Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Spark Therapeutics, Inc. | d116709d8k.htm |

Spark Therapeutics, Inc. J.P. Morgan Healthcare Conference January 11, 2016 Exhibit 99.1

Forward-looking statements This presentation includes ‘‘forward-looking statements,” within the meaning of the Private Securities Litigation Reform Act of 1995, that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, expectations regarding the clinical development of our product candidates, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words ‘‘anticipate,’’ ‘‘believe,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘plan,’’ ‘‘predict,’’ ‘‘project,’’ ‘‘target,’’ ‘‘potential,’’ ‘‘will,’’ ‘‘would,’’ ‘‘could,’’ ‘‘should,’’ ‘‘continue’’ and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make as a result of a variety of risks and uncertainties, including those described in the ‘‘Risk Factors’’ section of our public filings with the Securities and Exchange Commission. We do not assume any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.

First gene therapy platform validated by successful randomized, controlled pivotal trial of SPK-RPE65 (voretigene neparvovec) Phase 3 data statistically and clinically significant for RPE65-mediated blindness with >3 years of durability data from comparable Phase 1 cohort Expect to complete BLA filing for SPK-RPE65 in 2H16 and launch in 2017 Uniquely positioned to deliver 3 commercial or pivotal-stage programs from 10 clinical candidates by 2018 Proven capabilities across vector selection, design and manufacture A history of collaborating with regulators to enable optimal clinical development At the forefront of shaping a patient-centric, commercial model for gene therapies Resident expertise to develop internal and evaluate external candidates and maximize value through internal commercialization or external collaboration Data readouts expected across 5 clinical programs in 3 franchises by 1H17 First efficacy readout for SPK-CHM anticipated in 2H16 Capitalized through significant key inflection points into 2019, with >$300 million Spark is a fully integrated platform company developing one‐time, transformative therapies for severe genetic diseases
