, and (i) 1,153,000 shares of our common stock issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $1.50 per share, subject to adjustment to $0.80 per share upon the exercise of all Series A Warrants or all Series B Warrants.
The first column in the table below lists the selling stockholders. The second column lists the number of shares of common stock beneficially owned by each selling stockholder, based on such stockholder’s respective ownership of shares of our common stock, shares of our Series B Stock, warrants and options as of January 6, 2016, assuming conversion of the Series B Stock and exercise of all warrants and options held by the selling stockholders on that date, without regard to any limitations on conversions, amortizations, redemptions or exercises. The number of shares owned are those beneficially owned, as determined under the rules of the SEC, and such information is not necessarily indicative of beneficial ownership for any other purpose. Beneficial ownership includes any shares of our common stock as to which a person had sole or shared voting power or investment power and any shares of common stock which the person had the right to acquire within 60 days of January 6, 2016, through the exercise of any option, warrant or right, through conversion of any security or pursuant to the automatic termination of a power of attorney or revocation of a trust, discretionary account or similar arrangement, and such shares are deemed to be beneficially owned and outstanding for computing the share ownership and percentage of the person holding such options, warrants or other rights, but are not deemed outstanding for computing the percentage of any other person. Beneficial ownership percentages are calculated based on 49,172,026 shares of our common stock outstanding as of January 6, 2016. Unless otherwise set forth below, based upon the information furnished to us, (a) the persons and entities named in the table have sole voting and sole investment power with respect to the shares set forth opposite the selling stockholder’s name, subject to community property laws, where applicable, (b) no selling stockholder had any position, office or other material relationship within the past three years, with us or with any of our predecessors or affiliates, and (c) no selling stockholder is a broker-dealer or an affiliate of a broker-dealer. Selling stockholders who are broker-dealers or affiliates of broker-dealers are indicated by footnote. We have been advised that these broker-dealers and affiliates of broker-dealers purchased our common stock in the ordinary course of business, not for resale, and, at the time of purchase, did not have any agreements or understandings, directly or indirectly, with any person to distribute such common stock.
| 25 |
The third column lists the shares of common stock issuable upon the conversion of our Series B Stock being offered by this prospectus by the selling stockholders, and the fourth column lists the shares of common stock issuable upon exercise of the warrants being offered by this prospectus by the selling stockholders, which are, in each case, 150% of the securities purchased by such selling stockholders pursuant to the Securities Purchase Agreement. Under the terms of the Series B Stock and the warrants, a selling stockholder may not convert the Series B Stock or exercise the warrants to the extent (but only to the extent) such selling stockholder or any of its affiliates would beneficially own greater than 9.99% of our common stock.
Because the conversion price of the Series B Stock and the exercise price of the warrants may be adjusted, the number of shares that will actually be issued may be more or less than the number of shares being offered by this prospectus. The fifth and sixth columns assume the sale of all of the shares offered by the selling stockholders pursuant to this prospectus, and the sixth column identifies (if one percent or more) the percentage of our common stock to be beneficially owned by each selling stockholder after completion of the offering
The table below has been prepared based upon information furnished to us. We have not undertaken any independent research as to the stock holdings of any selling stockholders. The selling stockholders identified below may have sold, transferred or otherwise disposed of some or all of their shares in transactions exempt from or not subject to the registration requirements of the Securities Act. Information concerning the selling stockholders may change from time to time and, if necessary, we will amend or supplement this prospectus accordingly. We cannot give an estimate as to the number of shares of our common stock that will actually be held by the selling stockholders upon termination of this offering because the selling stockholders may offer some or all of their common stock under the offering contemplated by this prospectus or acquire additional shares of common stock. The total number of shares that may be sold hereunder will not exceed the number of shares offered hereby. Please read the section entitled “Plan of Distribution” in this prospectus.
| Selling Stockholder | Shares of common stock beneficially owned prior to the offering | Shares of Series A Stock beneficially owned prior to the offering | Maximum number of shares of common stock issuable upon conversion of Series B Stock owned prior to this offering and registered hereby(1) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby(1) | Shares of common stock beneficially owned upon completion of the offering(2) | Shares of Series A Stock beneficially owned upon completion of the offering | Percentage of common stock beneficially owned upon completion of the offering(2)(3) | Percentage of Series A Stock beneficially owned upon completion of the offering(3) | ||||||||||||||||||||||||
| Abeles, John(4) | 6,187,002 | 0 | 250,000 | 1,250,002 | 5,187,000 | 0 | 1.8 | * | ||||||||||||||||||||||||
| AIGH Investment Partners LLC(5) | 2,500,002 | 0 | 625,000 | 3,125,002 | 0 | 0 | * | * | ||||||||||||||||||||||||
| AIGH Investment Partners LP(6) | 5,000,004 | 0 | 1,250,001 | 6,250,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Allan Lipkowitz Revocable Living Trust U/A 8/26/05(7) | 500,004 | 0 | 125,001 | 625,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Alpha Capital Anstalt(8) | 7,500,000 | 0 | 1,875,000 | 9,375,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Altstiel, Larry, MD, PhD(9) | 250,002 | 0 | 62,500 | 312,502 | 0 | 0 | * | * | ||||||||||||||||||||||||
| American Capital Management LLC(10) | 2,000,004 | 0 | 500,001 | 2,500,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| 26 |
| Selling Stockholder | Shares of common stock beneficially owned prior to the offering | Shares of Series A Stock beneficially owned prior to the offering | Maximum number of shares of common stock issuable upon conversion of Series B Stock owned prior to this offering and registered hereby(1) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby(1) | Shares of common stock beneficially owned upon completion of the offering(2) | Shares of Series A Stock beneficially owned upon completion of the offering | Percentage of common stock beneficially owned upon completion of the offering(2)(3) | Percentage of Series A Stock beneficially owned upon completion of the offering(3) | ||||||||||||||||||||||||
| Anderson, Kent Tucker | 1,256,136 | 0 | 255,000 | 1,275,000 | 236,136 | 0 | * | * | ||||||||||||||||||||||||
| Armitage, Barclay | 129,221 | 0 | 25,000 | 125,002 | 29,219 | 0 | * | * | ||||||||||||||||||||||||
| Baker, Adrienne | 558,442 | 0 | 125,001 | 625,005 | 58,438 | 0 | * | * | ||||||||||||||||||||||||
| Baker, Christopher | 500,004 | 0 | 125,001 | 625,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Bell Family Trust, dtd 2/2/95, as amended(11) | 1,298,153 | 0 | 250,000 | 1,250,002 | 298,151 | 0 | * | * | ||||||||||||||||||||||||
| Benison, Jeffrey | 258,000 | 0 | 64,500 | 322,500 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Berkovits, Elliot | 200,004 | 0 | 50,001 | 250,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Berkowitz, Hershel | 300,000 | 0 | 75,000 | 375,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Blatt, Jonathan & Gina JTWROS | 120,000 | 0 | 30,000 | 150,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Blazier, John C. | 250,002 | 0 | 62,500 | 312,502 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Blum, Christopher J. and Denise M., JTWROS | 143,852 | 0 | 30,000 | 150,000 | 23,852 | 0 | * | * | ||||||||||||||||||||||||
| Bozarth LLC(12) | 559,634 | 0 | 125,001 | 625,005 | 59,630 | 0 | * | * | ||||||||||||||||||||||||
| Brenner, Andrew S. | 180,000 | 0 | 45,000 | 225,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Brescia, Rocco, Jr. | 129,221 | 0 | 25,000 | 125,002 | 29,219 | 0 | * | * | ||||||||||||||||||||||||
| Brio Capital Master Fund Ltd.(13) | 5,238,525 | 0 | 1,250,001 | 6,250,005 | 238,521 | 0 | * | * | ||||||||||||||||||||||||
| Cardone, Scott(14) | 190,987 | 79,149 | 0 | 65,449 | 147,354 | 79,149 | * | 6.0 | % | |||||||||||||||||||||||
| Cialone, Juli-Ann | 254,000 | 0 | 63,000 | 315,000 | 2,000 | 0 | * | * | ||||||||||||||||||||||||
| Chestler, Daniel | 618,072 | 0 | 125,001 | 625,005 | 118,068 | 0 | * | * | ||||||||||||||||||||||||
| Chicago Investments, Inc.(15) | 559,634 | 0 | 125,001 | 625,005 | 59,630 | 0 | * | * | ||||||||||||||||||||||||
| Codi, Joseph | 338,255 | 0 | 62,500 | 312,502 | 88,253 | 0 | * | * | ||||||||||||||||||||||||
| Cohen, Richard | 366,877 | 0 | 62,500 | 312,502 | 116,875 | 0 | * | * | ||||||||||||||||||||||||
| 27 |
| Selling Stockholder | Shares of common stock beneficially owned prior to the offering | Shares of Series A Stock beneficially owned prior to the offering | Maximum number of shares of common stock issuable upon conversion of Series B Stock owned prior to this offering and registered hereby(1) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby(1) | Shares of common stock beneficially owned upon completion of the offering(2) | Shares of Series A Stock beneficially owned upon completion of the offering | Percentage of common stock beneficially owned upon completion of the offering(2)(3) | Percentage of Series A Stock beneficially owned upon completion of the offering(3) | ||||||||||||||||||||||||
| Corbin, Lee Harrison | 395,000 | 0 | 67,500 | 337,500 | 125,000 | 0 | * | * | ||||||||||||||||||||||||
| Cotter, John A. & Wendy M. Cotter, JTWROS | 179,815 | 0 | 37,500 | 187,500 | 29,815 | 0 | * | * | ||||||||||||||||||||||||
| Cozzolino, Christopher(16) | 11,433 | 2,000 | 0 | 17,149 | 0 | 2,000 | * | * | ||||||||||||||||||||||||
| Currie Family Trust Dtd 6/26/1987, As Amended(17) | 654,327 | 0 | 125,001 | 625,005 | 154,323 | 0 | * | * | ||||||||||||||||||||||||
| Dailey, Robert Jackman | 500,004 | 0 | 125,001 | 625,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Dave Rickey & Daughters Foundation Charitable Trust Dtd 8/15/2002(18) | 179,700 | 0 | 37,500 | 187,500 | 29,700 | 0 | * | * | ||||||||||||||||||||||||
| David M. Rickey Trust Dtd 5/8/2002(19) | 359,300 | 0 | 75,000 | 375,000 | 59,300 | 0 | * | * | ||||||||||||||||||||||||
| DeAtkine, David Jr. | 252,000 | 0 | 63,000 | 315,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| DeLoach, Dennis R., Jr. | 281,815 | 0 | 63,000 | 315,000 | 29,815 | 0 | * | * | ||||||||||||||||||||||||
| DiChiara, Stephen A. | 250,002 | 0 | 62,500 | 312,502 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Dimitry, Theodore | 358,438 | 0 | 75,000 | 375,000 | 58,438 | 0 | * | * | ||||||||||||||||||||||||
| Dritz, James L. | 563,630 | 0 | 126,000 | 630,000 | 59,630 | 0 | * | * | ||||||||||||||||||||||||
| Dritz, Russell S | 261,250 | 0 | 63,000 | 315,000 | 9,250 | 0 | * | * | ||||||||||||||||||||||||
| Due Mondi Investments, Ltd.(20) | 208,438 | 0 | 37,500 | 187,500 | 58,438 | 0 | * | * | ||||||||||||||||||||||||
| EFD Capital. Inc.(21) | 60,000 | 0 | 0 | 75,000 | 10,000 | 0 | * | * | ||||||||||||||||||||||||
| Ehrenstein, Paul(22) | 30,000 | 0 | 0 | 45,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Ellis International LP(23) | 13,570,002 | 0 | 3,392,500 | 16,962,502 | 0 | 0 | * | * | ||||||||||||||||||||||||
| 28 |
| Selling Stockholder | Shares of common stock beneficially owned prior to the offering | Shares of Series A Stock beneficially owned prior to the offering | Maximum number of shares of common stock issuable upon conversion of Series B Stock owned prior to this offering and registered hereby(1) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby(1) | Shares of common stock beneficially owned upon completion of the offering(2) | Shares of Series A Stock beneficially owned upon completion of the offering | Percentage of common stock beneficially owned upon completion of the offering(2)(3) | Percentage of Series A Stock beneficially owned upon completion of the offering(3) | ||||||||||||||||||||||||
| Empery Asset Master, Ltd(24) | 4,163,520 | 0 | 1,040,880 | 5,204,400 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Empery Tax Efficient, LP(25) | 2,707,992 | 0 | 676,998 | 3,384,990 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Empery Tax Efficient II, LP(26) | 3,128,490 | 0 | 782,123 | 3,910,613 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Engel, Suzanne | 179,815 | 0 | 37,500 | 187,500 | 29,815 | 0 | * | * | ||||||||||||||||||||||||
| Ernest W. Moody Revocable Trust, dated Jan 14, 2009(27) | 5,000,004 | 0 | 1,250,001 | 6,250,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Firstfire Global Opportunities LLC(28) | 2,000,004 | 0 | 500,001 | 2,500,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Fischhoff, Brian & Andrea | 150,000 | 0 | 37,500 | 187,500 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Fisher, Melissa | 2,176,510 | 0 | 500,001 | 2,500,005 | 176,506 | 0 | * | * | ||||||||||||||||||||||||
| Foster Family Trust, dtd 04-13-2001(29) | 500,004 | 0 | 125,001 | 625,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Frankel, Robert D. | 208,849 | 0 | 30,000 | 150,000 | 88,849 | 0 | * | * | ||||||||||||||||||||||||
| Freeland, Charles | 417,698 | 0 | 60,000 | 300,000 | 177,698 | 0 | * | * | ||||||||||||||||||||||||
| Gentile, Albert & Hiedi, JTWROS | 100,002 | 0 | 25,000 | 125,002 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Gibralt Capital Corporation(30) | 1,250,004 | 0 | 125,001 | 625,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Gostanian, Justin | 250,002 | 0 | 62,500 | 312,502 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Gostanian, Nicholas | 250,002 | 0 | 62,500 | 312,502 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Gould, Peter C | 179,034 | 0 | 30,000 | 150,000 | 59,034 | 0 | * | * | ||||||||||||||||||||||||
| Greenberg, Dean A. | 246,754 | 0 | 50,001 | 250,005 | 46,750 | 0 | * | * | ||||||||||||||||||||||||
| Greenberger, Marc | 235,782 | 0 | 50,001 | 250,005 | 35,778 | 0 | * | * | ||||||||||||||||||||||||
| 29 |
| Selling Stockholder | Shares of common stock beneficially owned prior to the offering | Shares of Series A Stock beneficially owned prior to the offering | Maximum number of shares of common stock issuable upon conversion of Series B Stock owned prior to this offering and registered hereby(1) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby(1) | Shares of common stock beneficially owned upon completion of the offering(2) | Shares of Series A Stock beneficially owned upon completion of the offering | Percentage of common stock beneficially owned upon completion of the offering(2)(3) | Percentage of Series A Stock beneficially owned upon completion of the offering(3) | ||||||||||||||||||||||||
| Greene, Jonathan and Laura M., JTWROS | 30,000 | 0 | 7,500 | 37,500 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Greenover Group LP(31) | 1,394,458 | 0 | 125,001 | 625,005 | 894,454 | 0 | * | * | ||||||||||||||||||||||||
| Gubbay Investments, LLC(32) | 279,221 | 0 | 62,500 | 312,502 | 29,219 | 0 | * | * | ||||||||||||||||||||||||
| H Investment Company, LLC(33) | 698,004 | 0 | 125,001 | 625,005 | 198,000 | 0 | * | * | ||||||||||||||||||||||||
| Hackett Family Trust, dtd 07.27.98(34) | 659,630 | 0 | 150,000 | 750,000 | 59,630 | 0 | * | * | ||||||||||||||||||||||||
| Haddad, Souheil F | 250,002 | 0 | 62,500 | 312,502 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Haft, Jay(35) | 338,255 | 0 | 62,500 | 312,502 | 88,253 | 0 | * | * | ||||||||||||||||||||||||
| Hale, Allan L | 250,002 | 0 | 62,500 | 312,502 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Hanley, Kimberly(36) | 4,777 | 842 | 0 | 7,165 | 0 | 842 | * | * | ||||||||||||||||||||||||
| Harrigan, Todd(37) | 8,265 | 62,788 | 0 | 12,397 | 0 | 62,788 | * | 4.7 | % | |||||||||||||||||||||||
| Hart, Kara Lynn | 200,004 | 0 | 50,001 | 250,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Herrmann, Tim(38) | 133,862 | 52,303 | 0 | 200,793 | 0 | 52,303 | * | 3.9 | % | |||||||||||||||||||||||
| Hudson Bay Master Fund Ltd(39) | 10,000,002 | 0 | 2,500,000 | 12,500,002 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Hummel, Daniel W. and Allaire, JTWROS | 123,854 | 0 | 25,000 | 125,002 | 23,852 | 0 | * | * | ||||||||||||||||||||||||
| Iroquois Capital Investment Group LLC(40) | 1,000,002 | 0 | 250,001 | 1,250,003 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Iroquois Master Fund Ltd(41) | 12,000,000 | 0 | 3,000,000 | 15,000,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Iseli, Andre | 309,036 | 0 | 62,500 | 312,502 | 59,034 | 0 | * | * | ||||||||||||||||||||||||
| Janssen, Morgan(42) | 39,807 | 0 | 0 | 59,711 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Janssen, Peter(43) | 50,000 | 0 | 0 | 75,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Jaret, Alec H. | 309,632 | 0 | 62,501 | 312,503 | 59,630 | 0 | * | * | ||||||||||||||||||||||||
| 30 |
| Selling Stockholder | Shares of common stock beneficially owned prior to the offering | Shares of Series A Stock beneficially owned prior to the offering | Maximum number of shares of common stock issuable upon conversion of Series B Stock owned prior to this offering and registered hereby(1) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby(1) | Shares of common stock beneficially owned upon completion of the offering(2) | Shares of Series A Stock beneficially owned upon completion of the offering | Percentage of common stock beneficially owned upon completion of the offering(2)(3) | Percentage of Series A Stock beneficially owned upon completion of the offering(3) | ||||||||||||||||||||||||
| Joel L. Hochman Revocable Trust UAD 12/8/1994(44) | 299,630 | 0 | 60,000 | 300,000 | 59,630 | 0 | * | * | ||||||||||||||||||||||||
| Kadi Family Trust, dtd Aug 31, 2006(45) | 200,004 | 0 | 50,001 | 250,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Kagan, Gamliel | 200,010 | 0 | 50,003 | 250,013 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Kastner, Peter S | 250,002 | 0 | 62,501 | 312,503 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Kaul, Pradeep | 1,096,307 | 0 | 125,001 | 625,005 | 596,303 | 0 | * | * | ||||||||||||||||||||||||
| Kingsbrook Opportunities Master Fund LP(46) | 999,996 | 0 | 249,999 | 1,249,995 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Koch, Kevin & Susan, JTWROS | 179,815 | 0 | 37,500 | 187,500 | 29,815 | 0 | * | * | ||||||||||||||||||||||||
| Konik, Lisa Demarco & Randal | 200,004 | 0 | 50,001 | 250,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Konik, Ryan(47) | 13,901 | 0 | 0 | 20,852 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Lance & Dalia Nagel Family Trust U/A dtd 05/19/2009(48) | 366,877 | 0 | 62,501 | 312,503 | 116,875 | 0 | * | * | ||||||||||||||||||||||||
| Landskowsky, David(49) | 756,614 | 184,038 | 0 | 643,223 | 327,799 | 184,038 | * | 13.9 | % | |||||||||||||||||||||||
| Lebhar, Clay G. | 588,257 | 0 | 125,001 | 625,005 | 88,253 | 0 | * | * | ||||||||||||||||||||||||
| Lincoln Park Capital Fund, LLC(50) | 2,250,000 | 0 | 562,500 | 2,812,500 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Manzi, Joseph | 558,442 | 0 | 125,001 | 625,005 | 58,438 | 0 | * | * | ||||||||||||||||||||||||
| Mathieu, Michael J. | 400,002 | 0 | 100,001 | 500,003 | 0 | 0 | * | * | ||||||||||||||||||||||||
| McGrandy, Lindsey(51) | 12,974 | 7,554 | 0 | 19,461 | 0 | 7,554 | * | * | ||||||||||||||||||||||||
| McGregor, Clyde S & LeAnn P. Pope, JTWROS | 20,596,307 | 0 | 5,000,001 | 25,000,005 | 596,303 | 0 | * | * | ||||||||||||||||||||||||
| McGurk, Tom, Jr. | 100,002 | 0 | 25,001 | 125,003 | 0 | 0 | * | * | ||||||||||||||||||||||||
| 31 |
| Selling Stockholder | Shares of common stock beneficially owned prior to the offering | Shares of Series A Stock beneficially owned prior to the offering | Maximum number of shares of common stock issuable upon conversion of Series B Stock owned prior to this offering and registered hereby(1) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby(1) | Shares of common stock beneficially owned upon completion of the offering(2) | Shares of Series A Stock beneficially owned upon completion of the offering | Percentage of common stock beneficially owned upon completion of the offering(2)(3) | Percentage of Series A Stock beneficially owned upon completion of the offering(3) | ||||||||||||||||||||||||
| Mendelson, Alan | 100,002 | 0 | 25,001 | 125,003 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Meryle Evans Family Trust, dtd 12/22/2011(52) | 796,963 | 0 | 125,001 | 625,005 | 296,959 | 0 | * | * | ||||||||||||||||||||||||
| Michael, Daniel | 178,438 | 0 | 30,000 | 150,000 | 58,438 | 0 | * | * | ||||||||||||||||||||||||
| Northlea Partners LLLP(53) | 2,144,458 | 0 | 312,501 | 1,562,505 | 894,454 | 0 | * | * | ||||||||||||||||||||||||
| NTR21 Holdings, LLC(54) | 2,192,615 | 0 | 250,001 | 1,250,003 | 1,192,613 | 0 | * | * | ||||||||||||||||||||||||
| O’Connell, Edward | 173,071 | 0 | 30,000 | 150,000 | 53,071 | 0 | * | * | ||||||||||||||||||||||||
| Omenn, Gilbert | 416,875 | 0 | 75,000 | 375,000 | 116,875 | 0 | * | * | ||||||||||||||||||||||||
| Pallini, Larry H | 89,815 | 0 | 15,000 | 75,000 | 29,815 | 0 | * | * | ||||||||||||||||||||||||
| Peierls, Brian Eliot | 862,706 | 0 | 163,500 | 817,500 | 208,706 | 0 | * | * | ||||||||||||||||||||||||
| Peierls, E. Jeffrey | 1,438,521 | 0 | 300,000 | 1,500,000 | 238,521 | 0 | * | * | ||||||||||||||||||||||||
| Peierls Bypass Trust [The](55) | 192,000 | 0 | 48,000 | 240,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Peierls Foundation, Inc. (Non-Profit) [The](56) | 5,600,004 | 0 | 1,400,001 | 7,000,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Pierce, Michael J. | 1,616,875 | 0 | 375,000 | 1,875,000 | 116,875 | 0 | * | * | ||||||||||||||||||||||||
| Pruzansky, Joel | 250,002 | 0 | 62,501 | 312,503 | 0 | 0 | * | * | ||||||||||||||||||||||||
| R-Squared Partners, LLC(57) | 360,000 | 0 | 90,000 | 450,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Raza, Saiyed. Atiq & Nandini Saraiya, JTWROS | 499,998 | 0 | 125,000 | 624,998 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Renaud, Stephen(58) | 706,521 | 13,750 | 25,001 | 942,438 | 61,562 | 13,750 | * | 1.0 | % | |||||||||||||||||||||||
| Republic Construction Corp(59) | 149,219 | 0 | 30,000 | 150,000 | 29,219 | 0 | * | * | ||||||||||||||||||||||||
| Regan, Daniel | 100,002 | 0 | 25,001 | 125,003 | 0 | 0 | * | * | ||||||||||||||||||||||||
| 32 |
| Selling Stockholder | Shares of common stock beneficially owned prior to the offering | Shares of Series A Stock beneficially owned prior to the offering | Maximum number of shares of common stock issuable upon conversion of Series B Stock owned prior to this offering and registered hereby(1) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby(1) | Shares of common stock beneficially owned upon completion of the offering(2) | Shares of Series A Stock beneficially owned upon completion of the offering | Percentage of common stock beneficially owned upon completion of the offering(2)(3) | Percentage of Series A Stock beneficially owned upon completion of the offering(3) | ||||||||||||||||||||||||
| Revocable Trust Agreement between Roland F. Hartman and Roland F. Hartman Trust Dtd. 6/5/1998(60) | 147,706 | 0 | 25,001 | 125,003 | 47,704 | 0 | * | * | ||||||||||||||||||||||||
| Robert A. McCleeary Revocable Trust dtd 11/1/2006(61) | 98,852 | 0 | 18,750 | 93,750 | 23,852 | 0 | * | * | ||||||||||||||||||||||||
| RS & VS Ltd.(62) | 250,002 | 0 | 62,501 | 312,503 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Rogers, Dyke | 1,500,000 | 0 | 375,000 | 1,875,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Rubenstein, Eric(63) | 498,641 | 252,764 | 0 | 747,962 | 0 | 252,764 | * | 19.1 | % | |||||||||||||||||||||||
| Sack Family Investment Fund, LLC(64) | 1,000,002 | 0 | 250,000 | 1,250,002 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Schaffer, Mitchell(65) | 3,300 | 0 | 0 | 4,950 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Schlosser, Alyson D | 252,000 | 0 | 63,000 | 315,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| SDL Ventures, LLC(66) | 3,477,042 | 0 | 750,000 | 3,750,000 | 477,042 | 0 | * | * | ||||||||||||||||||||||||
| Segal, Aaron(67) | 152,559 | 103,884 | 0 | 228,839 | 0 | 103,884 | * | 7.8 | % | |||||||||||||||||||||||
| Seyburn, Bruce | 500,004 | 0 | 125,001 | 625,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Shymansky, J. Stephen | 700,404 | 0 | 175,101 | 875,505 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Silverman, Michael(68) | 674,902 | 13,750 | 30,000 | 982,353 | 0 | 13,750 | * | 1.0 | % | |||||||||||||||||||||||
| Skrzypczak, Casimir | 180,000 | 0 | 45,000 | 225,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Smith, Brian C | 250,002 | 0 | 62,501 | 312,503 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Stark, Michael | 500,004 | 0 | 125,001 | 625,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Strawbridge, William | 273,000 | 0 | 30,000 | 150,000 | 153,000 | 0 | * | * | ||||||||||||||||||||||||
| Strazzulla, Domenic | 279,817 | 0 | 62,501 | 312,503 | 29,815 | 0 | * | * | ||||||||||||||||||||||||
| Stringer, Howard | 368,070 | 0 | 62,501 | 312,503 | 118,068 | 0 | * | * | ||||||||||||||||||||||||
| Struve, Clayton A. | 1,478,893 | 0 | 325,001 | 1,625,003 | 178,891 | 0 | * | * | ||||||||||||||||||||||||
| 33 |
| Selling Stockholder | Shares of common stock beneficially owned prior to the offering | Shares of Series A Stock beneficially owned prior to the offering | Maximum number of shares of common stock issuable upon conversion of Series B Stock owned prior to this offering and registered hereby(1) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby(1) | Shares of common stock beneficially owned upon completion of the offering(2) | Shares of Series A Stock beneficially owned upon completion of the offering | Percentage of common stock beneficially owned upon completion of the offering(2)(3) | Percentage of Series A Stock beneficially owned upon completion of the offering(3) | ||||||||||||||||||||||||
| Trout Capital(69) | 25,000 | 0 | 0 | 37,500 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Trust of Paul E. Freiman & Anna Mazzuchi Freiman, Dated May 24, 1996(70) | 1,000,002 | 0 | 250,001 | 1,250,003 | 0 | 0 | * | * | ||||||||||||||||||||||||
| U.D. Ethel F. Peierls Charitable Lead Trust(71) | 719,150 | 0 | 145,500 | 727,500 | 137,150 | 0 | * | * | ||||||||||||||||||||||||
| U.D.E.F. Peierls for Brian E. Peierls(72) | 544,475 | 0 | 123,000 | 615,000 | 52,475 | 0 | * | * | ||||||||||||||||||||||||
| U.D.E.F. Peierls for E. Jeffrey Peierls(73) | 544,475 | 0 | 123,000 | 615,000 | 52,475 | 0 | * | * | ||||||||||||||||||||||||
| U.D.J.N. Peierls for Brian Eliot Peierls(74) | 672,749 | 0 | 150,000 | 750,000 | 72,749 | 0 | * | * | ||||||||||||||||||||||||
| U.D.J.N. Peierls for E. Jeffrey Peierls(75) | 672,749 | 0 | 150,000 | 750,000 | 72,749 | 0 | * | * | ||||||||||||||||||||||||
| U.D.E.S. Peierls for E.F. Peierls et al(76) | 330,000 | 0 | 82,500 | 412,500 | 0 | 0 | * | * | ||||||||||||||||||||||||
| U.W.E.S. Peierls for Brian E. Peierls – Accumulation(77) | 479,704 | 0 | 108,000 | 540,000 | 47,704 | 0 | * | * | ||||||||||||||||||||||||
| U.W.E.S. Peierls for E. Jeffrey Peierls – Accumulation (78) | 301,008 | 0 | 67,500 | 337,500 | 31,008 | 0 | * | * | ||||||||||||||||||||||||
| U.W.J.N. Peierls for Brian E. Peierls(79) | 591,208 | 0 | 132,000 | 660,000 | 63,208 | 0 | * | * | ||||||||||||||||||||||||
| U.W.J.N. Peierls for E. Jeffrey Peierls(80) | 591,208 | 0 | 132,000 | 660,000 | 63,208 | 0 | * | * | ||||||||||||||||||||||||
| Wagner, John V. | 588,257 | 0 | 125,001 | 625,005 | 88,253 | 0 | * | * | ||||||||||||||||||||||||
| Washburn, Christopher | 309,036 | 0 | 62,501 | 312,503 | 59,034 | 0 | * | * | ||||||||||||||||||||||||
| Weinstein, Robert(81) | 250,002 | 0 | 62,501 | 312,503 | 0 | 0 | * | * | ||||||||||||||||||||||||
| 34 |
| Selling Stockholder | Shares of common stock beneficially owned prior to the offering | Shares of Series A Stock beneficially owned prior to the offering | Maximum number of shares of common stock issuable upon conversion of Series B Stock owned prior to this offering and registered hereby(1) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby(1) | Shares of common stock beneficially owned upon completion of the offering(2) | Shares of Series A Stock beneficially owned upon completion of the offering | Percentage of common stock beneficially owned upon completion of the offering(2)(3) | Percentage of Series A Stock beneficially owned upon completion of the offering(3) | ||||||||||||||||||||||||
| Whiting Holdings LP(82) | 2,348,136 | 0 | 528,000 | 2,640,000 | 236,136 | 0 | * | * | ||||||||||||||||||||||||
| Whited, Craig | 700,000 | 0 | 75,000 | 375,000 | 400,000 | 0 | * | * | ||||||||||||||||||||||||
| Wiesenberg, James | 150,000 | 0 | 37,500 | 187,500 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Wilke, Susanne | 100,002 | 0 | 25,000 | 125,002 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Willis, Michael L. and Sharon D. Willis JTWROS | 300,004 | 0 | 50,001 | 250,005 | 100,000 | 0 | * | * | ||||||||||||||||||||||||
| Yanowitz, Joel | 200,004 | 0 | 50,001 | 250,005 | 0 | 0 | * | * | ||||||||||||||||||||||||
| Zahavi, Thomas | 120,000 | 0 | 30,000 | 150,000 | 0 | 0 | * | * | ||||||||||||||||||||||||
* Denotes less than 1%
| 1) | An aggregate of (a) 26,234,940 shares of our common stock are issuable upon conversion of 262,349.4 outstanding shares of our Series B Stock, (b) 26,234,940 shares of our common stock are issuable upon exercise of the outstanding Series A warrants to purchase our common stock having an exercise price of $0.80 per share, (c) 26,234,940 shares of our common stock are issuable upon exercise of the outstanding Series B warrants to purchase our common stock having an exercise price of $0.80 per share, (d) 26,234,940 shares of our common stock are issuable upon exercise of the outstanding Series C warrants to purchase our common stock having an exercise price of $1.25 per share, (e) 26,234,940 shares of our common stock are issuable upon exercise of the outstanding Series D warrants to purchase our common stock having an exercise price of $1.00 per share, (f) 26,234,940 shares of our common stock are issuable upon exercise of the outstanding Series E warrants to purchase our common stock having an exercise price of $1.50 per share, (g) 363,456 shares of our common stock are issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.01 per share, (h) 1,090,370 shares of our common stock are issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $0.60 per share, and (i) 1,153,000 shares of our common stock are issuable upon exercise of placement agent warrants to purchase our common stock having an exercise price of $1.50 per share, subject to adjustment to $0.80 per share upon the exercise of all Series A Warrants or all Series B Warrants. We are registering 150% of such securities. The total number of units sold includes 166,667 units granted to Dr. Abeles as compensation for his services as a consultant to the Company, which accounts for $100,000 that is not included in our calculation of gross proceeds. |
| 2) | Assumes all of the shares of common stock to be registered on the registration statement of which this prospectus is a part, including all shares of our common stock underlying shares of our Series B Stock and Series A through Series E purchase warrants held by the selling stockholders, are sold in the offering and that shares of our common stock beneficially owned by such selling stockholders but not being registered by this prospectus are not sold. |
| 3) | Percentages are calculated based on an aggregate of 49,172,026 shares of common stock outstanding and warrants to purchase up to an aggregate of 1,325,000 shares of Series A Stock outstanding as of January 6, 2016 and assumes the sale of all securities registered hereby. |
| 4) | John Abeles served on our Board of Directors until November 12, 2015. He continues to serve as a consultant to the Company. |
| 5) | Orin Hirschman is the president of AIGH Investment Partners LLC and has sole voting and dispositive power with respect to the shares. |
| 6) | Orin Hirschman is the general partner of AIGH Investment Partners LP and has sole voting and dispositive power with respect to the shares. |
| 7) | Allan Lipkowitz is the trustee of Allan Lipkowitz Revocable Living Trust 8/26/2005 and has sole voting and investment power over the shares owned thereby. |
| 8) | Konrad Ackerman has voting and dispositive control with respect to the securities being offered. |
| 9) | Larry Altstiel serves on our Board of Directors. |
| 10) | Kimberly Page has the authority and responsibility for the investments made on behalf of American Capital Management LLC and accordingly, has voting and dispositive power over the securities held by American Capital Management LLC. |
| 11) | Hester L. Bell is the Trustee of The Bell Family Trust as amended, and has sole voting and investment power over the shares owned thereby. |
| 12) | Donna Bozarth is the Manager of Bozarth LLC and has sole voting and investment power over the shares owned thereby. |
| 35 |
| 13) | Shaye Hirsch is the Director of Brio Capital Master Fund Ltd. and has sole voting and investment power over the shares owned thereby. |
| 14) | Includes warrants to purchase up to 79,149 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 150% of each of the following placement warrants: (1) warrants to purchase 31,912 shares of common stock at $0.60/share and (2) warrants to purchase 1,083 shares of common stock at $1.50/share. |
| 15) | Joshua S. Kanter is the President of Chicago Investments, Inc. and has sole voting and investment power over the shares owned thereby. |
| 16) | Includes warrants to purchase up to 2,000 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 150% of each of the following placement warrants: (1) warrants to purchase 2,858 shares of common stock at $0.01/share and (2) warrants to purchase 8,575 shares of common stock at $0.60/share. |
| 17) | Dr. Malcolm Currie and Barbara Currie are Co-Trustees of the Currie Family Trust and have shared voting and investment power over the shares owned thereby. |
| 18) | David M. Rickey is the Trustee of the Dave Rickey & Daughters Foundation Charitable Trust and has sole voting and investment power over the shares owned thereby. |
| 19) | David M. Rickey is the Trustee of the David M. Rickey Trust dtd 5/8/02 and has sole voting and investment power over the shares owned thereby. |
| 20) | Robert S. Beadle is the President of Due Mondi Investments Ltd. and has sole voting and investment power over the shares owned thereby. |
| 21) | Consists of 150% of each of the following placement warrants: warrants to purchase 50,000 shares of common stock at $1.50/share. |
| 22) | Consists of 150% of each of the following placement warrants: warrants to purchase 30,000 shares of common stock at $1.50/share. |
| 23) | The selling shareholder is controlled by Mendy Scheen who has the power and control to vote and sell the securities. |
| 24) | Empery Asset Management LP, the authorized agent of Empery Asset Master Ltd ("EAM"), has discretionary authority to vote and dispose of the shares held by EAM and may be deemed to be the beneficial owner of these shares. Martin Hoe and Ryan Lane, in their capacity as investment managers of Empery Asset Management LP, may also be deemed to have investment discretion and voting power over the shares held by EAM. EAM, Mr. Hoe and Mr. Lane each disclaim any beneficial ownership of these shares. |
| 25) | Empery Asset Management LP, the authorized agent of Empery Tax Efficient, LP ("ETE"), has discretionary authority to vote and dispose of the shares held by ETE and may be deemed to be the beneficial owner of these shares. Martin Hoe and Ryan Lane, in their capacity as investment managers of Empery Asset Management LP, may also be deemed to have investment discretion and voting power over the shares held by ETE. ETE, Mr. Hoe and Mr. Lane each disclaim any beneficial ownership of these shares. |
| 26) | Empery Asset Management LP, the authorized agent of Empery Tax Efficient, LP ("ETE II"), has discretionary authority to vote and dispose of the shares held by ETE II and may be deemed to be the beneficial owner of these shares. Martin Hoe and Ryan Lane, in their capacity as investment managers of Empery Asset Management LP, may also be deemed to have investment discretion and voting power over the shares held by ETE II. ETE II, Mr. Hoe and Mr. Lane each disclaim any beneficial ownership of these shares. |
| 27) | Ernest W. Moody is the Trustee of the Ernest W. Moody Revocable Trust, dated Jan 14, 2009. As a result of the foregoing, he may be deemed to have beneficial ownership (as determined under Section 13(d) of the Securities Exchange Act of 1934, as amended) of the shares of common stock beneficially owned by Ernest W. Moody Revocable Trust, dated Jan 14, 2009. |
| 28) | Seth Fireman is the managing partner of FirstFire Global Opportunities LLC and owns voting control of the membership interests in FirstFire Global Opportunities LLC, and Mr. Fireman has sole power to vote or to direct the vote and sole power to dispose or to direct the disposition of all securities owned directly by FirstFire Global Opportunities LLC. |
| 29) | Dwight D. Foster, III and Jane C. Foster are trustees of the Foster Family Trust, U.D.T. dated June 18, 2010, or the Foster Trust, and share voting and dispositive power over the shares held by the Foster Trust. |
| 30) | Ryan Chan is the Chief Financial Officer of Gibralt Capital Corporation and has sole voting and investment power over the shares owned thereby. |
| 31) | J. Kelley Williams, Jr. is a Member of Greenover Managers, LLC, which is the General Partner of Greenover Group, LP and has sole voting and investment power over the shares owned thereby. |
| 32) | David Gubbay is the Manager of Gubbay Investments LLC and has sole voting and investment power over the shares owned thereby. |
| 33) | Pamela M. Baker is the manager of the selling stockholder has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. Ms. Baker disclaims beneficial ownership with respect to such shares, except to the extent of her pecuniary interest therein, if any. |
| 36 |
| 34) | Terry Clinton Hackett is the Trustee of the Hackett Family Trust dtd 7.27.98 and has sole voting and investment power over the shares owned thereby. |
| 35) | Jay Haft is a director of the Company. Shares of our common stock beneficially owned prior to the offering includes 226,445 shares underlying stock options held by Mr. Haft that are vested as of January 6, 2016 or will vest within 60 days thereafter, but does not include 223,555 shares underlying stock options that will not vest within 60 days after January 6, 2016. |
| 36) | Includes warrants to purchase up to 842 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 150% of each of the following placement warrants: (1) warrants to purchase 1,038 shares of common stock at $0.01/share, (2) warrants to purchase 3,114 shares of common stock at $0.60/share and (3) warrants to purchase 625 shares of common stock at $1.50/share. |
| 37) | Includes warrants to purchase up to 62,788 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 150% of each of the following placement warrants: (1) warrants to purchase 5,388 shares of common stock at $0.60/share and (2) warrants to purchase 1,083 shares of common stock at $1.50/share. |
| 38) | Includes warrants to purchase up to 52,302 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 150% of each of the following placement warrants: (1) warrants to purchase 99,584 shares of common stock at $0.60/share and (2) warrants to purchase 1,083 shares of common stock at $1.50/share. |
| 39) | Hudson Bay Capital Management LP, the investment manager of Hudson Bay Master Fund Ltd., has voting and investment power over these securities. Sander Gerber is the managing member of Hudson Bay Capital GP LLC, which is the general partner of Hudson Bay Capital Management LP. Sander Gerber disclaims beneficial ownership over these securities. |
| 40) | Joshua Silverman and Richard Abbe are the natural persons with voting and dispositive power over the shares held by Iroquois Capital Investment Group LLC. |
| 41) | Iroquois Capital Management L.L.C. (“Iroquois Capital”) is the investment manager of Iroquois Master Fund, Ltd (“IMF”). Consequently, Iroquois Capital has voting control and investment discretion over securities held by IMF. As managing members of Iroquois Capital, Joshua Silverman and Richard Abbe make voting and investment decisions on behalf of Iroquois Capital in its capacity as investment manager to IMF. As a result of the foregoing, Mr. Silverman and Mr. Abbe may be deemed to have beneficial ownership (as determined under Section 13(d) of the Securities Exchange Act of 1934, as amended) of the securities held by IMF. |
| 42) | Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 150% of each of the following placement warrants: (1) warrants to purchase 7,017 shares of common stock at $0.60/share and (2) warrants to purchase 30,450 shares of common stock at $1.50/share. |
| 43) | Consists of 150% of each of the following placement warrants: (1) warrants to purchase 50,000 shares of common stock at $0.60/share. |
| 44) | Joel L. Hochman is the Trustee of the Joel L. Hochman Revocable Trust UAD 12/8/1994 and has sole voting and investment power over the shares owned thereby. |
| 45) | William Kadi and Sandra Kadi are the Trustees of the selling stockholder and have voting and investment power over the shares. |
| 46) | Kingsbrook Partners LP (“Kingsbrook Partners”) is the investment manager of Kingsbrook Opportunities Master Fund LP (“Kingsbrook Opportunities”) and consequently has voting control and investment discretion over securities held by Kingsbrook Opportunities. Kingsbrook Opportunities GP LLC (“Opportunities GP”) is the general partner of Kingsbrook Opportunities and may be considered the beneficial owner of any securities deemed to be beneficially owned by Kingsbrook Opportunities. KB GP LLC (“GP LLC”) is the general partner of Kingsbrook Partners and may be considered the beneficial owner of any securities deemed to be beneficially owned by Kingsbrook Partners. Ari J. Storch, Adam J. Chill and Scott M. Wallace are the sole managing members of Opportunities GP and GP LLC and as a result may be considered beneficial owners of any securities deemed beneficially owned by Opportunities GP and GP LLC. Each of Kingsbrook Partners, Opportunities GP, GP LLC and Messrs. Storch, Chill and Wallace disclaim beneficial ownership of these securities. |
| 47) | Consists of 150% of each of the following placement warrants: (1) warrants to purchase 3,475 shares of common stock at $0.01/share and (2) warrants to purchase 10,426 shares of common stock at $0.60/share. |
| 48) | Lance Director Nagel and Dalia Charnels Nagel are the Co-Trustees of the Lance & Dalia Nagel Family Trust and have shared voting and investment power over the shares owned thereby. |
| 49) | Includes warrants to purchase up to 184,038 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 150% of each of the following placement warrants: (1) warrants to purchase 317,279 shares of common stock at $0.60/share and (2) warrants to purchase 5,772 shares of common stock at $1.50/share. |
| 50) | Josh Scheinfeld and Jonathan Cope, the Managing Members of Lincoln Park Capital, LLC, are deemed to be beneficial owners of all of the shares of common stock owned by Lincoln Park Capital Fund, LLC. |
| 37 |
| 51) | Includes warrants to purchase up to 7,554 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 150% of each of the following placement warrants: warrants to purchase 9,731 shares of common stock at $0.60/share. |
| 52) | Steven Evans is the Trustee of the Meryle Evans Family Trust and has sole voting and investment power over the shares owned thereby. |
| 53) | John H. Abeles is the Managing Member of Northlea Partners, LLLP and has sole voting and investment power over the shares owned thereby. Dr. Abeles served on our Board of Directors until November 12, 2015. He continues to serve as a consultant to the Company. Shares of our common stock beneficially owned prior to the offering includes 1,078,514 shares underlying stock options held by Dr. Abeles that are vested as of January 6, 2016 or will vest within 60 days thereafter, but does not include 126,986 shares underlying stock options that will not vest within 60 days after January 6, 2016. |
| 54) | Charles S. Ramat is the President of NRT21 Equities Corp, which is the Managing Member of NTR21 Holdings, LLC and has sole voting and investment power over the shares owned thereby. Mr. Ramat is serving as President and Chief Executive Officer of the Company and also is a director of the Company. Shares of our common stock beneficially owned prior to the offering includes 1,191,226 shares underlying stock options held by Mr. Ramat that are vested as of January 6, 2016 or will vest within 60 days thereafter, but does not include 763,178 shares underlying stock options that will not vest within 60 days after January 6, 2016. |
| 55) | E. Jeffrey Peierls is the Trustee of The Peierls Bypass Trust and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 56) | E. Jeffrey Peierls is the President and Authorized Officer of The Peierls Foundation, Inc. and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 57) | Neil Rock has voting and dispositive power over shares held by R-Squared Partners, LLC. |
| 58) | Includes warrants to purchase up to 13,750 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 143,402 shares of our common stock issuable upon exercise of common stock purchase warrants received by Stephen Renaud, an affiliate of a broker-dealer which acted as placement agent in the 2013 PPO. Mr. Renaud received the warrants in in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. Also consists of 150% of each of the following placement warrants: (1) warrants to purchase 50,493 shares of common stock at $0.60/share and (2) warrants to purchase 474,276 shares of common stock at $1.50/share. |
| 59) | Richard Arnos is the President of the Republic Construction Corporation and has sole voting and investment power over the shares owned thereby. |
| 60) | Roland F. Hartman is the Trustee of Revocable Trust Agreement between Roland F. Hartman and Roland T. Hartman DTD 06/05/1998 and has sole voting and investment power over the shares owned thereby. |
| 61) | Robert A. McCleeary is the Trustee of the Robert A. McCleeary Revocable Trust dtd 11/01/06 and has sole voting and investment power over the shares owned thereby. |
| 62) | Rodney N. Schorlemmer is the Manager of RS & VS Ltd. and has sole voting and investment power over the shares held thereby. |
| 63) | Includes warrants to purchase up to 252,764 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 150% of each of the following placement warrants: (1) warrants to purchase 369,650 shares of common stock at $0.60/share and (2) warrants to purchase 15,771 shares of common stock at $1.50/share. |
| 64) | Burton M. Sack is the Managing Member of the Sack Family Investment Fund, LLC and has sole voting and investment power over the shares owned thereby. |
| 65) | Consists of 150% of each of the following placement warrants: (1) warrants to purchase 825 shares of common stock at $0.01/share and (2) warrants to purchase 2,475 shares of common stock at $0.60/share. |
| 66) | Donald R. Scifres is the Managing Director of SDL Ventures and has sole voting and investment power over the shares owned thereby. |
| 67) | Includes warrants to purchase up to 103,884 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 150% of each of the following placement warrants: (1) warrants to purchase 24,745 shares of common stock at $0.01/share, (2) warrants to purchase 74,232 shares of common stock at $0.60/share and (3) warrants to purchase 53,582 shares of common stock at $1.50/share. |
| 68) | Includes warrants to purchase up to 13,750 shares of Series A Stock. Maximum number of shares of common stock issuable upon exercise of warrants owned prior to this offering and registered hereby consists of 143,402 shares of our common stock issuable upon exercise of common stock purchase warrants received by Michael Silverman, an affiliate of a broker-dealer which acted as placement agent in the 2013 PPO. Mr. Silverman received the warrants in the ordinary course of business for his own account and, at the time of receipt, had no agreements or understandings with any person, directly or indirectly, to further distribute the securities. Also consists of 150% of each of the following placement warrants: (1) warrants to purchase 50,494 shares of common stock at $0.60/share and (2) warrants to purchase 474,275 shares of common stock at $1.50/share. |
| 38 |
| 69) | Consists of 150% of each of the following placement warrants: warrants to purchase 25,000 shares of common stock at $1.50/share. |
| 70) | Paul Freiman and Anna Mazzuchi Freiman are Co-Trustees of Trust of Paul E. Freiman & Anna Mazzuchi Freiman, dated May 24, 1996. Paul Freiman serves on our Board of Directors. |
| 71) | E. Jeffrey Peierls is the Trustee of UD Ethel F. Peierls Charitable Lead Trust and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 72) | E. Jeffrey Peierls is the Trustee of UD E.F. Peierls for Brian E. Peierls and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 73) | E. Jeffrey Peierls is the Trustee of UD E.F. Peierls for E. Jeffrey Peierls and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 74) | E. Jeffrey Peierls is the Trustee of UD J.N. Peierls For Brian E. Peierls and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 75) | E. Jeffrey Peierls is the Trustee of UD J.N. Peierls For E. Jeffrey Peierls and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 76) | E. Jeffrey Peierls is the Trustee of UD E.S. Peierls for E.F. Peierls et al and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 77) | E. Jeffrey Peierls is the Trustee of UW E.S. Peierls for Brian E. Peierls – Accumulation and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 78) | E. Jeffrey Peierls is the Trustee of UW E.S. Peierls for E. Jeffrey Peierls – Accumulation and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 79) | E. Jeffrey Peierls is the Trustee of UW J.N. Peierls for Brian E. Peierls and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 80) | E. Jeffrey Peierls is the Trustee of UW J.N. Peierls for E. Jeffrey Peierls and has sole voting and investment power over the shares owned thereby. Mr. Peierls is also a selling stockholder for his own account. |
| 81) | Robert Weinstein serves as our Executive Vice President, Chief Financial Officer, Secretary and Treasurer. |
| 82) | Mark S. Whiting is the Member of Whiting Management, G.P., which is the General Partner of Whiting Holdings, LP and has sole voting and investment power over the shares owned thereby. |
| 39 |
We will not receive proceeds from sales of our common stock made under this prospectus.
DETERMINATION OF OFFERING PRICE
There currently is a limited public market for our common stock. The selling stockholders will determine at what price they may sell the offered shares, and such sales may be made at prevailing market prices or at privately negotiated prices. See “Plan of Distribution” below for more information.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information and Holders
Our common stock is currently eligible for quotation and trades on the OTC Market under the symbol “NTRP.” The quotation of our common stock began on or about October 2, 2013. On January 6, 2016, the last reported sale price for our common stock was $0.50 per share. There has been very limited trading in our common stock to date.
As of January 6, 2016, we had 49,172,026 shares of our common stock issued and outstanding held by approximately 212 stockholders of record. To date, we have not paid dividends on our common stock.
