Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Kindred Biosciences, Inc. | form8k-1112016.htm |

1 KindredBio Best Medicines for Our Best Friends

2 Forward Looking Statements This presentation contains forward-looking statements, including but not limited to statements regarding the timing of development for our product candidates, expected commencement and completion of pivotal trials, prospective product candidates, anticipated regulatory approvals for our product candidates, anticipated commercialization of our product candidates, our financial position, business strategy, plans and objectives of management for future operations and other similar statements. These forward-looking statements are based on our current expectations and beliefs, as well as assumptions concerning future events. These statements are subject to risks, uncertainties, and other factors, many of which are outside of our control, that could cause actual results to differ materially from the results discussed in the forward-looking statements, including, but not limited to, our limited operating history and lack of profitability, our lack of product revenue and potential need to raise additional capital to achieve our goals, our dependence upon the success of our lead product candidates, other companies’ ability to develop substantially similar products that may compete with our product candidates, any inability to obtain regulatory approval for our existing or future product candidates, any delay or discontinuance of our current or future pivotal trials, any inability to achieve market acceptance or commercial success for our product candidates even if they are approved, inability to obtain adequate intellectual property protection covering our product candidates, our dependence on third-party manufacturers for supplies, and any inability to successfully identify, develop and commercialize additional product candidates. Any forward-looking statement made by us in this presentation speaks only as of the date of this presentation and represents our estimates and assumptions only as of the date of this presentation. Except as required by law, we assume no obligation to update these statements publicly, or to update the reasons actual results could differ materially from those anticipated in these statements, even if new information becomes available in the future. January 11, 2016

3 We Love Our Pet Family Members $15.25 billion on veterinary care $1.5 billion on dog knee surgeries $370 million on pet Halloween costumes $700 million on Valentine’s Day gifts Annually, U.S. pet parents estimated to spend:

4 Bring the very best science and medicine to our animal family members. KindredBio

5 KindredBio’s Strategy: Repurpose Human Drugs for Pets Pursue molecules already known to work Reduce technical risk Reduce timelines $3M-$5M to develop each pet drug Reduce financing risk Portfolio approach

6 KindredBio Highlights Less than 2 Years to Launch KIND-012 for fever in horses KIND-010 for weight loss in cats 2+ launches per year thereafter Attractive Markets Rapidly growing Few current competitors World Class Team Extensive drug development experience Human and veterinary experience Deep Pipeline ~20 small molecule and biologic candidates Strategic Approach Reduces technical risk Reduces financing risk

7 Richard Chin, M.D. Founder and Chief Executive Officer Former Head of Clinical Research, BioTherapeutics, Genentech Rhodes Scholar World Class Leadership Team 7 Denise Bevers Founder and Chief Operating Officer Founder, SD Scientific; 25 years in biotech/pharma Stephen Sundlof, D.V.M, Ph.D. Chief Scientific Officer and Executive Vice President, Regulatory Affairs & Quality Former Director, Center for Veterinary Medicine, FDA Wendy Wee Vice President, Finance 16 years of biotechnology finance experience Hangjun Zhan, Ph.D. Vice President, Biologics Research 20 years of drug discovery experience

8 Molecule Indication Discovery/Process Development Pilot Studies Pivotal Clinical Studies NADA & Launch Preparation Biologic Product Candidates Feline Epo Anemia in cats Anti-Interleukin Antibodies Atopic dermatitis in dogs Checkpoint Inhibitors Cancer in dogs Anti-CD20 antibody Cancer and autoimmune diseases in dogs KIND-Bodies Multiple indications KIND-502 – Anti-IgE antibody Allergic diseases in dogs KIND-501 – Anti-VEGF antibody Cancer in dogs Molecule Indication Formulation Laboratory Pilot Studies Field Pilot Studies Pivotal Clinical Study NADA & Launch Preparation Small Molecule Product Candidates KIND-010 Management of weight loss in cats KIND-012 Fever in horses KIND-014* Equine gastric ulcer syndrome KIND-015* Metabolic syndrome in horses Deep Product Pipeline * Initial pilot studies completed. Final formulation being developed. Rolling NADA Being Filed