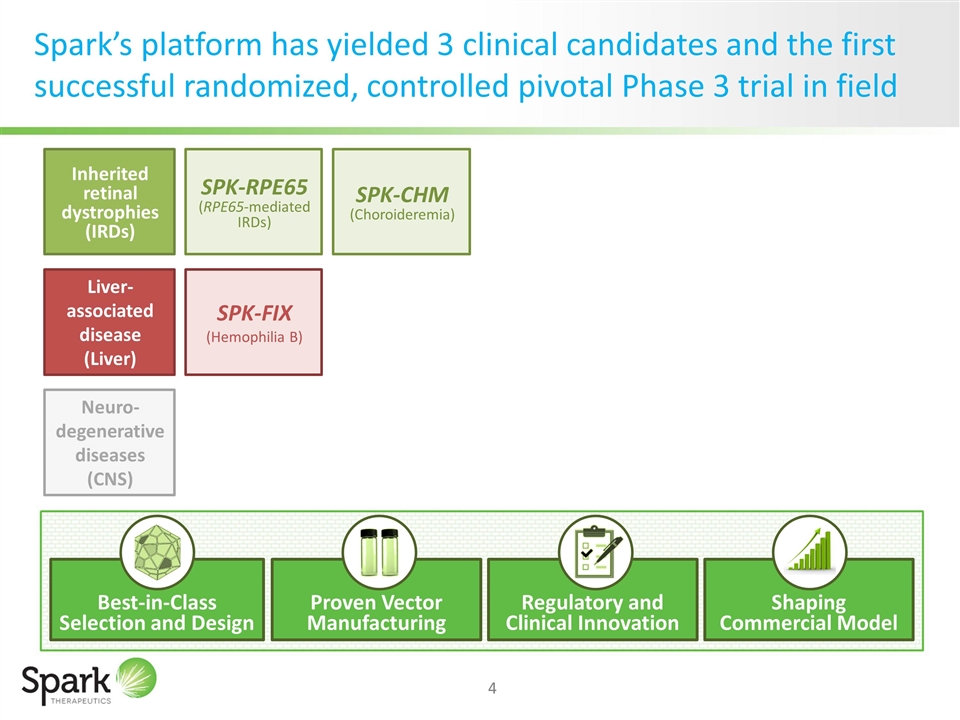
Spark’s platform has yielded 3 clinical candidates and the first successful randomized, controlled pivotal Phase 3 trial in field Inherited retinal dystrophies (IRDs) Liver-associated disease (Liver) Neuro-degenerative diseases (CNS) SPK-RPE65 (RPE65-mediated IRDs) SPK-CHM (Choroideremia) SPK-FIX (Hemophilia B) Best-in-Class Selection and Design Proven Vector Manufacturing Regulatory and Clinical Innovation Shaping Commercial Model
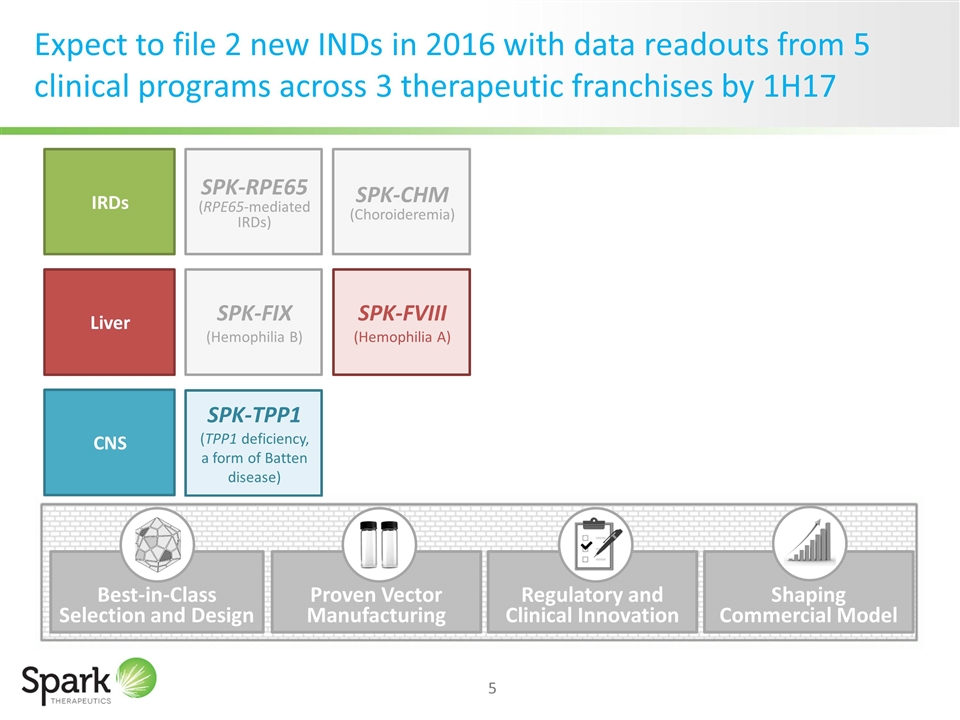
Expect to file 2 new INDs in 2016 with data readouts from 5 clinical programs across 3 therapeutic franchises by 1H17 IRDs Liver SPK-RPE65 (RPE65-mediated IRDs) SPK-CHM (Choroideremia) SPK-FIX (Hemophilia B) CNS SPK-FVIII (Hemophilia A) SPK-TPP1 (TPP1 deficiency, a form of Batten disease) Proven Vector Manufacturing Regulatory and Clinical Innovation Shaping Commercial Model Best-in-Class Selection and Design
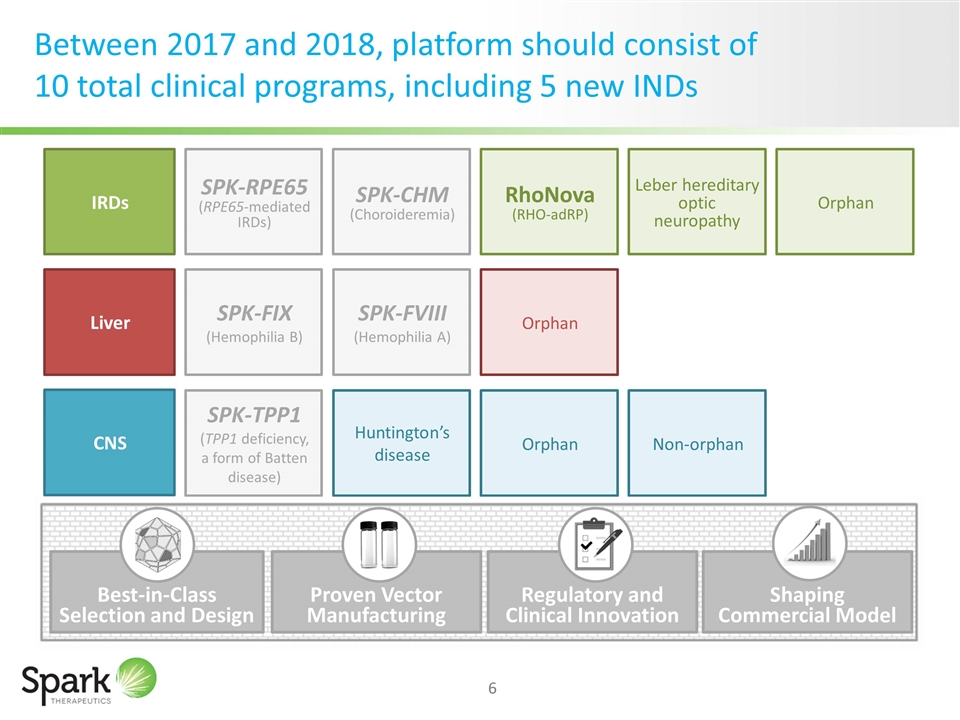
Between 2017 and 2018, platform should consist of 10 total clinical programs, including 5 new INDs IRDs Liver CNS SPK-FVIII (Hemophilia A) Huntington’s disease Leber hereditary optic neuropathy RhoNova (RHO-adRP) Orphan Orphan Orphan Non-orphan SPK-TPP1 (TPP1 deficiency, a form of Batten disease) SPK-RPE65 (RPE65-mediated IRDs) SPK-CHM (Choroideremia) SPK-FIX (Hemophilia B) Proven Vector Manufacturing Regulatory and Clinical Innovation Shaping Commercial Model Best-in-Class Selection and Design
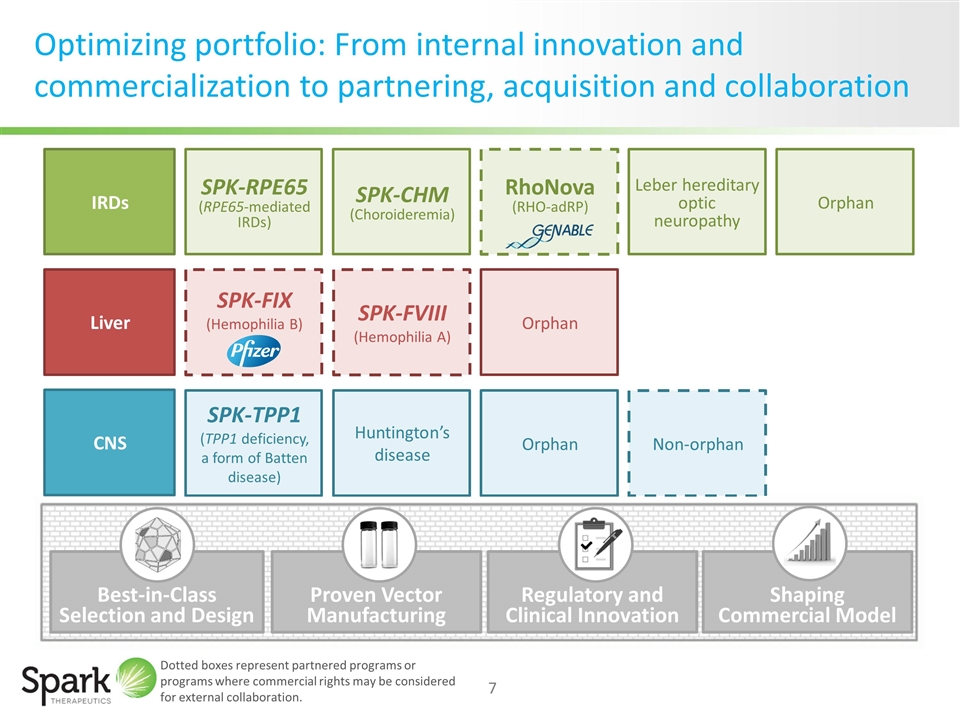
RhoNova (RHO-adRP) Optimizing portfolio: From internal innovation and commercialization to partnering, acquisition and collaboration IRDs Liver SPK-FIX (Hemophilia B) CNS SPK-FVIII (Hemophilia A) Non-orphan SPK-RPE65 (RPE65-mediated IRDs) SPK-CHM (Choroideremia) SPK-TPP1 (TPP1 deficiency, a form of Batten disease) Huntington’s disease Orphan Orphan Leber hereditary optic neuropathy Orphan Dotted boxes represent partnered programs or programs where commercial rights may be considered for external collaboration. Proven Vector Manufacturing Regulatory and Clinical Innovation Shaping Commercial Model Best-in-Class Selection and Design
