Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Vanda Pharmaceuticals Inc. | d102010d8k.htm |
| EX-99.1 - EX-99.1 - Vanda Pharmaceuticals Inc. | d102010dex991.htm |

2016 CORPORATE PRESENTATION January 2016 Exhibit 99.2

Forward-Looking Statements Various statements in this presentation, including, but not limited to Vanda’s preliminary financial results for the fourth quarter of 2015 and full year 2015, and financial guidance for 2015 and 2016, are “forward-looking statements” under the securities laws. Forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. Important factors that could cause actual results to differ materially from those reflected in Vanda’s forward-looking statements include, among others: the fact that Vanda’s preliminary financial results are unaudited and changes in such results may be required by Vanda’s accountants following their audit of the results, Vanda's ability to successfully commercialize HETLIOZ® (tasimelteon) for the treatment of Non-24-Hour Sleep-Wake Disorder (“Non-24”) in the U.S. and Europe; uncertainty as to the market awareness of Non-24 and the market acceptance of HETLIOZ®; Vanda’s ability to generate U.S. sales of Fanapt® (iloperidone) for the treatment of schizophrenia; the timing and costs of Vanda’s establishment of a sales and marketing, supply chain, distribution, pharmacovigilance, compliance and safety infrastructure to promote Fanapt® in the U.S.; Vanda’s dependence on third-party manufacturers to manufacture HETLIOZ® and Fanapt® in sufficient quantities and quality; Vanda’s limited sales and marketing infrastructure; the regulatory status of Fanapt® in Europe; Vanda’s ability to successfully commercialize HETLIOZ® and Fanapt® outside the U.S.; Vanda’s ability to prepare, file, prosecute, defend and enforce any patent claims and other intellectual property rights; Vanda’s ability to obtain the capital necessary to fund its research and development or commercial activities; a loss of rights to develop and commercialize Vanda’s products under its license agreements; the ability to obtain and maintain regulatory approval of Vanda’s products, and the labeling for any approved products; the timing and success of preclinical studies and clinical trials conducted by Vanda or its development