Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Viking Therapeutics, Inc. | vktx-8k_20160111.htm |

Corporate Presentation Biotech Showcase, January 2016 EXHIBIT 99.1

Forward-Looking Statements This presentation contains statements about our future expectations, plans and prospects that constitute forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including risks relating to: both our and our collaborators’ ability to successfully research, obtain regulatory approvals for, develop and commercialize products based upon our technologies; our ability to obtain and maintain proprietary protection for our technologies and product candidates; our reliance on third parties to manufacture our preclinical and clinical drug supplies; competitive pressures; our ability to obtain and maintain strategic collaborations; compliance with our in-license agreements; our ability to successfully execute on, and receive favorable results from, our proprietary drug development efforts; market acceptance of our drug candidates; retaining members of our senior management; and our ability to raise additional funds to finance our operations. The forward-looking statements included in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. While we may elect to update these forward-looking statements in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation.

Introduction Viking is a San Diego-based biotech company developing novel drugs for metabolic disorders Programs licensed from Ligand Pharmaceuticals Incorporated Our clinical-stage programs are targeting: Large, underserved markets Each with global product market opportunities exceeding $1B annually Data from major Phase 2 programs expected within 15 months
Investment Highlights Focused on first-in-class or best-in-class drugs for metabolic disorders Lead clinical programs have demonstrated preliminary efficacy in humans VK5211: In Phase 2 development for hip fracture Non-steroidal selective androgen receptor modulator (SARM) Promising efficacy in bone and muscle; aligns with post-injury biology Phase 2 results expected 2H16 TRb Program: Novel, selective thyroid receptor-b agonists VK2809: Entering Phase 2 development for hypercholesterolemia and fatty liver disease Phase 2 trial expected to complete in 2H16 VK0214: Preclinical promise in X-ALD; Orphan neurodegenerative disorder In vivo POC data expected in 2016 Three additional programs targeting diabetes, anemia, lipid disorders

