Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Juno Therapeutics, Inc. | d107426d8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Juno Therapeutics, Inc. | d107426dex991.htm |
| EX-2.1 - EXHIBIT 2.1 - Juno Therapeutics, Inc. | d107426dex21.htm |

Exhibit 99.2

Forward-looking statements This presentation and the accompanying oral commentary contain forward-looking statements that involve risks, uncertainties, and assumptions. If the risks or uncertainties ever materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such forward-looking statements. All statements other than statements of historical fact could be deemed forward-looking, including, but not limited to, any expectations regarding investment returns; any projections of financial information; any statements about historical results that may suggest trends for our business; any statements of the plans, strategies, and objectives of management for future operations, including our manufacturing and process development; any statements of expectation or belief regarding future events, potential markets or market size, technology developments, our product pipeline, clinical data, enforceability of our intellectual property rights, competitive strengths or our position within the industry; any statements regarding the anticipated benefits of our Celgene collaboration or other strategic transactions; and any statements of assumptions underlying any of the items mentioned. These statements are based on estimates and information available to us at the time of this presentation and are not guarantees of future performance. Actual results could differ materially from our current expectations as a result of many risks and uncertainties, including but not limited to, risks associated with: the success, cost, and timing of our product development activities and clinical trials; our ability to obtain regulatory approval for and to commercialize our product candidates; our ability to establish a commercially-viable manufacturing process and manufacturing infrastructure; regulatory requirements and regulatory developments; the effects of competition and technological advances; our dependence on third-party collaborators and other contractors in our research and development activities, including for the conduct of clinical trials and the manufacture of our product candidates; our dependence on Celgene for the development and commercialization outside of North America of product candidates for which Celgene exercises an option; our ability to obtain, maintain, or protect intellectual property rights related to our product candidates; among others. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 12, 2015 and our other periodic reports filed from time to time with the Securities and Exchange Commission. Except as required by law, we assume no obligation and do not intend to update these forward-looking statements or to conform these statements to actual results or to changes in our expectations.

Building the leading T cell company Significant advances in CD19-directed product candidates with a go-to-market strategy defined in ALL and NHL Progress with best-in-class product candidates and platform Broad program in solid organ tumors in significant areas of unmet need Building platform and self-sustaining research capability to support leadership in the engineered T cell space With Celgene, global manufacturing, development, and commercial capabilities to bring engineered T cells to market globally
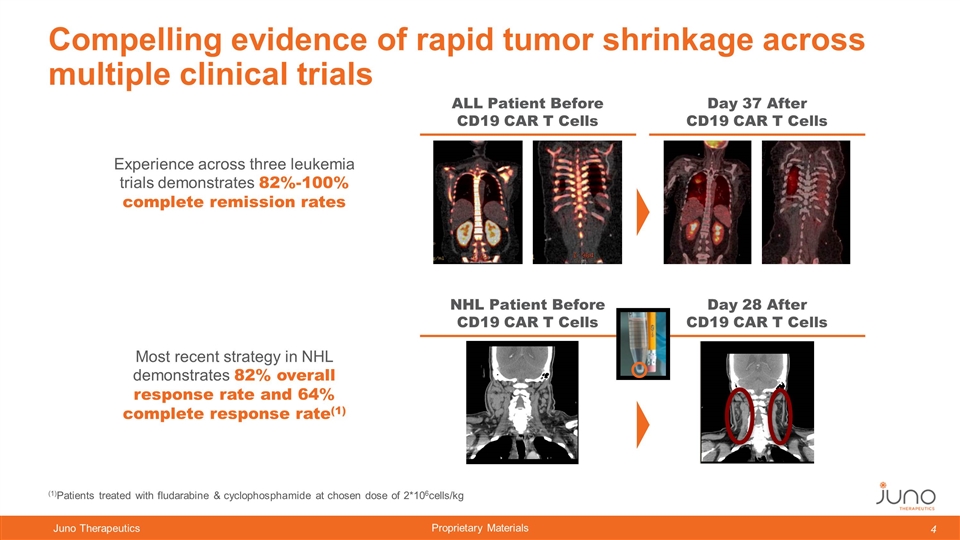