9 Key Strengths Validated, repurposed molecules Avoid risks associated with new chemical entities Reduced costs and timelines, including for API No royalties/milestones Expertise in biologics In-house clinical development capabilities Reduced costs and timelines by not having to rely on CROs Focus on lean cost structure and efficiency Strong cash position

10 Market Opportunity
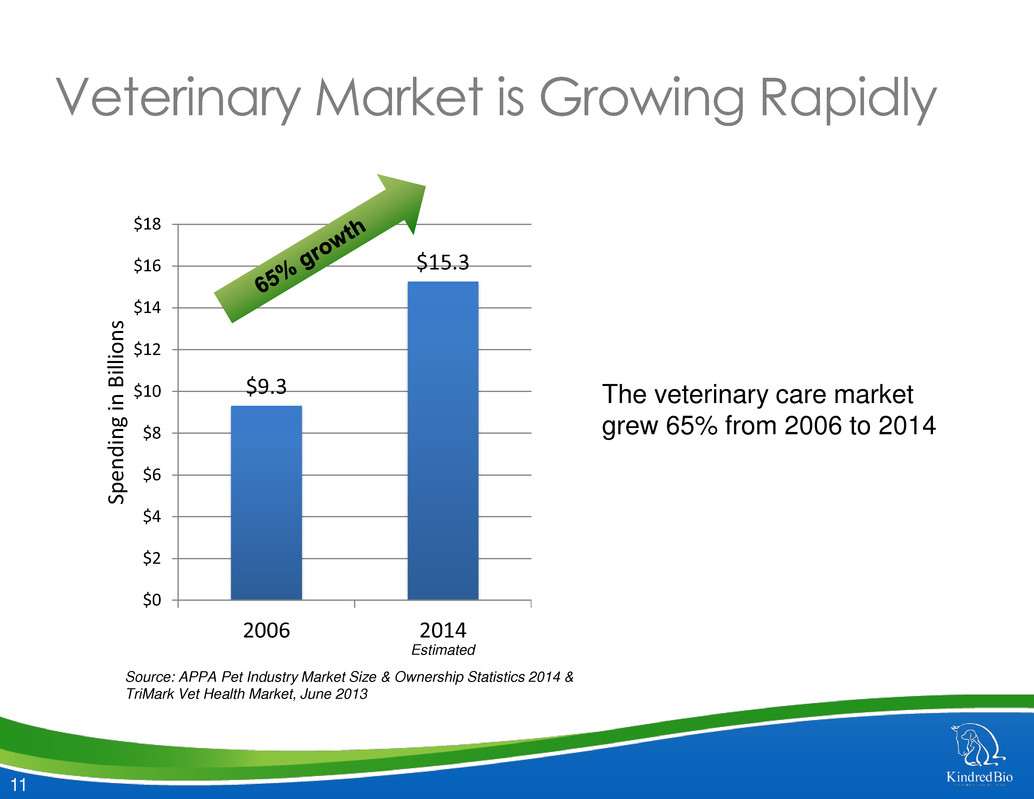
11 $9.3 $15.3 $0 $2 $4 $6 $8 $10 $12 $14 $16 $18 2006 2014 Sp e n d in g in B ill ion s Veterinary Market is Growing Rapidly The veterinary care market grew 65% from 2006 to 2014 Source: APPA Pet Industry Market Size & Ownership Statistics 2014 & TriMark Vet Health Market, June 2013 Estimated
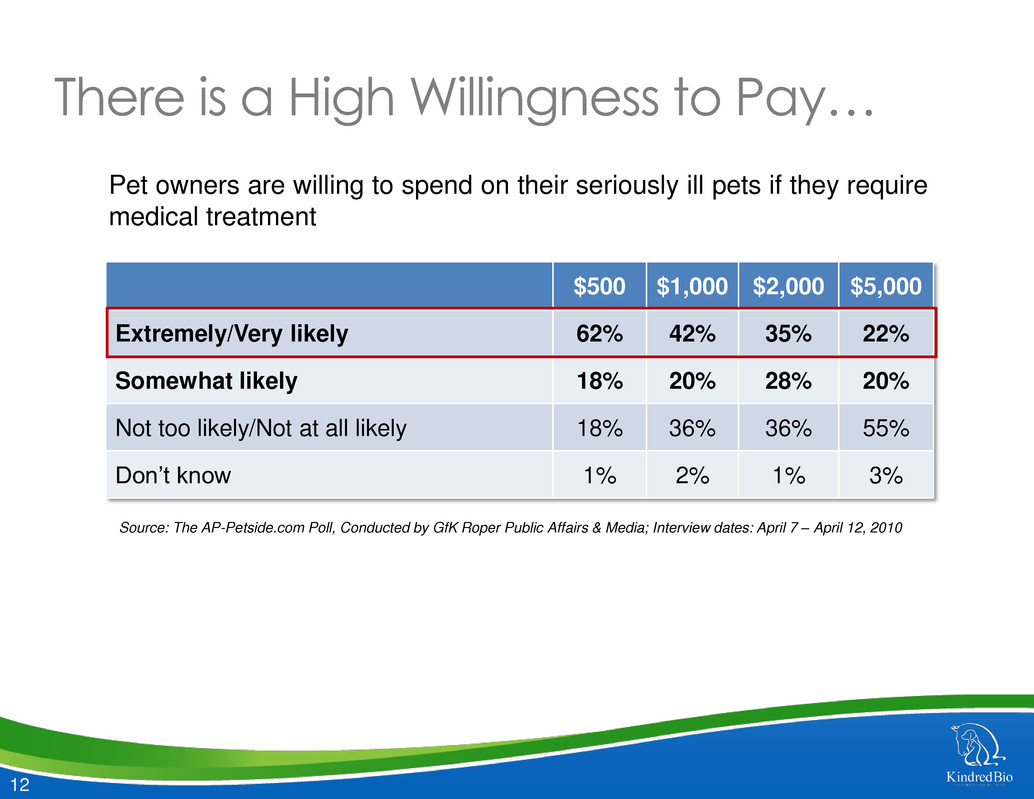
12 There is a High Willingness to Pay… $500 $1,000 $2,000 $5,000 Extremely/Very likely 62% 42% 35% 22% Somewhat likely 18% 20% 28% 20% Not too likely/Not at all likely 18% 36% 36% 55% Don’t know 1% 2% 1% 3% Pet owners are willing to spend on their seriously ill pets if they require medical treatment Source: The AP-Petside.com Poll, Conducted by GfK Roper Public Affairs & Media; Interview dates: April 7 – April 12, 2010