partners; a failure of Vanda’s products to be demonstrably safe and effective; the size and growth of the potential markets for Vanda’s products and the ability to serve those markets; Vanda’s expectations regarding trends with respect to its revenues, costs, expenses and liabilities; the scope, progress, expansion, and costs of developing and commercializing Vanda’s products; Vanda’s failure to identify or obtain rights to new products; a loss of any of Vanda’s key scientists or management personnel; limitations on Vanda’s ability to utilize some or all of its prior net operating losses and orphan drug and research and development credits; the costs and effects of litigation; losses incurred from product liability claims made against Vanda; use of existing cash, cash equivalents and marketable securities and other factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Vanda’s annual report on Form 10-K for the fiscal year ended December 31, 2014 and quarterly report on Form 10-Q for the quarter ended September 30, 2015, which are on file with the SEC and available on the SEC's website at www.sec.gov. Additional factors may be described in those sections of Vanda’s annual report on Form 10-K for the fiscal year ended December 31, 2015, to be filed with the SEC in the first quarter of 2016. In addition, other unknown or unpredictable factors could also affect Vanda’s results. There can be no assurance that the actual results or developments anticipated by Vanda will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Vanda. Therefore, no assurance can be given that the outcomes stated in such forward-looking statements and estimates will be achieved. All written and verbal forward-looking statements attributable to Vanda or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Vanda cautions investors not to rely too heavily on the forward-looking statements Vanda makes or that are made on its behalf. The information in this presentation is provided only as of the date of this presentation, and Vanda undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events, or otherwise.
Strategy for Long-Term Success Specialty pharmaceuticals Lifecycle management and product optimization Comprehensive customer support and engagement Novel therapies addressing high unmet needs Diversified pipeline in high- growth therapeutic markets