Compelling evidence of rapid tumor shrinkage across multiple clinical trials Experience across three leukemia trials demonstrates 82%-100% complete remission rates Most recent strategy in NHL demonstrates 82% overall response rate and 64% complete response rate(1) Day 37 After CD19 CAR T Cells ALL Patient Before CD19 CAR T Cells Day 28 After CD19 CAR T Cells NHL Patient Before CD19 CAR T Cells (1)Patients treated with fludarabine & cyclophosphamide at chosen dose of 2*106cells/kg

Significant progress with CD19-directed portfolio The Phase II ROCKET trial for JCAR015 is enrolling in adult relapsed or refractory ALL Multi-center Phase I JCAR017 trial in adult relapsed or refractory NHL is enrolling CLL is emerging as a potential near-term target market Manufacturing facility completing validation runs with expected supply online in 1Q16 Substantial progress across a number of trials in 2016 to further best-in-class strategy
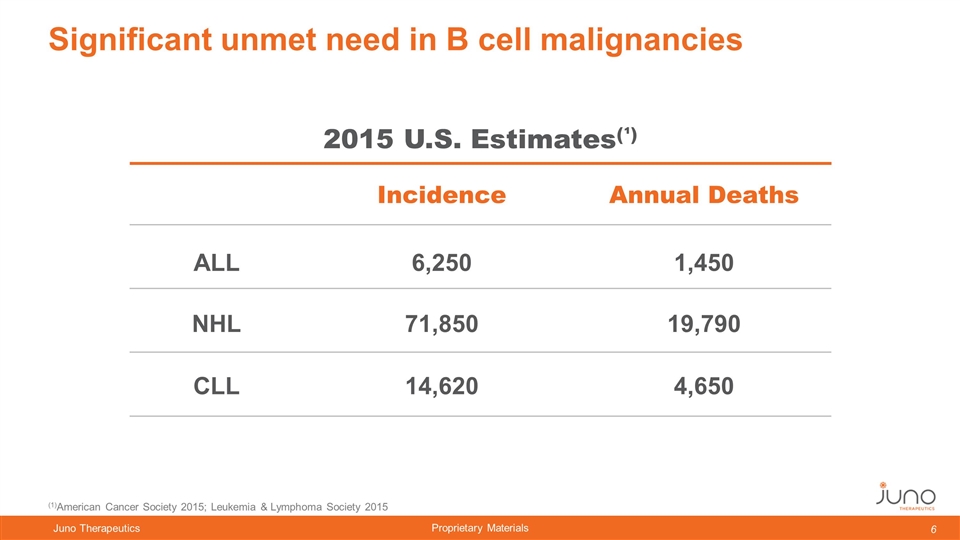
Significant unmet need in B cell malignancies 2015 U.S. Estimates(¹) Incidence Annual Deaths ALL NHL CLL 6,250 71,850 14,620 1,450 19,790 4,650 (1)American Cancer Society 2015; Leukemia & Lymphoma Society 2015
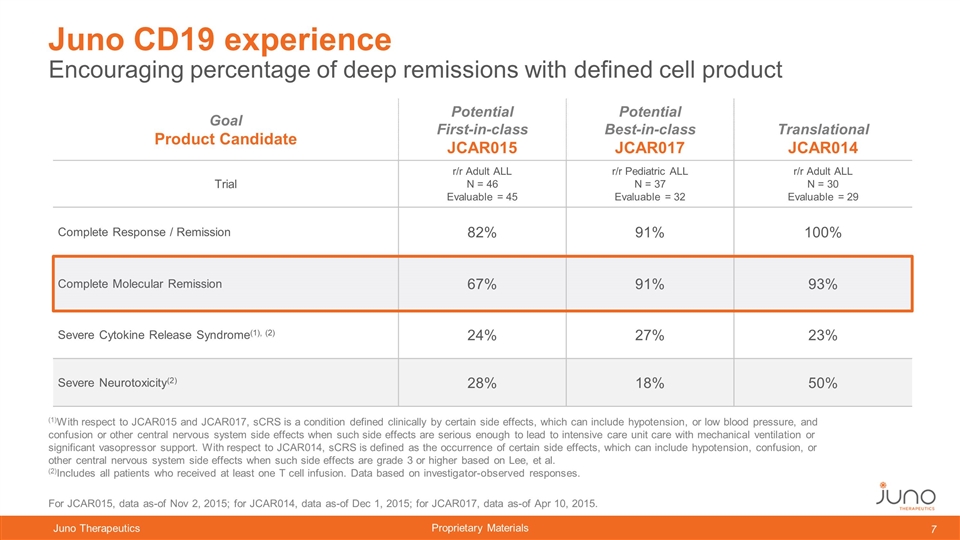
Encouraging percentage of deep remissions with defined cell product Juno CD19 experience Goal Product Candidate Potential First-in-class JCAR015 Potential Best-in-class JCAR017 Translational JCAR014 Trial r/r Adult ALL N = 46 Evaluable = 45 r/r Pediatric ALL N = 37 Evaluable = 32 r/r Adult ALL N = 30 Evaluable = 29 Complete Response / Remission 82% 91% 100% Complete Molecular Remission 67% 91% 93% Severe Cytokine Release Syndrome(1), (2) 24% 27% 23% Severe Neurotoxicity(2) 28% 18% 50% (1)With respect to JCAR015 and JCAR017, sCRS is a condition defined clinically by certain side effects, which can include