Preclinical Phase 1 Phase 2 Phase 3 Development Plans VK5211: SARM Hip Fracture Complete the ongoing trial in 2016 and report topline data in 2H16 VK2809: TRb Hypercholesterolemia, NASH Complete the Phase 2 trial in 2016 and report topline data in 4Q16/1H17 VK0214: TRb X-ALD Complete in vivo POC studies and report data in 2016 Pipeline Overview Two key POC clinical trials expected to complete in 2016; preclinical POC data for third program expected mid-2016
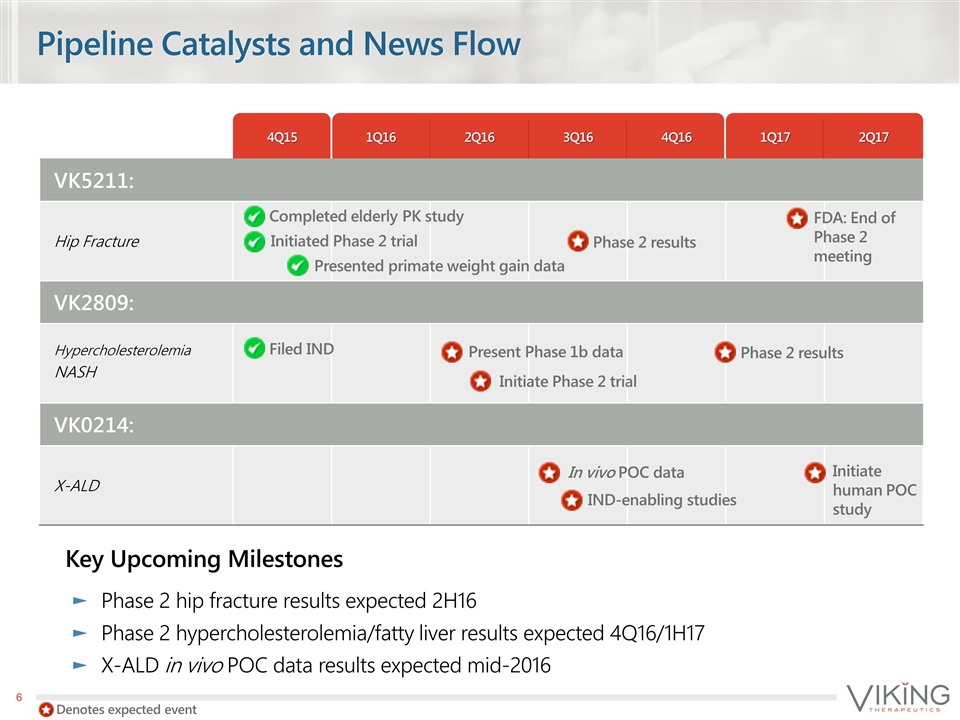
Pipeline Catalysts and News Flow Key Upcoming Milestones Phase 2 hip fracture results expected 2H16 Phase 2 hypercholesterolemia/fatty liver results expected 4Q16/1H17 X-ALD in vivo POC data results expected mid-2016 4Q15 1Q16 2Q16 3Q16 4Q16 1Q17 2Q17 VK5211: Hip Fracture VK2809: Hypercholesterolemia NASH VK0214: X-ALD Completed elderly PK study Initiated Phase 2 trial Presented primate weight gain data Phase 2 results FDA: End of Phase 2 meeting Filed IND Initiate Phase 2 trial Present Phase 1b data Phase 2 results In vivo POC data IND-enabling studies Initiate human POC study Denotes expected event

Clinical Programs VK5211: Selective Androgen Receptor Modulator Hip Fracture
VK5211: SARM Program for Hip Fracture Novel Selective Androgen Receptor Modulator (SARM), for hip fracture VK5211 is a potentially best-in-class small molecule SARM Promising efficacy signals Phase 1: Significant improvements in lean body mass Animals: Positive effects on bone Target indication: Rehabilitation post-hip fracture >300,000 patients per year, U.S.; expect continued growth Market opportunity exceeds $1B annually No approved therapies, no SARMs in development Phase 2 trial in hip fracture patients ongoing Data expected 2H16
VK5211: Potential Therapeutic Benefits of SARMs Goal: Retain beneficial properties of natural androgens with fewer side effects relative to anabolic steroids Muscle Mass Muscle Strength Bone Formation Bone Strength Bone Resorption Cognition Libido Energy MUSCLE BONE CNS