As of January 6, 2016, we also had the following securities outstanding:
| · | 262,349.4 shares of our Series B Stock held by 146 stockholders of record, convertible at any time into shares of our common stock. Our Series B Stock currently converts into our common stock on a one-hundred-for-one basis, but is subject to adjustment in certain circumstances as provided in the rights and designations of the Series B Stock, including upon the sale of certain securities at a price less than the current conversion price. |
| · | 26,234,940 Series A warrants to purchase shares of our common stock at an exercise price of $0.80 per share with an expiration date five years from the date of issuance. |
| · | 26,234,940 Series B warrants to purchase shares of our common stock at an exercise price of $0.80 per share with an expiration date of one year from the date of issuance. |
| · | 26,234,940 Series C warrants to purchase shares of our common stock at an exercise price of $1.25 per share with an expiration date of five years from the issuance date. |
| · | 26,234,940 Series D warrants, which are contingent upon the exercise of the Series B warrants, to purchase shares of our common stock at an exercise price of $1.00 per share with an expiration date that is five years from the date of the initial exercise of the Series B warrants. |
| · | 26,234,940 Series E warrants, which are contingent upon the exercise of the Series C warrants, to purchase shares of our common stock at an exercise price of $1.50 per share with an expiration date that is five years from the date of the initial exercise of the Series C warrants. |
| · | 32,941 placement agent warrants to purchase shares of our common stock at an exercise price of $0.01 per share, which reflects the exercise of 330,515 placement agent warrants. |
| · | 1,090,370 placement agent warrants to purchase shares of our common stock at an exercise price of $0.60 per share. |
| · | 1,153,000 placement agent warrants to purchase shares of our common stock at an exercise price of $1.50 per share, subject to adjustment to $0.80 per share upon the exercise of all Series A Warrants or all Series B Warrants. |
| · | Placement agent warrants to purchase 1,375,432 shares of our common stock, subject to adjustment in certain circumstances as provided therein, of which, 50,432 have an exercise price of $0.01 per share and 1,325,000 have an exercise price of $1.00. |
| 40 |
The following table sets forth the high and low closing bid prices for our common stock for the fiscal quarter indicated as reported on the OTC Market. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions. Our common stock is very thinly traded and, thus, pricing of our common stock on the OTC Market does not necessarily represent its fair market value.
| Period | High | Low | ||||||
| Quarter ended March 31, 2014 | $ | 2.80 | 1.47 | |||||
| Quarter ended June 30, 2014 | 1.98 | 1.25 | ||||||
| Quarter ended September 30, 2014 | 1.70 | 0.50 | ||||||
| Quarter ended December 31, 2014 | 1.22 | 0.51 | ||||||
| Quarter ended March 31, 2015 | 1.79 | 0.80 | ||||||
| Quarter ended June 30, 2015 | 1.29 | 0.51 | ||||||
| Quarter ended September 30, 2015 | 1.00 | 0.55 | ||||||
| Quarter ending December 31, 2015 | 0.66 | 0.30 | ||||||
| Quarter ending March, 31, 2016 (through January 6, 2016) | 0.60 | 0.50 | ||||||
Dividends
We have never paid any cash dividends on our capital stock and do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain future earnings, if any, to fund our ongoing operations and future capital requirements. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements and such other factors as the Board of Directors deems relevant. Other than provisions of the Nevada Revised Statutes requiring post-dividend solvency according to certain measures, there are no material statutory restrictions limiting, or that are likely to limit, our ability to pay dividends on our common stock.
Securities Authorized for Issuance under Equity Compensation Plan
On August 22, 2013, our Board of Directors adopted, and on August 22, 2013, our stockholders approved, our 2013 Equity Incentive Plan, which reserved a total of 7,000,000 shares of our common stock for issuance pursuant to awards granted under the plan. On July 23, 2014, our Board of Directors approved an amendment to our 2013 Equity Incentive Plan to increase the number of shares of common stock issuable thereunder by an additional 3,000,000 shares, so that the Company’s officers, key employees, consultants and directors can be granted stock options and other equity incentive awards with respect to an aggregate of 10,000,000 shares of our common stock. Pursuant to the Internal Revenue Code, this amendment must be approved by our stockholders within one year in order to allow for incentive stock option grants to be made. We hereinafter refer to our 2013 Equity Incentive Plan, as amended, as the 2013 Plan.
The following table provides information as of December 31, 2015, with respect to the shares of our common stock that may be issued under our existing equity compensation plan:
Equity Compensation Plan Information
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders (1) | 8,582,952 | $ | 1.327 | 1,417,048 | ||||||||
| Equity compensation plans not approved by security holders | 0 | 0 | 0 | |||||||||
| Total | 8,582,952 | $ | 1.327 | 1,417,048 | ||||||||
(1) The 2013 Plan
| 41 |
As described below, incentive awards authorized under the 2013 Plan include, but are not limited to, incentive stock options within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended, the Code. If an award granted under the 2013 Plan expires, terminates, is unexercised or is forfeited, or if any shares are surrendered to us in connection with the exercise of an incentive award, the shares subject to such award and the surrendered shares will become available for further awards under the 2013 Plan.
The number of shares of our common stock subject to the 2013 Plan or any number of shares subject to (a) any numerical limit in the 2013 Plan, (b) to the terms of any incentive award or (c) to any combination of the foregoing, is expected to be adjusted in the event of any change in our outstanding common stock by reason of any stock dividend, spin-off, split-up, stock split, reverse stock split, recapitalization, reclassification, merger, consolidation, liquidation, business combination or exchange of shares or similar transaction.
Administration
The Compensation Committee of our Board of Directors or our Board of Directors administers the 2013 Plan. Subject to the terms of the 2013 Plan, the Compensation Committee or our Board of Directors has complete authority and discretion to determine the terms of awards under the 2013 Plan.
Grants
The 2013 Plan authorizes the grant to participants of nonqualified stock options, incentive stock options, restricted stock awards, restricted stock units, performance grants intended to comply with Section 162(m) of the Code and stock appreciation rights, as described below:
| · | Options granted under the 2013 Plan entitle the grantee, upon exercise, to purchase a specified number of shares from us at a specified exercise price per share. The exercise price for shares of our common stock covered by an option generally cannot be less than the fair market value of our common stock on the date of grant unless agreed to otherwise at the time of the grant. In addition, in the case of an incentive stock option granted to an employee who, at the time the incentive stock option is granted, owns stock representing more than 10% of the voting power of all classes of stock of the Company or any parent or subsidiary, the per share exercise price will be no less than 110% of the fair market value of our common stock on the date of grant. |
| · | Restricted stock awards and restricted stock units may be awarded on terms and conditions established by the Compensation Committee or Board of Directors, which may include performance conditions for restricted stock awards and the lapse of restrictions on the achievement of one or more performance goals for restricted stock units. |
| · | The Compensation Committee or Board of Directors may make performance grants, each of which will contain performance goals for the award, including the performance criteria, the target and maximum amounts payable, and other terms and conditions. |
| · | The 2013 Plan authorizes the granting of stock awards. The Compensation Committee or Board of Directors will establish the number of shares of our common stock to be awarded and the terms applicable to each award, including performance restrictions. |
| · | Stock appreciation rights entitle the participant to receive a distribution in an amount not to exceed the number of shares of our common stock subject to the portion of the stock appreciation rights exercised multiplied by the difference between the market price of a share of our common stock on the date of exercise of the stock appreciation rights and the market price of a share of our common stock on the date of grant of the stock appreciation rights. |
The maximum aggregate number of shares of common stock that may be awarded and sold under the 2013 Plan is 10,000,000.
Duration, Amendment, and Termination
The Board of Directors has the power to amend, suspend or terminate the 2013 Plan without stockholder approval or ratification at any time or from time to time. No change may be made that increases the total number of shares of our common stock reserved for issuance pursuant to incentive awards or reduces the minimum exercise price for options or exchange of options for other incentive awards, unless such change is authorized by our stockholders within one year. Unless sooner terminated, the 2013 Plan will terminate ten years after it is adopted.
| 42 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with “Selected Financial Data” and our financial statements and the related notes appearing elsewhere in this prospectus. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included elsewhere in this prospectus.
On August 23, 2013, our wholly-owned subsidiary, Neurotrope Acquisition, Inc., a corporation formed in the State of Nevada on August 15, 2013 merged with and into Neurotrope BioScience. Neurotrope BioScience was the surviving corporation in the Reverse Merger and became Neurotrope, Inc.’s wholly-owned subsidiary. All of the outstanding Neurotrope BioScience Common Stock was converted into shares of the Company’s common stock on a one-for-one basis, and all of the outstanding shares of Neurotrope BioScience Series A Stock were converted into shares of our Series A Stock, in each case on a one-for-one basis. As the result of the Reverse Merger and the change in business and operations of the Company, from engaging in the business of providing software solutions to deliver geo-location targeted coupon advertising to mobile internet devices, to the biotechnology business, including the development of a drug candidate called bryostatin for the treatment of Alzheimer’s disease, or AD, a discussion of the past financial results of Neurotrope, Inc. (i.e., while operating as BlueFlash Communications, Inc.) is not pertinent, and under applicable accounting principles, the historical financial results of Neurotrope BioScience, the accounting acquirer, prior to the Reverse Merger, are considered our historical financial results.
The following discussion highlights our results of operations and the principal factors that have affected our financial condition as well as our liquidity and capital resources for the periods described, and provides information that management believes is relevant for an assessment and understanding of the statements of financial condition and results of operations presented herein. The following discussion and analysis are based on the unaudited financial statements contained in this report, which we have prepared in accordance with United States generally accepted accounting principles. You should read the discussion and analysis together with such financial statements and the related notes thereto.
Basis of Presentation
The audited financial statements for our fiscal years ended December 31, 2014 and 2013 and the unaudited financial statements for our fiscal quarters ended September 30, 2015 and 2014, include a summary of our significant accounting policies and should be read in conjunction with the discussion below and our financial statements and related notes included elsewhere in this prospectus. In the opinion of management, all material adjustments necessary to present fairly the results of operations for such periods have been included in financial statements. All such adjustments are of a normal recurring nature.
Overview
Neurotrope BioScience was founded as a Delaware corporation in October 2012. Our activities since Neurotrope BioScience’s inception through September 30, 2015 have been devoted primarily to the research and development of AD therapeutic products and related diagnostics using patented technology and raising capital. This technology is licensed by us from the Blanchette Rockefeller Neuroscience Institute, or BRNI, and its affiliate, NRV II, LLC pursuant to the Amended and Restated Technology License and Services Agreement, or BRNI License, entered into on February 4, 2015 and amended on November 12, 2015. Prior to being licensed to us, the technology had been under development by BRNI since 1999 and was financed by BRNI from a variety of non-investor sources including not-for-profit foundations, the National Institutes of Health and individual philanthropists up until March 2013. From March 2013 forward, development of the licensed technology has been funded principally through collaboration by BRNI with Neurotrope BioScience. See the description of Neurotrope BioScience financings below in “Financial Condition, Liquidity and Capital Resources – Sources and Uses of Liquidity.” The Company has not realized any revenues from its operations.
The BRNI License further amended and restated that certain Technology License and Services Agreement executed as of October 31, 2012, which was previously amended by Amendment No. 1 to the Technology License and Services Agreement as of August 21, 2013.
| 43 |
Pursuant to the BRNI License, Neurotrope BioScience maintained its exclusive (except as described below), non-transferable (except pursuant to the BRNI License’s assignment provision), world-wide, royalty-bearing right, with a right to sublicense (in accordance with the terms and conditions described below), under BRNI’s and NRV II’s respective right, title and interest in and to certain patents and technology owned by BRNI or licensed to NRV II, LLC by BRNI as of or subsequent to October 31, 2012 to develop, use, manufacture, market, offer for sale, sell, distribute, import and export certain products or services for therapeutic and diagnostic applications for AD and other cognitive dysfunctions in humans or animals (the “Field of Use”). Additionally, the BRNI License specifies that all patents that issue from a certain patent application, shall constitute licensed patents and all trade secrets, know-how and other confidential information claimed by such patents constitute licensed technology under the BRNI License. Furthermore, on July 10, 2015 under the terms of the Statement of Work and Account Satisfaction Agreement dated February 4, 2015, Neurotrope BioScience’s rights relating to an in vitro diagnostic test system reverted to BRNI. See Note 6 – Related Party Transactions and Licensing/Research Agreements to the Notes to Condensed Consolidated Financial Statements included above.
Notwithstanding the above license terms, BRNI and its affiliates retain rights to use the licensed intellectual property in the Field of Use to engage in research and development and other non-commercial activities and to provide services to Neurotrope BioScience or to perform other activities in connection with the BRNI License.
Under the BRNI License, BRNI is a preferred service provider in certain circumstances and Neurotrope BioScience may not enter into sublicense agreements with third parties except with BRNI’s prior written consent, which consent shall not be commercially unreasonably withheld. Furthermore, the BRNI License dated February 4, 2015 revises the agreement that was entered into as of October 31, 2012 and amended on August 21, 2013, in that it provides that any intellectual property developed, conceived or created in connection with a sublicense agreement that Neurotrope BioScience entered into with a third party pursuant to the terms of the BRNI License will be licensed to BRNI and its affiliates for any and all non-commercial purposes, on a worldwide, perpetual, non-exclusive, irrevocable, non-terminable, fully paid-up, royalty-free, transferable basis, with the right to freely sublicense such intellectual property. Previously, the agreement had provided that such intellectual property would be assigned to BRNI.
Under the BRNI License, BRNI and Neurotrope BioScience will jointly own data, reports and information that is generated on or after February 28, 2013, by Neurotrope BioScience, on behalf of Neurotrope BioScience by a third party or by BRNI pursuant to a statement of work that the parties enter into pursuant to the BRNI License, in each case to the extent not constituting or containing any data, reports or information generated prior to such date or by BRNI not pursuant to a statement of work (the “Jointly Owned Data”). BRNI has agreed not to use the Jointly Owned Data inside or outside the Field of Use for any commercial purpose during the term of the BRNI License or following any expiration of the BRNI License other than an expiration that is the result of a breach by Neurotrope BioScience of the BRNI License that caused any licensed patent to expire, become abandoned or be declared unenforceable or invalid or caused any licensed technology to enter the public domain (a “Natural Expiration”), provided, however, BRNI may use the Jointly Owned Data inside or outside the Field of Use for any commercial purpose following any termination of the BRNI License. Also, BRNI granted Neurotrope BioScience a license during the term and following any Natural Expiration, to use certain BRNI data in the Field of Use for any commercial purposes falling within the scope of the license granted to Neurotrope BioScience under the BRNI License.
The BRNI License further requires us to pay BRNI (i) a fixed research fee equal to a pro-rata amount of $1 million in the year during which we close on a $25 million round of financing, (ii) a fixed research fee of $1 million per year for each of the five calendar years following the completion of such financing and (iii) an annual fixed research fee in an amount to be negotiated and agreed upon no later than 90 days prior to the end of the fifth calendar year following the completion of such financing to be paid with respect to each remaining calendar year during the term of the BRNI License.
On November 12, 2015, we entered into an amendment to the BRNI License. The amendment eliminates the requirement that Neurotrope Bioscience pay BRNI prepaid royalties equal to five percent (5%) of financing proceeds received by Neurotrope Bioscience in any financing prior to a public offering and provides that Neurotrope Bioscience will deliver to BRNI, following each closing pursuant to a certain securities purchase agreement, an amount equal to 2.5% of the Post-PA Fee Proceeds received at such closing. In addition, the amendment provides that on or prior to December 31, 2016, Neurotrope Bioscience shall deliver to BRNI an amount equal to 2.5% of the aggregate Post-PA Fee Proceeds received at the closings. Each payment would constitute an advance royalty payment to BRNI and will be offset (with no interest) against the amount of future royalty obligations payable until such time that the amount of such future royalty obligations equals in full the amount of the advance royalty payments made. “Post-PA Fee Proceeds” means the gross proceeds received, less all amounts paid to the placement agent(s), in relation to such gross proceeds. No other expenses of Neurotrope Bioscience shall be subtracted from the gross proceeds to determine the “Post-PA Fee Proceeds.”
| 44 |
The term of the BRNI License continues until the later of the date (i) the last of the licensed patents expires, is abandoned or is declared unenforceable or invalid (in each case, determined in accordance with the BRNI License) and (ii) the last of the licensed technology enters the public domain. Either party has the right to terminate the BRNI License after 30 days prior notice in certain circumstances, including if either party were to materially breach any provisions of the BRNI License and does not cure such material breach within 60-days from notice of such material breach from the non-breaching party, for breaches that are capable of being cured, in the event of certain bankruptcy or insolvency proceedings or in the event of the termination of a certain Stockholders Agreement dated August 23, 2013 (which was terminated on November 12, 2015 and replaced with a letter agreement – see “Directors, Executive Officers, Promoters and Control Persons – Directors and Executive Officers”), with respect to us.
Concurrently with the November 12, 2015 amendment to the BRNI License, Neurotrope Bioscience entered into a new Statement of Work Agreement pursuant to the BRNI License Agreement (the “November 2015 SOW Agreement”). The November 2015 SOW Agreement replaced the February 2015 SOW Agreement, which was effective as of October 1, 2014 and expired on September 30, 2015.
Pursuant to the November 2015 SOW Agreement, Neurotrope Bioscience agreed to pay BRNI one million one hundred sixty-six thousand six hundred sixty-six dollars ($1,166,666) in service fees payable in the amount of eighty-three thousand three hundred thirty-three dollars ($83,333) per month for each month from November 1, 2015 through December 31, 2016. The payments under the November 2015 SOW Agreement will satisfy Neurotrope Bioscience’s obligations to reimburse BRNI pursuant to Section 5.6 of the BRNI License for any and all attorneys’ fees, translation costs, filing fees, maintenance fees, and other costs and expenses related to applying for, filing, prosecuting, and maintaining patents and applications for the licensed intellectual property incurred by BRNI during the term of the November 2015 SOW Agreement (but, for the avoidance of doubt, such payments shall not satisfy any attorneys’ fees, translation costs, filing fees, maintenance fees, or other costs or expenses related to applying for, filing, prosecuting, and maintaining patents and applications for the licensed intellectual property incurred by BRNI after the expiration or termination of the November 2015 SOW Agreement term), as well as any litigation costs which BRNI may incur related to any of the licensed intellectual property during the November 2015 SOW Agreement term. BRNI shall not commence any litigation to enforce the licensed intellectual property without the consent of Neurotrope (which consent shall not be unreasonably withheld, delayed, or denied).
In consideration for the payments made pursuant to the November 2015 SOW Agreement, BRNI shall perform the services requested by Neurotrope Bioscience for the further development of Neurotrope’s bryostatin drug platform. In addition, under the terms of the November 2015 SOW Agreement, BRNI may enroll one (1) additional compassionate use, in addition to the compassionate use patient currently enrolled, in trials of BRNI’s Alzheimer’s therapeutic drug platform during the November 2015 SOW Agreement term, and the payments set forth above, shall satisfy any and all of Neurotrope Bioscience’s obligation whatsoever to BRNI or to any other third party for costs incurred or to be incurred by BRNI relating to such trials. Neurotrope Bioscience and BRNI shall jointly review protocols which shall be established to the parties’ mutual satisfaction and contain appropriate safety measures to be employed by the treating physician. No additional compassionate use or expanded access patients will be enrolled by BRNI without the consent of Neurotrope Bioscience.
On May 12, 2014, we entered into a license agreement (the “Stanford Agreement”), with The Board of Trustees of the Leland Stanford Junior University (“Stanford”) pursuant to which Stanford granted us a revenue-bearing, world-wide right and exclusive license, with the right to grant sublicenses (on certain conditions), under certain patent rights and related technology for the use of bryostatin structural derivatives, known as “bryologs,” for use in the treatment of central nervous system disorders, lysosomal storage diseases, stroke, cardio protection and traumatic brain injury, for the life of the licensed patents. Pursuant to the Stanford Agreement, we paid a $60,000 license initiation fee to Stanford. We currently pay Stanford a $10,000 annual license maintenance fee, and will pay milestone payments in the aggregate amount of up to $3,700,000 upon the achievement of certain product development events commencing upon the filing of the first IND application through approval of an applicable product, as well as low single-digit royalties on net sales of the licensed products. Each party has the right to terminate the Stanford Agreement for an uncured material breach of the other party. Additionally, we may terminate the Stanford Agreement at any time upon 60 days written notice to Stanford.
| 45 |
On May 15, 2014, we entered into an agreement with a contract research organization, or CRO, to conduct a Phase 2a clinical trial relating to the evaluation of bryostatin for the treatment of AD. We agreed to pay fees to the CRO totaling $715,159 based upon signing of the agreement and the CRO achieving certain clinical trial milestones, plus reasonable out-of-pocket expenses. During November 2014, we amended the original agreement which reduced the number of subjects in the clinical trial from 15 to nine, resulting in a reduced total cost estimate of $657,236. The trial was completed in early 2015 and the results were reported as described below.
On July 14, 2014, Neurotrope BioScience entered into an Exclusive License Agreement, or the “Mount Sinai Agreement”, with the Icahn School of Medicine at Mount Sinai, or Mount Sinai. Pursuant to the Mount Sinai Agreement, Mount Sinai granted Neurotrope BioScience a revenue-bearing, world-wide right and (i) exclusive license, with the right to grant sublicenses (on certain conditions), under Mount Sinai’s interest in certain joint patent rights, as well as in certain test results and data, and (ii) non-exclusive license, with the right to grant sublicenses on certain conditions, to certain technical information. The Mount Sinai Agreement allows Neurotrope BioScience to research, discover, develop, make, have made, use, have used, import, lease, sell, have sold and offer for sale the Mount Sinai licensed products relating to the diagnostic, prophylactic or therapeutic use for treating diseases or disorders in humans relying on activation of PKC epsilon, which includes Niemann-Pick C Disease. Pursuant to the Mount Sinai Agreement, Neurotrope BioScience paid $25,000 license initiation fee to Mount Sinai. Neurotrope BioScience paid Mount Sinai the current $10,000 annual license maintenance fee which will continue to be paid until minimum royalty payments are due, and will pay milestone payments in the aggregate amount of up to $3,500,000 upon the achievement of certain product development events relating to the approval of a licensed product in the United States and other jurisdictions, as well as (a) low single-digit royalties on net sales of the Mount Sinai licensed products, and (b) a portion of sublicense fees ranging from a significant double-digit percentage based on sublicensing before completion of in vivo proof-of-concept experiments to a mid-single digit percentage after product approval. Each party has the right to terminate the Mount Sinai Agreement for an uncured material breach of the other party. Additionally, Neurotrope BioScience may terminate the Mount Sinai Agreement at any time upon 60 days written notice to Mount Sinai. Further, upon termination, Neurotrope BioScience may continue to sell any and all Mount Sinai licensed products, provided that Neurotrope BioScience will pay Mount Sinai a reduced royalty for Mount Sinai licensed products that are indicated as therapeutics or diagnostics for Niemann Pick disease which are sold following termination of the Mount Sinai Agreement.
On July 29, 2014, we announced that we initiated our Phase 2a clinical trial to evaluate bryostatin for the treatment of patients with AD. The trial was being conducted under an IND application filed by BRNI. BRNI transferred its rights and obligations arising under such IND application to us on February 4, 2015. We enrolled a total of nine patients in the randomized, double-blind, placebo-controlled, single dose study. Six patients were randomized to receive bryostatin by injection and three received a matching placebo control. The primary objective of the clinical trial was to assess the safety and tolerability of a single dose of bryostatin in the treatment of patients with AD. The secondary objectives of the study were the preliminary evaluation of the efficacy of a single dose of bryostatin in the treatment of patients with AD, its pharmacokinetics and pharmacodynamics and the evaluation of any correlation between changes in PKC epsilon with plasma levels of bryostatin and with improvement in cognitive function. On February 24, 2015, we announced that the Phase 2a clinical trial met its primary endpoint demonstrating preliminary safety and tolerability of bryostatin. On March 17, 2015, we announced that preliminary assessment of PKC epsilon levels in peripheral monocytes demonstrated a significant increase in total PKC protein levels at the end of the bryostatin infusion consistent with target engagement. An additional secondary objective of the study was the evaluation of efficacy following a single dose of bryostatin and there was no measurable improvement in cognition in this mildly impaired patient population.
Following on these results, on October 9, 2015, Neurotrope BioScience, Inc., our wholly-owned consolidated operating subsidiary, executed a Services Agreement with Worldwide Clinical Trials, Inc., or WCT, effective as of August 31, 2015. The Services Agreement relates to services for Neurotrope BioScience’s Phase 2b clinical study assessing the safety, tolerability and efficacy of bryostatin in the treatment of moderately severe to severe Alzheimer’s Disease, or the Study. Pursuant to the terms of the Services Agreement, WCT will provide services to enroll approximately one hundred and fifty (150) study subjects at approximately 30 sites across the United States. Depending upon our resources, we intend to begin enrollment at the initial sites by the end of 2015. Assuming prompt enrollment at the majority of the sites, we expect that within approximately 15 months, we will receive data from an interim analysis with final top line results available in approximately 18 months from the start of enrollment. This trial is designed to administer dosing of bryostatin for up to six months for moderately severe to severe AD patients. Among the trial’s endpoints are the measurement of improvement in cognition, activities of daily living and behavior. The Company’s goal is to show the robust treatment effect that the regulatory agencies, the marketplace and, most importantly, our patients and their caregivers are seeking.
| 46 |
The total estimated budget for the services, including pass-through costs, is approximately $11.6 million. Neurotrope BioScience paid WCT an advance payment of $200,000 upon execution of the Agreement. Neurotrope BioScience may terminate the Agreement without cause upon sixty (60) days’ prior written notice.
Recent Events
In the November 2015 Private Placement, we sold 26,234,940 units at $0.60 per unit, with each unit consisting of Series B Stock together with Series A through Series E warrants to purchase common stock, and certain placement agent warrants, resulting in gross proceeds of $15,640,963. The total number of units sold includes 166,667 units granted to Dr. Abeles as compensation for his services as a consultant to the Company, which accounts for $100,000 that is not included in our calculation of gross proceeds. The private placement was completed in two closings, which took place on November 13, 2015 and November 30, 2015. In connection with this private placement, effective as of November 13, 2015, the holders of all 16,656,894 shares of our Series A Stock converted their shares into 19,864,971 shares of our common stock, which included 3,208,077 shares of our common stock issued in accordance with anti-dilution provisions relating to the Series A Stock.
Going Concern
As shown in the consolidated financial statements contained in this report, we have generated no revenues to date, have incurred substantial losses, and have substantial contractual commitments pursuant to various agreements to which we are a party.
Our ability to continue existence is dependent upon our continuing efforts to obtain additional financing to carry out our business plan. We intend to fund our operations through equity and/or debt financing arrangements and any revenues generated in the future. However, there can be no assurance that these arrangements, if any, will be sufficient to fund our ongoing capital expenditures, working capital, and other cash requirements. The outcome of these matters cannot be predicted at this time.
There can be no assurance that any additional financings will be available to us on satisfactory terms and conditions, if at all. These conditions raise substantial doubt as to our ability to continue as a going concern.
The unaudited condensed consolidated financial statements contained in this report do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should we be unable to continue as a going concern.
Strategy
One of the central tenets of BRNI’s and our research and development is the belief that the neurodegeneration underlying these neurological diseases can be halted or reversed. We believe that the process of halting and reversing neurodegeneration may be achieved by an improvement in nerve cell viability and synaptic function through activation of an enzyme called PKC. This enzyme is actually a super family of isozymes (α, β, γ, δ, ε…) which have different activities in different tissues. The PKC epsilon variant has a very high concentration in the synapses of neurons, suggesting it plays a role in maintaining synaptic function. Deficient activity or low concentrations of PKC epsilon in aging subjects is proposed by BRNI to be one of the primary causes of the neurodegeneration seen in AD. Through a variety of the latest biomedical techniques and animal models developed to map and quantify neuroregeneration, BRNI has established a central role for PKC epsilon in re-modeling or restoring synaptic function in both healthy and diseased neurons in the central nervous system. In contrast to the industry’s historic focus on product candidates relating to either removal of amyloid plaque or Tau pathology in the brain, which have collectively resulted in numerous, failed clinical trials, Neurotrope is following a new and novel approach to AD drug development. Specifically, research conducted in animal models by the Company’s Chief Scientific Officer, Dr. Dan Alkon, of the Blanchette Rockefeller Neurosciences Institute, showed that PKCε, when stimulated, initiated a cascade of enzymatic events, ultimately improved synaptic function, increased vital neurotropic factors and induced formation of new synapses. The stimulation of PKCε is far upstream from the formation of plaques and tangles, which may be considered pathologic markers of AD, rather than causes. As a potent modulator of PKCε at low dose levels, bryostatin has been shown to stimulate this key enzyme in our animal models, thus showing potential promise in restoring memory and learning function.
Our lead product candidate in the Neurotrope armamentarium is bryostatin. This drug has previously been evaluated in over 1,400 patients at the National Cancer Institute, or NCI, for the treatment of various forms of cancer. While bryostatin did not show sufficient anti-cancer effects to warrant commercialization of the compound, much useful information on the safety, pharmacodynamics, and toxicity of the drug was gleaned from these human trials. The NCI has allowed BRNI access to the valuable chemical, animal and human data from its cancer studies, to which we, in turn, have access under the BRNI License to be used in our own research and regulatory programs.
| 47 |
It was discovered by BRNI that at a much lower dose than that which was used in these anti-cancer trials, bryostatin is a potent activator of PKC epsilon and thus may have efficacy in treating AD.
In addition, BRNI is conducting an expanded access program of Bryostatin-1 in patients with advanced AD. The purpose of the program is to provide experimental care to patients and to gain experience in understanding dosing, safety and efficacy of the drug in these patients. In this program, patients receive the drug, with informed consent, under the supervision of licensed physicians who are experts in the care of patients with advanced dementia. Treating physicians monitor safety and clinical outcomes. Thus far, four patients with advanced AD have been treated, three of which were treated under an Investigational New Drug Application, IND, cleared by the FDA which is held by BRNI. One of these patients, who had familiar AD, has died, but the death was not drug-related. The study for another one of these patients has concluded after almost one year on the protocol. A fourth patient has recently commenced treatment. On the basis of communication from caregivers and treating physicians, BRNI, with our support, has decided to enroll one additional patient in the extended access program. We are providing limited funding and personnel support under the terms of our agreement with BRNI for the modest expansion of this clinical effort in AD in the 2015 to 2016 timeframe. It is essential to note that this study is an open label study with no placebo control. We recognize that this study does not meet accepted scientific standards of a rigorous double blind placebo controlled clinical trial and does not provide rigorous data regarding safety and efficacy. Therefore, information derived from this program is purely anecdotal and cannot be used to support any scientific claims regarding either safety or efficacy.
Our strategy is to efficiently utilize our licensed proprietary and patented technologies to further the development of those technologies toward commercializing a therapeutic for AD and potentially utilize these technologies to treat other neurological diseases. We may also seek to acquire, by license or otherwise, other development stage products that are consistent with our product portfolio objectives and commercialization strategy. In addition, we plan to utilize technologies licensed from BRNI and Mount Sinai in order to pursue therapeutics for orphan drug indications, including Fragile X Syndrome and Niemann-Pick Type C disease. We license intellectual property from BRNI relating to Fragile X Syndrome (“FXS”). FXS is the most common cause of inherited intellectual disability and the most common known genetic cause of autism or autism spectrum disorders. Symptoms of FXS include a range from learning disabilities to more severe cognitive or intellectual disabilities. Delays in speech and language development are common, as are a variety of physical and behavioral characteristics. FXS is caused by a “full mutation” of the FMR1 Gene. There are approximately 135,000 Fragile X Syndrome patients in the United States and a similar number in Europe. Neurotrope BioScience receives support from the FRAXA Research Foundation, Inc. (“FRAXA”) to fund a pre-clinical Fragile X Syndrome (“FXS”) behavior study for bryostatin at FRAXA’s purpose built laboratory located at the University of Chile in Santiago, Chile. FRAXA will provide full funding for a preclinical study to evaluate the behavioral effects of bryostatin-1 in a FXS mouse model. We have formed an experienced clinical advisory board to assist us with the first protocol for a planned Phase 2a study in approximately 20 to 30 patients. We expect to complete the first clinical trial, depending upon our resources, by the end of 2016. We have been granted orphan drug designation by the FDA for the use of bryostatin in the treatment of Fragile X Syndrome.
Use of bryostatin to treat Niemann-Pick Type C Disease (“NP-C”) is being developed by the Company with collaborations with the Icahn School of Medicine at Mount Sinai in New York City, or Mt. Sinai, and with the organization Support of Accelerated Research (“SOAR”). NP-C mainly affects children who develop severe neurologic symptoms including gait disturbance and cognitive deficits early in life. There are approximately 3,500 NP-C patients in the United States and a similar number in Europe. Patients with NP-C have a gene defect that results in the loss of the “normal” NPC1 or NPC2 protein that is important for cholesterol trafficking. As a result of the gene defect, patients have accumulation of cholesterol and a number of other lipids such as sphingolipids, as well as an accumulation of gangliosides in various tissues, including the brain. Rates of disease progression and life expectancy vary widely; however, the majority of patients die between 10 and 25 years of age. Progressive neurological manifestations in NP-C patients have a profound effect on quality of life for both the patients and their caregivers.
Neurotrope Bioscience presented the findings of an in vitro study of bryostatin’s effects on Niemann Pick Type C1 cells, or NPC1 cells at the annual Michael, Marcia & Christa Parseghian Scientific Conference for Niemann-Pick Type C research at the University of Notre Dame in June 2015. The presentation reported the results of in vitro studies of three PKC activators, bryostatin, DCPLA and diazoxide, on their ability to correct the cholesterol transport defect in NPC1 cells. The studies, conducted at Mt. Sinai, in conjunction with Neurotrope, concluded that all three PKC activators were able to induce clearance of cholesterol from NPC1 cells to varying degrees and at different drug concentrations. The study demonstrated that bryostatin was the most potent compound showing statistically significant activity at the nanomolar concentration. A second patient cell line with a completely distinct NPC1 genotype was also tested with similar results. In vivo studies are currently underway at Mt. Sinai to evaluate the effect of bryostatin in an animal model of Niemann-Pick Type C. We are working towards completion of the necessary pre-clinical work in order to obtain FDA approval of an IND, or investigational new drug application. Assuming that the pre-clinical work shows positive activity, we expect to apply for orphan drug designation for this indication.
| 48 |
Following the conference, the Company announced in June 2015 that Neurotrope Bioscience would conduct an additional preclinical study, in collaboration with a leading research institution, to examine the effect of bryostatin in Niemann-Pick type C mice (NP-C Mice). The study is being funded by several family foundations under the auspices of SOAR-NPC. This planned study will examine the effects of various dosing regimens of bryostatin in NP-C Mice over a brief treatment period. Specifically, the in vivo study will seek to confirm previous in vitro studies that suggest bryostatin may be effective in correcting the cholesterol transport defect in NP-C. The Company expects the results of the study to be available in the fourth quarter of 2015. This study is designed to examine the ability of bryostatin treatment to remove cholesterol from the brain which is believed to be critical for an effective therapy of NP-C.
Critical Accounting Policies, Estimates, and Judgments
Our financial statements are prepared in accordance with accounting principles that are generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. We continually evaluate our estimates and judgments, the most critical of which are those related to accounting for equity compensation and our commitments to strategic alliance partners and the timing of the achievement of collaboration milestones. We base our estimates and judgments on historical experience and other factors that we believe to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known. Besides the estimates identified above that are considered critical, we make many other accounting estimates in preparing our financial statements and related disclosures. All estimates, whether or not deemed critical, affect reported amounts of assets, liabilities, revenues and expenses, as well as disclosures of contingent assets and liabilities. These estimates and judgments are also based on historical experience and other factors that are believed to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known, even for estimates and judgments that are not deemed critical.
Results of Operations
Comparison of the Years ended December 31, 2014 and December 31, 2013
Revenues
We did not generate any revenues for the years ended December 31, 2014 and 2013.
Operating Expenses
Overview
Total operating expenses for the year ended December 31, 2014 were $9,267,120 versus $7,590,782 for the year ended December 31, 2013, an increase of approximately 22%. The increase in operating expenses is due primarily to the acceleration of product research and development activities relating to its collaboration with BRNI and the treatment and diagnosis of neurodegenerative diseases, which was partially offset by a decrease in general and administrative expenses.
| 49 |
Research and Development Expenses
| Year ended December 31, 2014 | Year ended December 31, 2013 | |||||||||||||||||||||||||||||||
| Therapeutic | Diagnostic | Patents & Licenses | Total | Therapeutic | Diagnostic | Patents & Licenses | Total | |||||||||||||||||||||||||
| Related Party (BRNI) | $ | 789,172 | $ | 1,096,984 | $ | 337,487 | $ | 2,223,643 | $ | 245,830 | $ | 873,495 | $ | 248,319 | $ | 1,367,644 | ||||||||||||||||
| Non-Related Party | 1,183,534 | 0 | 59,172 | 1,242,706 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||
| Total | $ | 1,972,706 | $ | 1,096,984 | $ | 396,659 | $ | 3,466,349 | $ | 245,830 | $ | 873,495 | $ | 248,319 | $ | 1,367,644 | ||||||||||||||||
For the year ended December 31, 2014, we incurred $2,223,643 of research and development – related party expenses versus $1,367,644 for the year ended December 31, 2013. These expenses were incurred pursuant to our strategic alliance with BRNI for ongoing research and development principally relating to the development of our potential therapeutic and former diagnostic product. Of these expenses, for the years ended December 31, 2014 and 2013, $789,172 and $245,830, respectively, related to the development of our potential AD therapeutic product, $1,096,984 and $873,495, respectively, related to the continuing development of our former potential AD diagnostic product, and $337,487 and $248,319, respectively, was incurred for patent expenses associated with the two licensed technology platforms from BRNI.
For the year ended December 31, 2014, we incurred $1,242,706 in research and development expenses with non-related parties versus $0 for the year ended December 31, 2013. These expenses were incurred pursuant to developing the potential AD therapeutic product. Of these expenses, for the year ended December 31, 2014, $907,753 related to producing and storing drug product for clinical trial patient dosing for our Phase 2a clinical trial, which was completed and the drug product utilized during 2014, $185,700 for clinical consulting services, $90,081 was incurred for inventory management of drug product for clinical trials and $59,172 of licensing payment amortization relating to the Stanford and Mount Sinai license agreements.
The February 2015 SOW was effective as of October 1, 2014. For the year ended December 31, 2014, we incurred $600,000 in expenses relating to the February 2015 SOW versus $0 for the year ended December 31, 2013. Of this $600,000, we paid $400,000 in 2014, and we paid the remaining $200,000 in 2015.
General and Administrative Expenses
We incurred related party general and administrative expenses relating to BRNI and its affiliates totaling $108,693 and $1,446,986 for the years ended December 31, 2014 and 2013, respectively, a decrease of approximately 92%. The decrease is primarily attributable to the payment of a royalty of $0 and $1,038,750 to BRNI, respectively, such difference in the royalties due to BRNI is a result of royalties paid to BRNI in 2013 in connection with the closing of fundraising transactions that occurred in 2013 and; $108,694 versus $61,000 paid to our prior and current Chairmen for services in that capacity provided to us, respectively. In addition, for the year ended December 31, 2013, $347,236 of wages, taxes, insurance and consulting fees relating to our former President and Chief Executive Officer were classified as related party expenses.
We incurred $4,488,415 and $1,653,208 of other general and administrative expenses for the years ended December 31, 2014 and 2013, respectively, an increase of approximately 171%. Of the amounts for the years ended December 31, 2014 versus the comparable 2013 period: $2,123,189 was incurred for wages, bonuses, vacation pay and taxes for six employees versus $105,111, such difference due to the fact that we had one employee employed for a portion of 2013 versus six full-time employees in 2014; $691,867 for ongoing legal expenses versus $731,056 for ongoing legal expenses, plus one-time legal expenses for the BRNI License and closing of the merger and private placement financing; $876,704 was incurred for outside operations consulting services versus $424,971; $215,696 was incurred for travel expenses versus $0; $188,310 was incurred for investor relations services versus $0; $147,635 was incurred for professional fees associated with auditing, financial, accounting and tax advisory services versus $311,502, such difference is due to the merger and private placement transactions that occurred in 2013; $104,320 was incurred for insurance versus $47,094; $60,000 was incurred for payments to our Executive Chairman versus $0; and $80,694 was incurred for office supplies, license fees, filing costs, rent, advertising and other, mostly relating to new office facilities in New Jersey, versus $33,474.
| 50 |
Stock Based Compensation Expenses
We incurred related party non-cash expenses totaling $252,868 versus $2,854,745 for the years ended December 31, 2014 and 2013, respectively, in connection with the issuance of stock options. The decrease is a result of the issuance of stock options pursuant to the merger and private placement transactions that occurred in 2013.
We incurred $950,795 and $268,199 of non-related party non-cash expenses for issuance of stock options for the years ended December 31, 2014 and 2013, respectively.
Other Income
We earned $13,797 of interest income for the year ended December 31, 2014 versus $1,378 for the year ended December 31, 2013 on funds temporarily deposited in an interest bearing money market account.
Net loss and earnings (losses) per share
We incurred losses of $9,253,323 and $7,589,404 for the years ended December 31, 2014 and 2013, respectively. The increased loss was primarily due to our increased activities and new employee hiring during the current period. Earnings (losses) per share were ($0.49) and ($0.40) for the years ended December 31, 2014 and 2013, respectively. The increase in loss per share is primarily attributable to the increase in our operating loss for the current period.
Financial Condition, Liquidity and Capital Resources
Since the inception of Neurotrope BioScience, we have primarily devoted our efforts to the development of our therapeutic products, raising capital, negotiating the BRNI License and, until June 30, 2015, the license we formerly held under the BRNI License Agreement to certain technology, including rights relating to an in vitro test system based on examination of skin cells intended to predict the presence of Alzheimer’s disease in humans (the “AD Diagnostic Test”) toward commercialization while conducting business planning and recruiting executive management.
Cash and Working Capital
Since inception, we incurred negative cash flows from operations. As of December 31, 2014, we had an accumulated deficit of $18,282,689 and had working capital of $6,818,242 as compared to working capital of $14,890,387 as of December 31, 2013. The $8,072,146 decrease in working capital was attributable to our expenditures relating to development of a potential therapeutic and potential former diagnostic product and general and administrative expenses which resulted in a net loss of $9,253,323 and capital expenditures of $54,943 offset by non-cash expenses of $1,236,120 for the year ended December 31, 2014.
Sources and Uses of Liquidity
Since inception, we have satisfied our operating cash requirements from the private placement of Series A Preferred Stock sold principally to outside investors.
In February 2013, through a private placement, we issued 9,073,300 shares of Series A Preferred Stock at $1.00 per share, resulting in gross proceeds of $9,073,300. In connection with the closing of the private placement, we paid a placement agent $882,330. In May 2013, we issued an additional 1,313,325 shares of Series A Preferred Stock at $1.00 per share, resulting in gross proceeds of $1,313,325, on which we paid a placement agent $131,332. In August 2013, through an additional private placement, we issued 11,533,375 of Series A Preferred Stock at $1.00 per share, resulting in gross proceeds of $11,533,375. In connection with the closing of the August 2013 private placement, we paid a placement agent $1,103,338. Further, Neurotrope BioScience became a wholly-owned subsidiary of a publicly traded company in the Reverse Merger. In October 2013, through an additional private placement, we issued 1,080,000 of Series A Preferred Stock at $1.00 per share, resulting in gross proceeds of $1,080,000. In connection with the closing of the private placement, we paid a placement agent $108,000.
| 51 |
On November 13, 2015, we entered into a Securities Purchase Agreement to sell up to 26,234,940 units in a private placement at a per unit purchase price equal to $0.60. Each unit consisted of (i) one one-hundredth share of Series B Stock convertible into one share of our common stock, (ii) one warrant to acquire, at an exercise price of $0.80 per share with an expiration date five years from the date of issuance, one share of our common stock (the “Series A Warrant”), (iii) one warrant to acquire, at an exercise price of $0.80 per share with an expiration date of one year from the date of issuance, one share of our common stock (the “Series B Warrant”), (iv) one warrant to acquire, at an exercise price of $1.25 per share with an expiration date of five years from the issuance date, one share of our common stock (the “Series C Warrant”), (v) one warrant, which is contingent upon the exercise of the Series B Warrant, to acquire, at an exercise price of $1.00 per share with an expiration date that is five years from the date of the initial exercise of the Series B Warrant, one share of our common stock (the “Series D Warrant”), and (vi) one warrant, which is contingent upon the exercise of the Series C Warrant, to acquire, at an initial exercise price of $1.50 per share with an expiration date that is five years from the date of the initial exercise of the Series C Warrant, one share of our common stock (the “Series E Warrant”, and together with the Series A Warrant, the Series B Warrant, the Series C Warrant and the Series D Warrant, the “Investor Warrants”). The exercise prices of the Investor Warrants are initially subject to full protection for dilutive issuances. The Series A Warrant and Series B Warrant each contain a mandatory exercise right of ours to force exercise of the warrant if our common stock trades at or above $1.50 for 20 consecutive trading days (subject to certain conditions, including a $150,000 minimum daily volume requirement). The Series C Warrant contains a mandatory exercise right of the Company to force exercise of the warrant if our common stock trades at or above $2.00 for 20 consecutive trading days (subject to certain conditions, including a $150,000 minimum daily volume requirement). In addition, pursuant to the terms of the Securities Purchase Agreement, we may sell, in one additional closing on the same terms and conditions as those contained in the purchase agreement, additional units to one or more buyers (each, an “Additional Investor”), each of which is either (a) an institutional investor that focuses on the biotech industry, (b) an investor of our Series A Stock, (c) any investor investing under $5,000 or (d) any person approved in writing by each of those selling stockholders who are institutional investors and such selling stockholder’s purchase price (together with such selling stockholder’s institutional affiliates) equals or exceeds $1,000,000 (the “Large Buyers”). The Large Buyers also have certain consent rights with respect to any Additional Investor. In connection with the private placement, the holders of our Series A Stock consented to convert their holdings into common stock. The closing of the private placement was subject to customary closing conditions and was completed in two closings, which took place on November 13, 2015 and November 30, 2015. The gross proceeds from the closing of the initial portion of the private placement were approximately $15,330,000 and from the second portion of the private placement were $311,000.
Going Concern
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As shown in the accompanying consolidated financial statements, we have generated no revenues to-date have incurred substantial losses during the Company’s development stage, and have substantial contractual commitments pursuant to various agreements to which we are a party.
Our ability to continue existence is dependent upon our continuing efforts to obtain additional financing to carry out our business plan during the development stage. We intend to fund our operations through equity and/or debt financing arrangements and any revenues generated in the future. However, there can be no assurance that these arrangements, if any, will be sufficient to fund our ongoing capital expenditures, working capital, and other cash requirements. The outcome of these matters cannot be predicted at this time.
There can be no assurance that any additional financings will be available to us on satisfactory terms and conditions, if at all. These conditions raise substantial doubt as to our ability to continue as a going concern.
The accompanying consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should we be unable to continue as a going concern.
Off-Balance Sheet Arrangements
We did not engage in any “off-balance sheet arrangements” (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) as of December 31, 2014.
Results of Operations
Comparison of the nine months ended September 30, 2015 and September 30, 2014
Revenues
We did not generate any revenues for the nine months ended September 30, 2015 and 2014.
| 52 |
Operating Expenses
Overview
Total operating expenses for the nine months ended September 30, 2015 were $6,911,236 versus $6,870,607 for the nine months ended September 30, 2014, an increase of approximately 1%. The increase in operating expenses is due primarily to the acceleration of product research and development activities relating to efforts to commercialize pharmaceutical products for the treatment of neurodegenerative diseases and its collaboration with BRNI and an increase in general and administrative expenses to support product research and development activities offset by a decrease in non-cash stock compensation expenses.
Research and Development Expenses
| Nine months ended September 30, 2015 | Nine months ended September 30, 2014 | |||||||||||||||||||||||||||||||
| Therapeutic | Diagnostic | Patents
& Licenses | Total | Therapeutic | Diagnostic | Patents
& Licenses | Total | |||||||||||||||||||||||||
| Related Party (BRNI) | $ | NA | $ | NA | $ | NA | $ | 1,852,428 | $ | 474,892 | $ | 1,096,984 | $ | 337,487 | $ | 1,909,363 | ||||||||||||||||
| Non-Related Party | 1,295,065 | 0 | 53,078 | 1,348,143 | 740,107 | 0 | 34,082 | 774,189 | ||||||||||||||||||||||||
| Total | $ | 1,295,065 | $ | 0 | $ | 53,078 | $ | 3,200,571 | $ | 1,214,999 | $ | 1,096,984 | $ | 371,569 | $ | 2,683,552 | ||||||||||||||||
For the nine months ended September 30, 2015, we incurred $1,852,428 of research and development – related party expenses versus $1,909,363 for the nine months ended September 30, 2014. These expenses are incurred pursuant to our strategic alliance with BRNI for ongoing research and development principally relating to the development of our potential therapeutic under the terms of the February 2015 SOW. We paid a flat fee of $200,000 per month through September 30, 2015, which covered product development activities and maintenance of the licensed patent portfolio and paid separately for certain expenses relating to analytical testing and consulting fees.
For the nine months ended September 30, 2015, we incurred $1,348,143 in research and development expenses with non-related parties versus $774,189 for the nine months ended September 30, 2014, an increase of approximately 73%. These expenses were incurred pursuant to developing the potential AD therapeutic product and products relating to orphan drug indications. Of these expenses, for the nine months ended September 30, 2015, $1,108,509 related to conducting our AD Phase 2a clinical trial and related production, inventory management and storing of drug product versus $651,412 for the comparable 2014 period, $156,072 for clinical consulting services versus $88,695 for the comparable 2014 period, $41,078 of licensing payment amortization relating to the Stanford and Mount Sinai license agreements versus $34,082 for the comparable 2014 period, $30,484 for orphan drug development costs versus $0 for the comparable 2014 period, and $12,000 for development of alternative drug supply with Stanford University versus $0 for the comparable 2014 period. We expect our research and development expenses to increase, as our resources permit, in order to advance our potential products.
General and Administrative Expenses
We incurred related party general and administrative expenses totaling $81,000 and $81,693 for the nine months ended September 30, 2015 and 2014, respectively. This amount is attributable to the payments to our prior Chairman for services provided to us as a director.
| 53 |
We incurred $3,242,220 and $3,174,489 of other general and administrative expenses for the nine months ended September 30, 2015 and 2014, respectively, an increase of approximately 5%. Of the amounts for the nine months ended September 30, 2015 versus the comparable 2014 period: $2,017,166 was incurred for wages, bonuses, vacation pay, severance, taxes and insurance for five employees plus our Chairman, versus $1,443,445 for six employees for the 2014 comparable period; $597,096 for ongoing legal expenses versus $489,807; $171,736 was incurred for investor relations services versus $100,908; $177,800 was incurred for insurance versus $75,202; $0 was incurred for outside operations consulting services versus $714,953 based upon the Company either hiring the consultants or completing services provided by such consultants; $117,090 was incurred for travel expenses versus $175,769; $98,023 was incurred for professional fees associated with auditing, financial, accounting and tax advisory services versus $125,403; and $143,806 was incurred for office rent and utilities, supplies, license fees, filing costs, rent, advertising and other, mostly relating to office facilities in New Jersey offset by a credit of $80,497 relating to a settlement of a payable to a service provider versus $49,002. As further discussed in Note 4 (Contractual Commitments) and Note 12 (Subsequent Events) that are included in the Notes to Condensed Consolidated Financial Statements (Unaudited) above, with respect to the amounts that we incurred for severance, we have accrued a total of $265,000 of severance payments as of September 30, 2015, which are contingent upon us raising additional capital in excess of $7.5 million.
Stock Based Compensation Expenses
We incurred related party non-cash expenses totaling $131,453 versus $208,569 for the nine months ended September 30, 2015 and 2014, respectively, in connection with the issuance of stock options, and we incurred $255,774 and $722,304 of non-related party non-cash expenses for issuance of stock options for the nine months ended September 30, 2015 and 2014, respectively. The decrease is a result of canceling previously issued stock options terminated in 2014 partially offset by the issuance of new stock options during the nine months ended September 30, 2015.
Other Income
We earned $2,844 of net interest income for the nine months ended September 30, 2015 versus $11,590 for the nine months ended September 30, 2014 on funds temporarily deposited in an interest bearing money market account. This decrease is attributable to the reduction in cash balances.
Net loss and earnings (losses) per share
We incurred losses of $6,908,174 and $6,859,017 for the nine months ended September 30, 2015 and 2014, respectively. The increased loss was primarily due to our increased activities and new employee hiring during 2014 offset by a decrease in non-cash stock-based compensation expenses. Earnings (losses) per common share including dividends accruable on Series A Preferred Stock were ($0.30) and ($0.38) for the nine months ended September 30, 2015 and 2014, respectively. The increase in loss per share is primarily attributable to the decrease in our operating loss for the current period partially offset by the increase in the number of weighted average number of shares.