RhoNova (RHO-adRP) Spark aims to deliver 3 commercial or pivotal-stage programs from 10 clinical programs by 2018 IRDs Liver CNS SPK-FIX (Hemophilia B) SPK-FVIII (Hemophilia A) Non-orphan SPK-RPE65 (RPE65-mediated IRDs) SPK-CHM (Choroideremia) SPK-TPP1 (TPP1 deficiency, a form of Batten disease) Huntington’s disease Orphan Orphan Leber hereditary optic neuropathy Orphan Best-in-Class Selection and Design Proven Vector Manufacturing Regulatory and Clinical Innovation Shaping Commercial Model

SPK-RPE65: Statistically and clinically significant results for RPE65-mediated IRDs
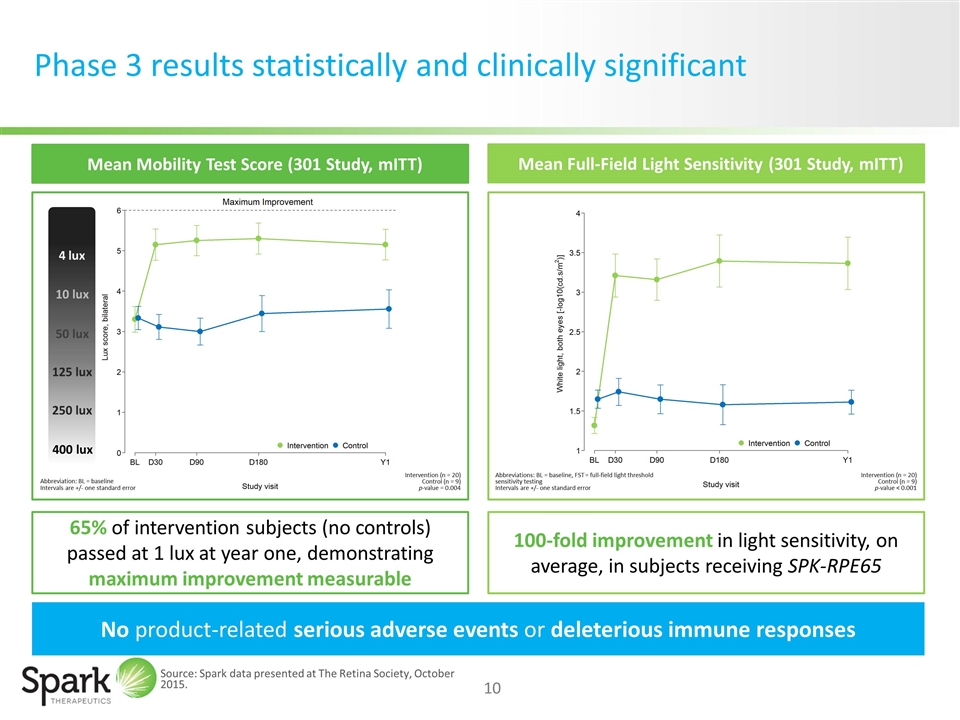
Phase 3 results statistically and clinically significant 65% of intervention subjects (no controls) passed at 1 lux at year one, demonstrating maximum improvement measurable 100-fold improvement in light sensitivity, on average, in subjects receiving SPK-RPE65 Mean Full-Field Light Sensitivity (301 Study, mITT) Mean Mobility Test Score (301 Study, mITT) No product-related serious adverse events or deleterious immune responses Abbreviation: BL = baseline Intervals are +/- one standard error Intervention (n = 20) Control (n = 9) p-value = 0.004 Intervention (n = 20) Control (n = 9) p-value < 0.001 Abbreviations: BL = baseline, FST = full-field light threshold sensitivity testing Intervals are +/- one standard error 4 lux 10 lux 50 lux 125 lux 250 lux 400 lux Source: Spark data presented at The Retina Society, October 2015.

Restoring functional vision through one-time administration of SPK-RPE65 CH-41: baseline visit at 4 lux (Fail) CH-41: 1-year visit after SPK-RPE65 administration at 4 lux (Pass) Note: The videos of CH-41 are representative of the mean improvement demonstrated in the intervention group in the Phase 3 trial. Source: Spark Phase 3 trial source data.