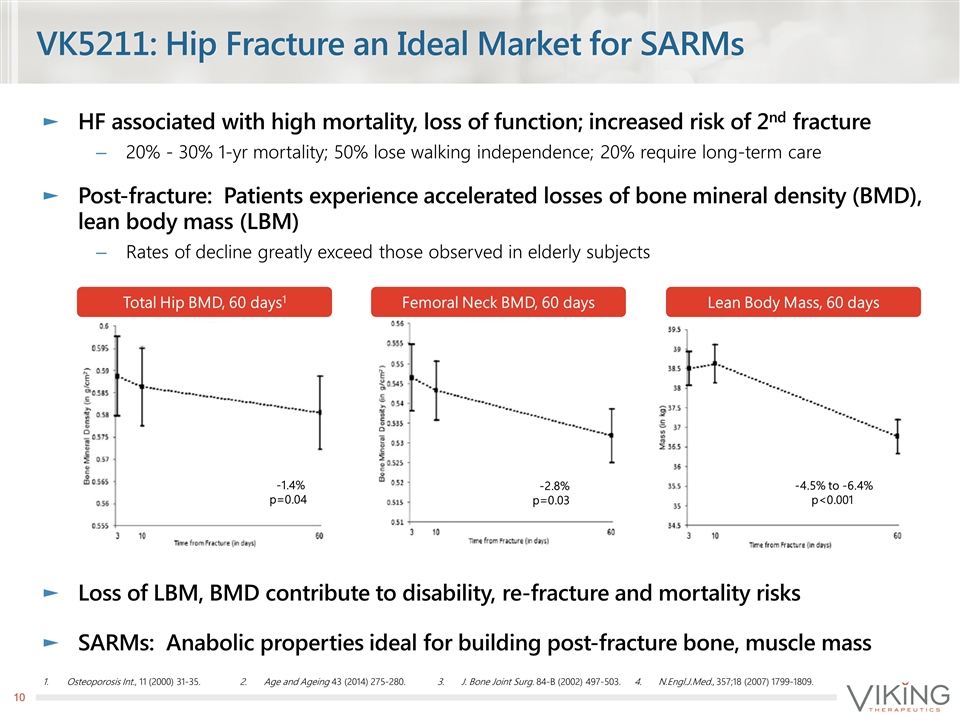
HF associated with high mortality, loss of function; increased risk of 2nd fracture 20% - 30% 1-yr mortality; 50% lose walking independence; 20% require long-term care Post-fracture: Patients experience accelerated losses of bone mineral density (BMD), lean body mass (LBM) Rates of decline greatly exceed those observed in elderly subjects Loss of LBM, BMD contribute to disability, re-fracture and mortality risks SARMs: Anabolic properties ideal for building post-fracture bone, muscle mass VK5211: Hip Fracture an Ideal Market for SARMs Osteoporosis Int., 11 (2000) 31-35. Age and Ageing 43 (2014) 275-280. J. Bone Joint Surg. 84-B (2002) 497-503. N.Engl.J.Med., 357;18 (2007) 1799-1809. Lean Body Mass, 60 days Femoral Neck BMD, 60 days Total Hip BMD, 60 days1 -4.5% to -6.4% p<0.001 -2.8% p=0.03 -1.4% p=0.04
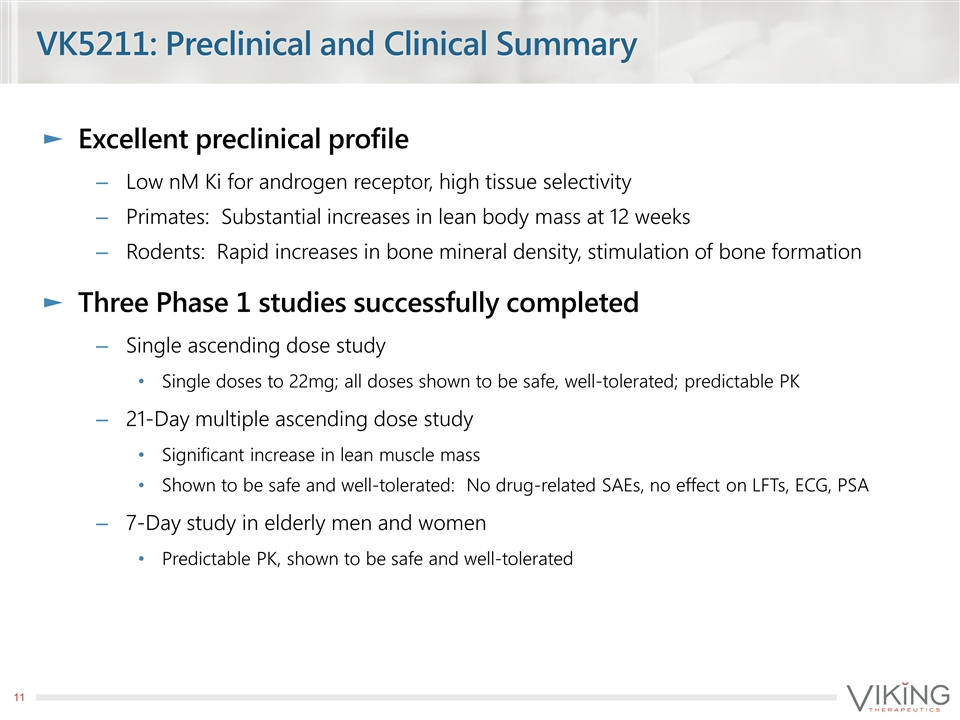
VK5211: Preclinical and Clinical Summary Excellent preclinical profile Low nM Ki for androgen receptor, high tissue selectivity Primates: Substantial increases in lean body mass at 12 weeks Rodents: Rapid increases in bone mineral density, stimulation of bone formation Three Phase 1 studies successfully completed Single ascending dose study Single doses to 22mg; all doses shown to be safe, well-tolerated; predictable PK 21-Day multiple ascending dose study Significant increase in lean muscle mass Shown to be safe and well-tolerated: No drug-related SAEs, no effect on LFTs, ECG, PSA 7-Day study in elderly men and women Predictable PK, shown to be safe and well-tolerated
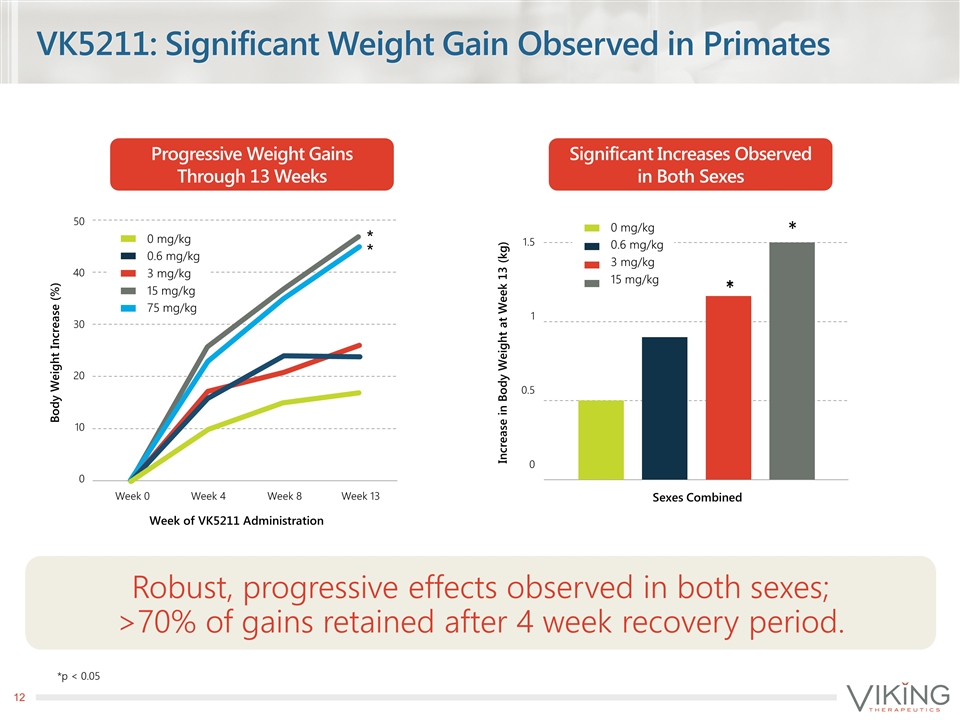
VK5211: Significant Weight Gain Observed in Primates Robust, progressive effects observed in both sexes; >70% of gains retained after 4 week recovery period. Progressive Weight Gains Through 13 Weeks Significant Increases Observed in Both Sexes Week 0 Week 4 Week 8 Week 13 Week of VK5211 Administration 50 40 30 20 10 0 Body Weight Increase (%) * * 0 mg/kg 0.6 mg/kg 3 mg/kg 15 mg/kg 75 mg/kg Increase in Body Weight at Week 13 (kg) 1.5 1 0.5 0 * 0 mg/kg 0.6 mg/kg 3 mg/kg 15 mg/kg Sexes Combined *p < 0.05
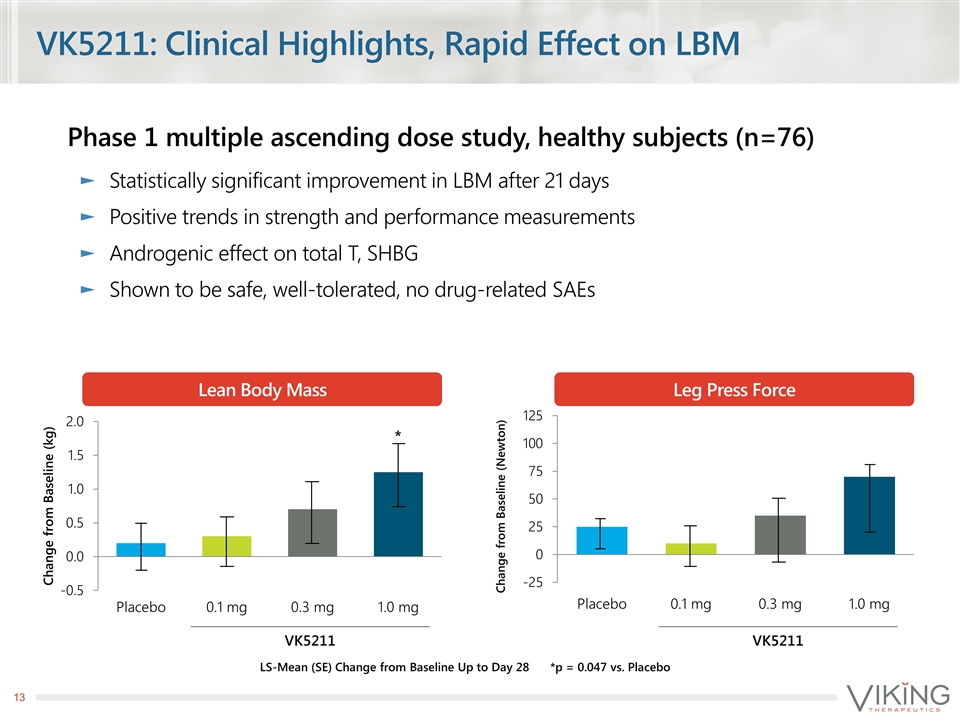