Comparison of the three months ended September 30, 2015 and September 30, 2014
Revenues
We did not generate any revenues for the three months ended September 30, 2015 and 2014.
Operating Expenses
Overview
Total operating expenses for the three months ended September 30, 2015 were $1,892,304 versus $3,144,315 for the three months ended September 30, 2014, a decrease of approximately 40%. The decrease in operating expenses is due primarily to reductions in research and development and general and administrative expenses and non-cash stock-based compensation expenses.
| 54 |
Research and Development Expenses
| Three months ended September 30, 2015 | Three months ended September 30, 2014 | |||||||||||||||||||||||||||||||
| Therapeutic | Diagnostic | Patents & Licenses |
Total | Therapeutic | Diagnostic | Patents & Licenses |
Total | |||||||||||||||||||||||||
| Related Party (BRNI) | $ | NA | $ | NA | $ | NA | $ | 636,547 | $ | 368,361 | $ | 274,246 | $ | 203,528 | $ | 846,135 | ||||||||||||||||
| Non-Related Party | 261,324 | 0 | 7,341 | 268,665 | 368,221 | 0 | 0 | 368,221 | ||||||||||||||||||||||||
| Total | $ | 261,324 | $ | 0 | $ | 7,341 | $ | 905,212 | $ | 736,582 | $ | 274,246 | $ | 203,528 | $ | 1,214,356 | ||||||||||||||||
For the three months ended September 30, 2015, we incurred $636,547 of research and development – related party expenses versus $846,135 for the three months ended September 30, 2014. These expenses were incurred pursuant to our strategic alliance with BRNI for ongoing research and development principally relating to the development of our potential therapeutic and the former AD Diagnostic Test. Under the terms of the February 2015 SOW, we paid BRNI a flat fee of $200,000 per month which covers product development activities and maintenance of the licensed patent portfolio and paid separately for certain expenses relating to analytical testing and consulting fees.
For the three months ended September 30, 2015, we incurred $268,665 in research and development expenses with non-related parties versus $368,221 for the three months ended September 30, 2014. These expenses were incurred pursuant to developing the potential AD therapeutic product and products relating to orphan drug indications. Of these expenses, for the three months ended September 30, 2015, $235,724 related to conducting our AD Phase 2a clinical trial and related production and inventory management and storing of drug product versus $610,279 for the comparable 2014 period, $17,331 for clinical consulting services versus $88,695 for the comparable 2014 period, $7,341 of licensing payment amortization relating to the Stanford and Mount Sinai license agreements versus $24,048 for the comparable 2014 period, $12,469 for orphan drug development costs versus $0 for the comparable 2014 period, and ($4,200) for development of alternative drug supply with Stanford University versus $0 for the comparable 2014 period. The credit balance relates to a reclassification and capitalization of licensing fees paid during the first half of 2015.
General and Administrative Expenses
We incurred related party general and administrative expenses totaling $27,000 and $27,693 for the three months ended September 30, 2015 and 2014, respectively. This amount is attributable to the payments to our prior Chairman for services provided to us as a director.
We incurred $817,880 and $1,307,877 of other general and administrative expenses for the three months ended September 30, 2015 and 2014, respectively, a decrease of approximately 30%. Of the amounts for the three months ended September 30, 2015 versus the comparable 2014 period: $506,818 was incurred for wages, bonuses, vacation pay, severance, taxes and insurance for five employees plus our Chairman versus $589,051 for six employees for the 2014 comparable period and other expenses totaling approximately $284,000 paid to the Company’s former President and Chief Executive Officer; $200,796 for ongoing legal expenses versus $249,970; $43,616 was incurred for investor relations services versus $34,228; $51,051 was incurred for insurance versus $33,652; $0 was incurred for outside operations consulting services versus $291,876 based upon the Company either hiring the consultants or completing services provided by such consultants; $35,061 was incurred for travel expenses versus $41,892; $22,521 was incurred for professional fees associated with auditing, financial, accounting and tax advisory services versus $31,608; and $38,514 was incurred for office rent and utilities, supplies, license fees, filing costs, rent, advertising and other, mostly relating to office facilities in New Jersey offset by a credit of $80,497 relating to a settlement of a payable to a service provider versus $35,600.
Stock Based Compensation Expenses
We incurred related party non-cash expenses totaling $44,299 versus $74,302 for the three months ended September 30, 2015 and 2014, respectively, in connection with the issuance of stock options, and we incurred $97,913 and $520,087 of non-related party non-cash expenses for issuance of stock options for the three months ended September 30, 2015 and 2014, respectively. During the three months ended September 30, 2014, several new stock options awards were granted with immediate vesting provisions.
Other Income
We earned $355 of net interest income for the three months ended September 30, 2015 versus $2,707 for the three months ended September 30, 2014 on funds temporarily deposited in an interest bearing money market account. This decrease is attributable to the reduction in cash balances.
| 55 |
Net loss and earnings (losses) per share
We incurred losses of $1,891,949 and $3,141,608 for the three months ended September 30, 2015 and 2014, respectively. The decreased loss was primarily due to decrease in head count during the second quarter of 2015 and decreased non-cash stock compensation expenses. Earnings (losses) per common share including dividends accruable on Series A Preferred Stock were ($0.08) and ($0.16) for the three months ended September 30, 2015 and 2014, respectively. The decrease in losses coincides with a decrease in losses and an increase in weighted average common shares outstanding.
Financial Condition, Liquidity and Capital Resources
Since the inception of Neurotrope BioScience, we have primarily devoted our efforts to the development of our therapeutic products, raising capital, negotiating the BRNI License and, until June 30, 2015, the former AD Diagnostic Test toward commercialization while conducting business planning and recruiting executive management.
Cash and Working Capital
Since inception, we incurred negative cash flows from operations. As of September 30, 2015, we had an accumulated deficit of $25,190,863 and had working capital of $292,490 as compared to working capital of $6,818,242 as of December 31, 2014. The $6,525,752 decrease in working capital was attributable to our expenditures relating principally to development of potential therapeutic products and potential former diagnostic product and general and administrative expenses, which resulted in a net loss of $6,908,174 and capital expenditures of $11,827 offset by non-cash expenses of $391,886 and proceeds from the exercise of common stock warrants of $2,363 for the nine months ended September 30, 2015.
Sources and Uses of Liquidity
Since inception, we have satisfied our operating cash requirements from the private placement of Series A Preferred Stock sold principally to outside investors.
In February 2013, through a private placement, we issued 9,073,300 shares of Series A Preferred Stock at $1.00 per share, resulting in gross proceeds of $9,073,300. In connection with the closing of the private placement, we paid a placement agent $882,330. In May 2013, we issued an additional 1,313,325 shares of Series A Preferred Stock at $1.00 per share, resulting in gross proceeds of $1,313,325, on which we paid a placement agent $131,332. In August 2013, through an additional private placement, we issued 11,533,375 of Series A Preferred Stock at $1.00 per share, resulting in gross proceeds of $11,533,375. In connection with the closing of the August 2013 private placement, we paid a placement agent $1,103,338. Further, Neurotrope BioScience became a wholly-owned subsidiary of a publicly traded company in the Reverse Merger. In October 2013, through an additional private placement, we issued 1,080,000 of Series A Preferred Stock at $1.00 per share, resulting in gross proceeds of $1,080,000. In connection with the closing of the private placement, we paid a placement agent $108,000.
| 56 |
On November 13, 2015, we entered into a Securities Purchase Agreement to sell up to 26,234,940 units in a private placement at a per unit purchase price equal to $0.60. Each unit consisted of (i) one one-hundredth share of Series B Stock convertible into one share of our common stock, (ii) one warrant to acquire, at an exercise price of $0.80 per share with an expiration date five years from the date of issuance, one share of our common stock (the “Series A Warrant”), (iii) one warrant to acquire, at an exercise price of $0.80 per share with an expiration date of one year from the date of issuance, one share of our common stock (the “Series B Warrant”), (iv) one warrant to acquire, at an exercise price of $1.25 per share with an expiration date of five years from the issuance date, one share of our common stock (the “Series C Warrant”), (v) one warrant, which is contingent upon the exercise of the Series B Warrant, to acquire, at an exercise price of $1.00 per share with an expiration date that is five years from the date of the initial exercise of the Series B Warrant, one share of our common stock (the “Series D Warrant”), and (vi) one warrant, which is contingent upon the exercise of the Series C Warrant, to acquire, at an initial exercise price of $1.50 per share with an expiration date that is five years from the date of the initial exercise of the Series C Warrant, one share of our common stock (the “Series E Warrant”, and together with the Series A Warrant, the Series B Warrant, the Series C Warrant and the Series D Warrant, the “Investor Warrants”). The exercise prices of the Investor Warrants are initially subject to full protection for dilutive issuances. The Series A Warrant and Series B Warrant each contain a mandatory exercise right of ours to force exercise of the warrant if our common stock trades at or above $1.50 for 20 consecutive trading days (subject to certain conditions, including a $150,000 minimum daily volume requirement). The Series C Warrant contains a mandatory exercise right of the Company to force exercise of the warrant if our common stock trades at or above $2.00 for 20 consecutive trading days (subject to certain conditions, including a $150,000 minimum daily volume requirement). In addition, pursuant to the terms of the Securities Purchase Agreement, we may sell, in one additional closing on the same terms and conditions as those contained in the purchase agreement, additional units to one or more buyers (each, an “Additional Investor”), each of which is either (a) an institutional investor that focuses on the biotech industry, (b) an investor of our Series A Stock, (c) any investor investing under $5,000 or (d) any person approved in writing by each of those selling stockholders who are institutional investors and such selling stockholder’s purchase price (together with such selling stockholder’s institutional affiliates) equals or exceeds $1,000,000 (the “Large Buyers”). The Large Buyers also have certain consent rights with respect to any Additional Investor. In connection with the private placement, the holders of our Series A Stock consented to convert their holdings into common stock. The closing of the private placement was subject to customary closing conditions and was completed in two closings, which took place on November 13, 2015 and November 30, 2015. The gross proceeds from the closing of the initial portion of the private placement were approximately $15,330,000 and from the second portion of the private placement were $311,000.
As of January 6, 2016, we had cash and cash equivalents totaling approximately $11.2 million. Management believes we may have sufficient capital to fund the Company through the next twelve months based upon our product development plans. However, as described in this prospectus, we are currently reviewing our strategic plans. We intend to adjust our operating plans, to the extent possible, by rationalizing non-contractual variable overhead expenses so that we have at least 12 months of cash for operations at the end of fiscal year 2015. In addition, we may choose to further reevaluate our strategic plan in the future (See “Going Concern” below).
| 57 |
Immediately following the Reverse Merger, the business of Neurotrope BioScience became our business. Neurotrope BioScience was formed for the primary purpose of commercializing the technologies initially developed by BRNI for therapeutic or diagnostic applications for AD or other cognitive dysfunctions.
History
As described in the Prospectus Summary above under the heading “Organizational History,” we were incorporated in Florida as BlueFlash Communications, Inc. on January 11, 2011. Prior to the Reverse Merger and Split-Off (each as defined above), our business was established to provide software solutions to deliver geo-location targeted coupon advertising to mobile internet devices. As a result of the Split-Off and the Reverse Merger, the Company discontinued its pre-Reverse Merger business and acquired the business of Neurotrope BioScience.
Our authorized capital stock currently consists of 300,000,000 shares of common stock, par value $0.0001, and 50,000,000 shares of “blank check” preferred stock, par value $0.0001, 24,325,000 of which has been designated as Series A Preferred Stock and 333,333 of which has been designated as Series B Preferred Stock. Our common stock is quoted on the OTC Market (OTCQB) under the symbol “NTRP.”
Our principal executive offices are located at 50 Park Place, Suite 1401, Newark, New Jersey 07102. Our telephone number is 1-973-242-0005. Our website address is www.neurotropebioscience.com.
Neurotrope BioScience was incorporated on October 31, 2012, under the laws of the State of Delaware, for the primary purpose of commercializing the technologies initially developed by BRNI for therapeutic and diagnostic applications for AD and other cognitive dysfunctions. The Company has been principally focused on developing two product platforms, a drug candidate called bryostatin for the treatment of AD and our diagnostic test for AD, both of which are in the clinical testing stage.
On October 31, 2012, Neurotrope BioScience entered into the Technology License and Services Agreement, the BRNI License, with BRNI and its affiliate NRV II, LLC, pursuant to which it was granted an exclusive non-transferable license to certain patents and technologies required to develop our proposed products. On February 4, 2015, the parties entered into an Amended and Restated Technology License and Services Agreement that further amended and restated the BRNI License. (For additional information, see “Certain Relationships and Related Transactions—BRNI License.”) Neurotrope BioScience was formed for the primary purpose of commercializing certain technologies, which were initially developed by BRNI, for therapeutic or diagnostic applications for AD or other neurodegenerative disorders. These technologies have been under development by BRNI since 1999 and, up until March 2013, were financed by BRNI through significant funding from a variety of non-investor sources (which included not-for-profit foundations, the National Institutes of Health (which is part of the U.S. Department of Health and Human Services) and contributions from individuals). From March 2013 forward, development of the licensed technology has been funded principally through collaboration by BRNI with Neurotrope BioScience.
Bryostatin is a potent modulator of the enzyme protein kinase C epsilon (“PKCε”). In preclinical in vivo models, this effect has been shown to play an important role in slowing or reversing AD and restoring cognition, memory and motor skills.
Bryostatin modulates the same enzyme target used by the former diagnostic test for the detection of AD. We believe bryostatin may restore synaptic structures and functions damaged by AD, leading to improvements in cognition and memory. Beyond AD, several other neurodegenerative diseases, such as ischemic stroke, traumatic brain injury, Fragile X mental retardation, depression and aging in the brain, may be amenable to treatment with the same approach.
BRNI is conducting an enhanced access program of Bryostatin-1 in patients with advanced AD. Thus far, three patients with advanced AD have been treated, two of which were treated under an IND application cleared by the FDA which is held by BRNI. The study for one of these patients concluded in 2014 and the other patient is still being treated after six months on the protocol. On the basis of communication from caregivers and treating physicians, BRNI, with our support, has decided to enroll additional patients in the extended access program. We are providing funding and personnel support under the terms of our agreement with BRNI for a modest expansion of this clinical effort in AD in 2015.
| 58 |
On July 29, 2014, we announced that we initiated our Phase 2a clinical trial to evaluate bryostatin for the treatment of patients with AD. The trial was being conducted under an IND application filed by BRNI. BRNI transferred its rights and obligations arising under such IND application to us on February 4, 2015. We enrolled a total of nine patients in the randomized, double-blind, placebo-controlled, single dose study. Six patients were randomized to receive bryostatin by injection and three received a matching placebo control. The primary objective of the clinical trial was to assess the safety and tolerability of a single dose of bryostatin in the treatment of patients with AD. The secondary objectives of the study were the preliminary evaluation of the efficacy of a single dose of bryostatin in the treatment of patients with AD, its pharmacokinetics and pharmacodynamics, and the evaluation of any correlation between changes in PKCε with plasma levels of bryostatin and with improvement in cognitive function. On February 24, 2015, we announced that the Phase 2a clinical trial met its primary endpoint demonstrating preliminary safety and tolerability of bryostatin. On March 17, 2015, we announced that preliminary assessment of PKCε levels in peripheral monocytes demonstrated a significant increase in total PKC protein levels at the end of the bryostatin infusion consistent with target engagement. An additional secondary objective of the study was the evaluation of efficacy following a single dose of bryostatin; there was no measurable improvement in cognition in this mildly impaired patient population.
In addition to bryostatin for AD, we intend to pursue development of selected other technology platforms with applications related to the treatment of AD and other neurodegenerative disorders based on our current licensed technology or technology available from third party licensors or collaborators.
AD and the Potential Market for our Products
The Epidemic of AD
According to the Alzheimer’s Association, it was estimated that 36 million people worldwide had AD in 2010. The prevalence of AD is independent of race, ethnicity, geography, lifestyle and, to a large extent, genetics. The most common cause of developing AD is living a long life. In developing countries where the median age of death is less than 65 years old, AD is rarely recognized or diagnosed. In the U.S. in 2014, 5.2 million people are estimated to have AD, and approximately 96% of these people are older than 65 years of age.
Researchers continue to explore a wide range of drug mechanisms in hopes of developing drugs to combat this disease. Figure 1 illustrates the range of mechanisms under consideration. Our approach, which involves the activation of the enzyme PKCε, represents a novel mechanism in the armamentarium of potential AD drug therapies.
Figure 1. Different Pharmacologic Targets being pursued for the Treatment of AD1
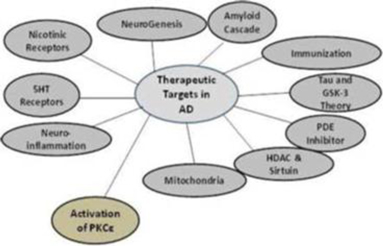
It has been shown that, during several years preceding the diagnosis of dementia associated with AD, there is a gradual cognition decline, which at first may have rather benign characteristics. Entering the mild cognitive impairment (“MCI”) phase of the disease marks progression of AD to the point where there is a significant loss of synapses (the junctions between nerve cells) preventing effective neurotransmission (Figure 2). This precursor phase transitions into mild, moderate and, finally, severe stages of the disease that are characterized by greater systemic loss of neurons in the brain tissue.
1 Business Insights: Reference Code B100040-005, Publication Date May 2011, “Advances in AD Drug Discovery”
| 59 |
Figure 2. Early Diagnosis of AD is Essential to Effective Treatment2
This progressive degeneration produces some abnormalities in the brain’s neurotransmitter systems. Multiple failures in acetylcholine and glutamate neurotransmitter systems (neurotransmitters) appear to underlie some of the symptomology of AD, and thus these systems have become targets for pharmacologic intervention.
The loss of neuronal function and neuronal cell death is also related to the abnormal processing of β amyloid (“Aβ”) peptide, ultimately leading to the formation of Aβ plaques (protein deposits) in the brain. As illustrated in Figure 2, this amyloid load in the brain usually becomes marked before the symptoms of the MCI phase appear in AD patients.
The conventional amyloid cascade hypothesis holds that amyloid pathology leads to tau proteins (a protein found in nerve cells) being deposited within neurons in the form of insoluble tangles, excitotoxicity (overstimulation of nerve cells by neurotransmitters), inflammation and finally synaptic depletion and neuronal death. The majority of drug development efforts to date have focused on stopping the production of Aβ or its fragments, and the elimination of these peptides from either intracellular or extracellular locations has represented the preponderance of drug design efforts to halt the progression of AD. However, these efforts have been largely unsuccessful.
We believe the current failures of therapies clearing formed amyloid come from an incorrect view of the process. In our view, amyloid plaques are one of the pathologic hallmarks of AD, but cognitive deficits and synaptic loss can often occur in AD patients in the absence of amyloid plaques. We believe the appearance of these plaques is not necessarily linked to the death of neurons or synapses, and that the elimination of the plaques does not restore cognitive function as already demonstrated in extensive clinical testing with pathologic correlates. However, we believe that the soluble amyloid pre-plaque oligomers do appear to be important in the progression of the disease.
In animal studies, BRNI found that PKCε activation in neurons targets the loss of synapses in the brains of animals with AD, and can delay or temporarily arrest other elements of the disease, i.e. the elevation of the toxic Aβ peptide, the loss of neurons, the appearance of plaques, and the loss of cognitive function.
Potential Market for Our Products
According to an article titled “Progress in AD” published in The Journal of Neurology in 2012, there has been a dearth of new product introductions in the last 20 years either for the treatment of AD symptoms or its definitive diagnosis in patients who begin exhibiting the memory and cognitive disorders associated with the disease. According to the Alzheimer’s Association, all of the products introduced to date for the treatment of AD have yielded negative or marginal results with no long-term effect on the progression of AD and no improvement in the memory or cognitive performance of the patients receiving these therapies. With 36 million people worldwide estimated to have had AD in 2010, there is significant commercial potential for a new therapeutic that is effective in delaying the progression of the disease.
We believe the markets for drugs or therapies to treat and analyze AD exist exclusively in the developed world and principally comprise the North American, European and Japanese markets. The aggregate AD market is subdivided into four distinct segments, which are shown in Figure 3, as are the projected compounded annual growth rates (“CAGRs”) for these segments over the 2009-2014 timeframe.
2 Lancet Neurol. 2010;9,119. CR Jack et al, “ Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade”
| 60 |
Sales of the major drug therapies available only by prescription are reported in Figure 3, which includes, among others, the acetylcholinesterase inhibitors (Exelon®, Razadyne®, and Aricept®) and the glutamate antagonist, Namenda®.3 These drugs are approved for the symptomatic treatment of the cognitive aspects of AD, but have no meaningful effect on disease progression, giving only temporary improvement in cognitive decline. Despite their limited efficacy, this group of drugs had collective worldwide sales in 2011 in excess of approximately $6 billion, according to a BBC Research Report. The negative CAGR for this segment reflects the fact that this class of drugs faces generic competition over the timeframe considered.
Figure 3. Global Market for AD ($ mm): 2009-20144
| Market Segments | 2009 | 2014 | CAGR% 2009-14 | |||||||||
| Prescription Drugs for AD | 5,947 | 5,211 | -2.6 | % | ||||||||
| Diagnostics / Biomarkers | 1,164 | 2,855 | 19.6 | % | ||||||||
| Therapeutics for Treatment of Symptoms | 567 | 726 | 5.1 | |||||||||
| Imaging | 361 | 852 | 18.7 | |||||||||
| Total | 8,039 | 9,644 | 3.7 | |||||||||
The “Therapeutics for Treatment of Symptoms” category cited in Figure 3 represents drugs from other classes that are being used to temporarily treat some of the symptoms of AD.5
Neurotrope’s Proposed Products
Challenges in Treating AD
One of the challenges in treating AD is that its symptoms become manifest only years after the disease process has actually commenced. Treatment strategies attempting to intervene once symptoms become apparent are focused on stimulating the neurotransmitter activity of still healthy neurons, or removing the amyloid plaque deposited in the brain. All drug development efforts to date that have targeted the removal of beta-amyloid or tau protein as their therapeutic mechanism of action have failed, and drugs approved for stimulating neurotransmitter activity offer short-lived, palliative results for AD patients. As such, these strategies have yielded negative or marginal results with no effect on the progression of AD and no improvement in the memory or cognitive performance of the patients receiving these therapies.
Dead or dying neurons cannot be returned to function, and many in the AD field currently believe that stemming the progression of the disease may only be possible with very early stage intervention. The FDA is encouraging the pharmaceutical industry to increase efforts to investigate such early stage interventional treatments by recommending that modified clinical endpoints, both functional and cognitive, be established to monitor the efficacy of drug prototypes being tested in early stage AD patients, according to an article published in The New England Journal of Medicine.6
In contrast, we believe that our data from various preclinical animal models demonstrates that activation of PKCε in central nervous system neurons improves neuronal vitality and function in areas of the brain damaged by AD, resulting in the improvement of memory and cognition.
3 Exelon is a registered trademark of Novartis AG Corporation; Razadyne is a registered trademark of Johnson & Johnson Corporation; Aricept is a registered trademark of Eisai R&D Management Co., Ltd. Corporation; and Namenda is a registered trademark of Merz Pharma GmbH & Co.
4 BCC Research Report PHM062A AD Therapeutics and Diagnostics: Global Markets, January 2010. Available at http://www.bccresearch.com/market-research/pharmaceuticals/alzheimers-disease-therapeutics-phm062a.html.
5 See footnote 3.
6 NEJM.org: The New England Journal of Medicine, March 15, 2013, page 1: Drug Development of Early AD, N. Kozauer, M.D., and Russell Katz, M.D.
| 61 |
Synaptogenesis
We believe that deficient activity or low concentrations of PKCε in aging subjects is one of the main causes of the neurodegeneration seen in AD. The schematic in Figure 4 illustrates only a portion of the changes mediated by PKCε, and how it may help reverse the neuronal damage and loss central to the pathogenic process in AD.
Figure 4. PKCε Activation Involves 5 Different Mechanisms to Stop the Progression of AD7
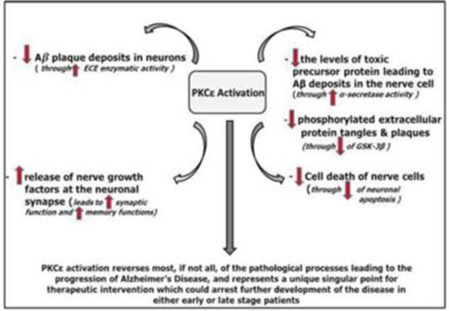
Activation of PKCε has been achieved with drug prototypes that mimic the activity of diacylglycerol and phosphatidylserine, which are the natural binding targets for this enzyme. In addition, a variety of in vitro and in vivo animal models have demonstrated that these drug prototypes are effective in restoring the structure and function of neuronal synapses. Our first clinical application of the PKCε activators is focused on the treatment of AD, but a number of other neurodegenerative diseases may be amenable to similar treatment. A list of these potential future drug targets is shown in Figure 5.
Figure 5. Therapeutic targets for neuroregeneration through PKCε activation
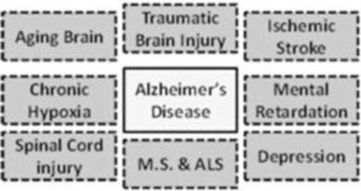
Treatment of AD by Stimulating Synaptic Regeneration and Prevention of Neuronal Death
BRNI’s research program in this area lies outside the conventional wisdom that has dominated research efforts in the industry. The pathology of AD likely has multiple layers in its development, and the accumulating presence of tau phosphorylated tangles and Aβ are causative factors in the poisoning of neurons and the resultant cognitive and memory disorders. However, once this process presents clinical manifestations of AD, restoring synaptic function thus far has not been effectively achieved by removing Aβ plaques with experimental drug interventions. Once neurons are poisoned with Aβ, the loss of function to the patient has been irreversible.
7 Based on unpublished BRNI research.
| 62 |
BRNI’s and our approach is to restore general viability and hence synaptic function in still-functioning neurons by stimulating the regeneration and growth of the dendritic branches in these neurons. (Dendrites are the branched projections of a neuron that act to propagate the electrochemical stimulation received from other neural cells.) This process can be visualized at the microscopic level in the neuronal cells of rats whose neurons have been damaged by ischemic shock (depriving oxygen) or traumatic injury to the brain. The morphology of the damaged neurons in these animal models looks strikingly different after they are treated with experimental drugs that activate PKCε. The new growth of dendritic trees on the damaged neurons creates a multiplicity of new synaptic connections, basically re-wiring the damaged neurons and restoring their function. Earlier therapeutic intervention with a PKCε activator produces better outcomes in tests measuring restored animal cognitive function.
PKCε Activation Stimulates the Formation of New Synaptic Connections
The new synaptic connections formed from the damaged neurons in rats can be demonstrated in various behavioral models for the animals that are used to measure memory functions. Treatment with bryostatin, for 12 weeks in genetically modified rodents pre-disposed to develop an AD-type of pathology showed that bryostatin promoted the growth of new synapses and preserved the existing synapses. In addition, this drug also stopped the decrease of PKCε and the increase of soluble amyloid.8
In cell tissue cultures, the difference in morphology between neurons damaged by the application of ASPD (a modified form of Aβ) as compared to neurons activated by the application of bryostatin + retinoic acid (a metabolite of vitamin A) is seen in Figure 6. Treatment with bryostatin, through PKCε activation, stimulates the regeneration of neurons and the formation of new synaptic connections.
Figure 6. Synaptogenesis in Hippocampus Neurons9
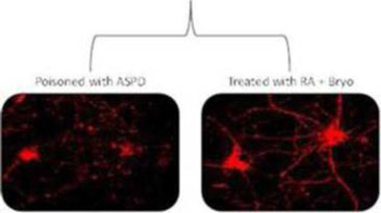
The Central Role of PKCε in Maintaining Neuron Structure and Function
Upon activation, PKCε migrates from the intracellular fluid to the cell membrane, where it activates signal-regulating enzymes (specifically the MAP kinases Erk1/2 and NF-κβ), causing a series of changes leading to increased DNA transcription, synaptic maturation, a consequent increase in levels of growth factor proteins (such as nerve growth factor and brain-derived neurotrophic factor), an inhibition of programmed cell-death and a reduction of β amyloid.
This myriad of events is orchestrated by PKCε, and prompts a number of secondary events occurring in both the pre- and post-synaptic portions of the neuron. Cellular visualization of this effect shows an increase in the number of pre-synaptic vesicles in the neurons, an increase in pre-synaptic levels of PKCε and an increase in the number of mushroom spines associated with individual synaptic boutons (knoblike enlargements at the end of a nerve fiber, where it forms a synapse), which spines may be important in memory. Their genesis in these neurons is responsible for the formation of new synapses.
8 Journal of Neuroscience 2011, 31 (2), 630, D. Alkon et al.
9 Based on unpublished BRNI research.
| 63 |
The central role of PKCε activation in these dynamic events does not contradict the amyloid hypotheses for AD, but offers an alternative target for therapeutic intervention which could prevent the formation of tangles and plaque.
Decreased amyloid formation from PKCε activation results from an increase in the rate of Aβ degradation by ECE (endothelin converting enzyme) and induction of αsecretase cleavage of amyloid precursor protein (the precursor molecule to Aβ) through phosphorylation of an enzyme known as Erk. In rodent models genetically predisposed to forming large amounts of amyloid deposits in their brains, PKCε activation was found to interrupt the ongoing formation of amyloid, suggesting that this approach may delay the progression of AD.
The key to BRNI’s innovation in this area has been in identifying highly potent drug prototypes that at low concentrations cause the specific and transient activation of PKCε, without interacting with the other isozyme variants of PKC whose inactivation would negate the synaptogenic properties of the e isoform.
Testing PKCε Activation in Humans
The basic drug mechanism invoking PKCε activation for neuronal regeneration has never been evaluated in man for any drug class or therapeutic application. We believe that the research in this field as described above is an ideal platform for testing this approach in human subjects.
We have licensed a body of biomedical research from BRNI that is comprised of new methods and drug prototypes designed to stimulate neuronal regeneration. For additional information, see “Certain Relationships and Related Transactions—BRNI License.” We believe the commercial application of this technology has potential to impact AD as well as traumatic brain injury, ischemic stroke, post-traumatic stress syndrome and learning disorders.
Drug Prototypes That Treat AD through Regeneration
BRNI has developed a new chemical family of polyunsaturated fatty acid (“PUFA”) analogs, which appear to be effective in the activation of PKCε. Representative structures of bryostatin and a lead PUFA analog are shown in Figure 7.
Figure 7. Structures of Bryostatin 1 and a PUFA Analog Effective in the Activation of PKCε10
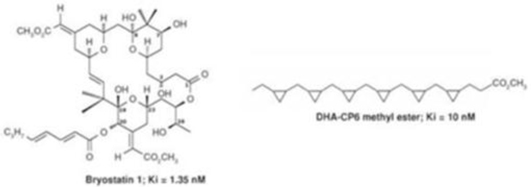
Ki values = effective concentration of the drug in achieving 50% activation of PKCε
These molecules activate PKCε by binding to two different and distinct active sites on the enzyme. The natural ligands that bind to these sites are diacylglycerol and phosphatidylserine. Bryostatin acts as a mimetic (mimic) for diacylglycerol by binding to the diacylglycerol site and, similarly, the PUFA analogs act as mimetics for phosphatidylserine by binding to the phosphatidylserine site.
Part of the hierarchal array of in vitro and in vivo tests useful in optimizing the potency of our potential drug prototypes is displayed in Figure 8.
10 Trends in Biochemical Sciences V. 34, #3, p.136. T.J. Nelson et al, “ Neuroprotective versus Tumorigenic protein kinase C activators”
| 64 |
Figure 8. Optimization of PKCε Activation Effects in Lead Drug Candidates: Array of in vitro and in vivo Test Models11
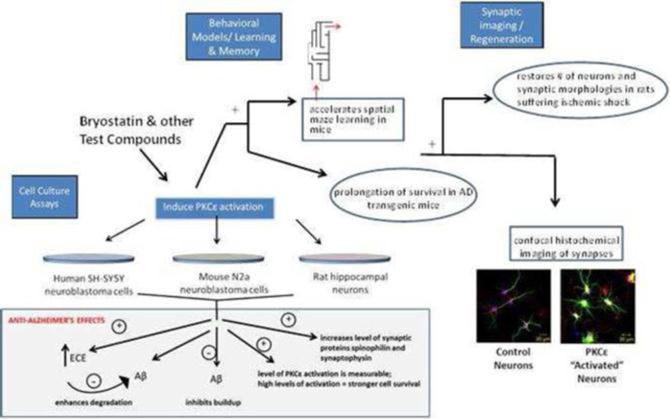
Bryostatin
Our lead product candidate is bryostatin. Bryostatin is a natural product isolated from a marine invertebrate organism, a bryozoan called Bugula neritina. Several total syntheses of this complex molecule have been achieved in recent years in various academic chemistry laboratories, and these approaches represent a possible alternative source of this drug. Bryostatin is a PKCα and e activator that was originally developed as a potential anticancer drug. According to Clinical Cancer Research, this drug candidate was previously evaluated in 63 clinical studies involving more than 1,200 patients at the NCI for the treatment of various forms of cancer. While having failed these studies as an experimental anti-cancer therapy, much useful information on the safety, pharmacodynamics and toxicity of the drug was obtained from these in-human trials.
It was discovered that at a much lower dose than what was used in these anticancer trials, bryostatin is a potent activator of PKCε and may have efficacy in treating AD. As described above, activation of PKCε has now been shown to partially restore synaptic function in neurons damaged by AD in in vitro and in vivo animal models.
The NCI has entered into a material transfer agreement with BRNI to provide the bryostatin required for pre-clinical research as well as the Phase 2 clinical trials planned by the Company. The clinical material transfer agreement specifies that BRNI retains all of the bryostatin intellectual property. Our license agreement with BRNI (see “Certain Relationships and Related Transactions—BRNI License”) permits our access to new bryostatin clinical trial data and information held by the NCI, as well as past clinical, safety and toxicity data compiled by the NCI during the time this drug was being evaluated for its anticancer properties. See “Risk Factors—We are dependent upon the NCI to supply bryostatin for our clinical trials.”
BRNI previously conducted an exploratory evaluation of bryostatin on a compassionate use basis in AD patients who have an inherited form of AD, frequently called familial AD, under an FDA-approved study protocol. Familial AD results from one of four major mutations in the genome, and this mutation is passed on from generation to generation within a family that carries the defective gene. The tragic consequence of familial AD is that it strikes its victims at an early age, often while they are in their twenties. The aggressive progression of familial AD can render these patients in the terminal stages of AD in their late 30s and early 40s.
11 Based on unpublished BRNI research.
| 65 |
Effective November 13, 2013, we agreed to a statement of work with BRNI pursuant to which we contracted for the further development of our potential therapeutic product. Pursuant to this statement of work, we paid BRNI $251,939 for related personnel and research services. BRNI completed the services pursuant to this statement of work in 2014.
As of March 12, 2014, we entered into a statement of work with BRNI to continue pre-clinical activities relating to the commercialization of our therapeutic product. We paid BRNI the entire total pursuant to this statement of work of approximately $465,000 during the year ended December 31, 2014. BRNI completed the services pursuant to this statement of work in 2014.
Bryologs
On May 12, 2014, we entered into a license agreement (the “Stanford License”) with The Board of Trustees of the Leland Stanford Junior University (“Stanford”) pursuant to which Stanford has granted to us a revenue-bearing, world-wide right and exclusive license, with the right to grant sublicenses (on certain conditions), under three issued U.S. patents and one pending U.S. patent and related technology for the use of bryostatin structural derivatives, known as “bryologs,” for use in the treatment of central nervous system disorders, lysosomal storage diseases, stroke, cardioprotection and traumatic brain injury, collectively referred to as the Licensed Field of Use, for the life of the licensed patents.
As mentioned above, our initial drug candidate, bryostatin, is a natural product isolated from a marine invertebrate organism, a bryozoan called Bugula neritina. However, it takes large quantities of biomass harvested from the oceans to produce even small quantities of bryostatin, and supply is limited.
Stanford researchers have synthesized a large family of bryologs over a number of years as part of a research program to define the essential molecular features critical to bryostatin’s biological activity. The bryologs are easier to produce than bryostatin due to their less complex chemical structures. They represent a collection of potential drug candidates, some of which we expect to advance to clinical trials for the treatment of several neurodegenerative diseases such as ischemic stroke, Fragile X Syndrome, traumatic brain injury and AD, although there can be no assurance that we will be successful in doing so. We plan to advance a bryolog drug candidate into clinical development in the 2016 timeframe.
We are required by the Stanford License to use commercially reasonable efforts to develop, manufacture, and sell products (“Licensed Products”) in the Licensed Field of Use. In addition, we must meet specific diligence milestones, and upon meeting such milestones, make specified milestone payments to Stanford. We will also pay Stanford royalties on net sales (as defined in the Stanford License), if any, of Licensed Products.
Stanford retains the right, on behalf of itself and all other non-profit research institutions, to practice the licensed patents and use the licensed technology for any non-profit purpose, including sponsored research and collaborations. The license is also subject to Title 35, Sections 200-204, of the United States Code, which governs patent rights in inventions made with U.S. government assistance. Among other things, these provisions provide the United States government with nonexclusive rights in the licensed patents. They also impose the obligation that product based on the licensed patents sold or produced in the United States be “manufactured substantially in the United States.”
PUFA Analogs
Several other drug prototypes termed the PUFA analogs have been synthesized at BRNI and evaluated for their PKCε activating properties in models of AD. The PUFA analogs are not structurally related to bryostatin and activate PKCε at a different site. We believe the PUFA analogs represent a potential source for follow-on drug candidates. PKCε activators from the PUFA family of drug prototypes have demonstrated neuroregeneration efficacy roughly equivalent to that of bryostatin. If the PUFA analogs show adequate potency in preclinical models of AD, we would plan to advance a drug prototype from this chemical family.
Other Potential Products
We may acquire, by license or otherwise, other development stage products that are consistent with our product portfolio objectives and commercialization strategy.
| 66 |
Discontinued Research
We had planned to develop two other lines of research related to learning and memory disorders: (i) drug prototypes that activate or inhibit the enzyme carbonic anhydrase to modulate the attention status of animals, which may have had applications for attention deficit disorder and post-traumatic stress disorder, and (ii) generalizing the application of a blood-brain-barrier delivery system to a variety of drugs through a contract research service to be offered to other pharmaceutical companies seeking to improve the penetration of their drug prototypes into the brain.
We have decided, however, to focus our efforts on neurodegenerative diseases, which are the most advanced programs in our portfolio, and therefore will not be pursuing either the drug candidate for activating carbonic anhydrase or the blood-brain-barrier delivery system.
We also relinquished rights to the AD diagnostic system under the terms of the February 2015 SOW (see below).
February 2015 SOW
On February 4, 2015, we entered into a Statement of Work and Account Satisfaction Agreement with BRNI (the “February 2015 SOW”), which was effective as of October 1, 2014 and continues until September 30, 2015.
Pursuant to the February 2015 SOW, we agreed, among other things, to pay BRNI twenty thousand dollars ($20,000) in quarterly payments during the twelve months from the date of the February 2015 SOW in exchange for advising and consulting services by BRNI’s chief scientist regarding our contract with Icahn School of Medicine at Mt. Sinai Hospital for the use of bryostatin in the treatment of Niemann-Pick Type C disease.
Under the February 2015 SOW, Neurotrope BioScience also agreed to pay BRNI two million four hundred thousand dollars ($2,400,000) in service fees and other amounts payable at a rate of two hundred thousand dollars ($200,000) per month for each month from October 1, 2014 through September 30, 2015. The parties agreed that the first six hundred thousand dollars ($600,000) of payments satisfy certain outstanding amounts owed to BRNI. In consideration for the February 2015 SOW, in addition to the terms described above, BRNI also agreed to (a) use commercially reasonable efforts to enroll, at no cost to Neurotrope BioScience, at least four (4) additional compassionate use or expanded access patients, in trials of BRNI’s Alzheimer’s therapeutic drug platform during the term of the February 2015 SOW, (b) perform certain services requested by Neurotrope BioScience for the further development of BRNI’s Alzheimer’s therapeutic drug platform, (c) perform certain services for the further development of BRNI’s Alzheimer’s diagnostic test, (d) to the extent permitted by applicable law, transfer all of its rights and regulatory obligations, except for those relating to the compassionate use expanded access trials, associated with BRNI’s IND 71,276 to Neurotrope BioScience, (e) conduct initial research on the application of its PKC epsilon platform to treat Fragile X disease, along with various other terms and conditions, (f) conduct initial research on PUFA derivatives for the purpose of developing a commercially usable PKCε activator and (g) provide assistance, advice and other similar services to us regarding our analysis of bryologs pursuant to our agreement with Stanford University, for the purpose of developing a commercially usable PKCε activator. Furthermore, BRNI agreed to transfer a certain amount of bryostatin drug substance and bryostatin kits containing drug substance for non-human use to a third-party for storage. In order for BRNI to perform certain of the services described in (c) above, Neurotrope BioScience reimbursed a third party for services BRNI received from such third party in the amount of $150,000 in connection with BRNI’s former diagnostic trial program with such third party. Also, under certain conditions, Neurotrope Bioscience has agreed to enter into an agreement with a specified research institution for the analysis of tissue samples and autopsy results for a validation trial as part of the development process for the diagnostic test. If Neurotrope Bioscience does not proceed with a certain portion of such agreement by June 30, 2015, then the rights to the diagnostic test will revert to BRNI and Neurotrope Bioscience will no longer be licensed thereto.
Services Agreement
On October 9, 2015, Neurotrope BioScience, Inc., our wholly-owned consolidated operating subsidiary, executed a Services Agreement with Worldwide Clinical Trials, Inc., or WCT, effective as of August 31, 2015. The Services Agreement relates to services for Neurotrope BioScience’s Phase 2b clinical study assessing the safety, tolerability and efficacy of bryostatin in the treatment of moderately severe to severe AD. Pursuant to the terms of the Services Agreement, WCT will provide services to enroll approximately one hundred and fifty (150) study subjects at approximately 30 sites across the United States. Depending upon our resources, we intend to begin enrollment at the initial sites by the end of 2015. Assuming prompt enrollment at the majority of the sites, we expect that within approximately 15 months, we will receive data from an interim analysis with final top line results available in approximately 18 months from the start of enrollment. This trial is designed to administer dosing of bryostatin for up to six months for moderately severe to severe AD patients. Among the trial’s endpoints are the measurement of improvement in cognition, activities of daily living and behavior. The Company’s goal is to show the robust treatment effect that the regulatory agencies, the marketplace and, most importantly, our patients and their caregivers are seeking.
| 67 |
Intellectual Property
Technology License and Services Agreement
On February 4, 2015, Neurotrope BioScience, BRNI and NRV II, LLC entered into an Amended and Restated Technology License and Services Agreement (the “BRNI License”), which further amended and restated the Technology License and Services Agreement dated as of October 31, 2012, as amended by Amendment No. 1 dated as of August 21, 2013.
Pursuant to the BRNI License, Neurotrope BioScience maintained its exclusive (except as described below), non-transferable (except pursuant to the BRNI License’s assignment provision), world-wide, royalty-bearing right, with a right to sublicense (in accordance with the terms and conditions described below), under BRNI’s and NRV II’s respective right, title and interest in and to certain patents and technology owned by BRNI or licensed to NRV II, LLC by BRNI as of or subsequent to October 31, 2012 to develop, use, manufacture, market, offer for sale, sell, distribute, import and export certain products or services for therapeutic and diagnostic applications for AD and other cognitive dysfunctions in humans or animals (the “Field of Use”). Additionally, the BRNI License specifies that all patents that issue from a certain patent application, shall constitute licensed patents and all trade secrets, know-how and other confidential information claimed by such patents constitute licensed technology under the BRNI License.
Notwithstanding the above license terms, BRNI and its affiliates retain rights to use the licensed intellectual property in the Field of Use to engage in research and development and other non-commercial activities and to provide services to Neurotrope BioScience or to perform other activities in connection with the BRNI License.
Under the BRNI License, Neurotrope BioScience may not enter into sublicense agreements with third parties except with BRNI’s prior written consent, which consent shall not be commercially unreasonably withheld. Furthermore, the BRNI License dated February 4, 2015 revises the agreement that was entered into as of October 31, 2012 and amended on August 21, 2013, in that it provides that any intellectual property developed, conceived or created in connection with a sublicense agreement that Neurotrope BioScience entered into with a third party pursuant to the terms of the BRNI License will be licensed to BRNI and its affiliates for any and all non-commercial purposes, on a worldwide, perpetual, non-exclusive, irrevocable, non-terminable, fully paid-up, royalty-free, transferable basis, with the right to freely sublicense such intellectual property. Previously, the agreement had provided that such intellectual property would be assigned to BRNI.
Pursuant to the terms of the November 12, 2015 amendment to the BRNI License, Neurotrope Bioscience will deliver to BRNI, following each closing pursuant to a certain securities purchase agreement, an amount equal to 2.5% of the Post-PA Fee Proceeds received at such closing. In addition, the amendment provides that on or prior to December 31, 2016, Neurotrope Bioscience shall deliver to BRNI an amount equal to 2.5% of the aggregate Post-PA Fee Proceeds received at the closings. Each payment would constitute an advance royalty payment to BRNI and will be offset (with no interest) against the amount of future royalty obligations payable until such time that the amount of such future royalty obligations equals in full the amount of the advance royalty payments made. “Post-PA Fee Proceeds” means the gross proceeds received, less all amounts paid to the placement agent(s), in relation to such gross proceeds. No other expenses of Neurotrope Bioscience shall be subtracted from the gross proceeds to determine the “Post-PA Fee Proceeds.”
Under the BRNI License, BRNI and Neurotrope BioScience will jointly own data, reports and information that is generated on or after February 28, 2013, pursuant to the license agreement dated October 31, 2012 and amended on August 21, 2013, by Neurotrope BioScience, on behalf of Neurotrope BioScience by a third party or by BRNI pursuant to a statement of work that the parties enter into pursuant to the BRNI License, in each case to the extent not constituting or containing any data, reports or information generated prior to such date or by BRNI not pursuant to a statement of work (the “Jointly Owned Data”). BRNI has agreed not to use the Jointly Owned Data inside or outside the Field of Use for any commercial purpose during the term of the BRNI License or following any expiration of the BRNI License other than an expiration that is the result of a breach by Neurotrope BioScience of the BRNI License that caused any licensed patent to expire, become abandoned or be declared unenforceable or invalid or caused any licensed technology to enter the public domain (a “Natural Expiration”) provided, however, BRNI may use the Jointly Owned Data inside or outside the Field of Use for any commercial purpose following any termination of the BRNI License. Also, BRNI granted Neurotrope BioScience a license during the term and following any Natural Expiration, to use certain BRNI data in the Field of Use for any commercial purposes falling within the scope of the license granted to Neurotrope BioScience under the BRNI License.
| 68 |
The BRNI License further requires us to pay BRNI (i) a fixed research fee equal to a pro-rata amount of $1 million in the year during which we close on a $25 million round of financing, (ii) a fixed research fee of $1 million per year for each of the five calendar years following the completion of such financing and (iii) an annual fixed research fee in an amount to be negotiated and agreed upon no later than 90 days prior to the end of the fifth calendar year following the completion of such financing to be paid with respect to each remaining calendar year during the term of the BRNI License.
The term of the BRNI License continues until the later of the date (i) the last of the licensed patents expires, is abandoned or is declared unenforceable or invalid (in each case, determined in accordance with the BRNI License) and (ii) the last of the licensed technology enters the public domain. BRNI has the right to terminate the BRNI License after 30 days prior notice in certain circumstances, including if we were to materially breach any provisions of the BRNI License and does not cure such material breach within 60-days from notice of such material breach from the non-breaching party, for breaches that are capable of being cured, in the event of certain bankruptcy or insolvency proceedings.
Our Licensed Intellectual Property
We have licensed from BRNI an extensive intellectual property portfolio that includes issued patents, pending patent applications and provisional patent applications, in the U.S. and elsewhere, which, we believe, together cover these key pharmaceutical markets. A method of use patent has been issued to BRNI that covers the use of the PUFA family of molecules for the same therapeutic applications.
We believe the BRNI License provides us rights to the patents and technologies required to develop our proposed products. The patents and technologies licensed to us pursuant to the BRNI License include, without limitation, the following:
| · | therapies based on bryostatin and PUFA chemical families; |
| · | methods for treating AD. |
A number of BRNI’s patent applications for treatment of neurological disorders have been under active prosecution for many years and have been the subject of multiple rejections for anticipation and/or obviousness based on prior art. There are no guarantees that BRNI’s pending patent applications will issue into commercially meaningful patents. If these patent applications are not approved or successfully prosecuted, then we will attempt to seek other means of protecting its proprietary position including, but not limited to, trade secrets, proprietary formulations and methods, etc.
A substantial amount of in-human data exists that was generated by the NCI that involves the earlier evaluation of bryostatin as an anticancer agent. The NCI also holds the existing inventory of the bryostatin drug product which is suitable for use in man. Our use of the substantial data package generated by the NCI on bryostatin, as well as access to the clinical supply of this substance, is permitted under a material transfer agreements entered into and between the NCI and BRNI.
There are no known patent conflicts or freedom to operate issues at this time which could encumber our ability to commercialize either the AD diagnostic system or the PKCε activators for the treatment of cognition and memory disorders. However, we cannot provide any assurance that such conflicts will not arise in the future. See the Risk Factors captioned “Our commercial success will depend, in part, on our ability, and the ability of our licensors, to obtain and maintain patent protection. Our licensors’ failure to obtain and maintain patent protection for our products may have a material adverse effect on our business.” and “Our licensed patented technologies may infringe on other patents, which may expose us to costly litigation.” under “Risk Factors”.
We also have the right to re-license certain patents and patent applications in certain jurisdictions that we had licensed under the BRNI License but had previously elected to relinquish. In the event that we decide to re-license any of such patents and/or patent applications, then we are required to reimburse BRNI for all of the attorneys’ fees, translation costs, filing fees, maintenance fees, and other costs and expenses related to such patents and/or patent applications that have been incurred since we elected to relinquish them under the BRNI License.
| 69 |
Governmental Regulation and Product Approval
The manufacturing and marketing of our potential products and our ongoing research and development activities are subject to extensive regulation by the FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries.
United States Regulation of Drugs
Before any drug product can be marketed in the United States, it must receive approval from the FDA. To receive this approval, any drug we develop must undergo rigorous preclinical testing and clinical trials that demonstrate the product candidate’s safety and effectiveness for each indicated use. This extensive regulatory process controls, among other things, the development, testing, manufacture, safety, efficacy, record keeping, labeling, storage, approval, advertising, promotion, sale, and distribution of pharmaceutical products.
In general, before any new pharmaceutical product can be marketed in the United States, the process typically required by the FDA includes:
| · | preclinical laboratory and animal tests; |
| · | submission of an IND, which must become effective before human clinical trials may begin; |
| · | adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug for its intended use; |
| · | pre-approval inspection of manufacturing facilities and selected clinical investigators; |
| · | submission of a New Drug Application (“NDA”) to the FDA; and |
| · | FDA approval of an NDA or an NDA supplement (for subsequent indications or other modifications, including a change in location of the manufacturing facility). |
Preclinical Testing
In the United States, drug candidates are tested in animals until adequate proof of safety and efficacy is established. These preclinical studies generally evaluate the mechanism of action and pharmacology of the product and assess the potential safety and efficacy of the product. Tested compounds must be produced according to applicable current good manufacturing practice requirements and preclinical safety tests must be conducted in compliance with FDA and international regulations regarding good laboratory practices. The results of the preclinical tests, together with manufacturing information and analytical data, are generally submitted to the FDA as part of an IND, which must become effective before human clinical trials may commence. The IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA requests an extension or raises concerns about the conduct of the clinical trials as outlined in the application. If the FDA has any concerns, the sponsor of the IND and the FDA must resolve the concerns before clinical trials can begin. Regulatory authorities may require additional preclinical data before allowing the clinical studies to commence or proceed from one phase to another, and could demand that the studies be discontinued or suspended at any time if there are significant safety issues. Furthermore, an independent institutional review board for each medical center proposing to participate in the conduct of the clinical trial must review and approve the clinical protocol and patient informed consent form before commencement of the study at the respective medical center.