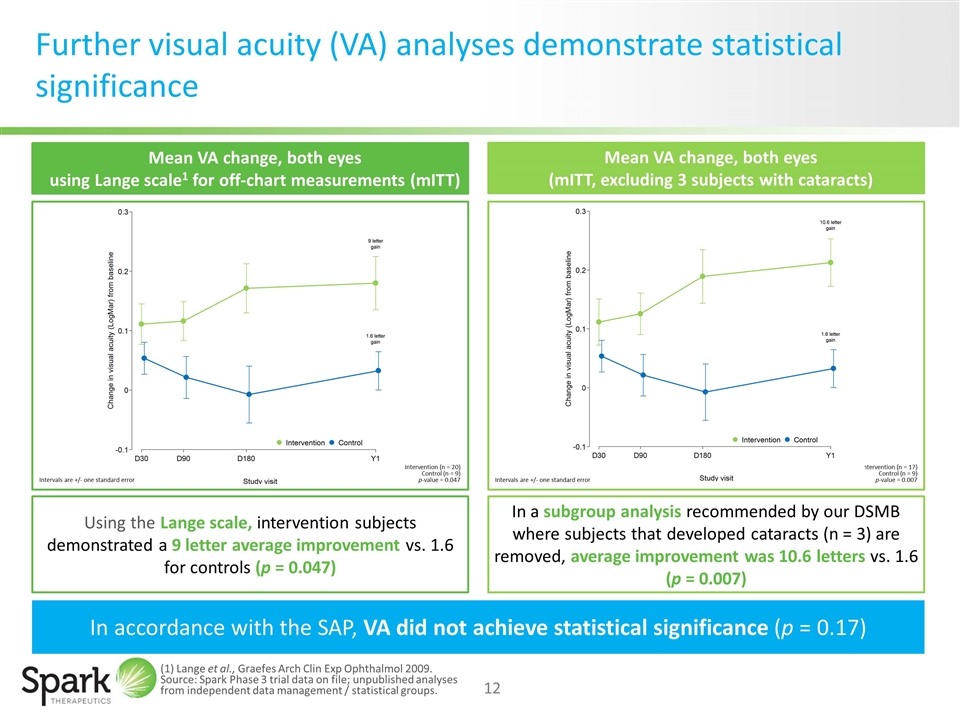
Further visual acuity (VA) analyses demonstrate statistical significance Using the Lange scale, intervention subjects demonstrated a 9 letter average improvement vs. 1.6 for controls (p = 0.047) In a subgroup analysis recommended by our DSMB where subjects that developed cataracts (n = 3) are removed, average improvement was 10.6 letters vs. 1.6 (p = 0.007) Mean VA change, both eyes (mITT, excluding 3 subjects with cataracts) Mean VA change, both eyes using Lange scale1 for off-chart measurements (mITT) In accordance with the SAP, VA did not achieve statistical significance (p = 0.17) Intervention (n = 17) Control (n = 9) p-value = 0.007 Intervention (n = 20) Control (n = 9) p-value = 0.047 Intervals are +/- one standard error Intervals are +/- one standard error (1) Lange et al., Graefes Arch Clin Exp Ophthalmol 2009. Source: Spark Phase 3 trial data on file; unpublished analyses from independent data management / statistical groups.
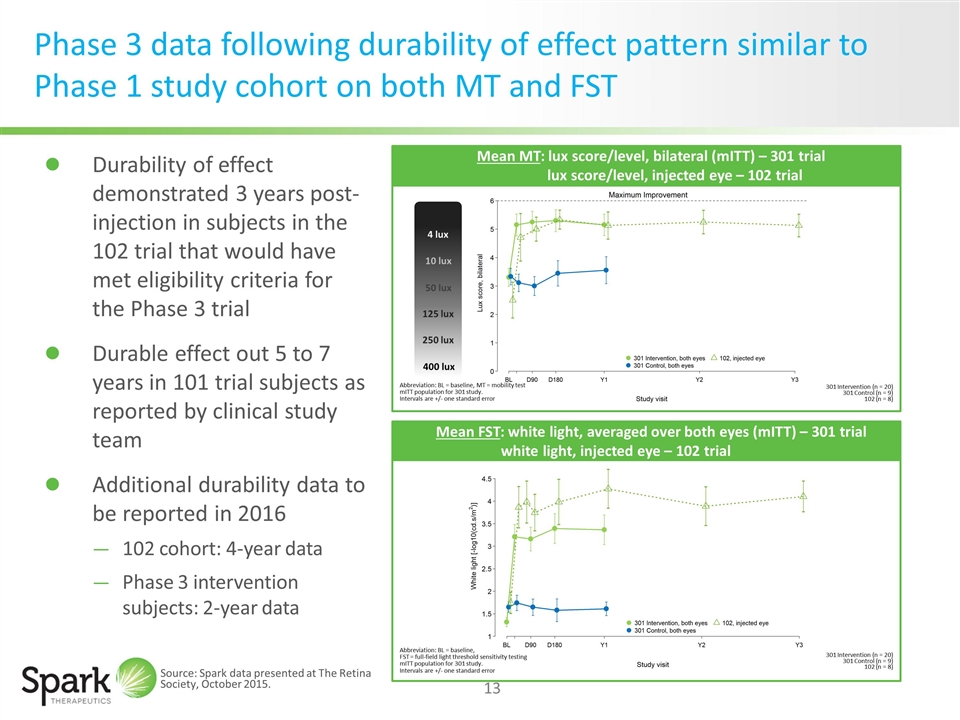
Phase 3 data following durability of effect pattern similar to Phase 1 study cohort on both MT and FST Abbreviation: BL = baseline, MT = mobility test mITT population for 301 study. Intervals are +/- one standard error Mean MT: lux score/level, bilateral (mITT) – 301 trial lux score/level, injected eye – 102 trial 301 Intervention (n = 20) 301 Control (n = 9) 102 (n = 8) Abbreviation: BL = baseline, FST = full-field light threshold sensitivity testing mITT population for 301 study. Intervals are +/- one standard error 301 Intervention (n = 20) 301 Control (n = 9) 102 (n = 8) Mean FST: white light, averaged over both eyes (mITT) – 301 trial white light, injected eye – 102 trial Durability of effect demonstrated 3 years post-injection in subjects in the 102 trial that would have met eligibility criteria for the Phase 3 trial Durable effect out 5 to 7 years in 101 trial subjects as reported by clinical study team Additional durability data to be reported in 2016 102 cohort: 4-year data Phase 3 intervention subjects: 2-year data Source: Spark data presented at The Retina Society, October 2015. 4 lux 10 lux 50 lux 125 lux 250 lux 400 lux