VK5211: Clinical Highlights, Rapid Effect on LBM Phase 1 multiple ascending dose study, healthy subjects (n=76) Statistically significant improvement in LBM after 21 days Positive trends in strength and performance measurements Androgenic effect on total T, SHBG Shown to be safe, well-tolerated, no drug-related SAEs LS-Mean (SE) Change from Baseline Up to Day 28 * VK5211 VK5211 *p = 0.047 vs. Placebo Lean Body Mass Leg Press Force
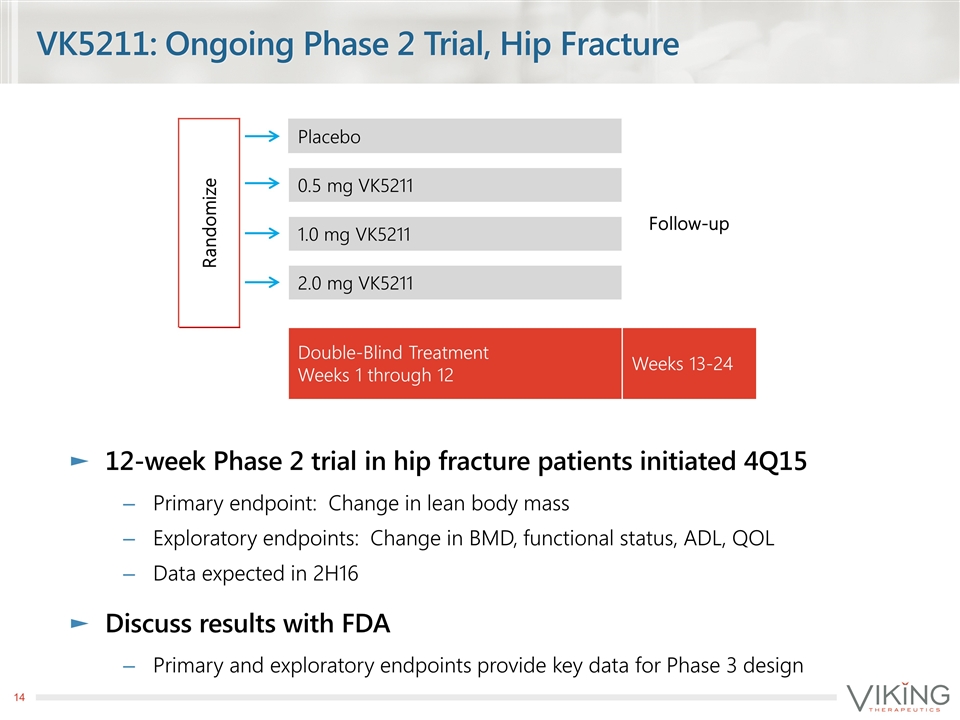
VK5211: Ongoing Phase 2 Trial, Hip Fracture 12-week Phase 2 trial in hip fracture patients initiated 4Q15 Primary endpoint: Change in lean body mass Exploratory endpoints: Change in BMD, functional status, ADL, QOL Data expected in 2H16 Discuss results with FDA Primary and exploratory endpoints provide key data for Phase 3 design Randomize Placebo Follow-up 0.5 mg VK5211 1.0 mg VK5211 2.0 mg VK5211 Double-Blind Treatment Weeks 1 through 12 Weeks 13-24

Clinical Programs VK2809: Selective Thyroid Receptor-β Agonist Lipid disorders
Thyroid-b Agonist Program Proprietary platform for small molecule thyroid mimetics Highly tissue and receptor selective Differentiated pro-drug technology, excellent oral bioavailability Unique chemical scaffolds, expected wider safety window vs. other approaches Biological profiles suggest potential benefit in multiple indications Broader: NASH, hypercholesterolemia, dyslipidemia Orphan: X-linked adrenoleukodystrophy (X-ALD) Lead molecules VK2809, VK0214 VK2809: Phase 2, hypercholesterolemia and fatty liver disease VK0214: Preclinical, X-ALD
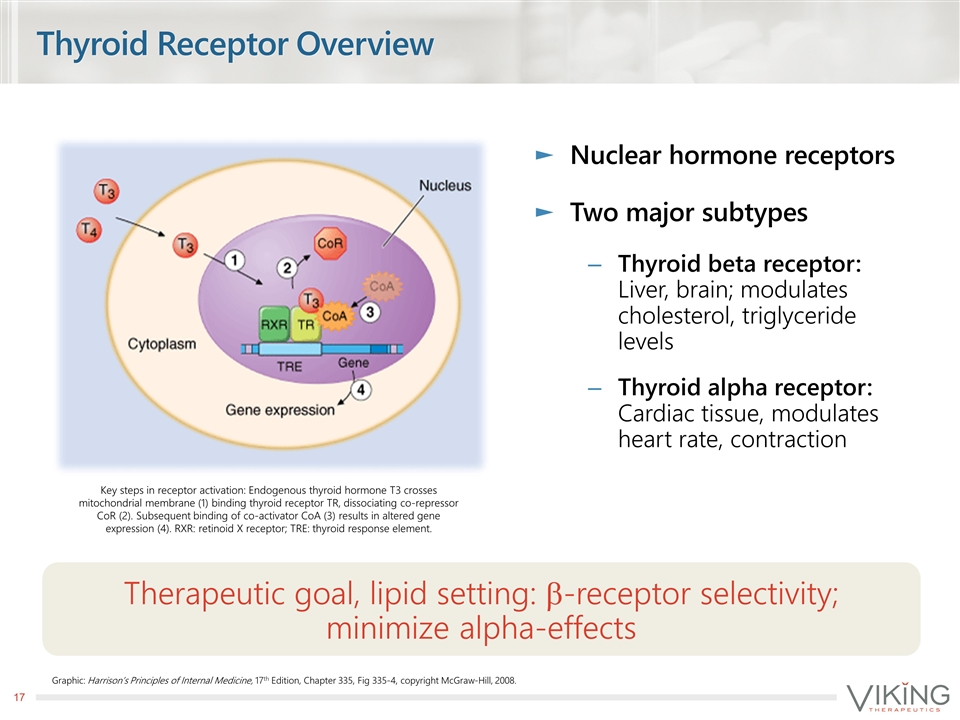
Thyroid Receptor Overview Nuclear hormone receptors Two major subtypes Thyroid beta receptor: Liver, brain; modulates cholesterol, triglyceride levels Thyroid alpha receptor: Cardiac tissue, modulates heart rate, contraction Therapeutic goal, lipid setting: b-receptor selectivity; minimize alpha-effects Graphic: Harrison’s Principles of Internal Medicine, 17th Edition, Chapter 335, Fig 335-4, copyright McGraw-Hill, 2008. Key steps in receptor activation: Endogenous thyroid hormone T3 crosses mitochondrial membrane (1) binding thyroid receptor TR, dissociating co-repressor CoR (2). Subsequent binding of co-activator CoA (3) results in altered gene expression (4). RXR: retinoid X receptor; TRE: thyroid response element.