Clinical Trials
Clinical trials for new drug candidates are typically conducted in three sequential phases that may overlap. In phase 1, the initial introduction of the drug candidate into healthy human volunteers, the emphasis is on testing for safety or adverse effects, dosage, tolerance, metabolism, distribution, excretion, and clinical pharmacology. Phase 2 involves studies in a limited patient population to determine the initial efficacy of the drug candidate for specific targeted indications, to determine dosage tolerance and optimal dosage and to identify possible adverse side effects and safety risks. Once a compound shows evidence of activity and is found to have an acceptable safety profile in phase 2 evaluations, pivotal phase 3 trials are undertaken to more fully evaluate clinical outcomes and to establish the overall risk/benefit profile of the drug, and to provide, if appropriate, an adequate basis for product labeling. During all clinical trials, physicians will monitor patients to determine effectiveness of the drug candidate and to observe and report any reactions or safety risks that may result from use of the drug candidate. The FDA, the trial sites institutional review board or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk.
| 70 |
The data from the clinical trials, together with preclinical data and other supporting information that establishes a drug candidate’s profile, are submitted to the FDA in the form of an NDA or NDA supplement (for approval of a new indication if the product candidate is already approved for another indication). Under applicable laws and FDA regulations, each NDA submitted for FDA approval is usually given an internal administrative review within 45 to 60 days following submission of the NDA. If deemed complete, the FDA will “file” the NDA, thereby triggering substantive review of the application. The FDA can refuse to file any NDA that it deems incomplete or not properly reviewable. The FDA has established internal substantive review goals of six months for priority NDAs (for drugs addressing serious or life threatening conditions for which there is an unmet medical need) and ten months for regular NDAs. The FDA, however, is not legally required to complete its review within these periods, and these performance goals may change over time. Moreover, the outcome of the review, even if generally favorable, is not typically an actual approval, but an “action letter” that describes additional work that must be done before the NDA can be approved. The FDA’s review of an NDA may involve review and recommendations by an independent FDA advisory committee. The FDA may deny approval of an NDA or an NDA supplement if the applicable regulatory criteria are not satisfied, or it may require additional clinical data and/or an additional pivotal phase 3 clinical trial. Even if such data are submitted, the FDA may ultimately decide that the NDA or NDA supplement does not satisfy the criteria for approval.
Data Review and Approval
Substantial financial resources are necessary to fund the research, clinical trials and related activities necessary to satisfy FDA requirements or similar requirements of state, local and foreign regulatory agencies. It normally takes many years to satisfy these various regulatory requirements, assuming they are satisfied. Information generated in this process is susceptible to varying interpretations that could delay, limit, or prevent regulatory approval at any stage of the process. Accordingly, the actual time and expense required to bring a product to market may vary substantially. We cannot assure you that we will submit applications for required authorizations to manufacture and/or market potential products or that any such application will be reviewed and approved by the appropriate regulatory authorities in a timely manner, if at all. Data obtained from clinical activities is not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. Success in early stage clinical trials does not ensure success in later stage clinical trials. Even if a product candidate receives regulatory approval, the approval may be significantly limited to specific disease states, patient populations and dosages, or have conditions placed on them that restrict the commercial applications, advertising, promotion or distribution of these products.
Once issued, the FDA may withdraw product approval if ongoing regulatory standards are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require testing and surveillance programs to monitor the effect of approved products which have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs. The FDA may also request additional clinical trials after a product is approved. These so-called phase 4 studies may be made a condition to be satisfied after a drug receives approval. The results of phase 4 studies can confirm the effectiveness of a product candidate and can provide important safety information via the FDA’s voluntary adverse drug reaction reporting system. Any products manufactured or distributed by us pursuant to FDA approvals would be subject to continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the drug. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with good manufacturing practices, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. We cannot be certain that we or our present or future suppliers will be able to comply with the good manufacturing practices regulations and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, require us to recall a drug from distribution or withdraw approval of the NDA for that drug. Furthermore, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market.
| 71 |
The FDA closely regulates the marketing and promotion of drugs. Approval may be subject to post-marketing surveillance and other record keeping and reporting obligations, and involve ongoing requirements. Product approvals may be withdrawn if compliance with regulatory standards is not maintained or if problems occur following initial marketing. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available drugs for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturers’ communications on the subject of such off-label use.
Fast Track Approval
The Federal Food, Drug, and Cosmetic Act, as amended, and FDA regulations provide certain mechanisms for the accelerated “Fast Track” approval of potential products intended to treat serious or life-threatening illnesses which have demonstrated the potential to address unmet medical needs. The procedures permit early consultation and commitment from the FDA regarding the preclinical and clinical studies necessary to gain marketing approval. Provisions of this regulatory framework also permit, in certain cases, NDAs to be approved on the basis of valid indirect measurements of benefit of product effectiveness, thus accelerating the normal approval process. In the future, certain potential products employing our technology might qualify for this accelerated regulatory procedure. Even if the FDA agrees that these potential products qualify for accelerated approval procedures, the FDA may deny approval of our drugs or may require additional studies before approval. The FDA may also require us to perform post-approval, or phase 4, studies as a condition of such early approval. In addition, the FDA may impose restrictions on distribution and/or promotion in connection with any accelerated approval, and may withdraw approval if post-approval studies do not confirm the intended clinical benefit or safety of the potential product.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process. If a product that has orphan drug designation subsequently receives FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same disease, except in very limited circumstances, for seven years. These very limited circumstances are (i) an inability to supply the drug in sufficient quantities or (ii) a situation in which a new formulation of the drug has shown superior safety or efficacy. This exclusivity, however, also could block the approval of our product for seven years if a competitor obtains earlier approval of the same drug for the same indication.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products in foreign countries. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Under European Union regulatory systems, we may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure, which is available for medicines produced by biotechnology or which are highly innovative, provides for the grant of a single marketing authorization that is valid for all EU member states. This authorization is a marketing authorization application. The decentralized procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may submit an application to the remaining member states. Within 90 days of receiving the applications and assessment report, each member state must decide whether to recognize approval. This procedure is referred to as the mutual recognition procedure.
| 72 |
The policies of the FDA and foreign regulatory authorities may change and additional government regulations may be enacted which could prevent or delay regulatory approval of our products and could also increase the cost of regulatory compliance. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
Other Government Regulation
Our research and development activities use biological and hazardous materials that are dangerous to human health and safety or the environment. We are subject to a variety of federal, state and local laws and regulations governing the use, generation, manufacture, storage, handling and disposal of these materials and wastes resulting from these materials. We are also subject to regulation by the Occupational Safety and Health Administration and federal and state environmental protection agencies and to regulation under the Toxic Substances Control Act.
In addition, once our products are marketed commercially, we will have to comply with the various laws relating to the Medicare, Medicaid and other federal healthcare programs. These federal laws include, by way of example, the following:
| · | The anti-kickback statute (Section 1128B(b) of the Social Security Act) prohibits certain business practices and relationships that might affect the provision and cost of healthcare services reimbursable under Medicare, Medicaid and other federal healthcare programs, including, among other things, the payment or receipt of remuneration for the referral of patients whose care or services will be paid by Medicare or other governmental programs; |
| · | The physician self-referral prohibition (Ethics in Patient Referral Act of 1989, as amended, commonly referred to as the Stark Law, Section 1877 of the Social Security Act), which prohibits referrals by physicians of Medicare or Medicaid patients to providers of a broad range of designated healthcare services in which the physicians (or their immediate family members) have ownership interests or with which they have certain other financial arrangements; |
| · | The anti-inducement law (Section 1128A(a)(5) of the Social Security Act), which prohibits providers from offering anything of value to a Medicare or Medicaid beneficiary to induce that beneficiary to use items or services covered by either program; |
| · | The False Claims Act (31 U.S.C. § 3729 et seq.), which prohibits any person from knowingly presenting or causing to be presented false or fraudulent claims for payment to the federal government (including the Medicare and Medicaid programs); |
| · | The Civil Monetary Penalties Law (Section 1128A of the Social Security Act), which authorizes the United States Department of Health and Human Services to impose civil penalties administratively for various fraudulent or abusive acts. |
| · | The Physician Payment Sunshine Act (Section 1128G of the Social Security Act), which requires manufacturers of drugs, medical devices and biologicals that participate in U.S. federal health care programs to report certain payments and items of value given to physicians and teaching hospitals. |
Sanctions for violating these federal laws include criminal and civil penalties that range from punitive sanctions, damage assessments, money penalties, imprisonment, denial of Medicare and Medicaid payments, or exclusion from the Medicare and Medicaid programs, or both. These laws also impose an affirmative duty on those receiving Medicare or Medicaid funding to ensure that they do not employ or contract with persons excluded from the Medicare and other government programs. Additionally, many states have laws and regulations that contain prohibitions that are similar to, and in many cases broader than, these federal laws and once our products are marketed commercially, we will have to comply with these various state laws as well.
| 73 |
Competition
We compete with many companies, research institutes, hospitals, governments and universities that are working to develop products and processes to treat or diagnose AD. Many of these entities have substantially greater financial, technical, manufacturing, marketing, distribution and other resources than we do. However, there has been a dearth of new product introductions in the last 20 years either for the treatment of AD symptoms or its definitive diagnosis in patients who begin exhibiting the memory and cognitive disorders associated with the disease. All of the products introduced to date for the treatment of AD have yielded negative or marginal results with little effect on the progression of AD and no improvement in the memory or cognitive performance of the patients receiving these therapies. We believe that there is currently no diagnostic test for AD that has achieved significant market penetration, and thus the absolute determination of AD in patients is currently achieved only upon autopsy. We believe we are the only company currently pursuing PKCε activation as a mechanism to treat AD and neurodegenerative disease. Although we believe that we have no direct competitors working in this same field at the present time, we cannot provide assurance that our competitors will not discover compounds or processes that may be competitive with our products and introduce such products or processes before us.
Employees
As of the date of this prospectus, we have four full-time personnel. They are: a President and Chief Executive Officer, an Executive Vice President and Chief Financial Officer, a Vice President—Regulatory Affairs and a Director of Clinical Operations. We have no part-time employees.
If we have sufficient financial resources, we plan to hire several additional employees within the next twelve months whose principal responsibilities will be the support of our clinical development activities, including a Chief Medical Officer.
Description of Properties
Our principal executive offices are currently located at 50 Park Place, Suite 1401, Newark, New Jersey 07102. The lease agreement for such office, which is an approximately 4,000 square foot facility, commenced on September 1, 2014. The lease agreement is for a period of approximately three years and three months at the basic rental rate of approximately $7,333 per month plus operating expenses. The lease agreement has an expiration date of November 30, 2017.
The 9,000 square foot BRNI lab that serves as the base of operations for the research related to our drug platform is located on the campus of John Hopkins University in Rockville, Maryland. We do not lease space in this laboratory; rather, BRNI provides services to us from this lab pursuant to arrangements entered into between us and BRNI under the BRNI License, for which we compensate BRNI.
| 74 |
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in any litigation matters that may arise from time to time that may harm business.
There are currently no pending legal proceedings that we believe will have, either individually or in the aggregate, a material adverse effect on our business, financial condition or operating results. As far as we are aware, no governmental authority is contemplating any proceeding to which we are a party or to which any of our properties is subject.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors and Executive Officers
The size of our Board of Directors is set at seven directors. Executive officers are appointed by the Board of Directors and serve at its pleasure. Below are the names of and certain information regarding the Company’s current executive officers and directors:
| Name | Age | Position | Date Named to Board of Directors or as Executive Officer | |||||
| Charles S. Ramat | 63 | Director, President, Chief Executive Officer | June 13, 2014 | |||||
| Robert Weinstein | 55 | Chief Financial Officer, Treasurer, Secretary and Executive Vice President | August 23, 2013 | |||||
| Paul E. Freiman | 80 | Director and Chairman of the Board | October 18, 2013 | |||||
| William S. Singer | 74 | Director; Vice-Chairman of the Board | August 23, 2013 | |||||
| Larry D. Altstiel | 65 | Director | September 12, 2014 | |||||
| James Gottlieb | 67 | Director | December 10, 2013 | |||||
| Jay M. Haft | 80 | Director | August 23, 2013 |
Directors are elected to serve until the next annual meeting of stockholders and until their successors are elected and qualified. Directors are elected by a plurality of the votes cast at the annual meeting of stockholders and hold office until the expiration of the term for which he or she was elected and until a successor has been elected and qualified.
The size of our Board of Directors is set at seven directors. Pursuant to the terms of the Merger Agreement (as defined below), the Company’s Board of Directors, as of the date of the closing of the Merger consisted of five (5) members, of whom four (4) members were designated by the controlling stockholders of Neurotrope BioScience prior to the Merger and one (1) other member who was independent (as defined in applicable SEC rules and the rules of The Nasdaq Stock Market). Pursuant to the Merger Agreement, the size of the Board of Directors was increased to seven (7) members, including two additional independent directors, one of whom, Jay Haft, was nominated by Hannah Rose Holdings, LLC (the “HRH Designee”). Pursuant to the Voting Agreement (as defined below), NRV, James New, Northlea Partners LLLP, and Dan Alkon agreed to vote all of the common stock that each beneficially owned in favor of electing the HRH Designee. For the avoidance of doubt, NRV, Northlea Partners LLLP, James New and Dan Alkon agreed to vote their common stock in favor of electing the HRH Designee only at the time of such individual’s initial appointment to the Company’s Board of Directors, and nothing obligated NRV, Northlea Partners LLLP, James New or Dan Alkon to vote in favor of the election of any other individual as an HRH Designee or in favor of the continuing service of the HRH Designee once elected to the Board. The Voting Agreement was terminated on November 12, 2015. See “Certain Relationships and Related Transactions—Voting Agreement” below.
| 75 |
On November 12, 2015, the Company and NRV entered into a letter agreement (the “Letter Agreement”) pursuant to which the Company agreed to take such reasonable actions within its control so that two (2) representatives designated by NRV (the “NRV Designees”) are nominated for election to the board of directors of the Company at each annual meeting of stockholders until such time as the BRNI License is no longer in effect. Furthermore, the Company will use its best efforts to ensure that (i) each NRV Designee is included in the Board’s slate of nominees to the stockholders for each election of directors, and (ii) each NRV Designee is included in the proxy statement for every meeting of the stockholders of the Company called with respect to the election of members of the Board. Subject to applicable law and stock exchange rules, no NRV Designee shall be removed from the Board unless such removal is for cause or requested in writing by NRV. In the event that any NRV Designee shall cease to serve for any reason, NRV shall be entitled to designate such person’s successor and the Board will promptly fill the vacancy with such successor nominee and such designee will serve the remainder of the term of the director whom such designee replaces. Under the Letter Agreement, if an NRV Designee is not appointed or elected to the Board because of such person’s death, disability, disqualification, withdrawal as a nominee or for other reason is unavailable or unable to serve on the Board, NRV is entitled to designate another nominee for such Board seat.
Pursuant to the above, our Board of Directors is currently comprised of six members: Mr. Haft, who was nominated as the Series A Preferred Stock designee prior to the conversion of the Series A stock; Messrs. Singer and Gottlieb, who are the NRV Designees (as defined below); Dr. Altstiel, who was an Abeles designee prior to the termination of both of the Common Stockholder’s Agreement and the Voting Agreement, and Mr. Freiman and Mr. Ramat who are nominated by the Board of Directors. Executive officers are appointed by the Board of Directors and serve at its pleasure.
The principal occupation and business experience during the past five years for our executive officers and directors is as follows:
Dr. Larry D. Altstiel – Director. Dr. Altstiel has been the Chief Executive Officer of Provectra Therapeutics, Inc., an early-stage biotechnology company developing novel gene therapy for neurodegenerative diseases, since February 2013. He also serves as a member of our Scientific Advisory Board. From 2007 to 2013, Dr. Altstiel was Vice President Neuroscience Clinical Development, Neuroscience Therapeutic Area Clinical Lead, at Pfizer Inc. Dr. Altstiel’s other positions included Senior Vice President, Head of Global Clinical Development, at Schwarz Biosciences, Inc.; Vice President for Research Operations at Eisai Medical Research Inc. and Schering-Plough; and Senior Clinical Research Physician and Group Leader for Neurodegenerative Diseases Clinical and Discovery Research for Alzheimer’s Disease, Parkinson’s Disease and Stroke at Eli Lilly and Company. Dr. Altstiel also has extensive experience in the fields of psychiatry and neurology from Mount Sinai School of Medicine, Columbia Presbyterian Medical Center and Hospital and The Bronx Veteran’s Administration Medical Center.
Paul E. Freiman –Director and Chairman of the Board of Directors. On July 16, 2014, the Board appointed Mr. Freiman to serve as Co-Chief Executive Officer, on an interim basis, of the Company and of Neurotrope BioScience. On September 12, 2014, Mr. Paul E. Freiman’s appointment as interim Co-Chief Executive Officer terminated and he became the sole Chairman of the Board. Mr. Freiman has extensive pharmaceutical and biotechnology industry operating experience as a board member and Chief Executive Officer of private and publicly-traded companies. Mr. Freiman also currently serves as an independent pharmaceutical and biotechnology industry consultant. He serves as Chairman of Chronix BioMedical, Inc. Since May 2002, he has also been a member of the Board of Directors of NovaBay Pharmaceuticals, Inc. In the past, Mr. Freiman served on the boards of Otsuka America, Inc., and several biotechnology companies based in the United States and Singapore. Prior to his current consulting and board member roles, Mr. Freiman was a partner of Burrill Brasil Investimentos based in Rio de Janiero, Brazil. He also served as President and Chief Executive Officer of Neurobiological Technologies, Inc., as well as a member of its Board of Directors.
| 76 |
Mr. Freiman also served as Chairman and Chief Executive Officer of Syntex Corporation, which was sold to The Roche Group during his tenure. He was involved with the marketing of Syntex’s lead product, Naprosyn®, and was responsible for moving the product to over-the-counter status, marketed as Aleve®.
Mr. Freiman served on the Board of the Pharmaceutical Research and Manufacturers Association of America (PhRMA) and was its Chairman. He served on a number of industry task forces both domestically and internationally. He also served as Chairman of the University of California (UCSF) Foundation, The United Way of Silicon Valley and a number of not-for-profit organizations over the years.
Mr. Freiman received a B.S. in pharmacy from Fordham University and an honorary doctorate from the Arnold & Marie Schwartz College of Pharmacy.
James Gottlieb – Director. Since 2010 to the present, Mr. Gottlieb has been a partner with Capitol Counsel LLC, where he leads the commerce team. His clients include entities representing a broad range of industries, including healthcare, aviation, telecommunications, e-commerce, oversight and investigations and mergers & acquisitions. Prior to joining Capitol Counsel LLC and based upon his in-depth experience in legislation, oversight and investigations (including with the FDA) and political roles on Capitol Hill, and foundation management, consulting and strategic advisory roles outside of government, Mr. Gottlieb formed Gottlieb Strategic Consulting, a government affairs firm.
In 1977, Mr. Gottlieb served as the chief of staff for Representative Ted Weiss (D-NY) and later the Subcommittee on Human Resources & Government Relations of the House Committee on Government Operations (now Committee on Oversight & Government Reform) from 1983 to 1992, where he directed a wide range of oversight investigation and legislation in health, education, and veterans’ matters. Mr. Gottlieb served as Senator Jay Rockefeller’s chief counsel and staff director for the Senate Committee on Veterans Affairs from 1992-2003. He also served as Senator Rockefeller’s Chief-of-Staff and was responsible for the Senator’s legislative community, economic development and political operations.
Mr. Gottlieb then served as chief management consultant to BRNI as a strategic policy and advocacy advisor and managed the restructuring of this multi-million dollar medical research facility for the study of brain disorders and continued to serve as legal counsel and policy advisor to Senator Rockefeller, BRNI’s Chairman, from 2003 to 2005. He serves as a Board of Directors member of BRNI and serves as Treasurer of Friends of Jay Rockefeller.
Mr. Gottlieb received a B.A. in Business Administration from Michigan State University in 1969, a Master of Education from New York University in 1970 and a law degree from New York Law School in 1974.
Jay M. Haft – Director. Since 2005 to the present, Mr. Haft has been a partner of Columbus Nova, a private investment arm of the Renova Group. Mr. Haft served as Chairman of the Board of Directors of DUSA Pharmaceuticals, Inc., a publicly-traded pharmaceutical company, from 2008 until December 2012, when the company merged with an affiliate of Sun Pharmaceutical Industries, Inc. From 1989 to 1994, he was a senior corporate partner of the law firm of Parker, Duryee, Rosoff and Haft, and was of counsel to that firm from 1994 until 2002. He is currently of counsel to the law firm of Reed Smith LLP. Mr. Haft has served on approximately 30 corporate boards, including his tenure as Chairman of the Executive Committee of Emerson Radio Corporation. He is also active in the non-profit sector as well, particularly in the areas of education and art. Mr. Haft earned his Bachelor’s degree and graduated Phi Beta Kappa from Yale University and earned his law degree from Yale Law School.
Charles S. Ramat –Director, President and Chief Executive Officer. On July 16, 2014, the Board appointed him to serve as Co-Chief Executive Officer, on an interim basis, of the Company and of Neurotrope BioScience. On September 12, 2014, Mr. Ramat’s appointment as interim Co-Chief Executive Officer terminated and he was appointed as our sole President and Chief Executive Officer. Additionally, on September 12, 2014, Mr. Ramat’s appointment as Co-Chairman of the Company’s Board of Directors terminated. Mr. Ramat remains a member of the Board. Mr. Ramat, a self-employed consultant, is currently a private investor and has extensive operational and general business experience in several industries including: biotechnology, medical devices, commercial finance, real estate and mobile communications. Until December 2013, Mr. Ramat was a principal investor and consultant to Continental Home Loans, the third largest FHA mortgage originator in New York State, with operations in 22 states and with annual originations and servicing portfolio both in excess of $1 billion. Since 2006, he has developed land, principally 1,000 acres of private gated communities, with a strong emphasis on environmentally friendly practices, in upstate New York. From 2002 to 2005, Mr. Ramat was the President and Chief Executive Officer of Kushner Companies, managing 25,000 apartment units and a commercial portfolio exceeding five million square feet.
| 77 |
From 1988 to 2002, Mr. Ramat was Chairman and Chief Executive Officer of Aris Industries, Inc., a publicly-traded apparel maker. Aris owned and licensed several major apparel brands and Mr. Ramat was active in the Company’s branding effort both domestically and internationally. During this tenure, annual sales rose from under $50 million to more than $400 million. Prior to 1988, Mr. Ramat had acquired various luxury residential buildings in Manhattan where he executed co-op conversions resulting in over $100 million in sales. Also, he was a principal in United Restaurant Corporation which held significant national restaurant franchise rights and a Vice President at Landall Corporation, a publicly-traded real estate development company.
Mr. Ramat has served on various business company boards, philanthropies and trade organizations. Mr. Ramat graduated cum laude with a B.A. from Yeshiva University and earned a Juris Doctor degree from Columbia Law School.
Robert Weinstein – Chief Financial Officer, Executive Vice President, Treasurer and Secretary. Mr. Weinstein joined the Company in June 2013 as its acting Chief Financial Officer. The Company is party to an employment agreement dated as of October 1, 2013, with Mr. Weinstein, pursuant to which he serves as the Company’s Chief Financial Officer and Executive Vice President. He has extensive accounting and finance experience, spanning more than 25 years, as a public accountant, investment banker, healthcare private equity fund principal and chief financial officer.
From September 2011 to the present, Mr. Weinstein has been an independent consultant for several healthcare companies in the pharmaceutical and biotechnology industries. From March 2010 to August 2011, he was the Chief Financial Officer of Green Energy Management Services Holdings, Inc., a publicly-traded energy consulting company. From August 2007 to February 2010, Mr. Weinstein served as Chief Financial Officer of Xcorporeal, Inc., a publicly-traded, development-stage medical device company which was sold in March 2010 to Fresenius Medical USA, the largest provider of dialysis equipment and services worldwide.
Mr. Weinstein received his MBA degree in finance and international business from the University of Chicago Graduate School of Business, is a Certified Public Accountant (inactive), and received his BS degree in accounting from the State University of New York at Albany.
William S. Singer – Director and Vice-Chairman of the Board of Directors. Mr. Singer serves as President of Blanchette Rockefeller Neurosciences Institute (“BRNI”) and is also on its board of directors. He was a partner in the Chicago office of the law firm of Kirkland & Ellis LLP from 1980 until 2006 and has been of counsel to that firm since that time, concentrating his practice on corporate, real estate, and legislative matters. Since 2006, he also has been the sole proprietor of Singer Consulting LLC, which advises clients on federal legislation. He has been listed in Crain’s Who’s Who in Chicago Business in the 2000, 2001, 2002, 2003, and 2004 editions.
Mr. Singer has been prominently active in Chicago public service, serving as an Alderman for several years and as a candidate for Mayoral office.
Other Key Personnel and Advisors
Dr. Daniel Alkon – Chief Scientific Officer (Advisor to Neurotrope BioScience). Dr. Alkon has been the founding Scientific Director of BRNI since 1999. He received his undergraduate degree in chemistry in 1965 at the University of Pennsylvania. After earning his M.D. at Cornell University and finishing an internship in medicine at the Mount Sinai Hospital in New York, he joined the staff of the National Institutes of Health where during his 30 year career he became a Medical Director in the U.S. Public Health Service at the NINDS and Chief of the Laboratory of Adaptive Systems. Dr. Alkon occupies the Toyota Chair in Neuroscience at BRNI. In this position, he and his team conduct multidisciplinary research on the molecular and biophysical mechanisms of memory and memory dysfunction in psychiatric and neurological disorders, particularly AD. He is also a Professor at BRNI and a Professor of Neurology at West Virginia University. Dr. Alkon is not an employee of Neurotrope BioScience.
| 78 |
David Crockford – Vice President, Regulatory Affairs of Neurotrope BioScience. Mr. Crockford has more than 30 years of professional experience in the biotechnology and pharmaceutical industries. From March 2005 to December 31, 2013, Mr. Crockford was the Vice President of Clinical and Regulatory Affairs of RegeneRX Biopharmaceuticals, Inc. There, he led the development and obtained marketing approval of 18 drug products, 17 immunodiagnostic tests, and an intraoperative medical device. Mr. Crockford has organized, presented and led discussions in many face-to-face meetings and teleconferences with a variety of Divisions and Centers (CBER, CDER and CDRH) at the FDA. He provided the regulatory strategy and direction and led the clinical development of a regenerative peptide for the treatment of patients with certain ailments including neurodegenerative diseases.
Mr. Crockford is the author of a number of articles and sole inventor/co-inventor of approximately twenty patents and applications disclosing compositions, methods of drug use and delivery. He earned a Bachelor of Arts degree in biology from Boston University’s College of Arts and Sciences and completed seminars in clinical chemistry, sponsored by Princeton University/Princeton Hospital, and reproductive medicine at Wayne State’s Mott Center for Human Growth and Development and UCLA Medical School.
Elaine Grenier – Director of Clinical Operations of Neurotrope BioScience. Ms. Grenier has more than 25 years of experience in the pharmaceutical industry, with management responsibility for clinical trials in oncology, cardiovascular, central nervous system and genetic disease indications. Ms. Grenier has served as Head of Clinical Operations of the Company since February 2015. Prior to joining us, Ms. Grenier served as a project manager at various companies, including Infacare Pharmaceutical, Inc. from June 2013 to February 2015, Ockham from November 2012 to May 2013, Covance from June 2011 to May 2012, and again at Infacare Pharmaceutical, Inc. from January 2010 to June 2011. Ms. Grenier participated in globalization of clinical development processes for a prominent pharmaceutical company to achieve uniform research standards across all global subsidiaries, with sensitivity to cultural and organizational differences. Ms. Grenier has provided management and leadership of multiple functional areas for integrated, comprehensive project oversight for US and international research programs. Ms. Grenier earned a Bachelor of Science degree from the University of Maryland.
Board of Directors
Our Board of Directors is authorized to consist of seven members and currently consists of six members.
Management has been delegated the responsibility for meeting defined corporate objectives, implementing approved strategic and operating plans, carrying on the Company’s business in the ordinary course, managing cash flow, evaluating new business opportunities, recruiting staff and complying with applicable regulatory requirements. The Board of Directors exercises its supervision over management by reviewing and approving long-term strategic, business and capital plans, material contracts and business transactions, and all debt and equity financing transactions and stock issuances.
Director Independence
We are not currently subject to listing requirements of any national securities exchange or inter-dealer quotation system which has requirements that a majority of the board of directors be “independent” and, as a result, we are not at this time required to have our Board of Directors comprised of a majority of “independent directors.” Nevertheless, our Board of Directors has determined that each of Messrs. Altstiel, Gottlieb and Haft are “independent” under the applicable federal securities laws and regulations and the rules of the NASDAQ Stock Market. The Company intends to fill the vacancy on the Board of Directors with a person who will be an independent member of the Board.
Board Committees
Our Board of Directors has established three committees, each of which is composed solely of independent directors:
| · | The Audit Committee consists of Mr. Haft, as Chairman, Dr. Altstiel and Mr. Gottlieb. |
| · | The Compensation Committee consists of Dr. Altstiel, as Chairman, and Mr. Haft. |
| · | The Nominating and Corporate Governance Committee consists of Dr. Altstiel, as Chairman, Mr. Gottlieb and Mr. Haft. |
| 79 |
Each of the Committees has a written charter adopted by the Board of Directors; a current copy of each such charter is available to security holders on our website, www.neurotropebioscience.com.
Compensation Committee Interlocks and Insider Participation
The Compensation Committee consists of Dr. Altstiel, as Chairman, and Mr. Haft. No member of the Compensation Committee has been an officer or employee of the Company. None of our executive officers serves on the board of directors or compensation committee of a company that has an executive officer that serves on our Board of Directors or Compensation Committee.
Family Relationships
There are no family relationships among our directors or executive officers.
Involvement in Certain Legal Proceedings
None of our directors or executive officers has been involved in any of the following events during the past ten years:
| · | any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time, except that Mr. Weinstein was the Chief Financial Officer of Able Laboratories, Inc. until November 2004, which filed for bankruptcy protection in July 2005; |
| · | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offences); |
| · | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his or her involvement in any type of business, securities or banking activities; or |
| · | being found by a court of competent jurisdiction (in a civil action), the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated. |
| 80 |
Summary Compensation Table
The following table sets forth information concerning the total compensation paid or accrued by us during the last two fiscal years ended December 31, 2015, to (i) all individuals that served as our principal executive officers or acted in a similar capacity for us at any time during the fiscal year ended December 31, 2015; (ii) the two most highly compensated executive officers other than the principal executive officer who were serving as executive officers at December 31, 2015; and (iii) up to two additional individuals for whom disclosure would have been required pursuant to clause (ii) above but for the fact that the individual was not serving as an executive officer at December 31, 2015 (collectively, the “named executive officers”). The Compensation Committee of the Board of Directors is responsible for determining executive compensation.
Name & Principal Position | Fiscal Year ended December 31 | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($)(7) | Non–Equity Incentive Plan Compensation ($) | Non– Qualified Deferred Compensation Earnings ($) | All Other Compensation ($)(8) | Total ($) | |||||||||||||||||||||||||
| Charles S. Ramat, President and CEO (1) | 2015 2014 | 450,000 206,667 | – – | – – | 366,175 477,916 | – – | – – | 15,420 – | 831,595 684,583 | |||||||||||||||||||||||||
| Paul Freiman, Chairman (2) | 2014 | 110,000 | – | – | 372,117 | – | – | – | 482,117 | |||||||||||||||||||||||||
| Robert Weinstein, CFO, Secretary and Executive Vice President (3) | 2015 2014 | 275,000 240,000 | – 50,000 | (6) | – – | – – | 52,252 – | – – | 29,203 524 | 356,455 290,524 | ||||||||||||||||||||||||
| Warren Wasiewski, Executive Vice President, Development and Chief Medical Officer of Neurotrope BioScience (4) | 2015 2014 | 288,875 54,167 | 135,070 17,014 | – – | – 155,583 | – – | – – | 27,005 – | 450,950 226,764 | |||||||||||||||||||||||||
| James New, Former President and CEO (5) | 2014 | 135,417 | – | – | – | – | – | 268,477 | 403,894 | |||||||||||||||||||||||||
| (1) | Mr. Ramat was appointed by our Board on July 16, 2014 to serve as our Co-Chief Executive Officer, on an interim basis. On September 12, 2014, Mr. Ramat’s appointment as interim Co-Chief Executive Officer terminated, and he was appointed as our sole President and Chief Executive Officer. Mr. Ramat’s 2014 amount includes consulting fees that Mr. Ramat received for his services as Co-CEO and CEO, as well consulting fees he receives under a consulting agreement with the Company. |
| (2) | Mr. Freiman was appointed by our Board on July 16, 2014 to serve as our Co-Chief Executive Officer, on an interim basis. On September 12, 2014, Mr. Freiman’s appointment as interim Co-Chief Executive Officer terminated. Mr. Freiman’s 2014 amount includes consulting fees that Mr. Freiman received for his service as Co-CEO and Chairman of the Board. |
| (3) | Mr. Weinstein became our Chief Financial Officer on August 23, 2013. The Company is party to an employment agreement dated as of October 1, 2013, with Mr. Weinstein, pursuant to which he serves as the Company’s Chief Financial Officer and Executive Vice President. |
| (4) | Dr. Wasiewski was appointed as our Executive Vice President, Development and Chief Medical Officer of Neurotrope BioScience on November 1, 2014. Dr. Wasiewski terminated his employment as Executive Vice President, Development with Neurotrope Bioscience on November 9, 2015. He currently serves as our interim Chief Medical Officer. For more information, see “Employment Agreements” below. |
| (5) | Reflects compensation received from Neurotrope BioScience, which was formed in 2012, through the date of the Reverse Merger in August 2013. Salary was accrued in 2012 but paid in 2013. On August 23, 2013, Dr. New was appointed as our Chief Executive Officer and President. Effective as of July 16, 2014, Dr. New’s employment as Chief Executive Officer and President of the Company was terminated. See “Employment Agreements; Separation Agreement—James New” below. |
| 81 |
| (6) | Represents a $50,000 bonus paid to Mr. Weinstein in 2015 for services rendered in 2014. |
| (7) | Option awards represent the grant date fair value of awards. Grant date fair value is based on the Black-Scholes option pricing model on the date of grant. For additional discussion on the valuation assumptions used in determining the grant date fair value, see Note 10 to the audited consolidated financial statements in our Annual Report on Form 10-K for the year ended December 31, 2014. |
| (8) | Dr. New’s 2014 amount represents (a) the amount paid to him pursuant to the Separation Agreement and General Release, effective as of July 16, 2014, which is comprised of severance, vacation and healthcare payments, and (b) life insurance premiums we paid on behalf of Dr. New. Mr. Weinstein’s 2014 amount represents life insurance premiums we paid on behalf of Mr. Weinstein. |
Outstanding Equity Awards at Fiscal Year-End
We have one compensation plan approved by our stockholders, the 2013 Plan. The following table provides information regarding 2013 Plan awards for each named executive officer outstanding as of December 31, 2015.
| Option awards | Stock awards | |||||||||||||||||||||||||||||||||
| Name | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable | Equity incentive plan awards: Number of securities underlying unexercised unearned options (#) | Option exercise price ($) | Option expiration date | Number of shares or units of stock that have not vested (#) | Market value of shares of units of stock that have not vested | Equity incentive plan awards: Number of unearned shares, units or other rights that have not vested | Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested ($) | |||||||||||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | |||||||||||||||||||||||||
| Charles S. Ramat | 230,302 | 69,698 | – | 1.00 | 08/23/2023(1) | – | – | – | – | |||||||||||||||||||||||||
| 54,404 | 0 | 1.75 | 08/23/2023(2) | |||||||||||||||||||||||||||||||
| 117,808 | 132,192 | 1.00 | 08/23/2023(3) | |||||||||||||||||||||||||||||||
| 272,055 | 127,945 | (9 | ) | 07/23/2024(4) | ||||||||||||||||||||||||||||||
| 250,000 | 0 | 0.60 | 09/12/2024(5) | |||||||||||||||||||||||||||||||
| 100,000 | 0 | – | 0.60 | 11/17/2025(6) | ||||||||||||||||||||||||||||||
| 150,000 | 450,000 | – | 0.80 | 11/19/2025(7) | ||||||||||||||||||||||||||||||
| Paul Freiman | 110,137 | 139,863 | – | $ | 1.50 | 10/18/2023(8) | – | – | – | – | ||||||||||||||||||||||||
| 20,575 | 29,425 | 1.55 | 12/10/2023(9) | |||||||||||||||||||||||||||||||
| 10,274 | 14,726 | 1.64 | 12/11/2023(10) | |||||||||||||||||||||||||||||||
| 272,055 | 127,945 | (9 | ) | 07/23/2024(11) | ||||||||||||||||||||||||||||||
| 50.000 | 0 | 0.60 | 09/12/2024(12) | |||||||||||||||||||||||||||||||
| 50,000 | 150,000 | – | 0.80 | 11/19/2025(13) | ||||||||||||||||||||||||||||||
| Robert Weinstein | 325,000 | 325,000 | – | $ | 1.00 | 10/01/2023(14) | – | – | – | – | ||||||||||||||||||||||||
| 25,000 | 75,000 | – | 0.80 | 11/19/2025(15) | ||||||||||||||||||||||||||||||
| Warren Wasiewski | 50,000 | 0 | – | $ | 0.71 | 11/15/2016(16) | – | – | – | – | ||||||||||||||||||||||||
| (1) | The options vested with respect to 88,864 shares as of the date of grant, with the balance vesting at a rate of 164 shares per day such that the option shall be fully vested as of February 28, 2017 (assuming continued service through such date). Vesting is subject to acceleration in the event of a change in control or termination of the consulting agreement between Neurotrope, Inc. and Mr. Ramat for good reason. |
| (2) | The options vested as of the date of grant. |
| (3) | The options vest daily over five years from the date of grant, such that the options will have vested with respect to all shares as of August 23, 2018. |
| (4) | One-half of the options granted to Mr. Ramat have an exercise price $1.11 per share and were fully vested upon the date of grant. The second-half of the options granted to Mr. Ramat have an exercise price of $2.22 per share and vest on a daily basis up to 25% per year through July 23, 2018. |
| (5) | The options vested as of the date of grant. |
| 82 |
| (6) | The options vested as of the date of grant. |
| (7) | The options have an exercise price of $0.80 per share and vest at a rate of 25% per year, with the initial 25% vested as of the date of grant. |
| (8) | The options vest daily over five years from the date of grant, such that the options will have vested with respect to all shares as of October 18, 2018 (assuming continued service through such date). Vesting is subject to the acceleration in the event of a change in control. |
| (9) | The options vest daily over five years from the date of grant, such that the options will have vested with respect to all shares as of December 10, 2018 (assuming continued service through such date). |
| (10) | The options vest daily over five years from the date of grant, such that the options will have vested with respect to all shares as of December 11, 2018 (assuming continued service through such date). |
| (11) | One-half of the amount of options granted to Mr. Freiman have an exercise price $1.11 per share and are fully vested upon date of grant. The second-half of the amount of options granted to Mr. Freiman have an exercise price of $2.22 per share and vest on a daily basis up to 25% per year through July 23, 2018. |
| (12) | The options vested as of the date of grant. |
| (13) | The options have an exercise price of $0.80 per share and vest at a rate of 25% per year, with the initial 25% vested as of the date of grant. |
| (14) | 25% of these options vest on each of the first four anniversaries of the date of grant (October 2, 2013). |
| (15) | The options have an exercise price of $0.80 per share and vest at a rate of 25% per year, with the initial 25% vested as of the date of grant. |
| (16) | 50,000 options vested on November 1, 2015. |
We have no plans in place and have never maintained any plans that provide for the payment of retirement benefits or benefits that will be paid primarily following retirement including, but not limited to, tax qualified deferred benefit plans, supplemental executive retirement plans, tax-qualified deferred contribution plans and nonqualified deferred contribution plans.
Except as indicated below, we have no contracts, agreements, plans or arrangements, whether written or unwritten, that provide for payments to the named executive officers listed above. In 2015, we did not hire any additional employees.
Employment Agreements; Separation Agreement
Warren Wasiewski. Neurotrope BioScience and Dr. Wasiewski executed an employment agreement dated as of November 1, 2014 pursuant to which Dr. Wasiewski served as Executive Vice President, Development and Chief Medical Officer (the “Wasiewski Employment Agreement”). The Wasiewski Employment Agreement provided for an initial term from November 1, 2014 to November 1, 2015 (the “Initial Term”), with automatic extensions for additional one-year periods (each, an “Extension Term”) unless Neurotrope BioScience or Dr. Wasiewski provided at least 90 days’ notice not to extend the term, or until terminated in accordance with termination provisions set forth in the Wasiewski Employment Agreement (the Initial Term and any Extension Term(s), collectively, the “Employment Term”). The Wasiewski Employment Agreement provided that Dr. Wasiewski receive an initial base salary of $325,000. During the Employment Term, Dr. Wasiewski’s salary was to be reviewed at least annually and could be adjusted as determined by the President of the Company. In addition, Dr. Wasiewski was eligible to receive an annual incentive bonus opportunity of 50% of his base salary commencing in 2015 to be earned and payable based upon attainment of both corporate and individual annual performance goals. Additionally, Dr. Wasiewski was entitled to receive a sign-on bonus in the amount of $102,083.40 payable in 12 equal monthly installments. In accordance with the terms and limitations in the Wasiewski Employment Agreement, Dr. Wasiewski was entitled to be reimbursed for his relocation expenses of up to $15,000 and his lodging expenses of up to $15,000. Dr. Wasiewski was also entitled to four weeks of paid vacation per annum and general expense reimbursement for pre-approved business related expenses incurred in the performance of Dr. Wasiewski’s job duties. Dr. Wasiewski was eligible for all benefits and retirement, life, disability, medical and dental plan benefits generally available to Neurotrope BioScience’s officers in accordance with the terms of those plans. Dr. Wasiewski’s employment with Neurotrope BioScience as Executive Vice President, Development and Chief Medical Officer terminated effective as of November 9, 2015. Dr. Wasiewski continues to serve as our interim Chief Medical Officer and as a consultant to the Company.
On November 1, 2014, Dr. Wasiewski was granted non-qualified stock options under the 2013 Plan to purchase 250,000 shares of our common stock at an exercise price equal to the fair market value of the our common stock on the grant date (the “Sign-On Options”). Twenty percent (20%) of the Sign-On Options will vest on each anniversary of the grant date for a period of 5 years from the grant date. As of January 6, 2016, 200,000 of the options originally granted to Dr. Wasiewski have been returned to the option pool upon the termination of his employment.
| 83 |
Robert Weinstein. The Company is party to an employment agreement dated as of October 1, 2013, with Robert Weinstein, pursuant to which he serves as the Company’s Chief Financial Officer and Executive Vice President. Under the terms of Mr. Weinstein’s employment agreement, the Company will pay Mr. Weinstein an annual base salary of not less than $240,000 per year for the period from the effective date to December 31, 2014; and $275,000 per year for the period January 1, 2015 to December 31, 2015. Such annual base salary may be reviewed annually and increased (but not decreased) at the discretion of the Board or a committee thereof, provided, however, that the salary will, at a minimum, be increased annually, beginning January 1, 2016, based upon the percentage increase in the Consumer Price Index for the immediately preceding year. The Company will pay Mr. Weinstein an annual incentive bonus of no less than $35,000 for the year ending December 31, 2013; an annual bonus of no less than $50,000 for the year ending December 31, 2014; and a discretionary annual bonus of up to 50% of his annual base salary for all years beginning January 1, 2015, to be earned and payable based upon attainment of annual performance goals as determined by the Board or a committee thereof. Mr. Weinstein’s annual bonus opportunity may be periodically reviewed and increased at the discretion of the Board or a committee thereof. Mr. Weinstein will also be eligible to participate in all Company benefits generally available to the Company’s officers in accordance with the terms of those benefit plans and all retirement, life, disability, medical and dental plan benefits generally available to the Company’s officers in accordance with the terms of those plans.
Pursuant to the employment agreement, the Company’s Board of Directors granted an incentive stock option to Mr. Weinstein under the 2013 Plan to purchase 650,000 shares of the Company’s common stock. The option vests with respect to 162,500 shares on each of the first, second, third and fourth anniversaries of October 1, 2013, subject to the executive’s continued employment with the Company on each such day. Mr. Weinstein will be entitled to additional options and/or equity-based awards as determined in the discretion of the Board or a committee thereof. All of his options and/or equity awards will cease vesting as of the date of termination of the employment agreement, provided that in the event of (i) Mr. Weinstein’s termination for good reason or (ii) termination of his employment by the Company without cause, his options and any other equity awards will be deemed to have vested as of the date of termination with respect to that number of shares that would have vested had his employment with the Company continued for a period of one year after the date of termination, and provided, further, that if Mr. Weinstein’s termination for Good Reason or the termination of his employment by the Company without Cause occurs within six months after the occurrence of a change of control of the Company, then all of his options and any other equity awards will be deemed to have vested as of the date of termination.
If Mr. Weinstein’s employment is terminated by the Company for a reason other than cause or by him for good reason, and subject to his compliance with other terms of Mr. Weinstein’s employment agreement, and certain other conditions, then the Company will pay him a severance amount equal to his annual base salary, payable in a single lump sum. In addition, if he elects health care continuation coverage under COBRA, the Company will pay for such health insurance coverage for a period of 18 months following the termination of his employment, as the same rate as it pays for health insurance coverage for its active employees (with Mr. Weinstein required to pay for any employee-paid portion of such coverage). If Mr. Weinstein’s employment is terminated by non-renewal or due to his death or disability, he will be entitled to any unpaid prorated Annual Bonus for the year in which his employment terminates.
The Company reimbursed Mr. Weinstein $4,800 for reasonable attorney’s fees and expenses that he incurred in connection with the negotiation, preparation and execution of his employment agreement.
Subject to earlier termination by Mr. Weinstein’s death or disability, or by the Company for Cause, the term of Mr. Weinstein’s employment agreement is four years and will be extended automatically for successive one-year periods, unless either party gives written notice of termination to the other party at least 90 days prior to the end of the then-current term.
James New. Effective as of July 16, 2014, Dr. James New’s employment as Chief Executive Officer and President of the Company was terminated. Accordingly, the employment agreement by and between Neurotrope BioScience and Dr. New dated February 25, 2013 (the “New Employment Agreement”) terminated as of July 16, 2014. On October 9, 2014, Neurotrope BioScience and Dr. New entered into a Separation Agreement and General Release (the “Separation Agreement”) pursuant to which Neurotrope BioScience paid or will pay or provide to Dr. New the following: (a) $2,684.02 within three days of the execution of the Separation Agreement by Dr. New, representing reimbursement of business expenses; (b) a lump sum equal to the total gross amount of $233,000 (less all applicable income and employment taxes and other required or elected withholdings, for which a Form W-2 will be issued to Dr. New); (c) a gross amount of $25,477.59 (less all applicable income and employment taxes and other required or elected withholdings) for Dr. New’s accrued, unused vacation; (d) reimbursement for the cost of continuing Dr. New’s current health care insurance in the same amount as the net reimbursement amounts paid to Dr. New on a monthly basis immediately prior to July 16, 2014 ($2,974 per month) until the earlier of the date on which Dr. New becomes eligible for medical insurance coverage with a new employer or July 16, 2015; (e) director’s and officer’s insurance coverage covering Dr. New’s actions while a director and/or officer of Neurotrope BioScience until July 16, 2020; and (f) $17,000 for reimbursement of Dr. New’s attorneys fees. In addition, the parties agreed that Dr. New’s ownership of any common shares of the company is not affected by the Separation Agreement and that any equity awards Dr. New received pursuant to the 2013 Equity Incentive Plan will be governed exclusively by the terms of such plan.
| 84 |
Consulting Arrangements
On July 16, 2014, the Board appointed Charles S. Ramat and Paul E. Freiman to serve as Co-Chief Executive Officers, on an interim basis, of the Company and of Neurotrope BioScience. In consideration of the services that Mr. Freiman and Mr. Ramat provided to the Company as interim Co-Chief Executive Officers, the Company paid each of them consulting fees in the amount of $20,000 per month. In addition, on July 23, 2014, Messrs. Freiman and Ramat were each granted non-qualified options to purchase 400,000 shares of the Company’s common stock for their roles as directors, Co-Chairmen of the Board and Co-Chief Executive Officers. On September 12, 2014, Messrs. Freiman’s and Ramat’s appointments as interim Co-Chief Executive Officers terminated effective on such date. See “Executive Compensation––Outstanding Equity Awards at Fiscal Year-End.”
On September 12, 2014, Mr. Ramat was appointed to serve as the Company’s President and Chief Executive Officer, effective as of such date. In consideration of Mr. Ramat’s services as President and Chief Executive Officer, the Company has agreed to pay Mr. Ramat a salary of $400,000 per year. This arrangement may be terminated by the Company or Mr. Ramat on 60 days’ notice. Mr. Ramat’s salary for his duties as President and Chief Executive Officer will be paid in addition to the consulting fees he receives under a Consulting Agreement with the Company pursuant to which he receives $50,000 per year. In addition, in connection with his appointment as President and Chief Executive Officer, Mr. Ramat was granted non-qualified stock options pursuant to the Company’s 2013 Equity Incentive Plan, with a term of ten years, to purchase 250,000 shares of common stock of the Company at an exercise price of $.60 per share, the closing price of the shares on September 12, 2014, the date of grant. All of the options vested as of September 12, 2014.
Also on September 12, 2014, Mr. Freiman was appointed to serve as the sole Chairman of the Company’s Board of Directors. In consideration of Mr. Freiman’s services as Chairman, the Company agreed to continue to pay Mr. Freiman $20,000 per month, being the same level as the consulting fees he was receiving as Co-Chief Executive Officer prior to September 12, 2014. In addition, in connection with his appointment as Chairman of the Company’s Board of Directors, Mr. Freiman was granted non-qualified stock options pursuant to the Company’s 2013 Equity Incentive Plan, with a term of ten years, to purchase 50,000 shares of common stock of the Company at an exercise price of $0.60 per share, the closing price of the shares on September 12, 2014, the date of grant. All of the options vested as of September 12, 2014. Mr. Freiman’s consulting fees will be reduced to $30,000 annually beginning February 1, 2016.
On November 19, 2015, Mr. Ramat received 100,000 ten-year options, all of which vested immediately, with an exercise price of $0.80 per share, and 600,000 ten-year options, 25% of which vested immediately with an additional 25% vesting over each of the next three anniversaries of the date of grant, with an exercise price of $0.80 per share. Also on November 19, 2015, Mr. Freiman received 200,000 ten-year options and Mr. Weinstein received 100,000 ten-year options, 25% of which, respectively, vested immediately with an additional 25%, respectively, vesting over each of the next three anniversaries of the date of grant.
Director Compensation
The Company currently does not pay any cash compensation to members of its Board of Directors for their services as directors of the Company. However, the Company reimburses its directors for all reasonable out-of-pocket expenses incurred in connection with their attendance at meetings of the Board of Directors. Historically, the Company has granted to each new director, at the time of such director’s appointment, a one-time option to purchase 250,000 shares of common stock. In addition, new directors who are expected to participate in one or more committees have been granted an additional option to purchase an additional 50,000 shares of common stock. See “Executive Compensation” above for a discussion regarding Messrs. Freiman’s and Ramat’s compensation for their services as executive officers and consultants of the Company. On November 19, 2015, each of Jay Haft and Larry Altstiel received 50,000 options, 25% of which vested immediately with an additional 25% vesting over each of the next three anniversaries of the date of grant.
| 85 |
The following table provides information concerning the compensation of our directors for the year ended December 31, 2015.