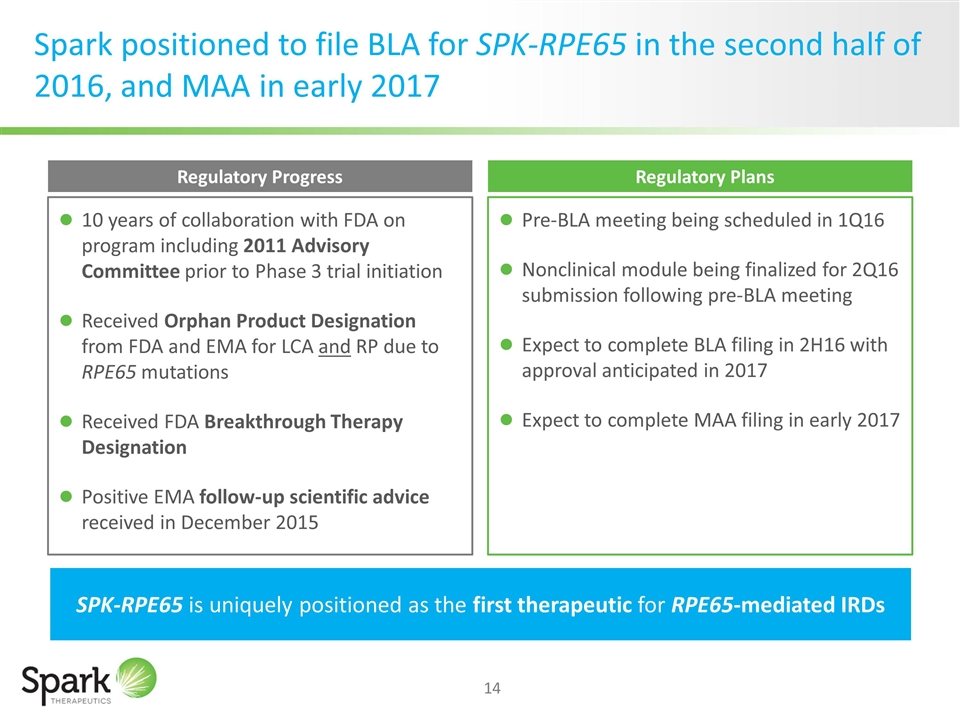
Spark positioned to file BLA for SPK-RPE65 in the second half of 2016, and MAA in early 2017 SPK-RPE65 is uniquely positioned as the first therapeutic for RPE65-mediated IRDs Regulatory Progress 10 years of collaboration with FDA on program including 2011 Advisory Committee prior to Phase 3 trial initiation Received Orphan Product Designation from FDA and EMA for LCA and RP due to RPE65 mutations Received FDA Breakthrough Therapy Designation Positive EMA follow-up scientific advice received in December 2015 Regulatory Plans Pre-BLA meeting being scheduled in 1Q16 Nonclinical module being finalized for 2Q16 submission following pre-BLA meeting Expect to complete BLA filing in 2H16 with approval anticipated in 2017 Expect to complete MAA filing in early 2017
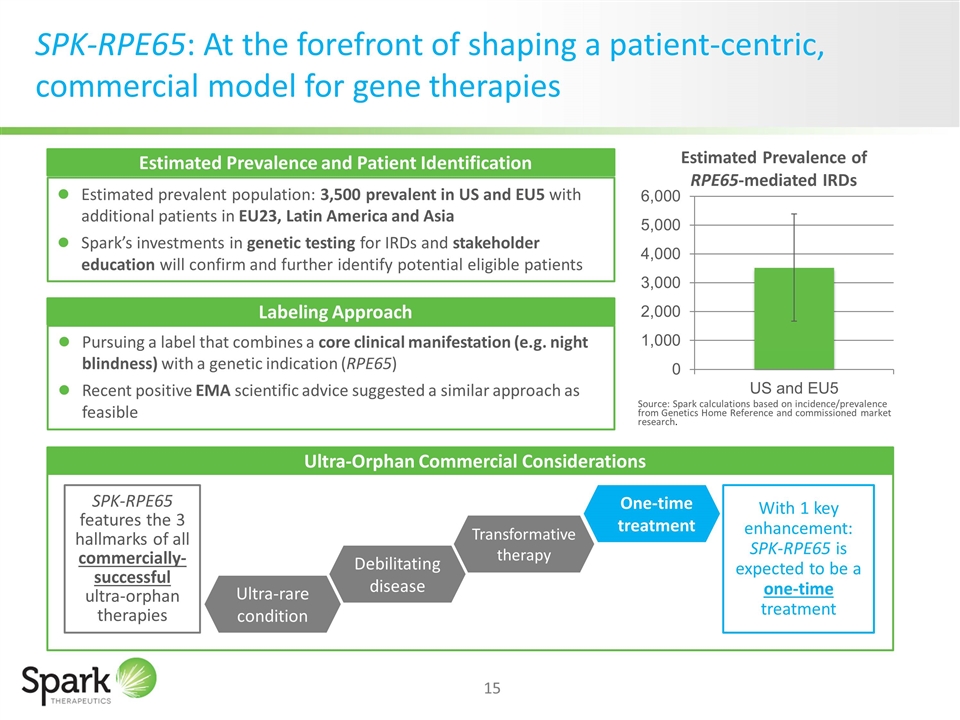
SPK-RPE65: At the forefront of shaping a patient-centric, commercial model for gene therapies Market Size Estimated prevalent population: 3,500 prevalent in US and EU5 with additional patients in EU23, Latin America and Asia Spark’s investments in genetic testing for IRDs and stakeholder education will confirm and further identify potential eligible patients Source: Spark calculations based on incidence/prevalence from Genetics Home Reference and commissioned market research. Ultra-rare condition Debilitating disease Transformative therapy One-time treatment SPK-RPE65 features the 3 hallmarks of all commercially-successful ultra-orphan therapies With 1 key enhancement: SPK-RPE65 is expected to be a one-time treatment Labeling Approach Pursuing a label that combines a core clinical manifestation (e.g. night blindness) with a genetic indication (RPE65) Recent positive EMA scientific advice suggested a similar approach as feasible Estimated Prevalence and Patient Identification Labeling Approach Ultra-Orphan Commercial Considerations