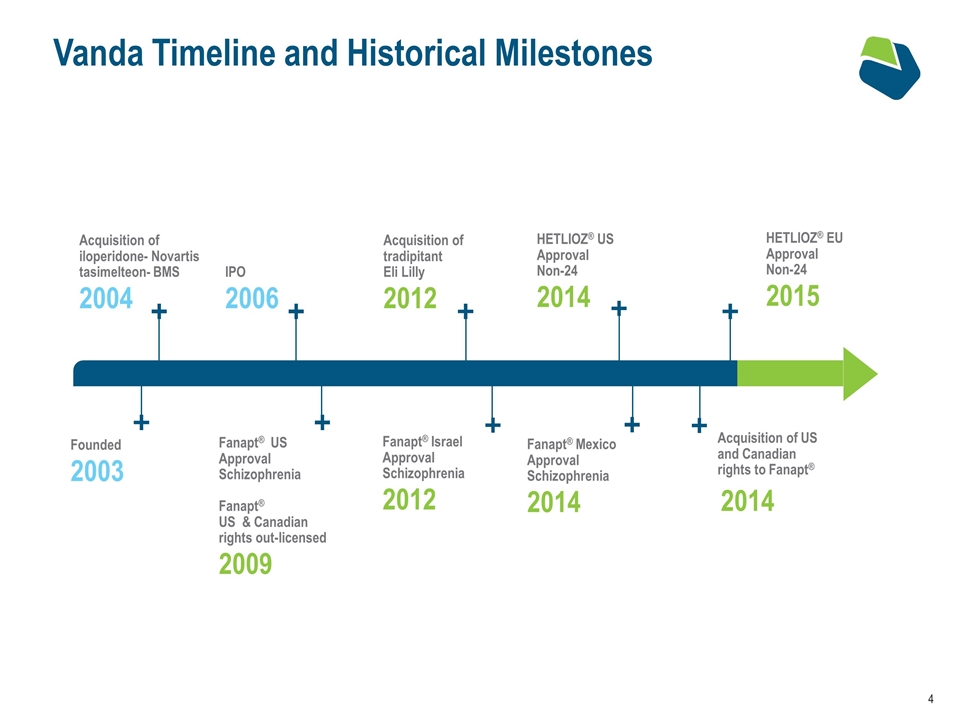
Vanda Timeline and Historical Milestones Acquisition of tradipitant Eli Lilly 2012 Acquisition of US and Canadian rights to Fanapt® 2014 Acquisition of iloperidone- Novartis tasimelteon- BMS 2004 IPO 2006 Founded 2003 Fanapt® US Approval Schizophrenia Fanapt® US & Canadian rights out-licensed 2009 HETLIOZ® US Approval Non-24 2014 Fanapt® Mexico Approval Schizophrenia 2014 Fanapt® Israel Approval Schizophrenia 2012 HETLIOZ® EU Approval Non-24 2015
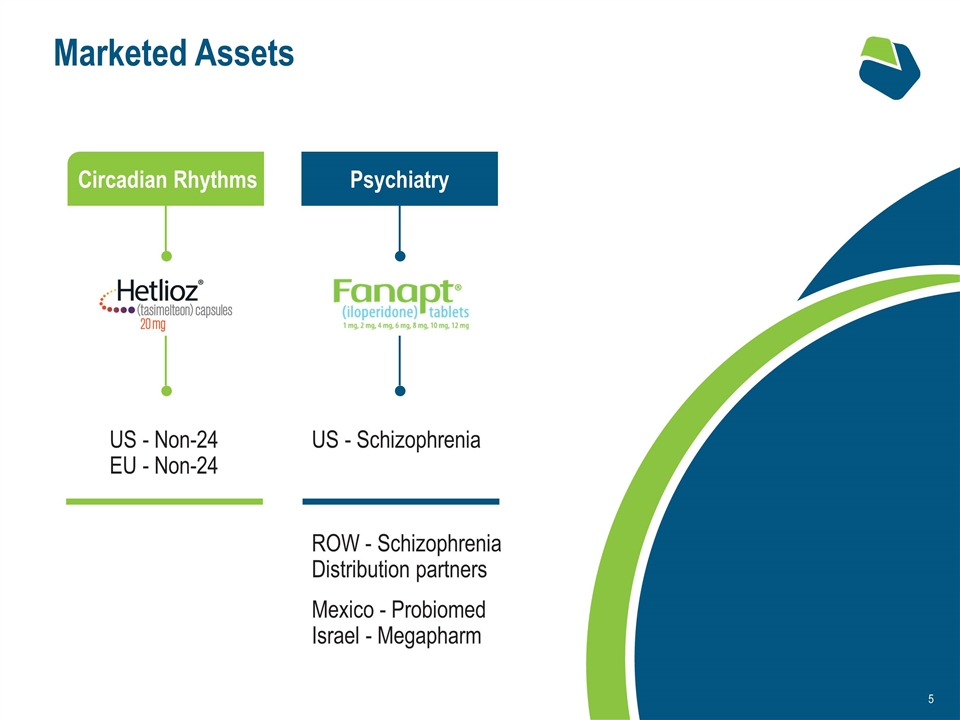
Marketed Assets US - Schizophrenia ROW - Schizophrenia Distribution partners Mexico - Probiomed Israel - Megapharm Circadian Rhythms Psychiatry US - Non-24 EU - Non-24
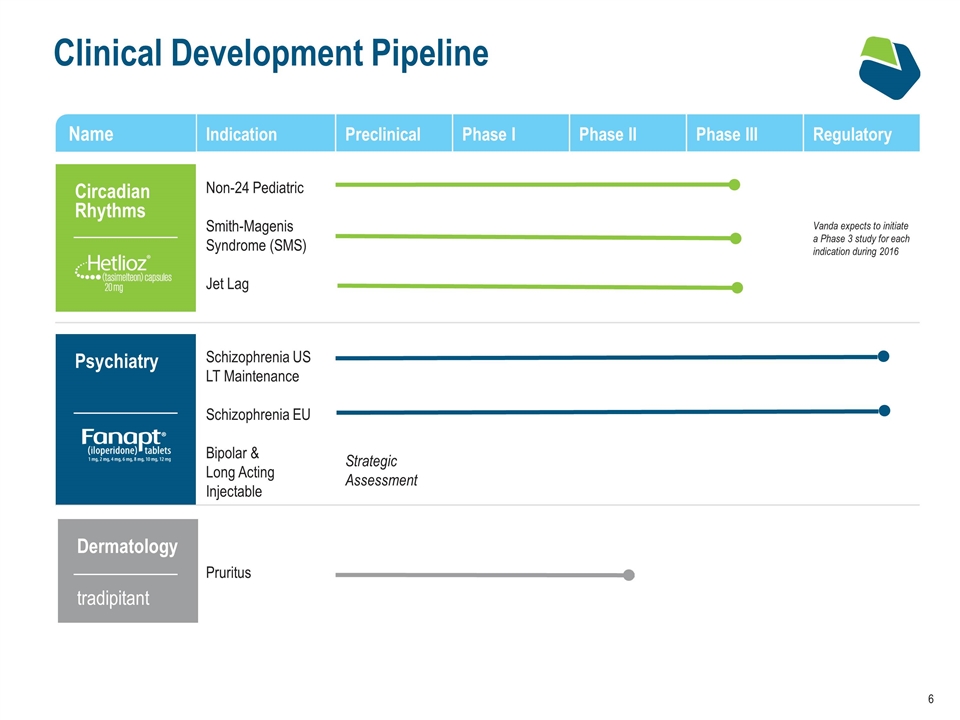
Clinical Development Pipeline Name Indication Preclinical Phase I Phase II Phase III Regulatory Non-24 Pediatric Smith-Magenis Syndrome (SMS) Jet Lag Vanda expects to initiate a Phase 3 study for each indication during 2016 Schizophrenia US LT Maintenance Schizophrenia EU Bipolar & Long Acting Injectable Strategic Assessment Pruritus Circadian Rhythms Dermatology tradipitant Psychiatry Name

Circadian Rhythms
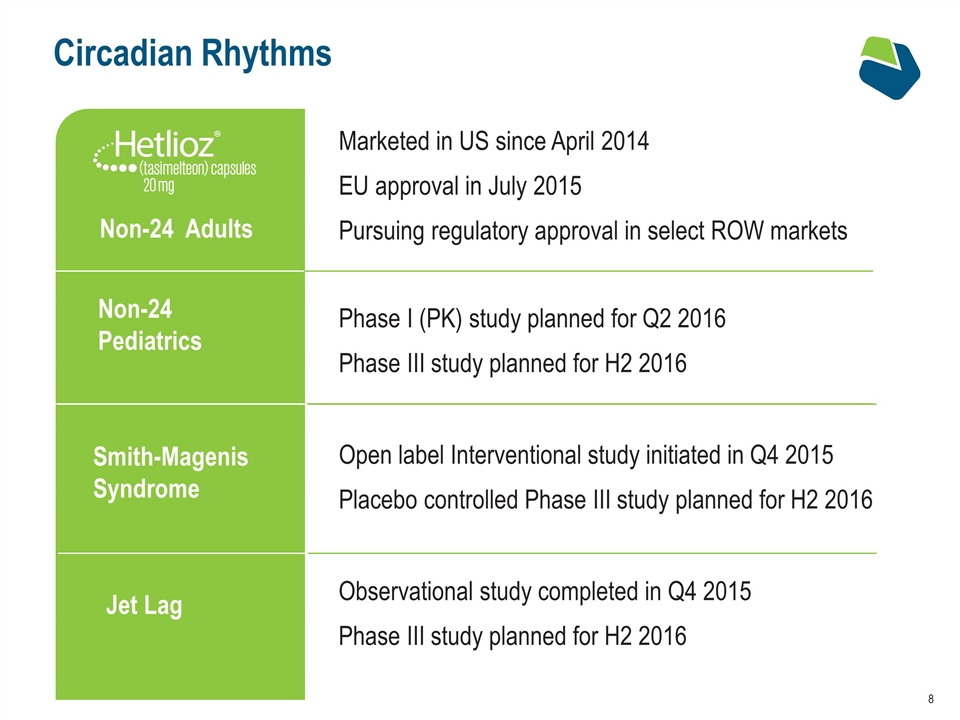
Circadian Rhythms Smith-Magenis Syndrome Non-24 Pediatrics Marketed in US since April 2014 EU approval in July 2015 Pursuing regulatory approval in select ROW markets Phase I (PK) study planned for Q2 2016 Phase III study planned for H2 2016 Open label Interventional study initiated in Q4 2015 Placebo controlled Phase III study planned for H2 2016 Jet Lag Observational study completed in Q4 2015 Phase III study planned for H2 2016 Non-24 Adults
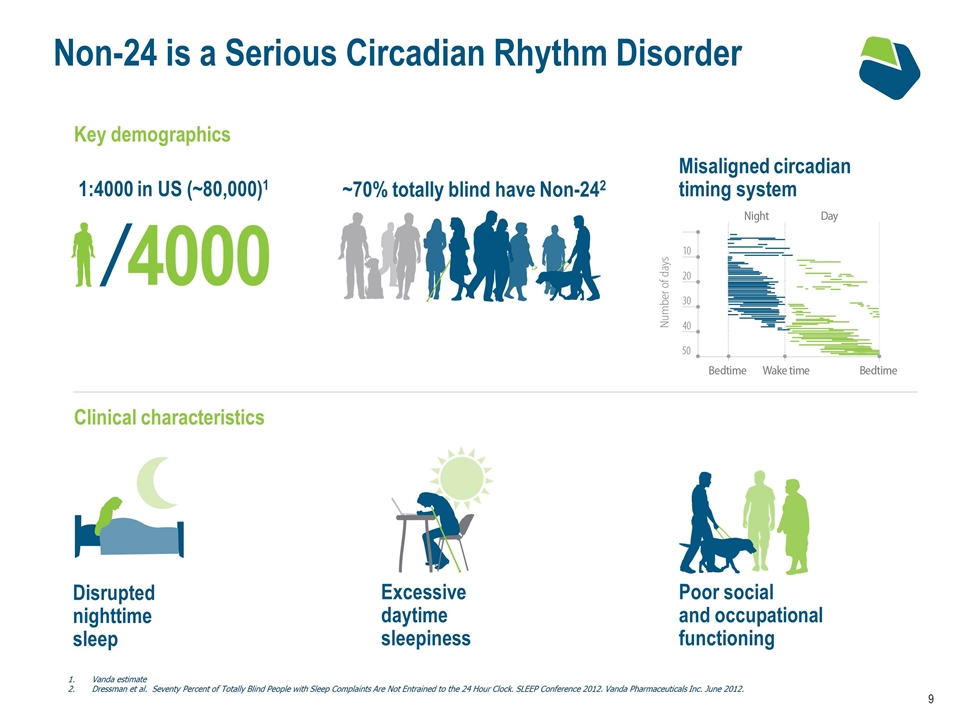
Non-24 is a Serious Circadian Rhythm Disorder Key demographics ~70% totally blind have Non-242 1:4000 in US (~80,000)1 Disrupted nighttime sleep Excessive daytime sleepiness Poor social and occupational functioning Clinical characteristics Misaligned circadian timing system Vanda estimate Dressman et al. Seventy Percent of Totally Blind People with Sleep Complaints Are Not Entrained to the 24 Hour Clock. SLEEP Conference 2012. Vanda Pharmaceuticals Inc. June 2012.
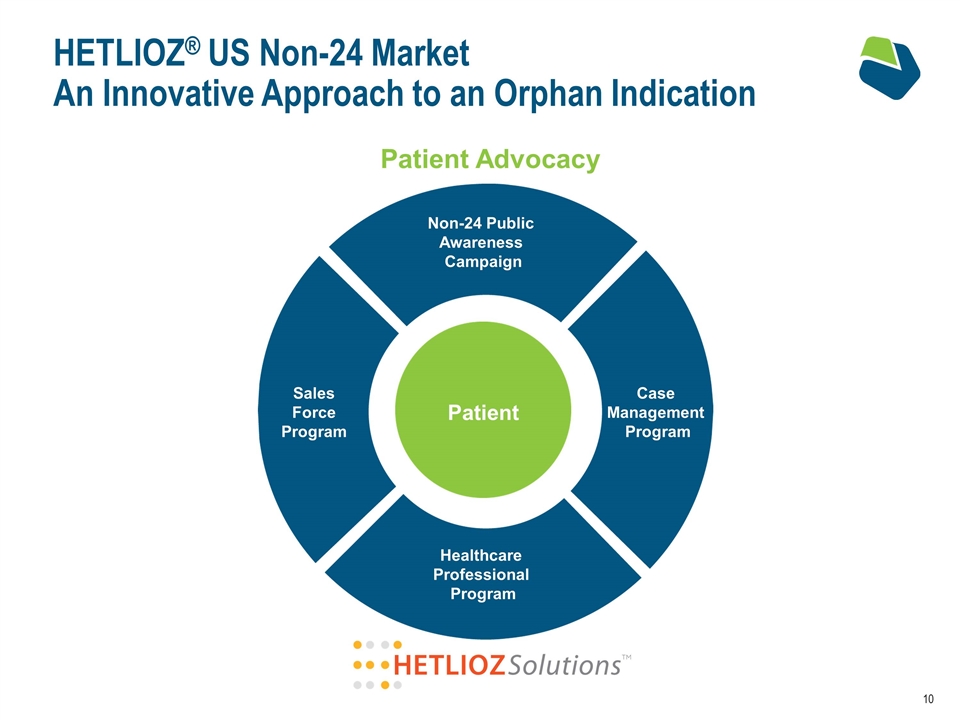