Fees earned | Non-equity incentive | Nonqualified deferred | ||||||||||||||||||||||||||
| or paid in | Stock | Option | plan | compensation | All other | |||||||||||||||||||||||
| cash | awards | awards | compensation | earnings | compensation | Total | ||||||||||||||||||||||
| Name | ($) | ($) | ($) | ($) | ($) | ($) | ($) | |||||||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | |||||||||||||||||||||
| John H. Abeles | 0 | - | 0 | - | - | - | 0 | |||||||||||||||||||||
| Larry Altstiel | 0 | - | 26,126 | - | - | - | 26,126 | |||||||||||||||||||||
| Paul E. Freiman1 | 0 | - | 104,504 | - | - | - | 104,504 | |||||||||||||||||||||
| James Gottlieb | 0 | - | 0 | - | - | - | 0 | |||||||||||||||||||||
| Jay M. Haft | 0 | - | 26,126 | - | - | - | 26,126 | |||||||||||||||||||||
| Charles S. Ramat2 | 0 | - | 0 | - | - | - | 0 | |||||||||||||||||||||
| William S. Singer | 0 | - | 0 | - | - | - | 0 | |||||||||||||||||||||
1 Mr. Freiman’s compensation for services on behalf of the Company is fully reflected in the Summary Compensation Table above.
2 Mr. Ramat’s compensation for services on behalf of the Company is fully reflected in the Summary Compensation Table above.
| 86 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information with respect to the beneficial ownership of our common stock as of January 6, 2016 (except as otherwise noted), by (i) each stockholder known by us to be the beneficial owner of more than 5% of our common stock, Series A Stock or Series B Stock (our only classes of voting securities), (ii) each of our directors and executive officers, and (iii) all of our directors and executive officers as a group. To the best of our knowledge, except as otherwise indicated, each of the persons named in the table has sole voting and investment power with respect to the shares of our common stock, Series A Stock or Series B Stock beneficially owned by such person, except to the extent such power may be shared with a spouse. To our knowledge, none of the shares listed below are held under a voting trust or similar agreement, except as noted. Other than the Reverse Merger, to our knowledge, there is no arrangement, including any pledge by any person of securities of the Company or any of its parents, the operation of which may at a subsequent date result in a change in control of the Company.
Unless otherwise indicated in the following table, the address for each person named in the table is c/o Neurotrope, Inc., 50 Park Place, Suite 1401, Newark, New Jersey 07102.
Name and Address of Beneficial Owner | Common Stock Beneficially Owned | Percent of Common Stock Beneficially Owned(1) | Shares
of | Percent of Series A Preferred Stock Beneficially Owned(1) | Shares of Common Stock Underlying Series B Stock Beneficially Owned(1) | Percent of Series B Preferred Stock Beneficially Owned(1) | ||||||||||||||||||
| Neurosciences
Research Ventures, Inc.(2) 364 Patteson Drive, #279 Morgantown, WV 26505 | 10,687,500 | 3.7 | % | 0 | 0 | % | 0 | 0 | % | |||||||||||||||
Dr. John H. Abeles/Northlea Partners LLLP(3) 2365 NW 41st Street Boca Raton, Florida 33431 | 10,783,383 | 3.7 | % | 0 | 0 | % | 375,001 | 1.4 | % | |||||||||||||||
Dr. James New(4) 10732 Hawk’s Vista Street Plantation, Florida 33324 | 3,838,000 | 1.3 | % | 0 | * | 0 | * | |||||||||||||||||
| Hannah Rose Holdings,
LLC(5) 101 Grovers Mill Road Suite #200 Lawrenceville, NJ 08648 | 4,957,030 | 1.7 | % | 0 | 0 | % | 0 | 0 | % | |||||||||||||||
| E. Jeffrey Peierls(6) 73 South Holman Way Golden, CO 80401 | 3,443,331 | 1.2 | % | 0 | 0 | % | 200,000 | 0.8 | % | |||||||||||||||
Charles S. Ramat(7) c/o Neurotrope, Inc. 50 Park Place, Suite 1401 Newark, New Jersey 07102 | 5,396,117 | 1.9 | % | 0 | * | 166,667 | * | |||||||||||||||||
| Paul E. Freiman(8) | 1,533,838 | * | 0 | * | 166,667 | * | ||||||||||||||||||
| Larry D. Altstiel (9) | 576,502 | * | 0 | * | 41,667 | * | ||||||||||||||||||
| Jay M. Haft(10) | 570,262 | * | 0 | * | 41,667 | * | ||||||||||||||||||
| William S. Singer(11) | 222,493 | * | 0 | * | 0 | * | ||||||||||||||||||
| Robert Weinstein(12) | 600,002 | * | 0 | * | 41,667 | * | ||||||||||||||||||
| James Gottlieb(13) | 193,370 | * | 0 | * | 0 | * | ||||||||||||||||||
| All directors and executive officers as a group (8 persons) | 19,875,967 | 6.9 | % | 0 | 0 | % | 833,336 | 3.2 | % | |||||||||||||||
| 87 |
* Denotes less than 1%
| (1) | Applicable percentage ownership is based on 49,172,026 shares of our common stock outstanding, 0 shares of our Series A Stock outstanding and 26,234,940 shares of our Series B Stock outstanding, together with securities exercisable or convertible into shares of our common stock within 60 days of January 6, 2016 for each stockholder. Shares of our common stock underlying the Series B Stock are calculated on a 100-for-one basis. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. The shares issuable pursuant to the exercise or conversion of such securities are deemed outstanding for the purpose of computing the percentage of ownership of the security holder, but are not treated as outstanding for the purpose of computing the percentage of ownership of any other person. |
| (2) | Includes 1,662,500 shares underlying stock options held by Neurosciences Research Ventures, Inc. that are vested as of January 6, 2016 or will vest within 60 days thereafter. |
| (3) | Includes 1,082,349 shares underlying stock options held by Dr. Abeles that are vested as of January 6, 2016 or will vest within 60 days thereafter, 375,001 shares of Series B Stock, and warrants to purchase 1,875,005 shares of our common stock but does not include 123,151 shares underlying stock options that will not vest within 60 days after January 6, 2016. 894,454 shares of our common stock indicated as beneficially owned by Dr. John H. Abeles and warrants to purchase 1,041,670 shares of our common stock are held by Northlea Partners LLLP. Dr. Abeles is an affiliate of Northlea Partners LLLP and has sole power to vote or direct the vote, and to dispose or direct the disposition, of such shares of common stock. |
| (4) | As of December 31, 2014, as reported on Schedule 13G filed with the SEC by Dr. New on February 17, 2015, the shares of our common stock indicated as beneficially owned include 3,646,100 shares of our common stock. |
| (5) | HRH is controlled by Matt Rosenblum. |
| (6) | Includes 2,000,000 shares of Series B Stock and warrants to purchase 1,000,000 shares of our common stock. |
| (7) | Includes 1,203,500 shares underlying stock options beneficially owned by Mr. Ramat that are vested as of January 6, 2016 or will vest within 60 days thereafter, 166,667 shares of Series B Stock and warrants to purchase 833,335 shares of our common stock, but does not include 750,904 shares underlying stock options that will not vest within 60 days after January 6, 2016. The shares of our common stock indicated as beneficially owned by Charles Ramat are held by Mr. Ramat, Ramat Consulting and NTR21 Holdings, LLC. Mr. Ramat is an affiliate of each of Ramat Consulting and NTR21 Holdings, LLC, and has sole power to vote or direct the vote, and to dispose or direct the disposition, of such shares of our common stock. |
| 88 |
| (8) | Includes 533,836 shares underlying stock options held by Mr. Freiman that are vested as of January 6, 2016 or will vest within 60 days thereafter, 166,667 shares of Series B Stock and warrants to purchase 833,333 shares of our common stock, but does not include 441,164 shares underlying stock options that will not vest within 60 days after January 6, 2016. The shares of our Series B Stock and warrants to purchase our common stock indicated as beneficially owned by Mr. Freiman are held by Trust of Paul E. Freiman & Anna Mazzuchi Freiman, Dated May 24, 1996. |
| (9) | Includes 326,500 shares underlying stock options held by Dr. Altstiel that are vested as of January 6, 2016 or will vest within 60 days thereafter, 41,667 shares of Series B Stock and warrants to purchase 208,335 shares of our common stock, but does not include 58,500 shares underlying stock options that will not vest within 60 days after January 6, 2016. |
| (10) | Includes 232,007 shares underlying stock options held by Mr. Haft that are vested as of January 6, 2016 or will vest within 60 days thereafter, 41,667 shares of Series B Stock and warrants to purchase 208,335 shares of our common stock, but does not include 217,993 shares underlying stock options that will not vest within 60 days after January 6, 2016. |
| (11) | Includes 222,493 shares underlying stock options held by Mr. Singer that are vested as of January 6, 2016 or will vest within 60 days thereafter, but does not include 177,507 shares underlying stock options that will not vest within 60 days after January 6, 2016. |
| (12) | Includes 350,000 shares underlying stock options held by Mr. Weinstein that are vested as of January 6, 2016 or will vest within 60 days thereafter, 41,667 shares of Series B Stock and warrants to purchase 208,335 shares of our common stock, but does not include 400,000 shares underlying stock options that will not vest within 60 days after January 6, 2016. |
| (13) | Includes 193,370 shares underlying stock options held by Mr. Gottlieb that are vested as of January 6, 2016 or will vest within 60 days thereafter, but does not include 181,630 shares underlying stock options that will not vest within 60 days after January 6, 2016. |
| 89 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
SEC rules require us to disclose any transaction or currently proposed transaction in which we are a participant and in which any related person has or will have a direct or indirect material interest involving the lesser of $120,000.00 or one percent (1%) of the average of the Company’s total assets as of the end of last two completed fiscal years. A related person is any executive officer, director, nominee for director, or holder of 5% or more of the Company’s common stock, or an immediate family member of any of those persons.
The descriptions set forth above under the captions “Executive Compensation—Employment Agreements”, “Executive Compensation—Director Compensation” and “Description of Business—Neurotrope’s Proposed Products” and below under “Description of Securities—Options” are incorporated herein by reference.
Merger Agreement
On August 23, 2013, the Company, Acquisition Sub and Neurotrope BioScience entered into an Agreement and Plan of Merger and Reorganization, or the Merger Agreement, which closed on the same date. Pursuant to the terms of the Merger Agreement, Acquisition Sub merged with and into Neurotrope BioScience, which was the surviving corporation and thus became a wholly-owned subsidiary of Neurotrope, Inc.
Pursuant to the Reverse Merger, we ceased to engage in the business of providing software solutions to deliver geo-location targeted coupon advertising to mobile internet devices and acquired the business of Neurotrope BioScience to engage in developing two product platforms, including our diagnostic test for AD and a drug candidate called bryostatin for the treatment of AD, both of which are in the clinical testing stage. See “Split-Off” below.
At the closing of the Reverse Merger, (a) each of the 19,000,000 shares of Neurotrope BioScience Common Stock issued and outstanding immediately prior to the closing of the Reverse Merger was converted into one share of our common stock, and (b) each of the 21,920,000 shares of Neurotrope BioScience Series A stock issued and outstanding immediately prior to the closing of the Reverse Merger was converted into one share of our Series A stock (See “Description of Securities – Preferred Stock – Series A Stock” below for a description of the voting powers, preferences and other rights of our Series A Stock). As a result, an aggregate of (a) 19,000,000 shares of our common stock were issued to the holders of Neurotrope BioScience Common Stock, and (b) 21,920,000 shares of our Series A Stock were issued to the holders of Neurotrope BioScience Series A stock. In addition, warrants issued to the placement agents and permitted sub-agents to purchase 900,000 shares of Neurotrope BioScience’s common stock were converted into Agent Warrants (as defined below) to purchase 900,000 shares of our common stock, and warrants issued to the placement agents and permitted sub-agents to purchase 1,217,000 shares of Neurotrope BioScience’s Series A stock were converted into Agent Warrants to purchase 1,217,000 shares of our Series A Stock. Neurotrope BioScience did not have any other stock purchase warrants or any stock options outstanding at the time of the Reverse Merger. The warrants to purchase 900,000 shares of our common stock and 1,217,000 shares of our Series A Stock issued on conversion of the warrants issued by Neurotrope BioScience as described above are referred to, collectively, as the “Agent Warrants.”
The Merger Agreement contained customary representations and warranties and pre- and post-closing covenants of each party and customary closing conditions. Breaches of the representations and warranties will be subject to customary indemnification provisions, subject to specified aggregate limits of liability.
The Merger Agreement provides that until the second anniversary of the closing date of the Reverse Merger, in the event that the Company incurs damages (a) with respect to liabilities, obligations or indebtedness of the Split-Off Subsidiary (as defined below), whenever accruing, or of the Company accruing prior to the Reverse Merger and not previously disclosed, or (b) that a pre-Merger common stockholder of Neurotrope BioScience is entitled to under the indemnification provisions of the Merger Agreement, then the Company will issue to, in the case of clause (a) above, all of the pre-Merger common stockholders of Neurotrope BioScience, or, in the case of clause (b) above, such pre-Merger common stockholder of Neurotrope BioScience so entitled to indemnification, a number of shares of our stock that (in addition to the merger consideration shares to which such person was entitled) would result from dividing the whole dollar amount representing such damages by $1.00 (subject to equitable adjustment in the event of any stock split, stock dividend, reverse stock split or similar event). The limit on the aggregate number of shares of our stock issuable under this provision is 500,000 shares.
| 90 |
Pursuant to the Merger Agreement, the pre-Merger common stockholders of Neurotrope BioScience received initially only 95% of the Company common stock that they were entitled to receive; the remaining 5% of such shares, totaling 950,000 shares (the “Indemnification Escrow Shares”) were placed in escrow pursuant to an Indemnification Escrow Agreement among the Company, Dr. James New, as the then Indemnification Representative, and Gottbetter & Partners, LLP, as escrow agent. The Indemnification Escrow Shares were be released from escrow and distributed to such holders on or about August 23, 2015, pursuant to the terms of the Indemnification Escrow Agreement.
The Reverse Merger has been treated as a recapitalization of the Company for financial accounting purposes. Neurotrope BioScience has been considered the acquirer for accounting purposes, and the historical financial statements of Neurotrope, Inc., before the Reverse Merger will be replaced with the historical financial statements of Neurotrope BioScience before the Reverse Merger in all future filings with the SEC.
The parties have taken all actions necessary to ensure that the Reverse Merger is treated as a tax-free exchange under Section 351 of the Code.
The issuance of shares of our common stock and Series A Stock to holders of Neurotrope BioScience’s capital stock in connection with the Reverse Merger was not registered under the Securities Act, in reliance upon the exemption from registration provided by Section 4(2) of the Securities Act, which exempts transactions by an issuer not involving any public offering, and Regulation D promulgated by the SEC under that section. These securities may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirement, and are subject to further contractual restrictions on transfer as described below.
We also agreed not to register under the Securities Act the resale of the shares of our common stock received in the Reverse Merger by our officers, directors and key employees and holders of 10% or more of our Common Stock for a period of two years following the closing of the Reverse Merger.
The form of the Merger Agreement is filed as an exhibit to the registration statement of which this prospectus is a part. All descriptions of the Merger Agreement herein are qualified in their entirety by reference to the text thereof filed as an exhibit hereto, which is incorporated herein by reference.
Split-Off
Upon the closing of the Reverse Merger, under the terms of a split-off agreement and a general release agreement, the Company transferred all of its pre-Reverse Merger operating assets and liabilities to its wholly-owned special-purpose subsidiary, Blue Flash Communications Corp., a Nevada corporation, Split-Off Subsidiary, formed on August 15, 2013. Thereafter, pursuant to the split-off agreement, the Company transferred all of the outstanding shares of capital stock of Split-Off Subsidiary to Marisa Watson, the pre-Reverse Merger majority stockholder of the Company, and the former sole officer and director of the Company (the “Split-Off”), in consideration of and in exchange for (i) the surrender and cancellation of an aggregate of 20,178,000 shares of our common stock held by Ms. Watson (which were cancelled and will resume the status of authorized but unissued shares of our common stock) and (ii) certain representations, covenants and indemnities. All descriptions of the split-off agreement and the general release agreement herein are qualified in their entirety by reference to the text thereof filed as exhibits hereto, which are incorporated herein by reference.
The PPO
Concurrently with the closing of the Reverse Merger and in contemplation of the Reverse Merger, Neurotrope BioScience held a closing of its private placement offering, or the PPO, in which it sold 11,533,375 shares of Neurotrope BioScience Series A stock, at a price of $1.00 per share, for gross proceeds (before deducting commissions and expenses of the offering) of $11,533,375. This closing of the PPO and the closing of the Reverse Merger were conditioned upon each other.
Neurotrope BioScience had previously held closings of the PPO between February and May 2013 for 10,386,625 shares of Neurotrope BioScience Series A stock, for a purchase price of $1.00 per share, for aggregate gross proceeds of $10,386,625 (before deducting placement agent fees and expenses of the offering). All of the outstanding shares of Neurotrope BioScience Series A stock were converted on a one-for-one basis into shares of our Series A Stock in connection with the Reverse Merger.
Subsequent to the closing of the Reverse Merger, we held a final closing for 1,080,000 additional shares of our Series A Stock at $1.00 per share, resulting in gross proceeds of $1,080,000, for a total of $23,000,000 of gross proceeds raised between February and October 2013.
| 91 |
Northlea Partners LLLP, which is controlled by a former director, purchased an aggregate of 750,000 shares of Neurotrope BioScience Series A stock in the PPO for an aggregate purchase price of $750,000. Jay Haft, our director, purchased an aggregate of 25,000 shares of Neurotrope BioScience Series A stock in the PPO for an aggregate purchase price of $25,000.
Neurotrope BioScience agreed to pay the placement agents in the PPO, EDI Financial, Inc. and Allied Beacon Financial, Inc., registered broker-dealers, a cash commission of 10% of the gross funds raised from investors in the PPO. In addition, the placement agents received (a) for the first $12,000,000 of aggregate gross PPO proceeds (including the prior closings), (i) warrants exercisable for a period of ten (10) years to purchase a number of shares of Neurotrope BioScience Common Stock equal to 7.5% of the number of shares of Neurotrope BioScience Series A stock sold to investors introduced by them, with a per share exercise price of $0.01, and (ii) warrants exercisable for a period of ten (10) years to purchase a number of shares of Neurotrope BioScience Series A stock equal to 2.5% of the number of shares of Neurotrope BioScience Series A stock sold to investors introduced by them, with a per share exercise price of $1.00; and (b) on aggregate gross PPO proceeds in excess of $12,000,000, warrants exercisable for a period of ten (10) years to purchase a number of shares of Neurotrope BioScience Series A stock equal to 10% of the number of shares of Neurotrope BioScience Series A stock sold to investors introduced by them, with an exercise price of $1.00 per share.
As a result of the foregoing, the placement agents and their permitted sub-agents were paid an aggregate commission of $2,225,000 and were issued warrants to purchase an aggregate of 900,000 shares of Neurotrope BioScience Common Stock and warrants to purchase an aggregate of 1,325,000 shares Neurotrope BioScience Series A stock, which were converted into Agent Warrants to purchase an aggregate of 900,000 shares of our common stock and an aggregate of 1,325,000 shares of our Series A Stock, as described more fully above. Subsequently, on February 9, 2015, the holders of placement agent warrants to purchase an aggregate of 1,325,000 shares of our Series A stock entered into Conversion Agreements with us pursuant to which such holders’ placement agent warrants to purchase Series A Stock were converted into common stock purchase warrants, for no additional consideration. The shares of common stock issuable upon exercise of such common stock purchase warrants are registered pursuant to this Registration Statement. Neurotrope BioScience was also required to reimburse the placement agents $25,000 of legal expenses incurred in connection with the PPO. Neurotrope BioScience agreed to indemnify the placement agents and their sub-agents to the fullest extent permitted by law, against certain liabilities that may be incurred in connection with this PPO, including certain civil liabilities under the Securities Act, and, where such indemnification is not available, to contribute to the payments the placement agents and its sub-agents may be required to make in respect of such liabilities.
The PPO was exempt from registration under Section 4(2) of the Securities Act in reliance upon the exemption provided by Rule 506(b) of Regulation D promulgated by the SEC thereunder. The PPO was sold to “accredited investors,” as defined in Regulation D.
A description of the voting powers, preferences and other rights of our Series A stock is set forth below under the caption “Description of Securities—Preferred Stock—Series A Stock,” which description is incorporated herein by reference. A copy of the Certificate of Designations, Preferences and Rights for the Series A Convertible Preferred Stock, as filed with the Nevada Secretary of State, is filed as an exhibit to the registration statement of which this prospectus is a part.
The holders of the Agent Warrants have agreed that, until the Company raises at least $25,000,000 in a financing subsequent to the PPO, our Board of Directors (acting through at least a majority) will have the right to vote any shares of our common stock issued upon exercise of Agent Warrants.
All descriptions of the Agent Warrants herein are qualified in their entirety by reference to the text thereof filed as exhibits hereto, which are incorporated herein by reference.
Several of our directors and executive officers participated in the PPO, including Messrs. Ramat and Freiman, who each invested $100,000, and Messrs. Weinstein, Haft and Altstiel, who each invested $25,000. Additionally, former director Dr. Abeles invested $125,000 through Northlea Partners, LLLP and has sole voting and investment power over the shares owned thereby. Dr. Abeles continues to serve the company as a consultant and has agreed to receive a total of 166,667 restricted units in lieu of receiving $9,000 per month (for up to $100,000). The securities underlying Dr. Abeles’s units will contain certain restrictions, including that such restrictions shall lapse with respect to 15,000 shares of Series B Stock and a corresponding portion of the warrants on a monthly basis, for services performed in the preceding month by Dr. Abeles.
| 92 |
Registration Rights
In connection with the PPO, we entered into a Preferred Stockholders Agreement, pursuant to which we agreed to file a registration statement registering for resale the shares of our common stock underlying the shares of our Series A Stock issued in the Reverse Merger in exchange for the shares of Neurotrope BioScience Series A stock sold in the PPO, or the Registrable Securities, as follows: If at any time after the earlier of (i) five years after August 23, 2013 or (ii) 180 days after the effective date of the registration statement for an underwritten public offering of our equity securities under the Securities Act with a price to the public of at least $5.00 per share and aggregate gross proceeds to us of at least $30,000,000, or a QPO, we receive a request from holders of at least 40% of the Registrable Securities then outstanding that we file a Form S-1 registration statement with the SEC with respect to Registrable Securities having an anticipated aggregate offering price, net of selling expenses, of at least $15,000,000, then we shall (x) within ten (10) days after the date such request is given, give notice thereof to all other holders of Registrable Securities; and (y) as soon as practicable, and in any event within sixty (60) days after the date such request is given, file a Form S-1 registration statement under the Securities Act covering all Registrable Securities that the initiating holders requested to be registered and any additional Registrable Securities requested to be included in such registration by any other holders, subject to certain limitations.
If at any time when we are eligible to use a Form S-3 registration statement, we receive a request from holders of at least thirty percent (30%) of the Registrable Securities then outstanding that we file a Form S-3 registration statement with respect to outstanding Registrable Securities of such holders having an anticipated aggregate offering price, net of selling expenses, of at least $1,000,000, then we shall (i) within ten (10) days after the date such request is given, give a notice to all other holders of Registrable Securities; and (ii) as soon as practicable, and in any event within forty-five (45) days after the date such request is given, file a Form S-3 registration statement under the Securities Act covering all Registrable Securities requested to be included in such registration, subject to certain limitations.
If we propose to register (including, for this purpose, a registration effected by us for stockholders other than the holders of Series A stock) any of its common stock under the Securities Act in connection with the public offering of such securities solely for cash (other than certain excluded registrations), we shall give each holder of Registrable Securities notice of such registration, and upon the request of each holder, shall, subject to certain limitations, cause to be registered all of the Registrable Securities that each such Holder has requested to be included in such registration.
We have agreed to maintain the effectiveness of any registration statement referred to above for at least 120 days after the date on which the registration statement is declared effective by the SEC, or, if earlier, until the distribution contemplated by the registration statement has been completed.
We will pay all expenses in connection with any registration obligation provided in the registration rights agreement, including, without limitation, all registration, filing, stock exchange fees, printing expenses, all fees and expenses of complying with applicable securities laws, and the fees and disbursements of our counsel and of our independent accountants. Each investor will be responsible for its own underwriting discounts and commissions, transfer taxes and the expenses of any attorney or other advisor such investor decides to employ.
Registration rights shall terminate upon the earliest of (i) the date when shares of registrable securities are eligible to be sold without restriction under Rule 144, (ii) the closing of a Deemed Liquidation Event, as such term is defined in our certificate of incorporation and (iii) the fifth anniversary of the effective date of the company’s first QPO after the closing date of the Reverse Merger.
All descriptions of the Preferred Stockholders Agreement herein are qualified in their entirety by reference to the text thereof filed as an exhibit hereto, which is incorporated herein by reference.
The holders of the Agent Warrants have “piggyback” registration rights for the shares common stock underlying the Agent Warrants (including shares of common stock issuable upon conversion of the Series A Stock underlying the Agent Warrants) with respect to any registration statement filed by the Company that would permit the inclusion of such underlying shares, and subject to customary limitations.
| 93 |
On September 3, 2014, we entered into Amendment No. 1 to the Preferred Stockholders Agreement with certain of our prior investors (“Amendment No. 1”), which removed provisions relating to the right of first refusal and certain prohibitions relating to transfers, which were set forth in the Preferred Stockholders Agreement. In connection with the November 2015 Private Placement, we entered into Amendment No. 2 to the Preferred Stockholders Agreement with certain of our prior investors (“Amendment No. 2”), which amended the Preferred Stockholders Agreement. Amendment No. 2 modified certain registration rights held by the stockholders party to such agreement, such as excluding the Securities Purchase Agreement, the Registration Rights Agreement and related agreements from certain “piggy-back” registration rights and limitations on subsequent registration rights. Pursuant to the Amendment No. 2, we also agreed to amend certain restrictions on transfer and waive the lock-up in the Preferred Stockholders Agreement. Further, we agreed that promptly following the filing of a registration statement pursuant to the Registration Rights Agreement, if we are then eligible to do so, we will prepare and file a post-effective amendment on Form S-3 with the SEC, to convert the registration statement on Form S-1 (SEC file number 333-200664) that we filed with the SEC on December 1, 2014, as amended, into a registration statement on Form S-3.
The Series B Offering
On November 13, 2015, we entered into a Securities Purchase Agreement to sell up to 26,234,940 units in a private placement at a per unit purchase price equal to $0.60. Each unit consisted of (i) one one-hundredth share of Series B Stock convertible into one share of our common stock, (ii) one warrant to acquire, at an exercise price of $0.80 per share with an expiration date five years from the date of issuance, one share of our common stock (the “Series A Warrant”), (iii) one warrant to acquire, at an exercise price of $0.80 per share with an expiration date of one year from the date of issuance, one share of our common stock (the “Series B Warrant”), (iv) one warrant to acquire, at an exercise price of $1.25 per share with an expiration date of five years from the issuance date, one share of our common stock (the “Series C Warrant”), (v) one warrant, which is contingent upon the exercise of the Series B Warrant, to acquire, at an exercise price of $1.00 per share with an expiration date that is five years from the date of the initial exercise of the Series B Warrant, one share of our common stock (the “Series D Warrant”), and (vi) one warrant, which is contingent upon the exercise of the Series C Warrant, to acquire, at an initial exercise price of $1.50 per share with an expiration date that is five years from the date of the initial exercise of the Series C Warrant, one share of our common stock (the “Series E Warrant”, and together with the Series A Warrant, the Series B Warrant, the Series C Warrant and the Series D Warrant, the “Investor Warrants”). The exercise prices of the Investor Warrants are initially subject to full protection for dilutive issuances. The Series A Warrant and Series B Warrant each contain a mandatory exercise right of ours to force exercise of the warrant if our common stock trades at or above $1.50 for 20 consecutive trading days (subject to certain conditions, including a $150,000 minimum daily volume requirement). The Series C Warrant contains a mandatory exercise right of the Company to force exercise of the warrant if our common stock trades at or above $2.00 for 20 consecutive trading days (subject to certain conditions, including a $150,000 minimum daily volume requirement). In addition, pursuant to the terms of the Securities Purchase Agreement, we may sell, in one additional closing on the same terms and conditions as those contained in the purchase agreement, additional units to one or more buyers (each, an “Additional Investor”), each of which is either (a) an institutional investor that focuses on the biotech industry, (b) an investor of our Series A Stock, (c) any investor investing under $5,000 or (d) any person approved in writing by each of those selling stockholders who are institutional investors and such selling stockholder’s purchase price (together with such selling stockholder’s institutional affiliates) equals or exceeds $1,000,000 (the “Large Buyers”). The Large Buyers also have certain consent rights with respect to any Additional Investor. In connection with the private placement, the holders of our Series A Stock consented to convert their holdings into common stock. The closing of the private placement was subject to customary closing conditions and was completed in two closings, which took place on November 13, 2015 and November 30, 2015. The gross proceeds from the closing of the initial portion of the private placement were approximately $15,330,000 and from the second portion of the private placement were $311,000.
Registration Rights Agreement
In connection with the signing of the Securities Purchase Agreement, the Company and the investors entered into a registration rights agreement (the “Registration Rights Agreement”) on November 13, 2015. Under the terms of the Registration Rights Agreement, the Company agreed to prepare and file with the SEC a registration statement covering the resale of 150% of the number of shares underlying the Series B Stock, the Investor Warrants and the placement agent warrants within 30 days following the date of the initial closing under the Securities Purchase Agreement. The Company will use its best efforts to have the Registration Statement declared effective by the SEC by the earlier of the (A) 90th calendar day after the initial closing date or, in the event that the SEC or the SEC staff cause a delay in the effectiveness of such Registration Statement due to comments regarding the number of shares being registered, then the 120th calendar day after the initial closing date and (B) 2nd Business Day after the date the Company is notified (orally or in writing, whichever is earlier) by the SEC that such Registration Statement will not be reviewed or will not be subject to further review. In the event that the Company fails to timely file or achieve effectiveness, maintain the effectiveness of the Registration Statement or to file any reports with respect to certain public information, then the Company must pay to each holder of the Registrable Securities an amount in cash equal to 1% of such holder’s Stated Value (as such term is defined in the Series B COD) of its Series B Stock on the date of such failure and 2% of such holder’s Stated Value of its Series B Stock on every 30 day anniversary until such failure is cured.
| 94 |
Common Stockholders’ Agreement
The Company entered into an Amended and Restated Stockholders Agreement, or the Common Stockholders Agreement, dated August 23, 2013, with all of the holders of Neurotrope BioScience Common Stock prior to the Reverse Merger: Neurosciences Research Ventures, Inc., or NRV, Dr. Daniel Alkon, Northlea Partners LLLP, or Northlea and Dr. James New, which amended and restated in its entirety the agreement between such stockholders and Neurotrope BioScience in effect prior to the Reverse Merger. Pursuant to the Common Stockholders Agreement, the parties agreed to certain corporate governance matters pertaining to the Company (including with respect to the composition of the Board of Directors) and to certain restrictions on the transfer of shares of common stock held by such parties.
On August 5, 2014, following receipt of a notice from Northlea Partners LLLP removing Dr. James New as one of Northlea’s director designees pursuant to the Common Stockholders Agreement, stockholders of the Company, representing not less than two-thirds of the voting power of the issued and outstanding common stock entitled to vote, acted by written consent to remove Dr. James New from the Company’s board of directors, effective as of August 5, 2014.
On November 12, 2015, the Company, NRV, Dan Alkon and Northlea Partners LLLP agreed to terminate the Amended and Restated Stockholders Agreement in its entirety. On November 12, 2015, the Company and NRV entered into a letter agreement pursuant to which the Company agreed to take such reasonable actions within its control so that two (2) representatives designated by NRV are nominated for election to the board of directors of the Company at each annual meeting of stockholders until such time as the BRNI License is no longer in effect. Furthermore, the Company will use its best efforts to ensure that (i) each NRV Designee is included in the Board’s slate of nominees to the stockholders for each election of directors, and (ii) each NRV designee is included in the proxy statement for every meeting of the stockholders of the Company called with respect to the election of members of the Board of Directors. Subject to applicable law and stock exchange rules, no NRV designee shall be removed from the Board unless such removal is for cause or requested in writing by NRV. In the event that any NRV designee shall cease to serve for any reason, NRV shall be entitled to designate such person’s successor and the Board will promptly fill the vacancy with such successor nominee and such designee will serve the remainder of the term of the director whom such designee replaces. Under the letter agreement, if an NRV designee is not appointed or elected to the Board because of such person’s death, disability, disqualification, withdrawal as a nominee or for other reason is unavailable or unable to serve on the Board, NRV is entitled to designate another nominee for such Board seat.
Voting Agreement
The Company, NRV, Northlea, Dr. New, Dr. Alkon, HRH, and Gottbetter entered into a Voting Agreement, or Voting Agreement, dated as of August 23, 2013, pursuant to which HRH and such other stockholder (which holds 488,079 shares of common stock) agreed to vote all of their common stock such that:
| · | the authorized number of directors of the Company will be seven, with six directors elected by the holders of a majority of the outstanding common stock, voting as a separate class (each, a “Common Director”) and one director elected by the holders of a majority of the outstanding Series A Stock, voting as a separate class on an as-converted basis; |
| · | five persons were nominated and elected to the Board as Common Directors as follows: |
| o | two NRV Designees, who are currently William S. Singer and James Gottlieb; |
| 95 |
| o | two Abeles Designees, who initially were Dr. John Abeles and Dr. James New; and |
| o | one independent Neurotrope Designee; and |
| · | subject to the provisions of applicable law, no NRV Designee, Abeles Designee or Neurotrope Designee shall be removed from the Board unless such removal is requested in writing by the party that designated such designee. |
In addition, each of NRV, Northlea, Drs. New and Alkon agreed to vote all of their common stock in favor of electing as a Common Director after the Reverse Merger one representative designated by HRH (the “HRH Designee”) as soon as practicable after such representative has been identified by HRH. NRV, Abeles, New and Alkon agreed to vote their common stock in favor of the election of the HRH Designee only at the time of such individual’s initial appointment to the Parent’s Board, and nothing obligates them to vote in favor of the election of any other individual as an HRH Designee or in favor of the continuing service of the HRH Designee once elected to the Board.
As mentioned above, on August 5, 2014, following receipt of a notice from Northlea Partners LLLP removing Dr. James New as one of Northlea’s director designees pursuant to the Common Stockholders Agreement, stockholders of the Company, representing not less than two-thirds of the voting power of the issued and outstanding common stock entitled to vote, acted by written consent to remove Dr. James New from the Company’s Board of Directors, effective as of August 5, 2014. On September 12, 2014, the Company’s Board of Directors elected Dr. Altstiel to fill the vacancy on the Board of Directors caused by Dr. New’s departure. Northlea nominated Dr. Altstiel to serve as one of the two designees to the Board appointed by the Majority Abeles Stockholder pursuant to the Common Stockholders’ Agreement.
The Voting Agreement was terminated on November 12, 2015.
For information on numbers of shares of our stock held by NRV, Abeles, New, Alkon and HRH, see “Security Ownership of Certain Beneficial Owners and Management” above.
2013 Equity Incentive Plan
Before the Reverse Merger, our Board of Directors adopted, and our stockholders approved, the 2013 Plan. On July 23, 2014, our Board of Directors approved an amendment to our 2013 Plan to increase the number of shares of our common stock issuable thereunder by an additional 3,000,000 shares, so that the Company’s officers, key employees, consultants and directors can be granted stock options and other equity incentive awards with respect to an aggregate of 10,000,000 shares of our common stock. See “Market Price of and Dividends on Common Equity and Related Stockholder Matters - Securities Authorized for Issuance under Equity Compensation Plans” below for more information about the 2013 Plan and the outstanding stock options.
Departure and Appointment of Directors and Officers
Our Board of Directors is authorized to consist of seven members. On the closing date of the Reverse Merger, Ronald Warren, our sole director before the Reverse Merger, resigned his position as a director, and John Abeles, James New, William Singer, Ralph Bean and Jay Haft were appointed to our Board of Directors. (Mr. Bean subsequently resigned and was replaced by James Gottlieb.) As mentioned above, on August 5, 2014, Dr. James New was removed from our Board of Directors. Also, on November 12, 2015, Dr. Abeles resigned from the Board of Directors.
Also on the closing date of the Reverse Merger, Mr. Warren, our President, Secretary, Treasurer and sole officer before the Reverse Merger, resigned from these positions, and James New was appointed as our Chief Executive Officer and President, Robert Weinstein was appointed as our Chief Financial Officer and Treasurer, William Singer was appointed as our Secretary and Dan Alkon was appointed our Chief Scientific Officer by the Board. On July 16, 2014, James New’s employment as Chief Executive Officer and President terminated effective on such date. Our Board of Directors appointed Charles S. Ramat and Paul E. Freiman to serve as Co-Chief Executive Officers, on an interim basis, of the Company, effective on July 16, 2014. On September 12, 2014, Mr. Ramat’s and Mr. Freiman’s appointments as interim Co-Chief Executive Officer terminated. On that date, Mr. Ramat was appointed as the Company’s sole President and Chief Executive Officer and Mr. Freiman became the sole Chairman of the Board.
| 96 |
See “Directors, Executive Officers, Promoters and Control Persons – Directors and Executive Officers” above for information about our directors and executive officers.
Lock-up Agreements and Other Restrictions
On November 12, 2015, we entered into a lock-up agreement with Dr. John Abeles and Northlea Partners LLLP. Pursuant to the terms of the lock-up agreement, Dr. John Abeles and Northlea Partners LLLP agreed not to pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of our Series B Stock and Series A through Series E warrants, or any securities underlying, convertible into or exercisable or exchangeable for our Series B Stock and Series A through Series E warrants. The lock-up agreement will automatically terminate upon the earliest to occur, if any, of (1) the second anniversary of the date of the final closing relating to the offering of our Series B Stock and Series A through Series E warrants, and (2) January 1, 2016, in the event that the final closing relating to the offering of our Series B Stock and Series A through Series E warrants has not occurred by that date.
BRNI License
On February 4, 2015, Neurotrope BioScience, BRNI and NRV II, LLC entered into an Amended and Restated Technology License and Services Agreement (the “BRNI License”), which further amended and restated the Technology License and Services Agreement dated as of October 31, 2012, as amended by Amendment No. 1 dated as of August 21, 2013. The BRNI License was subsequently amended on November 12, 2015.
Pursuant to the BRNI License, Neurotrope BioScience maintained its exclusive (except as described below), non-transferable (except pursuant to the BRNI License’s assignment provision), world-wide, royalty-bearing right, with a right to sublicense (in accordance with the terms and conditions described below), under BRNI’s and NRV II’s respective right, title and interest in and to certain patents and technology owned by BRNI or licensed to NRV II, LLC by BRNI as of or subsequent to October 31, 2012 to develop, use, manufacture, market, offer for sale, sell, distribute, import and export certain products or services for therapeutic applications for AD and other cognitive dysfunctions in humans or animals (the “Field of Use”). Additionally, the BRNI License specifies that all patents that issue from a certain patent application, shall constitute licensed patents and all trade secrets, know-how and other confidential information claimed by such patents constitute licensed technology under the BRNI License.
Notwithstanding the above license terms, BRNI and its affiliates retain rights to use the licensed intellectual property in the Field of Use to engage in research and development and other non-commercial activities and to provide services to Neurotrope BioScience or to perform other activities in connection with the BRNI License.
Under the BRNI License, BRNI is a preferred service provider in certain circumstances and Neurotrope BioScience may not enter into sublicense agreements with third parties except with BRNI’s prior written consent, which consent shall not be commercially unreasonably withheld. Furthermore, the BRNI License dated February 4, 2015 revises the agreement that was entered into as of October 31, 2012 and amended on August 21, 2013, in that it provides that any intellectual property developed, conceived or created in connection with a sublicense agreement that Neurotrope BioScience entered into with a third party pursuant to the terms of the BRNI License will be licensed to BRNI and its affiliates for any and all non-commercial purposes, on a worldwide, perpetual, non-exclusive, irrevocable, non-terminable, fully paid-up, royalty-free, transferable basis, with the right to freely sublicense such intellectual property. Previously, the agreement had provided that such intellectual property would be assigned to BRNI.
Under the BRNI License, BRNI and Neurotrope BioScience will jointly own data, reports and information that is generated on or after February 28, 2013, by Neurotrope BioScience, on behalf of Neurotrope BioScience by a third party or by BRNI pursuant to a statement of work that the parties enter into pursuant to the BRNI License, in each case to the extent not constituting or containing any data, reports or information generated prior to such date or by BRNI not pursuant to a statement of work (the “Jointly Owned Data”). BRNI has agreed not to use the Jointly Owned Data inside or outside the Field of Use for any commercial purpose during the term of the BRNI License or following any expiration of the BRNI License other than an expiration that is the result of a breach by Neurotrope BioScience of the BRNI License that caused any licensed patent to expire, become abandoned or be declared unenforceable or invalid or caused any licensed technology to enter the public domain (a “Natural Expiration”) provided, however, BRNI may use the Jointly Owned Data inside or outside the Field of Use for any commercial purpose following any termination of the BRNI License. Also, BRNI granted Neurotrope BioScience a license during the term and following any Natural Expiration, to use certain BRNI data in the Field of Use for any commercial purposes falling within the scope of the license granted to Neurotrope BioScience under the BRNI License.
| 97 |
The BRNI License further requires us to pay BRNI (i) a fixed research fee equal to a pro-rata amount of $1 million in the year during which we close on a $25 million round of financing, (ii) a fixed research fee of $1 million per year for each of the five calendar years following the completion of such financing and (iii) an annual fixed research fee in an amount to be negotiated and agreed upon no later than 90 days prior to the end of the fifth calendar year following the completion of such financing to be paid with respect to each remaining calendar year during the term of the BRNI License.
On November 12, 2015, we entered into an amendment to the BRNI License. The amendment eliminates the requirement that Neurotrope Bioscience pay BRNI prepaid royalties equal to five percent (5%) of financing proceeds received by Neurotrope Bioscience in any financing prior to a public offering and provides that Neurotrope Bioscience will deliver to BRNI, following each closing pursuant to a certain securities purchase agreement, an amount equal to 2.5% of the Post-PA Fee Proceeds received at such closing. In addition, the Amendment provides that on or prior to December 31, 2016, Neurotrope Bioscience shall deliver to BRNI an amount equal to 2.5% of the aggregate Post-PA Fee Proceeds received at the closings. Each payment would constitute an advance royalty payment to BRNI and will be offset (with no interest) against the amount of future royalty obligations payable until such time that the amount of such future royalty obligations equals in full the amount of the advance royalty payments made. “Post-PA Fee Proceeds” means the gross proceeds received, less all amounts paid to the placement agent(s), in relation to such gross proceeds. No other expenses of Neurotrope Bioscience shall be subtracted from the gross proceeds to determine the “Post-PA Fee Proceeds.”
The term of the BRNI License continues until the later of the date (i) the last of the licensed patents expires, is abandoned or is declared unenforceable or invalid (in each case, determined in accordance with the BRNI License) and (ii) the last of the licensed technology enters the public domain. Either party has the right to terminate the BRNI License after 30 days prior notice in certain circumstances, including if either party were to materially breach any provisions of the BRNI License and does not cure such material breach within 60-days from notice of such material breach from the non-breaching party, for breaches that are capable of being cured, in the event of certain bankruptcy or insolvency proceedings or in the event of the termination of a certain Stockholders Agreement dated August 23, 2013, with respect to us.
Our rights to develop, commercialize and sell our proposed products are dependent upon the BRNI License with BRNI. Neurosciences Research Ventures, Inc., which is an affiliate of BRNI, beneficially owns 21.9% of our outstanding common stock, and Dr. Dan Alkon, who is the Scientific Director of BRNI and the Chief Scientific Officer of the Company, beneficially owns 2.3% of our outstanding common stock. We are required to pay significant fees and royalties to BRNI pursuant to the BRNI License.
The Company’s director, William S. Singer, also currently serves as President of BRNI and serves as a member of BRNI’s board of directors.
Statements of Work
Effective August 28, 2013, we signed a statement of work (the “2013 SOW”) with BRNI pursuant to the BRNI License, whereby we contracted for the further development of its AD diagnostic product. The project is intended to validate each of three biomarkers in a heterogeneous patient population to determine sensitivity and selectivity parameters for each biomarker, or combination of biomarkers, to detect AD. The three biomarkers to be evaluated are: the PKCε levels, the Erk1/2 ratios, and the fibroblast morphology test. Pursuant to the 2013 SOW, we paid BRNI a total of $1,645,470 in twelve equal monthly installments of $137,123. These payments were for operating expenses associated with BRNI’s diagnostic laboratories.
Effective November 13, 2013, we agreed to a statement of work with BRNI pursuant in which we contracted for the further development of our potential therapeutic product. Pursuant to this statement of work, we paid BRNI $251,939 for related personnel and research services. The services provided pursuant to this statement of work were completed in 2014.
As of March 12, 2014, we entered into a statement of work with BRNI to continue pre-clinical activities relating to the commercialization of our therapeutic product. We paid BRNI the entire total pursuant to this statement of work of approximately $465,000 during the nine months ended September 30, 2014. The services provided pursuant to this statement of work were completed in 2014.
| 98 |
On February 4, 2015, we entered into a Statement of Work and Account Satisfaction Agreement with BRNI (the “February 2015 SOW”), which was effective as of October 1, 2014 and expired on September 30, 2015. Under the February 2015 SOW, we agreed, among other things, to pay BRNI twenty thousand dollars ($20,000) in quarterly payments during the twelve months from the date of the February 2015 SOW in exchange for advising and consulting services by BRNI’s chief scientist regarding our contract with Icahn School of Medicine at Mt. Sinai Hospital for the use of bryostatin in the treatment of Niemann Pick disease. The February 2015 SOW terminated and replaced the 2013 SOW and the parties agreed that neither party has or shall have any rights, claims damages or obligations for services or costs pursuant to the 2013 SOW.
On November 12, 2015, we entered into a Statement of Work Agreement pursuant to the BRNI License Agreement (the “November 2015 SOW Agreement”), which replaced the February 2015 SOW Agreement. Pursuant to the November 2015 SOW Agreement, Neurotrope Bioscience agreed to pay BRNI one million one hundred sixty six thousand six hundred sixty six dollars ($1,166,666) in service fees payable in the amount of eighty three thousand three hundred thirty three dollars ($83,333) per month for each month from November 1, 2015 through December 31, 2016. The payments under the November 2015 SOW Agreement will satisfy Neurotrope Bioscience’s obligations to reimburse BRNI pursuant to Section 5.6 of the BRNI License for any and all attorneys’ fees, translation costs, filing fees, maintenance fees, and other costs and expenses related to applying for, filing, prosecuting, and maintaining patents and applications for the licensed intellectual property incurred by BRNI during the term of the November 2015 SOW Agreement (but, for the avoidance of doubt, such payments shall not satisfy any attorneys’ fees, translation costs, filing fees, maintenance fees, or other costs or expenses related to applying for, filing, prosecuting, and maintaining patents and applications for the licensed intellectual property incurred by BRNI after the expiration or termination of the November 2015 SOW Agreement term), as well as any litigation costs which BRNI may incur related to any of the licensed intellectual property during the November 2015 SOW Agreement term. BRNI shall not commence any litigation to enforce the licensed intellectual property without the consent of Neurotrope (which consent shall not be unreasonably withheld, delayed, or denied).
In consideration for the payments made pursuant to the November 2015 SOW Agreement, BRNI shall perform the services requested by Neurotrope Bioscience for the further development of Neurotrope’s bryostatin drug platform. In addition, under the terms of the November 2015 SOW Agreement, BRNI may enroll one (1) additional compassionate use, in addition to the compassionate use patient currently enrolled, in trials of BRNI’s Alzheimer’s therapeutic drug platform during the November 2015 SOW Agreement term, and the payments set forth above, shall satisfy any and all of Neurotrope Bioscience’s obligation whatsoever to BRNI or to any other third party for costs incurred or to be incurred by BRNI relating to such trials. Neurotrope Bioscience and BRNI shall jointly review protocols which shall be established to the parties’ mutual satisfaction and contain appropriate safety measures to be employed by the treating physician. No additional compassionate use or expanded access patients will be enrolled by BRNI without the consent of Neurotrope Bioscience.
We are registering the shares of common stock issuable upon conversion of the Series B Stock and exercise of the warrants to permit the resale of these shares of common stock by the holders of the Series B Stock and warrants from time to time after the date of this prospectus. We will not receive any of the proceeds from the sale by the selling stockholders of the shares of common stock. We will bear all fees and expenses incident to our obligation to register the shares of common stock.
The selling stockholders may sell all or a portion of the shares of common stock held by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the shares of common stock are sold through underwriters or broker-dealers, the selling stockholders will be responsible for underwriting discounts or commissions or agent’s commissions. The shares of common stock may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions, pursuant to one or more of the following methods:
| · | on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale; |
| · | in the over-the-counter market; |
| 99 |
| · | in transactions otherwise than on these exchanges or systems or in the over-the-counter market; |
| · | through the writing or settlement of options, whether such options are listed on an options exchange or otherwise; |
| · | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| · | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| · | an exchange distribution in accordance with the rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | short sales made after the date the registration statement of which this prospectus is a part is declared effective by the SEC; |
| · | agreements between broker-dealers and the selling security holders to sell a specified number of such shares at a stipulated price per share; |
| · | a combination of any such methods of sale; and |
| · | any other method permitted pursuant to applicable law. |
The selling stockholders may also sell shares of common stock under Rule 144 promulgated under the Securities Act of 1933, as amended, if available, rather than under this prospectus. In addition, the selling stockholders may transfer the shares of common stock by other means not described in this prospectus. If the selling stockholders effect such transactions by selling shares of common stock to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling stockholders or commissions from purchasers of the shares of common stock for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved). In connection with sales of the shares of common stock or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the shares of our common stock in the course of hedging in positions they assume. The selling stockholders may also sell shares of our common stock short and deliver shares of our common stock covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. The selling stockholders may also loan or pledge shares of our common stock to broker-dealers that in turn may sell such shares.
The selling stockholders may pledge or grant a security interest in some or all of the Series B Stock, warrants or shares of our common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of our common stock from time to time pursuant to this prospectus or any amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending, if necessary, the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus. The selling stockholders also may transfer and donate the shares of common stock in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
To the extent required by the Securities Act and the rules and regulations thereunder, the selling stockholders and any broker-dealer participating in the distribution of the shares of common stock may be deemed to be “underwriters” within the meaning of the Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the shares of our common stock is made, a prospectus supplement, if required, will be distributed, which will set forth the aggregate amount of shares of our common stock being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling stockholders and any discounts, commissions or concessions allowed or re-allowed or paid to broker-dealers.
| 100 |
Under the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the shares of our common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any selling stockholder will sell any or all of the shares of our common stock registered pursuant to the registration statement, of which this prospectus forms a part.
The selling stockholders and any other person participating in such distribution will be subject to applicable provisions of the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder, including, without limitation, to the extent applicable, Regulation M of the Exchange Act, which may limit the timing of purchases and sales of any of the shares of common stock by the selling stockholders and any other participating person. To the extent applicable, Regulation M may also restrict the ability of any person engaged in the distribution of the shares of common stock to engage in market-making activities with respect to the shares of common stock. All of the foregoing may affect the marketability of the shares of common stock and the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.
We will pay all expenses of the registration of the shares of common stock pursuant to the registration rights agreement, estimated to be $72,000 in total, including, without limitation, Securities and Exchange Commission filing fees and expenses of compliance with state securities or “blue sky” laws; provided, however, a selling stockholder will pay all underwriting discounts and selling commissions, if any. We will indemnify the selling stockholders against liabilities, including some liabilities under the Securities Act in accordance with the registration rights agreements or the selling stockholders will be entitled to contribution. We may be indemnified by the selling stockholders against civil liabilities, including liabilities under the Securities Act that may arise from any written information furnished to us by the selling stockholder specifically for use in this prospectus, in accordance with the related registration rights agreements or we may be entitled to contribution.
Once sold under the registration statement, of which this prospectus forms a part, the shares of common stock will be freely tradable in the hands of persons other than our affiliates.