hypotension, or low blood pressure, and confusion or other central nervous system side effects when such side effects are serious enough to lead to intensive care unit care with mechanical ventilation or significant vasopressor support. With respect to JCAR014, sCRS is defined as the occurrence of certain side effects, which can include hypotension, confusion, or other central nervous system side effects when such side effects are grade 3 or higher based on Lee, et al. (2)Includes all patients who received at least one T cell infusion. Data based on investigator-observed responses. For JCAR015, data as-of Nov 2, 2015; for JCAR014, data as-of Dec 1, 2015; for JCAR017, data as-of Apr 10, 2015.
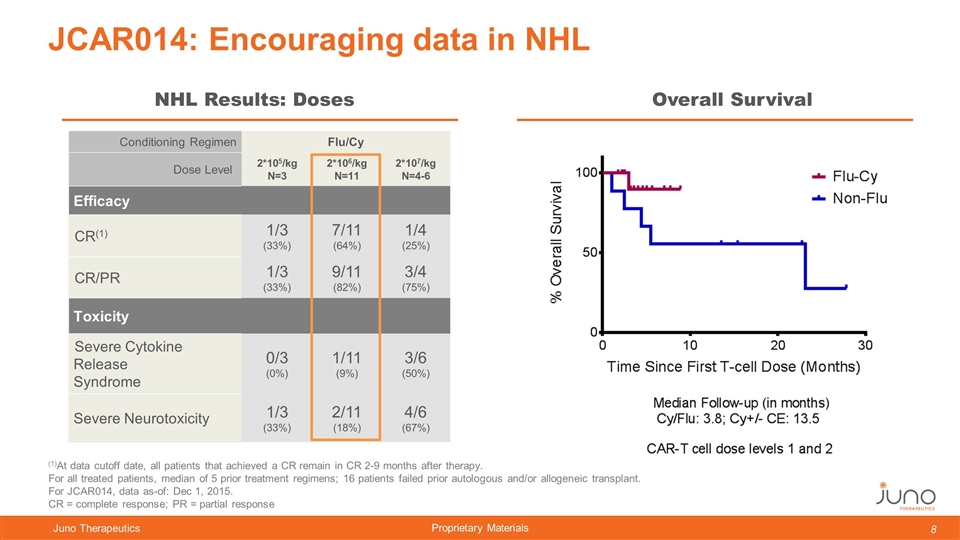
Conditioning Regimen Flu/Cy Dose Level 2*105/kg N=3 2*106/kg N=11 2*107/kg N=4-6 Efficacy CR(1) 1/3 (33%) 7/11 (64%) 1/4 (25%) CR/PR 1/3 (33%) 9/11 (82%) 3/4 (75%) Toxicity Severe Cytokine Release Syndrome 0/3 (0%) 1/11 (9%) 3/6 (50%) Severe Neurotoxicity 1/3 (33%) 2/11 (18%) 4/6 (67%) JCAR014: Encouraging data in NHL NHL Results: Doses Overall Survival (1)At data cutoff date, all patients that achieved a CR remain in CR 2-9 months after therapy. For all treated patients, median of 5 prior treatment regimens; 16 patients failed prior autologous and/or allogeneic transplant. For JCAR014, data as-of: Dec 1, 2015. CR = complete response; PR = partial response
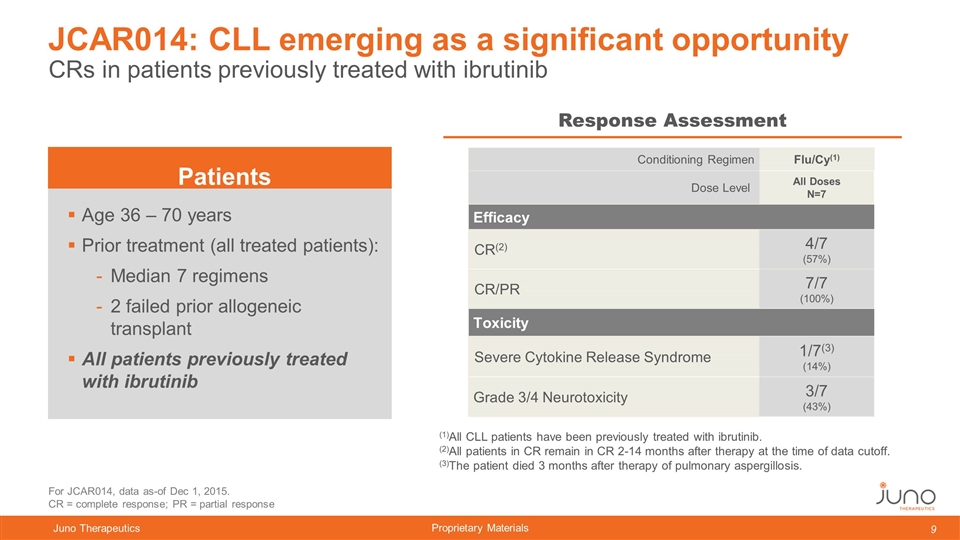
CRs in patients previously treated with ibrutinib JCAR014: CLL emerging as a significant opportunity Response Assessment Age 36 – 70 years Prior treatment (all treated patients): Median 7 regimens 2 failed prior allogeneic transplant All patients previously treated with ibrutinib (1)All CLL patients have been previously treated with ibrutinib. (2)All