13 …But Treatment Options are Limited Underserved market with attractive growth opportunities Few competing biotechs Large pharma focused on blockbusters On average, less than a dozen pet drugs are approved annually by the FDA In 2014 the FDA approved: 6 pet drugs 41 humans drugs

14 Veterinary Pharmaceuticals Field We believe there are similarities between veterinary pharmaceutical field now and the human pharmaceutical field in its early stages: Similar regulatory standards Similar commercial and reimbursement landscape Similar development costs Numerous untapped opportunities (low hanging fruit) Many years behind Pet Therapeutics Human Pharmaceuticals

15 Veterinary vs. Human Markets Can reach market in 3-5 years Faster Development Lower Development Cost Almost no biotechs, almost no generics Lower Competition Self-Pay Very few reimbursement hurdles Can develop for $3M-$5M per product

16 Programs

17 KindredBio Strategy Already validated in humans & established manufacturing Customize species- specific dosage and formulate flavored/convenient delivery Targets based on approved human drugs (e.g., Enbrel and Orencia) Create canine/ feline/equine versions of biologics with the same or similar target Small Molecules Biologics

18 Equine Products • High willingness to pay • Similar to human orphan drug market • Efficient commercial structure Biologics • Atopic dermatitis • Cancer KindredBio’s Priority Areas

19 Equine Market Fewer horses than cats or dogs, but higher willingness to pay – similar to human orphan drug markets Only 10-12 sales representatives required for full national coverage Underappreciated opportunity KindredBio’s goal is to become dominant company in the equine sector