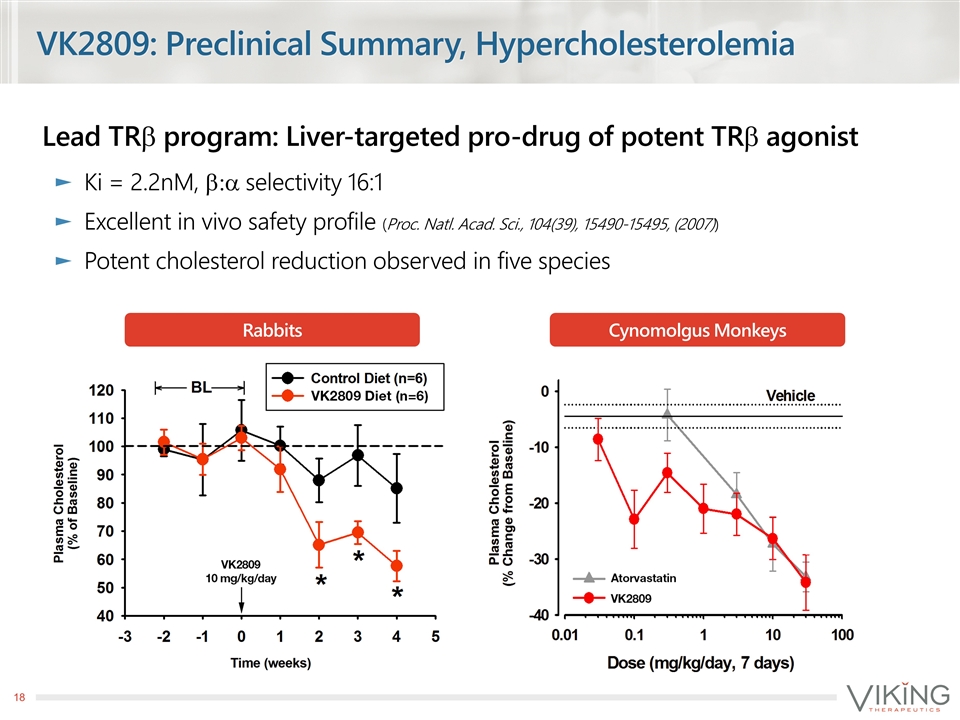
VK2809: Preclinical Summary, Hypercholesterolemia Lead TRb program: Liver-targeted pro-drug of potent TRb agonist Ki = 2.2nM, b:a selectivity 16:1 Excellent in vivo safety profile (Proc. Natl. Acad. Sci., 104(39), 15490-15495, (2007)) Potent cholesterol reduction observed in five species Rabbits Cynomolgus Monkeys
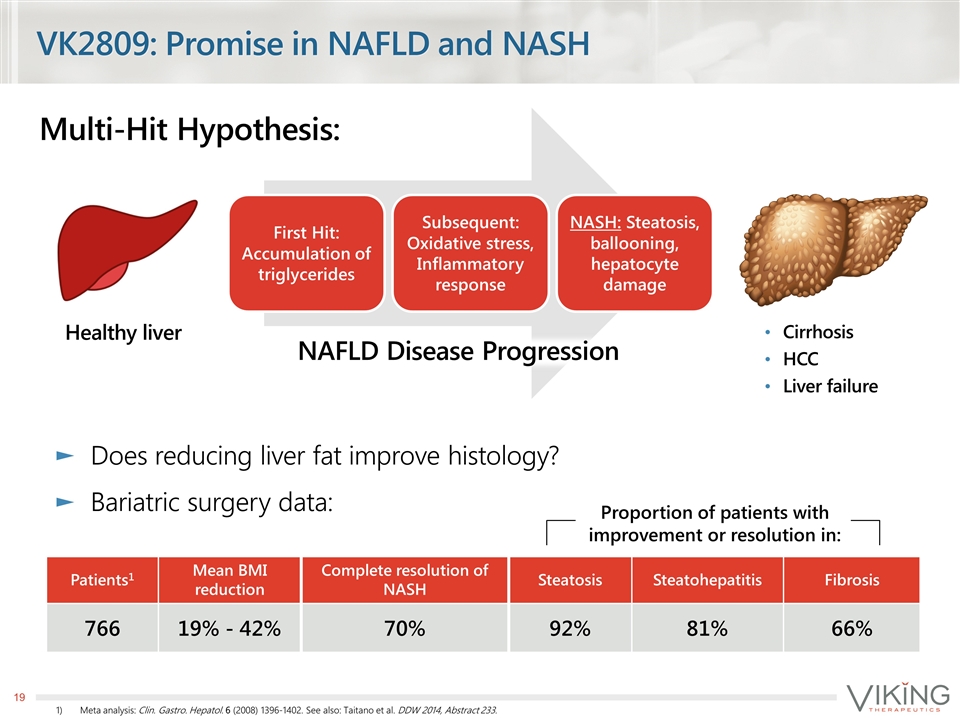
Multi-Hit Hypothesis: VK2809: Promise in NAFLD and NASH Proportion of patients with improvement or resolution in: Patients1 Mean BMI reduction Complete resolution of NASH Steatosis Steatohepatitis Fibrosis 766 19% - 42% 70% 92% 81% 66% Cirrhosis HCC Liver failure Healthy liver Meta analysis: Clin. Gastro. Hepatol. 6 (2008) 1396-1402. See also: Taitano et al. DDW 2014, Abstract 233. NAFLD Disease Progression Does reducing liver fat improve histology? Bariatric surgery data: First Hit: Accumulation of triglycerides Subsequent: Oxidative stress, Inflammatory response NASH: Steatosis, ballooning, hepatocyte damage
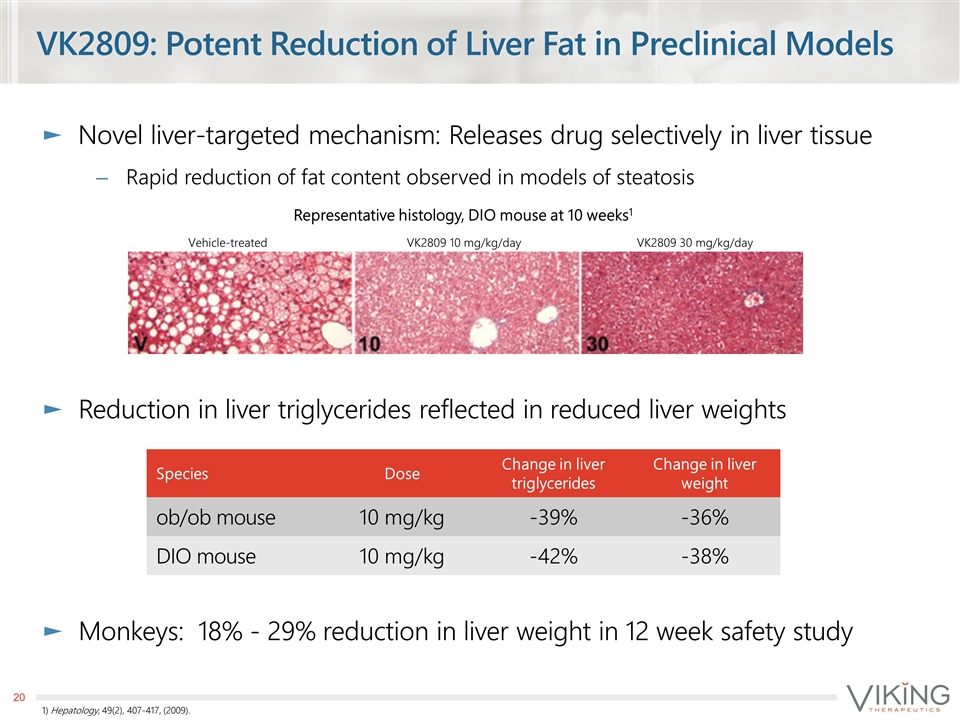