2016 clinical pipeline: 2 current programs and 2 new INDs
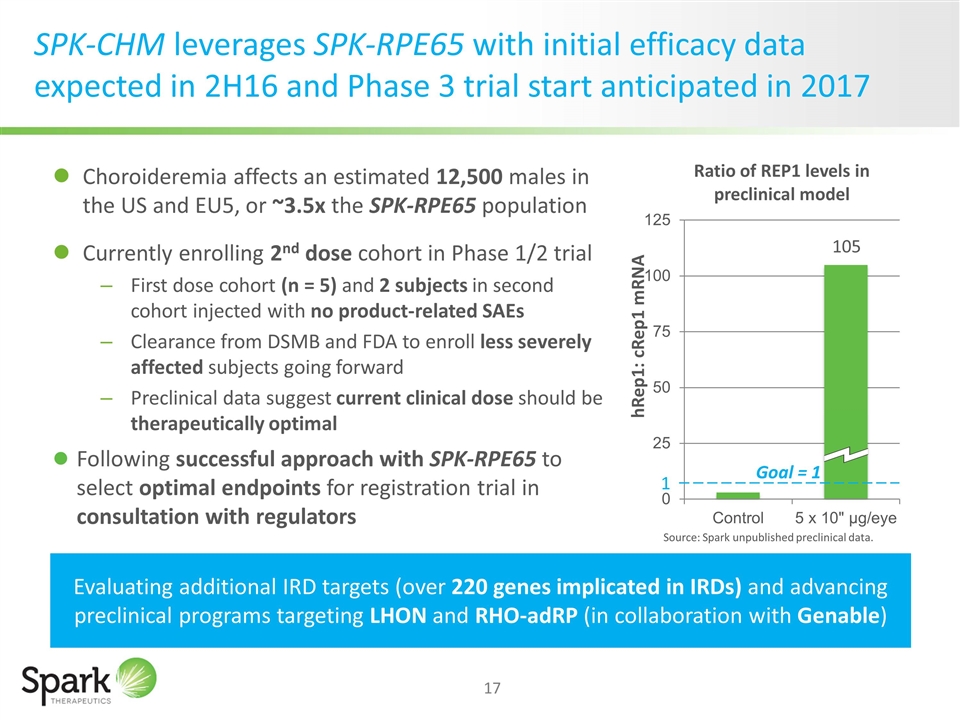
SPK-CHM leverages SPK-RPE65 with initial efficacy data expected in 2H16 and Phase 3 trial start anticipated in 2017 Choroideremia affects an estimated 12,500 males in the US and EU5, or ~3.5x the SPK-RPE65 population Currently enrolling 2nd dose cohort in Phase 1/2 trial First dose cohort (n = 5) and 2 subjects in second cohort injected with no product-related SAEs Clearance from DSMB and FDA to enroll less severely affected subjects going forward Preclinical data suggest current clinical dose should be therapeutically optimal Following successful approach with SPK-RPE65 to select optimal endpoints for registration trial in consultation with regulators Evaluating additional IRD targets (over 220 genes implicated in IRDs) and advancing preclinical programs targeting LHON and RHO-adRP (in collaboration with Genable) 1 Goal = 1 105 Source: Spark unpublished preclinical data.
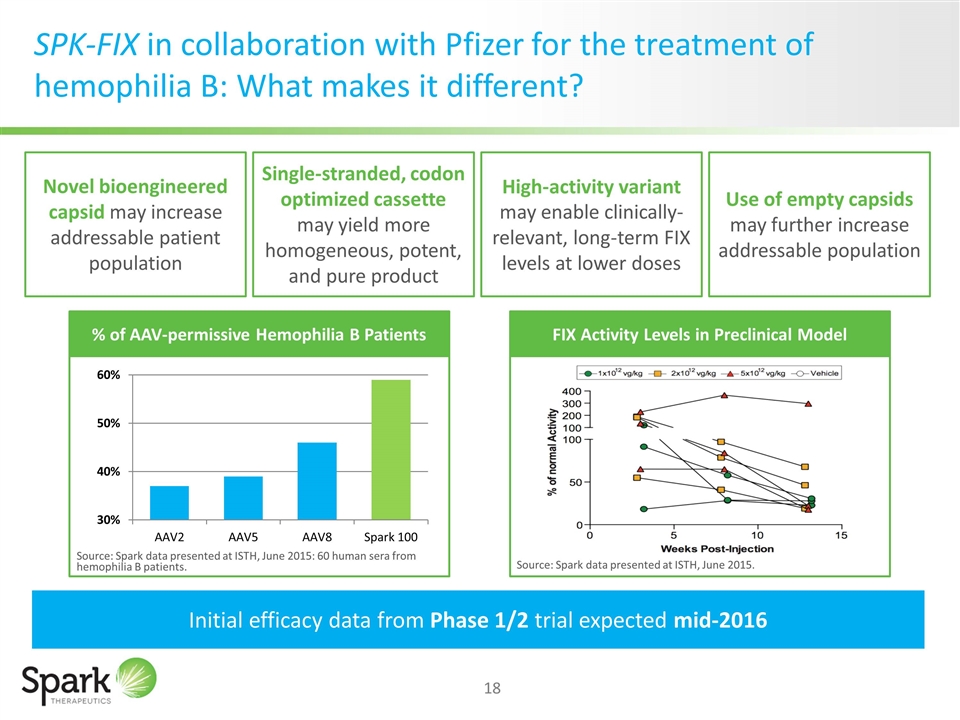
SPK-FIX in collaboration with Pfizer for the treatment of hemophilia B: What makes it different? Novel bioengineered capsid may increase addressable patient population Initial efficacy data from Phase 1/2 trial expected mid-2016 Single-stranded, codon optimized cassette may yield more homogeneous, potent, and pure product High-activity variant may enable clinically-relevant, long-term FIX levels at lower doses Use of empty capsids may further increase addressable population % of AAV-permissive Hemophilia B Patients Source: Spark data presented at ISTH, June 2015: 60 human sera from hemophilia B patients. FIX Activity Levels in Preclinical Model Source: Spark data presented at ISTH, June 2015.