20 KIND-012 IV and oral drug for treating fever in horses High unmet medical need Over 50% of equine veterinarians treat 1-3 fevers per month (~35% treat 6-10 fevers per month) IV: Positive pilot study completed Positive pivotal field study completed Target Animal Safety Study (TASS) in-life portion completed CMC technical section of NADA to be filed Q4 2015/Q1 2016 Oral: Formulation development ongoing Initial pilot studies underway
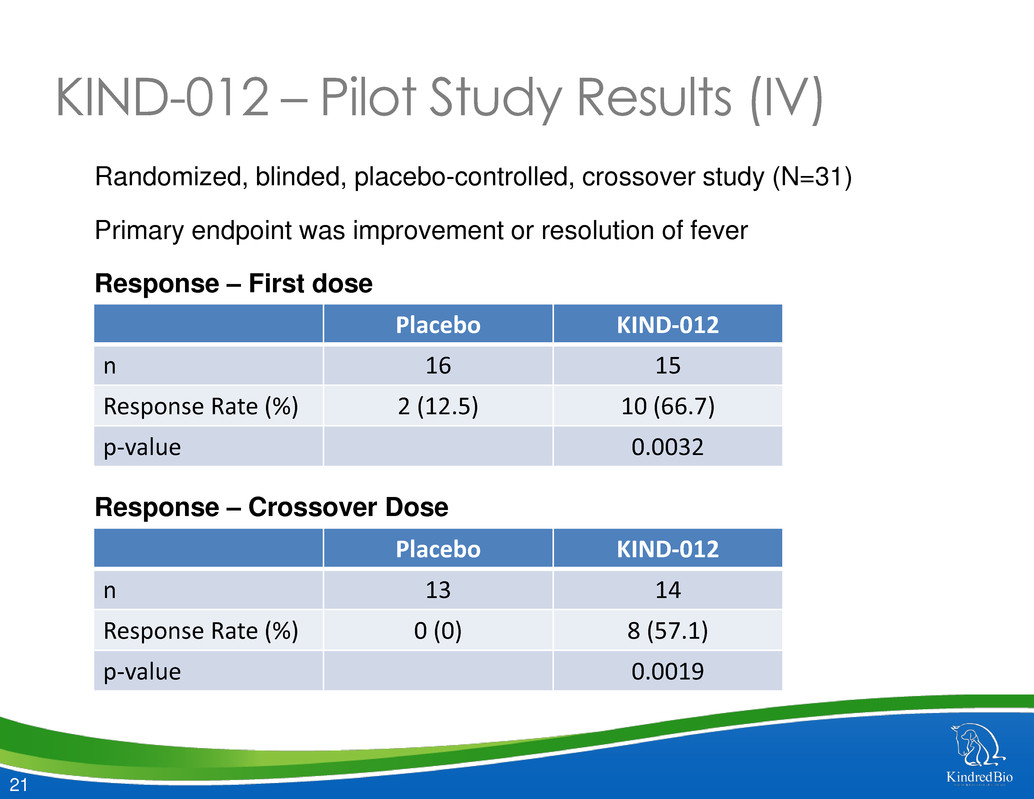
21 KIND-012 – Pilot Study Results (IV) Randomized, blinded, placebo-controlled, crossover study (N=31) Primary endpoint was improvement or resolution of fever Response – First dose Response – Crossover Dose Placebo KIND-012 n 16 15 Response Rate (%) 2 (12.5) 10 (66.7) p-value 0.0032 Placebo KIND-012 n 13 14 Response Rate (%) 0 (0) 8 (57.1) p-value 0.0019

22 KIND-012 – Pilot Study Results (IV) Mean Temp. over Time - First Dose and Crossover Dose (Treated Pts.) First Dose 6 Hours Post First Dose Crossover Dose 6 Hours Post Crossover Dose Te m pe ra tu re (F ) 97 98 99 100 101 102 103 104 105 106 Scheduled Visit (Hours Relative to First Dose) -5 0 5 10 15 20 25 30 35 40 45 50 55 First Treatment Placebo KIND-012

23 KB0120 Pivotal Field Study Results (IV) Randomized, double-blind, placebo-controlled (N=138) Primary endpoint was improvement or resolution of fever Placebo KIND-012 n 34 104 Success Rate (%) 7 (approx. 20%) 78 (approx. 75%) p-value < 0.0001