State Securities Blue Sky Laws
Transfers of our common stock may be restricted under the securities laws promulgated by various states and foreign jurisdictions, commonly referred to as “Blue Sky” laws. Absent compliance with such laws, our common stock may not be traded in such jurisdictions. Because our common stock registered hereunder has not been registered for resale under the “Blue Sky” laws of any state, the holders of our common stock and persons who desire to purchase our common stock in any trading market that might develop in the future, should be aware that there may be significant state “Blue Sky” law restrictions regarding the ability of investors to sell our common stock and of purchasers to purchase our common stock. Accordingly, investors may not be able to liquidate our common stock and should be prepared to hold our common stock for an indefinite period of time.
Thirty-three states have what is commonly referred to as a “manuals exemption” for secondary trading of securities such as those to be resold by selling stockholders under this registration statement. In these states, so long as we obtain and maintain a listing in Mergent, Inc. or Standard and Poor’s Corporate Manual, secondary trading of our common stock can occur without any filing, review or approval by state regulatory authorities in these states. These states are Alaska, Arizona, Arkansas, Colorado, Connecticut, District of Columbia, Florida, Hawaii, Idaho, Indiana, Iowa, Kansas, Maine, Maryland, Massachusetts, Michigan, Mississippi, Missouri, Nebraska, New Jersey, New Mexico, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Rhode Island, South Carolina, Texas, Utah, Washington, West Virginia and Wyoming. We cannot secure this listing, and thus qualification, until after this registration statement is declared effective. When we secure this listing, secondary trading of our common stock can occur in these states without further action.
In order to comply with the applicable securities laws of certain states, the securities may not be offered or sold, unless they have been registered or qualified for sale in such states or an exemption from such registration or qualification requirement is available and with which we have complied. The purchasers in this offering and in any subsequent trading market must be residents of such states where the shares have been registered or qualified for sale or an exemption from such registration or qualification requirement is available.
| 101 |
Authorized Capital Stock
We have authorized capital stock consisting of 300,000,000 shares of common stock, $0.0001 par value, and 50,000,000 shares of preferred stock, $0.0001 par value, 24,325,000 of which shares have been designated as Series A Stock and 333,333 of which shares have been designated as Series B Stock. As of January 6, 2016, there were 49,172,026 shares of common stock issued and outstanding, 0 shares of Series A Stock issued and outstanding and 262,349.4 shares of Series B Stock issued and outstanding.
Common Stock
The holders of our common stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends at such times and in such amounts as the Company’s Board of Directors from time to time may determine. Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. There is no cumulative voting of the election of directors then standing for election. Our common stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon liquidation, dissolution or winding up of the Company, the assets legally available for distribution to stockholders are distributable ratably among the holders of our common stock after payment of liabilities, accrued dividends and liquidation preferences, if any. Each outstanding share of our common stock is duly and validly issued, fully paid and non-assessable.
Preferred Stock
Shares of preferred stock may be issued from time to time in one or more series, each of which will have such distinctive designation or title as shall be determined by our Board of Directors prior to the issuance of any shares thereof. Preferred stock will have such voting powers, full or limited, or no voting powers, and such preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof, as shall be stated in such resolution or resolutions providing for the issue of such class or series of preferred stock as may be adopted from time to time by the Board of Directors prior to the issuance of any shares thereof. The number of authorized shares of preferred stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of all the then outstanding shares of our capital stock entitled to vote generally in the election of the directors, voting together as a single class, without a separate vote of the holders of the preferred stock, or any series thereof, unless a vote of any such holders is required pursuant to any preferred stock designation.
While we do not currently have any plans for the issuance of additional preferred stock, the issuance of such preferred stock could adversely affect the rights of the holders of common stock and, therefore, reduce the value of the common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of the common stock until the Board of Directors determines the specific rights of the holders of the preferred stock; however, these effects may include:
| · | Restricting dividends on the common stock; |
| · | Diluting the voting power of the common stock; |
| · | Impairing the liquidation rights of the common stock; or |
| · | Delaying or preventing a change in control of the Company without further action by the stockholders. |
Other than in connection with shares of preferred stock (as explained above), which preferred stock is not currently designated nor contemplated by us, we do not believe that any provision of our charter or By-Laws would delay, defer or prevent a change in control.
Series A Stock
Dividends. The holders of our Series A Stock are entitled to receive non-cumulative dividends, in preference to any dividend on our common stock, at the rate of 8% of the original purchase price ($1.00 per share) per annum, payable if, when and as declared by the Company’s Board of Directors.
| 102 |
Liquidation Preference. In the event of any liquidation, dissolution or winding up of the Company, or the sale of substantially all of the assets of the Company, the holders of our Series A Stock would be entitled to receive, in respect of each share of our Series A Stock, prior and in preference to any distribution of the assets of the Company to the holders of common stock, an amount equal to $1.00 per share plus all declared but unpaid dividends.
Automatic Conversion. Each share of Series A Stock plus declared but unpaid dividends will convert automatically into shares of common stock at the applicable conversion rate then in effect on the earlier of (i) a consent vote by a holders of a majority of shares of Series A stock or (ii) the consummation of a qualified underwritten public offering of our common stock with a price to the public of at least $5.00 per share and aggregate proceeds (net of the underwriting discount and commissions) in excess of $30,000,000.
The conversion rate for our Series A Stock is initially 1:1, subject to anti-dilution and other customary adjustments.
Optional Conversion. Each holder of our Series A Stock has the right to convert such holder’s Series A Stock, at the option of such holder, at any time, into shares of common stock at the applicable conversion ratio. Initially each share of our Series A Stock is convertible into one share of common stock, subject to adjustment in certain events.
Anti-dilution adjustments. Standard broad-based weighted average adjustment to our Series A Stock conversion price if any subsequent sale of stock or stock equivalents is done at a lower price per share, subject to customary exceptions.
Voting. The holders of our Series A Stock will vote on an as-if converted basis with the holders of the common stock and any future series of preferred stock or common stock that, by its terms, votes on an as-if converted basis with the common stock on all matters to be voted on or consented to by the stockholders, other than the election of the six Common Directors, and except as may otherwise be required by Nevada law.
Registration Rights. See “Certain Relationships and Related Transactions — Registration Rights” for a description of the registration rights granted to the holders of the Series A Stock, which description is incorporated herein by reference.
Other Rights of and Restrictions on Series A Stock.
The Preferred Stockholders Agreement also provides that:
Restrictions on Sales of Control of the Company. No holder of Series A Stock shall be a party to any transaction or series of related transactions in which a person, or a group of related persons, acquires from stockholders of the Company shares representing more than 50% of the outstanding voting power of the Company, unless all holders of Series A Stock are allowed to participate in such transaction and the consideration received pursuant to such transaction is allocated among the parties thereto in the manner specified, unless the holders of a majority of the Series A Stock elect otherwise by written notice given to the Company.
In connection with this private placement, effective as of November 13, 2015, the holders of all 16,656,894 shares of our then-outstanding Series A Stock converted their shares into 19,864,971 shares of our common stock, which included 3,208,077 shares of our common stock issued in accordance with anti-dilution provisions relating to the Series A Stock. As of January 6, 2016, warrants to purchase up to an aggregate of 1,325,000 shares of our Series A Stock remain outstanding.
Series B Stock
Dividends. The holders of our Series B Stock are entitled to receive non-cumulative dividends, in preference to any dividend on our common stock, at the rate of 8% of the original purchase price ($.60 per share) per annum, payable if, when and as declared by the Company’s Board of Directors.
Liquidation Preference. In the event of any liquidation, dissolution or winding up of the Company, or the sale of substantially all of the assets of the Company, the holders of our Series B Stock would be entitled to receive, in respect of each share of our Series B Stock, prior and in preference to any distribution of the assets of the Company to the holders of common stock, an amount equal to $.60 per share plus all declared but unpaid dividends.
| 103 |
Automatic Conversion. All of the Series B Stock will be automatically converted if (i) within 12 months of the date that the Series B Certificate of Designations is filed in the State of Nevada, the Company undertakes a reverse stock split of its Common Stock (the “Reverse Stock Split”) with the intention of listing its shares of Common Stock (or having its shares of Common Stock quoted on) a stock exchange registered as a “National Securities Exchange” pursuant to Section 6 of the Securities Exchange Act of 1934, as amended, and (ii) as a result of the Reverse Stock Split, the Company meets or exceeds the minimum listing requirements of a National Securities Exchange.
The conversion rate for our Series B Stock is initially 1/100:1, subject to anti-dilution and other customary adjustments.
Optional Conversion. Each holder of our Series B Stock has the right to convert such holder’s Series B Stock, at the option of such holder, at any time, into shares of common stock at the applicable conversion ratio. Initially each one-hundredth share of our Series B Stock is convertible into one share of common stock, subject to adjustment in certain events.
Anti-dilution adjustments. The Series B Stock contains standard broad-based full-ratchet adjustment to the conversion price if any subsequent sale of stock or stock equivalents is done at a lower price per share, subject to customary exceptions.
Voting. The holders of our Series B Stock will vote on an as-if converted basis with the holders of the common stock and any future series of preferred stock or common stock that, by its terms, vote on an as-if converted basis with the common stock on all matters to be voted on or consented to by the stockholders, except as may otherwise be required by Nevada law.
The Company may not enter into certain fundamental transactions without the consent of the holders of the majority of the outstanding shares of Series B Stock on the applicable date and certain enumerated holders (the “Required Holders”). In the event of a change of control of the Company, the holders of the Series B Stock are allowed to put their shares to the Company under certain terms. The Company also cannot, without first obtaining the prior written consent of the Required Holders, (i) increase the authorized number of shares of Series B Preferred Stock, (ii) amend, alter or repeal any provision of the Series B Certificate of Designations or the Company’s Articles of Incorporation, Bylaws or the Certificate of Designations, Preferences and Rights of Series A Convertible Preferred Stock in a manner that adversely affects the powers, preferences or rights of the Series B Stock, (iii) create, or authorize the creation of, or issue, or authorize the issuance of any debt security or any equity security or incur, or authorize the incurrence of any other indebtedness (iv) create, or authorize the creation of, or issue or obligate itself to issue shares of, any additional class or series of capital stock unless the same ranks junior to the Series B Stock with respect to the distribution of assets on the liquidation, dissolution or winding up of the Company, the payment of dividends and rights of redemption, or increase the authorized number of shares of Series B Stock or increase the authorized number of shares of any additional class or series of capital stock unless the same ranks junior to the Series B Stock with respect to the distribution of assets on the liquidation, dissolution or winding up of the Company, the payment of dividends and rights of redemption, or (v) in any manner, issue or sell any rights, warrants or options to subscribe for or purchase shares of our common stock or securities directly or indirectly convertible into or exchangeable or exercisable for shares of our common stock at a price which varies or may vary with the market price of the shares of our common stock, including by way of one or more reset(s) to any fixed price, unless the conversion, exchange or exercise price of any such security cannot be less than the then applicable conversion price.
Registration Rights. In connection with the signing of the Securities Purchase Agreement, the Company and the investors entered into a registration rights agreement (the “Registration Rights Agreement”) on November 13, 2015. Under the terms of the Registration Rights Agreement, the Company agreed to prepare and file with the SEC a registration statement covering the resale of 150% of the number of shares underlying the Series B Stock, the investor warrants and the placement agent warrants within 30 days following the date of the initial closing under the Securities Purchase Agreement. The Company will use its best efforts to have the registration statement declared effective by the SEC by the earlier of the (A) 90th calendar day after the initial closing date or, in the event that the SEC or the SEC staff cause a delay in the effectiveness of such registration statement due to comments regarding the number of shares being registered, then the 120th calendar day after the initial closing date and (B) 2nd Business Day after the date the Company is notified (orally or in writing, whichever is earlier) by the SEC that such Registration Statement will not be reviewed or will not be subject to further review. In the event that the Company fails to timely file or achieve effectiveness, maintain the effectiveness of the registration statement or to file any reports with respect to certain public information, then the Company must pay to each holder of the registrable securities an amount in cash equal to 1% of such holder’s Stated Value (as such term is defined in the Series B Certificate of Designations) of its Series B Stock on the date of such failure and 2% of such holder’s Stated Value of its Series B Stock on every 30 day anniversary until such failure is cured.
| 104 |
Options
Options to purchase an aggregate of 8,582,952 shares of our common stock have been issued under the 2013 Plan as of January 6, 2016. See “Market for Common Equity and Related Stockholder Matters—Securities Authorized for Issuance under Equity Compensation Plans” above for more information.
Warrants
As of January 6, 2016, the Agent Warrants entitle their holders to purchase 50,432 shares of our common stock, with a term of ten years (from August 23, 2013) and an exercise price of $0.01 per share, and 1,325,000 shares of common stock, with a term of ten years (from either August 23, 2013 or October 4, 2013, as applicable) and an exercise price of $1.00 per share. The Agent Warrants contain customary provisions for adjustment in the event of stock splits, subdivision or combination, mergers, etc.
The holders of the Agent Warrants have the right to exercise the warrant by means of a cashless basis.
See “Certain Relationships and Related Transactions—Registration Rights” for a description of the registration rights granted to the holders of the Agent Warrants, which description is incorporated herein by reference.
On November 13, 2015, we entered into a Securities Purchase Agreement to sell up to 26,234,940 units in a private placement at a per unit purchase price equal to $0.60. Each unit consisted of (i) one one-hundredth share of Series B Stock convertible into one share of our common stock, (ii) one warrant to acquire, at an exercise price of $0.80 per share with an expiration date five years from the date of issuance, one share of our common stock (the “Series A Warrant”), (iii) one warrant to acquire, at an exercise price of $0.80 per share with an expiration date of one year from the date of issuance, one share of our common stock (the “Series B Warrant”), (iv) one warrant to acquire, at an exercise price of $1.25 per share with an expiration date of five years from the issuance date, one share of our common stock (the “Series C Warrant”), (v) one warrant, which is contingent upon the exercise of the Series B Warrant, to acquire, at an exercise price of $1.00 per share with an expiration date that is five years from the date of the initial exercise of the Series B Warrant, one share of our common stock (the “Series D Warrant”), and (vi) one warrant, which is contingent upon the exercise of the Series C Warrant, to acquire, at an initial exercise price of $1.50 per share with an expiration date that is five years from the date of the initial exercise of the Series C Warrant, one share of our common stock (the “Series E Warrant”, and together with the Series A Warrant, the Series B Warrant, the Series C Warrant and the Series D Warrant, the “Investor Warrants”). The exercise prices of the Investor Warrants are initially subject to full protection for dilutive issuances. The Series A Warrant and Series B Warrant each contain a mandatory exercise right of ours to force exercise of the warrant if our common stock trades at or above $1.50 for 20 consecutive trading days (subject to certain conditions, including a $150,000 minimum daily volume requirement). The Series C Warrant contains a mandatory exercise right of the Company to force exercise of the warrant if our common stock trades at or above $2.00 for 20 consecutive trading days (subject to certain conditions, including a $150,000 minimum daily volume requirement).
The Investor Warrants may be exercised on any date on or after the issuance date and expire according to the following schedule: Series A Warrants expire on the five year anniversary of the issuance date, Series B Warrants expire on the one year anniversary of the issuance date, Series C Warrants expire on the five year anniversary of the issuance date, Series D Warrants expire on the five year anniversary of the issuance date of the Series B Warrants, and Series E Warrants expire on the five year anniversary of the issuance date of the Series C Warrants. The Investor Warrants contain customary provisions relating to certain limitations on exercise, mandatory exercise, restrictions on transfer and adjustment in the event of stock splits, subdivision or combination, mergers, etc.
| 105 |
In connection with the November 2015 Private Placement, the Company has agreed to deliver to Katalyst Securities LLC, the placement agent for the November 2015 Private Placement (“Katalyst”), warrants exercisable for a period of 5 years from the date of the initial closing to purchase a number of shares of the Company’s common stock equal to 10% of the number of units purchased by any entity specifically formed by a person directly introduced to the Company by Katalyst for the purpose of making an investment in the Company (collectively referred to as “Placement Agent Investors”), who are institutional investors (the “Institutional Placement Agent Investors”) with an exercise price of $1.50, which shall be lowered to $0.80 on the date of the exercise (if any) of all of the Series A Warrants or all of the Series B Warrants (the “Institutional Broker Warrant”). In addition, at each closing, the Company will deliver to Katalyst warrants exercisable for a period of five (5) years from the date of the initial closing to purchase a number of shares of the Company’s common stock equal to 10% of the number of units purchased by any Placement Agent Investors who are not Institutional Placement Agent Investors with exercise prices as apportioned as follows: (x) 25% of such number of warrants shall be exercisable for common stock at an exercise price of $0.01 per share (“Penny Broker Warrant”) and (y) 75% of such number of warrants shall be exercisable for common stock at an exercise price of $0.60 per share (the “IV Broker Warrant” and collectively with the Institutional Broker Warrant and the Penny Broker Warrant, the “Broker Warrants”). The Broker Warrants contain customary provisions, regarding lock-up periods, transferability, registration rights and adjustment in the event of stock splits, subdivision or combination, mergers, etc. The Broker Warrants may be exercised on a cash or cashless basis.
Convertible Securities
As of the date hereof, other than the common stock options, Agent Warrants, the Broker Warrants, Series B stock and Series A through Series E warrants described above, the Company does not have any outstanding convertible securities.
Transfer Agent
The transfer agent for our common stock is Philadelphia Stock Transfer, Inc. The transfer agent’s address is 2320 Haverford Rd., Suite 230, Ardmore, PA 19003 and its telephone number is (484) 416-3124.
Anti-Takeover Effects of Provisions of Nevada State Law
We may in the future become subject to Nevada’s control share laws. A corporation is subject to Nevada’s control share law if it has more than 200 stockholders of record, at least 100 of whom are residents of Nevada, and if the corporation does business in Nevada, including through an affiliated corporation. This control share law may have the effect of discouraging corporate takeovers. The Company currently has fewer than 100 stockholders of record who are residents of Nevada and does not do business in Nevada.
The control share law focuses on the acquisition of a “controlling interest,” which means the ownership of outstanding voting shares that would be sufficient, but for the operation of the control share law, to enable the acquiring person to exercise the following proportions of the voting power of the corporation in the election of directors: (1) one-fifth or more but less than one-third; (2) one-third or more but less than a majority; or (3) a majority or more. The ability to exercise this voting power may be direct or indirect, as well as individual or in association with others.
The effect of the control share law is that an acquiring person, and those acting in association with that person, will obtain only such voting rights in the control shares as are conferred by a resolution of the stockholders of the corporation, approved at a special or annual meeting of stockholders. The control share law contemplates that voting rights will be considered only once by the other stockholders. Thus, there is no authority to take away voting rights from the control shares of an acquiring person once those rights have been approved. If the stockholders do not grant voting rights to the control shares acquired by an acquiring person, those shares do not become permanent non-voting shares. The acquiring person is free to sell the shares to others. If the buyer or buyers of those shares themselves do not acquire a controlling interest, the shares are not governed by the control share law any longer.
If control shares are accorded full voting rights and the acquiring person has acquired control shares with a majority or more of the voting power, a stockholder of record, other than the acquiring person, who did not vote in favor of approval of voting rights for the control shares, is entitled to demand fair value for such stockholder’s shares.
| 106 |
In addition to the control share law, Nevada has a business combination law, which prohibits certain business combinations between Nevada corporations and “interested stockholders” for three years after the interested stockholder first becomes an interested stockholder, unless the corporation’s board of directors approves the combination in advance. For purposes of Nevada law, an interested stockholder is any person who is: (a) the beneficial owner, directly or indirectly, of 10% or more of the voting power of the outstanding voting shares of the corporation, or (b) an affiliate or associate of the corporation and at any time within the previous three years was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then-outstanding shares of the corporation. The definition of “business combination” contained in the statute is sufficiently broad to cover virtually any kind of transaction that would allow a potential acquirer to use the corporation’s assets to finance the acquisition or otherwise to benefit its own interests rather than the interests of the corporation and its other stockholders.
The effect of Nevada’s business combination law is to potentially discourage a party interested in taking control of the Company from doing so if it cannot obtain the approval of our Board of Directors.
| 107 |
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Under the Nevada Revised Statutes, our directors and officers are not individually liable to us or our stockholders for any damages as a result of any act or failure to act in their capacity as an officer or director unless it is proven that:
| · | His act or failure to act constituted a breach of his fiduciary duty as a director or officer; and |
| · | His breach of these duties involved intentional misconduct, fraud or a knowing violation of law. |
Nevada law allows corporations to provide broad indemnification to its officers and directors. At the present time, our Articles of Incorporation and Bylaws also provide for broad indemnification of our current and former directors, trustees, officers, employees and other agents.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons, we have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
The validity of the securities being offered by this prospectus will be passed upon for us by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C.
The consolidated financial statements of Neurotrope, Inc. as of December 31, 2014 and 2013, and for the years then ended, appearing in this registration statement, of which this prospectus forms a part, have been audited by Friedman LLP, an independent registered public accounting firm, as set forth in their report appearing elsewhere herein and are included in reliance on such report given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We file annual reports, quarterly reports, current reports and other information with the SEC. You may read or obtain a copy of these reports at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the public reference room and their copy charges by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains registration statements, reports, proxy information statements and other information regarding registrants that file electronically with the SEC. The address of the website is http://www.sec.gov.
We have filed with the SEC a Registration Statement on Form S-1 under the Securities Act to register the resale of shares offered by this prospectus. The term “registration statement” means the original registration statement and any and all amendments thereto, including the schedules and exhibits to the original registration statement or any amendment. This prospectus is part of that registration statement. This prospectus does not contain all of the information set forth in the registration statement or the exhibits to the registration statement. For further information with respect to us and the shares we are offering pursuant to this prospectus, you should refer to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract, agreement or other document referred to are not necessarily complete, and you should refer to the copy of that contract or other documents filed as an exhibit to the registration statement. You may read or obtain a copy of the registration statement at the SEC’s public reference facilities and Internet site referred to above.
| 108 |
NEUROTROPE, INC. AND SUBSIDIARY
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Neurotrope, Inc.
We have audited the accompanying consolidated balance sheets of Neurotrope, Inc. (the “Company”), as of December 31, 2014 and 2013, and the related consolidated statements of operations, changes in shareholders’ deficit, and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2014 and 2013, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 2 to the financial statements, the Company has sustained recurring losses from operations, has significant contractual commitments, and has not yet generated any revenues, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are described in Note 2. The financial statements do not include any adjustments that might result from the outcome of these uncertainties.
/s/ Friedman LLP
East Hanover, New Jersey
March 26, 2015
| F-2 |
Neurotrope, Inc. and Subsidiary
Consolidated Balance Sheets
| ASSETS | ||||||||||||
| December 31, | December 31, | |||||||||||
| 2014 | 2013 | |||||||||||
| CURRENT ASSETS | ||||||||||||
| Cash and cash equivalents | $ | 8,010,357 | $ | 15,211,744 | ||||||||
| Prepaid expenses | 97,073 | 87,059 | ||||||||||
| TOTAL CURRENT ASSETS | 8,107,430 | 15,298,803 | ||||||||||
| Fixed Assets, net of accumulated depreciation | 53,618 | - | ||||||||||
| TOTAL ASSETS | $ | 8,161,048 | $ | 15,298,803 | ||||||||
| LIABILITIES AND SHAREHOLDERS' DEFICIT | ||||||||||||
| CURRENT LIABILITIES | ||||||||||||
| Accounts payable | $ | 778,753 | $ | 119,608 | ||||||||
| Accrued expenses | 310,435 | 37,029 | ||||||||||
| Accounts payable and accrued expenses - related party | 200,000 | 251,779 | ||||||||||
| TOTAL CURRENT LIABILITIES | 1,289,188 | 408,416 | ||||||||||
| Commitments and contingencies | ||||||||||||
| Convertible redeemable preferred stock, Series A, $.0001 par value, 50,000,000 shares authorized; | ||||||||||||
| 21,363,000 and 23,000,000 shares issued and outstanding at December 31, 2014 and 2013, respectively. | ||||||||||||
| Liquidation preference of $21,363,000 plus dividends accruable at 8% per annum of $2,687,835 as of December 31, 2014 and | ||||||||||||
| Liquidation preference of $23,000,000 plus dividends accruable at 8% per annum of $1,023,616 at December 31, 2013 | 18,524,163 | 19,943,572 | ||||||||||
| SHAREHOLDERS' DEFICIT | ||||||||||||
| Common stock - 300,000,000 shares authorized, $.0001 par value; | ||||||||||||
| 24,034,094 issued and outstanding at December 31, 2014; | ||||||||||||
| 21,740,006 shares issued and outstanding at December 31, 2013 | 2,403 | 2,174 | ||||||||||
| Additional paid-in capital | 6,627,983 | 3,974,007 | ||||||||||
| Accumulated deficit | (18,282,689 | ) | (9,029,366 | ) | ||||||||
| TOTAL SHAREHOLDERS' DEFICIT | (11,652,303 | ) | (5,053,185 | ) | ||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS' DEFICIT | $ | 8,161,048 | $ | 15,298,803 | ||||||||
See accompanying notes to consolidated financial statements.
| F-3 |
Neurotrope, Inc. and Subsidiary
Consolidated Statements of Operations
| Year Ended | Year Ended | |||||||
| December 31, | December 31, | |||||||
| 2014 | 2013 | |||||||
| OPERATING EXPENSES: | ||||||||
| Research and development - related party | $ | 2,223,643 | $ | 1,367,644 | ||||
| Research and development | 1,242,706 | - | ||||||
| General and administrative - related party | 108,693 | 1,446,986 | ||||||
| General and administrative | 4,488,415 | 1,653,208 | ||||||
| Stock-based compensation - related party | 252,868 | 2,854,745 | ||||||
| Stock-based compensation | 950,795 | 268,199 | ||||||
| TOTAL OPERATING EXPENSES | 9,267,120 | 7,590,782 | ||||||
| OTHER INCOME: | ||||||||
| Interest income | 13,797 | 1,378 | ||||||
| Net loss before income taxes | (9,253,323 | ) | (7,589,404 | ) | ||||
| Provision for income taxes | - | - | ||||||
| Net loss | $ | (9,253,323 | ) | $ | (7,589,404 | ) | ||
| Preferred Stock dividends | $ | (1,664,219 | ) | $ | (1,023,616 | ) | ||
| Net loss attributable to common shareholders | $ | (10,917,542 | ) | $ | (8,613,020 | ) | ||
| PER SHARE DATA: | ||||||||
| Basic and diluted loss per common share | $ | (0.49 | ) | $ | (0.40 | ) | ||
| Basic and diluted weighted average common shares outstanding | 22,106,516 | 21,702,415 | ||||||
See accompanying notes to consolidated financial statements.
| F-4 |
Neurotrope, Inc. and Subsidiary
Consolidated Statement of Changes in Shareholders’ Deficit
| Additional | ||||||||||||||||||||
| Common Stock | Paid-In | Accumulated | ||||||||||||||||||
| Shares | Amount | Capital | Deficit | Total | ||||||||||||||||
| Balance, January 1, 2013 | 21,690,406 | 2,169 | - | (1,439,962 | ) | (1,437,793 | ) | |||||||||||||
| Issuance of common stock for consulting fees | 49,600 | 5 | 44,635 | - | 44,640 | |||||||||||||||
| Stock based compensation | - | - | 3,122,944 | - | 3,122,944 | |||||||||||||||
| Issuance of common stock warrants | - | - | 806,428 | - | 806,428 | |||||||||||||||
| Net loss | - | - | - | (7,589,404 | ) | (7,589,404 | ) | |||||||||||||
| Balance December 31, 2013 | 21,740,006 | $ | 2,174 | $ | 3,974,007 | $ | (9,029,366 | ) | $ | (5,053,185 | ) | |||||||||
| Conversion of Series A preferred stock to common stock | 1,637,000 | 164 | 1,419,246 | - | 1,419,410 | |||||||||||||||
| Stock based compensation | - | - | 1,203,663 | - | 1,203,663 | |||||||||||||||
| Issuance of common stock for consulting services | 43,860 | 4 | 24,996 | 25,000 | ||||||||||||||||
| Exercise of common stock warrants | 613,228 | 61 | 6,071 | 6,132 | ||||||||||||||||
| Net loss | - | - | - | (9,253,323 | ) | (9,253,323 | ) | |||||||||||||
| Balance, December 31, 2014 | 24,034,094 | $ | 2,403 | $ | 6,627,983 | $ | (18,282,689 | ) | $ | (11,652,303 | ) | |||||||||
See accompanying notes to consolidated financial statements.
| F-5 |
Neurotrope, Inc. and Subsidiary
Consolidated Statements of Cash Flows
| Year | Year | |||||||
| ended | ended | |||||||
| December 31, 2014 | December 31, 2013 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (9,253,323 | ) | $ | (7,589,404 | ) | ||
| Adjustments to reconcile net loss to net | ||||||||
| cash used by operating activities | ||||||||
| Stock based compensation | 1,203,663 | 3,122,944 | ||||||
| Consulting services paid by issuance of common stock | 25,000 | 44,640 | ||||||
| Depreciation expense | 1,325 | - | ||||||
| Change in assets and liabilities | ||||||||
| Increase in prepaid expenses | (10,014 | ) | (87,059 | ) | ||||
| Decrease in accounts payable | ||||||||
| and accrued expenses - related party | (51,779 | ) | (988,857 | ) | ||||
| Increase (decrease) in accounts payable | 659,146 | (72,734 | ) | |||||
| Increase in accrued expenses | 273,406 | 37,029 | ||||||
| Total adjustments | 2,100,747 | 2,055,963 | ||||||
| Net Cash Used in Operating Activities | (7,152,576 | ) | (5,533,441 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchase of property and equipment | (54,943 | ) | - | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Issuance of preferred stock, net of transaction costs | - | 20,750,000 | ||||||
| Proceeds from exercise of common stock warrants | 6,132 | - | ||||||
| Repayment of advances from related party | - | (4,815 | ) | |||||
| Net Cash Provided by Financing Activities | 6,132 | 20,745,185 | ||||||
| NET (DECREASE) INCREASE IN CASH | (7,201,387 | ) | 15,211,744 | |||||
| CASH AT BEGINNING OF YEAR | 15,211,744 | - | ||||||
| CASH AT END OF YEAR | $ | 8,010,357 | $ | 15,211,744 | ||||
| DISCLOSURE OF NON-CASH FINANCING ACTIVITIES: | ||||||||
| Conversion of Series A convertible redeemable preferred stock to common stock | $ | 1,419,410 | $ | - | ||||
See accompanying notes to consolidated financial statements.
| F-6 |
NEUROTROPE INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 – Organization and Nature of Planned Business:
References in these notes to the consolidated financial statements to “Neurotrope, Inc.,” “we,” “us,” “our Company” refer to Neurotrope, Inc. and its consolidated subsidiary Neurotrope Bioscience, Inc. (“Neurotrope BioScience”). Neurotrope BioScience was incorporated in Delaware on October 31, 2012. Neurotrope BioScience was formed to advance new therapeutic and diagnostic technologies in the field of neurodegenerative disease, primarily Alzheimer’s disease (“AD”). Neurotrope BioScience is collaborating with the Blanchette Rockefeller Neurosciences Institute (“BRNI”), a related party, in this process. The exclusive rights to the licensed technology transferred to the Company on February 28, 2013 (see Note 6).
On August 23, 2013, a wholly-owned subsidiary of Neurotrope, Inc. (formerly “BlueFlash Communications, Inc.”), Neurotrope Acquisition, Inc., a corporation formed in the State of Nevada on August 15, 2013 merged (the “Reverse Merger”) with and into Neurotrope BioScience. Neurotrope BioScience was the surviving corporation in the Reverse Merger and became the Company’s wholly-owned subsidiary. All of the outstanding Neurotrope BioScience common stock was converted into shares of Neurotrope, Inc. common stock on a one-for-one basis.
The transaction was accounted for as a reverse merger and recapitalization with Neurotrope BioScience as the acquirer for financial reporting purposes and Neurotrope, Inc. as the acquired company. Consequently, the assets and liabilities and the operations that are reflected in the historical financial statements are those of Neurotrope BioScience and are recorded at the historical cost basis of Neurotrope BioScience, and the consolidated financial statements after completion of the Reverse Merger include the assets and liabilities of Neurotrope BioScience and Neurotrope, Inc., and the historical operations of Neurotrope, Inc. and Neurotrope BioScience from the closing date.
As a result of the Reverse Merger, Neurotrope, Inc. discontinued its pre-Reverse Merger business and acquired the business of Neurotrope BioScience, which it is continuing to operate through Neurotrope BioScience. The common stock of Neurotrope, Inc. is traded under the ticker symbol “NTRP.”
Note 2 – Going Concern:
The consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As shown in the consolidated financial statements, we have generated no revenues to-date, have incurred substantial losses during the Company’s development stage and have substantial contractual commitments pursuant to various agreements to which we are a party.
Our ability to continue existence is dependent upon our continuing efforts to obtain additional financing to carry out our business plan during the development stage. We intend to fund our operations through equity and/or debt financing arrangements and any revenues generated in the future. However, there can be no assurance that these arrangements, if any, will be sufficient to fund our ongoing capital expenditures, working capital, and other cash requirements. The outcome of these matters cannot be predicted at this time.
There can be no assurance that any additional financings will be available to us on satisfactory terms and conditions, if at all. These conditions raise substantial doubt as to our ability to continue as a going concern.
The consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should we be unable to continue as a going concern.
Note 3 - Summary of Significant Accounting Policies:
Recently Adopted Accounting Standards:
In June 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2014-10, Development Stage Entities (Topic 915) – Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation (“ASU 2014-10”). ASU 2014-10 eliminates the concept of a development stage entity in its entirety from current accounting guidance. The new guidance eliminates the requirements for development stage entities to (i) present inception-to-date information in the statement of operations, stockholders’ equity and cash flows, (ii) label the financial statements as those of a development stage entity, (iii) disclose a description of the development stage activities in which the entity is engaged, and (iv) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. ASU 2014-10 is effective prospectively for public entities for annual reporting periods beginning after December 15, 2015, and interim periods within those annual periods; however, early adoption is permitted. The Company elected to adopt ASU 2014-10 as permitted, for the financial statements ended December 31, 2014 and, accordingly, has not included the inception-to-date disclosures and other previously required disclosures for development stage entities.
| F-7 |
Use of Estimates:
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Reclassifications:
Certain reclassifications have been made to the 2013 financial statements to conform to the consolidated 2014 financial statement presentation. These reclassifications had no effect on net loss or cash flows as previously reported.
Cash and Cash Equivalents:
The Company considers all highly liquid temporary cash investments with an original maturity of three months or less when purchased to be cash equivalents. At December 31, 2014, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation.
Property and Equipment:
Property and equipment is capitalized and depreciated on a straight line basis over the useful life of the asset, which is deemed to be between three and ten years. Property and equipment consist of the following at December 31:
| 2014 | 2013 | |||||||
| Property and equipment | $ | 54,943 | $ | 0 | ||||
| Accumulated depreciation | $ | (1,325 | ) | $ | 0 | |||
| Property and equipment, net | $ | 53,618 | $ | 0 | ||||
Depreciation expense for the year ended December 31, 2014 was $1,325 compared to $0 for 2013.
Research and Development Costs:
All research and development costs, including costs to maintain or expand the Company’s patent portfolio that do not meet the criteria for capitalization are expensed when incurred. FASB ASC Topic 730 requires companies involved in research and development activities to capitalize non-refundable advance payments for such services pursuant to contractual arrangements because the right to receive those services represents an economic benefit. Such capitalized advances will be expensed when the services occur and the economic benefit is realized. There were no capitalized research and development services at December 31, 2014 and 2013.
Income Taxes:
Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due and deferred taxes. Deferred taxes are recognized for differences between the bases of assets and liabilities for financial statement and income tax purposes and the tax effects of net operating loss and other carryforwards. The deferred tax assets and liabilities represent the future tax consequences of those differences and carryforwards, which will either be taxable or deductible when the related assets, liabilities or carryforwards are recovered or settled. Deferred tax assets are reduced by a valuation allowance when, based on the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
The Company applies the provisions of FASB ASC 740-10, Accounting for Uncertain Tax Positions, which clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The standard also provides guidance on de-recognition, classification, interest and penalties, and accounting in interim periods, disclosure and transitions.
The Company has concluded that there are no significant uncertain tax positions requiring recognition in the accompanying financial statements. The tax period that is subject to examination by major tax jurisdictions is from October 31, 2012 (inception) through December 31, 2014.
In the event the Company was to receive an assessment for interest and/or penalties, it will be classified in the financial statements as selling, general and administrative expense when assessed.
Risks and Uncertainties:
The Company operates in an industry that is subject to rapid technological change, intense competition and significant government regulation. The Company’s operations are subject to significant risk and uncertainties including financial, operational, technological, regulatory and other risk. Such factors include, but are not necessarily limited to, the results of clinical testing and trial activities, the ability to obtain regulatory approval, the ability to obtain favorable licensing, manufacturing or other agreements for its product candidates and the ability to raise capital to achieve strategic objectives.
| F-8 |
Stock Compensation:
The Company accounts for stock-based awards to employees in accordance with applicable accounting principles, which requires compensation expense related to share-based transactions, including employee stock options, to be measured and recognized in the financial statements based on a determination of the fair value of the stock options. The grant date fair value is determined using the Black-Scholes-Merton (“Black-Scholes”) pricing model. For all employee stock options, we recognize expense over the employee’s requisite service period (generally the vesting period of the equity grant). The Company’s option pricing model requires the input of highly subjective assumptions, including the expected stock price volatility, expected term, and forfeiture rate. Any changes in these highly subjective assumptions significantly impact stock-based compensation expense.
Options awarded to purchase shares of common stock issued to non-employees in exchange for services are accounted for as variable awards in accordance with applicable accounting principles. Such options are valued using the Black-Scholes option pricing model.
Note 4 – Contractual Commitments:
From January 1, 2013 through February 28, 2013, Neurotrope BioScience compensated its former President under an independent contractor agreement at the rate of $20,833 per month. On February 25, 2013, Neurotrope BioScience executed a four-year employment agreement, effective March 1, 2013, with its former President (the “Employment Agreement”). The Employment Agreement provided for an annual salary of $250,000 and an annual bonus of $50,000, which would have increased to an annual salary of $300,000 and an annual bonus of $100,000 effective as of the later of (a) February 28, 2015 or (b) the closing by the Company of a Series B Preferred Stock financing as described in Note 6 below. Effective as of July 16, 2014, the former President’s employment as Chief Executive Officer and President of Neurotrope BioScience and the Company was terminated.
In connection with his termination, effective as of July 16, 2014, Neurotrope BioScience and its former President entered into a separation agreement and general release on October 9, 2014 (the “Separation Agreement”).
The Separation Agreement states that Neurotrope BioScience will pay or provide to the former President the following: (a) $2,684 within three days of the execution of the Separation Agreement by him, representing reimbursement of business expenses; (b) a lump sum equal to the total gross amount of $233,000 (less all applicable income and employment taxes and other required or elected withholdings, for which a Form W-2 will be issued to him); (c) a gross amount of $25,478 (less all applicable income and employment taxes and other required or elected withholdings) for his accrued, unused vacation; (d) reimbursement for the cost of continuing his current health care insurance in the same amount as the net reimbursement amounts paid to him on a monthly basis immediately prior to July 16, 2014 ($2,974 per month) until the earlier of the date on which he becomes eligible for medical insurance coverage with a new employer or July 16, 2015; (e) director’s and officer’s insurance coverage covering his actions while a director and/or officer of Neurotrope BioScience until July 16, 2020; and (f) $17,000 for reimbursement of his attorneys fees. In addition, the parties agreed that his ownership of any common shares of the company is not affected by the Separation Agreement and that any equity awards he received pursuant to the 2013 Equity Incentive Plan will be governed exclusively by the terms of such plan. Approximately $282,000 was paid to him pursuant to the Separation Agreement in 2014.
Effective February 28, 2013, the Company executed an agreement with Ramat Consulting Corp. (“Ramat”), a related party to the Company’s current President and CEO Charles S. Ramat, for consulting services, including business development and marketing consulting, for a five-year period, subject to annual renewals thereafter. The Ramat agreement was amended on February 2, 2015 to add Mr. Ramat’s duties as the Company’s President and CEO. No other changes were made. Ramat's annual fee is $50,000, payable in monthly installments of $4,167, plus pre-approved travel and other reimbursable expenses. Ramat was issued a non-qualified option, with a term of ten years, to purchase 300,000 shares of common stock of the Company at an exercise price of $1.00 per share. The option vested with respect to 20% of the shares as of February 28, 2013, and the balance vest on a daily basis over the four-year period beginning on February 28, 2013. Vested options will terminate, to the extent not previously exercised, upon the occurrence of the first of the following events: (a) ten years from the date of grant; (b) the expiration of one year from the date of death or the termination of optionee as a service provider by reason of disability; or (c) the expiration of twelve months from the date of termination of optionee’s service as a service provider for any reason whatsoever other than termination of service for death or disability. An entity related to Ramat purchased 1,000,000 shares of the Company’s Series A Preferred Stock on the effective date of the consulting agreement described in this paragraph.
On October 1, 2013, the Company canceled its agreement with MCMS and executed a four-year employment agreement, effective October 1, 2013, with Robert Weinstein, its current Executive Vice President, Chief Financial Officer, Treasurer and Secretary. This agreement provides for an annual salary of $240,000, which shall increase to an annual salary of $275,000 beginning January 1, 2015. In addition, the agreement provides for a $35,000 bonus for the year ended December 31, 2013, a $50,000 bonus for the year ending December 31, 2014 and a targeted bonus of 50% of base salary for all subsequent years. On October 1, 2013, the Company’s Board of Directors (the “Board”) granted an incentive stock option to Mr. Weinstein under the Company’s 2013 Equity Incentive Plan (the “2013 Plan”) to purchase 650,000 shares of the Company’s common stock, with a term of ten years, exercisable at $1.00 per share. These options vest 25% per year over four years, with accelerated vesting of 25% upon a termination without cause or for good reason.
| F-9 |
On January 16, 2014, the Company executed a four-year employment letter with Neurotrope BioScience’s Vice President – Commercial Operations. This employment letter provides for an annual salary of $210,000 which shall increase at the discretion of the Company’s Compensation Committee. In addition, the employment letter provides for a discretionary bonus of up to 35% of base salary for all years. On January 23, 2014, the Board granted an incentive stock option to its Vice President – Commercial Operations under the 2013 Plan to purchase 100,000 shares of the Company’s common stock, with a term of ten years, exercisable at $1.76 per share. These options vest 20% per year over five years. Vested options will terminate, to the extent not previously exercised, upon the occurrence of the first of the following events: (a) ten years from the date of grant; or (b) the expiration of one year from the date of death, disability or the termination of employment.
On January 22, 2014, the Company executed a four-year employment agreement with Neurotrope BioScience’s Vice President and Chief Medical Officer. This agreement provided for an annual salary of $210,000. In addition, the employment agreement provided for a discretionary bonus of up to 35% of base salary for all years. On January 23, 2014, the Board granted an incentive stock option to its former Vice President and Chief Medical Officer under the 2013 Plan to purchase 125,000 shares of the Company’s common stock, with a term of ten years, exercisable at $1.76 per share. These options vest 25% per year over four years, with accelerated vesting of 25% upon a termination without cause or for good reason. On October 31, 2014, the Company terminated Neurotrope BioScience’s former Vice President and Chief Medical Officer without cause. Upon termination without cause and conditioned upon the execution of a release, he received severance and accelerated vesting of a portion of his incentive stock option to purchase 31,250 shares of the Company’s common stock. Approximately $217,000 was accrued for this severance arrangement in 2014 and paid in February 2015.
On June 1, 2014, the Company executed a four-year employment letter, with Neurotrope BioScience’s Executive Director – Pharmacology. This employment letter provides for an annual salary of $180,000 which shall increase at the discretion of the Company’s Compensation Committee. In addition, the employment letter provides for a discretionary bonus of up to 25% of base salary for all years. On July 16, 2014, the Board granted an incentive stock option to the Executive Director for the purchase of 50,000 shares of the Company’s common stock (see Note 10 – “Stock Options” for details).
On July 16, 2014, the Board appointed Charles S. Ramat and Paul E. Freiman to serve as Co-Chief Executive Officers, on an interim basis, of the Company and of Neurotrope BioScience. Mr. Freiman has served on the Board since October 18, 2013, and was appointed Co-Chairman of the Board on June 13, 2014. Mr. Ramat was elected to the Board on June 13, 2014 and was immediately appointed Co-Chairman of the Board.
In consideration of the services to be provided by Mr. Freiman and Mr. Ramat to the Company as Co-Chief Executive Officers, the Company agreed to pay each of them consulting fees in the amount of $20,000 per month for a term not to exceed six months, which fees would be reduced upon the employment of a permanent Chief Executive Officer to $10,000 per month for two months to aid in the transition of responsibilities to the new Chief Executive Officer. In addition, on July 23, 2014, Messrs. Freiman and Ramat were each granted non-qualified options to purchase 400,000 shares of the Company’s common stock for their roles as directors, Co-Chairmen of the Board and Co-Chief Executive Officers. Options to purchase 200,000 shares have an exercise price of $1.11 per share, or 110% of the Company’s last trading price on July 23, 2014 and were fully vested upon grant. Options to purchase the remaining 200,000 shares have an exercise price of $2.22 per share based upon two times the exercise price of $1.11 per share and vest on a daily basis up to 25% per year through July 23, 2018. See below regarding further changes to Messrs. Ramat and Freiman’s roles with the Company.
On September 4, 2014, the Company entered into a long-term lease for 4,000 square feet of office space in Newark, New Jersey. The lease commenced September 1, 2014 and expires December 1, 2017 and has two (2) one-year renewal options. The base rent is payable, commencing December 1, 2014, at an annual rate of $88,000 with no increases during the lease term and renewal terms. In addition, commencing September 1, 2014, the Company is obligated to pay its share of common area charges. The Company is amortizing the total base rent due over 39 months and has expensed $28,927 for 2014.
On September 12, 2014, the Board elected Mr. Ramat to be the Company’s President and Chief Executive Officer and Mr. Freiman to be the Company’s Chairman of the Board. In consideration of the services that Mr. Ramat will provide to the Company as President and CEO, the Company agreed to pay Mr. Ramat a consulting fee of $400,000 per year with a bonus opportunity of up to 50% of his consulting fee after one (1) year of service, which arrangement may be terminated by either party on 60 days’ notice. Mr. Ramat’s consulting fee replaces the $20,000 per month consulting fee of July 16, 2014. Mr. Freiman’s compensation remains at $20,000 per month as Chairman of the Board. In addition to his salary for services as President and Chief Executive Officer of the Company, Mr. Ramat will continue to be paid the consulting fees under a Consulting Agreement with the Company pursuant to which he receives $50,000 per year.
| F-10 |
In addition, on September 12, 2014, Messrs. Ramat and Freiman were granted non-qualified options to purchase 250,000 and 50,000 shares of the Company’s common stock, respectively, which have an exercise price of $0.60 per share, are fully vested upon grant, and have a ten-year life.
As of November 1, 2014, Neurotrope BioScience entered into an employment agreement for one (1) year with provisions for automatic renewals, with a new Executive Vice President, Development and Chief Medical Officer. This agreement provides for an annual salary of $325,000 which shall increase at the discretion of the Company’s President. In addition, the employment agreement provides for a signing bonus of $102,083 in total, payable $8,507 monthly (if employed during the applicable payment cycle) and a discretionary bonus of up to 50% of base salary. In connection with his employment and as of his date of hire, the Company’s Compensation Committee authorized a grant of a non-qualified stock option to Neurotrope BioScience’s new Executive Vice President and Chief Medical Officer under the 2013 Plan to purchase 250,000 shares of the Company’s common stock, with a term of ten years, exercisable at $0.71 per share. These options vest 20% per year over five years commencing November 1, 2015.
Note 5 – Collaborative Agreements:
On May 12, 2014, the Company entered into a license agreement (the “Stanford Agreement”) with The Board of Trustees of The Leland Stanford Junior University (“Stanford”), pursuant to which Stanford has granted to the Company a revenue-bearing, world-wide right and exclusive license, with the right to grant sublicenses (on certain conditions), under certain patent rights and related technology for the use of bryostatin structural derivatives, known as “bryologs,” for use in the treatment of central nervous system disorders, lysosomal storage diseases, stroke, cardioprotection and traumatic brain injury, for the life of the licensed patents.
Pursuant to the Stanford Agreement, in 2014, the Company paid Stanford a total of $60,850, of which $48,564 was expensed by the Company in 2014. The Company is also obligated to pay Stanford: (a) a $10,000 annual license maintenance fee; (b) milestone payments in the aggregate amount of up to $3,700,000 upon the achievement of certain product development events commencing upon the filing of the first IND application through approval of an applicable product; and (c) low single-digit royalties on net sales of licensed products.
Each party has the right to terminate the Stanford Agreement for an uncured material breach of the other party. Additionally, the Company may terminate the Stanford Agreement at any time upon 60 days’ written notice to Stanford.
On May 15, 2014, the Company entered into an agreement with a contract research organization (“CRO”) to conduct a clinical trial relating to AD. The Company has agreed to pay fees to the CRO totaling $715,159, based upon signing of the agreement and the CRO achieving certain clinical trial milestones, plus reasonable out-of-pocket expenses. On November 19, 2014, the CRO agreement was amended, requiring the Company to pay a reduced amount totaling $657,238. The Company has incurred a total of $417,877 in fees, all of which are included in the balance sheet as payables as of December 31, 2014.
On July 14, 2014, Neurotrope BioScience entered into an Exclusive License Agreement (the “Mount Sinai Agreement”) with Icahn School of Medicine at Mount Sinai (“Mount Sinai”). Pursuant to the Mount Sinai Agreement, Mount Sinai granted Neurotrope BioScience a revenue-bearing, world-wide right and (a) exclusive license, with the right to grant sublicenses (on certain conditions), under Mount Sinai’s interest in certain joint patents held by the Company and Mount Sinai (the “Joint Patents”) as well as in certain results and data (the “Data Package”) and (b) non-exclusive license, with the right to grant sublicenses on certain conditions, to certain technical information, both relating to the diagnostic, prophylactic or therapeutic use for treating diseases or disorders in humans relying on activation of PKCepsilon, which includes Niemann-Pick C Disease (the “Mount Sinai Field of Use”). The Mount Sinai Agreement allows Neurotrope BioScience to research, discover, develop, make, have made, use, have used, import, lease, sell, have sold and offer certain products, processes or methods that are covered by valid claims of Mount Sinai’s interest in the Joint Patents or an Orphan Drug Designation Application covering the Data Package (the “Mount Sinai Licensed Products”) in the Mount Sinai Field of Use.
Pursuant to the Mount Sinai Agreement, Neurotrope BioScience is obligated to pay a $25,000 license initiation fee to Mount Sinai. Neurotrope BioScience is also obligated to pay Mount Sinai (a) a $10,000 annual license maintenance fee until minimum royalty payments are due, (b) milestone payments in the aggregate amount of up to $3,500,000 upon the achievement of certain product development events relating to the approval of a licensed product in the United States and other jurisdictions, (c) low single-digit royalties on net sales of the Mount Sinai Licensed Products, and (d) a portion of sublicense fees ranging from a significant double-digit percentage based on sublicensing before completion of in vivo proof-of-concept experiments to a mid-single digit percentage after product approval. In 2014, the Company paid Mount Sinai $25,000, which represents the license initiate fee described above. Of this $25,000, the Company expensed $11,458 in 2014. Also in 2014, the Company accrued $12,000 as an expense to Mount Sinai for certain consulting services that did not get paid in 2014.
Each party has the right to terminate the Mount Sinai Agreement for an uncured material breach of the other party. Additionally, Neurotrope BioScience may terminate the Agreement at any time upon 60 days’ written notice to Mount Sinai. Further, upon termination, Neurotrope BioScience may continue to sell any and all licensed products, provided that Neurotrope BioScience will pay Mount Sinai a reduced royalty for licensed products that are indicated as therapeutics or diagnostics for Niemann Pick disease which are sold following the termination of the Mount Sinai Agreement.
| F-11 |
The term of the Mount Sinai Agreement began upon signing and will expire with respect to each Mount Sinai Licensed Product on a Mount Sinai Licensed Product-by-Mount Sinai Licensed Product basis and country-by-country basis, from first commercial sale until the later of: (a) expiration of the last to expire Joint Patent Rights covering such Mount Sinai Licensed Product in such country; (b) expiration of any market exclusivity period granted by a regulatory agency with respect to such Mount Sinai Licensed Product in such country; or (c) ten years after the first commercial sale of such Mount Sinai Licensed Product in such country.