patients in CR remain in CR 2-14 months after therapy at the time of data cutoff. (3)The patient died 3 months after therapy of pulmonary aspergillosis. Patients For JCAR014, data as-of Dec 1, 2015. CR = complete response; PR = partial response Conditioning Regimen Flu/Cy(1) Dose Level All Doses N=7 Efficacy CR(2) 4/7 (57%) CR/PR 7/7 (100%) Toxicity Severe Cytokine Release Syndrome 1/7(3) (14%) Grade 3/4 Neurotoxicity 3/7 (43%)
Best-in-class strategy Multiple trials to generate translational insights Advanced manufacturing capabilities with leading cell selection reagents to control the T cell population Multiple strategies for optimizing cell signaling, including: co-stimulatory domains armored CARs gene editing small molecule transcriptional modulators Clinical combinations including checkpoint inhibitors and others
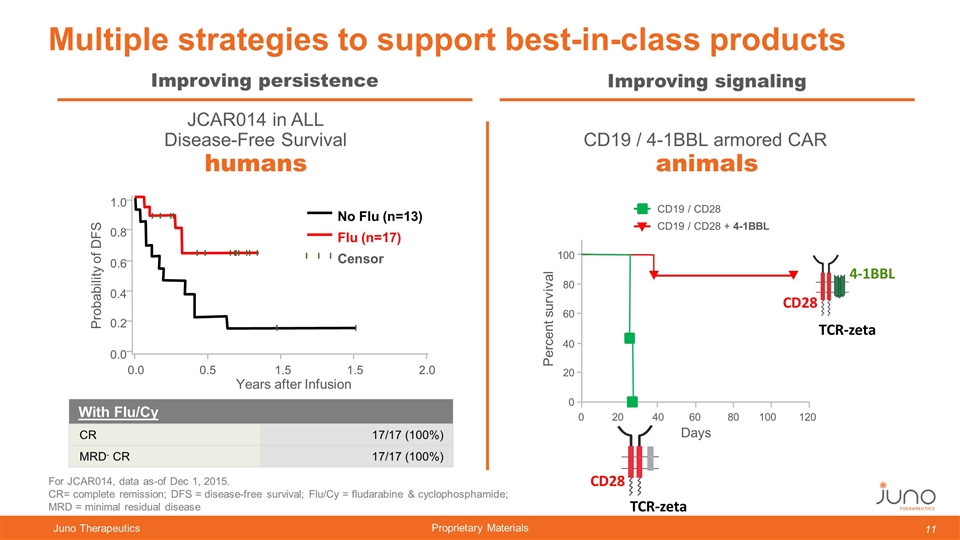
Multiple strategies to support best-in-class products JCAR014 in ALL Disease-Free Survival animals humans Improving persistence Improving signaling CD19 / 4-1BBL armored CAR Years after Infusion Probability of DFS 0.0 0.5 1.5 1.5 2.0 0.0 0.2 0.4 0.6 0.8 1.0 No Flu (n=13) Flu (n=17) Censor For JCAR014, data as-of Dec 1, 2015. CR= complete remission; DFS = disease-free survival; Flu/Cy = fludarabine & cyclophosphamide; MRD = minimal residual disease With Flu/Cy CR 17/17 (100%) MRD- CR 17/17 (100%) 100 80 60 40 20 0 0 20 40 60 80 100 120 Days CD19 / CD28 CD19 / CD28 + 4-1BBL Percent survival CD28 TCR-zeta CD28 TCR-zeta 4-1BBL
Increasing selection pressure with goal to reduce relapse rate JCAR018: Another important target in B cell malignancies The two mechanisms for relapse with CD19 CAR T cells are loss of CAR T cells and loss of CD19 CD22-directed CAR addresses CD19 epitope loss CD22 has the potential to be used alone or in combination with CD19 Early data are encouraging and the Phase I dose-escalation study is ongoing no dose-limiting toxicities 2 of 7 patients with complete response