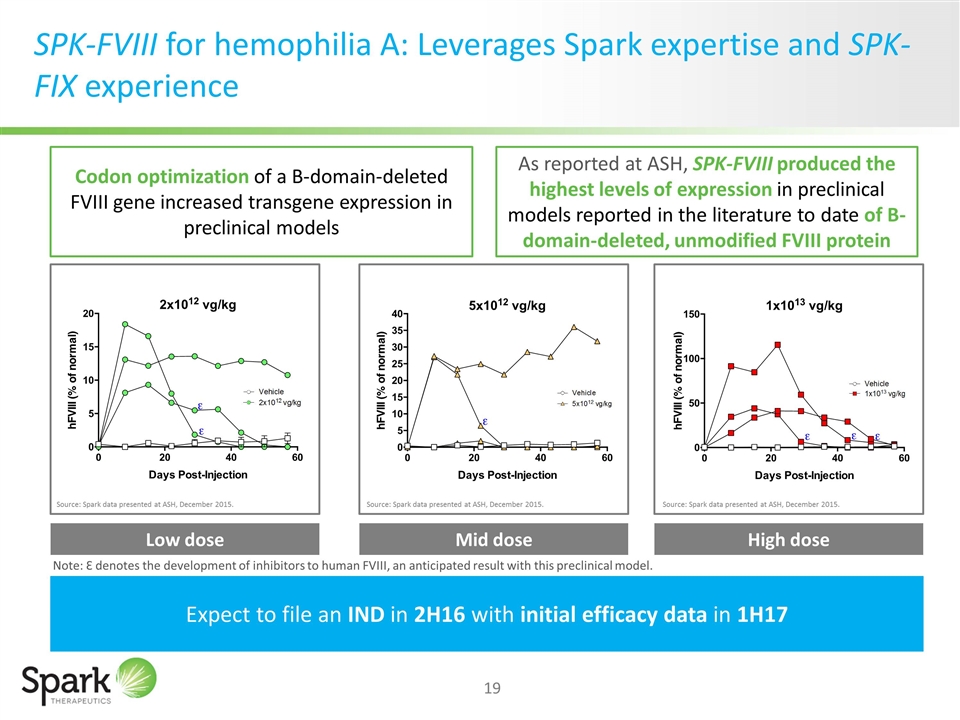
SPK-FVIII for hemophilia A: Leverages Spark expertise and SPK-FIX experience Expect to file an IND in 2H16 with initial efficacy data in 1H17 Low dose Mid dose High dose Source: Spark data presented at ASH, December 2015. Source: Spark data presented at ASH, December 2015. Source: Spark data presented at ASH, December 2015. Codon optimization of a B-domain-deleted FVIII gene increased transgene expression in preclinical models As reported at ASH, SPK-FVIII produced the highest levels of expression in preclinical models reported in the literature to date of B-domain-deleted, unmodified FVIII protein Note: Ɛ denotes the development of inhibitors to human FVIII, an anticipated result with this preclinical model.
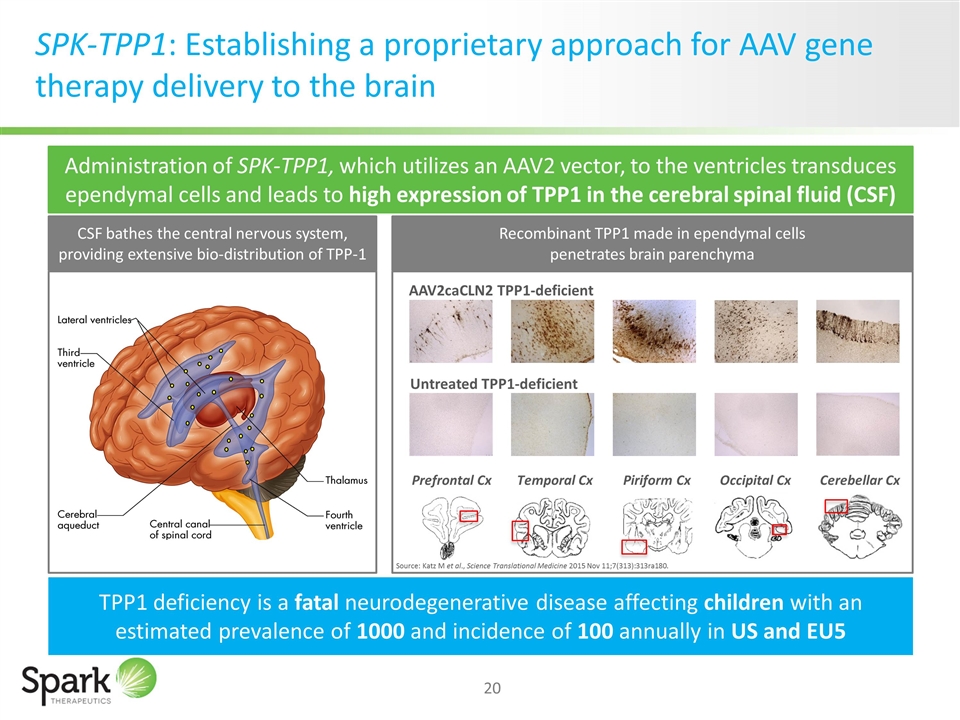
SPK-TPP1: Establishing a proprietary approach for AAV gene therapy delivery to the brain TPP1 deficiency is a fatal neurodegenerative disease affecting children with an estimated prevalence of 1000 and incidence of 100 annually in US and EU5 Source: Katz M et al., Science Translational Medicine 2015 Nov 11;7(313):313ra180. Untreated TPP1-deficient AAV2caCLN2 TPP1-deficient Prefrontal Cx Temporal Cx Piriform Cx Occipital Cx Cerebellar Cx Katz M et al., Science Translational Medicine 2015 Nov 11;7(313):313ra180 Administration of SPK-TPP1, which utilizes an AAV2 vector, to the ventricles transduces ependymal cells and leads to high expression of TPP1 in the cerebral spinal fluid (CSF) CSF bathes the central nervous system, providing extensive bio-distribution of TPP-1 Recombinant TPP1 made in ependymal cells penetrates brain parenchyma