VK2809: Potent Reduction of Liver Fat in Preclinical Models Novel liver-targeted mechanism: Releases drug selectively in liver tissue Rapid reduction of fat content observed in models of steatosis Reduction in liver triglycerides reflected in reduced liver weights Monkeys: 18% - 29% reduction in liver weight in 12 week safety study 1) Hepatology, 49(2), 407-417, (2009). Species Dose Change in liver triglycerides Change in liver weight ob/ob mouse 10 mg/kg -39% -36% DIO mouse 10 mg/kg -42% -38% Vehicle-treated VK2809 10 mg/kg/day VK2809 30 mg/kg/day Representative histology, DIO mouse at 10 weeks1
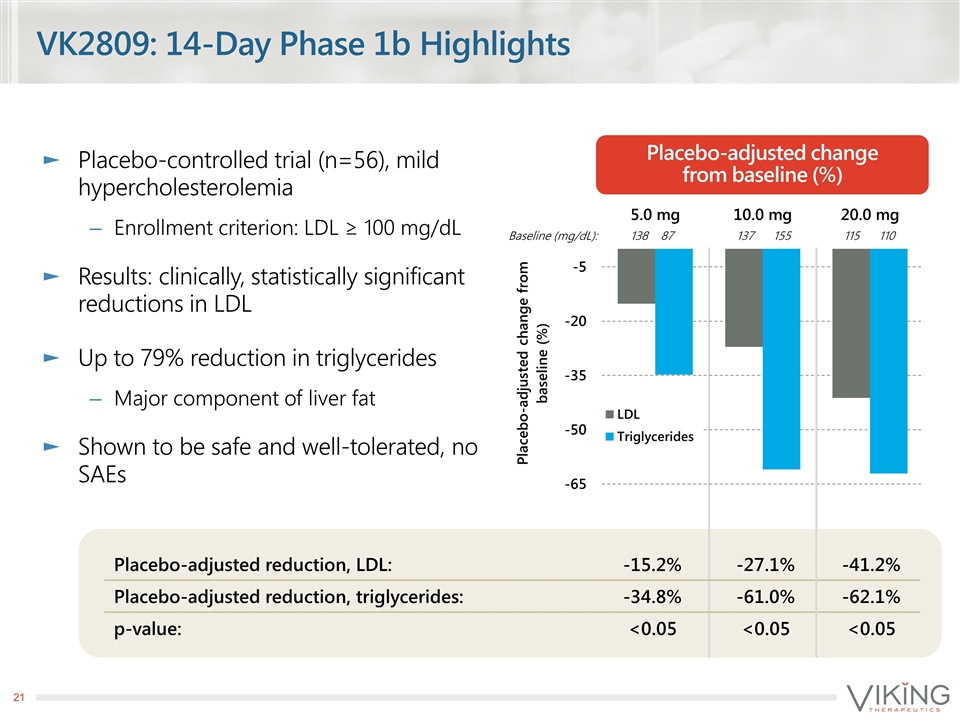
VK2809: 14-Day Phase 1b Highlights Placebo-controlled trial (n=56), mild hypercholesterolemia Enrollment criterion: LDL ≥ 100 mg/dL Results: clinically, statistically significant reductions in LDL Up to 79% reduction in triglycerides Major component of liver fat Shown to be safe and well-tolerated, no SAEs Placebo-adjusted change from baseline (%) Placebo-adjusted reduction, LDL: -15.2% -27.1% -41.2% Placebo-adjusted reduction, triglycerides: -34.8% -61.0% -62.1% p-value: <0.05 <0.05 <0.05 Baseline (mg/dL): 138 87 137 155 115 110
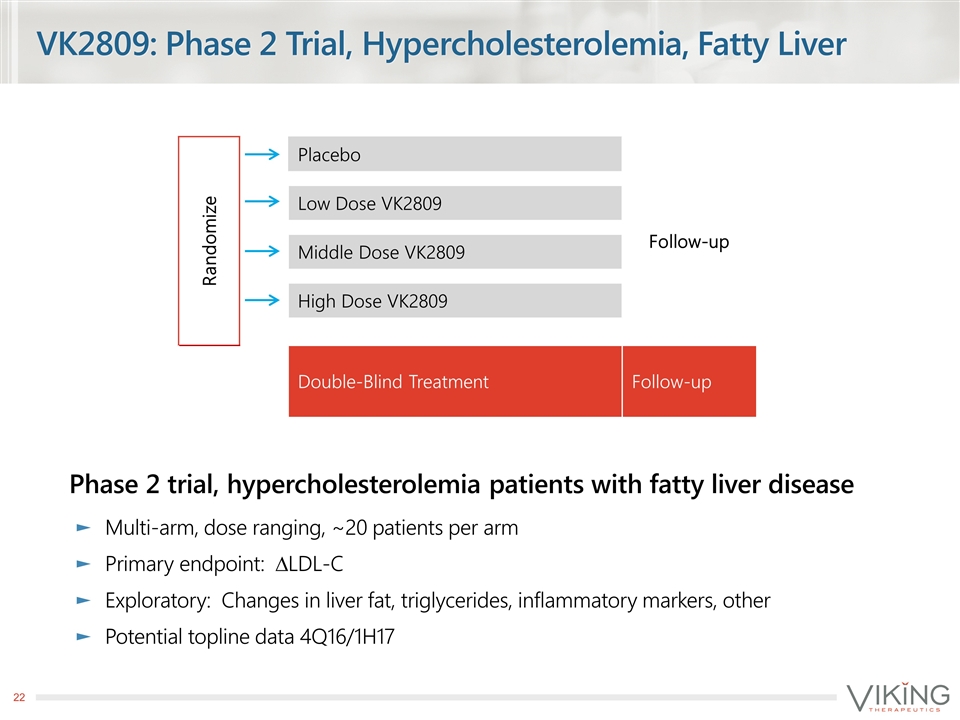