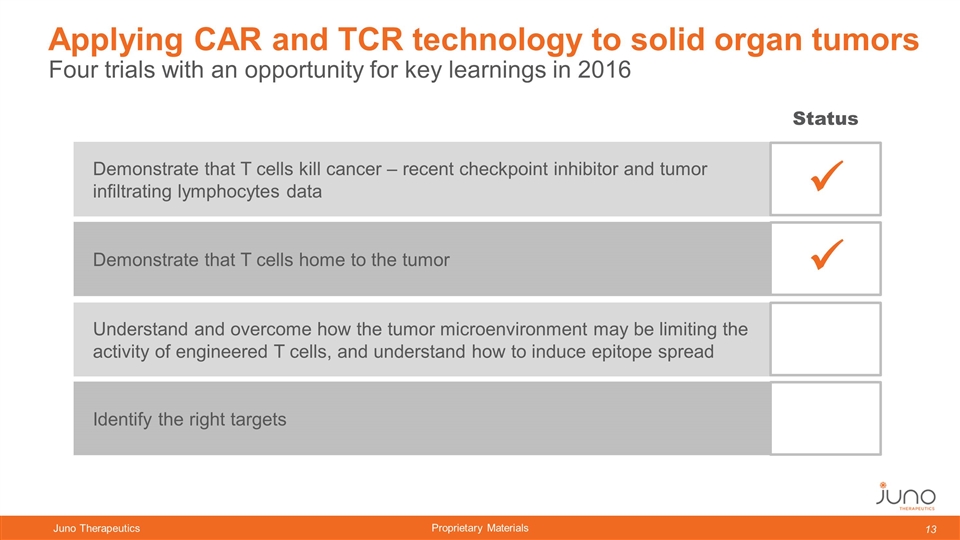
Four trials with an opportunity for key learnings in 2016 Applying CAR and TCR technology to solid organ tumors Demonstrate that T cells kill cancer – recent checkpoint inhibitor and tumor infiltrating lymphocytes data Demonstrate that T cells home to the tumor Understand and overcome how the tumor microenvironment may be limiting the activity of engineered T cells, and understand how to induce epitope spread Identify the right targets ü ü Status ü

Active and planned programs in clinical development The first set of solid tumor targets WT-1 (TCR) AML / NSCLC 15 patients with relapsed / high-risk AML treated with TCR product candidate after HSCT Generally well tolerated; demonstrated safety, persistence and encouraging clinical activity Ongoing studies in AML and NSCLC L1CAM (CAR) Neuroblastoma Overexpressed in neuroblastoma and a variety of solid tumors Preliminary pre-clinical data show encouraging safety profile ROR-1 (CAR) ROR-1 Expressed Tumors Overexpressed on a wide variety of cancers including NSCLC and B cell CLL Preliminary pre-clinical data show encouraging safety profile MUC-16 / IL-12 “Armored CAR” Ovarian First “Armored” CAR to advance into clinical testing MUC-16 is a protein cleaved to make CA125; overexpressed in the majority of ovarian cancers IL-12 is a pluripotent immune stimulator Multiple in-house and partner pre-clinical programs
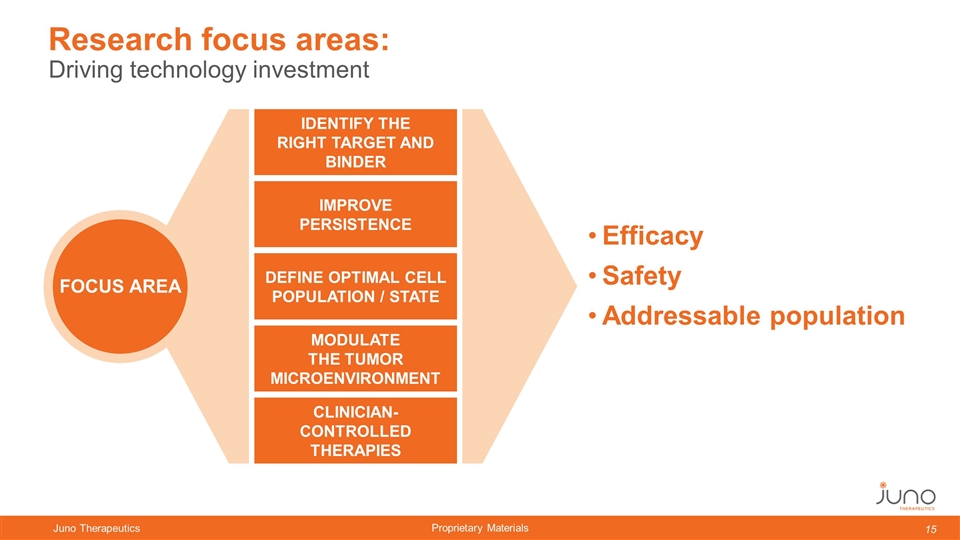
Driving technology investment Research focus areas: IDENTIFY THE RIGHT TARGET AND BINDER IMPROVE PERSISTENCE DEFINE OPTIMAL CELL POPULATION / STATE MODULATE THE TUMOR MICROENVIRONMENT CLINICIAN-CONTROLLED THERAPIES FOCUS AREA Efficacy Safety Addressable population