HETLIOZ® US Non-24 Market An Innovative Approach to an Orphan Indication Patient Advocacy Non-24 Public Awareness Campaign Case Management Program Healthcare Professional Program Sales Force Program Patient
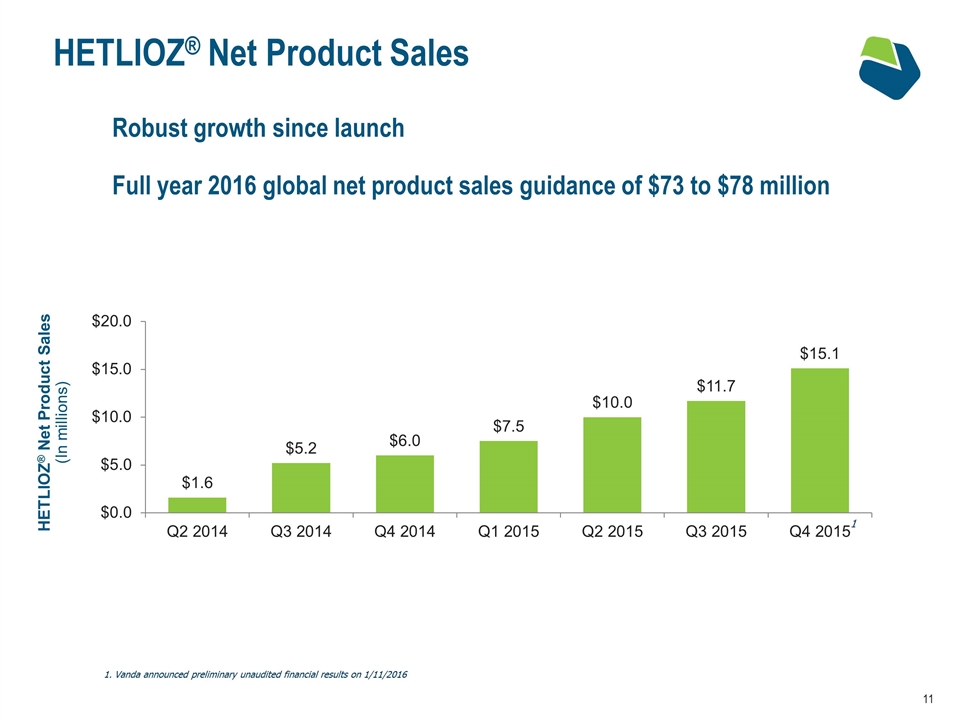
HETLIOZ® Net Product Sales HETLIOZ® Net Product Sales (In millions) Robust growth since launch Full year 2016 global net product sales guidance of $73 to $78 million Vanda announced preliminary unaudited financial results on 1/11/2016 1

HETLIOZ® European Non-24 Market Approximately 130,000 totally blind individuals in Europe have Non-241 EU Non-24 Market Pre - Launch Activities Similar prevalence to US market No approved circadian regulators in EU Engagement with blind advocacy groups Reimbursement & marketing preparations 2016 Priorities Germany – Q3 2016 planned product launch EU 5: Pricing dossier and strategy preparations for the 5 largest EU markets EU 6-28: Explore distribution partners for select remaining 23 EU markets Vanda estimate
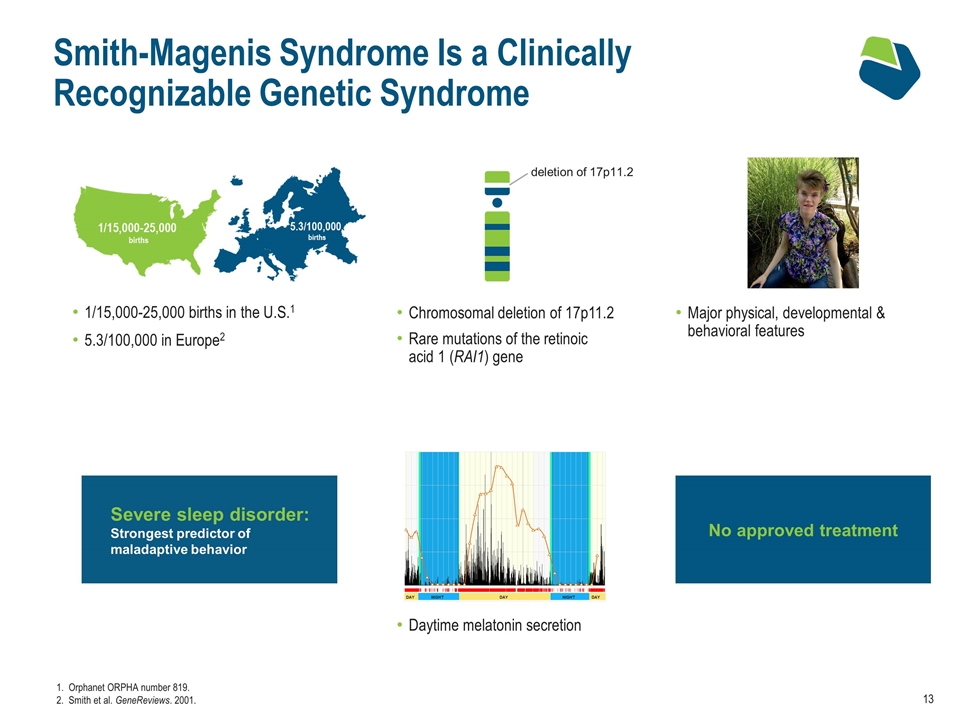
No approved treatment Smith-Magenis Syndrome Is a Clinically Recognizable Genetic Syndrome 1/15,000-25,000 births in the U.S.1 5.3/100,000 in Europe2 Chromosomal deletion of 17p11.2 Rare mutations of the retinoic acid 1 (RAI1) gene Daytime melatonin secretion Orphanet ORPHA number 819. Smith et al. GeneReviews. 2001. Severe sleep disorder: Strongest predictor of maladaptive behavior deletion of 17p11.2 Major physical, developmental & behavioral features
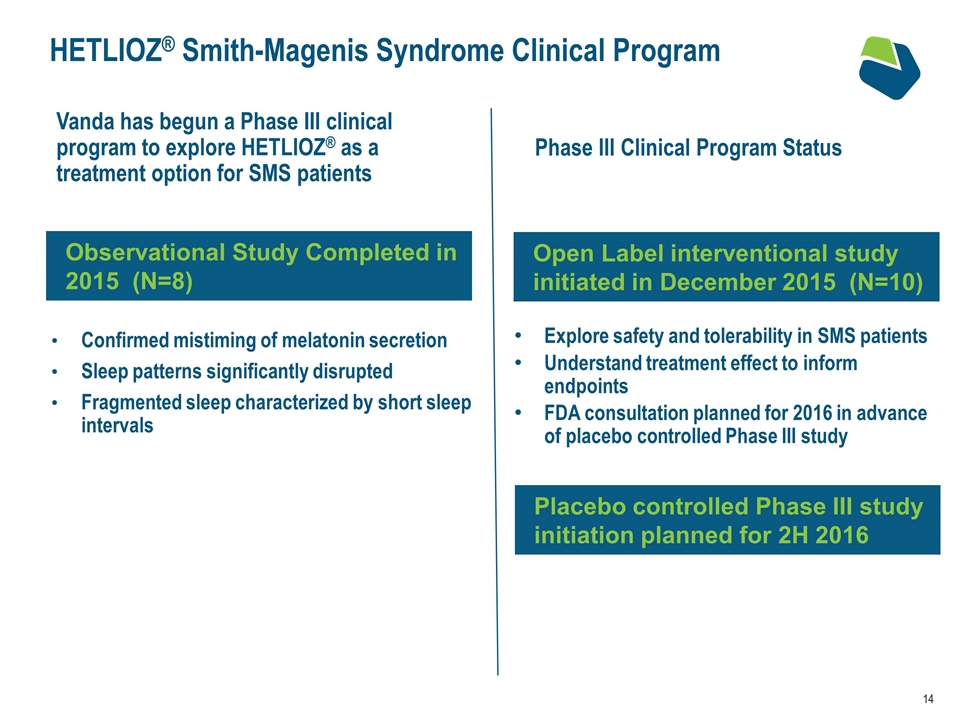
HETLIOZ® Smith-Magenis Syndrome Clinical Program Confirmed mistiming of melatonin secretion Sleep patterns significantly disrupted Fragmented sleep characterized by short sleep intervals Observational Study Completed in 2015 (N=8) Vanda has begun a Phase III clinical program to explore HETLIOZ® as a treatment option for SMS patients Phase III Clinical Program Status Explore safety and tolerability in SMS patients Understand treatment effect to inform endpoints FDA consultation planned for 2016 in advance of placebo controlled Phase III study Open Label interventional study initiated in December 2015 (N=10) Placebo controlled Phase III study initiation planned for 2H 2016
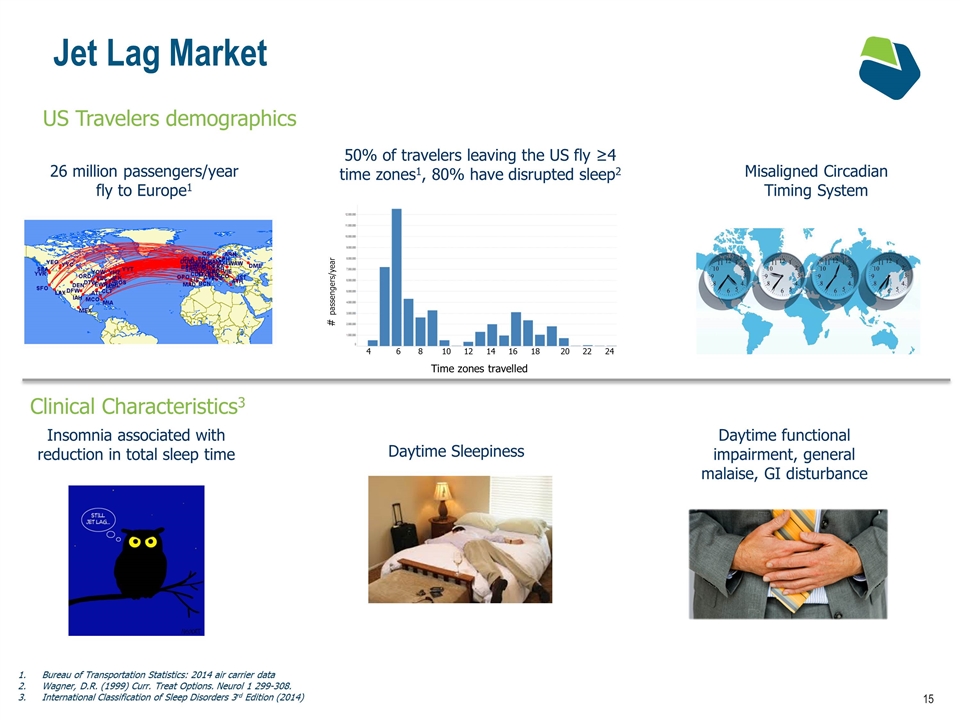