VK2809: Phase 2 Trial, Hypercholesterolemia, Fatty Liver Phase 2 trial, hypercholesterolemia patients with fatty liver disease Multi-arm, dose ranging, ~20 patients per arm Primary endpoint: DLDL-C Exploratory: Changes in liver fat, triglycerides, inflammatory markers, other Potential topline data 4Q16/1H17 Randomize Placebo Follow-up Low Dose VK2809 Middle Dose VK2809 High Dose VK2809 Double-Blind Treatment Follow-up
TRb Agonists in X-Linked Adrenoleukodystrophy, X-ALD Orphan neurodegenerative disorder X-linked: Carried by females, primarily manifesting in males EU, approximately 12,000 patients; U.S. 8,000 patients No cure, no approved therapy Most severe form: Cerebral ALD Rapidly progressive inflammatory demyelination; disruption of BBB Affects ~35% before age 12 (CCALD), ~20% between age 20 – 35 (CALD) Deterioration in speech, cognition; vegetative state within 3-5 years Most common form: Adrenomyeloneuropathy (AMN) Affects spinal cord, motor neurons; no inflammatory component or brain involvement Affects nearly all adult patients; considered “default” manifestation of ALD Progressive motor impairment; wheelchair confinement, leg paralysis common Biochimie, 98 (2014) 135-142.(4) Ann. Neurol. 49:512-517 (2001). Biochim. Biophys. Acta 1822 (2012) 1465-1474.(5) Orphanet J. Rare Dis. 7:51 (2012). Brain Pathol. 20(4): 845-856 (2010).