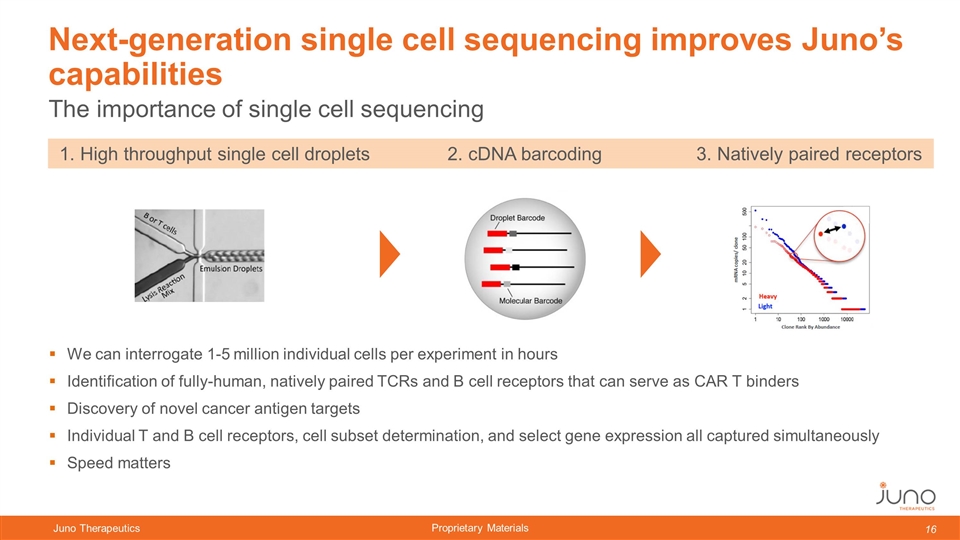
The importance of single cell sequencing Next-generation single cell sequencing improves Juno’s capabilities We can interrogate 1-5 million individual cells per experiment in hours Identification of fully-human, natively paired TCRs and B cell receptors that can serve as CAR T binders Discovery of novel cancer antigen targets Individual T and B cell receptors, cell subset determination, and select gene expression all captured simultaneously Speed matters 1. High throughput single cell droplets 2. cDNA barcoding 3. Natively paired receptors

Accelerating Juno’s pipeline Generate fully human TCRs Full sequence, fully-human, thymus-screened, α/β paired TCRs for our engineered T cell products Simultaneously capture TCRs restricted by multiple HLA-alleles (“moving beyond HLA-A2”) Identify novel targets Find cancer antigen tissue through interrogation of the immune repertoire Already discovered several novel targets with associated high quality binders Generate antibody/ScFv binders Identify and sequence high affinity, full natural, VH & VL paired antibodies including those existing inside tumors Natural screening for off-target toxicity Accelerate translational insights Apply to both pre-clinical and clinical development Interrogate immune repertoire and limited gene expression profile of individual cells (“form + function”)
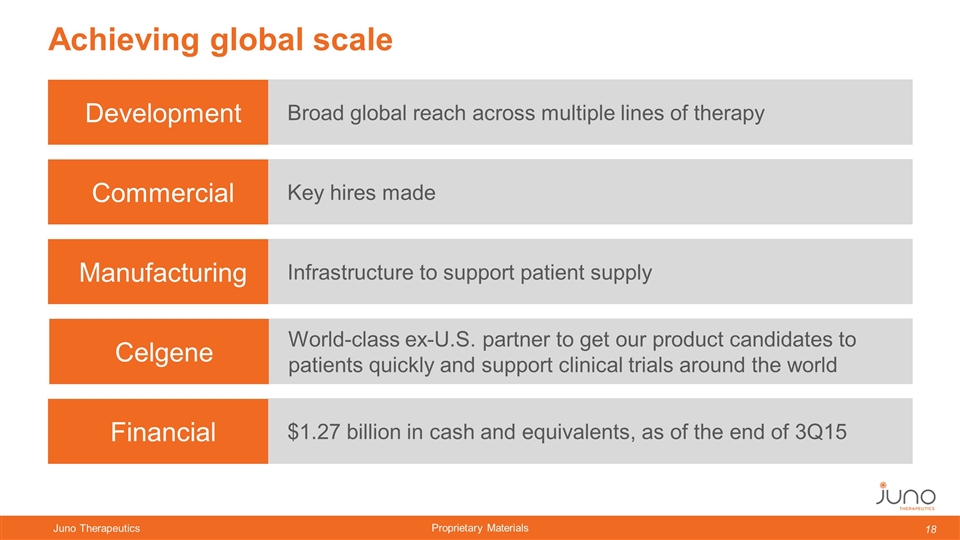
Achieving global scale Commercial Key hires made Manufacturing Infrastructure to support patient supply Celgene World-class ex-U.S. partner to get our product candidates to patients quickly and support clinical trials around the world Development Broad global reach across multiple lines of therapy Financial $1.27 billion in cash and equivalents, as of the end of 3Q15