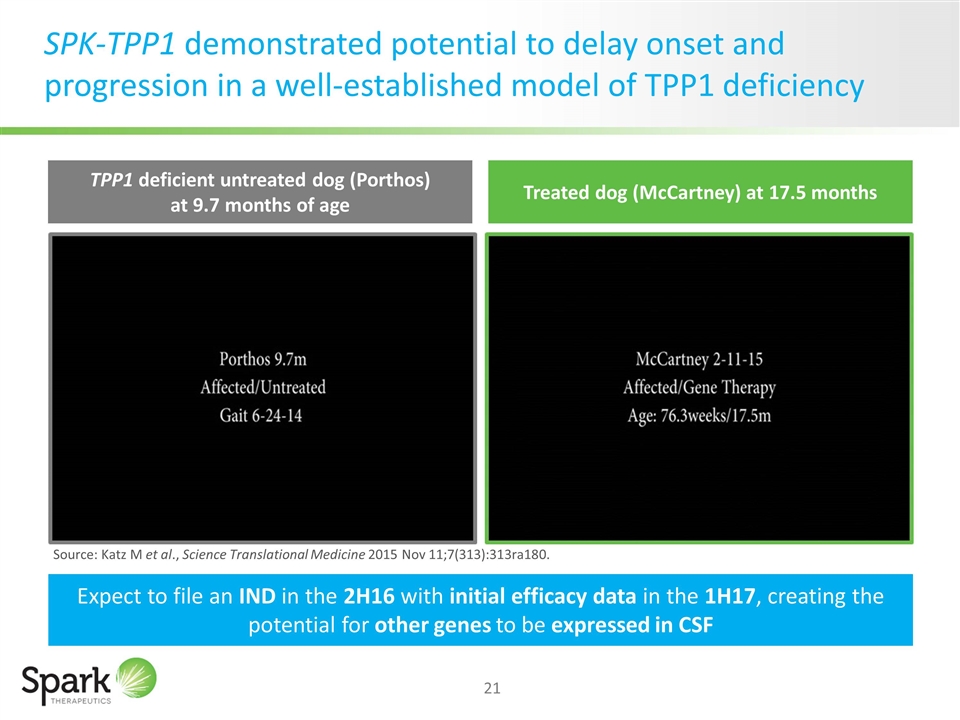
SPK-TPP1 demonstrated potential to delay onset and progression in a well-established model of TPP1 deficiency TPP1 deficient untreated dog (Porthos) at 9.7 months of age Treated dog (McCartney) at 17.5 months Expect to file an IND in the 2H16 with initial efficacy data in the 1H17, creating the potential for other genes to be expressed in CSF Source: Katz M et al., Science Translational Medicine 2015 Nov 11;7(313):313ra180.
A platform capable of generating a number of near-term catalysts
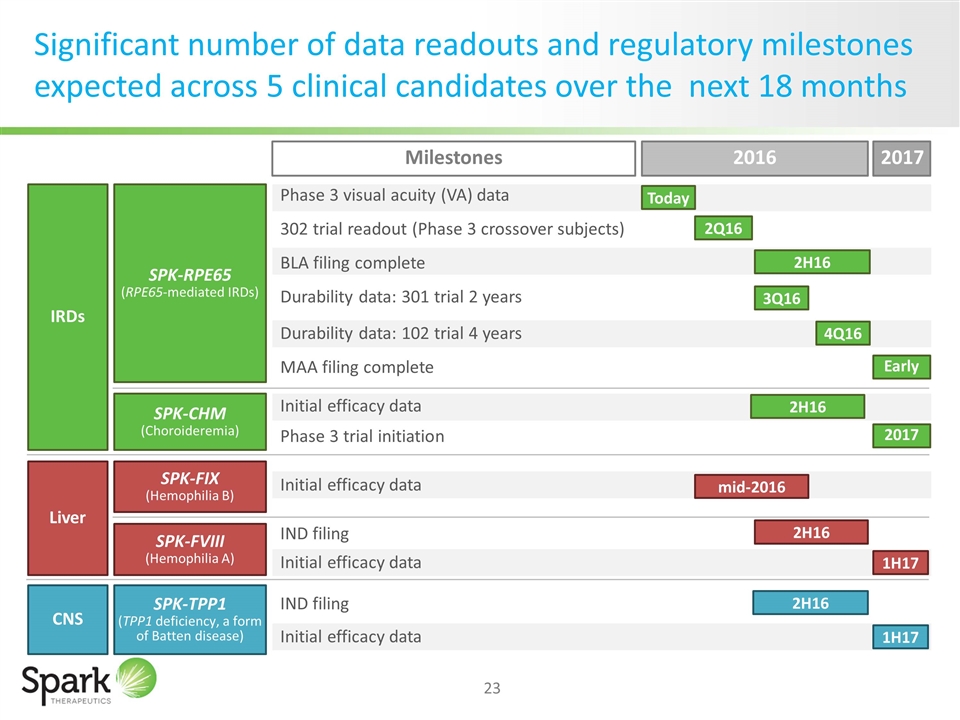
Significant number of data readouts and regulatory milestones expected across 5 clinical candidates over the next 18 months SPK-CHM (Choroideremia) SPK-FIX (Hemophilia B) SPK-FVIII (Hemophilia A) SPK-TPP1 (TPP1 deficiency, a form of Batten disease) Phase 3 visual acuity (VA) data 302 trial readout (Phase 3 crossover subjects) BLA filing complete Durability data: 301 trial 2 years Durability data: 102 trial 4 years MAA filing complete Initial efficacy data Phase 3 trial initiation Initial efficacy data IND filing Initial efficacy data IND filing Initial efficacy data 2016 2017 2Q16 3Q16 2H16 2H16 2017 mid-2016 2H16 1H17 2H16 1H17 Milestones SPK-RPE65 (RPE65-mediated IRDs) IRDs Liver CNS Today 4Q16 Early