Jet Lag Market US Travelers demographics 50% of travelers leaving the US fly ≥4 time zones1, 80% have disrupted sleep2 26 million passengers/year fly to Europe1 # passengers/year Time zones travelled 4 6 8 10 12 14 16 18 20 22 24 Misaligned Circadian Timing System Clinical Characteristics3 Insomnia associated with reduction in total sleep time Daytime Sleepiness Daytime functional impairment, general malaise, GI disturbance Bureau of Transportation Statistics: 2014 air carrier data Wagner, D.R. (1999) Curr. Treat Options. Neurol 1 299-308. International Classification of Sleep Disorders 3rd Edition (2014)
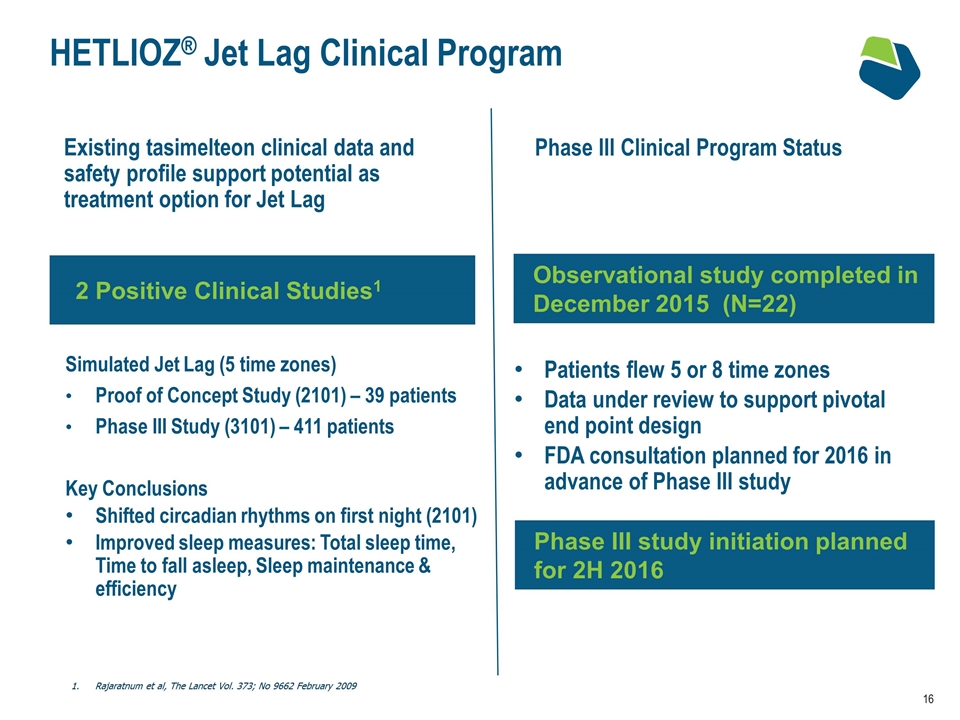
HETLIOZ® Jet Lag Clinical Program Simulated Jet Lag (5 time zones) Proof of Concept Study (2101) – 39 patients Phase III Study (3101) – 411 patients Key Conclusions Shifted circadian rhythms on first night (2101) Improved sleep measures: Total sleep time, Time to fall asleep, Sleep maintenance & efficiency 2 Positive Clinical Studies1 Existing tasimelteon clinical data and safety profile support potential as treatment option for Jet Lag Rajaratnum et al, The Lancet Vol. 373; No 9662 February 2009 Phase III Clinical Program Status Patients flew 5 or 8 time zones Data under review to support pivotal end point design FDA consultation planned for 2016 in advance of Phase III study Observational study completed in December 2015 (N=22) Phase III study initiation planned for 2H 2016

Psychiatry
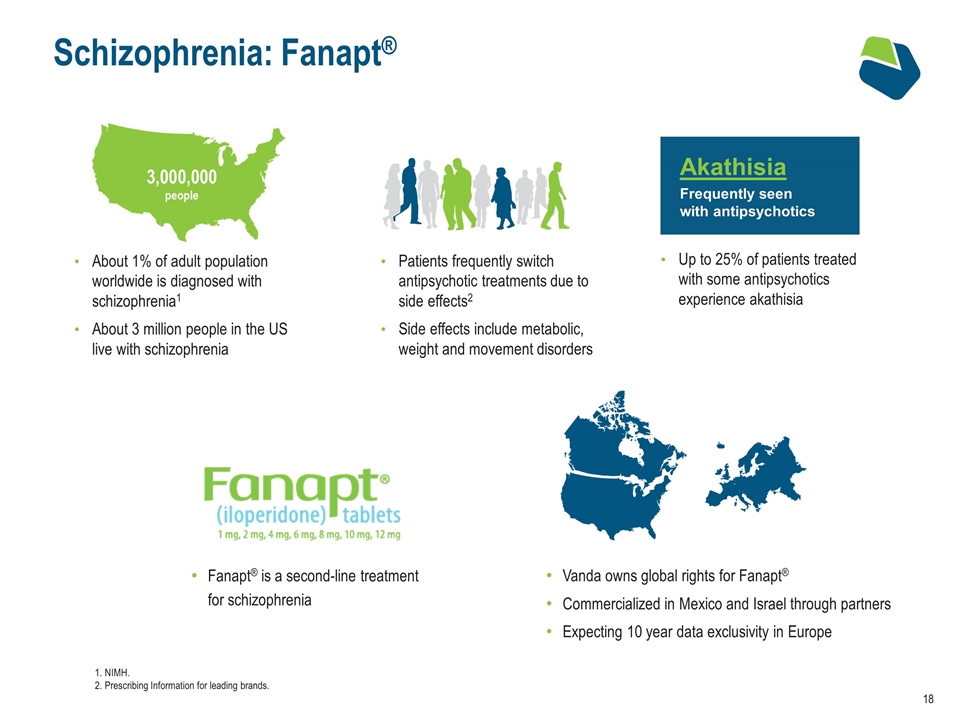
Vanda owns global rights for Fanapt® Commercialized in Mexico and Israel through partners Expecting 10 year data exclusivity in Europe Schizophrenia: Fanapt® Patients frequently switch antipsychotic treatments due to side effects2 Side effects include metabolic, weight and movement disorders About 1% of adult population worldwide is diagnosed with schizophrenia1 About 3 million people in the US live with schizophrenia Fanapt® is a second-line treatment for schizophrenia Up to 25% of patients treated with some antipsychotics experience akathisia 1. NIMH. 2. Prescribing Information for leading brands. Akathisia Frequently seen with antipsychotics
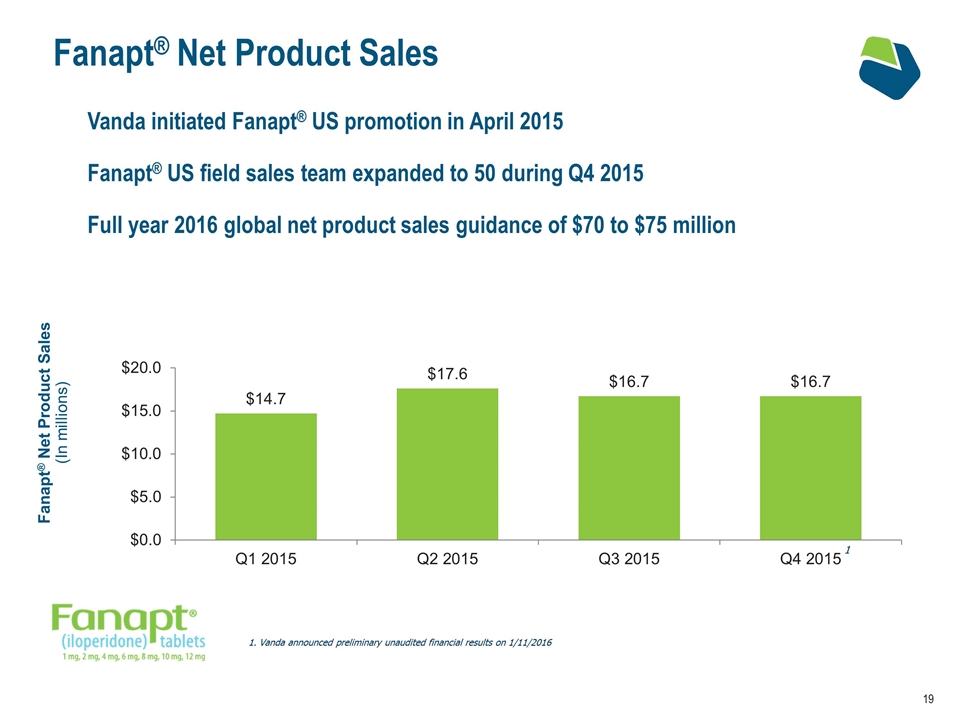