24 KIND-010 Transdermal drug for the management of weight loss in cats High unmet medical need Current drugs often not effective 90% of veterinarians treat cats with inappetence (average of 7 cats per week) PK studies completed - positive efficacy signal, as evidenced by increase in weight Pilot study completed - positive efficacy signal, as evidenced by increase in weight Pivotal field study underway

25 Biologics Highly experienced biologics team Extensive experience developing Lucentis, Xolair, Tysabri, Avastin, Rituxan, Herceptin, Enbrel, and multiple other biologics Internal caninization/felinization/equinization expertise Promising biologics candidates Feline erythropoietin Checkpoint inhibitors Interleukin antibodies New technologies, including KIND-Bodies

26 New Scaffold Technology: KIND-Body A proprietary, non-antibody scaffold technology being developed by KindredBio Binding affinity similar to antibodies Bispecific binding possible Could allow KindredBio to pursue biologics targets before other parties’ antibody patents expire

27 KIND-510 Feline Erythropoietin Proprietary recombinant feline erythropoietin Strong internal expertise in erythropoietin biology and engineering Initial laboratory study completed - positive efficacy signal, as evidenced by increased reticulocyte formation Pilot field study to begin shortly High unmet medical need Up to 30% of elderly cats (over 15 years) develop kidney failure, leading to anemia Human erythropoietin is immunogenic in cats

28 Key Focus Area: Atopic Dermatitis Atopic dermatitis is a large market Apoquel® projected peak sales >$200M Multiple antibody candidates Anti-IgE antibody Anti-IL17a antibody Anti-IL4R antibody Anti-IL31 antibody Anti-CD20 antibody Expect proof-of-concept data with some of the initial antibody variants potentially as early as this year

29 Key Focus Area: Cancer Multiple antibody candidates Anti-CTLA4 antibody Anti-PD1 antibody Anti-PDL1 antibody Anti-CD20 antibody

30 Strategic Shift Toward Biologics Will increase biologics headcount and increase laboratory space Counterbalanced by headcount reduction in other areas, including small molecule CMC (expect approximately $700K restructuring charge in Q1) Expect annual cash burn to remain at approximately $25M per year

31 Manufacturing Plant In-house biologics manufacturing plant nearly complete Single-use, state of the art system Will allow full GMP commercial production of feline epo Will allow full GMP clinical and early commercial production of antibodies

32 Commercialization

33 Commercialization Equine Commercialization: Launch and commercialize our U.S. products with ~10 person direct sales force Dog/Cat Commercialization Options: Self-Commercialization Out-license Partner for 3 – 5 years, and then transition to KindredBio salesforce

34 Few pet generic companies No automatic substitution Requires sales & marketing effort Rimadyl reached peak sales several years after loss of exclusivity Low Generic Penetration Note: Companion Animal dispensing rate within the veterinary clinic. Source: IMS Health, Putney, BofA Merril Lynch Global Research 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% Companion Animal Human 7% 81% G e n e ri c d is p e n s in g r a te s

35 Exclusivity and IP Position Full intellectual property protection anticipated for antibody portfolio Use and formulation patents for small molecules 20 years of patent protection from date of filing Regulatory Exclusivity 5 years in U.S. 10 years in E.U. Lifecycle Management New formulations, combinations and derivatives

36 Business Development In active discussions about acquisitions of businesses and/or assets, particularly of equine assets Ideal candidate: Revenue generating/accretive Commercial infrastructure Complementary assets

37 Finances

38 Select Summary Financials $ millions For the quarter ended September 30, 2015 Operating expenses: Research and development $5.0 General and administrative $2.1 Total cash operating expenses (excluding stock-based compensation) $6.1 Total operating expenses (including stock-based compensation) $7.1 Total cash and short-term investments (As of September 30, 2015) $83.6 We believe that our cash and equivalents are sufficient to fund operations until we start generating significant revenues.

39 Summary Validated Drugs and Targets $3M-$5M to Market Multiple Approvals Starting within 2 years World Class Team

40 Thank You