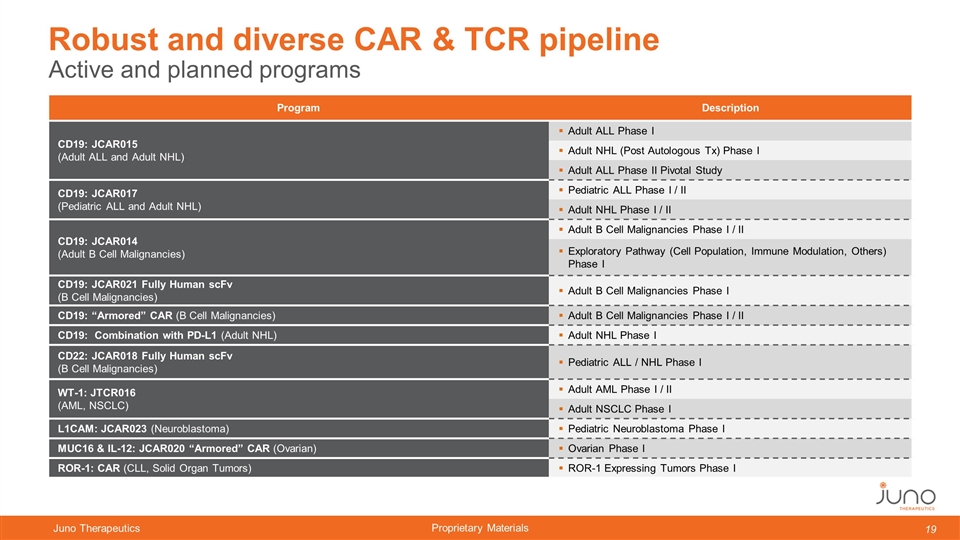
Program Description CD19: JCAR015 (Adult ALL and Adult NHL) Adult ALL Phase I Adult NHL (Post Autologous Tx) Phase I Adult ALL Phase II Pivotal Study CD19: JCAR017 (Pediatric ALL and Adult NHL) Pediatric ALL Phase I / II Adult NHL Phase I / II CD19: JCAR014 (Adult B Cell Malignancies) Adult B Cell Malignancies Phase I / II Exploratory Pathway (Cell Population, Immune Modulation, Others) Phase I CD19: JCAR021 Fully Human scFv (B Cell Malignancies) Adult B Cell Malignancies Phase I CD19: “Armored” CAR (B Cell Malignancies) Adult B Cell Malignancies Phase I / II CD19: Combination with PD-L1 (Adult NHL) Adult NHL Phase I CD22: JCAR018 Fully Human scFv (B Cell Malignancies) Pediatric ALL / NHL Phase I WT-1: JTCR016 (AML, NSCLC) Adult AML Phase I / II Adult NSCLC Phase I L1CAM: JCAR023 (Neuroblastoma) Pediatric Neuroblastoma Phase I MUC16 & IL-12: JCAR020 “Armored” CAR (Ovarian) Ovarian Phase I ROR-1: CAR (CLL, Solid Organ Tumors) ROR-1 Expressing Tumors Phase I Active and planned programs Robust and diverse CAR & TCR pipeline
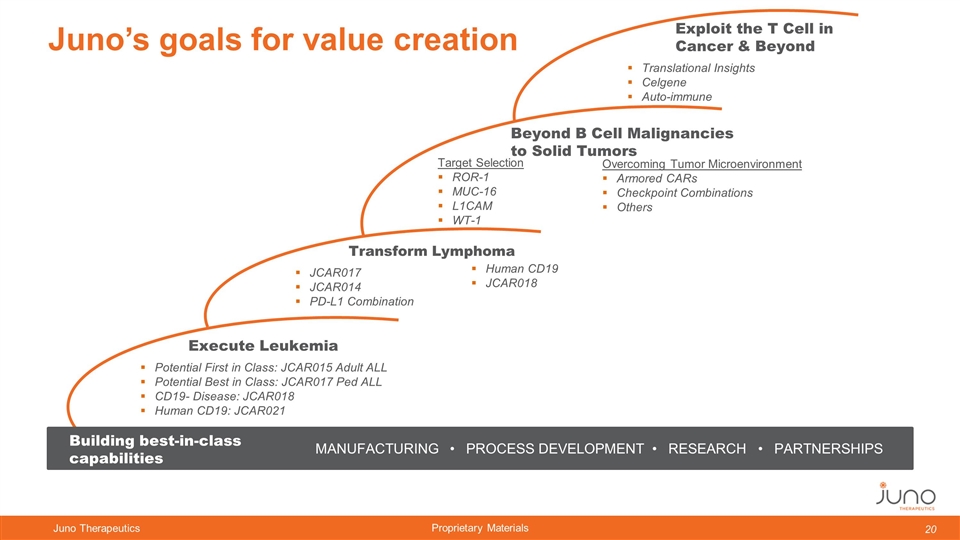
Juno’s goals for value creation Execute Leukemia Potential First in Class: JCAR015 Adult ALL Potential Best in Class: JCAR017 Ped ALL CD19- Disease: JCAR018 Human CD19: JCAR021 Transform Lymphoma JCAR017 JCAR014 PD-L1 Combination Beyond B Cell Malignancies to Solid Tumors Target Selection ROR-1 MUC-16 L1CAM WT-1 Exploit the T Cell in Cancer & Beyond Translational Insights Celgene Auto-immune MANUFACTURING • PROCESS DEVELOPMENT • RESEARCH • PARTNERSHIPS Building best-in-class capabilities Human CD19 JCAR018 Overcoming Tumor Microenvironment Armored CARs Checkpoint Combinations Others