Fanapt® Net Product Sales Vanda initiated Fanapt® US promotion in April 2015 Fanapt® US field sales team expanded to 50 during Q4 2015 Full year 2016 global net product sales guidance of $70 to $75 million Fanapt® Net Product Sales (In millions) Vanda announced preliminary unaudited financial results on 1/11/2016 1
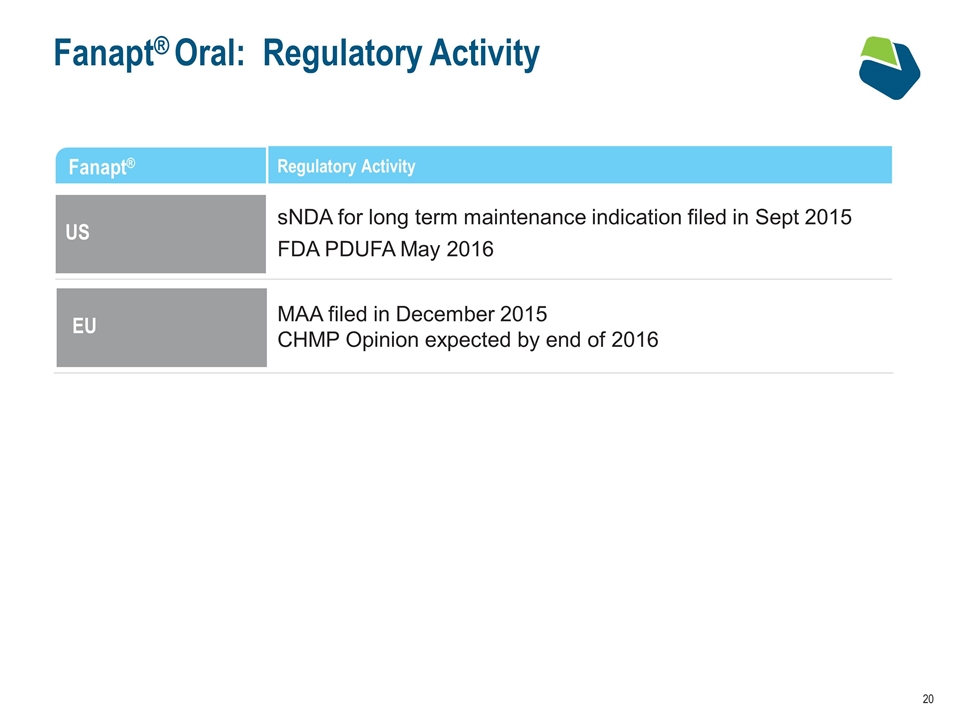
Fanapt® Oral: Regulatory Activity Fanapt® Regulatory Activity US sNDA for long term maintenance indication filed in Sept 2015 FDA PDUFA May 2016 EU MAA filed in December 2015 CHMP Opinion expected by end of 2016 EU US
Dermatology
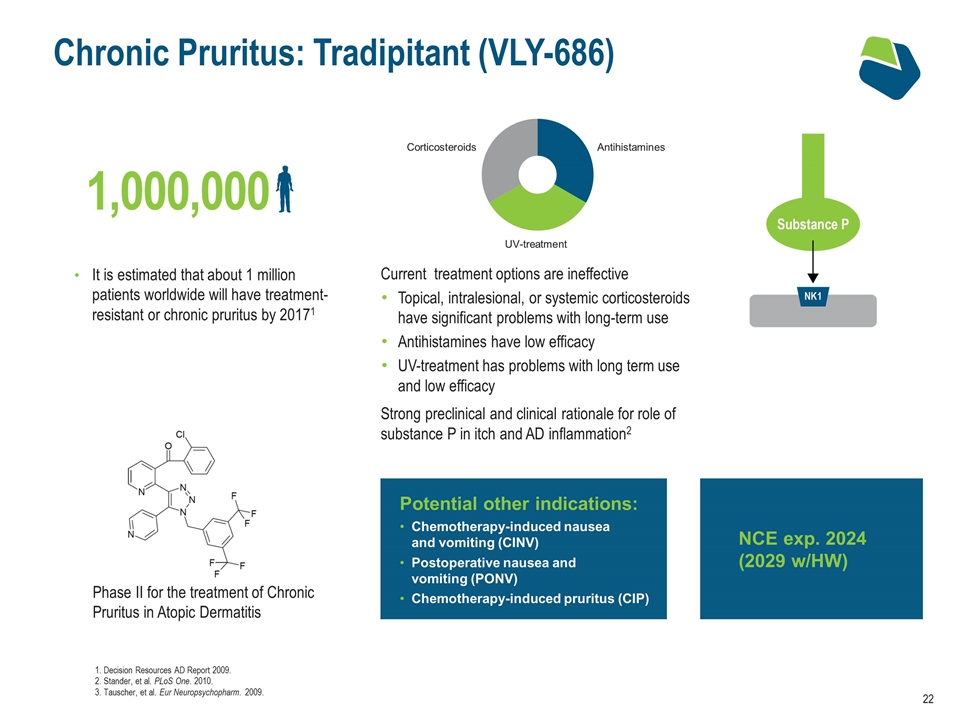
Chronic Pruritus: Tradipitant (VLY-686) Current treatment options are ineffective Topical, intralesional, or systemic corticosteroids have significant problems with long-term use Antihistamines have low efficacy UV-treatment has problems with long term use and low efficacy Strong preclinical and clinical rationale for role of substance P in itch and AD inflammation2 It is estimated that about 1 million patients worldwide will have treatment-resistant or chronic pruritus by 20171 Phase II for the treatment of Chronic Pruritus in Atopic Dermatitis 1. Decision Resources AD Report 2009. 2. Stander, et al. PLoS One. 2010. 3. Tauscher, et al. Eur Neuropsychopharm. 2009. Potential other indications: Chemotherapy-induced nausea and vomiting (CINV) Postoperative nausea and vomiting (PONV) Chemotherapy-induced pruritus (CIP) NCE exp. 2024 (2029 w/HW) Antihistamines UV-treatment Corticosteroids
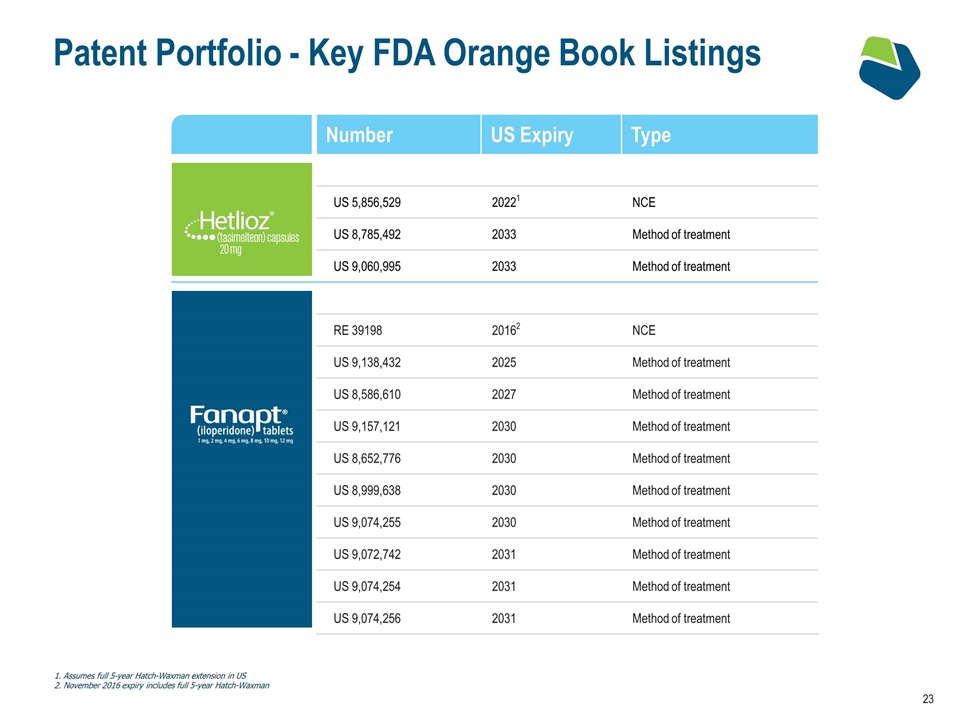
Patent Portfolio - Key FDA Orange Book Listings Name Number US Expiry Type US 5,856,529 20221 NCE US 8,785,492 2033 Method of treatment US 9,060,995 2033 Method of treatment RE 39198 20162 NCE US 9,138,432 2025 Method of treatment US 8,586,610 2027 Method of treatment US 9,157,121 2030 Method of treatment US 8,652,776 2030 Method of treatment US 8,999,638 2030 Method of treatment US 9,074,255 2030 Method of treatment US 9,072,742 2031 Method of treatment US 9,074,254 2031 Method of treatment US 9,074,256 2031 Method of treatment 1. Assumes full 5-year Hatch-Waxman extension in US 2. November 2016 expiry includes full 5-year Hatch-Waxman

For more information on HETLIOZ®, please visit www.HETLIOZ.com For more information on FANAPT®, please visit www.FANAPT.com
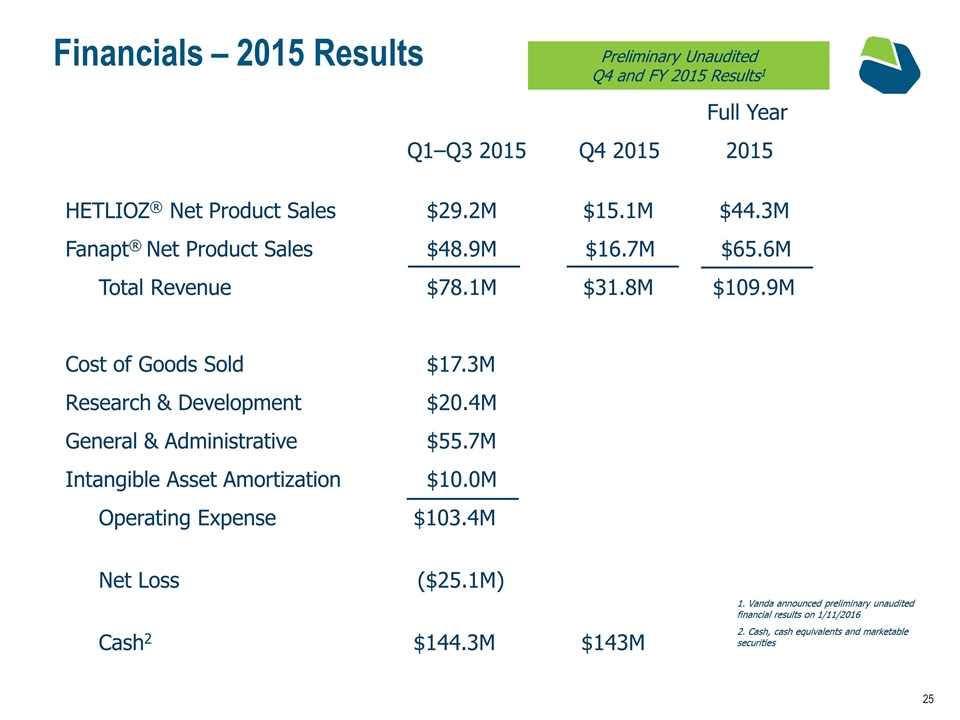