Note 6 – Related Party Transactions and Licensing / Research Agreements:
Two directors of the Company, James Gottlieb and William Singer, are also directors of BRNI. One of those directors, Mr. Singer, is the president of BRNI. BRNI is a stockholder of a corporation, Neuroscience Research Ventures, Inc. (“NRVI”), which owned 37.6% of the Company's outstanding common stock as of December 31, 2014.
Effective October 31, 2012, Neurotrope BioScience executed a Technology License and Services Agreement with BRNI, a related party, and NRV II, LLC, another affiliate of BRNI (the “BRNI License Agreement”). Under the terms of the BRNI License Agreement, BRNI provides research services and granted Neurotrope BioScience the exclusive and nontransferable license right to certain patent and intellectual property which became effective upon the Neurotrope BioScience’s completion of a Series A Preferred Stock financing generating net proceeds to Neurotrope BioScience of at least $8,000,000 on February 28, 2013. The BRNI License Agreement terminates on the later of the date (a) the last of the licensed patent expires, is abandoned, or is declared unenforceable or invalid or (b) the last of the intellectual property enters the public domain.
The research services provided under the BRNI License Agreement commenced on April 2, 2012. The BRNI License Agreement requires Neurotrope BioScience to reimburse BRNI for services rendered (the “Services Reimbursement”) on a pro-rated, 30-month basis, with respect to the period of time elapsed from April 2, 2012 through the date of the Series A Preferred Stock financing on February 28, 2013. BRNI charged Neurotrope BioScience $266,666 from January 1, 2013 to February 28, 2013.
After the initial Series A Preferred Stock financing, the BRNI License Agreement requires Neurotrope BioScience to enter into scope of work agreements with BRNI as the preferred service provider, for any research and development services or other related scientific assistance and support services. Neurotrope BioScience is not permitted to engage any other person other than BRNI to perform research or development services or other related scientific assistance without prior written consent of BRNI, except under certain circumstances. BRNI and Neurotrope may agree to have a third party provide services identical or similar to such services to Neurotrope in the case where BRNI is demonstrably unable to do so or such third party is demonstrably in a superior position to do so.
In addition, the BRNI License Agreement requires the Company to pay BRNI a “Fixed Research Fee,” commencing the date that the Company completes a Series B Preferred Stock financing resulting in net proceeds of at least $25,000,000 (the “Series B Financing”). The fixed research fee is (a) a pro-rata amount of $1,000,000 in the year the Company completes such financing (b) $1,000,000 per year for five calendar years subsequent to such financing and (c) an annual fixed research fee in an amount to be negotiated and agreed upon no later than 90 days prior to the end of the fifth calendar year following the completion of such financing to be paid with respect to each remaining calendar year during the term of the BRNI License Agreement. The Company has not yet completed this Series B Financing (see Note 9) and, accordingly, no such fees are due.
The BRNI License Agreement also requires the payment of royalties ranging between 2% and 5% of the Company’s revenues generated from the licensed patents and other intellectual property, dependent upon the percentage ownership that NRVI holds in the Company. Under the BRNI License Agreement, the Company is required to prepay royalty fees at a rate of 5% of all investor funds raised in the Series A or Series B Preferred Stock financings or any subsequent rounds of financing prior to a public offering, less commissions. On March 25, 2013, the Company prepaid $409,549 in royalties under the BRNI License Agreement and paid the remainder due of $60,349 in July 2013 relating to the May 7, 2013 Series A Preferred Stock financing. On August 29, 2013, the Company paid $520,252 in prepaid royalties relating to the August 23, 2013 Series A Preferred Stock financing. On October 4, 2013, the Company paid $48,600 in prepaid royalties relating to the Series A Preferred Stock financing. These royalties were expensed in “general and administrative expenses – related party” in the statement of operations as there is no guarantee that sales will be achieved and royalties warranted.
Effective August 28, 2013, Neurotrope BioScience signed a statement of work (“SOW”) with BRNI pursuant to the BRNI License Agreement for the further development of its Alzheimer’s disease (“AD”) diagnostic product. Pursuant to the SOW, Neurotrope BioScience paid BRNI a total of $1,645,470 in 12 equal monthly installments of $137,123, payable on the first business day of each month. These payments are for operating expenses associated with BRNI’s diagnostic laboratories. Operating expenses that are incurred in excess of this total amount are the responsibility of BRNI unless prior approval is obtained from Neurotrope BioScience. The SOW may be extended if BRNI provides Neurotrope BioScience with two months advanced notice that the SOW objectives are not met within the initial 12 month period. As of December 31, 2014, BRNI did provide Neurotrope BioScience with the requisite notice to extend the term of the SOW. The parties have negotiated the terms of such extension.
| F-12 |
Subsequent to the year ended December 31, 2014 and effective as of October 31, 2012, Neurotrope BioScience entered into an Amended and Restated Technology License and Services Agreement with BRNI and NRV II, LLC on February 4, 2015 (the “Amended and Restated BRNI License”). The agreement further amended and restated that certain Technology License and Services Agreement executed as of October 31, 2012. Pursuant to the Amended and Restated BRNI License, Neurotrope BioScience and BRNI entered into a new Statement of Work and Account Satisfaction Agreement dated February 4, 2015 (the “February 2015 SOW”). The February 2015 SOW is effective as of October 1, 2014 and continues until September 30, 2015. Neurotrope BioScience is obligated to pay $2.54 million pursuant to the February 2015 SOW (See Subsequent Events – Note 12). For the years ended December 31, 2014, Neurotrope BioScience incurred $600,000 in expenses relating to the February 2015 SOW, of which $400,000 was paid in 2014, and the remaining $200,000 was paid in 2015.
Effective November 13, 2013, Neurotrope BioScience agreed to another SOW with in which Neurotrope BioScience contracted for the further development of its AD therapeutic product. Pursuant to this SOW, Neurotrope BioScience paid BRNI $251,939 for related personnel and research services. Neurotrope BioScience expensed this entire amount in the year ended December 31, 2013. No additional expense relating to this SOW was incurred during the year ended December 31, 2014.
On March 12, 2014, Neurotrope BioScience signed an additional SOW with BRNI to continue pre-clinical activities relating to the commercialization of Neurotrope BioScience’s therapeutic product. Neurotrope BioScience paid BRNI the entire total pursuant to this SOW of approximately $465,000 for these services during the year ended December 31, 2014.
Note 7– Income Taxes:
The Company incurred a net operating loss for income tax purposes of $13,685,763 for the period from October 31, 2012 (inception) through December 31, 2014. This amount is available for carryforward for use in offsetting taxable income of future years through 2034. The net operating loss carryforward resulted in a deferred tax asset of $4,653,159 at December 31, 2014. Income tax effects of share-based payments are recognized in the financial statements for those awards that will normally result in tax deductions under existing tax law. Under current U.S. federal tax law, the Company would receive a compensation expense deduction related to non-qualified stock options only when those options are exercised. Accordingly, the financial statement recognition of compensation cost for non-qualified stock options creates a deductible temporary difference which results in a deferred tax asset of $221,098 as of December 31, 2014. The Company does not recognize a tax benefit for compensation expense related to incentive stock options (ISOs) unless the underlying shares are disposed of in a disqualifying disposition. Accordingly, compensation expense related to ISOs is treated as a permanent difference for income tax purposes. These deferred tax assets are reduced to zero by an offsetting valuation allowance. As a result, there is no provision for income taxes.
Note 8 – Common Stock:
On February 28, 2013, Neurotrope BioScience amended and restated its Certificate of Incorporation to authorize 45,000,000 common shares at a par value of $0.01. On that date, the Company issued 19,000,000 common shares. This recapitalization has been presented retroactively in the accompanying financial statements.
On June 20, 2013, BlueFlash Communications, Inc., a Florida corporation (“BlueFlash”), entered into an Agreement and Plan of Merger (the “Plan of Merger”) with Neurotrope, Inc., its wholly-owned Nevada subsidiary which was incorporated in Nevada on June 13, 2013, pursuant to which BlueFlash merged with and into Neurotrope, Inc. (the “Reincorporation Merger”). The Plan of Merger was amended by the Amendment to Agreement and Plan of Merger between the parties, dated July 10, 2013 (the “Amendment”). The purpose of the merger was to re-domicile BlueFlash from Florida to Nevada, and to effect a name change and recapitalization as described below.
On August 5, 2013, Articles of Merger were filed with both the Secretary of State of the State of Nevada and the Secretary of State of the State of Florida, pursuant to which the Reincorporation Merger was effective as of August 9, 2013. Upon effectiveness of the merger, Neurotrope, Inc.’s Articles of Incorporation and Neurotrope, Inc.’s Amended and Restated Bylaws became the Articles of Incorporation and Amended and Restated Bylaws of the registrant.
Neurotrope, Inc., has an authorized share capital of 300,000,000 shares of common stock, par value $0.0001 per share and 50,000,000 shares of “blank check” preferred stock, par value $0.0001 per share. Prior to the Reincorporation Merger, Neurotrope, Inc. had 100 shares of its common stock outstanding, held by BlueFlash, and therefore was a wholly-owned subsidiary of BlueFlash. Prior to the merger, Neurotrope, Inc. had no assets, liabilities or business.
Pursuant to the Plan of Merger, among other things, each share of common stock of BlueFlash, $0.0001 par value per share was automatically converted into 2.242 shares of Neurotrope’s common stock.
On August 23, 2013, the Reverse Merger occurred as described in Note 1 above, and all of the outstanding Neurotrope BioScience common stock was converted into shares of Neurotrope’s common stock on a one-for-one basis.
In connection with the Reverse Merger and pursuant to a Split-Off Agreement, Neurotrope, Inc. transferred its pre-Reverse Merger business to Marissa Watson, its pre-Reverse Merger majority stockholder, in exchange for the surrender by her and cancellation of 20,178,000 shares of Neurotrope’s common stock.
On September 25, 2014, the Company agreed to issue to a consultant 43,860 shares of common stock as compensation to assist the Company with investment banking and capital raising activities. The Company expensed $25,000 during the 2014 period as a general and administrative expense which represented the common stock issuable times the closing market price of the Company’s common stock on the date the consultant was engaged. During the year ended December 31, 2013, the Company issued (i) 9,600 shares of common stock to a consultant as compensation for advisor services in connection with the Reverse Merger and (ii) 40,000 shares of common stock to a consultant for investor relations services, and as a result, incurred expenses totaling $44,640.
During the year ended December 31, 2014, ten holders of 1,637,000 total shares of Preferred Stock converted that Preferred Stock into 1,637,000 shares of Neurotrope’s common stock and five holders of 613,228 warrants exercisable at $0.01 per share of common stock exercised their warrants into 613,228 shares of Neurotrope’s common stock.
| F-13 |
Note 9 – Preferred Stock:
Through a series of private placements closed between February and October 2013, Neurotrope BioScience issued 23,000,000 preferred shares at $1 per share, resulting in gross proceeds of $23 million. The Company agreed to pay the placement agent in the offering a cash commission of 10% of the gross funds raised from non-Company related investors in the private placement offering (“PPO”). As a result, the Company paid the placement agents cash totaling $2,225,000. In addition, the placement agent received (a) for the first $12,000,000 of gross PPO proceeds, (i) warrants exercisable for a period of ten (10) years to purchase a number of shares of common stock equal to 7.5% of the number of shares of Series A Preferred Stock sold to investors introduced by it, with a per share exercise price of $0.01, and (ii) warrants exercisable for a period of ten (10) years to purchase a number of shares of Series A Preferred Stock equal to 2.5% of the number of shares of Series A Preferred Stock sold to investors introduced by it, with a per share exercise price of $1.00; and (b) on gross PPO proceeds in excess of $12,000,000, warrants exercisable for a period of ten (10) years to purchase a number of shares of Series A Preferred Stock equal to 10% of the number of shares of Series A Preferred Stock sold to investors introduced by it, with an exercise price of $1.00 per share (collectively, the “Agent Warrants”). As a result of the foregoing aggregate placements, the placement agent was issued Agent Warrants to purchase 900,000 shares of the Company’s common stock and 1,325,000 shares of the Company’s Series A Preferred Stock.
The Series A Preferred Stock ranks senior with respect to liquidation preference and dividend rights to the common stock and any other class or series of stock that the Company may issue. The Series A Preferred Stock accrues a dividend at an annual rate of $0.08 per share, when and if declared by the Board of Directors of the Company. No dividends have been declared on the Series A Preferred Stock. In the event of any voluntary or involuntary liquidation, dissolution or winding-up of our affairs or similar event, a holder of Series A Preferred Stock will be entitled to be paid, before any distribution or payment may be made to any holders of common stock or other class or series of stock, the liquidation amount (which shall equal $1.00 per share) and the amount of any accruable and unpaid dividends as of such distribution or payment date. Each share of Series A Preferred Stock is convertible into common stock at the option of the stockholder at a price of $1.00 per share, subject to adjustment. Each share of Series A Preferred Stock will convert into more than one share of common stock in the event that the Company sells certain securities at a price per share that is less than the conversion price, which is currently set at $1.00 per share. The Series A shares are subject to mandatory conversion upon the vote of holders of a majority of the outstanding shares of Series A Preferred Stock at any given time, or upon the closing of a sale of common stock to the public at a price of at least $5.00 per share (subject to adjustment in the case of certain events) in a firm commitment underwritten public offering pursuant to an effective registration statement under the Securities Act, resulting in net proceeds to the Company of at least $30,000,000. The holders of Series A Preferred Stock are entitled to a class vote on certain Company actions, have the right to participate in future offerings and have the right to elect one member of the Company board of directors, in addition to certain additional rights and privileges as set forth in the Amended and Restated Certificate of Incorporation and in certain agreements between the Company and the holders thereof.
As of September 2, 2014, the Company and the holders of a majority of the Company’s Series A Preferred Stock entered into an amendment (the “Amendment”) to the Preferred Stockholders Agreement, dated as of August 23, 2013 (the “Preferred Stockholder Agreement”). Under the terms of the Amendment, the parties removed provisions relating to the right of first refusal and certain prohibitions relating to transfers, which were set forth in the Preferred Stockholder Agreement. In addition, the parties made other technical changes to the terms of the Preferred Stockholder Agreement.
During 2014, private placement investors converted a total of 1,637,000 shares of the Company’s Series A Preferred Stock into 1,637,000 shares of the Company’s common stock.
Note 10 – Stock Options:
Our Board of Directors adopted, and our stockholders approved, the 2013 Plan, which provides for the issuance of incentive awards of up to 7,000,000 shares of the Company’s common stock to officers, key employees, consultants and directors. Upon the closing of the Reverse Merger, options to purchase an aggregate of 5,154,404 shares of the Company’s common stock were issued. Of these options: (a) 3,500,000 options were issued to founding stockholders and 54,404 were granted to a consultant, each with a term of ten years, exercisable at $1.75 per common share, 100% of these options vested on the date of grant; (b) 1,050,000 options were issued to four directors (or their affiliates) and 250,000 to a consultant with a term of ten years, exercisable at $1.00 per common share, which vest 20% per year for each of five years after the date of grant; and, (c) 300,000 options were granted to a consultant with a term of ten years, exercisable at $1.00 per common share which vested 20% on the date of grant and vest 20% per year over the next four years. These options were issued in conversion of options issued by Neurotrope BioScience on February 28, 2013.
On January 23, 2014, the Company issued, under the 2013 Plan, 300,000 options to three employees, each with a term of ten years, exercisable at $1.76 per common share. 125,000 of these options vest 25% per year, with accelerated vesting of 25% upon a termination without cause or for good reason, with the remaining 175,000 vesting 20% per year. On March 21, 2014, the Company issued, under the 2013 Plan, 30,000 options to one employee with a term of ten years, exercisable at $2.30 per common share and vests 20% per year commencing on March 31, 2015.
| F-14 |
On July 16, 2014, the Company issued incentive stock options to purchase 50,000 shares of common stock under the 2013 Plan to one employee with a term of ten years, exercisable at $1.24 per common share which vest 20% per year commencing July 16, 2015.
On July 23, 2014, the Company’s Board approved increasing the total shares available under the 2013 Plan by 3,000,000 to 10,000,000. The 3,000,000 additional shares will be issuable as non-qualified stock options, unless shareholder approval is sought and received.
Also, on July 23, 2014, the Board granted non-qualified stock options to three directors, each in the amount of 100,000 shares of common stock; 50,000 of which have an exercise price equal to $1.11, being ten cents ($0.10) above the closing sales price of the common stock (or the closing bid, if no sales were reported) as listed on the OTC Market on July 23, 2014, which vested on the date of the grant hereof; and 50,000 of which have an exercise price equal to two times the value of the exercise price of the first 50,000 options, or $2.22 which vest daily over four (4) years (assuming continued service on behalf of the Company), up to 25% per year, such that 25% of the second 50,000 shall be vested on July 23, 2015 until the grant is fully vested on July 23, 2018.
On September 12, 2014, the Board granted the Company’s Chief Executive Officer and Chairman non-qualified options to purchase 250,000 and 50,000 shares of the Company’s common stock, respectively, which have an exercise price of $0.60 per common share, are fully vested upon grant and have a term of ten years. Also, the Board granted 300,000 non-qualified stock options to a newly appointed director with a term of ten years, exercisable at $0.60 per common share and vest daily over five years commencing September 12, 2014. The Company also granted incentive stock options to purchase 20,000 shares of the Company’s common stock to one employee with a term of ten years, exercisable at $0.60 per common share and vests 20% per year commencing September 12, 2015.
A summary of the Company’s stock option activity for the years ended December 31, 2014 and 2013 is as follows:
| Options Outstanding | Weighted-Average Exercise Price | |||||||
| Outstanding at January 1, 2013 | 0 | $ | 0 | |||||
| Options granted | 6,489,404 | $ | 1.47 | |||||
| Options granted | (239,452 | ) | $ | (1.00 | ) | |||
| Options exercised | 0 | 0 | ||||||
| Outstanding at December 31, 2014 | 6,249,952 | $ | 1.48 | |||||
| Options exercisable December 31, 2013 | 3,764,733 | $ | 1.71 | |||||
| Outstanding at December 31, 2013 | 6,249,952 | $ | 1.48 | |||||
| Options granted | 2,350,000 | $ | 1.29 | |||||
| Options granted | (830,750 | ) | $ | 1.77 | ||||
| Options exercised | 0 | 0 | ||||||
| Outstanding at December 31, 2014 | 7,769,202 | $ | 1.40 | |||||
| Options exercisable December 31, 2014 | 4,546,603 | $ | 1.49 | |||||
The weighted-average remaining contractual term of options exercisable and outstanding at December 31, 2014 was approximately 8.5 and 8.9 years, respectively.
The Company used the Black-Scholes valuation model to calculate the fair value of stock options. Stock-based compensation expense is recognized over the vesting period using the straight-line method.
The fair value of stock options issued for the year ended December 31, 2014 was estimated at the grant date using the following weighted average assumptions: Dividend yield 0%; Volatility 90.56%; Risk-free interest rate 2.53%; weighted average grant date fair value of $0.77 per common share.
The total stock option-based compensation recorded as operating expense was approximately $1,203,000 and $3,123,000 for the years ended December 31, 2014 and 2013, respectively. All of the stock option-based compensation expense was classified as stock based compensation expense.
| F-15 |
Note 11 – Common and Preferred Stock Reserved for Future Issuances:
Common stock and preferred stock reserved for future issuances consisted of the following at December 31, 2014:
| Common Stock Reserved | Preferred Stock Reserved | |||||||
| Common stock warrants outstanding | 286,772 | 0 | ||||||
| Preferred stock warrants outstanding | 1,325,000 | 1,325,000 | ||||||
| Common stock options outstanding | 7,769,202 | 0 | ||||||
| Conversion of Series A Preferred Stock | 21,363,000 | 0 | ||||||
| Total | 30,743,974 | 1,325,000 | ||||||
On February 9, 2015, the holders of the Preferred stock warrants outstanding agreed to convert these warrants to Common stock warrants with the same terms as the Preferred stock warrants (See Subsequent Events – Note 12)
Note 12 – Subsequent Events:
From January 1 through March 20, 2015, private placement investors converted a total of 2,580,606 shares of the Company’s Series A Preferred Stock (issued in 2013) into 2,580,606 shares of the Company’s common stock.
Additionally, from January 1 through March 26, 2015, private placement investors exercised common stock warrants (issued August 23, 2013) resulting in the issuance of a total of 180,558 shares of the Company’s common stock.
On February 9, 2015, the holders of placement agent warrants to purchase an aggregate of 1,325,000 shares of our Series A Preferred Stock entered into Conversion Agreements with us pursuant to which such holders’ placement agent warrants to purchase Series A Preferred Stock were converted, for no additional consideration, into common stock purchase warrants to purchase an aggregate of 1,325,000 shares of our common stock.
On February 4, 2015, Neurotrope BioScience entered into an Amended and Restated Technology License and Services Agreement with BRNI and NRV II, LLC. The agreement further amended and restated that certain Technology License and Services Agreement executed as of October 31, 2012, which was previously amended by Amendment No. 1 to the Technology License and Services Agreement as of August 21, 2013.
Pursuant to the Amended and Restated BRNI License, on February 4, 2015, Neurotrope BioScience and BRNI entered into a new Statement of Work and Account Satisfaction Agreement, or the February 2015 SOW, pursuant to the BRNI License. The February 2015 SOW is effective as of October 1, 2014 and continues until September 30, 2015.
The February 2015 SOW terminates and replaces the SOW dated August 28, 2013 and both parties agreed that neither party has or shall have any rights, claims damages or obligations for services or costs pursuant to the August 28, 2013 SOW.
Pursuant to the February 2015 SOW, Neurotrope BioScience agreed to pay BRNI two million four hundred thousand dollars ($2,400,000) in service fees and other amounts payable at a rate of two hundred thousand dollars ($200,000) per month for each month from October 1, 2014 through September 30, 2015. The parties agreed that the first six hundred thousand dollars ($600,000) of payments satisfy certain outstanding amounts owed to BRNI. Of this $600,000, $400,000 was paid in 2014, and the remaining $200,000 was paid in 2015. Neurotrope BioScience also agreed to pay BRNI twenty thousand dollars ($20,000) in quarterly payments during the twelve months from the date of the SOW in exchange for advising and consulting services by BRNI’s chief scientist regarding Neurotrope BioScience’s contract with the Icahn School of Medicine at Mt. Sinai Hospital for the use of bryostatin in the treatment of Niemann Pick disease.
| F-16 |
In consideration for the February 2015 SOW, BRNI agrees to (a) use commercially reasonable efforts to enroll at least four (4) additional compassionate use or expanded access patients, in trials of BRNI’s Alzheimer’s therapeutic drug platform during the term of the SOW, (b) perform certain services requested by Neurotrope BioScience for the further development of BRNI’s Alzheimer’s therapeutic drug platform, (c) perform certain services for the further development of BRNI’s Alzheimer’s diagnostic test, (d) to the extent permitted by applicable law, transfer all of its rights and regulatory obligations, except for those relating to the compassionate use expanded access trials, associated with BRNI’s IND 71,276 to Neurotrope BioScience, (e) conduct initial research on the application of its PKC epsilon platform to treat Fragile X disease, (f) conduct initial research on polyunsaturated fatty acid derivatives for the purpose of developing a commercially usable PKC epsilon activator, and (g) provide assistance, advice and other similar services to Neurotrope BioScience regarding Neurotrope BioScience’s analysis of bryologs pursuant to Neurotrope BioScience’s agreement with Stanford University, for the purpose of developing a commercially usable PKC epsilon activator. Furthermore, BRNI agreed to transfer a certain amount of bryostatin drug substance and bryostatin kits containing drug substance for non-human use to a third-party for storage and handling for Neurotrope BioScience’s pre-clinical and clinical use, which was completed on March 10, 2015.
In order for BRNI to perform certain of the services described in (c) above, Neurotrope BioScience agrees to reimburse a third party for services BRNI received from such third party in the amount of one hundred fifty thousand dollars ($150,000) in connection with BRNI’s former diagnostic trial program with such third party. Also, under certain conditions, Neurotrope BioScience has agreed to enter into an agreement with a specified research institution for the analysis of tissue samples and autopsy results for a validation trial as part of the development process for the diagnostic test. If Neurotrope BioScience does not proceed with a certain portion of such agreement by June 30, 2015, then the rights to the diagnostic test will revert to BRNI and Neurotrope BioScience will no longer be licensed thereto.
| F-17 |
Neurotrope, Inc. and Subsidiary
Condensed Consolidated Balance Sheets
(Unaudited)
ASSETS
| September 30, | December 31, | |||||||
| 2015 | 2014 | |||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents | $ | 1,543,537 | $ | 8,010,357 | ||||
| Prepaid expenses | 347,931 | 97,073 | ||||||
| TOTAL CURRENT ASSETS | 1,891,468 | 8,107,430 | ||||||
| Fixed Assets, net of accumulated depreciation | 60,786 | 53,618 | ||||||
| TOTAL ASSETS | $ | 1,952,254 | $ | 8,161,048 | ||||
| LIABILITIES AND SHAREHOLDERS' DEFICIT | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable | $ | 1,010,723 | $ | 778,753 | ||||
| Accrued expenses | 333,315 | 310,435 | ||||||
| Note payable | 18,393 | - | ||||||
| Accounts payable and accrued expenses - related party | 236,547 | 200,000 | ||||||
| TOTAL CURRENT LIABILITIES | 1,598,978 | 1,289,188 | ||||||
| Commitments and contingencies | ||||||||
| Convertible redeemable preferred stock, Series A, $.0001 par value, 50,000,000 shares authorized; | ||||||||
| 16,755,894 and 21,363,000 shares issued and outstanding at September 30, 2015 and December 31, 2014, respectively. Liquidation preference of $16,755,894 plus dividends accruable at 8% per annum of $3,139,966 as of September 30, 2015 and Liquidation preference of $21,363,000 plus dividends accruable at 8% per annum of $2,687,835 at December 31, 2014 | 14,522,010 | 18,524,163 | ||||||
| SHAREHOLDERS' DEFICIT | ||||||||
| Common stock - 300,000,000 shares authorized, $.0001 par value; | ||||||||
| 28,877,540 issued and outstanding at September 30, 2015; | ||||||||
| 24,034,094 shares issued and outstanding at December 31, 2014 | 2,888 | 2,403 | ||||||
| Additional paid-in capital | 11,019,241 | 6,627,983 | ||||||
| Accumulated deficit | (25,190,863 | ) | (18,282,689 | ) | ||||
| TOTAL SHAREHOLDERS' DEFICIT | (14,168,734 | ) | (11,652,303 | ) | ||||
| TOTAL LIABILITIES AND SHAREHOLDERS' DEFICIT | $ | 1,952,254 | $ | 8,161,048 | ||||
See accompanying notes to condensed consolidated financial statements.
| F-18 |
Neurotrope, Inc. and Subsidiary
Condensed Consolidated Statements of Operations
(Unaudited)
| Nine Months Ended | Nine Months Ended | Three Months Ended | Three Months Ended | |||||||||||||
| September 30, | September 30, | September 30, | September 30, | |||||||||||||
| 2015 | 2014 | 2015 | 2014 | |||||||||||||
| OPERATING EXPENSES: | ||||||||||||||||
| Research and development - related party | $ | 1,852,428 | $ | 1,909,363 | $ | 636,547 | $ | 846,135 | ||||||||
| Research and development | 1,348,143 | 774,189 | 268,665 | 368,221 | ||||||||||||
| General and administrative - related party | 81,000 | 81,693 | 27,000 | 27,693 | ||||||||||||
| General and administrative | 3,242,220 | 3,174,489 | 817,880 | 1,307,877 | ||||||||||||
| Stock-based compensation - related party | 131,453 | 208,569 | 44,299 | 74,302 | ||||||||||||
| Stock-based compensation | 255,774 | 722,304 | 97,913 | 520,087 | ||||||||||||
| TOTAL OPERATING EXPENSES | 6,911,018 | 6,870,607 | 1,892,304 | 3,144,315 | ||||||||||||
| OTHER INCOME: | ||||||||||||||||
| Interest income | 2,844 | 11,590 | 355 | 2,707 | ||||||||||||
| Net loss before income taxes | (6,908,174 | ) | (6,859,017 | ) | (1,891,949 | ) | (3,141,608 | ) | ||||||||
| Provision for income taxes | - | - | - | - | ||||||||||||
| Net loss | $ | (6,908,174 | ) | $ | (6,859,017 | ) | $ | (1,891,949 | ) | $ | (3,141,608 | ) | ||||
| Preferred Stock dividends | $ | (1,002,599 | ) | $ | (1,355,396 | ) | $ | (328,034 | ) | $ | (448,869 | ) | ||||
| Net loss attributable to common shareholders | $ | (7,910,773 | ) | $ | (8,214,413 | ) | $ | (2,219,983 | ) | $ | (3,590,477 | ) | ||||
| PER SHARE DATA: | ||||||||||||||||
| Basic and diluted loss per common share | $ | (0.30 | ) | $ | (0.38 | ) | $ | (0.08 | ) | $ | (0.16 | ) | ||||
| Basic and diluted weighted average common shares outstanding | 26,214,000 | 21,838,000 | 27,801,000 | 21,951,000 | ||||||||||||
See accompanying notes to condensed consolidated financial statements.
| F-19 |
Neurotrope, Inc. and Subsidiary
Condensed Consolidated Statement of Changes in Shareholders’ Deficit
(Unaudited)
| Additional | ||||||||||||||||||||
| Common Stock | Paid-In | Accumulated | ||||||||||||||||||
| Shares | Amount | Capital | Deficit | Total | ||||||||||||||||
| Balance, January 1, 2015 | 24,034,094 | $ | 2,403 | $ | 6,627,983 | $ | (18,282,689 | ) | $ | (11,652,303 | ) | |||||||||
| Conversion of Series A preferred stock to common stock | 4,607,106 | 461 | 4,001,692 | - | 4,002,153 | |||||||||||||||
| Stock based compensation | - | - | 387,227 | - | 387,227 | |||||||||||||||
| Exercise of common stock warrants | 236,340 | 24 | 2,339 | 2,363 | ||||||||||||||||
| Net loss | - | - | - | (6,908,174 | ) | (6,908,174 | ) | |||||||||||||
| Balance September 30, 2015 | 28,877,540 | $ | 2,888 | $ | 11,019,241 | $ | (25,190,863 | ) | $ | (14,168,734 | ) | |||||||||
See accompanying notes to condensed consolidated financial statements.
| F-20 |
Neurotrope, Inc. and Subsidiary
Condensed Consolidated Statements of Cash Flows
(Unaudited)
| Nine Months | Nine Months | |||||||
| ended | ended | |||||||
| September 30, 2015 | September 30, 2014 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (6,908,174 | ) | $ | (6,859,017 | ) | ||
| Adjustments to reconcile net loss to net | ||||||||
| cash used by operating activities | ||||||||
| Stock based compensation | 387,227 | 930,873 | ||||||
| Consulting services paid by issuance of common stock | - | 25,000 | ||||||
| Depreciation expense | 4,659 | 613 | ||||||
| Change in assets and liabilities | ||||||||
| Increase in prepaid expenses | (250,858 | ) | (67,553 | ) | ||||
| Increase in accounts payable | ||||||||
| and accrued expenses - related party | 36,547 | 51,749 | ||||||
| Increase in accounts payable | 231,970 | 183,919 | ||||||
| Increase in accrued expenses | 22,880 | 637,316 | ||||||
| Total adjustments | 432,425 | 1,761,917 | ||||||
| Net Cash Used in Operating Activities | (6,475,749 | ) | (5,097,100 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Purchase of property and equipment | (11,827 | ) | (48,720 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from note payable | 28,904 | - | ||||||
| Repayments on note payable | (10,511 | ) | - | |||||
| Proceeds from exercise of common stock warrants | 2,363 | - | ||||||
| Net Cash Provided by Financing Activities | 20,756 | - | ||||||
| NET DECREASE IN CASH | (6,466,820 | ) | (5,145,820 | ) | ||||
| CASH AT BEGINNING OF PERIOD | 8,010,357 | 15,211,744 | ||||||
| CASH AT END OF PERIOD | $ | 1,543,537 | $ | 10,065,924 | ||||
| DISCLOSURE OF NON-CASH FINANCING ACTIVITIES: | ||||||||
| Conversion of Series A convertible redeemable preferred stock to common stock | $ | 4,002,153 | $ | 304,515 | ||||
See accompanying notes to condensed consolidated
financial statements.
| F-21 |
NEUROTROPE INC. AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
Note 1 – Organization and Nature of Planned Business:
Neurotrope BioScience was incorporated in Delaware on October 31, 2012. Neurotrope BioScience was formed to advance new therapeutic and diagnostic technologies in the field of neurodegenerative disease, primarily Alzheimer’s disease (“AD”). Neurotrope BioScience is collaborating with the Blanchette Rockefeller Neurosciences Institute (“BRNI”), a related party, in this process. The exclusive rights to certain technology were licensed by BRNI to the Company on February 28, 2013 (see Note 6).
On August 23, 2013, a wholly-owned subsidiary of Neurotrope, Inc. (formerly “BlueFlash Communications, Inc.”), Neurotrope Acquisition, Inc., a corporation formed in the State of Nevada on August 15, 2013 merged (the “Reverse Merger”) with and into Neurotrope BioScience. Neurotrope BioScience was the surviving corporation in the Reverse Merger and became the Company’s wholly-owned subsidiary. All of the outstanding Neurotrope BioScience common stock was converted into shares of Neurotrope, Inc. common stock on a one-for-one basis.
The transaction was accounted for as a reverse merger and recapitalization with Neurotrope BioScience as the acquirer for financial reporting purposes and Neurotrope, Inc. as the acquired company. Consequently, the assets and liabilities and the operations that are reflected in the historical financial statements are those of Neurotrope BioScience and are recorded at the historical cost basis of Neurotrope BioScience, and the consolidated financial statements after completion of the Reverse Merger include the assets and liabilities of Neurotrope BioScience and Neurotrope, Inc., and the historical operations of Neurotrope, Inc. and Neurotrope BioScience from the closing date.
As a result of the Reverse Merger, Neurotrope, Inc. discontinued its pre-Reverse Merger business and acquired the business of Neurotrope BioScience, which it is continuing to operate through Neurotrope BioScience. The common stock of Neurotrope, Inc. is traded under the ticker symbol “NTRP.”
Certain information and footnote disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States (U.S. GAAP) have been condensed or omitted. These consolidated financial statements should be read in conjunction with the Consolidated Financial Statements and Notes to the Consolidated Financial Statements included in our Annual Report on Form 10-K for the year ended December 31, 2014.
Note 2 – Going Concern:
The unaudited consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As shown in the consolidated financial statements, we have generated no revenues to-date, have incurred substantial losses and have substantial contractual commitments pursuant to various agreements to which we are a party.
Our ability to continue existence is dependent upon our continuing efforts to obtain additional financing to carry out our business plan. We intend to fund our operations through equity and/or debt financing arrangements and any revenues generated in the future. However, there can be no assurance that these arrangements, if any, will be sufficient to fund our ongoing capital expenditures, working capital, and other cash requirements. The outcome of these matters cannot be predicted at this time.
There can be no assurance that any additional financings will be available to us on satisfactory terms and conditions, if at all. These conditions raise substantial doubt as to our ability to continue as a going concern.
The consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should we be unable to continue as a going concern.
Note 3 - Summary of Significant Accounting Policies:
Use of Estimates:
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
| F-22 |
Reclassifications:
Certain reclassifications have been made to the 2014 financial statements to conform to the consolidated 2015 financial statement presentation. These reclassifications had no effect on net loss or cash flows as previously reported.
Cash and Cash Equivalents:
The Company considers all highly liquid temporary cash investments with an original maturity of three months or less when purchased to be cash equivalents. At September 30, 2015, the Company’s cash balances may exceed the current insured amounts under the Federal Deposit Insurance Corporation.
Property and Equipment:
Property and equipment is capitalized and depreciated on a straight line basis over the useful life of the asset, which is deemed to be between three and ten years. Property and equipment consist of the following:
September 30, 2015 | December 31, 2014 | |||||||
| Property and equipment | $ | 66,770 | $ | 54,943 | ||||
| Accumulated depreciation | (5,984 | ) | (1,325 | ) | ||||
| Property and equipment, net | $ | 60,786 | $ | 53,618 | ||||
Depreciation expense for the nine months ended September 30, 2015 and 2014 was $4,659 and $613, respectively.
Note Payable:
The Company financed its general liability and workers compensation insurance premiums for the insurable period from May 16, 2015 to May 15, 2016. The total principal amount of the unsecured note payable relating to the premiums was $31,613 on May 16, 2015, which bears interest at an annual rate of 4.12%. The Company paid $2,709 as a down payment on May 16, 2015 and 11 additional monthly payments of $2,682 are due commencing June 15, 2015. The net balance of the note payable as of September 30, 2015 was $18,393.
Research and Development Costs:
All research and development costs, including costs to maintain or expand the Company’s patent portfolio licensed from BRNI that do not meet the criteria for capitalization are expensed when incurred. FASB ASC Topic 730 requires companies involved in research and development activities to capitalize non-refundable advance payments for such services pursuant to contractual arrangements because the right to receive those services represents an economic benefit. Such capitalized advances will be expensed when the services occur and the economic benefit is realized. There were no capitalized research and development services at September 30, 2015 and December 31, 2014.
Income Taxes:
Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due and deferred taxes. Deferred taxes are recognized for differences between the bases of assets and liabilities for financial statement and income tax purposes and the tax effects of net operating loss and other carryforwards. The deferred tax assets and liabilities represent the future tax consequences of those differences and carryforwards, which will either be taxable or deductible when the related assets, liabilities or carryforwards are recovered or settled. Deferred tax assets are reduced by a valuation allowance when, based on the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
The Company applies the provisions of FASB ASC 740-10, Accounting for Uncertain Tax Positions , which clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The standard also provides guidance on de-recognition, classification, interest and penalties, and accounting in interim periods, disclosure and transitions.
The Company has concluded that there are no significant uncertain tax positions requiring recognition in the accompanying financial statements. The tax period that is subject to examination by major tax jurisdictions is from October 31, 2012 (inception) through September 30, 2015.
In the event the Company was to receive an assessment for interest and/or penalties, it will be classified in the financial statements as selling, general and administrative expense when assessed.
| F-23 |
Risks and Uncertainties:
The Company operates in an industry that is subject to rapid technological change, intense competition and significant government regulation. The Company’s operations are subject to significant risk and uncertainties including financial, operational, technological, regulatory and other risk. Such factors include, but are not necessarily limited to, the results of clinical testing and trial activities, the ability to obtain regulatory approval, the ability to obtain favorable licensing, manufacturing or other agreements for its product candidates and the ability to raise capital to achieve strategic objectives. See Part II, Item 1A, “Risk Factors” of this quarterly report below.
Stock Compensation:
The Company accounts for stock-based awards to employees in accordance with applicable accounting principles, which requires compensation expense related to share-based transactions, including employee stock options, to be measured and recognized in the financial statements based on a determination of the fair value of the stock options. The grant date fair value is determined using the Black-Scholes-Merton (“Black-Scholes”) pricing model. For all employee stock options, we recognize expense over the employee’s requisite service period (generally the vesting period of the equity grant). The Company’s option pricing model requires the input of highly subjective assumptions, including the expected stock price volatility, expected term, and forfeiture rate. Any changes in these highly subjective assumptions significantly impact stock-based compensation expense.
Options awarded to purchase shares of common stock issued to non-employees in exchange for services are accounted for as variable awards in accordance with applicable accounting principles. Such options are valued using the Black-Scholes option pricing model.
Note 4 – Contractual Commitments:
Effective as of July 16, 2014, the Company’s former President’s employment as Chief Executive Officer and President of Neurotrope BioScience and the Company was terminated. In connection with the termination, effective as of July 16, 2014, Neurotrope BioScience and its former President entered into a separation agreement and general release on October 9, 2014 (the “Separation Agreement”).
The Separation Agreement states that Neurotrope BioScience will pay or provide to the former President the following: (a) $2,684 within three days of the execution of the Separation Agreement by him, representing reimbursement of business expenses; (b) a lump sum equal to the total gross amount of $233,000; (c) a gross amount of $25,478 for his accrued, unused vacation; (d) reimbursement for the cost of continuing his current health care insurance in the same amount as the net reimbursement amounts paid to him on a monthly basis immediately prior to July 16, 2014 ($2,974 per month) until the earlier of the date on which he becomes eligible for medical insurance coverage with a new employer or July 16, 2015; (e) director’s and officer’s insurance coverage covering his actions while a director and/or officer of Neurotrope BioScience until July 16, 2020; and (f) $17,000 for reimbursement of his attorneys fees. In addition, the parties agreed that his ownership of any common shares of the company is not affected by the Separation Agreement and that any equity awards he received pursuant to the 2013 Equity Incentive Plan (the “2013 Plan”) will be governed exclusively by the terms of such plan. Approximately $282,000 was paid to him pursuant to the Separation Agreement in 2014. All remaining payments due were paid during the nine months ended September 30, 2015.
Effective February 28, 2013, the Company executed an agreement with Ramat Consulting Corp. (“Ramat”), a related party to the Company’s current President and CEO Charles S. Ramat, for consulting services, including business development and marketing consulting, for a five-year period, subject to annual renewals thereafter. The Ramat agreement was amended on February 2, 2015 to add Mr. Ramat’s duties as the Company’s President and CEO. Ramat's annual fee is $50,000, payable in monthly installments of $4,167, plus pre-approved travel and other reimbursable expenses. Ramat was issued a non-qualified option, with a term of ten years, to purchase 300,000 shares of common stock of the Company at an exercise price of $1.00 per share. The option vested with respect to 20% of the shares as of February 28, 2013, and the balance vest on a daily basis over the four-year period beginning on February 28, 2013. Vested options will terminate, to the extent not previously exercised, upon the occurrence of the first of the following events: (a) ten years from the date of grant; (b) the expiration of one year from the date of death or the termination of optionee as a service provider by reason of disability; or (c) the expiration of twelve months from the date of termination of optionee’s service as a service provider for any reason whatsoever other than termination of service for death or disability. An entity related to Ramat purchased 1,000,000 shares of the Company’s Series A Preferred Stock on the effective date of the agreement described in this paragraph. (See below in this Note 4 and Note 12 – “Subsequent Events” for additional information regarding Mr. Ramat’s compensation arrangement).
On October 1, 2013, the Company executed a four-year employment agreement, effective October 1, 2013, with Robert Weinstein, its current Executive Vice President, Chief Financial Officer, Treasurer and Secretary. This agreement provides for an annual salary of $240,000, which increased to an annual salary of $275,000 beginning January 1, 2015 plus a targeted bonus of 50% of base salary for all subsequent years. On October 1, 2013, the Company’s Board of Directors (the “Board”) granted an incentive stock option to Mr. Weinstein under the 2013 Planto purchase 650,000 shares of the Company’s common stock, with a term of ten years, exercisable at $1.00 per share. These options vest 25% per year over four years, with accelerated vesting of 25% upon a termination without cause or for good reason.
| F-24 |
On January 16, 2014, the Company executed a four-year employment letter with Neurotrope BioScience’s former Vice President – Commercial Operations. This employment letter provided for an annual salary of $210,000. On January 23, 2014, the Board granted an incentive stock option to its Vice President – Commercial Operations under the 2013 Plan to purchase 100,000 shares of the Company’s common stock, with a term of ten years, exercisable at $1.76 per share. These options vest 20% per year over five years. Vested options will terminate, to the extent not previously exercised, upon the occurrence of the first of the following events: (a) ten years from the date of grant; or (b) the expiration of one year from the date of death, disability or the termination of employment. On May 15, 2015, the Vice President – Commercial Operations was terminated without cause. As a result, the Company paid a severance payment of approximately $40,000 during the nine months ending September 30, 2015. In addition, on June 17, 2015, the Vice President signed a separation agreement in which he deferred payment of his remaining severance due him of $175,000. Receipt of said amount is contingent upon the Company raising additional capital in excess of $7.5 million. As a result, the Company has accrued a severance payment of $175,000 as of September 30, 2015.
On January 22, 2014, the Company executed a four-year employment agreement with Neurotrope BioScience’s former Vice President and Chief Medical Officer. On October 31, 2014, the Company terminated him without cause. Approximately $217,000 was accrued for this severance arrangement in 2014 and paid in February 2015.
On June 1, 2014, the Company executed a four-year employment letter with Neurotrope BioScience’s former Executive Director – Pharmacology. This employment letter provided for an annual salary of $180,000. On July 16, 2014, the Board granted an incentive stock option to the Executive Director-Pharmacology for the purchase of 50,000 shares of the Company’s common stock with a term of tenyears, exercisable at $1.24 per share. These options vest 20% per year over five years. Vested options will terminate, to the extent not previously exercised, upon the occurrence of the first of the following events: (a) ten years from the date of grant; or (b) the expiration of one year from the date of death, disability or the termination of employment. On May 15, 2015, the Executive Director – Pharmacology was terminated without cause. As a result, the Company has accrued a severance payment of $90,000 as of September 30, 2015. Receipt of said amount is contingent upon the Company raising additional capital in excess of $7.5 million.
On July 16, 2014, the Board appointed Charles S. Ramat and Paul E. Freiman to serve as Co-Chief Executive Officers, on an interim basis, of the Company and of Neurotrope BioScience. Mr. Freiman has served on the Board since October 18, 2013, and was appointed Co-Chairman of the Board on June 13, 2014. Mr. Ramat was elected to the Board on June 13, 2014 and was immediately appointed Co-Chairman of the Board.
In consideration of the services to be provided by Mr. Freiman and Mr. Ramat to the Company as Co-Chief Executive Officers, the Company agreed to pay each of them consulting fees in the amount of $20,000 per month for a term not to exceed six months, which fees would be reduced upon the employment of a permanent Chief Executive Officer to $10,000 per month for two months to aid in the transition of responsibilities to the new Chief Executive Officer. In addition, on July 23, 2014, Messrs. Freiman and Ramat were each granted non-qualified options to purchase 400,000 shares of the Company’s common stock for their roles as directors, Co-Chairmen of the Board and Co-Chief Executive Officers. Options to purchase 200,000 shares have an exercise price of $1.11 per share, or 110% of the Company’s last trading price on July 23, 2014 and were fully vested upon grant. Options to purchase the remaining 200,000 shares have an exercise price of $2.22 per share based upon two times the exercise price of $1.11 per share and vest on a daily basis up to 25% per year through July 23, 2018. See below regarding further changes to Messrs. Ramat and Freiman’s roles with the Company.
On September 4, 2014, the Company entered into a long-term lease for 4,000 square feet of office space in Newark, New Jersey. The lease commenced September 1, 2014 and expires December 1, 2017 and has two (2) one-year renewal options. The base rent is payable, commencing December 1, 2014, at an annual rate of $88,000 with no increases during the lease term and renewal terms. In addition, commencing September 1, 2014, the Company is obligated to pay its share of common area charges. The Company is amortizing the total base rent due over 39 months.
On September 12, 2014, the Board elected Mr. Ramat to be the Company’s President and Chief Executive Officer and Mr. Freiman to be the Company’s Chairman of the Board. In consideration of the services that Mr. Ramat will provide to the Company as President and CEO, the Company agreed to pay Mr. Ramat $400,000 per year with a bonus opportunity of up to 50% of such amount after one (1) year of service, which arrangement could have been terminated by either party on 60 days’ notice. The compensation payments to Mr. Ramat replaced the $20,000 per month consulting fee of July 16, 2014. Mr. Freiman’s compensation remains at $20,000 per month as Chairman of the Board. In addition to his salary for services as President and Chief Executive Officer of the Company, Mr. Ramat continued to be paid under an agreement with the Company pursuant to which he received $50,000 per year until September 28, 2015 when the Company and Mr. Ramat entered into an Employment Agreement described below. Messrs. Ramat and Freiman voluntarily deferred part of their compensation in order for the Company to conserve cash (See Note 4 below – “Subsequent Events”).
In addition, on September 12, 2014, Messrs. Ramat and Freiman were granted non-qualified options to purchase 250,000 and 50,000 shares of the Company’s common stock, respectively, which have an exercise price of $0.60 per share, were fully vested upon grant, and have a ten-year life.
| F-25 |
As of November 1, 2014, Neurotrope BioScience entered into an employment agreement for one (1) year with provisions for automatic renewals, with its Executive Vice President, Development and Chief Medical Officer. This agreement provided for an annual salary of $325,000. In addition, the employment agreement provided for a signing bonus of $102,083 in total, payable $8,507 monthly (if employed during the applicable payment cycle) and a discretionary bonus of up to 50% of base salary. In connection with his employment and as of his date of hire, the Company’s Compensation Committee authorized a grant of a non-qualified stock option to such officer under the 2013 Plan to purchase 250,000 shares of the Company’s common stock, with a term of ten years, exercisable at $0.71 per share. These options vest 20% per year over five years commencing November 1, 2015. Vested options will terminate, to the extent not previously exercised, upon the occurrence of the first of the following events: (a) ten years from the date of grant; or (b) the expiration of one year from the date of death, disability or the termination of employment. As of November 9, 2015, this executive resigned as an officer from Neurotrope BioScience and will remain as Acting Chief Medical Officer until the earlier of December 31, 2015 or the appointment of his successor.
On April 10, 2015, the Board agreed to grant Mr. Ramat a stock option exercisable for 100,000 shares of common stock of Neurotrope (the “Financing Option”) in the event the Company successfully completes a financing pursuant to which it raises at least ten million dollars ($10,000,000). The Financing Option will be issued to Mr. Ramat on the date of the closing of the applicable financing (assuming Mr. Ramat’s continued service as the President and Chief Executive Officer of the Company as of such date), will be fully vested as of the date of grant, and will have an exercise price per share equal to the price per share paid by investors for securities of the Company in the applicable financing.
On July 1, 2015, Mr. Ramat agreed to defer $25,000 of his salary, and Mr. Freiman agreed to defer 50% of his consulting fees until the Company raises additional funds. Also, as of August 1, 2015, the former Chairman of the Board, who receives $9,000 a month in fees, agreed to defer 50% of his director fees until the Company raises additional funds. The Company accrues such fee deferral from July 1, 2015 and August 1, 2015, respectively, until they are paid. As of September 30, 2015, Mr. Freiman was paid $25,000 of his deferred amount.
On September 28, 2015, the Company and Neurotrope BioScience, Inc. consolidated its prior agreements, understandings and commitments into an Employment Agreement with Mr. Ramat to serve as the President and Chief Executive Officer of the Company (the “Employment Agreement). The Employment Agreement supersedes and replaces all such prior agreements, understandings and commitments with respect to Mr. Ramat’s employment and (until its expiration or termination) supersedes and replaces the consulting agreement effective February 28, 2013, as amended, between the Ramat Consulting Group, a related party to Mr. Ramat, and Neurotrope BioScience, Inc. (the “Consulting Agreement”). The consolidation of Agreements did not result in a change to Mr. Ramat’s cash compensation. The Employment Agreement is effective as of September 28, 2015, and has a term of one year with automatic renewals for successive one-year periods unless terminated by either party upon 60 days’ written notice prior to the expiration of the then-current term, or until such employment is earlier terminated in accordance with termination provisions set forth in the Employment Agreement (the “Employment Term”). During the Employment Term, so long as Mr. Ramat is willing to stand for re-election, the Company agreed to nominate him for re-election as a director at the annual meeting (and any other meeting) of stockholders for the election of directors.
Under the Employment Agreement, Mr. Ramat is entitled to five weeks of paid vacation per annum and general expense reimbursement for pre-approved business related expenses incurred in the performance of his duties. He will also be eligible for all benefits and retirement, life, disability, medical and dental plan benefits generally available to the Company’s officers in accordance with the terms of those plans.