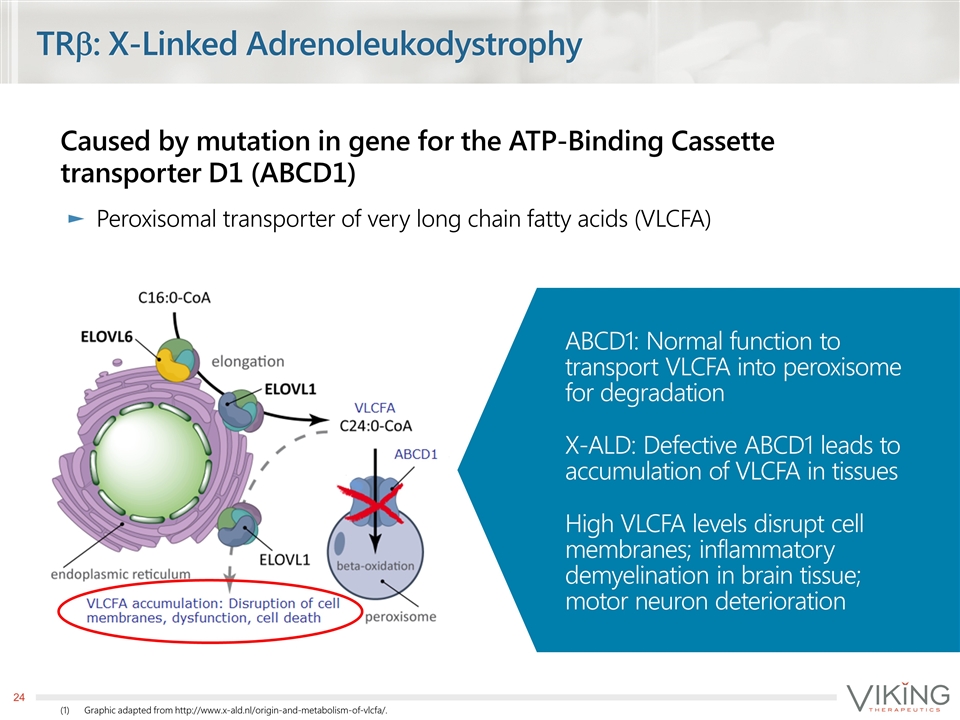
TRb: X-Linked Adrenoleukodystrophy Caused by mutation in gene for the ATP-Binding Cassette transporter D1 (ABCD1) Peroxisomal transporter of very long chain fatty acids (VLCFA) Graphic adapted from http://www.x-ald.nl/origin-and-metabolism-of-vlcfa/. ABCD1: Normal function to transport VLCFA into peroxisome for degradation X-ALD: Defective ABCD1 leads to accumulation of VLCFA in tissues High VLCFA levels disrupt cell membranes; inflammatory demyelination in brain tissue; motor neuron deterioration
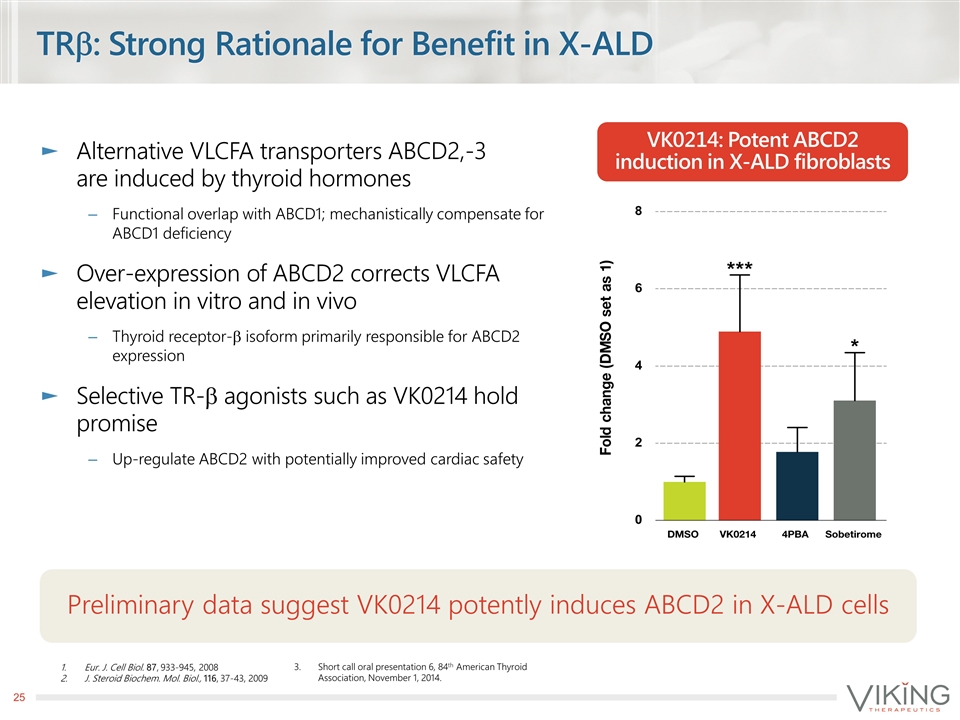
TRb: Strong Rationale for Benefit in X-ALD Alternative VLCFA transporters ABCD2,-3 are induced by thyroid hormones Functional overlap with ABCD1; mechanistically compensate for ABCD1 deficiency Over-expression of ABCD2 corrects VLCFA elevation in vitro and in vivo Thyroid receptor-b isoform primarily responsible for ABCD2 expression Selective TR-b agonists such as VK0214 hold promise Up-regulate ABCD2 with potentially improved cardiac safety Eur. J. Cell Biol. 87, 933-945, 2008 J. Steroid Biochem. Mol. Biol., 116, 37-43, 2009 VK0214: Potent ABCD2 induction in X-ALD fibroblasts Preliminary data suggest VK0214 potently induces ABCD2 in X-ALD cells Short call oral presentation 6, 84th American Thyroid Association, November 1, 2014.

TRb: X-Linked Adrenoleukodystrophy, Plans Current status Evaluation of VK0214 ongoing, encouraging in vitro results In vivo model in progress POC results expected 2016 IND-enabling work 2H16

Investment Highlights Focused on first-in-class or best-in-class drugs for metabolic disorders Lead clinical programs have demonstrated preliminary efficacy in humans VK5211: In Phase 2 development for hip fracture Non-steroidal selective androgen receptor modulator (SARM) Promising efficacy in bone and muscle; aligns with post-injury biology Phase 2 results expected 2H16 TRb Program: Novel, selective thyroid receptor-b agonists VK2809: Entering Phase 2 development for hypercholesterolemia and fatty liver disease Phase 2 trial expected to complete in 2H16 VK0214: Preclinical promise in X-ALD; Orphan neurodegenerative disorder In vivo POC data expected in 2016 Three additional programs targeting diabetes, anemia, lipid disorders

Corporate Presentation Biotech Showcase, January 2016