Financials – 2015 Results HETLIOZ® Net Product Sales $29.2M $15.1M $44.3M Fanapt® Net Product Sales $48.9M $16.7M $65.6M Total Revenue $78.1M $31.8M $109.9M Cost of Goods Sold $17.3M Research & Development $20.4M General & Administrative $55.7M Intangible Asset Amortization $10.0M Operating Expense $103.4M Net Loss ($25.1M) Cash2 $144.3M $143M Full Year Q1–Q3 2015 Q4 2015 2015 Vanda announced preliminary unaudited financial results on 1/11/2016 Cash, cash equivalents and marketable securities Preliminary Unaudited Q4 and FY 2015 Results1

Financials – Full Year 2015 Guidance1 Vanda expects to achieve the following financial objectives in 2015: Total net product sales from HETLIOZ® and Fanapt® of between $100 and $115 million. HETLIOZ® net product sales of between $40 and $45 million. Fanapt® net product sales of between $60 and $70 million. Non-GAAP Operating expenses, excluding cost of goods sold, of between $100 and $110 million2. Non-GAAP Operating expenses also excludes: Intangible asset amortization expense of $13.0 million. Stock-based compensation of between $8.5 and $10.5 million. 1. Guidance provided by Vanda Pharmaceuticals on and as of November 3, 2015 Vanda Pharmaceuticals undertakes no duty to update this guidance, and actual results may differ 2. A description of Vanda’s use of this Non-GAAP financial measure is included at the end of this presentation

Financials – Full Year 2016 Guidance1 Vanda expects to achieve the following financial objectives in 2016: Global net product sales from HETLIOZ® and Fanapt® of between $143 and $153 million. HETLIOZ® global net product sales of between $73 and $78 million. Fanapt® global net product sales of between $70 and $75 million. Non-GAAP Operating expenses, excluding cost of goods sold, of between $125 and $135 million2. Non-GAAP Operating expenses also excludes intangible asset amortization expense of $10.9 million and stock-based compensation of between $9 and $11 million. Cash is expected to decrease by less than $20 million during 2016. 1. Guidance provided by Vanda Pharmaceuticals on and as of January 11, 2016 Vanda Pharmaceuticals undertakes no duty to update this guidance, and actual results may differ 2. A description of Vanda’s use of this Non-GAAP financial measure is included at the end of this presentation