If Mr. Ramat’s employment is terminated for any reason, he is entitled to his accrued benefits. In addition, if his employment is terminated during the Employment Term by the Company for a reason other than Cause (as defined in the Employment Agreement), death or disability, or is terminated by Mr. Ramat for Good Reason (as defined in the Employment Agreement), or as a result of the Company not renewing the Employment Term, in each case, subject to Mr. Ramat’s compliance with certain conditions, then Mr. Ramat is entitled to receive a severance amount equal to his then-current base salary less $50,000 multiplied by 50%, payable in a single lump sum. If Mr. Ramat’s employment is terminated by: (i) the Company for Cause or due to Mr. Ramat’s death or disability, or (ii) by Mr. Ramat without Good Reason, then he is not entitled to any severance, and shall only be entitled to his accrued benefits.
The Employment Agreement contains non-competition, non-solicitation and non-disclosure covenants of Mr. Ramat.
Note 5 – Collaborative Agreements:
On May 12, 2014, the Company entered into a license agreement (the “Stanford Agreement”) with The Board of Trustees of The Leland Stanford Junior University (“Stanford”), pursuant to which Stanford has granted to the Company a revenue-bearing, world-wide right and exclusive license, with the right to grant sublicenses (on certain conditions), under certain patent rights and related technology for the use of bryostatin structural derivatives, known as “bryologs,” for use in the treatment of central nervous system disorders, lysosomal storage diseases, stroke, cardio protection and traumatic brain injury, for the life of the licensed patents.
Pursuant to the Stanford Agreement, in 2014, the Company paid Stanford a total of $60,850, of which $48,564 was expensed by the Company in 2014 with the balance expensed during the six month period ending June 30, 2015. The Company is also obligated to pay Stanford: (a) a $10,000 annual license maintenance fee; (b) milestone payments in the aggregate amount of up to $3,700,000 upon the achievement of certain product development events commencing upon the filing of the first IND application through approval of an applicable product; and (c) low single-digit royalties on net sales of licensed products. During the nine month period ending September 30, 2015, the Company paid Stanford the annual maintenance fee of $10,000 which will be amortized over the applicable one-year licensing period.
| F-26 |
Each party has the right to terminate the Stanford Agreement for an uncured material breach of the other party. Additionally, the Company may terminate the Stanford Agreement at any time upon 60 days’ written notice to Stanford.
On May 15, 2014, the Company entered into an agreement with a contract research organization (“CRO”) to conduct a clinical trial relating to AD. The Company had agreed to pay fees to the CRO totaling $715,159, based upon signing of the agreement and the CRO achieving certain clinical trial milestones, plus reasonable out-of-pocket expenses. On November 19, 2014, the CRO agreement was amended, requiring the Company to pay a reduced amount totaling $657,238 plus third party pass through expenses. The Company has incurred a total of $687,112 in fees, $377,225 and $417,877 of which are included in the balance sheet as payables as of September 30, 2015 and December 31, 2014, respectively.
On July 14, 2014, Neurotrope BioScience entered into an Exclusive License Agreement (the “Mount Sinai Agreement”) with the Icahn School of Medicine at Mount Sinai (“Mount Sinai”). Pursuant to the Mount Sinai Agreement, Mount Sinai granted Neurotrope BioScience a revenue-bearing, world-wide right and (a) exclusive license, with the right to grant sublicenses (on certain conditions), under Mount Sinai’s interest in certain joint patents held by the Company and Mount Sinai (the “Joint Patents”) as well as in certain results and data (the “Data Package”) and (b) non-exclusive license, with the right to grant sublicenses on certain conditions, to certain technical information, both relating to the diagnostic, prophylactic or therapeutic use for treating diseases or disorders in humans relying on activation of PKC epsilon, which includes Niemann-Pick C Disease (the “Mount Sinai Field of Use”). The Mount Sinai Agreement allows Neurotrope BioScience to research, discover, develop, make, have made, use, have used, import, lease, sell, have sold and offer certain products, processes or methods that are covered by valid claims of Mount Sinai’s interest in the Joint Patents or an Orphan Drug Designation Application covering the Data Package (“Mount Sinai Licensed Product(s)”) in the Mount Sinai Field of Use.
Pursuant to the Mount Sinai Agreement, Neurotrope BioScience is obligated to pay a $25,000 license initiation fee to Mount Sinai. Neurotrope BioScience is also obligated to pay Mount Sinai (a) a $10,000 annual license maintenance fee until minimum royalty payments are due, (b) milestone payments in the aggregate amount of up to $3,500,000 upon the achievement of certain product development events relating to the approval of a Mount Sinai Licensed Product in the United States and other jurisdictions, (c) low single-digit royalties on net sales of the Mount Sinai Licensed Products, and (d) a portion of sublicense fees ranging from a significant double-digit percentage based on sublicensing before completion of in vivo proof-of-concept experiments to a mid-single digit percentage after product approval. In 2014, the Company paid Mount Sinai $25,000, which represents the license initiation fee described above. Of this $25,000, the Company expensed $11,458 in 2014 and $13,542 during the nine month period ending September 30, 2015. In addition, the Company paid its annual license maintenance fee of $10,000 in September 2015 which will be amortized over the applicable one-year licensing period.
Each party has the right to terminate the Mount Sinai Agreement for an uncured material breach of the other party. Additionally, Neurotrope BioScience may terminate the Mount Sinai Agreement at any time upon 60 days’ written notice to Mount Sinai. Further, upon termination, Neurotrope BioScience may continue to sell any and all Mount Sinai Licensed Products, provided that Neurotrope BioScience will pay Mount Sinai a reduced royalty for Mount Sinai Licensed Products that are indicated as therapeutics or diagnostics for Niemann Pick disease which are sold following the termination of the Mount Sinai Agreement.
The term of the Mount Sinai Agreement began upon signing and will expire with respect to each Mount Sinai Licensed Product on a Mount Sinai Licensed Product-by-Mount Sinai Licensed Product basis and country-by-country basis, from first commercial sale until the later of: (a) expiration of the last to expire Joint Patent Rights covering such Mount Sinai Licensed Product in such country; (b) expiration of any market exclusivity period granted by a regulatory agency with respect to such Mount Sinai Licensed Product in such country; or (c) ten years after the first commercial sale of such Mount Sinai Licensed Product in such country.
On March 30, 2015, Neurotrope Bioscience signed a letter of intent with Worldwide Clinical Trials (“WCT”) to commence pre-patient enrollment activities for the conduct of a Phase 2b clinical trial of the Company’s compound, bryostatin-1, for the treatment of Alzheimer’s disease. The letter of intent, which has subsequently expired, outlined the scope of the services that WCT provided to the Company in relation to the expected Phase 2b clinical trial, as well as additional services that WCT expects to provide once the clinical trial is initiated after the Company obtains additional financing. The Company paid WCT approximately $300,000 for the services and related third-party costs pursuant to the terms of the letter of intent (See Note 12 – Subsequent Events, for additional information relating to WCT).
Note 6 – Related Party Transactions and Licensing / Research Agreements:
Two directors of the Company, James Gottlieb and William Singer, are also directors of BRNI. Mr. Singer is the president of BRNI. BRNI is a stockholder of a corporation, Neuroscience Research Ventures, Inc. (“NRVI”), which owned 31.1% of the Company's outstanding common stock as of September 30, 2015.
| F-27 |
Effective October 31, 2012, Neurotrope BioScience executed a Technology License and Services Agreement with BRNI, a related party, and NRV II, LLC, another affiliate of BRNI (the “TLSA”), which was previously amended by Amendment No. 1 to the TLSA as of August 21, 2013. As of February 4, 2015, the parties entered into an Amended and Restated Technology License and Services Agreement (the “BRNI License Agreement”). The BRNI License Agreement further amended and restated the TLSA, as amended. Under the terms of the BRNI License Agreement, BRNI provides research services and granted Neurotrope BioScience the exclusive and nontransferable license right to certain patent and intellectual property which became effective upon the Neurotrope BioScience’s completion of a Series A Preferred Stock financing generating net proceeds to Neurotrope BioScience of at least $8,000,000 on February 28, 2013. The BRNI License Agreement terminates on the later of the date (a) the last of the licensed patent expires, is abandoned, or is declared unenforceable or invalid or (b) the last of the intellectual property enters the public domain.
After the initial Series A Preferred Stock financing, the BRNI License Agreement requires Neurotrope BioScience to enter into scope of work agreements with BRNI as the preferred service provider, for any research and development services or other related scientific assistance and support services. Neurotrope BioScience is not permitted to engage any other person other than BRNI to perform research or development services or other related scientific assistance without prior written consent of BRNI, except under certain circumstances. BRNI and Neurotrope may agree to have a third party provide services identical or similar to such services to Neurotrope in the case where BRNI is demonstrably unable to do so or such third party is demonstrably in a superior position to do so.
In addition, the BRNI License Agreement requires the Company to pay BRNI a “Fixed Research Fee,” commencing the date that the Company completes a Series B Preferred Stock financing resulting in net proceeds of at least $25,000,000 (the “Series B Financing”). The fixed research fee is (a) a pro-rata amount of $1,000,000 in the year the Company completes such financing (b) $1,000,000 per year for five calendar years subsequent to such financing and (c) an annual fixed research fee in an amount to be negotiated and agreed upon no later than 90 days prior to the end of the fifth calendar year following the completion of such financing to be paid with respect to each remaining calendar year during the term of the BRNI License Agreement. The Company has not yet completed a Series B Financing (see Note 9) and, accordingly, no such fees are due.
The BRNI License Agreement also requires the payment of royalties ranging between 2% and 5% of the Company’s revenues generated from the licensed patents and other intellectual property, dependent upon the percentage ownership that NRVI holds in the Company. Under the BRNI License Agreement, the Company is required to prepay royalty fees at a rate of 5% of all investor funds raised in the Series A or Series B Preferred Stock financings or any subsequent rounds of financing prior to a public offering, less commissions.
Pursuant to the BRNI License Agreement, on February 4, 2015, Neurotrope BioScience and BRNI entered into a new Statement of Work and Account Satisfaction Agreement, or the February 2015 SOW. The February 2015 SOW is effective as of October 1, 2014 and ended on September 30, 2015.
Pursuant to the February 2015 SOW, Neurotrope BioScience agreed to pay BRNI $2,400,000 in service fees and other amounts payable at a rate of $200,000 per month for each month from October 1, 2014 through September 30, 2015. The parties agreed that the first $600,000 of payments satisfy certain outstanding amounts owed to BRNI. Of this $600,000, $400,000 was paid in 2014, and the remaining $200,000 was paid in 2015. Neurotrope BioScience also agreed to pay BRNI $20,000, in quarterly payments of $5,000 each, during the twelve months from the date of the SOW in exchange for advising and consulting services by BRNI’s chief scientist regarding Neurotrope BioScience’s contract with the Icahn School of Medicine at Mt. Sinai Hospital for the use of bryostatin in the treatment of Niemann Pick disease.
In consideration for the February 2015 SOW, BRNI agreed to (a) use commercially reasonable efforts to enroll at least four (4) additional compassionate use or expanded access patients, in trials of BRNI’s Alzheimer’s therapeutic drug platform during the term of the SOW, (b) perform certain services requested by Neurotrope BioScience for the further development of BRNI’s Alzheimer’s therapeutic drug platform, (c) perform certain services for the further development of BRNI’s Alzheimer’s diagnostic test, (d) to the extent permitted by applicable law, transfer all of its rights and regulatory obligations, except for those relating to the compassionate use expanded access trials, associated with BRNI’s Investigative New Drug Application (IND) 71,276 to Neurotrope BioScience, (e) conduct initial research on the application of its PKC epsilon platform to treat Fragile X disease, (f) conduct initial research on polyunsaturated fatty acid derivatives for the purpose of developing a commercially usable PKC epsilon activator, and (g) provide assistance, advice and other similar services to Neurotrope BioScience regarding Neurotrope BioScience’s analysis of bryologs pursuant to Neurotrope BioScience’s agreement with Stanford University, for the purpose of developing a commercially usable PKC epsilon activator. Furthermore, BRNI agreed to transfer a certain amount of bryostatin drug substance and bryostatin kits containing drug substance for non-human use to a third-party for storage and handling for Neurotrope BioScience’s pre-clinical and clinical use, which was completed on March 10, 2015.
In order for BRNI to perform certain of the services described in (c) above, Neurotrope BioScience reimbursed a third party for services BRNI received from such third party in the amount of $150,000 in connection with BRNI’s former diagnostic trial program with such third party.
Under the BRNI License Agreement, Neurotrope BioScience received a license to certain technology, including rights relating to an in vitro test system based on examination of skin cells intended to predict the presence of Alzheimer’s disease in humans (the “AD Diagnostic Test”). Further, under the February 2015 SOW, Neurotrope BioScience’s rights to the technology associated with the AD Diagnostic Test automatically reverted to BRNI based on certain contractual conditions on July 10, 2015. The remaining terms and conditions of the BRNI License Agreement, including the license of technology associated with Neurotrope BioScience’s PKC activator research (such as bryostatin), remain in full force and effect, in accordance with the conditions thereof.
| F-28 |
For the nine months ended September 30, 2015, Neurotrope BioScience incurred $1,852,428 in expenses relating to the February 2015 SOW and has recorded a payable of $200,000 on its balance sheet as of September 30, 2015.
See Note 4 for additional related party transactions.
Note 7– Income Taxes:
The Company incurred a net operating loss for income tax purposes of $20,184,163 for the period from October 31, 2012 (inception) through September 30, 2015. Income tax effects of share-based payments are recognized in the financial statements for those awards that will normally result in tax deductions under existing tax law. Under current U.S. federal tax law, the Company would receive a compensation expense deduction related to non-qualified stock options only when those options are exercised. Accordingly, the financial statement recognition of compensation cost for non-qualified stock options creates a deductible temporary difference which results in a deferred tax asset of approximately $388,000 as of September 30, 2015. The Company does not recognize a tax benefit for compensation expense related to incentive stock options (ISOs) unless the underlying shares are disposed of in a disqualifying disposition. Accordingly, compensation expense related to ISOs is treated as a permanent difference for income tax purposes. These deferred tax assets are reduced to zero by an offsetting valuation allowance. As a result, there is no provision for income taxes.
Note 8 – Common Stock:
During the nine months ended September 30, 2015, 36 holders of 4,607,106 total shares of Preferred Stock converted that Preferred Stock into 4,607,106 shares of Neurotrope’s common stock and seven holders of 236,340 warrants exercisable at $0.01 per share of common stock exercised their warrants into 236,340 shares of Neurotrope’s common stock. During the year ended December 31, 2014, ten holders of 1,637,000 total shares of Preferred Stock converted that Preferred Stock into 1,637,000 shares of Neurotrope’s common stock and five holders of 613,228 warrants exercisable at $0.01 per share of common stock exercised their warrants into 613,228 shares of Neurotrope’s common stock.
Note 9 – Preferred Stock:
As of September 2, 2014, the Company and the holders of a majority of the Company’s Series A Preferred Stock entered into an amendment (the “Amendment”) to the Preferred Stockholders Agreement, dated as of August 23, 2013 (the “Preferred Stockholder Agreement”). Under the terms of the Amendment, the parties removed provisions relating to the right of first refusal and certain prohibitions relating to transfers, which were set forth in the Preferred Stockholder Agreement. In addition, the parties made other technical changes to the terms of the Preferred Stockholder Agreement.
Note 10 – Stock Options:
Our Board of Directors adopted, and our stockholders approved, the 2013 Plan, which provided for the issuance of incentive awards of up to 7,000,000 shares of the Company’s common stock (until it was amended as provided below) to officers, key employees, consultants and directors. Upon the closing of the Reverse Merger, options to purchase an aggregate of 5,154,404 shares of the Company’s common stock were issued. Of these options: (a) 3,500,000 options were issued to founding stockholders and 54,404 were granted to a consultant, each with a term of ten years, exercisable at $1.75 per common share, 100% of these options vested on the date of grant; (b) 1,050,000 options were issued to four directors (or their affiliates) and 250,000 to a consultant with a term of ten years, exercisable at $1.00 per common share, which vest 20% per year for each of five years after the date of grant; and, (c) 300,000 options were granted to a consultant with a term of ten years, exercisable at $1.00 per common share which vested 20% on the date of grant and vest 20% per year over the next four years. These options were issued in conversion of options issued by Neurotrope BioScience on February 28, 2013.
On January 23, 2014, the Company issued, under the 2013 Plan, 300,000 options to three employees, each with a term of ten years, exercisable at $1.76 per common share. 125,000 of these options vest 25% per year, with accelerated vesting of 25% upon a termination without cause or for good reason, with the remaining 175,000 vesting 20% per year. On March 21, 2014, the Company issued, under the 2013 Plan, 30,000 options to one employee with a term of ten years, exercisable at $2.30 per common share and vests 20% per year commencing on March 31, 2015.
On July 16, 2014, the Company issued incentive stock options to purchase 50,000 shares of common stock under the 2013 Plan to one employee with a term of ten years, exercisable at $1.24 per common share which vest 20% per year commencing July 16, 2015.
On July 23, 2014, the Company’s Board, and on June 9, 2015, the stockholders, approved increasing the total shares available under the 2013 Plan by 3,000,000 to 10,000,000.
| F-29 |
Also, on July 23, 2014, the Board granted non-qualified stock options to three directors, each in the amount of 100,000 shares of common stock; 50,000 of which have an exercise price equal to $1.11, which vested on the date of the grant hereof; and 50,000 of which have an exercise price equal to two times the value of the exercise price of the first 50,000 options, or $2.22 which vest daily over four (4) years (assuming continued service on behalf of the Company), up to 25% per year, until the grant is fully vested on July 23, 2018.
On September 12, 2014, the Board granted the Company’s Chief Executive Officer and Chairman non-qualified options to purchase 250,000 and 50,000 shares of the Company’s common stock, respectively, which have an exercise price of $0.60 per common share, are fully vested upon grant and have a term of ten years. Also, the Board granted 300,000 non-qualified stock options to a newly appointed director with a term of ten years, exercisable at $0.60 per common share and vesting daily over five years commencing September 12, 2014.
On March 19, 2015, the Company issued stock options to purchase 20,000 shares of the Company’s common stock to one employee with a term of ten years, exercisable at $1.17 per common share and vesting 20% per year commencing March 19, 2016. In addition, the Company issued stock options to purchase 50,000 shares of the Company’s common stock to one consultant with a term of ten years, exercisable at $1.17 per common share and vesting over four years beginning September 15, 2014. As of September 30, 2015, the consultant’s options terminated with no vested options exercised based upon the consultant not renewing its consulting agreement with the Company.
A summary of the Company’s stock option activity for the nine months ending September 30, 2015 is as follows:
| Options Outstanding | Weighted-Average Exercise Price | |||||||
| Outstanding at January 1, 2015 | 7,769,202 | $ | 1.40 | |||||
| Options granted | 70,000 | $ | 1.17 | |||||
| Options forfeited | (101,250 | ) | $ | (1.24 | ) | |||
| Options exercised | 0 | 0 | ||||||
| Outstanding at September 30, 2015 | 7,737,952 | $ | 1.40 | |||||
| Options exercisable September 30, 2015 | 5,037,863 | $ | 1.48 | |||||
The weighted-average remaining contractual term of options exercisable and outstanding at September 30, 2015 was approximately up to 7.8 and up to 8.2 years, respectively.
The Company used the Black-Scholes valuation model to calculate the fair value of stock options. Stock-based compensation expense is recognized over the vesting period using the straight-line method.
The fair value of stock options issued for the nine months ended September 30, 2015 was estimated at the grant date using the following weighted average assumptions: Dividend yield 0%; Volatility 91.85%; Risk-free interest rate 1.98%; weighted average grant date fair value of $1.02 per common share. There is no current allowance for forfeitures.
The total stock option-based compensation recorded as operating expense was $387,227 and $930,873 for the nine months ended September 30, 2015 and 2014, respectively. All of the stock option-based compensation expense was classified as stock based compensation expense. On September 28, 2015, the Company’s Board of Directors amended options granted to all its officers and directors to reflect that all vested options expire at the end of the options term.
Note 11 – Common and Preferred Stock Reserved for Future Issuances:
Common stock reserved for future issuances consisted of the following at September 30, 2015:
| Common Stock Reserved | ||||
| Common stock warrants outstanding | 1,375,432 | |||
| Common stock options outstanding | 7,737,952 | |||
| Conversion of Series A Preferred Stock | 16,755,894 | |||
| Total | 25,869,278 | |||
On February 9, 2015, the holders of placement agent warrants to purchase an aggregate of 1,325,000 shares of our Series A Preferred Stock entered into Conversion Agreements with us pursuant to which such holders’ placement agent warrants to purchase Series A Preferred Stock were converted, for no additional consideration, into common stock purchase warrants to purchase an aggregate of 1,325,000 shares of our common stock.
In the November 2015 Private Placement, the Company sold 26,234,940 units at $0.60 per unit with each unit consisting of Series B convertible preferred stock, or the Series B Stock, together with Series A through Series E warrants to purchase common stock, and certain placement agent warrants, resulting in gross proceeds of $15,640,963. The total number of units sold includes 16,667 units granted to Dr. Abeles as compensation for his services as a consultant to the Company, which accounts for $100,000 that is not included in our calculation of gross proceeds. The private placement was completed in two closings, which took place on November 13, 2015 and November 30, 2015. In connection with this private placement, effective as of November 13, 2015, the holders of all 16,656,894 shares of our Series A Stock converted their shares into 19,865,109 shares of our common stock, which included 3,208,215 shares of our common stock issued in accordance with anti-dilution provisions relating to the Series A Stock.
On November 12, 2015, we entered into an amendment to the BRNI License. The amendment eliminates the requirement that Neurotrope Bioscience pay BRNI prepaid royalties equal to five percent (5%) of financing proceeds received by Neurotrope Bioscience in any financing prior to a public offering and provides that Neurotrope Bioscience will deliver to BRNI, following each closing pursuant to a certain securities purchase agreement, an amount equal to 2.5% of the Post-PA Fee Proceeds received at such closing. In addition, the amendment provides that on or prior to December 31, 2016, Neurotrope Bioscience shall deliver to BRNI an amount equal to 2.5% of the aggregate Post-PA Fee Proceeds received at the closings. Each payment would constitute an advance royalty payment to BRNI and will be offset (with no interest) against the amount of future royalty obligations payable until such time that the amount of such future royalty obligations equals in full the amount of the advance royalty payments made. “Post-PA Fee Proceeds” means the gross proceeds received, less all amounts paid to the placement agent(s), in relation to such gross proceeds. No other expenses of Neurotrope Bioscience shall be subtracted from the gross proceeds to determine the “Post-PA Fee Proceeds.”
| F-30 |
Note 12 – Subsequent Events:
From October 1 through November 13, 2015, holders of the Company’s Series A Preferred Stock converted a total of 99,000 shares of the Company’s Series A Preferred Stock (issued in 2013) into 99,000 shares of the Company’s common stock.
On October 9, 2015, Neurotrope BioScience executed a Services Agreement (the “Agreement”) with WCT, effective as of August 31, 2015. The Agreement relates to services for Neurotrope BioScience’s Phase 2b clinical study assessing the safety, tolerability and efficacy of bryostatin in the treatment of moderately severe to severe Alzheimer’s Disease (the “Study”). Pursuant to the terms of the Agreement, WCT will provide services to enroll approximately one hundred and fifty (150) Study subjects. The Company expects that the first Study site will be initiated during the fourth quarter of 2015. The total estimated budget for the services, including pass-through costs, is approximately $11.6 million. Neurotrope BioScience paid WCT an advance payment of $200,000 upon execution of the Agreement. On November 5, 2015, WCT will invoice Neurotrope BioScience for the following advance payments: (i) services fees of approximately $928,000, which will be due within ten (10) days of Neurotrope BioScience’s receipt of such invoice; (ii) pass-through expenses of approximately $268,000, which will be due within ten (10) days of Neurotrope BioScience’s receipt of such invoice; and (iii) investigator/institute fees of approximately $680,000, which will be due within twenty (20) days of Neurotrope BioScience’s receipt of such invoice. Neurotrope BioScience may terminate the Agreement without cause upon sixty (60) days’ prior written notice.
Unless earlier terminated under the provisions of the Agreement, the Agreement will expire upon WCT’s completed performance of the services thereunder (including delivery of all the deliverables) and WCT’s receipt of all payments from Neurotrope BioScience that are due under the Agreement. In addition to Neurotrope BioScience’s termination right described above, Neurotrope BioScience may terminate the Agreement immediately due to patient safety or regulatory request. Further, under the Agreement, either Neurotrope BioScience or WCT may terminate the Agreement if the other party materially breaches the Agreement and fails to cure such breach. Additionally, either Neurotrope BioScience or WCT may terminate the Agreement upon notice to the other party if the other party is adjudicated insolvent or petitions for relief under any insolvency, re-organization, receivership, liquidation, compromise, or any moratorium statute.
Also, in connection with the execution of the Agreement, Neurotrope BioScience received consent and is negotiating a Statement of Work pursuant to its BRNI License Agreement. The Statement of Work must be entered into by December 1, 2015 (which date can be extended by mutual agreement of the parties), or BRNI’s consent to the Agreement shall be withdrawn.
Beginning on December 17, 2015, certain holders of placement agent warrants issued as part of the November 2015 PPO exercised their warrants. As of January 6, 2016, holders had exercised placement agent warrants to purchase 330,515 shares of our common stock at an exercise price of $0.01 per share. Placement agent warrants to purchase 32,941 shares of our common stock at an exercise price of $0.01 per share remain outstanding.
| F-31 |
NEUROTROPE, INC.
240,024,699 Shares of Common Stock
PROSPECTUS
January , 2016
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
Set forth below is an estimate (except for registration fees, which are actual) of the approximate amount of the fees and expenses payable by us in connection with the issuance and distribution of the shares of our common stock. The selling stockholders will not be responsible for any of the expenses of this offering.
| EXPENSE | AMOUNT | |||
| SEC Registration fee | $ | 12,206 | ||
| Accounting fees and expenses | $ | 10,000 | ||
| Legal fees and expenses | $ | 50,000 | ||
| Printing and engraving expenses | $ | 5,000 | ||
| Miscellaneous | $ | 5,000 | ||
| Total | $ | 82,206 | ||
Item 14. Indemnification of Directors and Officers.
Nevada Revised Statutes (NRS) Sections 78.7502 and 78.751 provide us with the power to indemnify any of our directors, officers, employees and agents. The person entitled to indemnification must have conducted himself in good faith, and must reasonably believe that his conduct was in, or not opposed to, our best interests. In a criminal action, the director, officer, employee or agent must not have had reasonable cause to believe that his conduct was unlawful.
Under NRS Section 78.751, advances for expenses may be made by agreement if the director or officer affirms in writing that he has met the standards for indemnification and will personally repay the expenses if it is determined that such officer or director did not meet those standards.
Our Articles of Incorporation, provide a limitation of liability such that no director or officer of the Company shall be personally liable to the Company or any of its stockholders for damages for breach of fiduciary duty as a director or officer or for any act or omission of any such director or officer; however, the foregoing provision shall not eliminate or limit the liability of a director or officer for (a) acts or omissions which involve intentional misconduct, fraud or a knowing violation of law, or (b) the payment of dividends in violation of NRS Section 78.300.
Our By-Laws state that we shall indemnify every (i) present or former director or officer of us, (ii) any person who while serving in any of the capacities referred to in clause (i) served at our request as a director, officer, partner, venturer, proprietor, trustee, employee, agent or similar functionary of another foreign or domestic corporation, partnership, joint venture, trust, employee benefit plan or other enterprise, and (iii) any person nominated or designated by (or pursuant to authority granted by) the Board of Directors or any committee thereof to serve in any of the capacities referred to in clauses (i) or (ii), each an Indemnitee.
Our By-Laws provide that we shall indemnify an Indemnitee against all judgments, penalties (including excise and similar taxes), fines, amounts paid in settlement and reasonable expenses actually incurred by the Indemnitee in connection with any proceeding in which he was, is or is threatened to be named as defendant or respondent, or in which he was or is a witness without being named a defendant or respondent, by reason, in whole or in part, of his serving or having served, or having been nominated or designated to serve, if it is determined that the Indemnitee (a) conducted himself in good faith, (b) reasonably believed, in the case of conduct in his official capacity, that his conduct was in our best interests and, in all other cases, that his conduct was at least not opposed to our best interests, and (c) in the case of any criminal proceeding, had no reasonable cause to believe that his conduct was unlawful; provided, however, that in the event that an Indemnitee is found liable to us or is found liable on the basis that personal benefit was improperly received by the Indemnitee, the indemnification (i) is limited to reasonable expenses actually incurred by the Indemnitee in connection with the proceeding and (ii) shall not be made in respect of any proceeding in which the Indemnitee shall have been found liable for willful or intentional misconduct in the performance of his duty to us.
Other than in the limited situation described above, our By-Laws provide that no indemnification shall be made in respect to any proceeding in which such Indemnitee has been (a) found liable on the basis that personal benefit was improperly received by him, whether or not the benefit resulted from an action taken in the Indemnitee’s official capacity, or (b) found liable to us. The termination of any proceeding by judgment, order, settlement or conviction, or on a plea of nolo contendere or its equivalent, is not of itself determinative that the Indemnitee did not meet the requirements set forth in clauses (a) or (b) above. An Indemnitee shall be deemed to have been found liable in respect of any claim, issue or matter only after the Indemnitee shall have been so adjudged by a court of competent jurisdiction after exhaustion of all appeals therefrom. Reasonable expenses shall, include, without limitation, all court costs and all fees and disbursements of attorneys for the Indemnitee. The indemnification provided shall be applicable whether or not negligence or gross negligence of the Indemnitee is alleged or proven.
| II-1 |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Item 15. Recent Sales of Unregistered Securities.
All share and per share stock numbers in this section are after giving effect to the Reverse Merger on August 23, 2013, in which each share of Neurotrope BioScience Common Stock outstanding at the time of the Reverse Merger was automatically converted into one share of our common stock, and each share of Neurotrope BioScience Series A Stock outstanding at the time of the Reverse Merger was automatically converted into one share of our Series A stock.
The Series B Offering
The information regarding the Series B Stock and the warrants set forth in Part I under the caption “Certain Relationships and Related Transactions—The Series B Offering” is incorporated herein by reference. The Series B Offering was exempt from registration under Section 4(2) of the Securities Act in reliance upon the exemption provided by Rule 506(b) of Regulation D promulgated by the SEC thereunder. The offering was sold to “accredited investors,” as defined in Regulation D.
The PPO
The information regarding the private placement offering that was completed concurrently with the closing of the Reverse Merger set forth in Part I under the caption "Certain Relationships and Related Transactions - The PPO" is incorporated herein by reference. The PPO was exempt from registration under Section 4(2) of the Securities Act in reliance upon the exemption provided by Rule 506(b) of Regulation D promulgated by the SEC thereunder. The PPO was sold to “accredited investors,” as defined in Regulation D.
Shares Issued in Connection with the Reverse Merger
On August 23, 2013, pursuant to the terms of the Merger Agreement, all of the shares of Neurotrope BioScience Common Stock were exchanged for 19,000,000 restricted shares of our common stock, and all of the shares of Neurotrope BioScience Series A Stock were exchanged for 21,920,000 shares of our Series A Stock. This transaction was exempt from registration under Section 4(2) of the Securities Act as not involving any public offering. None of the securities were sold through an underwriter and, accordingly, there were no underwriting discounts or commissions involved.
Other Sales of Unregistered Securities
At the time of its formation on October 31, 2012, Neurotrope BioScience issued 1,000 shares of Neurotrope BioScience Common Stock to its founders, at a price of $0.01 per share, in a private placement offering exempt from registration requirements. The number of shares of Neurotrope BioScience Common Stock issued to each such founder in such offering are as follows:
| · | 475 to Neurosciences Research Ventures, Inc. |
| · | 273 to Northlea Partners LLLP |
| · | 202 to Dr. James New |
| · | 50 to Dr. Dan Alkon |
On February 28, 2013, Neurotrope BioScience effected a stock split in which each of the 1,000 issued and outstanding shares of Neurotrope BioScience Common Stock was converted into 19,000 shares of its common stock.
On August 28, 2013, the Company issued to a consultant 40,000 shares of common stock as compensation for services to the Company.
On January 23, 2014, the Company issued, under the 2013 Plan, 300,000 options to three employees, each with a term of ten years, exercisable at $1.76 per common share. 125,000 of these options vest 25% per year, with accelerated vesting of 25% upon a termination without cause or for good reason, with the remaining 175,000 vesting 20% per year.
On March 21, 2014, the Company issued, under the 2013 Plan, 30,000 options to one employee with a term of ten years, exercisable at $2.30 per common share and vests 20% per year commencing on March 31, 2015.
On July 16, 2014, the Company issued incentive stock options to purchase 50,000 shares of common stock to one employee with a term of ten years, exercisable at $1.24 per common share which vest 20% per year commencing July 16, 2015. These options were granted under the 2013 Plan.
| II-2 |
On July 23, 2014, Messrs. Ramat and Freiman, respectively, directors of the Company, then Co-Chairmen of the Board, and then Co-Chief Executive Officers of the Company on an interim basis, were each granted an option to purchase 400,000 shares of Common Stock for their extraordinary services as directors, Co-Chairmen of the Board, and Co-Chief Executive Officers of the Company, and Messrs. Haft, Gottlieb and Singer, directors of the Company, were each granted an option to purchase 100,000 shares of common stock for the extraordinary services they have been providing to the Company and their future services to the Company. The options were granted under the 2013 Plan.
One-half of the amount of options granted to each person will have an exercise price $1.11 per share being $.10 above, or 110% of, the Company’s last trading price on July 23, 2014 and are fully vested upon grant. The second half of the amount of options granted to each person have an exercise price of $2.22 per share based upon two times the exercise price of $1.11 per share and vest on a daily basis up to 25% per year through July 23, 2018.
On September 12, 2014, the Board granted the Company’s Chief Executive Officer and Chairman non-qualified options to purchase 250,000 and 50,000 shares of the Company’s common stock, respectively, which have an exercise price of $0.60 per common share, are fully vested upon grant and have a term of ten years. Also, the Board granted 300,000 non-qualified stock options to a newly appointed director with a term of ten years, exercisable at $0.60 per common share and vest daily over five years commencing September 12, 2014. The Company also granted incentive stock options to purchase 20,000 shares of the Company’s common stock to one employee with a term of ten years, exercisable at $0.60 per common share and vests 20% per year commencing September 12, 2015. In addition to the options described above for officers and directors, on November 19, 2015, options were issued to Elaine Grenier and David Crockford for 50,000 shares and 25,000 shares, respectively, 25% of which, respectively, vested immediately with an additional 25%, respectively, vesting over each of the next three anniversaries of grant.
On November 1, 2014, the Company granted Dr. Wasiewski granted non-qualified stock options to purchase 250,000 shares of common stock at an exercise price equal to $0.71 per share, the fair market value of the Company’s common stock on the grant date. 20% of the options will vest on each anniversary of the grant date for a period of 5 years from the grant date. As of his termination date, 20% had vested and will expire one year from November 9, 2015 if not exercised.
Additionally, from December 2, 2014 through January 6, 2016, private placement agents exercised common stock warrants, resulting in the issuance of a total of 1,180,083 shares of the Company’s common stock.
On February 9, 2015, the holders of placement agent warrants to purchase an aggregate of 1,325,000 shares of our Series A stock entered into Conversion Agreements with us pursuant to which such holders’ placement agent warrants to purchase Series A Stock were converted into common stock purchase warrants, for no additional consideration, to purchase an aggregate of 1,325,000 shares of our common stock.
On March 19, 2015, the Company issued, under the 2013 Plan, options to purchase up to 20,000 shares of common stock to Elaine Grenier, Director of Clinical Operations of Neurotrope Bioscience.
On September 28, 2015, the Company issued to a consultant 43,860 shares of common stock as compensation to assist the Company with investment banking and capital raising activities.
In connection with a private placement, effective as of November 13, 2015, the holders of all 16,656,894 shares of our Series A Stock converted their shares into 19,864,971 shares of our common stock, which includes 3,208,077 shares of our common stock issued in accordance with anti-dilution provisions relating to the Series A Stock.
On November 17, 2015, under the 2013 Plan, the Company issued options to purchase up to 100,000 shares of common stock to Mr. Ramat. On November 19, 2015, also under the 2013 Plan, the Company issued options to purchase up to an additional 600,000 shares of common stock to Mr. Ramat, as well as options to purchase up to 200,000 shares of common stock to Mr. Freiman, options to purchase up to 50,000 shares of common stock each to Messrs. Haft and Altstiel and Ms. Grenier, options to purchase up to 100,000 shares of common stock to Mr. Weinstein and options to purchase up to 25,000 shares to David Crockford, Vice President, Regulatory Affairs of Neurotrope Bioscience.
These issuances were exempt from registration under the Securities Act under Section 4(2) thereof as not involving any public offering of securities. None of the shares were sold through an underwriter, and accordingly, there were no underwriting discounts or commissions involved.
Item 16. Exhibits and Financial Statement Schedules.
The following exhibits are filed as part of this registration statement.
In reviewing the agreements included (or incorporated by reference) as exhibits to this registration statement, please remember that they are included to provide you with information regarding their terms and are not intended to provide any other factual or disclosure information about us or the other parties to the agreements. The agreements may contain representations and warranties by each of the parties to the applicable agreement. These representations and warranties have been made solely for the benefit of the parties to the applicable agreement and:
| · | should not in all instances be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate; |
| · | have been qualified by disclosures that were made to the other party in connection with the negotiation of the applicable agreement, which disclosures are not necessarily reflected in the agreement; |
| II-3 |
| · | may apply standards of materiality in a way that is different from what may be viewed as material to you or other investors; and |
| · | were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement and are subject to more recent developments. |
Accordingly, these representations and warranties may not describe the actual state of affairs as of the date they were made or at any other time. Additional information about us may be found elsewhere in this registration statement and our other public filings, which are available without charge through the SEC’s website at http://www.sec.gov.
| Exhibit Number |
Description | |
| 2.1 | Agreement and Plan of Merger, dated June 20, 2013, between BlueFlash Communications, Inc. and Neurotrope, Inc. (incorporated by reference from Exhibit 2.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on August 8, 2013) | |
| 2.2 | Amendment to Agreement and Plan of Merger, dated July 10, 2013, between BlueFlash Communications, Inc. and Neurotrope, Inc. (incorporated by reference from Exhibit 2.2 to the Registrant’s Current Report on Form 8-K filed with the SEC on August 8, 2013) | |
| 2.3 | Agreement and Plan of Merger and Reorganization, dated as of August 23, 2013, by and among the Registrant, Acquisition Sub and Neurotrope BioScience, Inc. (incorporated by reference from Exhibit 2.3 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 3.1 | Articles of Incorporation of the Registrant (incorporated by reference from Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on August 8, 2013) | |
| 3.2 | Florida Articles of Merger of BlueFlash Communications, Inc. with and into Neurotrope, Inc., filed August 5, 2013 (incorporated by reference from Exhibit 3.3 to the Registrant’s Current Report on Form 8-K filed with the SEC on August 8, 2013) | |
| 3.3 | Nevada Articles of Merger of BlueFlash Communications, Inc. with and into Neurotrope, Inc., filed August 5, 2013 (incorporated by reference from Exhibit 3.4 to the Registrant’s Current Report on Form 8-K filed with the SEC on August 8, 2013) | |
| 3.4 | Certificate of Merger of Neurotrope BioScience, Inc., with and into Neurotrope Acquisition, Inc., filed August 23, 2013 (incorporated by reference from Exhibit 3.4 to Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 3.5 | Certificate of Designations of Series A Convertible Preferred Stock of Neurotrope, Inc., filed with the Nevada Secretary of State on August 22, 2013 (incorporated by reference from Exhibit 3.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended July 31, 2013) | |
| 3.6 | Amended and Restated By-Laws of the Registrant (incorporated by reference from Exhibit 3.2 to the Company’s Current Report on Form 8-K filed with the SEC on August 8, 2013) | |
| 3.7 | Certificate of Designations Preferences and Rights of Series B Convertible Preferred Stock of Neurotrope, Inc. filed with the Nevada Secretary of State on November 13, 2015, including the Certificate of Corrections to Certificate of Designations, Preferences and Rights of Series B Preferred Stock filed November 19, 2015 (incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 5.1* | Legal Opinion of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. | |
| 10.1 | Split-Off Agreement, dated as of August 23, 2013, by and among the Registrant, Blue Flash Communications Corp. and Marissa Watson (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 10.2 | General Release Agreement, dated as of August 23, 2013, by and among the Registrant, Blue Flash Communications Corp. and Marissa Watson (incorporated by reference from Exhibit 10.2 filed to Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) |
| II-4 |
| Exhibit Number |
Description | |
| 10.3 | Form of Lock-Up and No Short Selling Agreement between the Registrant and the officers, directors and shareholders party thereto (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 10.4 | Form of Subscription Agreement between Neurotrope BioScience, Inc., and the investors party thereto (incorporated by reference from Exhibit 10.4 filed with the Company’s Current Report on Form 8-K to the SEC on August 29, 2013) | |
| 10.5 | Form of Agent Warrant for Common Stock of the Registrant (incorporated by reference from Exhibit 10.5 filed with the Company’s Current Report on Form 8-K filed with to SEC on August 29, 2013) | |
| 10.6 | Form of Agent Warrant for Series A Preferred Stock of the Registrant (incorporated by reference from Exhibit 10.6 filed with the Company’s Current Report on Form 8-K filed with to SEC on August 29, 2013) | |
| 10.7 | Placement Agency Agreement, dated June 25, 2013, between Neurotrope BioScience, Inc., and EDI Financial, Inc. (incorporated by reference from Exhibit 10.7 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 10.8 | Amendment to Placement Agency Agreement, dated August 12, 2013, between Neurotrope BioScience, Inc., and EDI Financial, Inc. (incorporated by reference from Exhibit 10.8 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 10.9 | Assignment of and Amendment to Placement Agent Agreement, dated as of August 23, 2013, among Neurotrope, Inc., Neurotrope BioScience, Inc., and EDI Financial, Inc. (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on October 9, 2013) | |
| 10.10† | Employment Agreement, dated February 25, 2013, between the Registrant (as successor to Neurotrope BioScience) and Dr. James New (incorporated by reference from Exhibit 10.9 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 10.11† | Consulting Agreement, dated as of June 2, 2013, between the Registrant and Medical Cash Management Solutions, LLC (assigned to the Registrant) (incorporated by reference from Exhibit 10.10 filed with the Company’s Current Report on Form 8-K to the SEC on August 29, 2013) | |
| 10.12† | The Registrant’s 2013 Equity Incentive Plan (incorporated by reference from Exhibit 10.11 filed with the Company’s Current Report on Form 8-K to the SEC on August 29, 2013) | |
| 10.13† | Form of Option Agreement under 2013 Equity Incentive Plan (incorporated by reference from Exhibit 10.12 filed with the Company’s Current Report on Form 8-K to the SEC on August 29, 2013) | |
| 10.14 | Technology License and Services Agreement, dated October 31, 2012, among the Registrant, BRNI and NRV II, LLC (incorporated by reference Exhibit 10.13 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 10.15 | Amendment #1 to Technology License and Services Agreement, dated August 21, 2013 among the Registrant, BRNI and NRV II, LLC (incorporated by reference from Exhibit 10.14 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 10.16 | Common Stockholders Agreement, dated August 23, 2013, among the Registrant and the stockholders party thereto (incorporated by reference from Exhibit 10.15 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 10.17 | Form of Preferred Stockholders Agreement, dated August 23, 2013, among the Registrant and the stockholders party thereto (incorporated by reference from Exhibit 10.16 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) | |
| 10.18 | Voting Agreement dated as of August 23, 2013, among the Registrant and the stockholders party thereto (incorporated by reference from Exhibit 10.17 to the Company’s Current Report on Form 8-K filed with the SEC on August 29, 2013) |
| II-5 |
| Exhibit Number |
Description | |
| 10.19 | Statement of Work Agreement dated August 20,2013, between Neurotrope BioScience and BRNI (incorporated by reference from Exhibit 10.18 filed with the Amendment No. 1 to the Company’s Current Report on Form 8-K filed with the SEC on August 30, 2013) | |
| 10.20† | Employment Agreement dated as of October 1, 2013, between Neurotrope, Inc., and Robert Weinstein (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on October 9, 2013) | |
| 10.21† | Amendment No. 1 to the Neurotrope, Inc. 2013 Equity Incentive Plan, dated as of July 23, 2014 (incorporated by reference from Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on August 14, 2014) | |
| 10.22 | Amendment No. 1 to the Neurotrope, Inc. Preferred Stockholders Agreement, dated as of September 3, 2014 (incorporated by reference from Exhibit 10.1 to Neurotrope Inc.’s Current Report on Form 8-K filed with the SEC on September 5, 2014) | |
| 10.23† | Confidential Separation Agreement and General Release, dated October 9, 2014 (incorporated by reference from Exhibit 10.1 to Neurotrope Inc.’s Current Report on Form 8-K filed with the SEC on October 10, 2014) | |
| 10.24† | Employment Agreement between Neurotrope BioScience, Inc. and Dr. Warren Wasiewski, made as of November 1, 2014 (incorporated by reference from Exhibit 10.1 to Neurotrope Inc.’s Current Report on Form 8-K filed with the SEC on November 6, 2014) | |
| 10.25 | Amended and Restated Technology License and Services Agreement among Neurotrope BioScience, Inc., Blanchette Rockefeller Neurosciences Institute and NRV II, LLC, made as of February 4, 2015 (incorporated by reference from Exhibit 10.1 to Neurotrope Inc.’s Current Report on Form 8-K filed with the SEC on February 10, 2015) | |
| 10.26 | Statement of Work Agreement dated February 4, 2015, and effective as of October 1, 2014, between Neurotrope BioScience and BRNI (incorporated by reference from Exhibit 10.2 to Neurotrope Inc.’s Current Report on Form 8-K filed with the SEC on February 10, 2015) | |
| 10.27 | Form of Conversion Agreement between Neurotrope, Inc. and the holders of Series A Preferred Stock Purchase Warrants, made as of February 9, 2015 (incorporated by reference from Exhibit 10.3 to Neurotrope Inc.’s Current Report on Form 8-K filed with the SEC on February 10, 2015) | |
| 10.28 | Form of Common Stock Purchase Warrant, dated February 9, 2015 (incorporated by reference from Exhibit 10.3 to Neurotrope Inc.’s Current Report on Form 8-K filed with the SEC on February 10, 2015) | |
| 10.29† | Consulting Agreement between Neurotrope BioScience, Inc. and Ramat Consulting Corp., as amended on December 18, 2013 and February 2, 2015 (incorporated by reference from Exhibit 10.29 to Neurotrope Inc.’s Annual Report on Form 10-K filed with the SEC on March 26, 2015) | |
| 10.30 | Services Agreement between Neurotrope BioScience, Inc. and Worldwide Clinical Trials, Inc., dated October 9, 2015 (incorporated by reference from Exhibit 10.1 to the Company's Current Report on Form 8-K filed with the SEC on October 15, 2015) | |
| 10.31 | Securities Purchase Agreement, dated November 13, 2015, by and among Neurotrope, Inc. and the buyers signatory thereto (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 10.32 | Registration Rights Agreement, dated November 13, 2015, by and among Neurotrope, Inc. and the buyers signatory thereto (incorporated by reference from Exhibit 10.7 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 10.33 | Form of Series A Warrant (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 10.34 | Form of Series B Warrant (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) |
| II-6 |
| Exhibit Number |
Description | |
| 10.35 | Form of Series C Warrant (incorporated by reference from Exhibit 10.4 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 10.36 | Form of Series D Warrant (incorporated by reference from Exhibit 10.5 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 10.37 | Form of Series E Warrant (incorporated by reference from Exhibit 10.6 the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 10.38 | Penny Broker Warrant (incorporated by reference from Exhibit 10.8 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 10.39 | IV Broker Warrant (incorporated by reference from Exhibit 10.9 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 10.40 | Institutional Broker Warrant (incorporated by reference from Exhibit 10.10 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 10.41 | Amendment No. 2 to Preferred Stockholders Agreement (incorporated by reference from Exhibit 10.11 to the Company’s Current Report on Form 8-K filed with the SEC on November 19, 2015) | |
| 10.42 | Amendment to Amended and Restated Technology License and Services Agreement among Neurotrope BioScience, Inc., Blanchette Rockefeller Neurosciences Institute and NRV II, LLC, dated November 12, 2015 (incorporated by reference from Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on November 13, 2015) | |
| 10.43 | Letter Agreement between the Neurotrope, Inc. and Neurosciences Research Ventures, Inc. regarding NRV Director Nominees, dated November 12, 2015 (incorporated by reference from Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on November 13, 2015) | |
| 10.44 | Statement of Work Agreement between Neurotrope BioScience, Inc. and Blanchette Rockefeller Neurosciences Institute, dated November 12, 2015 (incorporated by reference from Exhibit 10.3 to the Company’s Current Report on Form 8-K filed with the SEC on November 13, 2015) | |
| 21.1 | Subsidiaries of the Registrant (incorporated by reference from Exhibit 21.1 to the Company’s Annual Report on Form 10-K filed with the SEC on March 26, 2015) | |
| 23.1* | Consent of Independent Registered Public Accounting Firm | |
| 23.2* | Consent of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. (See Exhibit 5.1 above) | |
| 101* | The following financial information from the Registrant’s Annual Report on Form 10-K for the years ended December 31, 2014 and December 31, 2013, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets as of December 31, 2014 and December 31, 2013, (ii) Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2014 and December 31, 2013, (iii) Consolidated Statements of Stockholders’ Equity (Deficit) for the years ended December 31, 2014 and December 31, 2013, (iv) Consolidated Statements of Cash Flows for the years ended December 31, 2014 and December 31, 2013, and (v) the Notes to Consolidated Financial Statements; and the following financial information from the Registrant's Quarterly Report on Form 10-Q for the quarter ended September 30, 2015, formatted in XBRL (eXtensible Business Reporting Language): (i) Condensed Consolidated Balance Sheet at September 30, 2015 and September 30, 2014 (unaudited), (ii) Condensed Consolidated Statements of Operations and Comprehensive Loss for the nine month periods ended September 30, 2015 and September 30, 2014 (unaudited), (iii) Condensed Consolidated Statements of Cash Flows for the nine month periods ended September 30, 2015 and September 30, 2014 (unaudited) and (iv) Notes to Condensed Consolidated Financial Statements (unaudited). |
| II-7 |
| * | Filed herewith. |
| † | Management contract or compensatory plan or arrangement. |
Item 17. Undertakings.
(a) The undersigned registrant hereby undertakes:
| 1. | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| i. | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| ii. | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
| iii. | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| 2. | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| 3. | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (b) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities, other than the payment by the registrant of expenses incurred and paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding, is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue. |
| (c) | Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| II-8 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Newark, New Jersey, on January 14, 2016.
| NEUROTROPE, INC. | ||
| By: | /s/ Charles S. Ramat | |
| Name: | Charles S. Ramat | |
| Title: |
President and Chief Executive Officer (principal executive officer) | |
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
| Signature | Title | Date | ||
| /s/ Paul E. Freiman | Director and Chairman of the Board | January 14, 2016 | ||
| Paul E. Freiman | ||||
| /s/ Charles S. Ramat | Director, President and Chief Executive Officer | January 14, 2016 | ||
| Charles S. Ramat | (principal executive officer) | |||
| /s/ Larry D. Altstiel | Director | January 14, 2016 | ||
| Larry D. Altstiel | ||||
| /s/ James Gottlieb | Director | January 14, 2016 | ||
| James Gottlieb | ||||
| /s/ Jay M. Haft | Director | January 14, 2016 | ||
| Jay M. Haft | ||||
| /s/ William Singer | Director, Vice Chairman of the Board | January 14, 2016 | ||
| William Singer | ||||
| /s/ Robert Weinstein | Chief Financial Officer, Executive Vice President, | January 14, 2016 | ||
| Robert Weinstein | Treasurer and Secretary (principal financial officer and principal accounting officer) |