Non-GAAP Financial Information Vanda believes that the Non-GAAP financial information provided in this presentation can assist investors in understanding and assessing the ongoing economics of Vanda’s business and reflect how it manages the business internally and sets operational goals. This presentation includes projections of 2015 and 2016 Non-GAAP Operating expenses, excluding cost of goods sold, a forward-looking Non-GAAP financial measure. This Non-GAAP financial measure is determined by excluding cost of goods sold, stock-based compensation and intangible asset amortization. Vanda believes that excluding the impact of these items better reflects the recurring economic characteristics of its business, as well as Vanda’s use of financial resources and its long-term performance. Vanda is unable to reconcile this Non-GAAP guidance to GAAP because it is difficult to predict the future impact of these adjustments. This Non-GAAP financial measure, as presented, may not be comparable to a similarly titled measures reported by other companies since not all companies may calculate this measure in an identical manner and, therefore, it is not necessarily an accurate measure of comparison between companies. The presentation of this Non-GAAP financial measure is not intended to be considered in isolation or as a substitute for guidance prepared in accordance with GAAP. The principal limitation of this Non-GAAP financial measure is that it excludes significant elements that are required by GAAP to be recorded in Vanda’s financial statements. In addition, this Non-GAAP financial measure is subject to inherent limitations as it reflects the exercise of judgments by management in determining this Non-GAAP financial measure.

2016 Corporate Milestones* HETLIOZ® ¨ Non-24 – Germany product launch Q3 2016 ¨ Pediatric Non-24 Phase III study initiation H2 2016 ¨ SMS placebo controlled Phase III study initiation H2 2016 ¨ Jet Lag Phase III study initiation H2 2016 Fanapt ® ¨ Vanda v. Roxane (NCE & ‘610 Patents) Feb 2016 ¨ PDUFA - Long Term Maintenance sNDA May 2016 ¨ European MAA CHMP Opinion Q4 2016 Pipeline products ¨ Trichostatin A – IND submission H2 2016 ¨ Tradipitant – Pruritus POC study initiation H2 2016 * Reflects expected timing of select future milestones

